Detailed Cardiac - Superior Interatrial Fold
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Detailed Cardiac: Systemic Venous Sinus | Pulmonary Vein | Superior Interatrial Fold | Atrioventricular Cushions | Atrioventricular Canal | Interventricular Communication | Subpulmonary Infundibulum | Arterial Roots | Intrapericardial Arterial Trunks | Extrapericardial Arterial Channels | Sinus Node | Atrioventricular Conduction Axis |
Formation of the Superior Interatrial Fold and the Inferior Buttress of the Atrial Septum
Atrial Septation
The images shown in Figure 12 also demonstrate the initial stages of atrial septation. It is the completion of atrial septation, along with ventricular septation, which converts the initial heart tube, with its common lumen throughout its length, into the four-chambered arrangement as seen in postnatal life. Throughout embryonic life, however, there is the requirement for an interatrial communication. This is because the richly oxygenated placental blood, which enters only the right atrium subsequent to the rightward shift of the systemic venous tributaries, must gain access to the left side of the heart so that blood is able to reach the aorta, once this channel has achieved its definitive position above the morphologically left ventricle. The process of atrial septation, therefore, requires initial separation of the right and left sides of the common atrial chamber, but with the capacity for ongoing passage of blood from the right to the left side.
The initial stage of atrial septation is seen at Carnegie stage 13 in the human heart, comparable to the early part of E10.5 in the mouse, when a spur of myocardium grows down from the atrial roof (Figures 6, 13). As the atrial septum is growing initially from the atrial roof, the atrioventricular cushions have already formed superiorly and inferiorly within the atrioventricular canal. As already shown in the left hand panel of Figure 12, by the time the atrioventricular canal has expanded to permit the right atrium to communicate directly with the right ventricle, the primary atrial septum has extended well into the atrial chambers. This process is occurring during day 11.5 in the mouse. A comparable situation is seen at the beginning of the fifth week of development in man (Figure 14).
Fig. 13. The left hand image shows a cut through an episcopic dataset prepared from a mouse embryo at E10.5, while the right hand panel is a histological section from a human embryo at Carnegie stage 13 or 14, when the embryo is just over 4 weeks old. Both sections show the initial growth of the primary atrial septum (septum primum) from the atrial roof.
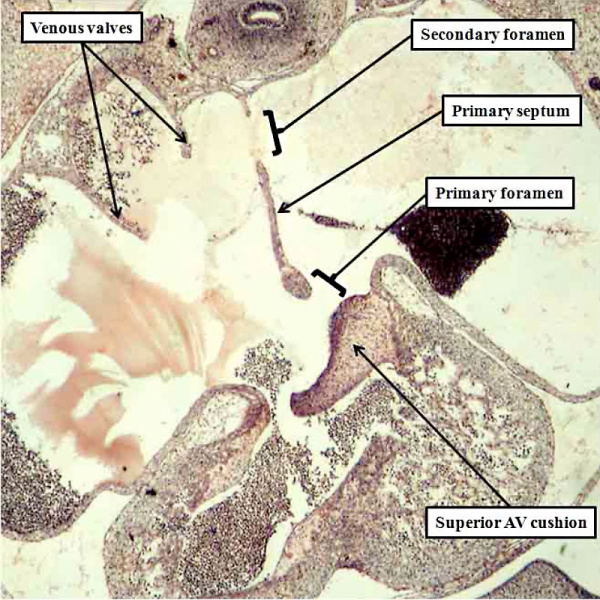
Fig. 14. The histological section, in four chamber plane, is from a human embryo at Carnegie stage 16, representing the turn from the fifth to the sixth week of development. The primary septum, carrying a mesenchymal cap, has extended well towards the atrial margin of the atrioventricular cushions, reducing the size of the primary foramen in the process. It has broken away from the atrial roof to form the secondary atrial foramen, which will permit the right atrial blood to continue to reach the left atrium subsequent to closure of the primary foramen. Note the remnant of the origin of the primary septum at the atrial roof.
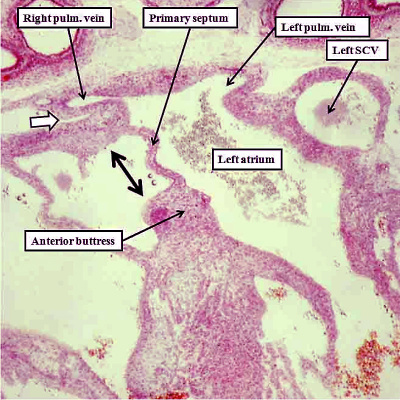
Much is written in current textbooks of human anatomy and development concerning the formation of a “secondary atrial septum”, or “septum secundum”. This structure is alleged to grow down from the atrial roof, paralleling the growth of the primary septum, but situated on its right side. There is no such growth of a secondary atrial septum from the atrial roof. By the time the primary septum has approached the atrial margins of the atrioventricular cushions, however, the veins have developed within the lungs, with canalization of the pulmonary vein within the dorsal mesocardium permitting the pulmonary venous blood directly to enter the heart (Figure 7). At first, the pulmonary vein, canalizing as it does within the pharyngeal mesenchyme, is a relatively midline structure. And, when it gains its initial entrance to the atrial cavity, this is adjacent to the developing atrioventricular junction, which contains the left sinus horn (Figure 7). Entering the atrial cavity as it does adjacent to the atrioventricular junction, and initially through a solitary orifice which drains the blood from both developing lungs, the pulmonary venous orifice is also adjacent to the base of the primary atrial septum. We have already seen that the dorsal mesocardium, which anchors the atrial part of the heart tube to the body of the embryo through the heart stalk, produces the bulges known as the pulmonary ridges, which flank the opening of the pulmonary vein (Figure 4). With growth of the embryo during the beginning of the fifth week of development, there is unequal growth of the rightward of the two pulmonary ridges, with the new tissues growing into the heart from the pharyngeal mesenchyme. This produces the structure known as the vestibular spine, and also called the dorsal mesenchymal protrusion. Growth of this structure ensures that the orifice of the pulmonary vein is committed to the left atrium (Figure 15). It is then the fusion of the vestibular spine, along with the mesenchymal cap carried on the leading edge of the primary septum, with the atrial aspect of the atrioventricular cushions, which themselves fuse during this process, which obliterates the primary foramen. Once the primary foramen is closed, the secondary foramen is the only route through which the richly oxygenated blood entering the right atrium can cross to the left side. At the time at which the primary foramen is obliterated, the atrial roof remains flat, with no formation of the alleged “secondary septum”.
Fig. 15. The images are serial sections from a human embryo at Carnegie stage 16 in four-chamber projection, with the right hand panel anterior to the section shown in the left hand panel. They show how the rightward border of the dorsal mesocardium has expanded, with tissue growing through the mesocardium to reinforce the base of the primary septum. It is the growth of the right ridge into the heart that produces the vestibular spine.
The persisting interatrial communication at the upper margin of the primary septum is now the primordium of the oval foramen, or foramen ovale. This will be required to close in postnatal life so as to remove the potential for interatrial shunting of blood. The arrangement permitting postnatal closure is achieved by the formation of a rim superiorly. The primary septum, which will form the floor of the oval fossa, will close against this rim once the left atrial pressure is higher than right atrial pressure in postnatal life. The superior rim is an infolding. It is formed concomitant with migration of the the pulmonary venous towards the roof of the left atrium, a process which occurs with ongoing atrial development. The process first becomes visible at the end of the seventh week of development (Figure 16). Incorporation of the pulmonary veins into the roof of the left atrium then continues beyond the completion of cardiac septation at Carnegie stage 23, when the embryo is 8 weeks old. It is at this stage that the roof of the atrium becomes fully infolded so as to produce the cranial rim of the oval fossa. By this time, an antero-inferior buttress, derived by muscularisation of the vestibular spine and the mesenchymal cap, has anchored the base of the primary septum to the insulating plane of the atrioventricular junction. This buttress forms the caudal rim and inferior rim of the oval fossa. These processes together establish the arrangement seen in postnatal life, whereby the flap valve forming the floor of the fossa, derived from the primary embryonic atrial septum, is able to close against its right atrial rims.
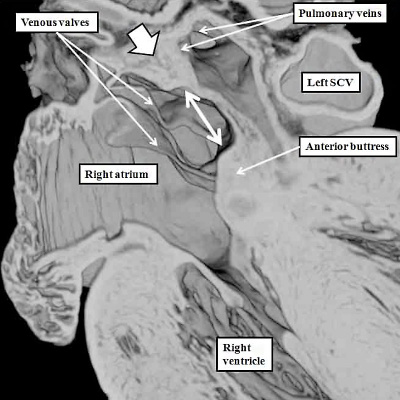
Fig. 16. The images are from human embryos, the left hand panel at CS20, and the right hand panel as CS21. The embryos are at the end of the seventh week of development. The pulmonary veins are migrating to the atrial roof, and as they do, a fold is produced between their atrial entrance and the right atrial structures (white arrows with black borders). By this time, the mesenchymal cap and vestibular spine have muscularised to form the inferior buttress of the atrial septum. The fold and buttress together form the right atrial rims of the oval foramen (double headed black arrow in right hand panel. The primary septum itself now forms the floor of the oval fossa. Abbreviations: SCV- superior caval vein; pulm. – pulmonary.
This situation is seen in the mouse heart just prior to term. A major difference, however, is that the pulmonary vein enters the mouse left atrium posteriorly, retaining its adjacency to the atrioventricular junction. The infolded rim of the fossa in the murine heart, therefore, is seen posteriorly (Figure 17), rather than cranially as in man (Figure 16).
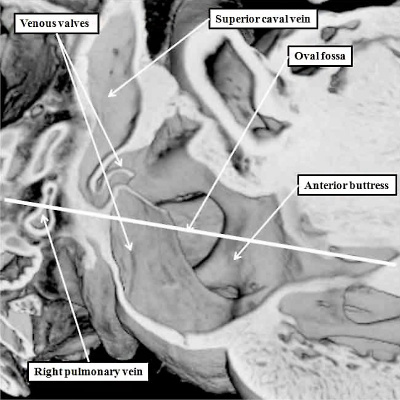
Fig. 17. The images are taken from an episcopic dataset prepared from a mouse embryo at E18.5, just prior to birth. The left hand panel shows the oval fossa as seen from the right side, with the venous valves obscuring its posterior margin. The right hand panel, which is a cross section along the white line in the left hand panel, reveals the infolding (white arrow with black borders) which forms the posterior rim of the fossa (double headed white arrow). The anterior rim is the buttress formed by muscularisation of the vestibular spine and mesenchymal cap. SCV-superior caval vein.
References
Cite this page: Hill, M.A. (2024, April 19) Embryology Detailed Cardiac - Superior Interatrial Fold. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Detailed_Cardiac_-_Superior_Interatrial_Fold
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G