Cardiovascular System - Blood Development
Introduction
Blood develops initially within the core of "blood islands" in the mesoderm.
During development, there follows a series of "relocations" of the stem cells to different organs within the embryo.
In the adult, these stem cells are located in the bone marrow. At the time when blood first forms, there are no bones!
| Blood initially develops along with the blood vessels in which it will flow. Blood itself is considered as a form of "liquid conective tissue" consisting of a fluid and cellular component.
Stem cells that form blood cells (Hematopoietic Stem Cells, HSCs) change their location during development moving from tissue to tissue until their adult mbone marrow location is formed and populated. Angioblasts initially form small cell clusters (blood islands) within the embryonic and extraembryonic mesoderm. These blood islands extend and fuse together making a primordial vascular network. Within these islands 2 populations of cells exist: peripheral and core. The peripheral cells form endothelial cells while the core cells form blood cells (haemocytoblasts). Blood formation occurs later (week 5) throughout embryoic mesenchyme, then liver, then spleen/thymus, bone marrow, lymph nodes. |
Some Recent Findings
[1] Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter.] Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Nature. 2009 Jan 7.
"It is thought that HSCs emerge from vascular endothelial cells through the formation of intra-arterial clusters and that Runx1 functions during the transition from 'haemogenic endothelium' to Haematopoietic stem cells (HSCs). ...Collectively these data show that Runx1 function is essential in endothelial cells for haematopoietic progenitor and HSC formation from the vasculature, but its requirement ends once or before Vav is expressed."
(More? OMIM - Runt-Related Transcription Factor 1 - Runx1 )
An in vitro model of vasculogenesis and hematopoiesis in mouse has been used to identify a separate developmental pathway in which the angioblast lineage forms from mesoderm prior to and independent of hemangioblast development. This result differs from our current understanding where hemangioblasts are considered the common progenitors of cells in vessels and in blood. [2]"It is thought that HSCs emerge from vascular endothelial cells through the formation of intra-arterial clusters and that Runx1 functions during the transition from 'haemogenic endothelium' to Haematopoietic stem cells (HSCs). ...Collectively these data show that Runx1 function is essential in endothelial cells for haematopoietic progenitor and HSC formation from the vasculature, but its requirement ends once or before Vav is expressed."
[3]"Hematopoietic system involves sequential transfers of hematopoietic stem cells (HSCs) generated in the yolk sac blood islands, to successive hematopoietic organs as these become active in the embryo (fetal liver, thymus, spleen and eventually bone marrow). 4.5 day gap between appearance of the yolk sac blood islands and the stage of a fully active fetal liver. Avian studies identified yolk sac produce only erythro-myeloid precursors that become extinct after emergence of a second wave of intra-embryonic HSCs from the region neighbouring the dorsal aorta." (text modified from paper abstract)
[4]"In the 1960s a series of ontogenetic studies in birds and subsequently in mice revealed that hematopoietic and lymphoid development involved migration streams of primitive cells that colonized developing primary lymphoid organs as well as spleen, marrow, and liver. The yolk sac was proposed as the ultimate origin of these lympho-hematopoietic precursors. Subsequent studies identified a region associated with the dorsal aorta as the primary site of "definitive" stem cells. These opposing views are currently achieving a compromise that recognizes that both sites contribute stem cells involved in seeding the developing tissues." (text from abstract)
Fetal Blood Facts
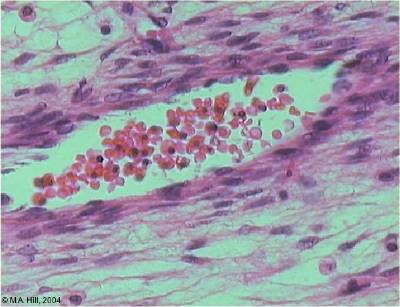 Fetal red blood cells (rbc) can also be identified by the presence of a nucleus that is absent in the adult red blood cell. Fetal red blood cells also contain a fetal haemoglobin which has different oxygen/carbon dioxide binding characteristics to adult red blood cell haemoglobin.
Fetal red blood cells (rbc) can also be identified by the presence of a nucleus that is absent in the adult red blood cell. Fetal red blood cells also contain a fetal haemoglobin which has different oxygen/carbon dioxide binding characteristics to adult red blood cell haemoglobin.
Maternal and fetal blood never mix, with exchange occuring across a number of membranes found in the placenta. (More? see [placenta.htm Placenta])
Haemolytic Disease of the Newborn (fetal erythroblastosis) is an immune problem arising from fetus Rh+ /maternal Rh-. Leakage of blood from fetus leads to maternal anti-Rh antibodies, which can then be dangerous for future pregnancies.
Red blood cells
Red blood cells (rbc) are the transporters of oxygen and carbon dixide in the blood.
When blood is centrifuged, the total % amount is known as the haemocrit. A low haemocrit or haemoglobin level leads to anemia (More? see [#Anemia Anemia]). Adult red blood cells contain no nucleus and have a limited lifespan. The lower oxygen tension at high altitudes leads to the body producing more rbc to compensate. (More? see [#Altitude Altitude])
White Blood Cells
White blood cells are a family of many different cell types that mediate many different functions including: immune defense, clotting, bacteria and virus destruction and cell debris scavanging.
These cells are not formed in the initial fetal bood and form much later in development.
Blood Progenitor Development
In the mouse, the yolk sac has an early important role in the provision of progeitor cells; before E8.0 all progenitors are found in the yolk sac, which remains enriched compared with the embryo from E9.5 to E10.5. (More? [../OtherEmb/mouse3.htm Mouse Heart])
4 to 8 somite stage (E8.25 - 8.5): small numbers of erythroblasts first enter the embryo (yolk sac-derived primitive erythroblasts)
26 to 30 somite stage (E10): 40% red cells steady state
Data from: [5]
(See also [6])
Anemia
The cut-offs for haemaglobin and haemocrit which are used to define anemia in people living at sea level:
(Data from- World Health Organization)
Altitude
The lower oxygen tension at high altitudes leads to the body producing more rbc to compensate. This means that people living at high altitudes have a higher haemocrit and/or haemoglobin level. This is also the reason why atheletes train at high altitude, to give them a higher gas carrying level when they return to sea level. This altitude effect on returning to sea level is gradually lost.
Alternately, this is also the basis of "altitude sickness" when people move rapidly from sea level to high altitude regions and their body has not yet been able to compensate.
Reading
- Human Embryology (2nd ed.) Larson Ch8 p189-228 Vasculature
- The Developing Human: Clinically Oriented Embryology (6th ed.) Moore and Persaud Ch14: p304-349
- Before we Are Born (5th ed.) Moore and Persaud Ch12; p241-254
- Essentials of Human Embryology Larson Ch8 p123-146 Vasculature
- Human Embryology Fitzgerald and Fitzgerald Ch13-17: p77-111
- [heart2.htm#References Additional References]
- Search [7]
- [8]
Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003 Mar 1;101(5):1669-76.
Search Pubmed Now
Click on the listed keywords below (used to search the external database) the most current references on Medline will be displayed.
[TITL+development[WORD]+review[WORD]&doptcmdl=DocSum blood[TITL]+development[WORD]+review[WORD]]
Cardiovascular Development Terms
- angioblasts- stem cells in blood islands generating endothelial cells
- angiogenesis- the formation of blood vessels also called vasculogenesis in the embryo
- anlage- (Ger. ) primordium, structure or cells which will form a future structure.
- atrial septal defects- (A.S.D.)
- blood islands- earliest sites of blood vessel and blood cell formation, seen mainly on yolk sac chorion
- branched villi- or terminal villi, grow from sides of stem villi, region of main exchange, surrounded by maternal blood in intervillous spaces
- cardinal veins- paired main systemic veins of early embryo, anterior, common, posterior.
- cardiogenic region- region above precordal plate in mesoderm where ceart tube initially forms.
- cord knotting- umbilical cord knotting occurs in 1%, prevents the passage of placental blood. pseudoknots also occur usually with no effect.
- cotyledons- on maternal face of placenta, form cobblestone appearance, originally placental septa formed grooves
- cytotrophoblast- extraembryonic cells of trophoblastic shell surrounding embryo, contribute to villi and placental membranes.
- decidua basalis-
- decidual reaction-
- ectoderm- the layer (of the 3 germ cell layers) which form the nervous system from the neural tube and neural crest and also generates the epithelia covering the embryo.
- endoderm- the layer (of the 3 germ cell layers) which form the epithelial lining of the gastrointestinal tract (GIT) and accessory organs of GIT in the embryo.
- endothelial cells- single layer of cells closest to lumen that line blood vessels
- extraembryonic mesoderm- mesoderm lying outside the trilaminar embryonic disc
- fetal erythroblastosis- see [#Haemolytic Disease Haemolytic Disease of the Newborn]
- haemocytoblasts- stem cells for embryonic blood cell formation
- Haemolytic Disease of the Newborn- fetal erythroblastosis, fetus Rh+ /maternal Rh-, fetus causes anti Rh antibodies, dangerous for 2nd child
- anastomose-
- chorionic villi- the finger-like extensions which are the functional region of the placental barrier and maternal/fetal exchange. Develop from week 2 onward as: primary, secondary, tertiary villi.
- estrogens- support maternal endometrium
- fetal drug addiction- occurs when drugs used maternally cross the placental barrier and can establish addiction in the unborn fetus.
- growth factor- usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg VEGF, shh)
- hCG- [#hCG see Human chorionic gonadotrophin]
- Human chorionic gonadotrophin- (hCG) like leutenizing hormone, supports corpus luteum
- Human chorionic somatommotropin- (hCS) or placental lactogen stimulate mammary development
- Human chorionic thyrotropin- (hCT) placental derived hormone equivilant to thyroid
- Human chorionic corticotropin- (hCACTH) placental derived hormone equivilant to
- maternal antibodies- immune molecules capable of crossing placental barrier
- maternal decidua- region of uterine endometrium where blastocyst implants. undergoes modification following implantation, decidual reaction.
- maternal sinusoids- placental spaces around chorionic villi that are filled with maternal blood. Closest maternal/fetal exchange site.
- mesoderm- the middle layer of the 3 germ cell layers of the embryo. Mesoderm outside the embryo and covering the amnion, yolk and chorion sacs is extraembryonic mesoderm.
- neural crest- cell region at edge of neural plate, then atop the neural folds, that remains outside and initially dorsal to the neural tube when it forms. These paired dorsal lateral streaks of cells migrate throughout the embryo and can differentiate into many different cell types(=pluripotential). Neural crest cells also contribute to major cardiac outflow vessels.
- patent ductus arteriosus- (P.D.A.)
- pharyngeal arches- (=branchial arches, Gk. gill) form structures of the head. Six arches form but only 4 form any structures. Each arch has a pouch, membrane and groove.
- placenta- (Gk. plakuos= flat cake) refers to the discoid shape of the placenta, embryonic (villous chorion)/maternal organ (decidua basalis)
- placenta accreta- abnormal adherence of placenta, with absence of decidua basalis
- placental arteries- paired, carry deoxygenated blood (from dorsal aorta) and waste products to the placental villi
- placental lactogen- see [#hCS Human chorionic somatommotropin]
- placenta percreta- villi of placenta penetrate myometrium
- placenta previa- placenta overlies internal os of uterus, abnormal bleeding, cesarian delivery
- placental veins- paired initially then only left at end of embryonic period, carry oxygenated blood to the embryo (sinus venosus)
- primary villi- week 2, first stage of chorionic villi development, trophoblastic shell cells (syncitiotrophoblasts and cytotrophoblasts) form finger-like extensions into maternal decidua.
- protein hormone- usually a protein distributed in the blood that binds to membrane receptors on target cells in different tissues. Do not easliy cross placental barrier.
- relaxin- hormone
- secondary villi- week 3, second stage of chorionic villi development, extraembryonic mesoderm grows into villi, covers entire surface of chorionic sac
- sinus venosus- cavity into which all major embryonic paired veins supply (vitelline, placental, cardinal)
- splanchnic mesoderm- portion of lateral plate mesoderm closest to the endoderm when coelom forms.
- stem villi- or anchoring villi, cytotrophoblast cells attached to maternal tissue.
- steroid hormone- lipid soluble hormone that easily crosses membranes to bind receptors in cytoplasm or nucleus of target cells. Hormone+Receptor then binds DNA activating or suppressing gene transcription. Easliy cross placental barrier.
- syncitiotrophoblast- extraembryonic cells of trophoblastic shell surrounding embryo, outside the cytotrophoblast layer, involved with implantation of the blastocyst by eroding extracellular matrix surrounding maternal endometrial cells at site of implantation, also contribute to villi. (dark staining, multinucleated)
- tetralogy of Fallot- Named after Etienne-Louis Arthur Fallot (1888) who described it as "la maladie blue". The syndrome consists of a number of a number of cardiac defects possibly stemming from abnormal neural crest migration.
- tertiary villi- third stage of chorionic villi development, mesenchyme differentiates into blood vessels and cells, forms arteriocapillary network, fuse with placental vessels, developing in connecting stalk
- frondosum-
- capsularis-
- electrolytes
- drugs-
- progesterone-
- umbilical cord-
- umbilical cord knotting- see [#cord knot cord knotting]
- vascular endothelial growth factor- (VEGF) protein growth factor family that stimulates blood vessel growth, a similar factor can be found in the placenta (PIGF).
- ventricular septal defects- (V.S.D.)
- virus- small infectious agent able to cross placental barrier. Can infect embryo and cause developmental abnormalities. (e.g. cytomegalovirus, rubella, measles)
- vitelline blood vessels- blood vessels associated with the yolk sac.
- waste products- products of cellular metabolism and cellular debris, e.g.- urea, uric acid, bilirubin
{Template:Glossary}}
Cite this page: Hill, M.A. (2024, April 25) Embryology Cardiovascular System - Blood Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Blood_Development
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G
- ↑ <pubmed>19129762</pubmed>
- ↑ <pubmed>16794034 Furuta C, Ema H, Takayanagi S, Ogaeri T, Okamura D, Matsui Y, Nakauchi H.] Discordant developmental waves of angioblasts and hemangioblasts in the early gastrulating mouse embryo. Development. 2006 Jul;133(14):2771-9.
Blood Stem Cells
A recent study in embryonic mouse development mapped the location of Hematopoietic stem cells (HSCs) during development. In the adult, blood cell formation is restricted to bone marrow, where a population of blood "stem cells" reside and differentiate into both red and white blood cells.
File:MouseHSC.gif Mouse hematopoietic stem cell locations (Image: Circulation and Chemotaxis of Fetal Hematopoietic Stem Cells Christensen JL, Wright DE, Wagers AJ, Weissman IL PLoS Biology Vol. 2, No. 3, e75 doi:10.1371/journal.pbio.0020075) Hematopoietic stem cells (HSCs) origins have been the source of some recent controversy, as to yolk sac and dorsal aorta contributions.
<ref><pubmed>19129762</pubmed>
- ↑ <pubmed>15906239</pubmed>
- ↑ <pubmed>15068689 </pubmed>
- ↑ <pubmed>12406884</pubmed>
- ↑ <pubmed>10529424</pubmed>
- ↑ <pubmed>medline.html PubMed- Medline]
Angiogenesis
- Introduction
- blood vessel formation
- vasculogenesis
- also occurs in adult and disease
- begins week 3 in extraembryonic mesoderm
- yolk sac
- connecting stalk
- chorion
- Growth Factors
- Vascular endothelial growth factor (VEGF), PIGF
- blood vessel formation
- Angiogenesis 2
- angioblasts form clusters - blood islands
- blood islands extend and fuse together
- forms a network
- 2 populations of cells
- peripheral- form endothelial cells
- core- form blood cells (haemocytoblasts)
- all vessels (arteries and veins) appear initially the same
- Blood formation
- blood formation occurs later (week 5)
- occurs throughout embryoic mesenchyme
- liver
- then spleen, bone marrow, lymph nodes
Blood flow through the Embryo
Maternal Blood | -> umbilical vein -> liver -> anastomosis -> sinus venosus -> atria ventricles-> truncus arteriosus -> aortic sac -> aortic arches-> dorsal aorta-> pair of umbilical arteries | Maternal Blood
This is shown on the [heart3.htm#Pig stage 13/14 pig G6 section].
References
- <ref><pubmed>10948449</pubmed>
- Introduction
- ↑ <pubmed>15094298</pubmed>
- ↑ <pubmed>12522770</pubmed>
- ↑ <pubmed>11563778</pubmed>
- ↑ <pubmed>10805260</pubmed>
- ↑ <pubmed>12406884</pubmed>
- ↑ <pubmed>10529424</pubmed>