Book - Vertebrate Embryology (1913) 9
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Jenkinson JW. Vertebrate Embryology. (1913) Oxford University Press, London.
- Vertebrate Embryology 1913: 1 Introduction | 2 Growth | 3 The Germ-Cells, their Origin and Structure | 4 The Germ- Cells, their Maturation and Fertilization | 5 Segmentation | 6 The Germinal Layers | 7 The Early Stages in the Development of the Embryo | 8 The Foetal Membranes of the Mammalia | 9 The Placenta | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter IX The Placenta
The placenta is that organ in which the blood-vessels of the embryo are brought into intimate anatomical and physiological relation with the spaces- which may be blood-vessels or lacunae of quite a different character- in which maternal blood is circulating. Though the apposition of foetal to maternal blood channels is very close, there is yet never any communication between the two ; an injection passed into the maternal mil not make its way into the foetal vessels, and conversely. At the same time the tissues that separate the two sets of channels are so thin that substances can readily travel by diffusion from the one to the other. In this way the embryo obtains its oxygen and probably food-stuffs, while by the same means it gets rid of its carbon dioxide and possibly of other waste products of its metabolism. The foetal blood is brought to the capillaries of the placenta by the allantois, which carries umbilical arteries and veins, while the maternal blood-supply is from the uterine vessels. The embryonic tissue which comes immediately in contact with the uterine wall is the trophoblast- the outer or ectodermal layer of the false amnion or chorion, - and it is the trophoblast which ensures the adherence of the embryo to the uterine wall and plays a part of conspicuous importance in the edification of the placenta, particularly in placentas of the so-called ' deciduate ' type.
In addition to the placenta - this organ formed by the trophoblast and vascularized by the capillaries of the allantois - the embryo has frequently other means of obtaining nutrition. Thus the trophoblast is often phagocytic- in early stages, before the allantois is developed, and in later stages in regions where it is not adherent to the uterine wall, the debris of dead maternal tissues and extravasated maternal corpuscles are devoured by it and passed on to the embryo inside. Again, in several forms, the yolk-sac with its absorptive epithelium and area vasculosa is instrumental in securing additional nutriment for the foetus. These processes we shall consider individually in the several groups.
Although it has been usual to separate the Eutheria as Placental Mammals from the Marsupials or Metatheria, it must yet be remembered that in the latter group there are arrangements by which the trophoblast is able to secure nourishment for the embryo from the walls of the uterus, which is handed on by means of the area vasculosa of the yolk-sac, and that in one case there is a true allantoic placenta, though it is of a peculiar type, not met with anywhere else.
The Marsupials thus stand apart in this as well as in other reproductive characters (the birth of the young in a very undeveloped condition, the large size of the egg, the presence of an egg-shell, the mode of segmentation, and the structure of the blastocyst), and we shall accordingly consider them separately.
The Marsupials
The yolk-sac, as we have seen, is large and its upper wall invaginated by the embryo. On this upper wall is an area vasculosa, which extends only a short way over the outer or lower wall, the greater part of the latter being directly in contact with the trophoblast.
In Didelphys the trophoblast opposite the area vasculosa of the yolk-sac is a columnar epithelium, thrown into folds. These folds fit into corresponding depressions m the uterine wall from which they appear to absorb nutrient material, which is then handed on to the vessels of the yolk-sac.
In Dasyurus the same region of the trophoblast is apphed closely to the uterine wall, and there is also beyond the limits of the area vasculosa a conspicuous annular zone of thickened trophoblast (Fig. 122, c). Cell-boundaries disappear and the syncytium so formed sends out pseudopodial processes which attack the uterine epithelium, grow in and enclose portions of it and the subjacent capillaries. The enclosed capillaries enlarge and maternal blood passes in between the trophoblast and the yolk-sac ; presumably it serves as food, for, as we shall see when we come to the Placentalia, maternal corpuscles are the source from which the embryo obtains its necessary iron.
In Perameles there is an allantoic placenta (Fig. 140). Where the trophoblast over the allantois touches the uterine wall the epithelium of the latter thickens to form a syncytium, from which processes grow down into the connective tissue ; the syncytium is soon invaded by maternal capillaries. Meanwhile the thin trophoblast has disappeared and the foetal capillaries of the aUantois, passing into the irregular depressions on the outside of the syncytium, are brought into fairly intimate relation with the maternal vessels.
Fig. 140.- Section through the placenta of PeromeZes. (After Hill.) all., allantoic epithelium ; m., mesoderm of allantois together with somatopleure of false amnion ; f.h.v., foetal blood-vessel ; ep.s., syncytium of uterine epithelium; m.6.u, maternal blood-vessels; c.«., subepithelial connective tissue.
At birth the allantois and its blood-vessels are left behind and absorbed by maternal leucocytes. This condition has been termed ' contra-deciduate '. The same fate befalls the syncytium.
The foetal tissues are similarly absorbed in Dasyurus.
The Placentalia
It has long been the custom to sharply distinguish two principal types of placenta from one another as the Indeciduate and Deciduate. In the former the connexion between foetal and maternal tissues is so slight that at parturition the first easily separate from the second, no maternal tissue is lost or 'deciduous', and the placenta is ' indeciduate '. In the other type, however, the union of foetal to maternal tissues was held to be so fast that at birth a considerable quantity of the latter was carried away by the former, and there was, in the language of a terminology which was invented when the histology of the placenta was not understood, a 'decidua'. This entirely erroneous conception of the structure of certain types of placenta (found, for example, in Rodents, Insectivora, and the human being), was based on the structure of the placenta in Ungulates. In the Ungulata, as was then well known, the * indeciduate ' placenta arises by the penetration of foetal (chorionic or trophoblastic) processes into crypts or depressions in the uterine wall, from v/hich crypts the processes or villi are readily pulled out at birth. Not unnaturally, in ignorance of the facts, it was surmised that the ' deciduate ' placenta originated in similar fashion, with the difference that the chorionic villi adhered so closely to the crypt walls that at birth they dragged away not only crypts but connective tissue and blood-vessels as well.
Thus the term ' decidua ' came to be applied to the tissue of the uterine wall, whether an embryo and placenta were present or not.
Now while it is true that the placenta of the Carnivora is developed in this kind of way, modern research has conclusively shown that in the majority of the so-called ' Deciduates ' the genesis of the placenta proceeds on an entirely different plan. If, therefore, we retain the name ' deciduate ' for the placenta of the Rodents, Insectivora, Cheiroptera, and some Primates {Tarsim, Monkeys, and Man), it must be on the distinct understanding that the word bears its original meaning no longer. The term ' indeciduate ' is not inapplicable to the Ungulate placenta, and there is no objection to its use.
We shall begin with the Ungulate as exhibiting structurally the simplest type.
Ungulata
In Ungulata the placenta is of the indeciduate form. At the surface of the chorionic sac there are produced finger-shaped processes or villi, formed of a single layer of trophoblast, and provided with a core of mesodermal tissue in whieh are the foetal capillaries. The endodermal epithelium of the allantois is not continued into the villi. These villi fit into depressions in the wall of the uterus known as crypts. The crypts are hned by an epithelium which is perfectly continuous with the ordinary epithelium of the uterus and persists throughout gestation. The persistence of the uterine epithelium is the real mark by which the indeciduate is distinguished from other placentas. Below the epithelium of the crypts are the maternal capillaries and connective tissue. The villi do not adhere closely to the crypt wails, and at birth are easily removed without damage to the maternal tissues.
Fig. 141. - Diagram of a foetal and maternal cotyledon of the cow. all., allantoic epithelium ; ir., trophoblast ; v., villus ; e'p., uterine epithelium continued into crypt ; c.w., wall of crypt. The maternal connective tissue is shaded.
The Ungulate Placenta may be Diffuse, or Cotyledonary, or of an intermediate type. In the first the whole surface of the chorionic sac is covered uniformly with villi which may be simple (as in the pig) or branched (as in the horse) (Fig. 131). In the cotyledonary placenta the villi are gathered together into bunches or cotyledons, the intervening regions of the chorion being smooth (Fig. 141). The villi - which are much branched - fit into crypts of a corresponding shape, the whole aggregation of crypts for the reception of the villi of a single cotyledon being termed a maternal cotyledon. The points in the wall of the uterus where these maternal cotyledons will be formed are predetermined and recognizable as raised areas - the cotyledonary caruncles - ^before gestation, can be seen indeed in the uterus of the unborn calf. The foetal cotyledons are scattered all over the surface of the chorion, except at its extreme ends, the ' diverticula allantoidis ' so called.
A cotyledonary placenta is characteristic of the Ruminants.
In some cases (Cervus, Giraffa, Oreas, Tetraceros) tho placenta is of an intermediate type, simple villi being found between the cotyledons.
As examples of indeciduate placentation we may take the cow and sheep.
Before describing the anatomy and physiology of the actual placenta it will be convenient first to consider the changes that take place in the wall of the uterus preparatory to the reception of the embryo, as well as the nutrition of the embryo while it is still free in the uterine cavity.
In the period known as the ' pro-oestrus ', which precedes • heat or 'oestrus', the subepithehal connective tissue of the uterus becomes h5rpertrophied, while the capillaries increase in number and become enlarged. Numbers of corpuscles - both haematids and leucocytes - are now extra vasated from these swollen blood vessels into the surrounding stroma of connective tissue, where many of the haematids are devoured and digested by leucocytes with the resultant deposition of pigment in the cytoplasm of the latter. This brown jjigment, derived from the haemoglobin of the extravasated corpuscles, may remain in the wall of the uterus for a considerable time. Meanwhile, as a result possibly of the pressure exerted by the congested capillaries, the uterine epithelium has given way ; patches of it degenerate and are cast into the uterine lumen along with some debris of subepithelial cells, haematids, and leucocytes. The fluid in the uterus already contains proteid, glycogen, and fat secreted by the uterine epithelium and glands. In this fat-secretion the outer ends of the cells, containing fat-globules, are nipped off and ejected. There are also present (in the sheep) rod-like or needleshaped bodies, composed of an albuminous substance and secreted by the epithelium. Iron, too, is found, derived from the digested haemoglobin of the extravasated haematids. To all this must be added the products of the cellular secretion of the glands (Fig 142) Small tracts of the epithelial wall become invaginated into the gland-lumen, are cut off, degenerate, and are thrown out by the mouths. The secretion of fat and proteid, of the albuminous rod-shaped bodies and of cell-masses by the glands, is not confined to the period of ' pro-oestrus ' but occurs throughout gestation.
Fig. 142.- Cellular secretion in the glands of the viterus. a, horse (after Kolster) ; B, clog (after Bonnet). In A a piece of the epithelium is being invaginated into the lumen of the gland. In B this has been nipped off. In A the secretion of fat (black globules) and pieces of cells is also shown.
The material thus provided is of a thick, viscid consistency and of a yellow colour, like pus, and is known as ' uterine milk ' . It is of the greatest importance for the nutrition of the embryo.
Ovulation or the escape of the ovum from the Graafian follicle occurs in ' oestrus ' : the ovum passes into the Fallopian tube, where fertihzation takes place. Development begins and the blastocyst enters the uterus. Here the trophoblast at once begins to absorb the nutriment prepared for it. The cells are phagocytic and ingest solid particles of the uterine milk : they also absorb fat and possibly iron.
The blastocyst next becomes attached by its trophoblast to the uterine wall, and the placenta is formed.
The uterine epithelium, where destroyed, has now been restored. In the cotyledonary caruncles it is continued into the crypts, which are now developed. If we may judge of what happens now by what is known of the manner in which accessory maternal cotyledons are formed in the later stages (in the cow), the crypts 'arise (Fig. 143) by a pitting of the columnar cihated epithelium, the cells which are at the bottom of the pits becoming shorter than the ordinary columnar cells around {a), followed by the outfolding of the epithelium between the pits (6) ; into these folds connective tissue soon penetrates (c), and later blood vessels.
Villi or finger-shaped processes of the trophoblast are now formed and enter the crypts. The epithelium lining the latter soon becomes modified, the cilia are lost, and the cells become cubical (d, e) (in the cow) or very flat (in the sheep). Even in the latter case, however, small patches of cubical cells remain, from which fresh crypts are formed by downgrowth into the subepithelial tissue (Fig. 144).
Fig 143. - a-e. Five stages in the formation of a crypt in tlie cow. a-c, pitting and folding of the epithelium; cl, the epithelium becomes cubical ; e, the cilia are lost.
Fig. 144. - Formation of accospory crypts in tlto sheep. In a and & the (lowngrowth of epithchuni i.s still solid ; in c it i« becoming hollow ; in e it is open to the old crypt.
With continued development the villi and crypts elongate and branch repeatedly, and the maternal cotyledon is raised above the level of the uterine wall (Fig. 141). The free surface is convex in the cow, but deeply concave in the sheep, where also the base of attachment is constricted to a narrow stalk.
In the cow the trophoblast covering the villi is composed of rounded or cubical elements, amongst which are gland-cells and curious oval binucleate cells (found also in the sheep). The core of each villus is occupied by connective tissue (somatopleure of the false amnion plus splanchnopleure of the allantois) and foetal capillaries, the latter very close to the epithehal cells (Fig. 145, 1).
The crypt is lined by cubical cells which secrete fat and proteid, the ends of the cells with the contained fat-globules being protruded, pinched off, and thrown into the space between crypt and villus. Fat can be demonstrated in the trophoblast, which doubtless absorbs the proteid also. The gland-cells may be of importance in this respect.
Below the epithelium in the crypt-walls are maternal connective tissue and maternal capillaries. The foetal and parental blood-streams are thus separated by the endothelium of the foetal capillaries, some connective tissue (not always), the trophoblast, the epithelium of the crypts, the cormective tissue, and the endothelium of the maternal vessels. Through these layers oxygen diffuses from maternal to foetal blood, and carbon dioxide in the reverse direction ; other substances may also pass. In the cotyledons, therefore, the respiratory exchange takes place and the absorption of fat and proteid.
It is, however, not merely by means of its cotyledonary villi that the embryo obtains nutrition. At the bases of the villi and therefore opposite the summits of the walls between the crypts the trophoblast is very tall and columnar (Fig. 145, 2). The outer ends of the cells are pseudopodial and ingest quantities of cell-debris and maternal red blood-corpuscles. The capillaries at the summits of the crypt-walls are gorged, blood is extravasated, and together with the remains of epithehal and subepithelial cells eagerly devoured by the trophoblast, and digested.
The ingested haematids get clumped together in the cells, and often surrounded by a food-vacuole (Fig. 145, 3). As intra
Fig. 145. - Histology of the placenta in the cow and sheep.
- Foetal and maternal tissues in a cotyledon, tr., trophoblast of a villus ; the cells are absorbing fat (black). In the trophoblast are two binucleate cells. Behind the trophoblast are the connective tissue and Tilood-vessels of the allantois. ep., uterine epithelium lining a crypt. Fat secretion is going on, the ends of the cells with fat-globules being pinched off and thrown into the lumen of the crypt. Below the epithelium are the maternal capillaries and connective tissue.
- Columnar trophoblast cells from between the bases of the cotyledonary villi. The cells are full of ingested matter (corpuscles, nuclear, and cell debris).
- Ingestion of extravasated maternal corpuscles by the trophoblast in the sheep. The corpuscles are seen inside the cells. The cells also contain pigment.
- Deposition of pigment after digestion of the haemoglobin of ingested corpuscles in trophoblast cells of the cow. The pigment-granules (black) are seen to be deposited on irregular masses in the cells. cellular digestion proceeds little granules of yellow-brown pigment appear on the surface of the mass, and gradually the whole assumes the same coloiar (Fig. 145, 4). The pigment probably contains no iron, at least when digestion is completed, the iron of the haemoglobin having been separated and carried o£E to the embryo by the blood of the allantois. Thus the foetus obtains its necessary iron in this as in earlier stages from the haemoglobin of extravasated maternal corpuscles, devoured by the trophoblast. The pigment remains in the trophoblast, where large quantities of it are accumulated by the end of gestation. In neutral solution it shows two bands very, nearly in the position of those of oxyhaemoglobin, in acid solution two bands almost exactly in the position of those of acid haematoporphyrin, but in alkaline solution not the four bands of alkaline haematoporphyrin, but only the two seen in the neutral solution. It is probably related to haematoporphyrin : it is certainly a haemoglobin derivative, and from it bile-pigments may be formed.
We have already had occasion to notice the curious roimded or elongated, often flattened bodies, sometimes soft, sometimes hard and brittle, found floating in the allantoic fluid and familiar for many centuries under the title of ' hippomanes '.
In the cow they are white or pale yellow, in the sheep a dirty brown. In the sheep they are formed by local accumulations of the viscid uterine milk, which get into pockets of the trophoblast, between the cotyledons. Gradually, pushing the trophoblast and allantois in front of them, they make their way into the cavity of the latter, in which they lie attached by a stalk to the wall ; the stalk narrows and breaks, and they are free in the cavity. At first they are surrounded by a membrane - the remains of their covering of allantois and trophoblast - and are soft : they are composed of a granular coagulable material, full of cell-detritus, degenerating nuclei, globules of fat and glycogen, and leucocytes. Later the membrane disappears, and the bodies become hard by being saturated with calcium oxalate in the form of ' envelope ' crystals. In the cow, when outside the chorion and still soft, they are a bright orange colour, due to the presence of bilirubin, doubtless derived from the extravasated corpuscles eaten by the trophoblast ; they are, indeed, found at the bases of the villi, just where these extravasations occur.
Large allantoic bodies impregnated with calcium oxalate are found in the horse.
Small quantities of glycogen are found in the uterine epithehura and subepithelial tissues, and in the uterine milk. Much larger quantities are found stored up in the amniotic thickenings - ^masses of stratified epithelium on the inner surface of the amnion. Towards the end of gestation the glycogen diminishes, and the cells undergo fatty degeneration and are impregnated with calcium oxalate. As the glycogen diminishes the dextrose in the amniotic fluid increases (from 1 % to 0-37 %).
Glycogen also occurs in the trophoblast, in the connective tissue of the chorion and in the umbilical cord round the blood-vessels and allantoic epithelium. In the body of the embryo it is abundant in all tissues, except in the liver, where it only appears late, when it is disappearing from the others.
The glycogen in the amniotic bodies appears to be a reserve store. We shall find a similar storage of glycogen in other cases.
Besides dextrose the amniotic fluid contains albumin, mucus, and chlorides of sodium and potassium.
In the allantoic fluid are dextrose (0-3 %), albumin, mucin, magnesium, sodium, and calcium phosphates, sodium chloride and sulphate, and ' envelope ' crystals of calcium oxalate ; further, a yellow pigment, and allantoin, the embryonic representation of urea.
It appears, therefore, that the allantois is a receptacle for the waste products of foetal metabolism.
Cetacea. In Orca the placenta appears to be indeciduate and diffuse, uniformly studded with villi. The chorionic sac extends into both corrnua of the uterus. The villi, which are branched, are only absent at the ends, opposite the Fallopian tubes, and again opposite the os uteri,
Sirenia. Halicore possesses an indeciduate, diffuse placenta. It is known that the uterine epithelium persists in the crypts. The villi, which are slightly branched, are limited to an annular area surrounding the chorionic sac, not qmte in the middle of the latter. When the region of the trophoblast, which enters into such intimate relations with the uterus as to form a placenta, is of this annular shape, the placenta is spoken of as zonary. (A zonary placenta may be of the deciduate type.)
Hippomanes are found in Hcdicore, but there are no amniotic bodies.
Proboscidea. In the elephant the shape of the placenta is zonary, though diffuse villi occur at the ends of the chorionic sac. These villi appear to be of the nature of those found in Ungulates. In the zonary region the villi appear to have become embedded in the wall of the uterus by their ends, while maternal blood is extravasated between their bases. In the absence of more exact information this placenta cannot be properly classified. Brown pigment abounds in the trophoblast of the villi, presumably a haemoglobin derivative.
Hyracoidea. In Hyrax the placenta is zonary in shape, with villous patches at the poles.
Edentata
The placenta is stated to be bell-shaped in Myrmecophaga and Tatnandua, zonary in Orycteropus, oval in Dasypus, diffuse in Manis and Choloepus, but we have no knowledge of its minute structure.
Lemuroidea
In this, the lower division of the Primates, the placenta is of the diffuse indeciduate type (except in Tarsius, which must certainly be placed with Monkeys and Man).
In Galago (Fig. 146) the chorionic sac is large and occupies both horns of the uterus. It is covered with short simple villi at the extremity of each of which is a sUght pit, the cells of which contain granular greenish masses (? haemoglobin derivatives). The villi lie in grooves lined by a persistent epithelium, from which they are easily pulled out. The chorionic vesicles are invaginations of the trophoblast opposite depressions in the uterine wall at the bottom of which glands open. Both chorionic vesicles and depressions are filled with a granular material - uterine milk - which appears to be absorbed by the villi which spring from the floor of the vesicle.
Fig. 146. - Placenta of the lemur Galago. (After Strahl.)
A, Section of a villus with the crypt in which it is lodged. The uteruie epithelium (ep.) lining the crypt persists ; m.h.v., maternal blood-vessel ; all, allantois epithelium ; f.b.v., foetal blood-vessel ; tr., trophoblast.
B, Section through a chorionic vesicle and the opposed depression of the uterine wall ; tr., trophoblast ; v., small branching vilh protruding into the chorionic vesicle ; gl., glands opening into the uterine depression ; m.h.v., maternal blood-vessels.
In the Carnivora we meet with a group which is from the comparative anatomical point of view of the greatest importance, since the placenta here holds an intermediate position between the Indeciduate and Deciduate (so-called) types. While in the disappearance of the uterine epithelium it must be ranked with the latter, it differs widely from them in the fact that the channels in which the blood of the mother circulates are the capillaries of the uterine wall, between which the trophoblast has penetrated after the destruction of the superficial epithelium. In this mutual apposition of foetal vessels and maternal vessels the Carnivorous does indeed resemble the Ungulate type, from which it may conceivably have been derived, and comes very near to fulfilling the original definition of a ' deciduate ' placenta, since at birth the maternal vessels and connective tissue are removed along with the foetal constituents. The shape of the placenta is always zonary. The genera most carefully investigated are the dog and cat.
We begin with a description of the processes preparatory to the reception of the embryo.
In the period of pro-oestrus prior to ' heat ' the uterus becomes swollen and hjrperaemic owing to the multiplication and enlargement of the blood-vessels and capillaries. Blood is extravasated first into the subepithelial tissue, and masses of brown pigment appear, as the result, presumably, of the digestion of the haemoglobin by the abundant leucocytes. Soon the superficial columnar epithelium gives way, and quantities of haematids with a certain amount of destroyed epithelial and connective tissue are discharged into the lumen of the uterus.
The uterine glands are long and twisted, and branch ; they apparently secrete some proteid material and masses of cells in the way already described in the Ungulata (Fig. 142). In addition there are the crypts, short tubular downgrowths of the epithelium.
During the following period of oestrus a regeneration of the destroyed epithelium takes place. Should fertilization have occurred the blastocyst is developed and makes its way into the uterus, in the placental regions of which the following changes now occur.
While the blastocyst is still unattached fat appears in the superficial epithelium of the uterus, and in that of the necks of the glands and crypts. The necks of the glands widen, so giving rise to the ' spongy ' layer. A thick layer of dense subepithelial tissue is formed, in which run the capillaries. The surface epithelium next becomes lower, while its nuclei begin to degenerate, and eventually the whole epithelium disappears. The openings of the crypts and glands are closed by masses of enlarged epithelial cells which, uniting to form syncytia, soon show signs of degeneration (Fig. 147, a).
Attachment now occurs. In the zonary placental region the trophoblast is produced into finger-shaped villi - which may be solid or provided with a core of mesoderm - and these villi make their way into the connective tissue from which the epithelium has now been removed, as well as into the plugs of degenerating syncytia closing the mouths of the crypts and glands. To these syncytia are added the cellular secretions of the glands.
The cells of the trophoblast are phagocytic and devour all this detritus. Where the trophoblast invades the connective tissue between the crypts and gland it comes into intimate relation with the capillaries there, and as soon as the villi have been penetrated by the foetal blood-vessels the placenta may be said to have been established (Fig. 147, b).
Below the placenta are the wide parts of the glands, separated only by thin lamellae of connective tissue in which run the larger blood-vessels. By these lamellae the placenta is attached to the muscularis.
The placenta so formed is at first thin, but soon grows in thickness by the simultaneous elongation of the trophoblastic villi and of the connective tissue which covers them and includes the maternal capillaries (Fig. 147, c). The villi meanwhile branch, the branches being thin sheets (perpendicular to the surface) and radiating out from the original villi : the foetal blood vessels of course branch correspondingly, as do, on the other side, the maternal connective tissue and capillaries. In the cat certain large cells are present in between the maternal capillaries which are possibly hypertrophied connective tissue-cells, but they may be trophoblastic (Fig. 148). The trophoblast at the base of the placenta continues to ingest and absorb the celldebris and fat supplied by the glands up to the end of gestation.
A feature of great physiological interest is the ' green border a system of pockets in the trophoblast along both edges of the placenta filled with masses of extravasated maternal blood, to which extravasation indeed the formation of the pockets is due (Pig. 147, d). Leucocytes are present, fibrin, and a green pigment (haematochlorine), a derivative of haemoglobin ; what its relation is to biliverdin is not known. There is also a yellow brown pigment, and, at the end of gestation, a black one. All this material is ingested by the trophoblast. The green border is poorly developed in the cat.
It will be evident that the placenta we have just considered is made of a compound tissue, foetal in the trophoblast and connective tissue and capillaries of the allantois, maternal in the connective tissue and capillaries surrounded and engulfed by the invading vilH.
The placentas we have still to study are not constructed on this plan, for though they have this much in common with that of the Carnivora, that the uterine epithelium disappears, yet they differ wholly from it in that the maternal blood circidates not in blood-vessels enclosed by the trophoblast but in lacunae, excavated in that tissue, into which extravasated maternal blood is poured. No maternal tissue, therefore, is lost at birth except the blood, apart from fragments of connective tissue adherent to the maternal side of the placenta.
A placenta of this kind is found in the Rodents, Insectivora, Cheiroptera, and, amongst the Primates, in Tarsius, Monkeys, and Man. We shall begin with the Rodents.
Rodentia
The placenta is always discoidal in shape, and attached to the mesometric side of the uterus.
As an example we may take the mouse. The uterine cavity is bounded by a columnar epithelium in which fat is secreted. Into it open glands with long necks. These secrete a coagulable, presumably proteid, material. These secretions are absorbed by the free blastocyst (Figs. 149, A ; 150, a). There is prepared for the reception of the blastocyst a pit on the antiinesonietric side of the uterus. This pit lies in the middle of a pronounced swelling, due to the hyi)ertroj)hy of the subepithelial connective tissue and enlargement and multiplication of the blood-vessels. To this tissue may be applied Hubrecht's term ' trophospongia '. By it the glands are driven away towards the muscularis, their necks stretched and eventually broken. The mouse produces a large litter of young, and there is a correspondingly large series of these swellings along the uterus. The pit in the middle of each swelling is open freely to the main cavity of the uterus (towards the mesometrium), and in each pit a blastocyst is lodged with its embryonic pole towards the opening of the pit. It is at this pole that the trophoblast will thicken to form the placenta, which is therefore on the mesometric side (Figs. 149, b ; 150, b).
Fig. 148. - Histology of the placenta of the cat. a, Earlier ; b, later stage (full time), f.c.t., foetal connection tissue ; tr., trophoblast (pale) ; m.c.t., maternal connective tissue (dark) ; f.h.v., foetal capillary ; m.b.v., maternal capillary.
Where the trophoblast touches the sides of the pit the epithelium, clothing the latter, now disappears, the cells becoming cubical, then flat, and finally vanishing. The nuclei are resolved into spherules of chromatin, the cytoplasm undergoes fatty degeneration. The fat is absorbed by the trophoblast. The trophoblast is thus brought into immediate contact with the subepithelial tissues.
The same degradation later attacks the epithelium at the bottom of the pit, and later still extends to that lining the main uterine lumen above it. This lumen then disappears and each embryonic pit is isolated, as also are the inter-embryonic regions of the viterus. At a subsequent stage a fresh lumen will be formed on the anti-mesometric side of the embryo, and this re-unites the inter-embryonic regions with one another and once more there is a continuous uterine lumen.
At the embryonic pole the trophoblast now thickens and drives the embryonic knob towards the opposite end, so mvagmating the upper wall of the yolk-sac ; the amnion is then developed and separated from the temporary cavity in the trophoblast as the extra-embryonic coelom extends between the two in the fashion already described.
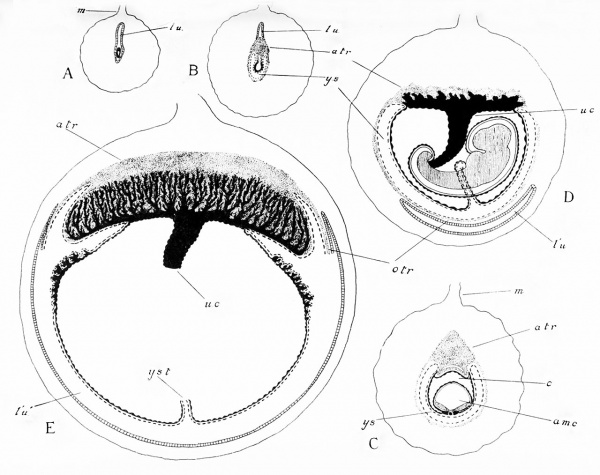
Fig. 149. Five stages in the formation of the placenta in the mouse. A, The blastocyst free in the uterus. B, The blastocyst attached and the placental thickening of the allantoidean trophoblast developed (a.tr.). C, Later stage, after elosure of the amniotic cavity (am.c.). D, The foetal blood-vessels beginning to penetrate the allantoidean trophoblast. E, Elaboration of the placenta. Disappearance of the distal Wall of the yolk-sac and omphaloidean trophoblast (o.tr.).
This thickening is the precursor of the placenta. It extends towards the mesometrium and is at first conical (Figs. 149, c ; 150, c), but soon becomes discoidal as the embryo in its amnion and extra-embryonic coelom enlarge. It is in contact with the distended uterine capillaries, and very quickly these burst and the extra vasated maternal blood is poured into irregular spaces or lacunae excavated in the trophoblast. Many of the haematids are phagocytically devoured by the trophoblast (Fig. 151, 8). The blood enters these spaces in the centre, leaves them by a number of wide vessels at the periphery. At its base this trophoblast remains cellular, but elsewhere it becomes syncytial by the disappearance of cell-boundaries ; the two regions have been termed respectively cyto- and plasmodi-trophoblast. Between the blood-vessels that supply these trophoblastic lacunae is the subepithehal connective tissue (Fig. 151, 7), and this soon undergoes an important modification. While some of the cells remain unaltered - ^fusiform or stellate in shape - as a supporting tissue, others become rounded and filled with globules of glycogen. The cells, though fairly closely packed, are distinct from one another. The nucleus is spherical, not very chromatic, and has one nucleolus. We shall speak of this tissue as the maternal glycogenic tissue (Fig. 151, 4). It is at about the zenith of its development by the time the foetal blood-vessels reach the placenta.
The future placental region of the trophoblast may be distinguished as ' allantoidean ' from the ' omphaloidean ' region, which lies immediately against the distal wall of the yolk-sac and therefore on the anti-mesometric side. The cells here become enormously hypertrophied and theix nuclei correspondingly enlarged (hence the term 'megalokaryocytes') : in the nuclei there are large nucleoli, and the chromatin is in irregular strings. They are incapable of mitosis. In contact with the subepithehal tissues they eagerly devour debris of degenerate cells, leucocytes and the haematids, which are abundantly extravasated in this region also (Fig. 151, 6, 9). They apparently play an important role in the nutrition of the embryo during this stage, prior to the development of the allantois, but later they are less important and disappear long before the end of gestation. Presumably the stuffs they have digested are passed on by means of the area vasculosa of the yolk-sac to the embryo.
Fig. 151. - Histology of the placenta of the mouse.
1, Foetal capillaries (with nucleated corpuscles) lying alongside the lacunae of the trophoblast (stage e).
2, 3, Early and late stages of glycogenesis of the trophoblast (stages E and later).
4, Maternal glycogenic cells with intervening connective tissue-cells (stage d).
5, Fold of epithelium on the proximal wall of the yolk-sac with bloodvessel (stage E).
6, Megalokaryocyte from the omphaloidean trophoblast. On the right extravasated maternal corpuscles, on the left the flat epithelium of the distal wall of the yolk-sac (stage d).
7, Closely packed maternal sub-epithelial tissue with blood-vessels (stage c).
8, Allantoidean trophoblast, the cells ingesting maternal corpuscles (stage c).
9, Melagokaryocyte cf the omphaloidean trophoblast ingesting corpuscles and detritus of maternal cells (stage c).
In the next stage (Figs. 149, d ; 150, d) the allantois is developed, grows with its blood-vessels across the coelom, reaches the so'matopleure at the base of the allantoidean trophoblast, and sends its capillaries into the latter in between the lacunae. The necessary relation between the foetal and maternal circulations which constitutes a placenta is now established. Further change is mainly one of growth.
Firmly fixed in the trophoblast the capillaries soon elongate and branch, mostly parallel to one another and perpendicular to the surface of the placenta. The trophoblast with its lacunae keeps pace, and so the whole organ, attaining a thickness many times greater than that which it originally possessed, comes ultimately to project button-like towards the centre of the uterus (Figs. 149, e ; 150, b). The trophoblast lining the lacunae becomes finally much attenuated except for the protrusions due to the rather large nuclei (Fig. 151, 1).
On the foetal side of the placenta are somewhat large lacunae to which blood is brought by chaimels passing directly through the centre of the placenta ; hence it passes into the smaller lacunae round the foetal capillaries and so into the efferent maternal vessels which leate the organ peripherally. The capillaries of the allantois, however, never penetrate the whole thickness of the trophoblast. On the maternal side there is a layer, increasingly broad, between the ends of the foetal vessels and the maternal tissues, a layer only traversed by the large channels which lead to and from the smaller lacunae (Fig. 150, e). In this layer the secretion of glycogen begins at the stage when the allantois has just reached the trophoblast, and soon attains enormous dimensions (Fig. 151, 2, 3). The whole tissue consequently appears highly vacuolated. The cells - ^if we may indeed speak of cell-boundaries - are oblong, the nuclei oval, rich in chromatin and provided with several nucleoli, thus differing from the maternal glycogen cells. We shall speak of this as the trophoblastic glycogenic tissue.
The previously differentiated maternal glycogenic tissue ceases to grow further, mth the enlargement of the whole uterus the constituent cells get separated, the glycogen cells having given up their glycogen collapse, disintegrate, and disappear, and only the supporting cells are left between the maternal blood-vessels. Upon the space so left vacant the trophoblastic glycogen tissue encroaches, engulfing the blood-vessels as it does so, and finally extends as far as the muscularis.
There can be no doubt that this tissue holds in reserve a store of food material for the use of the embryo . As sugar the glycogen passes into the maternal vessels and into the lacunae, and so is absorbed by the foetal capillaries. When the glycogen is used up the cells collapse, and their collapse may be a factor in determining the moment of parturition, since it is across this layer that the placenta breaks away. The trophoblastic is much more voluminous than the maternal glycogenic tissue ever was.
In the omphaloidean regions important changes have meanwhile occurred. A new lumen has been formed on the antimesometric side, placing the inter -placental portions of the uterus once more in communication with one another. This new lumen (Fig. 149, d, e, I'.u') is separated from the cavity of the yolk-sac by (1) the distal wall of the yolk-sac, (2) the omphaloidean trophoblast, (3) the subepithelial tissues, and (4) the epithelium. All these layers cease to grow, become passively stretched, and finally rupture, disintegrate, and disappear.
The yolk-sac now opens freely into the uterine lumen, and the richly folded columnar epithelium (Fig. 151, 5) of the upper wall is able to absorb the fat and proteid material secreted by the uterine epithelium and glands. Thus the yolk-sac acts and continues to act till the end of gestation as an accessory organ of nutrition. It also forms a protective envelope, since its edge is inserted into the margin of the placenta. This edge is l^ter carried up some little way on the outer surface of the placenta, the base of attachment of the latter to the uterine wall being narrowed, while at the same time the yolk-sac is inflected on the foetal side towards the insertion of the umbilical cord.
In a placenta of this type the foetal is only separated from the maternal blood by the endothelium of the capillaries and the trophoblastic lining of the lacunae, the foetal connective tissue being in the last stages negligible. There is no penetration of foetal tissues into maternal (except for the encroachment of the glycogenic tissue of the trophoblast on the space between the maternal blood-vessels), and there is no maternal tissue in the organ but the blood in the lacunae (except again the blood vessels in the glycogenic region). The relation between maternal and foetal blood-streams is brought about by the fastening of the trophoblast upon the subepithelial tissues after the destruction of the uterine epithelium ; once fixed there lacunae are excavated in it in which extravasated maternal blood circulates, and it is finally vascularized from the foetal side by the capillaries of the allantois.
In the guinea-pig {Cavia) the blastocyst is placed in a pit on the anti-mesometric side ; it comes into contact with the subepithelial tissues by burrowing beneath the epithelium, which is then destroyed. The original lumen of the uterus is obliterated in the embryonic swellings ; a new lumen is formed anti-mesometrically, and the tissues between it and the upper wall of the yolk-sac are distended and disintegrate, thereby placing the yolk-sac in contmuity with the uterine cavity, precisely in the way akeady described for the mouse, except that the lower wall of the yolk-sac has never been present. The placenta is discoidal and mesometricaUy placed ; it is developed from a thickening of trophoblast at the embryonic pole of the blastocyst. On its maternal side is an abundant glycogenic tissue, but whether this is of maternal or foetal origin, or both, has not been determined.
In the rabbit and squirrel no anti-mesometric pit is formed for the reception of the blastocyst. In the rabbit there are on the mesometric side two prominent folds, the placental folds, and in the future embryonic regions these become greatly thickened by the proliferation of the subepithelial tissue and blood-vessels (trophospongia). They have been termed 'cotyledons ', but the expression is here inapplicable. To these two swellings the blastocyst attaches itself by the trophoblast behind and at the sides of the embryonic plate ; the latter is at the surface when Rauber's cells have disappeared, but sinks inside when the amnion closes (Fig. 152).
The uterine epithelium, where touched by the trophoblast now disappears, and the latter is brought into immediate contact with the subepithelial tissue and blood-vessels. The blood vessels are to a very slight extent engulfed by the growing trophoblast, but their endothelial walls soon break down and their extravasated blood is discharged into lacunae excavated in the trophoblast, now much thickened and syncytial (plasmodi trophoblast), except at its base, where cell-bomidaries remain (cyto-trophoblast). The allantoic capillaries then make their way into the trophoblast and the placenta is established.
FiG. 162. - Foetal membranes and placenta of the rabbit, pr.am., proamnion. Other letters as before. (Aiter Duval and Van Beneden.)
The trophoblast with its lacunae and the foetal tissues grow pari passu ; the placenta thus increases in thickness and projects into the uterine cavity. In shape it is discoidal, but made up of two distinct halves or lobes, due to the attachment of the trophoblast to the two enlarged placental folds.
There is a voluminous glycogenic tissue on the maternal side, stated to be entirely of maternal origin. A good deal of it is, however, probably trophoblastic. It has been shown that the glycogen of the placenta increases up to the twenty-first day of gestation, but then diminishes till the end (twenty-ninth day). The glycogen in the foetal liver, which is at first almost negligible, increases rapidly during the last week of pregnancy. A glycogen splitting ferment has also been isolated from the placenta ; it is found, too, though less active, in the overlying maternal tissues. In the placenta, therefore, the embryo has a means of controlling the glycogen metabolism ; but this function is taken on by the foetal liver towards the close of gestation. The yolk sac in these forms also is an accessory organ of nutrition. The lower wall disappears, the cells of the upper wall then absorb material from the uterine cavity, and pass it on to the embryo by means of the area vasculosa.
Cheiroptera
In Vespertilio there is a discoidal placenta, or rather, since it is concave, saucer-shaped or bell-shaped (Fig. 153).
The blastocyst attaches itself by its embryonic pole to the anti-mesometric side of the right cornu of the uterus : only one is present at a time.
Below the epithelium the connective tissue has thickened, and the blood-vessels have increased in number and size. The uterine epithelium disappears, and the trophoblast then fixes itself by invading the subepithelial tissue and engulfing some of the superficial capillaries. The endothelium of these capillaries then degenerates, and they are indistinguishable from the lacunae formed in the way with which we have become familiar in the Rodent placenta.
The blood-vessels of the yolk-sac are at first applied to this mass of trophoblast, but as soon as the allantois is developed it pushes the yolk-sac away and sends its capillaries into the trophoblast. The placenta increases in thickness by the simultaneous growth of capillaries and lacunar trophoblast, and in area by an extension at the edges of the same process by which it was formed. After the first fixation there is no further penetration of the maternal by the foetal tissues.
Fig. 153.- Foetal membranes and placenta of the bat (V espertilio). (After Nolf.) Letters as before.
On the anti-embryonic side (mesometric) the uterme epithelium also disappears, the fatty debris, together with that of the underlying connective tissue, being eaten up by the trophoblast.
In Pteropus the placenta is discoidal but mesometric : the uterine epithelium seems to disappear.
Insectivora
In this order the placenta is again discoidal, and usually concave ; but in Tupaia there are two placentas, one right, the other left, at the sides of the uterus, and in Cenietes a large niimber. Where there is only one {Erinacells, Sorex, Taljia) it is anti-mesometric in position.
In all cases the uterine epithelium disappears in that region where the placenta is formed : the thickened trophoblast fastens on the subepithelial tissues, and lacunae are formed in it ; in
Fig. 154. - Two stages in the formation of the decidua reflexa of the hedgehog. (After Hubrecht.) d.r., decidua reflexa. Letters as before.
these the maternal blood circulates. The whole is then vascularized from the foetal side by the allantoic capillaries.
In Erinacells, the hedgehog, the most interesting feature is the formation of a ' decidua reflexa ' or ' capsularis ' resembling the structure known by that name in human embryology. ^ On the anti-mesometric side of the uterus there are formed two thick folds by the proliferation of the subepithelial vascular tissue (trophospongia). Between these two folds the blastocyst is lodged with its embryonic pole turned away from the mesometrium (Eig. 154, a). By the closure of the lips of the folds and obliteration of the cavity in front and behind this point the blastocyst is securely shut up in a coat of maternal tissue, the ' decidua reflexa ' (Fig. 154, b). The whole of the trophoblast now thickens enormously, becomes syncytial, destroys and devours the epithelium lining the cavity which lodges it, while into the lacunae hollowed out in it quantities of maternal blood are soon discharged from the adjacent swollen capillaries. The
- It is highly probable, however, that the human ' reflexa ' is formed in a different manner. (See below.)
Fig. 155. - Foetal membranes and placenta of the hedgehog. (After Hubrecht.) l.u., lumen uteri ; d.r., decidua reflexa. Other letters as before.
yolk-sac and omphaloidean trophoblast, against which its lower wall lies, are at the anti-embryonic end, that is, towards the covering ' decidua reflexa ', while towards the opposite end the allantois grows out and reaches the ' allantoidean ' trophoblast. It is from this part that the placenta is formed (Fig. 155), the foetal capiUaries being driven into the trophoblast between the lacunae. The whole grows in thickness.
The ' deciduofracts ' are phagocytic trophoblastic cells which eat up the maternal tissues adjoining the placenta.
In the omphaloidean region relations are at first established between the yolk-sac and the trophoblast with its lacunae. But as the allantoic placenta becomes increasingly functional the yolk-sac dwindles in importance and is folded up under the ' decidua reflexa '. By the extension of the uterine cavity round the base of the placenta the ' reflexa ' is enlarged, and surrounds the embryo on all sides except at the placenta. It becomes stretched, and the trophoblast beneath it much attenuated.
Fig. 156. - Foetal membranes and placenta of the shrew {Sorex). (After Hubrecht.) x, point where the omphaloidean trophoblast is in contact with the maternal tissues ; tr.an., trophoblastic annvdus, or thickening of trophoblast below x.
In the toole {Talpa) the uterine epithelium is also destroyed on the mesometric (non-placental) side ; the trophoblast comes into immediate contact with the subepithelial tissues. At birth the allantoic capillaries are pulled out of the placental trophoblast, which remains behind to be absorbed by the leucocytes of the mother. This arrangement is known as ' contra-deciduate '.
In Sorex (Fig. 156) there is, prior to the attachment of the trophoblast in the placental region, a conspicuous proliferation of the uterine epithelium with concomitant development of crypts between the glands on the anti-mesometric side. Into these the syncytial trophoblast makes its way, and then the epithelium is destroyed. The further stages in the development of the placenta are similar to those occurring in other forms.
Laterally there are also independent proliferations of the uterine epithelium to which the trophoblast becomes attached. The fused maternal and foetal tissues afterwards degenerate together and are dehisced from the wall ; the continuity of the uterine lumen is then restored. The area vasculosa of the yolksac which had been applied to this region is at the same time detached. Further towards the anti-embryonic polp there is an annular thickening of the trophoblast. The cells are here phagocytic and ingest quantities of extra vasated maternal haematids. Digestion of these presumably takes place in the trophoblast, since a bright-green pigment (? haemoglobin derivative) fills the yolk-sac. The iron would then be carried o£E by the blood vessels of the area vasculosa. At the anti-embryonic pole the trophoblast is thin and not attached to the uterus ; here the epithelium persists.
In Tupaia the yolk-sac, which has at first relations with the placental regions of the trophoblast, is later displaced by the allantois.
Tarsiits, Monkeys, and Man As we have already had occasion to see, the aberrant Lemur Tarsius agrees with Monkeys and the human being in the possession of a diminutive yolk-sac (provided, nevertheless, with an area vasculosa), a large and precociously developed extra-embryonic coelom, and a rudimentary allantois which only extends far enough outside the body of the embryo to penetrate the base of the ventral or body-stalk, which connects the embryo in its amnion and with its yolk-sac to the wall of the blastocyst and is to be developed into the umbilical cord. Such an arrangement of the foetal membranes is found nowhere else amongst the Mammalia. We have now to inquire whether in the origin and minute structure of the placenta there is an equally complete agreement.
Fig. 157. - Foetal membranes and placenta of Tarsius. (After Hubrecht.)
Letters as above.
In Tarsius alone is the complete history of the placenta kno^vn, and there is no doubt whatever here at any rate that the placenta is of that type which prevails in Rodents, Insectivores, and Cheiroptera. In form it is discoidal, or rather button-shaped, protruding into the uterine cavity ; it is developed at the antiembryonic pole of the blastocyst, and is placed on the mesometric side of the uterus (Fig. 157). Here there is, prior to fixation, a ' trophospongia ' or area of proliferating connective tissue and enlarged blood-vessels, and with this the placental trophoblast comes in contact as soon as, under its influence, the epithelium has been destroyed. Firmly fixed here, the tropho blast becomes hollowed out by lacunae, in which maternal blood circulates and is invaded from the other side by the foetal capillaries. The whole then grows into the lumen of the uterus until the complete thickness of the placenta is attained. An interesting feature is the conversion of much of the trophoblast into * megakaryocytes ', large cells with enormous nuclei containing big nucleoli, similar to those seen in the omphaloidean trophoblast of the mouse.
Unfortunately, we have no such thorough knowledge of the genesis of the placenta of Man and Apes, but the structure of the fully formed organ is known, and such early stages as have been described are comparable, without difficulty, with stages in the development of such placentas as those of Tarsius, Insectivores, and so on.
When completed, the placenta is discoidal in shape. Amongst the Platyrhine (New World) Monkeys it is double in Cebus, single (occasionally double) in Mycetes. The two placentas are placed respectively on the dorsal and ventral walls of the utenis, and are connected, of course, by blood-vessels. Where only one is present it is ventral, but there is on the dorsal wall a placentoid - a thickened region of widened blood-vessels - as though for the reception of a second placenta.
In the Catarrhines (Old World tailed Monkeys) there are usually two placentas, dorsal and ventral, as in Semnopithecus and Gercocebus (Macacus) (Fig. 136), but one (the ventral) may be absent. Either of the two may be the primary one. The umbilical cord in Gercocebus passes to the ventral placenta, whence blood-vessels travel to the other.
In the Simiidae {Hylobates, the gibbon) and Simia (the orang) and in Man there is but a single discoidal placenta, placed in the two Apes on the anterior (ventral) waU of the uterus, in Man usually on the posterior wall, though the position is variable. Further, in these forms the blastocyst or chorionic sac is always embedded in maternal tissue which forms, between it and the lumen uteri, a layer known as the ' decidua reflexa ', or, in more modern parlance, the ' capsularis ' (Fig. 158). What has been regarded as a precursor of this structure- a ridge running round the placenta - has been observed in Mycetes and Cercocebus.
Human anatomists distinguish from the ' decidua reflexa ' or ' capsularis ' that maternal tissue to which the placenta is attached as ' decidua serotina ' or ' basalis while the opposite wall of the uterus is known as the ' decidua vera '. The application of the term ' decidua ' to maternal tissues has already been alluded to ; it dates from the time when the type of placenta we are considering was supposed to include, and carry away at parturition, a considerable portion of the uterine wall.
Fig. 158. - ^Human foetal membranes and placenta. (After Balfour, after Longet.) The amniotic cavity (am.c.) has enlarged and occupies nearly the whole of the extra-embryonic coelom (c), the amnion being reflected over the umbilical cord (u.c.) and yolk-sac (y.s.). d.b., decidua basalis (serotina) ; d.r., decidua capsvdaris (reflexa) ; d.v., decidua vera ; l.u., lumen uteri ; am., amnion ; pi., placenta ; o.d., oviduct.
Structurally all these placentas resemble one another very closely. The maternal blood circulates in large spaces known as sinuses, which are supplied by the blood-vessels of the uterine wall (the decidua serotina or basalis in the Simiidae and in Man) (Fig. 158*). These sinuses are lined everywhere- not only over the foetal blood-vessels, but also on the maternal and on the foetal sides - by a syncytial layer, usually referred to as the syncytium, below which is a layer of cells- the cell-layer of Langhans of human embryology. These two layers separate the maternal blood in the sinuses from the foetal connective tissue and blood-capillaries (Figs. 159, 160). The more usual way, perhaps, of describing this arrangement is to say that the foetal villi - meaning by that the. capillaries, and coimective tissue and the cell-layer and syncytium covering them - branch about in sinuses filled with maternal blood. The expression ' villi ' dates, however, from the older conception of the origin of these structures from villi similar to those seen in an Ungulate, a conception which is almost certainly erroneous. The foetal capillaries do branch very considerably it is true, but the syncittium and cell layer are continued over the outer walls of the sinuses, next the tissues of the serotina. The sinuses, in fact, are lined everywhere by these two layers.
Fig. 158*. - Diagram of the structure of the human placenta, m.b.v., maternal blood-vessels in the decidua basalis {d.b.) opening into the sinuses of the placenta (s) in which the villi branch. The villi are covered and the sinuses lined on all sides by trophoblast (ir.) (syncytial layer and cell layer of Langhans). am., epithelium (ectoderm) of the amnion.
Fig. 160. - Structure of the insertion of a ' villus ' into the ' decidua basalis ' of the human placenta. d.b., large cells (' decidual cells ') of the basalis ; m.b.v., maternal blood-vessels ; s., sinus of the placenta ; si/., syncytial layer, and c, celllayer covering villus ; f.h.v., foetal bloodvessel in villus ; c'., mass of vacuolated cells continuous with the cell-layer and covering the extremity of the villus. The fat globules in the syncytium are rendered in black.
A, Large glycogen cells from tlia maternal side of the human placenta (5 months).
Fig. 159.- Middle strip of a section through the middle of the human placenta at 5 months, d.b., decidua basalis ; v'., - ua. "^i'li inserted into basalis; s., sinus; v., villi in sinus ; f.b.v., foetal vessel in villus ; - urn umbilical vein ; w.a., umbilical artery ;
am., epithelium of amnion. The synctium and cell-layer covering the villi and lining the inuses are stippled. Notice that this cell-layer is found between he end of the villus and the maternal tissue of the basalis.
Further, the cell-layer at the outer extremities of the villi is continued into a mass of cells which separate the villus from the tissue of the decidua basalis. These cells are vacuolated, containing glj^cogen.
In Mycetes there is a syncytial network between the ' villi cutting up the sinuses into smaller cavities (? lacunae) : there is no cell-layer.
In the human placenta the sjmcytium contains fat ; in late stages the cell-boundaries vanish in the layer of Langhans also.
On the maternal side of the placenta in the ' basalis ' there are in man, Simia, Hylobates, and the Catarrhines, enlarged coimective tissue-cells, known as ' decidual ' cells (Fig. 160). These decidual cells get intermingled with the masses of cells which, continuous with the layer of Langhans, cover the outer extremities of the villi and contain glycogen, the two together being disposed in a sheet known as the chorio-basahs. In Simia and in man there are also septa, that is, peninsulae of basalis tissue projecting into the placenta proper.
In man the layer of the basalis next the placenta is known as the compacta. In this are the necks of glands. As gestation proceeds the epithelial lining of these glands degenerates, the inter -glandular tissue undergoes a fibrinous degeneration, and there are extravasations of blood in between these cells and into the glands. Similar extravasations occur in Hylobates and Simia. The whole layer becomes stretched and thinned. Beyond the compacta is the spongiosa, a layer of maternal tissue in which the gland -necks are much enlarged. There is slight degeneration here also. A spongiosa is found in Simia, but not in Hylobates.
In the lower Monkeys which possess no decidua capsularis the chorion is smooth except in the placental region or regions, but in Hylobates, Simia, and Man the chorion which is covered by the capsularis is in an early stage produced into ' villi ' (which become poorly vascularized), as well as that opposite the basalis. Later the ' villi ' disappear, and this part of the chorion is then, to use an old term, the ' chorion laeve ', as distinct from the ' chorion frondosum ' of the placenta.
The capsularis is covered by a cubical epithelium (Fig. 158).
In it, at the sides only, are a few glands with openings into the lumen uteri. There are blood-vessels and extravasations. The whole layer gets distended by the growth of the embryo and eventually its tissues wholly degenerate.; the chorion is then immediately apposed to and united with the vera on the opposite side, and the uterine cavity Is obliterated. Only in the condition known as placenta reflexalis does the maternal circulation continue on this side.
Fig. 161. - Early human embryo with its membranes. (After Pet«rs.) d.h., decidua basalis (serotina); d.r.ep., uterine epithelium covering the decidua reflexa or capsularis ; I., lacuna in trophoblast (tr.) ; gl., uterine gland ; m.b.v., maternal blood-vessels opening here and there into lacunae ; cl., clot marking (probably) the point of entrance of the blastocyst ; here the epithelium is interrupted. Other letters as before.
In the decidua vera the epithelium ultimately disappears, the compacta is stretched and attenuated, and there are slight degenerative changes.
Such is the structure of the placenta in Man and Apes. We have still to discuss the mode of formation of the capsularis and the nature of the ' villi ' and ' sinuses '.
Fig. 162.- a small portion of the wall of the embryonic sac- on the side of the decidua basalis- of the human embryo shown in the last figure. (After Peters.) d.b., maternal connective tissue of decidua basalis ; end., endothelium of maternal capillary (m.c), opening into lacuna (/.) ; sy-^ sjaicytium (plasmodi-trophoblast) ; tr., cellular trophoblast (cyto-trophoblast) : the syncytium is pale, the cyto-trophoblast more deeply staimng ; m., somatopleure lining the extra-embryonic coelom (c).
In the hedgehog and mouse Ave have seen the blastocyst embedded in a pit in the uterine wall, in which it becomes securely enclosed. The pit is lined by a continuation of the uterine epithelium, which, however, soon disappears. The relation of the blastocyst to the enveloping maternal tissues is then very similar to the relation between the human chorionic sac and its capsularis, and it has not unnaturally been suggested that the latter is really developed in the same way.
There is, however, knoisTi to us another way by which the mammalian blastocyst may come into contact with subepithelial tissue, for, as we have seen, the blastocyst of the guinea-pig bores its way through the epithelium. In the earliest human embryos knoAvn to us - those described by Peters and Bryce ^ - there are very strong indications that the human capsularis is. formed in this way, for in both cases there is in the centre of this layer an interruption in the continuity of the epithelium, marked, in Peters's case, by a blood-clot (Fig. 161). This would then be the spot where the ovum effected its entrance. If so then there can never have been any uterine epithelium on the other side of the blastocyst, the side of the basalis where the placenta is developed. This should be borne in mind in considering the next question, the origin of the ' villi ' and ' sinuses '. In the embryos described by Peters and Bryce the somatopleuric wall of the extra-embryonic coelom is covered on the outside by a layer of cubical cells. Next to and perfectly continuous with this layer is a thick mass, composed of similar but polyhedral cells or in some places of a sjnacytium, with nuclei similar to those of the cellular tissue. In this mass are lacunae, and in these are maternal blood-corpuscles (Fig. 162). Outside all this is the subepithelial connective tissue of the uterus, with glands and blood-vessels : the latter open into the lacunae.
- The embryo described by Bryce is perhaps slightly the younger of the two, as the extra-embryonic coelom appears to be not yet ])roperly formed. That described by Peters was, however, obtained mi silii in the uterus, and so gives us more information as to the relation between the foetal and maternal tissues.
There can be no reasonable doubt that the whole of this lacunated mass, with a basal cellular layer next the somatopleure, is the trophoblast, which has become thickened and hollowed out for the reception of extravasated maternal blood. We have seen this occurring in a part only of the trophoblasl^ as in the mouse- or in the whole of it- as in the hedgehog, and there is no reason why any other interpretation should be put upon this stage in human ontogeny. The steps in its formation have, however, not yet been observed. The comparative anatomy of the placenta has taught us that in cases of this kind the necessary relation between foetal and maternal blood-streams is brought about by the penetration of the allantoic capillaries into the trophoblast. Exactly the same process occurs presumably in the human being : the embryonic blood-vessels, with their surrounding connective tissue, make their way into the trophoblast between the lacunae. There they branch repeatedly and become the ' villi ' of the completed placenta, while the sinuses are the transformed lacunae. The syncytial and cellular layers lining the sinuses and covering the villi are then both trophoblastic in origin, and similar to the plasmodi-trophoblast and cy to -trophoblast seen in other placentas of this type, in the mouse for example ; they may be derived respectively from the outer and inner layers of trophoblast in the early stage. It is now possible to understand why the sinuses are lined throughout, on the maternal side against the basahs, as well as over the ' villi ' and on the foetal side, by the syncytium and cell-layer, and why the outer extremities of the villi are separated from the decidual cells of the basahs by the cell-masses continuous with the layer of Langhans.
If this interpretation is correct then such hypotheses as that the sinuses are enlarged veins and the syncytium the endothehum of those veins, or that the sync3rtium is derived from uterine epithelium, must evidently be discarded ; the second of these views is indeed already ruled out of cotu-t if the implantation of the blastocyst is really effected in the way we have suggested, for there could be in that case no uterine epithelium on the side of the decidua basahs.
Such views as these date from the period when it was beheved that the human, like other ' deciduate ' placentas, was derived from the condition found in Ungulates by a simple adherence of the villi to the crypt-walls ; and this beUef was supported by the existence of ' chorionic villi that is, branching processes of the trophoblast, all over the outer surface of the early blastocyst, before the foetal vessels had appeared. But these ' chorionic vilh ' Avere observed only in blastocysts removed violently, perhaps after post-mortem changes, from the sac of the capsularis, and a proper histological examination of foetal and maternal tissues together has revealed their true nature ; they are not ' vilh' or processes plungmg into maternal tissues, but the irregular walls between the lacunae excavated in a thickened trophoblast.
The placenta of Man and Monkeys is, then, of the same kind as that seen in Tarsius, and in Rodents, Insectivora, and Cheiroptera, It contains no maternal tissue except the blood circulating in the sinuses or enlarged trophoblastic lacunae, and, in addition to the blood, no maternal tissue is lost at birth beyond the thin layer of the degenerate compacta - in both the deciduae basalis and vera - across which the break occurs, and such septa as may have forced their way into the placenta.
We may now briefly review the genesis of the Mammahan placenta in its varied types.
In Marsupials the placenta is wholly dissimilar from anything met with elsewhere, since the trophoblast degenerates while the syncytium is of uterine epithelial origin.
The Ungulates possess a typical Indeciduate placenta, with villi dipping loosely into crjrpts hned by a persistent epithelium, from which they may be readUy withdrawn without injury to the maternal tissues. Haemorrhage from the uterine blood vessels does, however, occur during gestation, and is of physiological importance in foetal nutrition.
The placenta is similar in Cetacea, Sirenia, and in the Lemuroidea (except Tarsius).
In the Proboscidea these haemorrhages are perhaps more extensive.
In the Camivora the conditions are different, for here the trophoblast does not send vilh into specially prepared crypts, but, after the destruction of the uterine epithelium, eats its way into the tissues, engulfing the maternal capillaries. These and the surrounding connective tissue grow pari passu with the trophoblast to produce the full thickness of the placenta. The foetal capillaries grow into the trophoblast. The placenta is therefore compounded of foetal and maternal tissues.
In the remaining orders this is no longer the case, for, after the destruction of the epithelium, the trophoblast merely fastens on to the underlying tissues ; only occasionally are the immediately adjacent capillaries engulfed (in the rabbit and in the bat), and even here their endothelium soon vanishes. Once fixed to the uterine wall the trophoblast grows not into the wall but from it towards the centre of the uterus, receiving into its lacunae the stream of maternal blood ; from the other side it is vascularized by the allantois.
But distinct though these three types of placentation are, it is yet possible that the third might have been derived from the second - if we inxagine the centripetal growth of the trophoblast to occur before the ingrowth into the maternal tissues has taken place - and the shght-' enclosure of maternal capillaries in the bat and rabbit almost demonstrates the change, while the insertion of the trophoblast into the newly formed cr3rpts in Sorex recalls another Carnivorous character. The second, in turn, may have sprung from the first by the suppression of the uterine epithelium.
These, however, are mere speculations, for which alternative hypotheses may without difficulty be substituted.
One other point requires brief consideration. It has been held that the characters of the placenta are a valuable criterion of genetic relationship, and may accordingly be used for classificatory purposes. Now while it must be pointed out that single characters in regard either to the gross or the minute anatomy cannot be employed legitimately in this way - there is no justification, for example, in grouping together the elephant, Hyrax, the Sirenia, Orycteropus, and the Carnivora, because they all possess a zonary placenta, nor on the other hand do we beUeve it is yet proposed to separate the Lemuroid Primates, with their typically Indeciduate, from the Anthropoids, with their Deciduate placenta - yet a combination of characters is often found to be a constant mark of a natural order - for instance, the large yolk-sac with its lower Avail lost and the mesometric discoidal placenta of Rodents, the zonary shape of the (histologically) peculiar placenta of Carnivora- £vnd it is for this reason that we hold that the remarkable structure of its foetal membranes and its placenta entitle Tarsius to be separated from the Lemurs and ranked with Monkeys and Man.
Literature
E. Van Beneden. Recherches Sur Les Premiers Stades Du D6Veloppement Du Murin {Vesperlilio Murinus). Anat. Anz. Xvi, 1899.
E. Van Beneden Et C. Julin. Recherches Sur La Formation Des Annexes Foetales Chez Les Mammiferes. Arch, De Biol, V, 1884.
R. Bonnet. Die Uterinmilch Und Ihre Bedeutung Fur Die Frucht Festschr.F. Bischoff, 1882.
R. Bonnet. Beitrage Zur Embryologie Der Wiederkauer Gewonnen Am Bchafei. Arch. Anat. U. Phys. {Anat.), 1884, 1889.
R. Bonnet. Beitrage Zur Embryologie Des Hundes. Anat. Hehe, L'â« Abt. Xvi, 1901.
T A Bryce And J. W. Teacher. The Early Embedding And Development Of The Human Ovum. Glasgow, 1908.
W. Cmpman. Observations On The Placenta Of The Rabbit, With Especial Reference To The Presence Of Glycogen, Fat, And Iron. Laboratory Reports, Hoy. Coll. Phys. Edinburgh, Viii, 1903.
M. Duval. Le Placenta Des Carnassiers. Joum. Dev Anat. Etde La Phys. Xxuc-Xxxi, 1893-5.
M. Duval. Le Placenta Des Rongeurs. Journ. De Vanat. El De La Phis Xxv-Xxvui, 1889-92.
R. Geime Dottersack Und Placenta Des Kalong {Pteropus Edulis). Belenka S Studien Zur Entwickelungsgeschichte Der Tiere, V, 2. Wiesbaden Xxm; Fm. Of The Mole. Quart. Journ. Micr. Sci.
1897.^' Placentation Of Perameles. Quart. Journ. Micr. Sci. Xl,
Membranes, Placentation And Parturition Of The Native Cat {Dasyurus Viverrinus). Anat. Anz. Xviii, 1900
Ht. Die Phylogenese Des Amnions Und Die Bedeutung Etensch. An.Sterda:,^ Tm2' Lefpzfg,T89F D.Keimblasevonj'a..-^.. Festsch.F. Gegenbaur.
J. W. Jenkinson. Notes On The Histology And Physiology Of The Placenta In Ungulata. Proc. Zool. Soc, 1906.
F. Keibel. Zur Vergleichenden Keimesgeschichte Der Primatcn. Selenlca'a Studien Iiber Enlwicklungsgeschichte Der Tiere, 10. Wiesbaden, 1903.
J. Kollmakn. Ueber Die Entwickelung Der Placenta Bei Dem Makaken. Anat. Anz. Xvii, 1900.
R. Kolstee. Die Embryotrophe Placentarer Sauger. Anat. Hefle, 1'8 Abt. Xviii, Xix, 1902, 1903.
R. Kolstee. Weitere Beitrage Zur Kenntniss Der Embryotrophe Bei Indeciduaten. Anat. Hefle, L'" Abt. Xx, 1903.
J. Lochhead And W. Ceamee. The Glycogenic Changes In The Placenta And The Foetus Of The Pregnant Rabbit. Proc. Roy. Soc. B. Ixxx, 1908.
F. H. A. Maeshall And W. A. Jolly. Contributions To The Physiology Of Mammalian Reproduction. Phil. Trans., Series B, Cxeviii, 1905.
F. H. A. Maeshall. The Physiology Of Reproduction. London, 1910.
F. H. A. Marshall. The Oestrous Cycle And The Formation Of The Corpus Luteum In The Sheep. Phil. Trans., Series B, Cxcvi, 1903.
P. Nolf. Etude Des Modifications De La Muqueuse Uterine Pendant La Gestation Chez Le Murin. Arch, De Biol, Xiv, 1896.
H. Petees. Die Einbettung Des Menschuchen Eies. Leipzig, 1899.
E. Selenka. Keimblatter Und Primitivorgane Der Maus. Wiesbaden, 1883.
E. Selenka. Die Blatterumkehrung Im Ei Der Nagethiere. Wiesbaden, 1884.
E. Selenka. Die Entwickelung Des Gibbon. Wiesbaden, 1899, 1900.
R. Semon. Die Embryonalhiillen Der Monotremen Und Marsupialier. Zool. Forschungsreise In Australien, Ii.
F. Geae Von Spee. Neue Beobachtungen Iiber Sehr Friihe Entwicklungsstufen Des Menschuchen Eies. Arch. Anat. U. Phys. {Anxit.), 1896.
F. Geaf Von Spee. Die Implantation Des Meerschweincheneies In Die Uteruswand. Zeitschr. Morph. U. Anthrop. Iii, 1901.
H. Steahl. Ueber Primaten-Placenten. Selenka' S Stvdien Vher Entwicklungsgeschichte Der Tiere, 12. Wiesbaden, 1903.
H. Steahl U. H. Happe. Ueber Die Placenta Der Schwanzafien. Selenka' S Studien Uher Die Entwicklungsgeschichte Der Tiere, 13. Wiesbaden, 1905.
Sie W. Tuenee. Lectures On The Comparative Anatomy Of The Placenta. Edinburgh, 1876.
Sie W. Tuenee. On The Foetal Membranes Of The Eland {Oreas Canna). Journ. Anat. And Phys. Xiv, 1879.
Sie W. Tuenee. On The Placentation Of Halicore Dv^ong. Trans. Roy. Soc. Edinburgh, Xxxv, 1890.
J. H. Veenhout. Ueber Die Placenta Des Maulwurfs. Anat. Hefle, I'e Abt. V, 1894.
C. Webster. Human Placentation. Chicago, 1901.
W. F. R. Weld On. Note On The Placentation Of Tetraceroa Quadricornis. Proc. Zool. Soc. London, 1884.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Jenkinson JW. Vertebrate Embryology. (1913) Oxford University Press, London.
- Vertebrate Embryology 1913: 1 Introduction | 2 Growth | 3 The Germ-Cells, their Origin and Structure | 4 The Germ- Cells, their Maturation and Fertilization | 5 Segmentation | 6 The Germinal Layers | 7 The Early Stages in the Development of the Embryo | 8 The Foetal Membranes of the Mammalia | 9 The Placenta | Figures
Cite this page: Hill, M.A. (2024, April 24) Embryology Book - Vertebrate Embryology (1913) 9. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Vertebrate_Embryology_(1913)_9
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G