Lecture - Ectoderm Development
Objectives
- Understanding of events during the third and fourth week of development
- Understanding the process of early neural development
- Brief understanding of neural crest formation
- Brief understanding of epidermis formation
- Understanding of the adult components derived from ectoderm
- Brief understanding of early neural abnormalities
References
The Developing Human: Clinically oriented embryology

|
Citation: The developing human : clinically oriented embryology 8th ed. Moore, Keith L; Persaud, T V N; Torchia, Mark G Philadelphia, PA : Saunders/Elsevier, c2008. |
Larsen's human embryology
|
Citation: Larsen's human embryology 4th ed. Schoenwolf, Gary C; Larsen, William J, (William James). Philadelphia, PA : Elsevier/Churchill Livingstone, c2009. |
UNSW Embryology

|
Hill, M.A. (2011) UNSW Embryology (11th ed.). Sydney:UNSW. |
Development Overview
Notochord
- forms initially as the Axial Process, a hollow tube which extends from the primitive pit , cranially to the oral membrane
- the axial process then allow transient communication between the amnion and the yolk sac through the neuroenteric canal.
- the axial process then merges with the Endodermal layer to form the Notochordal Plate.
- the notochordal plate then rises back into the Mesodermal layer as a solid column of cells which is the Notochord.
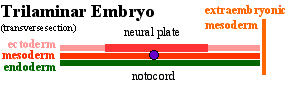
Ectoderm
- 2 parts
- midline neural plate
- columnar
- lateral surface ectoderm (MH - covered later in the course)
- cuboidal
- sensory placodes Placodes
- epidermis of skin, hair, glands, anterior pituitary, teeth enamel
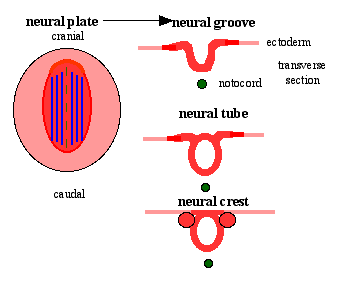
Neural Plate
| File:Neuralplate_001 icon.jpg</wikiflv> | Development of the neural plate region at the embryonic disc stage.
Dorsal view of the embryonic disc from the amniotic cavity side showing the ectoderm with the central region developing into the neural plate. The neural plate extends from buccopharyngeal membrane to primitive node and forms above the notochord and paraxial mesoderm.The neuroectodermal cells form a broad brain plate and narrower spinal cord region. Three specific regions medial to lateral can also be identified: midline region floor plate, neural plate, edge of neural plate neural crest
|
- extends from buccopharyngeal membrane to primitive node
- forms above notochord and paraxial mesoderm
- neuroectodermal cells
- broad brain plate
- narrower spinal cord
- 3 components form: floor plate, neural plate, neural crest
Neural Determination- neuronal populations are specified before plate folds
- signals from notochord and mesoderm - secrete noggin, chordin,follistatin
- all factors bind BMP-4 an inhibitor of neuralation
- bone morphogenic protein acts through membrane receptor
- lateral inhibition generates at spinal cord level 3 strips of cells
- expression of delta inhibits nearby cells, which express notch receptor, from becoming neurons
- Delta-Notch inetraction- generates Neural strips
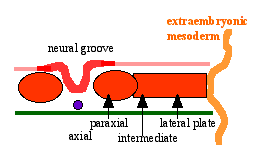
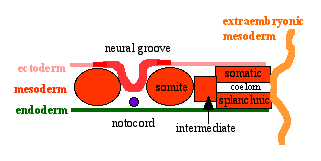
Neural Groove
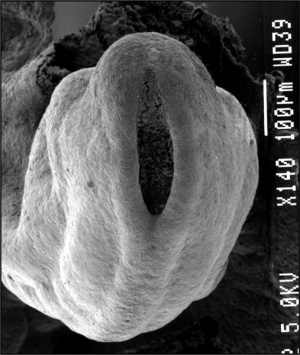
| File:Neuraltube_001 icon.jpg</wikiflv> | This animation of early neural development from week 3 onward shows the neural groove fusing to form the neural tube.
View - Dorsolateral of the whole early embryo and yolk sac. Cranial (head) to top and caudal (tail) to bottom. Yolk sac is shown to the left. Beginning with the neural groove initially fusing at the level of the 4th somite to form the neural tube and closing in both directions to leave 2 openings or neuropores: a cranial neuropore (anterior neuropore) and a caudal neuropore (posterior neuropore). The animation also shows as the embryo grows and folds it increases in size relative to the initial yolk sac. Note also the increasing number of somites over time. |
- forms in the midline of the neural plate (day 18-19)
- either side of which are the neural folds which continues to deepen until about week 4
- neural folds begins to fuse, beginning at 4th somite level
Neural Tube
- the neural tube forms the brain and spinal cord
- fusion of neural groove extends rostrally and caudally
- begins at the level of 4th somite
- closes neural groove "zips up" in some species.
- humans appear to close at multiple points along the tube.
- leaves 2 openings at either end - Neuropores
- cranial neuropore closes before caudal
Failure for the neural tube to close correctly or completely results in a neural tube defect.
Secondary Neuralation
| File:Secondary_neurulation_01 icon.jpg</wikiflv> | This animation shows the early developmental process often described as secondary neurulation.
Red - site of secondary neurulation | Blue - neural tube
|
Neural Crest
- a population of cells at the edge of the neural plate that lie dorsally when the neural tube fuses
- dorsal to the neural tube, as a pair of streaks
- pluripotential (forms many different types of cells)
- cells migrate throughout the embryo
- migration studied by quail-chick chimeras - transplanted quail cells have obvious nucleoli compared with chicken
Neural Crest Derivitives
- dorsal root ganglia (DRG)
- autonomic ganglia
- adrenal medulla
- drg sheath cells, glia
- pia-arachnoid sheath
- skin melanocytes
- connective tissue of cardiac outflow
- thyroid parafollicular cells
- craniofacial skeleton
- teeth odontoblasts
Early Brain Structure
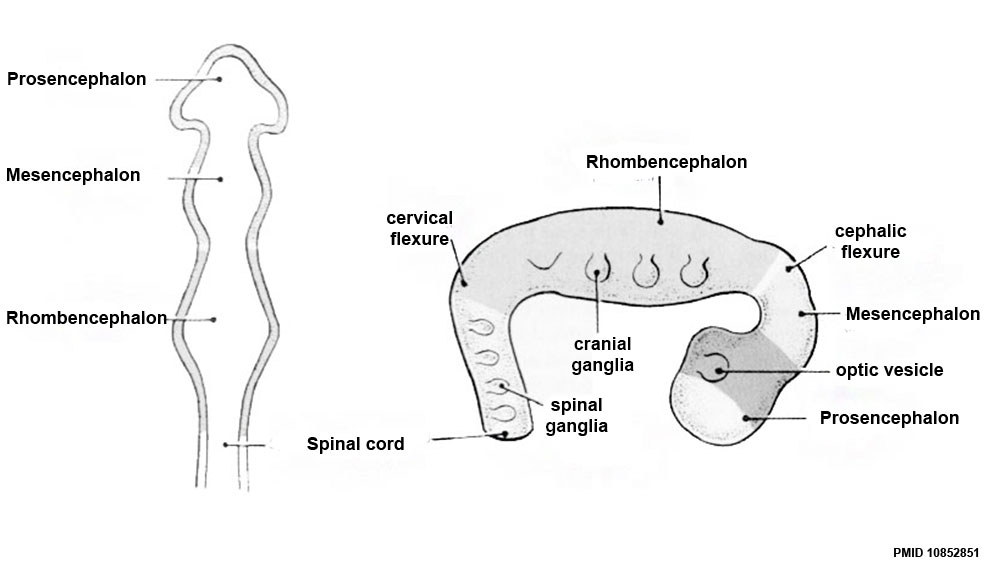
Primary Vesicles
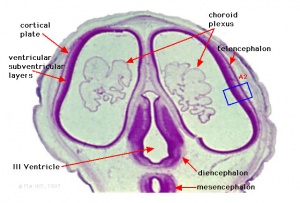
- rostral neural tube forms 3 primary brain vesicles (week 4)
- 3 primary vesicles: prosencephalon (forebrain), mesencephalon (midbrain), rhombencephalon (hindbrain)
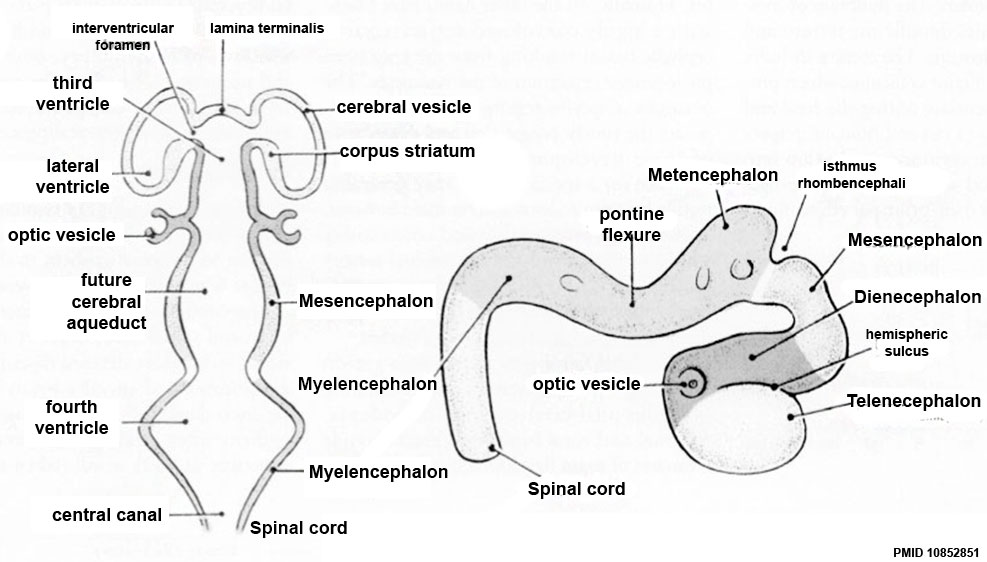
Secondary Vesicles
From the 3 primary vesicles developing to form 5 secondary vesicles
- prosencephalon- telencephalon (endbrain, forms cerebral hemispheres), diencephalon (betweenbrain, forms optic outgrowth)
- mesencephalon
- rhombencephalon- metencephalon (behindbrain), myelencephalon (medullabrain)
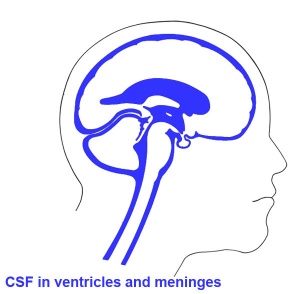
Ventricles
MH - this will be covered in detail in later neural development
- cavity within tube will form the contiguious space of the ventricules of the brain and central canal of spinal cord
- space is filled initially with amniotic fluid, later with CerebroSpinal Fluid (CSF)
- CSF is secreted by a modified vascular structure, the chorioid plexus, lying within the ventricles (More? Ventricular System)
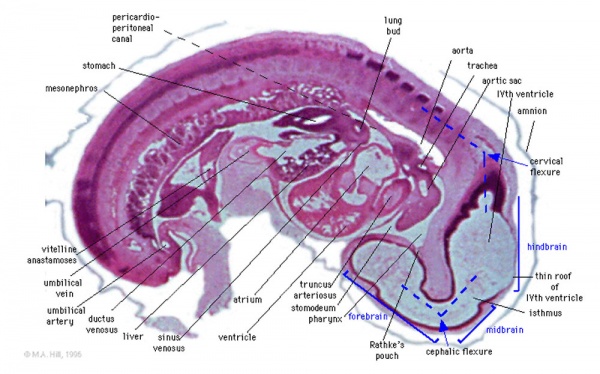
Brain Flexures
Rapid growth folds the neural tube forming 3 brain flexures
- cephalic flexure - pushes mesencephalon upwards
- cervical flexure - between brain stem and spinal cord
- pontine flexure - generates 4th ventricle
Carnegie stage 13 Embryo showing neural tube and brain flexures.
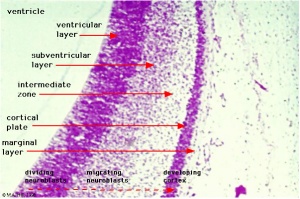
Neural Layers
- neural stem cells lie in the layer closest to the ventricular space, the ventricular layer
- this layer generates both neuroblasts and glioblasts
Neuroblasts - neurons arise first as neuroblasts and migrate along radial gial, their migration stops at cortical plate. Glioblasts - glia arise later as glioblasts
Both neurons and glia undergo a complex process of growth, differentiation and interaction over a long developmental time period.
Spinal Cord Axes
Identified by experimental manipulation of interactions.
- Initial experiments looked at how isolated tissues may influence the development of the spinal cord.
- Repositionining of specific tissues both in vivo and in vitro
- specific markers of or alteration of differentiation. Notocord Induction
Ventral Axis
- Sonic Hedgehog (SHH) - notochord secretes sonic hedgehog
- Gene expression studies (ISH) showed shh gene expression occured in a subset of inducing tissues
- has a patterning role elsewhere (limb, sclerotome, lung)
- 2 signaling activities acting (locally and at a distance) Ventral- Sonic Hedgehog
- Binds to cell surface receptor patched
- without shh, patched (Ptc) binds smoothened (Smo)
- with shh shh-Ptc releases Smo activating G protein pathway Gene Diseases
- shh Human mutation- holoprosencephaly 3
- characteristic faces of the severe form of HPE which included a single fused eye (cyclopia) and a nose-like structure (proboscis) above the eye
- Downstream targets of Sonic hedgehog signalling:
- transcription factors like Gli3 (responsible for Greigs polycephalosyndactyly in humans)
- d Hoxd13 (responsible for polysyndactyly)
Dorsal Axis
- Dorsalin - ectoderm secretes a growth factor shown to controls patterning in embryonic mesoderm (frog)
- Transforming Growth factor beta, (TGF b), related factors BMP-2, BMP-4, BMP-7, radar (flies related protein determines dorsoventral)
- homology search of vertebrate library identified protein of same family.
- dorsalin-1 (dsl-1) (Basler, Cell 73, p687, 1993) Dorsalin-1
- From overlying ectoderm
- Naming comes from the obvious reason that it promotes the differentiation of neural crest cells.
- Also signal for dorsal signal of neural tube.
- Inhibits the differentiation of motoneurons.
- Implication is that dsl-1 and shh act antagonistically, or competitively to establish d-v axis of neural tube.
Rostro-Caudal Axis
- Brain rostro-caudal axis is generated by differential expression of Hox genes (transcriptional activators)
- corresponding to genetic order on chromosome. (Wilkinson, Nature, 341, p405, 1989) Hox Genes
- Stands for Homeobox domain Genes
- A family of transcription factors
- Discovered in flies and conserved between all species. [../OtherEmb/fly.htm#antennapedia antennapedia]
- Expressed in sequence along the embryo rostro-caudal axis.
- Regulate many other aspects of development.
- 180aa region binds DNA and regulate gene expression
- large family of genes organized and expressed in sequence on the chromosome
- Nkx-2.2 first detected at 1 somite stage
- Lim hox gene expressed at spinal cord level
Ectodermal Placodes
- Specialized ectodermal "patches" in the head region
- Contribute sensory structures - otic placode (otocyst), nasal placode, lens placode
- Contribute teeth
Human Neuralation - Early Stages
The stages below refer to specific Carneigie stages of development.
- Carnegie stage 8 (about 18 postovulatory days) neural groove and folds are first seen
- Carnegie stage 9 the three main divisions of the brain, which are not cerebral vesicles, can be distinguished while the neural groove is still completely open.
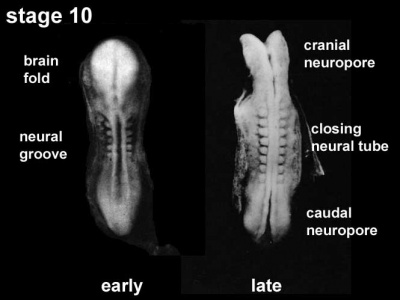
- Carnegie stage 10 (two days later) neural folds begin to fuse near the junction between brain and spinal cord, when neural crest cells are arising mainly from the neural ectoderm
- Carnegie stage 11 (about 24 days) the rostral (or cephalic) neuropore closes within a few hours; closure is bidirectional, it takes place from the dorsal and terminal lips and may occur in several areas simultaneously. The two lips, however, behave differently.
- Carnegie stage 12 (about 26 days) The caudal neuropore takes a day to close.
- the level of final closure is approximately at future somitic pair 31
- corresponds to the level of sacral vertebra 2
- Carnegie stage 13 (4 weeks) the neural tube is normally completely closed.
Secondary neurulation begins at stage 12 - is the differentiation of the caudal part of the neural tube from the caudal eminence (or end-bud) without the intermediate phase of a neural plate. Above text modified from[1]
- Carnegie Stages: 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | About Stages | Timeline
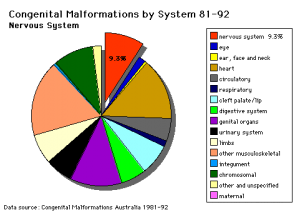
Abnormalities
See also Neural Abnormalities
Neural Tube Defects (NTD)
Failure of neural tube closure either incorrectly or incomplete
- Dysraphism is the term often used to describe the defective fusion of the neural folds. The position and degree of failure of fusion will result in either embryonic death or a range of different neural defects. The way (mode) in which the human neural tube fuses has been a source of contention. In humans, fusion appears to initiate at multiple sites but the mode is different from that found in many animal species used in developmental studies.
- severity dependent upon level within the tube and degree of failure
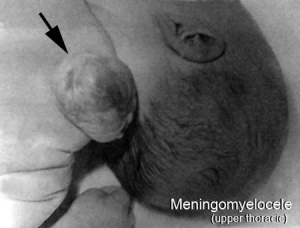
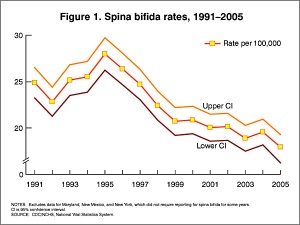
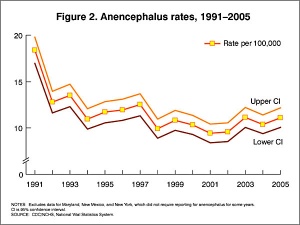
- caudal failure - spina bifida cranial failure - anancephaly
Maternal Diet
Found that supplementation of maternal diet with folate reduces incidence of NTDs (More? Folic Acid and Neural Tube Defects)
- A randomised controlled trial conducted by the Medical Research Council of the United Kingdom demonstrated a 72% reduction in risk of recurrence by periconceptional (ie before and after conception) folic acid supplementation (4mg daily).
- Women who have one infant with a neural tube defect have a significantly increased risk of recurrence (40-50 per thousand compared with 2 per thousand for all births)
In the U.S.A. the Food and Drug Administration in 1996 authorized that all enriched cereal grain products be fortified with folic acid, with optional fortification beginning in March 1996 and mandatory fortification in January 1998. The data in the above graphs show the subsequent changes in anencephaly and spina bifida rate over that period.
Holoprosencephaly
Holoprosencephaly (HPE) is developmental abnormality where the forebrain does not divide into the two separate hemispheres and ventricles.
Critical Periods of Human Development
Exposure to teratogens during these "critical periods" results in specific abnormalities.
- most systems are susceptible during embryonic development (first trimester)
- the earlier the exposure the more severe the effects
- each system has a different critical period
- longest critical periods
- longest developing systems (neural, genital)
- complicated developmental origins (sensory systems)
The table below identifies approximate windows of time, "critical periods", that following exposure to teratogens can lead to developmental abnormalities (anomalies, congenital). In general, the effects for each system are more severe (major anomalies) in the embryonic period during organogenesis in the first trimester. Later teratogen exposure are less severe (minor anomalies) in the fetal period during continued growth and differentiation in the second and third trimester.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Lecture - Ectoderm Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Lecture_-_Ectoderm_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- ↑ <pubmed>8005032</pubmed>