Neural - Medulla Oblongata Development: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Header}}[[File:Stage10_sem6.jpg|right|150px]] | {{Header}}[[File:Stage10_sem6.jpg|right|150px]] | ||
==Introduction== | ==Introduction== | ||
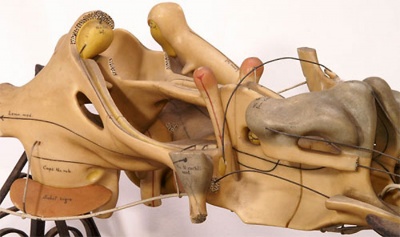
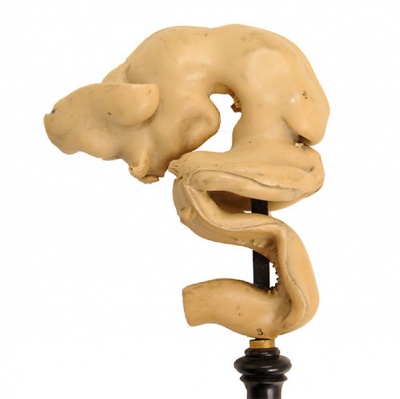
[[File:Ziegler model 10.jpg|thumb|Historic Model of the Medulla]] | [[File:Ziegler model 10.jpg|thumb|300px|alt=Historic Ziegler Model of the Medulla|Historic Ziegler Model of the Medulla]] | ||
The {{medulla}} or {{medulla oblongata}} develops from the secondary brain vesicle the [[Neural - Myelencephalon Development|myelencephalon]], that in turn formed from the earlier primary brain vesicle [[Neural - Rhombencephalon Development|rhombencephalon]]. The neural tube lateral walls have 2 halves (alar and a basal lamina) and are connected by a floor plate and roof-plate region. | |||
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system. | Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system. | ||
The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects). | The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects). | ||
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord. Neural development beginnings quite early, therefore also look at notes covering Week 3 neural tube and Week 4 early nervous system. | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Historic Medulla Embryology | |||
|- | |||
| {{Historic Disclaimer}} | |||
{{Ref-Sabin1901}} | |||
{{Ref-Essick1912}} | |||
|} | |||
{{Neural Links}} | {{Neural Links}} | ||
<br> | |||
{{Neural Links 2}} | {{Neural Links 2}} | ||
==Some Recent Findings== | ==Some Recent Findings== | ||
| Line 18: | Line 30: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
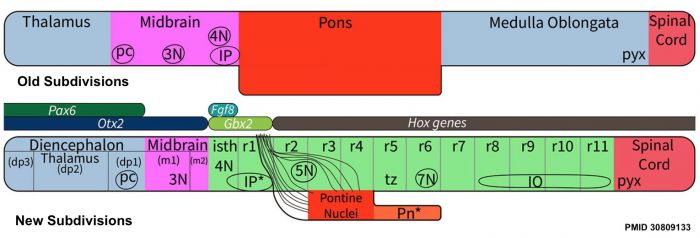
* '''A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei''' | [[File:Brain stem subdivisions 01.jpg|alt=Brain stem subdivisions|700px]] | ||
* '''Review - Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons From Developmental Gene Patterns'''{{#pmid:30809133|PMID30809133}} "The traditional subdivision of the brain stem into {{midbrain}}, {{pons}}, and {{medulla oblongata}} is based purely on the external appearance of the human brain stem. There is an urgent need to update the names of brain stem structures to be consistent with the discovery of rhomobomeric segmentation based on gene expression. The most important mistakes are the belief that the pons occupies the upper half of the hindbrain, the failure to recognize the isthmus as the first segment of the hindbrain, and the mistaken inclusion of diencephalic structures in the midbrain. The new nomenclature will apply to all mammals. This essay recommends a new brain stem nomenclature based on developmental gene expression, progeny analysis, and fate mapping." | |||
* '''Spatiotemporal expression of NDRG2 in the human fetal brain'''{{#pmid:30312765|PMID30312765}} "N-myc downstream-regulated gene 2 (NDRG2) has been implicated in the development of central nervous system and brain diseases such as brain tumors, ischemic stroke and neurodegenerative disorders. However, it remains unclear that the spatiotemporal distribution of NDRG2 in the human fetal brain. In this study, we examined the expression pattern of NDRG2 in different regions of human fetal brain at 16-28 gestational weeks (GWs) by using RT-PCR, western blot and immunohistochemistry. Firstly, RT-PCR revealed that mRNA of NDRG2 was detected in the human brain regions of fetuses at 16-28 GWs such as medulla oblongata (MdO), mesencephalon (MeE), cerebellum (Cbl), frontal lobe (Fr), ventricular (VZ)/subventricular zone (SVZ) and hippocampus (hip), and the expressions of NDRG2 mRNA in these human fetal brain regions were increased with gestational maturation. Furthermore, western blot and immunohistochemistry results revealed that at 28 GWs, the expression of NDRG2 protein was restricted to the MdO's olivary nucleus, MeE's aqueduct, cerebellar internal granular layers, cerebral cortex of the Fr, VZ/SVZ of lateral ventricle, and hippocampal dentate gyrus, and highest expression in the VZ/SVZ, and lowest in the MeE. Finally, double immunohistochemistry results showed that NDRG2 in the MdO, Cbl and VZ/SV at 28 GWS was mainly expressed in neurons (NeuN positive cells), and in some astrocytes (GFAP positive cells). Taken together, these results suggest that NDRG2 is mainly expressed in human fetal neurons of various brain regions during development, which may be involved in neuronal growth and maturation." | |||
* '''A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei'''{{#pmid:20495559|PMID20495559}} "Neurons usually migrate and differentiate in one particular encephalic vesicle. We identified a murine population of diencephalic neurons that colonized the telencephalic amygdaloid complex, migrating along a tangential route that crosses a boundary between developing brain vesicles. The diencephalic transcription factor OTP was necessary for this migratory behavior." | |||
|} | |} | ||
{| class="wikitable collapsible collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
! More recent papers | ! More recent papers | ||
|- | |- | ||
| Line 73: | Line 90: | ||
<references/> | <references/> | ||
===Reviews=== | ===Reviews=== | ||
===Articles=== | ===Articles=== | ||
{{#pmid:18986852}} | |||
{{#pmid:12850246}} | |||
{{#pmid:1867385}} | |||
===Search PubMed=== | ===Search PubMed=== | ||
| Line 89: | Line 107: | ||
{{ | {{Glossary}} | ||
{{ | {{Footer}} | ||
Latest revision as of 17:25, 8 June 2020
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The medulla or medulla oblongata develops from the secondary brain vesicle the myelencephalon, that in turn formed from the earlier primary brain vesicle rhombencephalon. The neural tube lateral walls have 2 halves (alar and a basal lamina) and are connected by a floor plate and roof-plate region.
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
The early central nervous system begins as a simple neural plate that folds to form a groove then tube, open initially at each end. Failure of these opening to close contributes a major class of neural abnormalities (neural tube defects).
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord. Neural development beginnings quite early, therefore also look at notes covering Week 3 neural tube and Week 4 early nervous system.
| Historic Medulla Embryology | ||
|---|---|---|
Sabin FR. and Knower H. An atlas of the medulla and midbrain, a laboratory manual (1901) Baltimore: Friedenwald. Essick CR. The development of the nuclei pontis and the nucleus arcuatus in man. (1912) Amer. J Anat. 13(1): -54. |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Medulla Oblongata Embryology <pubmed limit=5>Medulla Oblongata Embryology</pubmed> |
Development Overview
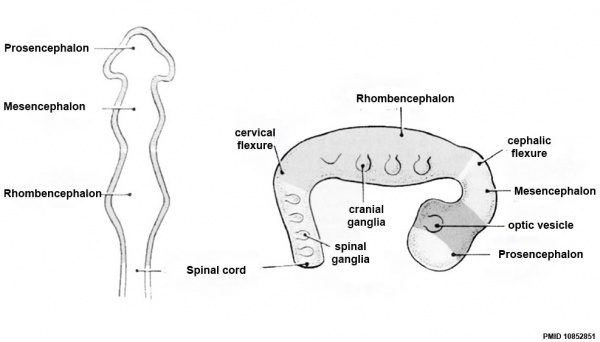
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
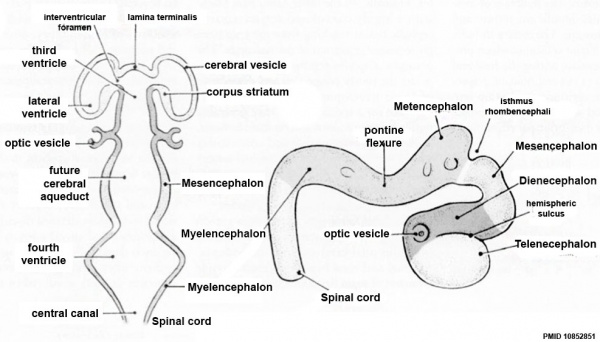
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
Early Brain Vesicles
Primary Vesicles
Secondary Vesicles
Historic Ziegler Model
- Links: Ziegler Models
References
- ↑ Watson C, Bartholomaeus C & Puelles L. (2019). Time for Radical Changes in Brain Stem Nomenclature-Applying the Lessons From Developmental Gene Patterns. Front Neuroanat , 13, 10. PMID: 30809133 DOI.
- ↑ Jin PP, Xia F, Ma BF, Li Z, Zhang GF, Deng YC, Tu ZL, Zhang XX & Hou SX. (2019). Spatiotemporal expression of NDRG2 in the human fetal brain. Ann. Anat. , 221, 148-155. PMID: 30312765 DOI.
- ↑ García-Moreno F, Pedraza M, Di Giovannantonio LG, Di Salvio M, López-Mascaraque L, Simeone A & De Carlos JA. (2010). A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nat. Neurosci. , 13, 680-9. PMID: 20495559 DOI.
Reviews
Articles
Duncan JR, Paterson DS & Kinney HC. (2008). The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Auton Neurosci , 144, 61-75. PMID: 18986852 DOI.
Lorke DE, Kwong WH, Chan WY & Yew DT. (2003). Development of catecholaminergic neurons in the human medulla oblongata. Life Sci. , 73, 1315-31. PMID: 12850246
Tan K & Le Douarin NM. (1991). Development of the nuclei and cell migration in the medulla oblongata. Application of the quail-chick chimera system. Anat. Embryol. , 183, 321-43. PMID: 1867385
Search PubMed
Search Pubmed: Medulla Embryology | Medulla Oblongata Development
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Neural - Medulla Oblongata Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Medulla_Oblongata_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G