Lecture - Stem Cells: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
2012 - This lecture will be presented by an expert guest lecturer | 2012 - This lecture will be presented by an expert guest lecturer Professor Edna Hardeman | ||
Here is a PDF for her lecture. | |||
{| | {| | ||
| Line 226: | Line 209: | ||
A set of 4 transcription factors when introduced into cells induces stem cell formation.<ref><pubmed>16904174</pubmed></ref> These four transcription factors can be expressed from doxycycline (dox)-inducible lentiviral vectors. The only culture difference in iPS cells and human embryonic stem cell culture is that iPS cell culture require 100ng/ml of bFGF in the culture media. | A set of 4 transcription factors when introduced into cells induces stem cell formation.<ref><pubmed>16904174</pubmed></ref> These four transcription factors can be expressed from doxycycline (dox)-inducible lentiviral vectors. The only culture difference in iPS cells and human embryonic stem cell culture is that iPS cell culture require 100ng/ml of bFGF in the culture media. | ||
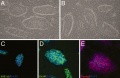
[http:// | [http://php.med.unsw.edu.au/embryology/index.php?title=File:Reprogramming_MEF_into_ES-like_cells_03.jpg Outline of the MEF reprogramming protocol 1] | ||
[http:// | [http://php.med.unsw.edu.au/embryology/index.php?title=File:Reprogramming_MEF_into_ES-like_cells_01.jpg Outline of the MEF reprogramming protocol 2] | [http://php.med.unsw.edu.au/embryology/index.php?title=File:Reprogramming_MEF_into_ES-like_cells_02.jpg stained with anti-Rex1, Sox2 and SSEA1 antibodies] | ||
Revision as of 14:29, 21 October 2013
Introduction
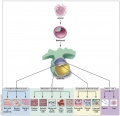
Embryology is all about stem cells, a single cell (zygote) divides and differentiates to form all the tissues throughout the body. Furthermore within some tissues, stem cells remain to continuously replace cells that are lost through the life of that tissue. In recent years Scientific and general interest in this topic has increased due to the many issues that surround this specialised cell type. This lecture will introduce the various types/sources of stem cells as well as their practical and therapeutic potentials. A brief discussion of the pros and cons of different types of stem cells currently investigated in the field of medical research will be discussed.
2012 - This lecture will be presented by an expert guest lecturer Professor Edna Hardeman Here is a PDF for her lecture.
|
The Nobel Prize in Physiology or Medicine 2012 was awarded jointly to Sir John B. Gurdon and Shinya Yamanaka "for the discovery that mature cells can be reprogrammed to become pluripotent"
|
| Lectopia Lecture Audio
Lecture Date: 2012-10-09 Lecture Time: 15:00 Venue: CLB 3 Speaker: Kuldip Sidhu |
Textbooks

|
Hill, M.A. (2011) UNSW Embryology (11th ed.). Sydney:UNSW. |
Objectives
- Understanding of stem cell history
- Understanding of stem cell types
- Understanding of stem cell identification/differentiation
- Understanding of advantages and disadvantages of different stem cell types
Why Stem Cells
Why are they in the News?
|
What are their uses?
|
Medline Search stem cell 2002 - 110,920 | 2004 - 128,485 | 2005 - 140,966 | 2006 - 154,176 | 2011 - 190,069 | 2012 - 210,393
Research that led to Stem Cells
- Human Diseases - Generation of “knock out” mice
- Human Development - Studying regulation of cell differentiation in development
- Human Reproduction - Disorders, sterility
Stem Cell Types
Tissue Stem Cells
- differentiated cells have short life spans continually replaced
- blood cells, epithelial cells of skin and digestive tract
- fully differentiated cells do not proliferate
- proliferation of less differentiated- stem cells
- produce daughter cells that either differentiate or remain as stem cells
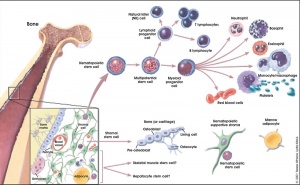
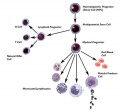
Blood Cells
- All different types of blood cells develop from a pluripotent stem cell in bone marrow
- Precursors of differentiated cells undergo several rounds of cell division as they mature
- proliferation ceases at terminal stages of differentiation
Embryonic Stem Cells
NIH - What are embryonic stem cells?
- What is a stem cell - Pluripotent (totipotent)
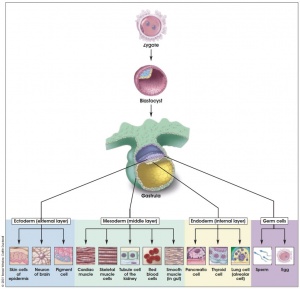
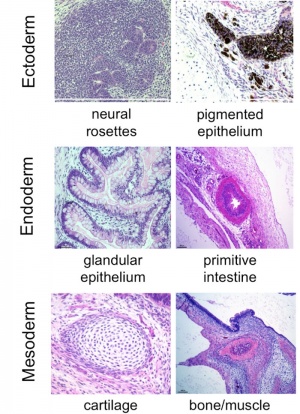
- Pluripotent - to describe stem cells that can give rise to cells derived from all 3 embryonic germ layers (Ectoderm, Mesoderm, Endoderm)
- layers are embryonic source of all cells of the body
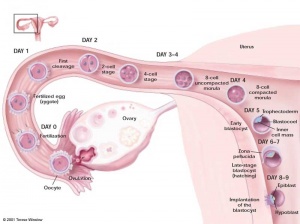
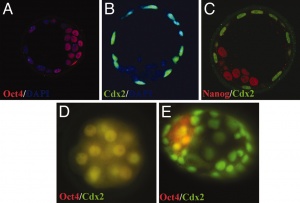
Blastocyst
- hollow structure composed of about 100 cells surrounding an inner cavity
- Only ES cells, which form inner cell mass, actually form the embryo.
- ES cells can be removed from the blastocyst and grown on lethally irradiated “feeder cells.” (See E. Robertson et al., 1986, Nature 323:445)
Stem Cell Definition
- cell that has ability to divide for indefinite periods
- self replicate
- throughout life of organism
- stem cells can differentiate
- conditions, signals
- to the many different cell types
Chimeric Mouse
- ES or teratocarcinoma - shows that stem cells can combine with cells of a normal blastocyst to form a healthy chimeric mouse
Embryoid Bodies
- spheroid cellular tissue culture structure
- mouse and human ES cells have the capacity to undergo controlled differentiation
- recapitulate some aspects of early development
- regional-specific differentiation program
- derivatives of all three embryonic germ layers
Historic References
Mouse<pubmed>6950406</pubmed> <pubmed>6714319</pubmed> <pubmed>3024164</pubmed> |
Pig and Sheep<pubmed>1843344</pubmed> |
Primate<pubmed>7544005</pubmed> |
Human<pubmed>9804556</pubmed> |
Stem Cell Lines ATCC - Embryonic Stem cell lines
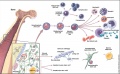
Cord Blood Stem Cells
- Blood collected from the placental umbilical cord of a newborn baby shortly after birth (about 90 ml)
- blood stem cells that can be used to generate red blood cells and cells of the immune system
- collected, typed, stored in Cord Blood Bank (public and private Banks have arisen)
- available for use by the donor and compatible siblings
- suggested use to treat a range of blood disorders and immune system conditions such as leukaemia, anaemia and autoimmune diseases
- cells also provide a resource for bone marrow replacement therapy in many diseases.
- can now also be transformed into induced stem cells
- Links: Cord Stem Cells
Adult Stem Cells
- Connective Tissue
- Bone marrow - Blood Cells, Osteoclasts, blasts
- Epithelia - Gut, Skin (Epidermis: Immortal Stem Cell)
- Neural?
Epithelium Stem Differentiation
- each generation at least 1 "immortal" stem cell - descendants present in patch in future
- Other basal cells - leave basal layer and differentiate
- Committed, born different or may be stem cells
- equivalent to immortal stem cell in character
- mortal in sense that their progeny jostled out of basal layer and shed from skin
Amplifying Cells
- Stem cells in many tissues divide only rarely
- give rise to transit amplifying cells
- daughters committed to differentiation that go through a limited series of more rapid divisions before completing the process.
- each stem cell division gives rise in this way to eight terminally differentiated progeny
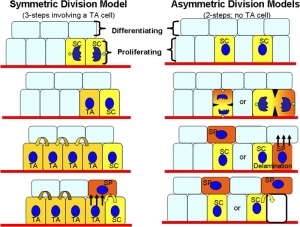
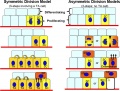
Stem Cell Production - Stem Cell Daughter Fates
- Environmental asymmetry
- daughters are initially similar
- different pathways according to environmental influences that act on them after they are born
- number of stem cells can be increased or reduced to fit niche available
- Divisional asymmetry
- stem cell has an internal asymmetry
- divides in such a way two daughters are already have different determinants at time of their birth
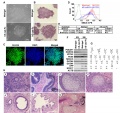
Induced Pluripotent Cells
- non-pluripotent cells engineered to become pluripotent (iPSC), a cell with a specialized function ‘reprogrammed’ to an unspecialized state
Yamanaka Factors
A set of 4 transcription factors when introduced into cells induces stem cell formation.[2] These four transcription factors can be expressed from doxycycline (dox)-inducible lentiviral vectors. The only culture difference in iPS cells and human embryonic stem cell culture is that iPS cell culture require 100ng/ml of bFGF in the culture media.
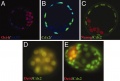
Outline of the MEF reprogramming protocol 1 Outline of the MEF reprogramming protocol 2 | stained with anti-Rex1, Sox2 and SSEA1 antibodies
- OCT4 Transcription factors containing the POU homeodomain
- MYC The MYC protooncogene encodes a DNA-binding factor that can activate and repress transcription. Ectopic expression of c-Myc can also cause tumorigenicity in offspring.
- SOX2 SRY-RELATED HMG-BOX GENE 2
- KLF4 Kruppel-like factor 4, zinc finger protein, transcription factor which acts as both an activator and repressor.
More recently shown that Oct4 together with either Klf4 or c-Myc is sufficient to generate iPS cells from neural stem cells.[3]
Thompson Factor
Stem Cell Markers
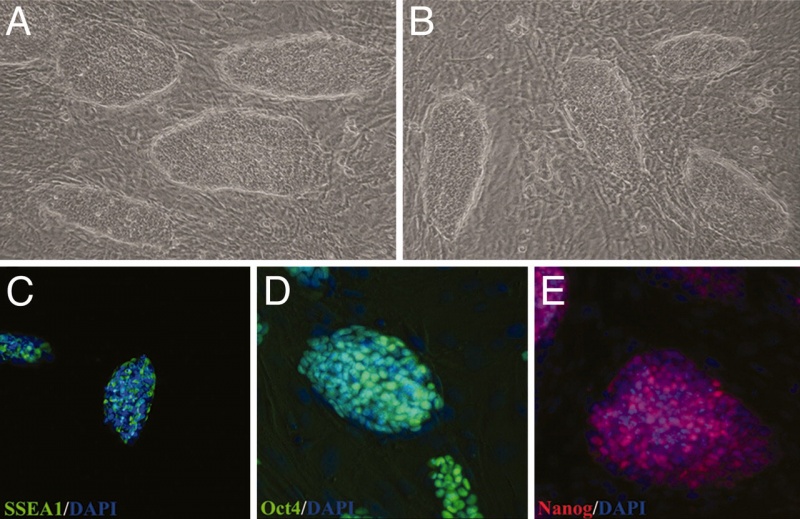
In order to carry out research on stem cells, it is important to be able to identify them. A number of different research groups in the late 90's generated several antibodies which specifically identified undifferentiated, differentiating or differentiated stem cells from a number of different sources and species. Note that the nomenclature in some cases is based upon the antibody used to identify the cell surface marker.
Morula cell lines express ES markers[4]
- Every cell surface has specialized proteins (receptors) that can selectively bind or adhere to other “signalling” molecules (ligands)
- Different types of receptors differ in structure and affinity for signalling molecules
- Cells use these receptors and molecules that bind to them as a way of communicating with other cells and to carry out their proper functions in the body
- Stage-specific embryonic antigen (SSEA)-1, -3 and -4 and tumor-rejection antigen (TRA)-1-60 and -1-81, are expressed in specific combinations by undifferentiated pluripotent cells.
- embryonic stem cells, induced pluripotent stem cells, embryonal carcinoma cells, primordial germ cells, mesenchymal progenitors in adult murine bone marrow, and embryonic germ cells.
- Stage-Specific Embryonic Antigen-1 (SSEA-1) cell surface glycan embryonic antigen which has a role in cell adhesion, migration and differentiation and is often differentially expressed during development. Can be identified by Davor Solter monoclonal antibody MC-480 (SSEA-1).
- Stage-Specific Embryonic Antigen-4 (SSEA-4) cell surface embryonic antigen of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES) which is down-regulated following differentiation of human EC cells. Antigen not expressed on undifferentiated murine EC, ES and EG cells but upregulated on differentiation of murine EC and ES cells. Can be identified by Davor Solter monoclonal antibody MC-813-70 (SSEA-4)
- Tumor Rejection Antigen (TRA-1-60) Sialylated Keratan Sulfate Proteoglycan expressed on the surface of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES).
- Tumor Rejection Antigen (TRA-1-81) antigen expressed on the surface of human teratocarcinoma stem cells (EC), human embryonic germ cells (EG) and human embryonic stem cells (ES).
- Both TRA antibodies identify a major polypeptide (Mr 240 kDa) and a minor polypeptide (Mr 415 kDa).
- Oct-4 (Pou5f1 – Mouse Genome Informatics) gene has an essential role in control of developmental pluripotency (Oct4 knockout embryo blastocysts die at the time of implantation). Oct4 also has a role in maintaining viability of mammalian germline.
- Stem Cell Antigen 1 (Sca-1) member of the Ly-6 family of GPI-linked surface proteins (Mr 18 kDa) and a major phenotypic marker for mouse hematopoietic progenitor/stem cell subset.
- CD133, AC133, prominin 5 transmembrane glycoprotein (865 aa) expressed on stem cells with hematopoietic and nonhematopoietic differentiation potential.
- Alkaline Phosphatase - embryonic stem cell is characterized by high level of expression alkaline phosphatase (undifferentiated state) ATCC ELF Phosphatase Detection Kit for Embryonic Stem Cells
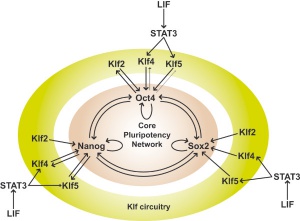
Expression of Zfp42/Rex1 Gene - used as a marker of undifferentiated stem cells[5]
- regulated by Nanog, Sox2, and Oct4, and by the Wnt pathway
- subject to epigenetic regulation by polycomb complexes and DNA methylation
Embryonic vs Adult Stem Cells
Embryonic Stem Cell Advantages
- Pluripotency - ability to differentiateinto any cell type.
- Immortal - one cell can supply endless amounts of cells.
- Easily available - human embryos from fertility clinics.
Embryonic Stem Cell Disadvantages
- Unstable - difficult to control differentiation into specific cell type.
- Immunogenic - potential immune rejection when transplanted into patients.
- Teratomas - tumor composed of tissues from 3 embryonic germ layers.
- Ethical Controversy - unethical for those who believes that life begins at conception.
Adult Stem Cell Advantages
- Already ‘specialised’ - induction of differentiation into specific cell types will be easier.
- Plasticity - Recent evidences suggest wider than previously thought ranges of tissue types can be derived.
- No Immune-rejection - if used in autologous transplantations.
- No Teratomas - unlike ES cells.
- No Ethical Controversy - sourced from adult tissues.
Adult Stem Cell Disadvantages
- Minimal quantity - number of isolatable cells may be small.
- Finite life-span - may have limited lifespan in culture.
- Ageing - stem cells from aged individuals may have higher chance of genetic damage due to ageing.
- Immunogenic - potential immune rejection if donor cells are derived from another individual.
Current stem cell research
How to:
- Isolate
- Grow
- Maintain, store
- Differentiate
- Therapeutic uses
References
Textbooks
- Molecular Biology of the Cell Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Raff, Martin; Roberts, Keith; Walter, Peter New York and London: Garland Science; c2002 Figure 22-4. The definition of a stem cell | Figure 22-19. Renewal of the gut lining | [http://www.ncbi.nlm.nih.gov:80/books/bv.fcgi?db=Books&rid=mboc4.figgrp.4092 Figure 22-5. Two ways for a stem cell to produce daughters with different fates
- Molecular Cell Biology Lodish, Harvey; Berk, Arnold; Zipursky, S. Lawrence; Matsudaira, Paul; Baltimore, David; Darnell, James E New York: W. H. Freeman & Co.; c1999 Figure 24-8. Formation of differentiated blood cells from hematopoietic stem cells in the bone marrow
- The Cell- A Molecular Approach Cooper, Geoffrey M. Sunderland (MA): Sinauer Associates, Inc.; c2000 Stem Cells
Search
- Online Textbooks "stem cell" Molecular Biology of the Cell | Molecular Cell Biology | The Cell- A molecular Approach
Images
- Stem cell therapy.jpg
Stem cell therapy
References