Detailed Cardiac - Arterial Roots
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
| Detailed Cardiac: Systemic Venous Sinus | Pulmonary Vein | Superior Interatrial Fold | Atrioventricular Cushions | Atrioventricular Canal | Interventricular Communication | Subpulmonary Infundibulum | Arterial Roots | Intrapericardial Arterial Trunks | Extrapericardial Arterial Channels | Sinus Node | Atrioventricular Conduction Axis |
Development of the Arterial Roots, with emphasis on Formation of the Leaflets and Sinuses
Development of the Ventricular Outflow Tracts
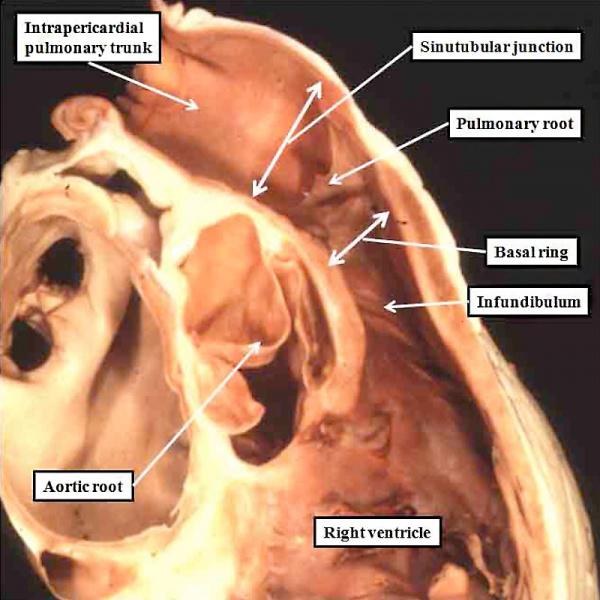
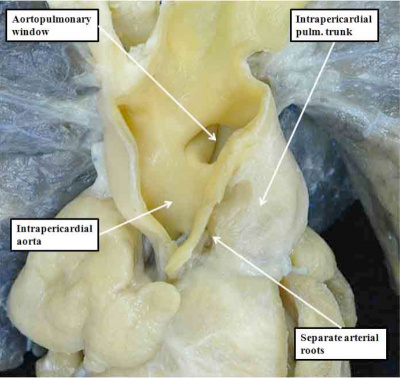
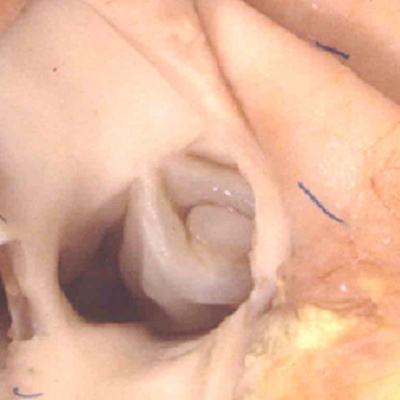
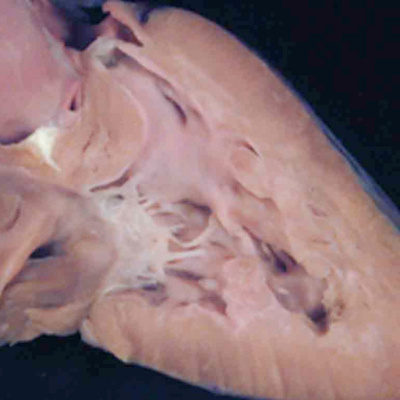
Understanding the development of the ventricular outflow tract, and relating the process of normal development to the congenital malformations that involve the outflow tract, is currently bedeviled by use of the term “conotruncal”. It is conventional wisdom that many, but not all, of the lesions involving the outflow tracts should be grouped under this banner. The use of the term itself reflect the suggestion made by Kramer, in a seminal work published in 1942, that the developing outflow tract could be considered in terms of two components, which he named the “truncus” and the “conus”. Currently, however, there is no consensus as to the boundaries of these two suggested components. The need for such definition becomes the more significant when we examine the postnatal outflow tracts, which possess three, rather than two, components. The normal arrangement is best seen in the right ventricular outflow tract, which is made up of the muscular subpulmonary infundibulum, the pulmonary root, and the intrapericardial component of the pulmonary trunk (Figure 36).
| Online Editor - Kramer 1942 |
|---|
| Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. (1942)
Amer. J Anat. 71(3): 343-370. |
Fig. 36. The image shows the outflow tract of the right ventricle in the postnatal heart, sectioned along its length. It has three discrete anatomic components. The intermediate component, made up of the pulmonary root, is limited distally by the sinutubular junction, and proximally by the basal ring created by joining together the nadirs of attachment of the pulmonary valvar leaflets. The outflow tract then has a subvalvar proximal component, the right ventricular infundibulum, and a distal component, the intrapericardial pulmonary trunk.
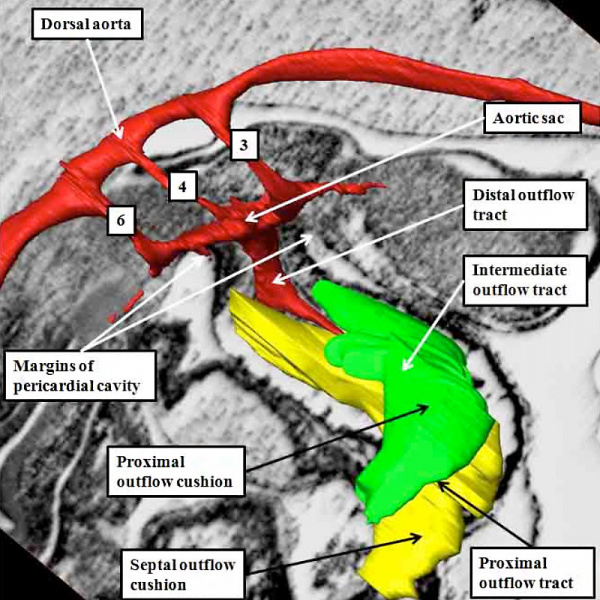
At the earliest stage of development, subsequent to looping of the heart tube (Figure 2), the developing outflow tract extends from the outlet of the primordium of the right ventricle to the margins of the pericardial cavity. The extent of the pericardial cavity is of particular value as an anatomic marker, since the cavity of outflow tract becomes continuous with that of the so-called aortic sac at this boundary. When reconstructed at this early stage of development, then the outflow tract, which has exclusively myocardial walls at this stage, also possesses three recognizable components. They are located in distal, intermediate, and proximal positions (Figure 37).
Fig. 37. The images shows a reconstruction of the developing heart in a human embryo at Carnegie stage 13, equivalent to four weeks of age subsequent to ovulation. The heart tube has already looped, with the outflow tract extending from the outlet of the developing right ventricle to the margins of the pericardial cavity, where its lumen becomes continuous with that of the aortic sac, shown in red. The reconstruction was made from an embryo in which myocardium was identified and coloured silver. The entirety of the outflow tract, at this stage, has myocardial walls, and is well described as possessing proximal, intermediate, and distal components.
The division of the outflow tract into three components according to its shape is more obvious in the human heart than in the mouse. Comparable reconstructions made in the mouse, nonetheless, at a slightly later stage, namely early on E11.5 (Figure 38), again reveal the three components of the developing outflow tract. The reconstruction also shows well how the solitary lumen of the distal outflow tract continues as the lumens of the arteries coursing through the segments of mesenchyme known as the pharyngeal arches. These arteries arise in symmetrical fashion from the aortic sac, passing round the trachea-esophageal pedicle to unify dorsally as the dorsal aorta (Figure 38).
Fig. 38. The image shows a reconstructed episcopic dataset from a mouse embryo early during E11.5. The reconstruction is viewed from the right side. The lumen of the outflow tract, and its continuation as the pharyngeal arch arteries, are shown in red. The right third, fourth, and sixth arch arteries are seen extending through the pharyngeal mesenchyme. They unite dorsally to form the dorsal aorta. The margins of the pericardial cavity mark the boundary between the distal component of the outflow tract and the aortic sac. By this stage, the myocardial walls have regressed minimally from the pericardial border, with the most distal walls of the outflow tract now formed by non-myocardial tissues. Note the cushions, derived from the cardiac jelly, which spiral through the intermediate and proximal part of the outflow tract. They end distally at the level of the regressing myocardial border. The so-called septal cushion is shown in yellow, with the parietal cushion colored green. The reconstruction was made by Dr. Simon Bamforth, and is reproduced with his kind permission.
At the stage of development shown in the mouse heart in Figure 37, which is early during the twelfth day of development (E11.5), new tissue has migrated from the heart-forming areas to enter the distal component of the outflow tract. The new tissue is non-myocardial. As it enters the heart, so the distal boundary of the initial myocardial walls regresses towards the right ventricle. As the myocardial border moves proximally, the spiraling cushions also regress. Eventually, the cushions occupy only the intermediate and proximal components, where they fuse to separate these parts into the arterial roots and the ventricular outflow tracts. The distal part of the outflow tract, in contrast, is separated into aortic and pulmonary components by growth of a protrusion from the dorsal wall of the aortic sac. The processes separating the outflow tract, and eventually placing the extrapericardial pulmonary channels in continuity with the right ventricle, and the systemic channels with the left ventricle, are complicated.
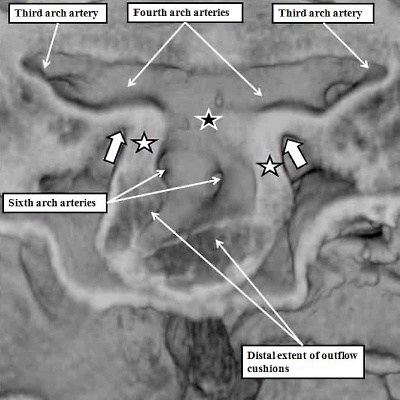
As we have seen, the outflow tract, when first formed, arises exclusively from the part of the ventricular loop that will become the right ventricle. The default option for the developing heart, therefore, is double outlet right ventricle (Figure 8B). We have also seen, nonetheless, that during the final part of ventricular development, the dorsal part of the proximal outflow tract is transferred to the left ventricle. This part becomes the subvalvar component of the aortic channel (Figure 32). To understand how this subvalvar component becomes connected to the systemic channels, we need to turn our attention to changes taking place earlier in development within the aortic sac. During the overall stages of development, there are five pairs of arteries that take origin from the sac, and extent symmetrically through the pharyngeal mesenchyme to join the dorsal aorta. Not all are present at the same time. Indeed, the first two pairs are relatively insignificant, and become transformed into small arteries within the head of the developing embryo. The significant sets are seen at the beginning of E11.5, as was shown in Figure 38. Of the three pairs of arteries recognisable at this stage, it is the third and fourth pairs which will become the systemic arterial channels. The right and left pulmonary arteries, by now also developing within the pharyngeal mesenchyme, take their origin from the undersurfaces of the sixth arch arteries. If we now examine the aortic sac at this stage, it can be seen that the systemic channels, namely the third and fourth arch arteries, arise from the cranial component, which the sixth arch arteries, feeding the pulmonary arteries, arise caudally from the base of the aortic sac (Figure 39, left hand panel). It is these caudal pulmonary channels that need to be connected, with ongoing development, with the right ventricle, leaving the left ventricle left supplying the systemic channels. One of the processes needed to achieve these connections is the regression of the right-sided dorsal channels extending between the aortic sac and the dorsal aorta (Figure 39 – right hand panel).
Fig. 39. The images show, to the left hand, a frontal section through the aortic sac of an embryonic mouse early on E11.5, and to the right hand, a reconstruction of the arch arteries seen frontally later on E11.5. The left hand panel shows how the distal walls of the distal outflow tract are now non-myocardial (white stars with black borders), the myocardial border being confluent with the distal extent of the outflow cushions. The black star with white borders shows the dorsal wall of the sac between the origins of the systemic third and fourth arch arteries and the pulmonary arteries, which arise from the sixth arch arteries. The right hand panel shows a later stage, when the distal outflow tract has divided into the aortic and pulmonary channels, and shows the beginning of regression of the right sixth arch artery. The pericardium surrounding the distal extent of the outflow tract is shown in green, with the newly formed intercalated cushions reconstructed in violet.
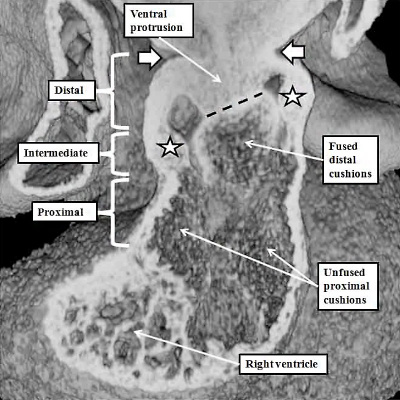
The process of separation of the channels within the distal outflow tract then requires growth of the protrusion from the dorsal wall of the sac. It grows obliquely into the distal outflow tract so as to separate the cranial origins of the third and fourth arch arteries from the caudal origin of the sixth arch arteries (Figure 40).
Fig. 40. The images show, in the left hand panel, how at E11.5 the growth of the protrusion from the dorsal wall of the aortic sac (black star in right hand panel of Figure 39) has grown ventrally into the cavity of the distal outflow tract, separating it into the intrapericardial aortic and pulmonary channels. The white arrows show the regressing myocardial border, which remains confluent with the ends of the distal outflow cushions. There is an embryonic aortopulmonary foramen at this stage of development. Later in day E11.5, as shown in the right hand panel, the protrusion fuses with the ends of the outflow cushions, which themselves have also fused by this stage. The processes of fusion divide the distal outflow tract into the intrapericardial aorta and pulmonary trunk, at the same time closing the embryonic aortopulmonary foramen. By this stage, additional cushions, known as intercalated cushions, have been formed within the intermediate part of the outflow tract (white stars). These cushions were also seen as the violet structures reconstructed in the right panel of Figure 39. Together with the major cushions, they will provide the primordiums for formation of the leaflets of the arterial valves. The cushions within the proximal outflow tract, in contrast, remain unfused at this stage.
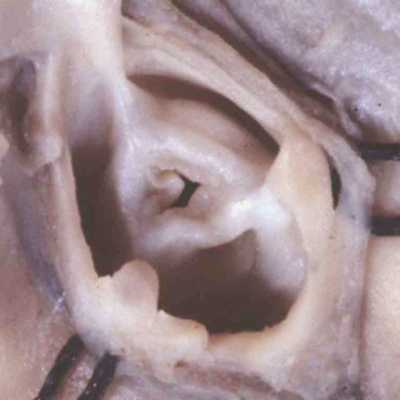
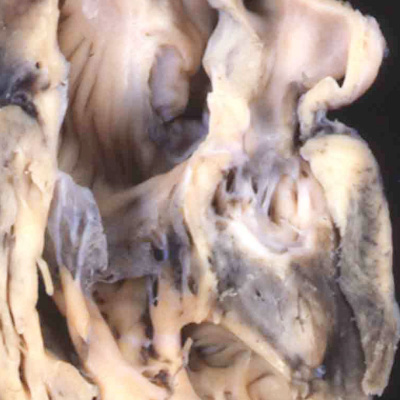
The fusion of the protrusion with the distal ends of the cushions, in combination with regression of the dorsal components of the initially bilaterally symmetrical arch arteries, has now connected the systemic extrapericardial channels with the right-sided and dorsal half of the distal outflow tract, leaving the pulmonary channels in continuity with the left-sided and ventral halft. It is then the spiraling configuration of the cushions within the intermediate and proximal parts of the outflow tract that places these distal channels in communication with the left and right ventricles, respectively. The fusion of the ventral protrusion from the dorsal wall of the aortic sac with the distal end of the outflow cushions also closes the embryonic aortopulmonary foramen (Figure 40). Should this process fail, but the cushions spiraling through the outflow tract succeed in fusing with each other, then there will be persistence of the foramen, producing the lesion known as an aortopulmonary window (Figure 41 – left hand panel).
Fig. 41. The images show the difference between aortopulmonary window (left hand panel) and common arterial trunk (right hand panel). The right hand panel shows an aortopulmonary window in a postnatal human heart . The intermediate and proximal cushions have fused to produce separate arterial roots, and separate ventricular outflow tracts, but the protrusion has failed to close the embryonic aortopulmonary foramen. The right hand panel is from a mouse genetically modified to perturb the Furin enzyme. The outflow cushions have failed to fuse throughout the length of the outflow tract. The distal outflow, nonetheless, has been separated into balanced intrapericardial aortic and pulmonary trunks by growth of the protrusion from the dorsal wall of the aortic sac (white arrow).
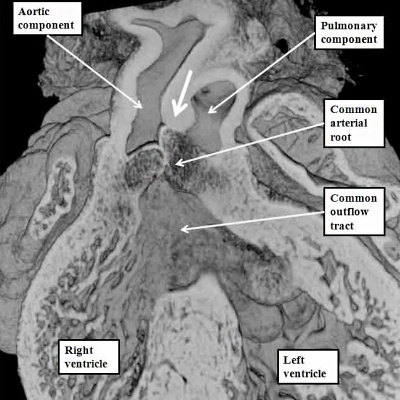
If, in contrast, the outflow cushions themselves have failed to fuse, then the end result is retention of a common arterial trunk. Both of these lesions can be related to abnormal separation of the components of the outflow tract. Common arterial trunk (Figure 40 – right hand panel) reflects failure of separation of the intermediate and proximal part of the tract. Separation of the distal aortic and pulmonary channels, which depends on the growth of the protrusion, is itself variable in the setting of the common trunk, serving to explain the variations known to exist with this lesion. The aortopulmonary window, in contrast, does require fusion of the intermediate and proximal cushions. The window, therefore, is likely to depend on an abnormality occurring at a slightly later stage than required to produce the common trunk. Both lesions imply abnormal development during the fifth and sixth weeks of human development, since the pulmonary and systemic components of the distal and intermediate parts of the outflow tract are normally separated by the beginning of the seventh week subsequent to ovulation.
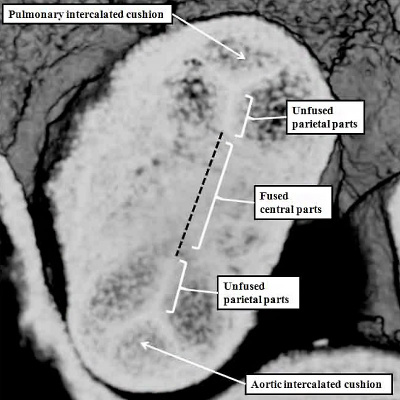
The growth of the new non-myocardial tissue into the distal outflow tract has effectively served to “push” the myocardial component, surrounding the outflow cushions, towards the right ventricle. This effective regression of the myocardial border is accompanied by the appearance of the intercalated cushions within the intermediate part of the outflow tract. The interdigitation of these newly formed intercalated cushions with the parietal ends of the major cushions then produces the scaffold for formation of the arterial valves. They appear during E11.5 (Figure 39 – right hand panel). By the end of E11.5, as we have seen, the central components of the major cushions have fused with each other, and also with the protrusion from the dorsal wall of the aortic sac (Figure 40 – right hand panel). By this stage, the cushions occupy the intermediate and proximal parts of the outflow tract. Within the intermediate part, it is only the central components of the major cushions that have fused. The peripheral parts remain unfused at their ends. It is the interdigitation of the intercalated cushions with these unfused margins that produces the scaffold for formation of the arterial valvar leaflets (Figure 42 – left hand panel). The leaflets are formed within the intermediate part of the outflow tract by excavation of the distal ends of the cushions (Figure 42 – right hand panel).
Fig. 42. The images show the stages involved in formation of the arterial roots within the intermediate component of the outflow tract. The left hand panel is a cross-section through the intermediate component at the end of E11.5. The central parts of the distal cushions have fused, but the parietal parts remain unfused. Together with the formation of the intercalated cushions, this sets the scene for formation of the leaflets of the arterial valves. This occurs during E12.5 and E13.5 by excavation of the distal ends of the cushions, as shown in the right hand panel for the pulmonary valve. At this relatively early stage, there has been minimal ingrowth of non-myocardial tissue to form the valvar sinuses, so that the distal myocardial border still remains adjacent to the tips of the developing valvar leaflets (white arrows with black borders). The sinuses continue to form subsequent to E13.5, which is the stage, in the mouse, of closure of the embryonic interventricular communication, equivalent to CS23, or eight weeks of development, in the human.
Ongoing migration of the non-myocardial tissues into the intermediate component then serves, over the next days of development, to produce the arterial walls of the valvar sinuses. It is the intermediate component of the outflow tract, therefore, that is the origin of the arterial valves and their supporting valvar sinuses. By the end of E12.5 in the mouse, the aortic and pulmonary roots will have separated one from the other, which is equivalent to six weeks after ovulation in the human embryo. If development proceeds normally, then as shown the ends of the major cushions will not have fused. Instead, they and the intercalated cushions will have cavitated so as to produce the leaflets of both arterial valves. Should the fusion of the peripheral margins of the cushions during these stages be excessive, or should one of the intercalated cushions fail to fuse, then the aortic or pulmonary valves will develop with two, rather than three leaflets. Such bicuspid valves are common congenital cardiac malformations, even though they may not produce symptoms until late in life. Excessive fusion of all three cushions during development then provides a similar explanation for so-called critical valvar stenosis, which can involve either the pulmonary or the aortic valves (Figure 43).
Fig. 43. The images show, to the left hand, a critically stenotic pulmonary valve, and to the right hand, a critically stenotic aortic valve. These lesions are well explained on the basis of excessive fusion of the distal outflow cushions and the intercalated cushion during the fifth or sixth week of development, or at any stage onwards to term. It is likely that the changes to produce the stenotic valves occur later in development, but the initial insult could occur during the fifth or sixth week.
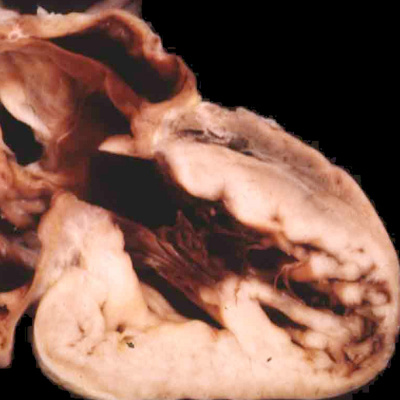
Since these valves, along with their supporting arterial roots, are formed within the central components of the developing outflow tracts, there is no valid reason to exclude them from the category of so-called “conotruncal” malformations. At the moment, however, they are not grouped in this fashion. Abnormal development during the fifth and sixth weeks can produce all the lesions that involve the arterial valves. Ongoing fusion of the valvar leaflets subsequent to closure of the embryonic interventricular communication, nonetheless, could provide an equally valid explanation. Lesions of the aortic or pulmonary valves, therefore, could represent abnormal development anytime after the sixth week of development in man. The lesions involving the arterial valves also include atresia. This again can involve either the aortic or pulmonary valve, and typically is found when the ventricular septum is intact. In the setting of the aortic valve, this produces the lesion known as “hypoplastic left heart syndrome”. This exists in two primary variations, one with stenosis of the mitral valve (Figure 44 – left hand panel), and the other with mitral atresia (Figure 44 – right hand panel).
Fig. 44. The images show the variations in ventricular morphology to be found in the hypoplastic left heart syndrome. The left had panel shows the small globular left ventricle, lined with fibroelastosis, as found in mitral stenosis with aortic atresia. The right hand panel shows the slit-like left ventricle, without fibroelastosis, found when there is mitral atresia and aortic atresia.
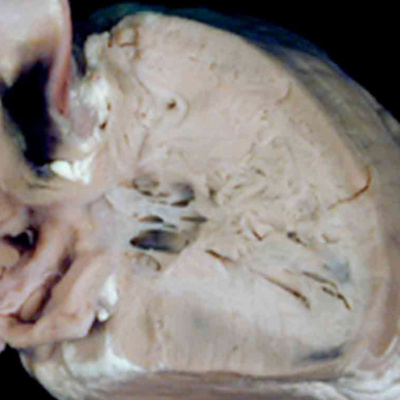
The differences in the two phenotypes point to the timing in development at which the abnormality was likely induced. In both variations, the ventricular septum is intact, implying that the lesion was produced subsequent to closure of the embryonic interventricular communication at the end of the eighth week of human development. The finding of mitral atresia, however, suggests that the initial insult occurred during maturation of the atrioventricular canal, during the fifth week of development. It is equally possible that an insult occurred at this early stage when aortic atresia is accompanied by mitral stenosis. Evidence now available from fetal echocardiographic studies, nonetheless, has proven that the heart can be normal at 12 weeks of development, and only subsequently progress to become part of the hypoplastic left heart syndrome. This situation is also the case when it is the pulmonary valve that is atretic in the setting of an intact ventricular septum. Such examples with hypoplasia of the right ventricle are comparable to the hypoplastic left heart syndrome. The evidence now available from fetal echocardiographic studies shows convincingly that these lesions are produced subsequent to the closure of the embryonic interventricular communication. Thus, the size of the right ventricle almost certainly reflects the timing of the abnormality, with the larger ventricles suggesting that atresia did not occur until perhaps the final trimester of pregnancy (Figure 45).
Fig. 45. The images show the right ventricle from two cases of pulmonary atresia with intact ventricular septum. In the left hand panel, the right ventricular cavity is no more that the inlet, with gross hypertrophy of the ventricular walls. In the right hand panel, the ventricular cavity shows well-formed inlet, apical, and outlet components, with less mural hypertrophy. The findings suggest development much later in gestation for the right hand lesion.
It cannot be denied with certainty, of course, that the initial insult had occurred prior to closure of the embryonic interventricular foramen. The anatomic evidence, nonetheless, combined with the fetal echocardiographic findings, indicates that the balance of probabilities favours an insult occurring after eight weeks of development, this being the timing of closure of the embryonic interventricular foramen.
The various forms of arterial valvar stenosis and atresia found with intact ventricular septum all depend on abnormal formation of the intermediate component of the outflow tract. Several more malformations can be explained on the basis of abnormal development of the proximal components of the outflow tract. We have already shown that the key feature in this regard is the transfer of the dorsal part of the outflow tract to the left ventricle. This process is a pre-requisite to permit closure of the embryonic interventricular foramen (Figure 32). As was also discussed during the explanation of this process, the closure of the foramen itself cannot take place until the proximal outflow cushions have fused. Subsequent to their fusion, the muscularised surface of the cushions is brought into alignment with the crest of the muscular interventricular septum, with closure of the foramen taking place during the eighth week of human development (Figure 32 – right hand panel).
The proximal components are the last parts of the spiraling cushions to close during normal development of the outflow tract. Proximal closure in the human begins during the seventh week of development, by which time the distal cushions have already closed to separate the aortic and pulmonary roots (Figure 46).
Fig. 46. The left hand panel shows a normal mouse embryo at E12.5. The intermediate cushions have already closed to separate the aortic and pulmonary roots. The proximal cushions, however, have yet to complete their fusion (white arrow with black borders). At this stage, furthermore, both arterial trunks retain their initial location above the cavity of the developing right ventricle. An abnormality at this stage will produce double outlet from the right ventricle. This can occur when the proximal cushions themselves remain hypoplastic, and the interventricular communication remains doubly committed, as shown in the right hand panel, which is from a mouse at E14.5, but genetically programmed by perturbation of the Furin enzyme.
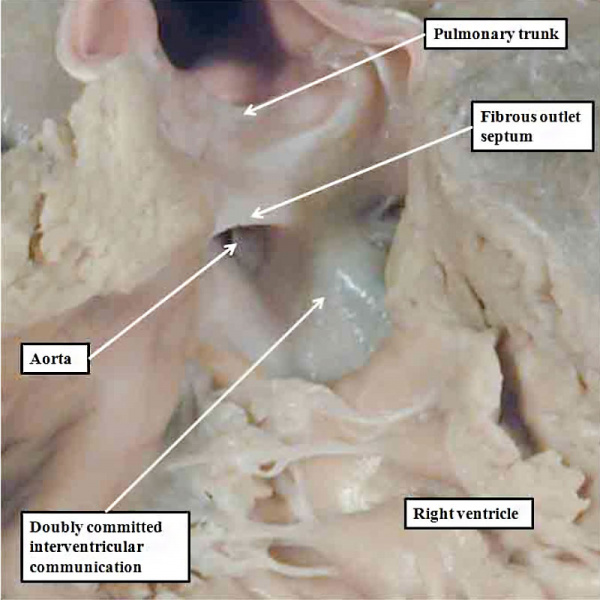
Should an abnormality occur at this stage, and the proximal cushion fuse, but fail to muscularise, then the arterial trunks will retain their location above the right ventricle, but the interventricular communication will be doubly committed, with a fibrous rather than a muscular outlet septum (Figure 47).
Fig. 47. The image shows a human heart with double outlet from the right ventricle, but with a doubly committed interventricular communication. The proximal outflow cushions have fused, but have failed to muscularise. Because of this, the outlet septum, derived from the fused cushions, is fibrous and hypoplastic. The arrangement is directly comparable to the lesion shown in the right hand panel of Figure 46.
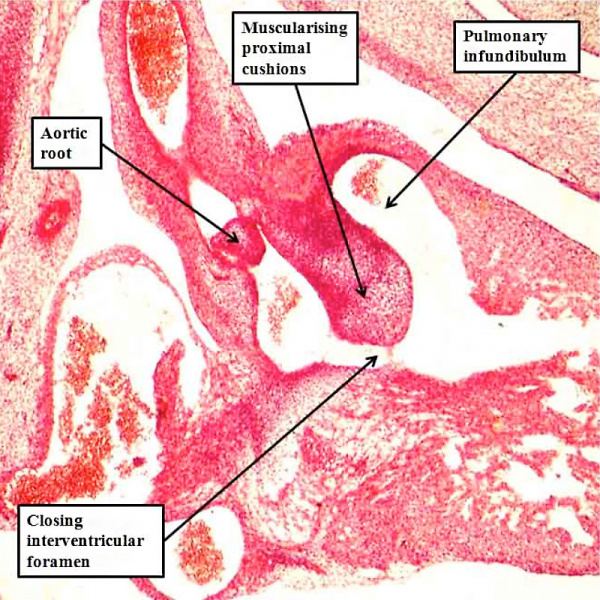
If development proceeds normally, the proximal cushions not only fuse, but also muscularise, with their surface then forming the free-standing subpulmonary infundibulum. It is the attenuation of the fused central component of the proximal cushions that eventually produces the extracavitary tissue plane that, in the postnatal heart, separates the pulmonary infundibular sleeve from the aortic root (Figure 36). The proximal cushions, during their development, nonetheless, begin the process of muscularisation before they are brought into line with the crest of the muscular interventricular septum. This process takes place during the eighth week of development in the human heart (Figure 48).
Fig. 48. The section is taken from a human embryo at Carnegie stage 20, in the eighth week of development, just prior to closure of the embryonic interventricular foramen. The proximal cushions have fused, and are beginning to muscularise. They are brought into alignment with the muscular ventricular septum as the aortic root is transferred to the left ventricle.
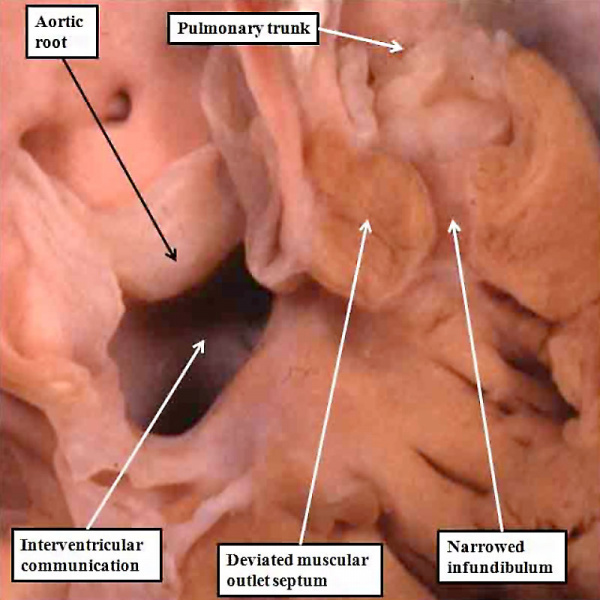
Should an abnormality occur around the stage of development shown in Figure 48, whilst the aortic root is being transferred to the left ventricle, but with the proximal cushions malpositioned within the outflow tract so that they obstruct the outlet to the pulmonary trunk, the resulting lesion is described as tetralogy of Fallot. The features of this lesion are an interventricular communication, overriding of the aortic root relative to the crest of the muscular ventricular septum, muscular obstruction of the subpulmonary outflow tract due to the malpositioned muscular outlet septum, and right ventricular hypertrophy. It is well accepted that the ventricular hypertrophy is an haemodynamic consequence of the embryonic malformation. The other three lesions are well explained on the basis of the abnormal location of the muscular outlet septum, derived by muscularisation of the proximal outflow cushions, coupled with an abnormal location of the parietal trabeculations formed with the right ventricle (Figure 49).
Fig. 49. The image shows the salient features of tetralogy of Fallot. The aortic root is overriding the crest of the muscular ventricular septum, in presence of a large interventricular communication. The muscular outlet septum is deviated antero-cranially, and is located exclusively within the right ventricle. Together with the hypertrophied trabeculations on the parietal wall of the right ventricle, it produces muscular subpulmonary obstruction. The walls of the right ventricle are hypertrophied, producing the fourth feature of the tetralogy.
There are several variable features found in hearts having the phenotypic feature of tetralogy, one of which is origin of both arterial trunks predominantly or exclusively from the right ventricle. This, along with the feature of the lesion shown previously with the doubly committed interventricular communication, show that double outlet right ventricle itself is no more than retention of the initial embryological situation, in which both arterial trunks began their development above the cavity of the developing right ventricle. There is, nonetheless, another variant of double outlet right ventricle that provides an important link to transposition, or discordant ventriculo-arterial connections. This is another of the lesions described under the banner of “conotruncal defects”, but better considered simply as a malformation of the outflow tracts. The essence of transposition, which can also be found in various phenotypic forms, is the presence of the aorta arising from the right ventricle, and the pulmonary trunk from the left ventricle. It is readily explained developmentally on the basis of straight, rather than spiraling, fusion of the outflow cushions (Figure 50).
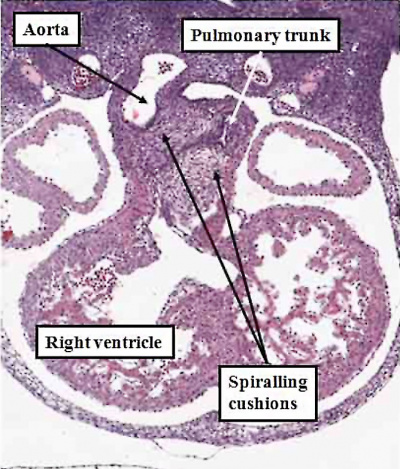
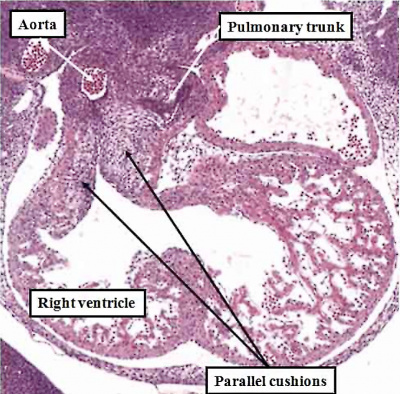
Fig. 50. The images, prepared from mouse embryos at E12.5, show the difference between a normal mouse (left hand panel), and a mouse that will develop with transposition (right hand panel). The mouse embryo shown in the right hand panel had been genetically modified by knocking out the Pitx2 gene. The outflow cushions are parallel, rather than being formed in spiraling fashion, as in the normal mouse.
Transposition in its various forms, therefore, can be explained on the basis of an abnormality occurring at or before E12.5 in the mouse heart, equivalent to the beginning of the seventh week of development in man. Subsequent changes can produce the variants, such as transposition with intact ventricular septum, or transposition with pulmonary stenosis. As can be seen from the right hand panel of Figure 49, in which the outflow cushions have fused in straight fashion, it is the pulmonary root, rather than the aortic root, which is closest to the embryonic interventricular communication. When development continues in this setting, it is then the pulmonary trunk that is transferred to the left ventricle, thus producing the transposition of the arterial trunks. Should, however, the pulmonary trunk retain its location above the morphologically right ventricle, then the end result will be yet another variant of double outlet right ventricle, specifically with a subpulmonary interventricular communication. This is the variant also known as the Taussig-Bing malformation.
With all these abnormalities, it is the location of the outlet septum, or its fibrous remnant, derived from the proximal cushions, and located within the proximal part of the outflow tract, which determines the presence of subaortic or subpulmonary obstruction. Excessive deviation of the outlet septum can produce pulmonary or aortic atresia, but then in association with a ventricular septal defect. These variants of arterial valvar atresia, therefore, require an abnormality to occur prior to normal closure of the embryonic interventricular communication, namely before the end of the eighth week of development of the human heart. Deviation of the outlet septum will, of necessity, obstruct the flow into either the pulmonary or the aortic outflow tracts, and hence reduce the flow into either the developing pulmonary or systemic circulations. In these situations, either the pulmonary arteries or the aortic arch will be hypoplastic. Hypoplasia of the aortic arch is described as coarctation, or in its extreme form is seen as interruption of the aortic arch. When found with an interventricular communication, these lesions reflect the timing of the ventricular lesion. Coarctation, and very rarely interruption, nonetheless, can also be found when the ventricular septum is intact. This then implies that the developmental abnormality occurred after closure of the embryonic interventricular communication, in other words subsequent to eight weeks after ovulation.
References
Cite this page: Hill, M.A. (2024, April 19) Embryology Detailed Cardiac - Arterial Roots. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Detailed_Cardiac_-_Arterial_Roots
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G