|
|
| Line 69: |
Line 69: |
| <references/> | | <references/> |
|
| |
|
| | ===Reviews=== |
| | <pubmed></pubmed> |
| | <pubmed></pubmed> |
| | <pubmed></pubmed> |
| | <pubmed>29330906</pubmed> |
|
| |
|
| ===Articles=== | | ===Articles=== |
Revision as of 21:09, 18 January 2018
Introduction

All-trans retinoic acid (atRA)
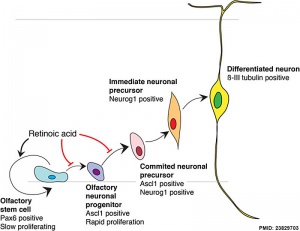
Model for retinoid acid in olfactory development
[1]
All-trans retinoic acid (atRA) is the transcriptionally active product of vitamin A and is known to play many roles in regulating embryo development. In the adult retinoid acid has additional signalling roles including spermatozoa maturation.
The compound has been used extensively postnatally in therapeutic treatments, for example in skin disease. As this compound also acts as a developmental signal, it has known teratogenic effects[2][3] following maternal to conceptus transfer.
- Retinoic Acid Links: retinoic acid | limb | Category:Retinoic acid | Molecular Development
| Category:Retinoic acid | Abnormal Development
Some Recent Findings
- Retinoic acid temporally orchestrates colonization of the gut by vagal neural crest cells[4] "The enteric nervous system arises from neural crest cells that migrate as chains into and along the primitive gut, subsequently differentiating into enteric neurons and glia. Little is known about the mechanisms governing neural crest migration en route to and along the gut in vivo. Here, we report that Retinoic Acid (RA) temporally controls zebrafish enteric neural crest cell chain migration. In vivo imaging reveals that RA loss severely compromises the integrity and migration of the chain of neural crest cells during the window of time window when they are moving along the foregut. After loss of RA, enteric progenitors accumulate in the foregut and differentiate into enteric neurons, but subsequently undergo apoptosis resulting in a striking neuronal deficit. Moreover, ectopic expression of the transcription factor meis3 and/or the receptor ret, partially rescues enteric neuron colonization after RA attenuation. Collectively, our findings suggest that retinoic acid plays a critical temporal role in promoting enteric neural crest chain migration and neuronal survival upstream of Meis3 and RET in vivo." Neural Crest Development | Zebrafish Development
- Cyp26 Enzymes Facilitate Second Heart Field Progenitor Addition and Maintenance of Ventricular Integrity[5] "Although retinoic acid (RA) teratogenicity has been investigated for decades, the mechanisms underlying RA-induced outflow tract (OFT) malformations are not understood. Here, we show zebrafish embryos deficient for Cyp26a1 and Cyp26c1 enzymes, which promote RA degradation, have OFT defects resulting from two mechanisms: first, a failure of second heart field (SHF) progenitors to join the OFT, instead contributing to the pharyngeal arch arteries (PAAs), and second, a loss of first heart field (FHF) ventricular cardiomyocytes due to disrupted cell polarity and extrusion from the heart tube." Zebrafish Development | Cardiovascular Abnormalities
- Retinoic acid signaling and neuronal differentiation[6] "The identification of neurological symptoms caused by vitamin A deficiency pointed to a critical, early developmental role of vitamin A and its metabolite, retinoic acid (RA). The ability of RA to induce post-mitotic, neural phenotypes in various stem cells, in vitro, served as early evidence that RA is involved in the switch between proliferation and differentiation. In vivo studies have expanded this "opposing signal" model, and the number of primary neurons an embryo develops is now known to depend critically on the levels and spatial distribution of RA. The proneural and neurogenic transcription factors that control the exit of neural progenitors from the cell cycle and allow primary neurons to develop are partly elucidated, but the downstream effectors of RA receptor (RAR) signaling (many of which are putative cell cycle regulators) remain largely unidentified. The molecular mechanisms underlying RA-induced primary neurogenesis in anamniote embryos are starting to be revealed; however, these data have been not been extended to amniote embryos. There is growing evidence that bona fide RARs are found in some mollusks and other invertebrates, but little is known about their necessity or functions in neurogenesis. One normal function of RA is to regulate the cell cycle to halt proliferation, and loss of RA signaling is associated with dedifferentiation and the development of cancer. Identifying the genes and pathways that mediate cell cycle exit downstream of RA will be critical for our understanding of how to target tumor differentiation. Overall, elucidating the molecular details of RAR-regulated neurogenesis will be decisive for developing and understanding neural proliferation-differentiation switches throughout development."
- Visualization of an endogenous retinoic acid gradient across embryonic development[7] "In vertebrate development, the body plan is determined by primordial morphogen gradients that suffuse the embryo. Retinoic acid (RA) is an important morphogen involved in patterning the anterior-posterior axis of structures, including the hindbrain and paraxial mesoderm. RA diffuses over long distances, and its activity is spatially restricted by synthesizing and degrading enzymes. ...Live imaging of endogenous concentration gradients across embryonic development will allow the precise assignment of molecular mechanisms to developmental dynamics and will accelerate the application of approaches based on morphogen gradients to tissue engineering and regenerative medicine." Zebrafish Development
|
| More recent papers
|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
- This search now requires a manual link as the original PubMed extension has been disabled.
- The displayed list of references do not reflect any editorial selection of material based on content or relevance.
- References also appear on this list based upon the date of the actual page viewing.
References listed on the rest of the content page and the associated discussion page (listed under the publication year sub-headings) do include some editorial selection based upon both relevance and availability.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References
Search term: Embryo Retinoic acid | Images
<pubmed limit=5>Embryo Retinoic acid</pubmed>
|
Organ Expression
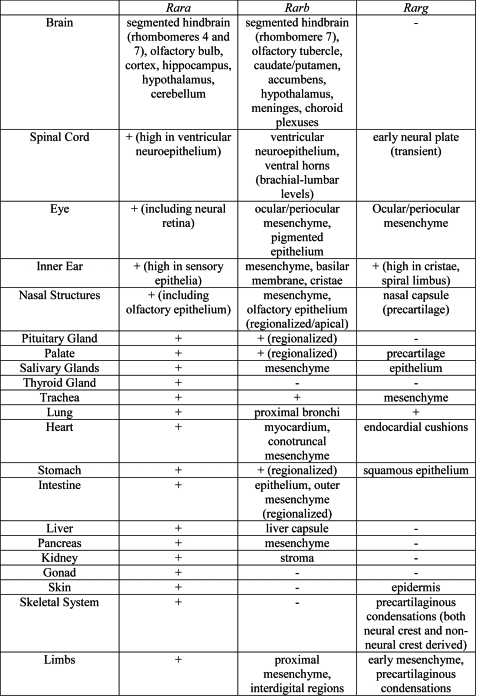
Summary of Rar gene expression patterns in mouse developing organ systems.[8]
Endoderm
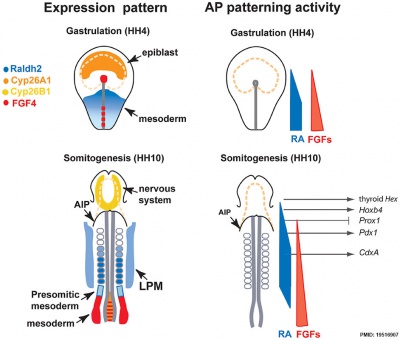
Chicken antero-posterior endoderm patterning[9]
- Links: Endoderm | Chicken Development
Fetal Gonad

Immunohistochemical localisation of retinoid receptor expression in the human fetal gonad[10]
Neural
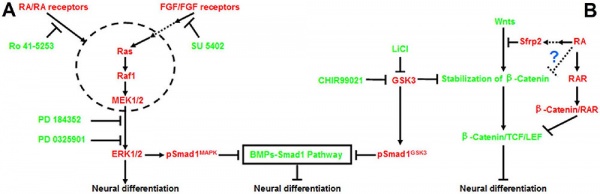
Model retinoic acid extracellular signal-regulated kinase and Wnt pathway interactions[11]

RA Is Not Required for Radial Expansion of the Embryonic Cortex
References
- ↑ <pubmed>23829703</pubmed>| Neural Dev.
- ↑ <pubmed>10331090</pubmed>
- ↑ <pubmed>11375780</pubmed>
- ↑ <pubmed>29108781</pubmed>
- ↑ <pubmed>27893754</pubmed>
- ↑ <pubmed>25558812</pubmed>
- ↑ <pubmed>23563268</pubmed>
- ↑ <pubmed>19471585</pubmed>
- ↑ 19516907</pubmed>| PLoS One.
- ↑ Childs AJ, Cowan G, Kinnell HL, Anderson RA, Saunders PTK (2011) Retinoic Acid Signalling and the Control of Meiotic Entry in the Human Fetal Gonad. PLoS ONE 6(6): e20249. PMID 21674038 | PLoS One
- ↑ <pubmed>19642999</pubmed>
Reviews
<pubmed></pubmed>
<pubmed></pubmed>
<pubmed></pubmed>
<pubmed>29330906</pubmed>
Articles
<pubmed>21673209</pubmed>| Can Fam Physician
Search Pubmed
Search Bookshelf Retinoic acid
Search Pubmed Now: Retinoic acid
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 19) Embryology Developmental Signals - Retinoic acid. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Signals_-_Retinoic_acid
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G







