Endoderm: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| (16 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
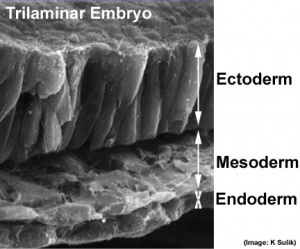
[[File:Trilaminar_embryo.jpg|thumb|300px|The trilaminar embryo]] | [[File:Trilaminar_embryo.jpg|thumb|300px|The trilaminar embryo]] | ||
The | The first germ layer generated in the early [[T#trilaminar embryo|trilaminar embryo]] germ layers ({{ectoderm}}, {{mesoderm}} and [[endoderm]]) formed by gastrulation. Note the historic name for {{endoderm}} was "entoderm". | ||
| Line 16: | Line 16: | ||
{| | {| | ||
|-bgcolor="F5FFFA" | |-bgcolor="F5FFFA" | ||
| '''Endoderm Links:''' | | '''Endoderm Links:''' {{endoderm}} | {{Mesoderm}} | {{Ectoderm}} | [[Lecture_-_Gastrointestinal_Development|Lecture - Endoderm, Early Gastrointestinal]] | [[Lecture - Respiratory Development]] | [[Gastrointestinal Tract Development]] | [[Respiratory System Development]] | [[:Category:Endoderm]] | ||
|} | |} | ||
==Some Recent Findings== | ==Some Recent Findings== | ||
{| | {| | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* ''' | * '''Dnmt1 is required for proximal-distal patterning of the lung endoderm and for restraining alveolar type 2 cell fate'''{{#pmid:31242446|PMID31242446}} "Lung endoderm development occurs through a series of finely coordinated transcriptional processes that are regulated by epigenetic mechanisms. However, the role of DNA methylation in regulating lung endoderm development remains poorly understood. We demonstrate that DNA methyltransferase 1 (Dnmt1) is required for early branching morphogenesis of the lungs and for restraining epithelial fate specification. Loss of Dnmt1 leads to an early branching defect, a loss of epithelial polarity and proximal endodermal cell differentiation, and an expansion of the distal endoderm compartment. Dnmt1 deficiency also disrupts epithelial-mesenchymal crosstalk and leads to precocious distal endodermal cell differentiation with premature expression of alveolar type 2 cell restricted genes. These data reveal an important requirement for Dnmt1 mediated DNA methylation in early lung development to promote proper branching morphogenesis, maintain proximal endodermal cell fate, and suppress premature activation of the distal epithelial fate." [https://www.omim.org/entry/126375 DNMT1] | ||
* '''Single origin of the epithelium of the human {{middle ear}}'''{{#pmid:31121244|PMID31121244}} "The epithelium lining the human middle ear and adjacent temporal bone cavity shows a varying morphological appearance throughout these cavities. Its embryologic origin has long been debated and recently got attention in a newly proposed theory of a dual embryologic origin. The epithelial morphology and its differentiating capabilities are of significance in future mucosa-targeted therapeutic agents and could affect surgical approaches of the temporal bone. This study aims to analyze reported {{murine}} histological findings that led to the theory of a dual epithelial embryological origin and immunohistochemically investigate whether such an epithelial embryological origin in the human fetal middle ear could be true. By combining a sagittal sectioning technique and immuno-histochemical staining, a comprehensive immuno-histological overview of the fetal human middle ear during a critical stage of tympanic cavitation was provided. A critical analysis of previously reported findings leading to the theory of a dual epithelial embryological origin and a comparison of these findings to the findings in the human fetal middle ear was performed. The reported findings and critical analysis provide multiple arguments for an entirely {{endoderm}}al embryonic origin of the epithelium lining the tympanic cavity. Different morphological epithelial appearances throughout the tympanic and temporal bone cavities could be explained by different stages of epithelial differentiation rather than different embryologic origin and endodermal rupture does not seem to be a necessity for these cavities to form." {{middle ear}} | |||
* '''Region-specific {{endoderm}}al signals direct {{neural crest}} cells to form the three {{middle ear}} ossicles'''{{#pmid:30630826|PMID30630826}} "Defects in the middle ear ossicles - {{malleus}}, {{incus}} and {{stapes}} - can lead to conductive hearing loss. During development, {{neural crest}} cells (NCCs) migrate from the dorsal hindbrain to specific locations in {{pharyngeal arch}} (PA) 1 and 2, to form the malleus-incus and stapes, respectively. It is unclear how migratory NCCs reach their proper destination in the PA and initiate mesenchymal condensation to form specific ossicles. We show that secreted molecules sonic hedgehog ({{SHH}}) and bone morphogenetic protein 4 ({{BMP}}4) emanating from the pharyngeal {{endoderm}} are important in instructing region-specific NCC condensation to form malleus-incus and stapes, respectively, in mouse. Tissue-specific knockout of {{Shh}} in the pharyngeal endoderm or Smo (a transducer of SHH signaling) in NCCs causes the loss of malleus-incus condensation in PA1 but only affects the maintenance of stapes condensation in PA2. By contrast, knockout of {{Bmp}}4 in the pharyngeal endoderm or Smad4 (a transducer of TGFβ/BMP signaling) in the NCCs disrupts NCC migration into the stapes region in PA2, affecting stapes formation. These results indicate that region-specific endodermal signals direct formation of specific middle ear ossicles." [http://dev.biologists.org/cgi/pmidlookup?view=long&pmid=30630826 Development] | |||
* ''' | * '''Enhancer, transcriptional, and cell fate plasticity precedes intestinal determination during endoderm development'''{{#pmid:30366903|PMID30366903}} "After acquiring competence for selected cell fates, embryonic primordia may remain plastic for variable periods before tissue identity is irrevocably determined (commitment). We investigated the chromatin basis for these developmental milestones in mouse endoderm, a tissue with recognizable rostro-caudal patterning and transcription factor (TF)-dependent interim plasticity. Foregut-specific enhancers are as accessible and active in early midgut as in foregut endoderm, and intestinal enhancers and identity are established only after ectopic cis-regulatory elements are decommissioned. Depletion of the intestinal TF CDX2 before this cis element transition stabilizes foregut enhancers, reinforces ectopic transcriptional programs, and hence imposes foregut identities on the midgut. Later in development, as the window of chromatin plasticity elapses, CDX2 depletion weakens intestinal, without strengthening foregut, enhancers. Thus, midgut endoderm is primed for heterologous cell fates, and TFs act on a background of shifting chromatin access to determine intestinal at the expense of foregut identity. Similar principles likely govern other fate commitments." | ||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 32: | Line 36: | ||
| [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | | [[File:Mark_Hill.jpg|90px|left]] {{Most_Recent_Refs}} | ||
Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Endoderm+Development ''Endoderm Development''] | [http://www.ncbi.nlm.nih.gov/pmc/?term=Endoderm+Development&report=imagesdocsum ''Images''] | Search term: [http://www.ncbi.nlm.nih.gov/pubmed/?term=Endoderm+Development ''Endoderm Development''] | [http://www.ncbi.nlm.nih.gov/pmc/?term=Endoderm+Development&report=imagesdocsum ''Endoderm Images''] | [http://www.ncbi.nlm.nih.gov/pubmed/?term=Endoderm ''Endoderm''] | | ||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Older papers | |||
|- | |||
| {{Older papers}} | |||
* '''Distinct mechanisms for PDGF and FGF signaling in primitive endoderm development'''{{#pmid:30026121|PMID30026121}} "{{FGF}} signaling is known to play a critical role in the specification of primitive endoderm (PrE) and epiblast (Epi) from the inner cell mass (ICM) during mouse preimplantation development, but how FGFs synergize with other growth factor signaling pathways is unknown. Because PDGFRα signaling has also been implicated in the PrE, we investigated the coordinate functions of PDGFRα together with FGFR1 or FGFR2 in PrE development. PrE development was abrogated in Pdgfra; Fgfr1 compound mutants, or significantly reduced in Pdgfra; Fgfr2 or PdgfraPI3K; Fgfr2 compound mutants. We provide evidence that both Fgfr2 and Pdgfra play roles in PrE cell survival while Fgfr1 controls PrE cell specification. Our results suggest a model where FGFR1-engaged ERK1/2 signaling governs PrE specification while PDGFRα- and by analogy possibly FGFR2- engaged PI3K signaling regulates PrE survival and positioning in the embryo. Together, these studies indicate how multiple growth factors and signaling pathways can cooperate in preimplantation development." | |||
* '''Nucleoporin 107, 62 and 153 mediate Kcnq1ot1 imprinted domain regulation in extraembryonic endoderm stem''' cells{{#pmid:30022050|PMID30022050}} "Genomic imprinting is a phenomenon that restricts transcription to predominantly one parental allele. How this transcriptional duality is regulated is poorly understood. Here we perform an RNA interference screen for epigenetic factors involved in paternal allelic silencing at the Kcnq1ot1 imprinted domain in mouse extraembryonic endoderm stem cells. Multiple factors are identified, including nucleoporin 107 (NUP107). To determine NUP107's role and specificity in Kcnq1ot1 imprinted domain regulation, we deplete Nup107, as well as Nup62, Nup98/96 and Nup153. Nup107, Nup62 and Nup153, but not Nup98/96 depletion, reduce Kcnq1ot1 noncoding RNA volume, displace the Kcnq1ot1 domain from the nuclear periphery, reactivate a subset of normally silent paternal alleles in the domain, alter histone modifications with concomitant changes in KMT2A, EZH2 and EHMT2 occupancy, as well as reduce cohesin interactions at the Kcnq1ot1 imprinting control region. Our results establish an important role for specific nucleoporins in mediating Kcnq1ot1 imprinted domain regulation. | |||
* '''Different murine-derived feeder cells alter the definitive endoderm differentiation of human induced pluripotent stem cells'''{{#pmid:30048506|PMID30048506}} "The crosstalk between cells is important for differentiation of cells. Murine-derived feeder cells, SNL76/7 feeder cells (SNLs) or mouse primary embryonic fibroblast feeder cells (MEFs) are widely used for culturing undifferentiated human induced pluripotent stem cells (hiPSCs). ... Taken together, our finding indicated that differences in murine-feeder cells used for maintenance of the undifferentiated state alter the expression of pluripotency-related genes in hiPSCs by the signaling pathways and affect DE differentiation from hiPSCs, suggesting that the feeder cells can potentiate hiPSCs for DE differentiation." | |||
* '''Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation and oral-aboral patterning Development'''{{#pmid:28705897|PMID28705897}} "The {{mesoderm}} is a key novelty in animal evolution, although we understand little of how the mesoderm arose. brachyury, the founding member of the T-box gene family, is a key gene in chordate mesoderm development. However, the brachyury gene was present in the common ancestor of fungi and animals long before mesoderm appeared. To explore ancestral roles of brachyury prior to the evolution of definitive mesoderm, we excised the gene using CRISPR/Cas9 in the diploblastic cnidarian Nematostella vectensis Nvbrachyury is normally expressed in precursors of the pharynx, which separates endoderm from ectoderm. In knockout embryos, the pharynx does not form, embryos fail to elongate, and endoderm organization, ectodermal cell polarity and patterning along the oral-aboral axis are disrupted. Expression of many genes both inside and outside the Nvbrachyury expression domain is affected, including downregulation of Wnt genes at the oral pole. Our results point to an ancient role for brachyury in morphogenesis, cell polarity and the patterning of both ectodermal and endodermal derivatives along the primary body axis." | |||
* '''Late stage definitive endodermal differentiation can be defined by Daf1 expression'''{{#pmid:27245320|PMID27245320}} "Definitive endoderm (DE) gives rise to the respiratory apparatus and digestive tract. Sox17 and Cxcr4 are useful markers of the DE. Previously, we identified a novel DE marker, Decay accelerating factor 1(Daf1/CD55), by identifying DE specific genes from the expression profile of DE derived from mouse embryonic stem cells (ESCs) by microarray analysis, and in situ hybridization of early embryos. Daf1 is expressed in a subpopulation of E-cadherin + Cxcr4+ DE cells. The characteristics of the Daf1-expressing cells during DE differentiation has not been examined. ...Daf1-expressing late definitive endoderm proliferates slowly and show low adhesive capacity." [http://www.omim.org/entry/125240 OMIM Daf1] | |||
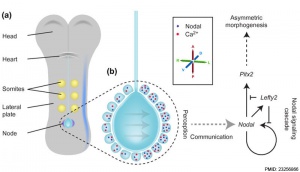
* '''Role of the gut endoderm in relaying left-right patterning in mice'''{{#pmid:22412348|PMID22412348}} "Establishment of left-right (LR) asymmetry occurs after gastrulation commences and utilizes a conserved cascade of events. In the mouse, LR symmetry is broken at a midline structure, the node, and involves signal relay to the lateral plate, where it results in asymmetric organ morphogenesis. How information transmits from the node to the distantly situated lateral plate remains unclear. ... Collectively, our data demonstrate that Cx43-mediated communication across gap junctions within the gut endoderm serves as a mechanism for information relay between node and lateral plate in a process that is critical for the establishment of LR asymmetry in mice." | |||
* '''Endoderm Review'''{{#pmid:19575677|PMID19575677}} "The endoderm germ layer contributes to the respiratory and gastrointestinal tracts and to all of their associated organs. Over the past decade, studies in vertebrate model organisms, including frog, fish, chick, and mouse, have greatly enhanced our understanding of the molecular basis of endoderm organ development. " | |||
|} | |||
<div id="Endoderm Overview"></div> | |||
==Overview== | |||
{{Endoderm table1}} | |||
<br> | |||
{{EndodermCells-table}} | |||
<br> | |||
{{Endoderm origin collapsetable1}} | |||
==Early Endoderm Cartoon== | ==Early Endoderm Cartoon== | ||
{| | {| | ||
| width=320px|< | | width=320px| | ||
<html5media height="360" width="360">File:Endoderm 003.mp4</html5media> | |||
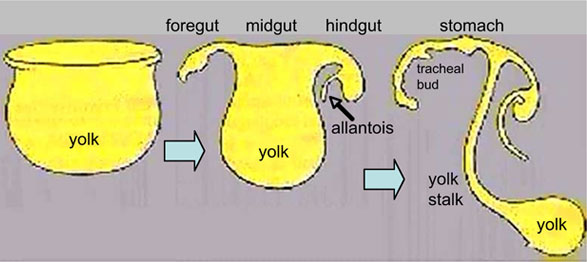
| This animation shows the early development of [[E#endoderm|endoderm]] forming the gastrointestinal tract, [[Y#yolk sac|yolk sac]] and [[A#allantois|allantois]]. The movie starts approximately week 3 and continues through week 4. | | This animation shows the early development of [[E#endoderm|endoderm]] forming the gastrointestinal tract, [[Y#yolk sac|yolk sac]] and [[A#allantois|allantois]]. The movie starts approximately week 3 and continues through week 4. | ||
| Line 60: | Line 91: | ||
* Klaatsch, H., (1898). Zur Frage nach der morphologiscrn Bedeutung der Hypochorda. Morphol. Jahrb. 25, 156-169. | * Klaatsch, H., (1898). Zur Frage nach der morphologiscrn Bedeutung der Hypochorda. Morphol. Jahrb. 25, 156-169. | ||
:'''Links:''' | :'''Links:''' {{frog}} | {{zebrafish}} | ||
==Molecular== | ==Molecular== | ||
| Line 67: | Line 99: | ||
===Markers=== | ===Markers=== | ||
Several studies have identified the following proteins as markers for endoderm in different species. | Several studies have identified the following proteins as markers for endoderm in different species. | ||
* '''Foxa2''' - Forkhead box A2 expressed by anterior primitive streak that forms endoderm. | * '''Foxa2''' - Forkhead box A2 expressed by anterior primitive streak that forms endoderm.{{#pmid:19234065|PMID19234065}} [http://www.omim.org/entry/600288?search=Foxa2&highlight=foxa2 OMIM FOXA2] | ||
* '''Sox17''' - Sry-Related HMG-Box gene 17 [[Developmental Signals - Sox|Sox]] | [http://www.omim.org/entry/610928 OMiM - SOX17] | * '''Sox17''' - Sry-Related HMG-Box gene 17 [[Developmental Signals - Sox|Sox]] | [http://www.omim.org/entry/610928 OMiM - SOX17] | ||
* '''Cxcr4''' - Chemokine, Cxc Motif, Receptor 4 a membrane receptor for neuropeptide Y [http://www.omim.org/entry/162643 OMIM CXCR4] | * '''Cxcr4''' - Chemokine, Cxc Motif, Receptor 4 a membrane receptor for neuropeptide Y [http://www.omim.org/entry/162643 OMIM CXCR4] | ||
| Line 79: | Line 111: | ||
:'''Links:''' | :'''Links:''' {{TGF-beta}} | [[Molecular Development]] | ||
===Patterning=== | ===Patterning=== | ||
| Line 90: | Line 122: | ||
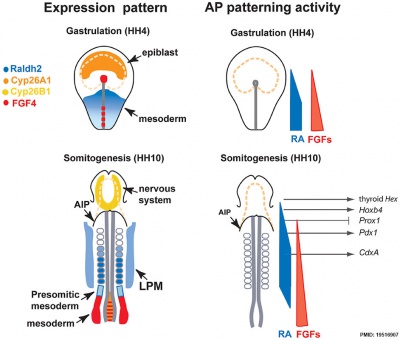
Chicken antero-posterior endoderm patterning{{#pmid:19516907|PMID19516907}} | Chicken antero-posterior endoderm patterning{{#pmid:19516907|PMID19516907}} | ||
| Line 131: | Line 164: | ||
{{Glossary}} | {{Glossary}} | ||
{{Footer}} | {{Footer}} | ||
[[Category:Endoderm]] [[Category:Gastrointestinal Tract]] [[Category:Respiratory]] | [[Category:Endoderm]] [[Category:Gastrointestinal Tract]] [[Category:Respiratory]] | ||
Latest revision as of 15:19, 16 July 2019
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
The first germ layer generated in the early trilaminar embryo germ layers (ectoderm, mesoderm and endoderm) formed by gastrulation. Note the historic name for endoderm was "entoderm".
The endoderm contributes the epithelia and glands of the gastrointestinal tract, respiratory tract and the renal bladder. This layer also contributes to the associated gastrointestinal tract organ development (liver and pancreas).
The layer appears to initially be influenced by the overlying notochord and subsequently by a range of growth factors regulating growth and differentiation.
Note that this layer also lines the extra-embryonic yolk sac and allantois, which are initially continuous with the intra-embryonic endoderm.
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Endoderm Development | Endoderm Images | Endoderm | |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Overview
|
| ||||||||
| Template Only - content to be added. Mesoderm has been completed. | |||||||||
| Overview: Ectoderm | Mesoderm | Endoderm Layers: ectoderm | mesoderm | endoderm | |||||||||
| Adult Cells - derived from Endoderm |
|---|
| Adult Cells Embryonic Origin: Endoderm | Mesoderm | Ectoderm |
| Exocrine secretory epithelial cells |
|
| Endocrine System Development - Hormone-secreting cells |
|
| Endoderm Structures | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Early Endoderm Cartoon
|
<html5media height="360" width="360">File:Endoderm 003.mp4</html5media> |
This animation shows the early development of endoderm forming the gastrointestinal tract, yolk sac and allantois. The movie starts approximately week 3 and continues through week 4.
Yellow shows the general lining of the yolk sac (bottom), continuous with the endoderm of the trilaminar embryonic disc (top) during week 3. As the trilaminar disc folds in this week, the foregut and hindgut regions become separated from the external yolk sac. The midgut region remains open to the yolk sac and will separate later. Foregut - Begins at the buccopharyngeal membrane, the foregut region in the head is now called the pharynx. At the lower end of the pharynx a ventral bud forms, that will later form the respiratory tract. Beneath this region the tube grows rapidly forming a dilation of the tube, that will later form the stomach. Beneath this region is the boundary of the foregut and ventrally lies the transverse septum. Midgut - Broadly open to the external yolk sac then with continued folding narrows to a "tube-like" connection the yolk stalk. This stalk will later degenerate and all connection will normally be lost. The yolk sac is pushed to the periphery by the growing amniotic sac, with its connecting yolk stalk in the umbilicus region. The midgut region also grows in length forming a loop lying outside the ventral body wall. Hindgut - The loop of midgut renters the body and the ventral portion of the hindgut extends as a blind-ended tube, or diverticulum, into the connecting stalk. This endoderm extension can be seen in histological sections of the initial placental cord and is called the allantois. The hindgut extends caudal (tailward) ending at the cloacal membrane. |
|
Hypochord
The hypochord (subnotochordal rod) is a transient endoderm structure found in the amphibian and fish embryo.[12] It forms a rod-like structure from a single row of cells lying immediately ventral to the notochord. The region is though to play a role in patterning the development of the dorsal aorta and degenerates by apoptosis.
It was first identified in the late 1900's:
- Stöhr, Ph. (1895). Ueber die Entwickelung der Hypochorda und des dorsalen Pankreas bei Rana temporaria. Morph. Jahrb. Bd. 23, 123-141.
- Bergfeldt, A. (1896). Chordascheiden und Hypochorda bei Alytes obstericans. Anat Hefte Bd. 7, 55-102.
- Klaatsch, H., (1898). Zur Frage nach der morphologiscrn Bedeutung der Hypochorda. Morphol. Jahrb. 25, 156-169.
Molecular
Markers
Several studies have identified the following proteins as markers for endoderm in different species.
- Foxa2 - Forkhead box A2 expressed by anterior primitive streak that forms endoderm.[14] OMIM FOXA2
- Sox17 - Sry-Related HMG-Box gene 17 Sox | OMiM - SOX17
- Cxcr4 - Chemokine, Cxc Motif, Receptor 4 a membrane receptor for neuropeptide Y OMIM CXCR4
- Daf1 - Decay-Accelerating Factor for complement.[9] OMIM DAF1
Nodal
- signaling pathway initiates endoderm and mesoderm development
- also required for proper gastrulation and axial patterning.
- ligands are members of the TGFβ family of secreted growth factors.
- promotes the expression of a network of transcription factors Mix-like proteins (Foxa2, Sox17, Eomesodermin, and Gata4–6).
- Links: TGF-beta | Molecular Development
Patterning
- foregut - (anterior) Hhex, Sox2, and Foxa2 transcription factors.
- hindgut - (posterior) also positioning of the foregut-hindgut boundary, homeobox genes Cdx1, 2, and 4.[15]
Chicken antero-posterior endoderm patterning[16]
References
- ↑ Liberti DC, Zepp JA, Bartoni CA, Liberti KH, Zhou S, Lu M, Morley MP & Morrisey EE. (2019). Dnmt1 is required for proximal-distal patterning of the lung endoderm and for restraining alveolar type 2 cell fate. Dev. Biol. , , . PMID: 31242446 DOI.
- ↑ van Waegeningh HF, Ebbens FA, van Spronsen E & Oostra RJ. (2019). Single origin of the epithelium of the human middle ear. Mech. Dev. , , 103556. PMID: 31121244 DOI.
- ↑ Ankamreddy H, Min H, Kim JY, Yang X, Cho ES, Kim UK & Bok J. (2019). Region-specific endodermal signals direct neural crest cells to form the three middle ear ossicles. Development , 146, . PMID: 30630826 DOI.
- ↑ Banerjee KK, Saxena M, Kumar N, Chen L, Cavazza A, Toke NH, O'Neill NK, Madha S, Jadhav U, Verzi MP & Shivdasani RA. (2018). Enhancer, transcriptional, and cell fate plasticity precedes intestinal determination during endoderm development. Genes Dev. , 32, 1430-1442. PMID: 30366903 DOI.
- ↑ Molotkov A & Soriano P. (2018). Distinct mechanisms for PDGF and FGF signaling in primitive endoderm development. Dev. Biol. , , . PMID: 30026121 DOI.
- ↑ Sachani SS, Landschoot LS, Zhang L, White CR, MacDonald WA, Golding MC & Mann MRW. (2018). Nucleoporin 107, 62 and 153 mediate Kcnq1ot1 imprinted domain regulation in extraembryonic endoderm stem cells. Nat Commun , 9, 2795. PMID: 30022050 DOI.
- ↑ Shoji M, Minato H, Ogaki S, Seki M, Suzuki Y, Kume S & Kuzuhara T. (2018). Different murine-derived feeder cells alter the definitive endoderm differentiation of human induced pluripotent stem cells. PLoS ONE , 13, e0201239. PMID: 30048506 DOI.
- ↑ Servetnick MD, Steinworth B, Babonis LS, Simmons D, Salinas-Saavedra M & Martindale MQ. (2017). Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation and oral-aboral patterning. Development , 144, 2951-2960. PMID: 28705897 DOI.
- ↑ 9.0 9.1 Ogaki S, Omori H, Morooka M, Shiraki N, Ishida S & Kume S. (2016). Late stage definitive endodermal differentiation can be defined by Daf1 expression. BMC Dev. Biol. , 16, 19. PMID: 27245320 DOI.
- ↑ Viotti M, Niu L, Shi SH & Hadjantonakis AK. (2012). Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. , 10, e1001276. PMID: 22412348 DOI.
- ↑ Zorn AM & Wells JM. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. , 25, 221-51. PMID: 19575677 DOI.
- ↑ Cleaver O, Seufert DW & Krieg PA. (2000). Endoderm patterning by the notochord: development of the hypochord in Xenopus. Development , 127, 869-79. PMID: 10648245
- ↑ Norris DP. (2012). Cilia, calcium and the basis of left-right asymmetry. BMC Biol. , 10, 102. PMID: 23256866 DOI.
- ↑ Burtscher I & Lickert H. (2009). Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development , 136, 1029-38. PMID: 19234065 DOI.
- ↑ Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J & Beck F. (2004). Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. U.S.A. , 101, 7641-5. PMID: 15136723 DOI.
- ↑ Bayha E, Jørgensen MC, Serup P & Grapin-Botton A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE , 4, e5845. PMID: 19516907 DOI.
Reviews
Zorn AM & Wells JM. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. , 25, 221-51. PMID: 19575677 DOI.
Zorn AM & Wells JM. (2007). Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. , 259, 49-111. PMID: 17425939 DOI.
Lewis SL & Tam PP. (2006). Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev. Dyn. , 235, 2315-29. PMID: 16752393 DOI.
Grapin-Botton A & Melton DA. (2000). Endoderm development: from patterning to organogenesis. Trends Genet. , 16, 124-30. PMID: 10689353
Wells JM & Melton DA. (1999). Vertebrate endoderm development. Annu. Rev. Cell Dev. Biol. , 15, 393-410. PMID: 10611967 DOI.
Articles
Bayha E, Jørgensen MC, Serup P & Grapin-Botton A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE , 4, e5845. PMID: 19516907 DOI.
Historic
Search PubMed
Search NLM Online Textbooks: "Endoderm" : Developmental Biology | The Cell- A molecular Approach | Molecular Biology of the Cell | Endocrinology
Search Pubmed: Endoderm
Additional Images
External Links
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 24) Embryology Endoderm. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Endoderm
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G