Developmental Signals - Fibroblast Growth Factor: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Header}} | {{Header}} | ||
==Introduction== | ==Introduction== | ||
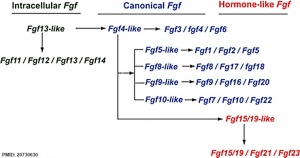
[[File:Fgf_gene_family_evolution.jpg|thumb|300px|Fgf gene family evolution | [[File:Fgf_gene_family_evolution.jpg|thumb|300px|Fgf gene family evolution{{#pmid:20730630|PMID20730630}}]] | ||
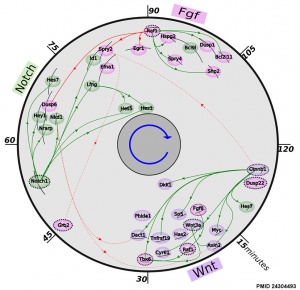
[[File:Mouse somitogenesis genes.jpg|thumb|Mouse somitogenesis genes | [[File:Mouse somitogenesis genes.jpg|thumb|Mouse somitogenesis genes{{#pmid:24304493|PMID24304493}}]] | ||
Fibroblast Growth Factors (FGF) were originally identified by their ability to stimulate fibroblast cell proliferation but have a role in a growing number of different tissues development and differentiation and continue to have a role in the adult. | Fibroblast Growth Factors (FGF) were originally identified by their ability to stimulate fibroblast cell proliferation but have a role in a growing number of different tissues development and differentiation and continue to have a role in the adult. | ||
| Line 32: | Line 32: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| | | | ||
* '''FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis''' | * '''FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis'''{{#pmid:26130757|PMID26130757}} "When a tubular structure forms during early embryogenesis, tubular elongation and lumen formation (epithelialization) proceed simultaneously in a spatiotemporally coordinated manner. We here demonstrate, using the Wolffian duct (WD) of early chicken embryos, that this coordination is regulated by the expression of FGF8, which shifts posteriorly during body axis elongation. FGF8 acts as a chemoattractant on the leader cells of the elongating WD and prevents them from epithelialization, whereas static ('rear') cells that receive progressively less FGF8 undergo epithelialization to form a lumen. Thus, FGF8 acts as a binary switch that distinguishes tubular elongation from lumen formation. The posteriorly shifting FGF8 is also known to regulate somite segmentation, suggesting that multiple types of tissue morphogenesis are coordinately regulated by macroscopic changes in body growth." [[Chicken Development]] | ||
* '''Tissue-specific roles of Fgfr2 in development of the external genitalia''' | |||
* '''Tissue-specific roles of Fgfr2 in development of the external genitalia'''{{#pmid:26081573|PMID26081573}} "Congenital anomalies frequently occur in organs that undergo tubulogenesis. Hypospadias is a urethral tube defect defined by mislocalized, oversized, or multiple openings of the penile urethra. Deletion of Fgfr2 or its ligand Fgf10 results in severe hypospadias in mice, in which the entire urethral plate is open along the ventral side of the penis. In the genital tubercle, the embryonic precursor of the penis and clitoris, Fgfr2 is expressed in two epithelial populations: the endodermally derived urethral epithelium and the ectodermally derived surface epithelium. Here, we investigate the tissue-specific roles of Fgfr2 in external genital development by generating conditional deletions of Fgfr2 in each of these cell types. These results demonstrate that urethral tubulogenesis, prepuce morphogenesis, and sexually dimorphic patterning of the lower urethra are controlled by discrete regions of Fgfr2 activity." [[Genital System Development]] | |||
|} | |} | ||
{| class="wikitable mw-collapsible mw-collapsed" | {| class="wikitable mw-collapsible mw-collapsed" | ||
| Line 48: | Line 49: | ||
|- | |- | ||
| {{Older papers}} | | {{Older papers}} | ||
* '''The precise timeline of transcriptional regulation reveals causation in mouse somitogenesis network''' | * '''The precise timeline of transcriptional regulation reveals causation in mouse somitogenesis network'''{{#pmid:24304493|PMID24304493}} "In vertebrate development, the segmental pattern of the body axis is established as somites, masses of mesoderm distributed along the two sides of the neural tube, are formed sequentially in the anterior-posterior axis. This mechanism depends on waves of gene expression associated with the Notch, Fgf and Wnt pathways." | ||
* '''Prolonged FGF signaling is necessary for lung and liver induction in Xenopus''' | |||
* '''FGF-signaling gradient maintains symmetrical proliferative divisions of midbrain neuronal progenitors''' | * '''Prolonged FGF signaling is necessary for lung and liver induction in Xenopus'''{{#pmid:22988910|PMID22988910}} "FGF signaling plays numerous roles during organogenesis of the embryonic gut tube. Mouse explant studies suggest that different thresholds of FGF signaling from the cardiogenic mesoderm induce lung, liver, and pancreas lineages from the ventral foregut progenitor cells. The mechanisms that regulate FGF dose in vivo are unknown. Here we use Xenopus embryos to examine the hypothesis that a prolonged duration of FGF signaling from the mesoderm is required to induce foregut organs....These results suggest that the liver and lungs are specified at progressively later times in development requiring mesoderm contact for different lengths of time. Our data suggest that this is achieved at least in part through prolonged FGF signaling. In addition to providing a foundation for further mechanistic studies on foregut organogenesis using the experimental advantages of the Xenopus system, these data have implications for the directed differentiation of stem cells into foregut lineages." | ||
* '''Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules''' | |||
* '''FGF-signaling gradient maintains symmetrical proliferative divisions of midbrain neuronal progenitors'''{{#pmid:21074523|PMID21074523}} "For the correct development of the central nervous system, the balance between self-renewing and differentiating divisions of the neuronal progenitors must be tightly regulated. To maintain their self-renewing identity, the progenitors need to retain both apical and basal interfaces. However, the identities of fate-determining signals which cells receive via these connections, and the exact mechanism of their action, are poorly understood. The conditional inactivation of Fibroblast growth factor (FGF) receptors 1 and 2 in the embryonic mouse midbrain-hindbrain area results in premature neuronal differentiation. Here, we aim to elucidate the connection between FGF-signaling and neuronal progenitor maintenance. Our results reveal that the loss of FGF-signaling leads to downregulation of Hes1 and upregulation of Ngn2, Dll1, and p57 in the ventricular zone (VZ) cells, and that this increased neurogenesis occurs cell-autonomously. Yet the cell-cycle progression, apico-basal-polarity, cell-cell connections, and the positioning of mitotic spindle in the mutant VZ appear unaltered. Interestingly, FGF8-protein is highly concentrated in the basal lamina. Thus, FGFs may act through basal processes of neuronal progenitors to maintain their progenitor status. Indeed, midbrain neuronal progenitors deprived in vitro of FGFs switched from symmetrical proliferative towards symmetrical neurogenic divisions. We suggest that FGF-signaling in the midbrain VZ is cell-autonomously required for the maintenance of symmetrical proliferative divisions via Hes1-mediated repression of neurogenic genes." | |||
* '''Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules'''{{#pmid:19741606|PMID19741606}} "It is widely accepted that tissue differentiation and morphogenesis in multicellular organisms are regulated by tightly controlled concentration gradients of morphogens. How exactly these gradients are formed, however, remains unclear. Here we show that Fgf8 morphogen gradients in living zebrafish embryos are established and maintained by two essential factors: fast, free diffusion of single molecules away from the source through extracellular space, and a sink function of the receiving cells, regulated by receptor-mediated endocytosis. Evidence is provided by directly examining single molecules of Fgf8 in living tissue by fluorescence correlation spectroscopy, quantifying their local mobility and concentration with high precision. By changing the degree of uptake of Fgf8 into its target cells, we are able to alter the shape of the Fgf8 gradient. Our results demonstrate that a freely diffusing morphogen can set up concentration gradients in a complex multicellular tissue by a simple source-sink mechanism." | |||
|} | |} | ||
| Line 58: | Line 62: | ||
[[File:Chicken_antero-posterior_endoderm_patterning_01.jpg|alt=Chicken antero-posterior endoderm patterning cartoon|400px]] | [[File:Chicken_antero-posterior_endoderm_patterning_01.jpg|alt=Chicken antero-posterior endoderm patterning cartoon|400px]] | ||
Chicken antero-posterior endoderm patterning | Chicken antero-posterior endoderm patterning{{#pmid:19516907|PMID19516907}} | ||
| Line 68: | Line 72: | ||
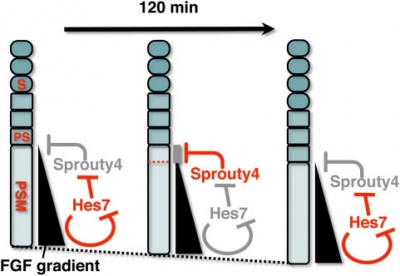
[[File:Model for Sprouty4 and FGF in mesoderm segmentation.jpg|400px]] | [[File:Model for Sprouty4 and FGF in mesoderm segmentation.jpg|400px]] | ||
A Putative Model for the role of Sprouty4 as a mediator that links the mouse segmentation clock to the gradient of FGF signaling. | A Putative Model for the role of Sprouty4 as a mediator that links the mouse segmentation clock to the gradient of FGF signaling.{{#pmid:19440349|PMID19440349}} | ||
The FGF signaling may be periodically inhibited by Sprouty4, by which temporal periodicity of Notch segmentation clock may be translated to spatial periodicity of the array of somites. | The FGF signaling may be periodically inhibited by Sprouty4, by which temporal periodicity of Notch segmentation clock may be translated to spatial periodicity of the array of somites. | ||
| Line 96: | Line 99: | ||
* new FGF10 signaling domains serve as two chemoattractant sources, leading to bifurcation of the epithelial tip. | * new FGF10 signaling domains serve as two chemoattractant sources, leading to bifurcation of the epithelial tip. | ||
Loop process mediated through FGF-activated transcription factor genes Etv4 and Etv5. | Loop process mediated through FGF-activated transcription factor genes Etv4 and Etv5.{{#pmid:26555052|PMID26555052}} | ||
:'''Links:''' [[Respiratory System Development]] | :'''Links:''' [[Respiratory System Development]] | ||
| Line 102: | Line 105: | ||
==Limb== | ==Limb== | ||
FGF soaked beads (FGF-1, FGF-2 and FGF-4) are capable of inducing additional limbs in chicken embryos. | FGF soaked beads (FGF-1, FGF-2 and FGF-4) are capable of inducing additional limbs in chicken embryos.{{#pmid:7889567|PMID7889567}} A later study{{#pmid:8674413|PMID8674413}} identified the endogenous signal as Fgf-8 from initially the intermediate mesoderm and then the prelimb field ectoderm for limb initiation and outgrowth, respectively. | ||
:'''Links:''' [[Musculoskeletal System - Limb Development|Limb Development]] | [[Chicken Development]] | :'''Links:''' [[Musculoskeletal System - Limb Development|Limb Development]] | [[Chicken Development]] | ||
| Line 125: | Line 128: | ||
===Articles=== | ===Articles=== | ||
{{#pmid:20582225}} | |||
===Search Pubmed=== | ===Search Pubmed=== | ||
Revision as of 20:38, 19 March 2018
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
Fibroblast Growth Factors (FGF) were originally identified by their ability to stimulate fibroblast cell proliferation but have a role in a growing number of different tissues development and differentiation and continue to have a role in the adult.
The first two identified factors were originally given the nomenclature of acidic or basic. We now know there to be at least 22 different human FGFs (Fgf1–Fgf23). These protein growth factors are bound by 4 different cell membrane receptors (FGFR1-4). FGFRs belong to the tyrosine kinase receptor family.
The mammalian Fgf family can be divided into the intracellular Fgf11/12/13/14 subfamily (iFGFs), the endocrine hormone-like Fgf15/21/23 subfamily (hFGFs), and the paracrine canonical Fgf subfamilies, including Fgf1/2/5, Fgf3/4/6, Fgf7/10/22, Fgf8/17/18, and Fgf9/16/20.
| Factor Links: AMH | hCG | BMP | sonic hedgehog | bHLH | HOX | FGF | FOX | Hippo | LIM | Nanog | NGF | Nodal | Notch | PAX | retinoic acid | SIX | Slit2/Robo1 | SOX | TBX | TGF-beta | VEGF | WNT | Category:Molecular |
Human FGF Family
| Table - Human Fibroblast Growth Factor Family FGF | ||||
| Approved Symbol |
Approved Name | Previous Symbols |
Synonyms | Chromosome |
|---|---|---|---|---|
| FGF1 | fibroblast growth factor 1 | FGFA | "AFGF, ECGF, ECGFA, ECGFB, HBGF1, ECGF-beta, FGF-alpha, GLIO703" | 5q31.3 |
| FGF2 | fibroblast growth factor 2 | FGFB | 4q28.1 | |
| FGF4 | fibroblast growth factor 4 | HSTF1 | "K-FGF, HBGF-4, HST, HST-1, KFGF" | 11q13.3 |
| FGF5 | fibroblast growth factor 5 | 4q21.21 | ||
| FGF6 | fibroblast growth factor 6 | 12p13.32 | ||
| FGF7 | fibroblast growth factor 7 | KGF | 15q21.2 | |
| FGF8 | fibroblast growth factor 8 | AIGF | 10q24.32 | |
| FGF9 | fibroblast growth factor 9 | 13q12.11 | ||
| Links: Developmental Signals - Fibroblast Growth Factor | OMIM Fgf1 | HGNC | Bmp Family | Fgf Family | Sox Family | Tbx Family | ||||
| Human FGF Family | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Protein Properties
Human FGF
- ~150–300 amino acids
- have a conserved ~120-residue core with ~30–60% identity
Some Recent Findings
|
| More recent papers |
|---|
|
This table allows an automated computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Fibroblast Growth Factor <pubmed limit=5>Fibroblast Growth Factor</pubmed> |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
|
Endoderm
Chicken antero-posterior endoderm patterning[8]
- Links: Endoderm | Chicken Development
Mesoderm
A Putative Model for the role of Sprouty4 as a mediator that links the mouse segmentation clock to the gradient of FGF signaling.[9]
The FGF signaling may be periodically inhibited by Sprouty4, by which temporal periodicity of Notch segmentation clock may be translated to spatial periodicity of the array of somites.
- In the PSM - FGF signaling establishes a posterior-to-anterior gradient, which is involved in the positioning of presumptive somite boundaries.
- Cyclic Sprouty4
- which is controlled by the Notch segmentation clock, the mechanism of which includes negative feedback loop of Hes7,
- may inhibit the FGF signaling possibly around the anterior border of the FGF signaling positive area
- where the FGF signaling is close to its threshold.
- S - somite
- PS - presumptive somite.
- Links: Somitogenesis | Axial Skeleton Development | Notch | FGF
Respiration
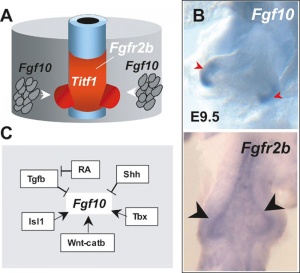
Lung Buds
Fibroblast growth factor 10 (FGF10) expression in mesoderm required for initial lung buds, through FGFR2IIIb transmembrane tyrosine kinase receptor protein.
Branching
Fibroblast growth factor 10 (FGF10) and sonic hedgehog (SHH) form a feedback loop for branching
- mesenchyme produced FGF10 signals to the distal epithelium to upregulate SHH expression.
- SHH then feeds back to inhibit Fgf10 expression in the adjacent mesenchyme, dividing in two the Fgf10 expression domain.
- new FGF10 signaling domains serve as two chemoattractant sources, leading to bifurcation of the epithelial tip.
Loop process mediated through FGF-activated transcription factor genes Etv4 and Etv5.[11]
Limb
FGF soaked beads (FGF-1, FGF-2 and FGF-4) are capable of inducing additional limbs in chicken embryos.[12] A later study[13] identified the endogenous signal as Fgf-8 from initially the intermediate mesoderm and then the prelimb field ectoderm for limb initiation and outgrowth, respectively.
- Links: Limb Development | Chicken Development
Hearing
- fibroblast growth factor 1 - (Fgf-1) a growth factor released from cochlea sensory epithelium which stimulates spiral ganglion neurite branching.
- fibroblast growth factor 8 - (Fgf-8) a growth factor released by inner hair cells which regulates pillar cell number, position and rate of development.
- fibroblast growth factor receptor 3 - (Fgfr-3) a tyrosine kinase receptor with a role in the commitment, differentiation and position of pillar cells in the organ of corti
- Links: Hearing
Abnormalities
- FGFR1 mutation has been associated with the relatively milder form of Pfeiffer syndrome type 1.
- FGFR2 and FGFR3 have been associated with the Apert, Crouzon and Pfeiffer syndromes.
References
- ↑ Itoh N. (2010). Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. , 342, 1-11. PMID: 20730630 DOI.
- ↑ 2.0 2.1 Fongang B & Kudlicki A. (2013). The precise timeline of transcriptional regulation reveals causation in mouse somitogenesis network. BMC Dev. Biol. , 13, 42. PMID: 24304493 DOI.
- ↑ Atsuta Y & Takahashi Y. (2015). FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis. Development , 142, 2329-37. PMID: 26130757 DOI.
- ↑ Gredler ML, Seifert AW & Cohn MJ. (2015). Tissue-specific roles of Fgfr2 in development of the external genitalia. Development , 142, 2203-12. PMID: 26081573 DOI.
- ↑ Shifley ET, Kenny AP, Rankin SA & Zorn AM. (2012). Prolonged FGF signaling is necessary for lung and liver induction in Xenopus. BMC Dev. Biol. , 12, 27. PMID: 22988910 DOI.
- ↑ Lahti L, Saarimäki-Vire J, Rita H & Partanen J. (2011). FGF signaling gradient maintains symmetrical proliferative divisions of midbrain neuronal progenitors. Dev. Biol. , 349, 270-82. PMID: 21074523 DOI.
- ↑ Yu SR, Burkhardt M, Nowak M, Ries J, Petrásek Z, Scholpp S, Schwille P & Brand M. (2009). Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature , 461, 533-6. PMID: 19741606 DOI.
- ↑ Bayha E, Jørgensen MC, Serup P & Grapin-Botton A. (2009). Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE , 4, e5845. PMID: 19516907 DOI.
- ↑ Hayashi S, Shimoda T, Nakajima M, Tsukada Y, Sakumura Y, Dale JK, Maroto M, Kohno K, Matsui T & Bessho Y. (2009). Sprouty4, an FGF inhibitor, displays cyclic gene expression under the control of the notch segmentation clock in the mouse PSM. PLoS ONE , 4, e5603. PMID: 19440349 DOI.
- ↑ [StemBook Cambridge (MA): Harvard Stem Cell Institute; 2008-. Specification and patterning of the respiratory system
- ↑ Herriges JC, Verheyden JM, Zhang Z, Sui P, Zhang Y, Anderson MJ, Swing DA, Zhang Y, Lewandoski M & Sun X. (2015). FGF-Regulated ETV Transcription Factors Control FGF-SHH Feedback Loop in Lung Branching. Dev. Cell , 35, 322-32. PMID: 26555052 DOI.
- ↑ Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK & Tickle C. (1995). Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell , 80, 739-46. PMID: 7889567
- ↑ Vogel A, Rodriguez C & Izpisúa-Belmonte JC. (1996). Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development , 122, 1737-50. PMID: 8674413
Reviews
Articles
Su N, Xu X, Li C, He Q, Zhao L, Li C, Chen S, Luo F, Yi L, Du X, Huang H, Deng C & Chen L. (2010). Generation of Fgfr3 conditional knockout mice. Int. J. Biol. Sci. , 6, 327-32. PMID: 20582225
Search Pubmed
Search Bookshelf Fibroblast Growth Factor
Search Pubmed Now: Fibroblast Growth Factor
Cite this page: Hill, M.A. (2024, April 19) Embryology Developmental Signals - Fibroblast Growth Factor. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Developmental_Signals_-_Fibroblast_Growth_Factor
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G