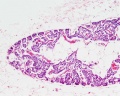
Endocrine - Parathyroid Development
| Embryology - 16 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
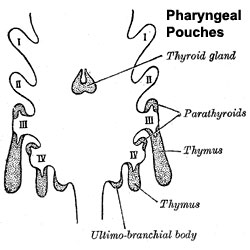
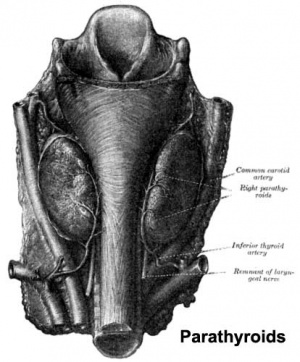
The parathyroid gland appears in the adult as a pair of inferior and a pair of superior "bumps" on the beside the (dorsal) thyroid (hence the name, "para"). The embryonic origin of this gland is from the third and fourth pharyngeal pouches endoderm, and could also have ectoderm and neural crest contributions.
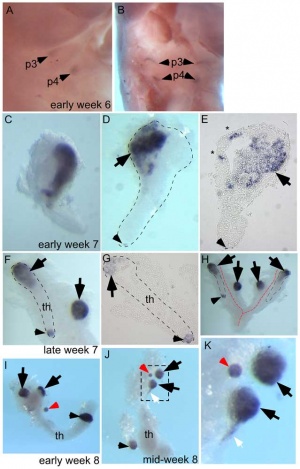
At 6 weeks a diverticulum elongates from the pouch, initially hollow and then solidifynig with cell proliferation.
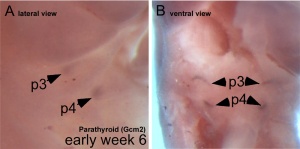
Interestingly, the inferior parathyroid originates from the third pharyngeal pouch and the superior arises from the fourth pharyngeal pouch, the adult anatomical position is the opposite of the pharyngeal rostro-caudal order. This occurs due to the third pharyngeal pouch also giving rise to the thymus, the superior pair descend along with the thymus.
The fetal parathyroids appear functional as they respond to calcium levels. The fetal calcium levels also higher than maternal levels.
| Lecture - Head Development |
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Parathyroid Embryology <pubmed limit=5>Parathyroid Embryology</pubmed> |
Development Overview
- Endoderm - third and fourth pharyngeal pouches, could also have ectoderm and neural crest
- 3rd Pharyngeal Pouch - inferior parathyroid, initially descends with thymus
- 4th Pharyngeal Pouch - superior parathyroid
- Week 6 - diverticulum elongate, hollow then solid, dorsal cell proliferation
- Fetal parathyroids - respond to calcium levels, fetal calcium levels higher than maternal
Parathyroid Hormone
(PTH, parathormone or parathyrin) A polypeptide (84 amino acids) hormone which increases the concentration of calcium ions in the blood. Its actions oppose the hormone calcitonin from the thyroid gland parafollicular cells (C cells), which decrease calcium. Acts through the parathyroid hormone receptor in bone, kidney and gastrointestinal tract.
- stimulate osteoclasts - degrade bone matrix, releasing calcium
- increase calcium gastrointestinal tract absorption
(PTHrP) Originally identified in the clinical syndrome humoral hypercalcemia of malignancy. It's developmental role is that of a regulatory protein expressed during the formation of many organs.
- mammary gland development - epithelial-mesenchymal interactions[3]
- chondrocyte differentiation[4]
Abnormalities
Hyperparathyroidism
Postnatal
- Postnatal adult ageing increased parathyroid hormone plasma levels are associated with cognitive decline and dementia.
- Parathyroid carcinoma (cancer) is a rare malignancy, occurring with an incidence of 0.5 to 4% of all cases of primary hyperparathyroidism.
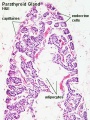
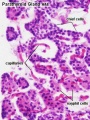
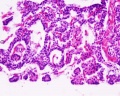
Adult Histology
References
Reviews
- The development of the parathyroid gland: from fish to human. Zajac JD, Danks JA. Curr Opin Nephrol Hypertens. 2008 Jul;17(4):353-6. Review. PMID: 18660669
- Parathyroid development and the role of tubulin chaperone E. Parvari R, Diaz GA, Hershkovitz E. Horm Res. 2007;67(1):12-21. Epub 2006 Sep 27. Review. PMID: 17008776
- Role of parathyroid hormone-related peptide and Indian hedgehog in skeletal development. Jüppner H. Pediatr Nephrol. 2000 Jul;14(7):606-11. Review. PMID: 10912527
Articles
Search PubMed
Search April 2010
- Parathyroid Development - All (3523) Review (768) Free Full Text (741)
Search Pubmed: parathyroid development
Additional Images
Terms
- parathyroid hormone - (PTH, parathormone or parathyrin) A polypeptide (84 amino acids) hormone secreted by the parathyroid gland, which increases the concentration of calcium ions in the blood. Its actions oppose the hormone calcitonin from the thyroid gland parafollicular cells (C cells), which decrease calcium. Acts through the parathyroid hormone receptor located mainly in bone, kidney and gastrointestinal tract. Hormone dual role is to: stimulate osteoclasts in bone to degrade bone matrix releasing calcium; increase gastrointestinal tract absorption of calcium.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 16) Embryology Endocrine - Parathyroid Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Endocrine_-_Parathyroid_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G