McMurrich1914 Chapter 16: Difference between revisions
(Created page with "{{McMurrich1914 header}} ==CHAPTER XVI. THE DEVELOPMENT OF THE ORGANS OF SPECIAL SENSE== Like the cells of the central nervous system, the sensory cells are all of ectoderma...") |
|||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{McMurrich1914 header}} | {{McMurrich1914 header}} | ||
= | =Chapter XVI. The Development of the Organs of Special Sense= | ||
Like the cells of the central nervous system, the sensory cells are all of ectodermal origin, and in lower animals, such as the earthworm, for instance, they retain their original position in the ectodermal epithelium throughout life. In the vertebrates, however, the majority of the sensory cells relinquish their superficial position and sink more or less deeply into the subjacent tissues, being represented by the posterior root ganglion cells and by the sensory cells of the special sense-organs, and it is only in the olfactory organ that the original condition is retained. Those cells which have withdrawn from the surface receive stimuli only through overlying cells, and in certain cases these transmitting cells are not specially differentiated, the terminal branches of the sensory dendrites e ding among ordinary epithelial cells or in such structures as the Pacinian bodies or the end-bulbs of Krause situated beneath undifferentiated epithelium. In other cases, however, certain specially modified superficial cells serve to transmit the stimuli to the peripheral sensory neurones, forming such structures as the hair-cells of the auditory epithelium or the gustatory cells of the taste-buds. | Like the cells of the central nervous system, the sensory cells are all of ectodermal origin, and in lower animals, such as the earthworm, for instance, they retain their original position in the ectodermal epithelium throughout life. In the vertebrates, however, the majority of the sensory cells relinquish their superficial position and sink more or less deeply into the subjacent tissues, being represented by the posterior root ganglion cells and by the sensory cells of the special sense-organs, and it is only in the olfactory organ that the original condition is retained. Those cells which have withdrawn from the surface receive stimuli only through overlying cells, and in certain cases these transmitting cells are not specially differentiated, the terminal branches of the sensory dendrites e ding among ordinary epithelial cells or in such structures as the Pacinian bodies or the end-bulbs of Krause situated beneath undifferentiated epithelium. In other cases, however, certain specially modified superficial cells serve to transmit the stimuli to the peripheral sensory neurones, forming such structures as the hair-cells of the auditory epithelium or the gustatory cells of the taste-buds. | ||
Thus three degrees of differentiation of the special sensory cells may be recognized and a classification of the sense-organs may be made upon this basis. One organ, however, the eye, cannot be brought into such a classification, since its sensory cells present certain developmental peculiarities which distinguish them from those of all other sense-organs. Embryologically the retina is a portion of the central nervous system and not a peripheral organ, and hence it will be convenient to arrange the other sense-organs | Thus three degrees of differentiation of the special sensory cells may be recognized and a classification of the sense-organs may be made upon this basis. One organ, however, the eye, cannot be brought into such a classification, since its sensory cells present certain developmental peculiarities which distinguish them from those of all other sense-organs. Embryologically the retina is a portion of the central nervous system and not a peripheral organ, and hence it will be convenient to arrange the other sense-organs according to the classification indicated and to discuss the" history of the eye at the close of the chapter. | ||
==The Development of the Olfactory Organ== | |||
The general development of the nasal fossa, the epithelium of which contains the olfactory sense cells, has already been described (pp. 99 and 283), as has also the development of the olfactory lobes of the brain (p. 406), and there remains for consideration here merely the formation of the olfactory nerve and the development of the rudimentary organ of Jacobson. | |||
===The Olfactory Nerve=== | |||
Very diverse results have been obtained by various observers of the development of the olfactory nerve, it having been held at different times that it was formed by the outgrowth of fibers from the olfactory lobes (Marshall), from fibers which arise partly from the olfactory lobes and partly from the olfactory epithelium (Beard), from the cells of an olfactory ganglion originally derived from the olfactory epithelium but later separating from it (His), and, finally, that it was composed of the prolongations of certain cells situated and, for the most part at least, remaining permanently in the olfactory epithelium (Disse). The most recent observations on the structure of the olfactory epithelium and nerve indicate a greater amount of probability in the last result than in the others, and the description which follows will be based upon the observations of His, modified in conformity with the results obtained by Disse from chick embryos. | |||
In human embryos of the fourth week the cells lining the upper part of the olfactory pits show a distinction into ordinary epithelial and sensory cells, the latter, when fully formed, being elongated cells prolonged peripherally into a short but narrow process which reaches the surface of the epithelium and proximally gives rise to an axis-cylinder process which extends up toward and penetrates the tip of the olfactory lobe to come into contact with the dendrites of the first central neurones of the olfactory tract (Fig. 252). These cells constitute a neuro-epithelium and in later stages of development retain their epithelial position for the most part, a few of them, however, withdrawing into the subjacent mesenchyme and becoming bipolar, their peripheral prolongations ending freely among the cells of the olfactory epithelium. These bipolar cells resemble closely in form and relations the cells of the embryonic posterior root ganglia, and thus form an interesting transition between these and the neuroepithelial cells. | |||
===The Organ of Jacohson=== | |||
In embryos of three or four months a small pouch-like invagination of the epithelium covering the lower anterior portion of the median septum of the nose can readily be seen. This becomes converted into a slender pouch, 3 to 5 mm. long, ending blindly at its posterior extremity and opening at its other end into the nasal cavity. Its lining epithelium resembles that of the respiratory portion of the nasal cavity, and there is developed in the connective tissue beneath its floor a slender plate of cartilage, distinct from that forming the septum of the nose. | |||
[[File:McMurrich1914 fig252.jpg|500px]] | |||
Fig. 252. Diagram Illustrating the Relations of the Fibers of the Olfactory Nerve. | |||
Ep, Epithelium of the olfactory pit; C, cribiform plate of the'ethmoid, G, glomerulus of the olfactory bulb; M, mitral cell. - (Van Gekuchten.) | |||
Ep, Epithelium of the olfactory pit; C, cribiform plate of the'ethmoid, G, glomerulus of | |||
the olfactory bulb; M, mitral cell. - (Van Gekuchten.) | |||
| Line 54: | Line 36: | ||
The olfactory neuro-epithelium, considered from a comparative standpoint, seems to have been derived from the system of lateral line organs so highly developed in the lower vertebrates (Kupffer). In higher forms the system, which is cutaneous in character, has disappeared except in two regions where it has become highly specialized. In one of these regions it has given rise to the olfactory sense cells and in the other to the similar cells of the auditory apparatus. | The olfactory neuro-epithelium, considered from a comparative standpoint, seems to have been derived from the system of lateral line organs so highly developed in the lower vertebrates (Kupffer). In higher forms the system, which is cutaneous in character, has disappeared except in two regions where it has become highly specialized. In one of these regions it has given rise to the olfactory sense cells and in the other to the similar cells of the auditory apparatus. | ||
==The Organs of Touch and Taste== | |||
Little is yet known concerning the development of the various forms of tactile organs, which belong to the second class of sensory organs described above. | |||
The taste | ===The Organs of Taste=== | ||
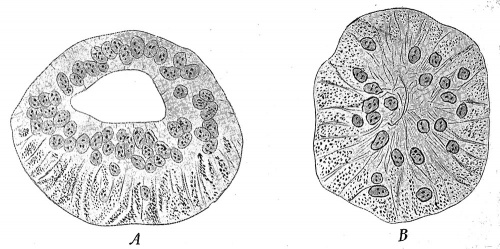
he remaining organs of special sense belong to the third class, and of these the organs of taste present in many respects the simplest condition. They are developed principally in connection with the vallate and foliate papillae of the tongue, and of the former one of the earliest observed stages has been found in embryos of 9 cm. in the form of two ridges of epidermis, lying toward the back part of the tongue and inclined to one another in such a manner as to form a V with the apex directed backward. From these ridges solid downgrowths of epidermis into the subjacent tissue occur, each downgrowth having the form of a hollow truncated cone with its basal edge continuous with the superficial epidermis (Fig. 253, A). In later stages lateral outgrowths develop from the deeper edges of the cone, and about the same time clefts appear in the substance of the original downgrowths (Fig. 253, B) and, uniting together, finally open to the surface, forming a trench surrounding a papilla (Fig. 253, C). The lateral outgrowths, which are at first solid, also undergo an axial degeneration and become converted into the glands of Ebner (b), which open into the trench near its floor. The various papillae which occur in the adult do not develop simultaneously, but their number increases with the age of the fetus, and there is, moreover, considerable variation in the time of their development. | |||
The taste-buds are formed by a differentiation of the epithelium which covers the papillae, and this differentiation appears to stand in intimate relation with the penetration of fibers of the glossopharyngeal nerve into the papillae. The buds form at various places upon the papillae, and at one period are especially abundant upon their free surfaces, but in the later weeks of intrauterine life these surface buds undergo degeneration and only those upon the sides of the trench persist, as a rule. | |||
[[File:McMurrich1914 fig253.jpg|500px]] | |||
Fig. 253. Diagrams Representing the Development of a Vallate Papilla. a, Valley surrounding the papilla; b, von Ebner's gland. - (Graberg.) | |||
Fig. 253. | |||
The foliate papillae do not seem to be developed until some time after the circumvallate, being entirely wanting in embryos of four and a half and five months, although plainly recognizable at the seventh month. | The foliate papillae do not seem to be developed until some time after the circumvallate, being entirely wanting in embryos of four and a half and five months, although plainly recognizable at the seventh month. | ||
==The Development of the Ear== | |||
It is customary to describe the mammalian ear as consisting of three parts, known as the inner, middle, and outer ears, and this division is, to a certain extent at least, confirmed by the embryonic development. The inner ear, which is the sensory portion proper, is an ectodermal structure, which secondarily becomes deeply seated in the mesodermal tissue of the head, while the middle and outer ears, which provide the apparatus necessary for the conduction of the sound-waves to the inner ear, are modified portions of the anterior branchial arches. It will be convenient, accordingly, in the description of the ear, to accept the usually recognized divisions and to consider first of all the development of the inner ear, or, as it is better termed, the otocyst. The Development of the Otocyst. - In an embryo of 2.4 mm. a pair of pits occur upon the surface of the body about opposite the middle portion of the hind-brain (Fig. 254, A). The ectoderm lining the pits is somewhat thicker than is the neighboring ectoderm | |||
[[File:McMurrich1914 fig264.jpg|500px]] | |||
Fig. 254. Transverse Section Passing through the Otocyst (ot) of Embryos of (A) 2.4 mm. and (B) 4 mm. (His.) | |||
of the surface of the body, and, from analogy with what occurs in other vertebrates, it seems probable that the pits are formed by the invagination of localized thickenings of the ectoderm. The mouth of each pit gradually becomes smaller, until finally the invagination is converted into a closed sac (Fig. 254, B), which separates from the surface ectoderm and becomes enclosed within the subjacent mesoderm. This sac is the otocyst, and in the stage just described, found in embryos of 4 mm., it has an oval or more or less spherical form. Soon, however, in embryos of 6.9 mm., a prolongation arises from its dorsal portion and the sac assumes the form shown in Fig. 255, A; this prolongation, which is held by some authors to be the remains of the stalk which originally connected the otocyst sac with the surface ectoderm, represents the ductus endolymphaticus , and, increasing in length, it soon becomes a strong club-shaped process, projecting considerably beyond the remaining portions of the otocyst (Fig. 255, B). In embryos of about 10.2 mm. the sac begins to show certain other irregularities of shape (Fig. 255, B, sc). Thus, about opposite the point of origin of the ductus endolymphaticus three folds make their appearance, representing the semicircular ducts, and as they increase in size the opposite walls of the central portion of each fold come together, fuse, and finally become absorbed, leaving the free edge of the fold as a crescentic canal, at one end of which an enlargement appears to form the ampulla. The transformation of the folds into canals takes place somewhat earlier in the cases of the two vertical than in that of the horizontal duct, as may be seen from Fig. 256, which represents the condition occurring in an embryo of 13.5 mm. | |||
of the surface of the body, and, from analogy with what occurs in other vertebrates, it seems probable that the pits are formed by the invagination of localized thickenings of the ectoderm. The mouth of each pit gradually becomes smaller, until finally the invagination is converted into a closed sac (Fig. 254, B), which separates from the surface ectoderm and becomes enclosed within the subjacent mesoderm. This sac is the otocyst, and in the stage just described, found in embryos of 4 mm., it has an oval or more or less spherical form. Soon, however, in embryos of 6.9 mm., a prolongation arises from its dorsal portion and the sac assumes the form shown in Fig. 255, A; this prolongation, which is held by some authors to be the remains of the stalk which originally connected the otocyst sac | |||
[[File:McMurrich1914 fig255.jpg|500px]] | |||
Fig. 255. - Reconstruction of the Otocysts of Embryo of (A) 6.9 mm. and (B) 10.2 mm. | |||
de, Endolymphatic duct; gc, ganglion cochleare; gg, ganglion geniculatum; gv, ganglion vestibulare; sc, lateral semicircular duct. - (His, Jr.) | |||
| Line 139: | Line 82: | ||
is closely associated with the geniculate ganglion of the seventh | is closely associated with the geniculate ganglion of the seventh | ||
nerve (Fig. 255, B), the two, usually spoken of as the acustico-facialis | nerve (Fig. 255, B), the two, usually spoken of as the acustico-facialis | ||
ganglion, forming a mass of cells lying in close contact with the | ganglion, forming a mass of cells lying in close contact with the anterior wall of the otocyst. The origin of the ganglionic mass has not yet been traced in the mammalia, but it has been observed that in cow embryos the geniculate ganglion is connected with the ectoderm at the dorsal end of the first branchial cleft (Froriep), and it may perhaps be regarded as one of the epibranchial placodes (see p. 417), and in the lower vertebrates a union of the ganglion with a suprabranchial placode has been observed (Kupffer), this union indicating the origin of the auditory ganglion from one or more of the ganglia of the lateral line system. | ||
[[File:McMurrich1914 fig256.jpg|500px]] | |||
Fig. 256. - Reconstruction of the Otocyst of an Embryo of | Fig. 256. - Reconstruction of the Otocyst of an Embryo of 13.5 MM. | ||
co, Cochlea; de, endolymphatic duct;.sc, semicircular duct. - (His Jr.) | co, Cochlea; de, endolymphatic duct;.sc, semicircular duct. - (His Jr.) | ||
[[File:McMurrich1914 fig257.jpg|500px]] | |||
Fig. 257. Reconstruction of the Otocyst of an Embryo of 20 mm., front view. cc, Common limb of superior and posterior semicircular ducts; eg, cochlear ganglion; co, cochlea; de, endolymphatic duct; s, sacculus; sdl, sdp, and sds x lateral, posterior and superior semicircular ducts; u, utriculus; vg, vestibular ganglion. - (Streeter.) | |||
At an early stage in the human embryo the auditory ganglion shows indications of a division into two portions, a more dorsal one, which represents the future ganglion vestibular e, and a ventral one, the ganglion cochleare. The ganglion cells become bipolar, in which condition they remain throughout life, never reaching the T-shaped condition found in most of the other peripheral cerebro-spinal ganglia. One of the prolongations of each cell is directed centrally to form a fiber of the auditory nerve, while the other penetrates the wall of the otocyst to enter into relations with certain specially modified cells which differentiate from its lining epithelium. | |||
â– In the earliest stages the ectodermal lining of the otocyst is formed of similar columnar cells, but later over the greater part of the surface the cells flatten down, only a few, aggregated together to form patches, retaining the high columnar form and developing hairlike processes upon their free surfaces. These are the sensory cells of the ear. In the human ear there are in all six patches of these sensory cells, an elongated patch (crista ampullaris) in the ampulla of each semicircular canal (Fig. 258, cr), a round patch (macula acustica, mii) in the utriculus and another (ms) in the sacculus, and, finally, an elongated patch which extends the entire length of the scala media of the cochlea and forms the sensory cells of the spiral organ of Corti. The cells of this last patch are connected with the fibers from the cochlear ganglion, while those of the vestibular ganglion pass to the cristas and maculae. | |||
[[File:McMurrich1914 fig258.jpg|500px]] | |||
Fig. 258. The Right Internal Ear of an Embryo of Six Months. ca, ce, and cp, Superior, lateral, and posterior semicircular ducts; cr, crista acustica; de, endolymphatic duct; Is, spiral ligament; mb, basilar membrane; ms and tnu, macula acustica sacculi and utriculi; rb, basilar branches of the cochlear nerve. - (Retzius.) | |||
In connection with the spiral organ certain adjacent cells also retain their columnar form and undergo various modifications, giving rise to a rather complicated structure whose development has been traced in the rabbit. Along the whole length of the cochlear duct the cells resting upon that half of the basilar membrane which is nearest the axis of the cochlea, and may be termed the inner half, retain their columnar shape, forming two ridges projecting slightly into the cavity of the scala (Fig. 259). The cells of the inner ridge, much the larger of the two, give rise to the membrana tectoria, either as a cuticular secretion or by the artificial adhesion of long hair-like processes which project from their free surfaces (Ayers). The cells of the outer ridge are arranged in six longitudinal rows (Fig. 259, 1-6); those of the innermost row (1) develop hairs upon their free surfaces and form the inner hair cells, those of the next two rows (2 and 3) gradually become transformed on their adjacent surfaces into chitinous | |||
[[File:McMurrich1914 fig259.jpg|500px]] | |||
Fig. 259. | Fig. 259. Section of the Cochlear Duct of a Rabbit Embryo of 55 mm. | ||
a, Mesenchyme; b to e, epithelium of cochlear duct; M.t, membrana tectoria; V.s.p, vein; 1 to 7, spiral organ of Corti. - (Baginsky.) | |||
p /''- /^ substance and form the rods of Corti, while the three outer rows | |||
p /''- /^ substance and form the rods of | |||
Corti, while the three outer rows | |||
| Line 237: | Line 132: | ||
While the various changes described above have been taking place in the otocyst, the mesoderm surrounding it has also been undergoing development. At first this tissue is quite uniform in character, but later the cells immediately surrounding the otocyst condense to give rise to a fibrous layer (Fig. 260, ep), while more peripherally they become more loosely arranged and form a somewhat gelatinous layer (s) , and still more peripherally a second fibrous layer is differentiated and the remainder of the tissue assumes a character which indicates an approaching conversion into cartilage. The further history of these various layers is as follows: The inner fibrous layer gives rise to the connective-tissue wall which supports the ectodermal lining of the various portions of the otocyst; the gelatinous layer undergoes a degeneration to form a lymph-like | While the various changes described above have been taking place in the otocyst, the mesoderm surrounding it has also been undergoing development. At first this tissue is quite uniform in character, but later the cells immediately surrounding the otocyst condense to give rise to a fibrous layer (Fig. 260, ep), while more peripherally they become more loosely arranged and form a somewhat gelatinous layer (s) , and still more peripherally a second fibrous layer is differentiated and the remainder of the tissue assumes a character which indicates an approaching conversion into cartilage. The further history of these various layers is as follows: The inner fibrous layer gives rise to the connective-tissue wall which supports the ectodermal lining of the various portions of the otocyst; the gelatinous layer undergoes a degeneration to form a lymph-like fluid known as the perilymph, the space occupied by the fluid being the perilymphatic space; the outer fibrous layer becomes perichondrium and later periosteum; and the precartilage undergoes chondrification and later ossifies to form the petrous portion of the temporal bone. | ||
fluid known as the perilymph, the space occupied by the fluid being the perilymphatic space; the outer fibrous layer becomes perichondrium and later periosteum; and the precartilage undergoes chondrification and later ossifies to form the petrous portion of the temporal bone. | |||
The gelatinous layer completely surrounds most of the otocyst structures, which thus come to lie free in the perilymphatic space, but in the cochlear region the conditions are somewhat different. In this region the gelatinous layer is interrupted along two lines, an outer broad one where the connective-tissue wall of the cochlear duct is directly continuous with the perichondrium layer, and an inner narrow one, along which a similar fusion takes place with the perichondrium of a shelf-like process of the cartilage, which later ossifies to form the lamina spiralis. Consequently throughout the cochlear region the perilymphatic space is divided into two compartments which communicate at the apex of the cochlea, while below one, known as the scala vestibuli, communicates with the space surrounding the saccule and utricle, and the other, the scala tympani, abuts upon a membrane which separates it from the cavity of the middle ear and represents a portion of the outer wall of the petrous bone where chondrification and ossification have failed to occur. This membrane closes what appears in the dried skull to be an opening in the inner wall of the middle ear, known as the fenestra cochlea (rotunda) ; another similar opening, also closed by membrane in the fresh skull, occurs in the bony wall opposite the utricular portion of the otocyst and is known as the fenestra vestibuli (ovalis) . | |||
[[File:McMurrich1914 fig261.jpg|500px]] | |||
Fig. 261. Diagrammatic Transverse Section through a Coil of the Cochlea showing the relation of the scal^e. c, Organ of Corti; co, ganglion cochleare; Is, lamina spiralis; SAT, cochlear duct; ST, scala tympani; SV, scala vestibuli. - (From Gerlach.) | |||
==The Development of the Middle Ear== | |||
The middle ear develops from the upper part of the pharyngeal groove which represents the endodermal portion of the first branchial cleft. This becomes prolonged dorsally and at its dorsal end enlarges to form the tympanic cavity, while the narrower portion intervening between this and the pharyngeal cavity represents the tuba auditiva (Eustachian tube). | |||
| Line 268: | Line 150: | ||
It has been seen in an earlier chapter that the axial mesoderm of each branchial arch gives rise to skeletal structures and muscles. The axial cartilage of the ventral portion of the first arch is what is known as Meckel's cartilage, but in that portion of the arch which forms the roof and anterior wall of the tympanic cavity, the cartilage becomes constricted to form two masses which later ossify to form the malleus and incus (Fig. 262, m and i), while the muscular tissue of this dorsal portion of the arch gives rise to the tensor tympani. Similarly, in the case of the second arch there is to be found, dorsal to | It has been seen in an earlier chapter that the axial mesoderm of each branchial arch gives rise to skeletal structures and muscles. The axial cartilage of the ventral portion of the first arch is what is known as Meckel's cartilage, but in that portion of the arch which forms the roof and anterior wall of the tympanic cavity, the cartilage becomes constricted to form two masses which later ossify to form the malleus and incus (Fig. 262, m and i), while the muscular tissue of this dorsal portion of the arch gives rise to the tensor tympani. Similarly, in the case of the second arch there is to be found, dorsal to the extremity of the cartilage which forms the styloid process of the adult, a narrow plate of cartilage which forms an investment for the facial nerve (Fig. 262, VII), and dorsal to this a ring of cartilage (st) which surrounds a small stapedial artery and represents the stapes. | ||
It has been found that in the rabbit the mass of cells from which the stapes is formed is at its first appearance quite independent of the second branchial arch (Fuchs), and it has been held to be a derivative of the mesenchyme from which the periotic capsule is formed. In later stages, however, it becomes connected with the cartilage of the second branchial arch, as shown in Fig. 262, and it is a question whether this connection, which is transitory, does not really indicate the phylogenetic origin of the ossicle from the second arch cartilage, its appearance as an independent structure being a secondary ontogenetic phenomenon. However that may be, the stapedial artery disappears in later stages and the stapedius muscle, derived from the musculature of the second branchial arch and therefore supplied by the facial nerve, becomes attached to the ossicle. | |||
[[File:McMurrich1914 fig262.jpg|500px]] | |||
Fig. 262. - Semi-diagrammatic Viewof the Auditory Ossicles of an Embryo of Six Weeks. i, Incus; j, jugular vein; m, malleus; mc, Meckel's cartilage; oc, capsule of otocyst; R, cartilage of the second branchial arch; st, stapes; VII, facial nerve. - (Siebenmann.) | |||
| Line 287: | Line 165: | ||
[[File:McMurrich1914 fig263.jpg|500px]] | |||
Fig. 263. | Fig. 263. Diagrams Illustrating the Mode of Extension of the Tympanic Cavity Around the Auditory Ossicles. | ||
M, Malleus; m, spongy mesenchyme; p, surface of the periotic capsule; T, tympanic cavity. The broken line represents the epithelial lining of the tympanic cavity. | M, Malleus; m, spongy mesenchyme; p, surface of the periotic capsule; T, tympanic cavity. The broken line represents the epithelial lining of the tympanic cavity. | ||
| Line 301: | Line 177: | ||
In the account of the development of the ear-bones given above it is held that the malleus and incus are derivatives of the first branchial (mandibular) arch and the stapes probably of the second. This view represents the general consensus of recent workers on the difficult question of the origin of these bones, but it should be mentioned that nearly all possible modes of origin have been at one time or other suggested. The malleus has very generally been accepted as coming from the first arch, and the same is true of the incus, although some earlier authors have assigned it to the second arch. But with regard to the stapes the opinions have been very varied. It has been held to be derived from the first arch, from the second arch, from neither one nor the other, but from the cartilaginous investment of the otocyst, or, finally, it has been held to have a compound origin, its arch being a product of the second arch while its basal plate was a part of the otocyst investment. | In the account of the development of the ear-bones given above it is held that the malleus and incus are derivatives of the first branchial (mandibular) arch and the stapes probably of the second. This view represents the general consensus of recent workers on the difficult question of the origin of these bones, but it should be mentioned that nearly all possible modes of origin have been at one time or other suggested. The malleus has very generally been accepted as coming from the first arch, and the same is true of the incus, although some earlier authors have assigned it to the second arch. But with regard to the stapes the opinions have been very varied. It has been held to be derived from the first arch, from the second arch, from neither one nor the other, but from the cartilaginous investment of the otocyst, or, finally, it has been held to have a compound origin, its arch being a product of the second arch while its basal plate was a part of the otocyst investment. | ||
==The Development of the Tympanic Membrane and of the Outer Ear== | |||
Just as the tympanic cavity is formed from the endodermal groove of the first branchial cleft, so the outer ear owes its origin to the ectodermal groove of the same cleft and to the neighboring arches. The dorsal and most ventral portions of the groove flatten out and disappear, but the median portion deepens to form, at about the end of the second month, a funnel-shaped cavity which corresponds to the outer portion of the external auditory meatus. From the inner end of this a solid ingrowth of ectoderm takes place, and this, enlarging at its inner end to form a disk-like mass, comes into relation with the gelatinous mesoderm which surrounds the malleus and chorda tympani. At about the seventh month a split occurs in the disk-like mass (Fig. 264), separating it into an outer and an inner layer, the latter of which becomes the outer epithelium of the tympanic membrane. Later, the split extends outward in the substance of the ectodermal ingrowth and eventually unites with the funnel-shaped cavity to complete the external meatus. | |||
[[File:McMurrich1914 fig264.jpg|500px]] | |||
Fig. 264. Horizontal Section Passing through the Dorsal Wall of the External Auditory Meatus in an Embryo of 4.5 cm. | |||
c, Cochlea; de, endolymphatic duct; i, incus; Is, transverse sinus; m, malleus; me, meatus auditorius externus; me' , cavity of the meatus; s, sacculus; sc, lateral semicircular | |||
canal; sc', posterior semicircular canal; st, stapes; t, tympanic cavity; u, utriculus; 7, facial nerve. - (Siebenmann.) | |||
The tympanic membrane is formed in considerable part from the substance of the first branchial arch, the area in which it occurs not being primarily part of the wall of the tympanic cavity, but being brought into it secondarily by the expansion of the cavity. The membrane itself is mesodermal in origin and is lined on its outer surface by an ectodermal and on the inner by an endodermal epithelium. | The tympanic membrane is formed in considerable part from the substance of the first branchial arch, the area in which it occurs not being primarily part of the wall of the tympanic cavity, but being brought into it secondarily by the expansion of the cavity. The membrane itself is mesodermal in origin and is lined on its outer surface by an ectodermal and on the inner by an endodermal epithelium. | ||
| Line 320: | Line 194: | ||
The auricle (pinna) owes its origin to the portions of the first and second arches which bound the entrance of the external meatus. Upon the posterior edge of the first arch there appear about the end of the fourth week two transverse furrows which mark off three tubercles (Fig. 258, A, 1-3) and on the anterior edge of the second arch a corresponding number of tubercles (4-6) is formed, while, in addition, a longitudinal furrow, running down the middle of the arch, marks ofT a ridge (c) lying posterior to the tubercles. From these six tubercles and the ridge are developed the various parts of the auricle, as may be seen from Fig. 265 which represents the transformation as described by His. According to this, the most ventral tubercle of the first arch (i) gives rise to the tragus, and the middle one (5) of the second arch furnishes the antitragus. The middle and dorsal tubercles of the first arch (2 and 3) unite with the ridge (c) to produce the helix, while from the dorsal tubercle of the second arch (4) is produced the anthelix and from the ventral one (6) the lobule. More recent observations, however, seem to indicate that the lobule is an accessory structure unrelated to the tubercles and that the sixth tubercle gives rise to the antitragus, while the fifth is either included in the anthelix or else disappears. It is noteworthy that up to about the third month of development the upper and posterior portion of the helix is bent forward so as to conceal the anthelix (Fig. 265, D); it is at just about a corresponding stage that the pointed form of the ear seen in the lower mammals makes its appearance, and it is evident that, were it not for the forward bending, the human ear would also be assuming at this stage a more or less pointed form. Indeed, there is usually to be found upon the incurved edge of the helix, some distance below the upper border of the auricle, a more or less distinct tubercle, known as Darwin's tubercle, which seems to represent the point of the typical mammalian ear, and is, accordingly, the morphological apex of the pinna. | The auricle (pinna) owes its origin to the portions of the first and second arches which bound the entrance of the external meatus. Upon the posterior edge of the first arch there appear about the end of the fourth week two transverse furrows which mark off three tubercles (Fig. 258, A, 1-3) and on the anterior edge of the second arch a corresponding number of tubercles (4-6) is formed, while, in addition, a longitudinal furrow, running down the middle of the arch, marks ofT a ridge (c) lying posterior to the tubercles. From these six tubercles and the ridge are developed the various parts of the auricle, as may be seen from Fig. 265 which represents the transformation as described by His. According to this, the most ventral tubercle of the first arch (i) gives rise to the tragus, and the middle one (5) of the second arch furnishes the antitragus. The middle and dorsal tubercles of the first arch (2 and 3) unite with the ridge (c) to produce the helix, while from the dorsal tubercle of the second arch (4) is produced the anthelix and from the ventral one (6) the lobule. More recent observations, however, seem to indicate that the lobule is an accessory structure unrelated to the tubercles and that the sixth tubercle gives rise to the antitragus, while the fifth is either included in the anthelix or else disappears. It is noteworthy that up to about the third month of development the upper and posterior portion of the helix is bent forward so as to conceal the anthelix (Fig. 265, D); it is at just about a corresponding stage that the pointed form of the ear seen in the lower mammals makes its appearance, and it is evident that, were it not for the forward bending, the human ear would also be assuming at this stage a more or less pointed form. Indeed, there is usually to be found upon the incurved edge of the helix, some distance below the upper border of the auricle, a more or less distinct tubercle, known as Darwin's tubercle, which seems to represent the point of the typical mammalian ear, and is, accordingly, the morphological apex of the pinna. | ||
[[File:McMurrich1914 fig265.jpg|500px]] | |||
Fig. 265. Stages in the Development of the Auricle. | |||
Fig. 265. | |||
A, Embryo of n mm.; B, of 13.6 mm.; C, of 15 mm.; D, at the beginning of the third month; E, fetus of 8.5 cm.; F, fetus at term. - (His.) | A, Embryo of n mm.; B, of 13.6 mm.; C, of 15 mm.; D, at the beginning of the third month; E, fetus of 8.5 cm.; F, fetus at term. - (His.) | ||
There seems to be little room for doubt that the otocyst belongs primarily to the system of lateral line sense-organs, but a discussion of this interesting question would necessitate a consideration of details concerning the development of the lower vertebrates which would be foreign to the general plan of this book. It may be recalled, however, that the analysis of the components of the cranial nerves described on page 415 refers the auditory nerve to the lateral line system. | There seems to be little room for doubt that the otocyst belongs primarily to the system of lateral line sense-organs, but a discussion of this interesting question would necessitate a consideration of details concerning the development of the lower vertebrates which would be foreign to the general plan of this book. It may be recalled, however, that the analysis of the components of the cranial nerves described on page 415 refers the auditory nerve to the lateral line system. | ||
==The Development of the Eye== | |||
The first indications of the development of the eye are to be found in a pair of hollow outgrowths from the side of the first primary brain vesicle, at a level which corresponds to the junction of the dorsal and ventral zones. Each evagination is directed at first upward and backward, and, enlarging at its extremity, it soon shows a differentiation into a terminal bulb and a stalk connecting the bulb with the brain (Fig. 232). At an early stage the bulb comes into apposition with the ectoderm of the side of the head, and this, over the area of contact, becomes thickened and then depressed to form the beginning of the future lens (Fig. 266). | |||
[[File:McMurrich1914 fig266.jpg|500px]] | |||
Fig. 266. Early Stages in the Development of the Lens in a Rabbit Embryo. The nucleated layer to the left is the ectoderm and the thicker lens epithelium, beneath which is the outer wall of the optic evagination; above and below between the two is mesenchyme. - (Rabl.) | |||
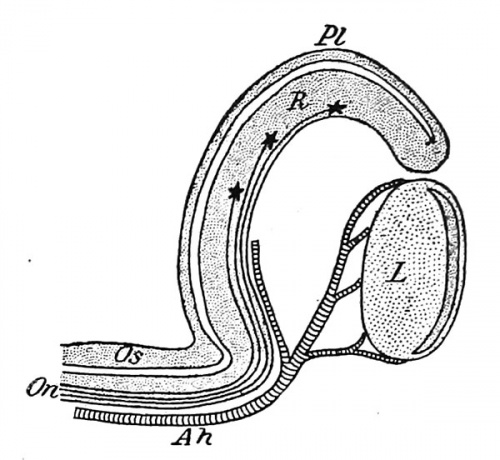
As the result of the depression of the lens ectoderm, the outer wall of the optic bulb becomes pushed inward toward the inner wall, and this invagination continuing until the two walls come into contact, the bulb is transformed into a double-walled cup, the optic cup, in the mouth of which lies the lens (Fig. 268). The cup is not perfect, however, since the invagination affects not only the optic bulb, but also extends medially on the posterior surface of the stalk, forming upon this a longitudinal groove and producing a defect of the ventral wall of the cup, known as the chorioidal fissure (Fig. 267). The groove and fissure become occupied by mesodermal tissue, and in this, at about the fifth week, a blood-vessel develops which traverses the cavity of the cup to reach the lens and is known as the arteria hyaloidea. | |||
[[File:McMurrich1914 fig267.jpg|500px]] | |||
Fig. 267. | Fig. 267. Reconstruction of the Brain of an Embryo of Four Weeks, showing the Chorioid Fissure. - (His.) | ||
In the meantime further changes have been taking place in the lens. The ectodermal depression which represents it gradually deepens to form a cup, the lips of which approximate and finally meet, so that the cup is converted into a vesicle which finally separates completely from the ectoderm (Fig. 268), much in the same way as the otocyst does. As the lens vesicle is constricted off, the surrounding mesodermal tissue grows in to form a layer between it and the overlying ectoderm, and a split appearing in the layer divides it into an outer thicker portion, which represents the cornea, and an inner thinner portion, which covers the outer surface of the lens and becomes highly vascular. The cavity between these two portions represents the anterior chamber of the eye. The cavity of the optic cup has also become filled by a peculiar tissue which represents the vitreous humor, while the mesodermal tissue surrounding the cup condenses to form a strong investment for it, which is externally continuous with the cornea, and at about the sixth week shows a differentiation into an inner vascular layer, the chorioid coat, and an outer denser one, which becomes the sclerotic coat. | In the meantime further changes have been taking place in the lens. The ectodermal depression which represents it gradually deepens to form a cup, the lips of which approximate and finally meet, so that the cup is converted into a vesicle which finally separates completely from the ectoderm (Fig. 268), much in the same way as the otocyst does. As the lens vesicle is constricted off, the surrounding mesodermal tissue grows in to form a layer between it and the overlying ectoderm, and a split appearing in the layer divides it into an outer thicker portion, which represents the cornea, and an inner thinner portion, which covers the outer surface of the lens and becomes highly vascular. The cavity between these two portions represents the anterior chamber of the eye. The cavity of the optic cup has also become filled by a peculiar tissue which represents the vitreous humor, while the mesodermal tissue surrounding the cup condenses to form a strong investment for it, which is externally continuous with the cornea, and at about the sixth week shows a differentiation into an inner vascular layer, the chorioid coat, and an outer denser one, which becomes the sclerotic coat. | ||
[[File:McMurrich1914 fig268.jpg|500px]] | |||
Fig. 268. Horizontal Section through the Eye of an Embryo Pig of 7 mm. Br, Diencephalon; Ec, ectoderm; I, lens; P, pigment, and R, retinal layers of the retina. | |||
Fig. 268. | |||
The various processes resulting in the formation of the eye, which have thus been rapidly sketched, may now be considered in greater detail. | The various processes resulting in the formation of the eye, which have thus been rapidly sketched, may now be considered in greater detail. | ||
===The Development of the Lens=== | |||
When the lens vesicle is complete, it forms a more or less spherical sac lying beneath the superficial ectoderm and containing in its cavity a few cells, either scattered or in groups (Fig. 268). These cells, which have wandered into the cavity of the vesicle from its walls, take no part in the further development of the lens, but early undergo complete degeneration, and the first change which is concerned with the actual formation of the lens is an increase in the height of the cells forming its inner wall and a thinning out of its outer wall (Fig. 269, A). These changes continuing, the outer half of the vesicle becomes converted into a single layer of somewhat flat cells which persist in the adult condition to form the anterior epithelium of the lens, while the cells of the posterior wall form a marked projection into the cavity of the vesicle and eventually completely obliterate it, coming into contact with the inner surface of the anterior epithelium (Fig. 269, B). | |||
These posterior elongated cells form, then, the principal mass of the lens, and constitute what are known as the lens fibers. At first those situated at the center of the posterior wall are the longest, the more peripheral ones gradually diminishing in length until at the equator of the lens they become continuous with and pass into the anterior epithelium. As the lens increases in size, however, the most centrally situated cells fail to elongate as rapidly as the more peripheral ones and are pushed in toward the center of the lens, the more peripheral fibers meeting below them along a line passing across the inner surface of the lens. The disparity of growth continuing, a similar sutural line appears on the outer surface beneath the anterior epithelium, and the fibers become arranged in concentric layers around a central core composed of the shorter fibers. In the human eye the line of suture of the peripheral fibers becomes bent so as to consist of two limbs which meet at an angle, and from the angle a new sutural line develops during embryonic life, so that the suture assumes the form of a three-rayed star. In later life the stars become more complicated, being either six-rayed or more usually nine-rayed in the adult condition (Fig. 270). | |||
[[File:McMurrich1914 fig269.jpg|500px]] | |||
Fig. 269. Sections through the Lens (4) of Human Embryo of Thirty Thirty-one Days and (B) of Pig Embryo of 36 Mu. - (Rabl.) | |||
As early as the second month of development the lens vesicle becomes completely invested by the mesodermal tissue in which blood-vessels are developed in considerable numbers, whence the investment is termed the tunica vasculosa lends (Fig. 278, tv). The arteries of the tunic are in connection principally with the hyaloid artery of the vitreous humor (Fig. 276), and consist of numerous fine branches which envelop the lens and terminate in loops almost at the center of its outer surface. This tunic undergoes degeneration after the seventh month of development, by which time the lens has completed its period of most active growth, and, as a rule, completely disappears before birth. Occasionally, however, it may persist to a greater or less extent, the persistence of the portion covering the outer surface of the lens, known as the membrana papillaris, causing the malformation known as congenital atresia of the pupil. | |||
[[File:McMurrich1914 fig270.jpg|500px]] | |||
Fig. 270. Posterior (Inner) Surface of the Lens from an Adult showing the Sutural Lines. - (Rabl.) | |||
Fig. 270. | |||
| Line 394: | Line 252: | ||
It is interesting from the standpoint of developmental mechanics to note that W. H. Lewis and Spemann have shown that in the Amphibia contact of the optic vesicle with the ectoderm is necessary for the formation of the lens, and, furthermore, if the vesicle be transplanted to other regions of the body of a larva, a lens will be developed from the ectoderm with which it is then in contact, even in the abdominal region, | It is interesting from the standpoint of developmental mechanics to note that W. H. Lewis and Spemann have shown that in the Amphibia contact of the optic vesicle with the ectoderm is necessary for the formation of the lens, and, furthermore, if the vesicle be transplanted to other regions of the body of a larva, a lens will be developed from the ectoderm with which it is then in contact, even in the abdominal region, | ||
The Development of the Optic Cup | |||
===The Development of the Optic Cup=== | |||
When the invagination of the outer wall of the optic bulb is completed, the margins of the resulting cup are opposite the sides of the lens vesicle (Fig. 268), but with the enlargement of the lens and cup the margins of the latter gradually come to lie in front of - that is to say, upon the outer surface of - the lens, forming the boundary of the opening known as the pupil. The lens, consequently, is brought to lie within the mouth of the optic cup, and that portion of the latter which covers the lens takes part in the formation of the iris and the adjacent ciliary body, while its posterior portion gives rise to the retina. | |||
| Line 403: | Line 263: | ||
The Development of the Iris and Ciliary Body | ===The Development of the Iris and Ciliary Body=== | ||
The first change noticeable in the ciliary portion of the retinal layer is its thinning out, a process which continues until the layer consists, like the pigment layer, of but a single layer of cells (Fig. 271), the transition of which to the thicker retinal portion of the layer is somewhat abrupt and corresponds to what is termed the ora serrata in adult anatomy. In embryos of 10.2 cm. the retinal layer throughout its entire extent is readily distinguishable from the pigment layer by the absence in it of all pigmentation, but in older forms this distinction gradually diminishes in the iris region, the retinal layer there acquiring pigment and forming the uvea. | The first change noticeable in the ciliary portion of the retinal layer is its thinning out, a process which continues until the layer consists, like the pigment layer, of but a single layer of cells (Fig. 271), the transition of which to the thicker retinal portion of the layer is somewhat abrupt and corresponds to what is termed the ora serrata in adult anatomy. In embryos of 10.2 cm. the retinal layer throughout its entire extent is readily distinguishable from the pigment layer by the absence in it of all pigmentation, but in older forms this distinction gradually diminishes in the iris region, the retinal layer there acquiring pigment and forming the uvea. | ||
| Line 413: | Line 273: | ||
Embedded in the substance of the iris stroma in the adult are non-striped muscle-fibers, which constitute the sphincter and dilatator iridis. It has long been supposed that these fibers were differentiated from the stroma of the iris, but recent observations have shown that they arise from the cells of the pigment layer of the optic cup, the sphincter appearing near the pupillary border (Fig. 271, Sph) while the dilatator is more peripheral. | Embedded in the substance of the iris stroma in the adult are non-striped muscle-fibers, which constitute the sphincter and dilatator iridis. It has long been supposed that these fibers were differentiated from the stroma of the iris, but recent observations have shown that they arise from the cells of the pigment layer of the optic cup, the sphincter appearing near the pupillary border (Fig. 271, Sph) while the dilatator is more peripheral. | ||
[[File:McMurrich1914 fig271.jpg|500px]] | |||
Fig. 271. Radial Section through the Iris of an Embryo of 19 cm. AE, Pigment layer; CC, ciliary folds; IE, retinal layer; I.Str, iris stroma; Pm, pupillary membrane; Rs, marginal sinus; Sph, sphincter iridis. - (Szili.) | |||
===The Development of the Retina=== | |||
The Development of the Retina | |||
Throughout the retinal region of the cup the pigment layer, undergoing the same changes as in the ciliary region, forms the pigment layer of the retina (Fig. 272, p). The retinal layer increases in thickness and early becomes differentiated into two strata (Fig. 268), a thicker one lying next the pigment layer and containing numerous nuclei, and a thinner one containing no nuclei. The thinner layer, from its position and structure, suggests an homology with the marginal velum of the central nervous system, and probably becomes converted into the nerve-fiber layer of the adult retina, the axis-cylinder processes of the ganglion cells passing into it on their way to the optic nerve. The thicker layer similarly suggests a comparison with the mantle layer of the cord and brain, and in embryos of 38 mm. it becomes differentiated into two secondary layers (Fig. 272), that nearest the pigment layer if) consisting of smaller and more deeply staining nuclei, probably representing the rod and cone and bipolar cells of the adult retina, while the inner layer, that nearest the marginal velum, has larger nuclei and is presumably composed of the ganglion cells. | Throughout the retinal region of the cup the pigment layer, undergoing the same changes as in the ciliary region, forms the pigment layer of the retina (Fig. 272, p). The retinal layer increases in thickness and early becomes differentiated into two strata (Fig. 268), a thicker one lying next the pigment layer and containing numerous nuclei, and a thinner one containing no nuclei. The thinner layer, from its position and structure, suggests an homology with the marginal velum of the central nervous system, and probably becomes converted into the nerve-fiber layer of the adult retina, the axis-cylinder processes of the ganglion cells passing into it on their way to the optic nerve. The thicker layer similarly suggests a comparison with the mantle layer of the cord and brain, and in embryos of 38 mm. it becomes differentiated into two secondary layers (Fig. 272), that nearest the pigment layer if) consisting of smaller and more deeply staining nuclei, probably representing the rod and cone and bipolar cells of the adult retina, while the inner layer, that nearest the marginal velum, has larger nuclei and is presumably composed of the ganglion cells. | ||
[[File:McMurrich1914 fig272.jpg|500px]] | |||
Fig. 272. Portion of a Transverse Section of the Retina of a New-born Rabbit. ch, Chorioid coat; g, ganglion-cell layer; r, outer layer of nuclei; p, pigment layer. (Falchi.) | |||
Fig. | Little is as yet known concerning the further differentiation of the nervous elements of the human retina, but the history of some of them has been traced in the cat, in which, as in other mammals, the histogenetic processes take place at a relatively later period than in man. Of the histogenesis of the inner layer the information is rather scant, but it may be stated that the ganglion cells are the earliest of all the elements of the retina to become recognizable. The rod and cone cells, when first distinguishable, are unipolar cells (Fig. 273, a and c), their single processes extending outward from the cell-bodies to the external limiting membrane which bounds the outer surface of the retinal layer. Even at an early stage the cone cells (a) are distinguishable from the rod cells (c) by their more decided reaction to silver salts, and at first both kinds of cells are scattered throughout the thickness of the layer from which they arise. Later, a fine process grows out from the inner end of each cell, which thus assumes a bipolar form (Fig. 2 73 , b and d) , and, later still, the cells gradually migrate toward the external limiting membrane, beneath which they form a definite layer in the adult. In the meantime there appears opposite the outer end of each cell a rounded eminence projecting from the outer surface of the external limiting membrane into the pigment layer. The eminences over the cone cells are larger than those over the rod cells, and later, as both increase in length, they become recognizable by their shape as the rods and cones. | ||
[[File:McMurrich1914 fig273.jpg|500px]] | |||
Fig. 273. Diagram showing the Development of the Retinal Elements. a, Cone cell in the unipolar, and b, in the bipolar stage; c, rod cells in the unipolar, and d, in the bipolar stage; e, bipolar cells; /and i, amacrine cells; g, horizontal cells; h, ganglion cells; k, Muller's fiber; I, external limiting membrane. - (Kallius, after Cajal.) | |||
| Line 448: | Line 301: | ||
In addition to the various nerve-elements mentioned above, the retina also contains neuroglial elements known as Muller's fibers (Fig. 273, k), which traverse the entire thickness of the retina. The development of these cells has not yet been thoroughly traced, but they resemble closely the ependymal cells observable in early stages of the spinal cord. | In addition to the various nerve-elements mentioned above, the retina also contains neuroglial elements known as Muller's fibers (Fig. 273, k), which traverse the entire thickness of the retina. The development of these cells has not yet been thoroughly traced, but they resemble closely the ependymal cells observable in early stages of the spinal cord. | ||
===The Development of the Optic Nerve=== | |||
The observations on the development of the retina have shown very clearly that the great majority of the fibers of the optic nerve are axis-cylinders of the ganglion cells of the retina and grow from these cells along the optic stalk toward the brain. Their embryonic history has been traced most thoroughly in rat embryos (Robinson), and what follows is based upon what has been observed in that animal. | |||
The | The optic stalk, being an outgrowth from the brain, is at first a hollow structure, its cavity communicating with that of the third ventricle at one end and with that of the optic bulb at the other. When the chorioid fissure is developed, it extends, as has already been described, for some distance along the posterior surface of the stalk and has lying in it a portion of the hyaloid artery. Later, when the lips of the fissure fuse, the artery becomes enclosed within the stalk to form the arteria centralis retina of the adult (Fig. 276). By the formation of the fissure the original cavity of the distal portion of the stalk becomes obliterated, and at the same time the ventral and posterior walls of the stalk are brought into continuity with the retinal layer of the optic cup, and so opportunity is given for the passage of the axis-cylinders of the ganglion cells along those walls (Fig. 274). At an early stage a section of the proximal portion of the optic stalk (Fig. 275, A) shows the central cavity surrounded by a number of nuclei representing the mantle layer, and surrounding these a non-nucleated layer, resembling the marginal velum and continuous distally with the similar layer of the retina. When the ganglion cells of the latter begin to send out their axis-cylinder processes, these pass into the retinal marginal velum and converge in this layer toward the bottom of the chorioidal fissure, so reaching the ventral wall of the optic stalk, in the velum of which they may be distinguished in rat embryos of 4 mm., and still more clearly in those of 9 mm. (Fig. 275, A). Later, as the fibers become more numerous, they gradually invade the lateral and finally the dorsal walls of the stalk, and, at the same time the mantle cells of the stalk become more scattered and assume the form of connective-tissue (neuroglia) cells, while the original cavity of the stalk is gradually obliterated (Fig. 275, B). Finally, the stalk becomes a solid mass of nerve-fibers, among which the altered mantle cells are scattered. | ||
[[File:McMurrich1914 fig274.jpg|500px]] | |||
Fig. | Fig. 274. Diagrammatic Longitudinal Section of the Optic Cup and Stalk passing through the chorioid fissure. | ||
| Line 466: | Line 318: | ||
[[File:McMurrich1914 fig275.jpg|500px]] | |||
Fig. 275. Transverse Sections through the Proximal Part of the Optic Stalk of Rat Embryos of (A) 9 mm. and (5) 11 mm. - (Robinson.) | |||
| Line 484: | Line 328: | ||
A justification of the exclusion of the optic nerve from the category which includes the other cranial nerves has now been presented. For if the retina be regarded as a portion of the central nervous system, it is clear that the nerve is not a nerve at all in the strict sense of that word, but is a tract, confined throughout its entire extent within the central nervous system and comparable to such groups of fibers as the direct cerebellar or fillet tracts of that system. | A justification of the exclusion of the optic nerve from the category which includes the other cranial nerves has now been presented. For if the retina be regarded as a portion of the central nervous system, it is clear that the nerve is not a nerve at all in the strict sense of that word, but is a tract, confined throughout its entire extent within the central nervous system and comparable to such groups of fibers as the direct cerebellar or fillet tracts of that system. | ||
===The Development of the Vitreous Humor=== | |||
It has already been pointed out (p. 448) that a blood-vessel, the hyaloid artery, accompanied by some mesodermal tissue makes its way into the cavity | |||
| Line 493: | Line 338: | ||
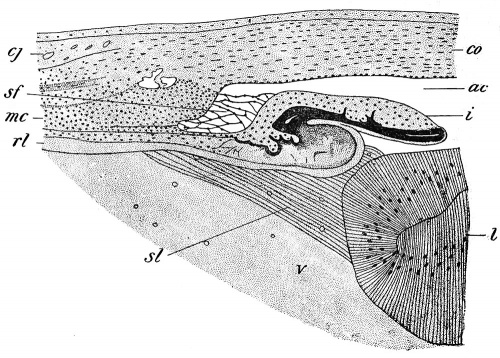
According to some authors, the formation of the vitreous humor is closely associated with the development of this artery, the humor being merely a transudate from it, while others have maintained that it is a derivative of the mesoderm which accompanies the vessel, and is therefore to be regarded as a peculiar gelatinous form of connective tissue. More recently, however, renewed observations by several authors have resulted in the deposition of the mesoderm from the chief role in the formation of the vitreous and the substitution in it of the retina. At an early stage of development delicate protoplasmic processes may be seen projecting from the surface of the retinal layer into the cavity of the optic cup, these processes probably arising from those cells which will later form the Muller's | According to some authors, the formation of the vitreous humor is closely associated with the development of this artery, the humor being merely a transudate from it, while others have maintained that it is a derivative of the mesoderm which accompanies the vessel, and is therefore to be regarded as a peculiar gelatinous form of connective tissue. More recently, however, renewed observations by several authors have resulted in the deposition of the mesoderm from the chief role in the formation of the vitreous and the substitution in it of the retina. At an early stage of development delicate protoplasmic processes may be seen projecting from the surface of the retinal layer into the cavity of the optic cup, these processes probably arising from those cells which will later form the Muller's (neuroglia) fibers of the retina. As development proceeds they increase in length, forming a dense and very fine fibrillar reticulum traversing the space between the lens and the retina and constituting the primary vitreous humor. The formation of the fibers is especially active in the ciliary portion of the retina and it is probable that it is from some of the fibers developing in this region that the suspensory ligament of the lens (zonula Zinnii) (Fig. 277, si) is formed spaces which occur between the fibers of the ligament enlarging to produce a cavity traversed by scattered fibers and known as the canal of Petit. | ||
(neuroglia) fibers of the retina. As development proceeds they increase in length, forming a dense and very fine fibrillar reticulum traversing the space between the lens and the retina and constituting the primary vitreous humor. The formation of the fibers is especially active in the ciliary portion of the retina and it is probable that it is from some of the fibers developing in this region that the suspensory ligament of the lens (zonula Zinnii) (Fig. 277, si) is formed spaces which occur between the fibers of the ligament enlarging to produce a cavity traversed by scattered fibers and known as the canal of Petit. | |||
[[File:McMurrich1914 fig277.jpg|500px]] | |||
'''Fig. 277.''' Transverse Section through the Ciliary Region of a Chick Embryo of Sixteen Days. ac, Anterior chamber of the eye; cj, conjunctiva; co, cornea; i, iris; I, lens; mc, ciliary muscle; rl, retinal layer of optic cup; sf, spaces of Fontana; si, suspensory ligament of the lens; v, vitreous humor. - (Angelucci.) | |||
| Line 512: | Line 351: | ||
The degeneration of the mesodermal elements which the latter view supposes is associated with the degeneration of the hyaloid artery. This begins in human embryos in the third month and is completed during the ninth month, the only trace after birth of the existence of the vessel being a more fluid consistency of the axis of the vitreous humor, this more fluid portion representing the space originally occupied by the artery and forming what is termed the hyaloid canal (canal ofCloquet). | The degeneration of the mesodermal elements which the latter view supposes is associated with the degeneration of the hyaloid artery. This begins in human embryos in the third month and is completed during the ninth month, the only trace after birth of the existence of the vessel being a more fluid consistency of the axis of the vitreous humor, this more fluid portion representing the space originally occupied by the artery and forming what is termed the hyaloid canal (canal ofCloquet). | ||
===The Development of the Outer Coat of the Eye, of the Cornea, and of the Anterior Chamber=== | |||
Soon after the formation of the optic bulb a condensation of the mesoderm cells around it occurs, forming a capsule. Over the medial portions of the optic cup the further differentiation of this capsule is comparatively simple, resulting in the formation of two layers, an inner vascular and an outer denser and fibrous, the former becoming the chorioid coat of the adult eye and the latter the sclera. | |||
| Line 525: | Line 365: | ||
[[File:McMurrich1914 fig279.jpg|500px]] | |||
Fig. 278. | '''Fig. 278.''' Transverse Section through the Ciliary Region of a Pig Embryo or 23 mm. ac, Anterior chamber of the eye; co, cornea; ec, ectoderm; I, lens; mc, ciliary muscle; p, pigment layer of the optic cup; r, retinal layer; tv, tunica vasculosa lentis. - (Angelucci.) | ||
23 | |||
| Line 539: | Line 376: | ||
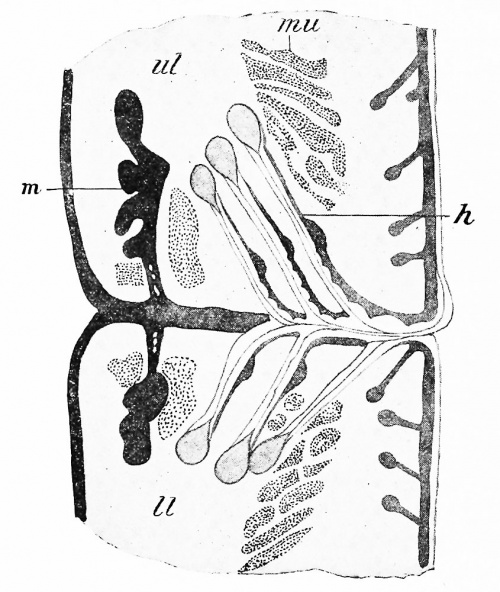
At about the beginning of the third month the lids have become sufficiently large to meet one another, whereupon the thickened epithelium which has formed upon their edges unites and the lids fuse together, in which condition they remain until shortly before birth. During the stage of fusion the eyelashes (Fig. 279, h) develop at the edges of the lids, having the same developmental history as ordinary hairs, and from the fused epithelium of each lid there grow upward or downward, as the case may be, into the mesodermic tissue, solid rods of ectoderm, certain of which early give off numerous short lateral processes and become recognizable as the tarsal (Meibomian) glands (m), while others retain the simple cylindrical form and represent the glands of Moll. When the eyelids separate, these solid ingrowths become hollow by a breaking down of their | At about the beginning of the third month the lids have become sufficiently large to meet one another, whereupon the thickened epithelium which has formed upon their edges unites and the lids fuse together, in which condition they remain until shortly before birth. During the stage of fusion the eyelashes (Fig. 279, h) develop at the edges of the lids, having the same developmental history as ordinary hairs, and from the fused epithelium of each lid there grow upward or downward, as the case may be, into the mesodermic tissue, solid rods of ectoderm, certain of which early give off numerous short lateral processes and become recognizable as the tarsal (Meibomian) glands (m), while others retain the simple cylindrical form and represent the glands of Moll. When the eyelids separate, these solid ingrowths become hollow by a breaking down of their central cells, just as in the sebaceous and sudoriparous glands of the skin, the tarsal glands being really modifications of the former glands, while the glands of Moll are probably to be regarded as specialized sudoriparous glands. | ||
[[File:McMurrich1914 fig279.jpg|500px]] | |||
'''Fig. 279.''' Section through the Margins of the Fused Eyelids in an Embryo of Six Months. ih, Eyelash; //, lower lid; m, tarsal gland; mu, muscle bundle; ul, upper lid. | |||
(Schweigger-Seidl.) | |||
| Line 560: | Line 388: | ||
The lachrymal gland is developed at about the third month as a number of branching outgrowths of the ectoderm into the adjacent mesoderm along the outer part of the line where the epithelium of the conjunctiva becomes continuous with that covering the inner surface of the upper eyelid. As in the other epidermal glands, the outgrowths and their branches are at first solid, later becoming hollow by the degeneration of their axial cells. | The lachrymal gland is developed at about the third month as a number of branching outgrowths of the ectoderm into the adjacent mesoderm along the outer part of the line where the epithelium of the conjunctiva becomes continuous with that covering the inner surface of the upper eyelid. As in the other epidermal glands, the outgrowths and their branches are at first solid, later becoming hollow by the degeneration of their axial cells. | ||
The naso-lachrymal duct is developed in connection with the groove which, at an early stage in the development (Fig. 62), extends | The naso-lachrymal duct is developed in connection with the groove which, at an early stage in the development (Fig. 62), extends from the inner corner of the eye to the olfactory pit and is bounded posteriorly by the maxillary process of the first visceral arch. The epithelium lying in the floor of this groove thickens toward the begining of the sixth week to form a solid cord, which sinks into the subjacent mesoderm. From its upper end two outgrowths arise which become connected with the ectoderm of the edges of the upper and lower lids, respectively, and represent the lachrymal ducts, and, finally, the solid cord and its outgrowths acquire a lumen and a connection with the mucous membrane of the inferior meatus of the nasal cavity. | ||
[[File:McMurrich1914 fig280.jpg|500px]] | |||
The eyelids are really fused at these stages but have been represented as | '''Fig. 280.''' Diagram showing the Insertions of the Lachrymal Ducts in embryos of 40 mm and 170 mm. The caruncula lacrimalis being formed in | ||
the latter. The eyelids are really fused at these stages but have been represented as separated for the sake of clearness. - (Ask.) | |||
The inferior duct connects with the border of the eyelid some distance lateral to the inner angle of the eye, and between its opening and the angle a number of tarsal glands develop. The superior duct, on the other hand, opens at first close to the inner angle and later moves laterally until its opening is opposite that of the inferior duct. During this change the portion of the lower lid between the opening of the inferior duct and the angle is drawn somewhat upward, and, with its glands, forms a small reddish nodule, resting upon the plica semilunaris and known as the caruncula lacrimalis (Fig. 280). | The inferior duct connects with the border of the eyelid some distance lateral to the inner angle of the eye, and between its opening and the angle a number of tarsal glands develop. The superior duct, on the other hand, opens at first close to the inner angle and later moves laterally until its opening is opposite that of the inferior duct. During this change the portion of the lower lid between the opening of the inferior duct and the angle is drawn somewhat upward, and, with its glands, forms a small reddish nodule, resting upon the plica semilunaris and known as the caruncula lacrimalis (Fig. 280). | ||
==Literature== | |||
Literature | |||
G. Alexander: "Ueber Entwicklung und Bau des Pars inferior Labyrinthi der hdheren Saugethiere," Denkschr. kais. wissench. Acad. Wien, Math.-Naturw. Classe, lxx, 1901. | G. Alexander: "Ueber Entwicklung und Bau des Pars inferior Labyrinthi der hdheren Saugethiere," Denkschr. kais. wissench. Acad. Wien, Math.-Naturw. Classe, lxx, 1901. | ||
A. Angeltjcci: "Ueber Entwickelung und Bau des vorderen Uvealtractus der Verte braten," Archiv fur mikrosk. Anat., xix, 1881. | |||
F. Ask: Ueber die Entwickelung der Caruncula lacrimalis beim Menschen, nebst Bemerkungen iiber die Entwickelung der Tranenrohrchen und der Meibom'schen Driisen," Anatom. Anzeiger, xxx, 1907. | |||
F. Ask: Ueber die Entwicklung der Lidrander, der Tranenkarunkel und der Nick haut beim Menschen, nebst Bemerkungen zur Entwicklung der Tranenabfuhrungswege," Anat. Hefte, xxxvi, 1908. | |||
B. Baginsky: "Zur Entwickelung der Gehorschnecke," Archiv fur mikrosk. Anat., xxviii, 1886. | |||
I. Broman: "Die Entwickelungsgeschichte der Gehorknochelchen beim Menschen," Anat. Hefte, xi, 189S. | |||
S. Ramon y Cajal: "Nouvelles contributions a l'etude histologique de la retine," Journ. de I' Anat. et de la Physiol., xxxii, 1896. | |||
G. Cirincione: "Ueber den gegenwartigen Stand der Frage hinsichtlich der Genese des Glaskorpers," Arch, fur Augenheilk., L, 1904. | |||
A. Contino: "Ueber Bau and Entwicklung des Lidrandes beim Menschen," Arch, fur Ophthalmol., lxvi, 1908. | |||
A. Contino: "Ueber die Entwicklung der Karunkel und der plica semilunaris beim Menschen," Arch, fur Ophthalmol, lxxi, 1909. | |||
J. Disse: "Die erste Entwickelung der Riechnerven," Anat. Hefte, ix, 1897. | |||
B. Fleischer: "Die Entwickelung der Tranenrohrchen bei den Saugetiere," Archiv fur Ophthalmol., lxii, 1906. | |||
H. Fuchs : " Bemerkungen iiber die Herkunft und Entwickelung der Gehorknochelchen bei Kaninchen-Embryonen (nebst Bemerkungen iiber die Entwickelung des Knorpelskeletes der beiden ersten Visceralbogen)," Archiv. fur Anat und Phys., Anat. Abth., Supplement, 1905. | |||
J. Graberg: "Beitrage zur Genese des Geschmacksorgans der Menschen," Morphol. Arbeiten, vn, 1898. | |||
J. A. Hammar: "Zur allgemeinen Morphologie der Schlundspalten des Menschen. Zur Entwickelungsgeschichte des Mittelohrraumes, des ausseren Gehorganges und des Paukenfelles beim Menschen," Anat. Anzeiger, xx, 1901. | |||
J. A. Hammar: " Studien iiber die Entwicklung des Vorderdarms und einiger angrenz ender Organe," Arch, fur mikrosk. Anat., Lix, 1902. | |||
C. Heerfordt: "Studien iiber den Muse, dilatator pupilke sammt Angabe von gemeinschaftlicher Kennzeichen einiger Falle epithelialer Musculatur," Anat. | |||
Hefte, xiv. J. Hegetschweiler: "Die embryologische Entwickelung des Steigbugels," Archiv fur Anat. und Physiol., Anat. Abth., 1898. | |||
F. Hochstetter: "Ueber die Bildung der primitiven Choanen beim Menschen," Verhandl. Anat. Gesellsch., VI, 1892. | |||
W. His, Jr.: "Die Entwickelungsgeschichte des Acustico-Facialisgebietes beim Menschen," Archiv fur Anat. und Physiol., Anat. Abth., Supplement, 1897. | |||
A. von Kolliker: "Die Entwicklung und Bedeutung des Glaskorpers," Zeitschr. fur wissensch. Zoolog., lxxvi, 1904. | |||
P. Lang: "Zur Entwicklung des Tranenausfiihrsapparates beim Menschen," Anat. Anzeiger," xxxvni, 1911. | |||
G. Leboucq: " Contribution a. l'etude de l'histogenese de la retine chez les mammiferes," Arch. Anat., Microsc, x, 1909. | |||
V. von Mihalkovicz: "Nasenhohle und Jacobsonsches Organ. Eine morphologische Studie." Anat. Hefte. xi, 1898. | |||
J. L. Paitlet: "Contribution a l'etude de l'organe de Jacobson chez l'embryon humain," Bibliogr. Anat., xvii, 1907. | |||
P. van Pee: "Recherches sur l'origine du corps vitre," Archives de Biol., xix, 1902. | |||
C. Rabl: "Ueber den Bau und Entwickelung der Linse," Zeitschrift fur wissensch. Zoologie, lxiii and lxv, 1898; lxviii, 1899. | |||
A. Robinson: "On the Formation and Structure of the Optic Nerve and Its Relation to the Optic Stalk," Journal of Anat. and Physiol., xxx, 1896. | |||
G. Speciale-Cirincione: "Ueber die Entwicklung der Tranendriise beim Menschen " Arch.filr Ophthalmol., LXIX, 1908. | |||
J. P. Schaefeer: "The Genesis and Development of the Nasolacrimal Passages in Man," Amer. Journ. Anat., xm, 1912. | |||
of the | |||
G. L. Streeter: "On the Development of the Membranous Labyrinth and the Acoustic and Facial Nerves in the Human Embryo," Amer. Journ. of Anat. vi, 1907. | |||
N. van der Stricht: "L'histogenese des parties constituantes du neuroepithelium acoustique, des taches et des cretes acoustiques et de l'organe de Corti " Arch. de Biol., xxiii, 1908. | |||
A. Szili: "Zur Anatomie und Entwickelungsgeschichte der hinteren Irisschichten mit besonderer Beriicksichtigung des Musculus sphincter iridis des Menschen " Anat. Anzeiger, xx, 1901. | |||
A. Szili: "Ueber das Entstehen eines fibrillares Stutzgewebes im Embryo und dessen Verhaltnis zur Glaskorperfrage," Anat. Hejte, xxxv, 190S. | |||
F. Tuckerman: "On the Development of the Taste Organs in Man," Journal of Anat. and Physiol., xxiv, 1889. | |||
R. Versari: "Ueber die Entwicklung der Blutgefasse des menschlichen Au^es " Anat. Anzeiger, xxxv, 1909. | |||
{{McMurrich1914 footer}} | {{McMurrich1914 footer}} | ||
Latest revision as of 22:53, 31 January 2017
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
McMurrich JP. The Development Of The Human Body. (1914) P. Blakiston's Son & Co., Philadelphia, Pennsylvania.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter XVI. The Development of the Organs of Special Sense
Like the cells of the central nervous system, the sensory cells are all of ectodermal origin, and in lower animals, such as the earthworm, for instance, they retain their original position in the ectodermal epithelium throughout life. In the vertebrates, however, the majority of the sensory cells relinquish their superficial position and sink more or less deeply into the subjacent tissues, being represented by the posterior root ganglion cells and by the sensory cells of the special sense-organs, and it is only in the olfactory organ that the original condition is retained. Those cells which have withdrawn from the surface receive stimuli only through overlying cells, and in certain cases these transmitting cells are not specially differentiated, the terminal branches of the sensory dendrites e ding among ordinary epithelial cells or in such structures as the Pacinian bodies or the end-bulbs of Krause situated beneath undifferentiated epithelium. In other cases, however, certain specially modified superficial cells serve to transmit the stimuli to the peripheral sensory neurones, forming such structures as the hair-cells of the auditory epithelium or the gustatory cells of the taste-buds.
Thus three degrees of differentiation of the special sensory cells may be recognized and a classification of the sense-organs may be made upon this basis. One organ, however, the eye, cannot be brought into such a classification, since its sensory cells present certain developmental peculiarities which distinguish them from those of all other sense-organs. Embryologically the retina is a portion of the central nervous system and not a peripheral organ, and hence it will be convenient to arrange the other sense-organs according to the classification indicated and to discuss the" history of the eye at the close of the chapter.
The Development of the Olfactory Organ
The general development of the nasal fossa, the epithelium of which contains the olfactory sense cells, has already been described (pp. 99 and 283), as has also the development of the olfactory lobes of the brain (p. 406), and there remains for consideration here merely the formation of the olfactory nerve and the development of the rudimentary organ of Jacobson.
The Olfactory Nerve
Very diverse results have been obtained by various observers of the development of the olfactory nerve, it having been held at different times that it was formed by the outgrowth of fibers from the olfactory lobes (Marshall), from fibers which arise partly from the olfactory lobes and partly from the olfactory epithelium (Beard), from the cells of an olfactory ganglion originally derived from the olfactory epithelium but later separating from it (His), and, finally, that it was composed of the prolongations of certain cells situated and, for the most part at least, remaining permanently in the olfactory epithelium (Disse). The most recent observations on the structure of the olfactory epithelium and nerve indicate a greater amount of probability in the last result than in the others, and the description which follows will be based upon the observations of His, modified in conformity with the results obtained by Disse from chick embryos.
In human embryos of the fourth week the cells lining the upper part of the olfactory pits show a distinction into ordinary epithelial and sensory cells, the latter, when fully formed, being elongated cells prolonged peripherally into a short but narrow process which reaches the surface of the epithelium and proximally gives rise to an axis-cylinder process which extends up toward and penetrates the tip of the olfactory lobe to come into contact with the dendrites of the first central neurones of the olfactory tract (Fig. 252). These cells constitute a neuro-epithelium and in later stages of development retain their epithelial position for the most part, a few of them, however, withdrawing into the subjacent mesenchyme and becoming bipolar, their peripheral prolongations ending freely among the cells of the olfactory epithelium. These bipolar cells resemble closely in form and relations the cells of the embryonic posterior root ganglia, and thus form an interesting transition between these and the neuroepithelial cells.
The Organ of Jacohson
In embryos of three or four months a small pouch-like invagination of the epithelium covering the lower anterior portion of the median septum of the nose can readily be seen. This becomes converted into a slender pouch, 3 to 5 mm. long, ending blindly at its posterior extremity and opening at its other end into the nasal cavity. Its lining epithelium resembles that of the respiratory portion of the nasal cavity, and there is developed in the connective tissue beneath its floor a slender plate of cartilage, distinct from that forming the septum of the nose.
Fig. 252. Diagram Illustrating the Relations of the Fibers of the Olfactory Nerve.
Ep, Epithelium of the olfactory pit; C, cribiform plate of the'ethmoid, G, glomerulus of the olfactory bulb; M, mitral cell. - (Van Gekuchten.)
This organ, which may apparently undergo degeneration in the adult, and in some cases completely disappears, appears to be the representative of what is known as Jacobson's organ, a structure which reaches a much more extensive degree of development in many of the lower mammals, and in these contains in its epithelium sensory cells whose axis-cylinder processes pass with those of the olfactor} sense cells to the olfactory bulbs. In man, however, it seems to be a rudimentary organ, and no satisfactory explanation of its function has as yet been advanced.
The olfactory neuro-epithelium, considered from a comparative standpoint, seems to have been derived from the system of lateral line organs so highly developed in the lower vertebrates (Kupffer). In higher forms the system, which is cutaneous in character, has disappeared except in two regions where it has become highly specialized. In one of these regions it has given rise to the olfactory sense cells and in the other to the similar cells of the auditory apparatus.
The Organs of Touch and Taste
Little is yet known concerning the development of the various forms of tactile organs, which belong to the second class of sensory organs described above.
The Organs of Taste
he remaining organs of special sense belong to the third class, and of these the organs of taste present in many respects the simplest condition. They are developed principally in connection with the vallate and foliate papillae of the tongue, and of the former one of the earliest observed stages has been found in embryos of 9 cm. in the form of two ridges of epidermis, lying toward the back part of the tongue and inclined to one another in such a manner as to form a V with the apex directed backward. From these ridges solid downgrowths of epidermis into the subjacent tissue occur, each downgrowth having the form of a hollow truncated cone with its basal edge continuous with the superficial epidermis (Fig. 253, A). In later stages lateral outgrowths develop from the deeper edges of the cone, and about the same time clefts appear in the substance of the original downgrowths (Fig. 253, B) and, uniting together, finally open to the surface, forming a trench surrounding a papilla (Fig. 253, C). The lateral outgrowths, which are at first solid, also undergo an axial degeneration and become converted into the glands of Ebner (b), which open into the trench near its floor. The various papillae which occur in the adult do not develop simultaneously, but their number increases with the age of the fetus, and there is, moreover, considerable variation in the time of their development.
The taste-buds are formed by a differentiation of the epithelium which covers the papillae, and this differentiation appears to stand in intimate relation with the penetration of fibers of the glossopharyngeal nerve into the papillae. The buds form at various places upon the papillae, and at one period are especially abundant upon their free surfaces, but in the later weeks of intrauterine life these surface buds undergo degeneration and only those upon the sides of the trench persist, as a rule.
Fig. 253. Diagrams Representing the Development of a Vallate Papilla. a, Valley surrounding the papilla; b, von Ebner's gland. - (Graberg.)
The foliate papillae do not seem to be developed until some time after the circumvallate, being entirely wanting in embryos of four and a half and five months, although plainly recognizable at the seventh month.
The Development of the Ear
It is customary to describe the mammalian ear as consisting of three parts, known as the inner, middle, and outer ears, and this division is, to a certain extent at least, confirmed by the embryonic development. The inner ear, which is the sensory portion proper, is an ectodermal structure, which secondarily becomes deeply seated in the mesodermal tissue of the head, while the middle and outer ears, which provide the apparatus necessary for the conduction of the sound-waves to the inner ear, are modified portions of the anterior branchial arches. It will be convenient, accordingly, in the description of the ear, to accept the usually recognized divisions and to consider first of all the development of the inner ear, or, as it is better termed, the otocyst. The Development of the Otocyst. - In an embryo of 2.4 mm. a pair of pits occur upon the surface of the body about opposite the middle portion of the hind-brain (Fig. 254, A). The ectoderm lining the pits is somewhat thicker than is the neighboring ectoderm
Fig. 254. Transverse Section Passing through the Otocyst (ot) of Embryos of (A) 2.4 mm. and (B) 4 mm. (His.)
of the surface of the body, and, from analogy with what occurs in other vertebrates, it seems probable that the pits are formed by the invagination of localized thickenings of the ectoderm. The mouth of each pit gradually becomes smaller, until finally the invagination is converted into a closed sac (Fig. 254, B), which separates from the surface ectoderm and becomes enclosed within the subjacent mesoderm. This sac is the otocyst, and in the stage just described, found in embryos of 4 mm., it has an oval or more or less spherical form. Soon, however, in embryos of 6.9 mm., a prolongation arises from its dorsal portion and the sac assumes the form shown in Fig. 255, A; this prolongation, which is held by some authors to be the remains of the stalk which originally connected the otocyst sac with the surface ectoderm, represents the ductus endolymphaticus , and, increasing in length, it soon becomes a strong club-shaped process, projecting considerably beyond the remaining portions of the otocyst (Fig. 255, B). In embryos of about 10.2 mm. the sac begins to show certain other irregularities of shape (Fig. 255, B, sc). Thus, about opposite the point of origin of the ductus endolymphaticus three folds make their appearance, representing the semicircular ducts, and as they increase in size the opposite walls of the central portion of each fold come together, fuse, and finally become absorbed, leaving the free edge of the fold as a crescentic canal, at one end of which an enlargement appears to form the ampulla. The transformation of the folds into canals takes place somewhat earlier in the cases of the two vertical than in that of the horizontal duct, as may be seen from Fig. 256, which represents the condition occurring in an embryo of 13.5 mm.
Fig. 255. - Reconstruction of the Otocysts of Embryo of (A) 6.9 mm. and (B) 10.2 mm.
de, Endolymphatic duct; gc, ganglion cochleare; gg, ganglion geniculatum; gv, ganglion vestibulare; sc, lateral semicircular duct. - (His, Jr.)
A short distance below the level at which the canals communicate with the remaining portion of the otocyst a constriction appears, indicating a separation of the otocyst into a more dorsal portion and a more ventral one. Later, the latter begins to be prolonged into a flattened canal which, as it elongates, becomes coiled upon itself and also becomes separated by a constriction from the remaining portion of the otocyst (Fig. 257). This canal is the ductus cochlearis (scala media of the cochlea), and the remaining portion of the otocyst subsequently becomes divided by a constriction into the utriculus, with which the semicircular ducts are connected, and the sacculus. The constriction which separates the cochlear duct from the sacculus becomes the ductus reuniens, while that between the utriculus and sacculus is converted into a narrow canal with which the ductus endolymphaticus connects, and hence it is that, in the adult, the connection between these two portions of H the otocyst seems to be formed by the ductus dividing proximally into two limbs, one of which is connected with the utricle and the other with the saccule.
When first observed in the human embryo the auditory ganglion
is closely associated with the geniculate ganglion of the seventh
nerve (Fig. 255, B), the two, usually spoken of as the acustico-facialis
ganglion, forming a mass of cells lying in close contact with the anterior wall of the otocyst. The origin of the ganglionic mass has not yet been traced in the mammalia, but it has been observed that in cow embryos the geniculate ganglion is connected with the ectoderm at the dorsal end of the first branchial cleft (Froriep), and it may perhaps be regarded as one of the epibranchial placodes (see p. 417), and in the lower vertebrates a union of the ganglion with a suprabranchial placode has been observed (Kupffer), this union indicating the origin of the auditory ganglion from one or more of the ganglia of the lateral line system.
Fig. 256. - Reconstruction of the Otocyst of an Embryo of 13.5 MM.
co, Cochlea; de, endolymphatic duct;.sc, semicircular duct. - (His Jr.)
Fig. 257. Reconstruction of the Otocyst of an Embryo of 20 mm., front view. cc, Common limb of superior and posterior semicircular ducts; eg, cochlear ganglion; co, cochlea; de, endolymphatic duct; s, sacculus; sdl, sdp, and sds x lateral, posterior and superior semicircular ducts; u, utriculus; vg, vestibular ganglion. - (Streeter.)
At an early stage in the human embryo the auditory ganglion shows indications of a division into two portions, a more dorsal one, which represents the future ganglion vestibular e, and a ventral one, the ganglion cochleare. The ganglion cells become bipolar, in which condition they remain throughout life, never reaching the T-shaped condition found in most of the other peripheral cerebro-spinal ganglia. One of the prolongations of each cell is directed centrally to form a fiber of the auditory nerve, while the other penetrates the wall of the otocyst to enter into relations with certain specially modified cells which differentiate from its lining epithelium.
â– In the earliest stages the ectodermal lining of the otocyst is formed of similar columnar cells, but later over the greater part of the surface the cells flatten down, only a few, aggregated together to form patches, retaining the high columnar form and developing hairlike processes upon their free surfaces. These are the sensory cells of the ear. In the human ear there are in all six patches of these sensory cells, an elongated patch (crista ampullaris) in the ampulla of each semicircular canal (Fig. 258, cr), a round patch (macula acustica, mii) in the utriculus and another (ms) in the sacculus, and, finally, an elongated patch which extends the entire length of the scala media of the cochlea and forms the sensory cells of the spiral organ of Corti. The cells of this last patch are connected with the fibers from the cochlear ganglion, while those of the vestibular ganglion pass to the cristas and maculae.
Fig. 258. The Right Internal Ear of an Embryo of Six Months. ca, ce, and cp, Superior, lateral, and posterior semicircular ducts; cr, crista acustica; de, endolymphatic duct; Is, spiral ligament; mb, basilar membrane; ms and tnu, macula acustica sacculi and utriculi; rb, basilar branches of the cochlear nerve. - (Retzius.)
In connection with the spiral organ certain adjacent cells also retain their columnar form and undergo various modifications, giving rise to a rather complicated structure whose development has been traced in the rabbit. Along the whole length of the cochlear duct the cells resting upon that half of the basilar membrane which is nearest the axis of the cochlea, and may be termed the inner half, retain their columnar shape, forming two ridges projecting slightly into the cavity of the scala (Fig. 259). The cells of the inner ridge, much the larger of the two, give rise to the membrana tectoria, either as a cuticular secretion or by the artificial adhesion of long hair-like processes which project from their free surfaces (Ayers). The cells of the outer ridge are arranged in six longitudinal rows (Fig. 259, 1-6); those of the innermost row (1) develop hairs upon their free surfaces and form the inner hair cells, those of the next two rows (2 and 3) gradually become transformed on their adjacent surfaces into chitinous
Fig. 259. Section of the Cochlear Duct of a Rabbit Embryo of 55 mm.
a, Mesenchyme; b to e, epithelium of cochlear duct; M.t, membrana tectoria; V.s.p, vein; 1 to 7, spiral organ of Corti. - (Baginsky.)
p /- /^ substance and form the rods of Corti, while the three outer rows
- (4 to 6) develop into the outer e -^~~ __ hair cells. It is in connection
with the hair cells that the peripheral prolongations of the cells of the cochlear ganglion ter™JS= d^t" 1 ™ mi «ate, and since these hair cells Rabbit Embryo of Twenty-four Days, are arranged in rows extending c, Periotic cartilage; ep, fibrous mem- .1 pnt j rp ] er ,a\h of the cochlear brane beneath the epithelium of the canal; me ermre lengin 01 me COCIliear p, perichondrium; s, spongy tissue. - (Von duct, the ganglion also IS drawn Kolliker.) . 1 .. .
out into a spiral following the coils of the cochlea, and hence is sometimes termed the spiral ganglion.
While the various changes described above have been taking place in the otocyst, the mesoderm surrounding it has also been undergoing development. At first this tissue is quite uniform in character, but later the cells immediately surrounding the otocyst condense to give rise to a fibrous layer (Fig. 260, ep), while more peripherally they become more loosely arranged and form a somewhat gelatinous layer (s) , and still more peripherally a second fibrous layer is differentiated and the remainder of the tissue assumes a character which indicates an approaching conversion into cartilage. The further history of these various layers is as follows: The inner fibrous layer gives rise to the connective-tissue wall which supports the ectodermal lining of the various portions of the otocyst; the gelatinous layer undergoes a degeneration to form a lymph-like fluid known as the perilymph, the space occupied by the fluid being the perilymphatic space; the outer fibrous layer becomes perichondrium and later periosteum; and the precartilage undergoes chondrification and later ossifies to form the petrous portion of the temporal bone.
The gelatinous layer completely surrounds most of the otocyst structures, which thus come to lie free in the perilymphatic space, but in the cochlear region the conditions are somewhat different. In this region the gelatinous layer is interrupted along two lines, an outer broad one where the connective-tissue wall of the cochlear duct is directly continuous with the perichondrium layer, and an inner narrow one, along which a similar fusion takes place with the perichondrium of a shelf-like process of the cartilage, which later ossifies to form the lamina spiralis. Consequently throughout the cochlear region the perilymphatic space is divided into two compartments which communicate at the apex of the cochlea, while below one, known as the scala vestibuli, communicates with the space surrounding the saccule and utricle, and the other, the scala tympani, abuts upon a membrane which separates it from the cavity of the middle ear and represents a portion of the outer wall of the petrous bone where chondrification and ossification have failed to occur. This membrane closes what appears in the dried skull to be an opening in the inner wall of the middle ear, known as the fenestra cochlea (rotunda) ; another similar opening, also closed by membrane in the fresh skull, occurs in the bony wall opposite the utricular portion of the otocyst and is known as the fenestra vestibuli (ovalis) .
Fig. 261. Diagrammatic Transverse Section through a Coil of the Cochlea showing the relation of the scal^e. c, Organ of Corti; co, ganglion cochleare; Is, lamina spiralis; SAT, cochlear duct; ST, scala tympani; SV, scala vestibuli. - (From Gerlach.)
The Development of the Middle Ear
The middle ear develops from the upper part of the pharyngeal groove which represents the endodermal portion of the first branchial cleft. This becomes prolonged dorsally and at its dorsal end enlarges to form the tympanic cavity, while the narrower portion intervening between this and the pharyngeal cavity represents the tuba auditiva (Eustachian tube).
To correctly understand the development of the tympanic cavity it is necessary to recall the structures which form its boundaries. Anteriorly to the upper end of the first branchial pouch there is the upper end of the first arch, and behind it the corresponding part of the second arch, the two fusing together dorsal to the tympanic cavity and forming its roof. Internally the cavity is bounded by the outer wall of the cartilaginous investment of the otocyst, while externally it is separated from the upper part of the ectodermal groove of the first branchial cleft by the thin membrane which forms the floor of the groove.
It has been seen in an earlier chapter that the axial mesoderm of each branchial arch gives rise to skeletal structures and muscles. The axial cartilage of the ventral portion of the first arch is what is known as Meckel's cartilage, but in that portion of the arch which forms the roof and anterior wall of the tympanic cavity, the cartilage becomes constricted to form two masses which later ossify to form the malleus and incus (Fig. 262, m and i), while the muscular tissue of this dorsal portion of the arch gives rise to the tensor tympani. Similarly, in the case of the second arch there is to be found, dorsal to the extremity of the cartilage which forms the styloid process of the adult, a narrow plate of cartilage which forms an investment for the facial nerve (Fig. 262, VII), and dorsal to this a ring of cartilage (st) which surrounds a small stapedial artery and represents the stapes.
It has been found that in the rabbit the mass of cells from which the stapes is formed is at its first appearance quite independent of the second branchial arch (Fuchs), and it has been held to be a derivative of the mesenchyme from which the periotic capsule is formed. In later stages, however, it becomes connected with the cartilage of the second branchial arch, as shown in Fig. 262, and it is a question whether this connection, which is transitory, does not really indicate the phylogenetic origin of the ossicle from the second arch cartilage, its appearance as an independent structure being a secondary ontogenetic phenomenon. However that may be, the stapedial artery disappears in later stages and the stapedius muscle, derived from the musculature of the second branchial arch and therefore supplied by the facial nerve, becomes attached to the ossicle.
Fig. 262. - Semi-diagrammatic Viewof the Auditory Ossicles of an Embryo of Six Weeks. i, Incus; j, jugular vein; m, malleus; mc, Meckel's cartilage; oc, capsule of otocyst; R, cartilage of the second branchial arch; st, stapes; VII, facial nerve. - (Siebenmann.)
The three ossicles at first lie embedded in the mesenchyme forming the roof of the primitive tympanic cavity, as does also the chorda tympani, a branch of the seventh nerve, as it passes into the substance of the first arch on the way to its destination. The mesenchyme in which these various structures are embedded is rather voluminous (Fig. 264), and after the end of the seventh month becomes converted into a peculiar spongy tissue, which, toward the end of fetal life, gradually degenerates, the tympanic cavity at the same time expanding and wrapping itself around the ossicles and the muscles attached to them (Fig. 263). The bones and their muscles, consequently, while appearing in the adult to traverse the tympanic cavity, are really completely enclosed within a layer of epithelium continuous with that lining the wall of the cavity, while the handle of the malleus and the chorda tympani lie between the epithelium of the outer wall of the cavity and the fibrous mesoderm which forms the tympanic membrane. The extension of the tympanic cavity does not, however, cease with its replacement of the degenerated spongy mesenchyme, but toward the end of fetal life it begins to invade the substance of the temporal bone by a process similar to that which produces the ethmoidal cells and the other osseous sinuses in connection with the nasal cavities (see p. 175). This process continues for some years after birth and results in the formation in the mastoid portion of the bone of the so-called mastoid cells, which communicate with the tympanic cavity and have an epithelial lining continuous with that of the cavity.
Fig. 263. Diagrams Illustrating the Mode of Extension of the Tympanic Cavity Around the Auditory Ossicles.
M, Malleus; m, spongy mesenchyme; p, surface of the periotic capsule; T, tympanic cavity. The broken line represents the epithelial lining of the tympanic cavity.
The lower portion of the diverticulum from the first pharyngeal groove which gives rise to the tympanic cavity becomes converted into the Eustachian tube. During development the lumen of the tube disappears for a time, probably owing to a proliferation of its lining epithelium, but it is re-established before birth.
In the account of the development of the ear-bones given above it is held that the malleus and incus are derivatives of the first branchial (mandibular) arch and the stapes probably of the second. This view represents the general consensus of recent workers on the difficult question of the origin of these bones, but it should be mentioned that nearly all possible modes of origin have been at one time or other suggested. The malleus has very generally been accepted as coming from the first arch, and the same is true of the incus, although some earlier authors have assigned it to the second arch. But with regard to the stapes the opinions have been very varied. It has been held to be derived from the first arch, from the second arch, from neither one nor the other, but from the cartilaginous investment of the otocyst, or, finally, it has been held to have a compound origin, its arch being a product of the second arch while its basal plate was a part of the otocyst investment.
The Development of the Tympanic Membrane and of the Outer Ear
Just as the tympanic cavity is formed from the endodermal groove of the first branchial cleft, so the outer ear owes its origin to the ectodermal groove of the same cleft and to the neighboring arches. The dorsal and most ventral portions of the groove flatten out and disappear, but the median portion deepens to form, at about the end of the second month, a funnel-shaped cavity which corresponds to the outer portion of the external auditory meatus. From the inner end of this a solid ingrowth of ectoderm takes place, and this, enlarging at its inner end to form a disk-like mass, comes into relation with the gelatinous mesoderm which surrounds the malleus and chorda tympani. At about the seventh month a split occurs in the disk-like mass (Fig. 264), separating it into an outer and an inner layer, the latter of which becomes the outer epithelium of the tympanic membrane. Later, the split extends outward in the substance of the ectodermal ingrowth and eventually unites with the funnel-shaped cavity to complete the external meatus.
Fig. 264. Horizontal Section Passing through the Dorsal Wall of the External Auditory Meatus in an Embryo of 4.5 cm.
c, Cochlea; de, endolymphatic duct; i, incus; Is, transverse sinus; m, malleus; me, meatus auditorius externus; me' , cavity of the meatus; s, sacculus; sc, lateral semicircular canal; sc', posterior semicircular canal; st, stapes; t, tympanic cavity; u, utriculus; 7, facial nerve. - (Siebenmann.)
The tympanic membrane is formed in considerable part from the substance of the first branchial arch, the area in which it occurs not being primarily part of the wall of the tympanic cavity, but being brought into it secondarily by the expansion of the cavity. The membrane itself is mesodermal in origin and is lined on its outer surface by an ectodermal and on the inner by an endodermal epithelium.
The auricle (pinna) owes its origin to the portions of the first and second arches which bound the entrance of the external meatus. Upon the posterior edge of the first arch there appear about the end of the fourth week two transverse furrows which mark off three tubercles (Fig. 258, A, 1-3) and on the anterior edge of the second arch a corresponding number of tubercles (4-6) is formed, while, in addition, a longitudinal furrow, running down the middle of the arch, marks ofT a ridge (c) lying posterior to the tubercles. From these six tubercles and the ridge are developed the various parts of the auricle, as may be seen from Fig. 265 which represents the transformation as described by His. According to this, the most ventral tubercle of the first arch (i) gives rise to the tragus, and the middle one (5) of the second arch furnishes the antitragus. The middle and dorsal tubercles of the first arch (2 and 3) unite with the ridge (c) to produce the helix, while from the dorsal tubercle of the second arch (4) is produced the anthelix and from the ventral one (6) the lobule. More recent observations, however, seem to indicate that the lobule is an accessory structure unrelated to the tubercles and that the sixth tubercle gives rise to the antitragus, while the fifth is either included in the anthelix or else disappears. It is noteworthy that up to about the third month of development the upper and posterior portion of the helix is bent forward so as to conceal the anthelix (Fig. 265, D); it is at just about a corresponding stage that the pointed form of the ear seen in the lower mammals makes its appearance, and it is evident that, were it not for the forward bending, the human ear would also be assuming at this stage a more or less pointed form. Indeed, there is usually to be found upon the incurved edge of the helix, some distance below the upper border of the auricle, a more or less distinct tubercle, known as Darwin's tubercle, which seems to represent the point of the typical mammalian ear, and is, accordingly, the morphological apex of the pinna.
Fig. 265. Stages in the Development of the Auricle.
A, Embryo of n mm.; B, of 13.6 mm.; C, of 15 mm.; D, at the beginning of the third month; E, fetus of 8.5 cm.; F, fetus at term. - (His.)
There seems to be little room for doubt that the otocyst belongs primarily to the system of lateral line sense-organs, but a discussion of this interesting question would necessitate a consideration of details concerning the development of the lower vertebrates which would be foreign to the general plan of this book. It may be recalled, however, that the analysis of the components of the cranial nerves described on page 415 refers the auditory nerve to the lateral line system.
The Development of the Eye
The first indications of the development of the eye are to be found in a pair of hollow outgrowths from the side of the first primary brain vesicle, at a level which corresponds to the junction of the dorsal and ventral zones. Each evagination is directed at first upward and backward, and, enlarging at its extremity, it soon shows a differentiation into a terminal bulb and a stalk connecting the bulb with the brain (Fig. 232). At an early stage the bulb comes into apposition with the ectoderm of the side of the head, and this, over the area of contact, becomes thickened and then depressed to form the beginning of the future lens (Fig. 266).
Fig. 266. Early Stages in the Development of the Lens in a Rabbit Embryo. The nucleated layer to the left is the ectoderm and the thicker lens epithelium, beneath which is the outer wall of the optic evagination; above and below between the two is mesenchyme. - (Rabl.)
As the result of the depression of the lens ectoderm, the outer wall of the optic bulb becomes pushed inward toward the inner wall, and this invagination continuing until the two walls come into contact, the bulb is transformed into a double-walled cup, the optic cup, in the mouth of which lies the lens (Fig. 268). The cup is not perfect, however, since the invagination affects not only the optic bulb, but also extends medially on the posterior surface of the stalk, forming upon this a longitudinal groove and producing a defect of the ventral wall of the cup, known as the chorioidal fissure (Fig. 267). The groove and fissure become occupied by mesodermal tissue, and in this, at about the fifth week, a blood-vessel develops which traverses the cavity of the cup to reach the lens and is known as the arteria hyaloidea.
Fig. 267. Reconstruction of the Brain of an Embryo of Four Weeks, showing the Chorioid Fissure. - (His.)
In the meantime further changes have been taking place in the lens. The ectodermal depression which represents it gradually deepens to form a cup, the lips of which approximate and finally meet, so that the cup is converted into a vesicle which finally separates completely from the ectoderm (Fig. 268), much in the same way as the otocyst does. As the lens vesicle is constricted off, the surrounding mesodermal tissue grows in to form a layer between it and the overlying ectoderm, and a split appearing in the layer divides it into an outer thicker portion, which represents the cornea, and an inner thinner portion, which covers the outer surface of the lens and becomes highly vascular. The cavity between these two portions represents the anterior chamber of the eye. The cavity of the optic cup has also become filled by a peculiar tissue which represents the vitreous humor, while the mesodermal tissue surrounding the cup condenses to form a strong investment for it, which is externally continuous with the cornea, and at about the sixth week shows a differentiation into an inner vascular layer, the chorioid coat, and an outer denser one, which becomes the sclerotic coat.
Fig. 268. Horizontal Section through the Eye of an Embryo Pig of 7 mm. Br, Diencephalon; Ec, ectoderm; I, lens; P, pigment, and R, retinal layers of the retina.
The various processes resulting in the formation of the eye, which have thus been rapidly sketched, may now be considered in greater detail.
The Development of the Lens
When the lens vesicle is complete, it forms a more or less spherical sac lying beneath the superficial ectoderm and containing in its cavity a few cells, either scattered or in groups (Fig. 268). These cells, which have wandered into the cavity of the vesicle from its walls, take no part in the further development of the lens, but early undergo complete degeneration, and the first change which is concerned with the actual formation of the lens is an increase in the height of the cells forming its inner wall and a thinning out of its outer wall (Fig. 269, A). These changes continuing, the outer half of the vesicle becomes converted into a single layer of somewhat flat cells which persist in the adult condition to form the anterior epithelium of the lens, while the cells of the posterior wall form a marked projection into the cavity of the vesicle and eventually completely obliterate it, coming into contact with the inner surface of the anterior epithelium (Fig. 269, B).
These posterior elongated cells form, then, the principal mass of the lens, and constitute what are known as the lens fibers. At first those situated at the center of the posterior wall are the longest, the more peripheral ones gradually diminishing in length until at the equator of the lens they become continuous with and pass into the anterior epithelium. As the lens increases in size, however, the most centrally situated cells fail to elongate as rapidly as the more peripheral ones and are pushed in toward the center of the lens, the more peripheral fibers meeting below them along a line passing across the inner surface of the lens. The disparity of growth continuing, a similar sutural line appears on the outer surface beneath the anterior epithelium, and the fibers become arranged in concentric layers around a central core composed of the shorter fibers. In the human eye the line of suture of the peripheral fibers becomes bent so as to consist of two limbs which meet at an angle, and from the angle a new sutural line develops during embryonic life, so that the suture assumes the form of a three-rayed star. In later life the stars become more complicated, being either six-rayed or more usually nine-rayed in the adult condition (Fig. 270).
Fig. 269. Sections through the Lens (4) of Human Embryo of Thirty Thirty-one Days and (B) of Pig Embryo of 36 Mu. - (Rabl.)
As early as the second month of development the lens vesicle becomes completely invested by the mesodermal tissue in which blood-vessels are developed in considerable numbers, whence the investment is termed the tunica vasculosa lends (Fig. 278, tv). The arteries of the tunic are in connection principally with the hyaloid artery of the vitreous humor (Fig. 276), and consist of numerous fine branches which envelop the lens and terminate in loops almost at the center of its outer surface. This tunic undergoes degeneration after the seventh month of development, by which time the lens has completed its period of most active growth, and, as a rule, completely disappears before birth. Occasionally, however, it may persist to a greater or less extent, the persistence of the portion covering the outer surface of the lens, known as the membrana papillaris, causing the malformation known as congenital atresia of the pupil.
Fig. 270. Posterior (Inner) Surface of the Lens from an Adult showing the Sutural Lines. - (Rabl.)
In addition to the vascular tunic, the lens is surrounded by a non-cellular membrane termed the capsule. The origin of 'this structure is still in doubt, some observers maintaining that it is a product of the investing mesoderm, while others hold it to be a product of the lens epithelium.
It is interesting from the standpoint of developmental mechanics to note that W. H. Lewis and Spemann have shown that in the Amphibia contact of the optic vesicle with the ectoderm is necessary for the formation of the lens, and, furthermore, if the vesicle be transplanted to other regions of the body of a larva, a lens will be developed from the ectoderm with which it is then in contact, even in the abdominal region,
The Development of the Optic Cup
When the invagination of the outer wall of the optic bulb is completed, the margins of the resulting cup are opposite the sides of the lens vesicle (Fig. 268), but with the enlargement of the lens and cup the margins of the latter gradually come to lie in front of - that is to say, upon the outer surface of - the lens, forming the boundary of the opening known as the pupil. The lens, consequently, is brought to lie within the mouth of the optic cup, and that portion of the latter which covers the lens takes part in the formation of the iris and the adjacent ciliary body, while its posterior portion gives rise to the retina.
The chorioidal fissure normally disappears during the sixth or seventh week of development by a fusion of its lips, and not until this is accomplished does the term cup truly describe the form assumed by the optic bulb after the invagination of its outer wall. In certain cases the lips of the fissure fail to unite perfectly, producing the defect of the eye known as coloboma; this may vary in its extent, sometimes affecting both the iris and the retina and forming what is termed coloboma iridis, and at others being confined to the retinal portion of the cup, in which case it is termed coloboma chorioidae.
Up to a certain stage the differentiation of the two layers which form the optic cup proceeds along similar lines, in both the ciliary and retinal regions. The layer which represents the original internal portion of the bulb does not thicken as the cup increases in size, and becomes also the seat of a deposition of dark pigment, whence it may be termed the pigment layer of the cup; while the other layer - â– that formed by the invagination of the outer portion of the bulb, and which may be termed the retinal layer - remains much thicker (Fig. 268) and in its proximal portions even increases in thickness. Later, however, the development of the ciliary and retinal portions of the retinal layers differs, and it will be convenient to consider first the history of the ciliary portion.
The Development of the Iris and Ciliary Body
The first change noticeable in the ciliary portion of the retinal layer is its thinning out, a process which continues until the layer consists, like the pigment layer, of but a single layer of cells (Fig. 271), the transition of which to the thicker retinal portion of the layer is somewhat abrupt and corresponds to what is termed the ora serrata in adult anatomy. In embryos of 10.2 cm. the retinal layer throughout its entire extent is readily distinguishable from the pigment layer by the absence in it of all pigmentation, but in older forms this distinction gradually diminishes in the iris region, the retinal layer there acquiring pigment and forming the uvea.
When the anterior chamber of the eye is formed by the splitting of the mesoderm which has grown in between the superficial ectoderm and the outer surface of the lens, the peripheral portions of its posterior (inner) wall are in relation with the ciliary portion of the optic cup and give rise to the stroma of the ciliary body and of the iris (Fig. 271), this latter being continuous with the tunica vasculosa lentis so long as that structure persists (Fig. 278). In embryos of about 14.5 cm. the ciliary portion of the cup becomes thrown into radiating folds (Fig. 271), as if by a too rapid growth, and into the folds lamellae of mesoderm project from the stroma. These folds occur not only throughout the region of the ciliary body, but also extend into the iris region, where, however, they are but temporary structures, disappearing entirely by the end of the fifth month. The folds in the region of the corpus ciliare persist and produce the ciliary processes of the adult eye.
Embedded in the substance of the iris stroma in the adult are non-striped muscle-fibers, which constitute the sphincter and dilatator iridis. It has long been supposed that these fibers were differentiated from the stroma of the iris, but recent observations have shown that they arise from the cells of the pigment layer of the optic cup, the sphincter appearing near the pupillary border (Fig. 271, Sph) while the dilatator is more peripheral.
Fig. 271. Radial Section through the Iris of an Embryo of 19 cm. AE, Pigment layer; CC, ciliary folds; IE, retinal layer; I.Str, iris stroma; Pm, pupillary membrane; Rs, marginal sinus; Sph, sphincter iridis. - (Szili.)
The Development of the Retina
Throughout the retinal region of the cup the pigment layer, undergoing the same changes as in the ciliary region, forms the pigment layer of the retina (Fig. 272, p). The retinal layer increases in thickness and early becomes differentiated into two strata (Fig. 268), a thicker one lying next the pigment layer and containing numerous nuclei, and a thinner one containing no nuclei. The thinner layer, from its position and structure, suggests an homology with the marginal velum of the central nervous system, and probably becomes converted into the nerve-fiber layer of the adult retina, the axis-cylinder processes of the ganglion cells passing into it on their way to the optic nerve. The thicker layer similarly suggests a comparison with the mantle layer of the cord and brain, and in embryos of 38 mm. it becomes differentiated into two secondary layers (Fig. 272), that nearest the pigment layer if) consisting of smaller and more deeply staining nuclei, probably representing the rod and cone and bipolar cells of the adult retina, while the inner layer, that nearest the marginal velum, has larger nuclei and is presumably composed of the ganglion cells.
Fig. 272. Portion of a Transverse Section of the Retina of a New-born Rabbit. ch, Chorioid coat; g, ganglion-cell layer; r, outer layer of nuclei; p, pigment layer. (Falchi.)
Little is as yet known concerning the further differentiation of the nervous elements of the human retina, but the history of some of them has been traced in the cat, in which, as in other mammals, the histogenetic processes take place at a relatively later period than in man. Of the histogenesis of the inner layer the information is rather scant, but it may be stated that the ganglion cells are the earliest of all the elements of the retina to become recognizable. The rod and cone cells, when first distinguishable, are unipolar cells (Fig. 273, a and c), their single processes extending outward from the cell-bodies to the external limiting membrane which bounds the outer surface of the retinal layer. Even at an early stage the cone cells (a) are distinguishable from the rod cells (c) by their more decided reaction to silver salts, and at first both kinds of cells are scattered throughout the thickness of the layer from which they arise. Later, a fine process grows out from the inner end of each cell, which thus assumes a bipolar form (Fig. 2 73 , b and d) , and, later still, the cells gradually migrate toward the external limiting membrane, beneath which they form a definite layer in the adult. In the meantime there appears opposite the outer end of each cell a rounded eminence projecting from the outer surface of the external limiting membrane into the pigment layer. The eminences over the cone cells are larger than those over the rod cells, and later, as both increase in length, they become recognizable by their shape as the rods and cones.
Fig. 273. Diagram showing the Development of the Retinal Elements. a, Cone cell in the unipolar, and b, in the bipolar stage; c, rod cells in the unipolar, and d, in the bipolar stage; e, bipolar cells; /and i, amacrine cells; g, horizontal cells; h, ganglion cells; k, Muller's fiber; I, external limiting membrane. - (Kallius, after Cajal.)
The bipolar cells are not easily distinguishable in the early stages of their differentiation from the other cells with which thy are mingled, but it is believed that they are represented by cells which are bipolar when the rod and cone cells are still in a unipolar condition (Fig. 273, e). If this identification be correct, then it is noteworthy that at first their outer processes extend as far as the external limiting membrane and must later shorten or fail to elongate until their outer ends lie in what is termed the outer granular layer of the retina, where they stand in relation to the inner ends of the rod and cone cell processes. Of the development of the amacrine (/", i) and horizontal cells (g) of the retina little is known. From their position in new-born kittens it seems probable that the former are derived from cells of the same layer as the ganglion cells, while the horizontal cells may belong to the outer layer.
In addition to the various nerve-elements mentioned above, the retina also contains neuroglial elements known as Muller's fibers (Fig. 273, k), which traverse the entire thickness of the retina. The development of these cells has not yet been thoroughly traced, but they resemble closely the ependymal cells observable in early stages of the spinal cord.
The Development of the Optic Nerve
The observations on the development of the retina have shown very clearly that the great majority of the fibers of the optic nerve are axis-cylinders of the ganglion cells of the retina and grow from these cells along the optic stalk toward the brain. Their embryonic history has been traced most thoroughly in rat embryos (Robinson), and what follows is based upon what has been observed in that animal.
The optic stalk, being an outgrowth from the brain, is at first a hollow structure, its cavity communicating with that of the third ventricle at one end and with that of the optic bulb at the other. When the chorioid fissure is developed, it extends, as has already been described, for some distance along the posterior surface of the stalk and has lying in it a portion of the hyaloid artery. Later, when the lips of the fissure fuse, the artery becomes enclosed within the stalk to form the arteria centralis retina of the adult (Fig. 276). By the formation of the fissure the original cavity of the distal portion of the stalk becomes obliterated, and at the same time the ventral and posterior walls of the stalk are brought into continuity with the retinal layer of the optic cup, and so opportunity is given for the passage of the axis-cylinders of the ganglion cells along those walls (Fig. 274). At an early stage a section of the proximal portion of the optic stalk (Fig. 275, A) shows the central cavity surrounded by a number of nuclei representing the mantle layer, and surrounding these a non-nucleated layer, resembling the marginal velum and continuous distally with the similar layer of the retina. When the ganglion cells of the latter begin to send out their axis-cylinder processes, these pass into the retinal marginal velum and converge in this layer toward the bottom of the chorioidal fissure, so reaching the ventral wall of the optic stalk, in the velum of which they may be distinguished in rat embryos of 4 mm., and still more clearly in those of 9 mm. (Fig. 275, A). Later, as the fibers become more numerous, they gradually invade the lateral and finally the dorsal walls of the stalk, and, at the same time the mantle cells of the stalk become more scattered and assume the form of connective-tissue (neuroglia) cells, while the original cavity of the stalk is gradually obliterated (Fig. 275, B). Finally, the stalk becomes a solid mass of nerve-fibers, among which the altered mantle cells are scattered.
Fig. 274. Diagrammatic Longitudinal Section of the Optic Cup and Stalk passing through the chorioid fissure.
Ah, Hyaloid artery; L, lens; On, fibers of the optic nerve; Os, optic stalk; PI, pigment layer, and R, retinal layer of the retina.
Fig. 275. Transverse Sections through the Proximal Part of the Optic Stalk of Rat Embryos of (A) 9 mm. and (5) 11 mm. - (Robinson.)
From what has been stated above it will be seen that the sensory cells of the eye belong to a somewhat different category from those of the other sense-organs. Embryologically they are a specialized portion of the mantle layer of the medullary canal, whereas in the other organs they are peripheral structures either representing or being associated with representatives of posterior root ganglion cells. Viewed from this standpoint, and taking into consideration the fact that the sensory portion of the retina is formed from the invaginated part of the optic bulb, some light is thrown upon the inverted arrangement of the retinal elements, the rods and cones being directed away from the source of light. The normal relations of the mantle layer and marginal velum are retained in the retina, and the latter serving as a conducting layer for the axis-cylinders of the mantle layer (ganglion) cells, the layer of nerve-fibers becomes interposed between the source of light and the sensory cells. Furthermore, it may be pointed out that if the differentiation of the retina be imagined to take place before the closure of the medullary canal - a condition which is indicated in some of the lower vertebrates - there would be then no inversion of the elements, this peculiarity being due to the conversion of the medullary plate into a tube, and more especially to the fact that the retina develops from the outer wall of the optic cup. In certain reptiles in which an eye is developed in connection with the epiphysial outgrowths of the diencephalon, the retinal portion of this pineal eye is formed from the inner layer of the bulb, and in this case there is no inversion of the elements.
A justification of the exclusion of the optic nerve from the category which includes the other cranial nerves has now been presented. For if the retina be regarded as a portion of the central nervous system, it is clear that the nerve is not a nerve at all in the strict sense of that word, but is a tract, confined throughout its entire extent within the central nervous system and comparable to such groups of fibers as the direct cerebellar or fillet tracts of that system.
The Development of the Vitreous Humor
It has already been pointed out (p. 448) that a blood-vessel, the hyaloid artery, accompanied by some mesodermal tissue makes its way into the cavity
Fig. 276. - Reconstruction of a Portion of the Eye of an Embryo of 13.8 mm. ah, Hyaloid artery; ch, chorioid coat; /, lens; r, retina. - (His.) of the optic cup through the chorioid fissure. On the closure of the fissure the artery becomes enclosed within the optic stalk and appears to penetrate the retina, upon the surface of which its branches ramify. In the embryo the artery does not, however, terminate in these branches as it does in the adult, but is continued on through the cavity of the optic cup (Fig. 276) to reach the lens, around which it sends branches to form the tunica vasculosa lentis.
According to some authors, the formation of the vitreous humor is closely associated with the development of this artery, the humor being merely a transudate from it, while others have maintained that it is a derivative of the mesoderm which accompanies the vessel, and is therefore to be regarded as a peculiar gelatinous form of connective tissue. More recently, however, renewed observations by several authors have resulted in the deposition of the mesoderm from the chief role in the formation of the vitreous and the substitution in it of the retina. At an early stage of development delicate protoplasmic processes may be seen projecting from the surface of the retinal layer into the cavity of the optic cup, these processes probably arising from those cells which will later form the Muller's (neuroglia) fibers of the retina. As development proceeds they increase in length, forming a dense and very fine fibrillar reticulum traversing the space between the lens and the retina and constituting the primary vitreous humor. The formation of the fibers is especially active in the ciliary portion of the retina and it is probable that it is from some of the fibers developing in this region that the suspensory ligament of the lens (zonula Zinnii) (Fig. 277, si) is formed spaces which occur between the fibers of the ligament enlarging to produce a cavity traversed by scattered fibers and known as the canal of Petit.
Fig. 277. Transverse Section through the Ciliary Region of a Chick Embryo of Sixteen Days. ac, Anterior chamber of the eye; cj, conjunctiva; co, cornea; i, iris; I, lens; mc, ciliary muscle; rl, retinal layer of optic cup; sf, spaces of Fontana; si, suspensory ligament of the lens; v, vitreous humor. - (Angelucci.)
A participation of similar protoplasmic prolongations from the cells of the lens in the formation of the vitreous humor has been maintained (von Lenhossek) and as strenuously denied. But it is generally admitted that at the time when the hyaloid artery penetrates the vitreous to form the tunica vasculosa lentis it carries with it certain mesodermal elements, whose fate is at present uncertain. It has been held that they take part in the formation of the definitive vitreous, which, according to this view, is of mixed origin, being partly ectodermal and partly mesodermal (Van P6e), and, on the contrary, it has been maintained that they eventually undergo complete degeneration, the vitreous being of purely ectodermal origin (von Kolliker).
The degeneration of the mesodermal elements which the latter view supposes is associated with the degeneration of the hyaloid artery. This begins in human embryos in the third month and is completed during the ninth month, the only trace after birth of the existence of the vessel being a more fluid consistency of the axis of the vitreous humor, this more fluid portion representing the space originally occupied by the artery and forming what is termed the hyaloid canal (canal ofCloquet).
The Development of the Outer Coat of the Eye, of the Cornea, and of the Anterior Chamber
Soon after the formation of the optic bulb a condensation of the mesoderm cells around it occurs, forming a capsule. Over the medial portions of the optic cup the further differentiation of this capsule is comparatively simple, resulting in the formation of two layers, an inner vascular and an outer denser and fibrous, the former becoming the chorioid coat of the adult eye and the latter the sclera.
More laterally, however, the processes are more complicated. After the lens has separated from the surface ectoderm a thin layer of mesoderm grows in between the two structures and later gives place to a layer of homogeneous substance in which a few cells,
The Anterior Chamber Of The Eye
more numerous laterally than at the center, are embedded. Still later cells from the adjacent mesenchyme grow into the layer, which increases considerably in thickness, and blood-vessels also grow into that portion of it which is in contact with the outer surface of the lens. At this stage the interval between the surface ectoderm and the lens is occupied by a solid mass of mesodermal tissue (Fig. 278, co and tv), but as development proceeds, small spaces (ac) filled with fluid begin to appear toward the inner portion of the mass, and these, increasing in number and size, eventually fuse together to form a single cavity which divides the mass into an inner and an outer portion. The cavity is the anterior chamber of the eye, and it has served to separate the cornea (co) from the tunica vasculosa lentis (tv) , and, extending laterally in all directions, it also separates from the cornea the mesenchyme which rests upon the marginal portion of the optic cup and constitutes the stroma of the iris. Cells arrange themselves on the corneal surface of the cavity to form a continuous endothelial layer, and the mesenchyme which forms the peripheral boundary of the cavity assumes a fibrous character and forms the ligamentum pectinatum iridis, among the fibers of which cavities, known as the spaces of Fontana (Fig. 277, sf), appear. Beyond the margins of the cavity the corneal tissue is directly continuous with the sclerotic, beneath the margin of which is a distinctly thickened portion of mesenchyme resting upon the ciliary processes and forming the stroma of the ciliary body, as well as giving rise to the muscle tissue which constitutes the ciliary muscle (Figs. 277 and 278, mc).
Fig. 278. Transverse Section through the Ciliary Region of a Pig Embryo or 23 mm. ac, Anterior chamber of the eye; co, cornea; ec, ectoderm; I, lens; mc, ciliary muscle; p, pigment layer of the optic cup; r, retinal layer; tv, tunica vasculosa lentis. - (Angelucci.)
The ectoderm which covers the outer surface of the eye does not proceed beyond the stage when it consists of several layers of cells, and never develops a stratum corneum. In the corneal region it rests directly upon the corneal tissue, which is thickened slightly upon its outer surface to form the anterior elastic lamina; more peripherally, however, a quantity of loose mesodermal tissue lies between the ectoderm and the outer surface of the sclerotic, and, together with the ectoderm, forms the conjunctiva (Fig. 277, cj).
The Development of the Accessory Apparatus of the Eye. - The eyelids make their appearance at an early stage as two folds of skin, one a short distance above and the other below the cornea. The center of the folds is at first occupied by indifferent mesodermal tissue, which later becomes modified to form the connective tissue of the lids and the tarsal cartilage, the muscle tissue probably secondarily growing into the lids as a result of the spreading of the platysma over the face, the orbicularis oculi apparently being a derivative of that sheet of muscle tissue.
At about the beginning of the third month the lids have become sufficiently large to meet one another, whereupon the thickened epithelium which has formed upon their edges unites and the lids fuse together, in which condition they remain until shortly before birth. During the stage of fusion the eyelashes (Fig. 279, h) develop at the edges of the lids, having the same developmental history as ordinary hairs, and from the fused epithelium of each lid there grow upward or downward, as the case may be, into the mesodermic tissue, solid rods of ectoderm, certain of which early give off numerous short lateral processes and become recognizable as the tarsal (Meibomian) glands (m), while others retain the simple cylindrical form and represent the glands of Moll. When the eyelids separate, these solid ingrowths become hollow by a breaking down of their central cells, just as in the sebaceous and sudoriparous glands of the skin, the tarsal glands being really modifications of the former glands, while the glands of Moll are probably to be regarded as specialized sudoriparous glands.
Fig. 279. Section through the Margins of the Fused Eyelids in an Embryo of Six Months. ih, Eyelash; //, lower lid; m, tarsal gland; mu, muscle bundle; ul, upper lid. (Schweigger-Seidl.)
A third fold of skin, in addition to^the two which produce the eyelids, is also developed in connection with the eye, forming the plica semilunaris. This is a rudimentary third eyelid, representing the nictitating membrane which is fairly well developed in many of the lower mammals and especially well in birds.
The lachrymal gland is developed at about the third month as a number of branching outgrowths of the ectoderm into the adjacent mesoderm along the outer part of the line where the epithelium of the conjunctiva becomes continuous with that covering the inner surface of the upper eyelid. As in the other epidermal glands, the outgrowths and their branches are at first solid, later becoming hollow by the degeneration of their axial cells.
The naso-lachrymal duct is developed in connection with the groove which, at an early stage in the development (Fig. 62), extends from the inner corner of the eye to the olfactory pit and is bounded posteriorly by the maxillary process of the first visceral arch. The epithelium lying in the floor of this groove thickens toward the begining of the sixth week to form a solid cord, which sinks into the subjacent mesoderm. From its upper end two outgrowths arise which become connected with the ectoderm of the edges of the upper and lower lids, respectively, and represent the lachrymal ducts, and, finally, the solid cord and its outgrowths acquire a lumen and a connection with the mucous membrane of the inferior meatus of the nasal cavity.
Fig. 280. Diagram showing the Insertions of the Lachrymal Ducts in embryos of 40 mm and 170 mm. The caruncula lacrimalis being formed in the latter. The eyelids are really fused at these stages but have been represented as separated for the sake of clearness. - (Ask.)
The inferior duct connects with the border of the eyelid some distance lateral to the inner angle of the eye, and between its opening and the angle a number of tarsal glands develop. The superior duct, on the other hand, opens at first close to the inner angle and later moves laterally until its opening is opposite that of the inferior duct. During this change the portion of the lower lid between the opening of the inferior duct and the angle is drawn somewhat upward, and, with its glands, forms a small reddish nodule, resting upon the plica semilunaris and known as the caruncula lacrimalis (Fig. 280).
Literature
G. Alexander: "Ueber Entwicklung und Bau des Pars inferior Labyrinthi der hdheren Saugethiere," Denkschr. kais. wissench. Acad. Wien, Math.-Naturw. Classe, lxx, 1901.
A. Angeltjcci: "Ueber Entwickelung und Bau des vorderen Uvealtractus der Verte braten," Archiv fur mikrosk. Anat., xix, 1881.
F. Ask: Ueber die Entwickelung der Caruncula lacrimalis beim Menschen, nebst Bemerkungen iiber die Entwickelung der Tranenrohrchen und der Meibom'schen Driisen," Anatom. Anzeiger, xxx, 1907.
F. Ask: Ueber die Entwicklung der Lidrander, der Tranenkarunkel und der Nick haut beim Menschen, nebst Bemerkungen zur Entwicklung der Tranenabfuhrungswege," Anat. Hefte, xxxvi, 1908.
B. Baginsky: "Zur Entwickelung der Gehorschnecke," Archiv fur mikrosk. Anat., xxviii, 1886.
I. Broman: "Die Entwickelungsgeschichte der Gehorknochelchen beim Menschen," Anat. Hefte, xi, 189S.
S. Ramon y Cajal: "Nouvelles contributions a l'etude histologique de la retine," Journ. de I' Anat. et de la Physiol., xxxii, 1896.
G. Cirincione: "Ueber den gegenwartigen Stand der Frage hinsichtlich der Genese des Glaskorpers," Arch, fur Augenheilk., L, 1904.
A. Contino: "Ueber Bau and Entwicklung des Lidrandes beim Menschen," Arch, fur Ophthalmol., lxvi, 1908.
A. Contino: "Ueber die Entwicklung der Karunkel und der plica semilunaris beim Menschen," Arch, fur Ophthalmol, lxxi, 1909.
J. Disse: "Die erste Entwickelung der Riechnerven," Anat. Hefte, ix, 1897.
B. Fleischer: "Die Entwickelung der Tranenrohrchen bei den Saugetiere," Archiv fur Ophthalmol., lxii, 1906.
H. Fuchs : " Bemerkungen iiber die Herkunft und Entwickelung der Gehorknochelchen bei Kaninchen-Embryonen (nebst Bemerkungen iiber die Entwickelung des Knorpelskeletes der beiden ersten Visceralbogen)," Archiv. fur Anat und Phys., Anat. Abth., Supplement, 1905.
J. Graberg: "Beitrage zur Genese des Geschmacksorgans der Menschen," Morphol. Arbeiten, vn, 1898.
J. A. Hammar: "Zur allgemeinen Morphologie der Schlundspalten des Menschen. Zur Entwickelungsgeschichte des Mittelohrraumes, des ausseren Gehorganges und des Paukenfelles beim Menschen," Anat. Anzeiger, xx, 1901.
J. A. Hammar: " Studien iiber die Entwicklung des Vorderdarms und einiger angrenz ender Organe," Arch, fur mikrosk. Anat., Lix, 1902.
C. Heerfordt: "Studien iiber den Muse, dilatator pupilke sammt Angabe von gemeinschaftlicher Kennzeichen einiger Falle epithelialer Musculatur," Anat.
Hefte, xiv. J. Hegetschweiler: "Die embryologische Entwickelung des Steigbugels," Archiv fur Anat. und Physiol., Anat. Abth., 1898.
F. Hochstetter: "Ueber die Bildung der primitiven Choanen beim Menschen," Verhandl. Anat. Gesellsch., VI, 1892.
W. His, Jr.: "Die Entwickelungsgeschichte des Acustico-Facialisgebietes beim Menschen," Archiv fur Anat. und Physiol., Anat. Abth., Supplement, 1897.
A. von Kolliker: "Die Entwicklung und Bedeutung des Glaskorpers," Zeitschr. fur wissensch. Zoolog., lxxvi, 1904.
P. Lang: "Zur Entwicklung des Tranenausfiihrsapparates beim Menschen," Anat. Anzeiger," xxxvni, 1911.
G. Leboucq: " Contribution a. l'etude de l'histogenese de la retine chez les mammiferes," Arch. Anat., Microsc, x, 1909.
V. von Mihalkovicz: "Nasenhohle und Jacobsonsches Organ. Eine morphologische Studie." Anat. Hefte. xi, 1898.
J. L. Paitlet: "Contribution a l'etude de l'organe de Jacobson chez l'embryon humain," Bibliogr. Anat., xvii, 1907.
P. van Pee: "Recherches sur l'origine du corps vitre," Archives de Biol., xix, 1902.
C. Rabl: "Ueber den Bau und Entwickelung der Linse," Zeitschrift fur wissensch. Zoologie, lxiii and lxv, 1898; lxviii, 1899.
A. Robinson: "On the Formation and Structure of the Optic Nerve and Its Relation to the Optic Stalk," Journal of Anat. and Physiol., xxx, 1896.
G. Speciale-Cirincione: "Ueber die Entwicklung der Tranendriise beim Menschen " Arch.filr Ophthalmol., LXIX, 1908.
J. P. Schaefeer: "The Genesis and Development of the Nasolacrimal Passages in Man," Amer. Journ. Anat., xm, 1912.
G. L. Streeter: "On the Development of the Membranous Labyrinth and the Acoustic and Facial Nerves in the Human Embryo," Amer. Journ. of Anat. vi, 1907.
N. van der Stricht: "L'histogenese des parties constituantes du neuroepithelium acoustique, des taches et des cretes acoustiques et de l'organe de Corti " Arch. de Biol., xxiii, 1908.
A. Szili: "Zur Anatomie und Entwickelungsgeschichte der hinteren Irisschichten mit besonderer Beriicksichtigung des Musculus sphincter iridis des Menschen " Anat. Anzeiger, xx, 1901.
A. Szili: "Ueber das Entstehen eines fibrillares Stutzgewebes im Embryo und dessen Verhaltnis zur Glaskorperfrage," Anat. Hejte, xxxv, 190S.
F. Tuckerman: "On the Development of the Taste Organs in Man," Journal of Anat. and Physiol., xxiv, 1889.
R. Versari: "Ueber die Entwicklung der Blutgefasse des menschlichen Au^es " Anat. Anzeiger, xxxv, 1909.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
McMurrich JP. The Development Of The Human Body. (1914) P. Blakiston's Son & Co., Philadelphia, Pennsylvania.
Cite this page: Hill, M.A. (2024, April 24) Embryology McMurrich1914 Chapter 16. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/McMurrich1914_Chapter_16
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G