Detailed Cardiac - Atrioventricular Cushions: Difference between revisions
(Created page with "==Mechanisms of Atrioventricular Septation== As we have already seen, obliteration of the primary atrial foramen requires growth of the vestibular spine by migration of tissu...") |
mNo edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Header}} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Online Editor | |||
|- | |||
|[[File:Mark_Hill.jpg|50px|left]] This content was contributed by [[Contributors#Prof_Robert_Anderson|Prof Robert H. Anderson]]. | |||
<br> | |||
{{Ref-Anderson2016}} | |||
Please note that the content is still at the early draft stage and will be the basis of updating the online cardiac development information with a more detailed description as shown by the page links below (shown in red if not yet existing). | |||
<br> | |||
{{Detailed Heart Links}} | |||
<br> | |||
{{Heart Links}} | |||
<br> | |||
Search: [https://www.ncbi.nlm.nih.gov/pubmed/?term=Anderson%20RH%5BAuthor%5D&cauthor=true&cauthor_uid=28011797 PubMed - Anderson RH] | |||
|} | |||
{{Detailed Heart Links}} | |||
=Fusion and Remodelling of the Atrioventricular Cushions= | |||
By [[Contributors#Prof_Robert_Anderson|Robert H. Anderson]] | |||
==Mechanisms of Atrioventricular Septation== | ==Mechanisms of Atrioventricular Septation== | ||
Latest revision as of 15:52, 18 February 2017
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Detailed Cardiac: Systemic Venous Sinus | Pulmonary Vein | Superior Interatrial Fold | Atrioventricular Cushions | Atrioventricular Canal | Interventricular Communication | Subpulmonary Infundibulum | Arterial Roots | Intrapericardial Arterial Trunks | Extrapericardial Arterial Channels | Sinus Node | Atrioventricular Conduction Axis |
Fusion and Remodelling of the Atrioventricular Cushions
Mechanisms of Atrioventricular Septation
As we have already seen, obliteration of the primary atrial foramen requires growth of the vestibular spine by migration of tissues into the heart through the right pulmonary ridge. This structure then combines with the mesenchymal cap to close the primary interatrial foramen, at the same time binding the atrial septum to the atrioventricular cushions. During this process, the atrioventricular cushions themselves must themselves fuse to divide the initially common atrioventricular canal seen during E11.5 (Figure 24 – left hand panel) into the mitral and tricuspid valvar orifices (Figure 24 – right hand panel).
Fig. 24. The images, from mice at E11.5 (left hand panel) and E12.5 (right hand panel) show how the vestibular spine and the mesenchymal cap fuse with the atrial surface of the atrioventricular cushions to divide the initially common atrioventricular canal (see also Figure 25) into the definitive right and left atrioventricular (AV) junctions.
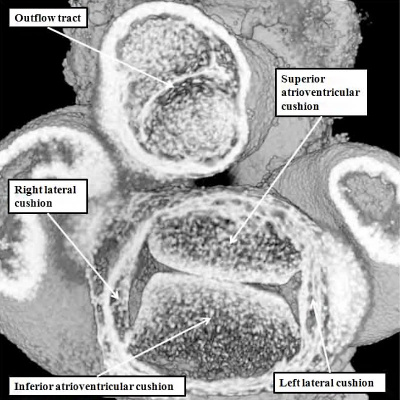
When assessing the developing heart in the so-called “four chamber projection” (Figure 24 – left hand panel), it is not immediately evident that, at E11.5, the major atrioventricular cushions have yet to fuse. The commonality of the canal at this stage is best appreciated when the heart is viewed in its short axis (Figure 25 – left hand panel). Images taken in the short axis then confirm that, at E11.5, the superior and inferior atrioventricular cushions have still to fuse along their facing surfaces. This process of fusion, along with the muscularisation of the vestibular spine and the mesenchymal cap, takes place during E12.5. Even subsequent to these stages, however, the aorta remains supported above the developing right ventricle. Then, although the inferior atrioventricular cushion has already fused dorsally with the crest of the muscular ventricular septum, thus obliterating the caudal component of the interventricular communication seen at E11.5, there remains of necessity a communication ventrally and cranially, so that the left ventricular blood is able to continue to flow into the aorta, and reach the developing brain (Figure 25 – right hand panel).
Fig. 25. The images are from mouse embryos at E11.5 (left hand panel) and E12.5 (right hand panel). Unlike those shown in Figure 23, these are sectioned to show the short axes of the atrioventricular junctions. At E11.5, as seen in the left hand panel, the superior and inferior atrioventricular cushions lie edge-to-edge, but have yet to fuse to each other. By the later stage (right hand panel – shown in a more oblique cut), the cushions have fused, but the aorta remains supported by the developing right ventricle. At this stage, therefore, the cranial and ventral part of the interventricular communication remains patent. This permits the blood entering the left ventricle to continue to reach the aortic root.
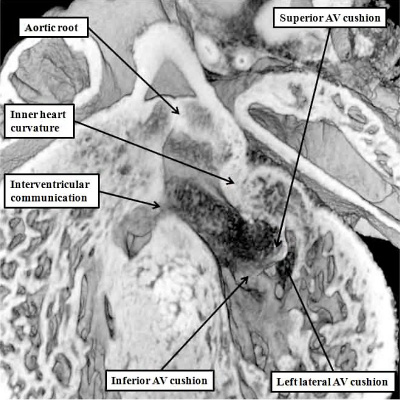
The completion of atrioventricular septation, therefore, cannot occur until the aortic root has been transferred from its initial location above the cavity of the right ventricle to its definitive position within the roof of the left ventricle. The transfer of the aortic root takes place during E13.5. Part and parcel of the transfer is the incorporation of the aortic root between the forming orifice of the mitral valve and the muscular ventricular septum. During this process, furthermore, there is expansion of the mural component of the developing mitral valve. The leaflet guarding this part of the mitral valvar orifice is derived from another cushion, itself formed leftward and laterally within the atrioventricular canal during E11.5 (Figure 25 – left hand panel). The process of fusion of the left ventricular components of the superior and inferior atrioventricular cushions has itself created the primordium of the aortic, or anterior, leaflet of the mitral valve (Figure 26).
Fig. 26. The images are from the same mouse heart at E13.5. The left hand panel, in the long axis, shows how the aortic root has become reorientated so as to be supported above the cavity of the left ventricle, although the interventricular communication remains patent. The superior and inferior atrioventricular (AV) cushions have now fused, and will eventually become the aortic, or anterior, leaflet of the mitral valve, which eventually achieves fibrous continuity with the developing leaflets of the aortic valve. At the stage shown in the left hand panel, however, the muscular inner heart curvature still interposes between the leaflets of the developing aortic and mitral valves. This is also well seen in the right hand panel, taken in short axis. The white dotted line shows the line of fusion between the left ventricular components of the superior and inferior atrioventricular cushions, with the white star showing the inner heart curvature. Note that, at this stage, the fused major cushions guard the larger part of the developing mitral valvar orifice, with the left lateral cushion being relatively small.
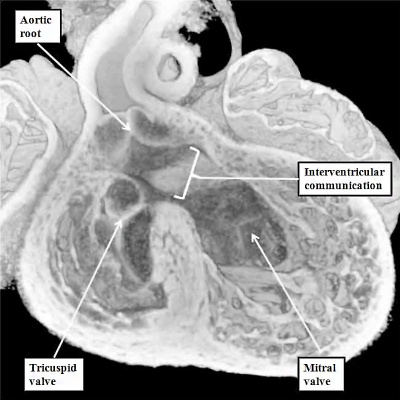
Subsequent to the fusion of the left ventricular components of the superior and inferior atrioventricular cushions to form the anterior component of the developing mitral valve, the valvar primordium derived from these components guards the greater part of the developing valvar orifice (Figure 25). As the aortic root is in the process of its transfer to the left ventricle, furthermore, the muscular inner heart curvature is interposed between the developing aortic and mitral valvar orifices within the roof of the left ventricle (Figure 25). This remains the situation after the persisting part of the interventricular communication is closed, completing the process of ventricular septation, and walling the aorta into the left ventricle (Figure 27).
Fig. 27. The images show how, at E14.5, when the aortic root is fully committed to the left ventricle, the persisting interventricular communication is closed by fusion of the rightward margins of the atrioventricular (AV) cushions (left hand panel). The right hand panel, which is from the same embryo but sectioned in short axis, shows how the left lateral atrioventricular cushion has expanded to form the mural leaflet of the mitral valve, which is now more extensive than the aortic leaflet, which is derived from the fused left ventricular components of the superior and inferior atrioventricular cushions (Compare with Figure 26). Note also that the aortic root is now fully wedged between the mitral valvar orifice and the muscular ventricular septum.
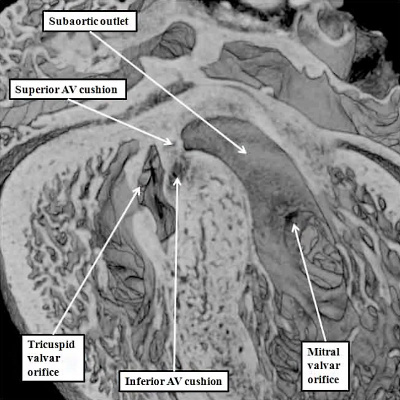
With closure of the interventricular communication, the aortic root itself is wedged more deeply between the mitral orifice and the muscular ventricular septum. Concomitant with the process of wedging, there is expansion of the leaflet of the mitral valve derived from the left lateral cushion formed within the atrioventricular canal (Figure 27 – right hand panel). At the stage of final closure of the interventricular communication, however, there has been no delamination of the septal leaflet of the developing tricuspid valve. This part of the tricuspid valve is formed from the larger parts of the rightward components of the superior and inferior atrioventricular cushions (Figure 26 – right hand panel), with smaller parts of the ventricular components of the cushions fusing together to close the embryonic interventricular communication (Figure 27 – left hand panel). As the atrioventricular junctions become separated, they also expand, with the mural leaflet of the mitral valve, derived from the left lateral cushion, eventually guarding two-thirds of the mitral valvar circumference. The right junction also expands, with the right lateral cushion forming the antero-superior and inferior leaflets of the tricuspid valve. This expansion of the right atrioventricular junction takes place inferiorly and caudally. The initial site of fusion of the cushions, reinforced on the atrial aspect by the site of fusion of the vestibular spine, then occupies a central location within the cardiac base (Figure 28).
Fig. 28. The images are from a mouse embryo at E15.5. Septation is now complete. The short axis cut, as seen in the left hand panel, shows how the right atrioventricular junction has expanded caudally and inferiorly, permitting formation of the septal, inferior, and anterro-superior leaflets of the tricuspid valve. The mitral valve now has aortic and mural leaflets, with the subaortic outlet interposed between the aortic leaflet and the septum. The star shows how the initial site of fusion of the cushions, the vestibular spine, and the mesenchymal cap now occupies a central location within the cardiac base. The four chamber section, taken along the plane showed by the dotted line, is illustrated in the right hand panel. The white double headed arrows show the separate nature of the right and left atrioventricular junctions. The septal leaflet of the tricuspid valve, however, has still to delaminate from the surface of the ventricular septum (white arrow with black borders).
It is the failure to separate the atrioventricular junctions that is the essence of the so-called “atrioventricular canal defects”. These are best considered as atrioventricular septal defects in the setting of a common atrioventricular junction. The situation is exemplified by the arrangement now identified in mouse embryos prior to term with the so-called “ostium primum” defect. Usually described as an “atrial” septal defect, the sections from the abnormal mice show that the essence of the malformation is retention of an atrioventricular septal defect, but with the leaflets derived from the major atrioventricular cushions fused to the crest of the muscular ventricular septum, so that shunting across the atrioventricular septal defect can take place only at atrial level (Figure 29).
Fig. 29. The images are from a mouse embryo at E18.5 with an “ostium primum” atrioventricular septal defect. The left hand panel shows a four chamber cut, revealing the persisting primary atrial foramen, with a common atrioventricular junction (double headed white arrow), but with shunting possible only at atrial level because the inferior atrioventricular cushion is firmly fused to the crest of the muscular ventricular septum. The mesenchymal cap has muscularised to form the leading margin of the oval fossa, but there has been no growth of the vestibular spine. The short axis cut from the same heart, shown in the right hand panel as seen from the ventricular aspect, reveals that the superior and inferior atrioventricular cushions have fused not only with the ventricular septum, but also with each other (white dotted line). The left atrioventricular valve now has three leaflets, retaining the initial figuration of the undivided atrioventricular canal (see Figure 25 – left hand panel). The aortic root is committed to the left ventricle, but is no longer wedged between the left atrioventricular valvar orifice and the muscular ventricular septum (compare with Figure 27 – right hand panel).
The arrangement as seen in the mouse embryo at E18.5 with the ostium primum defect then permits an understanding of the various types of “atrioventricular canal defect”. These lesions used to be thought of as representing failure of fusion of the major atrioventricular cushions during development. We now know that is not the case, since the cushions themselves have fused in the abnormal mouse so as to produce separate right and left valvar orifices within the common atrioventricular junction (Figure 29 – right hand panel). The lesion underscoring the formation of the defect is failure of growth of the vestibular spine, permitting us to pinpoint the timing of production of the defect to E11.5 in the mouse heart, and to Carnegie stage 17, or six weeks of development, in the human heart. It follows, therefore, that infants born with any variant of atrioventricular septal defect and common atrioventricular junction must have had their development disturbed prior to the beginning of the seventh week of normal development.
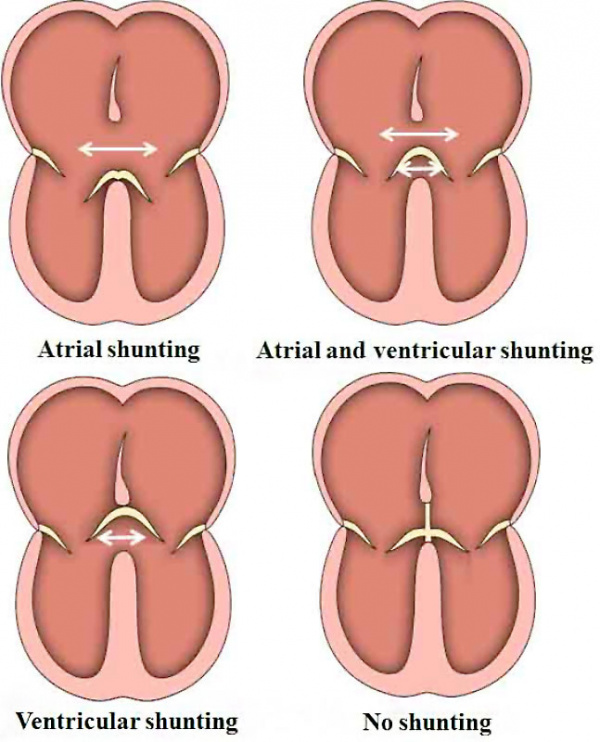
The various forms of atrioventricular septal defect with common atrioventricular junction are now recognized as representing the relationships between the bridging leaflets of the common atrioventricular valve, derived from the superior and inferior atrioventricular cushions, and the atrial and ventricular septal structures. When the bridging leaflets are attached to the crest of the muscular ventricular septum, as seen in the mouse heart with the “ostium primum” defect, then shunting across the atrioventricular septal defect can take place only at the level of the atrial chambers. In the commonest type of defect, however, the bridging leaflets are fused neither to the atrial nor ventricular septal structures, but are said to “float”. This means that shunting between the cardiac chambers can take place at both atrial and ventricular levels, with the extent of shunting reflecting the prevailing hemodynamic conditions, and the proximity of the floating leaflets to the septal structures. In the rarest type of defect, the bridging leaflets, rather than being attached to the crest of the ventricular septum, are tethered to the leading edge of the ventricular septum. This means that shunting can take place only at ventricular level. This parallels the situation seen typically in ventricular septal defects. Since the heart has a common atrioventricular junction, however, with a left atrioventricular valve having three rather than two leaflets, the lesion is properly described as the ventricular component of an atrioventricular septal defect (Figure 30).
Fig. 30. The cartoons show the variability in the structure of hearts with an atrioventricular septal defect and common atrioventricular junction depending on the relationship between the bridging leaflets of the common atrioventricular valve and the atrial and ventricular septal structures.
In all of the variants of atrioventricular septal defect with common atrioventricular junction, therefore, the problem during development must have occurred prior to separation of the common atrioventricular junction into its right and left components. This is not the case for patients having ventricular septal defects, although as we will see, there can be several phenotypic variants producing persistent interventricular shunting, including the lesion with a common atrioventricular junction. The more typical variants, however, have separate atrioventricular junctions. They too, nonetheless, can result from perturbations at different stages of normal development.
References
Cite this page: Hill, M.A. (2024, April 19) Embryology Detailed Cardiac - Atrioventricular Cushions. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Detailed_Cardiac_-_Atrioventricular_Cushions
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G