Neural - Cerebellum Development
| Embryology - 18 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
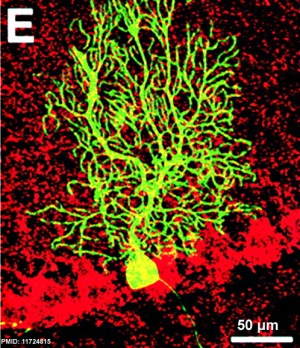
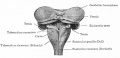
The cerebellum (Latin, ‘little brain’) major role is in role in sensory-motor processing that in the adult human contains half of all the brain's neurons.
Much of the basic structure of the cerebellum comes the historic histological studies and staining of Ramón Cahal (1852 - 1934) and Camillo Golgi (1843 - 1926).
| Cerebellum History | |
|---|---|
|
Santiago Ramon y Cahal (1852 - 1934) a Spanish researcher used then new histology Golgi staining techniques to identify the cerebellum cellular structure.
His work was a turning point in our understanding of the structure of the brain, that until then had been described as a "syncytium" and not consisting of discrete cellular elements. For this research and other work on defining the structure of the brain he, along with Camillo Golgi (1843 - 1926), received the 1906 Nobel Prize in Medicine. Camillo Golgi (1843 - 1926) developed the histology silver staining technique, though is best known today for the cellular organelle that bears his name, the Golgi apparatus.See also the early descriptive study by Palmgren (1921 )[3] |
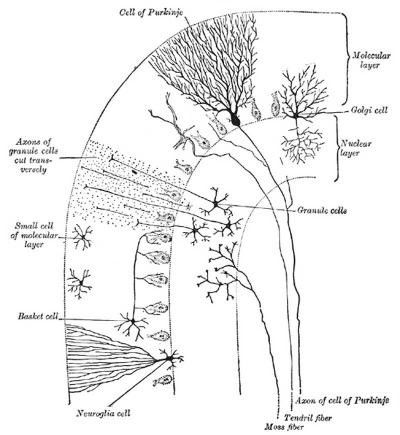
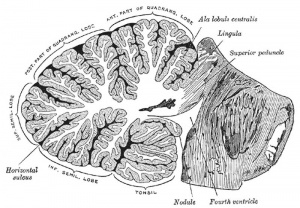
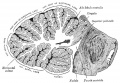
Transverse section of a cerebellar folium. |
| See also the description by Myers BD. A study of the development of certain features of the cerebellum. (1920) Contrib. Embryol., Carnegie Inst. Wash. 41: | |
Neural development is one of the earliest systems to begin and the last to be completed after birth. This development generates the most complex structure within the embryo and the long time period of development means in utero insult during pregnancy may have consequences to development of the nervous system.
- "For every neuron added to the cerebral cortex in evolution, four neurons are added to the cerebellum."[4]
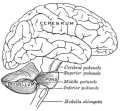
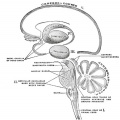
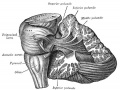
The adult cerebellum anatomy consists of three parts, the vermis (median) and the two hemispheres (lateral), which are continuous with each other.
Within the neural tube stem cells generate the 2 major classes of cells that make the majority of the nervous system : neurons and glia. Both these classes of cells differentiate into many different types generated with highly specialized functions and shapes. This section covers the establishment of neural populations, the inductive influences of surrounding tissues and the sequential generation of neurons establishing the layered structure seen in the brain and spinal cord.
- Neural development beginnings quite early, therefore also look at notes covering Week 3- neural tube and Week 4-early nervous system.
- Development of the neural crest and sensory systems (hearing/vision/smell) are only introduced in these notes and are covered in other notes sections.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Cerebellum Embryology <pubmed limit=5>Cerebellum Embryology</pubmed> |
| Older papers |
|---|
|
Development Overview
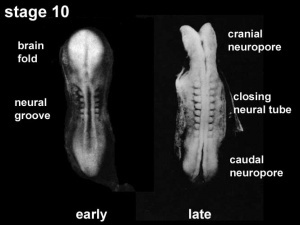
Neuralation begins at the trilaminar embryo with formation of the notochord and somites, both of which underly the ectoderm and do not contribute to the nervous system, but are involved with patterning its initial formation. The central portion of the ectoderm then forms the neural plate that folds to form the neural tube, that will eventually form the entire central nervous system.
- Early developmental sequence: Epiblast - Ectoderm - Neural Plate - Neural groove and Neural Crest - Neural Tube and Neural Crest
| Neural Tube | Primary Vesicles | Secondary Vesicles | Adult Structures |
|---|---|---|---|
| week 3 | week 4 | week 5 | adult |
| prosencephalon (forebrain) | telencephalon | Rhinencephalon, Amygdala, hippocampus, cerebrum (cortex), hypothalamus, pituitary | Basal Ganglia, lateral ventricles | |
| diencephalon | epithalamus, thalamus, Subthalamus, pineal, posterior commissure, pretectum, third ventricle | ||
| mesencephalon (midbrain) | mesencephalon | tectum, Cerebral peduncle, cerebral aqueduct, pons | |
| rhombencephalon (hindbrain) | metencephalon | cerebellum | |
| myelencephalon | medulla oblongata, isthmus | ||
| spinal cord, pyramidal decussation, central canal | |||
The following text description is from a study of 20 human embryos and fetuses between 6 weeks to 16 weeks from the Madrid Collection.[10]
- 6 weeks (CRL 12–16 mm) - anlage of the cerebellum was first identified as a pair of thickenings on the lateral site of the alar plate that faced the fourth ventricle.
- 7-9 weeks (CRL 28 mm) - rhombic lip (a pair of thickenings of the alar plate) protruded dorsally, bent laterally, extended ventrolaterally and fused with the medially located midbrain. During that process, the primitive choroid plexus appeared to become involved in the cerebellar hemisphere to form a centrally located eosinophilic matrix. At that stage, the inferior olive had already developed in the thick medulla. Thus, the term 'bulbo-pontine extension' may represent an erroneous labeling of a caudal part of the rhombic lip. The cerebellar vermis developed much later than the hemisphere possibly from a midline dark cell cluster near the aqueduct.
- 11–12 weeks (CRL 70–90 mm) - cerebellar hemisphere became as thick as the mid- brain. In the hemisphere, a laminar configuration became evident but the central eosinophilic matrix remained pres- ent. Fissures of the future vermis appeared in the midline area (Fig. 6): the developing fissures provided island-like structures in horizontal sections. The hemisphere and ver- mis, including the surfaces of the fissures, were covered by the external germinal cell layer.
- 15–16 weeks (CRL 110–130 mm) - cerebellar hemisphere contained the primitive dentate nucleus. The nodule and flocculus were identified, vermis became as thick as the hemisphere and it accompanied several deep fissures.
Early Brain Vesicles
| Primary Vesicles | Secondary Vesicles |
|---|---|
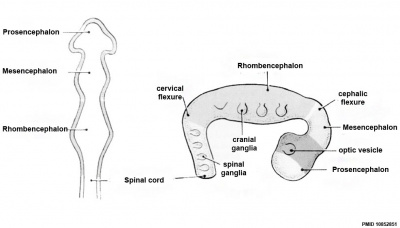
|
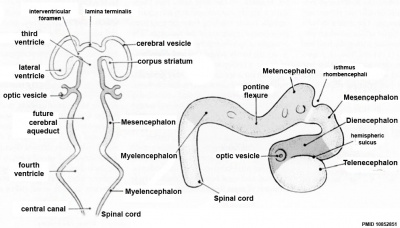
|
| week 3 to 4 | week 5 + |
Embryonic Cerebellum
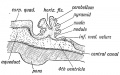
Week 4
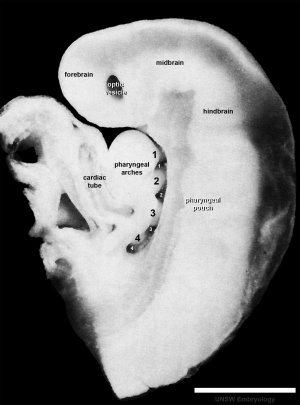
|
This midline section through the upper half of the embryo shows the 3 primary vesicle regions (unlabeled image).
The cerebellum will develop from the hindbrain (rhombencephalon) region. Adjacent to the forth ventricle (see as the enlarged dark dorsal region).
Human Embryo (Carnegie stage 13) 28 - 32 days. |
Week 8
| Carnegie stage 21 | |
|---|---|
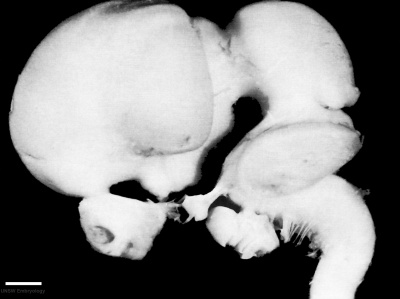
|
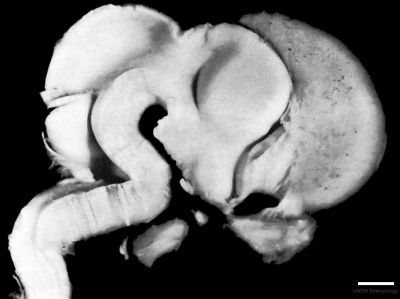
|
| lateral view (scale bar 1 mm) | Median view |
Fetal Cerebellum
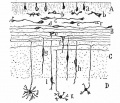
Week 10
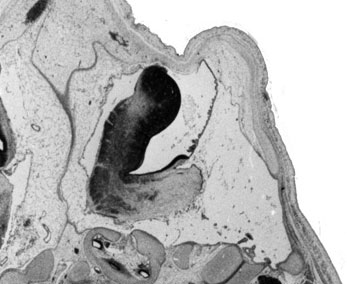
|
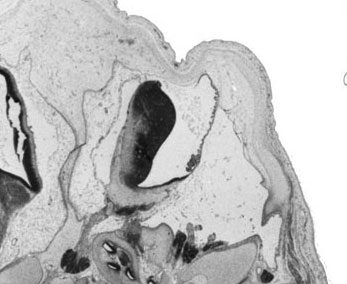
|
| Plane A (midline) | Plane B (medial) |
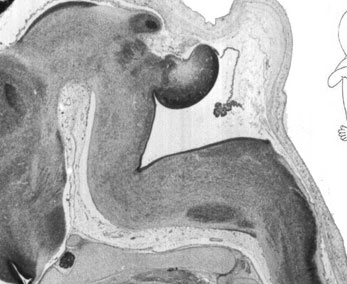
|
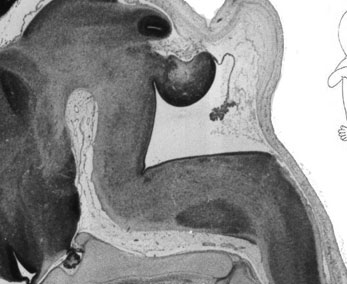
|
| Plane C (lateral) | Plane D (most lateral) |
- Links: 10 week Fetal | Fetal Development
Third Trimester
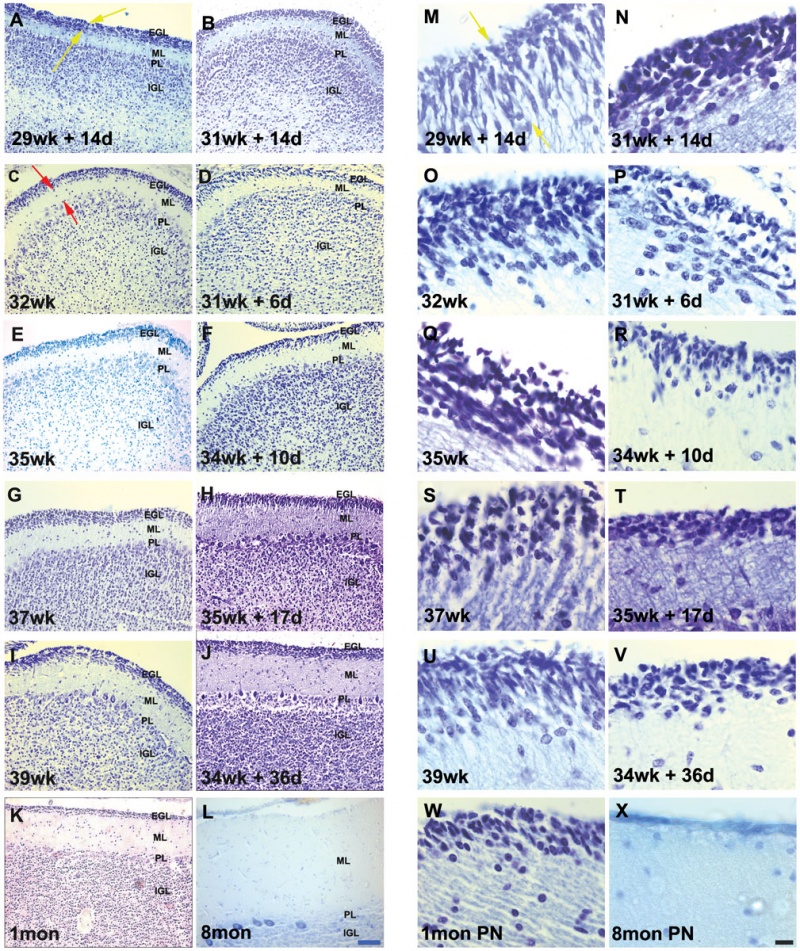
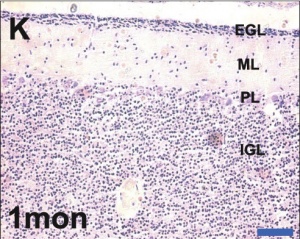
Developing human cerebellum preterm[1]
A greater EGL cell density and reduced EGL thickness were reported in preterms with ex-utero exposure, as compared to their age matched stillborn controls.
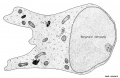
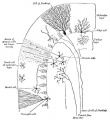
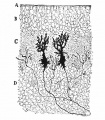
Cerebellar Cells
These drawings of cerebellar cells were based upon electron micrograph images from the rat cerebellum.[11]
Historic Description
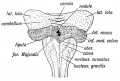
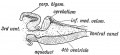
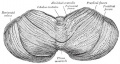
The cerebellum is developed in the roof of the anterior part of the hind-brain (Figs. 649 to 654). The alar laminæ of this region become thickened to form two lateral plates which soon fuse in the middle line and produce a thick lamina which roofs in the upper part of the cavity of the hind-brain vesicle; this constitutes the rudiment of the cerebellum, the outer surface of which is originally smooth and convex. The fissures of the cerebellum appear first in the vermis and floccular region, and traces of them are found during the third month; the fissures on the cerebellar hemispheres do not appear until the fifth month. The primitive fissures are not developed in the order of their relative size in the adult—thus the horizontal sulcus in the fifth month is merely a shallow groove. The best marked of the early fissures are: (a) the fissura prima between the developing culmen and declive, and (b) the fissura secunda between the future pyramid and uvula. The flocculus and nodule are developed from the rhombic lip, and are therefore recognizable as separate portions before any of the other cerebellar lobules. The groove produced by the bending over of the rhombic lip is here known as the floccular fissure; when the two lateral walls fuse, the right and left floccular fissures join in the middle line and their central part becomes the post-nodular fissure. (text from Gray's Anatomy 1918)
Precerebellar Neurons
Precerebellar neurons (PCNs) are born in the hindbrain alar plate in the specific region called the rhombic lip (for review see[12]). From there they migrate by a process termed nucleokinesis[13], extending a cytoplasmic process then move their nucleus.
In recent years a number of different chemotactic positive and negative factors have been suggested to have a role in driving the guided migration of these cells. Many signals are thought to be mediated through the Rho family GTPases links to the cytoskeleton.
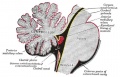
Cerebellar Pathways
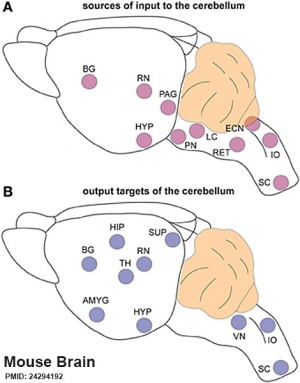
|
(A) Regions that send input to the cerebellum.
|
| Cerebellum connections to the brain and spinal cord (mouse).[15] |
Abnormalities
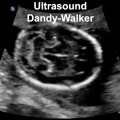
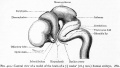
Dandy-Walker Syndrome
| Dandy-Walker Syndrome/Malformation (DWS) is a cerebellar hypoplasia and upward rotation of the cerebellar vermis with ventricular enlargement (cystic dilation of the fourth ventricle). Named in 1954 after the earlier identification by Walter Dandy (1914) and Arthur Earl Walker (1942), two USA neurosurgeons.
Walter Dandy (1886 – 1946) Arthur Earl Walker (1907 – 1995). International Classification of Diseases Q03 Congenital hydrocephalus Incl.: hydrocephalus in newborn Excl.: Arnold-Chiari syndrome (Q07.0) hydrocephalus: acquired (G91.-) due to congenital toxoplasmosis (P37.1) with spina bifida (Q05.0-Q05.4)
|
|
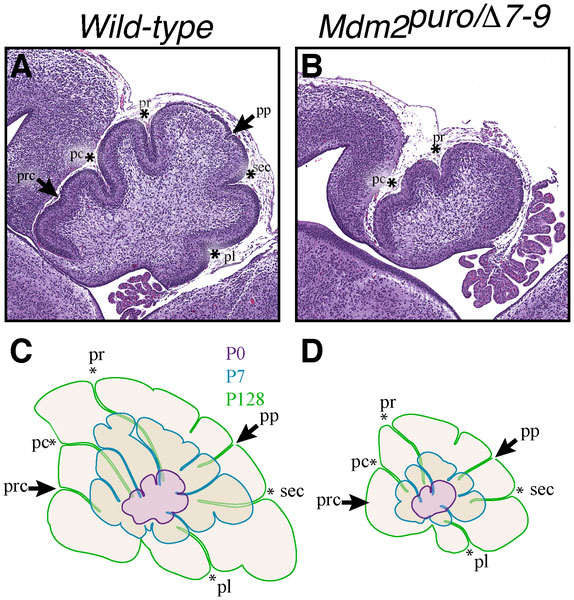
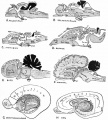
Foliation Defects
Mouse Cerebellar Foliation Defects[16]
(A–B) Midsagittal sections of newborn (P0) wild-type and Mdm2puro/Δ7-9 cerebella stained with H&E. (C–D) Superimposition of P0 (purple outline), P7 (blue outline), and adult (green outline) cerebella from wild-type (C) or Mdm2puro/Δ7-9 (D) mice. By P7, all four primary fissures, as well as two additional fissures, are evident in Mdm2puro/Δ7-9 mice. Moreover, even in adulthood, the mutant cerebellum does not reach the size or complexity of the wild-type cerebellum. Abbreviations are: prc, precentral; pc, pre-culminate; pr, primary; pp, prepyramidal; sec, secondary; pl, posterolateral fissures.
Pontocerebellar Hypoplasia
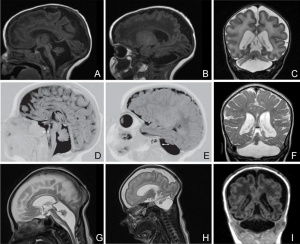
Pontocerebellar Hypoplasia (PCH) are very rare, inherited progressive neurodegenerative disorders with prenatal onset (for recent review see[17]). The major features are: hypoplasia or atrophy of cerebellum and pons, progressive microcephaly, and variable cerebral involvement. There is a further classification of 7 different subtypes (PCH1-7) and there is prenatal testing for the related inherited mutations.
- PCH2, PCH4, PCH5 - Mutations in the 3 tRNA splicing endonuclease subunit genes.
- PCH6 - Mutations in the nuclear encoded mitochondrial arginyl- tRNA synthetase gene.
- PCH1 - Mutations in the tRNA splicing endonuclease, the mitochondrial arginyl- tRNA synthetase and the vaccinia related kinase1.
Medulloblastoma
Medulloblastomas are the most common childhood primary central nervous system tumour. They are thought to arise in the developing cerebellum from the precursors of the granule cell.
References
- ↑ 1.0 1.1 1.2 <pubmed>21858122</pubmed>| PLoS One
- ↑ <pubmed>11724815</pubmed>| PMC2150878 | J Cell Biol.
- ↑ Palmgren A. Embryological and morphological studies on the mid-brain and cerebellum of vertebrates. (1921) Acta Zoologica. 64(5): 2-94.
- ↑ <pubmed>20300467</pubmed>| Front Neuroanat.
- ↑ <pubmed>26166429</pubmed>
- ↑ 6.0 6.1 <pubmed>25519244</pubmed>
- ↑ <pubmed>25050931</pubmed>
- ↑ <pubmed>24516532</pubmed>
- ↑ <pubmed>20835252</pubmed>
- ↑ <pubmed>21380713</pubmed>
- ↑ <pubmed>14222815</pubmed>| PMC2106521 | J Cell Biol.
- ↑ <pubmed>16111554</pubmed>
- ↑ <pubmed>15882636</pubmed>
- ↑ <pubmed>18801355</pubmed>
- ↑ <pubmed>24294192</pubmed>
- ↑ <pubmed>21437245</pubmed>| PLoS One.
- ↑ 17.0 17.1 <pubmed>21749694</pubmed>| Orphanet J Rare Dis.
Journals
- Cerebellum Springer Publishers
Reviews
<pubmed></pubmed> <pubmed></pubmed> <pubmed>27160001</pubmed> <pubmed>25336734</pubmed> <pubmed>22396330</pubmed>PMC3359460 <pubmed>21295689</pubmed> <pubmed>19732611</pubmed> <pubmed>17408845</pubmed> <pubmed>16243598</pubmed> <pubmed>15610138</pubmed> <pubmed>12843872</pubmed>
Articles
<pubmed></pubmed> <pubmed></pubmed> <pubmed>21380713</pubmed> <pubmed>20460306</pubmed> <pubmed>15496441</pubmed>
Search PubMed
Search Pubmed: Cerebellum Embryology | Cerebellum Development | Medulloblastoma
Terms
- Bergmann glia - a cerebellar radial glial population that in development act as scaffolds for the radial migration of granule cell precursors from the external germinal layer (EGL or outer granular layer) to the inner granular cell layer (IGL).
- cerebellar nuclei - clusters of glutamatergic and GABAergic neurons located in the cerebellar white matter that are the synaptic targets of the majority of Purkinje cells. Projection neurons within nuclei account for the output of the cerebellum. Cerebellar nuclei are often termed ‘deep’ although the designation is superfluous.
- External Germinal Layer - (EGL or outer granular layer) a developmental transient layer that is lost following granule cell migration.
- Granule cell - neurons in the internal granule layer that receive excitatory inputs from mossy fibres, that originate in the pons, medulla and spinal cord. These glutamatergic excitatory neutrons receive local inhibitory inputs from Golgi neurons. Granule cells axons are T-shaped and extend into the molecular layer synapsing on Purkinje cell dendrites.
- Inner Granular Layer - (IGL) granule neurons (granule cell) lies deep to the Purkinje cell layer.
- Purkinje cell - neurons located in the cerebellar cortex that receive excitatory inputs from granule cell parallel fibres and inhibitory input from climbing fibres of the inferior olive. These GABAergic inhibitory neurons axons mainly extend to the deep cerebellar nuclei, some axons also directly innervate hindbrain vestibular targets.
Additional Images
Historic Images
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Keith A. Human Embryology and Morphology. (1902) London: Edward Arnold.
Gray, Henry. Anatomy of the Human Body. Philadelphia: Lea & Febiger, 1918.
Bailey, F.R. and Miller, A.M. (1921). Text-Book of Embryology. New York: William Wood and Co. The Cerebellum
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 18) Embryology Neural - Cerebellum Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Neural_-_Cerebellum_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G