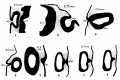Hearing - Inner Ear Development: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 500: | Line 500: | ||
===Reviews=== | ===Reviews=== | ||
<pubmed></pubmed> | |||
<pubmed>25040109</pubmed> | |||
<pubmed>20637105</pubmed> | <pubmed>20637105</pubmed> | ||
<pubmed>19750520</pubmed> | <pubmed>19750520</pubmed> | ||
| Line 505: | Line 507: | ||
* '''Bookshelf - Neuroscience''' [http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=neurosci.section.894 Neuroscience - The Inner Ear] | * '''Bookshelf - Neuroscience''' [http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=neurosci.section.894 Neuroscience - The Inner Ear] | ||
===Articles=== | ===Articles=== | ||
<pubmed></pubmed> | |||
<pubmed></pubmed> | |||
<pubmed>18603386</pubmed>| [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2628570 PMC2628570] | <pubmed>18603386</pubmed>| [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2628570 PMC2628570] | ||
Revision as of 11:26, 16 January 2016
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
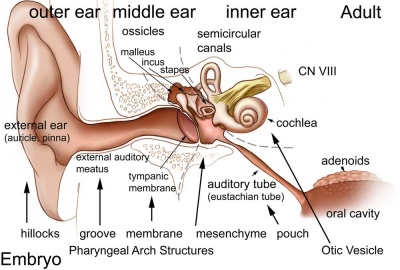
Introduction
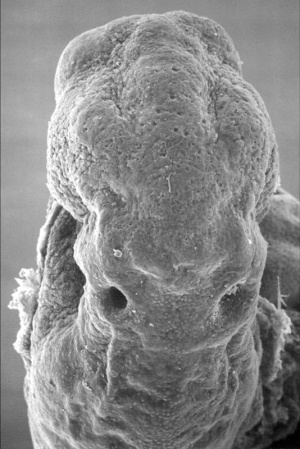
The inner ear is derived from a pair of surface sensory placodes (otic placodes) that appear in human development during week 4 (GA week 6) in the head region lying behind the second pharyngeal arch.
These otic placodes fold inwards forming initially a depression, then pinch off entirely from the surface forming an epithelium surrounding a fluid-filled sac or vesicle (otic vesicle, otocyst, auditory vesicle). The vesicle sinks into the head mesenchyme some of which closely surrounds the otocyst forming the otic capsule.
The otocyst finally lies close to the early developing hindbrain (rhombencephalon) and the developing vestibulo-cochlear-facial ganglion complex.
The otocyst epithelium then undergoes a series of morphological changes, forming the primitive membranous labyrinth. During the human fetal period this will differentiate into the inner ear components for hearing (cochlea) and balance (semi-circular canals).
The adult cochlear has a "snail-shell" appearance, with the total number of turns differing between species. The adult human cochlear is typically described as having 2.5 turns, but this can vary up to 2.75 or even 3 turns.[1]
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Inner Ear Development' <pubmed limit=5>Inner Ear Development</pubmed> |
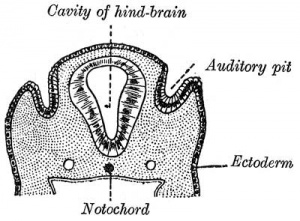
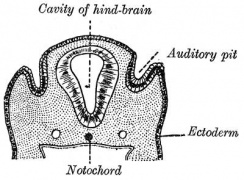
Otic Placode
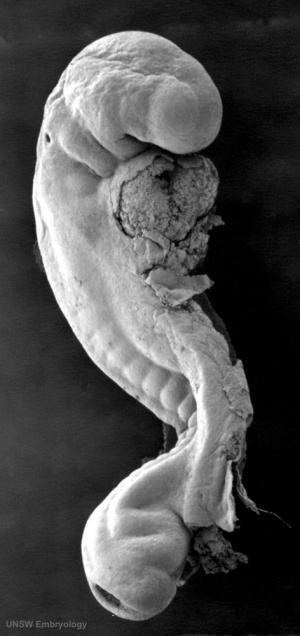
The embryonic surface sensory placode associated with hearing and balance. This will be lost from the embryo surface to form the otocyst or otic vesicle.
- Stage 11 - single layer of ectodermal cells organized in a columnar epithelium, which differs in cell shape from the surrounding cuboidal epithelia that will contribute the epithelia of the skin.
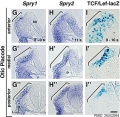
- zebrafish model - a number of specific genes are involved in initial induction of the otic placode including both growth factors (fgf3 and fgf8) and transcription factors (dlx3b, dlx4b, and foxi1).[6]
- Proliferation of the otic placode cells leads to an inward folding, or invagination, giving the external appearance of a depression on the lateral sides of the early developing neck region.
- The epithelium is still a single layer of cells, which continues to invaginate until the edges of the disc of cells come into apposition on the embryo surface.
- mouse model - placodal invagination but not specification requires placodal expression of the transcription factor Sox9.[7]
Sensory Placodes
- Week 4 a series of thickened surface ectodermal patches form in pairs rostro-caudally in the head region.
- Recent research suggests that all sensory placodes may arise from common panplacodal primordium origin around the neural plate, and then differentiate to eventually have different developmental fates.
- Each pair of sensory placodes will later contribute key components of each of our special senses (hearing, vision, smell and taste).
- Otic Placode - one of the first to form and contributes inner ear structures.
- Optic (Lens) Placode - lies on the surface, adjacent to the outpocketing of the nervous system (which will for the retina) and will form the lens.
- Nasal Placode - 2 components (medial and lateral) and will form the nose olefactory epithelium.
- Other species have a number of additional placodes which form other sensory structures (fish, lateral line receptor).
- Note that their initial postion on the developing head is significantly different to their final position in the future sensory system.
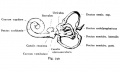
Otocyst
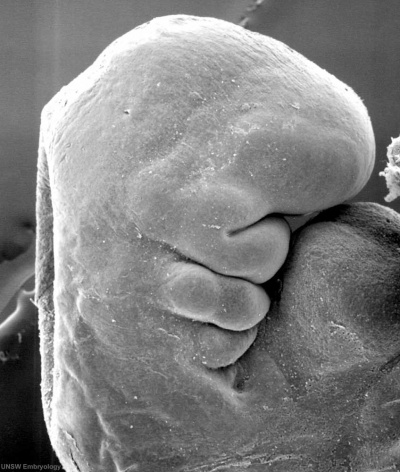
|
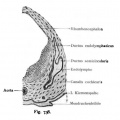
Stage 13 otic vesicle now lies beneath the embryo surface.
|
- Otocyst historic drawings
Week 5
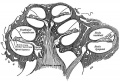
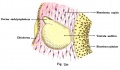
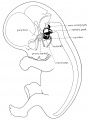
Stage 13 embryo (week 5) showing otocyst that will form the inner ear.
| A. Ventrolateral view of the whole embryo with 5-mm scale bar. At this stage of development no middle or external ear structures are apparent and will be derived later from pharyngeal arches one and two (labeled). | B. The gray bar through the head indicates the plane of cross-section, which is a cross-section of the head showing the size and position of the otic vesicles. At this stage of development they lie within the head mesenchyme behind pharyngeal arch one and two and in close apposition to the developing hindbrain. Note the close position of the otic vesicle to the rhombomeres, hindbrain folds that represent the initial segmentation of the hindbrain. Also shown are developing cranial ganglia and blood vessel lying adjacent to the otic vesicles. The wall of the otic vesicle at this stage is a simple epithelium. |
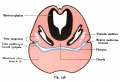
Week 7
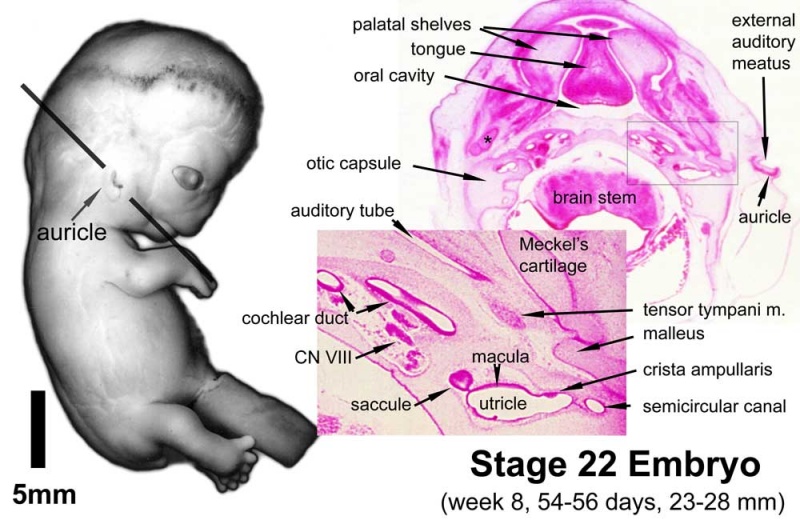
Week 8
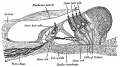
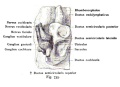
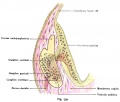
Stage 22 embryo (week 8) showing the embryo near the end of the embryonic period.
|
A. Lateral view of the whole embryo with 5 mm scale bar. Note the well developed external ear with simplified adult structure and narrower meatal opening. The grey bar through the head indicates the plane of cross-section for (B) and (C). |
B. Cross-section of the head at the plane of the skull base and oral cavity to the top. The otic capsule is well formed by this stage containing all the membranous labyrinth structures. It is still a cartilaginous structure ventral to the brainstem and lying behind the oral cavity. The tongue occupies the floor of the oral cavity with the unfused palatal shelves lying lateral and the auditory tubes clearly shown on the posterior wall. The external ear is visible on the right hand side of the head with a band of cartilage (dark stain) within the auricle. C. The gray box indicates this region: detail of inner and middle ear development. The middle ear cavity has not yet formed and the ossicles (malleus shown) are embedded in mesenchyme that is being lost. The tensor tympani muscle is differentiating in the adjacent mesenchyme. The inner ear membranous labyrinth has formed its adult external structure. The section through the turns of the cochlear duct shows the internal cochlea structure is still underdeveloped; in contrast, the balance region is more developed. |
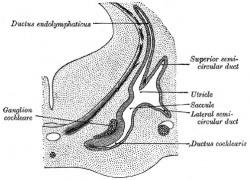
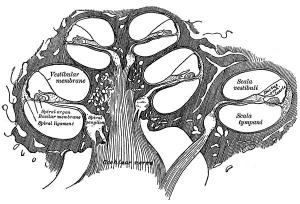
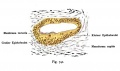
The Membranous Labyrinth
Fetal
Week 8.4
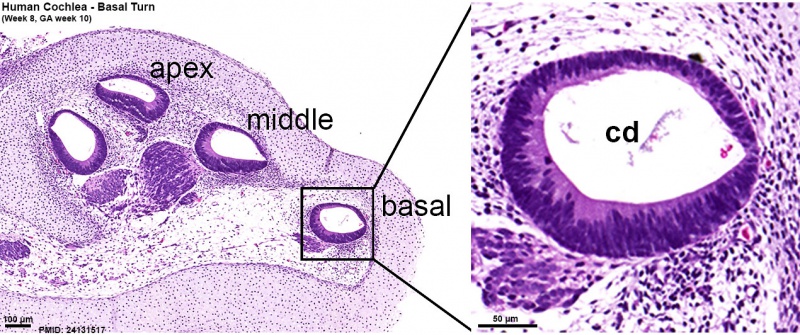
Human fetal cochlea basal turn week 8.4 Gestational Week GA 10.4 (Stain - Haematoxylin Eosin)[8]
Week 10
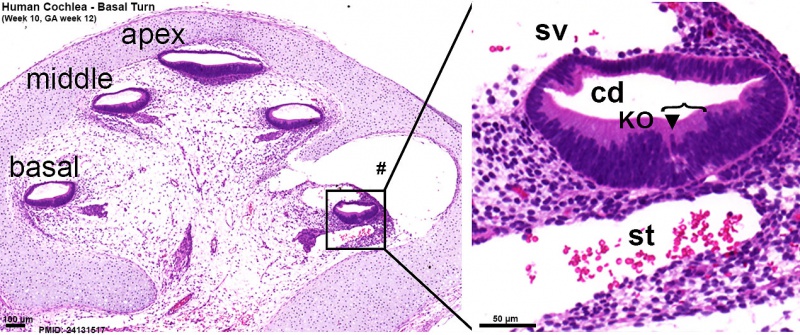
Human fetal cochlea basal turn week 10 Gestational Week GA 12 (Stain - Haematoxylin Eosin)[8]
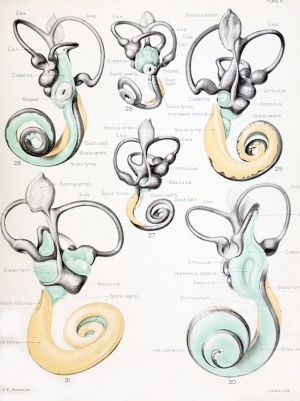
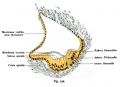
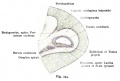
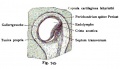
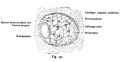
Historic
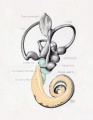
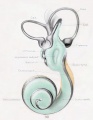
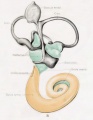
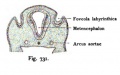
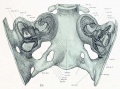
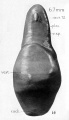
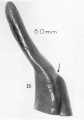
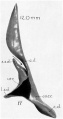
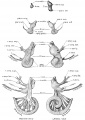
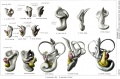
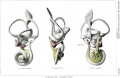
A series of historic wax-plate reconstructions of the membranous labyrinth and the surrounding periotic tissue-spaces showing development stages (median and lateral views) of these spaces at the same scale (ages are only approximations calculated on CRL).
| Human Fetal Membranous Labyrinth Development | |
|---|---|
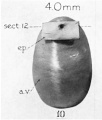
| Fetus 50 mm CRL 10 weeks (GA 12) | |
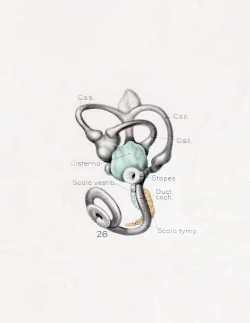
|
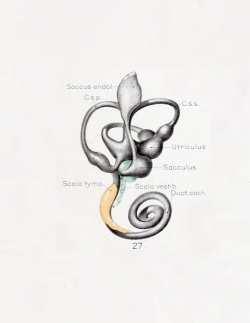
|
| lateral view | median view |
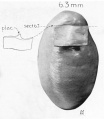
| Fetus 85 mm CRL 14 weeks (GA 16) | |
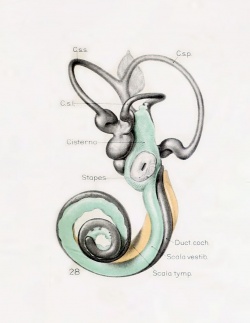
|
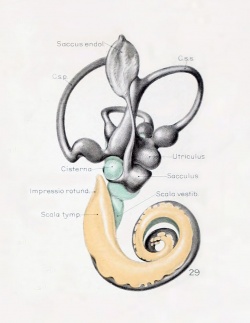
|
| lateral view | median view |
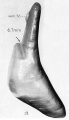
| Fetus 130 mm CRL 15 weeks (GA 17) | |
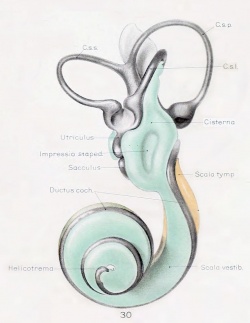
|
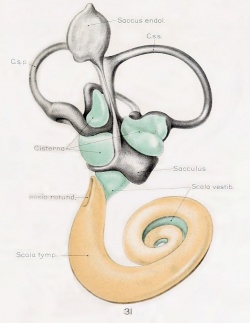
|
| lateral view | median view |
Fetal Cochlea Molecular
Human fetal cochlea basal turn by Gestational Week GA[8]
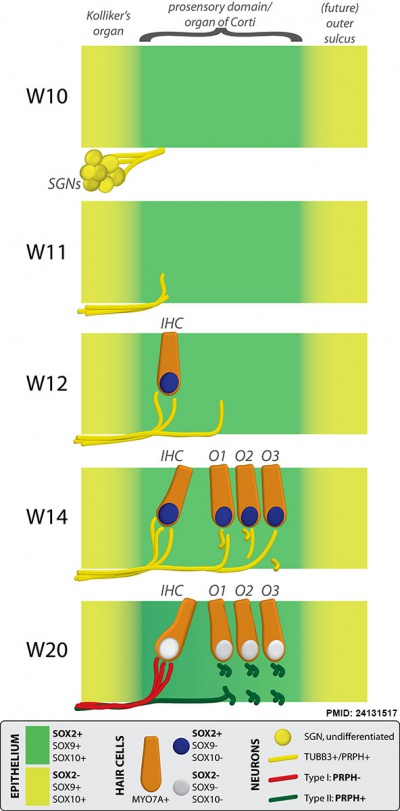
Abbreviations
|
Gestational Week GA
Week 10 (W10) - SOX2 identifies the prosensory domain within the SOX9/SOX10+ cochlear duct epithelium. Neurites from the adjoining TUBB3+/PRPH + SGNs do not yet penetrate into the epithelium.
|
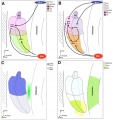
Other Species Overview
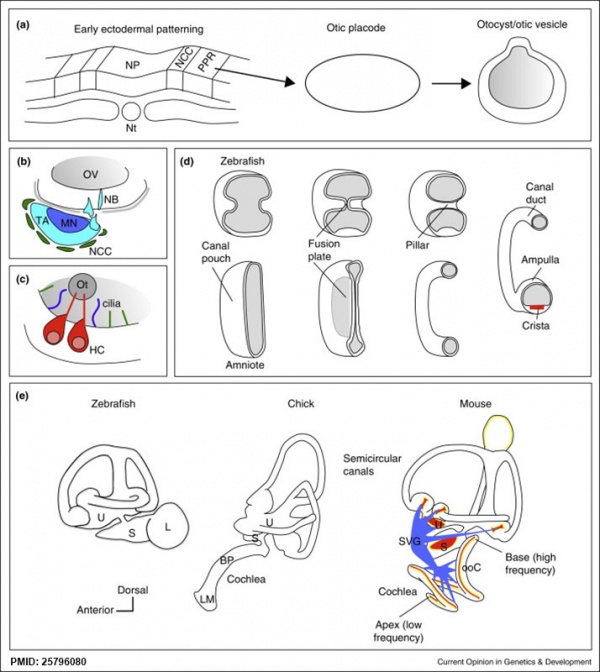
Comparison of zebrafish and late stage chick and mouse embryos[9] Zebrafish Development Chicken Development | Mouse Development |
(a) Formation of the pre-placodal region (PPR), otic placode and otocyst (otic vesicle) from cranial ectoderm. The otocyst is the source of nearly all cell types of the mature ear.
(b) Otic neurogenesis: neuroblasts are specified from otic vesicle epithelium, but delaminate from it and accumulate beneath the ear in a transit amplifying population (light blue). Neurons (dark blue) differentiate from this population, and innervate sensory hair cells in the overlying otic epithelium. The ganglion develops in close association with neural crest cells (green), which give rise to glia. (c) Early otolith formation in the zebrafish otic vesicle. At least three distinct populations of cilia can be distinguished: immotile hair cell kinocilia (red), which tether the otolith at early stages; motile cilia (blue) in the vicinity of the sensory hair cells, which do not bind otolithic material, and shorter immotile cilia (green). (d) Schematic comparison of semicircular canal formation in the zebrafish ear (top row) and a generalised amniote ear (bottom row). A single canal is illustrated for clarity. Epithelia adhere at a fusion plate, from which cells are cleared to make the duct. The end result of both events is the same (right hand image), but the fusion plate is much smaller in the zebrafish. (e) Comparative sketches of inner ears from adult zebrafish and late stage chick and mouse embryos. Sensory (red), neuronal (blue) and endolymph-regulating (yellow) cells are shown for the mouse ear.
(text from original figure legend) |
Mouse
- Dual embryonic origin of the mammalian otic vesicle forming the inner ear[10] "The inner ear and cochleovestibular ganglion (CVG) derive from a specialized region of head ectoderm termed the otic placode. During embryogenesis, the otic placode invaginates into the head to form the otic vesicle (OV), the primordium of the inner ear and CVG. Non-autonomous cell signaling from the hindbrain to the OV is required for inner ear morphogenesis and neurogenesis. In this study, we show that neuroepithelial cells (NECs), including neural crest cells (NCCs), can contribute directly to the OV from the neural tube. ...This study defines a dual cellular origin of the inner ear from sensory placode ectoderm and NECs, and changes the current paradigm of inner ear neurosensory development."
Endolymphatic Sac
- the adult endolymphatic sac is filled with endolymphatic fluid
- with a unique composition of high potassium and low sodium ions
- it is not known in humans at what stage of development this ionic status is achieved
- In the rat, adult sodium levels are seen in the first week after birth, while both potassium and chloride levels were below the normal adult levels.
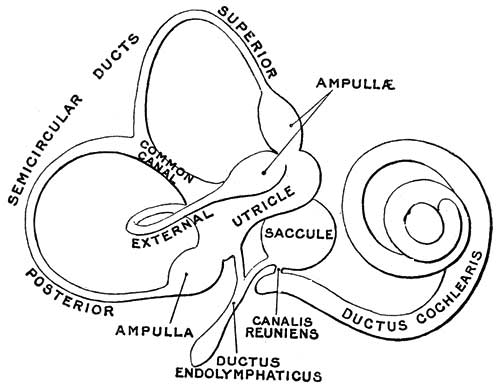
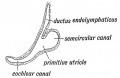
Vestibular Sac
- generates 3 expansions - form semicircular ducts
- remainder forms utricle
- epithelia lining generates - hair cells, ampullary cristae, utricular macula
- Vestibular - Otoconia, otoconin- inner ear biominerals
Cochlear Sac
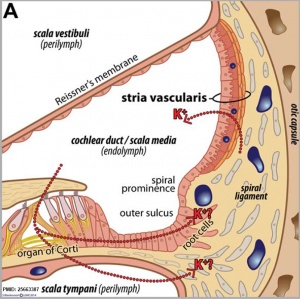
- generates coiled cochlear duct (humans 2 1/2 turns)
- remainder forms saccule
- epithelia lining generates
- hair cells
- structures of organ of corti
- saccular macula
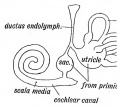
scala media
(Latin, medius = middle) spiral of middle cochlear duct lying between scala vestibuli and scala tympani, containing endolymph.
scala tympani
(Latin, tympanon = drum) the spiralling cochlear duct below spiral lamina, containing perilymph and ending at round window near tympanic membrane.
scala vestibuli
(Latin, vestibulum = cavity at beginning of canal) the spiralling cochlear duct above spiral lamina, containing perilymph, beginning near the vestbule and ending where it communicates with the scala tympani at the helicotrema.
endolymph
- extracellular fluid secreted by the stria vascularis.
- potassium is the main cation required for depolarizing electrical current in the hair cells.
perilymph
- extracellular fluid similar in composition to either plasma or cerebrospinal fluid.
- sodium is the main cation.
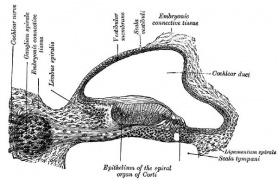
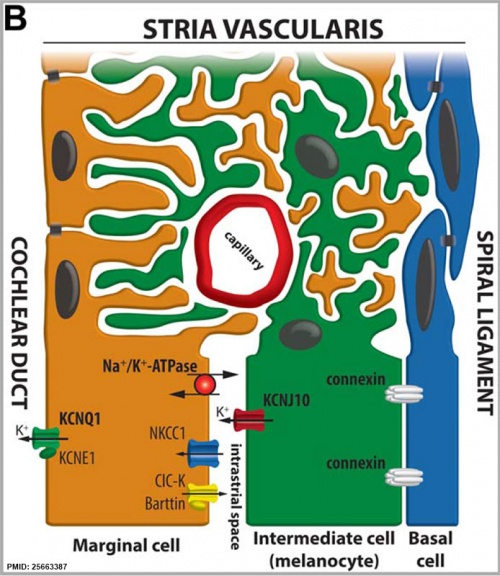
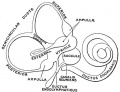
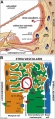
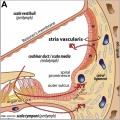
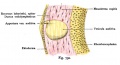
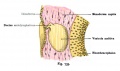
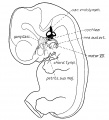
Stria Vascularis
In the adult the stria vascularis functions to synthesise and secretes endolymph. The three main cell types within the stria that have different embryological origins and are connected by different forms of cell junctions.
A schematic anatomical (upper half) and compartmental (lower half) model of the adult stria vascularis showing the three cellular layers and depicting the location of potassium regulating channels. The stria vascularis is electrochemically isolated from neighboring structures by tight junctions (black bars).[2]
| Marginal cells | Intermediate cells | Basal cells |
|---|---|---|
| line the lumen of the cochlear duct | melanocyte-like cells lie between the marginal and basal cell layers | mesenchymal spiral ligament fibrocytes |
| derived from epithelia. | derived from the neural crest. | derived from otic mesenchyme.[11] |
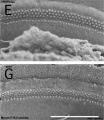
Mouse SEM
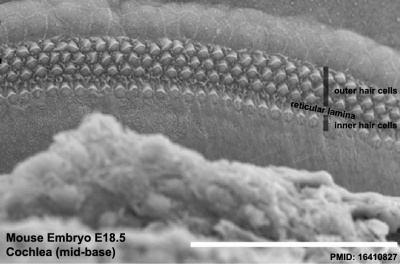
The gallery below shows scanning electron micrographs of the developing mouse (E18.5) cochlea.[12]
Adult Cochlea
| Magnetic Resonance Images of the Adult Cochlea[13] | |
|---|---|
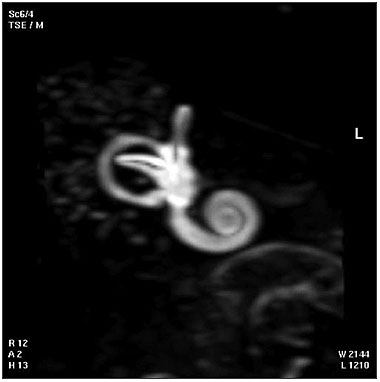
|
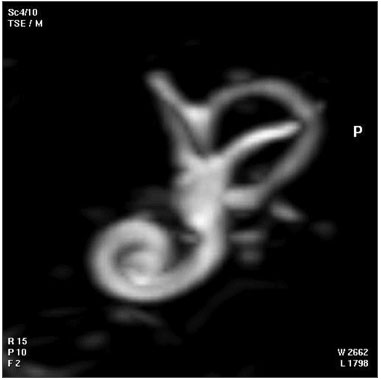
|
The investigators used a method to obtain magnetic resonance imaging (MRI) to measure cochlear length from the temporal bones of 6 cadavers. By overlapping digitalized rulers on these images it was possible to measure cochlear length. Adult cochlear length varied between 17 and 26.5 millimeters.[13]
Mouse cochlea (SEM) showing organization of the hair cells.[14]
- Mouse Cochlea Links: Cochlea overview SEM | Base region SEM | Mid-base and Apex region SEM | Mid-base region SEM | Mid-base hair cells SEM | Mouse Development
Bony Labyrinth
- formed from chrondified mesoderm
- Periotic Capsule
- mesenchyme within capsule degenerates to form space filled with perilymph
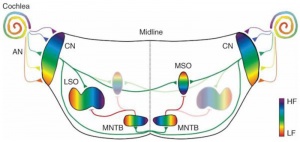
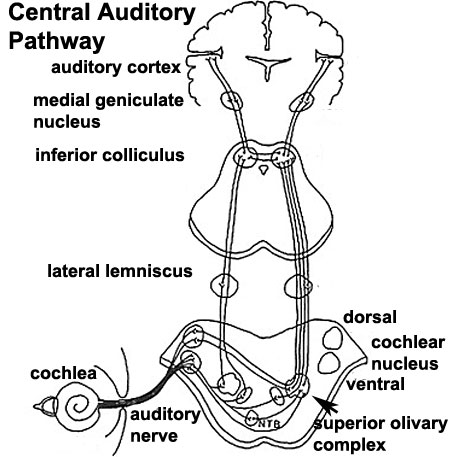
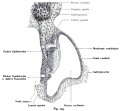
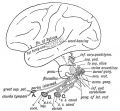
Auditory Neural Pathway
Vestibulocochlear Nerve
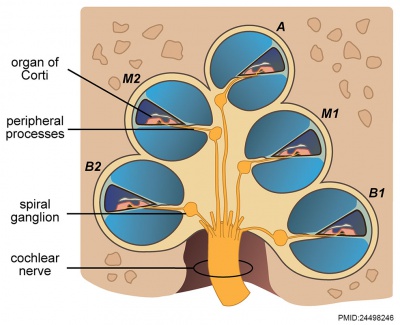
|
| Adult cochlea nerve glia cartoon[3] |
- forms beside otocyst
- from wall of otocyst and neural crest cells
- bipolar neurons
Vestibular Neurons
- outer end of internal acoustic meatus
- innervate hair cells in membranous labyrinth
- axons project to brain stem and synapse in vestibular nucleus
Cochlear Neurons
- cell bodies lie in modiolus
- central pillar of cochlear
- innervate hair cells of spiral organ
- axons project to cochlear nucleus
- Links: Hearing - Neural Pathway
Cochlea Glial
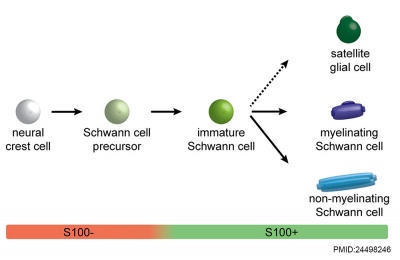
|
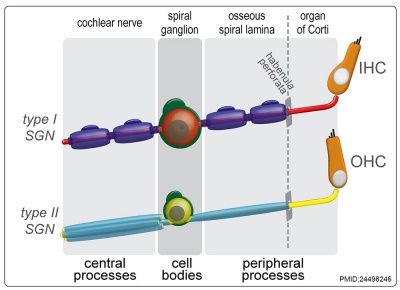
|
| Cochlea glial lineage [3] | Adult cochlea nerve glia cartoon[3] |
- Links: Hearing - Neural Pathway
Inner Ear Genes
- hindbrain segmentation occurs at same time placode arises
- otocyst adjacent to rhombomere 5
- may influence development
- Hoxa1, kreisler, Fgf3
- genes regulating neural crest cells (neural genes)
- Pax2 Ko affects cochlear and spiral ganglion, but not vestibular apparatus
- nerogenin 1 affects both ganglia
Semicircular canal
- Otx1- cochlear and vestibular normal
- Hmx3, Prx1, Prx2
Sensory Organs
- thyroid hormone receptor beta
- Zebrafish-mindbomb mutant has excess hair cells but not supporting cells, Notch-Delta signaling
- Gene Expression-inner ear
- Brn-3c and Hair cell development
- Supporting Cells- p27kip
- Thyroid Hormone
- Ganglion neurons require growth factors
- vestibular neurons- BDNF, NT3
- survival not development
Sox9 20346939 Sox2 20071536
Additional Images
Historic Images
References
- ↑ <pubmed>19225438</pubmed>
- ↑ 2.0 2.1 2.2 <pubmed>25663387</pubmed>| Dev Neurobiol.
- ↑ 3.0 3.1 3.2 3.3 <pubmed>24498246</pubmed>| PLoS One.
- ↑ <pubmed>23918393</pubmed>
- ↑ <pubmed>19476657</pubmed>
- ↑ <pubmed>15188428</pubmed>
- ↑ <pubmed>18377888</pubmed>
- ↑ 8.0 8.1 8.2 <pubmed>24131517</pubmed>| Neural Dev.
- ↑ <pubmed>25796080</pubmed>| Curr Opin Genet Dev.
- ↑ <pubmed>22110056</pubmed>
- ↑ <pubmed>21925491</pubmed>
- ↑ <pubmed>16410827</pubmed>| PMC1326221 | PLoS Genet.
- ↑ 13.0 13.1 <pubmed>19575114</pubmed>| Braz J Otorhinolaryngol.
- ↑ <pubmed>16410827</pubmed>| PMC1326221 | PLoS Genet.
Reviews
<pubmed></pubmed> <pubmed>25040109</pubmed> <pubmed>20637105</pubmed> <pubmed>19750520</pubmed>
- Bookshelf - Neuroscience Neuroscience - The Inner Ear
Articles
<pubmed></pubmed> <pubmed></pubmed> <pubmed>18603386</pubmed>| PMC2628570
Search PubMed
May 2010 "Inner Ear Development" All (4027) Review (452) Free Full Text (750)
Search Pubmed: Inner Ear Development Cochlea Development
Historic
- Keith, A. (1902) Human Embryology and Morphology. London: Edward Arnold. Development of the Organ of Hearing
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
- Mouse Genomic Database cochlea morphogenesis genes
- Acoustical Society of America ICA/ASA '98 Lay Language Papers
- Promenade around the Cochlea Organ of Corti
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Hearing - Inner Ear Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Hearing_-_Inner_Ear_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G