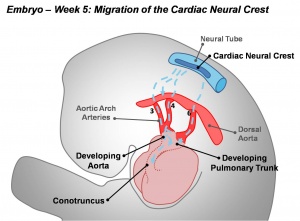
Neural Crest - Cardiac
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Draft Page (notice removed when completed).
Cardiac neural crest has an important contribution to the developing heart outflow tract.[1] It has been shown to not contribute to any of the heart conduction system.[2]
| Neural Crest Links: neural crest | Lecture - Early Neural | Lecture - Neural Crest Development | Lecture Movie | Schwann cell | adrenal | melanocyte | peripheral nervous system | enteric nervous system | cornea | cranial nerve neural crest | head | skull | cardiac neural crest | Nicole Le Douarin | Neural Crest Movies | neural crest abnormalities | Category:Neural Crest | |||
|
Cardiovascular System Development
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cardiac Neural Crest <pubmed limit=5>Cardiac Crest</pubmed> |
2013
Development of the Human Aortic Arch System Captured in an Interactive Three-Dimensional Reference Model
Am J Med Genet A. 2013 Apr 23. doi: 10.1002/ajmg.a.35881. [Epub ahead of print]
Rana MS, Sizarov A, Christoffels VM, Moorman AF. Source Heart Failure Research Center, Department of Anatomy, Embryology and Physiology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Abstract
Variations and mutations in the human genome, such as 22q11.2 microdeletion, can increase the risk for congenital defects, including aortic arch malformations. Animal models are increasingly expanding our molecular and genetic insights into aortic arch development. However, in order to justify animal-to-human extrapolations, a human morphological, and molecular reference model would be of great value, but is currently lacking. Here, we present interactive three-dimensional reconstructions of the developing human aortic arch system, supplemented with the protein distribution of developmental markers for patterning and growth, including T-box transcription factor TBX1, a major candidate for the phenotypes found in patients with the 22q11.2 microdeletion. These reconstructions and expression data facilitate unbiased interpretations, and reveal previously unappreciated aspects of human aortic arch development. Based on our reconstructions and on reported congenital anomalies of the pulmonary trunk and tributaries, we postulate that the pulmonary arteries originate from the aortic sac, rather than from the sixth pharyngeal arch arteries. Similar to mouse, TBX1 is expressed in pharyngeal mesenchyme and epithelia. The endothelium of the pharyngeal arch arteries is largely negative for TBX1 and family member TBX2 but expresses neural crest marker AP2α, which gradually decreases with ongoing development of vascular smooth muscle. At early stages, the pharyngeal arch arteries, aortic sac, and the dorsal aortae in particular were largely negative for proliferation marker Ki67, potentially an important parameter during aortic arch system remodeling. Together, our data support current animal-to-human extrapolations and future genetic and molecular analyses using animal models of congenital heart disease. © 2013 Wiley Periodicals, Inc. Copyright © 2013 Wiley Periodicals, Inc.
PMID 23613216
2011
FGF8 signaling is chemotactic for cardiac neural crest cells
Dev Biol. 2011 Jun 1;354(1):18-30. doi: 10.1016/j.ydbio.2011.03.010. Epub 2011 Mar 17.
Sato A, Scholl AM, Kuhn EN, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML. Source Department of Pediatrics (Neonatology), Duke University, Durham, NC 27710, USA. Erratum in Dev Biol. 2012 Oct 1;370(1):164. Kuhn, E B [corrected to Kuhn, E N]. Abstract Cardiac neural crest cells migrate into the pharyngeal arches where they support development of the pharyngeal arch arteries. The pharyngeal endoderm and ectoderm both express high levels of FGF8. We hypothesized that FGF8 is chemotactic for cardiac crest cells. To begin testing this hypothesis, cardiac crest was explanted for migration assays under various conditions. Cardiac neural crest cells migrated more in response to FGF8. Single cell tracing indicated that this was not due to proliferation and subsequent transwell assays showed that the cells migrate toward an FGF8 source. The migratory response was mediated by FGF receptors (FGFR) 1 and 3 and MAPK/ERK intracellular signaling. To test whether FGF8 is chemokinetic and/or chemotactic in vivo, dominant negative FGFR1 was electroporated into the premigratory cardiac neural crest. Cells expressing the dominant negative receptor migrated slower than normal cardiac neural crest cells and were prone to remain in the vicinity of the neural tube and die. Treating with the FGFR1 inhibitor, SU5402 or an FGFR3 function-blocking antibody also slowed neural crest migration. FGF8 over-signaling enhanced neural crest migration. Neural crest cells migrated to an FGF8-soaked bead placed dorsal to the pharynx. Finally, an FGF8 producing plasmid was electroporated into an ectopic site in the ventral pharyngeal endoderm. The FGF8 producing cells attracted a thick layer of mesenchymal cells. DiI labeling of the neural crest as well as quail-to-chick neural crest chimeras showed that neural crest cells migrated to and around the ectopic site of FGF8 expression. These results showing that FGF8 is chemotactic and chemokinetic for cardiac neural crest adds another dimension to understanding the relationship of FGF8 and cardiac neural crest in cardiovascular defects. Copyright © 2011 Elsevier Inc. All rights reserved.
PMID 2141976
2010
Factors controlling cardiac neural crest cell migration
Cell Adh Migr. 2010 Oct-Dec;4(4):609-21.
Kirby ML, Hutson MR. Source Department of Pediatrics, Duke University, Durham, NC, USA. mlkirby@duke.edu Abstract Cardiac neural crest cells originate as part of the postotic caudal rhombencephalic neural crest stream. Ectomesenchymal cells in this stream migrate to the circumpharyngeal ridge and then into the caudal pharyngeal arches where they condense to form first a sheath and then the smooth muscle tunics of the persisting pharyngeal arch arteries. A subset of the cells continue migrating into the cardiac outflow tract where they will condense to form the aorticopulmonary septum. Cell signaling, extracellular matrix and cell-cell contacts are all critical for the initial migration, pauses, continued migration, and condensation of these cells. This review elucidates what is currently known about these factors. PMID 20890117
Cardiac Neural Crest
<pubmed limit=5>Cardiac Neural Crest</pubmed>
References
- ↑ Creazzo TL, Godt RE, Leatherbury L, Conway SJ & Kirby ML. (1998). Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. , 60, 267-86. PMID: 9558464 DOI.
- ↑ Boullin J & Morgan JM. (2005). The development of cardiac rhythm. Heart , 91, 874-5. PMID: 15958352 DOI.
- ↑ Rosa AL, Alvarez ME, Lawson D & Maccioni HJ. (1990). A polypeptide of 59 kDa is associated with bundles of cytoplasmic filaments in Neurospora crassa. Biochem. J. , 268, 649-55. PMID: 2141976
Reviews
Creazzo TL, Godt RE, Leatherbury L, Conway SJ & Kirby ML. (1998). Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. , 60, 267-86. PMID: 9558464 DOI.
Articles
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 19) Embryology Neural Crest - Cardiac. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_Crest_-_Cardiac
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G