Neural - Prosencephalon Development: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 17: | Line 17: | ||
{{Neural Links 2}} | {{Neural Links 2}} | ||
==Some Recent Findings== | ==Some Recent Findings== | ||
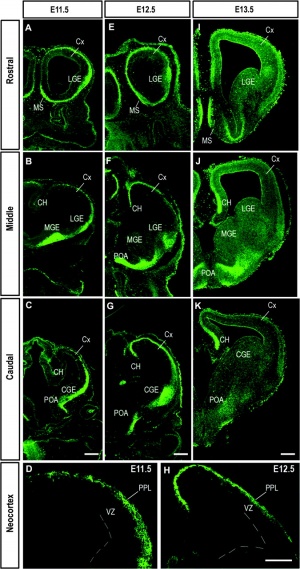
[[File:Mouse - forebrain Robo3 expression.jpg|thumb|Developing mouse forebrain Robo3 expression | [[File:Mouse - forebrain Robo3 expression.jpg|thumb|Developing mouse forebrain Robo3 expression{{#pmid:19366869|PMID19366869}}]] | ||
{| | {| | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* '''Development of laminar organization of the fetal cerebrum''' | * '''The genetic architecture of the human cerebral cortex'''{{#pmid:32193296|PMID32193296}} "The cerebral cortex underlies our complex cognitive capabilities, yet little is known about the specific genetic loci that influence human cortical structure. To identify genetic variants that affect cortical structure, we conducted a genome-wide association meta-analysis of brain magnetic resonance imaging data from 51,665 individuals. We analyzed the surface area and average thickness of the whole cortex and 34 regions with known functional specializations. We identified 199 significant loci and found significant enrichment for loci influencing total surface area within regulatory elements that are active during prenatal cortical development, supporting the radial unit hypothesis. Loci that affect regional surface area cluster near genes in Wnt signaling pathways, which influence progenitor expansion and areal identity. Variation in cortical structure is genetically correlated with cognitive function, Parkinson's disease, insomnia, depression, neuroticism, and attention deficit hyperactivity disorder." | ||
* '''Development of laminar organization of the fetal cerebrum'''{{#pmid:20981415|PMID20981415}} "Heads of 131 fetal specimens of 14-40 weeks gestational age (GA) were scanned by 3.0T MRI. Eleven fetal specimens of 14-27 weeks GA were scanned by 7.0T MRI. On T(1)-weighted 3.0T MRI, layers could be visualized at 14 weeks GA and appeared clearer after 18 weeks GA. On 7.0T MRI, four zones could be recognized at 14 weeks GA. During 15-22 weeks GA, when laminar organization appeared typical, seven layers including the periventricular zone and external capsule fibers could be differentiated, which corresponded to seven zones in histological stained sections. At 23-28 weeks GA, laminar organization appeared less typical, and borderlines among them appeared obscured. After 30 weeks GA, it disappeared and turned into mature-like structures. The developing lamination appeared the most distinguishable at the parieto-occipital part of brain and peripheral regions of the hippocampus. The migrating thalamocortical afferents were probably delineated as a high signal layer located at the lower, middle, and upper part of the subplate zone at 16-28 weeks GA on T(1)-weighted 3.0T MRI." | |||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
! More recent papers | ! More recent papers | ||
|- | |- | ||
| [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | | [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | ||
Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Prosencephalon+Embryology ''Prosencephalon Embryology''] | Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Prosencephalon+Development ''Prosencephalon Development''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Prosencephalon+Embryology ''Prosencephalon Embryology''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Prosencephalon ''Prosencephalon''] | ||
|} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Older papers | |||
|- | |||
| {{Older papers}} | |||
|} | |} | ||
== Development Overview == | == Development Overview == | ||
| Line 48: | Line 54: | ||
==Additional Images== | ==Additional Images== | ||
=== | ===Historic=== | ||
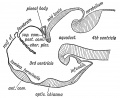
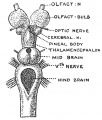
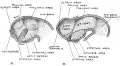
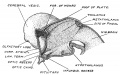
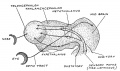
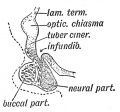
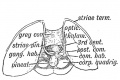
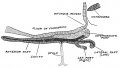
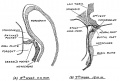
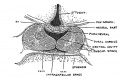
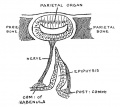
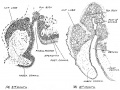
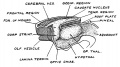
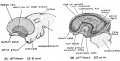
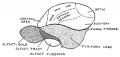
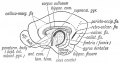
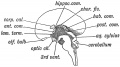
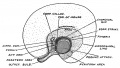
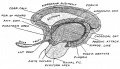
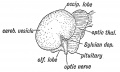
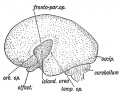
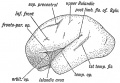
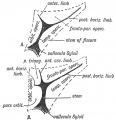
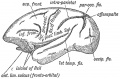
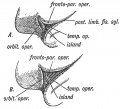
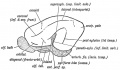
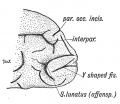
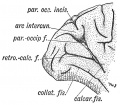
Keith, A. [[Book - Human Embryology and Morphology (1921)|'''Human Embryology And Morphology''']] (1921) Longmans, Green & Co.:New York. [[Human Embryology and Morphology_9|9 Fore-Brain]] and [[Human Embryology and Morphology_10|10 Fore-Brain Cerebral Vesicles]] | {| class="wikitable mw-collapsible mw-collapsed" | ||
! Human Embryology And Morphology (1921) | |||
|- | |||
| Keith, A. [[Book - Human Embryology and Morphology (1921)|'''Human Embryology And Morphology''']] (1921) Longmans, Green & Co.:New York. | |||
[[Human Embryology and Morphology_9|9 Fore-Brain]] and [[Human Embryology and Morphology_10|10 Fore-Brain Cerebral Vesicles]] | |||
<gallery> | <gallery> | ||
| Line 89: | Line 100: | ||
File:Keith1921 fig129.jpg|Fig. 129. The Primitive Vein of the Head and its tributaries in the 6th week | File:Keith1921 fig129.jpg|Fig. 129. The Primitive Vein of the Head and its tributaries in the 6th week | ||
</gallery> | </gallery> | ||
|} | |||
== References == | == References == | ||
| Line 95: | Line 106: | ||
===Reviews=== | ===Reviews=== | ||
{{#pmid:19560042}} | |||
{{#pmid:17275286}} | |||
{{#pmid:17030124}} | |||
{{#pmid:16418000}} | |||
{{#pmid:12626695}} | |||
{{#pmid:12461551}} | |||
===Articles=== | ===Articles=== | ||
{{#pmid:11955708}} | |||
{{#pmid:11803577}} | |||
{{#pmid:11734354}} | |||
{{#pmid:10473296}} | |||
{{#pmid:10375509}} | |||
{{#pmid:9810564}} | |||
{{#pmid:1661870}} | |||
{{#pmid:4018406}} | |||
===Search PubMed=== | ===Search PubMed=== | ||
Latest revision as of 09:17, 14 May 2020
| Embryology - 18 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects).
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
- Neural development beginnings quite early, therefore also look at notes covering Week 3- neural tube and Week 4-early nervous system.
- Development of the neural crest and sensory systems (hearing/vision/smell) are only introduced in these notes and are covered in other notes sections.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Prosencephalon Development | Prosencephalon Embryology | Prosencephalon |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page. |
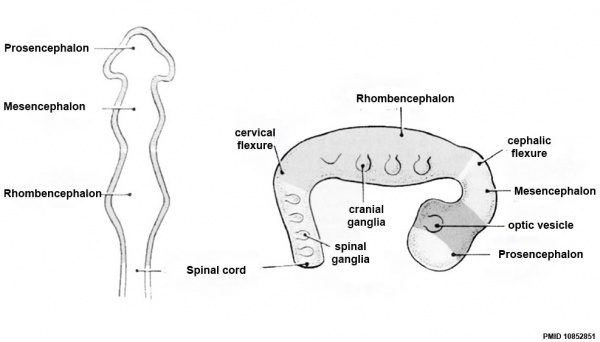
Development Overview
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
Primary Vesicles
Additional Images
Historic
| Human Embryology And Morphology (1921) |
|---|
| Keith, A. Human Embryology And Morphology (1921) Longmans, Green & Co.:New York.
9 Fore-Brain and 10 Fore-Brain Cerebral Vesicles |
References
- ↑ Barber M, Di Meglio T, Andrews WD, Hernández-Miranda LR, Murakami F, Chédotal A & Parnavelas JG. (2009). The role of Robo3 in the development of cortical interneurons. Cereb. Cortex , 19 Suppl 1, i22-31. PMID: 19366869 DOI.
- ↑ Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, Lind PA, Pizzagalli F, Ching CRK, McMahon MAB, Shatokhina N, Zsembik LCP, Thomopoulos SI, Zhu AH, Strike LT, Agartz I, Alhusaini S, Almeida MAA, Alnæs D, Amlien IK, Andersson M, Ard T, Armstrong NJ, Ashley-Koch A, Atkins JR, Bernard M, Brouwer RM, Buimer EEL, Bülow R, Bürger C, Cannon DM, Chakravarty M, Chen Q, Cheung JW, Couvy-Duchesne B, Dale AM, Dalvie S, de Araujo TK, de Zubicaray GI, de Zwarte SMC, den Braber A, Doan NT, Dohm K, Ehrlich S, Engelbrecht HR, Erk S, Fan CC, Fedko IO, Foley SF, Ford JM, Fukunaga M, Garrett ME, Ge T, Giddaluru S, Goldman AL, Green MJ, Groenewold NA, Grotegerd D, Gurholt TP, Gutman BA, Hansell NK, Harris MA, Harrison MB, Haswell CC, Hauser M, Herms S, Heslenfeld DJ, Ho NF, Hoehn D, Hoffmann P, Holleran L, Hoogman M, Hottenga JJ, Ikeda M, Janowitz D, Jansen IE, Jia T, Jockwitz C, Kanai R, Karama S, Kasperaviciute D, Kaufmann T, Kelly S, Kikuchi M, Klein M, Knapp M, Knodt AR, Krämer B, Lam M, Lancaster TM, Lee PH, Lett TA, Lewis LB, Lopes-Cendes I, Luciano M, Macciardi F, Marquand AF, Mathias SR, Melzer TR, Milaneschi Y, Mirza-Schreiber N, Moreira JCV, Mühleisen TW, Müller-Myhsok B, Najt P, Nakahara S, Nho K, Olde Loohuis LM, Orfanos DP, Pearson JF, Pitcher TL, Pütz B, Quidé Y, Ragothaman A, Rashid FM, Reay WR, Redlich R, Reinbold CS, Repple J, Richard G, Riedel BC, Risacher SL, Rocha CS, Mota NR, Salminen L, Saremi A, Saykin AJ, Schlag F, Schmaal L, Schofield PR, Secolin R, Shapland CY, Shen L, Shin J, Shumskaya E, Sønderby IE, Sprooten E, Tansey KE, Teumer A, Thalamuthu A, Tordesillas-Gutiérrez D, Turner JA, Uhlmann A, Vallerga CL, van der Meer D, van Donkelaar MMJ, van Eijk L, van Erp TGM, van Haren NEM, van Rooij D, van Tol MJ, Veldink JH, Verhoef E, Walton E, Wang M, Wang Y, Wardlaw JM, Wen W, Westlye LT, Whelan CD, Witt SH, Wittfeld K, Wolf C, Wolfers T, Wu JQ, Yasuda CL, Zaremba D, Zhang Z, Zwiers MP, Artiges E, Assareh AA, Ayesa-Arriola R, Belger A, Brandt CL, Brown GG, Cichon S, Curran JE, Davies GE, Degenhardt F, Dennis MF, Dietsche B, Djurovic S, Doherty CP, Espiritu R, Garijo D, Gil Y, Gowland PA, Green RC, Häusler AN, Heindel W, Ho BC, Hoffmann WU, Holsboer F, Homuth G, Hosten N, Jack CR, Jang M, Jansen A, Kimbrel NA, Kolskår K, Koops S, Krug A, Lim KO, Luykx JJ, Mathalon DH, Mather KA, Mattay VS, Matthews S, Mayoral Van Son J, McEwen SC, Melle I, Morris DW, Mueller BA, Nauck M, Nordvik JE, Nöthen MM, O'Leary DS, Opel N, Martinot MP, Pike GB, Preda A, Quinlan EB, Rasser PE, Ratnakar V, Reppermund S, Steen VM, Tooney PA, Torres FR, Veltman DJ, Voyvodic JT, Whelan R, White T, Yamamori H, Adams HHH, Bis JC, Debette S, Decarli C, Fornage M, Gudnason V, Hofer E, Ikram MA, Launer L, Longstreth WT, Lopez OL, Mazoyer B, Mosley TH, Roshchupkin GV, Satizabal CL, Schmidt R, Seshadri S, Yang Q, Alvim MKM, Ames D, Anderson TJ, Andreassen OA, Arias-Vasquez A, Bastin ME, Baune BT, Beckham JC, Blangero J, Boomsma DI, Brodaty H, Brunner HG, Buckner RL, Buitelaar JK, Bustillo JR, Cahn W, Cairns MJ, Calhoun V, Carr VJ, Caseras X, Caspers S, Cavalleri GL, Cendes F, Corvin A, Crespo-Facorro B, Dalrymple-Alford JC, Dannlowski U, de Geus EJC, Deary IJ, Delanty N, Depondt C, Desrivières S, Donohoe G, Espeseth T, Fernández G, Fisher SE, Flor H, Forstner AJ, Francks C, Franke B, Glahn DC, Gollub RL, Grabe HJ, Gruber O, Håberg AK, Hariri AR, Hartman CA, Hashimoto R, Heinz A, Henskens FA, Hillegers MHJ, Hoekstra PJ, Holmes AJ, Hong LE, Hopkins WD, Hulshoff Pol HE, Jernigan TL, Jönsson EG, Kahn RS, Kennedy MA, Kircher TTJ, Kochunov P, Kwok JBJ, Le Hellard S, Loughland CM, Martin NG, Martinot JL, McDonald C, McMahon KL, Meyer-Lindenberg A, Michie PT, Morey RA, Mowry B, Nyberg L, Oosterlaan J, Ophoff RA, Pantelis C, Paus T, Pausova Z, Penninx BWJH, Polderman TJC, Posthuma D, Rietschel M, Roffman JL, Rowland LM, Sachdev PS, Sämann PG, Schall U, Schumann G, Scott RJ, Sim K, Sisodiya SM, Smoller JW, Sommer IE, St Pourcain B, Stein DJ, Toga AW, Trollor JN, Van der Wee NJA, van 't Ent D, Völzke H, Walter H, Weber B, Weinberger DR, Wright MJ, Zhou J, Stein JL, Thompson PM & Medland SE. (2020). The genetic architecture of the human cerebral cortex. Science , 367, . PMID: 32193296 DOI.
- ↑ Zhang Z, Liu S, Lin X, Teng G, Yu T, Fang F & Zang F. (2011). Development of laminar organization of the fetal cerebrum at 3.0T and 7.0T: a postmortem MRI study. Neuroradiology , 53, 177-84. PMID: 20981415 DOI.
Reviews
Hoch RV, Rubenstein JL & Pleasure S. (2009). Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin. Cell Dev. Biol. , 20, 378-86. PMID: 19560042 DOI.
Lindwall C, Fothergill T & Richards LJ. (2007). Commissure formation in the mammalian forebrain. Curr. Opin. Neurobiol. , 17, 3-14. PMID: 17275286 DOI.
Bertrand N & Dahmane N. (2006). Sonic hedgehog signaling in forebrain development and its interactions with pathways that modify its effects. Trends Cell Biol. , 16, 597-605. PMID: 17030124 DOI.
Rhinn M, Picker A & Brand M. (2006). Global and local mechanisms of forebrain and midbrain patterning. Curr. Opin. Neurobiol. , 16, 5-12. PMID: 16418000 DOI.
Marín O & Rubenstein JL. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. , 26, 441-83. PMID: 12626695 DOI.
Rallu M, Corbin JG & Fishell G. (2002). Parsing the prosencephalon. Nat. Rev. Neurosci. , 3, 943-51. PMID: 12461551 DOI.
Articles
Ohkubo Y, Chiang C & Rubenstein JL. (2002). Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience , 111, 1-17. PMID: 11955708
Hidalgo-Sánchez M & Alvarado-Mallart RM. (2002). Temporal sequence of gene expression leading caudal prosencephalon to develop a midbrain/hindbrain phenotype. Dev. Dyn. , 223, 141-7. PMID: 11803577 DOI.
Crossley PH, Martinez S, Ohkubo Y & Rubenstein JL. (2001). Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience , 108, 183-206. PMID: 11734354
Ulfig N, Neudörfer F & Bohl J. (1999). Distribution patterns of vimentin-immunoreactive structures in the human prosencephalon during the second half of gestation. J. Anat. , 195 ( Pt 1), 87-100. PMID: 10473296
Hidalgo-Sánchez M, Simeone A & Alvarado-Mallart RM. (1999). Fgf8 and Gbx2 induction concomitant with Otx2 repression is correlated with midbrain-hindbrain fate of caudal prosencephalon. Development , 126, 3191-203. PMID: 10375509
Sztriha L, Várady E, Hertecant J & Nork M. (1998). Mediobasal and mantle defect of the prosencephalon: lobar holoprosencephaly, schizencephaly and diabetes insipidus. Neuropediatrics , 29, 272-5. PMID: 9810564 DOI.
Nakamura H, Matsui KA, Takagi S & Fujisawa H. (1991). Projection of the retinal ganglion cells to the tectum differentiated from the prosencephalon. Neurosci. Res. , 11, 189-97. PMID: 1661870
Couly GF & Le Douarin NM. (1985). Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev. Biol. , 110, 422-39. PMID: 4018406
Search PubMed
Search Pubmed: Prosencephalon Embryology | Prosencephalon Development |
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 18) Embryology Neural - Prosencephalon Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Prosencephalon_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G