Book - Physiology of the Fetus 6: Difference between revisions
| (One intermediate revision by the same user not shown) | |||
| Line 6: | Line 6: | ||
=Chapter VI Fetal Respiratory Movements= | =Chapter VI Fetal Respiratory Movements= | ||
It is commonly taught that the fetus in utero is apneic. I-Iowever, this concept has been chal1enged from time to time and some investigators have declared that respiratory movements occur regularly throughout prenatal life under normal physiologic conditions. No one doubts that mammalian fetuses are capable of performing rhythmical movements which talce the form. of shallow rapid breathing or even of dyspneic gasping. Movements of this nature are frequently encountered upon opening the uterus of a pregnant laboratory animal. The literature contains many articles in which mention of such activities has been made. The early studies were reviewed by Preyer.1 Human fetuses, aborted or removed surgically from the uterus, show the move— ments in question as early as the twelfth weelc of gestationk 0ne of the ·most strilcing examples of respiration in immature forms is encountered in the opossum.3 Although this animal is born after a sojourn« of only 1243 days in utero (or in the tubes, as a blastocyst) it is able to breathe and to make its way unassisted to the pouch where it reassumes dependence upon the mother. Most studies in other animals have employed fetuses removed from the uterus and one may well question whether any of them were representative of the normal. It is not permissible to conclude, on the basis of observations made under conditions which preiudice the placental exchange mechanism, that respiratory motor phenomena are indulged in normally by the fetus throughout prenatal life. | |||
investigators have declared that respiratory movements occur | |||
regularly throughout prenatal life under normal physiologic conditions. No one doubts that mammalian fetuses are capable of | |||
performing rhythmical movements which talce the form. of | |||
shallow rapid breathing or even of dyspneic gasping. Movements | |||
of this nature are frequently encountered upon opening the | |||
uterus of a pregnant laboratory animal. The literature contains | |||
many articles in which mention of such activities has been made. | |||
The early studies were reviewed by Preyer.1 Human fetuses, | |||
aborted or removed surgically from the uterus, show the move— | |||
ments in question as early as the twelfth weelc of gestationk 0ne | |||
of the ·most strilcing examples of respiration in immature forms is | |||
encountered in the opossum.3 Although this animal is born after | |||
a sojourn« of only 1243 days in utero (or in the tubes, as a blastocyst) it is able to breathe and to make its way unassisted to the | |||
pouch where it reassumes dependence upon the mother. Most | |||
studies in other animals have employed fetuses removed from the | |||
uterus and one may well question whether any of them were | |||
representative of the normal. It is not permissible to conclude, | |||
on the basis of observations made under conditions which preiudice the placental exchange mechanism, that respiratory motor | |||
phenomena are indulged in normally by the fetus throughout | |||
prenatal life. | |||
==Respiratory Movements in the Intact Animal== | |||
Let us examine the evidence of fetal movements resembling | Let us examine the evidence of fetal movements resembling respiration in the intact animal. In 1888 Ahlfeld, a German gynecologist, described certain rhythmical fetal movements which he and a pupil observed in patients during the latter weelcs of pregnancyxss Z superimposed upon the slow excursions of the gravid abdomen which resulted fronkmaternal respirations they could see more rapid rhythms which appeared to be due to activities of the fetus. Ahlfeld proposed that the ketus was making respiratory efforts and thought it must aspirate its amniotic fluid into the major respiratory passages. Many protested this theory, but as recently as 1905 he reiterated his views and published convincing graphic records of these movementsJk The intrauterine respiratory movements have been observed by other investigators,7 but the theory of« aspiration of fluid has not been generally accepted and it is usually concluded that the fetal glottis remains cJosed. The question has been raised again recently by investigatorss who are of the opinion that the human fetus executes rhythmical respiratory movements normally and that these serve to draw amniotic fluid into the lungs. It was even suggested that this fluid may assist in the development of the lung alveoli. Tracings illustrating the character of human» fetal respiratory movements are reproduced in Fig. se. A strilcing comparison of intrauterine fetal and early postnatal respiratory records will be seen in Fig. 33. | ||
respiration in the intact animal. In 1888 Ahlfeld, a German | |||
gynecologist, described certain rhythmical fetal movements which | |||
he and a pupil observed in patients during the latter weelcs of | |||
pregnancyxss Z superimposed upon the slow excursions of the | |||
gravid abdomen which resulted fronkmaternal respirations they | |||
[[File:Windle1940 fig32.jpg|600px|alt=Human intrauterine respiratory movements]] | |||
'''Fig. 32.''' Human intrauterine respiratory movements '''A,''' Intesrrupted rhythms at a frequency of about 52 per minute. '''B,''' Movements recorded from fetal abdomen and chest simultaneously about 80 per minute. (Ahlfeld: Monatschr. Geburtsh. Gynälsp Vol. 21, 1905.) '''C,''' Intrauterine fetal respiratory movements (upper) , maternal carotid Pulse, maternal respirations and time in 4 second intervals (lower) before labor be»gan. (K. Reifferscheid: Pflugers Arch., Vol. 140, 1911.) | |||
The principal objection to the current view is that the movements in question are not always, in fact not often, manifested by the human ketus. One must watch patiently to see them and when they do appear they are usually transient». If they were commonly encountered in the human, one should expect to see them in some other animals wo. Fetal respiratory-like activities are rarely apparent in cats and guinea pigs but have been seen a day or so before birth.9 The question of aspiration by the fetus will be considered later. It is pertinent first to invcstigate the factors which govern the manifestation of fetal respiratory-like movements. | |||
'''Fig. 33.''' Comparison of human intrauterine respiratory movements (upper) with those of a five day old inkanr. Time, 4 seoond intervals. (K. Reifferscheid: Pflugers Arch., Vol. 140, 1911.) | |||
Respiratory | ==Respiratory Movements Under Experimental Conditions== | ||
Respiratory movements have been studied recently in a number of laboratory animals including the rat, guinea pig, rabbit, sheep and cat.9-18 They have been observed in human beings under somewhat similar conditions.19-23 There is little doubt that their occurrence and characteristics are influenced by the experimental conditions. These must be evaluated critically. | |||
In the first place, anesthcsia seems to alter the threshold of the fetal respiratory center and for this reason most investigators have tried to avoid using it. Ether administered to the mother anesthetizes the fetuses which then rarely« exhibit respiratory activities until after placental circulation has been interruptedz at that time they talce the form of dyspneic gasping. Urethane lilcewise de— presses the rapid rhythms of the shallow respiratory movements.24 Doses of nembutal which anesthetize the mother similarly depress the fetusks Although sodium amytal is said to have no ekfect upon the fetus of the rat when given to the motherW and not to pass the placental barrier readily, further consideration should be given to this question. It is possible that some asphyxial types of anesthesia first stimulate respiratory movements in normally apneic fetuses,9 but there is little doubt that their continued use results in depression. Cyclopropane (3o per cent) is said to be about the only inhalation anesthetic which does not seriously affect the respiratory activities of fetuses and still gives surgical anesthesia.27 | |||
. | |||
Fig. 34. | '''Fig. 34.''' Two portions of a continuous crystograph record of uterine motility. maternal respiratjon and intrauterine fetal respiratory movements on the 67th day of gestation in the tat (end of gestation) . The animal had been decerebrated by the anemia method: no anesthesia was used during the experiment the abdomen was not opened. The major kluctuations at the beginning of each tracing indicate relaxation of the Uterus after a contraction occurring one minute before the arrow (↑). The large secondary waves are the maternal respirations (17 per minute) . The smallest waves are intrauterine fetal respiratory-like movements occurring at approximately 120 per minute, slower at first than when they are well under way. Five minutes between relaxations 1 and 2. | ||
maternal respiratjon and intrauterine fetal respiratory movements on the 67th day | |||
of gestation in the tat (end of gestation) . The animal had been decerebrated by | |||
the anemia method: no anesthesia was used during the | |||
was not opened. The major kluctuations at the beginning of each tracing indicate | |||
relaxation of the Uterus after a contraction occurring one minute before the arrow | |||
Various experimental procedures have been employed to avoid the use of general anesthetics spinal anesthesia can be used successfully in the larger species. spinal cord section if performed long enough beforehand to allow recovery from shoclc may be satisfactory in those animals which are rather docile anyway. Decerebration is to be recommended in other forms. After preparing an animal near term by one of these methods it is occasionally possible, without opening the .maternal abdomen, to see« movements of a rhythmical nature resembling fetal "breathing." In Fig. 34 such fetal respiratory movements are shown (after decerebration of the cat) superimposed upon maternal respirations This curvc was obtained by means of a crystograph activated by amplified potentials from an oscillating eircuit, any movements on the surface of the catss abdomen changing the frequencies of oscillation. To what extent decerebration, spinal cord Section or other preparatory surgical procedures were responsible for instituting the respiratory movements, is not known. | |||
When the pregnant animal is placed in a bath of warm saline solution, the abdomen opened and the uterus exposed, it is pos— sible to observe fetal activities more directly. Rabbit and guinea pig fetuses near term can be seen quite easily through the thin uterine wall. Body form of other species is readily distinguishable. During the course of zo minutes or more of observation, respiratory rhythms of fetal movements usually malce their appearance in the cat. The longer the uterus has been exposed and the nearer the end of gestation, the more frequently these activi· ties manifest themselvess They rarely continue for more than a few seconds but start and stop at fairly regular intervals. This intermittent occurrence of brief rhythms is associated with motility of the uterus in the cat. Mild contraction of uterine muscle is accompanied by a period of fetal quiescence relaxation of the muscle leads to a rhythm of respiratory Ihovements Fetal activities of a respiratory nature are rarely if ever seen in the unopened uterus before the last two weelcs of gestation in the cat. This is not because it is more difficult to visualize younger fetuses, for as we shall soon see it is possible to induce the movements in question by appropriate experimental methods as early as the middle of prenatal life. | |||
Most of the earlier studies on respiratory movements of fetuses were made after delivering the specimens from the uterus under warm saline solutions, leaving the placenta attached to the uterine wall. It has often been erroneously assumed that circulation and gaseous exchange can thus be maintained adequately and that observations made in the fetus under such conditions are representative of activities occurring normally in the intact animal. | |||
uterine wall. | |||
activities | |||
When a cat fetus has been determined to be apneic in utero | When a cat fetus has been determined to be apneic in utero and is then carefully but quiclcly delivered through a small incision in the least vascular portion of the uterus at one end of the zonary placenta it commonly begins to execute respiratory moves ments within a very short period of time; often this is a matter of one minute or less. The blood of the umbilical veins darlcens perceptibly at delivery and the— manifestation of respiratory movements is associated with such darlcening in cat fetuses during the third quarter of gestation. 18 | ||
and is then carefully but quiclcly delivered through a small incision in the least vascular portion of the uterus at one end of the | |||
zonary placenta it commonly begins to execute respiratory moves | |||
ments within a very short period of time; often this is a matter | |||
of one minute or less. The blood of the umbilical veins darlcens | |||
perceptibly at delivery and the— manifestation of respiratory | |||
Feta1 respiratory movements have been studied in other | Feta1 respiratory movements have been studied in other mammals under similar conditions.I3-14 It was found that the earliest respiratory movements of sheep fetuses can be elicited by mechanical stimulation at about 38-39 days of gestation. The first rhythms of movement seem to be enhanced by putting pressure upon the amnion and thus indirectly stimulating the fetus. Later they seem to occur spontaneous1y, but it is never possible to avoid some mechanical disturbance of the fetus and the manips ulation of the uterus itse1f may have been a factor in producing the «spontaneous" activities. Urethane or spinal anesthetics were employed for the ewes. | ||
mammals under similar conditions.I3-14 It was found that the | |||
earliest respiratory movements of sheep fetuses can be elicited by | |||
mechanical stimulation at about 38-39 days of gestation. The | |||
first rhythms of movement seem to be enhanced by putting pressure upon the amnion and thus indirectly stimulating the fetus. | |||
Later they seem to occur spontaneous1y, but it is never possible | |||
to avoid some mechanical disturbance of the fetus and the manips | |||
ulation of the uterus itse1f may have been a factor in producing | |||
the «spontaneous" activities. Urethane or spinal anesthetics were | |||
employed for the ewes. | |||
Rhythmical respiratory movements have been studied in rabbits after sectioning the spinal cords of the pregnant does« The activities were manifested both before and after delivering the fetuses from the exposed uterus. Most of the rabbits were at term or had passed the time birth should have occurred normally, labor having been inhibited by hormone treatment. It is possible that the post-mature fetuses suffered inefiicient placental exchange of oxygen and carbon dioxidez it has been shown that all rabbit fetuses die by the zöth day (normally delivery occurs at 31 or 32 days) when labor is inhibited. 28 | |||
and | |||
From the various experimental studies it is clear that mammalian fetuses are capable of exercising their respiratory muscles and of doing so rhythmically after the fashion of airbreathing animals rather early in prenatal like. But mammalian fetuses do not execute rhythmical respiratory movements continually in utero unless certain unfavorable conditions are set up. Even near the termination of life in utero when the eliiciency of the placenta in relation to the greatly enlarged fetus has declined and when one might expect to lind a physiologic anoxemia, it is surprising how seldom the activities in question can be observed in the intact animal. | |||
==Relation of Oxygen and Carbon Dioxide to Respiratory Movements== | |||
Since the blood of the fetus comes almost into equilibrium with that of its mother in respect to tensions of carbon dioxide and oxygen in the placental capillary bed one may well raise the question: why does the fetal respiratory center not respond as readily as that of its mother? It has been assumed that the threshold is higher than that of the mother.29- 30 If this is one of the main factors in preventing continuous fetal respiratory moves ments from manifesting themselves norma1ly in utero it should be possible to find a carbon dioxide level at- which the fetal center will respondz or perhaps the threshold ok the fetal center can be lowered experimentally until fetal respiratory movements begin. | |||
dioxide | |||
dioxide | |||
It has been suggested that accumulation of metabolic carbon dioxide may be the agent starting these movements in cat and rat fetuses. 10, 16 Barcroft and his colleaguess 31 administered atmospheres rich it; carbon dioxide to pregnant sheep without inducing respiratory movements in the fetuses. However, the animals had been anesthetized with urethane and it is possible that the threshold of the center had been raised by this drug to a Ievel which could not be reached by. the maximum concentration the blood could carry. The ekfect of giving carbon dioxide to pregnant rabbits has been investigated.12 The fetuses were already executing respiratory movements and the rate of the fetal rhythm was not greatly altered by the gas. The only positive results were obtained in cat fetuses 18 mostly in the third quarter of the gestation periodx the fetuses were apneic at the time of experimentas tion. Some were delivered from the uterus with placental circulation intact and others were observed through the unopened uterus. When the mother cats breathed atmospheres of oxygen containing 8 to 1o per cent carbon dioxide, rhythms of rapid respiratory movements appeared both in the delivered and the intact fetuses concomitantly with the increase in rate and amplitude of maternal breathing. substitution of 15 to 20 per cent carbon dioxide in oxygen led to depression of the fetal respiratory efforts, which became very deep gasps at a slow rate. The· cat was then allowed to breathe air again and a «rebound hyperpnea" often followed in the fetuses, presumably as the carbon dioxide level re— adjusted itself in the fetal blood. 0ne of these experiments is illustrated in Fig. 35. | |||
33 | Administration of mixtures of 5 to 8 per cent oxygen in nitrogen to the mother cats brought about cyanosis, indicated by darlcening of the uterine vesse1s, and respiratory movements of the previously apneic fetuses began promptlysz Compression of the umbilical cord produced similar results in exaggerated form and more quiclcly. In large fetuses of sheep it was found that compression of the umbilical arteries alone led to respiratory activity but that this activity was delayed and did not manikest itselk so soon as it did after compressing the veins too.31 This indicates that the fetus was able to draw upon blood in the placenta and obtain a little more oxygen while the veins were intact. Many other investigators have reported that interruption of the placental circulation brings about respiratory efforts in the fetus. 1, 10, 16, 32, 33, 34 Usually these were of the dyspneic gasping type. When asphyxia is avoided fetal respiratory movements of the cat occur at rates as high as 120 per Minute, commonly more than 60 per minute. | ||
Fig. 35. Effect of an 8 to 10 per cent carbon dioxide-oxygen mixture, breathed by the mother, in initiating rhythmical respiratory movements in a cat fetus delivered at caesarean Section but with placental circulation intact Respiratory rate in half minutes. (Wind1e, et al.: Physiol. Zool» Vol. 11, 1938.) | |||
Atmospheres defccient in oxygen breathed by full term guinea pigs on which no surgery had been performed and no anesthetick used caused rhythmical respiratory movements to start in the previously apneic fetuses.9 Usually the first activities to appear were rather fast rhythms of movement which resembled shallow respirations. With more marked cyanosis these became stronger and slower. Ultimately only slow rhythmical gasps were seen in the fetuses. Upon relieving the asphyxia the rapid rhythms returned and then they too stopped, the fetuses becoming apneic again with return of normal oxygenation. | |||
Atmospheres defccient in oxygen breathed by full term guinea | |||
Blood-gas analyses have been made from samples withdrawn anaerobically from the umbilical veins of cat fetuses delivered from the uterus but« in which the placental circulation was still intact. 35 It was found that the oxygen content was low. the blood being little more than so per cent saturated in those fetuses which were executing rhythmical respiratory movements at the time of sampling. When oxygen saturation dropped to about 25 per cent or less the fetuses gasped or became depressed to the point of complete inactivity. Under the conditions of the experiments it was less p0ssible to relate the presence or absence of the movernents in question to carbon dioxide than to oskcygen content. However, in the presence of the higher oxygen levels (4o-5o per cent saturation) the carbon dioxide content was greater than it was when the oxygen level was marlcedly loweredz in the former instances the fetuses executed rapid rhythms, whereas in the latter they became inactive and depressed. Arrhythmical deep gasps appear-ed when the carbon dioxide was high and the oxygen low. | |||
It was not possible to obtain blood from the cat fetuses with· out incising the uterus and disturbing relationships there to some extent; consequently one does not know what the oxygen level is in utero. However, it was evident that respiratory efforts often began as the fetus was»being delivered and as the umbilical vein blood was darlcening Probably a higher oxygen level is maintained in the normal undisturbed uterus than the highest value obtained at experimentation would indicate. This is true in other species. The sheep in late fetal life is apneic in uteroz correlatively its umbilical vein blood, obtained without removing the lamb from the uterus, is highly saturated, exceeding 90 per cent in somekss But when the lamb is delivered into a. bath of saline solution with its placental circulation intact the blood becomes reduced.37 | |||
in | |||
the | |||
In the human at normal birth, apnea prevails when the blood | In the human at normal birth, apnea prevails when the blood is about 50 per cent saturated with oxygen irrespective of the carbon dioxide contents, 38 but respiration starts readily. Some higher values have been obtained in apneic human fetuses 39, 40 as well as in the calf.41 0ne may infer from these experiments that the normal apnea ok ketuses in utero is associated with a degree ok oxygenation exceeding about 4o or 50 per cent saturation, that as this becomes reduced rhythms of active respiratory movements appear, but that depression ok the oxygen level much below 25 per cent saturation 1eads to gasping and ultimately to complete inactivity comparable with that in asphyxia neonatorum.* | ||
is about 50 per cent saturated with oxygen irrespective of the | |||
carbon dioxide | |||
higher values have been obtained | |||
* Barcrokt and his colleagues have demonstrated recently that apnea prevails in the sheep ketus when the blood going to the ketal brain is about So to so «per cent saturated with oxygen and when that leaving it is zo per cent or more Saturated. Lower values were encountered during the .1ast prenatal wee1c. Respiratory movetnents occurred when the blood leaving the brain was to to 25 pr cent aaturated (Barcrokt, J. D. H. Barron, A. T. cowie sc P. H. Forsham 1940, J. Physiol. 97: 338) | |||
the | |||
cent | |||
'''Fig. 36.''' Oxygen and carbon dioxide contents ok the atmosphere of the air space in two hencs eggs talcen at daily intervals during incubatiotx Respiratory moves ments begin at the plateau between 17 and 19 days. (Romijn and Roos: Jour. Physiol. vol. 94, 1938.) | |||
Experiments in mammalian fetuses have been coniirmed in the incubating eggs ok chiclcs and duck.42-45 It was possible to control physiologic conditions more precisely in the bird than in the mammaL Although space limitations do not allow discussion of these studies here it should be pointed out that our lcnowledge of the atmospheres breathed by the bird throughout incubation is reasonably complete. A nice correlation can be drawn between the decline in oxygen concentration, the elevation of carbon dioxide and the appearance of respiratory movements« This is illustrated in Fig. 36. | |||
We learn from the various experiments with carbon dioxide excess and oxygen want in mammals and in birds that the fetal respiratory center responds much as does that of the adult, but there is one very marlced difference between fetus and adult. Greater concentrations of carbon dioxide and more severe degrees of oxygen deliciency mustcome into play in the former before results are obtainable. In other words the fetal respiratory center seems to have a high threshold. | |||
==Other Factors in the Development of Respiration== | |||
Not all mammals are capable of performing rhythmical respiratory movements at the same period in prenatal life. One finds some species more precocious than others. As has already been indicated, the newborn opossum «embryo« breathes about 13 days after conception. In the more advanced fetus of the sheep respiratory movements have been observed at the end of the first quarter, but still at a relatively earlier time than in the cat. The latter reaches nearly the middle of gestation before rhythmical respiratory movements can be induced. The human fetus has accomplished necessary growth by the end of the twelfth weelc of intrauterine existence. | |||
Muscles and peripheral nerves are laid down long before fetal respiratory movements begin. It is quite clear that the accomplish— ment of rhythmical movements awaits further development of a central nervous mechanism. In the 15 mm. cat embryo intrinsic growth of spinal neurons and their connections have reached the point at which the first simple rellexes are possible.47 From this time onward the Process of nervous integration proceeds, new responses accompanying new nervous connections and simple activis ties becoming more complexsIs Most of the responses of which a cat embryo of 2o mm. length, delivered yvspith Flacental circulation intact, is capable appear to be almost purposeless. The rhythmical respiratory movements which make their appearance just before the 30 mm. stage are among the first to foretell a usefulness. However, the behavior pattern that makes possible rhythmical respiration is not a new thing; it is the rhythmicity that is new. Even before 30 mm., in fact as early as 20 mm., integkated but arrhythmical contractions of the future respiratory muscles occur when adequate stimuli are used to elicit them. Their resemblance to gasps is often strilcing It seems probab1e in all the species which have been studied (rat, rabbit, guinea pig, cat, sheep, goat and man) that the motor pattern responsible for respiration is a very fundamental one. It consists of the appropriate muscles with their motor neurons. Respiratory acts manifest themselves under experimental conditions as soon as the central nervous mechanism for simultaneous1y discharging these groups of eiferent neurons is developed. The period in fetal life at which this occurs is that during which there is an extensive development of connecting neurons of the reticular formation in the medu1la oblongata and downward growth into the spinal cord of many longitudina1 nerve übers. Ground bundles, reticu1ospinal and similar phylogenetically old pathways have been laid down. The appearance of rhythmicity has not been correlated with any specific change in the central nervous System. Growth there has gone so far at so mm. that many of the structures characterizing the adult tegmens tum have appeared. Multiplication of association neurons, and consequently of possible connections in neuron circuits, seems to characterize this stage in fetal brain growth. | |||
A very ingenious theory concerned with neural control of respiratory movements in the sheep fetus has been proposed.14 In the Erst place, it was assumed that the rapid rhythmical move— ments seen in 4o—day-old sheep fetuses delivered at experimental Caesarean section are physiologically normal and that they result from some poorly understood rhythmical (autochthonous?) discharge of the respiratory center. The progressive change in character of these movements which is encountered during further development of the fetus and which culminates in complete apnea at about 6o days gestation, it was suggested, is due to the progressive downgkowth of new inhibitory neurons from the higher nervous centers. Asphyxia or experimental transection of go the brain-stem between midbrain and pons appeared to abolish the inhibitory influences Jand leave a more primtive respiratory pattern like that seen in fetuses 50 days old or less. Although it is certain that all fetal activities change with the gradual maturation of nerve tracts in the brain, the experimental data do not prove the theory proposed and alternative theories may be equally well supported. It is quite possible that expiratory and pneumotaxic centers are dominant to the inspiratory center in fetal life. Inhibition of the inspiratory center by impulses from the pneumotaxic center or continual stimulation of the expiratory center would account for the unexpanded chest and collapsed lungs.49 Perhaps in the early sheep fetus the inspiratory center has not yet come under the dominance of higher neurons. | |||
in | |||
The importance of muscle tonus in establishing conditions favorable to lung ventilation at birth has been stressed by Some» If the fetus in utero possessed the tonus of a healthy newborn it is diflicult to see how it could iit itself to the space allowed and how it could keep from iilling its lungs with fluid. In one experimentZI action potentials from electrodes which were placed in the fetal muscles were demonstrated each time the specimen was lifted out of its warm saline bath. These stopped when it was returned to the bath (see Fig. 63, p. 176) . It seems probable that the increase in stimulation of afkerent neurons attendant upon delivering the fetus from a warm aqueous habitat to the outside air plays an important part in increasing the tonus of the muscles in consequence of setting up activity in neuron circuits. This neural activity may serve to lower the respiratory center’s threshold and allow a lower level of carbon dioxide to stimulate it to action. 0thers have found that the respiratory center of the fetal goat four to six weeks from full term responds to afferent nerve stimulation like that of the adult, but it requires much stronger stimulation.30 It was inferred that the fetal center has a higher threshold. By decerebration of the fetus it was demonstrated that the higher threshold was not due to inhibitory influences from the cerebrum. | |||
in the | |||
That the apnea of intrauterine life may be due to a physiologic anemia of developmental origin has been suggestedPI Occlusion of the umbilical arteries at birth is said to increase peripheral resistance in the aorta, raise the systemic pressure and induce a marked cerebral flow. This relieves the· anemia, the high carbon dioxide concentration prevailing acts as a respiratory stimulus and respiration is initiated. | |||
the | |||
of | |||
of the | |||
dioxide and | |||
==Aspiration of Amniotic Contents== | |||
In the early paragraphs of this chapter mention was made of the suggestion that the fetus normally aspirates amniotic fluid. Granting that respiratory movements can and do occur occasional1y in utero toward the end of gestation, what is the evidence that they cause aspiration of amniotic contents? It has been pointed out that dyes injected into the amniotic sac can be found in fetal lungs after removing the fetuses.8- IN« But in none of these experiments was anoxemia rigidly ruled out of consideration. Indeed, in some it is quite obvious that it may have been a factor in starting the respiratory activity. It is known that vernix caseosa is sometimes found in the lungs of infants which have survived birth a short time. In a large series of autopsies with microscopic study Farber and sweetss found that only 15 per cent of the lungs of infants surviving birth for five weeks or less contained signilicant amounts of döbris ascribable to fetal aspiration of amniotic contents, although 88 per cent showed at least a few desquamated epithelial cells. It is doubtful if as high a per— centage of healthy infants have to cope with birth condtions such as were encountered in those living but a short time. | |||
0ther evidence concerning fetal aspiration has been presented recently.9 Without using an anesthetic a thin hypodermic needle was passed through the abdorninal wall and into the amniotic sacs of guinea pig fetuses in the last weelc or two of gestation. small quantities of fluid were withdrawn (0.4 cc. to 1 cc.) from the region about the nostrils and replaced with colloidal solutions of thorium dioxide (thorotrast) or hydroxide (thorad) . In 27 fetuses so created a large series of x-ray lilms exposed after various intervals up to 14 days failed to show lung shadows which would signify that the fetuses had aspirated this opaque material. The thorotrast or thorad was observed in the stomach and intestines, for swallowing occurred regularly. | |||
Twenty—five additional experiments were performed. Films exposed after these injections showed no aspiration on the part of the fetuses. The pregnant guinea pigs were then allowed to breathe atmospheres low in oxygen or high in carbon dioxide or to rebreathe air until an anoxemia was set up. Some of the fetuses could be seen executing rhythmical movements resembling respiration. Roentgenograms were obtained subsequently. Of 14 experiments showing shallow, questionable or no feta1 respiratory movements, only two films revealed thorotrast in the fetal respiratory tract. ln the remaining 12, all showing strong movements of the type in question, seven positive results such as are illustrated in Fig. 37 were obtained. | |||
Fig. 37. Thorotrast (0.8 cc.) injected into the amniotic sacs of two guinea pig fetuses on the 63rd day of gestatiom The mother was rendered cyanotic with nitrogen containing a small amount of oxygen. Respiratory movements were observed in fetus B more clearly than in fetus A. Half an hour later, the roentgenogram was made. The bronchial tree of fetus B is well filled with thorotrast (arrows). (Windle, et al.: Sarg. Gyn. sc Obst» Vol. 69. 1939.) | |||
In addition to these experiments, three other fetuses dying in utero when the mothers died during diHicult labor or which were otherwise asphyxiated at the time of birth showed lungs filled with the thorotrast in consequence of liaviiig aspirated the amniotic contents (Fig. 38) . | |||
to | The literature contains a repQrt of a human fetus of the 6th month in which an experiment similar to those in the guinea pigs was performed. EhrhardtZS placed thorotrast in the amniotic sac 15 hours before he performed an hysterectoniy in a mentally defective woman requiring sterilization. Roentgenograms of the fetus talcen afterward showed that the thorotrast was concentrated in the stomach and small intestine, but none was present in the respiratory tract. A number of other observations in human fetuses at term, following the use of strontium iodide according to a method devised to outline the fetal Position, likewise failed to show fetal 1ung shadows.57 However, this materia1 gives a very weak visualization of fetal structures at best. 0ther experiments employing thorotrast, which have been described recently, seem to indicate that human fetuses of 4 to 6 months of gestation do aspirate amniotic lluid in utero.58 The total number of experiments performed was not stated but one particularly impressive one was described as illustrative of the series. In this instance (5 to 6 months) 25 cc. of thorotrast was injected into the amniotic sac 48 hours before the pregnancy was to be terminated surgicallv After Zo hours two films were exposed and these showed rather indefinite shadows which were probably cast by the opaque material aspirated by the fetsus. After the operation had been per— formed roentgenograms of the fetus showed very heavy lung shadows. In these experiments one cannot be certain that physiologically normal conditions prevai1ed because no specific data were given. The patients required surgical interruption of pregnancy, but it is not known whether they were tuberculous, suffered from cardiac disease, or from some uterine pathology which could have disturbed the normal uterine physiology Ehrhardt’s one patient was a mental defective and presumably had no pathology which would embarrass respiratory exchange in the placenta; correlative1y he found no aspiration of amniotic contents. In the more recent experiments there was no mention of preoperative medication and anesthetic. | ||
Fig. 38. Thorotrast (1 cc.) injected into the amniotic sacs on the Bist: day of gestation without using anesthesia. Daily roentgenograms showed no lung shadows Birth occurred on the 64th day. Fetus A was alive at birth. Fetus B died during birthz its lungs became filled with thorotrast. (W’indle, et al.: Sarg. Gyn. 8c Obst. Vol. 69, I939.) | |||
The experimental studies in guinea pigs demonstrated that as— piration of amniotic contents does not occur normally but that it may be brought about under asphyxial conditions. Furthermorex not all fetal movements which appear to be rhythmical and re· semble respiration serve to bring about aspiration of amniotic fluid. The recent human experiments whsich show aspiration in utero are unimpressive and open to question because proof that they represent the normal condition is laclcing. Although minor, atonic respiratory movements may occur during prenata1 life. there can be little doubt that the presence of vernix caseosa, lanugo hair, etc. in the lungs of infants at birth is unphysiologic and is associated with the deeper, dyspneic respiratory activity like that encountered in fetuses suffering from rather severe anoxemia. | |||
==Fetal Hiccup== | |||
The phenomenon of hiccup in utero was first described in 1880 by Mermann 59 and has been observed infrequently by many obstetricians since that time. DeLee 60 reported that hiccups can be identiiied as early as the ftfth month. They are short quiclc jerks of the shoulders and trunlc occurring about 15 to so times a minute and are regular (rhythmica1?) , visible, audible and palpable to the observer. In one instance, he declared, the hiccups were heard just before birth and within a minute after birth the infant was hiccuping so loudly it could be heard in the adjoining room. | |||
In our experience with fetuses of cats and other animals fetal hiccups have never been identiüed and to my lcnowledge no other investigators have found them except in the human. However, certain "jerky" movements of the head and trank, not distinctly of a respiratory nature, may be comparab1e with the hiccups of the human fetus. | |||
==Summary== | |||
The | Consideration of the recent experiments in feta1 respiratory movements leads to a more rationa1 conception of the subject than was formerly held. The physiology of respiration in utero is not so great1y different from that of the newborn or even of the adult organism as it might seem. | ||
Early in embryonic life, at a time which varies somewhat in different species, but rough1y at about the 1o mm. stage, the part of the somatic motor system which is to be concerned with breathing latet on has its genesis. soon higher neurons are formed and brought into a mutual1y integrated aggregate to comprise a respiratory center connected with the somatic motor system. Appropriate stimulation by neuronal afferent discharge into the center, by the chemical agent carbon dioxide acting upon it directly or by anoxial depression of its thresho1d can bring it into activity prematurelyx but it is doubtful if this newly formed respiratory mechanism actually functions in the strictly normal course of early intrauterine existence. The respiratory mechanism seems to be a dormant system charged with potentialities long in advance of the time it can be of any use to the fetus. | |||
of | |||
the | |||
This period of preparedness varies gkeatly in different animaIs. It must be exceedingly brief in the marsupials. Fortydive per cent of the gestation period passes in the cat before respiratory efforts can be induced. The figures are about 3o per cent for man and littIe more than 25 per cent for the sheep. | |||
It is possible to remove a fetus from the uterus and leave its circulation to the placenta intact, but it is impossib1e to maintain physiologic conditions comparable to those in utero before the experiment for very many minutes after this has been done. Partia1 anoxemia sets in promptly, the feta1 respiratory center thresh— old drops and its neurons begin to discharge in response to the usuaJ factors which are stimulatory in adult organisms. | |||
It | |||
Rhythms of shallow, rapid respiratory movements which are | Rhythms of shallow, rapid respiratory movements which are occasionally encountered in human fetuses late in the gestation period and which have been seen in a few laboratory animals at infrequent intervals signify that the fetus is momentarily experiencing either a depression of the threshold of its respiratory center or an elevation of the carbon dioxide in blood going to the brain. 0ne is justified in postulating a physiologic partial anoxemia in late fetal life associated with progressive decline in placental efliciency and with this, rninor rhythms of respiratory movements. Nevertheless, even these minor rhythms of shallow, rapid respiratory movements are not often encountered and when seen should give cause for apprehensiveness on the part of the obstetrician, for the danger of aspiration of amniotic contents then may become real if the partial anoxemia becomes a true asphyxia. | ||
That aspiration is not commonly encountered depends upon | That aspiration is not commonly encountered depends upon the fact that normally the fetus never sufkers a truly asphyxial state before it is born. It seems probable that the blood which it receives from the placenta is highly saturated with oxygen throughout the greater part of life in utero. If the oxygen saturation drops into the neighborhood of 50 per cent it is to be expected that shallow, rapid movements of the respiratory muscles will be inanifested. These are not accompanied by any changes in intrathoracic pressure because the normal hypotonicity of fetal muscles remains unchangedz consequently no aspiration results. But should the oxygen saturation decrease marlcedly, numerous motor neurons are activated, muscles tonus increases and dyspneic gasping movements ensue. These bring about aspiration of amniotic contents. 0ne can not doubt however that the ketus possesses a wide margin of safety in respect to the danger of «drowning" in its amniotic fluid. | ||
the fact that normally the fetus never sufkers a truly asphyxial | |||
state before it is born. It seems probable that the blood which it | |||
receives from the placenta is highly saturated with oxygen | |||
throughout the greater part of life in utero. If the oxygen saturation drops into the neighborhood of 50 per cent it is to be | |||
will be inanifested. These are not accompanied by any changes in | |||
intrathoracic pressure because the normal hypotonicity of fetal | |||
muscles remains unchangedz consequently no aspiration results. | |||
But should the oxygen saturation decrease marlcedly, numerous | |||
motor neurons are activated, muscles tonus increases and dyspneic | |||
gasping movements ensue. These bring about aspiration of | |||
amniotic contents. 0ne can not doubt however that the ketus | |||
possesses a wide margin of safety in respect to the danger of | |||
«drowning" in its amniotic fluid. | |||
==References Cited== | |||
i. Preyer, W. i885. specielle Physiologie des Embryo» Grieben, Leipzig. | i. Preyer, W. i885. specielle Physiologie des Embryo» Grieben, Leipzig. a. Windle, W. F» C. A. Dragstedy D. E. Murray s: R. R. Greene. i938. surg. Gynec. Obst» 66: 987. | ||
a. Windle, W. F» C. A. Dragstedy D. E. Murray s: R. R. Greene. i938. | |||
surg. Gynec. Obst» 66: 987. | |||
Z. Hartmary c. G. i92o. Anat. Ren, 192 Ist. « | Z. Hartmary c. G. i92o. Anat. Ren, 192 Ist. « | ||
| Line 758: | Line 184: | ||
4. Ahlfeld, F. i888. Verhandl. deutsch. Gesellsch Gynälsp g: 2o3. | 4. Ahlfeld, F. i888. Verhandl. deutsch. Gesellsch Gynälsp g: 2o3. | ||
z. Weber, H. i888. Inaugural dissertatiom Marburg. (cited by Ahlfeld.) | z. Weber, H. i888. Inaugural dissertatiom Marburg. (cited by Ahlfeld.) s. Ahlfeld, F. i9o5. Monatschr. Geburtsh. Gynälc., ei: i43. | ||
s. Ahlfeld, F. i9o5. Monatschr. Geburtsh. Gynälc., ei: i43. | |||
7. Reiikerscheich K. i9i«i. pliiiger’s Arcli., i4o: i. | 7. Reiikerscheich K. i9i«i. pliiiger’s Arcli., i4o: i. | ||
s. snyder. F. F. 8c M. Rosenfeld. 19377 J.A:M.A.« io8: i946. | s. snyder. F. F. 8c M. Rosenfeld. 19377 J.A:M.A.« io8: i946. MDO s Id Iiddd VII— occ IIICIIIIC VIII-III« PEPSVTIP THE | ||
MDO | |||
s | |||
Id Iiddd | |||
VII— occ | |||
IIICIIIIC VIII-III« | |||
PEPSVTIP THE | |||
. Minkowskh M. t9aa. | . Minkowskh M. t9aa. | ||
Windle, W. F» R. F. Becher, E. E. Barth sc M. D. schulz Gyn.« Obst» 69: 7o5. corey, E. L.: t93a. J. Exp. Zool., 6t: t. | |||
. cartttichaeh L. t934. Genetie Psychol. Monogr» t6: 337. . snyder, F. F. sc M. Rosenkeld. t937. Am. J. Physiol» ttg: t53. . Barcrokt, J. sc D. H. Barron. t936. J. Physiol» 88: 56. | |||
. cartttichaeh L. t934. Genetie Psychol. Monogr» t6: 337. | |||
. snyder, F. F. sc M. Rosenkeld. t937. Am. J. Physiol» ttg: t53. | |||
. Barcrokt, J. sc D. H. Barron. t936. J. Physiol» 88: 56. | |||
Bat-Gott, J. sc D. H. Barron. t937. Ibid., 9t: sag. | Bat-Gott, J. sc D. H. Barron. t937. Ibid., 9t: sag. | ||
Donat, B. E., c. M. Blutnenkeld sc c. Fenning. t938. Am. J. Dis. child» | Donat, B. E., c. M. Blutnenkeld sc c. Fenning. t938. Am. J. Dis. child» 55: t. » | ||
55: t. » | |||
Brot«-n, T. G. tgt5. J. Physiol» 49: ao8. | Brot«-n, T. G. tgt5. J. Physiol» 49: ao8. | ||
coronios, J. D. t933. Genetie Psychol. Monogr» t4: asz | coronios, J. D. t933. Genetie Psychol. Monogr» t4: asz Windle, W. F» M. Monnier sc A. G. steele. t938. Physiol. Zool» tt: 4a5. | ||
Windle, W. F» M. Monnier sc A. G. steele. t938. Physiol. Zool» tt: 4a5. | |||
Erbkam. t837. Neue Ztschn Geburtsk» z: 3a4. | Erbkam. t837. Neue Ztschn Geburtsk» z: 3a4. | ||
| Line 802: | Line 209: | ||
strassmanm P. t9o3. sammh Hin. Vortr» Gynälc» No. t3a: 947. | strassmanm P. t9o3. sammh Hin. Vortr» Gynälc» No. t3a: 947. | ||
schweitzer med. Wchnschr» No. ag and so: 7at, | schweitzer med. Wchnschr» No. ag and so: 7at, 75t. | ||
75t. | |||
Bolaflio, M. sc G. Artom. t9a4. Arch. d.i sei. Biol., Z: 457. | Bolaflio, M. sc G. Artom. t9a4. Arch. d.i sei. Biol., Z: 457. | ||
| Line 809: | Line 215: | ||
Walz, W. t9aa. Monatschn Geburtsh. Gynälc., 6o: 33t. | Walz, W. t9aa. Monatschn Geburtsh. Gynälc., 6o: 33t. | ||
Bart-mit, J» D. H. Barron, K. Kramer sc G. A. Millikarp | Bart-mit, J» D. H. Barron, K. Kramer sc G. A. Millikarp Physiol» 9o: 29 P. | ||
Physiol» 9o: 29 P. | |||
1937- J— | 1937- J— | ||
. Abel, s. sc W. F. Windle. t939. Anat. Ren, Es: 45t. | . Abel, s. sc W. F. Windle. t939. Anat. Ren, Es: 45t. . Bouceltz c. M. sc A. D. Renton. . Rosenkeld, M. sc F. F. sitz-der. | ||
. Bouceltz c. M. sc A. D. Renton. | |||
. Rosenkeld, M. sc F. F. sitz-der. | |||
t93t. surg. Gyn. Obst» Ha: 84t. | t93t. surg. Gyn. Obst» Ha: 84t. t939. Am. J. Obst. sc Gyn., 38: 4a4. KoiK A. K. sc M. E. Das-is. t937. Ibid., 34: as. | ||
t939. Am. J. Obst. sc Gyn., 38: 4a4. | |||
KoiK A. K. sc M. E. Das-is. t937. Ibid., 34: as. | |||
cohnstein, J. sc N. Zuntz t884. Pkliigeks Arch., 34: t73. | cohnstein, J. sc N. Zuntz t884. Pkliigeks Arch., 34: t73. | ||
. Huggett, A. St. G. t93o. J. Physiol» 69: t44. | . Huggett, A. St. G. t93o. J. Physiol» 69: t44. . Barcroky J. t935. Irish J. Med. sci., series 73 t: a89. | ||
. Barcroky J. t935. Irish J. Med. sci., series 73 t: a89. | |||
Flint, A. t88o. Am. J. Med. sei» so: 69. | Flint, A. t88o. Am. J. Med. sei» so: 69. | ||
. salmi, T. t93Z. Acta soc. Med. Fennicae (ser. B, fast. t, art. a) , t8: t. | . salmi, T. t93Z. Acta soc. Med. Fennicae (ser. B, fast. t, art. a) , t8: t. . Zuntz, N. t877. Plliigeks Arch» t4: 6o5. . steele, A. G. sc W. F. Windle. | ||
. Zuntz, N. t877. Plliigeks Arch» t4: 6o5. | |||
. steele, A. G. sc W. F. Windle. | |||
t939. J. Physiol» 94: Ist. | t939. J. Physiol» 94: Ist. Barcrofh J. and M. F. Mason. t938. Ibid., 93: aa P. Barcrokt, J» K. Kramer sc G. A. Millilcan. t939. Ibid» 94: 57t. | ||
Barcrofh J. and M. F. Mason. t938. Ibid., 93: aa P. | |||
Barcrokt, J» K. Kramer sc G. A. Millilcan. t939. Ibid» 94: 57t. | |||
. Eastmary N. J» E. M. K. Geiling sc A. M. DeLawder. t933. Johns Hop | . Eastmary N. J» E. M. K. Geiling sc A. M. DeLawder. t933. Johns Hop lcins Hosp. Bull., IF: a46. | ||
lcins Hosp. Bull., IF: a46. | |||
. Eastman, N. J. t93o. Ibid» 47: aat. | . Eastman, N. J. t93o. Ibid» 47: aat. | ||
. Bidone, M. t93t. Ann. di ostet. e ginec., 53: t97. | . Bidone, M. t93t. Ann. di ostet. e ginec., 53: t97. . Roos, J. sc c. Romijxx . Windle, W. F. and Barcrokr. t938. Arn. Physiol» tat: 684. | ||
. Roos, J. sc c. Romijxx | |||
. Windle, W. F. and | |||
. Windle, W. F» L. G. scbarpenbetg sc A. G. steele. t938. Ibid., tat: 69a. | . Windle, W. F» L. G. scbarpenbetg sc A. G. steele. t938. Ibid., tat: 69a. . wiud1e, w. F. s« D. N . Kuo, Z. Y. sc T. c. shet»t. t937. J. cornp. Psychol» a4: 4g. . Rotnijn, c. sc J. Roos. . Wind-le, W. F. t934. . Windle, W. F» D. W. Ort· sc W. L. Minean t934. Physiol. Zool» 7: 6oo. . Pitts. R. F» H. W. Magoun sc s. W. Ranson. t939. Am. J. Physiol» tas t938. J. Physiol» 9a: a49. elson. t"938. Ibid» tat: 7oo t938. J. Physiol» 94: 365. J! cotnp. Neur.,· 59: 487. | ||
. wiud1e, w. F. s« D. N | |||
. Kuo, Z. Y. sc T. c. shet»t. t937. J. cornp. Psychol» a4: 4g. | |||
. Rotnijn, c. sc J. Roos. | |||
. Wind-le, W. F. t934. | |||
. Windle, W. F» D. W. Ort· sc W. L. Minean t934. Physiol. Zool» 7: 6oo. | |||
. Pitts. R. F» H. W. Magoun sc s. W. Ranson. t939. Am. J. Physiol» tas | |||
t938. J. Physiol» 9a: a49. | |||
elson. t"938. Ibid» tat: 7oo | |||
t938. J. Physiol» 94: 365. | |||
J! cotnp. Neur.,· 59: 487. | |||
. | . | ||
. Hendetsom Y. t938. Adventures in Respiratiotr. Williatns sc Willcins, | . Hendetsom Y. t938. Adventures in Respiratiotr. Williatns sc Willcins, Baltitnore. | ||
Baltitnore. | |||
. Kraft-ca, J. t933. Am. J. Dis. child» 45: too7. | . Kraft-ca, J. t933. Am. J. Dis. child» 45: too7. | ||
52. Geyl, A. 188o. Arclx Gynäk., is: 385. | 52. Geyl, A. 188o. Arclx Gynäk., is: 385. | ||
| Line 879: | Line 258: | ||
56. Ehxhardy K. 1937. Münclx mecL Wchnschr., 84: 1699. | 56. Ehxhardy K. 1937. Münclx mecL Wchnschr., 84: 1699. | ||
57. Mem-es, T. 0., J. D. Miller sc: L. E. Hollzn 193o. Am. J. Roemz Rad. | 57. Mem-es, T. 0., J. D. Miller sc: L. E. Hollzn 193o. Am. J. Roemz Rad. Ther., 24: 363. | ||
Ther., 24: 363. | |||
58. Reifkerscheict W. sc R. schmiemantx 1939. lenkt-kühl. Gynälk 63: 146. | 58. Reifkerscheict W. sc R. schmiemantx 1939. lenkt-kühl. Gynälk 63: 146. | ||
| Line 886: | Line 264: | ||
59. Mermanty A. 188o. ZentralbL Gynäk., 4: 377. | 59. Mermanty A. 188o. ZentralbL Gynäk., 4: 377. | ||
so. DeLee, J. B. 1938. The Principles and Practice ok 0bstetrics, saunders, | so. DeLee, J. B. 1938. The Principles and Practice ok 0bstetrics, saunders, Phj1ade1phia. | ||
Phj1ade1phia. | |||
{{Footer}} | |||
Latest revision as of 15:39, 10 September 2018
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Windle WF. Physiology of the Fetus. (1940) Saunders, Philadelphia.
1940 Physiology of the Fetus: 1 Introduction | 2 Heart | 3 Circulation | 4 Blood | 5 Respiration | 6 Respiratory Movements | 7 Digestive | 8 Renal - Skin | 9 Muscles | 10 Neural Genesis | 11 Neural Activity | 12 Motor Reactions and Reflexes | 13 Senses | 14 Endocrine | 15 Nutrition and Metabolism | Figures
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter VI Fetal Respiratory Movements
It is commonly taught that the fetus in utero is apneic. I-Iowever, this concept has been chal1enged from time to time and some investigators have declared that respiratory movements occur regularly throughout prenatal life under normal physiologic conditions. No one doubts that mammalian fetuses are capable of performing rhythmical movements which talce the form. of shallow rapid breathing or even of dyspneic gasping. Movements of this nature are frequently encountered upon opening the uterus of a pregnant laboratory animal. The literature contains many articles in which mention of such activities has been made. The early studies were reviewed by Preyer.1 Human fetuses, aborted or removed surgically from the uterus, show the move— ments in question as early as the twelfth weelc of gestationk 0ne of the ·most strilcing examples of respiration in immature forms is encountered in the opossum.3 Although this animal is born after a sojourn« of only 1243 days in utero (or in the tubes, as a blastocyst) it is able to breathe and to make its way unassisted to the pouch where it reassumes dependence upon the mother. Most studies in other animals have employed fetuses removed from the uterus and one may well question whether any of them were representative of the normal. It is not permissible to conclude, on the basis of observations made under conditions which preiudice the placental exchange mechanism, that respiratory motor phenomena are indulged in normally by the fetus throughout prenatal life.
Respiratory Movements in the Intact Animal
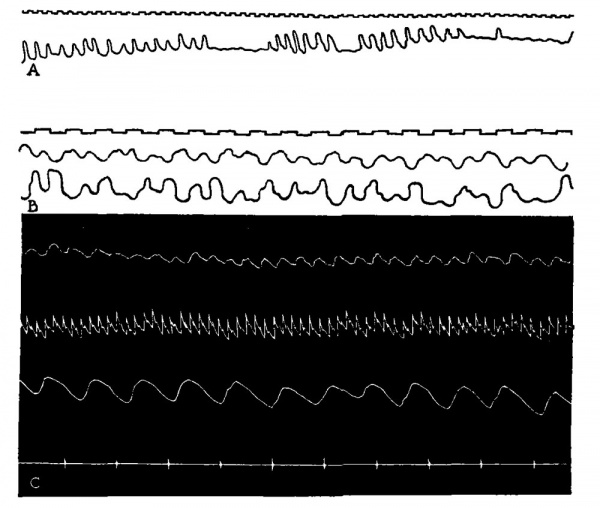
Let us examine the evidence of fetal movements resembling respiration in the intact animal. In 1888 Ahlfeld, a German gynecologist, described certain rhythmical fetal movements which he and a pupil observed in patients during the latter weelcs of pregnancyxss Z superimposed upon the slow excursions of the gravid abdomen which resulted fronkmaternal respirations they could see more rapid rhythms which appeared to be due to activities of the fetus. Ahlfeld proposed that the ketus was making respiratory efforts and thought it must aspirate its amniotic fluid into the major respiratory passages. Many protested this theory, but as recently as 1905 he reiterated his views and published convincing graphic records of these movementsJk The intrauterine respiratory movements have been observed by other investigators,7 but the theory of« aspiration of fluid has not been generally accepted and it is usually concluded that the fetal glottis remains cJosed. The question has been raised again recently by investigatorss who are of the opinion that the human fetus executes rhythmical respiratory movements normally and that these serve to draw amniotic fluid into the lungs. It was even suggested that this fluid may assist in the development of the lung alveoli. Tracings illustrating the character of human» fetal respiratory movements are reproduced in Fig. se. A strilcing comparison of intrauterine fetal and early postnatal respiratory records will be seen in Fig. 33.
Fig. 32. Human intrauterine respiratory movements A, Intesrrupted rhythms at a frequency of about 52 per minute. B, Movements recorded from fetal abdomen and chest simultaneously about 80 per minute. (Ahlfeld: Monatschr. Geburtsh. Gynälsp Vol. 21, 1905.) C, Intrauterine fetal respiratory movements (upper) , maternal carotid Pulse, maternal respirations and time in 4 second intervals (lower) before labor be»gan. (K. Reifferscheid: Pflugers Arch., Vol. 140, 1911.)
The principal objection to the current view is that the movements in question are not always, in fact not often, manifested by the human ketus. One must watch patiently to see them and when they do appear they are usually transient». If they were commonly encountered in the human, one should expect to see them in some other animals wo. Fetal respiratory-like activities are rarely apparent in cats and guinea pigs but have been seen a day or so before birth.9 The question of aspiration by the fetus will be considered later. It is pertinent first to invcstigate the factors which govern the manifestation of fetal respiratory-like movements.
Fig. 33. Comparison of human intrauterine respiratory movements (upper) with those of a five day old inkanr. Time, 4 seoond intervals. (K. Reifferscheid: Pflugers Arch., Vol. 140, 1911.)
Respiratory Movements Under Experimental Conditions
Respiratory movements have been studied recently in a number of laboratory animals including the rat, guinea pig, rabbit, sheep and cat.9-18 They have been observed in human beings under somewhat similar conditions.19-23 There is little doubt that their occurrence and characteristics are influenced by the experimental conditions. These must be evaluated critically.
In the first place, anesthcsia seems to alter the threshold of the fetal respiratory center and for this reason most investigators have tried to avoid using it. Ether administered to the mother anesthetizes the fetuses which then rarely« exhibit respiratory activities until after placental circulation has been interruptedz at that time they talce the form of dyspneic gasping. Urethane lilcewise de— presses the rapid rhythms of the shallow respiratory movements.24 Doses of nembutal which anesthetize the mother similarly depress the fetusks Although sodium amytal is said to have no ekfect upon the fetus of the rat when given to the motherW and not to pass the placental barrier readily, further consideration should be given to this question. It is possible that some asphyxial types of anesthesia first stimulate respiratory movements in normally apneic fetuses,9 but there is little doubt that their continued use results in depression. Cyclopropane (3o per cent) is said to be about the only inhalation anesthetic which does not seriously affect the respiratory activities of fetuses and still gives surgical anesthesia.27
Fig. 34. Two portions of a continuous crystograph record of uterine motility. maternal respiratjon and intrauterine fetal respiratory movements on the 67th day of gestation in the tat (end of gestation) . The animal had been decerebrated by the anemia method: no anesthesia was used during the experiment the abdomen was not opened. The major kluctuations at the beginning of each tracing indicate relaxation of the Uterus after a contraction occurring one minute before the arrow (↑). The large secondary waves are the maternal respirations (17 per minute) . The smallest waves are intrauterine fetal respiratory-like movements occurring at approximately 120 per minute, slower at first than when they are well under way. Five minutes between relaxations 1 and 2.
Various experimental procedures have been employed to avoid the use of general anesthetics spinal anesthesia can be used successfully in the larger species. spinal cord section if performed long enough beforehand to allow recovery from shoclc may be satisfactory in those animals which are rather docile anyway. Decerebration is to be recommended in other forms. After preparing an animal near term by one of these methods it is occasionally possible, without opening the .maternal abdomen, to see« movements of a rhythmical nature resembling fetal "breathing." In Fig. 34 such fetal respiratory movements are shown (after decerebration of the cat) superimposed upon maternal respirations This curvc was obtained by means of a crystograph activated by amplified potentials from an oscillating eircuit, any movements on the surface of the catss abdomen changing the frequencies of oscillation. To what extent decerebration, spinal cord Section or other preparatory surgical procedures were responsible for instituting the respiratory movements, is not known.
When the pregnant animal is placed in a bath of warm saline solution, the abdomen opened and the uterus exposed, it is pos— sible to observe fetal activities more directly. Rabbit and guinea pig fetuses near term can be seen quite easily through the thin uterine wall. Body form of other species is readily distinguishable. During the course of zo minutes or more of observation, respiratory rhythms of fetal movements usually malce their appearance in the cat. The longer the uterus has been exposed and the nearer the end of gestation, the more frequently these activi· ties manifest themselvess They rarely continue for more than a few seconds but start and stop at fairly regular intervals. This intermittent occurrence of brief rhythms is associated with motility of the uterus in the cat. Mild contraction of uterine muscle is accompanied by a period of fetal quiescence relaxation of the muscle leads to a rhythm of respiratory Ihovements Fetal activities of a respiratory nature are rarely if ever seen in the unopened uterus before the last two weelcs of gestation in the cat. This is not because it is more difficult to visualize younger fetuses, for as we shall soon see it is possible to induce the movements in question by appropriate experimental methods as early as the middle of prenatal life.
Most of the earlier studies on respiratory movements of fetuses were made after delivering the specimens from the uterus under warm saline solutions, leaving the placenta attached to the uterine wall. It has often been erroneously assumed that circulation and gaseous exchange can thus be maintained adequately and that observations made in the fetus under such conditions are representative of activities occurring normally in the intact animal.
When a cat fetus has been determined to be apneic in utero and is then carefully but quiclcly delivered through a small incision in the least vascular portion of the uterus at one end of the zonary placenta it commonly begins to execute respiratory moves ments within a very short period of time; often this is a matter of one minute or less. The blood of the umbilical veins darlcens perceptibly at delivery and the— manifestation of respiratory movements is associated with such darlcening in cat fetuses during the third quarter of gestation. 18
Feta1 respiratory movements have been studied in other mammals under similar conditions.I3-14 It was found that the earliest respiratory movements of sheep fetuses can be elicited by mechanical stimulation at about 38-39 days of gestation. The first rhythms of movement seem to be enhanced by putting pressure upon the amnion and thus indirectly stimulating the fetus. Later they seem to occur spontaneous1y, but it is never possible to avoid some mechanical disturbance of the fetus and the manips ulation of the uterus itse1f may have been a factor in producing the «spontaneous" activities. Urethane or spinal anesthetics were employed for the ewes.
Rhythmical respiratory movements have been studied in rabbits after sectioning the spinal cords of the pregnant does« The activities were manifested both before and after delivering the fetuses from the exposed uterus. Most of the rabbits were at term or had passed the time birth should have occurred normally, labor having been inhibited by hormone treatment. It is possible that the post-mature fetuses suffered inefiicient placental exchange of oxygen and carbon dioxidez it has been shown that all rabbit fetuses die by the zöth day (normally delivery occurs at 31 or 32 days) when labor is inhibited. 28
From the various experimental studies it is clear that mammalian fetuses are capable of exercising their respiratory muscles and of doing so rhythmically after the fashion of airbreathing animals rather early in prenatal like. But mammalian fetuses do not execute rhythmical respiratory movements continually in utero unless certain unfavorable conditions are set up. Even near the termination of life in utero when the eliiciency of the placenta in relation to the greatly enlarged fetus has declined and when one might expect to lind a physiologic anoxemia, it is surprising how seldom the activities in question can be observed in the intact animal.
Relation of Oxygen and Carbon Dioxide to Respiratory Movements
Since the blood of the fetus comes almost into equilibrium with that of its mother in respect to tensions of carbon dioxide and oxygen in the placental capillary bed one may well raise the question: why does the fetal respiratory center not respond as readily as that of its mother? It has been assumed that the threshold is higher than that of the mother.29- 30 If this is one of the main factors in preventing continuous fetal respiratory moves ments from manifesting themselves norma1ly in utero it should be possible to find a carbon dioxide level at- which the fetal center will respondz or perhaps the threshold ok the fetal center can be lowered experimentally until fetal respiratory movements begin.
It has been suggested that accumulation of metabolic carbon dioxide may be the agent starting these movements in cat and rat fetuses. 10, 16 Barcroft and his colleaguess 31 administered atmospheres rich it; carbon dioxide to pregnant sheep without inducing respiratory movements in the fetuses. However, the animals had been anesthetized with urethane and it is possible that the threshold of the center had been raised by this drug to a Ievel which could not be reached by. the maximum concentration the blood could carry. The ekfect of giving carbon dioxide to pregnant rabbits has been investigated.12 The fetuses were already executing respiratory movements and the rate of the fetal rhythm was not greatly altered by the gas. The only positive results were obtained in cat fetuses 18 mostly in the third quarter of the gestation periodx the fetuses were apneic at the time of experimentas tion. Some were delivered from the uterus with placental circulation intact and others were observed through the unopened uterus. When the mother cats breathed atmospheres of oxygen containing 8 to 1o per cent carbon dioxide, rhythms of rapid respiratory movements appeared both in the delivered and the intact fetuses concomitantly with the increase in rate and amplitude of maternal breathing. substitution of 15 to 20 per cent carbon dioxide in oxygen led to depression of the fetal respiratory efforts, which became very deep gasps at a slow rate. The· cat was then allowed to breathe air again and a «rebound hyperpnea" often followed in the fetuses, presumably as the carbon dioxide level re— adjusted itself in the fetal blood. 0ne of these experiments is illustrated in Fig. 35.
Administration of mixtures of 5 to 8 per cent oxygen in nitrogen to the mother cats brought about cyanosis, indicated by darlcening of the uterine vesse1s, and respiratory movements of the previously apneic fetuses began promptlysz Compression of the umbilical cord produced similar results in exaggerated form and more quiclcly. In large fetuses of sheep it was found that compression of the umbilical arteries alone led to respiratory activity but that this activity was delayed and did not manikest itselk so soon as it did after compressing the veins too.31 This indicates that the fetus was able to draw upon blood in the placenta and obtain a little more oxygen while the veins were intact. Many other investigators have reported that interruption of the placental circulation brings about respiratory efforts in the fetus. 1, 10, 16, 32, 33, 34 Usually these were of the dyspneic gasping type. When asphyxia is avoided fetal respiratory movements of the cat occur at rates as high as 120 per Minute, commonly more than 60 per minute.
Fig. 35. Effect of an 8 to 10 per cent carbon dioxide-oxygen mixture, breathed by the mother, in initiating rhythmical respiratory movements in a cat fetus delivered at caesarean Section but with placental circulation intact Respiratory rate in half minutes. (Wind1e, et al.: Physiol. Zool» Vol. 11, 1938.)
Atmospheres defccient in oxygen breathed by full term guinea pigs on which no surgery had been performed and no anesthetick used caused rhythmical respiratory movements to start in the previously apneic fetuses.9 Usually the first activities to appear were rather fast rhythms of movement which resembled shallow respirations. With more marked cyanosis these became stronger and slower. Ultimately only slow rhythmical gasps were seen in the fetuses. Upon relieving the asphyxia the rapid rhythms returned and then they too stopped, the fetuses becoming apneic again with return of normal oxygenation.
Blood-gas analyses have been made from samples withdrawn anaerobically from the umbilical veins of cat fetuses delivered from the uterus but« in which the placental circulation was still intact. 35 It was found that the oxygen content was low. the blood being little more than so per cent saturated in those fetuses which were executing rhythmical respiratory movements at the time of sampling. When oxygen saturation dropped to about 25 per cent or less the fetuses gasped or became depressed to the point of complete inactivity. Under the conditions of the experiments it was less p0ssible to relate the presence or absence of the movernents in question to carbon dioxide than to oskcygen content. However, in the presence of the higher oxygen levels (4o-5o per cent saturation) the carbon dioxide content was greater than it was when the oxygen level was marlcedly loweredz in the former instances the fetuses executed rapid rhythms, whereas in the latter they became inactive and depressed. Arrhythmical deep gasps appear-ed when the carbon dioxide was high and the oxygen low.
It was not possible to obtain blood from the cat fetuses with· out incising the uterus and disturbing relationships there to some extent; consequently one does not know what the oxygen level is in utero. However, it was evident that respiratory efforts often began as the fetus was»being delivered and as the umbilical vein blood was darlcening Probably a higher oxygen level is maintained in the normal undisturbed uterus than the highest value obtained at experimentation would indicate. This is true in other species. The sheep in late fetal life is apneic in uteroz correlatively its umbilical vein blood, obtained without removing the lamb from the uterus, is highly saturated, exceeding 90 per cent in somekss But when the lamb is delivered into a. bath of saline solution with its placental circulation intact the blood becomes reduced.37
In the human at normal birth, apnea prevails when the blood is about 50 per cent saturated with oxygen irrespective of the carbon dioxide contents, 38 but respiration starts readily. Some higher values have been obtained in apneic human fetuses 39, 40 as well as in the calf.41 0ne may infer from these experiments that the normal apnea ok ketuses in utero is associated with a degree ok oxygenation exceeding about 4o or 50 per cent saturation, that as this becomes reduced rhythms of active respiratory movements appear, but that depression ok the oxygen level much below 25 per cent saturation 1eads to gasping and ultimately to complete inactivity comparable with that in asphyxia neonatorum.*
- Barcrokt and his colleagues have demonstrated recently that apnea prevails in the sheep ketus when the blood going to the ketal brain is about So to so «per cent saturated with oxygen and when that leaving it is zo per cent or more Saturated. Lower values were encountered during the .1ast prenatal wee1c. Respiratory movetnents occurred when the blood leaving the brain was to to 25 pr cent aaturated (Barcrokt, J. D. H. Barron, A. T. cowie sc P. H. Forsham 1940, J. Physiol. 97: 338)
Fig. 36. Oxygen and carbon dioxide contents ok the atmosphere of the air space in two hencs eggs talcen at daily intervals during incubatiotx Respiratory moves ments begin at the plateau between 17 and 19 days. (Romijn and Roos: Jour. Physiol. vol. 94, 1938.)
Experiments in mammalian fetuses have been coniirmed in the incubating eggs ok chiclcs and duck.42-45 It was possible to control physiologic conditions more precisely in the bird than in the mammaL Although space limitations do not allow discussion of these studies here it should be pointed out that our lcnowledge of the atmospheres breathed by the bird throughout incubation is reasonably complete. A nice correlation can be drawn between the decline in oxygen concentration, the elevation of carbon dioxide and the appearance of respiratory movements« This is illustrated in Fig. 36.
We learn from the various experiments with carbon dioxide excess and oxygen want in mammals and in birds that the fetal respiratory center responds much as does that of the adult, but there is one very marlced difference between fetus and adult. Greater concentrations of carbon dioxide and more severe degrees of oxygen deliciency mustcome into play in the former before results are obtainable. In other words the fetal respiratory center seems to have a high threshold.
Other Factors in the Development of Respiration
Not all mammals are capable of performing rhythmical respiratory movements at the same period in prenatal life. One finds some species more precocious than others. As has already been indicated, the newborn opossum «embryo« breathes about 13 days after conception. In the more advanced fetus of the sheep respiratory movements have been observed at the end of the first quarter, but still at a relatively earlier time than in the cat. The latter reaches nearly the middle of gestation before rhythmical respiratory movements can be induced. The human fetus has accomplished necessary growth by the end of the twelfth weelc of intrauterine existence.
Muscles and peripheral nerves are laid down long before fetal respiratory movements begin. It is quite clear that the accomplish— ment of rhythmical movements awaits further development of a central nervous mechanism. In the 15 mm. cat embryo intrinsic growth of spinal neurons and their connections have reached the point at which the first simple rellexes are possible.47 From this time onward the Process of nervous integration proceeds, new responses accompanying new nervous connections and simple activis ties becoming more complexsIs Most of the responses of which a cat embryo of 2o mm. length, delivered yvspith Flacental circulation intact, is capable appear to be almost purposeless. The rhythmical respiratory movements which make their appearance just before the 30 mm. stage are among the first to foretell a usefulness. However, the behavior pattern that makes possible rhythmical respiration is not a new thing; it is the rhythmicity that is new. Even before 30 mm., in fact as early as 20 mm., integkated but arrhythmical contractions of the future respiratory muscles occur when adequate stimuli are used to elicit them. Their resemblance to gasps is often strilcing It seems probab1e in all the species which have been studied (rat, rabbit, guinea pig, cat, sheep, goat and man) that the motor pattern responsible for respiration is a very fundamental one. It consists of the appropriate muscles with their motor neurons. Respiratory acts manifest themselves under experimental conditions as soon as the central nervous mechanism for simultaneous1y discharging these groups of eiferent neurons is developed. The period in fetal life at which this occurs is that during which there is an extensive development of connecting neurons of the reticular formation in the medu1la oblongata and downward growth into the spinal cord of many longitudina1 nerve übers. Ground bundles, reticu1ospinal and similar phylogenetically old pathways have been laid down. The appearance of rhythmicity has not been correlated with any specific change in the central nervous System. Growth there has gone so far at so mm. that many of the structures characterizing the adult tegmens tum have appeared. Multiplication of association neurons, and consequently of possible connections in neuron circuits, seems to characterize this stage in fetal brain growth.
A very ingenious theory concerned with neural control of respiratory movements in the sheep fetus has been proposed.14 In the Erst place, it was assumed that the rapid rhythmical move— ments seen in 4o—day-old sheep fetuses delivered at experimental Caesarean section are physiologically normal and that they result from some poorly understood rhythmical (autochthonous?) discharge of the respiratory center. The progressive change in character of these movements which is encountered during further development of the fetus and which culminates in complete apnea at about 6o days gestation, it was suggested, is due to the progressive downgkowth of new inhibitory neurons from the higher nervous centers. Asphyxia or experimental transection of go the brain-stem between midbrain and pons appeared to abolish the inhibitory influences Jand leave a more primtive respiratory pattern like that seen in fetuses 50 days old or less. Although it is certain that all fetal activities change with the gradual maturation of nerve tracts in the brain, the experimental data do not prove the theory proposed and alternative theories may be equally well supported. It is quite possible that expiratory and pneumotaxic centers are dominant to the inspiratory center in fetal life. Inhibition of the inspiratory center by impulses from the pneumotaxic center or continual stimulation of the expiratory center would account for the unexpanded chest and collapsed lungs.49 Perhaps in the early sheep fetus the inspiratory center has not yet come under the dominance of higher neurons.
The importance of muscle tonus in establishing conditions favorable to lung ventilation at birth has been stressed by Some» If the fetus in utero possessed the tonus of a healthy newborn it is diflicult to see how it could iit itself to the space allowed and how it could keep from iilling its lungs with fluid. In one experimentZI action potentials from electrodes which were placed in the fetal muscles were demonstrated each time the specimen was lifted out of its warm saline bath. These stopped when it was returned to the bath (see Fig. 63, p. 176) . It seems probable that the increase in stimulation of afkerent neurons attendant upon delivering the fetus from a warm aqueous habitat to the outside air plays an important part in increasing the tonus of the muscles in consequence of setting up activity in neuron circuits. This neural activity may serve to lower the respiratory center’s threshold and allow a lower level of carbon dioxide to stimulate it to action. 0thers have found that the respiratory center of the fetal goat four to six weeks from full term responds to afferent nerve stimulation like that of the adult, but it requires much stronger stimulation.30 It was inferred that the fetal center has a higher threshold. By decerebration of the fetus it was demonstrated that the higher threshold was not due to inhibitory influences from the cerebrum.
That the apnea of intrauterine life may be due to a physiologic anemia of developmental origin has been suggestedPI Occlusion of the umbilical arteries at birth is said to increase peripheral resistance in the aorta, raise the systemic pressure and induce a marked cerebral flow. This relieves the· anemia, the high carbon dioxide concentration prevailing acts as a respiratory stimulus and respiration is initiated.
Aspiration of Amniotic Contents
In the early paragraphs of this chapter mention was made of the suggestion that the fetus normally aspirates amniotic fluid. Granting that respiratory movements can and do occur occasional1y in utero toward the end of gestation, what is the evidence that they cause aspiration of amniotic contents? It has been pointed out that dyes injected into the amniotic sac can be found in fetal lungs after removing the fetuses.8- IN« But in none of these experiments was anoxemia rigidly ruled out of consideration. Indeed, in some it is quite obvious that it may have been a factor in starting the respiratory activity. It is known that vernix caseosa is sometimes found in the lungs of infants which have survived birth a short time. In a large series of autopsies with microscopic study Farber and sweetss found that only 15 per cent of the lungs of infants surviving birth for five weeks or less contained signilicant amounts of döbris ascribable to fetal aspiration of amniotic contents, although 88 per cent showed at least a few desquamated epithelial cells. It is doubtful if as high a per— centage of healthy infants have to cope with birth condtions such as were encountered in those living but a short time.
0ther evidence concerning fetal aspiration has been presented recently.9 Without using an anesthetic a thin hypodermic needle was passed through the abdorninal wall and into the amniotic sacs of guinea pig fetuses in the last weelc or two of gestation. small quantities of fluid were withdrawn (0.4 cc. to 1 cc.) from the region about the nostrils and replaced with colloidal solutions of thorium dioxide (thorotrast) or hydroxide (thorad) . In 27 fetuses so created a large series of x-ray lilms exposed after various intervals up to 14 days failed to show lung shadows which would signify that the fetuses had aspirated this opaque material. The thorotrast or thorad was observed in the stomach and intestines, for swallowing occurred regularly.
Twenty—five additional experiments were performed. Films exposed after these injections showed no aspiration on the part of the fetuses. The pregnant guinea pigs were then allowed to breathe atmospheres low in oxygen or high in carbon dioxide or to rebreathe air until an anoxemia was set up. Some of the fetuses could be seen executing rhythmical movements resembling respiration. Roentgenograms were obtained subsequently. Of 14 experiments showing shallow, questionable or no feta1 respiratory movements, only two films revealed thorotrast in the fetal respiratory tract. ln the remaining 12, all showing strong movements of the type in question, seven positive results such as are illustrated in Fig. 37 were obtained.
Fig. 37. Thorotrast (0.8 cc.) injected into the amniotic sacs of two guinea pig fetuses on the 63rd day of gestatiom The mother was rendered cyanotic with nitrogen containing a small amount of oxygen. Respiratory movements were observed in fetus B more clearly than in fetus A. Half an hour later, the roentgenogram was made. The bronchial tree of fetus B is well filled with thorotrast (arrows). (Windle, et al.: Sarg. Gyn. sc Obst» Vol. 69. 1939.)
In addition to these experiments, three other fetuses dying in utero when the mothers died during diHicult labor or which were otherwise asphyxiated at the time of birth showed lungs filled with the thorotrast in consequence of liaviiig aspirated the amniotic contents (Fig. 38) .
The literature contains a repQrt of a human fetus of the 6th month in which an experiment similar to those in the guinea pigs was performed. EhrhardtZS placed thorotrast in the amniotic sac 15 hours before he performed an hysterectoniy in a mentally defective woman requiring sterilization. Roentgenograms of the fetus talcen afterward showed that the thorotrast was concentrated in the stomach and small intestine, but none was present in the respiratory tract. A number of other observations in human fetuses at term, following the use of strontium iodide according to a method devised to outline the fetal Position, likewise failed to show fetal 1ung shadows.57 However, this materia1 gives a very weak visualization of fetal structures at best. 0ther experiments employing thorotrast, which have been described recently, seem to indicate that human fetuses of 4 to 6 months of gestation do aspirate amniotic lluid in utero.58 The total number of experiments performed was not stated but one particularly impressive one was described as illustrative of the series. In this instance (5 to 6 months) 25 cc. of thorotrast was injected into the amniotic sac 48 hours before the pregnancy was to be terminated surgicallv After Zo hours two films were exposed and these showed rather indefinite shadows which were probably cast by the opaque material aspirated by the fetsus. After the operation had been per— formed roentgenograms of the fetus showed very heavy lung shadows. In these experiments one cannot be certain that physiologically normal conditions prevai1ed because no specific data were given. The patients required surgical interruption of pregnancy, but it is not known whether they were tuberculous, suffered from cardiac disease, or from some uterine pathology which could have disturbed the normal uterine physiology Ehrhardt’s one patient was a mental defective and presumably had no pathology which would embarrass respiratory exchange in the placenta; correlative1y he found no aspiration of amniotic contents. In the more recent experiments there was no mention of preoperative medication and anesthetic.
Fig. 38. Thorotrast (1 cc.) injected into the amniotic sacs on the Bist: day of gestation without using anesthesia. Daily roentgenograms showed no lung shadows Birth occurred on the 64th day. Fetus A was alive at birth. Fetus B died during birthz its lungs became filled with thorotrast. (W’indle, et al.: Sarg. Gyn. 8c Obst. Vol. 69, I939.)
The experimental studies in guinea pigs demonstrated that as— piration of amniotic contents does not occur normally but that it may be brought about under asphyxial conditions. Furthermorex not all fetal movements which appear to be rhythmical and re· semble respiration serve to bring about aspiration of amniotic fluid. The recent human experiments whsich show aspiration in utero are unimpressive and open to question because proof that they represent the normal condition is laclcing. Although minor, atonic respiratory movements may occur during prenata1 life. there can be little doubt that the presence of vernix caseosa, lanugo hair, etc. in the lungs of infants at birth is unphysiologic and is associated with the deeper, dyspneic respiratory activity like that encountered in fetuses suffering from rather severe anoxemia.
Fetal Hiccup
The phenomenon of hiccup in utero was first described in 1880 by Mermann 59 and has been observed infrequently by many obstetricians since that time. DeLee 60 reported that hiccups can be identiiied as early as the ftfth month. They are short quiclc jerks of the shoulders and trunlc occurring about 15 to so times a minute and are regular (rhythmica1?) , visible, audible and palpable to the observer. In one instance, he declared, the hiccups were heard just before birth and within a minute after birth the infant was hiccuping so loudly it could be heard in the adjoining room.
In our experience with fetuses of cats and other animals fetal hiccups have never been identiüed and to my lcnowledge no other investigators have found them except in the human. However, certain "jerky" movements of the head and trank, not distinctly of a respiratory nature, may be comparab1e with the hiccups of the human fetus.
Summary
Consideration of the recent experiments in feta1 respiratory movements leads to a more rationa1 conception of the subject than was formerly held. The physiology of respiration in utero is not so great1y different from that of the newborn or even of the adult organism as it might seem.
Early in embryonic life, at a time which varies somewhat in different species, but rough1y at about the 1o mm. stage, the part of the somatic motor system which is to be concerned with breathing latet on has its genesis. soon higher neurons are formed and brought into a mutual1y integrated aggregate to comprise a respiratory center connected with the somatic motor system. Appropriate stimulation by neuronal afferent discharge into the center, by the chemical agent carbon dioxide acting upon it directly or by anoxial depression of its thresho1d can bring it into activity prematurelyx but it is doubtful if this newly formed respiratory mechanism actually functions in the strictly normal course of early intrauterine existence. The respiratory mechanism seems to be a dormant system charged with potentialities long in advance of the time it can be of any use to the fetus.
This period of preparedness varies gkeatly in different animaIs. It must be exceedingly brief in the marsupials. Fortydive per cent of the gestation period passes in the cat before respiratory efforts can be induced. The figures are about 3o per cent for man and littIe more than 25 per cent for the sheep.
It is possible to remove a fetus from the uterus and leave its circulation to the placenta intact, but it is impossib1e to maintain physiologic conditions comparable to those in utero before the experiment for very many minutes after this has been done. Partia1 anoxemia sets in promptly, the feta1 respiratory center thresh— old drops and its neurons begin to discharge in response to the usuaJ factors which are stimulatory in adult organisms.
Rhythms of shallow, rapid respiratory movements which are occasionally encountered in human fetuses late in the gestation period and which have been seen in a few laboratory animals at infrequent intervals signify that the fetus is momentarily experiencing either a depression of the threshold of its respiratory center or an elevation of the carbon dioxide in blood going to the brain. 0ne is justified in postulating a physiologic partial anoxemia in late fetal life associated with progressive decline in placental efliciency and with this, rninor rhythms of respiratory movements. Nevertheless, even these minor rhythms of shallow, rapid respiratory movements are not often encountered and when seen should give cause for apprehensiveness on the part of the obstetrician, for the danger of aspiration of amniotic contents then may become real if the partial anoxemia becomes a true asphyxia.
That aspiration is not commonly encountered depends upon the fact that normally the fetus never sufkers a truly asphyxial state before it is born. It seems probable that the blood which it receives from the placenta is highly saturated with oxygen throughout the greater part of life in utero. If the oxygen saturation drops into the neighborhood of 50 per cent it is to be expected that shallow, rapid movements of the respiratory muscles will be inanifested. These are not accompanied by any changes in intrathoracic pressure because the normal hypotonicity of fetal muscles remains unchangedz consequently no aspiration results. But should the oxygen saturation decrease marlcedly, numerous motor neurons are activated, muscles tonus increases and dyspneic gasping movements ensue. These bring about aspiration of amniotic contents. 0ne can not doubt however that the ketus possesses a wide margin of safety in respect to the danger of «drowning" in its amniotic fluid.
References Cited
i. Preyer, W. i885. specielle Physiologie des Embryo» Grieben, Leipzig. a. Windle, W. F» C. A. Dragstedy D. E. Murray s: R. R. Greene. i938. surg. Gynec. Obst» 66: 987.
Z. Hartmary c. G. i92o. Anat. Ren, 192 Ist. «
4. Ahlfeld, F. i888. Verhandl. deutsch. Gesellsch Gynälsp g: 2o3.
z. Weber, H. i888. Inaugural dissertatiom Marburg. (cited by Ahlfeld.) s. Ahlfeld, F. i9o5. Monatschr. Geburtsh. Gynälc., ei: i43.
7. Reiikerscheich K. i9i«i. pliiiger’s Arcli., i4o: i.
s. snyder. F. F. 8c M. Rosenfeld. 19377 J.A:M.A.« io8: i946. MDO s Id Iiddd VII— occ IIICIIIIC VIII-III« PEPSVTIP THE
. Minkowskh M. t9aa.
Windle, W. F» R. F. Becher, E. E. Barth sc M. D. schulz Gyn.« Obst» 69: 7o5. corey, E. L.: t93a. J. Exp. Zool., 6t: t.
. cartttichaeh L. t934. Genetie Psychol. Monogr» t6: 337. . snyder, F. F. sc M. Rosenkeld. t937. Am. J. Physiol» ttg: t53. . Barcrokt, J. sc D. H. Barron. t936. J. Physiol» 88: 56.
Bat-Gott, J. sc D. H. Barron. t937. Ibid., 9t: sag.
Donat, B. E., c. M. Blutnenkeld sc c. Fenning. t938. Am. J. Dis. child» 55: t. »
Brot«-n, T. G. tgt5. J. Physiol» 49: ao8.
coronios, J. D. t933. Genetie Psychol. Monogr» t4: asz Windle, W. F» M. Monnier sc A. G. steele. t938. Physiol. Zool» tt: 4a5.
Erbkam. t837. Neue Ztschn Geburtsk» z: 3a4.
strassmanm P. t9o3. sammh Hin. Vortr» Gynälc» No. t3a: 947.
schweitzer med. Wchnschr» No. ag and so: 7at, 75t.
Bolaflio, M. sc G. Artom. t9a4. Arch. d.i sei. Biol., Z: 457.
Walz, W. t9aa. Monatschn Geburtsh. Gynälc., 6o: 33t.
Bart-mit, J» D. H. Barron, K. Kramer sc G. A. Millikarp Physiol» 9o: 29 P.
1937- J—
. Abel, s. sc W. F. Windle. t939. Anat. Ren, Es: 45t. . Bouceltz c. M. sc A. D. Renton. . Rosenkeld, M. sc F. F. sitz-der.
t93t. surg. Gyn. Obst» Ha: 84t. t939. Am. J. Obst. sc Gyn., 38: 4a4. KoiK A. K. sc M. E. Das-is. t937. Ibid., 34: as.
cohnstein, J. sc N. Zuntz t884. Pkliigeks Arch., 34: t73.
. Huggett, A. St. G. t93o. J. Physiol» 69: t44. . Barcroky J. t935. Irish J. Med. sci., series 73 t: a89.
Flint, A. t88o. Am. J. Med. sei» so: 69.
. salmi, T. t93Z. Acta soc. Med. Fennicae (ser. B, fast. t, art. a) , t8: t. . Zuntz, N. t877. Plliigeks Arch» t4: 6o5. . steele, A. G. sc W. F. Windle.
t939. J. Physiol» 94: Ist. Barcrofh J. and M. F. Mason. t938. Ibid., 93: aa P. Barcrokt, J» K. Kramer sc G. A. Millilcan. t939. Ibid» 94: 57t.
. Eastmary N. J» E. M. K. Geiling sc A. M. DeLawder. t933. Johns Hop lcins Hosp. Bull., IF: a46.
. Eastman, N. J. t93o. Ibid» 47: aat.
. Bidone, M. t93t. Ann. di ostet. e ginec., 53: t97. . Roos, J. sc c. Romijxx . Windle, W. F. and Barcrokr. t938. Arn. Physiol» tat: 684.
. Windle, W. F» L. G. scbarpenbetg sc A. G. steele. t938. Ibid., tat: 69a. . wiud1e, w. F. s« D. N . Kuo, Z. Y. sc T. c. shet»t. t937. J. cornp. Psychol» a4: 4g. . Rotnijn, c. sc J. Roos. . Wind-le, W. F. t934. . Windle, W. F» D. W. Ort· sc W. L. Minean t934. Physiol. Zool» 7: 6oo. . Pitts. R. F» H. W. Magoun sc s. W. Ranson. t939. Am. J. Physiol» tas t938. J. Physiol» 9a: a49. elson. t"938. Ibid» tat: 7oo t938. J. Physiol» 94: 365. J! cotnp. Neur.,· 59: 487.
.
. Hendetsom Y. t938. Adventures in Respiratiotr. Williatns sc Willcins, Baltitnore.
. Kraft-ca, J. t933. Am. J. Dis. child» 45: too7.
52. Geyl, A. 188o. Arclx Gynäk., is: 385.
53. Wis1ocki, G. B. 192o. Sankt. Brod» u: 47.
54. Zwecke, E. 1938. Beitr: path..Anat. allgem. Path. wo: 515.
55. Farben s. sc L. K. sweet. 1931. Am. J. Dis. child., 42: 1372.
56. Ehxhardy K. 1937. Münclx mecL Wchnschr., 84: 1699.
57. Mem-es, T. 0., J. D. Miller sc: L. E. Hollzn 193o. Am. J. Roemz Rad. Ther., 24: 363.
58. Reifkerscheict W. sc R. schmiemantx 1939. lenkt-kühl. Gynälk 63: 146.
59. Mermanty A. 188o. ZentralbL Gynäk., 4: 377.
so. DeLee, J. B. 1938. The Principles and Practice ok 0bstetrics, saunders, Phj1ade1phia.
Cite this page: Hill, M.A. (2024, April 19) Embryology Book - Physiology of the Fetus 6. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Physiology_of_the_Fetus_6
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G