|
|
| (11 intermediate revisions by the same user not shown) |
| Line 4: |
Line 4: |
| {{Windle1940 TOC}} | | {{Windle1940 TOC}} |
| {{Historic Disclaimer}} | | {{Historic Disclaimer}} |
| ==Chapter II The Fetal Heart==
| | =Chapter II The Fetal Heart= |
|
| |
|
| INITIÄTION OF« THE ITEART BEAT
| | ==Initiation of the Heart Beat== |
|
| |
|
| WHILE the embryo consists of an aggregate of re1ative1y few
| | While the embryo consists of an aggregate of relatively few cells there is no need of a special mechanism to circulate oxygen laden blood. Tissue respiration of the ovum is adequately supported by the gas tension gradients between maternal fluids and embryonal ce1ls at the site of implantationz nutritiona1 needs are slight. But with further growth in size the usefulness of a circulatory system becomes evident. |
| cells there is no need of a specia1 mechanism to circulate oxygen | |
| laden blood. Tissue respiration of the ovum is adequately sup— | |
| ported by the gas tension gradients between maternal fluids and
| |
| embryonal ce1ls at the site of implantationz nutritiona1 needs are | |
| slight. But with further growth in size the usefulness of a circu— | |
| 1atory system becomes evident.
| |
|
| |
|
| Unti1recent1y the earliest contractions ok the mammalian heart
| |
| had not been seen. Although many investigatorsH have studied
| |
| chiclc embryos incubated Iess than two days sabin4 and Johnstones
| |
| appear to have been the lirst to watch the initiation of the beat at
| |
| the ten somite stage. It was found that contractions begin on the
| |
| right side of the ventricle at a point near its junction with the
| |
| primordium of the atrium. It seemed to these observers that the
| |
| earliest contractions occurred rhythmically. More recently others«
| |
| have extended this worlc in a very painstalcing cinematographic
| |
| study. They discovered that the first beats are arrhythmical libri1—
| |
| lations of a few cells located in the bulbo-ventricular region of
| |
| nine somite embryos. The atrial myocardium shows no activity
| |
| until three or four hours after contractions have started in the
| |
| ventriclesz the sinus venosus begins to beat still 1ater. synchronized rhythmical contractions of the entire ventricle result from
| |
| coalescence of the early Hbrillations of right and-left sides. The
| |
| earliest agitation of ventricular contents is simply tida1. Blood is
| |
| not propelled directionally by the early cardiac activity but its
| |
| movement begins before the beat has involved the still incompletely fused sinus venosus. When contractions of the atrium start
| |
| they are faster than those of the ventricle. With coalescence of
| |
| activity in the atrium and ventricle there results an acceleration of
| |
| the rate of the ventricular beat. similarly the contractions of the
| |
| sinus venosus, when added to those of the ventricle and atrium,
| |
| bring about a second acceleration.
| |
|
| |
|
| II
| | Until recent1y the earliest contractions ok the mammalian heart had not been seen. Although many investigatorsH have studied chick embryos incubated Iess than two days sabin4 and Johnstones appear to have been the lirst to watch the initiation of the beat at the ten somite stage. It was found that contractions begin on the right side of the ventricle at a point near its junction with the primordium of the atrium. It seemed to these observers that the earliest contractions occurred rhythmically. More recently others« have extended this worlc in a very painstalcing cinematographic study. They discovered that the first beats are arrhythmical fibrillations of a few cells located in the bulbo-ventricular region of nine somite embryos. The atrial myocardium shows no activity until three or four hours after contractions have started in the ventriclesz the sinus venosus begins to beat still later. synchronized rhythmical contractions of the entire ventricle result from coalescence of the early fibrillations of right and left sides. The earliest agitation of ventricular contents is simply tida1. Blood is not propelled directionally by the early cardiac activity but its movement begins before the beat has involved the still incompletely fused sinus venosus. When contractions of the atrium start they are faster than those of the ventricle. With coalescence of activity in the atrium and ventricle there results an acceleration of the rate of the ventricular beat. similarly the contractions of the sinus venosus, when added to those of the ventricle and atrium, bring about a second acceleration. |
| 12 PHYSIOLOCY OF THE FETUs
| |
|
| |
|
| The genesis ok contractions ok the mamma1ian heart has been
| |
| observed in hangingdrops cu1tures of whole ernbryonic vesicles ok
| |
| katsJ The beat begins at the three somite stage before the primitive myocardial tubes have fused. Thus the heart starts to tunction at an earlier time in the rat, relativelzz than in the chiclc
| |
| Embryo. Three or kour cells of the lekt ventricular primordium,
| |
|
| |
|
| Perseus-Zins wo«
| |
|
| |
|
|
| | The genesis of contractions ok the mamma1ian heart has been observed in hanging drops cu1tures of whole ernbryonic vesicles of katsJ The beat begins at the three somite stage before the primitive myocardial tubes have fused. Thus the heart starts to tunction at an earlier time in the rat, relativelzz than in the chiclc Embryo. Three or four cells of the left ventricular primordium, near its junction with the future atrium, begin to contract rhythmically (Fig. 2, A) . About two hours later a s1ower rhythm starts in the right ventricular ruhe, retaining an independent beat until the two sides kuse (Fig. 2, B) . When this occurs the lekt side dominates the right and becomes the pacemalcer in establishing a single wavedike ventricular contraction; some variations were encountered Atrial contraxztionss begin. later than do those of the ventricular tubes. 1t was impossible to determine the existence of earlier, arrhythmical contractions lilce those seen in the chiclc embryo; perhaps failure to observe them can be explained on the basis of technical differences in the two investigations. |
|
| |
|
| |
|
| Lei- lxom ej· sitzt«
| |
| treuem-s
| |
|
| |
|
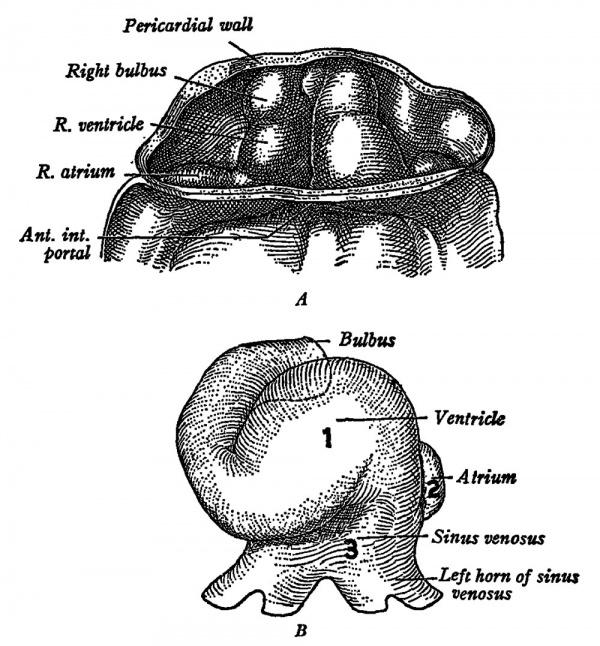
| Fig. s.-The heart ok human ernbryos ok («4) 6 sornites and (B) u somites.
| | [[File:Windle1940 fig03.jpg|600px]] |
| The heart begins to beat in mamrnalian embryos eornparable with L. The order
| |
| ok initiation ok the beat is shown in B by the iigures 1 (ventricle) , L (atriurn)
| |
| and Z (sinus venosus) . (Arey: «Developmental Anatomysj
| |
|
| |
|
| near its junction with the future atrium, begin to contract rhythmically (Fig. z, A) . About two hours later a s1ower rhythm starts
| | '''Fig. 3.''' The heart of human embryos of (A) 6 sornites and (B) 11 somites. The heart begins to beat in mamrnalian embryos eornparable with L. The order ok initiation ok the beat is shown in B by the figures 1 (ventricle) , 2 (atrium) and 3 (sinus venosus). (Arey: "Developmental Anatomy.") |
| in the right ventricular ruhe, retaining an independent beat until | |
| the two sides kuse (Fig. z, B) . When this occurs the lekt side | |
| dominates the right and becomes the pacemalcer in establishing
| |
| a single wavedike ventricular contraction; some variations were
| |
| encountered Atrial contraxztionss begin. later than do those ok the
| |
| THE FETAL HEART 13
| |
|
| |
|
| ventricular tubes. 1t was impossible to determine the existence
| |
| of earlier, arrhythmical contractions lilce those seen in the chiclc
| |
| embryo; perhaps failure to observe them can be explained on the
| |
| basis of technical differences in the two investigations.
| |
|
| |
|
| The initiation of the heart beat has been studied in amphibian
| |
| embryos where, in generaL the observations on birds and mams
| |
| mals have been coniirmedF The first activity occurs in different
| |
| parts of the ventricle in different specimens. Most of the embryonic amphibian hearts exhibit rhythmicity in the earliest stages
| |
| of beating. ·
| |
|
| |
|
| It was held for many years that the cardiac beat has its origin
| | The initiation of the heart beat has been studied in amphibian embryos where, in generaL the observations on birds and mams mals have been coniirmedF The first activity occurs in different parts of the ventricle in different specimens. Most of the embryonic amphibian hearts exhibit rhythmicity in the earliest stages of beating. |
| in the sino-atrial region and that this region remains the pace—
| |
| malcer thereafterks I» The recent studies in amphibian, bird and
| |
| mammalian embryos demonstrate that this is not the case. By curting the embryonic heart between ventricle and sinus venosus it
| |
| has been found that the beat of the ventricle remains unaItered
| |
| unless contractions have already begun in the other parts, in which
| |
| case the two pieces of tissue take up independent rhythmsks C» S« U
| |
| The intrinsic beat of the sinus venosus is faster than that of the
| |
| atrium, which in turn is faster than that of the ventricle. Each
| |
| newly acquired contracting portion when added to older parts sets
| |
| a faster pace for them. It may be concluded that although the
| |
| heart beat has its genesis in the ventricular region and not in the
| |
| sinus venosus, regulation of the ventricular beat is brought under
| |
| control of the sino-atrial region very early in development.
| |
|
| |
|
| THE PETAL ELECTROCARDIOGRAM
| |
|
| |
|
| A number of attempts have been made to record action currents of the human«fetal heart near term by means of the electrocardiograph, the investigators using leads placed on the mother’s
| | It was held for many years that the cardiac beat has its origin in the sino-atrial region and that this region remains the pace— malcer thereafterks I» The recent studies in amphibian, bird and mammalian embryos demonstrate that this is not the case. By curting the embryonic heart between ventricle and sinus venosus it has been found that the beat of the ventricle remains unaItered unless contractions have already begun in the other parts, in which case the two pieces of tissue take up independent rhythmsks C» S« U The intrinsic beat of the sinus venosus is faster than that of the atrium, which in turn is faster than that of the ventricle. Each newly acquired contracting portion when added to older parts sets a faster pace for them. It may be concluded that although the heart beat has its genesis in the ventricular region and not in the sinus venosus, regulation of the ventricular beat is brought under control of the sino-atrial region very early in development. |
| abdomen«and in the Wagina or rectum.12—I9 similar studies have
| |
| been undertaken in the horsesV The records obtained have been
| |
| of little value for interpreting events of conduction in the fetal | |
| heart because the dellections were small. The fetal electrocardio—
| |
| gram at 9 months is saick to exhibit a· simple upright monophasszic
| |
| curve.21 More recently however electrocardiograms have been
| |
| obtained from younger human fetuses removed at operationjVs 23
| |
| ln the three conventional leads the records showed äll deliections
| |
| of the adult. The most signiiicant difkerence between human | |
| 14 PHYSIOLVGY OF THE FETUS
| |
|
| |
|
| electrocardiograms at birth and in the adult is that the newborn
| | ==The Fetal Electrocardiogram== |
| right ventricle exhibits as definite functional preponderanceFÅ Z«
| |
| This is correlated with the fact that the right ventricle outweighs
| |
| the left by 13 per cent in full term fetusesks Left ventricular preponderance begins to become evident at about the second or third
| |
| postnatal month, and, by the sixth month of life one can scarcely
| |
| see any difference between electrocardiograms of infants and
| |
| adults. These observations signify that the left side of the heart
| |
| becomes larger than. the right by the second or third month. It
| |
| is doubtful if this comes about in those infants in which the ductus
| |
| arteriosus remains patent for several weelcs or months after birth
| |
| and consequently a1lows the blood pressure on the two sides to
| |
| equalize
| |
|
| |
|
| The developing bird’s heart offers the best opportunity to
| | A number of attempts have been made to record action currents of the human«fetal heart near term by means of the electrocardiograph, the investigators using leads placed on the mother’s abdomen«and in the Wagina or rectum.12—I9 similar studies have been undertaken in the horsesV The records obtained have been of little value for interpreting events of conduction in the fetal heart because the dellections were small. The fetal electrocardiogram at 9 months is saick to exhibit a· simple upright monophasszic curve.21 More recently however electrocardiograms have been obtained from younger human fetuses removed at operationjVs 23 ln the three conventional leads the records showed äll deliections of the adult. The most signiiicant difkerence between human electrocardiograms at birth and in the adult is that the newborn right ventricle exhibits as definite functional preponderanceFÅ Z« This is correlated with the fact that the right ventricle outweighs the left by 13 per cent in full term fetusesks Left ventricular preponderance begins to become evident at about the second or third postnatal month, and, by the sixth month of life one can scarcely see any difference between electrocardiograms of infants and adults. These observations signify that the left side of the heart becomes larger than. the right by the second or third month. It is doubtful if this comes about in those infants in which the ductus arteriosus remains patent for several weelcs or months after birth and consequently a1lows the blood pressure on the two sides to equalize. |
| examine action currents critically under controlled conditions.
| |
| A number of investigations have been made in the chiclc but only | |
| two merit consideration here. In one series of experiments27 small
| |
| holes were drilled through the shell of incubating eggs and electrodes were inserted without disturbing the embryos. The eggs
| |
| were then placed in a special incubator and allowed to remain
| |
| quietly for some time before electrical records were made. In the
| |
| other series28 the eggs were opened, blastoderms removed and
| |
| placed in a special chamber. A micromanipulator was used to
| |
| place the electrodes upon the embryo. Ampliftcation was employed in both cases.
| |
|
| |
|
| The first deflections of the galvanometer were obtained from
| |
| chicks of 15 somites (33 to 36 hours incubation) in which the heart
| |
| consists almost entirely of ventricle. This is not much later than
| |
| the time of initiation o·f rhythmical heart beaks. The first curves
| |
| showed none of the deflections which characterize the adult but
| |
| appeared as simple deflections first below, then above the isolec—
| |
| tric line (Fig. 4) . In slightly older, 16 somite embryos a sharp
| |
| downward deflection followed by a rapid return to or above the
| |
| line appeared; this resembled the Q R s complex. The auricular
| |
| deflection (P) did not appear until about 42 hours incubation,
| |
| is» soon after the auricular beats had become established, and it
| |
| was first seen as a downward deflection (Fig. z) . Later the P wave
| |
| reversed. These results correlate nicely with what has been
| |
| learned from direct- observations of. fhspe developing chiclc heart
| |
| THE FETAL HEART IZ
| |
|
| |
|
| By the fourth day of incubation the embryonic electrocardiogram
| | The developing bird’s heart offers the best opportunity to examine action currents critically under controlled conditions. A number of investigations have been made in the chiclc but only two merit consideration here. In one series of experiments27 small holes were drilled through the shell of incubating eggs and electrodes were inserted without disturbing the embryos. The eggs were then placed in a special incubator and allowed to remain quietly for some time before electrical records were made. In the other series28 the eggs were opened, blastoderms removed and placed in a special chamber. A micromanipulator was used to place the electrodes upon the embryo. Ampliftcation was employed in both cases. |
| was practically identical with that of the adult hen. This is a
| |
| remarlcable observation for there are no nerves in the heart at this
| |
|
| |
|
| III-I-s«I-D-III . IIIIIV-III
| |
| UI0«U-UH«w»--www -HumOO»Hwag
| |
|
| |
|
| , MMwMIIUUgINI-- -«-D—wwI | | The first deflections of the galvanometer were obtained from chicks of 15 somites (33 to 36 hours incubation) in which the heart consists almost entirely of ventricle. This is not much later than the time of initiation o·f rhythmical heart beaks. The first curves showed none of the deflections which characterize the adult but appeared as simple deflections first below, then above the isolec— tric line (Fig. 4) . In slightly older, 16 somite embryos a sharp downward deflection followed by a rapid return to or above the line appeared; this resembled the Q R s complex. The auricular deflection (P) did not appear until about 42 hours incubation, is» soon after the auricular beats had become established, and it was first seen as a downward deflection (Fig. z) . Later the P wave reversed. These results correlate nicely with what has been learned from direct- observations of. fhspe developing chick heart by the fourth day of incubation the embryonic electrocardiogram was practically identical with that of the adult hen. This is a remarlcable observation for there are no nerves in the heart at this time and the Special tissue of the cardiac conduction system is not as yet distinguishabla Time relationships taken from electrocardiograms of the chick will be found in Table 4.27 |
| ,-k»« -- ,p«s-Iss III«--I I-HMa-mcnwv | |
| II«-U UND-U U«--vIDI DO---us«-H
| |
| . --:- INTIIIII ImIIIUU--I» | |
| ;g:x;--- -:T:I0IMI IMM DUHMMwst
| |
| I-0s-—s0Ig-I--I--u-uNwaIU---« p«IcHIa.
| |
| ---II«---«www«IQkII
| |
|
| |
|
| « -IIUU-c-N::--«-I-IS« - II---IS-ICUIsIOIPI
| | [[File:Windle1940 fig04.jpg|600px]] |
| I:D-OsII- " TTIIIIQOIIOCIIICIHE ·--IOICIITIIICII-IKI
| |
|
| |
|
| "7IP··" - «-·IOCC«II--p.--...-. --. -- .
| |
|
| |
|
| -CsHsCJII — -, »« — -««-·««·«««""·"« -«"«««««E«« —WIIIII TTIIIBEHHIIM | | Fig. 4. Electrocardiogram from a 15 somite chick embryo (+/- 36 hours incubaction) . The diagram on the Iekt ok this and ok Fig. 5 shows the shape of the heart and position of the leads. (Hoff, et a1.: Am. Heart J. Vol. 17, 1939, C. V. Mosby Co) |
|
| |
|
| T«
| |
| --IIsI -SCwQ- III»-gVHoI -DssIUJD
| |
| - -VI» I--IIIIs-AMICI DII—sI
| |
| II I -UHSIII-I-oIGIMEmUA
| |
| I-IT- D«s0Ik-II Ia--III« ---mIUIUIII
| |
| UUsDI--J-sOIII-I I---nnmAIAg
| |
|
| |
|
| IIUICIPHIIC CIPIIIIIIITSKKII-JSKIII1QLIIIIEIIZDIIIS
| | [[File:Windle1940 fig05.jpg|600px]] |
|
| |
|
| EEZX-sss---IIONm-mIIms kIsmUIsIHUHII
| | Fig. 5. Electrocardiogram from a 20 somite chick embryo (+/- 42 hours incubation). Compare with Fig. 4. (Hoff, et a1.: Am. Heart J. Vol. 17, 1939, C. V. Mosby Co) |
| OI M UJSIIIIIIIIIOUDIIIHHSIC It— kfllsjcsxsisjcå
| |
| ÆIÄMIMIIGIGJ-GDMIi-- CSIIIIS SCFIIIUCJWIOII
| |
| II « II - IT IICIIZIIIIUIIULIWILISIGIIDII ZULLICIUDUL UUILXII
| |
| IN III ICIITFII-DIIPITIIIIIII-ssIIs·IIL--I!s!ssl- -IIIIJ
| |
| IIEIF HIIIUSILCIIIIlIIIIJIIFIIIIIMDIIIIIIIIIIIIHIIIMIIIII
| |
|
| |
|
| Ist!
| | ==The Fetal Pulse Rate== |
|
| |
|
| SESCIIPIQEIIUÜIMIUSWIZI CSIULII IFIIILIZQDILQCUIVEIIIIII I
| | The Pulse rate of chiclc embryos has been studied on a number of occasions, but most of the results are open to the criticism that temperature and mechanical kactorsjvere not controlled precisely during experiments The studies of Cohn and Wi1e29 and especially those of Bogue30 are the most significant. The latter recorded heart beats electrically from undisturbed eggs at a temperature which fluctuated no more than» o.250 C. It was found that the heart rate rises sharply in early stages of incubation, increases more s1ow1y from the tenth day onward to hatching, shows another rapid elevation at hatching and thereaftcr is maintained nearly at a constant 1evel throughout life. Average heart rates in chick embryos are illustrated graphically in Fig. 6. Bogue found that very slight temperature differences had a marked direct effect upon the embryonic pulse rate. He could find no relationship between the sex and pulse rate of chick embryos nor could a close correlation between metabolic rate and pulse rate be demonstrated. It may be signiiicant that a physiologic anoxemia is imposed upon the chiclc toward the end of incubationz this can have a direct inhibitory action upon the pacemalcer mechanism. The acceleration immediately after hatching may represent a release from the anoxemia. |
|
| |
|
| I - II Mk
| |
| ;?- -IX- KIIZIs-xkIUNUM-III-II-I---IN8I-uUhAIIINUI
| |
| ;«u2 I I I- U ---nm-IsI-n---O---c--III---üICUUD
| |
|
| |
|
| Fig. 4.—Electroca.rdiogram from a 15 sqmite chick embryo (z-36 hours incubas
| |
| tion) . The diagram on the Iekt ok this and ok Fig. 5 shows the shape of the heart
| |
| and position of the leads. (I-lokf, et a1.: Am. Heart J» Vol. i7, 1g3g, c. V. Mosby
| |
|
| |
|
| ca)
| | {{Windle1940 table4}} |
|
| |
|
| time and the Special tissue of the cardiac conduction system is not
| |
| as yet distinguishabla Time relationships talcen from electrocardiograms of the chiclc will be found in Table 4.27
| |
|
| |
|
| Ei? HFIITZIEEHEEJIJHPSIIJ
| |
|
| |
|
| - s , l: , » J
| |
| iskisjc Eis T»
| |
| - « « -
| |
|
| |
|
| - : zip-THE Jiiiczs III:
| | Fig. 6. Heart rate during incubation of the chick. The solid line: Bogue’s data; brolcen linke: Cohn and Wile. (Bogue: J. Exp. Bio1., Vol. g. 1933.) |
|
| |
|
| T» ·« sit-II
| |
| ' « - «:
| |
|
| |
|
| « , , », , , s ;" -:
| |
| " E« J Z Eis-Es« EINIGE-III III-MEPH-
| |
| ........... .... .. 3’"""« «« I « .... ·« · - . . «·- ·- - --«--- «.
| |
|
| |
|
| Fig. zskllectrocardiogkam from a so somite chiclc embryo E· 42 hours incubation). compare with Fig. 4. (I-Ioik, et a1.: Am. I-Ieart J» Vol. 17, 1939. C. V.
| | Because of its great importance in obstetrics, the human fetal heart rate has been studied extensively. It is well established that the average frequently at term is 130 to 150 beats per minute. Extremes of 110 and 180 are commonly observed but a rate of less than 1oo should be vieweLi with alarm. The average heart rate encountered in premature infants (about 120 per min.) is slower than that of infants at full term. The fetal heart slows during birth but accelerates when respirations begin and may be maintained at 17o to 180 beats per minute for iifteen to thirty minutes thereafter.92 |
| Mosby co.) »
| |
|
| |
|
| rmd Paris« puLsE Un;
| |
|
| |
|
| The Pulse rate of chiclc embryos has been studied on a number of occasions, but most of the results are open to the criticism
| | Few systematic correlations of the human heart rate with fetal age have been made. In fact very little reliable information is available for any mammal. It is impossible to detect human fetal heart sounds accurately before about the fifth month. The human fetal heart beats faster in mid-fetal life than it does just before birth.33 A steady decline from a mean of 156 beats per minute at the fifth lunar month to 142 at the ninth, and a slight subsequent increase during the tenth month have been observed in one series of thirty subjects. |
| that temperature and mechanical kactorsjvere not controlled pre16 Pnrsxohoci oF THE FETUs
| |
|
| |
|
| Tun: 4
| |
|
| |
|
| Tntu Rvrwtsroxssxxss cannot-Hm) niou Ehuornocanvxoomus o:- mv csxcx
| | In {{rat}} embryos which had been exposed by opening the uterus it was found« that the heart rate fluctuated widely throughout pregnancy and was slower than but ran parallel to that of the mother. Only roughly did the data indicate an increase in frequency from early to late prenatal stages. During late fetal life of the dog, ox, goat and man the heart beats faster than it does in the young offspring after birth. A gradual decline is observed from infancy to maturity. This is illustrated in Table z, compiled from observations of several investigators.29-33, 47 |
|
| |
|
|
| |
|
| |
|
| |
|
|
| | The adult rate appears to be higher than the fetal rate during the latter part of gestation in the rat« and» monlceyks resembling the bird in this respect. The possibility of asphyxial depression of the fetal heart in the rat experiments should be borne in rnind, although most of the fetaj heart rates were not abnormally slow in comparison with other species of animals. The adult rat, being an example of a small animal with rapid pulse rate, may not actu— ally develop the inhibitory mechanism to the same degree found in larger mamma1s. The rapid maternal pulse of the adult monkey was attributed to the anima1’s excitement and exertion. |
|
| |
|
| «« DIE« Ikzkxt THE. DIE« Essig-us: ZEIT-ZEIT«
| |
| heart m «« Use P e speed
| |
| per«
| |
|
| |
|
| mirs. »in. see. wo. · see. mmJsea
| | {{Windle1940 table5}} |
| 40 emkryo 0 8 120 uone .009—.012 0.28-0.s2 IF?
| |
| 60 .em ryo 1.2 168 0.11 .008-.010 0.20—0.21 .80
| |
| 120 Ins. embkyo 2.o 280 o.09 .o1o o.21—0.24 6.s5
| |
| Z day chick 14.0 295 0.06 .004-.006 0.24-0.s8 40.58
| |
| Adult hell 40. 0 320 O. 06 . 009 0 . III-O . 18 117 . 77
| |
|
| |
|
|
| | ==Nervous Control of the Fetal Heart and Circulation== |
|
| |
|
| cisely during experiments The studies of Cohn and Wi1e29 and
| | It is thought that the progressive slowing of the pulse in postnatal life is associated with improvement of control of the heart by the vagus nerves. It has been pointed out that animals with small hearts have rapid pulse rates and those with large hearts have slower rates.37 control by the vagus is less pronounced in the former than in the latter. The higher metabolic rate of small animals is thought to make it necessary for the heart to beat about as fast as it can under normal circumstances; consequently there should be no call for an active inhibitory mechanism. |
| especially those of Bogue30 are the most signiiicanh The latter
| |
| recorded heart beats electrically from undisturbed eggs at a temperature which Huctuated no more than» o.250 C. It was found
| |
|
| |
|
| 250
| |
| 240
| |
|
| |
|
| 280
| | Perhaps the fetal heart rate is rapid because nervous control has not become well established or is inhibited in Some way. Most of the evidence points to the conclusion that vagus function is not nearly so marked in the fetus as it is in the adult, although considerable species variation is encountered. stimulation of the vagus nerves in the chick had no effects at 18 to 20 days of incubation, but it slowed the beat seven to eight hours after hatching.38 Inhibition wsas ekkected by direct stimulation of the heart itself in embryos of Ave days incubation.39 Muscarin produced the same result in even younger chick embryos; it slowed the heart of small rat embryos and was antagonized by atropine. These results in— dicate that the failure to obtain inhibition of the heart by vagus stimulation is not due to factors inherent in the myocardium. |
| 220
| |
| 210
| |
| goo
| |
| 190
| |
| 180
| |
| 170 «
| |
| sco
| |
| 150
| |
| mo
| |
| 130
| |
| 120
| |
| I 10
| |
|
| |
|
|
| |
|
| |
|
| |
|
| Beste per minnt(
| | Most investigators who have studied the phenomenon of vagus inhibition in young postnatal mammals have been able to produce slowing or cessation of the heart beat by stimulating the peripheral cut end of the nerve, but in many instances the ekkects seem to have been weaker than would have been obtained in adults.40-45 This was especially true in experiments with kittensJU The nearer to birth, postnatally, the less the chance of producing inhibition by stimulating the vagus nerves. In one newborn rabbit which showed no cardiac slowing upon stimulation of the peripheral vagus, respiratory inhibition was demonstrated by stimulating the central end, indicating that the nerve is capable of conducting and that conduction can take place through medullary centers at birth.45 In prenatal life a few successes as well as many failures have been reported in several species.40- 4I- 45- 43 It is probable that the negative results were obtained in many instances because the vagus nerves had been stimulated only after a maximum decline in the rate of the heart beat had occurred in consequence of asphyxia. |
|
| |
|
| |
|
| 6 7 8 O l0lll2lsl4l5I6l7I8l9
| |
| Days of inculdiition of egg
| |
|
| |
|
| Fig. 6.-I-Ieart rate during incubation of the chic1c. The solid line: Bogue’s data;
| | In experiments with rabbits Bauer« has found that stimulation of the vagus nerve increases the decline in heart rate which is brought about by asphyxia. This was not demonstrable how— ever until about the fourth day after birth. Clamping the umbilical cord at experimental Caesarean section led almost immediately to asphyxial bradycardia, b·ut the phenomenon was delayed in specimens which had breathed air and in which asphyxia was sub— sequently produced by occluding the trachea. The amount of oxygen available in the blood was greater in the latter than in the former instance. The asphyxial slo.wing of the fetal heart rate appeared to be due, not to inHuence of the central nervous system efkected through the vagus nerves, but. to a direct chemical action upon the pacemaker of the heart. |
| brolcen linke: cohn and Wile. (Bogue: J. Exp. Bio1., Vol. g. 1933.)
| |
|
| |
|
| III-Ab
| |
|
| |
|
| that the heart rate rises sharply in early stages of incubation, increases more s1ow1y from the tenth day onward to hatching, shows | | Various theories have been proposed to explain the slowing of the human fetal heart at the time of delivery. Compression of the skull of young rabbits produces bradycardia and some have held that the passage of the fetal head through the birth canal may bring about enough pressure to cause a similar cardiac depression. However, a declining heart rate is not infrequently encountered during 1abor before the head becomes engaged in the 1ower part of the pelvis. |
| another rapid elevation at hatching and thereaftcr is maintained
| |
| nearly at a constant 1evel throughout life. Average heart rates in
| |
|
| |
|
| chiclc embryos are illustrated graphically in Fig. 6. Bogue found»
| |
| THE FETAL HEART 17
| |
|
| |
|
| that very slight temperature differences had a marked direct effect | | lt has been held also that the human fetal heart rate varies with uterine contractions and relaxationssfss I! 1t is probable that a greater volume of blood is forced into the fetal heart by each uterine contraction and that the pulse rate consequently declines as the blood pressure rises in accordance with Mareyks law. Clark so demonstrated experimentally that the fetal blood pressure under— goes a brief rise followed by a prolonged fall during contraction of the cat’s uterus. He thought that the fall was due to a diminished venous return to the fetal heart which resulted from increased peripheral resistance set up in the placenta by the uterine contractions. |
| upon the embryonic pulse rate. He could find no relationship
| |
| between the sex and pulse rate of chiclc embryos nor could a close
| |
| correlation between metabolic rate and pulse rate be demonstrated. It may be signiiicant that a physiologic anoxemia is im—
| |
| posed upon the chiclc toward the end of incubationz this can have
| |
| a direct inhibitory action upon the pacemalcer mechanism. The | |
| acceleration immediately after hatching may represent a release
| |
| from the anoxemia. | |
|
| |
|
| Because of its great importance in obstetrics, the human fetal
| |
| heart rate has been studied extensively. It is well established that
| |
| the average frequently at term is 130 to 150 beats per minute. Extremes of 11o and 18o are commonly observed but a rate of less
| |
| than 1oo should be vieweLi with alarm. The average heart rate
| |
| encountered in premature infants (about 120 per min.) is slower
| |
| than that of irifants at full termYI The fetal heart slows during
| |
| birth but accelerates when respirations begin and may be maintained at 17o to 180 beats per minute for iifteen to thirty minutes
| |
| thereafter.92
| |
|
| |
|
| Few systematic correlations of the human heart rate with fetal
| | Although there appears tobe no vagal tone before birth in sheep fetuses, ligating the umbilical cord results in an immediate elevation of the blood pressure and, in response to Marey’s law, an instantaneous bradycardia. When the vagi had been cut bradycardia followed occlusion of the cord, coming on gradually or suddenly as a 2:1 heart block. In either case it appeared only after an interval of about 25 seconds during which asphyxia had developed. |
| age have been made. In fact very little reliable information is
| |
| available for any mammal. It is impossible to detect human fetal
| |
| heart sounds accurately before about the iifth month. The human
| |
| fetal heart beats faster in mid-fetal life than it does just before
| |
| birth.33 A steady decline from a mean of 156 beats per minute
| |
| at the lifth lunar month to 142 at the ninth, and a slight subsequent increase during the tenth month have been observed in one
| |
| series of thirty subjects.
| |
|
| |
|
| In rat embryos which had been exposed by opening the uterus
| |
| it was found« that the heart rate fluctuated widely throughout
| |
| pregnancy and was slower than but ran parallel to that of the
| |
| mother. Only roughly did the data indicate an increase in frequency from early to late prenatal stages. During late fetal life of
| |
| the dog, ox, goat and man the heart beats faster than it does in the
| |
|
| |
|
| young offspring after birth-. A gradual decline is observed from .
| | It has been reported that the fetal heart rate is entirely unalfected by changes in the oxygen and carbon dioxide contents of the mother’s blood.32 However, amyl nitrite, which has a great relaxing effect on uterine and other smooth muscle, has an immediate influence on the mother’s heart rate and within a few seconds a similar slowing of the fetal heart occurs. This delay sug·gests a comparable phenomenon encountered during asphyxia in rabbit fetuses. |
|
| |
|
| infancy to maturity. This is illustrated in Table z, compiled from
| |
| observations of several investigators.29- 3347
| |
|
| |
|
| The adult rate appears to be higher than the fetal rate during | | The application of Marey’s law to explain· fetal bradycardia implies that ksascular reilex mechanisms are functioning in the fetus, at least near term. This is not borne out by experimental evidence in all species. Observations in cat and dog fetuses at experimental Caesarean section and in the young after birth led to the conclusion that pressor rellexes make their appearance about three or four days postnatally ksss No evidence of cardioaortic and carotid sinus reflexes was found before the fourth to sixth day in puppies and not before 1 1 days after birth in 1cittens. Faradic stimulation of the, depressor and carotid sinus nerves begins to elicit reflex inhibition ok the heart in 11 and 14 day old rabbits, respectively Asphyxiation fails to bring these reflexes into play unti1 about the kortieth day ok like because the blood pressure of -the young anima1 has not attained the necessary height until this time (Fig. 7) . The depressor reiiex hegins to be obtained when the systemic arteria1 pressure reaches 65 mm. Hg and the carotid sinus reliex appears at 8o mm. pressure. These are much higher pressures than are encountered in ketuses ok rabbits and other small anima1s. In the sheep and in man at the end of pregnancy ketal blood pressures are high enough to aikect responsive cardioaortic and carotid sinus mechanisms. |
| the latter part of gestation in the rat« and» monlceyks resembling | |
| 18 PHYsIoLoGY oF THE. FETUs
| |
|
| |
|
| TAELE Z
| |
| HEART RATE IN END« NDwEonN AND Adam« Anat-ans
| |
|
| |
|
|
| | Fig. 7. Blood pressure of rabbits at different ages after birth. J, Maximum blood pressure attained in asphyxiaz B, normal blood pressure (Bauer: Jour. Physiol» Vol. 95, i939.) |
|
| |
|
| I
| |
| Barly ketus Late ketus Newborn Aclult
| |
| Her! . . . . . . . . . . . . . 120—170 220«-264 295 320
| |
|
| |
|
| Rat . . . . . . . . . . . . . 96-114 1234248 . . . 184—280
| | The general conclusion reached from experimental studies and clinica1 observations is that the ketal heart pumps blood about as fast as it can without much control by the nervous System. The vagus nerves together with their endings and central connectionse are capable of conduction in some species near term, but in others which are born less completely equipped to cope with their en— vironment they may not be very well developed. Failure ok cardio-inhibitory and vascular reflex mechanisms to function at birth is related at least partially to low systemic blood pressures prevailing in the smalleiz less mature newborn animals. Asphyxia produces cardiac depression by direct chemical action upon the pacemaker. |
|
| |
|
| Goal: · . . . . · . . . . . , . . . . . . . . I 1204246 145-—240 . . .
| |
|
| |
|
| Ox . . . . . . .· . . . . . . . . . . . . . . 161 141 50
| | An explanation of the slowing of the human fetal heart during birth may be fortheoming from these studies. It is inferred that elevation of the blood pressure caused by uterine contraction brings about a reflex bradycardia. A decline in the oxygen saturation of the fetal blood during labor may induce an anoxial cardiac depression. |
|
| |
|
| Dog . . . . . . . · . . . » . . . . . . · . 120—17o 160 100
| |
|
| |
|
| Monkey . . · . . . . . . . . . . . . . 100—180 . . . l40-240?
| | The prompt but transient acceleration of the heart which occurs after breathing starts at birth may result from an awalcening, as it were, of sympathetic tone consequent upon the shower of new afferent impulses from the external environmenn Here too experimental evidence in· the sheep is available. The smooth muscle of the fetal spleen, which isinnervated by sympathetic neurons only, can be induced to contract reflexly by stimulating the central end of the cut vagus. splenic activity also follows ligation of the umbilical cord and is not related to the changes in blood pressure occasioned by this procedure. Furthermore, contraction of a smooth muscle sphincter of the ductus arteriosus occurs in the lamb at birth. In the chiclc sympathetic mechanisms are well formed at hatching time but vagus inhibitory function is deficient. 29 |
|
| |
|
| Plan , . . . . . . . . . . ." 156 l30-150 112 l 70
| | ==Arterial Blood Pressure== |
|
| |
|
| the bird in this respect. The possibility of asphyxial depression | | Determination of arterial blood pressure in fetuses of small animals involves diflicult technical problems and one can seldom be certain that results reflect the true condition in utero. Consequently few systematic studies have been reported. The force of the embryonic chiclc heart at two or three days incubation can lift a column of water only two centimeters during systole.34 , Pressures of 5 mm. to 20 mm. of mercury are necessary to obliterate How in the umbilical artery of rat fetuses 16.4 to 23.3 mm. 1ong.34 The variations in fetal carotid arterial pressure which accompany uterine contractions have been studiedfos I» Maximum estimations of zo mm. of mercury in the cat and 4o mm. in the dog fetus were reported during the latter part of gestation. The adult level was not reached in the dog until about 4o days after birth« and even Iater in the rabbit. 4 |
| of the fetal heart in the rat experiments should be borne in rnind, | |
| although most of the fetaj heart rates were not abnormally slow
| |
| in comparison with other species of animals. The adult rat, being
| |
| an example of a small animal with rapid pulse rate, may not actu—
| |
| ally develop the inhibitory mechanism to the same degree found
| |
| in larger mamma1s. The rapid maternal pulse of the adult | |
| monkey was attributed to the anima1’s excitement and exertion.
| |
|
| |
|
| NERVOIIS CONTROL OF THE« FETAL HEART AND CIRCULATION
| |
|
| |
|
| It is thought that the progressive slowing of the pulse in postnatal life is associated with improvement of control of the heart
| | Haselhorstss has studied blood pressures in human umbilical arteries. At one Caesarean Section he found it 68 mm. of mercury before delivering the child. At normal delivery in eight other experiments pressures varied between 46 mm. and no mm. of mercury, averaging 75 mm. It was concluded that there is no signiiicant ditference between the arterial pressure at full term in utero and after delivery. An initial umbilical arterial pressure of 60 to 70 mm. of mereury was found in one newborn infant before respiration started; this did not change with the advent of respiration but was temporarily elevated to 1oo mm. at the first cry. This experiment is illustrated in Fig. 8. |
| by the vagus nerves. It has been pointed out that animals with
| |
| small hearts have rapid pulse rates and those with large hearts
| |
| have slower rates.37 control by the vagus is less pronounced in
| |
| the former than in the latter. The higher metabolic rate of small | |
| animals is thought to make it necessary for the heart to beat about
| |
| as fast as it can under normal circumstances; consequently there
| |
| should be no call for an active inhibitory mechanism.
| |
|
| |
|
| Perhaps the fe-tal heart rate is rapid because nervous control
| |
| has not become well established or is inhibited in Some way. Most
| |
| of the evidence points to the conclusion that vagus function is not
| |
| nearly so marked in the fetus as it is in the adult, although considerable species variation is encountered. stimulation of the
| |
| vagus nerves in the chick had no effects at 18 to 20 days of incuba—
| |
| tion, but it slowed the beat seven to eight hours after hatching.38
| |
| Inhibition wsas ekkected by direct stimulation of the heart itself in
| |
| embryos of Ave days incubation.39 Muscarin produced the same
| |
| result in even younger chick embryos; it slowed the heart of small
| |
| THE! FETAL HEART 19
| |
|
| |
|
| rat embryos and was antagonized by atropine. These results in—
| |
| dicate that the failure to obtain inhibition of the heart by vagus
| |
| stimulation is not due to factors inherent in the myocardium.
| |
|
| |
|
| Most investigators who have studied the phenomenon of vagus
| | Fig. 8. Blood pressure in the human umbilical artery at birth. cannu1a in— serted at E and withdrawn at d. Respiration began at «! and the infant cried at s. (1-Iaselhorst: Ztschn Geburtsh. Gynälc.. Vol. g5, 1929.) |
| inhibition in young postnatal mammals have been able to produce
| |
| slowing or cessation of the heart beat by stimulating the peripheral
| |
| cut end of the nerve, but in many instances the ekkects seem to
| |
| have been weaker than would have been obtained in adults.40-45
| |
| This was especially true in experiments with kittensJU The
| |
| nearer to birth, postnatally, the less the chance of producing in—
| |
| hibition by stimulating the vagus nerves. In one newborn rabbit
| |
| which showed no cardiac slowing upon stimulation of the peripheral vagus, respiratory inhibition was demonstrated by stimulating the central end, indicating that the nerve is capable of
| |
| conducting and that conduction can take place through medullary
| |
| centers at birth.45 In prenatal life a few successes as well as many
| |
| failures have been reported in several species.40- 4I- 45- 43 It is probable that the negative results were obtained in many instances because the vagus nerves had been stimulated only after a maximum
| |
| decline in the rate of the heart beat had occurred in consequence
| |
| of asphyxia.
| |
|
| |
|
| In experiments with rabbits Bauer« has found that stimulation of the vagus nerve increases the decline in heart rate which
| |
| is brought about by asphyxia. This was not demonstrable how—
| |
| ever until about the fourth day after birth. Clamping the umbilical cord at experimental Caesarean section led almost immediately
| |
| to asphyxial bradycardia, b·ut the phenomenon was delayed in
| |
| specimens which had breathed air and in which asphyxia was sub—
| |
| sequently produced by occluding the trachea. The amount of
| |
| oxygen available in the blood was greater in the latter than in the
| |
| former instance. The asphyxial slo.wing of the fetal heart rate
| |
| appeared to be due, not to inHuence of the central nervous system
| |
| efkected through the vagus nerves, but. to a direct chemical action
| |
| upon the pacemaker of the heart.
| |
|
| |
|
| Various theories have been proposed to explain the slowing of
| |
| the human fetal heart at the time of delivery. Compression of the
| |
| skull of young rabbits produces bradycardia and some have held
| |
| that the passage of the fetal head through the birth canal may
| |
| bring about enough pressure to cause a similar cardiac.depression.
| |
| 20 PHYSIOLOGY OF THE FETUS
| |
|
| |
|
| However, a declining heart rate is not infrequently encountered
| | Fig. 9. Relation between blood pressure and fetal« age in the sheep. (Barcroft and lcennedzu Jour. Physiol» Vol. g5, 1g39.) |
| during 1abor before the head becomes engaged in the 1ower part
| |
| of the pelvis. ·
| |
|
| |
|
| lt has been held also that the human fetal heart rate varies
| |
| with uterine contractions and relaxationssfss I! 1t is probable that
| |
| a greater volume of blood is forced into the fetal heart by each
| |
| uterine contraction and that the pulse rate consequently declines
| |
| as the blood pressure rises in accordance with Mareyks law. Clarlcso
| |
| demonstrated experimentally that the fetal blood pressure under—
| |
| goes a brief rise followed by a prolonged fall during contraction
| |
| of the cat’s Uterus. He thought that the fall was due to a diminished venous return to the fetal heart which resulted from increased peripheral resistance set up in the placenta by the uterine
| |
| contractions. . ·
| |
|
| |
|
| Although there appears tobe no vagal tone before birth in sheep
| | Barcroft and Kennedyw have made the most comp1ete series of observations correlating blood pressure with the fetal age in the sheep and their data are reproduced in Fig. g. 0ther records of 39 mm. to 51 mm. of mercury at approximately 1 1o to 12o days gestation and 84 mm. near full term are available in this species. 58 |
| fetuses, ligating the umbilical cord results in an immediate elevation of the blood pressure and, in response to Marey’s law, an instantaneous bradycardia. When the vagi had been cut bradycardia
| |
| followed occlusion of the cord, coming on gradually or suddenly
| |
| as a 2:1 heart block. In either case it appeared only after an interval of about 25 seconds during which asphyxia had developedD
| |
|
| |
|
| It has been reported that the fetal heart rate is entirely unalfected by changes in the oxygen and carbon dioxide contents of
| |
| the mother’s blood.32 However, amyl nitrite, which has a great
| |
| relaxing effect on uterine and other smooth muscle, has an immediate influence on the mother’s heart rate and within a few
| |
| seconds a similar slowing of the fetal heart occurs. This delay sug·gests a comparable phenomenon encountered during asphyxia in
| |
| rabbit fetuses«
| |
|
| |
|
| The application of Marey’s law to explain· fetal bradycardia
| | {{Windle1940 table6}} |
| implies that ksascular reilex mechanisms are functioning in the
| |
| fetus, at least near term. This is not borne out by experimental
| |
| evidence in all species. Observations in cat and dog fetuses at
| |
| experimental Caesarean section and in the young after birth led
| |
| to the conclusion that pressor rellexes make their appearance
| |
| about three or four days postnatallyksss Z» No evidence of cardioaortic and carotid sinus reflexes was found before the fourth to
| |
| sixth day in puppies and not before 1 1 days after birth in 1cittens.
| |
| Faradic stimulation of the, depressor and carotid sinus nerves be—
| |
| THE FBTAL HBART 21
| |
|
| |
|
| gins to elicit reflex inhibition ok the heart in 11 and 14 day old
| |
| rabbits, respectivelykkk Asphyxiation fails to bring these reflexes
| |
| into play unti1 about the kortieth day ok like because the blood
| |
| pressure of -the young anima1 has not attained the necessary height
| |
| until this time (Fig. 7) . The depressor reiiex hegins to be obtained when the systemic arteria1 pressure reaches 65 mm. Hg
| |
| and the carotid si-nus reliex appears at 8o mm. pressure. These
| |
| are much higher pressures than are encountered in ketuses ok rabbits and other small anima1s. In the sheep and in man at the end
| |
|
| |
|
|
| |
|
| |
|
| 70
| | Left ventrieular systolie pressure. |
| Depkessok
| |
|
| |
|
| IOIOIIOHIOOOOIII IS
| | A marked elevation of the blood pressure has been observed to accompany respiration at birth of the sheepfls 59 It is thought to be caused by respiration but not necessarily to be permanently maintained by it. How this comes about is illustrated in Fig. 10. |
|
| |
|
| ihre-hold
| |
|
| |
|
| S«
| | Fig. 10. Femoral arterial blood pressure ok the lamb at birtlx The Erst: and subsequent respirations are indicated by the Signal (lower line); time in seconds. A diagratnmatie interpretation ok the eikect ok respirations (R1, R» etc.) in elevating the blood presst-re is shown below the tracinz (Barcrokt: «The Brain and Its Environment," Yale Univ. Press.) |
| å
| |
| E, 60
| |
| L
| |
| I
| |
| Z 50
| |
| n·
| |
| S
| |
| S 40
| |
|
| |
|
| 30
| |
|
| |
|
| 0 l0 20 30 40 50 60 70
| | It was suggested that the cardio-accelerator center ok the brain is set into activity by afkerent impulses from the lungs or diaphragm vasoconstriction, like the contraction ok the splenic smooth muscle, may be initiated similarly. |
| lduys
| |
|
| |
|
| Fig. 7.-Blood pressure ok rabbits at ditkerent ages after bitt-h. J, Maximum
| |
| blood pressure attained in asphyxiaz B, «norn1al" blood pressure (Bauer: Jour.
| |
|
| |
|
| Physiol» Vol. 95, i939.)
| | Another investigator32 has suggested that an elevation of systemic arterial pressures and the increase in the rate of heart beat after birth are kactors which operate to overcome the apnea of ketal like and initiate respiration by causing more blood of high carbon dioxide content to reach the respiratory center in the brain. But the truly signilicant elevation ok bIood pressure follows res.piration. It is probabIe that any marked rise in arterial pressure appearing upon establishment of respiration signikies that the fetus was previousIy depressed by asphyxia. Some experiments have demonstrated prenatal arterial pressures nearly as high as those after breathing has begun. |
|
| |
|
| of pregnancy ketal blood pressures are high enough to aikect responsive cardioaortic and carotid sinus mechanisms
| |
|
| |
|
| The general conclusion reached from experimental studies | | By means of a special high—speed hypodermic manometer and photographic recording, Hamilton, Woodbury and Woodsso have obtained left ventricular pressures of 45X2 and zoxo in dog fetuses near term before breathing started. In the specimen having a pressure of 45X2 this increased to zoxo after the umbilical cord had been«clamped and breathing had begun. Pressures were i4Xo in a premature rabbit fetus, 2oX i at term before breathing began, 28Xo at the end of inspiration at birth and Zoxz at the end of ex— piration. The newborn rabbit’s pressures were »so-i at inspiration and 4oXi at expirationz those of the two day old were 27X2 and 47X11. It is evident that the left ventricular pressure rises and falls with expiration and inspiration but the mean pressure increases only gradually toward the adult level after respiration is established. Hamilton and his colIeagues found that right and left ventricular pressures talcen simultaneously were similar before breathing. Clamping the umbilical cord caused little or no change, but they point out that the umbilical circuit may have been obliterated before they clamped the umbilical cord. Upon establishment of air breathing the right ventricuIar pressure dropped in inspiration more than. the left because a negative intrathoracic pressure was established, resulting in decreased peripheral resistance in the lungs. |
| and clinica1 observations is that the ketal heart pumps blood about | |
| as fast as it can without much control by the nervous System. The
| |
| vagus nerves together with their endings and central connectionse
| |
| are capab1e ok conduction «in some species near term, but in others
| |
| which are born less completely equipped to cope with their en—
| |
| vironment they may not be very well developed. Failure ok
| |
| cardio-inhibitory and vascular reAex mechanisms to kunction at
| |
| 22 PHYSIOLOGY OF THE FETUs
| |
|
| |
|
| birth is related at least partially to low systemic blood pressures
| | ==Venous Blood Pressure== |
| prevailing in the smalleiz less mature newborn animals. Asphyxia
| |
| produces cardiac depression by direct chemical action upon the
| |
| pacemalcen
| |
|
| |
|
| An explanation of the slowing of the human fetal heart during
| | Several investigators have reported venous pressures taken from the fetus at experimental hysterotomy. cohnstein and Zuntzss found pressures in the« lamb’s umbilical vein to vary between 16 mm. and 34 mm. of mercury at about Iio to 120 days gestation, and 33 mm. near term. The average of these values was about half the average of the arterial pressures. This gave the impression that thse venous pressure of the fetus is relatively much higher than that of the adult. Barcroft and KennedyM have estimated the pressure in the umbilical vein of the fetal sheep to be less than 1o mm. at 56 day gestation, about 1o mm. at 1 ro days and 18 mm. of mercury at 140 days (see Fig. g) . Blood passes through the umbilical veins of cat and guinea pig fetuses near term under pressures of 5 mm. to 13 mm. I-Ig, rising and falling with contraction and relaxation of the uterine musculatureJU Arterial pressures were not obtained in these animals but it is probable that they were considerably more than twice the venous pressures Because all the estimations of fetal venous pressures were made at experimental hysterotomy it is probable that many were higher than in -utero, being elevated by the force exerted upon the vascular bed of the placenta by the contracting uterus. Actua1ly a greater difkerential between systemic arterial and venous pressures is to be expected in the undisturbed fetus in utero than appears from the records obtained in the older experiments. It is diflicult to believe that there can be urine formation in the fetal lcidneys if the venous pressure is as much as half the arterial pressure (see Chapter VIII) . |
| birth may be fortheoming from these studies. It is inferred that
| |
| elevation of the blood pressure caused by uterine contraction
| |
| brings about a rellex bradycardia. A decline in the oxygen satura—
| |
| tion of the fetal blood during labor may induce an anoxial cardiac
| |
| depression.
| |
|
| |
|
| The prompt but transient acceleration of the heart which oc—
| |
| curs after breathing starts at birth may result from an awalcening,
| |
| as it were, of sympathetic tone consequent upon the shower of new
| |
| afferent impulses from the external environmenn Here too ex—
| |
| perimental evidence in· the sheep is available. The smooth
| |
| muscle of the fetal spleen, which isinnervated by sympathetic
| |
| neurons only, can be induced to contract reflexly by stimulating
| |
| the central end of the cut vagus. splenic activity also follows liga—
| |
| tion of the umbilical cord and is not related to the changes in
| |
| blood pressure occasioned by this procedureuss Furthermore, contraction of a smooth muscle sphincter of the ductus arteriosus
| |
| occurs in the lamb at birth. In the chiclc sympathetic mechanisms
| |
| are well formed at hatching time but vagus inhibitory function is
| |
| deöcientÆ
| |
|
| |
|
| ÄRTERIAL BLOOD PRESSURE
| | Haselhorstw recorded the pressure in the human umbilical vein at Caesarean Sections in three instances while the uterus was quiescen»t. It varied between 22 and 34 mm. of mercury. In one case the pressure in the vein increased from 24 to 7o mm. when pituitrin was injected into the uterus. A possible placental or uterine function in maintaining an adequate venous return to the, fetus should not be overloolced. |
|
| |
|
| Determination of arterial blood pressure in fetuses of small
| |
| animals involves diflicult technical problems and one can seldom
| |
| be certain that results reflect the true condition in utero. Consequently few systematic studies have been reported. The force
| |
| of the embryonic chiclc heart at two or three days incubation can
| |
| lift a column of water only two centimeters during systole.34 , Pres—
| |
| sures of 5 mm. to 20 mm. of mercury are necessary to obliterate
| |
| How in the umbilical artery of rat fetuses 16.4 to 23.3 mm. 1ong.34
| |
| The variations in fetal carotid arterial pressure which accompany
| |
| uterine contractions have been studiedfos I» Maximum estimations of zo mm. of mercury in the cat and 4o mm. in the dog fetus
| |
| were reported during the latter part of gestation. The adult level
| |
| was not reached in the dog until about 4o days after birth« and
| |
| even Iater in the rabbits
| |
| THE FETAL HEART 23
| |
|
| |
|
| Haselhorstss has studied blood pressures in human umbilical
| | ==References Cited== |
| arteries. At one Caesarean Section he found it 68 mm. of mercury
| |
| before delivering the child. At normal delivery in eight other
| |
| experiments pressures varied between 46 mm. and no mm. of
| |
| mercury, averaging 75 mm. It was concluded that there is no
| |
|
| |
|
|
| | . Fano, G. 1885. Lo sperirnentale, i: 143 (cited by F. Bottazzi sc G. Fano, 19oo, in Richet«s Dict. Physiol» 4: ask» Alcan, Paris) . |
| | |
| Fig. 8.—Blood pressure in the human umbilical artery at birth. cannu1a in—
| |
| serted at E and withdrawn at d. Respiration began at «! and the infant cried at s.
| |
| (1-Iaselhorst: Ztschn Geburtsh. Gynälc.. Vol. g5, 1929.)
| |
| | |
| signiiicant ditference between the arterial pressure at full term in
| |
| utero and after delivery. An initial umbilical arterial pressure
| |
| of 6o to 7o mm. of mereury was found in one newborn infant before respiration started; this did not change with the advent of
| |
| respiration but was temporarily elevated to 1oo mm. at the first
| |
| cry. This experiment is illustrated in Fig. 8.
| |
| | |
| sd
| |
| » Oshktsklsl present-e
| |
| S, Yo III-konsu- Faust«
| |
| · O
| |
| E
| |
| «· sc
| |
| Z
| |
| Z O
| |
| s
| |
| L so
| |
| Z 20
| |
| Z ld
| |
| | |
|
| |
| | |
| Mit) it) It) sc AIOUOIZOIIOICT
| |
| Fotslpgoindays
| |
| | |
| Fig. g.-Relation between blood pressure and fetal« age in the sheep. (Barcroft and
| |
| lcennedzu Jour. Physiol» Vol. g5, 1g39.)
| |
| | |
| Barcroft and Kennedyw have made the most comp1ete series.
| |
| | |
| of observations correlating blood pressure with the fetal age in
| |
| the sheep and their data are reproduced in Fig. g. 0ther records
| |
| of 39 mm. to 51 mm. of mercury at approximately 1 1o to 12o days
| |
| gestation and 84 mm. near full term are available in this speciesäss
| |
| 24 PHYSIOLOGY OF THE FETUs
| |
| | |
| Tilgt-I- 6
| |
| | |
| Print« Bsnoov Pnnsstrnn Nma Tnrku
| |
| | |
|
| |
|
| |
|
| |
| | |
|
| |
| Rat . . . . . . . . . . . . . . . . . . . . . . . . . . . . A) . · . . .
| |
| | |
| gäiåräea pig . . . . . . . . . . . . . . . . . . . . . . . . 5-10
| |
| | |
| it. . . . . . . . . . . . . . . . . . . . . . . . « . . . . .
| |
| | |
| Cis-l: . . . . . . . . . . . . . . . . . . . . . . . . . . . 80 7-I3
| |
| | |
| åog . . . . . . . . . . . . . . . . . . . . . . . . . . . W— X« . .
| |
| | |
| eep . . . . . . . . . . . . . . . . . . . . . . . . .
| |
| | |
| Mut! . . . . . . . . . . . . . . . . . . . . . . . . . cis-IN) 22-24
| |
| | |
|
| |
| | |
| « Lekt ventrieular systolie present-e.
| |
| | |
| A marked elevation ok the blood pressure has been observed to
| |
| accompany respiration at birth of the sheepfls 59 It is thought to
| |
| be caused by respiration but not necessarily to be permanently
| |
| maintained by it. How this comes about is illustrated in Fig. 1o.
| |
| | |
| -—« -j-.--«-L·--— »«-,j- « f,
| |
| | |
| JUC LUUULLLLLMHLEUUMLHULLLLLURHSHkijikscmuxuskisskksntttqtwtkiktktispcyiixxhf « « « « « «« « "
| |
| | |
| J— · TM- J ?1FU;·—"U-Ts——-Lrx1—-J--r—n« XICWTPI
| |
| | |
| Fig. 1o.—l-’ernoral arterial blood pressure ok the lamb at birtlx The Erst: and
| |
| subsequent respirations are indicated by the Signal (lower line); time in seconds.
| |
| A diagratnmatie interpretation ok the eikect ok respirations (R1, R» etc.) in elevating the blood presst-re is shown below the tracinz (Barcrokt: «The Brain and
| |
| Its Environment," Yale Univ. Press.)
| |
| | |
| It was suggested that the cardio-accelerator center ok the brain is
| |
| set into activity by afkerent impulses from the lungs or diaphragnx
| |
| Vasoconstriction, like the contraction ok the splenic smooth
| |
| muscle, may be initiated similarly.
| |
| | |
| Another investigator32 lys suggested that an elevation of sysTHE FETAL HEART 25
| |
| | |
| temic arterial pressures and the increase in the rate of heart beat
| |
| after birth are kactors which operate to overcome the apnea of
| |
| ketal like and initiate respiration by causing more blood of high
| |
| carbon dioxide content to reach the respiratory center in the brain.
| |
| But the truly signilicant elevation ok bIood pressure follows res.piration. It is probabIe that any marked rise in arterial pressure
| |
| appearing upon establishment of respiration signikies that the
| |
| fetus was previousIy depressed by asphyxia. Some experiments
| |
| have demonstrated prenatal arterial pressures nearly as high as
| |
| those after breathing has begun.
| |
| | |
| By means of a special high—speed hypodermic manometer and
| |
| photographic recording, Hamilton, Woodbury and Woodsso have
| |
| obtained left ventricular pressures of 45X2 and zoxo in dog fetuses
| |
| near term before breathing started. In the specimen having a
| |
| pressure of 45X2 this increased to zoxo after the umbilical cord
| |
| had been«clamped and breathing had begun. Pressures were i4Xo
| |
| in a premature rabbit fetus, 2oX i at term before breathing began,
| |
| 28Xo at the end of inspiration at birth and Zoxz at the end of ex—
| |
| piration. The newborn rabbit’s pressures were »so-i at inspiration and 4oXi at expirationz those of the two day old were 27X2
| |
| and 47X11. It is evident that the left ventricular pressure rises
| |
| and falls with expiration and inspiration but the mean pressure
| |
| increases only gradually toward the adult level after respiration is
| |
| established. Hamilton and his colIeagues found that right and
| |
| left ventricular pressures talcen simultaneously were similar before
| |
| breathing. Clamping the umbilical cord caused little or no
| |
| change, but they point out that the umbilical circuit may have
| |
| been obliterated before they clamped the umbilical cord. Upon
| |
| establishment of air breathing the right ventricuIar pressure dropped in inspiration more than. the left because a negative intrathoracic pressure was established, resulting in decreased peripheral
| |
| resistance in the lungs.
| |
| | |
| VENOUS BLOOD PRBSSURE
| |
| | |
| several investigators have reported venous pressures talcen.
| |
| | |
| from the fetus at experimental hysterotomy. cohnstein and
| |
| Zuntzss found pressures in the« lamb’s umbilical vein to vary between 16 mm. and 34 mm. of mercury at about Iio to 120 days
| |
| gestation, and 33 mm. near term. The average of these values
| |
| 26 PHYSIOLOGY OF THE« FETUS
| |
| | |
| was about half the average of the arterial pressures. This gave
| |
| the impression that thse venous pressure of the fetus is relatively
| |
| much higher than that of the adult. Barcroft and KennedyM have
| |
| estimated the pressure in the umbilical vein of the fetal sheep to
| |
| be less than 1o mm. at 56 day gestation, about 1o mm. at 1 ro days
| |
| and 18 mm. of mercury at 140 days (see Fig. g) . Blood passes
| |
| through the umbilical veins of cat and guinea pig fetuses near
| |
| term under pressures of 5 mm. to 13 mm. I-Ig, rising and falling
| |
| with contraction and relaxation of the uterine musculatureJU
| |
| Arterial pressures were not obtained in these animals but it is
| |
| probable that they were considerably more than twice the venous
| |
| pressures Because all the estimations of fetal venous pressures
| |
| were made at experimental hysterotomy it is probable that many
| |
| were higher than in -utero, being elevated by the force exerted
| |
| upon the vascular bed of the placenta by the contracting uterus.
| |
| Actua1ly a greater difkerential between systemic arterial and venous
| |
| pressures is to be expected in the undisturbed fetus in utero than
| |
| appears from the records obtained in the older experiments. It is
| |
| diflicult to believe that there can be urine formation in the fetal
| |
| lcidneys if the venous pressure is as much as half the arterial pressure (see Chapter VIII) .
| |
| | |
| Haselhorstw recorded the pressure in the human umbilical
| |
| vein at Caesarean Sections in three instances while the uterus was
| |
| quiescen»t. It varied between 22 and 34 mm. of mercury. In one
| |
| case the pressure in the vein increased from 24 to 7o mm. when
| |
| pituitrin was injected into the uterus. A possible placental or
| |
| uterine function in maintaining an adequate venous return to the,
| |
| fetus should not be overloolced.
| |
| | |
| REFERENCES CITED
| |
| | |
| . Fano, G. 1885. Lo sperirnentale, i: 143 (cited by F. Bottazzi sc G. | |
| Fano, 19oo, in Richet«s Dict. Physiol» 4: ask» Alcan, Paris) . | |
|
| |
|
| I-Ioolcer, D. 1911. J. Exp. Zool» u: 159. | | I-Ioolcer, D. 1911. J. Exp. Zool» u: 159. |
|
| |
|
| Lillie, F. R. 1919. The Development of the Chiclg Henry I-Iolt, N. Y. | | Lillie, F. R. 1919. The Development of the Chiclg Henry I-Iolt, N. Y. sabin, F. R. 192o. Contrib. Emb., g: arg. |
| sabin, F. R. 192o. Contrib. Emb., g: arg. | |
|
| |
|
| Johnstone, P. N. 1925. Johns Hoplcins Hosp. Bull., 363 299. | | Johnstone, P. N. 1925. Johns Hoplcins Hosp. Bull., 363 299. |
| Line 735: |
Line 183: |
| . Fano, G. 8c F. Bodano. 189o. Arch. Ital. Biol., is: 387. | | . Fano, G. 8c F. Bodano. 189o. Arch. Ital. Biol., is: 387. |
|
| |
|
| Piclcering, W. 18g3. Physiol» 143 383. | | Piclcering, W. 18g3. Physiol» 143 383. |
|
| |
|
| II« | | II« |
|
| |
|
| ZO Orts-wesse- kTHE FETAL HEART | | ZO Orts-wesse- |
|
| |
|
| ii. Pakk, G. H. i9s5. Anat. Rec., 6s: sos. | | ii. Pakk, G. H. i9s5. Anat. Rec., 6s: sos. |
| Line 745: |
Line 193: |
| is. Cremer, M. igo6. Mönch. med. Wochenschr., i: 8ii. | | is. Cremer, M. igo6. Mönch. med. Wochenschr., i: 8ii. |
|
| |
|
| is. Foä, C. i9ii. A.i·ch. 1tal. Biol., zö- i45. | | is. Foä, C. i9ii. A.i·ch. 1tal. Biol., zö- i45. i4. Nin-r, J. igsi. Ztschix Biol., 7s: iss. |
| i4. Nin-r, J. igsi. Ztschix Biol., 7s: iss. | |
|
| |
|
| is. sachs, H. i9ss. Pklügeks Arch., i97: 5s6. | | is. sachs, H. i9ss. Pklügeks Arch., i97: 5s6. |
| Line 770: |
Line 217: |
| 4i. | | 4i. |
|
| |
|
| s4. Kruinbhaaiz E. B. sc H. H. Jenlcs. i9i7. Heim, S: i89.
| | 44. Kruinbhaaiz E. B. sc H. H. Jenlcs. i9i7. Heim, S: i89. |
| | |
| | 45. Leu-is, T. i9i6. .Phil. Trans. Roy. soc» Lond. B» so7: ssi. |
|
| |
|
| s5. Leu-is, T. i9i6. .Phil. Trans. Roy. soc» Lond. B» so7: ssi.
| | 46. Patten, B. M. igss. In A. H. Curtis« Obstetrics and Gynecology, i: saunders, Philadelphia |
|
| |
|
| s6. Patten, B. M. igss. In A. H. Curtis« Obstetrics and Gynecology, i:
| | s7. Bogue, I. Y. i9ss. J. Exp. Biol» io: s86. |
|
| |
|
| 9o6, saunders, Philadelphia
| |
| s7. Bogue, I. Y. i9ss. J. Exp. Biol» io: s86.
| |
| s8. HolL E. C» T. C. Kramer, D. DuBois sc. B. M. Farren. i9s9. Anker. | | s8. HolL E. C» T. C. Kramer, D. DuBois sc. B. M. Farren. i9s9. Anker. |
|
| |
|
| Heart J., i7: 47o. | | Heart J., i7: 47o. Cohn, A. E. s: E. L. Wile. i9s5. J. Exp. Med., 4s: s9i. |
| Cohn, A. E. s: E. L. Wile. i9s5. J. Exp. Med., 4s: s9i. | |
|
| |
|
| Bogue, I. Y. i9ss. J. Eikp. Biol., g: s5i. | | Bogue, I. Y. i9ss. J. Eikp. Biol., g: s5i. Blaclckan, K. D. igss. Growth and Development ok the Childz White |
| Blaclckan, K. D. igss. Growth and Development ok the Childz White | |
|
| |
|
| House Conkerence Reports sect. I, Pt. i: P. 5s. Century Co» N. Y. | | House Conkerence Reports sect. I, Pt. i: P. 5s. Century Co» N. Y. |
| Line 796: |
Line 241: |
| Corey, E. L. i9ss. Am. J. Physiol» ioi: so4. | | Corey, E. L. i9ss. Am. J. Physiol» ioi: so4. |
|
| |
|
| Hartman, C. G» R. R. squier s: O. L. Tinlclepaugh i9so. Proc. soc. | | Hartman, C. G» R. R. squier s: O. L. Tinlclepaugh i9so. Proc. soc. Exp. Biol. s: Med., s8: s85. |
| Exp. Biol. s: Med., s8: s85. | |
|
| |
|
| . Barcrokh J. i9s6. Physiol. Reis» is: ios. | | . Barcrokh J. i9s6. Physiol. Reis» is: ios. . Clarlc, A.· J. i9s7. |
| . Clarlc, A.· J. i9s7. | |
|
| |
|
| Comparative Physiology ok the Heart, Cambridge | | Comparative Physiology ok the Heart, Cambridge Univ. Press. |
| Univ. Press. | |
|
| |
|
| Botta2zi, F. s: G. Fano. i9oo. In C. Richet’s Dictionnajre de Physiologia | | Botta2zi, F. s: G. Fano. i9oo. In C. Richet’s Dictionnajre de Physiologia 4: s5s. AIcan, Paris. |
| 4: s5s. AIcan, Paris. | |
|
| |
|
| Pickeiinz W. i896. Physiol» so: i65. | | Pickeiinz W. i896. Physiol» so: i65. |
|
| |
|
| . soltmann, O. i877.« Jahrb. Kinderhllc» ii: ioi. | | . soltmann, O. i877.« Jahrb. Kinderhllc» ii: ioi. |
| Line 816: |
Line 257: |
| . Heinrichs, G. i89o. «Ztschr. Biol., s6: i·97. | | . Heinrichs, G. i89o. «Ztschr. Biol., s6: i·97. |
|
| |
|
| . Meyer, E. i89s. Arcli. Physiol. Norm. et Path» z: 475. | | . Meyer, E. i89s. Arcli. Physiol. Norm. et Path» z: 475. . Buglia, G. igs6. Archq Fisiol» s4: 448. |
| . Buglia, G. igs6. Archq Fisiol» s4: 448. | |
|
| |
|
| . Kellogg, H. B. | | . Kellogg, H. B. . Clark, G. A. |
| . Clark, G. A. | |
|
| |
|
| . Bauer, D. i9s8. Ibid» 9s: gox 95: i87. | | . Bauer, D. i9s8. Ibid» 9s: gox 95: i87. . LeiL M. i9ss. Arn. J. Obst. Gyn., s4: 898. . Wissen, C. J. |
| . LeiL M. i9ss. Arn. J. Obst. Gyn., s4: 898. | |
| . Wissen, C. J. | |
|
| |
|
| i9s7. Pi·oc. soc. Exp. Biol. s: Med» s4: 8s9. | | i9s7. Pi·oc. soc. Exp. Biol. s: Med» s4: 8s9. i9s4. J. Physiol» As: ss9. |
| i9s4. J. Physiol» As: ss9. | |
|
| |
|
| i9s4. In I. A. Abiks Pediatrics, 4: i98, saunders Phila— | | i9s4. In I. A. Abiks Pediatrics, 4: i98, saunders Phila— delphia. |
| delphia. | |
|
| |
|
| . C1ar1c, G. A. i9ss. J. Physiol» 74: s9i. | | . C1ar1c, G. A. i9ss. J. Physiol» 74: s9i. |
| 28 PHYSIOLOGY OF THE FETUS
| |
|
| |
|
| Hi. Barcrokh J. 1938. The Brain ancl Its Environmenr. Yale Und. Frass, | | Hi. Barcrokh J. 1938. The Brain ancl Its Environmenr. Yale Und. Frass, |
| Line 841: |
Line 275: |
| se. Bach, W. 193i. Arclh Gynäk.,·i47: se. | | se. Bach, W. 193i. Arclh Gynäk.,·i47: se. |
|
| |
|
| 53. Tayloxz D. B. sc T. Gotsem 193s. citecl by J. Bat-Gott, 1938. | | 53. Tayloxz D. B. sc T. Gotsem 193s. citecl by J. Bat-Gott, 1938. |
| 54. Bill, L. sc Y. A2uma. 1927. Physiol» 62: 27P. | | |
| | 54. Bill, L. sc Y. A2uma. 1927. Physiol» 62: 27P. |
|
| |
|
| 55. Mark, G. A. sc H. E. Ho11ing. 1931. 1bicl., 73: 3o5. | | 55. Mark, G. A. sc H. E. Ho11ing. 1931. 1bicl., 73: 3o5. |
| Line 856: |
Line 291: |
| 6o. Hamilton, W. F» R. A. Wooclbury sc E. B. Woods. 1937. Am. J. Physiol» | | 6o. Hamilton, W. F» R. A. Wooclbury sc E. B. Woods. 1937. Am. J. Physiol» |
|
| |
|
| 119: 2o6. | | 119: 2o6. Si. DeMarslh B. sc W. F. Winde. 193g. Unpublishech |
| Si. DeMarslh B. sc W. F. Winde. 193g. Unpublishech | |
| CHAPTER III
| |
| | |
| THE FETAL CIRCULÄTION
| |
| | |
| VOLUUE OF« BLOOD AND RATE OF« CIRCULATION
| |
| | |
| BLooD volume is usually expressed as a function of body surkace or body weight in the adult. The fetus has no surface from
| |
| which heat is lost ·and therefore it is illogical to use surface relationships in indicating its blood volume. If we are to express it
| |
| in terms of the weight of fetal tissues through which the blood
| |
| passes we must consider not only the weight of the fetus itself but
| |
| also that of the placenta. The relationship between the two
| |
| changes greatly as gestation proceeds.
| |
| | |
| The blood volume of five huxnan infants 273 to 8 hours after
| |
| birth was found to vary between 144 and 173 cc. per kilogram
| |
| body weight, averaging 1»56.4 cc. per kilogram,1 but no data are
| |
| available for human fetuses. The amount of fetal blood in the
| |
| placenta is not known; but ioo cc. or more can be recovered
| |
| from the placenta when th«e umbilical cord is clamped immediately after birth. Much of this blood will return to the fetus if
| |
| clamping is delayed.
| |
| | |
| Determinations of blood volume have been made by Cohnstein
| |
| and Zuntz2 in rabbits and by Elliott, Hall and Huggetts in goats.
| |
| | |
| Tatar-I: 7
| |
| Bhoov Vor-wi- m Gou Pnrusns
| |
| | |
|
| |
|
| |
| | |
|
| |
|
| |
| | |
|
| |
| | |
| Total Blood volume Total volume
| |
| Fels« AS« htood Votum,- iu kskus ok htodd
| |
| w. X IW w. X IW
| |
| day« cc. ··W. in qmms wt in qmm
| |
| | |
| 68 ................ .. 1·6.3 21.8 8.0
| |
| | |
| 71 ............ 4p.4 « 40-4 8.8
| |
| | |
| 77 ................ . St» e» IT
| |
| | |
| 85 ................ .. 640 es« .
| |
| 101 ................ .. 112.0 l 17.s 9.s
| |
| us ................ . 145«..0 11.6 6.8
| |
| 126 ................ .. 2o4,.0 14.2 9.9
| |
| 136 ................ .. ist«-O« 14.2. », tu;
| |
| 144 ............ 428.0 I 14.7 11.95
| |
| | |
| ·« Triplet
| |
| 30 Isknsstococv or THE. FETUS
| |
| | |
| The latter investigators used the method of injecting a known
| |
| amount of dye into thefetal yessels and estimating its dilution in
| |
| the blood stream. Their results are summarized in Table 7.9
| |
| More recently Barcroft and Icennedy4 have employed an improved
| |
| colorimetric dye injection method (Evan’s Blue, T 1,824) to study
| |
| blood volume in sheep fetuses from the standpoint of develops
| |
| ment. Their results are illustrated in- Figs. 1 1 and Ia.
| |
| | |
| The placental cotyledons of the sheep reach maximal weight at
| |
| about the middle of gestation when the fetus weighs only about
| |
| | |
|
| |
|
| |
| | |
| Blood schone, me.
| |
| E
| |
| | |
|
| |
| | |
| I U) III) NO
| |
| Fern! age in days
| |
| | |
| Fig. 11.—The relation of blood volume in the ketus 4- the placenta to age in
| |
| single ketuses (O) and twin ketuses (o) . (I3arcrokt and lcennedze Jour. Physiol»
| |
| | |
| Vol. 95, i939.) «
| |
| | |
| IN)
| |
| | |
| 2oo grams. It is evident that the blood forms a. much larger part
| |
| of the fetal body weight at this time than it does toward full term.
| |
| The absolute amount of blood increases greatly in the fetal body
| |
| but that in the placenta remains nearly constant throughout the
| |
| last third of gestation. At ido days of gestation the«fetal blood is
| |
| divided about equally between fetal body and placenta; at approximately full term only about one-fourth of it is in the p1acenta.
| |
| Twin fetuses weigh a little less than single fetuses of comparable
| |
| age, and their volume of blood is even less than encountered in
| |
| | |
| single fetuses of comparable we·ig"h"t.
| |
| THE. FETAL CIRCULATJON .3I
| |
| | |
| Although the amount of blood in the uterus at any one time
| |
| during the last quarter of gestation remains about the same, the
| |
| quantity traversing the uterus per minute more than doubles be—
| |
| tween i io and i4o days. This may be related to the increase in
| |
| fetal blood pressure (see chapter II) . The rate of blood How to
| |
| and from the placenta is surprisingly great in the last quarter of
| |
| fetal life.5 At i i i days of gestation i i i cc. of blood traversed the
| |
| | |
| umbilical vessels per minute, and at 137 days this had increased to
| |
| 568 cc. per minute. similarly on the maternal side in the same
| |
| | |
| osvolunn in fetus snti placenta Osvolunis in fetus and placent
| |
| n· is » Tot-is alone
| |
| | |
| s· i· placent- alone
| |
| | |
| i- fetus aione
| |
| s· placenta alone
| |
| | |
| IS IIIkO-C-Hq
| |
| Iocscssssp --..
| |
| s.·.·.-.:«:k-·---0
| |
| IOOOOOOIH
| |
| | |
| IUO
| |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | |
| ä
| |
| | |
| Blum! wiss-ne, e.o.
| |
| Ist»
| |
| I IEJJJJI
| |
| ««;.·.·.-.·:«.-.
| |
| -t-«-»---««-o
| |
| -s----o
| |
| OO
| |
| xssossssso
| |
| its-»so—-t--------0
| |
| | |
| IW
| |
| | |
| .L7:«:«...
| |
| PCIHHH .
| |
| M- III- H
| |
| »Es-aq
| |
| O IWO NO FOR NO W sc 90 III) Ilc IZO IIO NO IN
| |
| Fett-l set. in g. Petri! aged in days
| |
| | |
| Fig. i2.—The blood volume in relation to (a) fetal weight and (d) fetal age in
| |
| single fetuses. (Barcroft and lcennedy: Jour. Physiol» Vol. 95. i939.)
| |
| | |
| animals the rates of How were 1o6 cc. per minute and 475 cc. per
| |
| minute. Lower values were obtained at fu«l1 term, and the Signiücance of this is not c1ear; contraction of the uterus upon the
| |
| placenta may have impeded the flow. Tab1e ·8 contains the data
| |
| which have been reported. «
| |
| | |
| The time taken for blood to circulate from the umbilical vein
| |
| through the body of the human fetus and back to the umbilical
| |
| artery has been estimated« The mean circulation time before
| |
| birth was found to be 30 seconds, and after birth, 60 seconds.
| |
| | |
| The cardiac output of the heart has been estimated in the
| |
| 32 PHYSIOLOGY OF THE FETUS
| |
| | |
| sheep fetus,7-9 but in view of the surprisingly rapid How through
| |
| the umbilical circuit it must be considerably greater than was reported in the earlier studies. More information is needed on this
| |
| question.
| |
| | |
| TABLE 8
| |
| RATE or Bhoon PLow Tktnoucin THE: PLACENTA m THE SIIDDP
| |
| | |
| jk
| |
| - H» sj jskjj H— j
| |
| | |
|
| |
| | |
| I Rate ok blood How
| |
| Age of ketus VVL of ketus Blosxs so!
| |
| d «
| |
| ( IN) Wams) Of kstus Umhi1ic21 cis-d Uterus
| |
| cc.,Jmin. cc.,Jmin.
| |
| 111 . . . . . . . . . . . . . . . . — 1,200 1;5 I 111 106
| |
| | |
| 126 . . . . . . . . . . . . . . . . 3,000 270 218 161
| |
| | |
| 127 . . . . . . . . . . . . . . . . 2850 265 162 179
| |
| | |
| 129 . . . . . . . . . . . . . . . . 2,750 270 600 . . .
| |
| | |
| 137 . « . . . . . . · . . . . . . . . 3,850 350 568 475
| |
| | |
| 138 . . . . . . . . . . . . . «. . . ZEIT) I 860 284 347
| |
| | |
| 152 . . . . . . . . . . . . . . 2800 412 300 I 268
| |
| | |
| THE COIJRSE OF THE FETAL BLOOD
| |
| | |
| The course of the blood through the fetal heart has been the
| |
| subject of controversy for more than a century. Most of the arguments need not be considered in this place, for readers who are
| |
| interested in the historical aspects of this subject can consult the
| |
| excellent review of Pohlman.10 Three principal theories are supported by experimental observations.
| |
| | |
| The one which has been favored by most writers until a few
| |
| years ago holds that oxygenated blood from the placenta is brought
| |
| by way of the umbilical vein, ductus venosus (part going through
| |
| Iiver sinuses) and inferior vena cava to the right atrium of the fetal
| |
| heart. A fold of endocardium known as the valve of the inferior
| |
| vena cava directs this stream of more richly oxygenated blood
| |
| across the blood ftlled right atrium and through the foramen ovale
| |
| into the left atrium. Pulmonary venous blood, said to be negligible in quantity before birth, is added to it there. This still
| |
| relatively well oxygenated mixture of blood enters the left ventricle, is pumped into the ascending aorta and the arch from
| |
| which spring the great vessels supplying the heart, head and upper
| |
| extremities A small quantity passes on through the aortic isthmus into the descending aorta. Venous blood returning from the
| |
| rostral parts of the body by way of the superior vena cava enters
| |
| THE FETAL CIRCULATION
| |
| | |
| the right atrium and is said to cross the stream from the inferior
| |
| vena cava without mixing with it. This reduced blood passes
| |
| into the right ventricle which forces it into the pulmonary arterial
| |
| trunkz a little goes to the lungs but the greater part is shunted by
| |
| the ductus arteriosus connecting the pulmonary artery with that
| |
| part of the aorta distal to its great branches. There a smaller
| |
| volume of the more highly oxygenated blood from the left side of
| |
| the heart joins it and passes to the lower extremities as well as
| |
| baclc to the placentas by way of the hypogastric and umbilical arteries. This is the sabatier (1791) doctrine, supported originally
| |
| only by anatomical observations.
| |
| | |
| 0pposed to it is the theory developed by PohlmanU and
| |
| lcelloggIY 12 which holds that two streams of Iluid entering a
| |
| common chamber from opposite directions must mix. Accotck
| |
| ingly, the well oxygenated umbilical vein blood, diluted once
| |
| when joined by venous blood of the abdominal inferior vena cava,
| |
| enters the right atrium and is diluted again by that arriving from
| |
| the upper part of the body in the superior vena cava. The re—
| |
| sulting complete mixture goes two ways. Part passes into the
| |
| right ventricle through the right atriowentricular oriücez the re—
| |
| mainder traverses the foramen ovale and left atrium to enter the
| |
| left ventricle. According to this view the aortic and the pulmonary ductus arteriosus streams of blood are approximately
| |
| alilce in respect to oxygen content, and the upper parts of the
| |
| body, including the heart which is the only organ working as hard
| |
| in fetal life as it does in the newborn, receive no better blood
| |
| than do the trunl(, inferior extremities and placenta. It is as—
| |
| sumed that no signiiicant quantity of blood enters the fetal heart
| |
| by channels other than the two venae cavae.
| |
| | |
| Let us defer consideration of the third hypothesis for the
| |
| moment and examine the experimental evidence in favor of the
| |
| sabatier and Pohlman-I(ellogg theories. The first study was made
| |
| more than a century agoU szby injecting pastes of contrasting color
| |
| into the superior and infexior venae cavae of three dead human
| |
| | |
| fetuses under equal pressures. 1n one fetus the injection of the
| |
| superior vena cava failed to reach the heart, in one the two colored
| |
| masses underwent some mixing, and in the third, a fetus of seven
| |
| months, the two crossed without mixing. The experiment was
| |
| accepted as a demonstration of the validity»of the sabatier theory.
| |
| 34 PHYSIOLOGY OF THE FETUs
| |
| | |
| Direct observation of the beating heart of guinea pig fetuses
| |
| whose placental circulation was intact has shown that the two sides
| |
| difker in color, the left being bright and the right being darlc.9
| |
| similar1y in cat fetuses we have observed that when the umbilical
| |
| vein blood is brilliantly red the carotid artery sometimes appears
| |
| bright and the umbilical artery dull. There are certain objec—
| |
| tions to accepting such observations. as these favoring the sabatier
| |
| doctrine, however. The left« ventric1e of the feta1 heart has a thinner wall than the right and may appear brighter even though the
| |
| oxygen content of the blood is the same in both chambers. The
| |
| bright red color of the umbilical vein of the cat fetus often results
| |
| from contraction of the uterus which sends blood to the heart
| |
| under higher pressures than are normally encountered. When
| |
| the intra-uterine pressure is unevenly distributed in a fetus which
| |
| has been removed from the Uterus, oxygenated blood may surge
| |
| through the foramen ovale and into the left side of the heart in
| |
| unusual proportions.
| |
| | |
| Experiments lending support to the second view, that the two
| |
| caval blood streams mix completely in the right atrium, were
| |
| initiated by Poh1manI0-I4 who was the first investigator to use
| |
| living fetuses in a study of the course of blood before birth. He
| |
| determined that both ventricles of the fetal pig heart have nearly
| |
| equal capacities and that the ventricular pressures are practically
| |
| identical. A saline suspension of corn starch was injected into the
| |
| umbilical vein or into the fetal superior vena cava. Thereafter
| |
| equal quantities of starch were recovered from samples of blood
| |
| withdrawn from tlie two ventricles.
| |
| | |
| KelloggU repeated and extended these experiments using a
| |
| much larger series of pig fetuses. He demonstrated that material
| |
| injected into either the umbilical vein or the fetal superior vena
| |
| cava appeared at the same instant in both ventricles. After simuItaneously withdrawing equal samples« under the same pressure
| |
| from the two ventricles equal numbers of starch grains per cc.
| |
| were counted in each sample. sedimentation of starch from larger
| |
| volumes of blood talcen from the two ventricles confirmed these
| |
| observations. similar results have been obtained in chiclc embryos.I5
| |
| | |
| It was thought that injection of a foreign substance may have
| |
| bloclced capillary beds besyond the heart, resulted in stasis and
| |
| THE« FETAL CIRCULATION
| |
| | |
| consequent churning of the blood within the heart. Therefore,
| |
| KelloggU resorted to direct manometric gas analyses of small
| |
| ventricular blood samples. For this investigation dog fetuses were
| |
| delivered under 1ocal anesthesia, care being talscen to maintain the
| |
| placenta1 circulation intact. It proved impossible to obtain adequate samples from the two ventricles simultaneous1y in the dog
| |
| without collapsing the heart. Consequently blood was iirst withdrawn from one ventricle, then after a few minutes, from the
| |
| other. The order of talcing was alternated. sixteen samples from
| |
| each ventricle were analyzed. The average values obtained from
| |
| right and left ventricular blood were 2.38 and 2.43 volumes per
| |
| cent of oxygen and 44.33 and 42.69 volumes per cent of carbon
| |
| dioxide. These data seemed to indicate that the two caval streams
| |
| of the dog fetus undergo rather thorough mixing in the right
| |
| atrium of the heart, assuming that the fetal pu1monary veins added
| |
| a negligible quantity of blood to the left atrium. It will be shown
| |
| presently however that the pulmonary return is much greater than
| |
| was previously supposed. Therefore the studies under discussion
| |
| do not prov complete mixture in the right atrium.
| |
| | |
|
| |
| | |
| 0ther s · s in goat and sheep fetuses by HuggettW and Barcroft9 see indicate that the blood takes a iigure of eight course
| |
| through etal body, first through an «upper" circulation and
| |
| | |
| « h a «lower," but without complete mixture in the
| |
| I— m. The evidence is based on a comparison of values
| |
| obtained by determining oxygen content of the blood drawn from
| |
| upper and lower Circulations, e.g., from the carotid and umbilical
| |
| arteries of the fetuses. The data in question are reproduced in
| |
| Table g. More recently additional blood—gas analyses have be
| |
| TAZLIJ 9
| |
| OXYSEN copy-kniest· or· Byoov n: Tag III-km! AND Izowsa Fig-kar- Cmomwistons
| |
| | |
| I
| |
| llJmbilical carotid Umbilical
| |
| | |
|
| |
|
| |
| | |
| Umbilical I carotid « Umbilical
| |
| | |
|
| |
| | |
| Gent vem artery arte Säsep vein artery I artery
| |
| no« (vol. W) I (vol. Z) H (vol. X) « I (vol. W) (vol. Z) (vol. NO,
| |
| .....—....-......-....---..-..-..-—..l-..—.B..... 7.o I 6.0 I c.0 11..... 11.3-12.3 10.3 l 6.9—9.2— 6.5 «: 025 14..... 15.7 1o.4 1o.4
| |
| D..... 5.o 4.5 0.9 16..... 17.4 9.1 - «:
| |
| M» 12.o H 9.0 I 4.0 19..... 6.8 337 1.7
| |
| 9.7 «« s.0 27..... 10.5 6.9 5.9
| |
| o..... 7.5 l 7.o , as I
| |
| Ave 7.96 5.9 2.94 Ave....l1.2.3—12.4 8.s 6.0-6.s
| |
| I
| |
| 36 PHYsmLocY oF THE FETUs
| |
| | |
| come available in sheep ketusesU and these are included in Fig. 13.
| |
| The data lend considerable support to the classical sabatier theory
| |
| but do not prove that a complete crossing ok the streams occurs
| |
| in the right atrium. ·
| |
| All data based upon fetal blood-gas analysis are open to criticism. No one can doubt that physiologic conditions are up—
| |
| set when fetuses are removed from the Uterus. Even though the
| |
| p1acentas were left intact the uterineplacental relationships were
| |
| altered and various degrees of anoxemia were set up in the fetuses.
| |
| | |
|
| |
| | |
|
| |
|
| |
| | |
|
| |
|
| |
| | |
|
| |
| | |
| ctypm i« Ost« ex. pes- lwr.c Hist!
| |
| | |
| o Ccfskfck »Ist)
| |
| L« "«·««««
| |
| | |
| W IW III) III) Ist) III)
| |
| Pein! OF« day«
| |
| | |
|
| |
| | |
| O
| |
| l
| |
| | |
| Fig. iz.—comparison ok the oxygen content ok blood from the urnbilical vein
| |
| (o) , carotid artery (o) and umbilical artery (x) at different ketal ages (sheep).
| |
| (l3arcrokt: «·The Brain and Its Environment," Yale Univn Press.)
| |
| | |
| -It is diflicult to believe that the blood ok the ketal heart contains
| |
| | |
| as little oxygen as was encountered in the dog at Caesarean Section,
| |
| and it is even questionable ik a proportional reduction could have
| |
| talcen place in the two ventricular samples. The withdrawal of
| |
| blood from only one ventricle at a time may have disturbed pressures and have led to serious errors.
| |
| | |
| The bl"ood-gas analyses in goats and sheep are not quite as convincing when all the data are scrutinized as they appear on super·
| |
| kicial inspection. In one ok the experimental animals (sheep No.
| |
| 1 I) the thoracic inferior vena cava blood appeared to contain less
| |
| THE FETAL cIRcULATIoN 37
| |
| | |
| oxygen than that of the carotid artery, which of course is quite
| |
| impossible; two different values were given for the blood of the
| |
| umbilical artery, one of which was not a great deal less than that
| |
| for the carotid artery. In other animals (sheep No. 14 and goat
| |
| B) the carotid and umbilical artery blood contained identical
| |
| amounts of oxygen.
| |
| | |
| Apparently no one has been able to obtain blood samples
| |
| simultaneously from the upper and lower Circulations. When this
| |
| has been done the results will have more meaning than those
| |
| available today. It should be pointed out that the oxygen content
| |
| | |
| Fig. 14.—Roentgenogram showing injection ok thorotrast into the superior vena
| |
| cava of a living sheep fetus. The passage of blood through the right side of the
| |
| heart, into the pulmonary arteries, ductus arteriosus and descending aorta may be
| |
| seen. Gar-day, et al.: Brit. J. Raclio1., Vol. te, 1939.)
| |
| | |
| of the umbilical vein blood of cat fetuses near birth fluctuates
| |
| widely from moment to moment and this appears to be related to
| |
| rhythmical uterine contractionsÄs «The inconsistencies in existing data may be related to this phenomenon. That species differences may exist must not be overloolced Perhaps less mixing
| |
| takes place in the sheep and goat than in the pig and dog fetuses,
| |
| | |
| as Patten has suggested.9
| |
| 0ne of the most significant studies of the fetal circulation is
| |
| | |
| that of Barc1ay and his colleagues.I9 By means of ac-ray cinephos
| |
| tography during injections of radio-opaque substances into the
| |
| 38 PHYsIoLocY oF THE FETUS
| |
| | |
| jugular or umbilical veins of sheep fetuses they were able to follow the course of the s two vena caval streams very clearly. The
| |
| superior vena cava blood appeared to pass directly through the
| |
| right atrium into the right ventricle (Fig. 14) as it should according to the sabatier theory. The blood from the inferior vena
| |
| cava took two courses. Most of it traversed the right atrium and
| |
| foramen ovale to the left atrium andleft ventriclez a small part of
| |
| it passed directly into the right ventricle (Fig. 15) . Barring the
| |
| possibility that the force of injection added susiicient impetus to
| |
| | |
| Fig. 15.—Roentgenogram showing injection of thorotrast into the inferior vena
| |
| cava of a Iiving sheep fetus. The passage of blood through the right atrium,
| |
| foramen ovale and left side of the heart may be seen. Its course through the
| |
| hrachiocephalic artery a11d aorta is showtr A small amount of blood enters the
| |
| right ventricle and may be seen in the pulmonary arteries. (Barclay, et a1.: Brit.
| |
| | |
| J. Radiol.. Vol. te, 1939.)
| |
| | |
| the blood to overcome a normal tendency to mix in the right
| |
| atrium, these experiments prove that a very significant proportion
| |
| of the more highly oxygenated blood from the placenta goes di—
| |
| rectly to the left side of the heart of the sheep fetus to be delivered,
| |
| after mixing with reduced pulmonary blood, to the heart, upper
| |
| extremities and head. This is precisely the arrangement proposed
| |
| by WolfkV more than 160 years ago and supported by experiments
| |
| in dead fetuses by ZiegenspeckJU
| |
| | |
| Patten and Toulmin22 liave approached the question of blood
| |
| flow through the heart from an entirely different standpoint.
| |
| THE FETAL CIRCULATION
| |
| | |
| After determining that the average weights of the left and right
| |
| ventricles are 7.11 and 8.o5 grarns, they measured the kunctional
| |
| areas ok all the heart apertures in 2o normal human still—born
| |
| ketuses at term. Their results expressed in average diameters and
| |
| areas are illustrated diagrammatically in Fig. 16. Although they
| |
| point out that it would be unwise to draw too specific conclusions
| |
| about the circulation merely from the sizes of vessels, they are in
| |
| Isfhmus
| |
| dss - U. Z sum.
| |
| S « «s.«söq«mm
| |
| | |
| Ducfus Arferiosus
| |
| me.- 44 tun!
| |
| S - «· XVIII-III
| |
| Deseending Aas-f«
| |
| | |
| . Les? PulArfery Es:
| |
| | |
|
| |
|
| |
| | |
|
| |
|
| |
|
| |
|
| |
| | |
| Puls-vonst- Ouflef »
| |
| do.- .7smn. Es« EIN«0 · H« HHH G s Mfqmm
| |
| Tofal Pulmons Nein
| |
| SUF vcns CZVI hielt; . IF« Jan.
| |
| disx DIE-sum.
| |
| Es III-III«- Funcfional Orjfica
| |
| | |
| For-man Ovale s ZU, aqu
| |
| coronary sinus
| |
| | |
| cis-«. us«
| |
| | |
| is, »F« Leff Ver-fruchtlos·
| |
| | |
| Wcsghfs »Es-m
| |
| las. Vena cavo
| |
| | |
| ckas A« mass08 III-kraus.
| |
| | |
| Ast-s Veuikiculsk
| |
| Veishf - spsgsu
| |
| | |
| Fig. 16.—Ditnensions ok the human heart and its orilices at term. (Patten s:
| |
| Toulmim White House conkerence Reports, D. Appletowcentury co.)
| |
| | |
| clined to believe that their results demonstrate a mechanism un
| |
| kavorable to the classica1 sabatier theory.
| |
| since lekt and right ventricular pressures are equal in the ketal
| |
| | |
| heargV the should equal .
| |
| lekt venttieular wetght are-I. ok aortte outlet
| |
| | |
| 8.05 85.0 «
| |
| FH should equal II. The rat1os are actually
| |
| | |
| in the proportion of o.938 to i. Ik the volume ok blood entering
| |
| the atria is proportional to the size ok the inlets the following
| |
| proposition should be true: «
| |
| | |
| From their data,
| |
| 4o Pnysxohooy oF THE. FETUs
| |
| | |
| Ente-ist«» III-« ais-sum Baker-Inn left ais-cumsupekiok veaa easy-o» s5.6 sq. mm.
| |
| Iaferior vekka easy-a. .. 69.7 sq. mm.
| |
| Coronaksy Sinne. . . . . IX? sq. mm.
| |
| Tote-l . . . . . . . . 111.0 sq. mm. Total pulmonary veias . . . . . . . . . . 86.9 sq. mm.
| |
| — 82.2 sq. mm.- Funetional okiäee ok foramen ovale. 82.2 sq. kam.
| |
| Net . . . . . . . . . 78.8·sq. mm. Tot-a! . . . . . . . . . . . . . . . . . . . . 69.1 sq. man,
| |
| Ente-sing seh« sent-Hol- Entmsng leis reitst-fel
| |
| If these figures are functionally significant the ratio of blood received by the two ventricles should equal both the ratio of right
| |
| ventricular to left ventricular weights and the ratio of pulmonary
| |
| to aortic outlet areas. Substituting Hgures obtained from heart
| |
| measurements:
| |
| | |
| THE-§ should equal T or 1.14o4 should equal 1.13o7, which
| |
| | |
| » »69.1 · ·7-.11
| |
| 1s in agreement 1n ratio of o.989 to 1.
| |
| | |
| 78.8 ss o
| |
| —- h l l-«—
| |
| s oud equa A»
| |
| | |
| 69.1
| |
| in agreement in ratio of o.941 to i.
| |
| | |
| or 1.14o4 should equal 1.21 1 i, which is
| |
| | |
| «Tentatively then," they conclude, ··a ratio of about 8 to 7 may
| |
| be accepted as expressing the relations between right and left di—
| |
| visions of the fetal heart."
| |
| | |
| It is generally assumed that the prenatal lungs, requiring but
| |
| a very small amount of blood for their own metabolism, are supplied with a negligible quantity until the moment of bikth when
| |
| they expand and take over respiratory function. At that— time a
| |
| radical rerouting of the blood is supposed to take place with dramatic suddenness. This conception has been questioned by Patten and his colleagues.22s IN« In normal still—born human hearts
| |
| they found the average combined cross-sectional area of the pulmonary arteries to be 22.8 sq. mm. and that of the pulmonary
| |
| veins, 36.9 sq. mm. The functional orilice of the« foramen ovale
| |
| was only 32.2 sq. mm. Comparison of the pulmonary vessel measurements with others from newborns which had died after breathing showed them to have virtually the same capacity. The total
| |
| cross—sectional a«rea of the pulmonary veins was found to be about
| |
| the same as that of the umbilical vein. The combined area of the
| |
| left and right pulmonary arteries about equals that of the um—
| |
| bilical arteries.
| |
| | |
| The okilice of the foramen ov··le i; so restkicted by attachments
| |
| THE« FETAL CIRCULATION 41
| |
| | |
| of its flap—lilce valve in late fetal life that it alone can not be as—
| |
| sumed to deliver enough blood to the left atrium to bring about
| |
| an equalization of pressure there with that in the right atrium.
| |
| And yet it has been proved that the two ventricular pressures are
| |
| practically equal in « the fetus before breathing startsPs 23 Although it is reasonable to assume from these facts that pulmonary
| |
| venous return makes up the difference the evidence from the
| |
| starch experiments casts doubt upon the «theory,I0- U for if there is
| |
| complete mixing in the right atrium and if there is a signiiicant
| |
| volume of blood returning from the fetal lungs to dilute the starch
| |
| laden blood from the foramen ovale less starch should have been
| |
| recovered from the left ventricle than from the right.
| |
| | |
| An attempt to settle the question of fetal pu1monary circu1ation has been made by comparing the total iron content of apneic
| |
| fetal lungs with that of the lungs of 1itter-mate lcittens which had
| |
| been allowed to breathe air for an hour or more after clamping the
| |
| umbilical cord.27 The average data from nine experiments are
| |
| presented in Table to. If we can assume that the surgical pro
| |
| Tznm 10
| |
| | |
| coupanrsodc or« Tun Tor-a« Pumonxnr InoN Am) Bsskruuixv Putz-rotem! Btoov m
| |
| c« Fmusns AND Tun-In Am Bank-Inn«- Lwrtm Maskns
| |
| | |
|
| |
| | |
| « bllcöscllzimtpjted «
| |
| No. ok Tot-il F« Totol III« o W Um«
| |
| speci- ikon P St« « Hbck per gut.
| |
| | |
| mens (mgs.) HEXE? (81n·s.) ashed Tom] in Pei- gut.
| |
| IUUS lun s ashed lung
| |
| COOZ (Oc·)
| |
| | |
|
| |
| | |
| No air. . . 12 0 .822 5 .695 00959 I .698 0.9086 15.546
| |
| Breatheck 15 0 .s195 Z . 988 0 . 0951 l .782 0.8690 15 .527
| |
| | |
| «· Assuming all iron to be in hemoglobin ok blood; amount of tissue iron is unknown
| |
| but would be equal in the two Krieg.
| |
| | |
| cedures involved in the experiinents did not destroy a real difference between the apneic fetuses and the air breathing lcittens it is
| |
| quite evident from Table Io that a circu1ation must be present
| |
| in the lungs d«uring late prenatal life which is wholly capable of
| |
| caring for oxygenation pending the assumption of respiration, and
| |
| that there is no sudden increase in its volume.
| |
| | |
| Direct observation of the fetal lungs before and after breathing
| |
| 42 Pnvsrohoov oF THE FETUs
| |
| | |
| lends support to this viewz the apneic fetal lungs present the appearance of highly vascular Organs. It has been estimated that
| |
| more than five per cent of the fetal blood is in the human lungs
| |
| before breathing beginsks Furthermore the recent studies of
| |
| Barclay and his colleaguesW in the sheep fetus have demonstrated
| |
| radiographically that the pulmonary arteries and veins carry a
| |
| very appreciable volume of blood. Perhaps the crucial evidence
| |
| favoring the concept of a significant pulmonary blood volume before birth is that afkorded by the exceedingly rare condition, congenital stenosis of the pulmonary veins, in which the left ventricle
| |
| develops only about half its normal capacity and muscular powerks
| |
| It seems probable that Patten’s original anatomical study portrays
| |
| the true state of the pulmonary circulation before birth.
| |
| | |
| Correlation of the various studies just discussed leaves us with
| |
| rather a different conception of the fetal circulation than is ex—
| |
| pressed by either of the two prevalent theories, although it must
| |
| be granted that there are points for disputation on both sides.
| |
| In the first place, one is justified in assuming some mixture of the
| |
| two caval streams in the right atrium, at least in respect to the
| |
| blood which goes to the right ventricle.. secondly, there can be
| |
| little doubt that a greater quantity of the highly oxygenated blood
| |
| from the placenta enters the left atrium than finds its way into
| |
| the right ventricle. Icnowing that the fetal pulmonary veins re—
| |
| turn a stream of blood to the left side of the heart approximately
| |
| as large as that of the umbilical vein, we fail to see how complete
| |
| mixing would have talcen place10-U in the right atrium and as
| |
| much starch have been recovered from the left ventricle as from
| |
| the right. More than half of the inferior caval blood must have
| |
| traversed the foramen ovale. As a matter of fact KelloggU did
| |
| recover more starch from the left ventricle in each of five dog
| |
| fetuses of one series in which all the injectionshad been made
| |
| into the umbilical veins. If there is a complete mixture of the
| |
| two caval streams in the right atrium and if there is any loss of
| |
| oxygen in the pulmonary circuit the lower half of the fetal body
| |
| must receive more highly oxygenated blood than the heart and
| |
| brain.
| |
| | |
| Perhaps after all the long discarded concept of Wolff and Ziegenspeclg which held that the superior vena cava blood goes entirely to the right ventricle but that the inferior caval stream
| |
| THE FETAL CIRCULATION
| |
| | |
| passes to both left and right sides, is mpre nearly correct than
| |
| either of the more prevalent theories of fetal circulation even
| |
| | |
| though the methods originally used to arrive at this conclusion
| |
| were not exactly physiologic It is this theory that the observa
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | |
| 1—D uctus ctrsvøriosus
| |
| Zfbrsarrxerx oval-z
| |
| . ZDuctus verxosus
| |
| 4«Unxbilical. vejrx
| |
| Iilmbiljcal Sirt-Trick
| |
| Fig. 17.—The fetal circulation and probable course of the blood through the
| |
| ketal hear-r. The most highly oxygenated blood is contained in the vessels least
| |
| shaded, the most completely reduced b1ood, in the darkest vessels, and four intermediate degrees of reduction due to mixing are indicated by kour intermediate
| |
| | |
| shades.
| |
| | |
| tions of Barcroft, Barclay and their colleagues support. The con
| |
| cept is illustrated in Fig. 17.
| |
| Again, a word ofcaution. There is no reason to believe that
| |
| | |
| the same conditions are met in all species. It is quite possible that
| |
| 44 PHYSIOLOGY oF THE FETUS
| |
| | |
| a greater proportion of the blood from the placenta reaches tl1e
| |
| left side of the heart wisthout dilution in the sheep fetus than in
| |
| | |
| man.««
| |
| | |
| CFIANGES IN· TW CIRCULATION AT BIIRTE
| |
| | |
| 0cclusion of the placental circulation is the most immediate
| |
| event at birth. The umbilical arteries continue to pulsate for a
| |
| few moments but very soon they constrict, alIowing no more blood
| |
| to leave the body of the newborn. Much of the blood which is
| |
| in the placenta drains back into the newborn if the umbilical cord
| |
| is not clamped immediately.29 It has been estimated on the basis
| |
| of 120 averaged cases that about 50 grams of blood returns to the
| |
| child in the first minute and 98 grams by the thirtieth minute
| |
| after birth.30 ·
| |
| | |
| It may be suggested that there is a reduction in the amount
| |
| of blood entering the right atrium when the umbilical vessels are
| |
| occluded. It is unsound to assume that development in fetal life
| |
| has given the child an abdominal vena cava larger than necessary
| |
| for the prenatal blood flow. This vessel probably does not accommodate immediately to the increased load placed upon it by
| |
| obliteration of the umbilical arteries, but only gradually increases
| |
| in diameter as postnatal growth takes place. What happens to
| |
| the extra blood? We may surmise that the capillary bed of the
| |
| newborn opens to receive more blood. This seems reasonable in
| |
| view of the increased muscular work and initiation of new activities, including tonus.
| |
| | |
| Less blood enters»the right atrium and its iilling pressure consequently decreases. Hamilton and his colleagues23 found no appreciable diikerence between intraventricular pressures before and
| |
| after clamping the umbilical cord, but they suggested that the
| |
| umbilical circuit may have been shut off before their observations
| |
| were made. If thefilling pressures of the two sides of the heart
| |
| are about equal after the umbilical cord has been occluded any fall
| |
| on the right side or any rise on the left will cause the valve of the
| |
| foramen ovale to close. In the dog and the rabbit another klaplilce valve guards the opening of the ductus arteriosus into the
| |
| | |
| « some recently completed injection experiments in living cat and guinewpig
| |
| fetuses provide a strilcing demonstmtion of the crossing of the mval streut-s. They
| |
| appear to support the sabatier doctrine even more completely than do Barclays
| |
| studies.
| |
| THE FETAL CIRCULATION 45
| |
| | |
| aortaz this too will close. With inspiration at birth in the dog
| |
| and rabbit a negative intrathoracic pressure brings about a direct
| |
| fall in pressure on both sides of the heart followed by a rise to the
| |
| original 1evel with expiration. As the negative intrathoracic pressure becomes permanent1y established it is thought to lead to a
| |
| decrease in the peripheral resistance within the blood channels
| |
| of the lungs, but of course not in the systemic capil1aries; consequently the right intraventricular systolic pressure declines more
| |
| than does the left which increases with development of systemic
| |
| vasomotor tonus. The diastolic pressures remain about equal
| |
| during the early hours after birth, but by two days in the rabbit
| |
| diastolic pressures are considerably higher on the left side than on
| |
| the right. As soon as the peripheral resistance is lowered, we may
| |
| assume an increased blood iiow through the lungs with increased
| |
| iilling pressure of the left atrium which renders the valve of the
| |
| foramen ovale permanently closed from a functional standpoinn
| |
| | |
| There is reason to believe that the events occurring at birth
| |
| vary in the different species. In the sheep we lcnow nothing about
| |
| intraventricular pressures but other information is at band«
| |
| When the umbilical cord is clamped there is a brief transient rise
| |
| in blood pressure followed by the establishment of respiration.
| |
| When breathing becomes regular the mean systemic blood pressure reaches a higher 1evel than it had before respiration began.
| |
| Deve1opment of sympathetic vaso-pressor tonus has been suggested
| |
| to take place concomitant with establishment of respiration. It
| |
| is known that the splenic smooth muscle begins to contract at
| |
| that time and, more important, the ductus arteriosus closes by
| |
| sphincter action.17-3I« These events seem to indicate that provision is made for the right side of the heart to function at lower
| |
| pressure levels than the left in the newborn lamb.
| |
| | |
| What are the conditions in man? Here we are faced with inadequate data. It has -been held that the systemic blood pressure
| |
| does not change signiiicantly with the advent of respiration at
| |
| birth.32 The ductus artjeriosus possesses no valve lilce that of the
| |
| rabbit and dogz nor has it been shown to occlude immediately
| |
| | |
| «« Barclay and his colleaguesP have found recently that they were mistalcen
| |
| about the identity of the ductus arteriosus in their iirst study. Nevertheless I am
| |
| convinced, from direct observations in Professor Barcrofcs laboratoryg that the ductus does occlude within a few minutes after breathing start-s.
| |
| 46 pkrYsmLooY oF THE FETus
| |
| | |
| by sphincter action like that of the lamb. 1ts closure is said to
| |
| be accotnplished very gradually over a period of several weelcs or
| |
| months by a process resembling that encountered in endarteritis
| |
| obliteransRE ZHH Intimal pads are present in the vessel during
| |
| late fetal life« If the ductus arteriosus remains open after birth
| |
| it must be assumed that pressures on the two sides of the heart
| |
| will tend to equalize and that blood can flow from the aorta into
| |
| the pulmonary artery. 0ne should expect to hear the character
| |
|
| |
|
| |
| | |
| O
| |
| C
| |
| | |
| S
| |
| | |
| No« pxesswe f« ask-». o! Ave-ev»
| |
|
| |
| | |
| Itsdssfsssdltsc IS II pp U«
| |
| - Doy of hospitolixotion
| |
| | |
| Fig. 18.—Dai1y blood pressures (upper: systolicx lower: diastolic) before and
| |
| after ligating a patent ductus arteriosus in a «7 year old chi1d. Day of operation
| |
| indicated by the arrow. (Gross and Habt-card: J.A.M.A., Vol. us. 1939.)
| |
| | |
| istic murmur of a patent ductus in every infant, but this is not the
| |
| case. It would seem that there must be some mechanism as yet un—
| |
| discovered in the human to bring about functional closure of the
| |
| ductus arteriosus at birth. It is common to find an anatomically
| |
| patent ductus at autopsy in early life, but it is questionable if
| |
| postmortem ductus arteriosus patency soon after birth can be
| |
| talcen as proof of a true physiologic patency existing before
| |
| death. continued patency of the ductus arteriosus several years
| |
| after birth is a relatively cornmon .occurrence. But the condition
| |
| THE FETAL GIRCULATION 47
| |
| | |
| predisposes toward bacterial endocarditis and is in itselk a danger
| |
| because ok the additional load placed upon the lekt ventricle which
| |
| may lead to cardiac decompensation. successkul surgical ligation
| |
| ok a patent ductus arteriosus in a 7 year old chi1d has been re—
| |
| ported recently.3·« The ekkects ok this operation upon the diastolic
| |
| | |
| blood pressure are shown in Fig. i8.
| |
| | |
| l.
| |
| | |
| ZO III-k- ssssisssxsszs e«
| |
| | |
| II«
| |
| | |
| TO.
| |
| | |
| ei.
| |
| . Patten, B. M. sc K. Toulmin. 1932 cited by K. D. Blaclckam Growth
| |
| | |
| Ho.
| |
| Hi.
| |
| | |
| Its h« Ists-Ists—TP PÄRCHEN
| |
| | |
| . Abel, s. sc W. F. Windle.
| |
| . lcraklca, J. i933. Am. J. Dis. Ghild» 45: ioo7.
| |
| | |
| REFERENCES CITED
| |
| | |
| Lucas, W. P. sc B. F. Dearing. i92i. Am. J. Dis. child.. ei: 96.
| |
| Cohnstein, J. sc N. Zuntz i888. Pllijgers Arch., 42: 342.
| |
| | |
| Elliott, R. H., F. G. Hall, sc A. st. G. Huggetr. i934. J. Physiol» se: i6o.
| |
| Barcrokt, J. sc J. A.«lcennedy. i939. Ibid., 95: i73.
| |
| | |
| Baker-oft, J., J. A. lcennedy sc M. F. Mason. i939. ibid» 95: 269.
| |
| Hasellioisy G. sc K. strombergen 1932 Ztschn Geburtsh. Gynä1c., me:
| |
| | |
| i6
| |
| | |
| cohnsteim J. sc N, Zuntz iss4. Pflüger-D Arch., 34: i73.
| |
| Baker-oft, J., L. B. Flexner, T. Mccurlcim i934. Physiol» 82: 498.
| |
| . Barcrokh i9Z6. Physiol. Reis» is: io3.
| |
| Pohlman, A. G. igo9. Anat. Rec., Z: 75.
| |
| | |
| lcellogz H. B. i928. Am. J. Anat» 42: 443.
| |
| lcelloggy I-I. B. i93o. Am. J. Physiol» 9i: 637.
| |
| | |
| . Reid, J. i835. Edin. Med. Sarg. J., 43: u; 3o8.
| |
| | |
| Pohlman, A. G. i9o7. Johns Hoplcins Hosp. Bull., is: 4o9.
| |
| | |
| . Magrudeiy s. R. i932. Anat. Rec., 54: i37.
| |
| | |
| Huggeth A. St. G. i927. J. Physiol» Ha: 373. ·
| |
| Bann-oft, J. i938. The Brain and Its Environmenh Yale Unm Press,
| |
| | |
| New Haven.
| |
| | |
| Windle, W. F. sc A. G. steele.
| |
| 39: 246.
| |
| | |
| Barclay, A. E» J. Barcroky D. H. Barron sc K. J. Franlclin.
| |
| J. Radiol., is: 5o5.
| |
| | |
| Wolkh c. F. i778. cited by Pohlman, i9o9.
| |
| | |
| Ziegenspecltz R. i882. cited by Pohlman, 19o9.
| |
| | |
| i938. Proc. soc. Eis-per. Biol. sc Med.,
| |
| | |
| i939. Brit.
| |
| | |
| and Development ok the childx White House conkerence Reports,
| |
| | |
| sect. l, Vol. s, F. esse. century Co» N. Y.
| |
| | |
| Hami1ton, W. F., R. A. Woodbury sc E. B. Woods. -.i937. Am. J. Physiol»
| |
| ii9: Los.
| |
| | |
| Patten, B. M., W. A. sommerlield sc G. H. PaE i929. Anat. Rec., 44: i65.
| |
| | |
| . Patten, B. M. i93i. Am. J. Anat» 48: i9.
| |
| | |
| Patten, B. M. i933. In A. H. cui-cis' 0bstetrics and Gynecology, i: 9o6,
| |
| saunders, Philadelphia. g
| |
| i939. Anat. Rec., 75: 45i.
| |
| | |
| Frischlcorm H. B. sc M. P.« Rucken i93g. Am. J. Obst. Gyxi., zst 592
| |
| Haselhorsh G. sc A. Allmeling. i9·3o. Ztschn Geburtsh. Gynälc., 98: io3.
| |
| Barclay, A. E., J. Barcrokt, D. H. Barron sc K. Franlclim i938. Brit.
| |
| | |
| J. Radiol., ii: 57o.
| |
| 48 PHYSIOLOGY OF THE. FETUs
| |
| | |
| se. Haselhorsh G. 1929. Zucht. Geburtslx Gynä1c., 95: 4oo.
| |
| | |
| II. scammoth R. E. s: E. I-I. Narr-is. 1918. Anat. Ren, is: 165.
| |
| 34. Schaeffer, J. P. 1914. «J. Exp. Mal» 19: 129.
| |
| | |
| 35. Möllckh J. 1926. Anat. Am» 61: 348.
| |
| | |
| zö- Gross R. E. Z: J. P. Huhbartt 1939. J.A.M.A., 112 (1): 729.
| |
| CHAPTER IV
| |
| | |
| THE BLO0D OF THE PETUS
| |
| | |
| sEyERAL opinions regarding development of the blood are reviewed in textbooks of embryology and histology1s 2 and a more
| |
| extensive consideration of this subject will be found in Downey’s
| |
| Handboolc of HematologyÆ The genesis of blood cells begins in
| |
| all mammals shortly after the formation of germ layers. It starts
| |
| in the wall of the yollc sac where mesenchymal cells retract their
| |
| processes to assume more compact rounded forms free«d from
| |
| attachment to other cells. All types of blood cells arise from these
| |
| hemoblasts. A similar transformation is encountered later in the
| |
| body mesenchyme, liver, spleen and bone marrow gis these Structures develop. The first red blood corpuscles, derived from the
| |
| primitive stem-cells of the yollc sac, play a transient functional
| |
| role and are ultimately replaced by blood elements derived from
| |
| other regions. Not until late embryonic stages do we normally
| |
| find a division of blood forming tissues into myeloid for elaboration of red corpuscles and granulocytes, and lymphatic tissues for
| |
| production of lymphocytes. Abnormally even after the adult
| |
| stage has been reached lymphatic tissues and the loose connective
| |
| tissue may lose their speciftcity and give ·rise to types of blood cells
| |
| other than usually ·formed by them.
| |
| | |
| THE RED BLOOD CORPIJSCLES
| |
| | |
| During the early part of prenatal life oxygen transport is effected by nucleated erythrocytes. As time goes on these are re—
| |
| placed by red blood corpuscles which have lost. their nuclei, but
| |
| some immature elements, the reticulocytes, are still observed in
| |
| blood smears at birth. The total number of hemoglobin containing cells and corpuscles is small at first, for the blood is highly
| |
| fluid, but increases as the end of gestation approaches. The diameter of the fetal red corpuscles is greater than in the adult, di—
| |
| minishing as development proceedsxHs
| |
| | |
| These few facts have been known for many years and it is
| |
| surprising that no one accepted their challenge to investigate this
| |
| | |
| 49
| |
| 50 PHYSIOLOGY OF THE FETUs
| |
| | |
| interesting subject systematically until recentlzkHs All but the
| |
| most recentIHs contributions have been reviewed by Wintrobe
| |
| and shumaclcerlss 20 who described their own careful comparative
| |
| study in the pig, rat, rabbit, cat, dog and man.
| |
| | |
| At a period as early as it was possible to obtain adequate blood
| |
| samp1es from fetuses they found that the number of red blood
| |
| corpuscles, the amount of hemoglobin and the volume of paclced
| |
| red corpuscles (hematocrit) are low in comparison with adult
| |
| blood of the same species. The corpuscles are large, chiefly nuc1eated, and contain a correspondingly large amount of hemoglobin.
| |
| | |
| sc sc sc CI sc II kc sc
| |
| IAYI AITII IIIICATIOI I Isfs VIII Iisflsl
| |
| | |
| Fig. 19.—Nurnber of red blood corpuscles (0) in millions, leulcocytes CI) in
| |
| thousand-s, volume (hematocrit) of paclced red oorpuscles (0) in per cent, amount
| |
| | |
| of hemoglobin (.1-) in grams per too cc., and specific grayity (0) of the blood of
| |
| cats during prenatal and postnatal life until maturityc Adult values for males are
| |
| | |
| higher than for females in.all except specific gravitzu Each point represents an
| |
| average.
| |
| | |
| As development proceeds, the number of corpuscles, amount of
| |
| hemoglobin and volume of paclced corpuscles increase, not in a
| |
| linear order, but more rapidly in early stages than later on near
| |
| term. The mean corpuscular volume, mean corpuscular hemoglobin and proportion ok immature korms of red blood corpuscles
| |
| decrease similarly. But the meanrorpuscular hemoglobin concentration remains about the same throughout the period studied.
| |
| The amount of hemoglobin available for transporting oxygen is a
| |
| function ok the size and number of red blood corpuscles and its
| |
| concentration does not vary within individual corpuscles. It was
| |
| observed that the period during which the greatest changes toolc
| |
| THE BLOOD OF THE« FETUS 51
| |
| | |
| place seemed to correspond to the time during which hemopoiesis
| |
| is most active in the fetal liver. The development of blood corpuscles and of hemoglobin in the cat, not only during prenatal
| |
| life but onward to maturity, is illustrated in Fig. 19.21
| |
| | |
| In the species whose gestation periods are short, such as the
| |
| rat,18 the blood at birth is less lilce that of the adult than in species
| |
| with long gestation periods, such as man. Even in the human
| |
| fetus at birth, the number of red blood corpuscles in umbilical
| |
| cord blood does not exceed that of the adult.19- 20 This view may
| |
| seem to bö opposed to that which is generally taught, that the
| |
| newborn has a higher red corpuscle count than the adult.22 Actually it is not, for the infant has more red corpuscles per cubic millimeter of blood in circulation twenty minutes to half an hour
| |
| after birth than it had in utero.23 «« Some investigators have reported more than seven million per cmm.24- 25 and six million is
| |
| not an excessive average iigure for the lirst day of life. If there is
| |
| any delay between birth and talcing blood samples, a true picture
| |
| of the condition in utero at the end of fetal life will not be ob·
| |
| tained.
| |
| | |
| A sharp increase in red blood corpuscles is encountered in the
| |
| cat on the day of birth.2I This persists for a weelc, but the number
| |
| declines to nearly the birth level by two weelcs, rising again only
| |
| after the lcittens begin to feed themselves at three weelcs (Fig. 19) .
| |
| Wintrobe and shumaclcer suggested that high human red corpuscle counts after birth may be explained on the basis of dehydration, but ·we believe that the role played by dehydration on the
| |
| first day of life of the cat is negligible. The specific gravity of the
| |
| lcitten’s blood does not increase at birth, but on the contrary, de—
| |
| creases.21 The transient rise in red corpuscle counts may be partly
| |
| explained on the basis of splenic contractions, which have been
| |
| demonstrated to start at· birth in the sheep.2·3
| |
| | |
| The human fetus has about as many red blood corpuscles at
| |
| the end of gestation as the adult· but the corpuscles are of greater
| |
| sizez consequently the mean» hemoglobin content and the total«
| |
| amount of hemoglobin in the blood are higher than in the adult..
| |
| The amount of hemoglobin in the blood of the newborn has been
| |
| reported by most investigators to be high; 2o gm. per ioo cc. of
| |
| blood or even more is not uncommon.27 The most recent estima
| |
| «« statement based partly on a study now in progress.
| |
| 52 PHYSIOLOGY OF THE« FETUs
| |
| | |
| tions made in blood drawn from the umbilical cord gave an average of 15.36 gm. per 1ooJcc.28 This value correlates well with the
| |
| oxygen capacity of human cord blood (see" Chapter V) . Hemoglobin decreases rapidly after birth. The high values for hemoglobin and the great number of corpuscles in the early postnatal
| |
| period are undoubtedly related but there are other, poorly understood factors involved in this difference In the lcitten the curves
| |
| for hemoglobin and corpuscles are not parallel until the fourth
| |
| weelc of life. Perhaps this may be related to a substitution of new
| |
| adult hemog1obin for the older fetal type.
| |
| | |
| An important conception of the development of blood has
| |
| been proposed by Wintrobe and shumaclcen They observed that
| |
| the developing fetal blood resembles that of patients with per—
| |
| nicious anemia who are.being subjected to an eikective, continuous
| |
| and extremely potent stimulus to blood formation. They suggest
| |
| that the anti-pernicious anemia factor of Castle may be the same
| |
| or very similar to the substance which causes the blood of the
| |
| fetus to develop. It is possible that the fetus obtains this from
| |
| the mother. If too little is available to supply the needs of both
| |
| mother and fetus a deiiciency should manifest itself. Apparently
| |
| it does so in «pernicious anemia of pregnancy" which can be control1ed by administering liver extract and which even when untreated is relieved spontaneously by birth.29- Z? If an inadequate
| |
| amount of the anti-pernicious anemia factor is available to the
| |
| fetus one might expect to observe efkects upon the infant at birth.
| |
| It has been shown that the incidence of primary anemia of the
| |
| newborn is more common in multiple births, especially in prema—
| |
| ture multiple births, than in single births at full term.31
| |
| | |
| Wintrobe and his colleagues were unable to bring about
| |
| changes in the blood of rabbit fetuses by feeding liver extract to
| |
| the m0thers or by injecting it into the placentas.32 They concluded that the fetuses were talcing up all the anti-pernicious
| |
| anemia principle they could from normal maternal sources. 0ther
| |
| investigators have created pregnant rats with human and swine
| |
| gastric juices, which contain the antdpernicious anemia factor,
| |
| and found that the diameter and mean volume of the fetal red
| |
| blood corpuscles were reduced signilicantly at birthFHZ When
| |
| the gastric juice had been inactivated by heating it produced no
| |
| effect. 0n the other hand Wigodslcy and 1vy, using large doses
| |
| THE nLooD oF THE FETUs 53
| |
| | |
| of potent anti-pernicious anemia factor, have been unable to coniirm these results«
| |
| | |
| Wintrobe and his colleagues made assays of fetal hog liver and
| |
| p1acenta and found these Organs laclcing in physiologically demonstrab1e amounts of anti-pernicious anemia factor.32 This suggested that a true physiological deliciency exists in the fetus and
| |
| must be made up by drawing upon maternal sources. More recently however the livers of fetal calves have been demonstrated
| |
| to contain large amounts of the active princip1e.37
| |
| | |
| OXYGEN CARRYING POWER OF FETAL BLO0D
| |
| | |
| Although oxygen transport will be considered in greater detail
| |
| in the next chapter it should be pointed out now that a true difference between fetal and adult hemoglobin has been found in several species of animals. This was suspected for some time38-4I and
| |
| | |
| soo
| |
| | |
| oo oo
| |
| Tot) oo
| |
| Yo
| |
| koo so
| |
| Z« so
| |
| E« «Jzo zo
| |
| E« 2o
| |
| | |
| so to
| |
| | |
|
| |
| | |
|
| |
| | |
|
| |
| | |
|
| |
| | |
|
| |
| | |
| O
| |
| | |
| 3456
| |
| | |
| 0I23456cl2
| |
| | |
| Pressa:- of etwas: mm As
| |
| Fett! lscmoglobin Mutes-ital hemoslobiti
| |
| | |
| shscp 44437 day:
| |
| Fig. aokscxygen dissociation curves for hernoglobin (c) of sheep fetuses, 44 to
| |
| 137 days gestatiorh and (b) of their mothers. Limits fall within the shaded are-as.
| |
| Approximate temperature 190 c; pH 9.3. (I-Iill; from Barcrofn Proc. Roy. soc»
| |
| Vol. us, 1935 London.)
| |
| | |
| Mccarthy has proved that fetal hemoglobin of the goat takes up
| |
| | |
| oxygen more readily at low gas tensions than does maternal hemoc
| |
| globin. This is illustrated in the oxygen dissociation curves of«
| |
| fetal and maternal hemoglobin reproduced in Fig. 2o. The difference becomes less noticeable progressively until about five weelcs
| |
| after birth when the kid’s hemoglobin attains adult characteristicsfs
| |
| Ha11 obtained similar results in fetal goats, rabbits and even
| |
| 54 PHYsmLooY or THE FETUs
| |
| | |
| chiclcsfs A very pronounced avidity for oxygen at low gas tensions
| |
| has been demonstrated szin whole blood of calves near term.«
| |
| It was shown that dilkerences in shape and Position of fetal oxygen
| |
| dissociation curves of whole blood in relation to those of the
| |
| mother are inexplainable solely on the basis of greater alkalinity
| |
| of the mother’s blood; they are governecl by true chemical dilkerences between the hemoglobins .
| |
| | |
| The human fetus at full term difkers from all others which
| |
| have been studied, for it has been shown that its hemoglobin when
| |
| studied in clilute solution takes up oxygen less readily than does
| |
| that of the motherksss 40 This will be seen in Fig. 2 1 which should
| |
| be contrasted with Fig. 2o. However, bufkered (PH 7.4) suspens
| |
| | |
| U) 20 Ida-am O«
| |
| | |
| Fig. suxscxygen dissociation curves for hemoglobin of the human ketus (tight)
| |
| and mother (left) . stiaurowitzx Ztschn Physiol. check, Vol. EIN, 1g35.)
| |
| | |
| sions of the fetal corpuscles, lilce whole fetal blood, become more
| |
| highly saturated with oxygen at low partial pressure than similar
| |
| suspensions of maternal corpusclesÆ The oxygen dissociation
| |
| curve of human fetal whole blood (see Fig. 27, F. 66) lies to the
| |
| left of that of the mother which in turn lies to the right of that for
| |
| normal human subjectstkk
| |
| | |
| species difkerences in avidity of the hemoglobin for oxygen
| |
| may be related to the degree of maturity reached at birth. It
| |
| would be interesting to construct dissociation curves for human
| |
| fetal hemoglobin at the iifth or sixth month of gestatiow One can
| |
| not doubt that the fetus of every species possesses a meehanism for
| |
| assuring adequate intrauterjne respiratory function throughout
| |
| THE izLoon oF THE; FETUS 55
| |
| l
| |
| | |
| prenatal life. Even though the number of red blood corpuscles
| |
| may be less than that of the adult they are larger and contain more
| |
| hemoglobin. 1n those species with great deftciency in number
| |
| of corpuscles at birth there is a compensatory increase in the avidity with which their hemoglobins combine with oxygen.
| |
| | |
| THE LEUKOCYTES AND PLATELETS
| |
| | |
| At the time in early embryonic life when secondary erythrocytes are being formed by the yolk sac mesenchyme a few atypical
| |
| megakaryocytes and leukocytes are si1nilarly produced. Later,
| |
| with intensive production of erythroblasts in the embryonic liver,
| |
| granular leukocytes proliferate between the endothelium and the
| |
| liver cells and a few megakaryocytes are formed there. The
| |
| pseudopodia of the megakaryocytes produce the blood platelets.
| |
| Neither granular leukocytes «nor platelets are formed in great numbers until fetal hemopoietic activity shifts to the bone marrow
| |
| about the third month. The lymphocytes of the body develop
| |
| relatively 1ater. They are formed by transformation of mesenchymal cells in the neighborhood of 1ymphatic vesse1s or in the
| |
| walls of primitive lymph sacs.
| |
| | |
| Little is lcnown about the number of leulcocytes and platelets
| |
| or of the numerica1 relations between granular and nongranular
| |
| leukocytes. Kindred and coreyls attempted to determine the
| |
| number of leukocytes in rat fetuses of different ages but found
| |
| difliculty in difkerentiating them from immature forms of the red
| |
| blood ce11s.- 1t was apparent however that they increase about
| |
| seven fold between the 16 and 35 mm. stages. It has been reported
| |
| that both lymphocytes and polymorphonuclear cells are present in
| |
| dog embryos at the time the limbs begin to grow out. But in
| |
| fetuses 26 to 41 mm. long, at which time true neutrophilic lenke—
| |
| cytes made their Hrst appearancq the total» white cell count was
| |
| only 1,5oo to 2,5oo per cmm. Jof blood, with lymphocytes forming
| |
| the greatest proportionks Total nucleated cell counts at the middle of gestation in the cat seem to be about 16,ooo per cmmEI
| |
| The number decreases toward full term reaching 7,5oo to 9,ooo
| |
| at birth. Thereafter it remains fairly constant at 8,ooo to 9,ooo
| |
| until 120 days, as compared with 1o,5oo to 12,7oo in the adult
| |
| (Fig. 19) . Bruner and his colleagues found less than one third
| |
| as many leulcocytes in the rat at birth as in the adult rat.18
| |
| 56 Pnrstotoor oF THE FETUs
| |
| | |
| At the time ok birth ok the human inkant it has been reported
| |
| that the blood contains gsreat numbers ok leulcocytesz in kact one
| |
| okten hears the term, «leulcocytosis ok birth." Counts greater than
| |
| 19,ooo on the Iirst day after birth, decreasing to about 9,ooo by the
| |
| tenth day, have been reported-Z Japha found 36,ooo leulcocytes
| |
| in the human newborn.24 What kactors, other than inkections,
| |
| may be responsible kor the elaboration ok such great numbers ok
| |
| leulcocytes are unlcnown. Bayer has reported that inhalation ok
| |
| carbon dioxide led to increasing the number ok granulocytes and
| |
| breathing oxygen resulted in the kormation ok more lymphocytesfs
| |
| | |
| The number ok blood platelets at birth has been reported to
| |
| Huctuate widely. Results ok a number ok investigators have been
| |
| summarized by Merritt and Davidson.22 Nothing is known about
| |
| them in prenatal like. The clotting time ok the blood in early
| |
| prenatal like appears to be long.
| |
| | |
| THE PLÄSMÄ
| |
| | |
| The blood has a high«water content which declines as the time
| |
| ok birth approaches. It has been reported that water korms about
| |
| 88 per cent ok the human ketal blood at the third month ok gestation and only 67 per cent at the ninth lunar month.49 The umbilical vein blood has a water content ok 79.5 per cent, as
| |
| compared with 8 1 .5 per cent in the mothersko A sharp postnatal increase in water was observed, reaching a maximum ok about 83
| |
| per cent 6o days akter birth.
| |
| | |
| A kew observations in the cat indicate that the speciiic gravity
| |
| ok the ketal blood is lower (about 1.o4) at about the middle ok
| |
| ketal like than it is at kull term ·(i.o52). It is as high or slightly
| |
| higher near the end oksgestation than in the adult, diminishing
| |
| gradually thereakter to reach its lowest level (1.o43) about two
| |
| weelcs postnatally.21
| |
| | |
| The osmotic pressure ok ketal blood colloids appears to be low
| |
| in some species ok animals, but data are incomplete. The subject
| |
| will be discussed in Chapter VIII in relation to urine kormation
| |
| in the ketal lcidneys.
| |
| | |
| TIIE OEREBROSPINAL FLIJID
| |
| | |
| A recent study ok the cerebrospinal iluid in dog ketuses has
| |
| shed light upon the nature ok its kormation in the adultFI The
| |
| THE nLooD oF TkiE FETUs 57
| |
| | |
| blood plasma and· the cerebrospinal liuid are alilce in respect to
| |
| their contents ok chlorine, sodium and urea during the early part
| |
| ok ketal like. Between 4o and 43 days (ketuses 5o to 6o mm. long)
| |
| | |
| the two Huids cease to be in balance.
| |
| | |
| It is at this time that the
| |
| | |
| cerebrospinal fluid is thought to change from an ultraiiltrate ok
| |
| the blood to a true s«ecretion.
| |
| | |
| IO
| |
| | |
| e9.
| |
| | |
| so.
| |
| . Parsons, L. G. 1931. J.A.M.A» 97 (e): 973.
| |
| | |
| IF.
| |
| | |
| . Bloom, W.
| |
| | |
| . Downey. H.
| |
| | |
| ZOOs99eO
| |
| | |
| . Wintrobe, M. M. se I-I. B. shumaclcen
| |
| . sweet, M. se W. F. Windle. 194o. Unpub1ished.
| |
| . Merritt, K. K. se L. T. Davidson. I933. Am. Dis. Child» 46: 99o.
| |
| | |
| . Japha, A.
| |
| | |
| . Waugh, T. R» F. T. Merchant se G. B. Maughan.
| |
| | |
| REFERENcEs CITED
| |
| | |
| Arey, L. B. 194o. Developmental Anatomy. saunders, Philadelphia
| |
| 1938. Maximows Textbook ok Histology, saunders, Philas
| |
| delphia. «
| |
| | |
| 1938. Handboolc ok I-Iematology, Hoeberz N. Y.
| |
| | |
| Malassez L. i875. Arch. Physiol. norm. path» set. e, e: est.
| |
| | |
| cohnsteim J. se N. Zuntz 1884. Pklügeks Arch» 34: 173.
| |
| | |
| Malassez L. 1889. Comph Rend. soc. Biol., 4I: e.
| |
| | |
| Jo1ly, J. 19o6. Ibid» 6o: 564.
| |
| | |
| . Jolly, J. 19o9· Ibid» 66: I36. «
| |
| . Nicho1as, J. s. se E. B. Boswortlx 19e8. Am. J. Physiol» 83: 499.
| |
| | |
| KnolL W. 19e9. Ztschn milc.—anat. Forsch» is: IV.
| |
| | |
| . Zeidberg, L. D. 19e9. Am. J. Physiol» 9o: —i7e.
| |
| . Deseö, D. 19e9. Pllügeks Arch» ee1: ges.
| |
| . lcindred, J. E. se E. L. Corey. 193o. Anat. Rec» 47: e13.
| |
| | |
| Kindred, J. E. se E. L. Corey. I931. Physiol. Zool» 4: e94.
| |
| | |
| . srnith, c. 193e. J. Path. Bact» 35: 717.
| |
| | |
| Jones, J. M» M. E. shipp se T. A. sonder.
| |
| se Med., 34: 873.
| |
| | |
| Baker-oft, J. se J. A. Kennedzk 1939 J. Physiol» 95: i73.
| |
| | |
| Brunerx H. D» J. van de Erve se A. J. Carlson. I938. Am. J. Physiol»
| |
| 1e4: 6eo.
| |
| | |
| Wintrobe, M. M. se I-I. B. shumaclcen 1935. clin. Inv» 14: 837.
| |
| | |
| 1936. Am. J. Anat» 58: gis.
| |
| | |
| 1936. Proc. soc. Exp. Bio1.
| |
| | |
| Schiff, E. 189e. Jahrlx Icinderh1lc» 34: 159.
| |
| 191e. In Pkaundler se schlossmanns Diseases ok chi1dren, e:
| |
| Ist, Lippincoth Philadelphia. »
| |
| Feldmam W. M. 19eo. Ante-Nara! and Post-Nara! child Physiologyz
| |
| Longmans Circen, London.
| |
| | |
| Taylor, D. B. se T. Gotsew 1938. Cited hy Barcrokh in The Brain and
| |
| . Its Environmenh Yale Univ. Press, New I-Iaven.
| |
| | |
| Uppmam H. s. 19e4. Am. J. Dis. child» e7: 473. ·
| |
| I939. Am. Med.
| |
| sei» 198: 646. - «
| |
| Bland, P. B» L. Goldstein se A. First. I93o. Ibid» 179: 48.
| |
| Beard, I-I. I-I. se V. C. Me«yers. 1933. Arn. Physiol» Io6: 449.
| |
| | |
| Wintrobe, M. M» R. E. Kinsey, R. c. B1ount se W. Trager. 1937. Am.
| |
| J. Med. sei» IV: 449.
| |
| . stasney, J. sc G. M. Eis-Zins.
| |
| . stasney, J. sc F. C. Mann« 1937. Ibicl., is: 699.
| |
| | |
| . Schliche, C. P. 1939. Ibid., 14: 145.
| |
| | |
| . Wigodslcx I-I. s. sc A. C. 1vy. 1938. Proc. soc. Exp. Biol. sc Med., 38: 787.
| |
| . Wigodslcy, H. s.,· 0. Richter sc A. C. Ivy. 19Z8. Am. Physiol» us: 21 z.
| |
| . Kriigetz F. sc W. Gerlach. 1927. Ztschtx f. ges. expetz Med., 54: 653.
| |
| | |
| . Njcoletti, F. 193o. Axch. AntropoL Crim. Psictiiac Mal. Legale, so: 386.
| |
| . Perrier, C. sc P. Janelli. 1931. Arch. Fisiol., 29: 289.
| |
| | |
| . Hentscheh I-I. 1928. Münclx mal. Wchnschty 75: 1237.
| |
| | |
| . McCarthy, E. F. 19Z3. J. Physiol» 8o: Los.
| |
| | |
| . Ball, F. G. 1934.
| |
| . Roos, J. sc C. Romijtx
| |
| . Haurowitz F. 1935. Hoppesey Ztschtz Physiol. Chem., Erz-s: us.
| |
| | |
| . Eastmarh N. J., E. M. K. Geiling sc A. M. DeLawder.
| |
| | |
| PHYSIOLOCY OF THE FETUS
| |
| | |
| 1937. stakk Proc., Mayo Clin., is: 49o.
| |
| | |
| 1bid., Sz- ges.
| |
| 1938. 1bid., ge: 249.
| |
| | |
| I-Iill, R. 1935. Cited by J. Baker-oft. Proc. Roy. soc. Loncl., B, us: 242.
| |
| | |
| 1933. Johns Hoplcins Hosp. Ball» 53: 246.
| |
| | |
| . Bayen W. 1932 Jahrb. Kinclerhlk., 134: 3o4.
| |
| . Talcakusm s., K. Kuroda sc K. Li.
| |
| . Kurocla, K. sc K. Li.
| |
| | |
| . Flexnety L. B. 1938. Am. J. Physiol» 124»: ist.
| |
| | |
| 1937. Keijo J. Med., 8: 58.
| |
| 1937. Ibid., 8: 4o.
| |
| CHAPTER V
| |
| | |
| FETÄL RESPMÄTION
| |
| | |
| THE MECHÄNISM OF GASEOUS EXCHANGE
| |
| | |
| THE placenta is the organ kor respiration in the fetus. Its
| |
| rnaternal portions develop somewhat in advance of the parts con—
| |
| tributed by the fetus with the result that the materna1 vascular bed
| |
| in the p1acenta reaches a large size while that of the ketus is al
| |
|
| |
| | |
|
| |
| | |
| Das;
| |
| Wes« 6 s lcs 12 14 kö IS 20 Z? 24 26 28 30 32 ? 4
| |
| I T I
| |
| I I i i
| |
| 90 se . «.—-—--4 « « » « — —I I s ! Z : .
| |
| | |
| sc- s ,———- « —- .—ä l « I I »« osss , « · I .
| |
| «; -0———L——--«—s- pas-s—- , .— «.
| |
| «« « « « « X i(
| |
| I Ho— « l « -» X i l —·.· X
| |
| s « i- s« i « r —» so « « ——-——-—" --—« -——-gs l i« J
| |
| »O—- « -s:
| |
| g «« Ja— is.
| |
| | |
| ZU—-
| |
| so— —————
| |
| | |
| c!
| |
| | |
| tu)
| |
| | |
| tot)
| |
| | |
| 90
| |
| | |
| St)
| |
| E
| |
| «: It)
| |
| S«
| |
| Es»s§
| |
| ·» 50
| |
| c«
| |
| Ǥ 40
| |
| E
| |
| s JO
| |
| s«
| |
| 20
| |
| | |
| «)
| |
| | |
| o.
| |
| | |
| Fig. 22.—Percentage saluration ok blood from thsze uterine veins during pregnancy in the rabbit. Notppxegnant uterine horn (a) , Pregnant horn (b). Birkh
| |
| indicated by the double 1ine. Two series ok experiments shown (0 and X) . (Bar·
| |
| croft, et al.: Jour. Physiol» Vol. 83, 1934.)
| |
| | |
| most negligib1e. At the time the fetus is vers! small the maternal
| |
| b1ood leaving the· placenta is red because it has given up little
| |
| oxygen to the ketus.1-2 As development oft the fetus proceeds
| |
| more and more oxygen is talcen up in the p1acenta and the ma
| |
| 59
| |
| 6o pHYsmLocY oF THE: FETUs
| |
| | |
| ternal veins leaving it become darlcer and darlcer, containing little
| |
| oxygen indeed at the end of gestation. This is illustrated in ex—
| |
| periments designed to preventpregnancy in one uterine horn ok
| |
| the rabbit. · The oxygen content ok uterine vein blood remains
| |
| constant throughout gestation on the nowpregnant side but declines progressively on the pregnant side (Fig. ge) .
| |
| | |
| 0ne can picture the placental circulation rather simply. A
| |
| well oxygenated stream ok the mother’s blood enters the placenta
| |
| | |
| Frsom Frsom Prsom To
| |
| | |
| mothers Fetus . mothcr ketus
| |
| g «:- —-——d
| |
| 0 Z
| |
| | |
| o,4 o,2 o,·4—" » o,4
| |
| coze coz1o co,8 coza
| |
| 023 , 022 oza
| |
| Co« 9 Co, 9 Co, 10 Co,1o
| |
| | |
| To mothers A To Fecus To mothers B Frsom Fetus
| |
| | |
| Fig. 23.—The exchange ok oxygen and carbon dioxide in the placenta. lk the
| |
| maternal and ketal Capillary streams course in the same directions U) , blood re—
| |
| turning to the ketus will be as venous as that returning to the mother. But ik
| |
| the streams course in opposite directions (B) , it will come into equilibrium with
| |
| that ok the mother’s arterial blood. (Redrawn from Mossmam Am. J. Anat»
| |
| Vol. 37, 1926.)
| |
| | |
| and comes into intimate contact with the ketal blood stream, poor
| |
| in oxygen. The two do not mix, but the degree ok contact dikkers
| |
| in various mammals. 0ne might thinlc that the two streams
| |
| should come into equilibrium in respect to the tensions ok their
| |
| blood gases, the ketal umbilical vein blood being as saturated or
| |
| unsaturated as the uterine vein blood of the mother, and that the
| |
| umbilical vein blood ok the fetus should become progressively
| |
| FETAL anspuutrtou 61
| |
| | |
| darlcer as gestation proceeds towards its termination. Actually
| |
| this does not happen. The umbilical vein blood which courses
| |
| toward the fetus is observed to be of much brighter color at term
| |
| than that leaving the placenta on the maternal side. 0ne explanation of this lies in an anatomical arrangement of the fetal and maternal vessels of the placenta. Mossmans has found that the fetal
| |
| and maternal capillary streams, instead of coursing parallel to one
| |
| another, actually flow in opposite directions in the rabbit placenta.
| |
| A similar arrangement has been observed in the cat, dog, and other
| |
| animals, but may not be present in all species. Thus the fetal
| |
| blood has the opportunity to come into equilibrium, not with
| |
| maternal venous blood, but with that at the arterial end o«f the
| |
| maternal capillary bed. This mechanism is illustrated diag’ram—
| |
| matically in Fig. es.
| |
| | |
| The placenta reaches its maximum size while the fetus is still
| |
| growing at a rapid rate. Nevertheless the volume of fetal blood
| |
| traversing it increases in proportion to the weight of the fetus.4
| |
| An increase in fetal blood pressure throughout pregnancy helps
| |
| regulate the amount of blood passing through the placenta per
| |
| minute. 0n the maternal side of the placenta, there is a somewhat smaller increase in the volume of How, except at the end
| |
| of gestation when it appears to be reduced (see Table s, p. se) .
| |
| | |
| The fetus is provided with another mechanism to enable it to
| |
| obtain oxygen readily in the placenta. — Its hemoglobin difkers
| |
| from that of the mother’s blood and the diiference is such in most
| |
| species that oxygen is talcen up with greater ease at the low partial
| |
| pressures which occur in the placenta. The total amount of
| |
| oxygen talcen up by the fetus increases progressively as the total
| |
| number of corpuscles and their number per cubic centimeter of
| |
| blood increase. The amount of oxygen utilized per gram of rissue remains nearly constant throughout the latter part of gestation
| |
| in the sheeps at approximately o.oo43 cc. per minute.
| |
| | |
| THE OXYGEN CAPACITY OF« THE BLOOD
| |
| | |
| A number of attempts have been made in recent years to shed
| |
| light upon the question of fetal respiration by analyzing the blood
| |
| on both sides of the placenta for oxygen and carbon dioxide
| |
| since the original study of Cohnstein and Zuntzs several important investigations have been undertalcen in sthe goat, sheep and
| |
| 62 PHYSIOLOGY oF THE FETus
| |
| | |
| human fetus and newborn.7-17 The hemoglobin of healthy adult
| |
| systemic blood leaving thesheart is approximately saturated (95 to
| |
| 97 per cent) with oxygenJs The blood contains on the average
| |
| about 19 volumes of this gas per ioo cc. in the human female.
| |
| The amount of oxygen carried in each cubic centimeter of the
| |
| mother’s blood in the latter part of gestation is somewhat less than
| |
| this. The amount of oxygen which the fetal blood can take up at
| |
| any partial pressure does not depend entirely upon the capacity
| |
| of the mother’s blood for oxygen but upon other factors which
| |
| will become evident when the nature of the maternal and fetal
| |
| dissociation curves are considered. Before discussing these relationships it will be of interest to compare the capacities of maternal and fetal bloods as found experimentally. Results obtained
| |
| at the end of gestation in human subjects are summarized in Table
| |
| l hie, is, 17
| |
| | |
| Tatar-n 11
| |
| Avniuen Oxronn Gar-zerrt- ots III-rat. Arn) Mast-annu- Bhoov n: Max«
| |
| | |
|
| |
| | |
| Fetal Maternal
| |
| l (vol. Yo) (vol. Z)
| |
| Adult (non-pregnant) . . . . . . . . . . . . . . . . . . . . . . . . . 19 . 00
| |
| At 8 moaths (1 caesakequ Section) . . .. ...I 18.14 17420
| |
| At term (1 caesarean Section) . . . . . . . . . . . . 20.9 15.6
| |
| | |
| At normal birth 4 cases) . . . . . . . . . . . . . . . . . 2042 15 . 82
| |
| | |
| At normal birth 15 eases). . . . . . . . . . . . . . . . 20.8 15 .4
| |
| | |
| Ät normal birth ksc enges) . . . . . . . . . . . . . . .. 2l.28-2l.6«
| |
| | |
| At normal birth 80 cis-see) . . . . . . . . . . . . . . . . 21.92 . . .
| |
| | |
|
| |
| | |
| «« Umbilieal artery and umbilieal vein blood.
| |
| | |
| It will be seen that the capacity of the mother’s blood to carry
| |
| oxygen at the end of gestation is low in comparison with normal
| |
| non-pregnant individuals. This may be related to changes in the
| |
| reaction of the blood (reduced allcalinity) , decrease in number
| |
| of maternal corpuscles in each cubic centimeten and other factors.
| |
| A marked decline in the oxygen content of cats’ blood during the
| |
| latter part of gestation has been observed.19
| |
| | |
| The capacity of the human fetal blood is higher than that of
| |
| the mother and is about the same as that of the average normal
| |
| adult male. 0ne might expect the fetal blood, which contains
| |
| larger red corpuscles and considerably more hemoglobin than the
| |
| adult, to be able to take up even. gcegter quantities of oxygen.
| |
| FETAL RESPIRATION 63
| |
| | |
| However, it· has been shown that human full-term fetal hemo—
| |
| globin in dilute solutions takes up oxygen, gram for gram, less
| |
| readily than does that of the adult.20- «! The increased amount of
| |
| hemoglobin little more than compensates for this deiiciency. This
| |
| observation is in contrast with the determinations in other mainmals.
| |
| Oxygen capacities have been determined in lower anima1s by
| |
| a number of investigators, but most of the data are insullicient to
| |
| -demonstrate the relation to adults of the same species. The studies
| |
| in sheep and goats have shown that the mother’s blood carries
| |
| more oxygen·per cubic centimeter than that of the fetusH Re
| |
| Oxygen eapscity stolz. per cent
| |
| | |
| Z(
| |
| 22
| |
| 20
| |
| IS
| |
| IS
| |
| U
| |
| IT
| |
| sc
| |
| s
| |
| 6
| |
| 4
| |
| 2
| |
| O
| |
| | |
| s 9 Wll I! UHISIHU DIRECT! U «Weclcs of ketal life
| |
| | |
| Fig. 24.—comparison of oxygen capacities ok mater-nat (o) and fetal (x) blood in
| |
| the goat. (Batcrokt: Proc. Roju soc. London, Vol. us, 19350
| |
| | |
| cent experiments in the ox and horseU suggest that the feta1 blood
| |
| at term has a slightly greater capacity than that of the mother
| |
| and is therefore comparable with that of man. The relationship
| |
| between oxygen capacities in mothers and fetuses which Barcrofts
| |
| found in the goat is illtistrated in Fig. 24. It will be seen that the
| |
| fetal values increase as the maternal values decrease with the approach of birth. Thiscan be correlated with changing hemoglobin yalues .
| |
| | |
| Roos and Romijn folslowed the changes in oxygen capacity
| |
| during the Erst few days after normal birth in cows and their
| |
| calvesJI They found greater values for blood of the calves at birth
| |
| than at 73H to 835 months gestation. The oxygen capacity of the
| |
| 64 PHYstoLooY oF THE FETUS
| |
| | |
| mother cows akter delivery was greater, not only than those in late
| |
| pregnanczz but also greater than the normal non-pregnant cows.
| |
| The data are surnmarized in Table 12.
| |
| | |
| Tun-u 12
| |
| | |
| Avuxuou Oxrow ckrzerrr or Bpoov or· Pisa-us, Ort-stumm un) Morgen m srnu O:
| |
| | |
|
| |
|
| |
| | |
| calves cows
| |
| ee. 0-X100 ee. blood ee. 0-X100 ee. blood
| |
| II norkpregnant eows . . . . . . . . . . . . . . . . . . . . . . 14.6
| |
| 8 caesarean Sections at 7å to 85 rnonths
| |
| gestation . . . . . . . . . . . . . . . . . . . . . . . . . 12 . 6 12 .2
| |
| 5 eows and 6 ealves one hour or less after
| |
| birtb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . I8.3 15 .8
| |
| | |
|
| |
| | |
| DISSOOIATION OURVES
| |
| | |
| The usekulness ok a Special fetal type ok hemoglobin becomes
| |
| apparent when the oxygen dissociation curves ok ketal and maternal
| |
| blood are eompared. conditions under which the fetal blood
| |
| | |
| »IIIIIIx
| |
| IIIIIZIIIs5IIII»-I
| |
| staatsIIHmIII
| |
| IImII
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | |
|
| |
| | |
| Pesscentaqe satufatioa with oxykcn
| |
| | |
| Oxykkq pkcssukc mklhligi
| |
| | |
| Fig. 25.-0xygen dissoeiation curves ok (a) ketal and (b) maternal blood ok
| |
| the goat at 18 to 19 weelcs' gestation. Brolcen lines indicate lirnits ok normal non·
| |
| pregnant adult goat blood. (Barcrokt, et a1.: Jour. Physiol» Vol. Es, 1934.)
| |
| | |
| takes up oxygen are not nearly so advantageous as those oceurring
| |
| in tlie adult lungs. An oxygen tension of about ioo mm. Hg is
| |
| kound in the lungs but the tension, although not delinitely de—
| |
| | |
| terrnined, is considerably reduced in the placenta. Furthermore,
| |
| FETAL REsP1RAT1oN 65
| |
| | |
| the surface area of the human chorionic villi has been estimated
| |
| to be only about half that of the lung alveoli at birth.22
| |
| | |
| Oxygen dissociation curves of maternal and fetal blood at the
| |
| time of birth have been prepared by a number of investigators,11s I2s 1«3-23- 24s 25 but Barcroft and his colleaguess are the only
| |
| ones who have compared the curves at different times during prenatal life. They studied the goat. It was found that the curve for
| |
| | |
| UVJO «« ·
| |
| | |
| P.c. satukation with oxygen
| |
| | |
|
| |
|
| |
| | |
| s
| |
| f
| |
| | |
| 0 I 20 30 40 O 0
| |
| Oxygen pressute, mm. AS.
| |
| Fig. 26.—0xygen dissociation curves ok the blood of a newborn goat (lekt) and
| |
| the mother (right). Broken lines indicate limits of normal nonspregnant adult
| |
| goat blood. (Barcrokt, et a1.: Jour. Physiol» Vol. 83, 1934.)
| |
| | |
| maternal blood is displaced to the right of the normal adult curve
| |
| from ten weeks on to birth, while that of the fetus gradually shifts
| |
| to the left between the 1oth and the 18th or rgth weeks and then
| |
| returns at full term to approximately the position characterizing
| |
| normal adult blood. The shape of the fetal cjurve difkers in important respects from that of the mother. It is steeper at low
| |
| oxygen pressures and crosses the maternal curve at about 7o mm.
| |
| Hg. These results are illustrated in Figs. 25 and 26.
| |
| | |
| 5
| |
| 66 PHYs1oLooY«oF THE FETUs
| |
| | |
| Oxygen dissociation curves for the human at full term are
| |
| shown in Fig. 27. Here ioo the maternal curve 1ies to the right
| |
| of that of the normal nowpregnant woman and the fetal curve is
| |
| almost within the range of the non-pregnant woman, but lies to
| |
| the 1eft of the maternal curve and is of a different shapeJEI some
| |
| investigators24 have found variations in human fetal oxygen dissociation curves at bitt-h; the extremesare of two types, one of
| |
| which is displaced farther to the left than the other and is less de
| |
|
| |
|
| |
|
| |
| | |
| O
| |
| O
| |
| | |
| s
| |
| | |
| U
| |
| O
| |
| | |
| s
| |
| | |
| II II
| |
| | |
| II fern. stooo 0--0
| |
| | |
| «« etooo o« essen-sm- wouasi 0-·-0
| |
| | |
| I otooo os- nowpnsousnr souv- —io so
| |
| | |
| ao ao so so 7o oo oo
| |
| aktiver-as oxvosn ten-Don
| |
| | |
| Ists eins« ok ncuootoem oitroeoutco
| |
| Z
| |
| | |
|
| |
| | |
| Fig. 27.-0xygen dissociation curves of the blood of the human fetus at bit-m,
| |
| its mother, and notkpregnant human adult. (Eastman, et al.: Johns Hoplcins Iiosp.
| |
| | |
| Ball» Vol. 53. 1933.)
| |
| | |
| ilected, crossing the maternal curve at higher oxygen tensions (85
| |
| mm. I—Ig) than the other (7o mm. Hg).
| |
| | |
| There is general agreement that the shift of the maternal oxygen dissociation curve to the right is due to a reduced allcalinity
| |
| of the maternal blood. If this is the only factor governing the
| |
| shift, the amount of displacement of the maternal curve must be
| |
| deiinitely limited, for the reaction of the blood can not be greatly
| |
| altered toward the acid side and still maintain normal health.
| |
| | |
| The shape of the fetal oxygen dissociation curve was found
| |
| to be very different from that of the mother by most investigators.
| |
| FETAL xtgspttulriox 67
| |
| | |
| Roos and RomijnU have obtained the most strilcing demonstration of this difkerence in the fetal calf. They found that the fetal
| |
| blood can attain 5o per cent saturation with oxygen at an oxygen
| |
| pressure of 1 1.5 mm. I-Ig, whereas that of the maternal blood becomes only about 8 per cent saturated at this tension. The maternal curve lies within the rather wide range of the normal non—
| |
| pregnant cow, but the fetal curve is so far to the left that go per
| |
| cent saturation with oxygen is reached when the mother’s blood
| |
| is less than 7o per cent saturated. Because the carbon dioxide
| |
| dissociation curves of the fetus and mother almost coincide in the
| |
| ox, the leftward displacement of the fetal oxygen dissociation
| |
| curve cannot be explained on the basis of blood pH changes during pregnancy It must be explained by a different type of hemoglobin in the fetus.
| |
| | |
| What is the signilicance of the observations outlined above?
| |
| They show that there are species’ diiferences, that the blood of
| |
| some animals at birth is able to take up oxygen at approximately
| |
| the same tension as that. in the adult, but in others the fetal blood
| |
| near term still takes it up with greater avidity than the adult and
| |
| gives it up less readily. They demonstrate a mechanism which
| |
| must be of inestimable value to successful intrauterine life. The
| |
| spread between maternaf and fetal oxygen dissociation curves indicates that oxygenation of the fetal blood is facilitated at the partial pressures which are physiologic in the placenta; the fetal blood
| |
| can takeup oxygen at a tension which causes the mother’s blood
| |
| to lose oxygen. It also makes the giving up of oxygen to the tissues
| |
| of the fetus less easily efkectedz but this is not a serious obstacle to
| |
| the fetus in utero which is relatively inactive and can get along
| |
| with a slower tissue respiratioxr After birth it is desirable that
| |
| these relationships be·reversed. When the blood is oxygenated in
| |
| the lungs it fmds a greater oxygen tension prevailing there than
| |
| in the placenta and the avidity of the fetal hemoglobin is no longer
| |
| needed. 0n the other hand an· active tissue metabolism calls for
| |
| more eilicient transfer of oxygen in the other direction at the
| |
| peripheral capillaries. It; would seem that there is an anticipation
| |
| of this need some time before it becomes acute in the goat, for the
| |
| fetal oxygen dissociation curve shifts gradually to the right and
| |
| assumes a less inllected form during late gestation. The fctus is
| |
| thus prepared in late fetal life for conditions it will meet after
| |
| 68 pnYsroLooY oF THE FETUs
| |
| | |
| birth. In some newborn human infants the shift has not gone as
| |
| far to the right as that of the newbom goat, and one may say that
| |
| they correspond to goat fetuses about a month before birth.«
| |
| carbon dioxide dissociation curves of fetal blood have been
| |
| constructed in the goat, ox and man.7-I1-I9-24-27-2S The results
| |
| of Eastman and his colleagues show that the fetal blood takes up
| |
| carbon dioxide less readily and gives it up with greater ease than
| |
| the nowpregnant adult blood at any given partial pressure of the
| |
| gasU However, the diiference between mother and fetus in this
| |
| respect is not marked. At about six weeks before term in the goat
| |
| | |
| votuuc Ist« etus unt-ou umso:
| |
| | |
|
| |
| | |
| soaososososososovotoo
| |
| usttiurkkns can-on via-not: reusiou
| |
| | |
| Fig. ask-carbon dioxidesdissociation curves of the blood of the human fetus
| |
| at birth, its mother and notppregnant human adult. (Eastman, et al.: Johns Hopkins I-Iosp. Bull., Vol. 53, 1933.)
| |
| | |
| the fetal carbon dioxide dissociation curve lies on the left of the
| |
| maternal curve.27 By full term it has shifted well to the rights«
| |
| The carbon dioxide dissociation curve of the pregnant cow was
| |
| found to be displaced slightly to the right of that of the eightmonth fetus.U The human carbon dioxide dissociation curves
| |
| at birth are illustrated in Fig. 2820
| |
| | |
| Calculations of the blood pH have been made from the carbon
| |
| dioxide dissociation curves of goat’s blood. Direct measurements
| |
| in the blood of goats (glass electrode) coincide with the calculated
| |
| pH va1ues.8 The fetal blogdis lesssallcaline than that of the nor—
| |
| FETAL nEsPmATIoN 69
| |
| | |
| mal non-pregnant goat, falling within the range of pH 7.27!-7.38,
| |
| although it is more allcaline than that of the mother six weelcs
| |
| before the end of gestation. The lowered pH (decreased alkalin—
| |
| ity) of the maternal blood·is adequate to explain the shift to the
| |
| right of the maternal oxygen dissociation curve. In normal hu—
| |
| man newborns the average pI-I of the umbilical artery blood has
| |
| been found to be 7.32 and that of the umbilical vein blood, 7.35.
| |
| The latter is about the pH of the mother’s cubital vein blood,
| |
| which is less allcaline than blood of normal nonpregnant individuals.29
| |
| | |
| A deficiency of carbonic anhydrase in fetal blood may explain
| |
| the higher allcali reserve of the fetal blood plasma in goats six
| |
| weeks before term.30 The gradual shift to the right of the fetal
| |
| dissociation curve for carbon dioxide during the latter part of
| |
| gestation in the goat may indicate a gradual depletion of the
| |
| allcali reserve.
| |
| | |
| OXYGEN AND CÄRBON DIOXIDE OONTENTS OF FETÄL KLOOD
| |
| | |
| It is essential to learn how much oxygen the fetal blood actually contains as it leaves the placenta, at various places in the fetal
| |
| circulation and when it returns again to the placenta. In the iirst
| |
| place, how does the blood of the umbilical vein compare with that
| |
| of the umbilical artery? A number of studies have been made in
| |
| human fetuses at the moment of birth. Results fluctuate widely
| |
| but this can be minimjzed to some extent by discarding cases of
| |
| asphyxia at birth. 0ne must bear in mind the technical diköcuL
| |
| ties encountered in obtaining fetal blood under physio1ogic conditions. There can be no doubt that maximum values for oxygen
| |
| are more representative of the true condition than are averages
| |
| which contain values obtained under partial anoxemia. Table 13
| |
| summarizes the more recent and reliable data from human infants obtained at the« moment of birth.12-I5-25-31 In addition
| |
| EastmanIs found 6.3 volumes per cent oxygen in the umbilical
| |
| artery and 13.3 volumes per cent in the vein at one Caesarean
| |
| section. 0thers who have drawn blood at Caesarean sections have
| |
| obtained very low values for oxygen.
| |
| | |
| In half of Noguchks experiments the infants had talcen their
| |
| first breath before blood samples could be drawn, but it was
| |
| demonstrated that this did not signiiicantly alter the analyses.
| |
| 70 PHYSIOLOGY OF THE FETUS
| |
| | |
| Haselhorst and stromberger obtained similar results. Noguchi
| |
| found no dilference betwesen 16 male and 14 female fetuses in the
| |
| oxygen capacity of the blood. ·
| |
| | |
| Tun: 18
| |
| | |
| Uppxn Latr-re am) Avaov Var-uns rot: Oxrogn am) cannot«- Dxoxrvs IN Btoov
| |
| or« Eurem· Its-Danks U« Mosis-Nr or« Bank:
| |
| | |
|
| |
|
| |
| | |
|
| |
|
| |
|
| |
|
| |
|
| |
| | |
| Umbilieal artery Umbilieal vein
| |
| | |
|
| |
| | |
| No.
| |
| I ti t f
| |
| «« S« «« »He, vor A, o, m. A, co- m. A, o, v0I. A, cohigh ave. high ave. high we. high ave.
| |
| Haselhorst « s
| |
| and Strom- l
| |
| bot-get. . . . . 22 . . . . . . . . . . . . . . . . . . . . 14.88 I0.I4 47.88 40.71
| |
| 28 8.02 840 52.85 46.21 . . . . . . . . . . - . . . . . · . . . ..
| |
| Biclone . . . . . . 9 15.60 12.95 62.26 50.06 17.57 I4.94 46.67 , 41.21
| |
| Eastman.... 15 5.90 8.30 . . . . . . . . .. I8.20 l0.50 . . . . . . . . ..
| |
| Nosuchi..... 80 10.00 4.40· 58.70 4420 I5.90 11.00 47.70 38.00
| |
| | |
| The percentage saturation of umbilical blood has been determined by the investigators cited above and the values are recorded
| |
| in Table 14. We wish to stress the point that these are averages
| |
| | |
| Tat-m 14
| |
| Avnnaon Print-ausknei- Oxrotm saturation or« Humm- Fnsrat Bnoov Ast« Bank:
| |
| Umb. vem l Froh. artery
| |
| A) sat 70 set.
| |
| Heselhort and stromberger . . . . . . . . . . . . .. 45 . 6 15 . s
| |
| | |
| Easttnan . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50.5 15.8
| |
| | |
| Bastman (caesarean) . . . . . . . . » . . . . . . . . 034 l s0.2
| |
| | |
| Noguchi . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57 .7 21 .7
| |
| | |
| Bidone (estimation) . . . . . . . . . . . . . . . . . . . . 7813 63-8
| |
| | |
| and not upper limits. If we accept the figure 2 1 volumes per cent
| |
| as the average oxygen capacity of human fetal blood at full te«rm
| |
| normal birth, and limit consideration to the highest values for
| |
| oxygen content (Table is) , the umbilical vein blood appears to
| |
| be between 63 and 84 per cent saturated at birth depending upon
| |
| whose data are used. This assumption is not entirely valid because
| |
| individual variation is to be expected in the amount of hemoglobin and oxygen capacity, but it may more nearly express the
| |
| true condition of the fetal blood than-do the mean iigures in
| |
| Table 14. Eastman found no rnore than 63 per cent saturation
| |
| FETAL RESPIRATIoN 71
| |
| | |
| with oxygen in the umbilical veinss but it is apparent that a
| |
| much higher saturation was encountered by Bidone.3I
| |
| | |
| The data available in lower animalzs are similar to those ob—
| |
| tained in man. Great variation in the content of oxygen and
| |
| carbon dioxide i·n the umbilical vessels has been reported in the
| |
| goat, sheep, ox, dog and cat,«· 7- V« U« IN« but if we admit only the
| |
| highest values and consider that technical limitations may have
| |
| reduced other values we are left with quite a different conception
| |
| of respiratory conditions than we get from the average values. It
| |
| would seem that the fetus does not thrive on venous blood.
| |
| | |
| The greatest number of analyses have been made by Barcroft
| |
| and his col1eagues who found that the superior limits of saturation
| |
| with oxygen in the umbilical vein blood of fetal goats vary between
| |
| 6o per cent and 87 per cent from the tenth to the nineteenth weelcs
| |
| of gestation, fa1ling ofk to about 45 per cent in the last two weelcsk
| |
| similarly in the sheep, blood going to the fetus (umbilica1 vein)
| |
| was found to be as much as 86 per cent saturated before the 137tl1
| |
| day; after that lower values were the rules« One of the most im—
| |
| portant observations on oxygen content of the blood of sheep
| |
| fetuses 75 to 14o days gestation was made recent1y by Barcroft and
| |
| Mason,35 who devised a technique for obtaining blood from the
| |
| umbilical vein or artery without delivering the fetus from the
| |
| uterus and with a minimum of manipulationsf When this was
| |
| done and the tendency toward placental separation avoided the
| |
| blood going to the fetuses was found to be more than 90 per cent
| |
| saturated with oxygen in half (five) their experiments and not
| |
| less than 7o per cent saturated in the others. These results are
| |
| shown in Table is.
| |
| | |
| Tun-n 15
| |
| Piave-Durham Oxford-N Hektorn-icon« or« Ums-text« Am) Ums-Zum Bhoov m srmv sitt-Et
| |
|
| |
|
| |
| | |
| 9073 or As— 79- SO- l IV— 49
| |
| · over 8075 7098 6073 5095 4073
| |
| No. ok easesk Utah. vein . . . . . . - 5 s 2 . . l . . . .
| |
| No. ok case-s: Urnb. arterzc . . . . . . . . . 3 6 I
| |
| No. ok case-s: Ufer. venule · l
| |
| (Ple.cental vein) . . . . . . . . . . . . . . . 8 I 2
| |
| | |
|
| |
| | |
| «« In the final paper (Barcroft, J» J. A. Kennedy sc M. F. Mai-on, 1g4o, J.
| |
| Physiol» 97: 347) it was reported that the highest values were found between the
| |
| 75th and Iooth days ok gestation and that they dropped considembly in the final
| |
| weelc (a physiologic anoxemia).
| |
| 72 PHYsIoLocY oF THE. FETUs
| |
| | |
| 1t is evident that the oxygen content of the umbilical vein
| |
| during life in utero is not as low as most studies lead one to be—
| |
| lieve. The better the teczhnical procedures, the higher the values
| |
| obtained. ·0ther investigators have encountered 85 to go per cent
| |
| saturation in the umbilical vein blood occasionally. BidonesI re—
| |
| ported 17.6 volumes per cent oxygen (approximately 84 per cent
| |
| saturated) in one human fetus before respiration started at birth.
| |
| Roos and RomijnU found that the umbilical vein blood of the
| |
| fetal calf of HH to 8175 months was saturated to a rather high degree, go per cent in one experiment.
| |
| | |
| Granting during fetal life that the blood coming from the
| |
| placenta to the fetus by way of the umbilical vein is as much as
| |
| 90 per cent saturated with oxygen does not entitle us to conclude
| |
| that the fetal tissues are bathed continuous1y in blood so rich in
| |
| this gas. The umbilical vein blood is diluted by reduced blood
| |
| from the lower parts of the fetal body-when it enters the fetal
| |
| inferior vena cava. The degree of dilution is an unlcnown and
| |
| perhaps not a constant factor. The inferior vena caval blood
| |
| enters the right atrium of the heart and most of it passes into the
| |
| left atrium by way of the foramen ovale, where it may be diluted
| |
| a second time by blood returning from the fetal lungs (see Fig.
| |
| 17, p. 43) . This pulmonary blood is reduced because, even if
| |
| little oxygen is removed by the tissues of the fetal lungs, much of
| |
| the blood has already traversed the heart and upper parts of the
| |
| body before passing to the lungs. The blood which is sent through
| |
| the ascending aorta and the great vessels which spring from the
| |
| aortic arch, i.e., the blood which goes to the heart and brain, cannot be as saturated constantly with oxygen as that which enters
| |
| the liver and inferior «vena cava from the umbilical vein and
| |
| ductus venosus.
| |
| | |
| Barcroft, Icramer and MillilcanW have attempted to determine
| |
| the degree of oxygen saturation in the carotid artery blood of
| |
| sheep fetuses near the end of the gestation period. They used
| |
| an ingenious mechanism consisting of a photoelectric cell and
| |
| micro-lamp between which the carotid artery was placed. The
| |
| blood acted as a red älter, oxyhemoglobin transmitting more light
| |
| in the red end of the spectrum than reduced hemoglobin. Variations in the current from the photoelectric cell deflected a galvan—
| |
| ometer mirror, and a beam of light from the mirror was used to
| |
| FETAL RESPUIATION 73
| |
| | |
| record continuous1y on moving photographic paper. Respirations
| |
| were recorded simultaneously. The mechanism was calibrated
| |
| | |
| by analyses made with the Van slyke manometric apparatus. Two
| |
| records are reproduced in Figs. 29 and 30 and their interpretation
| |
| in Fig. 31. It will be seen that complete saturation of the fetal
| |
| blood was eikected more readily when the 1ambs breathed oxygen
| |
| than when they breathed air. In other experiments it was found
| |
| that several hours were required for a complete saturation of the
| |
| newborn blood without the aid of 0xygen. The carotid artery
| |
| | |
|
| |
| | |
| Fig. 29.
| |
| | |
|
| |
| | |
| Fig. so.
| |
| | |
| Figs. 29, 3o.—Two Photographie records ok the changes in the oxygen saturation of the carotid blood during the initiation of respiration at birth in the sheep
| |
| fetus. The sensitivity of the apparatus diifered in the two experiments For in—
| |
| | |
| terpretation, see Fig. 31. (Barcrokt, et al.: Dur. Physiol» Vol. 94, 1939.)
| |
| | |
| blood of sheep fetuses studied at Caesarean Section was found ini—
| |
| tially to be between 32 and 70 per cent saturated with oxygen.
| |
| How much higher than this the saturation is in the normal un—
| |
| disturbed fetus in utero we do not lcnow.’«·
| |
| | |
| It has been very dikiicult to obtain- satisfactory data on the
| |
| blood gas contents of fetal blood because of technical dikiiculties
| |
| No one can doubt that operative procedures in exposing and open
| |
| « It has been demonstrated recently that the carotid arterial blood is as much
| |
| as 88 per cent saturated with oxygen in the sheep fetus prior to the last weelc of
| |
| gestation (Barcrokt, J» D. H. Barron, A. T. Cowie 8c P. I-I. Forsham, 194o, J. Physiol»
| |
| | |
| 972 gas) - r
| |
| 74 PHYSIOLOCY OF THE« FBTUS
| |
| | |
| ing the Uterus bring about changes in the respiratory relationships
| |
| in the placenta. Any series of experiments will give some results
| |
| below 25 per cent saturation witsh oxygen in umbilical vein blood,
| |
| but one is justiiied in concluding that these values are not physiologic. The blood can actually be observed to darlcen before a
| |
| sample can be drawn for analysis
| |
| | |
|
| |
| | |
| jkkjgsss
| |
| j111I-i1TTII—II
| |
| Tjsssss
| |
| junge-Jst
| |
| Ins-TUTTI
| |
| | |
|
| |
|
| |
| | |
| ·8·
| |
| | |
|
| |
|
| |
| | |
| Esaus-Utica Hi,
| |
| " B 8 8 s s Z 8 8
| |
| «!
| |
| l:
| |
| | |
| IO
| |
| | |
| --IIVIE
| |
| O, Untat-stinkt R,
| |
| » s g s· s s s a.- 8 8
| |
| s
| |
| F
| |
| I
| |
| | |
|
| |
| | |
|
| |
| | |
| | |
| I·
| |
| | |
| H
| |
| U
| |
| | |
| 0 - l 2 I H
| |
| Minutos altes« ligation of umbilical ookd
| |
| | |
| Fig. 31.—Ikxtckpkekation M) of Fig. 29 and G) ok Fig. so, showing percentage
| |
| saturation ok the lamb’s bloock in the carotid artery at birth. Umbilical cokd tted
| |
| zk T ; Iamh hkezchiqg oxygen at o—o. (Barcroft. et a1.: Jour. Physiol» Vol. 94
| |
| 19399
| |
| | |
| At the end of fetal life there is some evidence that the oxygen
| |
| carried by the umbilical vein blood is normally more reduced in
| |
| goats and in cats than it was earlier.9- 38 This constitutes a physiologic anoxemia. A kactor which may play a part in regulating
| |
| the amount of oxygen available to the fetus in the latter part of
| |
| gestation in the cat, and which has been generally overloolcecL is
| |
| the motor activity of the Uterus itself.30
| |
| FETAL nEsptnarxoN 75
| |
| | |
| Prelabor contractions of the Uterus are of physiologic occurrence. It has usually been assumed that should they become severe
| |
| they would block the fetal circulation through the placenta and
| |
| lead to anoxemia in the fetus. clarlc’s37 studies of blood pressure
| |
| reilexes in cat fetusespointed in this direction. In our laboratory
| |
| we have observed that the contractions, providing they are not
| |
| severe, are accompanied by improvement in color of the umbilical veins and that relaxation of the uterus results in darkening
| |
| of. the blood. Furthermore, analyses of the blood leaving the
| |
| pIacenta on the maternal side, drawn from placental tributaries of
| |
| the uterine vein, showed iluctuation in oxygen content, less oxygen
| |
| being present during and just following a uterine contraction than
| |
| in the interval of the relaxation. These values are the reciprocaIs
| |
| of those observed on the fetal side of the placenta (umbilical
| |
| vein) . Blood was drawn from the placental tributaries without
| |
| interfering at all with the fetuses and with little manipu1ation of
| |
| the uterus. The pregnant animals were not anesthesized but had
| |
| been decerebrated some time before. It is apparent that one can
| |
| not spealc of the conditions of fetal respiration, at least during the
| |
| last few days of pregnancy, as though they were constant. They
| |
| Auctuate normally from moment to moment; and whereas 95 per
| |
| cent saturation with oxygen may be attained at times, it is doubts
| |
| ful if the average amount available to the fetus during the latter
| |
| part of fetal life can be as great as it was earlier. The physiologic
| |
| anoxemia of the fetus in late gestation will be given consideration
| |
| in succeeding chapters. 0ther mechanisms have been suggested
| |
| whereby dilation and contraction of uterine vessels play a part in
| |
| regulating blood Aow through the placentaks
| |
| | |
| ASPEYXIA AT BIRTII
| |
| | |
| Much has been written concerning the conditions under which
| |
| the fetus at birth fails to begin breathing. Asphyxia is the cause
| |
| of death in an alarming number of cases, especially so as the demands for obstetricaI anesthesia become more urgent. In recent
| |
| years much light has been cast upon this subject by studies in the
| |
| blood gases at birth. some investigators have held to the old conception of asphyxia as an accumulation of carbon dioxide and
| |
| have supposed that this was the case in the blood of newborns
| |
| which failed to breathePD In view of I-«Ienderson’-s40 conception
| |
| 76 PHYsioLooY oF THE FETUs
| |
| | |
| of asphyxia as a decrease in carbon dioxide as well as in oxygen
| |
| one should expect that in the asphyiciated ketus, as well as in adults
| |
| subjected to asphyxial conditions, the blood carbonates would
| |
| shift to the tissues and hemoglobin, and the blood would contain
| |
| less carbon dioxide.
| |
| | |
| Eastman4ls 42 appears to be the iirst investigator to demonstrate
| |
| the truth of Henderson’s theory in asphyxia neonatorum. He
| |
| found that there is a reduction in oxygen content of umbilical
| |
| vein blood to very low levels (sometimes less than one volume per
| |
| cent) , that the carbon dioxide is lilcewise lowered and that the pH
| |
| of the blood may fall below 7.oo in fatal cases. Noguchirks 29 determined that there was some shift of plasma fluid in the fetus,
| |
| probably to the fetal tissues but not across the placenta, that the
| |
| oxygen was greatly lowered and that the carbon dioxide shifted
| |
| to the cells from the plasma but was not greatly lowered in total
| |
| amount. I-le also found a marked lowering of the pH of the blood
| |
| and concluded that asphyxia neonatorum is «a state of uncompensated allcali delicit in consequence of oxygen want. Cat fetuses
| |
| depressed at experimental Caesarean section resemble human infants in asphyxia neonatorum. The oxygen content of their
| |
| blood drops severely, and so does the carbon dioxide content.
| |
| | |
| REFERENCES cITED
| |
| | |
| i. Dator-oft, J» W. Herlcel 8c R. M. I-Iill. i933. Physiol» 77: i94.
| |
| e. Barcrofy J» L. B. Flexneiy W. I-Ierlcel, E. F. Mccarthy sc T. Mochi-Lin.
| |
| i934. 1bid» Sz- Fig.
| |
| z. Mossmath H. W. i926.» Am. J. Anat» 37: 433.
| |
| 4. Bann-oft, J. 8c J. A. Kennedyc i939. J. Physiol» 95: i73.
| |
| z. Bart-ruft, J» J. A. Kennedy Z: M. F. Masotr iggg Ibid» 95: Läg.
| |
| S. Gohnstein, J. sc N. Zuntz i884. Pflüger-T Arch» 34: i73.
| |
| 7. Huggett A. sc. G. i927. J. Physiol» se: 373.
| |
| 8. Bann-oft, J» R. H. E. Elliott, L. B. Flexney F. G. I-Iall, W. Herlceh E. F.
| |
| Mccarthyz T. Mcclurkin s: M. Talaat. i934. Ibid» As: i92.
| |
| g. Baker-oft. J. i935. Proc. Roy. soc» Lond. B, ii8: 242.
| |
| io. Barcrofh J» K. Kramer s: G. A. Millilcan. i939. J. Physiol» 94: 57i.
| |
| ii. Roos, J. sc G. Romijix i938. Ibid» ge: 249.
| |
| is. I-Iaselhorst, G. 8cK. strombeisgen i93o. Ztscliin Geburtsh. Gynäk» 98: 49.
| |
| is. Haselliorsh G. se K. strombeigen i93i. Ibid., ioo: 48; i932. Ibid»
| |
| io2: i6.
| |
| i4. I-Iaselhorst, G. i93i. Arch. Gynälc» i44: 558.
| |
| i5. Eastmam N. J. i93o. Johns Hopkins Hosp. Bull» 47: 22 i.
| |
| is. Eastman, N. J» E. M. K. Geiling s: A. M. DeLawdeia i933. Ibid» 53:
| |
| 246.
| |
| ooeo
| |
| Tkscp 023
| |
| | |
| mmwwwwmvnu nnnnnv«- QMAWPKPOPF PYUPCVPPPV IV«
| |
| | |
| 77
| |
| | |
| PETAL KESPIRATION
| |
| | |
| . Noguchi, M. 1937. Jap. Obst. Gyn» so: s18.
| |
| | |
| Bat-Gott, J. 19s5. The Respiratory Function ok the Blood, cambriclge
| |
| Univ. Press.
| |
| | |
| . steele, A. G. s: W. F. Winclle. 1g38. J. Physiol» 94: 5s5.
| |
| | |
| Haurowitz F. 1935. Hoppesey Ztschn physiol. chekn» s3s: 1s5.
| |
| Hill, R. 1935. cited by J. Barcrokh Pkoe. Roy. soc» LoncL B, us: s4s.
| |
| christotkeisem A. K. 1934. compr. read. soc. Biol» u7: 641.
| |
| | |
| . Anselmino, K. J. s: F. Hoffmann. 193o. Arch. Gynä1c» 143, 477.
| |
| | |
| Leibson, R. G., I. I. Lilchnitzlcy sc M. G. sank. 1g36. J. Physiol» 87: 97.
| |
| NoguchL M. 1937. Jap. J. Obst. Gyn» so: 358.
| |
| | |
| Bat-most, J. 1938. The Brain and Its Environmenh Yale Univ. Press,
| |
| | |
| New I-Iaven. .
| |
| Mccarthy, E. F. 1933. J. Physiol» so: so6.
| |
| Iceys, A. B. 1934. Ibicl» so: 491.
| |
| | |
| . Noguchi. M. 1937. Jap. J. Obst. Gyn» so: s48.
| |
| | |
| Roughtoty F. J. W. 1935. Physiol. Ren, is: s41.
| |
| Bidone, M. igzh Ann. ostet. since» 53: 197.
| |
| Kellogg, H. B. 193o. Am. J. Physiol» 91: 637.
| |
| | |
| . Bat-Gott, 1936. Physiol. Reif» is: 1o3.
| |
| | |
| . steele, A. G. sc: W. F. Windle. 193g. Physiol» 94: Ist.
| |
| . Baker-oft, J. s: M. F. Mason. 1938. Ibicl» 93: ssP.
| |
| | |
| . Winde, W. F. B: A. G. steele.
| |
| | |
| 1938. Proe. soc. Exp. Biol. sc Med» Zg:
| |
| s46.
| |
| | |
| . Oliv-le, G. A. . 1934. J. Physiol» 83: ss9.
| |
| . schnitt, W. 19s5. Deut. mecl. Wehnsehr» 51(1): 189.
| |
| | |
| Kam, I-I. F. s: J. Kreiselmatx 193o. Am. J. Obst. Gyn., so: 8s6.·
| |
| | |
| . I-Ienderson, Y. 19s8. J.A.M.A» go: 583.
| |
| . Eastmam N. J. 193s. Johns Hoplcins I-Iosp. Bull., so: 39.
| |
| . Eastmath N. sc c. M. McLane.
| |
| | |
| 1931. 1bicl» 48: s61.
| |
| CHAPTER VI
| |
| | |
| FETAL RESPIRATORY MOVEMENTS
| |
| | |
| IT is commonly taught that the fetus in utero is apneic. I-Iowever, this concept has been chal1enged from time to time and some
| |
| investigators have declared that respiratory movements occur
| |
| regularly throughout prenatal life under normal physiologic conditions. No one doubts that mammalian fetuses are capable of
| |
| performing rhythmical movements which talce the form. of
| |
| shallow rapid breathing or even of dyspneic gasping. Movements
| |
| of this nature are frequently encountered upon opening the
| |
| uterus of a pregnant laboratory animal. The literature contains
| |
| many articles in which mention of such activities has been made.
| |
| The early studies were reviewed by Preyer.1 Human fetuses,
| |
| aborted or removed surgically from the uterus, show the move—
| |
| ments in question as early as the twelfth weelc of gestationk 0ne
| |
| of the ·most strilcing examples of respiration in immature forms is
| |
| encountered in the opossum.3 Although this animal is born after
| |
| a sojourn« of only 1243 days in utero (or in the tubes, as a blastocyst) it is able to breathe and to make its way unassisted to the
| |
| pouch where it reassumes dependence upon the mother. Most
| |
| studies in other animals have employed fetuses removed from the
| |
| uterus and one may well question whether any of them were
| |
| representative of the normal. It is not permissible to conclude,
| |
| on the basis of observations made under conditions which preiudice the placental exchange mechanism, that respiratory motor
| |
| phenomena are indulged in normally by the fetus throughout
| |
| prenatal life.
| |
| | |
| RESPIRÄTORY MOVEMENTS IN« THE INTACT ANIMAL
| |
| | |
| Let us examine the evidence of fetal movements resembling
| |
| respiration in the intact animal. In 1888 Ahlfeld, a German
| |
| gynecologist, described certain rhythmical fetal movements which
| |
| he and a pupil observed in patients during the latter weelcs of
| |
| pregnancyxss Z superimposed upon the slow excursions of the
| |
| gravid abdomen which resulted fronkmaternal respirations they
| |
| | |
| 78
| |
| FETHL nEsPInATonY MovEMENTs 79
| |
| | |
| could see more rapid rhythms which appeared to be due to activities of the fetus. Ahlfeld proposed that the ketus was making
| |
| respiratory efforts and thought it must aspirate its amniotic fluid
| |
| into the major respiratory passages. Many protested this theory,
| |
| but as recently as 1905 he reiterated his views and published convincing graphic records of these movementsJk The intrauterine
| |
| respiratory movements have been observed by other investiga
| |
| Fig. 32.—I-luman intrauterine respiratory movementa A, Intesrrupted rhythms
| |
| at a frequency of about 52 per minute. B, Movements recorded from fetal ab—
| |
| domen and chest simultaneouslys about so per minute. (Ahlfeld: Monatschr.
| |
| Geburtsh. Gynälsp Vol. at, 19o5.) c, Intrauterine fetal respiratory movements
| |
| (upper) , maternal carotid Pulse, maternal respirations and time in 4 second intervals (lower) before labor be»gan. (lc. Reilkerscheich Piliigeks Arch., Vol. 14o, 1gu.)
| |
| | |
| tors,7 but the theory of« aspiration of fluid has not been generally
| |
| accepted and it is usually concluded that the fetal glottis remains
| |
| cJosed. The question has been raised again recently by investigatorss who are of the opinion that the human fetus executes
| |
| rhythmical respiratory movements normally and that these serve
| |
| to draw amniotic fluid into the lungs. It was even suggested that
| |
| this fluid may assist in the development of the lung alveoli. Tracings illustrating the character of human» fetal respiratory move—
| |
| 8o pnvstoLocv oF THE FETUs
| |
| | |
| ments are reproduced in Fig. se. A strilcing comparison of intrauterine fetal and early postnatal respiratory records will be
| |
| seen in Fig. 33. «
| |
| | |
| The principal objection to the current view is that the move—
| |
| ments in question are not always, in fact not often, manifested by
| |
| the human ketus. One must watch patiently to see them and when
| |
| they do appear they are usually transient». If they were commonly
| |
| encountered in the human, one should expect to see them in
| |
| | |
|
| |
| | |
|
| |
| | |
| Fig. zzpcomparison of human intrauterine respiratory movements (upper)
| |
| with those of a five day old inkanr. Time, 4 seoondsintervalsx (Ic. Reifkerscheich
| |
| | |
| Ptliigeks Arch., Vol. 14o, 1gu.)
| |
| | |
| some other animals wo. Fetal respiratory-like activities are rarely
| |
| apparent in cats and guinea pigs but have been seen a day or so
| |
| before birth.9 The question of aspiration by the fetus will be
| |
| considered later. It is pertinent Erst toinvcstigate the factors
| |
| which govern the manifestation of fetal respiratory-like moveInstit-s.
| |
| | |
| RESPIRÄTORY MOVEMDNTS UNDER EXPERIMENTAL CONDITIONS
| |
| | |
| Respiratory movements have been studied recently in a number of laboratory animals including the rat, guinea pig, rabbit,
| |
| sheep and cat.9-18 They have been observed in human beings
| |
| under somewhat similar conditions.19-23 There is little doubt
| |
| that their occurrence and characteristics are influenced by the
| |
| experimental conditions. These must be evaluated critically.
| |
| | |
| In the Erst place, anesthcsia seems to alter the threshold of the
| |
| fetal respiratory center and for this reason most investigators have
| |
| tried to avoid using it. Ether administered to the mother anesthetizes the fetuses which then rarely« exhibit respiratory activities
| |
| FETAL nnsptnaronv MovEMENTs 81
| |
| | |
| until after placental circulation has been interruptedz at that time
| |
| they talce the form of dyspneic gasping. Urethane lilcewise de—
| |
| presses the rapid rhythms of the shallow respiratory movements.24
| |
| Doses of nembutal which anesthetize the mother similarly depress
| |
| the fetusks Although sodium amytal is said to have no ekfect
| |
| upon the fetus of the rat when given to the motherW and not
| |
| to pass the placental barrier readily, further consideration should
| |
| be given to this question. It is possible that some asphyxial types
| |
| of anesthesia first stimulate respiratory movements in normally
| |
| apneic fetuses,9 but there is little doubt that their continued use
| |
| results in depression. Cyclopropane (3o per cent) is said to be
| |
| about the only inhalation anesthetic which does not seriously
| |
| | |
| N.»—»,.«—W»D-.F—HAW»DHWWWMW»JX"
| |
| .—..-.IZ——- o
| |
| | |
| N A
| |
| | |
|
| |
| | |
|
| |
| | |
| Fig. 34.-Tuso portions of a continuous crystograph record of uterine motility.
| |
| maternal respiratjon and intrauterine fetal respiratory movements on the 67th day
| |
| of gestation in the tat (end of gestation) . The animal had been decerebrated by
| |
| the anemia method: no anesthesia was used during the experirnentx the abdomen
| |
| was not opened. The major kluctuations at the beginning of each tracing indicate
| |
| relaxation of the Uterus after a contraction occurring one minute before the arrow
| |
| | |
| (1«) . The large secondary waves are the maternal respirations (17 per minute) .
| |
| The smallest waves are intrauterine fetal respiratory-like movements occurring at
| |
| | |
| approximately 120 per minute, slower at first than when they are well under way.
| |
| Five minutes between relaxations i and a.
| |
| | |
| aikect the respiratory activities of fetuses and still gives surgical
| |
| anesthesia.27
| |
| | |
| Various experimental procedures have been employed to avoid
| |
| the use of general anesthetics spinal anesthesia can be used
| |
| successfully in the larger species. spinal cord section if performed
| |
| long enough beforehand to allow recovery from shoclc may be
| |
| satisfactory in those animals which are rather docile anyway.
| |
| Decerebration is to be recommended in other forms. After preparing an animal near term by one of these methods it is occasionally possible, without opening the .maternal abdomen, to see«
| |
| movements of a rhythmical nature resembling fetal «breathing.".
| |
| In Fig. 34 such fetal respiratory movements are shown (after de—
| |
| cerebration of the cat) superimposed upon maternal respirations
| |
| This curvc was obtained by means of a crystograph activated by
| |
| amplified potentials from an oscillating eircuit, any movements
| |
| | |
| 6
| |
| 82 PHYsioLooY oF THE: FETUS
| |
| | |
| on the surface of the catss abdomen changing the frequencies of
| |
| oscillation. To what extent decerebration, spinal cord Section or
| |
| other preparatory surgical procedures were responsible for instituting the respiratory movements, is not known.
| |
| | |
| When the pregnant animal is placed in a bath of warm saline
| |
| solution, the abdomen opened and the uterus exposed, it is pos—
| |
| sible to observe fetal activities more directly. Rabbit and guinea
| |
| pig fetuses near term can be seen quite easily through the thin
| |
| uterine wall. Body form of other species is readily distinguish—
| |
| able. During the course of zo minutes or more of observation,
| |
| respiratory rhythms of fetal movements usually malce their appearance in the cat. The longer the uterus has been exposed and
| |
| the nearer the end of gestation, the more frequently these activi·
| |
| ties manifest themselvess They rarely continue for more than a
| |
| few seconds but start and stop at fairly regular intervals. This
| |
| intermittent occurrence of brief rhythms is associated with
| |
| motility of the uterus in the cat. Mild contraction of uterine
| |
| muscle is accompanied by a period of fetal quiescencex relaxation
| |
| of the muscle leads to a rhythm of respiratory Ihovements Fetal
| |
| activities of a respiratory nature are rarely if ever seen in the unopened uterus before the last two weelcs of gestation in the cat.
| |
| This is not because it is more difficult to visualize younger fetuses,
| |
| for as we shall soon see it is possible to induce the movements in
| |
| question by appropriate experimental methods as early as the
| |
| middle of prenatal life.
| |
| | |
| Most of the earlier studies on respiratory movements of fetuses
| |
| were made after delivering the specimens from the uterus under
| |
| warm saline solutions, leaving the placenta attached to the uterine
| |
| wall. It has often been erroneously assumed that circulation and
| |
| gaseous exchange can thus be maintained adequately and that observations made in the fetus under such conditions are representative of activities occurring normally in the intact animal.
| |
| | |
| When a cat fetus has been determined to be apneic in utero
| |
| and is then carefully but quiclcly delivered through a small incision in the least vascular portion of the uterus at one end of the
| |
| zonary placenta it commonly begins to execute respiratory moves
| |
| ments within a very short period of time; often this is a matter
| |
| of one minute or less. The blood of the umbilical veins darlcens
| |
| perceptibly at delivery and the— manifestation of respiratory moves
| |
| FETAL RESPIKATOKY MovEMENTs 83
| |
| | |
| ments is associated with such darlcening in cat fetuses during the
| |
| third quarter of gestationss
| |
| | |
| Feta1 respiratory movements have been studied in other
| |
| mammals under similar conditions.I3-14 It was found that the
| |
| earliest respiratory movements of sheep fetuses can be elicited by
| |
| mechanical stimulation at about 38-39 days of gestation. The
| |
| first rhythms of movement seem to be enhanced by putting pressure upon the amnion and thus indirectly stimulating the fetus.
| |
| Later they seem to occur spontaneous1y, but it is never possible
| |
| to avoid some mechanical disturbance of the fetus and the manips
| |
| ulation of the uterus itse1f may have been a factor in producing
| |
| the «spontaneous" activities. Urethane or spinal anesthetics were
| |
| employed for the ewes.
| |
| | |
| Rhythmical respiratory movements have been studied in rabbits after sectioning the spinal cords of the pregnant does« The
| |
| activities were manifested both before and after delivering the
| |
| fetuses from the exposed uterus. Most of the rabbits were at
| |
| term or had passed the time birth should have occurred normally,
| |
| labor having been inhibited by hormone treatment. It is possible
| |
| that the post-mature fetuses suffered inefiicient placental exchange
| |
| of oxygen and carbon dioxidez it has been shown that all rabbit
| |
| fetuses die by the zöth day (normally delivery occurs at 31 or 32
| |
| days) when labor is inhibited-S
| |
| | |
| From the various experimental studies it is clear that mammalian fetuses are capable of exercising their respiratory muscles
| |
| and of doing so rhythmically after the fashion of airbreathing
| |
| animals rather early in prenatal like. But mammalian fetuses do
| |
| not execute rhythmical respiratory movements continually in
| |
| utero unless certain unfavorable conditions are set up. Even near
| |
| the termination of life in utero when the eliiciency of the placenta
| |
| in relation to the greatly enlarged fetus has declined and when
| |
| one might expect to lind a physiologic anoxemia, it is surprising
| |
| how seldom the activities in question can be observed in the intact
| |
| animal.
| |
| | |
| RELATION OF OXYGEN END CARBON DIOXIDB TO RESPIRATORY «
| |
| MOVEMENTS
| |
| | |
| since the blood of the fetus comes almost into equilibrium
| |
| with that of its mother in respect to tensions of carbon dioxide
| |
| and oxygen in the placental capillary bed one may« well raise the
| |
| 84 PHYs1oLocY oF THE FETUs
| |
| | |
| question: why does the fetal respiratory center not respond as
| |
| readily as that of its mother? It has been assumed that the threshold is higher than that of the mother.29- 30 If this is one of the
| |
| main factors in preventing continuous fetal respiratory moves
| |
| ments from manifesting themselves norma1ly in utero it should
| |
| be possible to find a carbon dioxide level at- which the fetal center
| |
| will respondz or perhaps the threshold ok the fetal center can be
| |
| lowered experimentally until fetal respiratory movements begin.
| |
| | |
| It has been suggested that accumulation of metabolic carbon
| |
| dioxide may be the agent starting these movements in cat and rat
| |
| fetusesJos I« Barcroft and his colleaguessI administered atmospheres rich it; carbon dioxide to pregnant sheep without inducing respiratory movements in the fetuses. However, the animals
| |
| had been anesthetized with urethane and it is possible that the
| |
| threshold of the center had been raised by this drug to a Ievel
| |
| which could not be reached by. the maximum concentration the
| |
| blood could carry. The ekfect of giving carbon dioxide to pregnant rabbits has been investigatedN The fetuses were already
| |
| executing respiratory movements and the rate of the fetal rhythm
| |
| was not greatly altered by the gas. The only positive results were
| |
| obtained in cat fetuses-IS mostly in the third quarter of the gestation periodx the fetuses were apneic at the time of experimentas
| |
| tion. Some were delivered from the uterus with placental circulation intact and others were observed through the unopened
| |
| uterus. When the mother cats breathed atmospheres of oxygen
| |
| containing 8 to 1o per cent carbon dioxide, rhythms of rapid respiratory movements appeared both in the delivered and the intact
| |
| fetuses concomitantly with the increase in rate and amplitude of
| |
| maternal breathing. substitution of 15 to 20 per cent carbon
| |
| dioxide in oxygen led to depression of the fetal respiratory efforts,
| |
| which became very deep gasps at a slow rate. The· cat was then
| |
| allowed to breathe air again and a «rebound hyperpnea" often followed in the fetuses, presumably as the carbon dioxide level re—
| |
| adjusted itself in the fetal blood. 0ne of these experiments is
| |
| illustrated in Fig. 35.
| |
| | |
| Administration of mixtures of 5 to 8 per cent oxygen in nitrogen to the mother cats brought about cyanosis, indicated by darlcening of the uterine vesse1s, and respiratory movements of the
| |
| previously apneic fetuses began promptlysz Compression of the umpar-u. RESPIRATORY MovEMENTs 85
| |
| | |
| I
| |
| | |
| bilical cord produced similar results in exaggerated form and
| |
| more quiclcly. In large fetuses of sheep it was found that compression of the umbilical arteries alone led to respiratory activity
| |
| but that this activity was delayed and did not manikest itselk so
| |
| soon as it did after compressing the veins too.31 This indicates
| |
| that the fetus was able to draw upon blood in the placenta and
| |
| obtain a little more oxygen while the veins were intact. Many
| |
| other investigators have reported that interruption of the placental circulation brings about respiratory efforts in the fetusJs W·
| |
| | |
| 33 b? OZTFCOH F 024
| |
| | |
| 30
| |
| cat: 17
| |
| Bot-us!
| |
| | |
| Z 23 60 sum.
| |
| s 44 days
| |
| Z« 20
| |
| Z
| |
| Z .Z«
| |
| 10
| |
| s s
| |
| | |
| — H
| |
| Tirne in half« minnt-es
| |
| | |
| Fig. 35.-Etkect of an 8 to to per cent carbon dioxideoxygen mixture, breathed
| |
| by the mothcks It! itütistivg khythmical respiratory movements in a cat fetus delivered at caesarean Section but with placental circulation intact Respiratory rate in
| |
| half minutes. (Wind1e, et al.: Physiol. Zool» Vol. u, 19z8.)
| |
| | |
| 1«-32-33-34 Usually these were of the dyspneic gasping type.
| |
| When asphyxia is avoided fetal respiratory movements of the cat
| |
| occur at rates as high as 120 per Minute, commonly more than 6o
| |
| | |
| per minute.
| |
| Atmospheres defccient in oxygen breathed by full term guinea
| |
| | |
| pigs on which no surgery had been performed and no anesthetick
| |
| | |
| used caused rhythmical respiratory movements to start in the
| |
| previously apneic fetuses.9 Usually the first activities -to- appear
| |
| were rather fast rhythms of movement which resembled shallow
| |
| respirations. With more marked cyanosis these became stronger
| |
| 86 PHYsIoLocY oF THE: FETUs
| |
| | |
| and slower. Ultimately only slow rhythmical gasps were seen in
| |
| the fetuses. Upon relieving the asphyxia the rapid rhythms returned and then they too stopped, the fetuses becoming apneic
| |
| again with return of normal oxygenation.
| |
| | |
| Blood-gas analyses have been made from samples withdrawn
| |
| anaerobically from the umbilical veins of cat fetuses delivered
| |
| from the uterus but« in which the placental circulation was still
| |
| intact» It was found that the oxygen content was low. the blood
| |
| being little more than so per cent saturated in those fetuses which
| |
| were executing rhythmical respiratory movements at the time of
| |
| sampling. When oxygen saturation dropped to about 25 per cent
| |
| or less the fetuses gasped or became depressed to the point of com—
| |
| plete inactivity. Under the conditions of the experiments it was
| |
| less p0ssible to relate the presence or absence of the movernents
| |
| in question to carbon dioxide than to oskcygen content. However,
| |
| in the presence of the higher oxygen levels (4o-5o per cent saturation) the carbon dioxide content was greater than it was when
| |
| the oxygen level was marlcedly loweredz in the former instances
| |
| the fetuses executed rapid rhythms, whereas in the latter they
| |
| became inactive and depressed. Arrhythmical deep gasps appear-ed
| |
| when the carbon dioxide was high and the oxygen low.
| |
| | |
| It was not.possible to obtain blood from the cat fetuses with·
| |
| out incising the uterus and disturbing relationships there to some
| |
| extent; consequently one does not lcnow what the oxygen level is
| |
| in utero. However, it was evident that respiratory efforts often
| |
| began as the fetus was»being delivered and as the umbilical vein
| |
| blood was darlcening Probably a higher oxygen level is maintained in the normal undisturbed uterus than the highest value
| |
| obtained at experimentation would indicate. This is true in
| |
| other species. The sheep in late fetal life is apneic in uteroz correlatively its umbilical vein blood, obtained without removing the
| |
| lamb from the uterus, is highly saturated, exceeding 9o per cent
| |
| in somekss But when the lamb is delivered into a. bath of saline
| |
| solution with its placental circulation intact the blood becomes
| |
| reduced.37
| |
| | |
| In the human at normal birth, apnea prevails when the blood
| |
| is about 50 per cent saturated with oxygen irrespective of the
| |
| carbon dioxide contents« but respiration starts readily. some
| |
| higher values have been obtained iii apneic human fetuses-W« 40 as
| |
| FETAL KESPIKATORY MovEMENTs 87
| |
| | |
| well as in the calfKI 0ne may infer from these experiments that
| |
| the normal apnea ok ketuses in utero is associated with a degree ok
| |
| oxygenation exceeding about 4o or 50 per cent saturation, that as
| |
| this becomes reduced rhythms of active respiratory movements appear, but that depression ok the oxygen level much below 25 per
| |
| cent saturation 1eads to gasping and ultimately to complete inactivity comparable with that in asphyxia neonatorumds
| |
| | |
| Respivatovy mai-e«- Haar«
| |
| xrxønxs begin-·«- 2 4 H s
| |
| | |
| Isgssssssssss
| |
| IvIIIIaa
| |
| | |
| Itiissssssss
| |
| Isxwssstss
| |
| | |
| l2
| |
| | |
|
| |
|
| |
| | |
| 18
| |
| | |
| Z
| |
| | |
|
| |
|
| |
|
| |
| | |
|
| |
| | |
|
| |
|
| |
| | |
| F »
| |
| g «« III-Insp- F
| |
| s»IIIm-;Ja».
| |
| H III-C ja ;
| |
| 8 Ists-sit Ist «»IIUIIIIIII
| |
| Ists-Iststratst-strick
| |
| «, III-Ists
| |
| | |
|
| |
| | |
| It) 12 14 If) IS 20 21
| |
| Days of inoubation
| |
| | |
| Fig. 36.—0xygen and carbon dioxide contents ok the atmosphere ok the air space
| |
| in two hencs eggs talcen at daily intervals during incubatiotx Respiratory moves
| |
| ments begin at the plateau between 17 and 19 days. (Romijn and Roos: Jour.
| |
| Physiol.- vol. 94, 1938.)
| |
| | |
| Experiments in mammalian fetuses have been coniirmed in the
| |
| incubating eggs ok chiclcs and duc .42-45 It was possible to con—
| |
| | |
| I« Barcrokt and his colleagues have demonstrated recently that apnea prevails
| |
| in the sheep ketus when the blood going to the ketal brain is about So to so «per
| |
| cent saturated with oxygen and when that leaving it is zo per cent or more Saturated. Lower values were encountered during the .1ast prenatal wee1c. Respiratory
| |
| movetnents occurred when the blood leaving the brain was to to 25 «pr cent Saturated (Barcrokt, J» D. I-I. Bari-on, A. T. cowie sc P. H. Forsham 194o, J. Physiol-»
| |
| | |
| g7s sie-S)88 rsktysxotocis or THE rETUs
| |
| | |
| trol physiologic conditions more precisely in the bird than in
| |
| the mammaL Although space limitations do not allow discussion
| |
| of these studies here it should be pointed out that our lcnowledge
| |
| of the atmospheres breathed by the bird throughout incubation is
| |
| reasonably complete. A nice correlation can be drawn between
| |
| the decline in oxygen concentration, the elevation of carbon
| |
| dioxide and the appearance of respiratory movements« This is
| |
| illustrated in Fig. 36.
| |
| | |
| We learn from the various experiments with carbon dioxide
| |
| excess and oxygen want in mammals and in birds that the fetal
| |
| respiratory center responds much as does that of the adult, but
| |
| there is one very marlced difference between fetus and adult.
| |
| Greater concentrations of carbon dioxide and more severe degrees
| |
| of oxygen deliciency mustcome into play in the former before results are obtainable. In other words the fetal respiratory center
| |
| seems to have a high threshold.
| |
| | |
| OTHER PACTORS IN THE DEVELOPMENT OF RESPIRATION
| |
| | |
| Not all mammals are capable of performing rhythmical respiratory movements at the same period in prenatal life. One
| |
| finds some species more precocious than others. As has already
| |
| been indicated, the newborn opossum «embryo« breathes about
| |
| 13 days after conception. In the more advanced fetus of the sheep
| |
| respiratory movements have been observed at the end of the first
| |
| quarter, but still at a relatively earlier time than in the cat. The
| |
| latter reaches nearly the middle of gestation before rhythmical
| |
| respiratory movements can be induced. The human fetus has
| |
| accomplished necessary growth by the end of the twelfth weelc of
| |
| intrauterine existence.
| |
| | |
| Muscles and peripheral nerves are laid down long before fetal
| |
| respiratory movements begin. It is quite clear that the accomplish—
| |
| ment of rhythmical movements awaits further development of a
| |
| central nervous mechanism. In the 15 mm. cat embryo intrinsic
| |
| growth of spinal neurons and their connections have reached the
| |
| point at which the first simple rellexes are possible.47 From this
| |
| time onward the Process of nervous integration proceeds, new re—
| |
| sponses accompanying new nervous connections and simple activis
| |
| ties becoming more complexsIs Most of the responses of which a cat
| |
| embryo of 2o mm. length, delivered yvspith Flacental circulation inFETAL RESPIRATORY MovEMENTs 89
| |
| | |
| tact, is capable appear to be almost purposeless. The rhythmical
| |
| respiratory movements which make their appearance just before
| |
| the 30 mm. stage are among the first to foretell a usefulness. However, the behavior pattern that makes possible rhythmical respiration is not a new thing; it is the rhythmicity that is new. Even
| |
| before 30 mm., in fact as early as 20 mm., integkated but arrhyth—
| |
| mical contractions of the future respiratory muscles occur when
| |
| adequate stimuli are used to elicit them. Their resemblance to
| |
| gasps is often strilcing It seems probab1e in all the species which
| |
| have been studied (rat, rabbit, guinea pig, cat, sheep, goat and
| |
| man) that the motor pattern responsible for respiration is a very
| |
| fundamental one. It consists of the appropriate muscles with
| |
| their motor neurons. Respiratory acts manifest themselves under
| |
| experimental conditions as soon as the central nervous mechanism
| |
| for simultaneous1y discharging these groups of eiferent neurons
| |
| is developed. The period in fetal life at which this occurs is that
| |
| during which there is an extensive development of connecting
| |
| neurons of the reticular formation in the medu1la oblongata and
| |
| downward growth into the spinal cord of many longitudina1 nerve
| |
| übers. Ground bundles, reticu1ospinal and similar phylogenetically old pathways have been laid down. The appearance of
| |
| rhythmicity has not been correlated with any specific change in
| |
| the central nervous System. Growth there has gone so far at so
| |
| mm. that many of the structures characterizing the adult tegmens
| |
| tum have appeared. Multiplication of association neurons, and
| |
| consequently of possible connections in neuron circuits, seems to
| |
| characterize this stage in fetal brain growth.
| |
| | |
| A very ingenious theory concerned with neural control of
| |
| respiratory movements in the sheep fetus has been proposed.14 In
| |
| the Erst place, it was assumed that the rapid rhythmical move—
| |
| ments seen in 4o—day-old sheep fetuses delivered at experimental
| |
| Caesarean section are physiologically normal and that they result
| |
| from some poorly understood rhythmical (autochthonous?) discharge of the respiratory center. The progressive change in
| |
| character of these movements which is encountered during further
| |
| development of the fetus and which culminates in complete
| |
| apnea at about 6o days gestation, it was suggested, is due to the
| |
| progressive downgkowth of new inhibitory neurons from the
| |
| higher nervous centers. Asphyxia or experimental transection of
| |
| go PHYSIOLOGY oF THE FETUs
| |
| | |
| the brain-stem between midbrain and pons appeared to abolish
| |
| the inhibitory influences Jand leave a more primtive respiratory
| |
| pattern like that seen in fetuses 50 days old or less. Although it
| |
| is certain that all fetal activities change with the gradual maturation of nerve tracts in the brain, the experimental data do not
| |
| prove the theory proposed and alternative theories may be equally
| |
| well supported. It is quite possible that expiratory and pneumotaxic centers are dominant to the inspiratory center in fetal
| |
| life. Inhibition of the inspiratory center by impulses from the
| |
| pneumotaxic center or continual stimulation of the expiratory
| |
| center would account for the unexpanded chest and collapsed
| |
| lungs.49 Perhaps in the early sheep fetus the inspiratory center
| |
| has not yet come under the dominance of higher neurons.
| |
| | |
| The importance of muscle tonus in establishing conditions
| |
| favorable to lung ventilation at birth has been stressed by Some»
| |
| If the fetus in utero possessed the tonus of a healthy newborn it is
| |
| diflicult to see how it could iit itself to the space allowed and how
| |
| it could keep from iilling its lungs with fluid. In one experimentZI action potentials from electrodes which were placed in the
| |
| fetal muscles were demonstrated each time the specimen was
| |
| lifted out of its warm saline bath. These stopped when it was
| |
| returned to the bath (see Fig. 63, p. 176) . It seems probable that
| |
| the increase in stimulation of afkerent neurons attendant upon delivering the fetus from a warm aqueous habitat to the outside air
| |
| plays an important part in increasing the tonus of the muscles in
| |
| consequence of setting up activity in neuron circuits. This neural
| |
| activity may serve to lower the respiratory center’s threshold and
| |
| allow a lower level of carbon dioxide to stimulate it to action.
| |
| 0thers have found that the respiratory center of the fetal goat
| |
| four to six weeks from full term responds to afferent nerve stimulation like that of the adult, but it requires much stronger stimulation.30 It was inferred that the fetal center has a higher threshold.
| |
| By decerebration of the fetus it was demonstrated that the higher
| |
| threshold was not due to inhibitory influences from the cerebrum.
| |
| | |
| That the apnea of intrauterine life may be due to a physiologic
| |
| anemia of developmental origin has been suggestedPI Occlusion
| |
| of the umbilical arteries at birth is said to increase peripheral
| |
| resistance in the aorta, raise the systemic pressure and induce a
| |
| marked cerebral flow. This relieves the· anemia, the high carbon
| |
| FETAL RESPIRAToRY MovEMENTs 91
| |
| | |
| dioxide concentration prevailing acts as a respiratory stimulus
| |
| and respiration is initiated.
| |
| | |
| ASPIIRATION OF AMNIOTIC CONTENTS
| |
| | |
| In the early paragraphs of this chapter mention was made of
| |
| the suggestion that the fetus normally aspirates amniotic fluid.
| |
| Granting that respiratory movements can and do occur occa—
| |
| sional1y in utero toward the end of gestation, what is the evidence
| |
| that they cause aspiration of amniotic contents? It has been
| |
| pointed out that dyes injected into the amniotic sac can be found
| |
| in fetal lungs after removing the fetuses.8- IN« But in none of
| |
| these experiments was anoxemia rigidly ruled out of consideration. Indeed, in some it is quite obvious that it may have been
| |
| a factor in starting the respiratory activity. It is known that
| |
| vernix caseosa is sometimes found in the lungs of infants which
| |
| have survived birth a short time. In a large series of autopsies
| |
| with microscopic study Farber and sweetss found that only 15 per
| |
| cent of the lungs of infants surviving birth for five weelcs or less
| |
| contained signilicant amounts of döbris ascribable to fetal aspiration of amniotic contents, although 88 per cent showed at least a
| |
| few desquamated epithelial cells. It is doubtful if as high a per—
| |
| centage of healthy infants have to cope with birth condtions such
| |
| as were encountered in those living but a short time.
| |
| | |
| 0ther evidence concerning fetal aspiration has been presented
| |
| recently.9 Without using an anesthetic a thin hypodermic needle
| |
| was passed through the abdorninal wall and into the amniotic sacs
| |
| of guinea pig fetuses in the last weelc or two of gestation. small
| |
| quantities of fluid were withdrawn (o.4 cc. to 1 cc.) from the
| |
| region about the nostrils and replaced with colloidal solutions of
| |
| thorium dioxide (thorotrast) or hydroxide (thorad) . In 27
| |
| fetuses so created a large series of x-ray lilms exposed after various
| |
| intervals up to 14 days failed to show lung shadows which would
| |
| signify that the fetuses had aspirated this opaque material. The
| |
| thorotrast or thorad was observed in the stomach and intestines,
| |
| for swallowing occurred regularly.
| |
| | |
| Twenty—live additional experiments were performed. Films
| |
| exposed after these injections showed no aspiration on the part
| |
| of the fetuses. The pregnant guinea pigs were then allowed to
| |
| breathe atmospheres low in oxygen or high in carbon dioxide or
| |
| 92 PHYSIOLOGY OF THE. FETUS
| |
| | |
| to rebreathe air until an anoxemia was set up. Some of the fetuses
| |
| could be seen executing rhythmical movements resembling respiration. Roentgenograms were obtained subsequently. Of
| |
| 14 experiments showing shallow, questionable or no feta1 respiratory movements, only two films revealed thorotrast in the fetal
| |
| respiratory tract. ln the remaining 12, all showing strong movements of the type in question, seven positive results such as are
| |
| illustrated in Fig. 37 were obtained.
| |
| | |
| TH
| |
| ifJ »
| |
| o«
| |
| | |
| i - -: «.
| |
| i · -
| |
| i ·. « «. . s «· «-.--,-r
| |
| « H« «» d— · s,
| |
| «7’ G - Ist) « s s—- PI I« » - « - «· - «·-
| |
| Fig. 37.-Thor0trast (o.8 cc.) injected into the amniotic sacs of two guinea pig
| |
| ketuses on the 63rd day of gestatiom The tnother was rendered cyanotic with nitros
| |
| gen containing a small amount of oxygem Respiratory movements were observed
| |
| in fetus B more clearly than in fetus A. Half an hour later, the roentgenogram was
| |
| made. The bronchial tree of fetus B is well Hlled with thorotrast (arrows) .
| |
| | |
| (Windle, et al.: Sarg. Gyn. sc Obst» Vol. 69. 1939.)
| |
| | |
| In addition to these experiments, three other fetuses dying in
| |
| utero when the mothers died during diHicult labor or which were
| |
| otherwise asphyxiated at the time of birth showed lungs filled
| |
| with the thorotrast in consequence of liaviiig aspirated the amniotic contents (Fig«. 38) .
| |
| | |
| The literature contains a repQrt of a human fetus of the 6th
| |
| month in which an experiment similar to those in the guinea pigs
| |
| was performed. EhrhardtZS placed thorotrast in the amniotic
| |
| sac 15 hours before he performed an hysterectoniy in a mentally
| |
| FETAL REsPUzAToRY MovEMENTs 93
| |
| | |
| defective woman requiring sterilization. Roentgenograms of the
| |
| fetus talcen afterward showed that the thorotrast was concentrated
| |
| in the stomach and small intestine, but none was present in the
| |
| respiratory tract. A number of other observations in human
| |
| fetuses at term, following the use of strontium iodide according to
| |
| a method devised to outline the fetal Position, likewise failed to
| |
| show fetal 1ung shadows.57 However, this materia1 gives a very
| |
| weak visualization of fetal structures at best. 0ther experiments
| |
| | |
| ». .
| |
| . -.....-s-,·.
| |
| . ·(·
| |
| L ·
| |
| «« - «....4i
| |
| | |
| A« i i? B
| |
| | |
| Fig. 38.—Thorotrast («1 cc.) injected into the amniotic sacs on the Bist: day of
| |
| gestation without using anesthesia. Daily roentgenograms showed no lung shadows
| |
| Birth occurred on the 64th day. Fetus A was alive at birth. Fetus B died during
| |
| birthz its lungs became filled with thorotrast. (W’indle, et al.: Sarg. Gyn. 8c Obst»
| |
| | |
| Vol. 69, I939.)
| |
| | |
| employing thorotrast, which have been described recently, seem
| |
| to indicate that human fetuses of 4 to 6 months of gestation do
| |
| aspirate amniotic lluid in utero.58 The total number of experiments performed was not stated but one particularly impressive
| |
| one was described as illustrative of the series. In this instance (5
| |
| to 6 months) 25 cc. of thorotrast was injected into the amniotic
| |
| sac 48 hours before the pregnancy was to be terminated surgicallv
| |
| After Zo hours two films were exposed and these showed rather
| |
| 94 Pnystohoov oF THE: FETUs
| |
| | |
| indefinite shadows which were probably cast by the opaque material aspirated by the fetsus. After the operation had been per—
| |
| formed roentgenograms of the fetus showed very heavy lung
| |
| shadows. In these experiments one cannot be certain that physiologically normal conditions prevai1ed because no specific data were
| |
| given. The patients required surgical interruption of pregnancy,
| |
| but it is not known whether they were tuberculous, suffered from
| |
| cardiac disease, or from some uterine pathology which could have
| |
| disturbed the normal uterine physiology Ehrhardt’s one patient
| |
| was a mental defective and presumably had no pathology which
| |
| would embarrass respiratory exchange in the placenta; correlative1y he found no aspiration of amniotic contents. In the more
| |
| recent experiments there was no mention of preoperative medication and anesthetic.
| |
| | |
| The experimental studies in guinea pigs demonstrated that as—
| |
| piration of amniotic contents does not occur normally but that it
| |
| may be brought about under asphyxial conditions. Furthermorex
| |
| not all fetal movements which appear to be rhythmical and re·
| |
| semble respiration serve to bring about aspiration of amniotic
| |
| fluid. The recent human experiments whsich show aspiration in
| |
| utero are unimpressive and open to question because proof that
| |
| they represent the normal condition is laclcing. Although minor,
| |
| atonic respiratory movements may occur during prenata1 life.
| |
| there can be little doubt that the presence of vernix caseosa,
| |
| lanugo hair, etc. in the lungs of infants at birth is unphysiologic
| |
| and is associated with the deeper, dyspneic respiratory activity like
| |
| that encountered in fetuses suffering from rather severe anoxemia.
| |
| | |
| FETAL EIccIJP
| |
| | |
| The phenomenon of hiccup in utero was first described in
| |
| 188o by MermannID and has been observed infrequently by many
| |
| obstetricians since that time. DeLeeCO reported that hiccups can
| |
| be identiiied as early as the ftfth month. They are short quiclc
| |
| jerlcs of the shoulders and trunlc occurring about 15 to so times
| |
| a minute and are regular (rhythmica1P) , visible, audible and’palpable to the observer. In one instance, he declared, the hiccups
| |
| were heard just before birth and within a minute after birth the
| |
| infant was hiccuping so loudly it could be heard in the adjoining
| |
| | |
| 1·00m.
| |
| Frist-u. nssprtuxrotur MovEMENTs 95
| |
| | |
| In our experience with fetuses of cats and other animals fetal
| |
| hiccups have never been identiüed and to my lcnowledge no other
| |
| investigators have found them except in the human. However,
| |
| certain «jerlcy" movements of the head and trank, not distinctly
| |
| of a respiratory nature, may be comparab1e with the hiccups of
| |
| the human fetus.
| |
| | |
| SUMMARY
| |
| | |
| Consideration of the recent experiments in feta1 respiratory
| |
| movements leads to a more rationa1 conception of the subject than
| |
| was formerly held. The physiology of respiration in utero is not
| |
| so great1y different from that of the newborn or even of the adult
| |
| organism as it might seem.
| |
| | |
| Early in embryonic life, at a time which varies somewhat in
| |
| different species, but rough1y at about the 1o mm. stage, the part
| |
| of the somatic motor system which is to be concerned with breathing latet on has its genesis. soon higher neurons are formed and
| |
| brought into a mutual1y integrated aggregate to comprise a res—
| |
| piratory center connected with the somatic motor system. Ap—
| |
| propriate stimulation by neuronal afferent discharge into the
| |
| center, by the chemical agent carbon dioxide acting upon it
| |
| directly or by anoxial depression of its thresho1d can bring it into
| |
| activity prematurelyx but it is doubtful if this newly formed respiratory mechanism actually functions in the strictly normal
| |
| course of early intrauterine existence. The respiratory mech—
| |
| anism seems to be a dormant system charged with potentialities
| |
| long in advance of the time it can be of any use to the fetus.
| |
| | |
| This period of preparedness varies gkeatly in different animaIs.
| |
| It must be exceedingly brief in the marsupials. Fortydive per cent
| |
| of the gestation period passes in the cat before respiratory efforts
| |
| can be induced. The figures are about 3o per cent for man and
| |
| littIe more than 25 per cent for the sheep.
| |
| | |
| It is possible to remove a fetus from the uterus and leave its
| |
| circulation to the placenta intact, but it is impossib1e to maintain
| |
| physiologic conditions comparable to those in utero before the
| |
| experiment for very many minutes after this has been done. Partia1 anoxemia sets in promptly, the feta1 respiratory center thresh—
| |
| old drops and its neurons begin to discharge in response to the
| |
| usuaJ factors which are stimulatory in adult organisms.
| |
| | |
| Rhythms of shallow, rapid respiratory movements which are
| |
| 96 PHYsioLocY oF Tini: FETUS
| |
| | |
| occasionally encountered in human fetuses late in the gestation
| |
| period and which have been seen in a few laboratory animals at
| |
| infrequent intervals signify that the fetus is momentarily ex—
| |
| periencing either a depression of the threshold of its respiratory
| |
| center or an elevation of the carbon dioxide in blood going to the
| |
| brain. 0ne is justified in postulating a physiologic partial
| |
| anoxemia in late fetal life associated with progressive decline in
| |
| placental efliciency and with this, rninor rhythms of respiratory
| |
| movements. Nevertheless, even these minor rhythms of shallow,
| |
| rapid respiratory movements are not often encountered and when
| |
| seen should give cause for apprehensiveness on the part of the
| |
| obstetrician, for the danger of aspiration of amniotic contents
| |
| then may become real if the partial anoxemia becomes a true
| |
| asphyxia.
| |
| | |
| That aspiration is not commonly encountered depends upon
| |
| the fact that normally the fetus never sufkers a truly asphyxial
| |
| state before it is born. It seems probable that the blood which it
| |
| receives from the placenta is highly saturated with oxygen
| |
| throughout the greater part of life in utero. If the oxygen saturation drops into the neighborhood of 50 per cent it is to be ex—
| |
| pected that shallow, rapid movements of the respiratory muscles
| |
| will be inanifested. These are not accompanied by any changes in
| |
| intrathoracic pressure because the normal hypotonicity of fetal
| |
| muscles remains unchangedz consequently no aspiration results.
| |
| But should the oxygen saturation decrease marlcedly, numerous
| |
| motor neurons are activated, muscles tonus increases and dyspneic
| |
| gasping movements ensue. These bring about aspiration of
| |
| amniotic contents. 0ne can not doubt however that the ketus
| |
| possesses a wide margin of safety in respect to the danger of
| |
| «drowning" in its amniotic fluid.
| |
| | |
| REFERENCFS CITED
| |
| | |
| i. Preyer, W. i885. specielle Physiologie des Embryo» Grieben, Leipzig.
| |
| a. Windle, W. F» C. A. Dragstedy D. E. Murray s: R. R. Greene. i938.
| |
| surg. Gynec. Obst» 66: 987.
| |
| | |
| Z. Hartmary c. G. i92o. Anat. Ren, 192 Ist. «
| |
| | |
| 4. Ahlfeld, F. i888. Verhandl. deutsch. Gesellsch Gynälsp g: 2o3.
| |
| | |
| z. Weber, H. i888. Inaugural dissertatiom Marburg. (cited by Ahlfeld.)
| |
| s. Ahlfeld, F. i9o5. Monatschr. Geburtsh. Gynälc., ei: i43.
| |
| | |
| 7. Reiikerscheich K. i9i«i. pliiiger’s Arcli., i4o: i.
| |
| | |
| s. snyder. F. F. 8c M. Rosenfeld. 19377 J.A:M.A.« io8: i946.
| |
| MDO
| |
| s
| |
| Id Iiddd
| |
| VII— occ
| |
| IIICIIIIC VIII-III«
| |
| PEPSVTIP THE
| |
| | |
| . Minkowskh M. t9aa.
| |
| | |
| COCOOOCOOOOOOOOOOOIOIOIOIIV VII»
| |
| | |
| 97
| |
| t939. surg.
| |
| | |
| FETAL RESPIRATORY MOVEMENTS
| |
| | |
| Windle, W. F» R. F. Becher, E. E. Barth sc M. D. schulz
| |
| Gyn.« Obst» 69: 7o5.
| |
| corey, E. L.: t93a. J. Exp. Zool., 6t: t.
| |
| | |
| . cartttichaeh L. t934. Genetie Psychol. Monogr» t6: 337.
| |
| . snyder, F. F. sc M. Rosenkeld. t937. Am. J. Physiol» ttg: t53.
| |
| . Barcrokt, J. sc D. H. Barron. t936. J. Physiol» 88: 56.
| |
| | |
| Bat-Gott, J. sc D. H. Barron. t937. Ibid., 9t: sag.
| |
| | |
| Donat, B. E., c. M. Blutnenkeld sc c. Fenning. t938. Am. J. Dis. child»
| |
| 55: t. »
| |
| | |
| Brot«-n, T. G. tgt5. J. Physiol» 49: ao8.
| |
| | |
| coronios, J. D. t933. Genetie Psychol. Monogr» t4: asz
| |
| Windle, W. F» M. Monnier sc A. G. steele. t938. Physiol. Zool» tt: 4a5.
| |
| | |
| Erbkam. t837. Neue Ztschn Geburtsk» z: 3a4.
| |
| | |
| strassmanm P. t9o3. sammh Hin. Vortr» Gynälc» No. t3a: 947.
| |
| | |
| schweitzer med. Wchnschr» No. ag and so: 7at,
| |
| 75t.
| |
| | |
| Bolaflio, M. sc G. Artom. t9a4. Arch. d.i sei. Biol., Z: 457.
| |
| | |
| Walz, W. t9aa. Monatschn Geburtsh. Gynälc., 6o: 33t.
| |
| | |
| Bart-mit, J» D. H. Barron, K. Kramer sc G. A. Millikarp
| |
| Physiol» 9o: 29 P.
| |
| | |
| 1937- J—
| |
| | |
| . Abel, s. sc W. F. Windle. t939. Anat. Ren, Es: 45t.
| |
| . Bouceltz c. M. sc A. D. Renton.
| |
| . Rosenkeld, M. sc F. F. sitz-der.
| |
| | |
| t93t. surg. Gyn. Obst» Ha: 84t.
| |
| t939. Am. J. Obst. sc Gyn., 38: 4a4.
| |
| KoiK A. K. sc M. E. Das-is. t937. Ibid., 34: as.
| |
| | |
| cohnstein, J. sc N. Zuntz t884. Pkliigeks Arch., 34: t73.
| |
| | |
| . Huggett, A. St. G. t93o. J. Physiol» 69: t44.
| |
| . Barcroky J. t935. Irish J. Med. sci., series 73 t: a89.
| |
| | |
| Flint, A. t88o. Am. J. Med. sei» so: 69.
| |
| | |
| . salmi, T. t93Z. Acta soc. Med. Fennicae (ser. B, fast. t, art. a) , t8: t.
| |
| . Zuntz, N. t877. Plliigeks Arch» t4: 6o5.
| |
| . steele, A. G. sc W. F. Windle.
| |
| | |
| t939. J. Physiol» 94: Ist.
| |
| Barcrofh J. and M. F. Mason. t938. Ibid., 93: aa P.
| |
| Barcrokt, J» K. Kramer sc G. A. Millilcan. t939. Ibid» 94: 57t.
| |
| | |
| . Eastmary N. J» E. M. K. Geiling sc A. M. DeLawder. t933. Johns Hop
| |
| lcins Hosp. Bull., IF: a46.
| |
| | |
| . Eastman, N. J. t93o. Ibid» 47: aat.
| |
| | |
| . Bidone, M. t93t. Ann. di ostet. e ginec., 53: t97.
| |
| . Roos, J. sc c. Romijxx
| |
| . Windle, W. F. and Barcrokr. t938. Arn. Physiol» tat: 684.
| |
| | |
| . Windle, W. F» L. G. scbarpenbetg sc A. G. steele. t938. Ibid., tat: 69a.
| |
| . wiud1e, w. F. s« D. N
| |
| . Kuo, Z. Y. sc T. c. shet»t. t937. J. cornp. Psychol» a4: 4g.
| |
| . Rotnijn, c. sc J. Roos.
| |
| . Wind-le, W. F. t934.
| |
| . Windle, W. F» D. W. Ort· sc W. L. Minean t934. Physiol. Zool» 7: 6oo.
| |
| . Pitts. R. F» H. W. Magoun sc s. W. Ranson. t939. Am. J. Physiol» tas
| |
| t938. J. Physiol» 9a: a49.
| |
| elson. t"938. Ibid» tat: 7oo
| |
| t938. J. Physiol» 94: 365.
| |
| J! cotnp. Neur.,· 59: 487.
| |
| | |
| .
| |
| | |
| . Hendetsom Y. t938. Adventures in Respiratiotr. Williatns sc Willcins,
| |
| | |
| Baltitnore.
| |
| | |
| . Kraft-ca, J. t933. Am. J. Dis. child» 45: too7.
| |
| | |
| 7
| |
| 98 PHYsIoLooY OF THE FETUs
| |
| | |
| 52. Geyl, A. 188o. Arclx Gynäk., is: 385.
| |
| | |
| 53. Wis1ocki, G. B. 192o. Sankt. Brod» u: 47.
| |
| | |
| 54. Zwecke, E. 1938. Beitr: path..Anat. allgem. Path. wo: 515.
| |
| | |
| 55. Farben s. sc L. K. sweet. 1931. Am. J. Dis. child., 42: 1372.
| |
| | |
| 56. Ehxhardy K. 1937. Münclx mecL Wchnschr., 84: 1699.
| |
| | |
| 57. Mem-es, T. 0., J. D. Miller sc: L. E. Hollzn 193o. Am. J. Roemz Rad.
| |
| Ther., 24: 363.
| |
| | |
| 58. Reifkerscheict W. sc R. schmiemantx 1939. lenkt-kühl. Gynälk 63: 146.
| |
| | |
| 59. Mermanty A. 188o. ZentralbL Gynäk., 4: 377.
| |
| | |
| so. DeLee, J. B. 1938. The Principles and Practice ok 0bstetrics, saunders,
| |
| Phj1ade1phia.
| |
| CHAPTER VII
| |
| | |
| THE FETAL DIGESTIVE SYSTEM
| |
| | |
| As we find elsewhere in studying the nature of vital activities
| |
| before birth the new individual is prepared for postnatal functions
| |
| far in advance of the time of need. Nowhere is this better illustrated than in the digestive System. Motor activities associated
| |
| with obtaining and assimilating food are present in prematurely
| |
| delivered human infants and in late fetal life of many other animals. The digestive secretions appear to be supplied at this time
| |
| although information concerning them is meager. 0n the other
| |
| hand much has been learned about fetal swallowing and gastrointestinal movements in recent years.
| |
| | |
| FETAL SWALLOWING
| |
| | |
| That amniotic fluid is swallowed by the fetus and that it may
| |
| even serve some useful function during intrauterine life is not a
| |
| new idea. PreyerI summarized the view held in 1885 when he
| |
| wrote: «That amniotic fluid is a food for the fetus is certain but
| |
| if it is not swallowed plentifully it contributes little to nourishment beyond mere water feeding." swallowing was inferred be—
| |
| cause of the presence of squamous epithelial cel1s, lanugo hair and
| |
| vernix caseosa in the digestive tract before birth.
| |
| | |
| Preyer reviewed experiments which had been performed to
| |
| determine the source of amniotic Iiuid but which incidentally
| |
| provided some evidence of fetal swallowing. certain chemicals
| |
| administered by mouth or by injection into the maternal blood
| |
| stream appeared later in the amniotic fluid although not in the
| |
| fetal tissues. However, .urine samples col1ected from the infants
| |
| after birth often contained these substances. It was assumed that
| |
| the fetus had swallowed the amniotic fluid.2-3
| |
| | |
| Others have demonstrated that rabbit and dog fetuses swallow
| |
| | |
| actively and that absorption of the swallowed fluid takes place«
| |
| | |
| readily through the gastric and intestinal epitheliak Two or threex
| |
| hours after injecting calcium ferrocyanide into the amniotic
| |
| | |
| 99
| |
| 100 PHYSIOLOGY OF THE FETUs
| |
| | |
| cavity it was possible to induce the prussian blue reaction in all
| |
| the fetal tissues, especially in the stomach, intestines, lcidneys and
| |
| slcin. More recent1y injections of colloida1 dyes into the amniotic
| |
| cavity in guinea pigs and cats have demonstrated that absorption
| |
| talces place through the gastroäntestinal mucosa and respiratory
| |
| epithelium as well as through the amnion itselfJ Although one
| |
| must conclude that the fetuses did swallow the amniotic Huid it
| |
| was not proved that swallowing is a normal physiologic function
| |
| of the fetus. The experiments were conducted under general
| |
| anesthesia and under conditions which may have caused a.certain
| |
| amount of anoxemia in the fetuses which in turn could have induced the swallowing. « « ·« ,
| |
| | |
| An ingenious method for treating patients suffering from the
| |
| efkects of the distention of the abdomen attendant upon polyhydramnios has been described.8 The fetus was apparently induced to swallow more actively by« sweetening the amniotic Huid.
| |
| This was accomplished under local anesthesia by injecting saccharine solution directly through the maternal abdomen into the
| |
| amniotic cavity. Girth of the abdomen diminished, the disagreeable symptoms of the mother gradually subsided and normal in—
| |
| fants were borne by all but one patient. In this one instance in
| |
| which treatment had been ineffective the child was born alive but
| |
| a congenital atresia of the esophagus had prevented it from swallowing its amniotic Huid. Polyhydramnios associated with tera—
| |
| toma of the neclc which occluded the esophagus has been reported
| |
| by others.9
| |
| | |
| In the experimentsdescribed above saccharine was present in
| |
| the umbilical vein blood and in the first urine of the infants which
| |
| had swallowed it. Moreover, by catheterizing the mothers and
| |
| collecting urine samples regularly after injecting methylene blue
| |
| together with saccharine it was possible to obtain evidence that
| |
| the fetus swallowed intermittently. The patients reported an in—
| |
| | |
| creased incidence of fetal movements at times coinciding with
| |
| the appearance of dye in their urine. The conclusion reached
| |
| | |
| was that the child apparently sleeps for many hours in utero and,
| |
| becoming wakefuL begins to move and drinlc the sweet amniotic
| |
| | |
| Huid.
| |
| 0ther evidence of fetal swallowing in humans has been ob
| |
| tained by injecting materials impervious to Iorays into the amnioTHE FETAL DIGESTIVE sYsTEM 101
| |
| | |
| tic sac after withdrawing an amount of Huid equal to that
| |
| injected. The first investigatorsw to pursue this type of experimentation used solutions of strontium iodide which were swal—
| |
| lowed by the fetus near term. Others l1ave employed «diodrast.« U
| |
| Much more striking results have been obtained recently in specimens of Hve and six months’ gestation by means of «thorotrast."
| |
| U« 13 Very clear shadows of the fetal stomach were seen in recent
| |
| | |
| (
| |
| -»sp
| |
| | |
| - -« « - · P» - z» ·,-s-- . e——-«.-—-- -..
| |
| | |
| Fig. 39.—Pregnant guinea pig on the 48th day ok gestation Co1loida1·thoriun1 .
| |
| | |
| hydroxide (o.6 cc.) was injected into one amniotic sac on the 46th day; some of it:
| |
| has been swallosved and is in the stomach and intestines (Becker, et al.: Burg.
| |
| | |
| Gyn. 8c Obst» Vol. 70, 1940.)
| |
| | |
| roentgenograms of the uterus and its contents taken after hysterectomy 15 hours or more following« injections From the experiments employing direct injection into the amnion we may conclude that the human fetus can svvallow its amniotic fluid at least
| |
| as early as the 5th month. How much earlier is not known.
| |
| swallowing and gastrointestinal activity have been studied
| |
| | |
| from the developmental standpoint in the guinea pig.14 Colloidal
| |
| 102 PHYSIOLOGY OF THE FETUS
| |
| | |
| solutions of non-absorbabl»e and relatively inert thorium dioxide
| |
| (thorotrast) and thorium hydroxide (thorad) were injected
| |
| through the intact abdominal wall into the amniotic cavity in
| |
| small amounts (o.6 cc. to 1.o cc.) after withdrawing equivalent
| |
| volumes of amniotic iluid at various times during the gestation
| |
| period. No anesthesia was used, no surgical procedures were
| |
| necessary and it was therefore possibles to maintain practically
| |
| normal physiologic conditions. The guinea pig fetuses began to
| |
| swallow the thorium compounds mixed with amniotic tluid about
| |
| the forty-second day of gestation, for at that time the first stomach
| |
| shadows appeared in roentgenograms such a shadow can be
| |
| seen in Fig. 39, a roentgenogram talcen on the 48th day. The
| |
| rapidity with which the material reached the fetal stomach in—
| |
| creased with age, talcing about 36 hours at 42 days but only about
| |
| 2 hours in fetuses near term (66 to 67 days) . so much fluid was
| |
| drunlc that all the thorium dioxide or hydroxide was flushed out
| |
| of the amniotic cavity. It toolc nearly 41;H days for this to be accomplished in the early part of the Zrd quarter of fetal life but
| |
| only about 18 hours near term. Fetal swallowing seemed to be in—
| |
| creased when the mother guinea pigs were subjected to conditions
| |
| of anoxemia for brief intervals. That swallowing begins at about
| |
| the time the fetus is growing most rapidly was apparent. The
| |
| relation of this function to absorption and possibly to fetal water
| |
| utilization will be discussed latet.
| |
| | |
| FBTAL GASTRIC MOTILITY
| |
| | |
| Movement of the stomach musculature of human fetuses may
| |
| be implied from the presence of amniotic constituents in th·e intestines as early as the «4kh or zth month. F urthermore, several
| |
| investigators have observed gastric motility directly in mammalian
| |
| fetuses but the extent to which asphyxia occasioned by the ex—
| |
| perimental procedures induced or inlluenced the movements is
| |
| not lcnownJss I« Cat fetuses delivered with placental circulation
| |
| intact, but of course not functioning as eföciently as normally,
| |
| showed marked peristaltic movements of the stomach by about the
| |
| middle of prenatal life, at which time they were only 35 mm.
| |
| long.I7 After they had grown to 7o mm. the behavior of the
| |
| stomach appeared to be no different from that in unanesthetized
| |
| lcittens a day or two old (12o-1-4o mm.) . Peristalsis was very acTHE FBTAL DIGBsTlVB sYsTEM 103
| |
| | |
| tive and rhythmica1, began on the fundic side of the py1oric
| |
| antrum and spread over the pylorus. Emptying of the stomach
| |
| contents into the duodenum could be seen, especially when the
| |
| specimens were a1lowed to swallow a little air.
| |
| | |
| Xsray studies in guinea pigs have revealed gastric movements
| |
| in a few of the fetuses« Furthermore, it was found that stomach
| |
| emptying time decreased greatly between the 42nd day and the
| |
| time of birth. Experiments in incubating chiclcs substantiate these
| |
| observationsss By injecting lycopodium powder into the amnion
| |
| at various times and examining stomach and intestinal contents
| |
| 1ater, it was found that swallowing began about the gth day and
| |
| it toolc Io hours for the material to reach the intestines. At 12
| |
| days it required 8 hours and at 16 days, only 2 hours.
| |
| | |
| Rhythmical waves of movement directed toward the cardiac
| |
| end of the stomach have been observed in cat fetuses at hyster—
| |
| otomy on a number of occasionsss We have seen regurgitation of
| |
| stomach contents and even of bile colored iluid in cat fetuses during the last third of prenatal life. This seemed to be associated
| |
| with strong respiratory efforts and perhaps it was caused by com—
| |
| pression of the stomach by the abdominal muscle contractions.
| |
| | |
| Hunger contractions have been studied in newborn infants
| |
| and in puppies delivered 8 to 1o days prematurelyIY They were
| |
| found to be vigorous and more frequent than in the adult.
| |
| | |
| FISTAL LNTESTINAL MOVEMENTS
| |
| | |
| some of the earliest experiments in fetal intestinal activity are
| |
| those of Preyer, reviewed in his book in 1885. Between that time
| |
| and the present only one systematic developmental study has
| |
| been made. YanaseAs «« described intestinal movements which he
| |
| observed in freshly lcilled fetuses of the guinea pig and human.
| |
| He saw the earliest peristalsis in guinea pigs on about the 26th
| |
| or 27th day (19 mm. long) at which time the longitudinal muscle
| |
| of the intestine had been laid down. Earlier (15 mm.) only circular muscle was present -and he could elicit only local constrictions
| |
| by pinching and by faradic stimulation. Peristalsis could not be
| |
| observed in human fetuses before the iith weelc although longitudinal muscle and its nerves had been present since the 7th weelc.
| |
| Faradic stimulation elicited local contractions during the 6th
| |
| weelc when the circular layer alone was present. It was believed
| |
| 104 PHYSIOLOGY OF« THE FETUS
| |
| | |
| that the early intestinal movements were of neurogenic origin.
| |
| Yanase’s observations tell us little about what actually takes place
| |
| in the normal fetus in utero because he studied asphyxiated materiaL
| |
| | |
| We have examined cat fetuses delivered by hysterotomy with
| |
| placental circu1ation intact.17- 19 The cats had been decerebrated
| |
| previously by the anemia method and required no anesthetic dur
| |
| -. .-.«-—.--.-» ·—,-—...
| |
| | |
| Fig. 4o.—same guinea pig on the 57th day of gestatiom Most of the thorium
| |
| hydroxide has been swallowetL has passed out of the stomach and is concentrated
| |
| in the intestines. Intestinal constrictions indicating activity can be seen. (Becker,
| |
| | |
| et al.: Burg. Gyn. 8c 0bst., Vol. 7o, 194o.)
| |
| | |
| --:-----«-—-..-.
| |
| | |
| ing the experiments with their fett1ses. Local constrictions of the
| |
| intestinal wall were induced in embryos 18.5 mm. long (about 1
| |
| gram) by mechanical and faradic sti1nulation. Movements appeared spontaneously upon opening the abdomens of 25 to 35
| |
| mm. specimens (2-3 gms.) at about the middle of the gestation
| |
| period. There seemed to be no well established directional control of the early gut movements. Ikrequently waves of contracTHE FETAL DlGEsTlVE SYSTEM 105
| |
| | |
| tion progressed both orally and aborally from one point of constriction. sometimes a movement passed in one direction around
| |
| a loop of intestine and then reversed itself. strength of move—
| |
| ments increased rapidly after midfetal life and by 7o 1nn1. (18
| |
| grams) the behavior of the intestine Evas very much like that seen
| |
| in unanesthetized kittens a day or two old (1 1o—12o gms.) . Po—
| |
| larity became better established with advancement in age.
| |
| | |
| .o.«·.-- .- .....«-l
| |
| | |
| ». .--....
| |
| | |
| Fig. zip-same guinea pig on the day of birth. Defecation in amnio is occur—
| |
| rings; a pool of meconium is seen in the aniniotic sxic at the arrow. (Becl(er, et al.:
| |
| | |
| sur-g. Cyn. sc Obst» Vol. 7o, 1940.)
| |
| | |
| Intestinal peristalsis of the digestive type involved seginentap
| |
| tion and propagation of contents. In sotne instances spastic
| |
| rhythmical segmentatioti of the intestine replaced the« digestivse
| |
| type of n1ovement. All specimens vvere studied under conditions
| |
| involving a certain amount of anoxemia even though the placentas
| |
| were intact. It was found that cligestive peristalsis tended to
| |
| prevail when the unibilical vein blood contained on the averagje
| |
| 106 PHYsIoLooY or THE FETUS
| |
| | |
| 6.5 volumes of oxygen per 1oo cc. and that when this decreased
| |
| to about 2.1 volumes perscent the rhythmical segmentations were
| |
| more apt to occur. When the fetuses were deeply asphyxiated
| |
| some time after clamping the umbilical cord, tonus of intestinal
| |
| musculature diminished and agonal, pendulous writhing movements were manifested. similar changes in activity of the in—
| |
| testines were observed under profound asphyxia in lcittens one or
| |
| two days old.
| |
| | |
| It would appear from the roentgenologic study in the guinea
| |
| pig14 that intestinal peristalsis is a perfectly normal function of
| |
| the fetus, but its incidence before swallowing occurs has not been
| |
| proved in the intact animal. The rate of passage of material along
| |
| the fetal intestine increases with age. Near term it toolc only one—
| |
| jifth the time necessary -early in the third prenatal quarter for
| |
| material to pass from stomach to large intestine. The x-ray Iilms
| |
| demonstrated segmentations of the intestines very clearly and it
| |
| was possible to determine that they are more vigorous in late fetal
| |
| life than they are earlier. Movement of intestinal contents will
| |
| be seen in Figs. 4o and 41.
| |
| | |
| ABSORPTION IN« THE FETAL DIGEHTIVE TRACT
| |
| | |
| That absorption of chemicals and dyes can occur throughout
| |
| the gastrointestinal tract of mammalian fetuses was well estab—
| |
| lished by the experiments to which reference was made on
| |
| page 99.S- 7 It was proved by the x-ray studies of guinea pig fetuses
| |
| that large quantities of fluid from the amniotic sac are actually
| |
| absorbed under normal physiologic conditions34 Only a small
| |
| amount of thorotrast or thorad, usually less than o.8 cc., was added
| |
| to many times its volume of amniotic fluid with which it mixed
| |
| thoroughly. After having been swallowed this dilute solution be—
| |
| came concentrated in the stomach and intestines until it cast a
| |
| darlc shadow of these Organs. In roentgenograms of one human
| |
| experiment at 6 months of gestation,I2 it may be seen that a high
| |
| degree of concentration had been elfected in the stomach alone
| |
| in 15 hours time, for little material passed into the small intestine
| |
| and yet the amniotic sac was nearly clear. Otherss have suggested
| |
| that much of the absorption takes place in the fetal stomach and
| |
| our own experiments bear this out. Material traverses the
| |
| duodenum so rapidly that little absorption can occur there; but
| |
| THE« FETAL DIGESTIVE SYSTEM 107
| |
| | |
| other parts of the small and especially the large bowel retain their
| |
| contents for long periods during prenatal life. considerable absorption undoubtedly takes place in them.
| |
| | |
| The significance of fetal swallowing, gastrointestinal movements and absorption is not entirely c1ear. It has been suggested
| |
| however that these activities serve an important function in fetal
| |
| life. Some of the water may be of real use to the fetus. ReynoldsV
| |
| has shown that the volume of fetal Huid reaches a pealc at about
| |
| the 24th day of gestation in the rabbit and then drops olf rapidly
| |
| until birth, which occurs on the send day. At the time the iluids
| |
| begin to diminish precipitously the fetal growth curve is rising
| |
| rapidly. Concurrently, placental growth rate and efkiciency of
| |
| maternal blood llow through the placenta are declining. Thesö
| |
| facts suggest that some of the water needed for metabolism begins
| |
| to be talcen by mouth from the amniotic reservoir at this point
| |
| and that during further growth the fetus draws more and more
| |
| upon this source as it becomes less easy to get sufkicient fluids
| |
| from the placental blood stream. This concept was suggested by
| |
| Dr. Reynolds who, however, has pointed out that complete occlus
| |
| sion of the human fetal esophagus is not necessarily accompanied
| |
| by po1yhydramnios. There are not enough data on the guinea
| |
| pig24- 23 to allow accurate comparison with the rabbit. Fetal
| |
| growth does begin to increase rapidly about the time (42nd day)
| |
| swallowing can be demonstrated. Until approximately the 46th
| |
| or 48th days the volume of amniotic fluid increases steadily.
| |
| Thereafter conflicting data are encountered.
| |
| | |
| DEPECATION AND MECONIOPEAGY IN AMNIO
| |
| | |
| The contents of the fetal intestines are derived from the
| |
| amniotic fluid swallowed by the fetus. from various secretory
| |
| products and from desquamated epithelium, both external and
| |
| internal. They form a darlc greenish, viscid, odorless substance
| |
| passed from the bowel at birth, to which the name meconium
| |
| has been given. The green color is due largely to the presence
| |
| of bile pigments and has been reported to be laclcing in rare
| |
| cases of congenital obliteration of the bile ducts, meconium being
| |
| then light gray or yellow instead of green. Meconium is regularly
| |
| observed in the fifth month and we have seen it as early as the
| |
| fourth. It is of lighter color than in later months because
| |
| 108 Pnrsrohoor or« THE FETUs
| |
| | |
| biliverdin is not formed in significant amount before the sixth
| |
| monthks »«
| |
| | |
| concerning defecation in amnio there are few observations
| |
| save our recent ones. Some« hold that it occurs occasionally in
| |
| the human fetus before birth. Meconium is passed at birth or
| |
| soon thereaften but the amniotic kluid is usually said to be clear
| |
| except after prolonged labor or in cases of asphyxia neonatorum.
| |
| Some evidence has been presented that passage of meconium at
| |
| birth is due to an increase in the carbon dioxide tension consequent upon lowering of the blood pI—I and not to a simple accumulation of carbon dioxide.27 Perhaps it· may be related to the
| |
| estab1ishment of a general viscera1 motor hyperactivity lilce that
| |
| ·which closes the ductus arteriosus and starts splenic contractions.
| |
| Although active peristalsis was commonly seen in the large in—
| |
| testine of cat fetuses with placental circulation intact» the act of
| |
| defecation was never observed while the intestines were exp0sed.
| |
| The passage of meconium by intact cat fetuses has been seen in a
| |
| few instances. The amniotic Auid of many species of animals is
| |
| found to contain meconium after anesthesia has been used. It has
| |
| been observed at the time of hysterotomy under local anesthesia
| |
| in human fetuses of 4 months gestation.
| |
| | |
| Defecation in amnio is a normal physiologic activity in the
| |
| mature fetus of the guinea pig, occurring about a week before
| |
| term. Furthermore, the xsray studies demonstrate that meconium
| |
| mixes with the amniotic fluid and is swallowed by the fetus«
| |
| The same material passes through the gastrointestinal tract again
| |
| and again during the "last weelc of prenata1 life. Defecation in
| |
| amnio on the last day of gestation is i11ustrated in Fig. 41. Meconiophagy has not been« observed in man.
| |
| | |
| THE FETÄL DIGESTIVE GLANDSZ ENZYMES
| |
| | |
| The subject of functional activity in prenatal digestive glands
| |
| is poorly understood in spite of the fact that many investigators
| |
| have discussed it. For the most part experiments have been un—
| |
| controlled, methods inadequate and results discordant and confused. For a more complete review of the principal studies on
| |
| enzymes in the various portions of the gastrointestinal tract the
| |
| reader is referred e1sewhere.29
| |
| | |
| secretion of the salivary glands has been studied extensively
| |
| THE« FETAL DIGBSTIVE sYsTEM 109
| |
| | |
| at the time of birth, but little is known about their prenatal function. Ptyalin has been identitied as early as the fourth month in
| |
| the human fetus. Mucous glands develop later than serous and
| |
| contribute little secretion to the saliva of the newborn infant.
| |
| The nervous mechanism for salivary seeretion seems to be at
| |
| least partially in order at birth.
| |
| | |
| Laclc of agreement concerning presence or absence of hydros
| |
| chloric acid in the fetal stomach prevails in man as well as in other
| |
| mammals. The human gastric glands seem to be capable of forming acid about as soon as they diikerentiate in the fourth month.30
| |
| The presence of free acid has been denied even at the time of
| |
| birth.« spontaneous seeretion appears to be taking place in
| |
| guinea pig fetuses near terms« and elaboration of free acid seems
| |
| to occur in the stomach of rabbit fetuses, the quantity increasing
| |
| with ageks
| |
| | |
| several gastric ferments have been identiiied in the fetal
| |
| stomach of man and other animals. Pepsin may be present during
| |
| the fourth or iifth months, rennin appears a little later and
| |
| amylase has been found at birth. The question whether the fetal
| |
| stomach actually performs any chemical digestive function or not
| |
| has been debated. Some have held that the presence of protein
| |
| curds indicates activity and have made the suggestion that some
| |
| little nutriment may be gained from the contents of the amnion
| |
| which are swallowedJs T« «» s»
| |
| | |
| The fetal intestinal glands are said to secrete. Many enzymes
| |
| have been declared to be present by some investigators and to be
| |
| absent by others. Maltase, invertase, lactase, erepsin and trypsin
| |
| have been identiiied before the lifth month in man; rennin and
| |
| enterokinase are said to be present by the sixth month and others
| |
| at full term. The present state of our lcnowledge is too insecure
| |
| to justify anything like a complete consideration of intestinal
| |
| secretions here.29 However, there is little. doubt that the fetal
| |
| small intestines are prepared for some of the chemical as well as
| |
| the mechanical digestive processes well in advance of the time
| |
| they will be called upon to employ them. «
| |
| | |
| The large size of the fetal liver and the fact that it is the only
| |
| fetal organ which receives undiluted the highly oxygenated blood
| |
| from the umbilical vein suggests that it performs some important
| |
| functions before birth. Its hemopoietic role in early fetal life has
| |
| 110 PHYSIOLOGY OF THE. FBTUs
| |
| | |
| been mentioned in the chapter on fetal blood. Its relationship to
| |
| prenatal carbohydrate aisid lipid metabolism will be deferred to
| |
| the last chapter. It begins its secretory activity early. Between
| |
| the third and lifth months of gestation the human gall bladder
| |
| contains a thin mucoid material which is practically colorless, but
| |
| at about the lifth month the fetal bile begins to take on a yellow
| |
| appearance. The pigment bilirubin is·said to be present at that
| |
| time and formation of biliverdin is thought to start about a month
| |
| laterks During the last four months of fetal life the bile pigments
| |
| are produced extensively and the contents of the intestines be—
| |
| come deep green in consequence.
| |
| | |
| The production of bilirubin in the human fetus is related to
| |
| the commonly encountered pathologic condition known as icterus
| |
| neonatorum. The mechanism of formation of the pigment and its
| |
| escape into the blood stream t·o give rise to hyperbilirubinemia34
| |
| is not understood. Any thorough discussion of this subject
| |
| would lead us to questions of possible placental function in the
| |
| capacity of a «-uterine liver."95 Fetal iron metabolism will be
| |
| considered brielly in the last chapten
| |
| | |
| It is well known that the pancreas elaborates secretions in late
| |
| fetal life. In the calf its, proteolytic activity is slight szbefore the
| |
| üfth or sixth monthskss s« Nevertheless some have been able to
| |
| identify enzymes in the human fetal gland much earlier than this.
| |
| Trypsinogen, lipase and amylase are present near term and the
| |
| first two are said to have been detected as early as the fourth
| |
| month.29
| |
| | |
| REFERENQES cITED
| |
| | |
| . Preyer, W. i885. specielle Physiologie des Embryo» Grieben, Leipzig.
| |
| . Fehlingz H. i879. Arch. Gynäk., i4: sei.
| |
| | |
| . Zuntz N. i878. Piliigeks Arch., is: 548.
| |
| | |
| . Wiens-r, M. i88i. Arch. Gynäk., i7: 24.
| |
| | |
| Icrukenberz G. i884. Ibid., es: i.
| |
| | |
| . Wiens-r, M. i883. ZentralbL Gynäk., 7: 4o9.
| |
| | |
| . Wislockh G. B. i92o. contr. Emb., ii: 47.
| |
| | |
| De sn00- K· 1937- M0natschr. Geburt-h. Gynäk.. ioz: 88.
| |
| | |
| Wilson, J. se. G. i939. J. Obst. Gyn. Brit. Emp., 46: 44.
| |
| | |
| MERMIS« T— O» J. D. Miller 8c L. E. I-Iolly. i93o. Am. J. Roenh Rad.
| |
| | |
| Thier» 24: 363.
| |
| . case, J. T. i933. In A. H. curtis’ 0bstetrics and Gyneco1ogy, z: 762,
| |
| saunders Philadelphizh
| |
| . Ehrhardh K. i937. Mund-i. nied- Wchnschr., 84: i699.
| |
| | |
| Zcp you— Ietzt-do- «- s.
| |
| III
| |
| T
| |
| | |
| sM
| |
| UND-Ists- ItsZOFPCPPEIOM AS«
| |
| | |
| . Reitkerscheich W. sc R. schmjemann.
| |
| . Becher, R. F» W. F. Winde, E. E. Barth sc M. D. Schule. 194o. Sorg.
| |
| | |
| . Gundobin, N. P.
| |
| | |
| THE! FETAL DIGESTIVE sYsTEM 111
| |
| | |
| 19z9. 2entra1bl. Gynäk., 63: 146.
| |
| | |
| Gyn. Obst» 7o: 6o3.
| |
| | |
| . Tani, K. 1927. Jap. J. Obst. Gynsp to: e.
| |
| . Friedman, M. I-I. F. 1936. Proc. soc. Expetx Bio1. sc Med., 34: 495.
| |
| . Windle, W. F. sc C. L. Bishop.
| |
| | |
| 1939 Ibid., 4o: L.
| |
| | |
| Vrbitcly s. 1924. cotnpt. Rend. soc. Bio1., g« 6o4
| |
| Becken R. F. sc W. F. Wind1e. 194o. Unpublished
| |
| | |
| Car1son, A. J. sc H. Ginsburg. 1915. Am. J. Physiol-« 382 TO·
| |
| Yanase, J. 19o7. Pllügeks Arch., 117: 345.
| |
| | |
| Yanase, J. 19o7. Ibid., no: 451.
| |
| | |
| Reyno1ds, s. R. M. 1939. Physiology ok the Uterus- HOODOII N« YIbsen, I-I. L. 1928. J. Exp. Zool» Hi: Hi.
| |
| | |
| . Drapen R. L. 192o. Anat. Rec., is: z6g.
| |
| | |
| Feldman, W. M. 192o. Ante—nata1 and Posvnatal child Physiologzg
| |
| Longtnans, Green sc Co» London.
| |
| | |
| . Noguchh M. 1937. Jap. J. Obst. Gyn., so: 248.
| |
| | |
| Ibrahim, J. 19o9. Biochenx Zucht» es: 24.
| |
| | |
| Needhanx J. Igzn chemical Embryologzz catnbridge Und-s. Press.
| |
| 1912 Die Besonderheiten des Kinder-sahen. Allgemeine med. Verlag» Berlin.
| |
| | |
| . schmidh R. 1g14. Biochem Ztschr., 63: 287.
| |
| . sutherlanch G. F. 1921. Am. J. Physiol» 55: 3g8.
| |
| . Van Puteren, M. 188o. cited by Needhanh tgzh
| |
| | |
| Ylppö, A. 1913. Ztschxx Icinderh1k., g: 208 (1914, Obern. Abst., s: 941) .
| |
| | |
| . Schick, B. 1921. Ztschr. Kinderhllsp 27: est.
| |
| . Banting, F. G. sc c. I-I. Best. 1922. J. Lab. clin. Mec- 7t 464·
| |
| CHAPTER VII!
| |
| | |
| THE FETAL KDNEYS AND FLUDS THE FBTÄL SKIN
| |
| | |
| DEVELOPMENT OF· KIDNEY FVNCTION
| |
| | |
| DEvELoPMENT of renal function is related to the formation of
| |
| amniotic and allantoic fluids so closely that a consideration of one
| |
| invariably involves the other. The most casual observation in
| |
| fetuses of laboratory animals reveals the fact that a clear fluid
| |
| lills the bladder. This is known to be true in human fetuses during the kourth month. Analysis of the bladder contents later in
| |
| prenatal life demonstrates that the Auid is indeed a dilute
| |
| urine.1-3 An appreciable .amount of uric acid (1oo mg.) was
| |
| found in the human ketal kidneys at 7 months by one investi
| |
| Fig. 42.—Re1ation of the« bladder, amnion and allantois in the ketal Calf
| |
| gatorf Urea has been detected in human amniotic fluid as early
| |
| as LZ months gestationk We may infer from these observations
| |
| that the fetal lcidneys do function, at 1east to a limited extent,
| |
| well before they are required to perform all elimination.
| |
| Excretion of nitrogenous wastes is accomplished entirely by the
| |
| fetus in birds. A large allantoic sac is formed to receive the Urweconcentrate it and salvage the water which is essential to the fetus
| |
| for other metabolic processes The rigid economy of water en—
| |
| countered in birds is not necessary in« the mammals. The placenta
| |
| | |
| 112
| |
| FETAL KIDNIZYS AND FLUIDs. FETAL sxxkq 113
| |
| | |
| provides a mechanism for turning over the end-products of ketal
| |
| metabolism to the maternal blood and lcidneys in a11 the true
| |
| mammals. An allantois would seem to be unneeessary but one
| |
| korms neverthe1ess, and kunctions to a variahle extent. The
| |
| al1antois is vestigial in some mamma1s and man but is exceedingly
| |
| 1arge« in others, opening into the ketal bladder through the
| |
| urachus. The urethra communicates with the amniotic vesie1e.
| |
| Thus both allantoic and amniotic Huids can receive the fetal
| |
| urine. These relations are illustrated in Figs. 42 and 43.
| |
| | |
| THH
| |
| | |
| «« Ursexchrsa
| |
| Placenta
| |
| | |
| Fig. 43.-Relation ok the bladdeix amnion and vestigial allantejs jh the humeh kenn,
| |
| | |
| Although the placenta is the chiek excretory organ of mainmalian ketuses it seems probable that elimination through
| |
| it varies with the intimaqy of the ketal and maternal hlood strearnz
| |
| One should not expect the ketal waste products to pass through six
| |
| tissue layers in the epitheliochorial placenta of? the pig as readiiy
| |
| as through only three layers in the hemoschoriiil plaeenta of xnan
| |
| or the single membrane which is found in the herno-endothe1ia1
| |
| p1acenta of the higher rodents. Some believe that there is no
| |
| placental elimination at all in the pig, sheep and eat, anirnals in
| |
| | |
| 8
| |
| 114 PHYSlOLOGY OF THE. FETUS
| |
| | |
| which a large allantois is present« In general it appears to be true
| |
| that the species with the: simplest placentas are those with the
| |
| largest allantoic vesicles, and the mammals with highly developed
| |
| placentas in which the two bloods are more intimately related
| |
| | |
| have very small or vxstigial allantoic sacs.
| |
| Moreover it has been thought that the size of the embryonic
| |
| | |
| mesonephros is related to the intimacy .of the two bloods in the
| |
| placenta as well as to the development of an allantois. Bremers
| |
| found that the trophoblast epithelial layer of the higher placentas
| |
| becomes thin in certain places and covers the chorionic capillaries
| |
| lilce Bowman’s capsule invests adult renal glomerular capillaries.
| |
| He suggested that this formation of trophoblast plates takes place
| |
| when the mesonephric tubules cease to function and before the
| |
| true lcidney (metanephros) takes over excretion. He found no
| |
| trophoblast plates in species whose mesonephric and metanephric
| |
| function overlapped and concluded that in others the placenta
| |
| had been forced to adapt itself to more eliicient excretory activity
| |
| in order to bridge a gap in renal function. However, we shall
| |
| soon learn that mesonephros and metanephros actually overlap
| |
| functionally not only in lower mammals but in those with ·the
| |
| most eliicient placentas as well; the precise relationship just
| |
| postulated has been questioned.
| |
| | |
| PHYSIOLOGIO DEVELOPMENT OF« THE NEPERIC TUBVLES
| |
| | |
| There is no laclc of evidence that the placenta is the principal
| |
| excretory organ of the fetus. It can perform its duties in abnormal fetuses laclcing lcidneys and in specimens with congenital
| |
| occlusion of the urinary passagesJs S Nevertheless, the fact is
| |
| well established that both mesonephric and metanephric tubules
| |
| are capable of elimination very early in prenatal life» of the normal
| |
| fetus.
| |
| | |
| Among the older studies on fetal renal function there are a
| |
| number in which chemical solutions were injected into the mother
| |
| and sought in the fetal lcidneys or bladder. Preyers and NeedhamI have reviewed these. 1t may be said that placental permeability varies directly with the intimacy of contact between
| |
| maternal and fetal blood streams and is highest in the hemos
| |
| chorial and hemoendothelial placentas. The chem-icals used ex—
| |
| perimentally were recoverable in the fetal urine after introduc—
| |
| FETAL IclDNEYs AND FLUlDs· FETAL sIclN 115
| |
| | |
| tion int·o the maternal blood partly because their small molecular
| |
| size made them capable of passing the placental membranes and
| |
| the fetal nephric epithelium.
| |
| | |
| Attempts have been made to inject materials directly into the
| |
| fetus in order to determine their concentration in the fetal kidney
| |
| or bladder. A number of experiments in rabbits, cats, guinea pigs
| |
| and swine have contributed little specific information concerning
| |
| | |
| intrinsic functions of the nephronEIs chiclc embryos have been'
| |
| | |
| used more extensively than mammals, but it is well known of
| |
| course that their early nephrons do excrete, for there is no other
| |
| accessory mechanism like the placenta to function in elimination.
| |
| Experimental occlusion of the mesonephric (Wolfk1an) duct leads
| |
| to hydronephrosis as early as the fourth day of incubationU
| |
| because of pressure of the urine in the mesonephric tubules.
| |
| Indigo red solutions injected into the vascular systems of chiclc
| |
| embryos incubated five days and more« appeared in the lumen- of
| |
| the nephric tubules. Trypan blue has been used in the chick
| |
| by a number of investigatorsIS49 and it too made its appearance
| |
| in the mesonephric and metanephric tubules. Chambers and his
| |
| colleagues20s 21 have confirmed and extetided these earlier experiments. They found that elimination of phenol red begins in the
| |
| chiclc mesonephrons at about 41zs days of incubation. They grew
| |
| pieces of embryonic lcidneys in tissue cultures and used these
| |
| preparations to study activities of the nephrons. The proximal
| |
| convoluted tubule of both meso- and metanephrons passed an in—
| |
| dicator dye, phenol red, into the lumen and the distal portions of
| |
| the tubule resorbed waret. Some similar experiments have been
| |
| reported in the duclc.22
| |
| | |
| The most signilicant study of functional capabilities of the
| |
| mammalian mesonephric and metanephric tubules is that of
| |
| GershW who employed carefully controlled histochemical methods.
| |
| He used a nonstoxic solution of sodium ferrocyanide as an indicator of glomerular elimination and phenol red as a test for elimination in the proximal convoluted tubule. These chemicals
| |
| had been employed previously to study similar functions in adult
| |
| nephrons« Administration was effected by placental transmission in the rabbit, intravenously in the chiclc embryo· and by
| |
| direct injection into the fetal bodies (intraperitoneally?) of rabbits, cats, pigs and pouch—young opossums. Results indicate that
| |
| 116 PHYSIOLOCY oF THE FETUs
| |
| | |
| the ferrocyanide was eliminated with water into the glomerular
| |
| space from which it passed through the lumen of the remaining
| |
| portions of the tubules of both mesonephros and metanephros
| |
| just as in adult ltidneys. The phenol red was never found in the
| |
| glomerular space but did appear in the cells of the proximal part
| |
| of the tubule and the Iumen of the remaining portions in both
| |
| meso- and metanephros. The criticism may be raised that sonie
| |
| dye was passed by the glomerulus but in such a dilution that it
| |
| could not be detected histologically in the Bowman’s capsule.
| |
| Gersh however considered it doubtful if phenol red was eliminated at all by the glomerulus. Its presence within the cells of the
| |
| proximal tubules suggested secretion. Concentration of the test
| |
| substances was suAiciently marked to justify the conclusion that
| |
| urine was being formed in the mesonephric as well as the
| |
| metanephric tubules of the embryo. «
| |
| | |
| In all the species studied the mesonephros functioned in elimi—
| |
| nating ferrocyanide and phenol red for a shorter or longer time
| |
| after the metanephric tubules had talcen on the same functions.
| |
| Water resorption in the loop began later than did glomerular and
| |
| tubular eliminationks The high uric acid content encountered
| |
| in the fetal lcidney in one instance suggests that much of the
| |
| water dialysate together with urea and other soluble compounds
| |
| which enter the embryonic nephrons is resorbed before reaching
| |
| the ca1yces.4 Fetal nephric excretion appears to be a slow cons
| |
| tinuous process.
| |
| | |
| structural difkerentiation of the nephric tubule could be
| |
| correlated with onset "of elimination in Gersh’s study, but the
| |
| growth of new blood vessels along the tubules bore no re1ationship to it. In the glomeruli on the other hand no immediate
| |
| change in structure appeared to be related to onset of ferrocyanide
| |
| elimination which, it was suggested, must be due to «some extraglomerular or extrarenal factor such as change in blood pressure,
| |
| andXor osmotic pressure of the blood colloids or with a sub—
| |
| microscopic change in energy capacity or permeability of the
| |
| glomerular membrane." 23 The assumption of ability on the part
| |
| of the lcidney to do thermodynamic worlc is accompanied by increased oxygen consumption as measured by the Warburg
| |
| method«FETAL IcIDNEYs AND FLUIDS. FETAL sIcIN 117
| |
| | |
| That conditions in the human fetal nephrons are similar to
| |
| those in the other mammals seems certain. Histologically their
| |
| development is the same. 0n the basis of difkerential staining
| |
| HewerW conc1uded that an histologic change which may be associated with assumption of kunctional activity talces place in the
| |
| convoluted tubules at 12 weelcs and possib1y as early as 9 weelcs.
| |
| Gersh23 believes that the human metanephrons begin to eliminate
| |
| at the 32—mm. stage, which is about 9 weelcs fertilization age,27
| |
| and that mesonephric tubules are still functional at that time.
| |
| | |
| Cameron and ChambersW studied tissue cultures of bits of
| |
| human fetal kidneys at ZH months. They observed that the cut
| |
| ends of tubu1e segments healed over in the cultures, and that
| |
| phenol red and orange G dye solutions which were present in the
| |
| culture medium passed into and accumulated in the lumens of the
| |
| proximal tubules. Furthermore, it was determined that the fluid
| |
| accumulating in these tubules had a pH of approximately 7.o while
| |
| that of the culture medium was from 7.4 to 7.6. In the chiclc acidification of the cultures with carbon dioxide to a pH of 5.o failed
| |
| to prevent the secretion of phenol red.29
| |
| | |
| CONDITIONS RBGULATING RENAL PUNOTION
| |
| | |
| 0ne important deduction made from the experiments with
| |
| mammalian fetuses is that urine formation is remarlcably slow.23
| |
| As a matter of fact it might be considered remarlcable that urine
| |
| is formed at all in fetuses of some species, in which it has been
| |
| reported that a small gradient between arterial and venous blood
| |
| pressures exists. In cat fetuses at term arterial pressures of at
| |
| least so mm. and venous pressures somewhat less than 1o mm. of
| |
| mercury may be physiologic in the large vessels. Filtration in
| |
| the glomeruli must be carried out at a rather low pressure
| |
| especially in view of the fact that intraureteral pressure is appreci—
| |
| able.23 If these observations are correct osmotic pressure of the
| |
| fetal blood colloids must be low. No information is available in
| |
| the cat, but it has been found that the osmotic pressure in the dog
| |
| at birth (with an arterial pressure of« 4o mm. mercury) is low.3-0
| |
| | |
| The protein nitrogen of the dog’s blood at birth amounts to
| |
| 375 mg. per ioo cc. It increases, with a proportional risein blood
| |
| pressure, to 8oo or goo mg. in the adult. Each gram of the newborn dog’s plasma protein has only about »three-fourths the osmotic
| |
| 118 Pnvstohoov or THE FETUs
| |
| | |
| equivalent of each gram in the adult. The osmotic pressure of the
| |
| fetal blood has not been jneasured, but in the young puppy it is
| |
| 16.7 cm. of water when the plasma protein nitrogen concentra—
| |
| tion reaches 5 16 mg. per cent, rising to 37.7 cm. of water when the
| |
| concentration attains a value of 866 mg. per cent.
| |
| | |
| In contrast to these data from carnivorous mammals the
| |
| gradient between arterial and venous pressures is high in fetal
| |
| sheep near term (artery 75 to 8o mm.; vein 18 mm. mercury) ,
| |
| and there is some reason to believe that the osmotic pressure of the
| |
| fetal blood is considerably greater than in the dog. The serum
| |
| of the sheep fetus has a high proportion of albumin (62 per cent)
| |
| and a low proportion of globulin (38 per cent) , which would
| |
| make the blood protein have, gram for gram, a higher osmotic
| |
| pressure than that of the mother. But the total protein con—
| |
| tent is approximately only about iiveeighths that of the mother
| |
| per cc. of serumkIs Z« Consequently the osmotic pressure of the
| |
| blood plasma of the sheep fetus may be no higher, if as high, as
| |
| that of the mother.
| |
| | |
| In the human fetus arterial pressures as high as 1 1o mm. have
| |
| been recorded at delivery and the pressure in the veins is no
| |
| greater than 2o or 25 mm., perhaps much less under normal conditions within the uterus before labor starts (see Chapter II) .
| |
| This allows enough of a pressure gradient to account for filtration
| |
| in the fetal glomeruli without assuming a reduced osmotic pressure
| |
| of the fetal blood colloids. In adult man we know that systemic
| |
| arterial pressures greater than 75 mm. and intraureteral pressures
| |
| less than zo mm. of niercury are compatible with urine formation.33
| |
| | |
| In summarizing the present state of our lcnowledge concerning renal function in prenatal life the following observations appear to be of greatest signiiicancm Excretion begins early in the
| |
| true lcidney (9 weelcs in the human) and is continuous but sl»ow.
| |
| The mesonephros functions before the true lcidney (metanephros)
| |
| and for some time after the latter has talcen over urine production.
| |
| The fetal glomerulus (capillary tuft and Bowman’s capsule) gives
| |
| rise to a dialysate from the blood and the rapidity with which
| |
| this forms is related to fetal capillary pressure, osmotic pressure of
| |
| the fetal blood col1oids, carbon dioxide level of the fetal blood
| |
| and other factors. some substances may» be contributed to the
| |
| FETAL IcIDNBYs AND FLUIDs. FETAL sIcIN 119
| |
| | |
| glomerular Huid» by secretory activity of the cells of the proximal
| |
| convoluted tubules. shortly after elimination begins in the
| |
| glomerulus and proximal tubule, resorption of water and highly
| |
| soluble compounds occurs in the proximal tubules and the t.hin
| |
| segments of Henle’s loop in response to an altered osmotic balance
| |
| in the blood which has passed through the glomeruli. It seems
| |
| probable that elimination in the fetus can be explained large1y
| |
| on the basis of factors which bring about excretion in the adult.
| |
| although secretion of tubular epithelium may play a more important röle in the fetus than in the adult.
| |
| | |
| The placenta is the chief excretory organ of the human fetus,
| |
| but its importance appears to vary in other species with the degree
| |
| of intimacy of the fetal and maternal blood streams. An allantoic
| |
| vesicle provides a receptacle for fetal urine in some mammals,
| |
| notably those with the less highly developed types of placentas.
| |
| The amniotic vesicle can and does receive fetal urine during part
| |
| of prenatal life in all mammals.
| |
| | |
| cEANGEs IN ELIMINATION AT BIRTE
| |
| | |
| Provisions are made for rapidly altering many of the organism’s vital activities at the time of birth. changes are encountered in the circulatory and nervous systems and especially in the
| |
| respiratory mechanism where a sudden shift from placental to
| |
| lung breathing takes place. It is therefore not surprising to lind
| |
| dramatic structural and functional changes in the kidneys at the
| |
| time of birth.
| |
| | |
| High columnar epithelium invests the fetal renal glomerulus.
| |
| This, the visceral layer of Bowman’s capsule, forms a heavy sac
| |
| which coniines the glomerular tufts. Its comparatively low permeability must serve as an impediment to rapid glomerular elimination. The fetus not only does not need a mechanism for rapid
| |
| tiltration in its lcidneys, but such a mechanism might actually be
| |
| detrimentaL for it would seriously disturb the Huid balance in
| |
| utero. When placental elimination is suddenly abolished at
| |
| birth the columnar epithelial investments of the renal glomeruli
| |
| burst, allowing the glomerular vascular loops to expand and to
| |
| come directly into contact with the capsular space. Henceforth
| |
| glomerular liltration is greatly enhanced.34
| |
| 120 PHYSIOLOGY OF THE FETUS
| |
| | |
| THE FETAL VRINE
| |
| | |
| Although it has been Jdetermined that urine formation is slow
| |
| nothing is lcnown about the amount produced in any period of
| |
| time. It is said that the human fetal bladder capacity varies from
| |
| 4o to 7o cc. between the seventh month and birthFZ certainly the
| |
| amount contained within the bladder at any moment is no measure ok the total formed because the amniotic fluid receives an
| |
| indeterminable quantity.
| |
| | |
| Tut-s- 16
| |
| | |
| cktknzcsknntssktcs or· Hwzu Fmszh Unmi
| |
| At term At tertn At 6 months
| |
| Free-ins point («) . . . . . . . . . . . . . . . . —0.141 . . . . . -0.174
| |
| Total N . . . . . . . . . . . . . . . . . .gm. R, 0.043 0.041 0.061
| |
| NaCI . . . . . . . . . . . . . . . . . . ..gm. Cz, 0.1s6 04263 0.171
| |
| | |
| A few chemical analyses have been made on human fetal
| |
| urine. Makepeace and his colleagues2 examined samples collected at birth in two specimens and at six months gestation in
| |
| one other. Jacques analyzed the bladder urine of fetal sheep 36-—47
| |
| cm. long and compared the values with adult sheep urine and
| |
| other fetal Auids. Some of their data appear in Tables 161 and 172
| |
| | |
| TAVLD 17
| |
| | |
| cnknaosknntssktos or« Fhurvs IN Ins sang?
| |
| | |
| Adult Fetal Allantoie l Anmiotie
| |
| | |
| « urine urine l fluid tlutd
| |
| Free-sing point («) . . . . . . . . . »-1.959 -0.255 —0.«545 I -0.4,70
| |
| | |
| Protein . . . . . . . . . ..gm.7?s, 0.044 l 0.054
| |
| | |
| NQCI . . . . . . . . . . . »Hm. F, 022 0.17 0.16 l 0.64
| |
| | |
| Total ash . . . . . . . . .gm. N, 0.689 0.37 0.924. 0.84
| |
| | |
| Insol. ash . . . . . . . . .g«tn. N, 0.13 0.011 0.074 0.017
| |
| | |
| sptssh . . . . . . . . . ..g1n.9;, use) o.s4 J 0.85 0.82
| |
| I.
| |
| | |
|
| |
| | |
| That the contents of the bladder is urine rather than some
| |
| simple transudate may be assumed from the fact that it is isotonic
| |
| neither with the blood of the fetus nor with that of the motherz
| |
| it is low in sodium chloride. Jacquss data show some resemblance between fetal urine and the allantoic Huid of the sheep,
| |
| and it may be concluded that the latter is partly concentrated
| |
| FETAL KIDNEYS AND FLUIDS. FBTAL sIcIN 121
| |
| | |
| fetal urine. However, the feta1 fluids of the sheep do not contain
| |
| all the excreted waste products because it is probable that some
| |
| elimination takes place through the placenta. The freezing point
| |
| of the sheep urine decreases after birth; at the end of 36 hours
| |
| it was —1.o42" in one case, in contrast to —o.255«’ before birth.
| |
| In other words the hypotonicity of fetal urine in respect to blood
| |
| (fetal blood = —o.6230; maternalsheep blood = —o.5780) disappears after birth with the assumption of full elimination by
| |
| the lcidneyss
| |
| · THE Art-Aurora Frau)
| |
| | |
| Few additional facts concerning the allantois and its contents
| |
| need be mentioned. The vesicle develops as a ventral diverticulum of the embryonic bladder and maintains a patent connection with the bladder during the first half of fetal life or longer
| |
| by -means of a duct, the urachus. In those animals with well
| |
| developed allantois such as the sheep the amount of Huid contained
| |
| in it rises sharply in early prenatal life, then dec1ines and subsequently increases again toward the end of gestation. It has a
| |
| greater volume than the amniotic Huid in early prenatal life and
| |
| may have more toward the end of gestation in individual cases,
| |
| but the relationship does not hold throughout the middle of the
| |
| period when the volume of amniotic iluid overtalces it. NeedhamT
| |
| believes, on the basis of the data of a number of other investiga—
| |
| tors, that there is an exchange of- Huids between amnion and
| |
| allantois (the former giving up Huid to the latter) made possible
| |
| by the fac·t that the contents of allantoic and amniotic sacs are
| |
| separated by nothing more than a double membrane. The function of the allantois is most clearly indicated in birds where the
| |
| organ receives all nitrogenous wastes, concentrates and precipitates
| |
| the uric acid, and salvages the water for other uses.
| |
| | |
| L sTHE AMNIOTIC FLUID
| |
| | |
| The most important function performed by the amniotic fluid
| |
| is the provision of an aquatic environmenkfor the developing
| |
| embryo.30 Were it not for this it is doubtful if uniformly even
| |
| growth could take place because the very soft embryonic tissues
| |
| would be molded by pressure from the surrounding structures.
| |
| The Huid is said also to prevent embryonic adhesions. Protection from shoclcs and drying is provided by the Chorion, uterus and
| |
| 122 PHYSIOLOGY OF THE. FETUS
| |
| | |
| body wa11 more than by the amniotic fluid and the delicate membrane enclosing it. The «fluid-lilled amniotic sac acts as an hydraulic wedge for the descending fetal head at the time of birth
| |
| and helps malce fetal postural adjustments to birth possible.
| |
| | |
| The composition of amniotic fluid has been studied in man
| |
| and several other animals2s Z· E« 3749 but a detailed account of its
| |
| chemistry would be out of place here.I It has a specific gravity of
| |
| about hooögss in man and is defmitely hypotonic both to the
| |
| maternal and fetal blood, containing less sodium chloride and
| |
| other salts. Its urea and uric acid content gives a clue to the
| |
| origin in part. The quantity of these two substances increases
| |
| during prenatal life, as may be seen in Table 18.5 It follows that
| |
| the amniotic iluid receives a significant contribution from the
| |
| fetal lcidneys throughout most of the gestation period.
| |
| | |
| Takt-s 18
| |
| | |
| casnsortgnxswrcs or« Eos-tm Arnsirowro Fvmv «
| |
| | |
|
| |
| | |
| Month of Volume Free-sing oint Urea Urie acid
| |
| gestation (oe.) (" Cl) l Aug. W) (mg. W)
| |
| 2.5 . . . . . . . . . . . . . . .. 40 -0.520 - 84 sal4.5 .............. .. 140 -o.51s l 88 4.o
| |
| 7.5 . . . . . . . . . . . . . . . . I,050 -0.482 40 H .5
| |
| I0.0 . . . . . . . . . . . . . . .. I,800 -0.467 44 5.1
| |
| | |
| This brings us to the still unsettled controversy regarding
| |
| the source and method of formation of amniotic fluid. stated in
| |
| the simplest terms, two theories have developed: (a) that the fluid
| |
| is a transudate or dialysate of the mother, and (b) that it is
| |
| formed entirely by the fetus, some investigators holding that it is
| |
| a secretory product of fetal kidneys and amniotic epithelium. A
| |
| detailed consideration of all information bearing upon the subject
| |
| can not be given here; and once more the reader must turn to
| |
| more extensive reviews.1
| |
| | |
| In the sheep, an animal with a large allantois as well "as
| |
| amnion, it has been found that the fetal urine is passed from the
| |
| bladder through the urachus to the allantois (see Fig. 42) up to
| |
| a little past the middle of the gestation period.3 In late fetal life
| |
| however the urethra transmits urine into the amnion and the
| |
| urachus ceases to supply it to the allantois. A mid-interval exists
| |
| during which both vesicles receive the fetal urine. «
| |
| FETAL lcIDNBYs AND FLUIDs. FBTAL sIcIN 123
| |
| | |
| 0ne of the strongest arguments favoring the theory of fetal
| |
| origin of amniotic fluid is based on experiments of Watson40
| |
| who found that rabbit fetuses died when the amniotic fluid was
| |
| withdrawn from the pregnant does, but the maternal part of the
| |
| placenta continued to grow and was vascularized normally. There
| |
| was no regeneration of the amniotic fluid. 0ther evidence for
| |
| at Ieast a partial fetal origin is of course the proved fact of fetal
| |
| renal activity and the presence of an open pathway from the
| |
| lcidneys to the amniotic space. The presence of fructose and of
| |
| certain proteins in fetal urine of some species of animals and in
| |
| the amniotic and allantoic Huids of the same but not in others
| |
| adds weight to the theory of fetal originJU
| |
| | |
| It has been pointed out that the chorion of the cat and the
| |
| vitelline membrane of the rabbit are vascularized by fetal vessels
| |
| which are interposed between the fetal Auids and Uterus. « Further—
| |
| more, the cat’s endometrial epithelium is rather thoroughly re—
| |
| stored during the last half of pregnancy except at the placental
| |
| site. These facts make it seem unlilcely that fetal fluids can arise
| |
| as a transudate from the mother’s endometrium to the amniotic
| |
| sac during the last half of gestation in the cat and rabbit.
| |
| | |
| some interesting experiments leading to production of poly—
| |
| hydramnios in rabbits provide rather convincing evidence that the
| |
| amniotic fluid is formed by fetal structures.42- 43 After double
| |
| nephrectomy of pregnant rabbits it was found that a signilicant
| |
| increase in amniotic fluid volurne occurred during the latter part
| |
| of the gestation period when the fluid volume is normally decreased. No edema, ascites or other transudate was encountered
| |
| in the mother’s body. Average data from this study are presented
| |
| | |
| in Table 19.42
| |
| Tand-z 19
| |
| Ast-may Vor-Fuss or« Awrortc Hort) m Rai-ans
| |
| | |
|
| |
|
| |
| | |
| Ave. Ist. ketus cotitrols Nephrectomized
| |
| | |
| (gms.) (cc.) (cc.)
| |
| Lessthanlc . . . . . . . . . . . . . . . . . . . . . . . . .. 24
| |
| | |
| 10-20 . . . . . . . . . . . . . . . . . . . . . . 4.9 4.1
| |
| | |
| 20430 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. 3.8 4.8
| |
| | |
| Z0-40 . . . . . . . . . . . . . . . . . . . . . . . . »« . . . . .. I.7 5»6
| |
| | |
| 4o-5o . . . . . . . . . . . . . . . . . »» .......... .. o s 1o.75
| |
| 124 Pnvsrohoov or THE FETUs
| |
| | |
| Injection of large amounts of saline so1ution into nonnephrectomized pregnants animals leads to formation of transu—
| |
| dates in the mother’s body« cavities without increasing · the
| |
| amount of amniotic fluid.44
| |
| | |
| Although the strongest evidence favors the concept that
| |
| amniotic Huid is formed by fetal structures it does not prove that
| |
| this is its only source in all species. It is certainly not formed
| |
| entirely by the fetal kidneys. Early in embryonic life before renal
| |
| function becomes established we do not lcnow its origin. The
| |
| amniotic epithelium and blood vessels of the embryo have been
| |
| suggested The possibility of maternal origin can not be dismissed entire1y. More than 2oo cc. may be present in the pig
| |
| when the fetus is only 30 mm. longss certain chemical compounds, enzymes and antibodies present in the mother’s body
| |
| appear in the placenta and amniotic fluid but not in the fetal
| |
| tissues themselvess
| |
| The quantity of amniotic fluid increases sharply during the
| |
| early part of prenatal life in all species of animals. In some, such
| |
| as the cat and« guinea pig, the rise has been found to continue to
| |
| full term, but with considerable individual variations« In
| |
| others, probably man and the sheep, it attains a maximum some
| |
| time before the end of gestation and this volume is maintained
| |
| until term. A remarlcable diminution in the quantity of amniotic
| |
| fluid is encountered in the latter part of prenatal life of the rabbit. A careful comparative study of the minute volume of blood
| |
| flow in the uterus correlated with the quantity of fetal fluids at
| |
| different ages may help explain species differences. The gTeat
| |
| individual and species variations in volume of amniotic f1uid
| |
| seen during late pregnancy may be related in part to the
| |
| phenomenon of fetal swallowingkss It has been reported« that
| |
| polyhydramnios in human subj ects can be reduced by stimulating
| |
| | |
| swallowing by the fetus.
| |
| | |
| THE FETAL SKIN
| |
| | |
| Little can be said regarding the physiology of the fetal slcin
| |
| and its, associated structures. The sweat g1ands are present and
| |
| have developed lumens by the seventh month but it is questionable whether they actually secrete in -utero. The sebaceous glands
| |
| | |
| do function before birth, adding their oily secretion to desqua—
| |
| FETAL KIDNEYS AND FLUIDS. FETAL sIcIN 125
| |
| | |
| mated epithelium and lanugo hair to form the vernix caseosa
| |
| which covers the fetus. It is usually said that this material serves
| |
| to protect the living epithelial cells from becoming macerated in
| |
| the amniotic Huid, a statement for which there does not appear to
| |
| be the least justiftcation. 0thers have held on the basis of unsatisfactory evidence that the vernix caseosa is a deposit upon
| |
| the slcin from lipids excreted by the amnion.48 The vernix is
| |
| usually removed for esthetic reasons at the time of birth, but
| |
| when allowed to remain it has been found that it will disappear
| |
| of its own accord in about 8 hoursfs probably by absorption during drying and corniftcation of the outer epithelial layer.
| |
| | |
| 0ther cutaneous glands are capable of functional activity at
| |
| birth. A most curious transient phenomenon is encountered
| |
| in the mammary glands (see Chapter XIV). A small quantity
| |
| of secretion is often observed in both sexes and to this the name
| |
| «witch milk" has been applied. Lacrimal glands are well formed
| |
| in prenatal life but apparently they do not function. The newborn child is said to cry without tears. ·
| |
| | |
| skin pigmentation is deftcient at birth even though melanin
| |
| production starts early elsewherez pigment granules start to form
| |
| in the optic cup of the 7 mm. human embryo. Melanin appears
| |
| to be manufactured by the fetal tissues with the aid of an oxydase.
| |
| Although pigment is not found in the hair primordia of human
| |
| fetuses until the ftfth month and is not present in the epidermal
| |
| cells before the sixth, the enzyme is there earlier and can pro—
| |
| duce pigrnent when the dopa reagent (dihydroxyphenyla1anine)
| |
| is added experimentallyåo The developmental chemistry of the
| |
| skin should be an interesting and prolitable study.
| |
| | |
| The fetal skin can have no importance from the standpoint of
| |
| heat regulation in utero, but it is of interest to know whether
| |
| or not this function is present at birth. For more than a century
| |
| it has been known that the ofkspring of some animals are incap—
| |
| able of maintaining their birth temperature when removed from
| |
| a warm environmentPo Young rabbits and kittens acquire ability
| |
| to fully regulate their temperatures ·a"bout 15 days after birth.
| |
| 0ther animals, such as the guinea pig, are born with a good coat
| |
| of hair and possess a well formed heat regulatory mechanism at
| |
| that time. similar species difkerences are encountered in birds,
| |
| with the chick of the domestic fowl falling into the class of the
| |
| 126
| |
| | |
| PHYSIOLOGY OF THE! FETUS
| |
| | |
| guinea pigPIs 52 Many anima1s can compensate for a drop in
| |
| temperature by increasing their metaboIism but are incapable
| |
| ok meeting the conditions imposed by a high tempctatuke III the
| |
| external environmentz the rabbit, cat and man are in this class.
| |
| 0thers, such as the mouse, possess no form ok heilt kegulation
| |
| while still others, the guinea pig being an examplh alt« com—
| |
| pletely homothermic at birthFs
| |
| | |
| . Needhaxxk J.
| |
| . Malcepeacq A. W., F. Fremontsmith M. E. Dailey Z: M. P. C3UV1I«
| |
| | |
| . saridstrorry C. J.
| |
| . Gersh, I.
| |
| . Gersh, I.
| |
| . Flexner, L. B. E I. Gersli. i9s7. Contr. Emb., 26: ieiä
| |
| | |
| . Heu-er, E. E. i924. Quart. J. Exp. Physiol., i4: 49.
| |
| | |
| . Altschu1e, M. D. i9so. Anat. Rec., 46: 8i.»
| |
| | |
| . cameron, G. E R. chambers i9s8. Am. J. Physiol» ins: 482.
| |
| | |
| . chambers, R. E R. T. Icemptoix i9ss. J. Cel1. comp. Physiol» s: i si.
| |
| . Stark, G. A. E I-I. E. I-Io1ling. i9si. J. Physiol» 7s: so5.
| |
| | |
| . Mccarthy, E. F. i9s8. Ibid., 9s: 8i.
| |
| | |
| . I-Iowe, P. E. ig25. Physiol. Reis» z: 4s9.
| |
| | |
| . smith, I-I. W. i9s7. The Physiology ok the Icidney, Oxford Univ. Press.
| |
| | |
| REFERENCES CITED
| |
| i9si. Chemical Embryology, Cambridge Und« Ptess.
| |
| | |
| i9si. Sarg. Gyn. E 0bst., 5s: 6s5.
| |
| | |
| Jacque, L. i9o6. Art-h. Internat. Physiol» s: 46s.
| |
| | |
| Leu-is, J. I-I. i9i6. J. Bio1. Chem., 24: 249.
| |
| | |
| Guthinanm H. E W. May. i9so. Arch. Gynä1(., i4it 450.
| |
| Bremer, J. L. i9i6. Arn. Anat., i9: i79.
| |
| | |
| Engliseh, i88i. Arch. Icinderhllk L: 85.
| |
| | |
| . Preyek W. i885. speeielle Physiologie des Embryo. Gliedert- Leipzig.
| |
| . Bat, P. i88i. , Recherches pour servir a Phistoire de khydkamnidss Paris«
| |
| | |
| (cited by I. Gersh, i9s7.)
| |
| Fritscheltz F. i928. Ztschr. mi1c.—anat. Forsch., is: öl
| |
| . Firlr.et, J. i92o. Comph Rend. soc. Biol., 8s: i2so. Wisloclci, G. B. i92i. Johns Hoplcins I-Iosp. Bul1., se: 9s.
| |
| . Franlcenbergen Z. i92i.
| |
| | |
| Rozpravy Ceslce Alcademiep 302 47« (Cited by
| |
| | |
| I. Gersh, i9s7.)
| |
| | |
| . Boyden, E. A. i924. J. Exp. Zool» 4o: 4s7.
| |
| . Balcounine, s. i895. Arch. Ital. Biol., es: s5o.
| |
| . Zaretslcy, s. i9io. Virchows Art-h» 2oi: as.
| |
| . AtwelL W. E E. B. I-Ianan.
| |
| . I-Iurd, M. C. i928. Am. J. Anat., 42: i 55.
| |
| | |
| i926. Anat. Rec., se: suppL ges.
| |
| | |
| Wisloclch G. B. i92i. Anat. Rec., ge: 267.
| |
| | |
| chambers, R. E G. camerom i9s2. J. cell. comp. Physiol» 22 99chambers, R., L. V. Beclc E M. Bellcin. i9s5. Ibid.- S: 425.
| |
| | |
| i9s5. Anat. Rec., Sie: 7.
| |
| | |
| i9s7. contn Emb., 26: ss.
| |
| | |
| i9s4. Am. Physiol» io8: s55.
| |
| | |
| Gruenwald, P. E I-I. Popper. i94o. J. Urol., 4s: 45Z.
| |
| Feldman, W. M. i92o. Anteqiatal and Posvnatal child Physi0l0gyLongmans, Green, N. Y.
| |
| . Ginglingen A. sc. c. Ray-set.
| |
| | |
| FYEITAL KIDNBYS AND FLUIDs. FETAL sIcIN 127
| |
| | |
| . Mossman, H. W. 1937. contra Kind» as: i29.
| |
| . Döderlein, A. i89o. Arclr. Gynälc., 37: i41.
| |
| . Uyeno, D. 19i9. J. Biol. chern., 37: 77.
| |
| | |
| cantarom A» H. stuckert sc R. c. Das-is. i933. Sarg» Gyn. se Obst»
| |
| 57- Sz
| |
| . Watson, B. P. i9o6. J. Obst. Gyn. Brit. Emp., g: is.
| |
| . Paton, D. H» B. P. Watson sc J. Kett.
| |
| | |
| i9o7. Trans. Roy. soc. Edin.,
| |
| | |
| 46: 7i.
| |
| | |
| . Wolih B. 19o4. Arch. Gynälc., 71: 224.
| |
| . Wolfh B. 19o9. Ibid., 89: i77.
| |
| | |
| . Bakounine, s. 19oo. Atti d. r. Accad. med.-chik., Napoli, 54: i. (cited
| |
| by Needham, i931.")
| |
| | |
| . Wislocki, G. B. 1935. Anat. Rec., Cz: 183.
| |
| | |
| . Becken R. F» W. F. Windle, E. E. Barth sc. M. D. Schuh. i94o. Sarg.
| |
| | |
| Gyn. s: Obst» 7o: sog.
| |
| | |
| . De snoo, K. i937. Monatschtu Gebuktsh. Gynälsp los: As.
| |
| | |
| . Iceiiketz H. i926. Gynee et Obstet., 14: I.
| |
| | |
| . Bloclh B! i921. Arch. f. DermatoL syphilis, i35: 77.
| |
| | |
| . Edwakdsh F. 1824. Traite de Pinlluence des agents physiques sur la vie,
| |
| | |
| Paris. (Cited by M. s. Pembrezs 1895.)
| |
| | |
| . Pembkezs M. s. i895. J. Physiol» is: 363.
| |
| . Giaja,
| |
| | |
| 1925. Amt. d. Physiol. et Phys.-chem. Biolsp i: 628.
| |
| by Needham, i93i.)
| |
| | |
| (cited
| |
| | |
| 1929. compt Rend. soc. Biol., tot: 71 I.
| |
| CHAPTER IX
| |
| | |
| THE FETAL MUscLEs
| |
| | |
| TIssIJE CULTURBS AND INLVITZO EXPERIMENTS
| |
| | |
| HISToGENIJSIS of muscle tissue has been carefully investigatedI
| |
| and a few observations have been made from the standpoint of
| |
| functionx Although it is diHicult to make physiologic studies on
| |
| the earliest embryonal muscles in vivo, it has been possible to obtain growth from small pieces of muscle in tissue culture and to
| |
| study the behavior of new cells in this altered environment.
| |
| Thus, valuable information concerning the activity of very young
| |
| cells of smooth, cardiac and slceletal muscle has been gained.
| |
| | |
| At one time discussion centered about the question whether or
| |
| not muscle tissue can contract in the absence of nerve iibers supplying it. We now know that it can, and it does so under normal
| |
| physiologic conditions in some locations. For example the
| |
| amnion of the chiclc embryo contains sheets of smooth muscle
| |
| in which nerve fibers have never been demonstrated? This tissue
| |
| functions actively to churn the embryo about in a rhythmical
| |
| fashion. Then too, the embryonic heart begins to beat well in
| |
| advance of the time nerves grow into it. Tissue cultures give
| |
| conclusive proof that nerves are not essential. Recent histologic studies of amphibian and mammalian heart primordia -have
| |
| demonstrated that specialized cytologic structures, iibri11ae, and
| |
| cross striations, are not formed until after contractility has become well establishedPs 4
| |
| | |
| Nerve-free cultures of both cardiac muscle· and amniotic
| |
| smooth muscle from chiclc embryos demonstrate spontaneous
| |
| rhythmical contraction in isolated cells as well as in groups
| |
| of cells.5- C« simi1arly, pieces from the legs of 4 to 1o day chiclcs,
| |
| grown in Lewis-Loclce so1ution, contain functional myoblasts
| |
| and young slceletal muscle übers? All these young isolated elements do not contract at any one time and the period of the
| |
| rhythm varies in different ones, some slceletal iibers contracting as
| |
| often as 120 times a minute, some in slower rhythms and others
| |
| only once in one to ten minutess
| |
| | |
| 128
| |
| THE FETAL Musen-Es 129
| |
| | |
| Interesting difkerences have been observed in the nature of
| |
| the contractions of smooth, cardiac and skeletal muscle cells in tissue cultures. Individual contractions of amniotic smooth muscle
| |
| cells are slow and seem to be produced by currents of protoplasm
| |
| flowing toward a center of active change in the cell. This results
| |
| in a 1ocal pi1ing up of the protoplasm at the center of active
| |
| change. In embryonic cardiac muscle cells the contractions appear as quiclc rhythmical beats, the cell bellying out at its center
| |
| with each beat. Individual cells as well as groups showed this
| |
| phenomenon. contractions of skeletal muscle Hbers and myoblasts resemble straight twitches rather than the flowing and
| |
| beating movements of the smooth and cardiac e1ements. In no
| |
| type of muscle could myotibrils be demonstrated in the living
| |
| cellsk Recent studies have shown that it is impossible to relate
| |
| the genesis of contractility in rat embryonic slceletal muscle in
| |
| vivo to any intrinsic structural change in the cell.8
| |
| | |
| The cause of the spontaneous contractions of embryonic muscle cells and myoblasts in cultures is not known, but autochthonicity is not necessarily assumed. Many cells are inactive while one
| |
| contracts. Activity may depend upon some change in the tissue it—
| |
| self or in the surrounding medium. A certain Optimum balance
| |
| of sodium, potassium and calcium ions (Lewis-Locke solution)
| |
| has proved to be favorable. slight changes in this ionic balance
| |
| and dilution of the medium sometimes stimulates an increase in
| |
| activity for»a brief period but soon leads to degeneration of the
| |
| growth.
| |
| | |
| In the amniotic smooth muscle a laclc of oxygen or an accumulation of carbon dioxide appea«red to bring about cessation of
| |
| spontaneous rhythmical activity in vitrok This seems to be
| |
| equally true of adult smooth muscle, although some believe that
| |
| carbon dioxide may act as a stimulus to rhythmical spontaneous
| |
| activity.9- I« In the intact egg of the chick the amnion becomes
| |
| progressively more active during the course of incubation until
| |
| about the izth day, after which contractions are less frequent.
| |
| From analyses of the atmosphere within the air—space of the incubating eggII we know that cessation of amniotic activity coincides with the time a physioIogic partial anoxemia becomes well
| |
| established.
| |
| 130 PIIYSIOLOGY OF THE« FETUS
| |
| | |
| SPONTANEOUS AOTIVITY OF INTÄCT SKELETAL MUscLE
| |
| | |
| skeletal muscle Ebers and myob1asts begin to develop in 1 1
| |
| mm. to 12 mm. cat embryos«about 23 days after insemination.
| |
| 0ne should expect to encounter functional activity at that time
| |
| and, to be sure, faradic stimulation with micro-electrodes does result in contractions.32 However, spontaneous movements have
| |
| never been observed in small embryos when studied with their placental circulation intact, and we may conclude that the early skeletal
| |
| muscle cells possess no autochthonicity in vivo. Furthermorek re—
| |
| mova1 of the specimens from the uterus with consequent asphyxia
| |
| does not bring about automatic muscular activity. Embryos only
| |
| a litt1e larger than these (13.5 mm.) do show spontaneous move—
| |
| ments of the fore1imbs under certain unnatura1 conditions. When
| |
| removed from the uterus they remain perfectly inactive so long
| |
| as the surrounding medium contains sodium, potassium and calcium ions in the proportion found in« Locike’s solution. When
| |
| an ionic unbalance is introduced by substituting solutions deficient in ca1cium or potassium or both, the forelimbs begin to
| |
| move rhythmically in a waving manner.I3-14-I5 In larger specimens the rhythmical movements occur first in the tail and limbs,
| |
| structures from which diffusion can take place most readily. We
| |
| may conclude that spontaneous contractility of new muscle übers
| |
| is stimulated by ionic unbalance in the young tissues. But
| |
| since nerve libers are already present in the intact specimens,
| |
| one does not know whether the muscle itself responds to direct
| |
| stimulation, whether the nervous elements are stimulated or
| |
| whether some elementary nervous inhibitory control over the
| |
| new muscle has been removed by the change of medium, thus al1owing the muscle übers to exhibit contractility which is their
| |
| inherent property and which never manifests itself in the presence of neu-es.
| |
| | |
| small mammalian embryos stand in marked contrast with those
| |
| of lower vertebrates in respect to the absence of observable contractions of intact skeletal muscles. some investigators have re—
| |
| ported muscle twitching and even rhythmical contractions before
| |
| external stimulation elicits responses of a reflex natura« ln some
| |
| experiments activity of the nervous centers seems to have been ex—
| |
| cluded, the movements being entirely aneural but occurring only
| |
| upon direct stimulation.17s IS- «
| |
| THE FETAL MUscLEs 131
| |
| | |
| FÄRADIC AND MECHÄNICAL STIMULÄTION
| |
| | |
| Development of contraction of skeletal muscles in response to
| |
| stimu1ation has been studied in several species of animals. That
| |
| contractility can be induced before a fetal reflex mechanism can
| |
| activate it has been well established in mammals.12s19«22 The
| |
| first muscu1ar responses were obtain-ed in cat embryos only 11
| |
| mm. long (Fig. 44) . These were brought about by faradic
| |
| stimu1ation wich micro—e1ectrodes applied to points directly over
| |
| developing muscle masses. The forelimb is scarcely more than
| |
| a limb bud at this time but it did move outward, forward or backward, depending on the position of the electrodes. These forelimb movements were the first responses which could be obtained.
| |
| soon the head could be caused to Hex to one side or the other,
| |
| and at the 12 mm. stage separate movements of the proximal and
| |
| | |
|
| |
| | |
| Fig. 44.—-Cat embryo 11 mm. C. R. length. The first muscle contractions were
| |
| elicited by stimulating the region marked with the asterisk (««)».
| |
| | |
| distal parts of the forelimb were induced. At this time contractions of the entire trunk and even the tail muscles appeared.
| |
| Further development was very rapid and soon a large number of
| |
| muscles could be brought into activity by properly placed stimuli.
| |
| It is apparent from these studies that in a general way the course
| |
| of physiologic development proceeds caudally and rostrally from
| |
| the shoulder region and distally and ventrally from the dorsal part
| |
| of the trank.
| |
| | |
| Faradic stimuli were more eifective than other kinds during
| |
| the earIiest stages of muscle deve1opment. strong mechanical
| |
| stimuli produced contractions, but they could not be app1ied with
| |
| as much linesse and the passive movement brought about by
| |
| such means made it diflicult to see muscle contractions. As de—
| |
| velopment proceeds, the strength of stimulus needed to induce a
| |
| 132 Pnrsxotoor OF THE FETUs
| |
| | |
| response decreases. several factors other than strength of stimu1i
| |
| determine whether or not responses to direct musc1e stimulation
| |
| will be obtained. Movements were at their best in specimens
| |
| whose p1acenta1 circulation was intact and which had been delivered into a saline bath at body temperature. Death of the
| |
| embryo abo1ished responses only after a considerable length of
| |
| time. Contractions of the embryonic muscles persisted after 1ow—
| |
| ering the bath temperatures 1o- or 15 degrees Centigrade but
| |
| were abolished quiclcly by raising it Iess than 5 degrees above
| |
| normal body temperature
| |
| | |
| Fig. 45.-Photomicrograph of nerve übers ending upon myoblasts in the shoulder
| |
| region of a 7-week human embrytx Pyridinesilver stainx X 45o.
| |
| | |
| The induced embryonic muscIe contractions possess certain
| |
| characteristics unlilce those of reiiexes A minima1 stimu1us gives
| |
| rise to a quick, sharp, immediate contraction moving the fore—
| |
| limb, let us say, outward. Upon cessation of the stimulation, the
| |
| 1imb tends to maintain this contraction momentarilzg returning
| |
| more slowly to the original Position (see «fetal tetanic reactionsk
| |
| below). A second stimu1ation following closely upon this in—
| |
| duces another response, for there appears to be no grossly perTHE FETAL MUscLEs 133
| |
| | |
| ceptible fatigue period. The direction of movement can be controlled by placing the electrodes in different positions. This indicates that the stimuli were well localized and suggests that
| |
| rather small groups of muscle Ebers were being stimulated. Histologically it was found that a few primitive nerve über terminations are already present in the embryonic muscles at the time
| |
| early contractions can be obtained (Fig. 45) . Whether or not
| |
| these nerve endings have anything to do with the nature of re—
| |
| sponses to direct stimulation is undetermined.
| |
| | |
| THE FETAL TETANIC REACTION
| |
| | |
| A most interesting study of fetal muscle physiology was made
| |
| by MinlcowslciW who tested the excitability of nerves and of muscles to galvanic and faradic stimulation in 2o human fetuses 15
| |
| mm. to 350 mm. crown-heel length after removal from the Uterus.
| |
| By applying galvanic stimuli directly to the muscles, he found
| |
| only two specimens which did not react. In four others the
| |
| anodal closing contractions were sharp and were followed by
| |
| slow, often incomplete relaxation while the current was still passing. Brealcing the circuit produced a new sharp contraction.
| |
| Fourteen fetuses responded to closing the circuit both at the anode
| |
| and cathode with a quiclc contraction of the muscle; upon reach—
| |
| ing a certain magnitude, they remained contracted so long as the
| |
| current llowed. There followed a slower relaxation after brealcing the circuit. To this phenomenon the name «fetal tetanic re—
| |
| action" was given. Faradic stimulation, as has been observed in
| |
| young infra-human fetuses, produced a similar quiclc contraction
| |
| followed by a slow relaxation.
| |
| | |
| Minlcowslci was not the first to make a study of the electrical
| |
| responses in fetal muscles but his emphasis of the tetanic reaction
| |
| was new. 0thers24 experimented with human fetuses of about
| |
| 7 months gestation and reported no tetanic reaction. However,
| |
| Bichat25 recognized that slceletal muscles of fetal guinea pigs can
| |
| be induced to contract in response to electrical stimulation and
| |
| found that the more mature the fetus, the better and more rapid
| |
| the reaction. PreyerI9 spolce of tonic contraction of the muscles
| |
| of rabbit fetuses in which he had stimulated the spinal cord with
| |
| | |
| faradic shoclcs.
| |
| 134 PHYSIOLOGY OF THE« FETUS
| |
| | |
| 1n the newborn rabbit, cat and dog, excitability to electrical
| |
| stimulation is less than insadultsks The muscle ok the newborn
| |
| acts like that ok a katigued animal, as may be seen in Fig. 46. Although it toolc 7o stimuli to bring about tetanus in the adult, only
| |
| 16 were required to do so in the newborn, conditions being the
| |
| same. These studies have been confirmed in human inkants.27- 23
| |
| | |
|
| |
| | |
| Fig. 46.-Myograms in the cat- a, adultx b, 7-day-o1d kirren; c, neu-horn- Time
| |
| in second-s. Arrow indicates direction ok movement ok the paper. (soltmann:
| |
| Jahr-b. Kinder-Mk» Vol. te, 1878.)
| |
| The character ok the myogram was studied in one healthy premature inkant weighing 1,26o grams and comparisons were made
| |
| with normal new»borns and with inkants sufkering from several
| |
| diseases.29 We are interested in the results obtained in the healthy
| |
| individuals and have reproduced them in the following Table 2o.
| |
| | |
| TAVLV 20
| |
| | |
| cnzmornnrssrrcs or Mroonms IN klomm Brut-re.
| |
| | |
|
| |
| | |
| Luteltt Mlmmlkm Dotation of Hei ht of
| |
| Ase · period Los-ZEIT« eontraetion eontkaction
| |
| (s1gma) Cletus) (sigma) (mm.)
| |
| Premature . . . . . . . . ..L 81.6 61.5 688.9" 20.5
| |
| shouks ........... .. 17.2 l 50.8 8984 · 15.0
| |
| sweelcs . . . . . . . . . . . .. 18.8 52.5 804.2 19.0
| |
| Cmonths . . . . . . . . . .. 21.8 ] 58.8 ) 2726 l8.5
| |
| | |
| 1t will be seen that the latent period was longer, the contraction
| |
| a little slower and its duration much greater in the one premature
| |
| inkant than in the newborn; but no signiticance should be placed
| |
| on a study ok one individual. According to another investigatorso
| |
| the latent period ok muscle in young rat ketuses is betwen 5oo and
| |
| 1,ooo sigma. How this was determinexl is not stated.
| |
| THE« FETAL MUscLEs IZZ
| |
| | |
| EXOITATION 017 FETAL MUscLE BY NERVE STIMULATION
| |
| | |
| Fetal muscle responds to direct stimulation, as we have said,
| |
| in advance of the time it can be activated by stimulating nerves.
| |
| | |
| All investigators agree upon this point. In cat» rat and sheep
| |
| fetuses, responses to nerve stimulation can be elicited soon after
| |
| | |
| the first direct muscle contractions. In the human it has been reported to be about a month later.31 Contractions following
| |
| human nerve stimulation were not obtained until about the
| |
| fourth month of gestatioxy at which time the responses were less
| |
| lively and less constant than those following direct muscle stimulation. They should be manifested much earlier than this« It
| |
| has been found that asphyxia prejudices the results obtained
| |
| from stimulation of the nervous systemFs since all of the human
| |
| fetuses were studied under asphyxial conditions it is doubtful if
| |
| results with indirect activation of the fetal muscles can be of great
| |
| significance.
| |
| | |
| The type of motor response obtained by stimulating the spinal
| |
| motor centers of small cat fetuses differs from that which results
| |
| from stimulating the muscle itself with faradic shoclcs. VVhen the
| |
| point of a line dental broach is passed into the spinal cord or when
| |
| a faradic shoclc is applied to the cord by microselectrodes the
| |
| movements which occur are quiclcer and their tetanic or maintained characteristic is not so pronounced as it is when the muscle
| |
| itself is stimulated. The direction of movement is less easily
| |
| molded than it is when the electrodes are shifted about over the
| |
| surface of muscle masses. Furthermore, fatigue enters in when
| |
| motor centers are stimulated directly, malcing a second response
| |
| diflicult to elicit after obtaining the first one.
| |
| | |
| EFFECTS OF CURARE
| |
| | |
| Recently an attempt was made to curarize small rat fetuses of
| |
| 17 days gestation delivered by experimental Caesarean Section«
| |
| It was reported that three minutes after injecting a minute
| |
| amount of a one per cent solution of this drug, reflexes were
| |
| abolished but direct muscle stimulation still produced contractions. These results are very different from those obtained 50
| |
| years earlier in guinea pig fetuses« At that time it was reported
| |
| that a dosage adequate to curarize an adult guinea pig in 1o minutes failed to akfect the fetuses when injected into them in utero
| |
| 136 PHYsIoLocY OF THE FETUs
| |
| | |
| unti1 52 minutes had elapsedz complete feta1 curarization did not
| |
| come about for 8o mintites. » Furthermore, the drug passed
| |
| through the placenta1 barrier from fetal to maternal side and
| |
| killed the mother before alfecting the fetus. It seems doubtful
| |
| if the observations on such small fetuses as were used in the recent
| |
| experiments can rnean’ more than that reflexes had died out be—
| |
| cause of asphyxial conditions prevailing cluring the experiments
| |
| | |
| FETAL RIGOR KCORTIS
| |
| | |
| There has been discussion from time to time as to whether or
| |
| not fetuses dying in utero exhibit the phenomenon of rigor mortis.
| |
| Ballantyness reported several cases and gave references to the eaxly
| |
| literature on this subject. From what is known of the chemistry
| |
| and physiology of fetal skeletal Inuscle there is no reason to doubt
| |
| that rigor does take place but it may not be as pronounced as in
| |
| the adult. The phenomenon has been described in kittens dying
| |
| in uteroIs In no case was the degree of rigidity as marked in the
| |
| fetus as in the mother eat.
| |
| | |
| REFERENCES cITED .
| |
| | |
| i. Arey, L. B. i940. Developmental Anatomzt 4th ed. saundets philadelphja
| |
| | |
| L. clark, E. L. sc E. R. clark. i9i4. Exp. Zool» i7: 373.
| |
| | |
| s. copenhaven W. M. ig39. J. Exp. Zool» so: i93.
| |
| | |
| 4. Goss, c. M. ig4o. Anat. Ren, 76: is.
| |
| | |
| z. Baums-s, M. T. igim Miinch. med. Wchnschrsp 59: i473.
| |
| | |
| S. Leu-is, Maigaret R. i9i5. Am. J. Physiol» 38: i53.«
| |
| | |
| 7. Leu-is, Margaret R. i92o. contr. Einb., g: i9i.
| |
| | |
| s. straiis, W. L. ig39. Anat. Ree., 73: supph Ho.
| |
| | |
| g. Hoolcer, D. R. i9i2. Am. J. Physiol» zi- 47.
| |
| io. Mansfelch G. i92i. Pfliigeks Arch., i88: 24i.
| |
| ii. Romijn, c. sc J. Rom. i938. J. Physiol» 94: 365.
| |
| is. Windle, W. F., D. W. Orr sc W. L. Mineaix i934. Physiol. Zool» 7: «6oo.
| |
| is. Angulo y contain, A. W. i93o. Proc. soc. keep. Bio1. sc Med.. 27: 579.
| |
| i4. Angulo y Gonzalez A. W. i934. Anat. Ren, 58: suppL 45.
| |
| is. Winde, W. F. i939. Physiol. Zool» is: 39.
| |
| is. Tracjh H. c. i926. J. comp. Neur., 4o: 253.
| |
| i7. Wintreberg P. i92o. Arch. Zool. Exp. Gen» so: sei.
| |
| | |
| is. I-looker, D. igi i. J. Exp. Zool» ii: i59.
| |
| i9. Preyer, W. i885. specielle Physiologie des Embryo. crieben, Leipzig.
| |
| so. Angiilo y Gon2alez, A. W. i933. Proe. soc. Exp. Bio1. sc Med., Hi: iii.
| |
| Si. Ranezs E. T. sc L. carmichaeL i934. J. Genetic Psyehol., 45: z.
| |
| ge. Barcroft J., D. H. Barron s- W. F. Windle. ig36. J. Physiol» 87: 73.
| |
| its. Minkowskh M. i928. sehst. Areh. Neun Psyehjat., es: 64.
| |
| 24. Bo1atiio, M. »so G. Artoixiz Jgsixx Arclt d? sei. Bio1., z: 457.
| |
| . Zieh-it, X. 1822.
| |
| | |
| THE FETAL MUscLEs 137
| |
| | |
| General Anatomy. Trans. by G. Raps-arti, Richard·
| |
| son s« Lord, Boston.
| |
| Solon-Inn, O. 1878. Jalirlx Icinderhllg re: r.
| |
| Westphah c. 1886. Neun centralbh z: 361.
| |
| | |
| . Westphah A. 1894. Arch. Psychiat., es: r.
| |
| . Icrasnogorskh N. 1914. Jahrb. lcinderhlk., 79: est.
| |
| . Angulo y com-des, A. W. i936. Cited by D. Hoolcen Yale J. Biol. 8c
| |
| | |
| Magd» 8: 579.
| |
| | |
| . Minkowskh M. 1922. seine. mal. Wchnschr., He: 721, 751.
| |
| . Windle W. F. 8c J. E. Fitzgeralct 1937. J. comp. Neun, 67: 493.
| |
| . Winde, W. F. Z: R. F. Becher.
| |
| | |
| 1g4o. Arch. Neun Psychiat., 43: 9o.
| |
| Angulo y Gonzalez A. W. 1935. Proc. soc. Exp. Biol. F: Meist» sie: Hist.
| |
| Ballantyncz J. W. i9o2. Manual ok Antenatal Pathology and Hygiene.
| |
| | |
| The Foetus. William Green 8c sonst, Eclinburglx
| |
| Tissoh J. 1894. Arch. Physiol. vorm. packt» ser. z, S: 86o.
| |
| » CHAPTER x
| |
| | |
| THE GENESIS OF FUNCTION IN THE NERVOIJS SYSTEM
| |
| | |
| THE beginning of functional activity in the nervous system has
| |
| been investigated most thoroughly in embryos of the rat,I-4 guinea
| |
| pig,5- S sheepfs 8 and cat,9-13 and less completely in man14s 15 and
| |
| other mammals.10-I7 studies in the lower vertebrates,18-22 especially amphibia, have influenced conceptions of behavioral de—
| |
| velopment in mammals to a very considerable extent. A complete
| |
| review of all articles on the subject of early fetal movements
| |
| would require far more space than is available in the present chap—
| |
| tclc
| |
| | |
| A B C
| |
| | |
| Fig. 47.—Embryo.s of the (.-1) rat, (B) cat and (C) human at approximately
| |
| the stage in development at which simple rellexes are expected for the first time.
| |
| crownckump length: 12 mm. rat; 14 mm. cat; 18 mm. human (7 weeks) . Magni—
| |
| ficatiom X Z.
| |
| | |
| 1t is impossible to say precisely when nerve cells attain the
| |
| ability to discharge or when kibers can conduct nervous impulses
| |
| for the first time. However, muscle contractions induced by
| |
| nervous activity can be elicited surprisingly early in prenatal life.
| |
| The stage at which nervous function is first observable in mammals varies to some extent, but in all species that have been in—
| |
| | |
| 138
| |
| oENEs1s or FUNCTION IN NEnvoUs sYsTEM 139
| |
| | |
| vestigated it is before body form has talcen on the characteristic
| |
| appearance of the species. In other words, somatic movements
| |
| start before the close of the embryonic, rather than in the fetal
| |
| period. This is illustrated by the accompanying photographs of
| |
| rat, cat and human embryos talcen at about the time simple reHexes can first be induced (Fig. 47) .
| |
| | |
| The initiation of function in slceletal muscle cells was considered in the preceding chapten Myogenic xesponses precede
| |
| reiiexes by at least one day in most mamma1s. The development
| |
| of muscle iibers and of motor nerves with simple epilemmal motor
| |
| nerve endings goes hand -in hand, but there does not seem to be
| |
| an immediate correlation between the appearance of motor end—
| |
| ings and Csunctional reflexes as some have suggestedks From the
| |
| structural point of view it is possible that muscle contractions can
| |
| be induced by discharge of motor neurons before reflex arcs have
| |
| been completely formed. With the advent of conduction from
| |
| afferent to efkerent neurons through synaptic centers, reHex re—
| |
| sponses to stimulation are manifested. At this point in deve1opment behavior may be said to have its genesis.
| |
| | |
| MYOGENIC RESPONSES
| |
| | |
| Let us examine the antecedents of behavioral genesis in somewhat gyeater detail. spontaneous muscle twitching characterizes
| |
| embryos of certain lower vertebrates before reHex excitation becomes eifective. 0bservations in fishes are especially notable.I9
| |
| The similarity to spontaneous contractions of myob1asts and
| |
| muscle iibers in cultures of embryonic chiclc tissues is striking. It
| |
| is possible that some of the earliest spontaneous movements »observed in the intact living chick embryo1S-21-24-25 are of this natake. ·
| |
| | |
| No comparable phenomenon has been seen in .mammalian
| |
| embryos studied under normal« physiologic conditions. However,
| |
| the movements which manifest themselves in ionically unbalanced
| |
| saline solutions probably are myogenic responsesKs Why spontaneous muscle twitching is not encountered normally in mammalian embryos has not been determined. Musculature is laid
| |
| down well in advance of- the time it can be activated reflexly in
| |
| the rat, guinea pig, sheep and cat.
| |
| | |
| Mechanical and electrical stimuli applied directly to muscle
| |
| 140 PHYSIOLOGY OF THE FETUs·
| |
| | |
| masses of intact embryoX efkect contractions readily. Microselecs
| |
| trodes constructed of Hne nichrome wires insulated except at the
| |
| tips serve admirably for deliveringdocalized faradic shoclcs. Contractions of the embryonic slceletal muscles induced in this manner possess certain characteristics which distinguish them from
| |
| other types of somatic movement. They are as follows: (a) a
| |
| minimal stimulus, just adequate to produce a response, gives rise
| |
| to a quiclc contraction followed by a slower relaxationx (b) each
| |
| succeeding stimulation produces a similar contraction, for there
| |
| is no noticeable interval of fatigue during which the muscle is re—
| |
| fractoryz (c) the contraction is remarlcably well localized in a
| |
| small region immediately around the tips of the electrodes and
| |
| consequently -movements in several planes can usually be brought
| |
| about by shifting the Position of the electrodes; (d) the embryonic muscle tissue retains a high degree of excitability irrespective of great changes in metabolic conditions. In fact, specimens which have been allowed to bleed until white, which have
| |
| cooled to room temperature and in which the heart has practically
| |
| stopped beating still respond to direct stimulation of the slceletal
| |
| musculature. «
| |
| | |
| All the muscles of an embryo do not simultaneously reach a
| |
| state of development in which contractions are possible. The
| |
| first in which such direct responses can be observed are those ok
| |
| the forelimbs at the attachment to the body. With advancement
| |
| of growth,.excitability spreads both rostrally and caudally as well
| |
| as distally from these po»ints.
| |
| | |
| NEUROMOTOR RESPONSES
| |
| | |
| The second step in development of behavior is thought to be
| |
| the appearance of muscle contraction in response to. excitation of
| |
| motor neurons.27 Nerve endings of a primitive lcind (Fig. 45)
| |
| are present upon developing muscle übers at a time when the
| |
| only somatic movements are those which follow direct skimulation
| |
| of the muscleskss 29 Although it has been impossible to obtain
| |
| direct evidence in the youngest cat embryos that purely neuromuscular contractions precede rellexes, such contractions can be
| |
| demonstrated in specimens a little more advanced, in which reflex
| |
| responses are already obtainable. After the reflexes have died
| |
| away with deterioration of the· plsysiologic oonditions of the
| |
| cENEsIs OF kUNcTlcN IN NERVOUS sYsTEM 141
| |
| | |
| embryo, stimulation of motor centers produces movements. A
| |
| s1ender and sharp dental broach was used to pierce the tissues of
| |
| the back and the spinal cord of the embryos. It was found that
| |
| a baclcward movement of the forelimb followed when the instrument was passed into the spinal cord at the level between C.7
| |
| and T.1 and a forward movement resulted in the same specimens
| |
| when it was inserted at the level between c.4 and c.6. The true
| |
| reflexes which had been obtained previously were all baclcward
| |
| and outward movements of the 1imbs. Thus it is apparent that a
| |
| new forward movement of the arm had been induced by direct
| |
| stimulation of a motor center before such a movement occurred
| |
| as the result of retlex stimulation of afferent nerves. It is especially noteworthy that the responses were localized and that there
| |
| was no diffuse spreading of excitation through the center even
| |
| with this rather crude form of stimulation. The segmentaL nonintegrated character of the motor ce11 column of the embryonic
| |
| spinal cord, so clearly evident in silver-stained histologic preparations, is demonstrable by physiologic methods.
| |
| | |
| GENESIS OF REFLEX BEEAVIOR
| |
| | |
| The third step in behavioral development is characterized by
| |
| the appearance of reflexes. These do not manifest themselves
| |
| unti1 atkerent and efferent neurons, simple nerve endings in
| |
| peripheral tissues, connector neurons in the central nervous system and functional synaptic central mechanisms have been
| |
| formed. ·
| |
| | |
| There are essentially two conceptions of the development of
| |
| behavior in mammalian embryos. 0ne group of investigatorsY
| |
| II· IS believe that they have demonstrated the genesis of reflexes
| |
| by a process of individuation from a fully integrated mass reaction or «total Patter·n." In other words, they believe that more
| |
| or less discrete movements are not the primary units of behavior
| |
| but that local reflexes difkerentiate from a more fundamental
| |
| baclcground of massive movement. Another group of investigatorsws 30 hold the opposjng view that the basic elements in the
| |
| genesis of mammalian behavior are relatively simple reliex re—
| |
| sponses. They lind that the more complex reactions of older
| |
| fetuses are formed by progressive neuronal integration of the less
| |
| complicated activities of the embryo. some other investigatorss
| |
| 142 PHYSIOLOGY OF THE FETUS
| |
| | |
| maintain that both theories are partially true, but are inclined to
| |
| kavor the form-er.
| |
| | |
| THE CONCEPT OF Ä TOTAL PATTERN
| |
| | |
| The doctrine of development of behavior from a total pattern
| |
| is based on a long series of correlated physiologic and histologic
| |
| studies by coghill in the urode1e amphibiam Amblystoma, ap—
| |
| pearing frequently since 1913 and summarized in his London lec
| |
| x Ifloor PMB cslls
| |
| | |
| Fig. 48.—Diagram of the sensoryimotor mechanism of the Salamander embryo
| |
| | |
| which acoounts for the cephalwcaudal progkession of movement away from the
| |
| side stimulated Arrows indicate direction of conductiom (coghi11: «Anatomy and
| |
| | |
| the Problem of Behavior," Cambridge Unim Press.)
| |
| | |
| turesEs It was found that a motor mechanism develops on either
| |
| side of the embryonic floor plate as a longitudinally conducting
| |
| system of neurons. Each neuron extends a process caudally to
| |
| the next one; from this Process branches run to the muscles of the
| |
| baclc and later to the 1imbs. Thesseries of neurons constitutes an
| |
| oENEsrs or FUNoHoN 1N NEnvoUs sYsTEM 143
| |
| | |
| integrating motor system which is laid down before kunction appears. An integrating s«ensory system is kormed by temporary
| |
| neurons, the Rohon-Beard ce11s in the dorsal portion ok the spinal
| |
| cord. They too send out processes, but in a rostral direction, with
| |
| branches running to the epithelium and the muscles. The motor
| |
| and sensory systems become connected by commissural fioovplate
| |
| | |
| Spinoi cord
| |
| -(-—«-’——
| |
| | |
|
| |
| | |
| T Gkk Moses· Post)
| |
| · —0 s» Riqht mofor YOU«
| |
| | |
| Xsksoor Plds cell
| |
| | |
| Fig. 49.—Diagrarn ok the neuromotor mechanism ok the Salamander embryo
| |
| which allows an initial irnpulse (a, w) to be kollowed by a oontralatetal secondary
| |
| Impulse (c, X) through an intermediate connecting neuron (b) . This makes pos—
| |
| sible the swimming rnovernents like those in Fig. He. (coghi11: «Anatomy and the
| |
| Problem ok Behavior," cambridge Univ. Press.)
| |
| | |
| neurons, appearing iirst near the rostral end ok the embryo.
| |
| These relations are illustrated in Figs. 48 and 49.
| |
| | |
| A stimulus which is applied to one side of the embryonic
| |
| salamander sets up impulses which are conducted rostra11y in the
| |
| sensory System, across the· kloopplate neurons near its rostral end,
| |
| and then caudally in the integrating motor System. contralateral flexion is thereupon the iirst true» behavioral responsez it«
| |
| 144 Pktysxoxock oF THE FETUs
| |
| | |
| is a mass movement or fully integrated response ’from the very
| |
| start. This reaction is iliustrated in a series of drawings taken
| |
| from a motion-picture record« (Fig. Ho) . The single flexion
| |
| stage is followed shortly by bilateral ilexion and then by typical
| |
| swimming. The latter depends upon the appearance of collateral branches of motor neurons (Fig. 49) which allow the
| |
| caudal1y flowing impulses of one side ·to precede those of the
| |
| | |
| Fig. 5o.-seria1 tracings from motion pictures (frame numhers indicatety of
| |
| the earliest contralateral movement in response to stimulation of a Salamander
| |
| embryo. The neural mechanism involved is illustrated in Fig. 48. (coghill:
| |
| | |
| «Anatomy and the Problem of Behavior," cambridge Univ. Press.)
| |
| | |
| other. In this way two waves of movement can course down the
| |
| trunlc as shown in Figs. 51 and 52. 0ne response coming upon
| |
| | |
| ·another in this manner produces forward propulsion of the
| |
| | |
| embryo, establishing aquatic locomotion.
| |
| | |
| Later, as the 1imbs grow out they first move with the trunlc
| |
| passively but ultimately acquire independence. Limb movements may thus be said to individuatefrdm the mass movement
| |
| oENEs1s oF FUNcTmN 1N NERvoUs sYsTEM 145
| |
| | |
| Fig. 51.—serial tracings from motion pictures of the early swimming stage of a
| |
| Salamander embryo. Resting position in 1 and to. (coghi11: «Anatomy and the
| |
| Problem of Behavior," cambridge Unim Press.)
| |
| | |
| Fig. Hex-Three diagrams to; show the development of the first and subsequent
| |
| waves of contraction which result in swimming in the Salamander embryo. (coghill: «Anatomy and the Problem of Behavior," cambridge Unim Press.)
| |
| | |
| of the trank. Terrestrial locomotion, the feeding reaction and
| |
| other activities are made possible by brealcing up of the original
| |
| total pattern or by the formation of segondary patterns within
| |
| | |
| 10
| |
| 146 Pnvssror.ocv oF THE FETUs
| |
| | |
| it. 0ther more discrete reflexes individuate from these patterns
| |
| as development proceeds. « An independent and integrated motor
| |
| system is present in fishesfor some time before it is captured by
| |
| developing sensory mechanisms. During this period of independence the motor system can respond to changes in the internal
| |
| but not the external environmenr.U
| |
| | |
| In 1929 Coghillsl lattempted to explain behavior in human
| |
| embryos studied by Minlcowslci32 and others in terms of the total
| |
| pattern of the Salamander. He concluded that behavior in man
| |
| follows a developmental plan of a similar type. Some of the
| |
| earlier systcespmatic studies on development of fetal movements in
| |
| other mammals2s 9 suggested very strongly that nearly all embryonic motility develops from mass reactions resembling total
| |
| patterns. The more recent interpretations of these observations
| |
| will be discussed in the latter part of the present chapter.
| |
| | |
| Human and other mammalian embryos are so very different
| |
| from Amblystoma at the time movements and reflexes first appear
| |
| that it is surptising to find any functional similarities. The larval
| |
| Salamander develops its total reaction and precisely integrated
| |
| side to side waves of movement within this integrated pattern
| |
| before limbs and mouth have been formed. Mammalian embryos
| |
| are far from having attained functional age at a comparable stage
| |
| in morphologic dilferentiation (compare Figs. 47 and Ho) .
| |
| Muscle is entirely l·aclcing. Within the embryonic central nervous
| |
| system of mammals no structures exist which are comparable
| |
| with the chains of transient afkerent Rohon-Beard cells of lower
| |
| forms. Furthermore, the motor neurons are arranged segmentally
| |
| rather than in longitudinal series and they do not appear to be
| |
| connected with one another. The ear1iest secondary neurons
| |
| of the spinal cord build tracts that are predominantly ascending
| |
| pathways. 1n the brain, the descending tracts send few übers into
| |
| the spinal cord until spinal behavioral responses have become
| |
| establishedkHs « In other word-s, there is no longitudinally integrating mechanism in the spinal cord of mammalian embryos at
| |
| the stage in development which corresponds to the early motile
| |
| | |
| « Recently Aug-Mo« has reported that the media1 longitudinal fascicle of the
| |
| | |
| spinal cord is the descending integrating tract for the mass reaction of the rat
| |
| embryo and that its termination forms the ventral spinal commissure. This is at
| |
| variance with our own observations which show that the ventral commissure übers
| |
| | |
| arise in the spinal cord and ascend in the vgntraltuniculus
| |
| GENESIS OF FUNCTION IN NERVOUS sYsTEM 147
| |
| | |
| Amblystomaz nor is there until some time later. By the time
| |
| dikkerentiation of structure makes functional activities possible
| |
| the head and limbs have become prominent structures.
| |
| | |
| In the further course of development of mammalian embryos
| |
| these parts exert an ever increasing dominance over the trunk,
| |
| and growth in the nervous system responds to this dominance.
| |
| There is never a need for the type of aquatic locomotor total pattern which is found in the iishes and amphi«bians.
| |
| | |
| EARLY REFLEXES IN MÄMMALIAN BMBRYOS
| |
| | |
| True behavior makes its appearance about one ·««day after it
| |
| was Erst possible to elicit muscle contractions in embryos of the
| |
| rat, guinea pig, cat and sheep. It is essential to emp1oy experimental methods which do not impair the physiologic conditions of pregnant animals and their embryos to observe the tirst
| |
| reflexes. F urthermore, studies must be conducted immediately
| |
| upon opening the uterus because this operation invariably interferes with placental respiratory exchange, resulting in anoxemia.
| |
| The procedures used in such studies have been discussed in the
| |
| first chapter.
| |
| | |
| Although the description of early reflex responses which follows pertains primarily to cat embryos about 14 mm. crownrump lengthP similar results have been obtained in other mammals.3- «« C« «« When an embryo with placenta still attached to the
| |
| uterine wall has been brought quickly into view and the intact
| |
| amniotic sac is percussed witli some blunt instrument, pressure
| |
| transmitted to the embryo through the amniotic fluid results in
| |
| quick outward and backward movement of the forelimb. The
| |
| movement may be called a twitch or jerk. similar responses are
| |
| obtainable in other ways. Flipping the limb gently with a needle
| |
| or a hair passed through the amnion serves equally well. In a
| |
| few instances it was even possible to elicit this reaction by lightly
| |
| touching certain points upon the forelimb. Furthermore, faradic
| |
| shocks applied to approximately the same points by means of
| |
| micro-electrodes gave rise to similar quick outward and backward
| |
| movements of the limb.
| |
| | |
| Another reaction is frequently elicitable in embryos 14 mm.
| |
| long. stimulation of the forward end of the head. especially the
| |
| snoutz results in extension of the head. When the stimulus is
| |
| 148 PHYSIOLOGY or THE FETUs
| |
| | |
| applied to one side ok the midline the head moves toward the opposite side and baclcwardH When the tip ok the snout is stimu1ated
| |
| it moves baclcward. Because this head response is more resistant
| |
| to changing physiologic conditions than that of the korelimb it
| |
| usually persists a little longer. Under the best conditions it too
| |
| is a quiclc movementz It has been observed by all investigators,
| |
| although some have encountered it at an earlier stage than others.
| |
| | |
| The head and korelimb responses are entirely separate and
| |
| distinct from one another when they iirst appear. They difker
| |
| somewhat in respect to the types ok stimulation eliciting them but
| |
| both seem to require stronger stimuli at first than they do at a
| |
| slightly later stage in development. The head movements, being
| |
| contralateral and involving muscles some little distance away
| |
| | |
| from the site of stimulation, are certainly reflexes. All observers
| |
| agree on this point. However, some have doubted the reflex nature ok the korelimb reactions, holdingsthat they may be due to
| |
| stimulation ok the muscles directly. The evidence, which will be
| |
| reviewed brieilyy kavors the view that they too are simple spinaltype reHexes.
| |
| | |
| Although the limb muscles can be induced to contract by
| |
| direct stimulation in asphyxiated embryos, the response held to be
| |
| a reflex is (a) elicitable kor only a briek interval (okten only a
| |
| kew seconds) while« metabolic conditions ok the embryo are at
| |
| their best. states of anoxemia set up experimentally make it impossible to obtain the reactions. Akter adequate direct stimulation the new muscle tends to relax slowly, whereas the reHex-like
| |
| response (b) seems to be a quiclcer movement with more rapid relaxation. Embryonic muscle appears to respond instantaneously
| |
| to directly app1ied stimuli, but (c) there is an interval just per—
| |
| ceptible· between stimulus and response ok thexeiiex type. 0ne
| |
| muscle contraction after another can be induced by direct stimulation, but (d) a second reflezklilce reaction cannot be made to kollow the Erst one until a briek interval ok time, a rekractory or
| |
| katigue period, has elapsed. Finally, (e) the responses believed to
| |
| be reilexes are stereotyped and do not show the molding characterizing direct contractions when the position ok the stimulus is
| |
| varied.
| |
| | |
| 0ther experimental evidence demonstrates that the korelimb
| |
| movements are reflexes. When xtiicroelectrodes are used to stimGENEsIs OF FUNCTION IN NERVOUS sYsTEM 149
| |
| | |
| ulate an embryo over the spinal cord some distance caudal to the
| |
| forelimb, the same quiclc outward and baclcward twitch results.
| |
| This is due to conduction up the spinal cord; stimuIation of other
| |
| parts of the embryo, equally distant from the limbs, does not pro—
| |
| duce this movement. At least one synapse is involved as indicated in Fig. 53.
| |
| | |
| The conception of the refiex nature of the early forelimb response iinds further confirmation in histologic studies in the
| |
| spinal cords and peripheral nerves of the very specimens which
| |
| showed the reaction and which were subsequently stained by the
| |
| | |
| Fig. zssdiagram illustrating the probable nervous elements involved in elicits
| |
| ing forelimb movement by faradic stimulation of the spinal cord of an early main
| |
| malian embryo.
| |
| | |
| Ranson pyridine-silver techniquekss THE All nervous elements
| |
| essential for reflex action are present in the embryonic spinal cord
| |
| but their intrinsic synaptic connections are incomplete at the
| |
| time muscles can be made to contract by stimulating them directly
| |
| and previous to the appearance of the reliexdilce responses. 0ne
| |
| iinds alferent neurons whose peripheral Ebers pass to the tissues
| |
| immediately beneath the' epithelium of the forelimb and whose
| |
| central branches constitute the dorsal roots and dorsal funiculus
| |
| of the spinal cord. The efkerent neurons are assembled in two
| |
| groups in the ventral gray matter of the spinal cord, a medial
| |
| 150 PHYSIOLOGY OF THE« FETUS
| |
| | |
| nucleus for trunk innervation and a lateral nucleus for the arm.
| |
| The efferent axons courses into the muscles of the shoulder region
| |
| and end in simple terminations upon some of the muscle übers.
| |
| Commissural and associationa1 neurons are present in the dorsal
| |
| column. ,The former are numerous in the region just beneath the
| |
| dorsomedial border of the dorsa1 funiculus, while the latter tend
| |
| to accumulate nearer the ventrolateral border of this afferent
| |
| pathway. Commissural axons pursue a course ventrally through
| |
| | |
| J« « »F. - «. v · « ·s - fis, F; - »,«’-«»; J l -,E"-Z««
| |
| | |
|
| |
| | |
|
| |
| | |
| -..-«. ·« JHJL J»
| |
| Fig. 54.-—Photo1nicrograph of the fifth cervical Segment of the spina1 cord of a
| |
| 13 mm. cat embryo just before the time the Erst forelimb reflexes can be elicitedx
| |
| as» Ventral funiculus; tut» association neurons; c» cornmissurex ern» Commissural
| |
| neurons; ihn, dorsal root and ganglionx M» Iateral funiculusx m.l., motor nucleus
| |
| for the limb muscles; Max» motor nucleus for the trunlc musclesz p.f., dorsal funiculus. Compare with Fig. 56. Pyridinesjlver stainz X so.
| |
| | |
| - s « -. »«
| |
| J» I ««
| |
| | |
| the gray matter and cross the kloor p1ate, to become an ascending
| |
| tract close to the motor nerve cells which supply the trank. Associational axons pass close to the motor nucleus for limb muscles
| |
| and enter the Iateral funicu1us. This relation of associational
| |
| neurons and primary motor forelimb neurons appears to be very
| |
| intimate, with axons of the former coursing parallel with dendrons
| |
| of the latter. However, up to the time of appearance of the forelimb response there is no close re1ationship between the primary
| |
| afferent and the secondary neurons (Fig. 54) . A few collatera1
| |
| CENESIS OF FUNCTION IN NERVOUS SYSTEM 151
| |
| | |
| branches of dorsal funiculus ftbers do pass for a very short distance
| |
| toward the associational group, but not many have reached it.
| |
| | |
| In the early motile embryos in which reilezklike twitches of the
| |
| forelimb occurred when the limbs were flipped or when the
| |
| amniotic sac was percussed, connections have been comp1eted be—
| |
| tween the primary afkerent and the associati0na1 neurons. This is
| |
| | |
| ..
| |
| | |
| « Z«
| |
| | |
| Fig. 55.-—Photoicrograph of the dorsal funiculus (zt).j.) f the spinal cord of:
| |
| a 13 mm. cat embryo showing the Erst collateral branches (col.) of primary aikerent
| |
| neuronsz these serve to complete the first spinal reflex arcs. Pyridinesilver stainx
| |
| | |
| X Hin.
| |
| | |
| accomplished when collateral branches grow in among the cells
| |
| of the dorsal gray horn from the sensory Hbers nearest the lateral
| |
| border of the dorsal funiculus Some of the longest collaterals
| |
| enter the nucleus of motor cells supplying the forelimb musc1es.
| |
| These relationships will be seen in accompanying photomicros
| |
| graphs (Figs. 54 and 55) and diagrams (Figs. 56 and 57) . The
| |
| organization of the first structural reflex mechanism is clearly such
| |
| 152 PHYSIOLOGY OF THE FETUS
| |
| | |
| that when it begins to kunction the response will not only be
| |
| homolateraI but will be conlined to the segments at which the
| |
| impulses enter the spinal cordx The greatest number and the
| |
| | |
| Fig. 56.-camera lucida tracings ok the dorsal roots (d.s·.) and dorsal kuniculus
| |
| (d.f.) ok sheep embryos M) Do mai» (B) 23 kam» and (c) 24 nun. long. The
| |
| developrnent ok collaterals (col.) ok the dorsal kuniculus which curve rnedially
| |
| around the gelatinous substance (gel.) ok the gras matter is correlated with the
| |
| appearance ok the tirst korelitnb retiexes. Pyridinesilver stainz X 72.
| |
| | |
| Fig. 57.—Diagrammatic cross Section through the embryonic spinal cord just
| |
| before (lekt side) and just after (right side) the completion ok reilex arcs malte
| |
| the earliest retiexes e1icitable. The earliest connections ok aiketent neurons (·a)
| |
| appear at the lateral end ok the dorsal kuniculus and complete unisegtnental reklex
| |
| arm. some ok these involve an interneuron but others (c) make direct connections
| |
| with the pritnary motor neurons kor the limb rnuscles. Only later do the dorsal
| |
| kuniculus übers krom lower segtnents ok the spinal c0rd connect with the Commissural interneurons by means ok collaterals O) and thus etkect contralateral trunk
| |
| | |
| tnovements.
| |
| | |
| longest new collateraIs lirst grow into the gray matter in the
| |
| brachial region and, correlatively, one linds the first reklex re—
| |
| | |
| sponse involving the korelimbs.
| |
| osksinsrs ori- FUNcUoN 1N NERvoUs sYsTEM 153
| |
| | |
| The correlation between completion of anatomical reflex
| |
| arcs in the spinal cords of cat embryos and the manifestation of
| |
| forelimb reiiex function has been confirmed in the chiclc, rat and
| |
| sheep. The stage of development reached in the spinal cord of
| |
| these four species at the time reiiexes appear varies to some extent,
| |
| but the responses occur in all at the time reflex arcs are ready
| |
| irrespective of other structural variations.
| |
| | |
| such evidence as has been disclosed by the correlated histologic and physiologic experiments reviewed here briefly leads to
| |
| the conclusion that the early foreIimb movements are local, uni—
| |
| segmentah homolateral two- and three—neuron reflexes. Mammalian behavior has its genesis, not in a mass reaction or total
| |
| pattern like that of lower vertebrates, but in these relatively Simple reilexes which are at first entirely nonintegrated.
| |
| | |
| OTHER SIUPLE REFLEXES AND TBZEIR MTEGRAT10N
| |
| | |
| During the course of development of cat embryos, many re—
| |
| flexes malte their appearance. Just as the first responses at the
| |
| shoulder can be elicited before the limbs move spontaneously and
| |
| before they move with the neclc and trank, local reilexes appear
| |
| at the elbow and wrist joints as separate entities before the distal
| |
| portions of the« limbs become integrated with other parts of the
| |
| body. Local liexion at the elbows occurs at about the 16—mm.
| |
| steige. It is often followed by other more distant movements,
| |
| such as bending at the shoulder or extension of the head and
| |
| Iiexion of the trank. However, these more proximal and cephalic
| |
| movements are not followed by rnovement at the elbow at this
| |
| time. similarly, local wrist movements seen in embryos about
| |
| 17 mm. long, are at lirst unrelated to other movements.
| |
| | |
| Local movements at the proximal hind-leg joint, unintegrated
| |
| with trunlc responses, are encountered in specimens between 15
| |
| and 16 mm. long. Those at the lcnee appear at 17.5 mm. The
| |
| earliest independent motility of the tail is found at the same
| |
| steige.
| |
| | |
| Although the first head reilexes can be elicited by stimulating
| |
| only a small area near the tip of the snout in embryos 13—14 mm.
| |
| long, it is but a short time later that they occur in response to
| |
| stimulation of most of the facial area. In specimens 15—16 mm.
| |
| long, contralateral head flexion is obtainable from all parts of the
| |
| 154 PHYSIOLOGY OF THE« FETUS
| |
| | |
| face except that supplied by the ophthalmic division of the trigem—
| |
| inal nerve. From the ophthalrnic region, extension with flexion
| |
| to the same side occurs. With further developmenh stimulation
| |
| of more and more Portions of the face leads to the homolateral
| |
| response until, at about the 20 mm. stage, only the ear gives a contralateral head flexionk
| |
| | |
| These and other interesting reflex sactivities have been ob—
| |
| served in embryos. All possess an element of individuality at
| |
| first, but ultimately most of the local responses are brought together into more generalized movements. This comes about by
| |
| integration within the frameworlc of a growing central nervous
| |
| mechanism. Longitudinal tracts of nerve ftbers develop within
| |
| the spinal cord, and as they make connections with afkerent and
| |
| efkerent neurons they begin to exert an integrating function over
| |
| the local, isolated reactions.»
| |
| | |
| The earliest secondary pathway in the spinal cord is a ventral
| |
| longitudinal bundle, primarily an ascending tract formed by the
| |
| axons of commissural neurons. Impulses carried by it apparently
| |
| are able to discharge motor neurons supplying trunlc and neck
| |
| musc1es at more rostral 1evels. Cel1 bodies of the commissural
| |
| neurons, lying near the medial border of the dorsal funiculus,
| |
| receive impulses from primary afferent neurons which have been
| |
| coursing rostrally for some distance in the dorsal funiculus. Consequently the local homo1ateral forelimb reflex is sometimes followed, at the 15 to 1 6 mm. stage, by a contraction of neclc muscles
| |
| similarly at a later period, hind-limb reflexes are followed progressively by responses of the forelimbs and the neck. A progressive discharge of neurons from caudal to rostral regions of the
| |
| spinal cord is broughtlabout through integration of ascending
| |
| neurons of the primary afferent dorsal funiculus with the commissural secondary tract.
| |
| | |
| Nerve Ebers grow caudally into the spinal cord from centers
| |
| in the medulla oblongata and midbrain. Many of these occupy
| |
| | |
| »positions in the ventral funiculus but they do n’ot reach any given
| |
| | |
| point in the spinal cord until after ascending Ebers from a lower
| |
| spinal segment have reached the medulla oblongata. consequently stimulation of structures near the caudal end of an
| |
| embryo of about 18 mm. (Fig. 58) can result sequentially in (a)
| |
| local reflexes, (b) reflexes of more rostral .parts, (c) head moves
| |
| | |
| GENESIS OF FUNCTION IN NERVOUS sYsTEM 155
| |
| | |
| ments and then (d) the trunk activities which are integrated with
| |
| the head and which always fall just short of the most recently
| |
| acquired local responses. The descending integrating tracts of
| |
| nerve ftbers are so placed in the ventral portion of the spinal
| |
| cord that when activated they can more eifectively bring about
| |
| discharge of motor cells which supp1y the trunk muscles than
| |
| neurons for the limb muscles. As a result of such an arrangement
| |
| the limhs seem not to be completely integrated with the trunk
| |
| during the early embryonic period. Later the association be—
| |
| comes more intimate. 0n the other hand, the trunlc movements
| |
| become integrated with those of the neclc almost as soon as they
| |
| | |
| Fig. 58.-—Cat embryo 18.5 mm. C. R. 1ength, from which local reHexes as well
| |
| as early integrated movements could be elicited. Magniiication is the same as that
| |
| | |
| in Figs. 44 and 47.
| |
| | |
| begin to appear at the 15 mm. stage. With growth in size of the
| |
| individual, more and more muscles of the back are added to the
| |
| trunk activities in a caudal1y expanding Progression.
| |
| | |
| Some recent experiments involving the production of states of
| |
| anoxemiaU have added important information to our conception
| |
| of the relation of simple reHexes to the more massive integrated
| |
| activities of cat embryos. When decerebrate cats were allowed to
| |
| breathe atmospheres low in oxygen before the uterus was opened
| |
| it was found that local reflexes became depressetd and the integrated head and trunk movements were exaggerated and more
| |
| sustained or tonic than normally. Irritability of the embryos wszas
| |
| is6 isiiYsiohocY or THE Fixsrus
| |
| | |
| diminished during anoxemia. Extended use ok the gas mixture
| |
| brought about more complete anoxemia and all responses ceased.
| |
| spontaneous movements and responses to stimulation ok embryos
| |
| studied under conditions ok partial anoxemia resemble mass reactions and might easily lead one to the conclusion that behavior
| |
| develops in mammalss in a manner very similar to that in the
| |
| Salamander. Only when such conditions are avoided can the
| |
| | |
| various simple reflexes be observed as separate elements.
| |
| | |
| REFERENCES CITED
| |
| | |
| i. swenson, E. A. i9e6. Thesis, Univ. Kansas.
| |
| | |
| e. Angulo y Gonzalez A. W. i93e. comp. Neur., ss: 39s.
| |
| | |
| z. Raney, E. T. sc L. carmichaeL i934. Genetic Psychol., 4s: Z.
| |
| | |
| 4. Windle, W. F., W. L. Minear, M. F. Austin sc D. W. 0rr. i93s. Physiol.
| |
| | |
| Zool» s: i s6.
| |
| | |
| s. carmichaels L. i934. Genetic Psychol. Mono., is: 337.
| |
| | |
| 6. Bridgmaix c. s. sc L. cariiiichaeL i93s. J. Genetic Psychol., 47: e47.
| |
| 7. Barcrokh J» D. H. Bari-on sc W. F. Windle. i936. J. Physiol» s7: 73.
| |
| s. Barcroktz sc D. I-I. Barron. i939. comp. Neur., 7o: 477.
| |
| | |
| 9
| |
| o
| |
| | |
| . Windle, W. F. sc A. M. Griklin. i93i. J. comp. Neur., se: i49.
| |
| . Windle, W. F., J. E. 0’Donnell sc E. E. Glasshagle i933. Physiol. Zool.,
| |
| | |
| 6- sei.
| |
| | |
| ii. Windle, W. F., D. W. 0rr sc W. L. Minear. i934. Physiol. Zool» 7: 6oo.
| |
| ie. Windle, W. F. sc R. F. Becken i94o. Arch. Neun Psychiat., 43: ge.
| |
| | |
| i Z. coronios, J. D. i933. Genetic Psychol. Mono., i4: es3.
| |
| | |
| i4. Minlcowslti. M. i93s. Abderhaldecks Handb. biol. Arbeitsmeth, Abt. V,
| |
| | |
| Teil sB: si i.
| |
| | |
| is. I-Iooker, D. i936. Yale J. Biol. Med., s: s79.
| |
| | |
| i6. Preyeix W. isss. specielle Physiologie des Embryo. Stichen, Leipzig.
| |
| i7. Pankratz D. s. i93i. Anat. Rec., 49: 3i.
| |
| | |
| is. coghilh G. E. i9e9. Anatomy and the Problem ok Behavioic Macmib
| |
| | |
| lau, New York.
| |
| | |
| i9. Tracy, I-I. C. i9e6. J. comp. Neur., 4o: es3.
| |
| | |
| eo. Tuge, H. i93i. Proc. soc. Exp. Biol. sc Med., e9: se.
| |
| | |
| ei. Kuo, Z. Y. i93e. J. Expen Zool» Si: 39s.
| |
| | |
| ee. Youngstrom, K. A. i93s. J. comp. Neur., 6s: 3si.
| |
| | |
| es. East, E. W. i93i. Anat. Rec., so: eoi.
| |
| | |
| e4. clarlc E. L. sc E. R. Clarlh igi4. J. Exp. Zool» i7: 373.
| |
| | |
| es. 0rr, D. W. sc W. F. Windle. i934. J. comp. Neur., 6o: e7i.
| |
| | |
| es. Windle, W. F. i939. Physiol. Zool» ie: 39.
| |
| | |
| e7. Angulo y Gonealez A. W. i933. Proc. soc. Exp. Biol. sc Med., zi- ii»i.
| |
| es. Windle, W. F. i937. 1bid., Zö- 64o.
| |
| | |
| e9. scharpenberg, L. G. sc W. F. Windle. ig3s. Anat» 7e: 344.
| |
| | |
| so. carmichaeL L. i933. 1n C. Murchison’s I-Iandb. child Psychol» end ed»
| |
| | |
| chap. e, P. 3i, clarlc Univ. Press, Worcestetx Mass
| |
| | |
| 3i. coghilh G. E. i9e9. Arch. Neun sc Psychiat., ei: 989.
| |
| GENEsIs OF FUNCTION IN NERVOUS sYsTEM 157
| |
| | |
| ge. Minlcowslch M. 1922. Schweiz. mecL Woche-weht» He: 721, 751.
| |
| 33. Windle, W. F. 1934. J. comp. Neun, 59: 487.
| |
| | |
| 34. Windle, W. F. 8c D. W. Ort. i934. Ibid., so: 287.
| |
| | |
| 35. Winde, W. F. sc R. E. Bauer. 1936. Ibic1., Eis: 189.
| |
| | |
| 36. Winde, W. F. 8c J. E. Finger-sich i937. Ibid., 67: 493.
| |
| | |
| 37. Angulo y Gonzalez A. W. 1939. Ibic1., 71: 325.
| |
| CHAPTER x1
| |
| | |
| CONDPDIONS REQULATING FETAL NERVOUS ACTIVITY
| |
| | |
| THERE is no doubt that the progressive development of nervous function is related to difkerentiation of structure in the fetal
| |
| nervous System. 1t was hoped that many correlations like those
| |
| discussed in Chapter X could be drawn between specific responses
| |
| and the appearance histologically of new neura1 connections.
| |
| This is not an easy task because structural growth proceeds with
| |
| great rapidity and results in the establishment very early of complexities defying microscopic ana1ysis. 0n the other hand, function in the central nervous system of the fetus is not regulated
| |
| solely by structural factors. 0ne must not lose sight of metabolic
| |
| influencesz it is quite clear that variations in fetal activities are
| |
| closely related to changing respiratory conditions. The parts
| |
| played by the blood, the heart and vascular system and even the
| |
| endocrine glands have to be determinedh Many problems in
| |
| physiology of the fetal nervous system await solution, but it will
| |
| be well to view some of them, even though questions raised
| |
| thereby can not be given satisfactory answers.
| |
| | |
| THE PLAN OF STRIJCTURAL DEVELOPMENT OF« THE FETAL BRAM
| |
| | |
| lcnowledge of development of intrinsic brain structure is far
| |
| from complete, for it ha’s been within the past few years only that
| |
| systematic studies in specimens prepared by adequate methods
| |
| were undertaken. The« usual histologic procedures are unsatisfactory to demonstrate embryonic nerve übers, their terminations
| |
| and relations to one another inside the brain and spinal cord.
| |
| Cajal’s silver stains, especially the Ranson modification, bring out
| |
| details of this nature incomparably better than any other known
| |
| techniqueJ Procedures of this type are being used extensively
| |
| for studying the prenatal mammalian nervous system in this2-I2
| |
| and the Madrid13-I7 laboratories, as well as in a few other
| |
| places.18-22
| |
| | |
| By the time fetal movements can be elicited for the Hrst time in
| |
| any species, a surprisingly exfensive organization ok neuron groups
| |
| | |
| 158
| |
| CONDITIONS REGULATING NERVOUS ACTIVITY 159
| |
| | |
| Fig. so.
| |
| | |
| Fig. St.
| |
| | |
| Fig-I. 59-61.-—I)iagrams ok the brains ok cat embryos 7 nun. (Fig. 59) , to nun.
| |
| Eis. so) and 15 sum. (Fig. Si) c. R. length showing the principal über tracts
| |
| present in each. crossing neurons are clotted Eines. Questionable courses: dash
| |
| lines.
| |
| 160 rnrstotocr or THE: rrsrus
| |
| | |
| and fiber tracts has already formed within the central nervous
| |
| System. We have discussed certain correlations between intrinsic
| |
| spinal connections and the first· forelimb reilexes. In the brain
| |
| equally specific correlations have not been worlced out completely.
| |
| Most of the tracts and nuclei have been identilied in cat embryos
| |
| and those that are present at the time« behavior has its genesis are
| |
| | |
| Tznm 21
| |
| | |
| cusstrtcssrrou or N rnvr Prnnns or srnr Trnnncnrnznou AND Drndronrnznon or
| |
| Wanst. c« Bank-we Aoconnnco sro Arrnoxmsskr Onnrn or Arrrsnzuon
| |
| | |
| «·I · - size of smallest
| |
| Abbrevtations «
| |
| Name of Aber group used in figs. WJTDIYFHL
| |
| ZHT komd
| |
| | |
| Z
| |
| Hasses-Heda doch-H?
| |
| | |
| Medial Iongitudinal kaseiele . . . . . . . . . . . . . . . . .
| |
| supraoptie system- direct preoptie eomponenh
| |
| supraoptie System: eommissural eomponent.. .
| |
| Olkaetmhypothalamie Ebers . . . . . . . . . . . . . . . . .
| |
| Olfaetwsubthalamie fibers . . . . . . . . . . . . . . . . . .
| |
| striosisubthalnmie Ebers . . . . . . . . . . . . . . . . . . . . .
| |
| Direet snbthalamwtegjnental Ebers (dilXuse) . .
| |
| Crossed preteetotegmental and thalamw
| |
| tegmental fibers (ventral eomrnissure) . . . . . .
| |
| | |
| Lemniseus system . . . . . . . . . . . . . . . . . . . . . . . . . .
| |
| | |
| Terminal nerv·e libers . . . . . . . . . . . . . . . . . . . . . . .
| |
| | |
| Posterior eommissure Ebers . . . . . . . . . . . . . . . . .
| |
| | |
| Eabenulwpeduneular fibers . . . . . . . . . . . . . . . . .
| |
| | |
| Iateral olkaetory traet fibers . . . . . . . . . . . . . . . .
| |
| | |
| Mammillwtegmental traet Ebers . . . . . . . . . . . . .
| |
| | |
| Optie nerve Ebers . . . . . . . . . . . . . . . . . . . . . . . . . .
| |
| Thalamostrial and thalatnosseortieal übers. . .. .
| |
| Mammillosthalamie Ebers . . . . . . . . . . . . . . . . . . .
| |
| Olkactory nerve Ebers . . . . . . . . . ». . . . . . . . . . . .
| |
| | |
| s-«-«-«-«-«-«-«-«-« GOOHHHHPOOOO PAGA-todt
| |
| | |
| listed in Table 2 I. Figss 59 to 61 illustrate diagrammatically and
| |
| incomp1etely this extensive development of tracts ich the rostral
| |
| portions of the embryonic brain.
| |
| | |
| (a) ORDER or Dnvaormnr n( Ptmcnonu srsrrus
| |
| | |
| Within the growing tangle of nerve iibers it is possible to distinguish several systems of functionally related tracts. They appear to be growing in anticipation of the time they will conduct
| |
| impulses to distant elfector organs. such systems of tracts are laid
| |
| down economicallyz for the most part they pursue the shortest
| |
| possible courses from place to place. 0ne .of the most interesting
| |
| coNInrIoNs nEcULATINc NEnvous Acrrvrrr 161
| |
| | |
| features of growth of each System is the order in which its component tracts are formed. This is from motor toward SenSory
| |
| side.
| |
| | |
| The very Erst neurons which can be recognized in the embryo
| |
| are primary motor e1ements of the spinal and cranial nerveS, present in rat embryos only z mm. long and appearing in the other
| |
| species at comparable Stages. These are final common path
| |
| neurons over which impulses must ultimately pass to bring about
| |
| responses in the muscleS of. the body. They are the first, local
| |
| connecting neurons are second and the primary afferent tracts appear third.
| |
| | |
| The earliest secondary connecting neurons make their appearance in 17 day old cat embryos in the portion of the medulla
| |
| oblongata which will become the reticular formation. Growth
| |
| very soon spreads into the upper spinal cord segmentS. There,
| |
| nerve cells give rise to crossed and direct axons which make local
| |
| connections with primary motor elementS. Some in the medulla
| |
| oblongata form the earliest reticuloSpinal übers, but they do not
| |
| extend their procesSes far along the nerve axis. secondary elementS of the spinal cord enter into the formation of local conduction Systems, all components of which are not present how—
| |
| ever until primary afferent neurons begin to build the sensory
| |
| tracts. Even then, the local Systems do not become functional retlex mechanisms until Synaptic relations between the three elements-—motor, connector and sensory—have been eStablished on
| |
| the 24th day in the cat embryos. similar reflex arcs are completed during the 8th week in man.
| |
| | |
| Almost simu1taneously with the appearance of secondary
| |
| neurons in the medulla oblongata, another group begins to be
| |
| laid down in front of the mesencephalon. FiberS from this source
| |
| descend to the lower part of the brain stem without crossing and
| |
| form one component of- the medial longitudina1 kascicle. 1t seems
| |
| probable that the early secondary neurons are related to more
| |
| than one baSic rellex System. Just as the lower motor neurons are
| |
| not an exclusive component of any one System, so these interneurons may be shared by several.
| |
| | |
| secondary neurons malce their appearance in still another location in .the early embryo. crossing and direct ftbers arise
| |
| in the mesencephalic tectum and form the tectobulbar and tecto
| |
| II
| |
| 162 PHYstoLooY or THE FETUS
| |
| | |
| spinal tracts. Thus we lind essential1y four groups of secondary
| |
| neurons undergoing developrnent in the Central nervous system
| |
| between the 18th and 2oth days-of gestation in the cat and during
| |
| the 5th weelc in man. These are (a) local intraseginental and
| |
| intersegmental elements, such as later constitute the ground
| |
| bundles of the spinal cord, (b) reticulospinal tracts, (c) medial
| |
| longitudinal fascic1es and (d) tectobulbar and tectospinal tracts.
| |
| Few übers course farther caudally than the lower end of the
| |
| medulla oblongata until a day or so after initiation of the first individual head and forelimb ref1exes· However, it can not be
| |
| doubted that one or more of these tracts play a part in the earliest
| |
| cephalocaudal integration of movements which occurs on the 25th
| |
| day in cat embryos.
| |
| | |
| secondary neurons of .the spinal cord, especially commissural,
| |
| course rostrally in the ventral and lateral funiculi. These are
| |
| present before a signiiicant number of descending iibers from the
| |
| brain reach the cord. They account for conduction of impulses
| |
| from caudal to rostral segments and explain the observation of
| |
| forelimb and head reflexes which sometimes follow stimulation
| |
| of more caudal structures.
| |
| | |
| (b) Gaovrn or Ort-Es: Crskcurrs For: Rmixxss AND Etext-n lnrsoaartos
| |
| | |
| Just as the intrasegmental neurons of the spinal cord enter
| |
| into the structure of basic short reflex circuits, reticulospinah
| |
| tectospinal and medial iongitudinal iibers constitute Iinlcs in
| |
| Ionger circuits which becotne functional much later than the local
| |
| ones. Examples of such- systems are the olfactory and optic reflex
| |
| mechanisms of the early embryo. Analysis of the growth of these
| |
| systems shows that theircomponent tracts too are laid down from
| |
| efferent to afkerent side. For example, during development of
| |
| the optioreflex conduction mechanism the primary spinal motor
| |
| and the oculomotor neurons begin to form Erst, secondary
| |
| neurons represented by the medial longitudinal and tectospinal
| |
| tracts are next, the posterior commissure follows, the optic tract
| |
| itseIf is fourth, and linally the retinal bipolar neurons and visua1
| |
| cells appear. similar sequential development seems to be the
| |
| rule in other reiiex conduction Systems.
| |
| | |
| Within any conduction system such as the optic or olfactory,
| |
| the simplest reflex pathways are« formt-d« before those which have
| |
| CONDITIONS REGULATING NERVOUS ACTIVITY 163
| |
| | |
| to do with perception and higher integrative activities. Neurofibrillar development is late in the cerebellum, corpus striatum
| |
| and cerebral cortex. A primitive secondary aiferent tract, medial
| |
| lemniscus in part, reaches the thalamus of cat embryos from spinal
| |
| and bulbar centers by the 2 ist day of gestation. Fibers begin to
| |
| pass from the thalamus to the cerebrum on the 22nd day, but not
| |
| until late in fetal life does the cerebral cortex exert any influence
| |
| over lower motor parts of the nervous System. The pyramidal
| |
| tracts are last to form.
| |
| | |
| correlated with the tardy development of cerebral cortex, it
| |
| has been determined that cerebral electric potentials are absent
| |
| throughout most of prenatal life. Even in the guinea pig, an
| |
| animal which is much more mature than the rat, cat or man at
| |
| the time of birth, they do not manifest themselves until about
| |
| 2 or z weelcs before the end of the gestation periodks A cortical
| |
| control of the motor mechanism for forelimbs has been established
| |
| at the time of birth in the cat, but the hind limbs laclc it until 16
| |
| days later.24
| |
| | |
| (c) Amor-Erst! as RELATED ro koste-non m tm: Nmvous srsrm
| |
| | |
| Many neurologists adhere to the theory that the initiation and
| |
| maturation of function of the nervous system depends upon the
| |
| formation of myelin sheaths. FlechsigV called attention to the
| |
| fact that the progress of deposition of myelin is orderly and that
| |
| tracts having definite functions become myelinated at different
| |
| times in the human infant’s brain. Some investigatorsW who have
| |
| studied the course of development of behavior in lcittens and have
| |
| attempted to correlate it with myelin formation have suggested
| |
| functional relationships. 0thers24-27-31 have carried out similar
| |
| studies in pouch-young opossum, Icitten and human infant
| |
| brains as well as in the fetal nervous system of the cat and man.
| |
| In a general way it seemed that maturation of behavior and the
| |
| acquisition of myelin sheaths of certain fiber tracts were related,
| |
| but it was impossible to draw specific correlations in all cases. For
| |
| example, the corticospinal tract is still unmyelinated at birth but
| |
| cortical areas for control of forelimbs are electrically excitable.
| |
| | |
| They can be a great deal of well organized activity in the
| |
| brain before any nerve libers become myelinated« In the cat,
| |
| myelin is present neither in the peripheral nerve roots nor in the
| |
| 164 Pnvsrohoev oF THE. FETUs
| |
| | |
| tracts of the spinal cord and brain before the 42nd day of fetal
| |
| life.33 But 30 day old cat· fetuses can execute rhythmical respiratory and other coordinated reflex movements similar to those employing myelinated tracts at a later time. The early behavioral
| |
| reactions of the rat are certainly executed in the absence of
| |
| myelinated nerve fibers.32- 34
| |
| | |
| An attempt was made to correlate specific righting reflexes of
| |
| cat fetuses with development of myelin sheaths on the nerve fibers
| |
| which were involvedks It was demonstrated that the vestibular
| |
| righting reaction appears coincidentally with sheaths upon the
| |
| iibers of the vestibular nerve and that conduction pathways used
| |
| in the reaction are partially myelinated when the reflex first occurs. However, the righting response to sensory impulses from
| |
| the slcin and deep tissuesof the body, i. e» a body righting reflex,
| |
| is manifested before that employing the labyrinthine apparatus.
| |
| Its neural mechanism is incompletely myelinated at the time.
| |
| | |
| It is possible that myelination is more closely related to the
| |
| order of development of tracts in the embryonic nervous system
| |
| than to specific functional activities. Thus we find parts of the
| |
| medial longitudinal and reticulospinal systems of neurons appearing early and receiving their myelin sheaths first. But the
| |
| correlation is not absolute and many discrepancies can be ob—
| |
| served. About all that one can say is that the first tracts to develop in the embryo are the first to begin to be myelinated and
| |
| the last to form in the late fetus are the last to receive sheaths.
| |
| Some tracts never develop signiftcant numbers of myelin sheaths.
| |
| It is quite probable that conduction of impulses may ,be improved
| |
| with the acquisition of» myelin, but myelination is certainly not
| |
| an essential corollary of function. With increasing fetal si2e,
| |
| distances between points in the nervous system become greaten
| |
| Perhaps myelin is laid down to compensate by increasing the conduction speed of the iibers.
| |
| | |
| FAOTORS OTHER TIIAN STRIJCTIIRAL GROWTE
| |
| (a) THE: QmEscENcE oF IIITUJJTERME Lan!
| |
| It is relatively easy to elicit nervous activities throughout the
| |
| greater part of prenatal life in the guinea pig, cat, sheep and man
| |
| | |
| under certain experimental conditions. But it should not be assumed that all responses which can— sbe induced occur spontaneCONDITIONS REGILLATING NBRVOUS ACTIVITY 165
| |
| | |
| eously within the uterus of the normal intact individual. As a
| |
| matter of fact, there is scanty evidence that any of them occur
| |
| normally during the early part of the gestation period.
| |
| | |
| Human fetal movements can be detected as early as the 14th
| |
| weelc by means of a stethoscope, but the mother is usually unable
| |
| to feel them before the 17th weelc of gestation. It is difhcult to
| |
| diagnose them accurately much before the latter time without
| |
| considerable experiencttz for they are often confused with the
| |
| sounds produced by movements of intestinal gases. Fetuses of
| |
| experimental animals appear to be singularly quiet until late in
| |
| prenatal life when occasional quiclc jerlcs or twitches can be ob—
| |
| served upon the maternal abdomen. surprisingly little fetal activity is seen even when the thin-wal1ed uterus is delivered under
| |
| local anesthesia.
| |
| | |
| Although the few fetal movements which are readily visib1e
| |
| in the intact individual seem to be purposeless, we know that some
| |
| well coordinated and useful activities do talce place normally during the second half of prenata1 life. For examplq intrauterine
| |
| swallowing has been proved to be a normal physiologic func—
| |
| tionks This is an activity engaged in with great regularity during the last third of gestation in the guinea pig. We do not lcnow
| |
| its cause (see Chapter VII) .
| |
| | |
| (b) AFFERENT srmuxanon m Urciio
| |
| | |
| The relative quiescence of the normal fetus in utero is somewhat surprising when one considers all the activities of which the
| |
| growing specixnen is capable when removed from the uterus. The
| |
| reason seems to be at least twofold: laclc of adequate stimulation
| |
| and high thresholds in the fetal central nervous System. The
| |
| fetus is adequately nourished and warmed in a medium laclcing
| |
| practically all the stimulating iniluences of the environment with
| |
| which it will have to cope later on. No signiftcant excitation of
| |
| the external receptors occurs.
| |
| | |
| Experimental evidence in the cat supports the view that there
| |
| is little spontaneous motor discharge in the absence of afferent
| |
| impulses. In several hundred embryos and young fetuses de1ivered under good physiologic conditions and without using
| |
| anesthesia. spontaneous movements have rarely been seen at the
| |
| moment of delivery. They make their appearance within a few
| |
| 166 PHYSIOLOGY or THE: FErUs
| |
| | |
| seconds or minutes. apparently because placental exchange has
| |
| been jeopardized or becaIuse changes in the environment cause
| |
| stimulation. One can not avoid manipulation entirely, and even
| |
| though every effort is made to maintain the placental circulation
| |
| intact, incision of the uterus disturbs the relationship between
| |
| uterus and placenta. .The resulting anoxemia accounts partially
| |
| for the movements. .
| |
| | |
| If there were a true automaticity of embryonic motor neurons,
| |
| spontaneous movements should be observed in many instances at
| |
| the moment the specimens are brought into view. But there is
| |
| very little motor discharge without afkerent stimulation. Rhythmical movements have been seen in sheep embryos 40 to 5o days
| |
| | |
| old at the moment of delivery and it has been suggested that the
| |
| responses are automatic« Some are- defmitely initiated by mechanical stimulation in the younger embryos. It was proposed
| |
| that the automatic movements of the sheep fetus become inhibited
| |
| as soon as descending secondary tracts grow down into the spinal
| |
| cord from the brain stem. In the cat. no rhythmical movernents
| |
| can be obtained until after tracts have. grown down; responses
| |
| between 23 and 28 days of gestation are neither automatic nor
| |
| rhythmic.
| |
| (c) Kannst· Tkmssnoxvs ro srmvunorst
| |
| | |
| Later in prenatal life all fetuses become less responsive to
| |
| stimulation than they were at first. How much this may be due
| |
| to inhibition over newly developed descending pathways from
| |
| the brain we do not know. Guinea pig and cat fetuses near term
| |
| do not respond activeIy to ordinary manual palpation through
| |
| the intact abdomen but they can be aroused from their profound
| |
| «slu1nber« by priclcing or prodding them with a needle thrust
| |
| into the abdomen and uterus. It seems reasonable to conclude
| |
| that the fetal nervous system has developed high thresholds. At
| |
| | |
| « any rate, fetal motor centers are less excitable than they were
| |
| | |
| earlier in prenatal life and less excitable than those of newborn
| |
| individuals.
| |
| | |
| But the excitability of fetuses in utero can be enhanced ex—
| |
| perimentally. One way to do so is to reduce the oxygen available
| |
| in the fetal brain without creating complete asphyxia Partial
| |
| anoxemia at all times in prenatal life predisposes toward an in—
| |
| crease in fetal movementsz but- most activities are not actually
| |
| induced through the internal environment b"y chemical stimuli.
| |
| CONDITIONS REGULATING NBRVOUS AcTlVlTY 167
| |
| | |
| They follow mechanical stimuli which were subliminal before
| |
| the anoxemia was set up. This was demonstrated in experiments
| |
| like the following one.
| |
| | |
| At 63 days of gestation, fetuses of a decerebrated cat were
| |
| observed to be very quiet in uter0. When a needle was passed
| |
| through the abdominal wall and into the Uterus, the fetuses responded to proddingx they executed brief kicks on non-respira—
| |
| tory jerks of the head which stopped almost immediately after
| |
| stimulation ceased. With the needle still in place, the cat was allowed to rebreathe air in a rubber tube with wide bore. stimulation of the fetuses was repeatedz this time the responses were much
| |
| | |
| Fig. Sau-Three Portions of a crystograph record showing maternal respirations
| |
| (1arge was-es, 16 per minute) broken up by intrauterine fetal movementsh The
| |
| cat (63 days gestation) had been decerebrated by the anemia method; no anesthesia
| |
| was used during the experimentz the abdornen was not opened but a long needle
| |
| had been passed through the abdominal wa11 into one amniotic sac. At the heavy
| |
| solid lines, the fetus was stimulated with the needle and the irregular defiections
| |
| on the record at these points are due to this mechanical eikech Fetal rnovenients
| |
| were observed at the points indicated by the broken linesz these movements were
| |
| siight in records i and z, during which the cat was breathing air. In reoord e, the
| |
| cat was rebreathing to induce a partia1 anoxemiaz stimulation led to marked proionged fetal rnovements of a tonic squirming type (double broken 1ines) which
| |
| caused considerable interference in the maternal respiratory reoord.
| |
| | |
| H
| |
| | |
| more active, the fetuses kicked and squirmed for some time after
| |
| the cessation of the stimulus and the movements were tonic and
| |
| sustained. The rebreathing tube was then removed and as soon as
| |
| the mother’s breathing had become normal again the fetuses were
| |
| restimulated. Results were obtained like those before the
| |
| anoxemia. One experiment of this type is illustrated in the parts
| |
| of a continuous record reproduced in Fig. 62.
| |
| | |
| certain very rapid rhythmica1 movements of respiratory
| |
| muscles do appear to be elicited by endogenous chemical stimulation of the fetal respiratory center.37 They can be induced by
| |
| raising the carbon dioxide level in the blood during the early part
| |
| of active fetal life, but can not be called forth by this method in
| |
| the late fetal period unless a rather marked oxygen deiiciency is
| |
| 168 Pknsstohooy oF THE FETus
| |
| | |
| brought on too. These observations suggest a rising threshold in
| |
| the fetal nervous system of the cat with advancing prenatal age.
| |
| | |
| Respiratory rhythms are quite independent of most other
| |
| somatic movements in the cat. At the time they are first obtain—
| |
| able, they involve only the muscles which are normally used by
| |
| the adult for breathing, i. e» the diaphrag·m, intercostals and abdominals A little later in fetal life, they can be made to involve
| |
| neclc and trunlc muscles if the degree of anoxemia used to elicit
| |
| them is increase-d. Respiratory rhythms often set off other somatic movements in cat fetuses but are themselves less frequently
| |
| started by some non-respiratory twitch. Indeed, the cat fetus
| |
| when stimulated in the intact uterus can be induced to lcick or
| |
| move its head vigorously without any movement of a respiratory
| |
| nature following.
| |
| | |
| (c1) Muse-DE Tonus AND Uass Movzminrs
| |
| | |
| Not only does anoxemia facilitate the efkectiveness of subliminal mechanical stimulation and induce automatic rhythmical
| |
| respirationdilce movements but it also brings about changes in the
| |
| character of motor responses when its severity is increased beyond
| |
| the point of facilitation. Early in the gestation period of the cat,
| |
| local reflexes of the limbs are abolished more readily than those of
| |
| the trunlc and neclc under anoxemia. Throughout the middle of
| |
| prenatal life, stimulation of anoxemic fetuses leads to responses
| |
| which resemble mass reactions, any adequate stimulus eliciting
| |
| not local movements but generalized activities such as squirmingÆs
| |
| | |
| Movements of anoxemic cat fetuses laclced the «jerlcy" quality
| |
| they had shown beforehand and became more sustained and tonic.
| |
| A single stimulus often results in repeated movements suggestive
| |
| of considerable aftepdischarge in motor centers. Under marked
| |
| anoxemia, such as that following ·occlusion of the umbilical cord,
| |
| fetal muscles sometimes become so hypertonic that the fetus resembles a decerebrate animaL similar postures have been ob—
| |
| served in lcittens and rabbits (see Fig. 64) decerebrated by sectioning the brain at the rostral border of the mesencephalon.39
| |
| The increase in muscle tonus under asphyxial conditions may be
| |
| interpreted as a protective mechanism. It acts to insure the ex—
| |
| pansion of the chest which is necessakyfor air breathingfo
| |
| codxvtrtodcs Kraut-Arme Nsnnous Acrtvtrv 169
| |
| | |
| To what extent anoxemia is normal and physiologic in prenatal life is not lcnownX 0bservations in early stages of several
| |
| species of animals suggest that it may follow experimental procedures more readily in some than in others. This may be due in
| |
| part to species variation in the placenta as an organ for oxygen
| |
| exchange in early fetal life. It is possible that embryos of animals
| |
| like the sheep, which have rather primitive syndesmochorial
| |
| p1acentas, may tolerate operative procedures involving sdme
| |
| manipulation of the uterus less well than other forms such as the
| |
| cat or guinea pig, which have their maternal and fetal blood
| |
| streams in more intimate contact (see Chapter I) . Theoretically,
| |
| an equal amount of trauma would be more disastrous in the
| |
| former than in the latter. The fact that it was more diilicult to
| |
| observe the very first reliexes in sheep than in cat and guinea pig
| |
| embryos and that there is more of a tendency for movements,
| |
| sekjningly automatic, to manifest themselves in sheep than in cat
| |
| embryos, would iit into such a conception.
| |
| | |
| (e) suscsisrxgnxrr Gut-Eins ro Ast-Inn«
| |
| | |
| The exact nature of changes which depress neural thresholds
| |
| under oxygen deliciency is unknown. They may be chemical or
| |
| physical. some investigators have suggested that certain fetal
| |
| movements result from stimulation of the nervous system by
| |
| accumulating metabolic end-products, principally carbon dioxide.41-44 Although it is true that automatiq rhythmicaL respiratory
| |
| movements can be initiated by increasing the carbon dioxide content of fetal blood experimentally, those induced by asphyxiation
| |
| may be related less to an increase in the chemical stimulus than
| |
| to a depression of thresholds (increase of neuron excitability) .
| |
| | |
| It has been postulatedWs 49 that endogenous (chemical) stimulation afkects the motor centers directly and acts first on the most
| |
| recently developed units, that the new neurons have the highest
| |
| physiologic gradient and are consequently stimulated first by accumulating metabolites in the blood. The evidence is open to
| |
| question because experimental conditions were not well controlled. The embryos were studied in unbalanced saline solutions. It has been demonstrated more recently« that the waving
| |
| of embryonic limbs and tail, which suggested the theory, occurs
| |
| | |
| «« see J. Barcrofh et a1., 194o, J. Physiol» 97: 338, 347.
| |
| 17o PHYsmLooY oF THE: FETUS
| |
| | |
| only in embryos placed in solutions delicient in calcium and
| |
| potassium and is not necessarily related to accumulating metabo—
| |
| lites. ·
| |
| | |
| Under asphyxia, the behavior of fetal sheep tends to revert to
| |
| a type characterizing younger specimens but a clear cut recapitulation of development of reilex movements was not found« simple
| |
| reflexes most recently acquired apparently were not called forth
| |
| during asphyxia. In the catss it has been observed that asphyxia
| |
| abolishes activities in a rather orderly manner. It has a more
| |
| destructive action upon the appendicular motor mechanism than
| |
| upon that of the neclc ånd trunlc. The last activity to disappear
| |
| under asphyxia is a respiratory movement of the chest. This conlirms a previous observation in bird fetuses-S; it was found that
| |
| deep rhythmical gasping could be induced repeatedly by tying
| |
| and untying the allantoic vessels. It seems probable that motor
| |
| centers show gradients of susceptibility to asphyxia but there is
| |
| no evidence that the oldest reflexes, i. e» those of the neclc and
| |
| forelimb, are the last to be afkected by the asphyxia.
| |
| | |
| (k) Intmrrroa or Moroa Hammer-s n! mer-rast can-rats
| |
| | |
| It has been suggested that the changes ·in behavior of sheep
| |
| fetuses under asphyxia may result from the removal of inhibitory
| |
| inliuences of new descending nerve tracts of higher order upon
| |
| lower motor neurons« This view conforms to the conception of
| |
| a gradient of susceptibility to destruction by asphyxia, the new
| |
| higher order neurons being thrown out of function before the
| |
| older motor neurons. It also assumes some sort of automaticity
| |
| in the lower motor centers.
| |
| | |
| Transection of the fetal brain stem and spinal cord at various
| |
| levels below the mesencephalon was performed in ten fetuses zo to
| |
| 76 days old without removing them from the uterusås 0ne to 13
| |
| days later the abdomens of the ewes were opened again and the
| |
| fetuses delivered by Caesarean Section. Fetal movements at that
| |
| time resembled those characterizing unoperated specimens at 40
| |
| to 50 days gestation. It was suggested that the operations had released lower motor mechanisms from inhibitory influences of
| |
| nervous centers above the transection level in the region of the
| |
| red nucleus. 0n the other hand one investigator49 encountered
| |
| no qualitative difference in behavior of newborn rats whose cerebrum (alone or with other parts of the brain above the medulla
| |
| CONDITIONS REGULATING NERVOUS ACTIVITY 171
| |
| | |
| oblongata) had been destroyed in utero and their normal litter
| |
| mate controls.
| |
| | |
| It is probably true that sheep as well as other mammalian
| |
| fetuses are endowed with relatively lower thresholds in early fetal
| |
| life than later on toward term, and that structural growth within
| |
| the brain plays an important part in determining the nature of
| |
| thresholds The theory that motor centers are held in checlc by
| |
| the descending tracts is a very attractive one and deserves careful
| |
| study, but more experiments in other species of animals must be
| |
| performed before it can be proved.
| |
| | |
| 0ther studies have been made with rssults suggestive of the
| |
| phenomenon in question. Minlcowslciso observed a reversal of
| |
| the type of response in the human plantar reflezc During the
| |
| course of its development plantar iiexion preceded dorsal flexion
| |
| from stimulating the sole, and after the latter had become well
| |
| established it commonly changed baclc to plantar ilexion under
| |
| narcosis as well as progressive asphyxia. 0thers have disagreed
| |
| with his interpretationsJU and Personal experience has shown that
| |
| the Babinslci reflex (dorsal ilexion) of the human fetus is remarlcably resistant to asphyxia. When it does succumb, the plantar flexion which remains appears to result from direct stimulation
| |
| of the muscles in the sole of the foot.
| |
| | |
| The earliest head movemen of cat embryos are contralateral.
| |
| With further development theyschange to homolateral responses,
| |
| but under asphyxia they sometimes become contralateral again.
| |
| Extension of the head of the early sheep fetus usually accompanies the respiratory-lilce rhythms of movement, but after a time,
| |
| during which a partial anoxemia builds up, extension changes to
| |
| ilexionR These and other examples show how progressive
| |
| asphyxia exerts selective action upon the central nervous System.
| |
| similar results have been observed in adult animalssss
| |
| | |
| REFERENCES C1TED
| |
| | |
| . Davenport, I-I. A» W. F. Windle s: R. I-I. Beech. 1934. stain Tech., g: Z.
| |
| . Windle, W. F. 1931. J. comp. Neur., Zzx 71.
| |
| | |
| . Windle, W. F. 1932 Ibid., 55: gg.
| |
| | |
| . Winde, W. F. 1932 Ibi·d., 55: gis.
| |
| | |
| . Windla W. F. 1g33. Ibid., 58: 643.
| |
| | |
| . Windle, W. F. 1934. Ibid., 59: 487.
| |
| | |
| . Windle, W. F. 1g35. 1bid., 63: 139.
| |
| | |
| . Windle, W. F. s« R. F» Baxten 1936. Ibids Eis: 173.
| |
| | |
| ON! CAN-Poe v s—172
| |
| . Windle, W. F. sc R. E. Baxten
| |
| | |
| . Tello, J. F.
| |
| | |
| . Trillo, F. i938.
| |
| | |
| . Lanyworthy, O. R.
| |
| . Langworthy, O. R. i933. contn Ernb» e4: Z.
| |
| | |
| . Angulo y Gonzalen A. W. i9e9. J. Cornp. Neur., 48: 459.
| |
| . Windle, W. F., M. W. Fish sc J. E. O’Donnell.
| |
| | |
| . Beclcen R. F., W. F. Windle, E. E. Barth sc M. D. Schule.
| |
| | |
| iskiYsionocY or· THE. Fisrus
| |
| | |
| i936. Ibid.- As: i89.
| |
| Windle, W. F. i937. Proc.· soc. Expen Biol. sc Med., 36: 64o.
| |
| | |
| . Windle, W. F. sc J. E. Fitzgeralch ·i937. J. Comp. Neur., 67: 493.
| |
| | |
| scharpenbergx L. G. sc W. F. Windle. i938. J. Anat» 7e: 344.
| |
| Tello, J. F. i934. Ztschn inilc.—anat. For-seh» 36: See.
| |
| | |
| Tello, J. F. i934. Trav. Lab. Rech. Biol., Univ. Madrid, e9: 339.
| |
| i935. Ibid» so: 447.
| |
| | |
| i936. Ibid» Si: 77.
| |
| | |
| Ibid., se: i. .
| |
| | |
| Bok, s. T. i9e8. in W. von Möllendorkks Handbuch mik. Anat. Mensch.,
| |
| | |
| 4 (I)- 478—
| |
| shanen R. F. i93e. J. cotnp. Neun, H: 493.
| |
| shanen R. F. i934. Ibid» 6o: z.
| |
| | |
| Tello, J. F.
| |
| | |
| . shanen R. F. i934. Anat» 68: 3i4.
| |
| . Eos-g, l. D. Cited by D. Hooken
| |
| | |
| i936. Yale J. Biol. sc Med., 8: 579.
| |
| | |
| Jaspen H. H» C. s. Bridgman sc L. catmichaeL i937. Expen Psychol»
| |
| ei: 63.
| |
| | |
| Langworthy, O. R. i9e7. 'contr. Emb» i9: i77.
| |
| | |
| Flechsig, P. i876. Die Leitungsbahnen im Gehirn und Rüclcenmarlc
| |
| des Menschen auk Grund entwiclcelungsgeschichtlicher Untersuchungen. W. Engelmann, Leipzig.
| |
| | |
| Tilney, F. sc L. casainajon i9e4. Arch. Neun sc Psychiat» ie- i.
| |
| | |
| Lang-worthy, O. R. i9e6. Contn Kind» i7: ie5.
| |
| | |
| Langworthy, O. R. i9e8. J. Comp. Neun, 46: eoi.
| |
| | |
| Lang-worthy, O. R. i9e9. contn Emb., so: ie7.
| |
| Use. Arch. Neurol. sc Psychiat» es: i365.
| |
| | |
| i934. Ibid., 59: iZ9.
| |
| Watson, J. B. i9o3. Animal Education. Univ. chicago Press.
| |
| i94o. surg»
| |
| | |
| Gyn. sc Obst., 7o: sog.
| |
| Barcrokn J. sc D. H. Barron. i937. J. Physiol» 9i: 3e9.
| |
| | |
| Windle, W. F» M. Monnier sc A. G. steeles i93s. Physiol. Zool» it: 4e5.
| |
| | |
| . Windle, W. F. sc R. F. Becken i94o. Arch. Neun sc Psychiat, 43: 9o.
| |
| . Windle, W. F.
| |
| . Hendersom Y. i937. science, 85: 89.
| |
| | |
| . Zuntn N. i877. Pklügens Arch., i4: sog.
| |
| . Brown, T. G.
| |
| | |
| i9e9. Cornp. Neun, 48: 2e7.
| |
| | |
| i9i5. Physiol» 49: 2o8.
| |
| | |
| Grahain, E. A. i9i3-i9i5. Trans. Chicago Path. soc» g: ie3.
| |
| | |
| . Walz, W. i9ee. Monatschn Geburt. Gyn., 6o: zzn
| |
| . Angulo y Gonzalen A. W.
| |
| . Angulo y Gonzalen A. W. i934. Anat. Rec., 58: suppL 45.
| |
| | |
| i930. Proc. soc. Exp. Biol. sc Med., e7: 579.
| |
| | |
| Windle, W. F. i939. Physiol. Zool» re: 39.
| |
| | |
| . Windle, W. F. sc J. Barcrokn i938. Am. J. Physiol» iei: 684.
| |
| . corey, E. L.
| |
| . Minlcowskh M. i9e3. schweizer Arch. Neun Psychiat» i z: 475.
| |
| | |
| . Bolaklio, M. sc G. Artom. i9e6. Zeitschr. Neun Psychiat., io3: 3eo.
| |
| . Barcrokn J. sc D. H. Barron.
| |
| . Kaban H. sc c. Dennis.
| |
| | |
| i934. Proc. soc. Exp. Biol. sc Med., Zi- 95i.
| |
| | |
| ig36. J. Physiol» 88: 56.
| |
| Proc. soc. Exp. Biol. sc Med., 38: 864.
| |
| CHAPTER Xll
| |
| | |
| FETAL MOTOR REACTION s AND REFLBXES
| |
| | |
| MANY provisions are made during intrauterine like to assure
| |
| survival akter birth. A number ok these depend upon development of the ketal nervous system. 0ne function ok vital im—
| |
| portance to all species is respiration and this has been emphasized
| |
| by separate consideration in another chapter. 0ther kundamental
| |
| activities involving the somatic motor mechanisms ok the body
| |
| are sucking and swallowing which are well developed in all mammals at birth, crying which is encountered in most ok them, and
| |
| locomotion which occurs to a variable extent. ln addition to
| |
| these instinctive motor reactions, a number ok reflexes which some
| |
| have thought ok as purposekul or protective have their genesis
| |
| during prenatal like. The amount of kunctional independence at—
| |
| tained within the uterus depends upon the degree ok matssdtion
| |
| reached in the nervous system and varies within wide limits.
| |
| | |
| DEVELOPUENT OF FEEDING RBÄCTIONS
| |
| | |
| Coordinated movements ok suclcing and swallowing are kully
| |
| kormed in viable premature human inkants, and may be seen in
| |
| the common laboratory animals considerably before birth. In—
| |
| deed, suclcing appears to be one ok the kew reactions endowing
| |
| the tiny newborn opossumKs 2 Feeding reactions may be said to
| |
| begin with the iirst movements ok the jaw.3- 4 Opening and closing ok the mouth appear -in cat fetuses only about 25 mm. long,
| |
| roughly comparable with the 9 weelcs human. The tongue can be
| |
| protruded almost this early but its sides do not. curl until several
| |
| days later. similar observations have been made in guinea pigss
| |
| and sheepäs In the latter species, closure ok the jaws is the only
| |
| response obtained by touching the tongue between the 41st and
| |
| 49th days ok gestation. Throat movements occurring in briek
| |
| rhythms follow jaw closure at 49 days. The tongue ok the fetal
| |
| sheep curls at about 70 days. These early simple activities are the
| |
| korerunners ok a number ok more complex movements whose
| |
| | |
| 173
| |
| 174 PHYSIOLOGY OF THE« FETUS
| |
| | |
| integration ultimately accomplish suclcing, chewing and biting
| |
| in the cat, sheep and guinea pig. The early components of the
| |
| feeding reacti0n have not been thoroughly studied in human
| |
| fetuses but lip movements which may be related to suclcing have
| |
| been seen at about 1o weelcs and later.7- Es 9
| |
| | |
| True suclcing is rhythmical in the cat and involves a coordins
| |
| ation or integration of tongue, lip, jaw and throat movements.
| |
| Furthermore, alternate forward thrusts of the limbs and side to side
| |
| head movements enter into the feeding reaction to a remarlcable
| |
| extent in the newborn kirren. However, the rhythrnical suclcing
| |
| of the fetus is accomplished without participation of the limbs until late in the fetal period. Coronios4 observed pursing of the lips
| |
| around the tip of a glass stimulator in specimens 43 mm. long,
| |
| and at 54 mm. the first deftnite sucking coordinated with head and
| |
| forelimb movements was encountered. The response continues
| |
| to improve and appears to be fully developed and vigorous at
| |
| least one weelc before birth of the lcitten.
| |
| | |
| The actual contractions of muscles which occur in swallowing
| |
| are diiIicult to observe and can be seen only in late fetuses. These
| |
| movements have been reported in sheep of so days gestation and
| |
| we have seen them in the human fetus at 14 weelcs. Undoubtedly
| |
| they occur even earlie»1·. When the abdominal wall of a cat fetus
| |
| 25 to zo mm. long is opened the stomach may be seen to be well
| |
| lilled with iluid. In specimens a little larger, brought out into the
| |
| air, bubbles soon appear in this Organ. This early fetal swallowing of the cat is in no way remarlcable when a comparison is
| |
| made wich ehe aik-hkeekhihg, suekihz 23 day «emhkye" ek khe
| |
| opossum. »
| |
| | |
| swallowing of amniotic lluid does not seem to occur under
| |
| perfectly normal physio1ogic conditions in the fetal guinea pig
| |
| until about the 42nd day of gestation. This has been demonstrated by roentgenologic studiesso made after injecting thorotrast into the amniotic sac (see chapter VII) . The frequency of
| |
| swallowing and the amount of iluid talcen into the fetal stomach
| |
| increase with age. It may be concluded that the feeding mechan
| |
| —ism, lilce that for respiration, has its genesis in the early part of
| |
| the fetal period well in advance of the time it is normally called
| |
| | |
| into use.
| |
| FETAL MOTOR RBACTIONS AND REFLEXES 175
| |
| | |
| DEVELOPMENT OF POSTURE AN D PROGRESSION
| |
| | |
| Mammalian locomotion is a complicated act requiring cooperation of many groups of muscles and exquisite integrative development within the Central nervous System. Nevertheless, it too
| |
| has its genesis in ear1y prenatal life. Establishment of erect
| |
| posture is prerequisite for wallcing. It involves the coming into
| |
| action of righting reflexesz afkerent impulses for these arise in
| |
| the slcin and muscles of the neclc and body, in the labyrinths and,
| |
| for higher mammals including the cat, in the retina. Maintenance of erect posture requires the presence of static or postural
| |
| tonus to establish the proper neurologic balance between flexor
| |
| and extensor muscle groups in order that the force of gravity be
| |
| successfully opposed. After these conditions have been met, progression becomes possible by alternately and rhythmically changing the balance in such a way that flexion-extension stepping
| |
| movements of the limbs are performed. Developmentally, however, the various components of a locomotor reaction are not laid
| |
| down in utero in the sequence just stated.3
| |
| | |
| 1t is evident that alternation of trunlc movements is fairly well
| |
| developed in the fetus before there is evidence of a postural
| |
| mechanism. At the time bilateral flexion of the neclc and upper
| |
| trunk can be induced in cat embryos less than 20 mm. long, the
| |
| forelimbs move at the shoulders with the contractions of the
| |
| trunlc muscles. No rhythmicity manifests itself in these bilateral
| |
| trunk movements before the 25 to 28 mm. stage. Active,
| |
| arrhythmic alternation of the forelimbs can be induced in 3o to
| |
| 35 mm. fetuses with considerable regularityz in fact, crossed ex—
| |
| tension responses begin to appear at 25 mm.
| |
| | |
| The bilateral trunk flexions, synchronized more or less pas—
| |
| sively with the limbs, are forerunners of squirming movements.
| |
| Early in development the resemblance between squirming and
| |
| aquatic locomotion is rather strilcing At birth the lcitten utilizes
| |
| side to side movements in its crawling-search reaction. The reflex crossed extension following active flexopwithdrawal of one
| |
| forelimb foreshadows the act of steppi·ng. squirming and stepping
| |
| are distinct from one another until the cat fetus has reached a
| |
| length of about 50 mm., at which time the first integrated activity vaguely resembling the act of crawling can be seen. synchronized stepping movements of all fourjegs are not encountered
| |
| 176 pnYsrohooY oF THE FETUs
| |
| | |
| before 8o mm.,I1 and even at birth the hind limbs are imperfectly
| |
| coordinated with the forelimbsU Rhythmicity of forelimb stepping movements improves as the time of birth approaches. Kittens 95 to ioo mm. long delivered two weelcs prematurely manage
| |
| to crawl very credibly.
| |
| | |
| Development of Progression difkers according to species in
| |
| respect to late stages of developmenh but there is a surprising
| |
| amount of similarity at Erst. 1t requires no great imagination to
| |
| see a resemblance between the side to side head movements and
| |
| coordinated forelimb activities by means of which the opossum
| |
| | |
| »· -«.
| |
| | |
| »« «.»» . « ih ».·- --». »» -»·-,», - · s 1
| |
| »«"«·--:«-»«,-«·-s.cå4 ANY« ;-,4,!" «,««’ JdzpVsstwsJt «Q4.Ez7:-G« IN« «« «« «« Es« «« - "·««7 V H ««
| |
| | |
| Fig. 63.-Record of muscle tonus in a sheep fetus at 144 days gestation delivered
| |
| at caesarean Section with placental circulation intact. In the Erst, third and iifth
| |
| | |
| records, the fetus lay quietly in a warm saline bath. In the second and fourth
| |
| records it was lifted into the air and impulses began to flow to the muscle-s. (Bak
| |
| croft: Irish Joutx Med. sei» 1935.)
| |
| | |
| «embryo" reaches the mother’s pouch at birth and the side to side
| |
| necl(, trunlc and forelimb movements of the so mm. cat fetus.
| |
| similar reactions have been observed in rat13- «« and guinea pig
| |
| fetuses.5 The records are very incomplete in the human, but we
| |
| have seen Hexor withdrawal of one leg accompanied by crossed
| |
| extension of the opposite leg in 4o mm. specimens Rhythmicity
| |
| of stepping has not been reported until much later in fetal life
| |
| and even at birth behavior of a locomotor type is most ineffectuaL
| |
| | |
| Development of postural tonus has not been studied thorough1y.
| |
| 1t is probable that muscle tone as we know it in the adult is not
| |
| present at all in utero under nor-mal physiologic conditions. The
| |
| FETAL Mosron KEACTIONS AND nEFLExEs 177
| |
| | |
| fact that rhythmical respiratory movements can occur toward the
| |
| end of gestation without aspiration of a signiftcant amount of
| |
| amniotic fluid demonstrates that the fetal chest is not held tonically in an elevated position which it must assume after birthLE
| |
| The absence of afferent stimulation in utero is unquestionably an
| |
| important factor in maintaining tonus at a low level. As illus—
| |
| trated in Fig. 63, it was found that action potentials from fetal
| |
| muscles appear when a fetus is lifted out of its warni saline bath
| |
| and disappear again when it is returned to the bathJC The relation of anoxemia to thresholds of nervous activity and to tonus
| |
| has been discussed in the preceding chapter.
| |
| | |
| How early in prenatal life postural tonus can be induced ex—
| |
| perimentally is not deiinitely known, but an indication of it may
| |
| be seen in cat fetuses about 50 mm. long. It was noticed that re
| |
| » lease from their membranes was followed by straightening of the
| |
| | |
| back and extension of all limbs in such a way that they appeared
| |
| to stretch. Full term birth posture, i.e., extension of the head
| |
| and forelimbs," seem to be induced in part by a similar release
| |
| of tension when the membranes burst. A factor in development
| |
| of muscle tonus may be observed in the sustained fetal movements
| |
| during anoxemia. sustained extensor movements are sometimes
| |
| so marked in cat fetuses only 30 mm. long that they resemble the
| |
| decerebrate condition.
| |
| | |
| Decerebration of cat fetuses results in hyperextension of the
| |
| limbs during the last three weeks of prenatal life.3- IS 0ne or two
| |
| weeks before birth deiinite decerebrate rigidity appears in the
| |
| forelegs of specimens in which the brain has been cut through
| |
| from the rostral border of the mesencephalon to the rostral border
| |
| of the pons. When the level of the transection passes farther for—
| |
| ward, leaving the region of the red« nucleus intact, decerebrate
| |
| rigidity fails to appearRss IV Postural tonus can be called forth in
| |
| the human fetus by decerebration20 and there too it seems to be
| |
| more especially related to the midbrain and lower centers than to
| |
| the higher parts of the nervous System. Decerebrate rigidity in
| |
| the rabbit after birth is illustrated in« Fig. 64.
| |
| | |
| The mechanism by which the righting reaction evolves likewise has its development in the early fetus, but the actual accomplishment of righting has not been observed until after postural
| |
| | |
| «tonus and alternate stepping movements can be induced« Cat
| |
| 178 PHYs1oLooY OF THE FETUs
| |
| | |
| fetuses 75 to 1oo mm. long try to hold their heads up and turn
| |
| their jaws parallel to the ground when they are placed upon a Hat
| |
| surface. The general impression gained is that they could right
| |
| themselves save for the weakness of their muscles. Between 1oo
| |
| and 1 Io mm., they are actually able to right the head in respect
| |
| to the surface on which they 1ie, but they are completely disoriented when placed in warm water beyond their depth. No
| |
| evidence of vestibular function was obtained in cat fetuses less
| |
| than iio mm. in lengtlr Neither rotation of the specimens nor
| |
| destruction of one or both labyrinths experimentally had any
| |
| efkect before this time. It was concluded that the vestibular right
| |
| Fig. 64.———l)ecerebrate rigidity in a young rabbiL The midbrajn was sectioned through the rostral border of the superior colliculus and rostral third of
| |
| | |
| the pons.
| |
| | |
| ing reflex appears about the 54th day of prenata1 life in the cat
| |
| and that a body righting reklex precedes it by at least four days.
| |
| The latter is activated by akferent impulses from the skin and
| |
| deep tissues of the body and necl(. Visual impulses play no part
| |
| in righting reactions until Some time after birth of the kitten
| |
| whose eyes remain closed for several days. ln the newborn kitten
| |
| the vestibular righting reflex is still incompletely developed.22
| |
| Development of a body righting reaction before the vestibular
| |
| mechanism begins to function has been conHrmed in other species.
| |
| In the sheep, which is more mature than the cat at birth and has a
| |
| longer gestation period, righting is accomplished relatively ears
| |
| FETAL MOTOR REACTIONS AND RBFLBXES 179
| |
| | |
| lier.3 0rientation in respect to gravity, seen in the opossum
| |
| at the time of birth, which is long before the vestibular mechanism
| |
| is functional, is an interesting related phenomenonks
| |
| | |
| In all animals in which fetal studies have been made, three
| |
| components essential for locomotion—righting, postural tonus
| |
| and alternate synchronous limb movements—have been developed
| |
| by the time of birth. All three are present in some form well
| |
| before the end of the gestation in most species, thus providing a
| |
| factor of safety against the danger of premature interruption of
| |
| | |
| intrauterine life.
| |
| DEVELOPMENT OF EYE REFLEXES
| |
| | |
| We have considered the prenatal development of motor mechanisms for respiration, feeding, posture and locomotion in some
| |
| detail. 0ther equally interesting fetal activities could be studied
| |
| profttably, but at the present time little is known about them.
| |
| some observations on movements of the eyes and eyelids have
| |
| been made but they are incomplete.
| |
| | |
| The eyeballs move behind closed lids in the fetuses of several
| |
| species. such movements can be seen at the middle of the gesta—
| |
| tion period in guinea pigss when the face is stimulated in the
| |
| neighborhood of the eyes. somewhat later, postural changes
| |
| elicit eye movements. Reactions to light appear during the third
| |
| quarter of prenatal life. In the cat, eye movements in response to
| |
| vestibular stimulation were not obtained before birth.« Most
| |
| l(ittens 5 to 7 days old showed ocular nystagmus during and after
| |
| stimulation of the labyrinthine afferents by rotation.
| |
| | |
| Contraction of the orbicularis oculi muscle can be elicited
| |
| in cat and guinea pig fetuses at about the middle of the gestation
| |
| period. The palpebral reflexes for protection of the eye have
| |
| their genesis in these movements and are well developed in late
| |
| fetal life, as can be determined by opening the eye experimentally
| |
| and stimulating the cornea. Contraction of the human orbicularis oculi was seen at about 12 weelcs (4o mm.) when the eye
| |
| region was touched.
| |
| | |
| Very little is known about the early development of light reflexes. The iris of guinea pig fetuses contracts in response to light
| |
| early in the last third of prenatal life. The visual mechanism of
| |
| this animal is more advanced than that of the cat or man at birth.
| |
| 180 pnvstohoov oF THE: Fnrus
| |
| | |
| DEVELOPMENT OF PALMAR AND PLANTAR REFLEXES
| |
| | |
| several investigatorsUss 28 have studied the movements of the
| |
| digits of the hand which ente·r into the prehension reaction of
| |
| the fetus. These constitute the palmar or grasp reflex. HoolcerV
| |
| observed Hexion of the iingers but not the thumb when the
| |
| palm of a human fetus of 11 weelcs was touched, and it may
| |
| occur even earlier. At 12 weelcs the. fetus formed a true list
| |
| by flexing the thumb and fingers. Later, around 16 weelcs, when
| |
| the iingers were held in a flexed posture stimulation of the palm
| |
| brought about tightening of the iingerss Thumb movements
| |
| were never as marked as those of the iingers and apposition of the
| |
| thumb did not occur until after birth. Eikective sustained gripping of objects with the lingers began to manifest itself around
| |
| 18 weelcs but even at 25 weelcs it was not strong. The grasp reflex appears to have two componentst iinger closure and gripping
| |
| | |
| The genesis of the human plantar reflex has interested many
| |
| because of its practical importance in neurologic diagnosis. Although several have studied it in prenatal life,25-29 Minlcows
| |
| slci’s7s S« 30 observations have been the most complete. spontaneous
| |
| dorsal ilexion of the great toe characterized the early fetal period.
| |
| No responses to stimulating the sole of the foot were obtained
| |
| before Io weelcsz at this time one specimen exhibited a plantar
| |
| flexion of the foot right after delivery. This was of very brief
| |
| duration and when it was no longer elicitable, due to progressive
| |
| asphyxiation of the specimen, only direct muscle responses could
| |
| be obtained.
| |
| | |
| In fetuses of 1 1 to« 15 weelcs gestation, stimulating the sole of
| |
| the foot sometimes produced dorsal flexion of the foot or of the
| |
| great toe with spreading of the others. These responses, which
| |
| constitute the Babinski phenornenon, were obtainable only when
| |
| local anesthesia had been used and in the first few minutes of the
| |
| observations. General anesthesia and progkessive asphyxia led
| |
| to an inversion of the response, in which the same type of stimulus
| |
| brought about plantar instead of dorsal Hexion. section of the
| |
| cervica1 spinal cord or brain stem did not alter the plantar re—
| |
| flexesz they could be observed for longer periods before they
| |
| changed from the dorsal to the ventral type of response. There
| |
| was only slight indication that higher centers participated in the
| |
| dorsal form of plantar reflext before— 6 months gestation.
| |
| FETAL MOTOR REAcTIONs AND REFLEXES 181
| |
| | |
| Minlcowslcis named the earIy period up to i6o mm. C. H.
| |
| length the neuromuscular stagez the period between 160 and i8o
| |
| mm. he called the spinal stagez the period between 190 and 270
| |
| mm., the tegmento-spinal stagez and the remainder up to birth,
| |
| | |
| the pal1ido—cerebello-teg-inentosspinal stage. These divisions were»
| |
| | |
| set somewhat arbitrarily, for the number of observations was
| |
| limited and the physiologic conditions of specimens varied to a
| |
| great extent. The phenomenon of inversion of the response has
| |
| been discussed in chapter XI. The subject of plantar responses
| |
| of infants and young children has been thoroughly reviewed recemiy by Richakds and Ikwixxss «
| |
| | |
| OTHER REFLEXES
| |
| | |
| Throughout the literature, mention has been made of many
| |
| other reactions and reflexes not only in experimental animals but
| |
| also in human fetusesF For the most part, the course of development of the slcin, tendon, deep neclc and other reflexes has not
| |
| been followed completelsy and these activities do not Iend themselves to signiücant discussion for this reason. Here are Helds
| |
| which future investigations may be expected to explore very
| |
| proütably.
| |
| | |
| REFERENCES CJITED
| |
| | |
| i. Hartman, c. G. i9eo. Anat. Ren, i9: e5i.
| |
| e. Mccradzz E. i938. The Embryology of the Opossunn Wistar Press,
| |
| Philadelphia
| |
| | |
| . Windle, W. F. sc A. M. Griklin igzn J. comp. Neur., se: i49.
| |
| | |
| . Coronios, J. D. i933. Genetic PsychoL Monog., i4: e83.
| |
| | |
| . carmichaeh L. i934. Ibid., i6: 337.
| |
| | |
| . Barcrofn J. sc D. I-I. Bari-on. i93g. J. comp. Neur., 7o: 477.
| |
| Minlcowskh M. i9ee. sehr-v. med. Wchnschn, se: 7ei, 75i.
| |
| Minkowskh M. i938. Abderhaldecks I-Iandb. biol. Arbeitsmetlk Abt.
| |
| | |
| V, Teil 5 B: zu.
| |
| g. Hooken D. i936. Yale J. Biol. sc Med., s: 579.
| |
| o. Becken R. F., W. F. Windle, E. E. Barth sc M. D. Schule. i94o. sur-g.
| |
| Gyn. sc Obst., 7o: sog.
| |
| | |
| ii. Brown, T. G. i9i5. J. Physiol» 49: Los.
| |
| | |
| te. Tilney, F. sc L. casamajon i9e4. Arch. Neur. sc Psychjat., ie: i.
| |
| | |
| i Z. swenson, E. A. i9e6. Thesis, Unin Kam.
| |
| | |
| i4. Angulo y Gonzalen A. W. i93e. J. comp. Neur., 55: 395.
| |
| | |
| i z. I-Ienderson, Y. i938. Adventures in Respiration, Williams sc Willcins,
| |
| | |
| Baltimore.
| |
| is. Barcroln J. i935. Irish J. Med. sei» series 7, i: e89.
| |
| i7f Rudolpln L. sc A. c. 1vy. i933. Am. J. Obst. Gyn., es: 74.
| |
| | |
| Pf« EVEN«
| |
| | |
| l
| |
| 182 PHYsmLooY oF THE. FETUs
| |
| | |
| 18. Windle, W. F. 1929. J. comp. Neun, 48: 227.
| |
| | |
| 19. Langworthzz 0. R. 19291 Contrilx Emb., so: 127.
| |
| | |
| so. Minlcowslcjs M. I921. Ren Neun, igsu I1o5, I235.
| |
| | |
| tu. Winde, W. F. Z: M. W. Fish. 1932 J. comp. Neun, 54: 85.
| |
| | |
| u. Gen-Michael, L. i934. Genetic Psychoh 44: 453.
| |
| | |
| as. Larsell, 0., E. Mccrady s: A. A. Zimmermann. 1935. J. Comp. Neun,
| |
| 632 os
| |
| 24. Fish, M. W. s: W. F. Winde. 1932. J. Comp. Neun, 54: 1o3.
| |
| | |
| es. Bot-cito, M. sc G. Artom. 1924. Ast-eh. di sei. Bio1., z: 457.
| |
| | |
| es. I-Iool(et, D. i938. Proc. Am. PhiL soc» 79: 597.
| |
| | |
| 27. Krabbe, K. «1912. Rest. Neuro1.. 24: 434.
| |
| | |
| as. Bersoh I-I. 192i. sehn« Arclx Neun Z: Psychiat., s: 47.
| |
| | |
| 29. I-Ioo1:er, D. 1939. Atlas ok Early Human Fetal Behavior (Pkivate1y
| |
| Printed).
| |
| | |
| so. Minkowslch M. 1926. c. R. cong. Med. Alienistes Neurologistes
| |
| Geneve so: got.
| |
| | |
| 31. Richards T. W. Z: O. c. Irwin. 1935. Unisn Iowa stud. child Welkare, u (No. 1): I. «
| |
| CHAPTER XIII
| |
| | |
| THE FETAL sENsEs
| |
| | |
| IN discussing sensory mechanisms before birth the be1ief is
| |
| not implied that the fetus is consciously aware of any sensation
| |
| either in utero or after removal. Interest lies solely in whether
| |
| or not the various endiorgans and afkerent neurons can function
| |
| before birth. This is determinable by observation of the reflex
| |
| motor effects of stimuli of different kinds applied to the fetus. It
| |
| is seldom possible to be certain of the nature of neurons stimu1ated. Whether they conduct painful or tactile atkerent impulses,
| |
| or even whether exteroceptive or proprioceptive, following a given
| |
| stimulus is usually undeterminable.
| |
| | |
| Almost everyone who has studied fetal movements has contributed information to this subject, but it is impossible to
| |
| evaluate and coordinate all the data. We are faced in most in—
| |
| stances with the task of trying to synthesize experiments performed
| |
| under good and bad conditions to arrive at some lmowledge of
| |
| the- subject. No one would thinlc of accepting results in adult
| |
| physiology of sense org-ans obtained in animals under narcosis
| |
| and asphyxia Yet that is the lcind of data predominating in
| |
| respect to the fetus.
| |
| | |
| It would seem that variations among species as well as in animals of the same species by different investigators are explainable
| |
| td a very great extent on the basis of experimental methods used
| |
| to study fetuses. When specimens are examined without using a
| |
| general anesthetic and when they are exposed very quickly it is
| |
| found that they are excitable by much milder forms of stimuli
| |
| than must be used a minute or so later when anoxemia has begun
| |
| to afkect them.I Furthermore, grossly undetectab1e deterioration
| |
| of the physio1ogic condition of fetuses after delivery, even when
| |
| the placental circulation remains intact, aEects some sensory
| |
| nerves more than others and appears to alter synaptic mechanisms
| |
| in such a way that motor responses change their Character. These
| |
| efkects are especially well illustrated in human fetuses at hyster
| |
| 183
| |
| 184 PHYsmLooY oF THE: FETUs
| |
| | |
| otomy performed under local anesthesia. The human fetus of the
| |
| third and fourth monthsslies quietly within its crystal clear amnion. A very little pressure, such as follows tapping the membrane lightly, causes it to malce quiclc jerlcy movements of arms,
| |
| legs and other parts. When the stimulus stops, the movements
| |
| stop. 0n the other hand, when the placenta is detached and the
| |
| fetus removed from its membranes it· executes «spontaneous"
| |
| squirming movements which are more sustained and tonic than
| |
| those seen at first. Fewer slcin regions are sensitive to the lighter
| |
| forms of stimulation than was the case in amnio. Excitability
| |
| diminishes rapidly. Most human fetuses have been studied after
| |
| removal from the uterus2s Z« 4 and it was only recently that the
| |
| opportunity arose to observe them at the moment of delivery
| |
| while the placenta was intact and before the amnion was rupturedP consequently our lcnowledge of sensation in human
| |
| fetuses is still very incomplete. ·
| |
| | |
| THE FETAL sKIN As A RECBPTOR ORGAN
| |
| | |
| (a) Pnsssunx Touckt Am) Pan(
| |
| | |
| Motor function precedes sensibility.·3—8 1n mammalian embryos it is always possible to obtain contractions of slceletal muscles by stimulating them directly before any of the sensory neurons
| |
| can be activated. spontaneous movements of the chick embryo
| |
| occur in advance of the reactions which follow stimulating the
| |
| surface of the body,9- 10 but this is not the case in mammals.
| |
| | |
| The superlicial epithelium with its underlying mesenchymal
| |
| connective tissue serves as a receptor organ in early fetal life.
| |
| Nerves course beneath the epithelium and end under it in primitive free terminations before any reflexes can be elicited in mammalian embryos.1I-I2 They appear in the face and forelimbs before they can be seen in the hind limbs and tail. Reflex responses
| |
| follow stimulating them in cat embryos about 14 mm. long, Ibut
| |
| the stimuli must be somewhat stronger at that time than later.
| |
| Mild faradic shoclcs call forth responses before it is possible to
| |
| obtain them by touching the epithelium with a single hair or a
| |
| soft brush. A little later, in specimens about 15 mm. or 16 mm.
| |
| long, touching with a single hair often serves adequately, providing the stimulus is placed directly over a spot supplied by
| |
| primitive afkerent nerve übers. stimulation with a little brush
| |
| THE FETAL sENsEs 185
| |
| | |
| made up of soft hairs is more often effective than a single punctate stimu1us,7-8-13-I4 because there is greater chance of pressing
| |
| upon one of the sparse endings with it.
| |
| | |
| It is relatively easier to lind reflexogenous spots upon the face
| |
| than upon the limbs. Furthermore, -responses to stimulating endings in the forelimb disappear before those from the face, under
| |
| the influence of progressive anoxemiaJ Consequently, the failure
| |
| to elicit fetal movements by punctate stimulation of the epithelium of a limb does not prove the absence of a response-producing mechanism in the limb unless careful consideration is given
| |
| to experimental conditions.
| |
| | |
| With further growth the number of nerve endings in the con—
| |
| nective tissue beneath the epithelium increases and the Ebers
| |
| begin to penetrate the epithelium itself. It becomes progressively
| |
| easier to lind points whose stimulation elicits motor responses.
| |
| Development proceeds in a cephalo-caudal direction in the body
| |
| and proximodistally in the limbs. 0n the other band, rising
| |
| thresholds, either in the endings themselves or in the central nervous System, soon bring about a condition of diminishing excitability of many cutaneous surfacesJs
| |
| | |
| 0ne is scarcely justiiied in classifying the early sensory func·
| |
| tions as touch or pain. strictly spealcing, the fetus experiences no
| |
| sensation whatsoever; it simply responds automatically, reflexly,
| |
| in the early part of prenatal life. It is true that the neurons are
| |
| activated by external environmental changes and may be considered exteroceptive, but there is nothing about their structure
| |
| and nothing about the response itself which would indicate that
| |
| some subserve pain and others touch or cutaneous pressure.
| |
| | |
| It is the opinion of some investigators that both pain and touch
| |
| are difkerentiated in late fetal life. Very little difkerence could
| |
| be observed in cat fetuses between responses elicited by coarse
| |
| but innocuous stimuli and ones which produced demonstrable
| |
| trauma until after the 45th day of gestationJ Even at full term,
| |
| pain, touch and pressure are not well differentiated Raney and
| |
| carmichaels have dealt with the question of localization to tactual
| |
| stimuli in relation to the genesis of space perception in the rat.
| |
| They found greater speciiicity of response as the time of birth
| |
| approached.
| |
| 186 PHYsIoLooY or THE FETUS
| |
| | |
| (b) Tauf-www ssnsmvrrr
| |
| | |
| Only one attempt has been made to study the eifect of tempera—
| |
| ture stimulation systematically throughout the entire fetal
| |
| periodss Physiological saIine solutions of different temperatures
| |
| were applied in drops upon six representative cutaneous areas of
| |
| guinea pig fetuses and the motor responses were recorded with a
| |
| motion—picture camera. The parts stimulated were vibrissae
| |
| area, ear, shoulder, rump, forepaw and hind paw. Control tests
| |
| were made with the solution at body temperature. Responses
| |
| were obtained throughout most of the fetal period but the warmer
| |
| or cooler the solution the greater their number. Cold solutions
| |
| | |
|
| |
| | |
| kraus-o mous- ussde am)
| |
| Ein-o sum» tsssas now
| |
| Usoio eng-«- (so-s3 Im)
| |
| | |
|
| |
|
| |
| | |
| 95
| |
| | |
| o; Tom. «
| |
| Its-sonstcticireo
| |
| m net( I
| |
| se! est-up
| |
| | |
| lIlIIIIIllllllIlt(lIIIIlIllIllllllI
| |
| Illllllllsltlllllltl
| |
| | |
| lllIllIllll
| |
| | |
| Illcsllllllllislllk
| |
| IIIIIIIIIIIIIIIIIIIIIIIIIIIII
| |
| tlttttIttlIlitt«lIItIsIItIIstIItIItstIists
| |
| | |
| s s n« II« «« es« as« c
| |
| senken-nun:
| |
| | |
| Essen-Ists o- cvinekpsc kkrusks w meinst. STZIUIII
| |
| | |
| Fiz 65.—Temperature sensitivity in guinea pig fetuses. (carmichae1 s: Lehnen
| |
| J. Genetic Psycholsp Vol. so, 1g37.)
| |
| | |
| IIIlIlIlIIIIIlIllllIIIIIIllIIIIlllIlIlIIIIII
| |
| | |
|
| |
| | |
| appeared to be a little nsore effective than warm during the early
| |
| part of the motile period. sensitivity increased with age to some
| |
| extent but the growth of hair modified the effectiveness of stimuIation in the older specimens Furthermore, there »was evidence
| |
| of sensitivity spreading from cephalic to caudal and from pro-c—
| |
| imal to distaI parts as development progressed. Fig. 65 illustrates
| |
| | |
| the relative eEectiveness of solutions of Various temperatures used
| |
| in three age groups of fetuses.
| |
| | |
| PROPRIOCEPTIVE FUNCTION IN THE PETUS
| |
| It is quite possible that afferent nerves of the deeper fetal cis—
| |
| sues such as muscles and joints become functionaI very early.
| |
| Some of the first responses of mammglian embryos may resu1t
| |
| THE FETAL sENsEs 187
| |
| | |
| krom their activation. Movements ok the primitive limbs can be
| |
| induced by bending the limbs or by tapping on them. They can
| |
| lilcewise be obtained without touching the embryos at all by
| |
| tapping lightly upon the fluid filled amniotic sac containing a
| |
| specimen, but one does not know what nerves are being stimulated. since akkerent neurons are present in the connective tissue
| |
| just underneath the epithelium, it is just as likely that the response is due to exteroceptive as to proprioceptive stimulation.
| |
| | |
| Nevertheless there are many observations suggesting that
| |
| primitive endings in the muscle are capable ok being stimulated
| |
| by the middle ok the gestation period or a little latet. The stretch—
| |
| ing ok the ketus upon opening the amniotic vesicle and thus
| |
| changing the pressure upon the specimen is a case in point. 0ther
| |
| observations on the development ok muscle tonus and the tonic
| |
| neclc and body righting rellexes leave no doubt that proprioception is present, and well kormed, considerably bekore birth.
| |
| | |
| 1n newborn rats whose spinal cords were sectioned completely
| |
| during intrauterine like it was often very diklicult to determine
| |
| by physiologic tests that nerve pathways had been interr"upted.
| |
| The animals responded to stimulation ok points below the level
| |
| ok section much as did their unoperated litter mates.I7 It was
| |
| suggested that in these very immature animals reflex movements
| |
| below the lesion were responsible kor stimulating proprioceptive
| |
| endings in muscles above, setting up proprioceptive reilex movements ok which the rats were aware and in this way acquainting
| |
| | |
| «the rats, as it were, with what was happening in a part ok the body
| |
| | |
| from which no direct messages could be received. The spread ok
| |
| activity krequently seen in much less mature mammalian ketuses
| |
| ok other species suggests a mechanism ok a similar sort. Ik proprioceptive kunction plays a part in the responses ok the early human
| |
| ketus it is certain that it does not require highly specialized
| |
| neuromuscular spindles because these structures do not appear
| |
| until about the third monthÄs
| |
| | |
| Function ok the vestibular mechanism begins rather late in
| |
| ketal like ok the can« In other species it may be present relatively
| |
| earlier, as seems to« be thes case in the sheepJs However, caution
| |
| must be exercised in attempting to determine its presence, kor
| |
| righting reflexes and eye movements can be induced by stimulat188 PHYsmLoeY oF THE FETUs
| |
| | |
| ing other receptors such as those of the neck and body. The
| |
| righting reiiexes have beens discussed in the preceding chapter.
| |
| | |
| OLPACTORL GUSTATORY AND VISCERAL sENsEs
| |
| | |
| The olfactory apparatus is made ready during prenatal life,
| |
| but it is doubtful if adequate stimuli are ever present in utero
| |
| where no air comes into contact with the nasal recept0rs. In the
| |
| newborn and prematurely born human infant, various observers
| |
| have obtained evidence of olfactory function.20s 21 It has been
| |
| pointed out, however, that common chemical receptors of the
| |
| trigeminal nerve are readily stimulated by strongly aromatic materials and they must not be confused with true olfactory
| |
| phenomena.
| |
| | |
| Taste has been demonstrated at birth in man as well as in
| |
| lower animals, but it is doubtful if dikkerentiation between sour,
| |
| salt and bitter is very well formed. The day old lcitten can distinguish between millc and a mixture of millc and sodium chloride.22 The experiments of De snooks who injected saccharin
| |
| solution into the amniotic sac of women sukkering from polyhydramnios, seem to indicate that the fetus responded to the sweet
| |
| taste and swallowed unusually large quantities of the amniotic
| |
| fluid.
| |
| | |
| Regarding other visceral aiferent stimulation, nothing is
| |
| known. 0ne can speculate that the normally occurring intestinal
| |
| movements stimulate afkerent neurons. Perhaps the active swallowing of amniotic Auid by fetuses in the last months of gestation
| |
| is reflexly controlled by» such a mechanism. Vigorous «hunger"
| |
| contractions of the stomach are found in prematurely delivered
| |
| mammals.24 .
| |
| | |
| IIEARING AND VISION
| |
| | |
| Hearing has been considered to be imperfect «at birth but
| |
| seems to improve within a short time after the amniotic fluid and
| |
| secretions drain from the middle ear.25 — Prematurely delivered
| |
| infants show evidence of audition a little while after birth. Some
| |
| investigators hold that the respiratory changes observed in the
| |
| human infant concomitant with the production of sounds signify
| |
| a functional auditory mechanism. «
| |
| | |
| Attempts were made by PeiperW to observe changes in intra—
| |
| uterine activity associated with Hund. »Alt.hough it was diflicult
| |
| THE FETAL sENsEs 189
| |
| | |
| to rule out stimulation of the fetus by the mother’s own responses,
| |
| the evidence suggests that strong sounds may have initiated reflex
| |
| movements of the fetus near term. 0thers have confirmed these
| |
| results« We have found that very slight tapping upon the amnion at the time of hysterotomy under local anesthesia results in
| |
| similar quiclc fetal movements even at a much earlier time in
| |
| prenatal life) such stimuli need not be thought of as sound producing. It is possible that the strong sounds, especially in the
| |
| case of tapping upon a metal bath tub in which the pregnant
| |
| woman 1ay, were not in themselves the cause of the fetal responses,
| |
| but that pressure was transmitted to the fetus as in our own ex—
| |
| periments. 0n the other hand, sontag and Wallace have presented good evidence that the human fetus does react in utero
| |
| to sound producing stimuli app1ied externally to the mother’s
| |
| abdomen. The fetal responses (movements) became more
| |
| marked as term approachedks Electrical responses have been re—
| |
| corded from the fetal cochlea after auditory stimulation in guinea
| |
| pigs of 52 days gestation.29 This was the earliest period at which
| |
| this species reHd overtly to such stimuli.
| |
| | |
| Although there can be no stimulation by 1ight before birtln
| |
| it is probable that the visual mechanism is functional t«o an imperfect degree in late prenatal life. 0bservations in premature
| |
| infants indicate that some sort of differentiation of light and dark
| |
| is present. Pupillary responses to strong light can be obtained
| |
| in late fetal life.
| |
| | |
| REFERENCES cITED
| |
| | |
| r. Windle, W. F. sc R. F. Becken 194o. Arch. Neur. sc Psychiat., 43: ge.
| |
| . Minkowslci. M. 1938. Abderhaldecks Handb. biol. Arbeitsmeth., Abt.
| |
| V, Teil 5 B: Zu.
| |
| | |
| . Bolaflio, M. sc G. Artom. 1924. Arch. di sei. Biol., H: 457.
| |
| | |
| . Hoolcen D. 1936. Yale biol. sc Med., s: 579.
| |
| | |
| . Fit2gerald, E. sc W. F. Windle, unpublished observations
| |
| | |
| . Preyer, W. 1885. spedielle Physiologie des Embryo. Gnaden, Leipzig.
| |
| . Windle, W. F. sc A. M. Griiiiw xgsn J. comp. Neun, se: 149.
| |
| | |
| . Raney, E. T. sc L. carrnichaeL 1934. J. Genetic Psychol., 45: Z.
| |
| | |
| g. 0rr, D. W. sc W. F. Windle. 1934. J. comp. Neur., 6o: 271.
| |
| | |
| to. lcuo, Z. Y. 1932 J. Exp. Zool» Hi: 395.
| |
| | |
| u. Windle, W. F. 1937. Proc. soc. Exp. Ziel. sc. Med., 36: 64o.
| |
| | |
| is. Windle, W. F. sc E. Fitzgerald. 1937. comp. Neun, 67: 493.
| |
| | |
| is. coronios, J. D. 1933. Genetic PsychoL Monog., I4: 283.
| |
| | |
| i4. carmichaeh L. 1933. In c. Murchisocks Handb. child Psychol., and
| |
| | |
| ed., clarlc Univ. Press, Worcester.
| |
| | |
| U
| |
| | |
| IN! GIVE-Abs
| |
| 190
| |
| | |
| IOIAIOIOIIIAIOU Obschon-HiYPOVPSOPEVP PPYPOJTSIWI
| |
| | |
| PHYSIOLOGY OF THE FETUS
| |
| | |
| . Bat-Gott, J. s- D. H. Bau-on. 1939. J. comp. Neun, 7o: 477.
| |
| | |
| cartnichaeh L. s- G. F. J. Lehnen 1937. J. Genetic Psychol., so: 217.
| |
| Hoolten D. s- J. s. Nicholas 193o. J. Comp. Neun, so: 413.
| |
| | |
| . cuajunco, F. 1927. contn Emb., 19: 45.
| |
| | |
| Winde, W. F. s- M. W. Fish. Igzn J. comp. Neun, 54: 85.
| |
| | |
| Peipetx A. 1928. Ergebw inn. Med. Kinderhlh 33: 5o4.
| |
| | |
| Frau, K. c» A. K. Nelson s- K. H. sun. 193o. The Behavior of the
| |
| | |
| Newborn Inkanr. Ohio state Univ. studies, No. to.
| |
| | |
| Pkalkmanm C. 1936. J. Genetic Psychol., 49: Si.
| |
| | |
| De snoo, K. 1937. Monatschtz Geburtsh. Gyn., 1o5: As.
| |
| | |
| carlson, A. J. s- I-I. Ginsburg. Ists. Am. J. Physiol» 38: 29.
| |
| | |
| Peterson, F. s- I.. I-I. Rainezu 191o. Bu11. N. Y. Lyingsin I-Iosp., 7: 99.
| |
| Peipen A. 1925. Monatschtn Kind-Ethik» sey: 236.
| |
| | |
| . Fort-es, H. s. s- I-l. B. Fort-es. 1927. J. Gott-F. Psychol., 7: 353.
| |
| . sontag, L. W. and R. Wallacn
| |
| | |
| child Developmeny 6: 253.
| |
| 1938. Exp.
| |
| | |
| I935Rawdonismith A. F» L. carmichael and B. We1Iman.
| |
| | |
| Psychol., es: zzn
| |
| CHAPTER XIV
| |
| | |
| THE FETAL ENDOCRINE GLANDS
| |
| | |
| Qui: lcnowledge of endocrine functions during prenatal life is
| |
| fragmentary as may be expected from the fact that adult glands of
| |
| internal secretion are still incompletely understood and their
| |
| relationship to one another only partly determine-d. There
| |
| seems to be little doubt that a few of the maternal hormones do
| |
| influence embryonic deve1opment, but not all can pass the pla—
| |
| cental barriers The present deftciency of information concem—
| |
| ing placental transmission of hormones is a factor limiting any
| |
| discussion of their activities in the fetus. Perhaps the secretions
| |
| of the fetus itself are equally or more important than those of the
| |
| mother for the well being and normal metabolism of the new
| |
| individual. It is with their functions that we shall be especially
| |
| concerned.
| |
| | |
| THE SUPRARENAL OORTBX
| |
| | |
| Among all the endocrine glands of the humazfetus the
| |
| suprarenals manifest the most remarlcable peculiaritiesks 3 Exam—
| |
| ination of them in the still—born infant reveals that they are proportionately very much larger than at any time after birthx in fact
| |
| they form o.2 per cent of the entire body weight. Those of the
| |
| adult constitute only o.o1 per cent.4 The reason for their great
| |
| size is found in an hypertrophy of the innermost cortical cells
| |
| | |
| sforming a layer to which the names, X-zone, fetal cortex and
| |
| | |
| androgenic zone have been applied. Only the outer rim of the
| |
| embryonic gland difkerentiates into the characteristic suprarenal
| |
| cortex of the adult, and it does not come into prominence until
| |
| after prenatal life.
| |
| | |
| The androgenic zone of the fetal suprarenal undergoes involution rapidly after birthF and as it disappears the size of the gland
| |
| becomes actually -as well as relatively smaller. The growth curve
| |
| of the human suprarenal gland is reproduced in Fig. 66.C The
| |
| gland loses one—third of its birth weight during the first postnatal
| |
| week, one-half in the first three months and fouplifths by the
| |
| | |
| 191
| |
| 192 PHYSIOLOGY OF THE FETUS
| |
| | |
| » end of the first year. Thereafter, a slow growth takes place and at
| |
| puberty the suprarenal again attains the weight it had at the end
| |
| of the fetal life ; but the androgenic Zone is no longer recognizable. This characteristically fetal part of the suprarenal gland
| |
| has been identiiied in the cat,7 Inouse,8 rabbit9 and in one strain of
| |
| rats.10 It seems to be absent, or at least not present as a comparable distinct layer of cells, in the albino rat and some other
| |
| anitnals.
| |
| | |
| The physiologic signiHcance of the hypertrophiecl fetal cortex
| |
| of the suprarenal gland is not understood. That it is closely re—
| |
| | |
| c« B «? 4 6 F J« ZZ « 36 II ZU
| |
| . Ase f« Yes-·.
| |
| Fig. 66.-—Growth of the human suprarenal glands (weight) during fetal like (c—B)
| |
| and after birth. (scammon: «The Measurement of Maus« Und: dünn. Press.)
| |
| | |
| lated to other endocrine organs is quite certain. A possible influence of Inaternal sex hormones upon the growing feta1 suprarenal is suggestecl by the closely parallel growth curve of the
| |
| uterus in prenatal and early postnatal life (Fig. 67) . Involution
| |
| of the X-2one of young male Ihice is accomplished under the in—
| |
| fluence of testicular horrnoneU
| |
| | |
| It has been suggested that the fetal suprarenaLgland elaborates an anclromirnetic substance.I2- I« Its ability to maintain the
| |
| prostates of the castrated immature mouse and rat, which degenerate when gonads and suprarenals are removed, demonstrates
| |
| THE FETAL ENDOCRINE GLANDS lgs
| |
| | |
| an andromimetic property quite clearly-P«- 15 «« Recentlzy however,
| |
| evidence has been advanced which indicates that carefully prepared extracts of fetal and of other X—2one—bearing glands do not
| |
| have androgenic propertiesss but it is possible that the amount
| |
| of suprarenal tissue extracted was too small to produce eifects.
| |
| should it prove that androgens are laclcingx one would have to
| |
| discard the attractive hypothesis that the androgenic cortex serves
| |
| directly to protect the fetus against an excessive iniluence of maternal estrogens reaching it through the placental barrier.
| |
| | |
| a---»»!
| |
| OF« Z Z 4 6 J« E) «? ««- Jö X r«AXJD Bär-«.
| |
| Fig. 67.-Growth of the hutnan Uterus (length) during ketal like (c-—B) and after
| |
| birth. (scammon: «The Measurement of Man," Univ. Minn. Press.)
| |
| | |
| 75
| |
| | |
|
| |
|
| |
| | |
|
| |
| | |
|
| |
| | |
| The possibility that cortin or a cortin-like hormone is forrned
| |
| by the feta1 suprarenal gland has received attention. Some
| |
| investigators have reported that the survival times of adrenalectomized cats and dogs are prolonged during advanced pr«eg—
| |
| nancy.I7szI9 Others failed to substantiate this at the end of gestation,20 but even if it is true there is no proof that a fetal secretion
| |
| protected the mother. Progesterone maintains life and growth
| |
| in ferrets and rats in the absence of suprarenal glands,2I-««’3 and
| |
| the functional corpus luteum of pregnant adrenalectomized anirnals does the Same« Adrenalectomy of pregnant rats during
| |
| gestation results in an increase in weight of the fetal glandsW as
| |
| will be seen in Table ge.
| |
| | |
| IZ
| |
| 194 Pnrsxoroor or THE: rETUs
| |
| | |
| Tanm 22
| |
| | |
| Tun Bringe-IS or Aussicht-Demut Denn-ro Pagen-mer III-on kur- Wntenks or rat;
| |
| Fast-Hi« sur-Hauptn- Gunvs
| |
| | |
| Time of adrenaleetomy No. of Average Ist. Ave. set. of suprarenal
| |
| of mothek litters of fetuses (gm.) glands of fetuses (mg.)
| |
| « d« 9 o« 9
| |
| Unoperated eontrols . . . . . . 18 5 .84 5.t»)8 0.90 0.82
| |
| 14th day of FeSUItIOIL . . . . 15 4 .96 4.78 LLZ I .l8
| |
| 7th day of Feste-tion . . . . . . 10 4 .70 4.51 1.17 Lls
| |
| | |
|
| |
| | |
| Attempts have been made to destroy the suprarenal g1ands by
| |
| means of intrauterine surgery to observe effects on other fetal
| |
| | |
| endocrine organskk but it proved impossible to obtain clear—cut
| |
| results because of the magnitude of technical difkiculties
| |
| | |
| THE SUPRARENAL MEDULLA
| |
| | |
| The medulla of the suprarena1 gland has an embryonic origin
| |
| very different from that of the cortex. It is formed by cells which
| |
| arise from the primordia of sympathetic ganglia and which begin
| |
| to migrate into the already prominent cortical bodies at about
| |
| seven weeks gestation in man. cells ok the suprarena1 medulla
| |
| as well as of certain other small glandular bodies of similar embryonic origin (e.g., the aortic paraganglia) possess a retnarld
| |
| able afkinity for chrome compounds with which they take on a
| |
| brown color. This chromafkin reaction has been demonstrated
| |
| to be elicitable iirst at about the time extracts of embryonic suprarenal tissue begin to produce pharmacologic responses characteristic of epinephrink749
| |
| | |
| Many have investigated the activities ok the embryonic -and
| |
| fetal suprarenal medulla by this histochemical method as well
| |
| as by other chemical and sensitive physiologic techniques Epinephrindilce reactions are obtainable from suprarenal extracts pre—
| |
| pared from chiclc embryos as early as the eighth day of incubation although similar extracts of other embryonic tissues give
| |
| negative resultsFHI Epinephrin is formed, or at least stored,
| |
| in the medulla of the glands in many fetal mammals before the
| |
| middle of gestation.32-39 The medullary cells show the chromaliin reaction at the 17th to 18th day in the pig and both physiologic and histochemical tests reveal the presence of an epinephrins
| |
| lilce substance at the time migration of. iznedullary cells into the
| |
| THE« FETAL ENDOCRINE GLANDS 195
| |
| | |
| cortical bodies is first observablekssfs The epinephrin content
| |
| of fetal glands has been reported to be greater than in the adult;
| |
| more was found in female than in rnale fetusesPs A correlation
| |
| between appearance of. epinephrin in the suprarena1 of th«e rat
| |
| and the origin of fetal movements has been suggestedxm but this
| |
| seems to be coincidental.
| |
| | |
| In sharp contrast with results obtained in most rnamma1s,
| |
| human fetal suprarenal extracts give negative or only very
| |
| slightly positive tests for epinephrinJHss 4144 However, in- full
| |
| term infants as well as prematures which lived for a short time
| |
| somewhat more definite reactions were obtained. The near fai1—
| |
| ure to obtain epinephrin-lilce responses from human fetal suprarenal extracts may be contrasted with the observation that the
| |
| paraganglia yielded definite amounts of epinephrin in one in—
| |
| stance:43
| |
| | |
| kluman suprarenal at birth - 0.0I arg. epinephrin per OR? Hm. Flur-d.
| |
| | |
| Etunan paraganglion at birth - 0.24 rag- epinephria per 0.1I Hm. sind.
| |
| | |
| Any relationship between low content of epinephrin and the
| |
| presence of a very prominent androgenic cortical zone in man is
| |
| | |
| undetermined
| |
| Im: sEx Blond-muss
| |
| | |
| An excellent consideration of embryologic development of
| |
| sex with a review of all but the latest literature has appeared
| |
| recentlyxss We are limited here to only a small part of this interesting subject.
| |
| | |
| The male gonads produce substances with androgenic properties in prenatal life. 1t was demonstrated that extracts prepared
| |
| from the testes of fetal calves are similar to those from the adult
| |
| and the hormonal yield per unit weight of tissue is greaterKS It
| |
| is probable that the male sex glands begin to elaborate secretions
| |
| about as soon as their sex can be differentiated, which is the sixth
| |
| day in the incubating chiclc and the seventh weelc in man. The
| |
| ovary is recognizable as such about a weelc later than the testes.
| |
| | |
| The best indication we have that fetal androgens are active
| |
| in early prenatal life is that forthcoming from a study of freemartins in cattle.47s 48 The freemartin is an intersexed or mascu—
| |
| linized female calf which deve1ops under conditions of chorionic
| |
| fusion in which vascu1ar anastomoses are estab1ished between the
| |
| 196 PHYSIOLOGY oF THE. FETus
| |
| | |
| placentas of adjacent male and female fetuses. The male is always a normal individual: It is believed that the hormone elab—
| |
| orated by the fetal male gonadscirculates in the conjoined blood
| |
| streams, acting upon the female twin’s Miillerian or female duct
| |
| derivatives to inhibit their normal development and upon its
| |
| Wolllian or masculine duct derivatives to stimulate their abnormal
| |
| dilferentiatiom When vascular connections are not established
| |
| between adjacent fetuses of opposite sex no freemartin results,
| |
| but the calves are normal male and female.
| |
| | |
| A. similar freemartin condition has been described in swine.49
| |
| It should be noted that the placentas of both cattle and swine are
| |
| relatively ineflicient from the standpoint of permeabi1ity. A high
| |
| degree of placental fusion, apparently with vascular union, was
| |
| observed in one instance of synchorial twinning in the cat.50 The
| |
| fetuses were of opposite sexes, were sexually normal in every way,
| |
| and were sulliciently advanced in development to make it appear
| |
| certain that the female twin would not have become a freemartin.
| |
| similarly synchorial twins of opposite sexes are encountered in
| |
| other animals and man,51- 32 but freemartins have not been re—
| |
| ported. It will probably be prolitable to- learn how the transmission of fetal male sex hormones across the placental barrier
| |
| is related to the phenomenon in question. It is diflicult to see how
| |
| the freemartin condition can be so limited unless the diffusibility
| |
| of embryonic testicular hormones is greater in the deciduate types
| |
| of placentas which therefore never allow hormones to accumulate in suilicient amounts to stimulate the Wolflian derivatives of
| |
| the genetically female twin.
| |
| | |
| It would carry us too far alield to inquire deeply into the ex—
| |
| tensive experimental studies on production of pseudohermaphrodism in the lower animals« success has been attained in mams
| |
| mals at several laboratories recentlyks Injections of pregnant rats
| |
| with large doses of testosterone and related preparations bring
| |
| about abnormal development of the potentially male ducts of
| |
| genetically female young. It is necessary to administer the hormone before the 16th day of gestation to obtain the most marked
| |
| effects.54 This is about one day before the WolHian ducts begin
| |
| to regress The intersexed individuals produced experimentally
| |
| resemble the naturally occurring freemartins in certain particu
| |
| lars.
| |
| THE! FETAL ENDOCRINB GLANDS 197
| |
| | |
| Male ofkspring of rats receiving large doses of estrogens before
| |
| the Izth day of gestation have been markedly feminizedPs Thus
| |
| a converse of nature’s freemartin has been induced with excessive
| |
| female sex hormones. The extent to which the mother’s own
| |
| hormones may inliuence normal development of sex in the fetus
| |
| is not understood. It is known that the fetal uterus exhibits a
| |
| marked hypertrophy and diminishes in size after intimate contact with the mother is abolished by birth. The mammary glands
| |
| of newborn infants of both sexes show enlargement and may
| |
| secrete transientlys It is possible that this production of «witch
| |
| milk" is stimulated by the same maternal hormonal mechanism
| |
| that leads to the preparation of the mother’s breasts for lactation.
| |
| | |
| THE TEYROID GLAND
| |
| | |
| The ability of the fetal thyroid to secrete at an early period
| |
| seems to have been established. Iodine has been identilied in the
| |
| gland at the 2nd or zrd month of gestation in cattle, sheep and
| |
| swiness and in man at least as early as the 6th month» The
| |
| amount is said to increase toward the end of prenatal life but to
| |
| be low as compared with the adult gland, perhaps because storage
| |
| of colloid is not so marked in the fetus« There is no close correlation between the maternal and fetal blood content of hormone
| |
| iodine, a fact which suggests that the fetus is secreting its own
| |
| hormoneIs The presence of thyreoglobulin in the human fetus at
| |
| the zrd and 4th months has been established by means of an
| |
| immunologic precipitin reaction.59
| |
| | |
| Amphibian metamorphosis and growth can be inlluenced by
| |
| extracts and transplants of avian and mammalian fetal thyroid
| |
| glands. In several, it may be said that the thyroid« becomes active
| |
| at about the time its structure begins to resemble that of the
| |
| adult. This is on the iith day of incubation in the chickW In
| |
| calves colloid is present as early as the end« and differentiation is
| |
| comp1eted between the 4th and 6th prenatal monthsz at this time
| |
| extracts serve to bring about metamorphosis in the axolotl, a
| |
| salamander which normally retains the larval state throughout
| |
| life.30 Extracts prepared from the glands of pig fetuses 7 cm.
| |
| long proved to be inactive, but those from 9 cm. pig fetuses produced reactions comparable with adult thyroids; correlatively,
| |
| the adult structure was nearly attained at 9 cm.» When bits of
| |
| 198 PHYsmLooY oF THE: FETUS
| |
| | |
| the thyroid gland from a 3-months-old human fetus (1o cm. C. R.
| |
| length) were transplanted into larvae of a toad, accelerated development took place, and trarisplants from 5-months-old human
| |
| fetuses had more marked efkectsYs Control experiments with bits
| |
| of fetal muscle gave negative results. It was found that the thyroid
| |
| gland of the youngenfetus had already deve1oped col1oid Iilled
| |
| vesicles.
| |
| | |
| Little is known about placenta! transmission of the thyroid
| |
| secretions. In swine, horses, cattle and sheep, animals with
| |
| adeciduate placentas, it appears that there is no transmission. In
| |
| geographical regions where iodine deliciency is prevalent the offspring of these animals are born in a state of athyreosis while the
| |
| mothers show little or no evidence of the iodine lack.C4- S« It
| |
| seems sthat the fetal requirements of iodine are greater than those
| |
| of the mother and that the fetus cannot draw upon the mother’s
| |
| hormone but must manufacture its own. Iodine feeding during
| |
| pregnancy corrects this deliciency, and the newborn pigs are then
| |
| normal. In man, on the other hand, it seems probable that the
| |
| mother’s hormone is available to the fetus because it can traverse
| |
| the placental barrier. Human infants born without or with
| |
| atrophic thyroid glands exhibit none of the symptoms of myxe—
| |
| dema, but a latent athyreosis soon manifests itself.S3-71
| |
| | |
| THE PARATIIYROID GLANDS
| |
| | |
| Practically nothing is known of function of fetal parathyroid
| |
| glands. Injections of parathyroid hormone into dog fetuses bring
| |
| on an elevation of the calcium level of the fetal, but not the maternal blood. This suggests that the parathyroid secretion does
| |
| not pass the placenta in the species studied.72 Attempts have been
| |
| made to determine the effects of fetal glands of dogs after thyroparathyroidectomy of the mothers. It was found that tetany de—
| |
| veloped just as soon as it did in nonpregnant animalsJss ««
| |
| | |
| THE TIIYMUS
| |
| | |
| Although the thymus is usually considered with the glands of
| |
| internal secretion, it is doubtful if it logically belongs there. By
| |
| 3 months in man, the thymus has the appearance of a lymphoid
| |
| organ with cortex and medulla already in evidence. There is no
| |
| anatomical basis for the belie,f that the sgland elaborates a hormone
| |
| THE« FBTAL BNDOCRINE GLANDS 199
| |
| | |
| and few attempts have been made to study the fetal thymus from
| |
| the standpoint ·of its endocrine function.30s «« ««
| |
| | |
| Extracts of thymus seem to exert no elfects when fed to tadPoles, although opinion has been divided on this questions«- ««
| |
| An extract of calf thymus, to which the name «thymocrescin" was
| |
| given, has been reported to produce marked acceleration of
| |
| growth in young rats when injected in daily doses as small as
| |
| 1 .mg.79
| |
| | |
| Another extract prepared in an entirely different way resulted
| |
| in even more marked effects in the hands of Rowntree and his
| |
| colleaguesko This material was injected intraperitoneally in i cc.
| |
| doses into rats over long periods including gestation and lactation; the young of succeeding generations were similarly treated.
| |
| Elfects on the olkspring of the first animals were not signijicant
| |
| but the second and subsequent generations showed remarkable
| |
| changes. They were larger at birth, more of them survived and
| |
| their postnatal development was delinitely speeded. The young
| |
| rats became sexually mature precociously. Maximum effects
| |
| were found in the eighth and tenth generations. It was necessary to keep giving the treatments and not miss a generation or
| |
| the effects were promptly dissipated. From the more recent re—
| |
| ports it seems that it was necessary to inject the extracts into
| |
| females onlyFI
| |
| | |
| 0ther investigators have attempted to reproduce these very
| |
| interesting results. but so far no adequate confirmation has been
| |
| reported» The biologic effects of certain iodine-reducing sub—
| |
| stances (glutathione, ascorbic acid, cysteine) have been found to
| |
| simulate those of the thymus extracts in certain particularsPI
| |
| | |
| Im; nrpopkkrsxs
| |
| | |
| A few studies have been made on placental transmission of
| |
| hypophyseal extracts but we know Iittle about hormone elaboration by the fetus itself. When pituitrin was injected into rabbit
| |
| fetuses no muscular contractions were observed in the mother.83
| |
| This suggests, but does not prove, that the substance failed to
| |
| pass the placenta. Anterior lobe extract did not produce any
| |
| evidence of its usual gonadotropic activity in the mother when
| |
| it was introduced into the fetuses« Furthermore, this hormone
| |
| failed to appear in the fetal fluids after it had been injected into
| |
| 200 PHYSIOLOGY OF THE FETUS
| |
| | |
| the «mother; at least, the administration of these fluids to other
| |
| adult rabbits fai1ed to bring about ovulatory changesss These
| |
| experiments seem to show that there is very Iittle if any transmission of the large molecules of the anterior lobe gonadotropic
| |
| factor even in the hemosendothelial type of p1acenta.
| |
| | |
| The fetal hypophysis seems to be capable of elaborating several active principlesPss VHV A pressor substance has been found
| |
| at 6 months in man. similar studies have been made in fetuses
| |
| of cattle, sheep and swine in which the response was found relatively earlier. The guinea pig uterine strip method served to
| |
| demonstrate the oxytocic princip1e about as early as the pituitary
| |
| glarid can be recognized macroscopicallzn It was found in appreciable amounts in pigs and sheep at term.
| |
| | |
| The melanophoreexpanding hormone has been identified in
| |
| the fetal hypophysis It was found in the glands from calf fetuses
| |
| of 3 months gestation but was not there at 2 months. It was present in pigs of only Zo mm. c. R. length.30- 88
| |
| | |
| Gonadotropic and growth promoting factors of the anterior
| |
| lobe seem to make their appearance rather late in fetal life, and
| |
| the former is later than the latter.90 In fetal pigs the gonadotropic
| |
| response was obtained from glands at the 2o to 21 ern. stage, a
| |
| short time before the end of gestation but was not found earlier.
| |
| The general body growth response could be obtained at the 9 to
| |
| 13 cm. stage which was just about the same time the thyroid hormone made its appearancesEs 90
| |
| | |
| SECRETIN
| |
| | |
| Extracts of the proximal portion of the fetal small intestine
| |
| have been found to cause secretion of pancreatic juice when in—
| |
| jected into adult animals with pancreatic Hstu1as.9I·9f The earliest
| |
| period at which secretin has been obtained from the human fetus
| |
| is 414 months. The exact source of the hormone is unknown
| |
| and attempts to ascribe it to the chromalkn cells of the duodenum93 seem to be entirely unjustiöed
| |
| | |
| THE ENDOCRINE PANCREAS
| |
| | |
| The endocrine function of the pancreas is vested in the cells
| |
| of the islands of Langerhans These make their appearance in the
| |
| third month of human gestation but— it-.is not known how early
| |
| THE FETAL ENDOCRINE GLANDS 201
| |
| | |
| they become capable of secreting. The acinar portion of the
| |
| gland does not begin to produce its proteolytic ferment before
| |
| about the 5th month,93 and Banting and Best took advantage of
| |
| the fact that island tissue is functional earlier when they chose the
| |
| pancreas of the fetal calf as a source of antidiabetic principle in
| |
| their early search for insulin.97 Many have discussed the possibility that fetal insulin plays an important röle in carbohydrate
| |
| metabolism of the fetus and have pointed to a correlation between
| |
| the appearance of glycogen in the liver and the development of
| |
| island tissue in the pancreassss 99 but the relationship is still somewhat unsatisfactorily established because the influence of maternal
| |
| secretion acting through the placenta is diflicult to evaluate. It is
| |
| said not to pass the placenta from fetal to maternal sides.83 Administration of insulin to pregnant cats failed to reduce the blood
| |
| sugar level of the fetuses near term. This suggests that the pla—
| |
| centa is impervious at the time, but at earlier stages similar results
| |
| were not obtainedEoo Further discussion of this question will be
| |
| found in Chapter XVI »
| |
| | |
| In birds, where all metabolic processes must be managed by
| |
| the fetus itself, an insulin-like substance has been found in the
| |
| unincubated eggJOI However, it is not present in the tissues of
| |
| the early chick embryo until after the pancreatic islets are formed.
| |
| | |
| The ofkspring of diabetic animals are not diabetic and as a ruIe
| |
| seem to possess healthy glandsW This is not always true in man
| |
| where hypertrophy and hyperplasia of islands and postpartum
| |
| hypoglycemic deaths are encountered in infants born of diabetic
| |
| womenJos Although hyperplastic pancreatic islands are not.
| |
| found in all instances, careful searching might show the condition
| |
| to be more prevalent.
| |
| | |
| The possibility that during prenatal life fetal insulin can
| |
| protect the diabetic mother has been discussed by several investigators. It was discovered by Carlson and his colleaguesIM W
| |
| that the urine of completely pancreatectomized dogs remained
| |
| free from sugar when the .operation was performed in late stages
| |
| of pregnancy. This suggested that fetal island tissue had, supported both the mother and fetuses, for after parturition the
| |
| mother exhibited glycosuria. These experiments have been adequately confirmedW and similar conditions app«arently occur
| |
| in the human.I07 Completely depancreatized dogs maintained in
| |
| 202 PHYSIOLOGY OF THE FETUs
| |
| | |
| good hea1th by diet and insu1in therapy can conceive and give
| |
| birth to normal pups. They show an increased carbohydrate tolerance kor only about two weeks prior to labor. However, an even
| |
| greater tolerance appears after birth during lactationz it would
| |
| seem that the results previously ascribed entirely to ketal insulin
| |
| are more probab1y due largely to increased utilization ok carbohydrates by the fetuses and, after birth, by the nursing puppies.
| |
| We cannot be sure that the ketal insulin plays any part in protecting the diabetic mother. It is quite reasonable to suppose that
| |
| it is more important kor the utilization ok sugar received by the
| |
| | |
| ketus krom the mother.
| |
| | |
| Glycogen appears in the liver ok the deve1oping chick at 7 days
| |
| ok incubation. This is about three days before delinitive islands
| |
| ok Langerhans make their appearance. Between the tenth and
| |
| thirteenth days the glycogen content ok liver cells diminishes and
| |
| | |
| the metabolic rate and respiratory quotient increase, although
| |
| there is no rise in the blood sugar concentration. Thus it appears that an increased utilization ok carbohydrate by the embryo
| |
| is correlated with the advent ok kunction in suprarenal medulla.
| |
| pancreatic islands and thyroid glandsÄss «
| |
| | |
| REFERENCES CITED
| |
| | |
| i. Needham, J. i93i. chemical Embryology, Macmillan, N. Y.
| |
| | |
| g. Gro11man, A. i93s. The Adrena1s, Williartis sc Wi1kins, Baltimore
| |
| | |
| z. Rogokk, J. M. 1932 chap. as, vol. 2 in cowdry: Special cytology,
| |
| I-Ioeber, N. Y.
| |
| | |
| 4. scammon, R. E. i923. Chap. z, vol. i in Abt: Pediatrics, saunders,
| |
| Philadelphia. «
| |
| | |
| z. Elliott, T. R. sc R. G. Arnioun igi i. J. Path. sc Bach, is: 4si.
| |
| | |
| s. scammon, R. E. i93o.» Pt. IV in The Measurement ok Man. Univ.
| |
| ok Minn. Press, Minneapolis
| |
| | |
| 7. Davies, s. ig37. Quart. J. Mic. sci., so: si.
| |
| | |
| s. I-Ioward-Mil1er, E. i927. Am. J. Anat» 4o: 25i.
| |
| | |
| g. Roak, R. i935. J. Anat., 7o: ists.
| |
| | |
| io. Bacsich, P. sc s. J. Folley. i939. J. Anat» 73: 432.
| |
| | |
| ii. Martin, s. J. ig3o. Proc. soc. Exp. Bio1. sc Med., Es: 4i..
| |
| | |
| i2. Reichstein, T. i93s. I-Ielv. chi. Acta, i9: Les.
| |
| | |
| is. I-Ioward, E. i937. Am. J. Physiol» ii9: 339.
| |
| | |
| i4. BurrilL M. W. sc R. R. Greene. i93g. Proc. soc. Exp. Bio1. sc Meds.,
| |
| 403 IS?
| |
| i5. I-Ioward, E. i939. Am. J. Anat., s5: io5.
| |
| | |
| is. Gersh, I. sc,A. Gro1lman. i939. Am. Physiol» us: 3ss.
| |
| | |
| i7. stewarch I-I. A. i9i3. Proc. i7th latet-nat. Gang. Magd» secr. z, Pt. g,
| |
| | |
| p- I73THE. FETAL ENDOGRINE GLANDS 203
| |
| | |
| ts. stewart, G. N. sc J. M. Rogolk t9es. Pt·oc. soc. Exp. Biol. sc Med» ee:
| |
| sg4 and es: tgo.
| |
| | |
| t9. Rogokh M. sc G. N. stewarr. t9e7. Am. Physiol» 79: so8.
| |
| | |
| eo. cotsey, E. L. tge7. Proc. soc. Exp. Biol. sc Med» es: t67.
| |
| | |
| et. Gaunt, R» W. 0. Nelson sc E. Loomis. t9s8. Ibid» s9: st9.
| |
| | |
| ee. Gaum, R. sc H. W. I-Iays. t9s8. Science, 88: s76.
| |
| | |
| es. Greene. R. R., J. A. Wells sc A. c. Ivy. t9s9. Pt·oc. soc. Exp. Biol. sc
| |
| | |
| Med» 4o: 8s.
| |
| | |
| e4. Rogokh J. M. sc G. N. stewarr. tgeg. Am. J. Physiol» 88: t6e.
| |
| | |
| es. Ing1e, D. J. sc G. T. Fishetn t9s8. Proc. soc. Exp. Biol. sc Med» s9: t49.
| |
| e6. Tobin, C. E. t9s9. Am. J. Anat» 6s: tst.
| |
| | |
| e7. Weymanth M. F. t9ee. Anat. Rec» e4: e99.
| |
| | |
| es. Howard-Miller, E. t9e6. Am. J. Physiol» 7s: e67.
| |
| | |
| es. 0lcuda, M. t9e8. Endocrin» te: s4e.
| |
| | |
| so. I-Iogben, L. T. sc F. A. E. Stets. t9es. Brit. Exp. Biol., t: t.
| |
| | |
| st. Lutz, B. R. and M. A. case. tges. Am. J. Physiol» 7s: 67o.
| |
| | |
| se. Langlois. J. P. and J. Rehns. t899. comp. Rend. soc. Biol., st: t46.
| |
| ss. Fengetx F. t9te. J. Biol. chem., it: 489 and te: ss.
| |
| | |
| s4. cevolotto, G. t9t6. chem. Abstt·» to: tete.
| |
| | |
| ss. Mccord, c. P. t9ts. J. Biol. chem., es: 4ss.
| |
| | |
| s6. svehla, K. t9oo. A1ch. exp. Path. u. Phaxm» 4s: set.
| |
| | |
| s7. Moore, B. sc c. 0. Purinton. t9oo. Am. J. Physiol» 4: s7.
| |
| | |
| s8. Lewis, J. I-I. tgt6. J. Biol. chem., e4: e49.
| |
| | |
| s9. saito, s. t9e9. Toholcu J. Exp. Med» te: es4.
| |
| | |
| 4o. Panlusatz D. s. t9st. Anat. Rec» 49: st.
| |
| | |
| 4t. Ingietx A. sc G. schmorL 191 t. Miinch. med. Wochenschr» t9tt, e4os
| |
| | |
| (Abstt·.) .
| |
| 4e. samelson, P. t9te. Ztschtn i. Kinderhllc» s: 6s.
| |
| 4s. Elliott, T. R. Personal to G. Bat-get, t9t4. The simpler Natural
| |
| Bases, Longmans Green, London (p. 9s).
| |
| 44. Krametx D. t9t8. Monatschtx f. Kinderhllg t4: sst.
| |
| 4s. Williety B. H. t9s9. chap. s in E. Allen’s «·sex and Internal secres
| |
| tions," Williams sc Wilkins, Baltimore.
| |
| 46. Womaclh E. B. sc F. c. Koch. t9st. Proc. end 1nternat. cong. set:
| |
| Res» t9so, P. se9.
| |
| | |
| 47. Linie, F. R. t9t7. Etcp. Zool» es: s7t.
| |
| | |
| 48. Linie, F. R. t9e3. Biol. Ball» 44: 47.
| |
| | |
| 49. I-Iughes, W. t9e9«. Anat. Rec» 4t: et s.
| |
| | |
| so. Wisloclus G. B. sc G. W. D. Hamletr. t9s4. Ibid» 6t: 97.
| |
| | |
| st. Hamlett. G. W. D. sc G. B. Wisloclti. t9s4. Ibiii» St: st..
| |
| | |
| se. Wisloclci, G. B. t9s9. Am. Anat» 64: 44s.
| |
| | |
| ss. Gt·eene, R. R» M. W. Burrill sc A. c. Iyy. t9s8. Am. Obst. sc Gyn»
| |
| | |
| s6: tos8.
| |
| | |
| s4. Butrill, M. W. sc R. R. Greene. tgs9. Am. J. Physiol» te6: 4se.
| |
| | |
| ss. Greene, R. R. sc M. W. Burrill. t9s9. Ibid» te6: sto.
| |
| | |
| s6. Nosalca, T. t9e7. chem. Abs» et: s9ts.
| |
| | |
| s7. Mauretx E. t9e7. Ztschtu i. Kinderhllc» 4s: t6s.
| |
| | |
| s8. Mcclendon, J. F. sc c. E. McLennam t9s9. Proc. soc. Exp. Biol. sc
| |
| | |
| Med., 4o: sss.
| |
| sg. I-Ielctoett, L. sc K. schulhoL t9es. Proc. »Natl. Acad. sei» tt: 48t.
| |
| eo4
| |
| | |
| . Wegelin, C. sc J. Abelin.
| |
| . Thomas, E. sc E. Delhougne
| |
| | |
| . snyder, F. F. s: F. M. Hoskins.
| |
| | |
| . Leu-is, D.
| |
| . snydeiy F. F. i9es. Am. J. Anat» 4i: s99.
| |
| | |
| . Bell, G. H. sc M. Robson. i9s7. Quart. J. EZcp. Physiol» e7: eo5.
| |
| . sinith, P. sc c. Dortzbaclx
| |
| . Hallion, L. 8c P. Lequeux.
| |
| . camus, L. i9o6. Ibid., 6i: 59.
| |
| . Pringle, H.
| |
| | |
| . Banting, F. G. s: c. H. Best.
| |
| . Aron, M.
| |
| . Port-in, R. sc M. Aron. i9e7. coinp. rend. soc. Biol., 96: e67.
| |
| . Britton, s. W. i9so. Am. J. Physiol» 95: i78.
| |
| | |
| . shilcinamh Y. i9es. Tohoku J. Exp..Med.,sp io: i.
| |
| | |
| PHYSIOLOGY OF THE« FETUs
| |
| | |
| . Willieiy B. H. Personal to J. Needham. i9si. cheiii. Emb., Mac
| |
| millan, N. Y.
| |
| | |
| . Abbott, A. C. 8c J. Prendergasr. . i9s7. can. Med. Assoc. J., s6: ees.
| |
| . Rumph, P. 8c P. E. smith. i9e6. Anat. Ren, ss- es9.
| |
| | |
| . Schuhe, W» W. schmitt sc K. Hölldobler.
| |
| . smith, G. E. s: I-I. Welch. i9i7. J. Biol. chem., e9: ei z.
| |
| . smith, G. E.
| |
| . siegen.
| |
| | |
| i9es. Endolcrin., e: e.
| |
| | |
| i9i9. Endocrin., s: e6e.
| |
| | |
| i9ei. Verh.«deuts. Gesel. Kinderhllsp ee: s64.
| |
| | |
| Kot-her, T. is9e. Deuts. Ztschr. chirurg.,-s4: 556.
| |
| | |
| i9ei. Arch. exp. Path. u. Pharm., sg: ei9.
| |
| i9e4. Virchow’s Arch. path. Anat. u.
| |
| | |
| Physiol» e4s: eoi .
| |
| | |
| . Kraus, E. J. i9e9. Beitr. path. Anat. allg. Path., se: e9i.
| |
| . sgalitzey K. i9ss. Ibid., ioo: es5.
| |
| . Hoslcins, F. M. sc F. F. snyder.
| |
| | |
| i9e7. Proc. soc. Eis-P. Biol. sc Med.,
| |
| es: e64. ·
| |
| | |
| . Vassale, G. i9o5. Art-h. ital. biol., 4s: i77.
| |
| . Werelius, A. i9is. Sarg. Gyti. sc Obst» i6: i4i.
| |
| . Trinlca, L.
| |
| | |
| i9i4. Publ. biol. Ecole Hautes Etudes Vet. de Brno (in
| |
| czechz cited by J. Needham, i9si) . —
| |
| | |
| . Macchiariilo, 0. i9so. Riv. ital. di Gin., ii: s57.
| |
| . Gudernatsch, F.
| |
| . Allen, B. M. i9eo. J. Exp. Zool., so: is9.
| |
| | |
| . Asher, L. i9ss. Abderhalden’s Handb. biol. Arbeits-method. Abt. s,
| |
| | |
| i9i4. Am. J. Anat., is: 4si.
| |
| | |
| Teil sB (e): 9e9.
| |
| | |
| . Rowntree, L. G., J. H. clarlc sc A. M. Hans-on. i9s4. science, so: e74.
| |
| . Rowntree, L. G., A. steinberg, N. H. Einhorn 8c N. K. schaffen
| |
| | |
| i9ss.
| |
| Endocrin., es: 584.
| |
| | |
| . Nelson, W. 0. i9s9. chap. ei, in E. Allen’s «sex and Internal Secre
| |
| tions," Williams 8c IVillcinsk Baltimore
| |
| i9e7. Anat. Rec., s5: es.
| |
| | |
| Wisloclci, G. B. sc F. F. snydeix i9se. Proc. soc. Exp. Biol. sc Med.,
| |
| so: i96.
| |
| . Goodman, L. s: G. B. Wisloclci. i9ss. Am. J. Physiol» io6: ses.
| |
| schlimperh H. i9is. Monatschix Geh. u. Gyn., ss: s.
| |
| | |
| i9i6. J. Exp. Med., es: 677.
| |
| | |
| i9e9. Anat. Rec., 4s: e77. i9o6. comp. rend. soc. Biol., 6i: ss.
| |
| | |
| i9ii. Physiol» 4e: 40 P.
| |
| | |
| . Koschtojanz c. i9si. Pllügeks Arch., ee7: s59.
| |
| . parat, M. i9e4. comp. rend. soc. Biol., 9o: wes.
| |
| . Ibrahim, J. i9o9. Biochem. Ztschr., ee: e4.
| |
| | |
| i9ee. J. Lab. s: Glitt. Med., 7: 464.
| |
| i9es. comp. rend. soc. Biol., s9: is7, is9.
| |
| THE FETAL ENDOCRINE GLANDS 205
| |
| | |
| me. Jos1in, E. P. 1915. Boston Mai. sc: Sarg. J., 173: 841.
| |
| | |
| 1o3. smyth, F. s. 8e M. B. 01ney. 1938. J. Pediat., is: 772.
| |
| | |
| 1o4. Car1son, A. J. 8e F. M. Drennam 1g11. Am. J. Physiol» es: 3g1.
| |
| | |
| 1o5. car1son, A. J» J. s. Ort· 8e W. s. Jones. 1914. J. BioL Odem» 17: 19.
| |
| 1o6. cuthberiz F. P., A. C. Ivy, B. L. Isaacs se: Gray. 1936. Am. J. Physiol»
| |
| | |
| us: 480.
| |
| 1o7. Lawrence, R. D. 1929. Quart. J. Mai» es: 191.
| |
| Los. Daltorh A. J. 1937. Anat. Ren, 68: 393.
| |
| CHAPTER XV
| |
| | |
| FETAL NUTRITION AN D METABOLISM
| |
| | |
| PARAPLACENTAL NUTRITION
| |
| | |
| THE maternal organism not only breathes and excretes for the
| |
| ketus but it also digests kood and kurnishes nutriments needed kor
| |
| the growth ok the new individual. In early stages ok development
| |
| the intimacy between embryo and mother is slight, and until a
| |
| close approximation to the endometrium is ekkected nutrition can
| |
| not be accomplished by direct processes which characterize the
| |
| older and more advanced types ok placental circulatory Systems.
| |
| An intermediate substance ok transient nutritional value to the
| |
| early embryo is provided by secretion ok the uterine glands, by
| |
| transudation and especially by erosion ok the endometrium and
| |
| production ok tissue detritus in response to implantation ok the
| |
| newly arrived blastocyst. To this paraplacental nutriment, the
| |
| name histotrophe may be applied.I-4
| |
| | |
| In mammals with relatively simple epithelio-chorial and syn—
| |
| desmo-chorial p1acentas, such as the horse and sheep, quite a
| |
| different histotrophic material bathes the chorionic surkace
| |
| throughout gestation. This, the «uterine mi11c," is composed predominantly ok secretions and transudates from the intact uterine
| |
| epithelium. Its high kat- content gives it the appearance ok dilute
| |
| millc.
| |
| | |
| It is doubtkul ik histotrophic nutrition can be ok real signikicance in man and other primates for more than a kew days during
| |
| implantation) A yollc-sac placenta develops early in the rat, and
| |
| with it a more eiiicient mechanism kor nutrition. The passage
| |
| ok substances through the yollcssac epithelium ok the rat has been
| |
| demonstrated very clearly.0- 7 0ne can not consider that the processes involved are entirely histotrophic in those animals in which
| |
| uterine millc is laclcing.
| |
| | |
| With the kormation ok endothelio-chorial, hemo—chorial and
| |
| hemo-endothelial (deciduate) placentas in carnivores, primates
| |
| and rodents, histotrophe plays only a. Jninor and transient part
| |
| | |
| 206
| |
| FETAL NUTRITION AND MBTABOLISM 207
| |
| | |
| and nutrition becomes possible largely by processes similar to
| |
| those occurring in the tissues ok the body itseltl The substances
| |
| which pass more directly from one blood stream to the other have
| |
| been designated hemotrophe. Thus Bonet’s term «embryotrophe" has given way to a more usekul classiiicatiom
| |
| | |
| Bmbryofrophe
| |
| Eistotrophe klemotrophe
| |
| l . I . I . I « I
| |
| Ikansient Uterme milk. Diikusible Nitrogenous substances
| |
| endometrial Nutrition substances substances (e.y., lipids)
| |
| detritus, etc. throughout (gases, ok high truly
| |
| Nutrition kor gestation in dextrose and molecular absorbed b)implanting ungulatea inorganic weight which trophoblast
| |
| blastoeyssz eompounds) are diikuss and yolkssac
| |
| requiring no ible but epitheliunh
| |
| resynthesia require
| |
| | |
| resynthesia
| |
| | |
| PLACENTAL PERMEABEITY
| |
| | |
| N utrition ok the fetus is closely dependent upon the manner
| |
| and ekkiciency with which materials are transmitted across the
| |
| placental barrier. It must be born in mind that the physiologic
| |
| characteristics ok the placenta are not constant throughout development, nor are they the same in all species ok mammals. Furthermore, the chorio-allantoic attachment is not the only means
| |
| ok contact between the ketus and motherz in some species, e.g.,
| |
| the rat, a yollosac placenta ok very dilkerent structure is known
| |
| to serve concomitantly throughout gestationsk The term «the
| |
| placenta« rekers as a rule to the combined ectoplacenta and yollc—
| |
| sac placenta when used in rekerence to kunctioxx Density and
| |
| number ok tissue layers separating maternal and fetal blood
| |
| streams vary, as was pointed out in Chapter I. For these reasons
| |
| one must exercise caution in attempting to draw conclusions regarding the permeability ok one type of placenta based upon experiments with a different type.
| |
| | |
| The subject ok placental permeability is much too extensive ito
| |
| be discussed in detail, but a few signiiicant observations can be
| |
| considered such observations inquire into the characteristics ok
| |
| materials which make their way across the living membranes, and
| |
| into the nature ok the processes involved in their passage.
| |
| | |
| Particulate matten even when microscopic in size, kails to pass
| |
| 208 PHYSIOLOGY oF THE: FETUs
| |
| | |
| the most advanced hemo-endothelial placentas. Formerly there
| |
| was much discussion of this, but the fact is now well establishedF
| |
| certain ultræmicroscopic particles in colloidal solutions do pass,
| |
| and the dye trypan blue seems to be one lying on the borderline
| |
| between the transmissible and non—transmissible substances in the
| |
| hemo-endothelial placentas. This dye does not traverse the
| |
| endothelio-chorial type found in the cat.?
| |
| | |
| That a relationship exists between the molecular weight of
| |
| substances and their ability to pass through placentas can scarcely
| |
| be doubted. 0xygen, carbon dioxide and many chemical com—
| |
| pounds of low molecular weight traverse membranes of all placentas. Tabulation of data available ·up to 1931 will be found
| |
| in N eedham’s10 «Chemical Embryology" (Table 227) . From this
| |
| it appears that the thinner the barrier, the more permeable it is
| |
| to materials of large molecular size. 0n the other hand, it has
| |
| been demonstrated that the thin chorionic trophoblast plates of
| |
| the early rat placenta are actually less permeable than the much
| |
| thiclcer yoll(-sac epithelium to the dye, toluidin blue.
| |
| | |
| A very close parallelism exists between the ability of colloidal
| |
| dye solutions to diffuse in iilms of 2o to 30 per cent gelatin and the
| |
| efliciency of their transmission from the mother to the fetuses of
| |
| rats and mice.U These and other observationsW have frequently
| |
| been cited as evidence that the placenta acts largely in the capacity of an ultra-iilter. There is ample evidence, however, that the
| |
| fat solubility of materials in the blood streams, their pH and
| |
| ionic charges at the membrane play important röles in governing
| |
| placental permeability. "Furthermore, one can not tell whether
| |
| the experiments with colloidal dyes demonstrate passage through
| |
| the ectoplacenta, the yolldsac placenta or a combination of both.
| |
| | |
| Although a number of investigations have led» to the conclusion that species differences exist at term in respect to permes
| |
| ability of dye solutions,9 other chemical solutions« and antibodiesss few experiments have been concerned with the changes
| |
| talcing place throughout the course of development of any one
| |
| species. Recently, however, it was demonstrated that the permeability of the rabbit’s placenta to agglutinins and hemolysins in—
| |
| creases during the course of gestation.14 The ratio of the titre of
| |
| the fetuses to that of the mother plotted against the gestation-age
| |
| forms a sigmoid curve (Fig. 68)'. Permeability is clearly related
| |
| FETAL NUTRITION AND METABOLISM 209
| |
| | |
| to the changing histologic structure of the placenta during gestationss «
| |
| | |
| The two principal theories concerning the nature of the placental barrier merit further study. Many recent observers have
| |
| favored the view that it is essentially an inert semipermeable
| |
| membrane, but advocates of the concept of a vital function are
| |
| not lacking. A preformed rcgulatory mechanism, inferring a
| |
| secretory process, has been suggcstedJs
| |
| | |
| Those who favor the ultra-iilter theory believe that substances
| |
| pass from mother to fetus, or in the reverse direction, by diffusion
| |
| and filtration, that physical processes alone govern the transmis
| |
| tIIIII--I
| |
| tIIIIgIII
| |
| ssIIZIIIs
| |
| tgtZIIIIs
| |
| I-IssUII
| |
| | |
| II Z( 2
| |
| | |
|
| |
| | |
|
| |
| | |
| O
| |
| G
| |
| | |
|
| |
|
| |
| | |
| Z
| |
| | |
|
| |
| | |
| Rotte-Pers! Fuss-Hofstaat Tit-se
| |
| | |
|
| |
| | |
| Period of Gestaden-days
| |
| | |
| Fig. 68.—Permeability of the rabbit placenta to antibodies at different times during
| |
| prenatal life. (Rodo1fo: J. Exp. Zool» Vol. 68, 1934.)
| |
| | |
| sion and that molecular size plays an important part in determining which shall and which shall not cross the barrier.10-U-17- 18
| |
| This seems to be well established for gases, dextrose and a number
| |
| of chemical compounds of relatively low molecular weight, including some of the products of fetal metabolism. The subject
| |
| has been reviewed by schlossmanW who concludes that, aside from
| |
| endocrine activities, there is not the slightest reason to believe that
| |
| the placenta and its chorionic epithelium has a truly secretory
| |
| function. He believes that even in the instances of seemingly
| |
| vitalistic activities, physical principles in the last analysis can ex—
| |
| plain transmission. Nevertheless it is reasonable to adhere to the
| |
| concept of absorption in the trophoblast.. The recent studies on
| |
| | |
| 14
| |
| 210 PHYSIOLOGY OF THE FETUs
| |
| | |
| metabolism of lipids provide strong circumstantial evidence for
| |
| the vitalistic theoryäs
| |
| | |
| With the recognition of functional properties of the yollc-sac
| |
| in some animals, the question of secretory function talces on re—
| |
| newed interest. In structure as well as in physiologic reaction to
| |
| perfusion of the maternal blood vessels with various chemical and
| |
| dye solutions, the yollcssac placenta of the rat appears to be an
| |
| organ for absorptionks 7 When more attention has been paid to
| |
| the functions of this organ, as well as to factors of age and species
| |
| differences, our lcnowledge of the passage of the nutriment from
| |
| mother to fetus will undoubtedly be advanced far beyond its
| |
| | |
| present state.
| |
| UETAZOLISM oF cumoknmmrns
| |
| | |
| The body of the fetus is bui-It from chemical substances which
| |
| are available in the mother’s blood. Energy needed by the fetus
| |
| is derived from the same source. Indeed, all the prenatal require·
| |
| ments are met by processes of intermediary metabolism. The
| |
| most readily available substance is carbohydrate in the form of
| |
| dextrose which serves as the important energy source for the fetus.
| |
| | |
| Dextrose can pass from mother to fetus across the placental
| |
| barrier in all mammalskHs Human fetal bloodssugar concentration is always a little lower than that of the mother near term.
| |
| For example, Morriss found averages of 1 15 mg. per cent in the
| |
| fetus and 132 Ins. per cent in the motherkl indicating that there
| |
| is a gradient of flow towards the fetus. A similar condition pre—
| |
| vails in most mammalssks 23 but not in the pig and cow in which
| |
| the concentration is lower in maternal than in fetal blood.23- 24
| |
| The reason for these species differences is not clear, but they may
| |
| be related to placental structural variations and to histotrophe as
| |
| an intermediate nutriment. Much of our information has been
| |
| obtained at the end of gestation or is based on averaged data of
| |
| different prenatal ages. A more complete study is available in the
| |
| incubating chiclc.25 The blood-sugar concentration maintains a
| |
| reasonably constant level up. to the 16th day; thereafter it rises
| |
| and surpasses the adult level at about hatching time, which is the
| |
| 21st day. Fetal blood—sugar concentration varies with gestation
| |
| age in mammals too. For example, it decreases toward term in
| |
| the cow but increases marlcedly in the spguinea pig and rabbitkss 27
| |
| FETAL NUTRITION AND METABOLISM 211
| |
| | |
| It is apparent that a simple filtration across the placental barrier is
| |
| not the only mechanism governing the blood-sugar level in the
| |
| fetus.
| |
| | |
| The placenta and liver are important depots for carbohydrate
| |
| storage in prenatal life. As early as 1858, Claude Bernard demonstrated that the placenta contains glycogen and serves as a «transitory liver« for the embryoks It has been clearly shown that the
| |
| glycogen content of the placenta is high in the early part of fetal
| |
| life whenlittle or none is in the liver. Only the maternal portion of the placenta contains it.29 A time is reached, however,
| |
| when glycogen storage becomes active in the fetal liver; when this
| |
| occurs there is a corresponding reduction of storage in the placenta. This crossing over takes place after the elapse of 75 per
| |
| cent of the total gestation time in the rat, 82 per cent in the chiclc
| |
| and 91 per cent in the rabbitPHI The glycogen content of the
| |
| fetal liver rises rapidly toward the end of gestation and is especially high a few days before birth.22 Nevertheless, the amount of
| |
| liver glycogen is inconstant at any given period in the fetus and.
| |
| varies with the food intalce of the mother. Following 24 hours
| |
| of fasting in the rat at full term, the pealc of maternal liver glycogen was reached about four hours sooner than that of the fetuses«
| |
| When the average glycogen content in the mother’s liver was o.29
| |
| per cent of the total liver weight (fasting1eve1) , that of the fetuses
| |
| was 4.95 per cent. At the pealc of storage after feeding, average
| |
| values of 3.1 per cent and 1o.6 per cent were found in the mothers
| |
| and fetuses respectively. These differences are truly remarlcable.
| |
| | |
| Glycogenic function begins after secretion of bile has startedFs
| |
| It has been thought to begin at almost precisely the time the is—
| |
| lands of Langerhans differentiate and begin to supply insulin to
| |
| the fetal bloodZss ZHC However, it has been demonstrated re—
| |
| cently that the livers of incubating chiclcs contain glycogen at
| |
| Ieast as early as the 7th day of incubation, although the pancreatic
| |
| islands do not appear until the Iith day.3·3 In livers of pig and
| |
| sheep fetuses, likewise, glycogen has been observed before the
| |
| pancreatic islands are formed. Glycogen is distributed evenly
| |
| throughout the chiclc liver after island tissue makes its appearance, but is conlined to the cells around large veins in earlier
| |
| embryos. Nearly all livers show glycogen on the seventh, eighth
| |
| and ninth days and again after thirteen days, but in the interven212 PHYSIOLOGY oF THE. FETUs
| |
| | |
| ing period fewer contain it. There is no detectable change in the
| |
| blood-sugar concentration at this time of rapid glycolysis, even
| |
| though the metabolic rate increases and the respiratory quotient
| |
| approaches unity. These facts suggest very strongly a relative increase in utilization of carbohydrates as an energy source between
| |
| the Ioth and izth days of incubation, and they indicate that the
| |
| fetal liver supplies some of this material at this period during
| |
| which endocrine functions of thyroid, suprarenal and pancreas
| |
| are just becoming established. «
| |
| | |
| The passage of carbohydrate from mother to fetus is a s1ow
| |
| process under normal conditions, but it can be accelerated greatly
| |
| by injecting insulin into the fetus.37 When this was done it was
| |
| found that the glycogen or total carbohydrate content of livers
| |
| and muscles of dog fetuses was not infiuenced significantly However, the lactic acid concentration of the fetal blood was raised
| |
| a few hours after insulin injection and the difference between its
| |
| level in the umbilical artery and vein was increased many times
| |
| above the normal. About three-fourths of the dextrose which
| |
| passed from the dog to its fetuses to compensate for the experimental fetal insulin hypoglycemia returned to the mother as lactic
| |
| acid.22 "
| |
| | |
| The fetal dog is reported to be highly resistant to insulin and
| |
| the fetal sheep and goat scarcely respond at all to doses as great
| |
| as 415 units per lcilogram. An insulin antagonistic substance
| |
| seems to be present in their blood.22- 23 Although insulin fails to
| |
| deplete the fetal liver glycogen in dogs, sheep and goats when
| |
| given to the fetuses, it·has been observed to do so in rats when
| |
| injected into the mother-PS Adrenalectomy of pregnant rats simii
| |
| lar1y leads to depletion of the fetal liver glycogenks Apparently
| |
| the fetus practices a rigid glycogen economy at theexpense of its
| |
| mother’s dextrose, drawing upon its own liver store only in einer—
| |
| | |
| gencies.
| |
| METABOLISM 017 LIPIDs
| |
| | |
| Well nourished fetuses are supplied with good stores of fat.
| |
| Do they acquire this by transfer through the placenta or must it
| |
| be synthesized from simpler materials? Perhaps the fetus is able
| |
| to synthesize some from carbohydrates or amino acids, but these
| |
| are not the only sources. Certaimlipids are passed from the
| |
| FETAL NUTRITION AND METABOLISM 213
| |
| | |
| mother’s blood to that of the fetus by poorly understood mechanisms and thus become available for construction of fat. To what
| |
| extent lipids are oxidized by the mammalian fetus for energy is
| |
| not known. They form the principal source of energy in birds
| |
| during the greater part of the period of incubationKo
| |
| | |
| It has been quite deiinitely determined that the fat which is
| |
| fed to a pregnant animah and which is absorbed and stored in its
| |
| tissues, does not pass through the placenta unchanged When
| |
| stained by sudan III or some other similar dye before feeding,
| |
| the body fat becomes intensely colored, but there is not the least
| |
| color in the fetuses.39-4I Regardless of the degree of saturation
| |
| of the fatty acids available from material fed to the mother, the
| |
| fetal fat has an almost unalterable degree of saturation.42 It is
| |
| quite different from that of the mother in respect to melting point
| |
| and chemical composition, in that it contains much more palmitic
| |
| and less oleic and stearic acids.43
| |
| | |
| 0ther lines of evidence suggest that there is no direct passage
| |
| of lipids across the placenta! membranes. A marked dilkerence
| |
| | |
| «in fatty acid content of maternal and fetal blood has been found.
| |
| | |
| The lipid content of red blood corpuscles is about the same in
| |
| infant and adult. However, the blood plasma contained on the
| |
| average 948 mg. per cent in the former and 737 mg. per cent in
| |
| the latter in one series of estimations.44 Average values in another
| |
| series of human newborn infants appear in Table 23345
| |
| | |
| Tut-D 23
| |
| Lusrv couposrrron or Gut-arm) Fuss«
| |
| | |
| kapu- Nswkom Isgsggskezkszks
| |
| mg.Xl00 ce. mg.Xl00 Oe. »Ja« value«
| |
| Tom! lipids ........... .. 589 -i- 87 198 -s- 80 84
| |
| Neutral fat . . . . . . . . . . . . . 154 - 42 90 - 50 58
| |
| Tom! kam— acids ...... .. 353 -i- 56 140 - 57 40
| |
| Totul eholesteroL . . . : . . . 162 -I- 32 84 -l- 15 2l»
| |
| Bster eholesterol . . . . . . . . 115 -I- 27 A) -s- 12 l7
| |
| Iüsee eholesterol . . . . . . . . . 47 -I- 7 14 -s- 7 80
| |
| Phospholipid . . . . . . . . . . . . 196 -l- 23 6l- -I- 32 31
| |
| | |
| 0n the other band, the blood passing to the fetus from the
| |
| placenta is richer in certain lipids than that returning from the
| |
| fetusKC The difference must represent lipids used or stored by
| |
| 214 PITYSIOLOCLIY OF THE FETUS
| |
| | |
| the growing fetus. Averages of 15 analyses are given in Table
| |
| 24310
| |
| Tut-n 24
| |
| Lan) contes-im« or« Ema-m Unten-Iehr- conv Bnoov
| |
| | |
| Artery Vein
| |
| | |
| Ins! 100 Oe. mgjlllll ee.
| |
| Phospholipid . . . . . . . . . . . . . . . . . . . . . . . . 16«0 204
| |
| Free cholesterol . . . . . . . . . . . . . . . . . . . . . . 55 64
| |
| Ester cholesterol . . . . . . . . . . . . . . . . . . . . . 8 18
| |
| | |
| Neutral kat . . . . . . . . . . . . . . . . · . . . . . . . . 116 121
| |
| | |
| Phospholipids are always talcen up in large amounts, and
| |
| smaller amounts of free cholesterol may be absorbed by the human placenta. cholesterol esters pass to the fetus when they are
| |
| present in sulficient quantities in the mother’s blood. Neutral
| |
| fats have been thought to pass in both directions. Lipids continue to be added to the static placental blood by the placenta
| |
| after birth of the child. Without malcing a positive statement
| |
| concerning the mechanism involved, it may be said that a significant passage of lipids across the placental barrier takes place in
| |
| the human near term. It has been estimated that a well nourished
| |
| fetus takes up about 50 grams of lipids a day at full term, 40 grams
| |
| of which are in the form of phospholipidsås
| |
| | |
| The iipid eempesikieh ek khe pieeehkä and kekus ek the kahhik
| |
| at various stages in gestation has been reported« The phospho—
| |
| lipid and free cholesterol concentration increase rapidly in the
| |
| fetus up to the middle of gestation and then more slowly until
| |
| about the final weelc at which time the rate increases again. Up
| |
| to the middle of gestation, the placental concentration of« phos—
| |
| pholipid decreases while that of ester cholesterol increases. Beyond the midpoint in gestation, the reverse was found. slight increases in neutral fat and free cholesterol were encountered in
| |
| the placenta throughout gestation. The results suggest that there
| |
| is a greater demand for phospholipid by the fetus than can be met
| |
| by the placenta in the middle of gestation. and a late secondary
| |
| demand which is compensated near term by increased placental
| |
| ability to supply it.
| |
| | |
| Large amounts of fatty acids are accumulated in the liver by
| |
| the guinea pig fetus.48 Early irxgestation the liver contains about
| |
| FETAL NUTRITION AND METABOLISM 215
| |
| | |
| the same proportion as that of the adult, which is approximately
| |
| 2 to 4 gramsx its liver fatty acid concentration is already increasing,
| |
| and at a few days before birth (8o grams weight) the value reaches
| |
| 15 grams per cent, while the mother’s liver shows no change. It
| |
| drops again to the adult level within 3 or 4 days after birth.
| |
| | |
| The lipids of the fetal liver are much more unsaturated than
| |
| those in other fetal tissues and less so than those of the mother’s
| |
| liverfs 0ne wonders whether the fetal liver is endowed with
| |
| greater ability to desaturate fatty acids than is the mother’s liver
| |
| or if it simply receives already desaturated acids from the plaöenta.
| |
| The latter seems the more likely, and for the following reasons.
| |
| | |
| When the pregnant animals were fasted and then given
| |
| phloridzin it was found that the fetal liver storage of fatty acids
| |
| was increased in the early period of gestation (fetuses weighing
| |
| 30 grams and less) , but no signiticant change occurred in the maternal liver with the dosage used. Furthermore, the fatty acid was
| |
| less unsaturated than normal, as would be expected under influence of phloridzin with Inobilization of the connective tissue
| |
| fat to the liver. Evidently the fatty acid in the fetal liver, norma1ly encountered, is not transported from the other fetal tissues
| |
| but comes from the placenta.
| |
| | |
| Evidence has been presented recently that esterification of
| |
| cholesterol by fatty acids takes place in the liver cells of the chick
| |
| embryofk Histochemical tests indicated the presence of free
| |
| cholesterol, ester cholesteroland cholesterol-fatty acid mixtures in
| |
| the liver on the e1eventh day; but in chorio-allantoic grafts of the
| |
| liver, in which the host was several days older than the graft, these
| |
| substances appeared during the latter part of the seventh day. It
| |
| is evident, therefore, that the fetal liver is prepared for its röle
| |
| in lipid metabolism some time in advance of the day it actually
| |
| begins to work.
| |
| | |
| METABOLISM OF PROTEIN«
| |
| | |
| A great deal of information has been obtained in recent years
| |
| regarding the metabolism of proteins in bird fetusesEo but we
| |
| still know little about this process in mammals. There are three
| |
| principal methods for approaching the question. The chemical
| |
| composition of maternal and fetal blood can be compared, the
| |
| composition of the embryo itself at different stages of development can be determined, and Hnally the initrogenous waste prod216 Ptivsxotocv oF THE FErUs
| |
| | |
| ucts of combustion in the fetus can be analyzed. We shall ex—
| |
| amine evidence obtained in these ways.
| |
| | |
| Food proteins are digested and brolcen down into amino acids
| |
| which are absorbed into the mother’s blood. These are used, not
| |
| only for tissue metabolism of the mother’s own body, but they
| |
| serve as a readily available material out of which the fetus builds
| |
| its tissues. Some of the nitrogenous food material together with
| |
| nitrogenous waste products can be determined analytically as the
| |
| non-protein nitrogen of the fetal blood. It has been found that
| |
| non-protein nitrogen concentration of maternal and fetal blood is
| |
| practically identicaIZU This suggests that the compounds in question pass through the placenta by simple diffusion.
| |
| | |
| Amino acids of use to the fetus are relatively simple nitrogi
| |
| enous compounds which are soluble in the blood p1asma, and it
| |
| is known that they are highly ditkusible The human fetal plasma
| |
| at term contains about 2 mg. of amino-acid nitrogen per Ioo cc.
| |
| more than does that of its mother. In one 8 month premature infant the dilkerence was greater. This makes it seem probable that
| |
| simple physical processes are not the only mechanisms involved
| |
| in the passage of amino acids through the« placenta.
| |
| | |
| In the case of the nitrogenous waste products, ammonia, urea,
| |
| uric acid and creatinine, the concentration in the two blood
| |
| streams is almost identical and they probably pass from fetus to
| |
| mother by purely physical processes.44 Table 25 summarizes some
| |
| of the data on human subjects.
| |
| | |
| , TAVLD 25
| |
| | |
| Armut-n cost-knister- ops Ntstsnoonuovs coupounvs m sum« Bnoov or« IIUUAN Mosknmis
| |
| AND Fsskusns ask Fuhr« Tut-tu«
| |
| | |
|
| |
| | |
| Motheks blood Fetal blood No. of
| |
| | |
| mg.XI00 cc. mgJ 100 cc.« cases
| |
| Nonsprotein nitrogen . . . . . . . . . . . . . 25.2 24 . 9 85
| |
| Amino-acid nitrogen (plqsma) . . . . . . 5.5 7.4 10
| |
| | |
| 7 . 2 II .9 I premature
| |
| | |
| Urea and ammonia . . . . . . . . . . . . . . . 10.5 I0. 4 16
| |
| Uric acid . . . . . . . . . . . . . . . . . . . . . . .. s. 8 8.7 IT
| |
| creatinine (plasma) . . . . . . . . . . . . . .« I .67 I .75 I8
| |
| - I . 70 I . 78 12
| |
| | |
| Results of analysis of embryonic tissues throughout the course
| |
| of gestation demonstrate that the pig. builds very largely with
| |
| FETAL NUTRlTlcN AND METABOLISM 217
| |
| | |
| nitrogenous compounds during its early prenatal like. The total
| |
| nitrogen content ok the body per unit of dry weight decreases
| |
| gradually from the 6 mm. to the so mm. stage and then remains
| |
| constant throughout the remaining portion ok the gestation
| |
| period. The decrease may be related to an increase ok other nonnitrogenous solids such as carbohydrates, lipids and inorganic
| |
| salts. At the so mm. stage, when total nitrogen becomes constant,
| |
| the embryo may be said to have attained chemical maturityFo
| |
| | |
| Tut-ti- 26
| |
| Aventin Witten-r am) Pvacnivsrhen contain« or· Wann, Ast! am) Nrsraoonu n: PreKarosse«
| |
| l
| |
| Embryo Ash Nitrogen
| |
| Weiter«
| |
| Lenglzh Weight P« com; Wes; Dry Wet Dry Ashckree
| |
| mm. ging. per cent per eent per eent per eent per eent
| |
| 24 . . . . . . . . . . . . . . 97 .4 . . . . . . . . . . . . . . . . . . . . . . . . .
| |
| | |
| 647 . . . . . . .. 0 81 94.07 . . . . . . . . ·. 0 699 18 18
| |
| | |
| l0 . . . . . . . . 0 50 98.87 0 558 8.48 0 861 12 99 14 18
| |
| | |
| 15 . . . . . . . . 0 98 91.88 0 775 9.00 l 061 12 81 18 52
| |
| | |
| R) . . . . . . . . 2 21 91.14 0 708 8.00 l 108 12 45 18 58
| |
| | |
| 50 . . . . . . . . 6 55 91.65 l 086 12.41 0 910 10 91 12 45
| |
| | |
| 60 . . . . . . .. 014 85 91.05 . . . . . . . . .. 0 966 l0 80
| |
| | |
| 80 . . . . . . .. 26 00 91.59 . . . . . . . . .. 0 915 10 88
| |
| | |
| 100 . . . . . . ·. 722 t 91.l8 . . . . . . . . .. 095 1078
| |
| | |
| 110 . . . . . . .. 82 2 91.02 l so 14.50 0 972 10 82 12 65
| |
| | |
| 120 . . . . . . .. 962 91.26 . . . . . . . . .. 0950 1087
| |
| | |
| 160 . . . . . . .. 288 57 91.71 l 849 i 16.28 0 891 l0 75 12 84
| |
| | |
| 200 . . . . . . .. 488 0 90.84 . . . . . . . . .. l 014 10 50
| |
| | |
| 240 . . . . . . . . 725 0 88.7 2 58 2309 l 288 11 01 14 29
| |
| | |
| Interesting changes in the various kractions ok the total nitrogen have been observed. No signiiicant variation was apparent in
| |
| amide, humin and cystine nitrogen but amino nitrogen concentration was increased and that ok the non-amino nitrogen decreased correspondingly during the early stages. There was a kall
| |
| in arginine and histidine nitrogen and a deiinite rise in lysine
| |
| nitrogen before the 30 mm. steige. Tyrosine showed a gradual
| |
| decline throughout development. Glutathione, which is thought
| |
| to aid in synthesis ok proteins, increased sharply until 30 mm.
| |
| had been reached, after which it gradually decreased. Reciprocal
| |
| ontogenetic variations in the nitrogenous substances arginine,
| |
| histidine and lysine have been compared with somewhat similar
| |
| 218 PHYsIoLocY oF THE FETUS
| |
| | |
| phylogenetic variationsPfs E! They may be correlated to some ex—
| |
| tent with observations on tumor tissues, from which it appears
| |
| that the younger types of neoplasms have the greater content of
| |
| arg1n1ne.
| |
| | |
| Many attempts to study nitrogenous excretion in 1namma1ian
| |
| fetuses have been made without notable success. It is impossible
| |
| to account for all the nitrogen excretion because the greater part
| |
| is passed through the placenta, dissipated in the mother’s blood
| |
| and removed by her lcidneys. some, but only a small part, is
| |
| excreted by the fetal mesonephros and metanephros (see Chapter
| |
| VI1l) and passed into the allantoic and amniotic fluids which can
| |
| be recovered for analysis.
| |
| | |
| The urea content, in milligrams per ioo grams of human embryo, has been estimated to decrease as the gestation period advancesLo The amount of nitrogen per gram of fetus which is
| |
| excreted into the fetal fluid of the ruminating mammals is lilcewise
| |
| high in ear1y prenatal life, but decreases sharply and then remains
| |
| at a low level throughout the greater part of gestationFfs I» A much
| |
| clearer picture of nitrogenous metabolism of the embryo has been
| |
| obtained from studies in the chiclc. There. a closed system makes
| |
| it possible to obtain all the nitrogenous wastes which- accumulate
| |
| in the allantoic sac.59-10 Uric acid begins to collect in the allantois on the iifth day of incubation. The chiclc makes eflicient
| |
| use of the available protein, for about 96 per cent of that absorbed
| |
| from the egg by the embryo during the first 13 days of incubation
| |
| is retained in the embryonic tissues. Some protein is burned by
| |
| the chiclcz in fact about« 6 per cent of all organic matter used for
| |
| energy during the first two weelcs of incubation is protein.
| |
| | |
| 0ne of the most interesting aspects of fetal protein metabolism
| |
| is its comparative embryologysw Protein materials are used for
| |
| energy in much greater amounts by embryos with an aquatic
| |
| habitat than by those which are terrestrial. We may classify
| |
| mammalian embryos in the aquatic group with those of ftshes,
| |
| amphibians and many invertebrates, for they pour out their ex—
| |
| cretions through the placenta into the limitless aqueous environment of the mother’s blood stream and l(idneys. The terrestrial
| |
| group includes birds, some reptiles (e.g., lizards, snakes) , arthro—
| |
| pods (e.g., insects) and molluslcs (e.g., land snails) . Aquatic
| |
| embryos excrete nitrogen principally in« the— forth of ammonia and
| |
| FETAL NUTRITION AND METABOLISM 219
| |
| | |
| urea which are very so1ub1e and diffusible end-products and require excessive use of water for their e1imination. From a teleo1ogical viewpoint, one may say that the terrestrial forms must conserve water and consequently have had to devise other methods
| |
| of excreting nitrogen. Uric acid is the end-product in these
| |
| embryos. If birds had retained urea excretion instead of resorting
| |
| to uric acid, and if they had to store it all, their tissues would soon
| |
| become high1y saturated with urea because this substance can
| |
| dilfuse through the allantois into the body whereas uric acid is
| |
| retained, concentrated and precipitated within the allantoic sac
| |
| as the water is being absorbed and utilized. In their early development birds recapitulate aquatic stages in respect to their
| |
| protein metabolism. During the first 5 days of incubation ammonia and urea are excreted, but on the lifth day a shift is made
| |
| to uric acid and the embryo is thus spared a uremic fate.
| |
| | |
| IN ORGANIO IVIETABOLISM
| |
| | |
| It is lcnown that copper is stored in the human Iiver and its
| |
| concentration and absolute amount is higher there at birth than
| |
| at any subsequent time. Its concentration is greater at birth than
| |
| at earlier prenata1 periods.34 copper is essential for hemoglobin
| |
| synthesis and its mobilization in the fetal liver is thought to assure
| |
| normal blood formation in the postnata1 nursing period during
| |
| which the diet is delicient in this element. In contrast to conditions in man it has been reported that the late fetal pig liver
| |
| shows no increase in percentage of copper as growth proceedsPsH
| |
| The copper reserve of the liver is unusually low in the goat at
| |
| birthFS In the incubating chick too the percentage of copper
| |
| in the liver declines from the izth day to hatching, although
| |
| there is an increase in the actual amount present in the liver
| |
| throughout» The difference between pig, goat and chick on the
| |
| one hand and man on the other may be explained ,on the basis
| |
| of placental permeability. The chiclc must utilize what store it
| |
| has in the egg, the pig and goat get their copper from the histotrophe, but man, having a true placenta in which contact between
| |
| maternal and fetal blood streams is intimate, may be able to draw
| |
| heavily upon maternal stores.
| |
| | |
| It has been suggested that catabolism of maternal hemoglobin
| |
| talces place in the human placenta to supply the pigment fraction
| |
| 220 PHYSIOLOGY OF THE FETUS
| |
| | |
| of the hemoglobin molecule intact to the fetal circulationKs The
| |
| iron content of the human placenta gradually increases during development.59 Iron is stored in« the liver during fetal life and for
| |
| about two months after birth during which time there is an active physiologic postnatal hemolysis. Thereafter it declines in
| |
| amount until the nursing period has passed.s4 Iron is excreted in
| |
| the bile but is absorbed again in the fetal intestines.«4 As is true
| |
| of copper, the iron reserve of the goat is low at birth," and the percentage concentration of iron declines in the liver of the incubating chicl(.37 The ratio of copper to iron in the chiclc’s tissues,
| |
| other than the liver, stays constant throughout incubation. Nonhematin iron in the tissues is small. The metals are utilized and
| |
| not stored in such large quantities in the liver for postnatal use
| |
| as they are in the human fetus.
| |
| | |
| The efkect on fetal rats of iron deficient diets fed to the
| |
| mothers has been investigated recently.·3«0- S! The first pregnancy
| |
| brought on marked depletion of maternal liver iron but there
| |
| was no anemia; with the advent of a second pregnancy an anemia
| |
| did appear. The first litter of rat pups had normal hemoglobin
| |
| values, but a reduction in total iron content of the entire body
| |
| by about one-half the normal was evident. The second litter
| |
| exhibited a reduction of the hemoglobin of the blood and the
| |
| total iron content was only one-fourth normal. studies in the
| |
| human« reveal that iron deficiency of the fetus may be related
| |
| to that of the mother. Infants which are born of anemic mothers
| |
| may exhibit hypochromic anemia during the first year. The normal full term infant has a good reserve of liver iron which is
| |
| probably fully as important to it as the iron it may salvage from
| |
| catabolism of its excess hemoglobin during the early postnatal
| |
| period. If it were not for this fact, the human infant would probably exhibit more symptoms than it does when deprived of placental blood by the commonly practiced prompt c1amping of the
| |
| umbilical cord at birth.
| |
| | |
| A large series of chemical analyses of human fetuses has been
| |
| summarized recently by swanson and Iob.02 content of nitrogen,
| |
| calcium, iron and phosphorus throughout the greater part of prenatal life is illustrated in Fig. 69. The retention of these materials
| |
| shows a similar pattern of gradually increasing quantities. The
| |
| results indicate that there, can— be lit·kle··demand upon the mother’s
| |
| FETAL NUTRITION AND METABOLISM 221
| |
| | |
| « zoo
| |
| M
| |
| | |
|
| |
| | |
| PHOSPHORUS
| |
| 2»L
| |
| | |
|
| |
| | |
| s o
| |
| 34587690345670910
| |
| Dom-r« »oui«-ZFig. 69.- tent ok nitrogen, iu1n,iron and phospho ok the hutnan ketus
| |
| | |
| between the « c! th and bitt . (swanson sc lob: Am. . bst. sc. cyn., Vol. 38,
| |
| | |
| 1939. c. V. Mosby .
| |
| | |
| Fig. 7o.-E.tk ok changes in the cliet ok pregnant rats on calciu ncl phoss
| |
| phorus content o eolksprinz (swanson Z: lob: Arn. J. Obs . sc Gynsp . 38, 1939,
| |
| c. V. Mosby co.)
| |
| 222 PHYSIOLOGY OF THE« FETUS
| |
| | |
| reserve of the elements in question during the first halk ok presnancy. In kact it is not Juntil the last two or three months ok
| |
| gestation that the ketal requirements become large.
| |
| | |
| calcium and phosphorus are concerned in building skeletal
| |
| structures. Their content in the ketus is iniiuenced by vitamin D
| |
| in the mother’s diet and apparently by the amount ok exposure to
| |
| sunlight.s«4 The eEects ok changing the diet ok pregnant rats
| |
| in respect to vitamin D are illustrated in Fig. 7o. When the
| |
| mother’s diet, fortiiied by vitamin D, is low in the required
| |
| minerals the calcium and phosphorus content ok the ketal body
| |
| approaches the normal level, but when the diet has in it the required amounts ok the minerals plus the vitamins the ketal calcium
| |
| and phosphorus content exceeds the normal. Thus the ketal
| |
| metabolism ok calcium and phosphorus is dependent upon that
| |
| ok the mother and the transmission 'ok these substances to the ketus
| |
| can be increased by vitamin D adminis·tration. In the human
| |
| subject occurrence ok early congenital riclcets is illustrative ok
| |
| maternal deiicienciesXE
| |
| | |
| Metabolism ok inorganic substances other than those we have
| |
| already considered has been studied less eictensively. The most.
| |
| marked changes in all inorganic compounds are encountered in
| |
| the kourth lunar month in man. Bekore that time the ketus contains relatively little chlorine, potassium, sodium and magnesium,
| |
| but these elements show a marked increase at the kourth monthFs
| |
| | |
| ENERGY METÄBOLISM
| |
| | |
| Oxygen consumption in the ketus has been studied in various
| |
| species and by various methods. The most signikicant data relative to amount and rateok utilization have been obtained in the
| |
| incubating chiclc and in the sheep ketus. The amounts ok oxygen
| |
| used and ok carbon dioxide given oE by the incubating chicl(·increase in proportion to growth in size ok the embryo. At six days
| |
| ok incubation oxygen is consumed by the embryo (exclusive ok
| |
| its membranes) at the rate ok o.o2 cc.Jgrn.Jmin. This rate de—
| |
| clines as growth proceeds and by the nineteenth day reaches
| |
| o.oi34 ccJgrnJminFC Barcrokt and his colleagueswi have estimated the rate ok oxygen consumption in the sheep ketus recently
| |
| by a direct method. They obtained samples ok ketal blood at
| |
| timed intervals akter occludingthei umbilica1 cord and determined
| |
| FETAL NUTRITION AND METABOLISM 223
| |
| | |
| its oxygen content. In this way they observed the loss of oxygen
| |
| in respect to time and could calculate its utilization per gram ok
| |
| the ketal tissue without the complicating kactors ok the placenta
| |
| and ketal rnembranes. Their data appear in Table 27. It will
| |
| | |
| Tat-m 27
| |
| Oxford: conswrrtorc m sauer« Ferner-s
| |
| | |
| Oxygen eonsumption
| |
| | |
|
| |
|
| |
| | |
| Fetal age Fetal weight
| |
| days grams
| |
| cc.-mirs. cc.Xgm.Xtain.
| |
| III . . . . . . . . . . . . . . . . . . ·. I,200 4.6 0.0038
| |
| | |
| 126 . . . . . . . . . . . . . . . . . . . .I 8,000 I2·s 0.004I
| |
| | |
| 127 . . . . . . . . . . . . . . . . . . . . 2850 II .2 0. 0089
| |
| | |
| 129 . . . . . . . . . . . . . . . . . . 2750 SZL 0.008I·«
| |
| | |
| Is7 . . . . . . . . . . . . . . . . . . . . 8,850 20.0 00052
| |
| | |
| 138 . . . . . . . . . . . . . . . . . . . .k FOR) I5.5 0.0042
| |
| | |
| 152 . . . . . . . . . . . . . . . . . . . . 2800 I6.4s 0.0048·«
| |
| | |
|
| |
| | |
| ’«'Authors’ values; errors present but souree unless-wo.
| |
| | |
| be seen that, although the total amount ok oxygen consumed
| |
| each minute rises sharply at the beginning ok the last quarter
| |
| ok gestation, the rate ok utilization remains nearly constant
| |
| throughout the period studied and averages o.oo43 cc.xgrn.jmin.
| |
| (excepting the 129 day ketus) . This is a higher value than was
| |
| obtained in earlier less satiskactory experiments in the Cambridge
| |
| laboratoryss and by other investigators who have used indirect
| |
| methods o»k estimation. The oxygen consumption ok human
| |
| ketuses at terms bekore labor starts has been estimated to be
| |
| 1.25 ccJkiloJminO
| |
| | |
| The ratio ok the amount ok carbon dioxide given olk to that ok
| |
| cc. carbon dioxide
| |
| | |
| cc. oxygen
| |
| embryos ok several species. This, the respiratory quotient, varies
| |
| signiHcantly in the chiclc. Most deterrninations during the Hrst
| |
| tive days ok incubation have given values in excess ok o.7, some
| |
| ok them approaching unity. In vitro experiments on the iive
| |
| day chiclc have demonstrated that the quotient is 1.o at this
| |
| time« Between the sixth and ninth days ok incubation the respiratory quotient declines to approximately o.7, but it rises again
| |
| toward unity between the tenth and thirteenth days. These fluc
| |
| oxygen consumed ( ) has been determined in
| |
| 224 PHYSIOLOGY OF THE« FETUs
| |
| | |
| tuations have been talcen to signify that the embryo utilizes carbohydrates almost exclusively for combustion up to five days, burns
| |
| proteins very largely for the next few days and after the tenth
| |
| day of incubation resorts to combustion of fat supplemented by
| |
| rather large quantities of carbohydrateJo It should .be pointed
| |
| out that in the chiclc, which excretes uric acid instead of urea after
| |
| the lifth day, a respiratory quotient of o.7 rather than o.8 should
| |
| be expected during combustion of proteins. For more detailed information, the reader should refer to original articlesJos W- ««
| |
| | |
| The respiratory quotient of guinea pig fetuses has been determined74 by direct measurement of the oxygen consumption and
| |
| carbon dioxide evolution of the mother and her fetuses in utero
| |
| before and after occluding the umbilical cords. Quotients of o.9
| |
| to 1.2 were obtained for the fetuses in most instances, and o.7 to
| |
| o.9 in the mother. Coniirmatory results have been obtained by
| |
| others« in experiments using whole rat embryos in vitro (mean
| |
| R. O. .—.—. I.o4) . Respiratory quotients of this nature indicate that
| |
| mammalian fetuses consume carbohydrates almost exclusively in
| |
| their energy metabolism.
| |
| | |
| Many investigators have studied metabolism during pregnancy.
| |
| especially in man, by indirect calorimetric methods.10 Mur1in75
| |
| observed a dog during two consecutive pregnancies, one pup being
| |
| produced at the lirst and live pups at the second birth. An in—
| |
| crease in caloric energy production due to the single fetus could
| |
| be detected at the sixth weelcz it amounted to 9 per cent between
| |
| the sixth and eighth weelcs. The total energy produced at full
| |
| term was proportional to’ the weight of the offspring and was about
| |
| equal to that required by the newborn pups (when calculated according to the law of slcin area·) . It amounted to 16.4 gm. cal.J1oo
| |
| gar. in the single pregnancy and 16.8 gm. cahxioo gar. in the
| |
| multiple pregnancy. The curve of total energy production of the
| |
| dog and her pups showed no deflection at birth. The number of
| |
| calories formed by the resting pregnant dog plus fetuses, placentas
| |
| and membranes was very much the same as the sum of that produced after delivery by the lactating dog and her resting pups.
| |
| | |
| In the human not all investigators have found relationships
| |
| quite so simple as those occurring in Mur1in’s experiments in the
| |
| dog. some« have reported that excessive heat production in
| |
| pregnancy results from some factors other than those of fetal
| |
| FBTAL NUTRITION AND METABOLISM 225
| |
| | |
| growth. 0thers"- 78 have been able to account for all of the ex—
| |
| cess on the basis of fetal heat production, fmding that the energy
| |
| produced by the woman plus her fetus and its accessory structures
| |
| at full term is equal to that produced by the lactating woman
| |
| and the infant after birth. Recently, Enright and her associatessp
| |
| reported that a greater post-partum drop in energy metabolism
| |
| than is accountable on the basis of that produced by the fetus
| |
| alone occurs in i 5-year-old females, amounting to about three
| |
| times the probable basal energy requirements of infants. They
| |
| concluded that in pregnant adolescents there appears to be some
| |
| factor stimulating metabolism which results in a greater rise than
| |
| occurs in more mature women. They suggested that this excessive energy production of immature -girls may be related to thyroid
| |
| function, and have presented some evidence that feeding iodized
| |
| salt diminished the rise in metabolism during pregnancy.
| |
| | |
| One point which has not been emphasized is worth consideration. The fetus in utero is quiescent and hypotonic whereas the
| |
| newborn infant is active and its muscles possess good tonus. If
| |
| the energy produced by the newborn is commensurately greater
| |
| than that of the full term apneic, hypotonic, quiescent fetus use
| |
| will have to conclude that more energy is produced by the woman
| |
| (plus the accessory fetal structures but minus the fetus) than is
| |
| produced by the post—partum lactating woman. The alternative
| |
| assumption is that basal requirements of the hypotonic fetus are
| |
| fully as great as in the newborn infant, and this seems unreason—
| |
| able.
| |
| | |
| The various calorimetric studies suggest that the fetal metabolic rate remains fairly constant throughout the latter part of
| |
| gestation, but during the early period while the embryo is very
| |
| small no data are available« Postnatally the rate rises and reaches
| |
| a pealc at about the Erst or second year in the human. similar
| |
| postnatal pealcs have been observed in other animals such as the
| |
| rabbit, mouse and some breeds of pigz others, notably the guinea
| |
| pig, show an already declining metabolic rate at birth. These
| |
| facts may be related to the maturity of the heat regulating mechanisms in the different species (see chapter VIII) . They suggest
| |
| | |
| «« Oxygen consumption of mammalian eggs during the one— to eightscell stages
| |
| ainounts to o.ooo7z c.mm. per egg per hour. When gkowth in size begins the oxygen
| |
| oonsumption increasesz on the eighth day of gestation in the rat it amounts to about
| |
| «o.o1 c.mm. per hour. This increases to about o.2 drum. in the next two days«
| |
| | |
| Is
| |
| 226
| |
| | |
| PHYSIOLOGY OF THE FETUS
| |
| | |
| that the metabo1ic rate may in rea1ity be increasing to some ex—
| |
| tent throughout prenatal like in man and in the other animals
| |
| with a postnatal pealc ok heat production and may have begun to
| |
| decline in the others before birth.
| |
| | |
| REFERENCES CITED
| |
| | |
| . Meyer, R. i9e5. CentralbL Gyn» 49: i866.
| |
| . Grossen 0. i9e7. Frähentwiclälung Eihautbildung und Placentatiom
| |
| | |
| Bergmanm München.
| |
| | |
| Grossen O. i933. Lancet, ee4: 999.
| |
| | |
| Bryce, T. H. i937. Edin. Med. J» 44: 3i7.
| |
| | |
| Wislockb G. B. sc G. L. streeten i938. conti·. Kind» 27- tBrunschwigz A. E. ige7. Anat. Rec» 34: e37.
| |
| | |
| Everett, J. W. i935. J. Exp. Zool» 7o: e43.
| |
| | |
| Wisloclch G. B. i9ei. Anat. Rec., ei: e9.
| |
| | |
| Wislockh G. B. i9ei. «Contr. Erim» i z: 9i.
| |
| | |
| N eedham, J. i93i. chemical Embryology, Macmillanp N. Y.
| |
| shimidzm Y. i9ee. Am. ·J. Physiol» He: -eoe.
| |
| | |
| . cunningham, R. s. i9e3. Proc. soc. Exp. Biol. sc MAX» eot 343.
| |
| . Römer, P. H. i9o5. Beitr. exper. Therap» g: is.
| |
| | |
| . Rodolio, A. i934. J. Exp. Zool» 68: ei z.
| |
| | |
| . Mossman, H. W. ige6. Am. J. Anat» 37: 433.
| |
| | |
| . Cunningham, R. s. igee. Am. J. Physio1»,6o: 448.
| |
| | |
| . Bremer, J. L. i9i6. Am. J. Anat» i9: i.79.
| |
| | |
| . Anselmino, K. J. i9e9. Arch. Gynäk.. i 38: 7io.
| |
| | |
| . schlossmann, H. i93e. Ergebn. Physiol» 34: 74i.
| |
| | |
| . cohnstein, J. sc N. Zuntz. i884. Plliigeks Arch., 34: i73.
| |
| | |
| Morriss, W. H. igi7. Johns Hoplcins Hosp. Ball» es: i4o.
| |
| | |
| . schlossmanm H. i938. J. Physiol» ge: eig.
| |
| | |
| . Passmore, R. sc H. schlossmann. i938. J. Physiol» 922 459.
| |
| | |
| . Aron, M. i9eo. compr. Rend. soc. Biol., 83: 63i, 1445- l447i
| |
| . Zorn, C. M. sc A. J. Dalton. ig37. Am. J. Physiol» ii9: 6e7.
| |
| . Aron, M. ige4. Arch. Internat. Physiol» ee: e73.
| |
| | |
| . snydeiy F. F. sc F. M. Hoslcins.
| |
| . Bernard, C. i858. Ann. sei. Nat. (ser 4), io: in.
| |
| | |
| . Chipman, W. i9oe. stud. Roy. Victorian Hosp» Montreah WI- 1- M)
| |
| i9e8. Anat. Rec» 38: es.
| |
| | |
| 4, Gynec. i, pp. i-e6i. (Reprinted in Rep. Lab. Roy. Goll. Physicians
| |
| Edin» vol. s, i9o3.)
| |
| | |
| . Lochhead, J. sc W. Cramer. i9o6. J. Physiol» 35: ii P.
| |
| . Corey, E. L. i935. Am. J. Physiol» ne: e63.
| |
| | |
| . staat-r, H. A. sc G. M. Higgins 1935. Ibid., iii: 59o.
| |
| | |
| . sandstrom, R. H. ig34. Physiol. Zool» 7: Les.
| |
| | |
| . Aron, M. i9ee. Arch. Anat. Hist. Emb., i: 69.
| |
| | |
| . Potvin, R. sc M. Aron.
| |
| . Dalton, A. J. i937. Anat. Rec., 68: IV.
| |
| | |
| . schlossmann, H. ig3i. Arch. Exp. Path. Pharmalm Izgk As.
| |
| . Gorey, E. L. i935. Am. J. Physiol» its: 45o.
| |
| | |
| . Gage. s. H. sc s. P. Gage. · i»9g9. --Anat..Rsze·c» z: eo3.
| |
| | |
| i9e7. Comp. Rend. soc. Biol.. 96: e67.
| |
| FETAL NUTRITION AND METABOLISM 227
| |
| | |
| 4o. Meiidel, L. B. sc A. L. Daiiiels i9ie. Biol. chem., i z: 7i.
| |
| | |
| 4i.
| |
| . Wessoti, L. G. t9e6. Johns Hoplcins Hosp. Bull., 38: e37.
| |
| 43«
| |
| . slemons, J. M.
| |
| . Boych E. M. t936. Am. J. Dis. child., He: i 3i9.
| |
| . Boyd, E. M. sc K. M. Wilson.
| |
| . Boyd, E. M. i935. Biochem. J» e9: 985.
| |
| | |
| . linrie, c. G. sc s. G. Graham. i9eo. J. Biol. chem» 44: e43.
| |
| | |
| . Dalton, A. i937. Anat. Rec» 67: 43i. «
| |
| | |
| . Willcersom V. A. sc R. A. Gorrtien i93e. Am. J. Phys10I-- tue: i53.
| |
| . Rosedale, L. sc P. Morris. i93o. Biochetrx J., e4: ie94.
| |
| | |
| . ljnclsazy D. E. t9ie.
| |
| . Fislce, C. I-I. sc E. A. Boyclen. i9e6. J. Biol. chem» 7o: 535.
| |
| | |
| . Ramagcz I-I., J. l-I. shelclon sc W. sheldoix i933. Proc. Roy. soc., Lotid.
| |
| | |
| Baum-um, E. J. sc O. M. l-lolly. t9e6. Am. J. Physiol» 75: 633.
| |
| | |
| Knopkelmachetz W. i897. Jahrb. Kitiderhllc., 45: i77.
| |
| i9i9. Atti. J. Obst» so: i94.
| |
| | |
| i935. clin. Ins-est» 142 7
| |
| 1hid., s: 79.
| |
| | |
| B., its: 3o8.
| |
| | |
| . Willcersoih V. A. i934. Biol. chem., io4: 54i.
| |
| . Ramage, I-I. i934. Bioclietix J., es: i5oo.
| |
| | |
| . McFarlatie, W. D. sc l-I. l. Milne.
| |
| . Schick, B. i9ei. Ztsclir. Kindes-thue, e7: est.
| |
| | |
| . I-lilgenbei·g, F. c. i93o. Ztschr. Geburtslx Gytiäk., 98: e9i.
| |
| | |
| . Parsoiis, L. G., E. M. Hiclcmans sc E. Fluch. ig37. Axch. Dis. chilclli»
| |
| | |
| t934. J. Biol. chem» io7: zog.
| |
| | |
| te: 369.
| |
| | |
| . L. i938. Am. J. Dis. child., 56: 975.
| |
| | |
| . swanson, W. W. sc V. lob. tg39. Am. J. Obst. sc Gyn» 38: 38e.
| |
| . swanson, W. W. sc V. lob. i935. Am. J. Dis. chilc1» 49: 43.
| |
| | |
| . sontag, L. W» P. Munson sc E. I-Iukk. i936. lbicl., Hi: 3oe.
| |
| | |
| . lob, V. sc W. W. swatison.
| |
| . Neeclham, J. t93e. Proc. Roy. soc» Loiicl» B, iio: 46.
| |
| | |
| . Barcroky J» J. A. Kennecly sc M. F. Masoix i939. J. Physiol» 95: e6g.
| |
| . Baxcivktz J» L. B. Flexner sc T. Mcclurlciix
| |
| . I-laselhorst, G. sc K. strombergen
| |
| | |
| t934. lbicl., 47: 3oe.
| |
| | |
| i934. lbicl., se: 49s.
| |
| i93e. Ztsckm Geburtsh. Gynäk., ioe:
| |
| i6
| |
| | |
| . Dicke-Ins, F. sc F. simeia i93o. Biocheitx J» e4: i got.
| |
| | |
| . Bohig c. sc K. A. Hasselbalch igo3. slcath Arch. Physiol» i4: 398.
| |
| . l-lasselbalch, K. A.
| |
| . Murray, H. A» Ji·.
| |
| . Rohr, c. i9oo. Rand. Arch. Physiol» to: 4i3.
| |
| | |
| . Mut-litt, J. R. i9io. Am. J. Physiol» e6: i34.
| |
| | |
| . Rost-e, A. W. sc W. c. tzoyci. igzsr. J. Nutritioiy z: 55i.
| |
| | |
| . carpentetz T. M. sc J. R. Mut-Un. i9i i. Arch. litt. Mai» 7: i84.
| |
| | |
| . saiidikorch l» T. Wlieeler sc W. M. Boothbyc i93i. Am. J. Physiol»
| |
| | |
| i9oo. lbicl., to: III.
| |
| i9e7. J. Gen. Physiol» to: 337.
| |
|
| |
|
| 96: tgi.
| |
|
| |
|
| . Ein-ishr, L» V. V. cole sc F. A. Hitchcoclg t935. lbicl» its: eei.
| | {{Footer}} |
| . Bot-II, E. J. sc J. s. Nicholas i939. scieiice, 9o: 4i i.
| |