Organoids: Difference between revisions
(Created page with "{{Header}} == Introduction == thumb|Human Blastocyst (Carnegie Stage 3) Organoids stem cell derived self-organized three-dimensional ''in vitro'' tissue cul...") |
mNo edit summary |
||
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Header}} | {{Header}} | ||
== Introduction == | == Introduction == | ||
[[ | [[File:Organoids overview 01.jpg|thumb|alt=Organoids overview|Organoids overview{{#pmid:30219074|PMID30219074}}]] | ||
Organoids stem cell derived self-organized three-dimensional ''in vitro'' tissue cultures. These cultures have been be manipulated to replicate some of the complexity of an ''in vivo'' organ, or to produce only certain types of organ related cells. | |||
Organoids stem cell derived self-organized three-dimensional ''in vitro'' tissue cultures. These cultures have been be manipulated to replicate some of the complexity of an ''in vivo'' organ, or to produce only certain types of organ related cells. This stem cell research technique has many potential applications in understanding tissue and organ development, clinical therapeutics and cancer medicine. | |||
{{Stem Cell Links}} | {{Stem Cell Links}} | ||
==Some Recent Findings== | ==Some Recent Findings== | ||
{| | {| | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* ''' | * '''Review - Organoids-on-a-chip'''{{#pmid:31171693|PMID31171693}} "Recent studies have demonstrated an array of stem cell-derived, self-organizing miniature organs, termed organoids, that replicate the key structural and functional characteristics of their in vivo counterparts. As organoid technology opens up new frontiers of research in biomedicine, there is an emerging need for innovative engineering approaches for the production, control, and analysis of organoids and their microenvironment. In this Review, we explore organ-on-a-chip technology as a platform to fulfill this need and examine how this technology may be leveraged to address major technical challenges in organoid research. We also discuss emerging opportunities and future obstacles for the development and application of organoid-on-a-chip technology." | ||
* '''Review - Organoids by design'''{{#pmid:31171692|PMID31171692}} "Organoids are multicellular structures that can be derived from adult organs or pluripotent stem cells. Early versions of organoids range from simple epithelial structures to complex, disorganized tissues with large cellular diversity. The current challenge is to engineer cellular complexity into organoids in a controlled manner that results in organized assembly and acquisition of tissue function. These efforts have relied on studies of organ assembly during embryonic development and have resulted in the development of organoids with multilayer tissue complexity and higher-order functions. We discuss how the next generation of organoids can be designed by means of an engineering-based narrative design to control patterning, assembly, morphogenesis, growth, and function." | |||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 20: | Line 22: | ||
| [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | | [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | ||
Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Organoids ''Organoids''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Organoid Culture ''Organoid Culture''] | Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Organoids ''Organoids''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Organoid+ Culture ''Organoid Culture''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Kidney+Organoids ''Kidney Organoids''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Pancreas+Organoids ''Pancreas Organoids''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Neural+Organoids ''Neural Organoids''] | ||
Search: [https://journals.plos.org/plosone/browse/organoids PLOS] | |||
|} | |} | ||
| Line 27: | Line 31: | ||
|- | |- | ||
| | | | ||
* '''The use of brain organoids to investigate neural development and disease'''{{#pmid:28878372|PMID28878372}} "Understanding the development and dysfunction of the human brain is a major goal of neurobiology. Much of our current understanding of human brain development has been derived from the examination of post-mortem and pathological specimens, bolstered by observations of developing non-human primates and experimental studies focused largely on mouse models. However, these tissue specimens and model systems cannot fully capture the unique and dynamic features of human brain development. Recent advances in stem cell technologies that enable the generation of human brain organoids from pluripotent stem cells (PSCs) promise to profoundly change our understanding of the development of the human brain and enable a detailed study of the pathogenesis of inherited and acquired brain diseases." | |||
* '''Kidney Organoids: A Translational Journey'''{{#pmid:28188103|PMID28188103}} "Human pluripotent stem cells (hPSCs) are attractive sources for regenerative medicine and disease modeling in vitro. Directed hPSC differentiation approaches have derived from knowledge of cell development in vivo rather than from stochastic cell differentiation. Moreover, there has been great success in the generation of 3D organ-buds termed 'organoids' from hPSCs; these consist of a variety of cell types in vitro that mimic organs in vivo. The organoid bears great potential in the study of human diseases in vitro, especially when combined with CRISPR/Cas9-based genome-editing. We summarize the current literature describing organoid studies with a special focus on kidney organoids, and discuss goals and future opportunities for organoid-based studies." | |||
* '''Modeling Development and Disease with Organoids'''{{#pmid:27315476|PMID27315476}} "Recent advances in 3D culture technology allow embryonic and adult mammalian stem cells to exhibit their remarkable self-organizing properties, and the resulting organoids reflect key structural and functional properties of organs such as kidney, lung, gut, brain and retina. Organoid technology can therefore be used to model human organ development and various human pathologies 'in a dish." Additionally, patient-derived organoids hold promise to predict drug response in a personalized fashion. Organoids open up new avenues for regenerative medicine and, in combination with editing technology, for gene therapy. The many potential applications of this technology are only beginning to be explored." | |||
|} | |} | ||
==Inner Ear Organoids== | |||
[[File:Organoids - inner ear 01.jpg|thumb|alt=CHIR-treated aggregates give rise to inner ear organoids harboring mechanosensitive hair cells|CHIR-treated aggregates give rise to inner ear organoids harboring mechanosensitive hair cells{{#pmid:27607106|PMID27607106}}]] | |||
'''Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture'''{{#pmid:27607106|PMID27607106}} | |||
:"Stem cell-derived inner ear sensory epithelia are a promising source of tissues for treating patients with hearing loss and dizziness. We recently demonstrated how to generate inner ear sensory epithelia, designated as {{inner ear}} organoids, from {{mouse}} embryonic stem cells (ESCs) in a self-organizing 3D culture. Here we improve the efficiency of this culture system by elucidating how Wnt signaling activity can drive the induction of otic tissue. We found that a carefully timed treatment with the potent {{Wnt}} agonist CHIR99021 promotes induction of otic vesicles-a process that was previously self-organized by unknown mechanisms. The resulting otic-like vesicles have a larger lumen size and contain a greater number of Pax8/Pax2-positive otic progenitor cells than organoids derived without the Wnt agonist. Additionally, these otic-like vesicles give rise to large inner ear organoids with hair cells whose morphological, biochemical and functional properties are indistinguishable from those of vestibular hair cells in the postnatal mouse inner ear. We conclude that Wnt signaling plays a similar role during inner ear organoid formation as it does during inner ear development in the embryo." | |||
{{inner ear}} | |||
==Kidney Organoids== | |||
[[File:Organoids - renal glomerulus.jpg|thumb|alt=Renal glomerulus orgaonids|Renal glomerulus orgaonids{{#pmid:30048451|PMID30048451}}]] | |||
'''Kidney Organoids: A Translational Journey'''{{#pmid:28188103|PMID28188103}} | |||
:"Human pluripotent stem cells (hPSCs) are attractive sources for regenerative medicine and disease modeling in vitro. Directed hPSC differentiation approaches have derived from knowledge of cell development in vivo rather than from stochastic cell differentiation. Moreover, there has been great success in the generation of 3D organ-buds termed 'organoids' from hPSCs; these consist of a variety of cell types in vitro that mimic organs in vivo. The organoid bears great potential in the study of human diseases in vitro, especially when combined with CRISPR/Cas9-based genome-editing. We summarize the current literature describing organoid studies with a special focus on kidney organoids, and discuss goals and future opportunities for organoid-based studies." | |||
{{renal}} | |||
==Liver Organoids== | |||
{{#pmid:31300517}} | |||
{{#pmid:30948332}} | |||
{{liver}} | |||
==Pancreas Organoids== | |||
'''Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation.'''{{#pmid:27560176|PMID27560176}} | |||
:"Adult somatic tissues have proven difficult to expand in vitro, largely because of the complexity of recreating appropriate environmental signals in culture. We have overcome this problem recently and developed culture conditions for adult stem cells that allow the long-term expansion of adult primary tissues from small {{intestine}}, {{stomach}}, {{liver}} and {{pancreas}} into self-assembling 3D structures that we have termed 'organoids'. We provide a detailed protocol that describes how to grow adult mouse and human liver and pancreas organoids, from cell isolation and long-term expansion to genetic manipulation in vitro. Liver and pancreas cells grow in a gel-based extracellular matrix (ECM) and a defined medium. The cells can self-organize into organoids that self-renew in vitro while retaining their tissue-of-origin commitment, genetic stability and potential to differentiate into functional cells in vitro (hepatocytes) and in vivo (hepatocytes and endocrine cells). Genetic modification of these organoids opens up avenues for the manipulation of adult stem cells in vitro, which could facilitate the study of human biology and allow gene correction for regenerative medicine purposes. The complete protocol takes 1-4 weeks to generate self-renewing 3D organoids and to perform genetic manipulation experiments." | |||
{{Pancreas}} | |||
==Neural Organoids== | |||
'''The use of brain organoids to investigate neural development and disease'''{{#pmid:28878372|PMID28878372}} | |||
:"Understanding the development and dysfunction of the human brain is a major goal of neurobiology. Much of our current understanding of human brain development has been derived from the examination of post-mortem and pathological specimens, bolstered by observations of developing non-human primates and experimental studies focused largely on mouse models. However, these tissue specimens and model systems cannot fully capture the unique and dynamic features of human brain development. Recent advances in stem cell technologies that enable the generation of human brain organoids from pluripotent stem cells (PSCs) promise to profoundly change our understanding of the development of the human brain and enable a detailed study of the pathogenesis of inherited and acquired brain diseases." | |||
{{neural}} | |||
==Testicular Organoids== | |||
{{#pmid:31328437}} | |||
{{testis}} | |||
== References == | == References == | ||
<references/> | <references/> | ||
| Line 37: | Line 79: | ||
===Reviews=== | ===Reviews=== | ||
{{#pmid: | {{#pmid:31718893}} | ||
{{#pmid:31071759}} | |||
{{#pmid:31783130}} | |||
{{#pmid:31274205}} | |||
{{#pmid:31328437}} | |||
{{#pmid:31310823}} | |||
{{#pmid:31300517}} | |||
===Articles=== | ===Articles=== | ||
{{#pmid:31872998}} | |||
{{#pmid:31778651}} | |||
{{#pmid:20369364}} | {{#pmid:20369364}} | ||
| Line 72: | Line 129: | ||
{{Footer}} | {{Footer}} | ||
[[Category:Organoids]] | |||
Revision as of 20:01, 30 December 2019
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
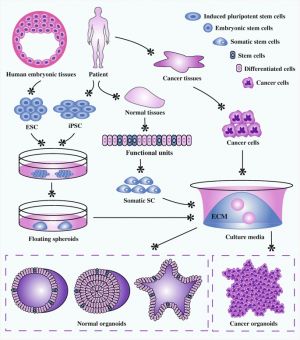
Organoids stem cell derived self-organized three-dimensional in vitro tissue cultures. These cultures have been be manipulated to replicate some of the complexity of an in vivo organ, or to produce only certain types of organ related cells. This stem cell research technique has many potential applications in understanding tissue and organ development, clinical therapeutics and cancer medicine.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Organoids | Culture Organoid Culture | Kidney Organoids | Pancreas Organoids | Neural Organoids Search: PLOS |
| Older papers |
|---|
|
Inner Ear Organoids
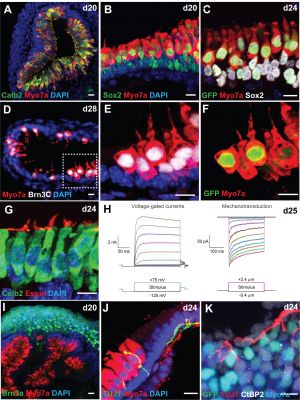
Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture[7]
- "Stem cell-derived inner ear sensory epithelia are a promising source of tissues for treating patients with hearing loss and dizziness. We recently demonstrated how to generate inner ear sensory epithelia, designated as inner ear organoids, from mouse embryonic stem cells (ESCs) in a self-organizing 3D culture. Here we improve the efficiency of this culture system by elucidating how Wnt signaling activity can drive the induction of otic tissue. We found that a carefully timed treatment with the potent Wnt agonist CHIR99021 promotes induction of otic vesicles-a process that was previously self-organized by unknown mechanisms. The resulting otic-like vesicles have a larger lumen size and contain a greater number of Pax8/Pax2-positive otic progenitor cells than organoids derived without the Wnt agonist. Additionally, these otic-like vesicles give rise to large inner ear organoids with hair cells whose morphological, biochemical and functional properties are indistinguishable from those of vestibular hair cells in the postnatal mouse inner ear. We conclude that Wnt signaling plays a similar role during inner ear organoid formation as it does during inner ear development in the embryo."
Kidney Organoids
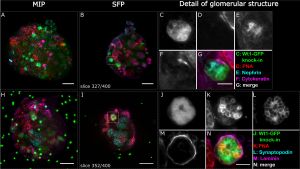
Kidney Organoids: A Translational Journey[5]
- "Human pluripotent stem cells (hPSCs) are attractive sources for regenerative medicine and disease modeling in vitro. Directed hPSC differentiation approaches have derived from knowledge of cell development in vivo rather than from stochastic cell differentiation. Moreover, there has been great success in the generation of 3D organ-buds termed 'organoids' from hPSCs; these consist of a variety of cell types in vitro that mimic organs in vivo. The organoid bears great potential in the study of human diseases in vitro, especially when combined with CRISPR/Cas9-based genome-editing. We summarize the current literature describing organoid studies with a special focus on kidney organoids, and discuss goals and future opportunities for organoid-based studies."
Liver Organoids
Prior N, Inacio P & Huch M. (2019). Liver organoids: from basic research to therapeutic applications. Gut , , . PMID: 31300517 DOI.
Günther C, Brevini T, Sampaziotis F & Neurath MF. (2019). What gastroenterologists and hepatologists should know about organoids in 2019. Dig Liver Dis , 51, 753-760. PMID: 30948332 DOI.
Pancreas Organoids
Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation.[9]
- "Adult somatic tissues have proven difficult to expand in vitro, largely because of the complexity of recreating appropriate environmental signals in culture. We have overcome this problem recently and developed culture conditions for adult stem cells that allow the long-term expansion of adult primary tissues from small intestine, stomach, liver and pancreas into self-assembling 3D structures that we have termed 'organoids'. We provide a detailed protocol that describes how to grow adult mouse and human liver and pancreas organoids, from cell isolation and long-term expansion to genetic manipulation in vitro. Liver and pancreas cells grow in a gel-based extracellular matrix (ECM) and a defined medium. The cells can self-organize into organoids that self-renew in vitro while retaining their tissue-of-origin commitment, genetic stability and potential to differentiate into functional cells in vitro (hepatocytes) and in vivo (hepatocytes and endocrine cells). Genetic modification of these organoids opens up avenues for the manipulation of adult stem cells in vitro, which could facilitate the study of human biology and allow gene correction for regenerative medicine purposes. The complete protocol takes 1-4 weeks to generate self-renewing 3D organoids and to perform genetic manipulation experiments."
Neural Organoids
The use of brain organoids to investigate neural development and disease[4]
- "Understanding the development and dysfunction of the human brain is a major goal of neurobiology. Much of our current understanding of human brain development has been derived from the examination of post-mortem and pathological specimens, bolstered by observations of developing non-human primates and experimental studies focused largely on mouse models. However, these tissue specimens and model systems cannot fully capture the unique and dynamic features of human brain development. Recent advances in stem cell technologies that enable the generation of human brain organoids from pluripotent stem cells (PSCs) promise to profoundly change our understanding of the development of the human brain and enable a detailed study of the pathogenesis of inherited and acquired brain diseases."
Testicular Organoids
Sakib S, Goldsmith T, Voigt A & Dobrinski I. (2019). Testicular organoids to study cell-cell interactions in the mammalian testis. Andrology , , . PMID: 31328437 DOI.
References
- ↑ Xu H, Lyu X, Yi M, Zhao W, Song Y & Wu K. (2018). Organoid technology and applications in cancer research. J Hematol Oncol , 11, 116. PMID: 30219074 DOI.
- ↑ Park SE, Georgescu A & Huh D. (2019). Organoids-on-a-chip. Science , 364, 960-965. PMID: 31171693 DOI.
- ↑ Takebe T & Wells JM. (2019). Organoids by design. Science , 364, 956-959. PMID: 31171692 DOI.
- ↑ 4.0 4.1 Di Lullo E & Kriegstein AR. (2017). The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. , 18, 573-584. PMID: 28878372 DOI.
- ↑ 5.0 5.1 Morizane R & Bonventre JV. (2017). Kidney Organoids: A Translational Journey. Trends Mol Med , 23, 246-263. PMID: 28188103 DOI.
- ↑ Clevers H. (2016). Modeling Development and Disease with Organoids. Cell , 165, 1586-1597. PMID: 27315476 DOI.
- ↑ 7.0 7.1 DeJonge RE, Liu XP, Deig CR, Heller S, Koehler KR & Hashino E. (2016). Modulation of Wnt Signaling Enhances Inner Ear Organoid Development in 3D Culture. PLoS ONE , 11, e0162508. PMID: 27607106 DOI.
- ↑ Held M, Santeramo I, Wilm B, Murray P & Lévy R. (2018). Ex vivo live cell tracking in kidney organoids using light sheet fluorescence microscopy. PLoS ONE , 13, e0199918. PMID: 30048451 DOI.
- ↑ Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK & Huch M. (2016). Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc , 11, 1724-43. PMID: 27560176 DOI.
Journals
Reviews
Merenda A, Fenderico N & Maurice MM. (2020). Wnt Signaling in 3D: Recent Advances in the Applications of Intestinal Organoids. Trends Cell Biol. , 30, 60-73. PMID: 31718893 DOI.
Kyrousi C & Cappello S. (2020). Using brain organoids to study human neurodevelopment, evolution and disease. Wiley Interdiscip Rev Dev Biol , 9, e347. PMID: 31071759 DOI.
Chhibber T, Bagchi S, Lahooti B, Verma A, Al-Ahmed A, Paul MK, Pendyala G & Jayant RD. (2019). CNS organoids: an innovative tool for neurological disease modeling and drug neurotoxicity screening. Drug Discov. Today , , . PMID: 31783130 DOI.
Gopalakrishnan J. (2019). The Emergence of Stem Cell-Based Brain Organoids: Trends and Challenges. Bioessays , 41, e1900011. PMID: 31274205 DOI.
Sakib S, Goldsmith T, Voigt A & Dobrinski I. (2019). Testicular organoids to study cell-cell interactions in the mammalian testis. Andrology , , . PMID: 31328437 DOI.
van den Hurk M & Bardy C. (2019). Single-cell multimodal transcriptomics to study neuronal diversity in human stem cell-derived brain tissue and organoid models. J. Neurosci. Methods , 325, 108350. PMID: 31310823 DOI.
Prior N, Inacio P & Huch M. (2019). Liver organoids: from basic research to therapeutic applications. Gut , , . PMID: 31300517 DOI.
Articles
Mukherjee N, Nandi S, Ghosh S, Garg S & Ghosh S. (2019). Three-Dimensional Microfluidic Platform with Neural Organoids: Model System for Unraveling Synapses. ACS Chem Neurosci , , . PMID: 31872998 DOI.
Muchnik SK, Lorente-Galdos B, Santpere G & Sestan N. (2019). Modeling the Evolution of Human Brain Development Using Organoids. Cell , 179, 1250-1253. PMID: 31778651 DOI.
Pekkanen-Mattila M, Pelto-Huikko M, Kujala V, Suuronen R, Skottman H, Aalto-Setälä K & Kerkelä E. (2010). Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem. Cell Biol. , 133, 595-606. PMID: 20369364 DOI.
Search PubMed
May 2006 "stem cell" 154,176 reference articles of which 16,449 were reviews.
Search PubMed Now: stem cell | embryonic stem cell | adult stem cell |
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- NIH (USA) Human Embryonic Stem Cell Registry | [feed://hescregapp.od.nih.gov/hesc.xml RSS]
- StemBook - Table of Contents
- International Society for Stem Cell Research (ISSCR) is an independent, nonprofit organization formed in 2002 to foster the exchange of information on stem cell research.
- University of Michigan Stem Cells Explained
- Transcript of discussion on ABC Radio (Dr. J Kahn , Dr. JWagner) on Genetic Technology And Ethics
- A brief article on Cord Blood stem cells and their therapeutic potential from the BBC.
- Monash University (Australia) Monash Immunology and Stem Cell Laboratories (MISCL)
- Europe - ESTOOLS DATA@HAND "resource contains human gene expression array data from 97 GEO and ArrayExpress sample sets, which involve altogether 1674 Affymetrix, Illumina and Agilent arrays. The source of the biological samples is mainly pluripotent stem cells, their differentiated progeny, and their parent cells. All data has been preprocessed so as to enable computational analysis with analysis workflows and tools provided."
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Organoids. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Organoids
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
