Book - Outlines of Chordate Development 6
| Embryology - 20 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Kellicott WE. Outlines of Chordate Development (1913) Henry Holt and Co., New York.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter 6 The Early Development of the Mammal - The Mammalian Embryonic Membranes and Appendages
Introduction
WE shall not undertake, in the present chapter, to give an account, however brief, covering the whole embryonic history of a Mammal. We shall rather attempt to describe certain phases or aspects of mammalian development, selected on account of their interest or importance for the general student. We shall give first a description of the mammalian ovum, its formation, the early processes of cleavage and the formation of the embryonic layers, and the formation of the embryo and its chief rudiments. This will be followed by a brief account of the development of external form of the human embryo. Then in conclusion we shall outline the more salient facts regarding the embryonic membranes and appendages, and the establishment of those relations between the embryo and the maternal organism which are such fundamental characteristics of the true (Eutherian) Mammals. For the whole subject of mammalian organogeny the student may be referred to the excellent and recent accounts given in such texts as those of 0. Hertwig, Keibel-Mall, Minot, McMurrich, etc.
The whole life-history of the Mammal may be roughly divided into four periods, each marked by one or two striking characteristics, but often not otherwise clearly separated. First is the true embryonic period or period of gestation, during the greater part of which the organism is retained within the uterine cavity of the mother, drawing its nourishment from the uterine walls. This period extends from the time of fertiliza tion to the time of birth, and its duration is widely variable ir different species, though usually quite constant in any single form, due, perhaps, in part, to the fact that conditions of temperature, nutrition, etc., are subject to only slight variation. To mention a few examples, the period of gestation is, in the mouse twenty to thirty days (Daniel), rat about twenty-one days, in the rabbit thirty to thirty-two days, guinea-pig sixty-four to seventy days, cat about nine weeks, dog fifty-nine to sixtythree days, sheep about twenty-one weeks, pig about four months, cow about nine months, man about nine months (270-280 days), deer ten months, horse about eleven months, elephant about twenty months.
The time of birth, or parturition, marks the most abrupt physiological and morphological transition in the entire lifehistory. There is wide variation, among different forms, of the comparative stage to which development has proceeded when this event occurs. Some organisms, like the calf or the colt, may be termed precocious, since they are able, within a few hours after birth, to run about actively and to live with a minimum of parental protection and care. Others, such as the kitten or young rabbit, are born in a much less advanced stage and, with unopened eyes and uncoordinated movements, remain almost helpless for several days. Some of the marsupial Mammals (Metatheria) are quite remarkable in that the young are born after a very brief period of gestation (about eight days in the opossum (Didelphys) and in Dasyurus), at a relatively very early stage of development, not able even to perform the simple act of sucking. In these forms there are special adaptations to this condition, and the young are at once transferred to a special external cavity of the mother, the marsupium or pouch, where development proceeds.
In no case is the young Mammal entirely independent of the maternal organism for some time after parturition, for there follows the second general period in the life-history, that of lactation, during which the young organism is wholly or partly dependent for its nourishment upon the mammary secretion of the mother'. During this period development continues, of course, but at a slower rate, and toward its close there is a gradual transition to conditions of complete independence, save that some degree of parental care may be exercised for a . time longer. The duration of the period of lactation is variable, even in a given species.
Two other periods in the life-cycle of the Mammal need only to be mentioned; these are the period of adolescence, during which growth and development continue at a still slower rate, and the period of adult life or sexual maturity. The transition between these periods is often marked by a series of structural and physiological alterations in characteristics other than those of the reproductive system.
Embryologically as well as morphologically, the Mammalia present many similarities to the Sauropsida. The mammalian ovum is nearly yolkless, and yet in its development it exhibits many of the phenomena of yolk-influence characteristics of eggs of the extreme telolecithal (meroblastic) type such as the formation of a modified germ disc, of a (yolkless) yolk-sac, and other less striking characteristics. While the mammalian egg resembles that of Amphioxus in size and deutoplasmic relations, it exhibits almost none of the regularities of cleavage, blastula formation, and gastrulation that we should expect to be associated with a small, homolecithal ovum.
The origin of the most fundamental modifications of the sauropsid type of development lies in the replacement of the intra-oval yolk-mass by a source of food and energy lying outside of the ovum and embryo, i.e., the maternal uterine walls, and in the early and extensive relation between the embryo and this new source of nutrition.
In the following account of certain phases of mammalian development, we shall not be limited to any single form throughout, but shall describe in general, elementary terms, the mammalian type of development, using various forms as illustrations of the topics considered.
The Egg and its Formation
The Reproductive Organs of the Female
In the Mammals there is always a single parr of ovaries, suspended in the postero-dorsal region of the body cavity by peritoneal mesovaria (Fig. 143). They are whitish, rounded or ovoid bodies, of rather small dimensions (human, 3-4 cm. long, by 2-3 cm. wide, by 0.7 1.2 cm. thick; rabbit, about 2 X 0.8 cm. The ovaries are not directly connected with the gonoducts, although the openings of the oviducts are suspended in the same peritoneal mesovaria, and are located very near to the ovaries, so that the ova, when discharged from the ovary pass only a short distance through the body cavity before entering the oviduct (Fig. 143).
The Miillerian ducts, or oviducts in the broad sense, are muscular tubes, highly differentiated into three regions. The upper or anterior portion forms the Fallopian tube or oviduct, stricto sensu (Fig. 143). The inner end of this, where it opens out of the body cavity near the ovary, is expanded and its margin is drawn out into finger-like or fringe-like processes; this is the infundibulum, ostium or fimbriated opening. The second section is the uterus, thicker walled than the Fallopian tube, and of greatly varying extent in different Mammals, correlated with the number of young produced at one time, for this is the part of the oviduct occupied by the developing embryos. Lastly is the terminal vagina, which opens directly to the outside in all placental Mammals.
The vaginal region is practically always a single, median structure, formed by the fusion of the lower ends of the two oviducts. Different groups of Mammals exhibit various degrees in the extent of the fusion of the uterine sections also. Thus in the Rodents the vaginae alone are fused, the uteri remaining entirely distinct (uterus duplex), in the Garni vors and most Ungulates the uteri are partly fused, partly free (uterus bicornis), and in the Primates the uteri are completely fused and only the Fallopian tubes remain paired (uterus simplex).
Fig. 143. Diagrammatic representation of the human female reproductive organs. Dorsal (posterior) view. From Quain's Anatomy. The posterior walls of the uterus and vagina have been removed to show their cavities, c, Cervix of uterus; fi, fimbriated opening or ostium of oviduct; h, hydatid; i, wider distal part of oviduct; I, round ligament; II, the broad ligament; lo, ligament of ovary; o, ovary (naturally the ovary has an oblique or nearly vertical position); od, oviduct or Fallopian tube; po, parovarium; u, fundus of uterus; v, upper part of vagina.
The Ovum and its Ovarian History
The ova of the placental Mammals are among the smallest known. When fully formed they are usually 0.1-0.3 mm. in diameter, although these limits are occasionally exceeded; for example, the ovum of the mouse measures about 0.06 mm., deer 0.07-0 . 10 mm., guinea-pig 0.09 mm., dog about 0.18 mm., human 0.22-0.32 mm., cat 0.135-0.15 mm., rabbit 0.1 1-0. 12 mm. The cytoplasm of the ovum ordinarily exhibits two general regions, a clear exoplasm or cortical layer surrounding an opaque endoplasm containing small granules of deutoplasmic material (Figs. 144, 146). The nucleus or germinal vesicle of the fully formed oocyte is relatively large, usually 0.025-0.040 mm. in diameter; it is spherical, possesses a definite nuclear membrane, a large nucleolus (karyosome), and has a slightly eccentric position.
Fig. 144. Fully grown human oocyte just removed from the ovary. Outside the oocyte are the clear zona pellucida and the follicular epithelium (corona radiata). The central part of the oocyte contains deutoplasmic bodies and the eccentric nucleus (germinal vesicle). Superficially there is a well-marked exoplasm, or cortical layer. From Waldeyer (Hertwig's Handbuch, etc.).
The presence of a vitelline membrane is not definitely known; surrounding the ovum, however, is a thick transparent membrane apparently of chorionic nature (i.e., of follicular origin secondary egg membrane), known as the zona pellucida. This often has, either throughout or at least peripherally, the appearance of being perforated by minute pores or canals, and hence is often called the zona radiata. A micropyle is not known. The zona pellucida is usually separated from the surface of the fully grown egg by a narrow perivitelline space. In many Mammals, at the time the ovum escapes from the ovary it is, and for a time remains .surrounded by a few layers of regularly arranged cells forming the corona radiata (Fig. 144). This is a part of the ovarian egg follicle, and in order to understand its relations we must outline the earlier ovarian history of the ovum.
The formation of the ova begins in the ovary before the time of birth, and in the case of the Mammals all of the ova which are to be produced during the period of fertility, are at that time definitely established, although only partly differentiated. In other words the period of the multiplication of the oogonia is completed during embryonic life. Subsequently there occur the phases of oogonial growth and the maturation processes. In the embryonic ovary the primordial germ cells divide repeatedly and soon form large numbers of cells arranged in small groups or " nests" (Fig. 145). The cells composing these have all had a similar history, but are destined to have very different fates. In each group one cell enlarges and becomes a definitive ovum, while its sister cells and their descendants are to form the egg follicle. All of the stages in the history of the formation of the ovum and follicle can be found in the ovary of a fertile individual.
The follicle appears first as a small group of flattened cells, forming a single layer around the slightly enlarged central oogonium (Fig. 145). As the follicle cells multiply they become cubical and then columnar, forming a definite epithelium. As the ovum increases steadily in size the follicle more than keeps pace with it, so that as the follicular epithelium becomes three to four cells deep, spaces begin to appear within the follicle, usually toward one side. On the side opposite these spaces the follicular cells multiply more rapidly and form a definite accumulation known as the discus proligerus, in the midst of which the ovum is buried (Fig. 145). The follicle cells immediately surrounding the ovum become markedly elongated forming the corona radiata, mentioned above/ These cells appear to be directly connected with the ovum by fine pseudopodial processes affording the pathways by which substances enter the ovum, providing for its growth. The other cells of the follicle form what is known as the stratum granulosum.
Fig. 145. Section through part of the ovary of a dog. After Waldeyer. a. "Germinal epithelium"; b, egg tubes; c, small ovarian follicles; d, older ovarian follicles; e, ovum surrounded by discus proligerus; /, second ovum in follicle with e. (Only rarely are two ova thus found in a single follicle.) g. Outer capsule of the follicle; h, inner capsule of the follicle; i, membrana granulosa; k, collapsed, degenerating follicle; I, blood-vesesls; m, sections through tubes of the parovarium; y, involuted portion of superficial epithelium; z, transition to peritoneal epithelium.
As the follicle and egg approach maturity the follicular cavity, containing the liquor folliculi, becomes very large, and the whole structure becomes enclosed in a definite capsule consisting externally of connective-tissue fibers and cells formed from the stroma of the ovary, and internally of a thick layer of cells, blood-vessels, .and nerves. Within this lies the basement membrane of the follicular epithelium (granulosa cells). At the close of the oogonial growth period, the cells of the corona radiata form a thick membrane (zona pellucida) around the egg, and the protoplasmic processes remain only partially and indistinctly (zona radiata). The full-grown follicle is very large (9-14 mm. in man) forming a well-marked projection from the surface of the ovary. The mammalian follicle is usually known as the Graafian follicle. It was first described in 1677 by Regnerus de Graaf and was regarded as what we should now call the ovum, until Von Baer's description in 1827 of the true mammalian ovum.
As to the history of the egg itself during the growth stage, little need be said here. A differentiated region around the nucleus appears very early, before the follicle has definitely formed. This becomes a sort of "yolk nucleus" giving rise to the deutoplasmic content of the egg; it disappears while the follicle is still single layered. During the growth period, which is also a period of "organization" of the ovum, the nucleus appears to give off chromatic substance into the cytoplasm. A pair of small centrioles with surrounding centrosphere may be seen during the early stages of growth.
Maturation
At the close of the growth period the nucleus forms a large clear vesicle, with very little chromatin other than that of the large chromatin reservoir or karyosome. It is now ready to enter upon the period of maturation. As a rule, in the Mammals, the first polar body is given off while the ovum is still within the ovary (in the mouse about one hour before ovulation), and the second polar spindle is established; the second polar division is then completed only after fertilization, which occurs, of course, in the oviduct. It is not necessary to describe the details of the maturation process here, for in most respects it offers nothing unusual. With respect to the size of the polar spindles and polar bodies, however, the Mammals are rather remarkable, for these, especially the first polar body, are very large, often one-fourth the diameter of the ovum itself, and in some cases even larger. Occasionally an abnormally large first polar body may be formed so that the egg divides almost equally; the later history of such cases is not known. The first polar body usually divides soon after its formation; amoeboid movements have been observed in the first polar body of the mouse. Centrioles are distinctly present, but the asters are only slightly indicated or absent.
A large second polar spindle is formed at once and moves toward the surface of the egg (secondary oocyte,) when the process of maturation is inhibited while the processes of ovulation and fertilization occur. The second polar body is then formed while the ovum is in the upper part of the oviduct; it is smaller than the first. The polar bodies do not remain attached to the surface of the ovum (Fig. 147) and are easily lost sight of entirely. The number of chromosomes can be most easily determined during these phases; some of the somatic numbers determined are the following: man, twentytwo in the male, twenty-four in the female (Guyer), mouse twenty-four, cat between twenty-eight and thirty-two.
Ovulation
The escape of the ovum (secondary oocyte) from the Graafian follicle and the ovary, is termed ovulation. The capsule of the maturing follicle becomes very vascular, and at one place very thin and easily ruptured; this is the cicatrix or stigma. It hag been suggested that the rupture of the follicle may be due to the continued accumulation of the liquor folliculi. However this may be, when the follicle bursts the liquor flows out into the periovarial cavity, carrying along the ovum, still surrounded by the corona radiata. The fimbriaB of the oviduct are also enclosed in this periovarial cavity, and through the ciliary action of the epithelium covering these and lining the upper part of the oviduct, and probably also through peristaltic contractions of the oviducal walls, the ovum is carried through the ostium and into the oviduct. During this passage the first polar body frequently breaks through the chorion (zona pellucida or radiata) and through the corona radiata as well, so that it is entirely lost from the region of the ovum (mouse, Kirkham).
Returning to the history of the follicle itself, we find it undergoing very important changes, as the result of which it is converted into the corpus luteum. The emptied follicle soon becomes a nearly solid mass of cells, known as lutein cells, large rounded cells containing quantities of pigmented granules or lutein. The origin of these cells is somewhat uncertain; they appear to be derived from the stratum granulosum cells of the follicle, although they may come from the inner capsule of the follicle (stroma cells). Their pigment is yellowish in man, hence the name corpus luteum; in other Mammals it may be pinkish (pig, rabbit), red (mouse), brown (sheep), etc.
In cases of non-pregnancy following ovulation, the corpus luteum is rapidly converted into fibrous connective tissue and is absorbed, but when pregnancy follows, the corpus luteum retrogresses very slowly and disappears only after parturition. There is considerable evidence (Marshall, L. Loeb) that the corpus luteum produces an internal secretion (hormone) of great physiological importance in effecting the fixation of the ovum to the walls of the uterus (implantation, see below).
The general conditions determining the occurrence of ovulation are unknown in most instances. In the lower Mammals it is associated with a general physiological condition known as cestrus or heat, which may possibly itself be determined by internal ovarian secretions (hormones). Usually, too, the period of oestrus is preceded by a procestrus, or menstruation. In man, however, these two conditions are more or less independent, and no very constant relation is apparent. In the rabbit, cat, and ferret, ovulation occurs only after coitus (nine to ten hours after, in the rabbit, and usually within fifty hours in the cat), while in the dog, rat, and mouse, and in many Ungulates and Primates, it occurs independently of coitus. In many cases ovulation follows upon parturition, in the mouse about fourteen hours after the birth of a litter, and in the rat within about eighteen hours.
Fertilization and the Early Phases of Development
Fertilization
In most of the Mammals fertilization occurs in the upper part of the oviduct, almost immediately upon the entrance of the ovum. After the introduction of the spermatozoa into the vagina, they make their way to the upper ends of the oviducts, where they may remain alive and capable of functioning for several days or even weeks. Apparently the ovum too may remain in the oviduct, alive and capable of development, for several days or even a fortnight, in case fertilization does not occur at once.
The details of the sperm entrance, the formation of the second polar body, the establishment and fusion of the egg and sperm pronuclei, and the formation of the first cleavage figure, are not unusual and need not be described here (Fig. 146). Monospermy is typical of the Mammalia, and since there is no micropyle, the spermatozoon has to penetrate the zona pellucida (radiata), as well as the corona radiata, which may remain surrounding the egg during fertilization and even until a late cleavage stage. Long before it finally breaks down it becomes soft and easily penetrated (Fig. 146) . In the rat the vigorous movements of the spermatozoa within and among the cells of the corona tear this to pieces and leave the egg naked after twenty to twenty-five minutes. In the mouse, at least, the entire spermatozoon enters the ovum, but the tail soon disappears. Some of the details of the process of fertilization in the cat, are shown in Fig. 146.
Fig. 146. Reconstruction of four sections through the fertilized ovum of the cat. From Longley (combined from two figures). No zona pellucida is visible in these sections. The corona radiata is disintegrating, s, Remains of second polar spindle; /, first polar body; //, second polar body; d", sperm pronucleus; 9 , egg pronucleus.
Cleavage
Before taking up the details of cleavage and early development, we should say, in preface, that in the placental Mammals these processes of cleavage, formation of the blastoderm and early cell layers, are in many respects unique and often very difficult of comparison with the corresponding processes in other forms. As mentioned above, many of these peculiarities result from the fact that the mammalian ovumw r as originally markedly telolecithal, probably like that of the Sauropsida, and we are already familiar with the fact that the presence of a large yolkmass profoundly modifies the simple processes of cleavage, gastrulation, etc. Accompanying the return of the mammalian egg to the nearly alecithal condition, however, we do not find a corresponding return to the simpler early developmental processes characteristic of the primarily alecithal egg. On the contrary these processes are modified in new directions, not known elsewhere.
Added to these modifications is another series of alterations resulting from the very early development of a mechanism by which the segmenting ovum becomes intimately related with the uterine walls. Altogether, then, we find conditions here that are very special and not closely paralleled in other animals. So profoundly have the early stages of development become modified that there is sometimes difficulty in clearly identifying and homologizing with other' types, certain structures of the early mammalian embryo, such as the germ layers, primitive streak, etc. In the following description we shall adopt the convenient and more customary descriptive terms, but it should be clearly recognized that there are possible contradictions, and some students of mammalian embryology would say, positive errors, in applying the customary terminology to the early history of the mammalian embryo.
In most of the Mammals the early development is very slow. The cleavage stages usually occur as the egg is passing slowly down the oviduct. In the mouse the first and second cleavages occur about twenty-four hours and forty-eight hours, respectively, after coitus, and about eighty hours elapse before the ovum reaches the uterus. In the rabbit the first cleavage occurs fourteen to fifteen hours after ovulation, and nearly four days are occupied in the passage to the uterus. In the dog eight to ten days elapse before the ovum reaches the uterus. A few instances are known, on the other hand, where the egg arrives in the uterus just as cleavage begins (bat, hedgehog, and other Insectivors). In the European roe-deer, where fertilization occurs in the autumn, or perhaps in mid-summer, the ovum develops only as far as a cleavage stage that season; its development then continues the following spring.
There is considerable variation in the details of cleavage in the Mammalia, and since we are not describing any single form, we must be limited to very general terms; many of the details may be learned from the accompanying figures (Figs. 147, 148). Cleavage is total and at first adequal, very early becoming quite unequal, and in many cases very irregular, so that stages of three-, four-, five-cells, etc., may be found. The first cleavage plane, and usually the second, pass nearly through the chief egg axis, but the promorphological relations of these cleavages are not known.
Fig. 147. Cleavage of the ovum of the rabbit. After Assheton. A. Twocell stage, twenty-four hours after coitus, showing the two polar bodies separated. B. Four-cell stage, twenty-five and one-half hours after coitus. C. Eight-cell stage, a, Albumenous layer derived from the wall of the oviduct; z, zona radiata.
Fig. 148. Morula and early blastodermic vesicles of the rabbit. After Assheton. The zona radiata and albumenous layer are not shown. A. Section through morula stage, forty-seven hours after coitus. B. Section through very young vesicle, eighty hours after coitus. Taken from uterus; ordinarily the ova have not reached the uterus at this age. C. Section through more advanced vesicle, eighty-three hours after coitus. Taken from uterus, c, Cavity of blastodermic vesicle; i, inner cell mass; w, wall of blastodermic vesicle (subzonal layer, trophoblast).
In a comparatively early stage the cells take on a fairly definite arrangement. Thus on the surface of the cleavage group we may see a regular and usually continuous, epitheliumlike layer, surrounding, or nearly surrounding, a central mass of large, irregularly arranged cells (Fig. 148). The egg usually remains closely surrounded by the zona pellucida during these stages and the superficial cell layer is known as the subzonal layer, the central mass as the inner cell mass. This arrangement of cells forms what is known as the morula stage, equivalent to the blastula stage of other forms. (The identification of this stage as a gastrula ("metagastrula," Van Beneden) leads to some difficulties in the interpretation of the homologies of the layers of later stages.) The fully formed morula consists of thirty-six to seventy-two cells, of which twenty-four to thirty compose the inner cell mass.
The Blastodermic Vesicle
As cleavage continues in the cells of the morula, vacuoles appear among the cells of the inner cell mass, toward one side of the morula only. These vacuoles rapidly enlarge and flow together toward one pole, while the cells of the inner mass remain grouped together at the opposite pole (Fig. 148). The structure thus formed is termed the blastodermic vesicle; the fluid of the cavity is supposed to represent the yolk-mass of the sauropsid blastula or gastrula.
In all cases the uterus is reached by the time the blastodermic vesicle is formed, and the immediately subsequent events in development may be described under two heads, (a) the growth or enlargement of the blastodermic vesicle; (6) the formation of the embryonic rudiment and its layers (germ layers). The implantation or embedding of the vesicle in the uterine wall will be described in connection with the history of the foetal membranes. Although these processes overlap to a considerable extent we may describe them separately, as a matter of convenience.
A. The Growth Of The Blastodermic Vesicle
The enlargement of the vesicle results from the flattening of the cells of the subzonal layer, as well as from their multiplication, and the wall of the vesicle thus becomes very thin (Fig. 149). Growth of the vesicle is always rapid, and often it becomes very large. In the rabbit the ovoid vesicle reaches dimensions of about 4.5X3.5 mm. by the seventh day of development (third day within the uterus), when implantation commences. In the mouse the spherical vesicle is much smaller when implantation begins. In the Ungulates the vesicle becomes elongated and tapered at each end, and very large; in the sheep the twelve-days vesicle (Bonnet) reaches a length of more than 20 cm., its diameter being only 1-2 mm. Ultimately the Ungulate vesicle may extend through the entire uterus, and may even be folded, so that a total length of 1 m. may be reached. In all these cases the embryonic portion of the early vesicle is limited to a small, almost microscopic mass about in its middle.
Fig. 149. Section through the fully formed blastodermic vesicle of the rabbit. From Quain's Anatomy, after Van Beneden. fcm, Granular cells of the inner cell mass; troph, trophoblast cell,s; zp, zona pellucida.
B. The Formation Of The Embryo And The Embryonic Layers
We may now return to a stage where the small blastodermic vesicle is just established and consists of a single layer of subzonal cells, with the inner cell mass suspended from its inner surface like a hanging drop (Figs. 148, C; 149). The first step in the differentiation of this structure consists in the rearrangement of the cells of the inner mass so that those bordering the cavity of the vesicle are formed into a definite, continuous layer, known as the embryonic endoderm (Figs. 152, 155). The relative time at which the endoderm becomes distinct may vary greatly. The endoderm cells multiply rapidly, and typically they spread distally over the entire inner surface of the subzonal layer, converting the blastodermic vesicle into a two
Fig. 150. Diagrams of the formation of the amnion in the Insectivors. After Keibel. Black, embryonic ectoderm; heavy stipples, trophoblast; light stipples, endoderm; oblique ruling, mesoderm. A. Before the appearance of the amnionic cavity. Inner cell mass differentiated into embryonic ectoderm and mesoderm; endoderm extending completely around the wall of the vesicle. B. The amnionic cavity (a) appearing in the ectoderm. C. Enlargement of the amnionic cavity. Mesoderm expanded and split into somatic and splanchnic layers, separated by the ccelom. s, Primitive streak.
layered structure, the gastrula (Figs. 150, 153, 155). In the Primates, however, the formation of the endoderm does not keep pace with the enlargement of the blastodermic vesicle, so that the endoderm forms a smaller second vesicle suspended below the inner cell mass (Fig. 161).
After the separation of the endoderm, the remainder of the inner cell mass is known as the embryonic ectoderm. In most instances this remains quite distinct from the original subzonal layer, which, in view of its future function of attaching the vesicle to the uterine mucosa, may now be termed the trophoblast (Hubrecht). The inner cell mass is now clearly differentiated into embryonic ectoderm and endoderm, but before we continue the later history of these layers, we must consider briefly two other matters, (a) the relation between the ectoderm of the embryonic shield and the trophoblast cells
Fig. 151. Sections through four stages in the early development of the Insectivor, Tupaija javanica. From Hubrecht. A. Blastodermic vesicle completely closed; endoderm still continuous with the embryonic ectoderm. B, C. Embryonic ectoderm split and folding out upon the surface of the vesicle, pushing away the trophoblast cells. D. Embryonic ectoderm forming a flat disc on the surface of the blastodermic vesicle. E. Inner cell mass ("ectodermal shield"); ec, embryonic ectoderm; en, endoderm; tr, trophoblast.
(subzonal layer), and (6) the establishment of an important cavity, the amnionic cavity, which first appears about this time. There is a great deal of variation among the Mammals in the details of these relations, and we shall attempt to give only a very general statement of the more important conditions.
We may make a preliminary distinction between those instances where the trophoblast is interrupted in a circumscribed area, just above the embryonic ectoderm, the embryonic disc then moving up and occupying this space, and thus acquiring a superficial position; and others where the trophoblast remains continuous above the embryonic ectoderm, forming a complete enveloping layer around the endoderm and embryonic shield. The latter condition is known as the entypy of the germ.
Fig. 152. Three stages in the formation of the embryonic shield in the deer. After Keibel. A. Spaces appearing in the inner cell mass. B. Fully formed cavity (embryocyst) in the inner cell mass, which is still covered with a thin trophoblast. C. Embryocyst opened out upon the surface, c, Ectoderm ; i, inner cell mass; n, endoderm; s, embryonic shield; t, trophoblast; y, embryocyst.
Among the former instances we find again quite a variety of methods by which the embryonic layers acquire their superficial position. (1) In the Insectivor, Tupaija, the inner cell mass forms a cup-shaped structure which opens out upon the surface of the vesicle, pushing the trophoblast away (Fig. 151). (2) More commonly, as in most Ungulates, in Tarsius, and the opossum, the ectoderm moves up irregularly, gradually pushing away the trophoblast cells (Figs. 152, 153, 157). (3) In the rabbit, shrew, and probably in the dog, the trophoblast remains for a time continued as a very thin layer (Rauber's layer) over the surface of the ectoderm of the embryonic region. Apparently the thin cells of the trophoblast finally disappear, leaving the ectoderm upon the surface, but it is possible that they mingle indistinguishably with the cells of the ectoderm itself ; in either case the trophoblast cells here disappear as such (Figs. 154, 157, G, H).
Fig. 153. Transverse sections through the early blastodermic vesicle of the hedgehog, Erinaceus. After Hubrecht. A. Embryonic ectoderm forming a solid mass. Endoderm lining the entire trophoblast wall. Trophoblast becoming trophodermic. B. Amnionic cavity established. Trophoderm formed completely around the blastodermic vesicle, a, Amnionic cavity; c, ectoderm; d, trophoderm; n, endoderm; s, embryonic shield; t, trophoblast.
Fig. 154. A. Section through part of the blastodermic vesicle of a six-day rabbit. From Quain's Anatomy, after Van Beneden. B. Transverse section through the embryonic shield of a dog of unknown age (between eleven and fifteen days). After Bonnet, a, Trophoblast (in A, Rauber's layer); 6, embryonic ectoderm; c, endoderm; s, embryonic shield (embryonic ectoderm); x, space in embryonic shield.
In the second condition mentioned, where the trophoblast remains continuous above the embryonic layers (entypy), we find a still greater variety in the details of the relations, which may become very complicated. This general type of development is found in many Primates (probably in man), many Rodents including the mouse, rat, and guinea-pig, in the Chiroptera (Fig. 155), and in some Insectivora including the hedgehog (Erinaceus) Galeopithecus and Gymnura. In all these forms a space appears in the ectoderm, known as the amnionic cavity. This space may result from a definite splitting apart of two cell masses (e.g., hedgehog), or it may result from the gradual confluence of irregular spaces (e.g., bat, Fig. 155). It is important to bear in mind that in these instances of entypy, the true embryo develops only from the cells (ectoderm and endoderm) lying in the floor of the amnionic cavity.
Very often the trophoblast in this region becomes thickened, forming a trophoblastic knob ("trdger"), which enlarges and pushes the embryonic shield down into the cavity of the blastodermic vesicle (rat and mouse, for example). An additional cavity, the false amnionic cavity, may develop within this trophoblastic knob. This should not be confused with the true amnionic cavity, which here forms a completely closed vesicle, with ectodermal wall, entirely separate from the trophoblastic knob, and often of considerable size, even in these early stages (Fig. 156). A rather more special condition is found in the guinea-pig, where the small amnionic vesicle separates widely from the trophoblast, leaving a large space known as the interamnionic cavity between the true and the false amnionic cavities. The false amnionic cavity of the trophoblastic knob becomes very large (Fig. 156, B), while during these early stages the true amnionic cavity remains very small.
It is among these forms (e.g., rat, guinea-pig, etc.) that the phenomenon of the so-called "inversion of the germ layers" was described. It is now clear that no genuine inversion takes place, but the basis of the phrase is clear. The enlargement of the trophoblastic knob with its false amnionic cavity, pushes the embryonic ectoderm cells far down within the cavity of the blastodermic vesicle; this is especially marked in the guineapig. Then as the endoderm cells spread out over the inner surface of the embryonic ectoderm (Fig. 156) they may extend over the walls of both the true and the false amnionic cavities, so that the embryonic ectoderm appears to lie within an endodermal sac (Fig. 156, A). When the embryo itself begins to be differentiated in the floor of the true amnionic cavity it is thus already surrounded by this endodermal layer, and the appearance of an inversion of the layers is produced. Consideration of the entire series of conditions mentioned above demonstrates the absence of a true inversion of the germ layers.
Fig. 155. Sections through the blastodermic vesicle, or its embryonic portion, of the bat, Vespertilio, showing the formation of the amnionic cavity. After Van Beneden (Brachet). A. Section passing through the inner cell mass showing the very beginning of the amnionic cavity. B. Amnionic cavity clearly indicated as irregular spaces. The endoderm is torn away from its normal position under the embryonic ectoderm. C. Thickened trophoblast now a syncytiotrophoblast. D. Amnionic cavity fully established. The endoderm is torn away from the wall of the vesicle at its ends, a, Amnionic cavity; am, amnion; 6, capillaries of the uterine wall; c, embryonic ectoderm; e, endoderm; i, inner cell mass; s, syncytiotrophoblast; t, trophoblast; v, cavity of blastodermic vesicle.
Fig. 156. Diagrams of the relations of the cavities and layers in the rat, showing the "inversion" of the germ layers. After Selenka. Median sagittal sections. Embryo and amnion, black; ectodermal knob or "trager" in light tone; endoderm and mesoderm in darker tone. A. Early stage before the formation of the false amnionic cavity. B. Late stage showing false and true amnionic cavities and the interamnionic cavity, a, Amnion; ac, true amnionic cavity; c, chorion; E, embryo (anterior end); ea, endodermal rudiment of allantois; /, false amnionic cavity; i, interamnionic cavity; m, mesoderm; ma, mesoderm of allantois; n, endoderm; o, trophoblast (ectoderm); p, anterior intestinal portal; ra, rudiment of true amnionic cavity; rf, rudiment of false amnionic cavity; s, marginal sinus; t, "trager" (ectoderm); y, yolk-sac; ye, yolksac endoderm; x, amnionic folds.
Fig. 157. Diagrams of the formation of the amnion. After Keibel. A-F, in Ruminants, pig, and Mammals, with entypy of the germ. G, H, in the Carnivora. Black, embryonic ectoderm; heavy stipples, trophoblast; light stipples, endoderm; oblique shading, mesoderm. A. Morula. B. Early blastodermic vesicle; endoderm limited to embryonic region. C. Formation of cavity in the embryonic ectoderm (embryocyst). Extension of the endoderm. D. Embryocyst opening out; embryonic disc becoming superficial. Endoderm completely lining the vesicle. E. Embryonic disc or shield superficial in position (compare H). F. Formation of amnionic folds, primitive streak, and exocoelom. G. Early carnivor blastodermic vesicle. H. Embryonic shield superficial in position (compare Fig. 154, B.) A later stage would be identical with F. a, Amnionic folds; c. embryocyst; e, exocoelom; p, primitive streak; s, embryonic shield.
An interesting condition, transitional between the two general classes described above, is found in the mole. Here spaces form between the ectoderm and the continuous trophoblast, which flow together as in the bat, forming a definite amnionic cavity. This cavity is then obliterated by the refusion of the ectoderm and trophoblast, resulting in a condition similar to that of the shrew and rabbit. Then the superficial cells of the trophoblast disappear, leaving the ectoderm on the surface of the blastodermic vesicle.
The formation of the amnionic cavity has not been mentioned in forms where the embryonic disc becomes superficial in position. In these instances the amnionic cavity is formed in a manner entirely different from that described above. Here a system of amnionic folds, much like those of the chick, appears just outside the embryonic region proper. These folds grow up over the embryo, establishing an amnionic cavity in which the embryo is enclosed (Figs. 157, 176). The history of the amnion will be taken up later, in connection with the other embryonic membranes.
We may consider now the processes leading to the formation of the definitive embryo. To give a comparative account of these processes as they occur in the whole group of Mammals would carry us far into details, as there is wide variation even within this single class, and we shall, therefore, describe these phenomena as they are found in such a form as the rabbit or dog, forms whose early history is comparatively well known.
Of the entire blastodermic vesicle, with its complicated associated structures, the only truly embryonic portion is found in a restricted portion of the embryonic ectoderm and endoderm known as the embryonic shield (Figs. 151-155, 157, 158, A). (We should note that in the rabbit the endoderm does not come to line the entire blastodermic vesicle until a relatively late stage, so that throughout early development only the upper portion of the vesicle is two-layered.) In the rabbit the embryonic shield first becomes visible about the fifth day, when the blastodermic vesicle is still spherical and only about 1.5 mm. in diameter. By the seventh day the embryonic shield forms a well-marked oval thickening about 1.5 X 1.0 mm., the entire vesicle at this time measuring about 4.5 X 3.5 mm.
Fig. 158. A. Surface view of embryonic shield of a dog of thirteen to fifteen days. B. Surface view of embryonic shield of dog showing medullary plate, etc. From Minot (Laboratory Text-Book of Embryology), A, after Bonnet. A.o., Area opaca; A. p., area pellucida; Kn., Hensen's knot or node; Md., medullary plate; md.F., medullary furrow; ps., pr.s., primitive streak; Sh. t embryonic shield.
Sections through the embryonic shield show that it is formed largely by a circumscribed thickening of the embryonic ectoderm, three or four cells deep, beneath which the endoderm remains only one cell in thickness (Fig. 154). Over the surface of the shield the original trophoblast cells (Rauber's layer) are no longer distinguishable. Peripherally the shield passes into the thinner extra-embryonic ectoderm, or trophoblast of these forms.
The next phase of development is indicated (rabbit, about the end of the seventh day, dog, about thirteen to fifteen days) by the appearance of a slight opacity toward the middle of the embryonic shield. This is known as the primitive knot or Hensen's node. Usually the primitive knot is eccentric in position, toward what proves to be the anterior margin of the shield. At about this same time, or even before Hensen's node is distinctly visible, an opaque line appears across the shield, extending from the node to the posterior margin of the shield, where it joins an opaque crescent-shaped region (Fig. 158, A). This line is the primitive streak, and usually, along its middle can be seen the clearer primitive groove (Figs. 157, F; 159). A little later a less distinct opaque line may be seen extending a short distance forward from Hensen's node; this is the head process.
These superficial appearances in the embryonic shield may be understood only by the examination of sections. Sections across the primitive streak of the rabbit or mole, for instance, show that the opacity of the region is due to a very marked thickening of the ectoderm, with which is associated the formation of the middle layer or mesoderm. A section like that illustrated in Fig. 159, A, shows the primitive streak to be a region of rapid cell proliferation in the ectoderm. From the sides and inner surface of the primitive streak cells are given off which gradually take on the arrangement of a definite layer quite distinct from the ectoderm, and between the ectoderm and endoderm. This is the mesoderm which here, therefore, as in the chick, is in its origin more closely associated with the ectoderm than with endoderm. Sections through Hensen's node show that this is a region of thickened ectoderm and mesoderm, less clearly differentiated from one another than in the primitive streak region. The endoderm, however, is not fused with the other cells, as it is in the chick. And in front
Fig. 159. A. Transverse section through the primitive streak of the mole. J5. Transverse section through a human embryo of 1.54 mm. (Graf von Spec's Embryo Gle.) From Minot (Laboratory Text-book of Embryology, after Heape (A), and Graf von Spec (B). ch, Notochord; ct, somatic mesoderm; df, splanchnic mesoderm; EC, ek, ectoderm; en, En, endoderm;/, dorsal furrow; g, junction of extra-embryonic somatic and splanchnic mesoderm; me, mes, mesoderm; p, rudiment of embryonic ccelom; p.gr., primitive groove; Pr, primitive streak.
of Hensen's node, in the region of the head process, mesoderm is present, though entirely separate from the ectoderm, indicating its origin from the region of Hensen's node and not directly from the ectoderm of the head process region.
Later on conditions are found which make possible very close comparison between the primitive streak and associated structures of the Mammal with those of the chick. Within the head process, or in the region of Hensen's node, a rearrangement of cells produces a small cavity known as the notochordal canal. This varies greatly in extent. In such forms as the guinea-pig and bat, the canal is comparatively long, extending from Hensen's node, where it opens upon the surface by a definite perforation through the ectoderm, anteriorly and ventrally ; finally either opening through the endoderm into the cavity of the endodermal vesicle (Fig. 160, C), or simply ending blindly among the cells of the head process. In other forms, such as the sheep, pig, or hedgehog, and in man, the notochordal canal is simply a vertical perforation through the embryonic layers, connecting the cavity of the endodermal vesicle directly with the outside (amnionic cavity) (Figs. 160, A; 161, ). And in still other forms the canal may never quite perforate the layers of the shield (mole) ; or it may be reduced to a simple groove on the lower surface of the shield (rabbit, Fig. 160, B).
Fig. 160. A. Sagittal section through the embryonic shield of the hedgehog, showing the transitory blastopore. After Hubrecht. B. Posterior part of a sagittal section through the embryonic disc of the mole. C. Diagram of a aagittal section through the embryonic disc of the mole. From McMurrich (Development of the Human Body), after Heape. am, Amnion; bl, blastopore; ce, chorda endoderm; ec, ectoderm; en, endoderm; nc, neurenteric canal; prm, prostomial mesoderm; ps, primitive streak; t, trophoderm.
Sections through the notochordal canal reveal an arrangement of the germ layers which is practically that found around the more typical blastopore of the yolk-filled egg. In this region the layers are continuous with one another and the primitive groove may be deepened into a sort of "primitive pit" (compare chick). Altogether then we may recognize in the primitive streak of the Mammal the essential equivalent of the similarly named structure in the Sauropsida. The notochordal canal therefore becomes the modified equivalent of the blastoporal remains.
When the notochordal canal has the form of a simple perforation it may therefore correctly be termed the neurenteric canal, marking the posterior limit of the embryonic rudiment. The primitive streak, representing the modified and fused blastoporal lips, is here, as in the chick, a region from which the structures of the embryo are derived and differentiated in the antero-posterior direction, again much as in the chick.
All details regarding the formation of the embryonic layers and organs are to be omitted from the present account. It must suffice to say. that a typical medullary plate is formed, medullary folds appear and fuse, forming a neural tube (Figs. 158, B; 159, B). A typical notochord is. differentiated, running forward from the primitive knot. The mesodermal layer rapidly extends laterally from the axial region, finally passing widely beyond the embryonic region and taking a very important part in the development of the extra-embryonic structures. The mesodermal sheet is very early split into somatic and splanchnic layers by the appearance of a coelomic cavity; both embryonic and extra-embryonic.
The Development of the External Form of the Human Embryo
The earliest phases in the development of the human organism, up to the formation of the primitive streak, are not known at all, and it is not until the medullary plate stage is reached that the structure of the human embryo is fully known. Scarcely half a dozen embryos younger than this have been described. It is clear, however, that the processes leading to the formation of the embryonic layers, the amnionic cavity, etc., are in general similar to those found among the rat, mouse and bat. That is, the amnionic cavity is formed by delamination, between the continuous trophoblast and the embryonic ectoderm. The endodermal vesicle is much smaller than the ectodermal trophoblastic vesicle, leaving a wide space between the two layers (Fig. 161). When the mesoderm forms it extends rapidly through the extra-embryonic region of the blastodermic vesicle, one layer (somatic) applied to the inner surface of the trophoblast, the other (splanchnic) applied to the outer surface of the endodermal vesicle, so that the large cavity of the blastodermic vesicle becomes, in effect, an extra-embryonic coelomic space. The layer of endoderm with the splanchnic mesoderm is at first the yolk-sac, although of course entirely yolkless, while the ectodermal trophoblast with the somatic mesoderm forms the chorion. (Further reference to the chorion, as well as to the amnion and the allantois, is deferred until a later section.)
The reader should note that students of mammalian development use the term "ovum" to designate any early stage in development. In this connection the "ovum" includes not only the embryo proper, but also all of the associated structures of the blastodermic vesicle.
As the embryo enlarges, its posterior end remains attached to the inner surface of the trophoblastic wall by a mass of mesoderm cells. This attachment is known as the body stalk or belly stalk. Sections through an early embryo would give, therefore, the appearance shown in Figs. 161, D; 179, A, B.
A slightly later stage is shown in sagittal section in Fig. 161, E. At this time the embryo (Graf Spec's embryo Gle) measures 1.54 mm. in length by about 0.7 mm. in greatest breadth, while the entire vesicle ("ovum") is approximately 10 mm. in diameter. Its dorsal surface is occupied by the large neural plate, with a distinct neural groove. Toward the posterior end is the small neurenteric canal, behind which may be seen a short primitive streak and groove. A short thick body stalk attaches the embryo to the chorionic wall of the blastodermic vesicle. The wall of the ovoid yolk-sac, whose diameters are about equal to the length of the whole embryo, is already quite after Graf von Spee. In all the figures the anterior end is toward the left Black, embryonic ectoderm; heavy stipples, trophoblast and trophoderm; light stipples, endoderm; oblique ruling, mesoderm. A. Hypothetical early stage; mesoderm a solid mass. B. Amnionic cavity and wide exoccelom established; endoderm limited to a small vesicle beneath the embryonic ectoderm. The exoccelom in reality contains scattered mesenchyme cells. C. Blastodermic vesicle enlarged and covered with trophodermic villi, into which the mesoderm is extending. Endodermic vesicle (yolk-sac) very small (stage of Peter's ovum). D. Embryonic portion only, of an older vesicle showing the neurenteric canal, primitive streak (in the plane of the section), and body stalk. The mesoderm of the yolk-sac is becoming vascular. E. Sagittal section through the human embryo of 1.54 mm. (Graf von Spec's embryo Gle). a, Amnionic cavity; al, allantois; am, amnion; B, body stalk; ch, chorion; e, exoccelom; h, heart region; nc, neurenteric canal; V, chorionic villi; Y, yolk-sac.
Fig. 161. Diagrams of sagittal sections through the human blastodermic vesicle, showing the formation of the amnion and trophoderm. A-D, after Keibei and Elze. E, From McMurrich (Development of the Human Body),
Fig. 162. Young human embryos. After Keibel and Elze. A. Keibel and Elze's Embryo Klb, X 25. B. Kollmann's Embryo Bulle, X 20. For description, see text.
vascular. The notochord is distinctly differentiated posteriorly, and the embryonic mesoderm is only incompletely separated into somatic and splanchnic layers (Fig. 159, B), although in the extra-embryonic region there is a very wide coelom.
A sagittal section through this embryo shows that it is somewhat arched over the dorsal surface of the yolk-sac , and that an endodermal outgrowth, the rudiment of the allantois, is extending into the mesoderm of the body stalk (Fig. 161, E).
Shortly after this, in an embryo measuring 1.8 X 0.9 mm. (Keibel and Elze's embryo Klb.) the neural folds become very prominently elevated and the head and tail regions project slightly above the surface of the yolk-sac, as shown in Fig. 162, A. This figure shows also the persistent neurenteric canal, and the very short primitive streak. Five or six pairs of mesodermal somites are now present.
The head region now commences to enlarge rapidly although the neural groove is still open. In an embryo of 2.36 mm. length (Kollmann's embryo, Bulk) illustrated in Fig. 162, B, the body is concavely arched toward the yolk-sac, while the head and tail regions show distinct downward flexures. The elementary divisions of the brain are already indicated, and the fore-brain is protecting downward from the anterior end of the neural axis. Though not shown in the figure the paired rudiment of the heart is present. About fifteen pairs of somites are visible externally.
As the embryo now begins to elongate rapidly it becomes clearly folded off from the extra-embryonic structures, and the opening of the yolk-sac out of the endodermal gut cavity of the embryo becomes relatively, though not actually, narrower. The yolk-sac thus appears to be attached to the embryo proper by a narrow stalk, the yolk stalk, the connection of which, with the embryo, is the yolk stalk umbilicus.
By the time the embryo reaches a length of. 2.5 mm. (Kollmann's embryo, 2.5 mm., age given as thirteen to fourteen days, but probably much older) the high neural folds have begun to close together posteriorly (Fig. 163). The head region is considerably enlarged and extends downward in front of the heart, which is now very large and clearly differentiated into regions by the development of flexures.
The length of the embryo now affords a very unsatisfactory index of the age or degree of development on account of the considerable variability, and because of the bendings which appear in the longitudinal axis. Apparently, shortly after this time, the body becomes sharply bent downward into a U-form just opposite the umbilicus, producing what has been called the dorsal flexure. That this is entirely normal is, however, still open to question.
Fig. 163. Human embryo of thirteen or fourteen days. From Minot (Laboratory Text-book of Embryology), after Kollmann. Al, Body stalk; Am, amnion; Ht, heart; Md, medullary groove; ST, seventh somite; Yks, yolk
Figure 164 illustrates an embryo, enclosed in the amnion and with yolk-sac attached, whose length, in a straight line, is 2.6 mm. and whose age was originally estimated at eighteen to twenty-one days, though very probably it was approximately one month (His's embryo, M). The entire blastodermic vesicle or chorionic vesicle ("ovum") still measures approximately 10 mm. in diameter. This embryo shows many important advances. No trace of the dorsal flexure remains, while both the anterior and posterior extremities of the embryo are now bent downward and inward. The body is also slightly twisted so that the head lies toward the left, the tail toward the right (the direction of this twisting is not fixed, for in other embryos it may be in the opposite direction). The yolk-sac is some what shrunken and elongated, and the yolk stalk is clearly distinguishable. Topographically the most anterior part of the embryo is formed by the mid-brain, beneath which the forebrain is now folded back toward the heart. The heart is very prominent and on the sides of the neck region three pairs of gill clefts are indicated, decreasing in size posteriorly. Four pairs of visceral arches (mandibular, hyoid, two branchial) are thus marked out, and the most anterior (mandibular) already shows signs of its transverse division into upper and lower portions, the maxillary and mandibular processes. Later a fourth cleft and fifth arch are indicated. Of course in the Mammal actual gill clefts are not present as perforations; the so-called clefts are vestigial structures and, excepting the first, merely form superficial grooves, opposite corresponding pockets out of the pharyngeal cavity. An anterior depression, the oral sinus (stomodaBum) between the mandibular arches, marks the position of the future mouth, which is perforated very shortly after this time.
Fig. 164. Human embryo of 2.6 mm. From Minot (Laboratory Text-book of Embryology), after His. The embryo is enclosed in the amnion and shows the maxillary and mandibular processes, the rudiments of three gill clefts, and the large heart. The large yolk-sac extends ventrally, while posterior to its origin the root of the body stalk is shown turned dorsally.
Just a few words may be added concerning the internal structure of this embryo (Fig. 165). The slender notochord extends the entire length of the body and tail. Optic vesicles are distinct and the otocysts are entirely closed off below the surface. The expanded pharynx shows an anterior hypophysial outgrowth, in addition to the lateral branchial pockets. A small rudiment of the lung is indicated, and at the posterior end of the narrowed oesophagus the liver rudiment is well marked. Posterior to this the gut is open into the yolk-sac by way of the yolk stalk, and continuing posteriorly from this is the narrow intestine, which, near its extremity, sends a small allantoic outgrowth into the body stalk. Rudiments of the mesonephros (Wolffian body) and its duct are slightly indicated.
The vascular system is very well developed. Opening into the posterior end of the heart Aortic limb of y hea /' AUi body sta i k ' ;
Fig. Reconstruction of a human embryo of 2.6 mm. (See Fig. 164). From Minot (Laboratory Textji ^ i f ji ^ i , book of Embryology), after His. A,
there are, the paired ductuS ^> dorsal aorta; Au, umbilical arteries; Car, posterior cardinal vein; Jg, an LUVien, lormed by anterior terior cardinal vein (jugular vein). Om,
flnH nn^terinr oarHinal vpinq omphalomesenteric vein; op, optic ves
an(1 P lmal V1QS > icle; ot, otocyst; Vh, right umbilical
the paired vitelline or omphalo- vein. mesenteric veins, coming from
the yolk-sac by way of the yolk stalk, and the paired allantoic or umbilical veins coming from the allantoie region by way of the body stalk. Opening out of the anterior end of the heart is the ventral aorta, which immediately divides into right and left halves from each of which arise five aortic arches passing through the visceral arches to the dorsal side of the pharynx, where they reunite into the dorsal aorta. A small anterior carotid artery appears a little later. From the dorsal aorta is given off a pair of small mtelline arteries supplying the yolk-sac, while al posteriorly the dorsal aorta divides into a pair of umbilical or allantoic arteries, passing through the body stalk and supplying the allantois.
As the head now enlarges rapidly a wellmarked flexure (cervical about n ' exure ) appears just back of the gill cleft region, while through the entire body and tail a strongly marked curvature appears, so that head and tail are almost brought in contact, and the entire embryo is almost circular in general outline (Fig. 166). The foreand hind-limb buds have appeared as low, extended elevations, and the abdominal region is becoming prominent on account of the growth of the liver and other viscera. The ventral body wall now becomes more completely closed together, and the tissues (somatopleural) at the base of the body stalk grow forward enclosing the root of the yolk stalk for single stalk from the embryo now carries both the yolk stalk and the allantoic stalk. This is known as the umbilical stalk or cord, and its attachment to the embryo is the umbilicus. Ultimately the entire yolk stal becomes enclosed in the umbilical cord, and the yolk-sac itself is surrounded by the tissues of the placenta as described below.
Fig. 167. Human embryo of about 9.3 , ?? mm. After Hochstetter, X 6 2/3. a Short distance, SO that a For description see text.
Fig. 166. Human embryo of twenty-three days (4.0 mm.). From Minot (Laboratory Text-book of Embryology), after His (Embryo a), al, Fore-limb bud; BS, body stalk; Op, optic vesicle; pi, hindlimb bud; IV, fourth ventricle of brain; 1, mandibular process; 2, hyoid arch; 3, 4, third and fourth visceral arches.
Up to this time the correlation between age and size of the embryo is very uncertain. According to the careful studies of Mall the ages of most of the early embryos have been underestimated, and it is very probable that the events thus far described have occupied about the first month of development. From this time on, however, the age of the human embryo is more certainly determined.
During the sixth week of development the embryo measures 9.0-10.0 mm. in a straight line drawn from the apex of the mid-brain to the sacral flexure or rump ("crown-rump" length) (Fig. 167). The head, still the largest part of the embryo, is beginning to be elevated on account of the straightening out of the cervical flexure, and the whole body shows considerably less curvature than before. A lense has been formed opposite the small optic vesicle, and a pair of wellmarked olfactory pits has appeared on the under side of the head, in front of the maxillary processes. Both maxillary and mandibular processes are more prominent, while the posterior visceral arches and clefts have become sunk in a depression, the margins of which have nearly closed together forming a cavity below the surface known as the cervical sinus. The most anterior gill cleft (hyomandibular) is not included in this cervical sinus, but in part remains on the surface of the neck, as the rudiment of the external auditory meatus. The cervical sinus later disappears entirely, along with the posterior gill clefts.
The ventral body region is still protuberant, and anteriorly three elevations can often be observed, marking the underlying auricle, ventricle, and liver. The limbs are somewhat elongated, and the fore-limbs, which are always in a more advanced stage than the hind-limbs, show some indications of differentiation of the hand. The umbilical cord is elongating and the tail has reached nearly to the condition of its greatest development.
The further development of the general bodily topography may be sketched very briefly with the aid of the accompanying figures (Figs. 168, 169, 170). During the latter part of the second month (Fig. 169) the head continues to be elevated rapidly, and the body to straighten. The head is now at its greatest relative size, constituting about 45 per cent, of the total weight of the embryo (Jackson). The pinna of the ear is formed from elevations of the first and second visceral arches around the external auditory meatus. The rudiments of the eye are fully established and the eyelids are formed. The ventral body wall still remains protuberant. The proximal end of the umbilical cord becomes considerably expanded, and into its extra-embryonic coelomic cavity extend several coils of the embryonic intestine and even a portion of the liver. This characteristic extension of the intestine (intestinal hernia) reaches its maximum during the second month. The intestine is rapidly withdrawn later and at about nine or ten weeks is completely retracted into the embryonic body cavity. Beyond this expanded region the umbilical cord is considerably elongated and begins to show its characteristic spiral twisting. The yolk stalk is correspondingly elongated and. now loses its endodermal cavity.
Fig. 168. Human embryo of 14.5 mm. (thirty-six days) showing thick umbilical cord, and yolk-sac at the end of the slender yolk stalk. After Minot. X 4.3. For description see text.
Fig. 169. Human embryo of 22.8 mm. (fifty-three days). X4. For description see text. After Minot,
The limbs grow rapidly during this month; they become differentiated into their three chief regions, and in the hand and foot the digits become clearly differentiated (Figs. 168, 169). By the end of this month the limbs project beyond the outlines of the body. The tail gradually recedes and by the time the embryo is two months old is scarcely visible externally. The structures of the facial region develop rapidly during this month (see below), and at its close the embryo acquires adistinctly "human" aspect and is generally known as a "foetus" in distinction from the earlier "embryo."
Fig. 170. Outlines of human embryos of 106 to 110 days (118 to 120 mm.). From Minot (Laboratory Text-book of Embryology). The figure to the left shows the most frequent position of the embryo in utero; that to the right shows the position assumed when removed from the embryonic membranes.
The external form changes now become relatively slower. The outlines of the head become more rounded; the facial characters are more fully established; the eyelids close together. The abdominal region recedes, the limbs become slender, elongated, and flexed. The foetus is in a position of constraint within the uterus, as shown in Fig. 170 from Minot.
The general changes in size and weight of the embryo and foetus during the entire intra-uterine period are summarized in the accompanying table.
Table Showing The Average Weight And Length Of The Human Embryo And Foetus
Compiled from Jackson (weight) and Mall (length). (The column headed CH gives the length as measured in a straight line from the crown of the head to the heel; that marked CR gives the "sitting height," or length from the crown to rump or sacral flexure.)
Length
Weight
CH
CR
Ovum (estimated).
0. 000004 grm.
28 days. .
0.04
2.5 mm.
2.5 mm.
56 days.
3.0
30.0
25.0
84 days.
36.0
98.0
68.0
112 days.
120.0
180.0
121.0
140 days.
330.0
250.0
167.0
168 days.
600.0
315.0
210.0
196 days.
1000.0
371.0
245.0
224 days.
1500.0
425.0
284.0
252 days.
2200.0
470.0
316.0
270 days.
500.0
336.0
280 days.
3200.0
- Age probably underestimated.
Before leaving the subject of the development of external form we should add a few details regarding the development of the facial characteristics and of the external genitalia.
The Development Of The Face
We may take as our starting point here, a stage of 2.6 mm. (probably about thirty days) already described and figured (Fig. 164). At this time the first gill cleft is unreduced, the otocyst is not yet closed, and the optic vesicles are entirely lateral in position. The fore-brain region hangs down over the deep oral sinus (stomodseum), the floor of which is still formed by the im perforate oral membrane. Within the next few days this membrane becomes perforated by the mouth opening. The oral sinus is bordered posteriorly by the mandibular processes, which do not quite meet in the mid-line, and antero-laterally by the maxillary processes, which are widely separated medially, the interval being occupied by the frontal process, a ridge over the surface of the fore-brain.
Fig. 171. Early stages in the development of the head and face. After Rabl. A. Head of a human embryo of 8.3 mm., seen from in front (ventrally). B. Head of human embryo of about 12 mm., seen from in front. For explanation see text.
An important advance is to be seen in the development of the olfactory pits, which appear at the ends of the frontal process. Bordering the olfactory pits are inner or medial and outer or lateral elevations or olfactory processes (Fig. 171, A). The maxillary and mandibular processes are now closer together so that the opening of the oral sinus (now called the mouth) becomes a transversely elongated slit.
In the embryo of the fifth to sixth week (Fig. 171, B) the olfactory pits have deepened and have moved in toward the mid-line, thus separating the mouth from the fore-brain or forehead region. At the same time the medial and lateral olfactory processes become more prominent, and the former are uniting with the maxillary processes of the first visceral arch to form the rudiments of the upper jaw and lip. The mandibular processes are still separated medially by a groove. During the sixth week the eyes become visible from in front, the upper lip begins to enlarge though still indented medially, the mandibular processes fuse completely forming the completed rudiment of the lower lip and jaw, and the chin appears. The medial olfactory processes (globular processes) soon fuse together forming the nasal septum (Fig. 172, A), and the nose becomes slightly marked off from the forehead by a groove.
Fig. 172. The development of the face of the human embryo. After Retzius. A. 18 mm. embryo, X4. B. 25 mm. embryo, X4. C. 42.5 mm. embryo, X2. D. 117 mm. embryo, X4/5. For explanation see text.
The chin gradually enlarges, and the lips, now both complete medially, continue to enlarge (seventh week). At about eight weeks (Fig. 172, B) the eyelids are forming, and the eyes, now rapidly approaching one another, are separated from the forehead by oblique supraorbital folds. The ears now are marked by well-developed pinnae, but still lie far down toward the neck, below the level of the mouth. The mouth is less extended transversely and the nose is completely separated from the forehead.
During the next week or ten days (Fig. 172, C), the eyelids close, the eyes move closer together, and the height of the forehead increases. The nose, though still very broad, begins to project slightly, and the external nares become temporarily closed by epidermal proliferations. The ear gains a somewhat higher position. The mouth is smaller, the lips thinner, and the lower jaw quite prominent. During the third month (Fig. 172, D), the pinna reaches nearly its adult position, the nose projects markedly, the lips, especially the upper, become thinner and protruded, and the essentials of the adult physiognomy are fairly established.
The Development Of The External Genitalia
The end of the gut posterior to the origin of the allantois (see below) forms the dilated cloaca, which is separated from the surface of the body by a thin portion of the body wall known as the cloacal membrane (Fig. 173, A). This membrane is later depressed below the surface of the body, at the bottom of a shallow depression (proctodseum). The cloacal cavity becomes divided into a ventral portion, the urinogenital sinus, receiving the openings of the excretory and reproductive ducts and the allantois, and the rectal portion. The cloacal membrane is correspondingly divided into the urinogenital membrane and the anal membrane, the two being separated by a narrow bridge of tissue forming the perineal rudiment.
In order to find the earliest traces of the external genitalia, we must go back to the embryo of the early part of the second month. Here we find a pair of ridges, either side of the cloacal membrane (Fig. 173, A), which gradually fuse and enlarge anteriorly, forming, toward the close of this month, a distinct cloacal tubercle. The uronogenital membrane is perforated about this time by the urinogenital aperture. (The time at which the anal opening is formed is quite variable, but usually is also toward the close of the second month.)
Fig. 173. The development of the external genitalia. A, -After Keibel. B-E, After Felix, from Meyer. A. Model of the cloacal region of a human embryo of 3 mm. B. Ventral view of the caudal end of a human embryo of 18mm. C. Same of 28 mm. Indifferent stage. D. Same of 32.5 mm. Female. E. Same of three and one-half months. Male, a, Anal opening; g, genital ridge; gc, glans clitoridis; gp, glans penis; h, hind-limb; Im, labia majora; m, cloacal membrane; ms, median scrotal rudiment; p, phallus; r, cloacal ridge; s, scrotal ridge; t, coccygeal tubercle; u, umbilical cord (in A, umbilicus;) ug, urinogenital aperture.
Upon the cloacal tubercle, and toward its posterior or anal side, there grows out quite rapidly a definitely circumscribed process called, at this stage, the phallus (Fig. 173, B). The remainder of the cloacal tubercle, at the base of the phallus and mostly anterior and lateral to it, is now known as the genital tubercle. The urinogenital aperture is continued forward upon the posterior (ventral) surface of the phallus as a narrow groove, the lateral margins of which are somewhat elevated as the genital folds, which gradually enlarge and so reduce the urinogenital aperture to a narrow elongated slit.
By the beginning of the third month (Fig. 173, C) the phallus has enlarged considerably, and its extremity has dilated as the rudiment of the glans. Lateral to each genital fold a second, larger ridge, the genital swelling, has appeared. This marks the end of the so-called indifferent period, during which there is but very slight external differentiation between the sexes. As a matter of fact, the sex of the individual is determined at the time of fertilization, and even during the latter part of this "indifferent period " the female embryo can be distinguished by the presence of a groove around the base of the phallus, which is lacking in the male.
The later development may be sketched very briefly. In the female, where the modifications are less extensive, the glans and the anterior (oral) portion of the phallus are transformed into the clitoris (Fig. 173, D), while the posterior (anal) portion of the phallus together with the lateral margins of the urinogenital aperture, become the labia minora. The labia major a are formed from the genital swelling and the genital tubercle (basal portion of the cloacal tubercle).
In the male (Fig. 173, E), the entire phallus is transformed into the penis, composed of the glans plus the shaft, the posterior (anal) portion of which is therefore equivalent to the labia minora. The anterior extension of the urinogenital aperture upon the male phallus is enclosed by the fusion of the genital folds and so added to the lower part of the urethra. The genital swellings in part fuse and are transformed into the scrotal sac, and in part disappear, to be replaced by other scrotal swellings which form the remainder of the scrotal sac. The essentials in the history of the external genitalia may be summarized as follows
Indifferent Period
Female
Male
Early
Late
Urinogenital aperture
Vestibule
Terminal portion urethra
1 Anterior
In part, portion of
Ger
ital Genital ! portion
Mons Veneris 1
scrota! sat;
Scrotal
tub Cloacal
ercle swelling f Lateral J portions
Labia majora J
In part, replaced by scrotal swellings
sac
tubercle
Glans
Glans and anterior
Phs
illus ' [ Anterior
Clitoris
portion shaft
e , ,, J portion I Shaft } Posteriori
Penis
( portion } Genital folds J
Labia minora
Posterior portion shaft J
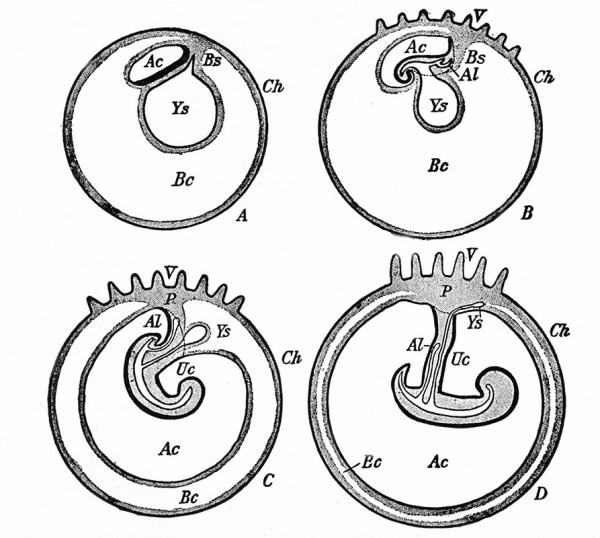
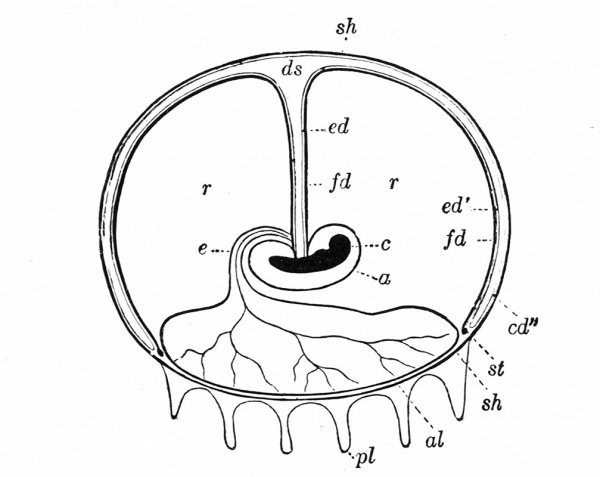
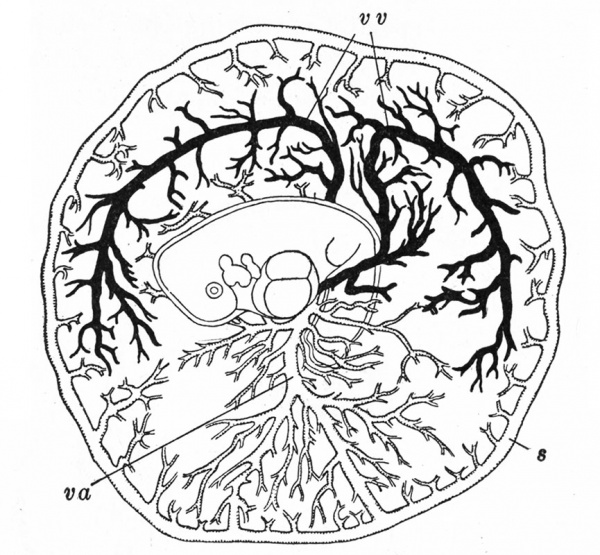
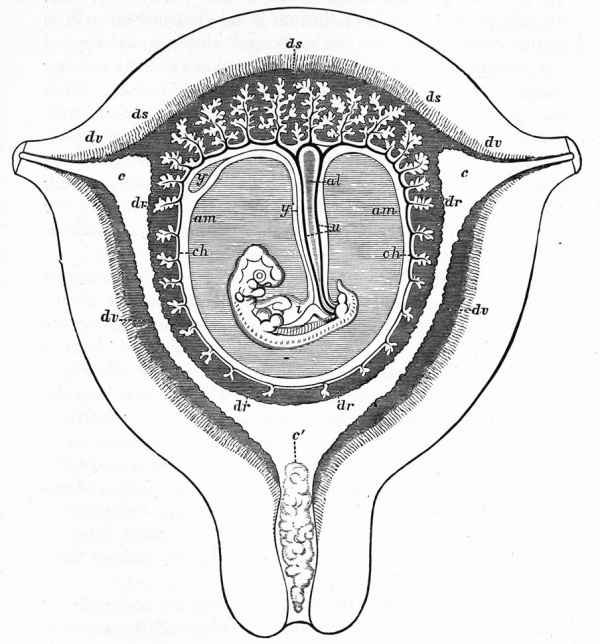
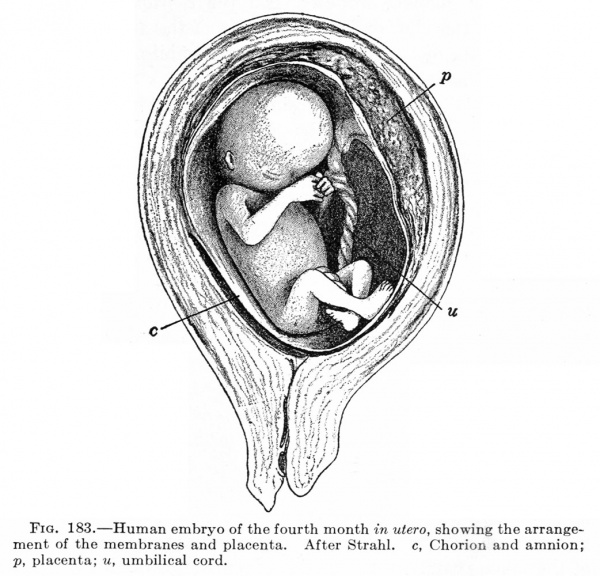
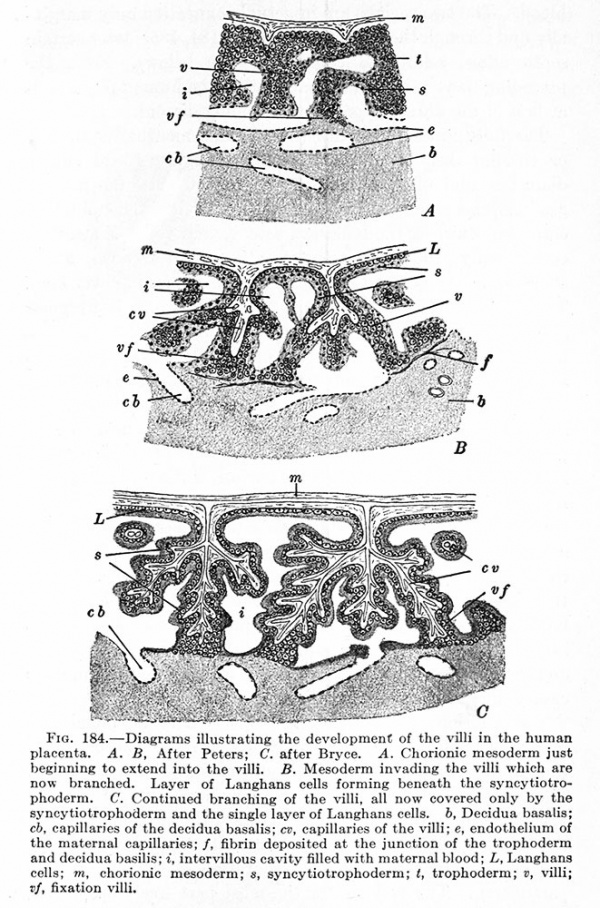
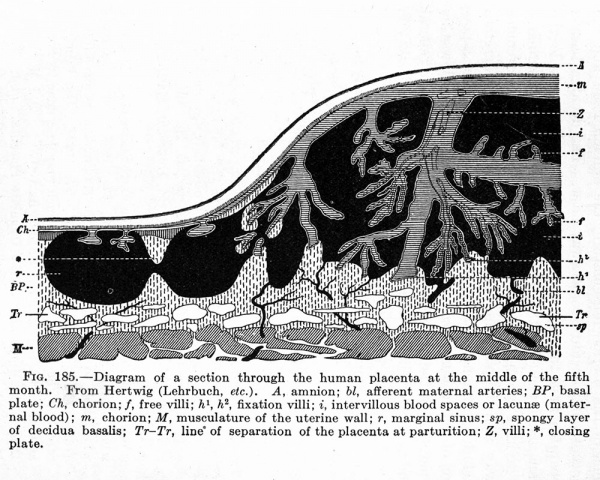
The Embryonic Membranes And Appendages Of The Eutherian Mammals
Scattered references have been made in the preceding pages to various details regarding the development of the amnion, the chorion, the allantois, and yolk-sac, and we must now give, possibly with some repetition, a more connected, though brief, account of the development of these structures. There is in general a remarkable similarity between the early history of the embryonic appendages of the Mammals and those of the Sauropsida, a similarity that is the more remarkable when the eggs of the two groups are compared. As mentioned in the introductory paragraphs of this chapter, these similarities are to be explained upon an historical basis, that of relationship through descent. But while there is, in the early stages, such close agreement between Mammal and Sauropsid, in these respects, during their later history, these mammalian membranes undergo profound changes in function associated with the intrauterine development of the embryo and the consequent substitution, as a source of nutritive substance, of the maternal tissues in place of the intra-oval yolk-mass or albuminous egg membranes of the Sauropsida.
In the chick the amnion serves, among other functions, to protect the embryo from drying and from the deforming pressure of the rigid shell; the yolk-sac contains a large part of the food substance for the developing embryo; the allantoic wall is the embryonic respiratory organ, while its cavity serves as an excretory reservoir; and the chorion (serosa) appears to have little, if any, physiological importance. In the Mammal this is all changed. The amnion is a membrane of secondary protective importance; the yolkless yolk-sac is a vestigial organ, often of little functional value; the allantois loses its respiratory and excretory significance and is usually concerned in relating the embryo to the source of its food supply; while the chorion, either as a whole or in part, becomes the chief organ concerned in the exchange of nutritive materials and excreted substances between the embryo and the maternal uterine circulation.
These characteristic relations of the mammalian embryonic membranes do not appear in this group in a fully established condition; there is, on the contrary, a long series of intermediate conditions, transitional in almost every respect, between the Sauropsid condition and that found in the highest Mammals, the Primates, where these relations are most highly developed. It is a familiar fact that in the lowest Mammals, the Prototheria (Monotremata or Ornithodelphia) including only the genera Ornithorhynchus, Echidna, and Proechidna, essentially Sauropsid conditions obtain here, as well as in so many morphological and physiological characteristics of the adults. Here the developing embryo has no organic relation with the mother, for the fully formed eggs are deposited outside the body of the parent, either in an integumentary fold (Echidna), or in a "nest" (Ornithorhynchus), where the young develop independently, enclosed within a tough parchment-like egg shell. Usually only a single egg is produced at one time in Echidna, while Ornithorhynchus normally produces two at a time. The eggs too are reptilian in character, much larger than any other mammalian egg, and yolk laden. As laid, they measure about 15-16.5X12-13 mm. (in Echidna); the egg cell proper, as it leaves the ovary is, of course, smaller than this, but even so is much larger than the egg cell of the higher Mammals, being 3.0-4.0 mm. in diameter in Echidna, 2.5 mm. in Ornithorhynchus.
Among the Metatheria (Didelphia or Marsupialia) many of the typical mammalian conditions are found. The ova, though commonly somewhat larger than in the higher Mammals, are sometimes of no greater size. The embryo has a brief intrauterine period of development, during which nutritive relations are established with the uterine wall by means of the surface of the chorion, which, however, retains its originally smooth surface and merely comes closely into contact with the vascular uterine epithelium, without acquiring a close organic union. The yolk-sac is very large in these forms and underlies nearly the entire chorion (serosa). Nutritive substances from the maternal circulation may thus pass, with some difficulty, through the chorion and the wall of the yolk-sac, into the blood of the latter.
Conditions suggestive of the higher Mammals are by no means lacking, however, for in Dasyurus (Hill) the yolk-sac in certain areas becomes very vascular and forms a close relation with the uterine wall. The ectoderm cells of the chorion, between the two, aid in establishing this relation between the maternal and the embryonic blood, a relation which is very different from the mere contact relation of the typical Metatheria. And in Perameks (Hill) the allantois takes up a similar relation with the uterine mucosa, sending into the latter well-developed vascular outgrowths (Fig. 174). We have here then, a condition that in many respects resembles closely the relation found in many of the "placental" Mammals. Indeed, these structures are known as the " yolk-sac" and the "allantoic placenta" respectively.
Among the remaining Mammalian orders, or Eutheria (Monodelphia or Placentalia), there is the greatest diversity in the mode and degree of the relation between embryonic membranes and appendages, and the uterine wall, or, in other words, in the character of the placentation. In the simpler cases (pig, horse, and many others) the relation is much like that described in Perameks in its essentials, while in the more highly specialized instances (apes and man) the relation becomes very complex, involving considerable modification of what is regarded as the typical arrangement of the embryonic appendages. Between these two extremes there is the greatest variety of conditions of placentation, which frequently do riot parallel the usual ordinal classification, so that even within a single order (e.g., Ungulata, Primates) there may be divergences which considerably exceed the range of the morphological traits upon which the orders are based.
Before attempting to describe any of the actual details of the structure and development of the placenta, we must give a general outline of the ontogenetic history of the embryonic membranes and appendages. We may consider first the method by which the young embryo or "ovum" (blastodermic vesicle) effects its primary relation with the uterine wall. In the remaining pages it is understood that what is said is limited to the Eutheria.
Fig. 174. Diagram of the arrangement of the foetal membranes in the Marsupial, Perameles. From Hill. The ectoderm is represented by a light continuous line, the endoderm by a dotted line, and the mesoderm by a heavy line. amn, Amnion; all. c., allantoic cavity; all. mes., allanto-chorionic mesenchyme; all. s., allantoic stalk; bil, omph.. ectodermal and endodermal wall of yolk-sac; ch., margin of true chorion; cce., exocoelom; cce. w., inner wall of allantois; proa, r., persisting remnant of proamnion; s. t., sinus terminalis; vase, omph., three-layered portion of yolk-sac wall; y. c., cavity of yolk-sac; y. spl., invaginated splanchnopleural wall of yolk-sac.
Implantation
Through the process of menstruation, or procestrous, its equivalent in the lower Mammals, the mucous membrane lining the uterus is kept in an active, wholly living condition, and as the ovum or blastodermic vesicle enters this cavity it almost immediately becomes attached or implanted upon the wall. It may become superficially attached to the wall of the main uterine cavity, so that as it grows it projects freely into the lumen of the uterus; this is known as central implantation and is found in the Ungulates and Carnivors, the lower Primates and some Rodents such as the rabbit. In other forms the vesicle may come to lie in a furrow or groove in the uterine wall, which is then closed off from the main cavity by the fusion of the margins of the furrow, enclosing the vesicle. This is eccentric implantation and is found in such forms as the mouse and some Insectivors. Or the vesicle may burrow into the substance of the mucous membrane lining the uterus, the mucosa then closing together over the point of entrance, as in man and some Rodents, such as the guinea pig and the gopher. This type of implantation is known as interstitial.
The structure primarily concerned in effecting this early connection between the vesicle and the uterine mucosa is the trophoblast (Hubrecht). We have already described the formation of this superficial layer of ectoderm cells which covers the entire blastodermic vesicle as this passes down the oviduct and enters the uterus (Figs. 154, A; 175). The trophoblast may remain for a short time the only component of the peripheral wall of the blastodermic vesicle. But extra-embryonic mesoderm usually forms very early around the wall of the vesicle, and the entire extra-embryonic wall may then be known as the chorion; the trophoblast may then be called the chorionic ectoderm.
It is convenient to distinguish two general types of behavior on the part of the trophoblast or chorionic ectoderm. In most instances of central implantation (e.g., pig, horse) it merely forms an adhesive layer which comes closely into contact, over practically its entire surface, with the uterine mucosa. In other instances of central implantation and in the eccentric and interstitial types, a part or even the whole of the trophoblast becomes highly specialized, physiologically, as the trophoderm (Minot), in which the cells proliferate rapidly forming a layer of considerable thickness (Figs. 175, 176). It is the function of the trophoderm to dissolve or digest the uterine mucosa, with which it is in contact. The trophoderm, probably through the action of specific enzymes, rapidly erodes the uterine wall, and the blastodermic vesicle becomes either partially or wholly embedded in the maternal tissue, so that the embryo bears a relation to the maternal organism which is quite that of an internal parasite.
In most cases a part of the trophoblast is thus specialized as trophoderm. In the rabbit, for example, the trophoderm forms a horse-shoe shaped area just around the embryonic rudiment, lateral and posterior to it; this region alone becomes embedded in the uterine tissue, while the remainder of the blastodermic vesicle, projecting into the lumen of the uterus, remains covered with the relatively unmodified trophoblast (chorion). In the spermophile (Rejsek) the trophoderm forms a thickened mass, in the wall of the vesicle, opposite the inner cell mass or embryonic rudiment. As the trophoderm erodes the mucosa, the vesicle is carried down and partially embedded in the uterine wall (Fig. 175). In forms like the hedgehog, apes, and man, the entire trophoblast becomes trophodermal (Figs. 153, 161). Here, then, the maternal tissues on all sides of the vesicle are eroded, and the "ovum" becomes completely embedded and surrounded by a mass of dissolved tissue.
The cells of the trophoderm very early begin to fuse together forming either small masses, known as multinuclear giant cells, or extensive protoplasmic masses known then as syncytia, or better, the syncytiotrophoderm (syncytiotrophoblast) (Figs. 175, 184). In the trophoblast, which is less intimately associated with the maternal tissues, the cell boundaries usually remain, and this is then distinguished as the cytotrophoblast.
It is evident from this description that the trophoderm or Syncytiotrophoderm, forms the boundary between the embryonic and the maternal tissues, and not only effects the implantation of the "ovum," but at the same time establishes the primary element in the placenta. The trophoderm later becomes vascularized from the mesoderm of the chorion or allantois (yolk-sac in some cases), and acts as the chief absorptive or resorptive surface, taking in materials from the maternal tissues and blood.
Fig. 175. Early stages in the implantation of the blastodermic vesicle of the spermophile (Spermophilus citillus) . After Rejsek. A. Unattached vesicle. B. Syncytiotrophoderm just penetrating the epithelium of the uterine mucous membrane. C. Syncytiotrophoderm extending along the basement membrane of the uterine epithelium. D. More highly magnified view of the Syncytiotrophoderm after it has penetrated the basement membrane and entered the connective tissue of the uterine mucosa. b, Basement membrane of uterine epithelium; ct, connective tissue of the uterine mucosa; e, epithelium of the uterine mucosa; en, endoderm; t, inner cell mass; s, Syncytiotrophoderm; t, trophoblast.
The extent to which the trophoderm erodes the uterine lining varies greatly. Of course where no trophoderm is differentiated, little or no actual erosion occurs. And when the trophoderm is present, the erosion may affect only the epithelium of the mucosa, or it may involve the connectivetissue elements, or even the walls of the uterine blood-vessels. The degree of erosion has been suggested as a basis for the classification of the types of placentae (Grosser), but this and many other facts regarding the later history of the trophoderm are better considered later, in connection with the placenta itself.
The Amnion and Chorion
Our description of the formation of these membranes may be very brief on account of their general similarity to those of the chick fully described in an earlier chapter (Fig. 178).
We must distinguish, at the very outset, between two general types of amnion formation found among the Mammals, a distinction that has already been noted above in describing the formation of the embryonic disc and its relation to the trophoblast. In the Carnivors, Ruminants, many Insectivors and some Rodents, such as the rabbit, the amnion is formed from a series of folds of the extra-embryonic somatopleure (wall of the blastodermic vesicle) much as in the chick. In other forms, such as the mouse, guinea-pig, bat, some Insectivors, and many Primates, including man, the amnionic cavity arises in situ (entypy), above the embryonic disc, through a splitting of the ectoderm or through the confluence of gradually enlarging spaces (Figs. 152, 153, 155).
The amnion and chorion of the rabbit may be described as a fair representative of the first type. Here, as in the chick, the mesoderm very early extends posteriorly and laterally from the embryo, but immediately anterior to it the wall of the blastodermic vesicle remains for a considerable period devoid of mesoderm, and therefore composed of ectoderm and endoderm only. This mesoderm-free region is called the proamnion (Fig. 176). The amnionic folds appear, toward the end of the ninth day, between the embryo and the horse-shoe shaped implantation area described above. At this stage of development the embryo is well established, its head- and tail-folds are formed, and the head is beginning to enlarge. In the wall of the blastodermic vesicle the endoderm has extended nearly or quite completely around the inside of the vesicle (trophoblast), while the mesoderm with its exoccelom extends through only the upper third of the vesicle, which is nearly as far as it ever goes in the rabbit.
Fig. 176. Diagrams of the formation of the embryonic membranes and appendages in the rabbit. After Van Beneden and Julin (partly after Marshall). Sagittal sections. A. At the end of the ninth day. B. Early the tenth day. C. At the end of the tenth day. Ectoderm black; endoderm dotted; mesoderm gray, al, Allantois; as, allantoic stalk; b, tail bud; c, heart; d, trophoderm; e, endoderm; ex, exocoelom; /, fore-gut; h, hind-gut; m, mesoderm; N, central nervous system; p, pericardial cavity; pa, proamnion; s, marginal sinus (sinua terminalis); /, trophoblast; ta, tail-fold of amnion; v, trophodermal villi; vb, trophoblastic villi; y, cavity of yolk-sac; ys, yolk-stalk.
Fig. 177. Transverse section through the rabbit embryo of eight days and two hours. From Minot (Laboratory Text-book of Embryology). Am, Amnion; Ao, lateral dorsal aorta; Ch, notochord; Cho, chorion; Cce, coelom; Ent, endoderm; Md, medullary tube (nerve cord); Seg, myotome; Som. somatopleure; Spl, splanchnopleure.
The tail-fold of the amnion appears first, composed of ectoderm and somatic mesoderm of the extra-embryonic region, and therefore containing an extension of the exoccelom; the mesodermal layer is unusually thick in the tail-fold. Laterally folds soon appear as anterior extensions of the extremities of the tail-fold. The head of the embryo rapidly enlarges, and as it sinks down into the proamnionic region this flows up to or above the level of the surface of the embryo forming the rudiment of the head-fold of the amnion, which is thus composed at first of the ectodermal and endodermal wall of this part of the vesicle. The posterior and lateral folds rapidly come together, close above the embryo, in the anterior direction (Fig. 177). Finally all four of the folds fuse together in front of the middle of the embryo. The region of their fusion, the seroamnionic connection, is a small knot, quite in contrast to the elongated seam of the chick; it should also be noted that the direction of the closure of the folds is just the reverse of what it is in the chick, for here the tail-fold, rather than the headfold, grows the more rapidly.
Following the complete fusion of the amnionic folds, occurs the separation of the inner and outer layers of the folds, thus establishing (1) an outer membrane, really an extension over the embryo of the wall of the blastodermic vesicle, known as the chorion; (2) an inner membrane, the amnion, separated from the embryo itself by (3) the amnionic cavity; and (4) an extension completely around the dorsal and lateral sides of the embryo, of the exoccelom (Fig. 176).
From the relation of these folds (Fig. 178), it is clear that the chorion is ectodermal superficially, lined with extra-embryonic somatic mesoderm, while the amnion is ectodermal internally with its somatic mesodermal layer turned away from the embryo. The exoccelom is of course entirely lined with mesoderm, while the amnionic cavity is wholly lined with ectoderm, embryonic and extra-embryonic. The amnion is a thin, semitransparent membrane, while the chorion is thicker and quite opaque. The amnion is non-vascular, while the chorion is, in the higher Mammals, often richly supplied with blood vessels.
As the embryo enlarges, the attachment of the amnion, and therefore the region where the amnionic ectoderm becomes continuous with the embryonic ectoderm, remains restricted to the region just around the origin of the yolk stalk and allantois or umbilicus (Fig. 176, B, C).
The proamnion finally becomes invaded by mesoderm, which has from a very early stage been present in front of the proamnion, and the entire amnion and chorion then have a mesodermal lining. In the rabbit the amnionic cavity remains relatively small, while the exocoelom expands so as to fill the cavity of the blastodermic vesicle, save for the space occupied by the yolk-sac and allantois (Fig. 180). The tail- fold appears extremely early in some forms, such as the mole (Talpa) and in Tarsius, so that the point of the final closure of the amnionic folds is far forward over the head. The extent of the proamnion is also extremely variable; in some forms (e.g., Ruminants, bats, many Primates) it is nearly or quite absent, while in the opossum it forms nearly the entire amnion.
Fig. 178. Diagram of the embryonic membranes and appendages of the Mammals in general. From Hertwig (Lehrbuch, etc.), after Turner. AC, Amnionic cavity; al, allantois; ALC, allantoic cavity; am. amnion; E, embryonic ectoderm; H, embryonic endoderm (the reference letter is placed in the gut cavity); M, embryonic mesoderm; pc, trophoblast or trophoderm with villi; sz, chorion; UV, yolk-sac.
Turning to the second type, where the amnionic cavity is formed in situ, as a closed cavity from the very beginning, we may describe the human amnion as an example. The earliest stages in its formation have not been observed as yet, in man, but a relatively early condition has been illustrated in Fig. 161, D. Here the embryo itself remains connected with the wall of the blastodermic vesicle by the body stalk, described above, and in this respect the human embryo is not a satisfactory example of this type of amnion formation. Usually this attachment represents the remains of a thickening in the wall of the vesicle ("trager" see above), to which is added later the definitive attachment of the allantois growing out from the embryo through the cavity of the vesicle to the inner surface of the chorion.
In the human vesicle, however, the amnionic cavity appears directly above the embryo, so that the embryonic disc itself forms its floor, while the body stalk bounds it posteriorly (Figs. 161, 179). At first the roof of the amnionic cavity is simply the trophoblast, but as the cavity enlarges it becomes partly free, below the trophoblast, so that its roof is a separate structure. The attachment of the amnion is around the margin of the embryonic disc (Fig. 162, A}] its roof and sides are composed of a thin layer of ectoderm toward the embryo, outside of which is a thin mesodermal layer, for the extraembryonic mesoderm has already been formed throughout the entire vesicle. A proamnion is therefore lacking in forms whose amnion is developed in this manner.
As the embryo enlarges and extends in every direction the origin of the amnion appears to be carried ventrally, so that, as in the rabbit, it connects with the embryo just around the umbilicus. At the posterior end, however, it passes around the sides of the body stalk, to its posterior surface, so that this structure finally becomes wrapped in a part of the amnionic membrane (Fig. 179). In man the yolk stalk and sac, and the allantois, are also bound up in this body stalk or umbilical stalk (Fig. 179). The final disposition and character of the amnion and chorion are thus essentially the same as when formed from folds.
Fig. 179. Diagrams illustrating the formation of the umbilical cord and the relations of the allantois and yolk-sac in the human embryo. From McMurrich (Development of the Human Body). The heavy black line represents the embryonic ectoderm; the dotted line marks the line of the transition of the body (embryonic) ectoderm into that of the amnion. Stippled areas, mesoderm. Ac, Amnionic cavity; Al, allantois; Be, exocoelom; Bs, body stalk; Ch, chorion; P, placenta; Uc, umbilical cord; V, chorionic (trophodermic) villi; Ys, yolk-sac.
In man the amnionic cavity grows rapidly and by the third month becomes so large as completely to fill the cavity of the The exoccelom is thereby entirely obliterated, the amnion and chorion being brought into contact with one another over their mesodermal surfaces, and finally they may fuse together. The amnionic cavity is filled with a fluid known as the liquor amnii, now thought to be formed by the amnionic epithelium. It contains solids, mostly albumins, grape sugar, and urea, to the extent of about 1 per cent. The amount of the liquor amnii in man varies considerably, but usually between 0.5 and 1.0 liter at the period of its maximum amount, which is some time before parturition. At parturition, of course, both the amnion and chorion normally are ruptured and the amnionic fluid escapes with or before the foetus.
The more important aspects of the chorion are those concerned with placentation and will be considered in connection with the development of the placenta.
The Yolk-sac
We may summarize what has already been said concerning the yolk-sac and add a few facts of importance, chiefly from the comparative standpoint. We are to think of the yolk-sac of the Mammals as typically occupying the chief part of the early blastodermic vesicle, its cavity opening widely into the embryonic gut by the broad yolk stalk, and its wall separated from the chorion by the exoccelom (Figs. 161, 179). The yolksac is thus a splanchnopleuric structure, in contrast to the somatopleuric amnion and chorion. This typical relation, however, is subject to varied and often profound modification. In the rabbit we have seen that the endodermal wall of the vesicle develops slowly, so that for a considerable early period the yolksac is incomplete ventrally. Finally the endoderm does form a complete sac, in contact with the chorionic ectoderm; the extra-embryonic mesoderm then develops very slowly pushing down between the ectoderm and endoderm, but limited to the upper half of the vesicle (Fig. 176). The yolk-sac in the rabbit is therefore strictly splanchnopleural only in its upper half; below this the chorionic ectoderm is in direct contact with the yolk-sac endoderm. When the exoccelom expands so considerably as it does in the rabbit, the yolk-sac is compressed and reduced to an umbrella-shaped structure with a very narrow cavity, connected with the embryonic gut by a long narrow yolk stalk, the wall of which includes mesoderm as well as endoderm (Fig. 180).
Fig. 180. Diagrammatic section through the fully formed blastodermic vesicle of the rabbit, showing the reduced yolk-sac. From Hertwig (Lehrbuch,.eJc.) after Bischoff. o, Amnion; al, allantois; ds, yolk-sac; e, embryo; ed, ed f , ed" yolk-sac endoderm; fd, vascular layer (mesoderm) of yolk-sac; pi, villi; r, exoccelom; st, sinus terminalis; u, allantoic stalk.
Fig. 181. Area vasculosa (yolk-sac circulation) of an eleven-day rabbit. After Van Beneden and Julin. Veins black, arteries white, s, Marginal sinus (sinus terminalis) ; va, vitelline artery; vv, vitelline veins.
As in the chick the mesodermal wall of the yolk-sac becomes very vascular. Its rich network of blood vessels is supplied by the vitelline or omphalomesenteric arteries arising from the dorsal aorta (Fig. 181) . After spreading over the surface of the sac these collect into a well-marked sinus terminalis, which is a complete ring in the rabbit, and from which the blood is returned to the embryo through the vitelline or omphalomesenteric veins. These empty into the posterior end of the heart after penetrating the liver; later the extra-embryonic portions of these veins disappear, while their embryonic portions are transformed into the portal (hepatic portal) vein.
In the Carnivors the yolk-sac is very large and has a complete mesodermal investment which is unusually vascular. At first it is closely in contact with the chorion, and thus may have temporarily a placental relation, but soon it is in part separated from the chorion by the extension, between the two membranes, of the allantois which then assumes the definitive placental relation. The primarily placental character of the yolk-sac in the marsupial, Dasyurus, has been mentioned. In the mole, Talpa, a similar relation has been described, the vascular mesoderm of the upper part of the yolk-sac, with the endodermal wall of its lower part, coming into close organic relation with the chorion and uterine epithelium.
In many other Mammals the yolk-sac is from the beginning a relatively small organ (Tarsius, hedgehog, Primates), or it may early be reduced from an originally well-developed state (horse). In man, which may be described as in a general way representative of this type, the yolk-sac at all stages comes far short of filling the cavity (exocoelom) of the blastodermic vesicle. It grows slowly out into the exocoelom during the first month or more of development, its diameter about equal to the length of the embryo. Its wall is richly vascular even in its very early stages, the blood vessels producing a characteristic roughness of its outer (mesodermal) surface (Fig. 164). After reaching a size of about 11X7 mm. it begins to diminish in size. A yolk stalk becomes clearly differentiated and elongates rapidly, and we have already seen how it becomes enclosed proximally within the umbilical stalk or cord. Finally the entire yolk stalk is thus enclosed and, as the amnionic membrane expands, filling the entire cavity of the "ovum," wiping out the exocoelom, the yolk-sac itself disappears from view, embedded in the mesodermal tissues of the placental region (Fig. 179).
The greater part of the yolk stalk becomes a solid cord of endoderm during the latter part of the second month, and finally it disappears entirely; its proximal end may occasionally remain tubular as Meckel's diverticulum of the intestine. The yolksac remains as a very small vestige, ordinarily 5 mm. or less in diameter, even up to the the time of parturition.
The Allantois
The allantois is no less variable among the Eutheria, both in its mode of development and in its definitive relations, than are the other embryonic appendages. And again there is little parallel between allantoic characters and the usual ordinal classification of the Eutheria. Among the Carnivora and the lower Primates (Lemurs) the allantois becomes very large and fills the exocoelomic space, while, as an opposite condition, among the other Primates it forms not even a free vesicle, but remains as a vestigial structure, enclosed within the umbilical cord. Between these two extremes there is a great variety of conditions.
The earlier embryonic history of the allantois is considerably less variable than its later history and final relations. At first there is essential similarity to the condition already described in the chick, so that the earlier phases in its development need not be described here. Many of the later modifications of the avian type of allantois are correlated with the fact that among the Mammals the allantoic circulation functionally takes the place of the avian yolk-sac circulation, or in other words, the allantois takes an essential, though secondary, part in the formation of the embryonic placental structures, indeed it may even be limited to this relation, as in the rabbit. From the functional relations of the allantois, among the Mammalia, it is clear that its mesodermal structures, in particular its blood vessels, are its most important elements, and there is relatively slight variation in their arrangement.
As in describing the amnion we may consider two of the important types of allantoic formation and history, and then mention briefly a few comparative points of interest.
In the rabbit the allantois appears on the eighth day of development, as a small mass of mesoderm cells extending into the exoccelom opposite the posterior end (primitive streak) of the embryo (Fig. 176, -A). At its base the tail-fold of the amnion is just appearing, and there is already a slight indication of the evagination of the endoderm into it. By the ninth day the tail of the embryo has begun to grow out, and the allantoic rudiment is forced into a ventral position; the amnion therefore appears to arise posteriorly to the allantois, beneath the base of the tail. By this day the allantois has enlarged and its extremity has dilated forming a vesicle which extends freely into the exoccelom, while the narrower allantoic stalk is attached beneath the embryo, just back of the attachment of the yolk-sac, from the cavity of which the allantoic cavity is now clearly marked off.
As the allantois grows out it comes immediately into relation with the inner surface of the chorion, in the region where the chorionic ectoderm has become trophodermic (Fig. 176, 5), and since the trophoderm is the beginning of the placental structure, the allantoic stalk thus becomes the direct pathway between the embryo and the placental region.
During the tenth to twelfth days the allantois expands rapidly beneath the chorionic trophoderm, its mesodermal wall thickens and in it a rich vascular network is developed (Fig. 176, C). Blood vessels have been present in the allantoic mesoderm from a very early period, and by the tenth day there are present a pair of umbilical arteries, and a pair of umbilical veins, having relations similar to those already described in the human embryo. It is through the allantoic blood vessels, therefore, that the embryonic circulation is related with the placental, and in the rabbit this appears to be the only important function of the whole allantoic structure.
In man the history of the allantois is a very different story. Here, as in all Primates, the primitive connection of the hinder end of the embryo with the chorion is never interrupted, and this connection, known as the body stalk (see above), composed of mesoderm, may be regarded as the modified equivalent of the allantoic stalk of such a form as the rabbit. Into this body stalk there early extends a small tubular outgrowth from the endodermal lining of the yolk-sac, from which at this time the hind-gut is not distinguishable (Fig. 161, D, E).
The essential relation thus established is never extensively altered. When the hind-gut forms, it is dorsal or posterodorsal to the opening of the allantoic canal, and as the body stalk elongates the allantoic canal continues to extend up through it, finally reaching the region of the chorionic mesoderm (Fig. 179). The allantois never expands into a free vesicle in the exoccelom, but remains as a vestigial structure, wholly embedded within the tissues of the body stalk, or of what is later the umbilical stalk (see above). The endodermally lined allantoic canal within the umbilical cord, remains present in this condition throughout the foetal period. As the ventral body wall of the embryo is formed it encloses the proximal portion of the allantoic stalk, and this becomes enlarged, forming the rudiment of the urino-genital sinus and the urinary bladder; between this and the body wall it is reduced to a solid strand of connective tissue called the urachus.
The blood vessels of the allantois (umbilical arteries and veins) remain typically developed here, in spite of the vestigial character of the endodermal portions of the allantois, and as in the rabbit these are the only functional elements of the whole allantoic structure. The vessels are very large and form a very rich network in the placental region, beyond the limits of which the chorion becomes almost non-vascular, although in earlier stages the entire chorion is vascular.
Only among the higher Primates is the allantois vestigial to such a degree; and it is not often that it has as limited an extent as in the rabbit. In other forms, such as the Lemurs, Carnivors, and Ungulates, the allantois extends completely around the inner surface of the chorionic vesicle; among the Ungulates this seems to be correlated with the simple type of placenta (see below). In such cases, and in some other instances where the allantois is more nearly limited to a definite placental region, a definite allantoic cavity is present, small and compressed in forms like the sheep and pig, or large and filling a large part of the cavity of the entire vesicle.
The Placenta
Among the Eutherian Mammalia placentation may be defined as an intimate relation between a portion of the uterine mucosa and a part or the whole of the chorionic membrane (trophoblast) of the blastodermic vesicle. This relation may involve merely the close apposition of these two tissues, or their actual fusion. Further, in order that this relation may be effective in the nutrition of the embryo, which is in fact its whole raison d'etre, the blood vessels of the allantois become closely associated with the related chorionic and maternal tissues.
All of the structures thus associated in effecting the nutritive, respiratory and excretory interchanges between embryo and maternal organism, may collectively be termed the placenta. It is thus immediately evicbent that the placenta is a complicated structure and one that is extremely variable, including as it does, these several elements, themselves individually variable.
Many of the essential facts regarding the establishment and the grosser morphological relations of the placenta have been mentioned in other connections. In the section on the implantation of the "ovum" we have seen that the earliest step in placentation is to be found in the relation established between the chorionic ectoderm, whether trophoblast or trophoderm, and the uterine wall (Fig. 175). This is followed considerably later by the vascularization of the chorion thus related to maternal tissues, by the blood vessels of the yolk-sac, as in certain Marsupials (Fig. 174), or of the allantois, as in the Ungulates and rabbit (Fig. 176), or by the vessels of the chorionic mesoderm itself, as in man.
Upon the character and completeness of the relation between chorionic ectoderm and the uterine tissues depends the fundamental character or type of the placenta developed later. Among most of the Marsupial Mammalia the surface of the chorion retains its smooth surface and is ordinarily not vascularized, either by the yolk-sac or allantois, and since it forms only simple contact with the uterine wall, nutritive interchanges between embryo and parent are carried on only with some difficulty. These forms have been termed the achoria or aplacentalia, although strictly speaking these terms are misnomers, for a simple chorion is present and a placental relation does exist, although to a very limited degree.
All of the Eutherian Mammalia may then be termed choriata or placentalia, and it is characteristic of these forms that upon the outer surface of the chorion there develop elevations or papilla? known as the villi (Figs. 153, 161, 184).
The villi are the organs of primary importance in effecting the nourishment of the embryo, and the essential characters, as well as many of the minor characters of the placenta, depend upon the form, mode of distribution, and other characteristics of the villi.
The chief variations in the characteristics of the villi may be enumerated as follows: they may be trophoblastic or trophodermic in origin; they may be simple papilla? or complexly branching, dendritic outgrowths; they may develop very early in embryonic history or very late after a considerable period of intra- uterine life; they may be almost non- vascular or very highly vascularized from the embryonic circulation (umbilical arteries and veins); they may be distributed quite uniformly over the greater part of the chorionic surface or definitely grouped and restricted to certain areas; when restricted they may be grouped in definite patches or cotyledons, scattered over the chorion, like polka-dots, or they may be restricted to certain general zones or bands, or to single large circular areas, or arranged in still other ways; they may be in contact with the maternal mucous epithelium lining the uterine cavity, or with its connective tissue stroma, or with the endothelium of the uterine vessels, or actually bathed directly in the maternal blood stream.
Several classifications of placenta? have been formulated; based upon one or another of these conditions. While none of these represents a natural classification, we may outline certain of the more important, as a convenient way of stating the essential facts of placental arrangement. The earlier classifications, such as those of Owen, Huxley and Kolliker, based upon the type of villous distribution and the degree of intimacy between the villi and the uterine mucous membrane, may be summarized as follows, following Hertwig in the main:
I. Achoria. Chorion with few or no villi. Monotremes and Marsupials.
II. Choriata. Chorion with many villi.
A. With uniformly distributed villi, not intimately related with the maternal tissues. Most Ungulates except the Ruminants (e.g., horse, pig, camel, deer, etc.),
B. With villi localized in definite areas, and more or less closely related with maternal tissues. The true Placentalia.
1. Villi in numerous small patches or cotyledons. Cotyledonary placenta. Ruminants.
2. Villi in a band or zone around the chorion. Zonary placenta. Carnivora.
3. Villi in a single large circular area. Discoid placenta. Insectivors, Bats, Rodents, higher Primates including man.
The terms deciduate and non-deciduate in the classification above require a word of explanation. In several groups of Mammals the epithelium of the mucous membrane lining the uterine cavity becomes greatly thickened during pregnancy, or preceding menstruation. This thickening during pregnancy is termed the decidua, and usually the relations of the villi to the decidua are such that the separation of the placenta at parturition involves a certain loss of maternal tissue (deciduata). In other forms, however, the uterine epithelium shows no such proliferation, and the chorionic villi, at parturition are simply withdrawn from the pits in the mucosa where they have been lodged, and no destruction of maternal tissues results (non-deciduata). An intermediate condition, known as contra-deciduate, is found in the mole where, although a decidua is formed, the placental tissues are not lost at parturition, but are absorbed by the walls of the uterus.
A more recent and more detailed classification of placental arrangement, again based upon the morphological arrangement of the villi, is that of Strahl, as follows:
I. Mammalia ovipara. Monotremata. II. Mammalia vivipara.
A. Achoria (Aplacentalia) . Most Marsupialia (the exceptions are Perameles, Dasyurus, and perhaps Phascolarctos) .
B. Choriata (Placentalia) .
1. Semiplacentalia (Partial placenta). Chorionic and uterine structures in close apposition but not fused; simple separation at parturition.
a. Semiplacenta avillosa. Chorion without villi. Perameles, Dasyurus.
b. Semiplacenta diffusa. Simple villi uniformly distributed. Horse, pig, tapir, hippopotamus, camel, deer, whale, Manis, Lemurs.
c. Semiplacenta multiplex. Villi in groups or cotyledons. (Cotyledonary placenta.) Ruminants.
d. Semiplacenta zonaria. Villi in a zone or band around the chorion. Only in the Dugong (Halicore) .
2. Placentalia vera (Complete placenta). More or less complete fusion of chorionic and uterine tissues, involving tissue destruction at parturition.
e. Placenta zonaria. Placental fusion in the form of a broad band or zone, completely around the chorion. Most Carnivora. In Hyrax and the elephant combined with cotyledons.
f. Placenta zono-discoidalis. Placental fusion in the form of an incomplete band or zone. Mustelidce (e.g., marten, weasel, ferret, etc.).
g. Placenta discoidalis. Placental fusion in the form of a circular disc, usually simple in outline, though sometimes lobed. Insectivors, bats, Rodents, Tarsius, apes, man.
Of the many other classifications* of placental types we may mention only one, that of Grosser, based upon an entirely different relation, namely, the extent of the erosion of the maternal uterine tissues effected by the trophodermal cells of the chorion. From this point of view placentae are arranged in four groups, as follows:
I. Placenta epitheliochorialis. All maternal tissues retained, uneroded. Chorionic epithelium (of the villi) in contact with the uterine epithelium. Pig.
II. Placenta syndesmochorialis. Uterine epithelium nearly or wholly eroded. Chorionic epithelium in contact with the connective tissue of the uterine mucosa. Ruminants.
III. Placenta endotheliochorialis. Epithelium and connective tissue of the uterine mucosa eroded. Chorionic epithelium in contact with the endothelial walls of the uterine blood vessels. Carnivora.
IV. Placenta hcemochorialis. Uterine epithelium, connective tissue, and vascular endothelium eroded. Chorionic epithelium in contact with. maternal blood stream. Man.
Variation in the extent of the erosion of the maternal tissues determines to some extent also the character of the nutritive substances absorbed or resorbed by the chorionic epithelium. The nutritive materials taken into the foetal circulation are of two classes. (A) Food substances already dissolved in the maternal blood. These, known as hcemotrophe, may pass by diffusion or by active resorption directly into the embryonic circulation. Obviously this process is easier, and this type of nutritive substance more important, in those placenta where the chorionic epithelium is more closely related with the maternal blood (Placenta endotheliochorialis and PL risemochorialis) . (B) Products of the uterine mucous membrane including the secretion of the uterine glands, the products of the erosive or dissolving action of the trophoderm, and the resultant extravasated blood. This is known as the ernbryotrophe or pabulum. Frequently these materials undergo a sort of digestive process before their absorption into the embryonic circulation. In forms whose chorionic (villous) epithelium is less closely related to the maternal blood stream (PI. epitheliochorialis, PI. syndesmochorialis) the embryotrophe is the more important source of nutrition. In the other types it may be of great importance during the early period of development, and gradually give place to the haBmotrophe as the relation between the villous and uterine blood streams becomes more intimate.
With these general facts regarding the variety of placental arrangement in mind, we may describe the development and structure of but a single type the human placenta (Placenta discoidalis, hamochorialis) . (For a description of the placental relations in other forms, the student may be referred to Marshall's " Physiology of Reproduction.")
The very early stages in the history of the human ovum are not yet known, but from comparisons with other similar forms, and from the conditions of the youngest embryos known (see Peters, Bryce-Teacher) the characters of the early human blastodermic vesicle may be inferred with a high degree of probability. It is entirely probable that the entire trophoblastic surface of the vesicle becomes trophodermic (Fig. 161), and digests or erodes the uterine mucosa all around itself, when it becomes attached to the uterine wall after entering the uterine cavity. This attachment and implantation of the "ovum' 7 usually takes place on the anterior (upper) wall of the cavity, between the openings of the oviducts, in what is known as the fundus of the uterus. Attachments to other parts of the wall are not infrequent, however, and do not affect the normality of development.
The "ovum" or vesicle apparently eats its way a short distance into the mucosa, which closes behind it, and by destroying the adjacent tissues becomes surrounded by a narrow space filled with fluid extravasated blood and the products of erosion (embryotrophe). This space is the rudiment of what is later known as the intervillous cavity. The uterine cells in this region then begin to proliferate rapidly, so that as the blastodermic vesicle enlarges it remains covered with a distinct layer of the uterine mucosa; this, as we shall see, is the beginning of the decidua capsularis or reflexa. In some of the Rodents the injury of the mucosa, when combined with the presence of an internal secretion from the ovary (or corpus luteum) serves as the stimulus to this proliferation (L. Loeb).
The chorionic villi are formed very early and at first develop all over the surface of the vesicle. Some of the villi simply extend freely into the intervillous cavity, while others grow across it and reach the undisturbed tissues of the uterine mucosa, to which they become definitely attached as the fixation villi.
Before tracing further the history of the villi and the formation of the placenta, we must consider some facts regarding the general epithelial lining of the uterine cavity (Fig. 182). During pregnancy this epithelium is not sloughed off as it is during menstruation; on the contrary it thickens rapidly and considerably, over the entire wall of the uterine cavity forming the decidua. Its later history varies in different regions. That part of the decidua not directly related with the blastodermic vesicle, and therefore its greater part, is known as the decidua vera. The region covering the vesicle and separating it from the uterine cavity is the decidua capsularis or decidua reflexa; while the portion beneath the vesicle, between it and the muscular layer of the uterine wall, is the decidua basalis or decidua serotina.
As the decidua vera thickens, during the early phases of pregnancy, its vascularity increases and the uterine glands within it elongate, becoming branched and anastomosing, extending down into the deeper layers of the mucosa. Toward their openings upon the surface of the mucosa they dilate considerably and the connective tissue matrix in which they are embedded is correspondingly reduced, so that a superficial layer of the decidua may be distinguished, as the spongy layer. The deeper region is known as the compact layer; here the glands do not dilate and the decidual cells multiply and enlarge. The decidua vera reaches its maximum development during the second or third month of development, when it may be 6-10 mm. in thickness; degenerative changes have already begun at this time, however.
At first the structure of the decidua capsularis is not markedly unlike that of the decidua vera, save where all its structure has been destroyed by the entering vesicle, but its glands soon atrophy. As the blastodermic vesicle rapidly enlarges the decidua capsularis becomes extended, and by the fifth month it is pushed out into contact with the decidua vera, and the original cavity of the uterus is obliterated. The decidua capsularis now becomes non-vascular, gradually thins out, and finally disappears completely, leaving the chorionic surface of the vesicle in contact with the decidua vera. But this too has partly degenerated; it has become less vascular, its superficial epithelium and spongy layer have disappeared, and there remain only a part of its compact layer and the deeper portions of the uterine glands.
Fig. 182. Diagrammatic section through the gravid human uterus and the embryo at the seventh or eighth week. From Quain's Anatomy, after Allen Thomson, al, Allantois; am, amnion; c, c, openings of the oviducts (Fallopian tubes) into the uterine cavity; c', cervix filled with mucous plug (the reference letters c,c,c', are placed in the cavity of the uterus; ch, chorion with vascular villi growing into the decidua capsularis and decidua basalis; in the decidua capsularis the villi are becoming atrophied (chorion Iseve) ; dr, decidua capsularis; ds, decidua basalis; dv, decidua vera; i, embryo; u, umbilical cord; y, yolk-sac; y r , yolk-stalk;
The early history of the decidua basalis is not essentially unlike that of the decidua vera, save that its glands disappear, but later, instead of exhibiting any phenomena of atrophy its importance increases; its capillaries dilate, its decidual interglandular cells continue to multiply, and it takes an essential part in the formation of the placenta, forming in fact the whole maternal portion of this organ. To understand the origin and structure of the placenta we must return to the early blastocyst, upon the surface of which the villi are forming. The villi are formed at first wholly of the ectodermal trophoderm, and as we have seen, extend into or across the intervillous cavity from all surfaces of the blastodermic vesicle. As the vesicle enlarges the villi on the sides toward the decidua capsularis disappear along with the capsularis itself, and the smooth chorion thus left is the chorion Iceve, which then comes into contact with the decidua vera on the opposite side of the uterus (Figs. 182, 183). The villi in relation with the decidua basalis alone remain, forming then the chorion frondosum; this part of the chorion is in the region of the attachment of the body stalk of the embryo, where the umbilical (allantoic) blood vessels are distributed.
Fig. 183. Human embryo of the fourth month in utero, showing the arrangement of the membranes and placenta. After Strahl. c, Chorion and amnion; p, placenta; u, umbilical cord.
The villi of the chorion frondosum enlarge and branch, many of them finally assuming a dendritic appearance, extending irregularly through the intervillous cavity (Fig. 184). While at first simply ectodermal, the mesoderm of the chorion soon pushes out into them and becomes extremely vascular. Even before this the superficial cells of the trophoderm (and villi) have fused into a syncytial layer known as the syncytiotrophoderm, which is lined internally, for a time, by a simple epithelium of the cells of Langhans, also apparently derived from the chorionic ectoderm. The vascular mesoderm then forms the core of the villus (Fig. 184).
As the villi grow out they continue to erode the substance of the decidua basalis, and the intervillous cavity is consequently enlarged and filled with maternal blood from the opened capillaries and vessels of the region. Finally the whole intervillous space is occupied by large vascular sinuses or lacunae, and when the Langhans cells disappear, the embryonic and maternal blood streams are separated only by the endothelium of the villous capillaries and the syncytiotrophoderm covering them (placenta haBmochorialis). Of course the two blood streams are nowhere in direct communication.
The formation of the placenta has now been described. It is a structure combined from two distinct sources, a maternal portion, the decidua basalis, and an embryonic portion, the chorion frondosum; the two are separated by the intervillous, or as we may now say, intra-placental cavity, filled with maternal blood. The two regions are in actual connection only marginally and through the fixation villi (Figs. 184, 185); later certain septa afford additional connection (see below). From the preceding description it is apparent that the human placenta is at first of the diffuse type, later becoming discoid.
Fig. 184. Diagrams illustrating the development of the villi in the human placenta. A. B, After Peters; C. after Bryce. A. Chorionic mesoderm just beginning to extend into the villi. B. Mesoderm invading the villi which are now branched. Layer of Langhans cells forming beneath the syncytiotrophoderm. C. Continued branching of the villi, all now covered only by the syncytiotrophoderm and the single layer of Langhans cells, b, Decidua basalis; cb, capillaries of the decidua basalis; cv, capillaries of the villi; e, endothelium of the maternal capillaries; /, fibrin deposited at the junction of the trophoderm and decidua basilis; i, intervillous cavity filled with maternal blood; L, Langhans cells; m, chorionic mesoderm; s, syncytiotrophoderm; t, trophoderm; v, villi; vf, fixation villi.
The fully formed placenta (i.e., at nine months) is an oval or circular disc of irregular outline, roughly 16-20 cm. in diameter, and about 3 cm. in thickness toward the middle, gradually becoming thinner toward the margin. The umbilical cord transmitting the umbilical arteries and veins, is attached eccentrically; the cord itself measures, at full term, about 50-60 cm. in length and has a diameter of something over 1 cm. The villous surface of the placenta is marked out in irregular areas by partitions or septa, extending upward from the decidua basalis, some of which, when the placenta is in situ, attach to the chorion and so divide the intra-placental or inter villous cavity into chambers. These areas are known as the loculi, or sometimes as cotyledons, and they must not be confused with the groups of villi of the cotyledonary placenta of the Ruminants. The villi extend from the surface of the septa as well as from the general chorionic surface.
Fig. 185. Diagram of a section through the human placenta at the middle of the fifth month.
Examination of Fig. 185, illustrating diagrammatically a section through the placenta at its margin, will serve to make clear the structure of the placenta. The embryonic surface of the placenta, which is toward the top of the page, is seen to be covered with the thin amnion; that is, the exoccelom has been obliterated by the apposition of the amnion and chorion, and the cavity of the embryonic vesicle is therefore the amnionic cavity, filled with the amnionic fluid.
The chorion presents first a connective tissue membrane (close vertical ruling) beneath which are the vascular villi (horizontal ruling) which in reality form a dense spongy mass. The vessels extend through the complexly branched villi, both types of which are shown. Toward the close of gestation the fixation villi become very loosely attached to the decidua basalis, in preparation for the separation of the placenta at parturition. The villi are for the most part freely suspended in the intra-placental cavity filled with maternal blood (black in Fig. 185). Beneath this are the greatly reduced layers of the decidua basalis, traversed by the maternal blood vessels communicating with the intra-placental cavity. In the deeper layer of the decidua basalis portions of the uterine glands remain present. Finally the decidua basalis rests upon the muscular layer of the uterine wall (oblique ruling).
Peripherally the irregular chambers of the intra-placental cavity are nearly or quite free from villi, and form the marginal sinus. This is incompletely separated from the remainder of the cavity by an irregular septum known as the closing plate.
At parturition, when the amnion has been ruptured and the amnionic fluid and foetus have been expelled by the contractions of the uterine musculature, the arnnion itself and the placenta and deciduse come away later. The decidua basalis separates first through the spongy region, just outside the glandular layer (Fig. 185, Tr-Tr). The decidua vera similarly is divided through the remains of its spongy layer and comes away with the placenta. The so-called " after-birth " therefore includes amnion, chorion, decidua vera, placenta, and a portion of the decidua basalis. The hemorrhage which follows this separation of the maternal tissues is diminished by the general contraction of the uterine walls. The entire uterine cavity is then lined with the deeper decidual layer containing vestiges of uterine glands, and from this tissue the decidua is formed anew.
References To Literature
Chapter VI
This list contains but a very few of the important works bearing directly upon the topics of the chapter. For very full references to the literature' of Mammalian development see especially the works in the following list, by O. Hertwig, Handbuch, etc., 0. Hertwig, Lehrbuch, etc., F. Keibel and F. P. Mall, Handbuch, etc., Hubrecht, Marshall, and Minot, 1893.
ASSHETON, R., A Re-investigation into the Early Stages of the Development of the Rabbit. Q. J. M. S. 37. 1894. On the Causes which lead to the Attachment of the Mammalian Embryo to the Walls of the Uterus. Q. J. M. S. 37. 1894. The Primitive Streak of the Rabbit ; the Causes which may determine its Shape, and the Part of the Embryo formed by its Activity. Q. J. M. S. 37. 1894. The Morphology of the Ungulate Placenta. Phil. Trans. Roy. Soc. 198. 1906. The Segmentation of the Ovum of the Sheep, with Observations on the Hypothesis of a Hypoblastic Origin for the Trophoblast. Q. J. M. S. 41. 1898. The Development of the Pig during the First Ten Days. Q. J. M. S. 41. 1898. Early Ontogenetic Phenomena in Mammals. Q. J. M. S. 54. 1909.
VAN BENEDEN, E., Recherches sur Tembryologie des mammiferes La formation des feuillets chez le Lapin. Arch. Biol. 1. 1880. Recherches sur les premiers stades du developpement du Murin (Vespertilio murinus). Anat. Anz. 16. 1899. (Brachet, ed.) Recherches sur Fembryologie des Mammiferes. I. De la segmentation, de la formation de la cavit6 blastodermique et de 1'embryon didermique chez le Murin. Arch. Biol. 26. 1911. II. De la ligne primitive, du prolongement cephalique de la notochorde et du mesoblaste chez la lapin et chez le murin. Arch. Biol. 27. 1912.
VAN BENEDEN, E. and JULIN, C., Observations sur la maturation, la fe*condation et la segmentation de Tceuf chez les Cheiropteres. Arch. Biol. 1. 1880.
BONNET, R., Beitrage zur Embryologie des Hundes. I. Anat. Hefte. 9. 1897. II. Anat. Hefte. 16. 1901. III. Anat. Hefte. 20. 1902.
BRYCE, T. H. and TEACHER, J. H., Contributions to the Study of the Early Development and Imbedding of the Human Ovum. I . An Early Ovum imbedded in the Decidua. Glasgow. 1908.
BURCKHARD, G., Die Implantation des Eies der Maus in die Uterusschleimhaut und die Umbildung derselben zur Decidua. Arch, mikr. Anat. 57. 1901.
DANIEL, J. F., Observations on the Period of Gestation in White Mice. Jour. Exp. Zool. 9. 1910.
ETERNOD, A.-C.-F., L'ceuf humain. Implantation et gestation, trophoderm et placenta. Geneva. 1909.
FELIX, W., See Keibel-Mall, Handbuch, etc.
GROSSER, O., Vergleichende Anatomic und Entwicklungsgeschichte der Eihaute und der Placenta, mit besonderer Beriicksichtigung des Menschen. Wien. 1909. See also Keibel-Mall, Handbuch, etc.
GUYER, M. F., Accessory Chromosomes in Man. Biol. Bull. 19. 1910.
HEAPE, W., The Development of the Mole (Talpa Europea). The Formation of the Germinal Layers, and Early Development of the Medullary Groove and Notochord. Q. J. M. S. 23, 1883. 27. 1887.
HERTWIG, 0., Lehrbuch der Entwickelungsgeschichte des Mensch und den Wirbeltiere. 9 Aufl. Jena. 1910. (Editor.) Handbuch der vergleichenden und experimentellen Entwickelungslehre der Wirbeltiere. Jena. 1906.
HILL, J. P., The Placentation of Perameles. (Contributions to the Embryology of the Marsupialia. I.) Q. J. M. S. 40. 1897. On the Fcetal Membranes, Placentation and Parturition of the Native Cat (Dasyurus viverrinus). Anat. Anz. 18. 1900.
His, W., Unsere Korperform und das physiologische Problem ihrer
Entstehung. Leipzig. 1874. Anatomie menschlicher Embryo
nen. I-III. Leipzig. 1880-1885. HOCHSTETTER, F., Bilder der ausseren Korperform einiger menschlicher
Embryonen aus den beiden ersten Monaten der Entwickelung.
Miinchen. 1907. HUBRECHT, A. A. W., Early Ontogenetic Phenomena in Mammals and
their Bearing on our Interpretation of the Phylogeny of the Vertebrates.. Q. J. M. S. 63. 1908. JACKSON, C. M., On the Prenatal Growth of the Human Body and the
Relative Growth of the Various Organs and Parts. Amer. Jour.
Anat. 9. 1909. KEIBEL, F., Zur Entwickelungsgeschichte des Rehes. Verh. Anat.
Gesell. Tubingen. 1899. Die Gastrulation und die Keimblatt
bildung der Wirbeltiere. Ergeb. Anat. u. Entw. 10. 1900 (1901).
See also Hertwig's Handbuch, etc. KEIBEL, F. and ELZE, C., Normentafeln zur Entwicklungsgeschichte
des Menschen. Jena. 1908. KEIBEL, F. and MALL, F. P., (Editors and contributors). Handbuch
der Entwicklungsgeschichte des Menschen. Leipzig. 1910, 1911.
American Edition, Manual of Human Embryology. Philadelphia.
1910, 1912. KIRKHAM, W. B., Maturation of the Egg of the White Mouse. Trans.
Connecticut Acad. Arts and Sciences. 13. 1907. (See also Biol.
Bull. 12. 1907.) Ovulation in Mammals, with Special Reference
to the Mouse and Rat. Biol. Bull. 18. 1910. KOLLIKER, A. VON, Entwicklungsgeschichte des Menschen und der
hoheren Thiere. 2 Aufl. Leipzig. 1876, 1879. Grundriss der
Entwicklungsgeschichte des Menschen und der hoheren Thiere.
2 Aufl. Leipzig. 1884. KOLLMANN, J., Die Korperform menschlicher normaler und patholog
ischer Embryonen. Arch. Anat. Physiol. Suppl. 1889. Handatlas der Entwickelungsgeschichte des Menschen. Jena. 1907. KOLSTER, R., Weitere Beitrage zur Kenntnis der Embryotrophe. IV.
Zur Kenntnis des Chorionepithels. Anat. Hefte. 40. 1909. LANE-CLAYPON, J. E., On the Origin and Life-history of the Interstitial
Cells of the Ovary of the Rabbit. Proc. Roy. Soc. B. 77. 1905. LOEB, L., Beitrage zur Analyse des Gewebwachstums. III. Die Erz
eugung von Deciduen in dem Uterus des Kaninchens. Arch.
Entw.-Mech. 27. 1909. The Function of the Corpus Luteum,
the Experimental Production of the Maternal Placenta, and the
Mechanism of the Sexual Cycle in the Female Organism. Medical
Record. 77. 1910. LONG, J. A., The Living Eggs of Rats and Mice, etc. Univ. of California
Publications in Zool. 9. 1912.
LONG, J. A. and MARK, E. L., The Maturation of the Egg of the Mouse. Publ. Carnegie Institution. Washington. No. 142. 1911.
LONGLEY, W. H., The Maturation of the Egg and Ovulation in the Domestic Cat. Amer. Jour. Anat. 12. 1911.
McMuRRiCH, J. P., The Development of the Human Body. 3rd. ed. Philadelphia. 1909.
MALL, F. P., Early Human Embryos and the Mode of their Preservation. Bull. Johns Hopkins Hospital. 4. 1893. See also Keibel-Mall, Handbuch, etc.
MARSHALL, F. H. A., The Physiology of Reproduction. London. 1910.
MELISSINOS, K., Die Entwickelung des Eies der Mause von den ersten Furchungs-Phsenomen bis zur Festsetzung der Allantois an der Ectoplacentarplatte. Arch. Mikr. Anat. 70. 1907.
MINOT, C. S., Human Embryology. New York. 1892. A Bibliography of Vertebrate Embryology. Mem. Boston Soc. Nat. Hist. 4. 1893. A Laboratory Text-Book of Embryology. 2nd. ed. Philadelphia. 1911.
NAGEL, W., Das menschliche Ei. Arch. mikr. Anat. 31. 1888.
NEWMAN, H. H., The Ovum of the Nine-banded Armadillo. Growth of the Ovocytes, Maturation and Fertilization. Biol. Bull. 23. 1912.
PETERS, H., Ueber die Einbettung des menschlichen Eies und das friiheste bisher bekannte menschliche Placentationsstadium. Leipzig and Wien. 1899.
RABL, C., Die Entwickelung des Gesichtes. Das Gesicht der Saugethiere. Leipzig. 1902.
REJSEK, J., Anheftung (Implantation) des Saugetiereies an die Uteruswand, insbesondere des Eies von Spermophilus citillus. Arch, mikr. Anat. 63. 1904.
RETZIUS, G., Weitere Beitrage zur Kenntniss der Spermien des Menschen und einiger Saugethiere. Biol. Unters. N. F. 10. 1902. Zur Kenntnis der Entwicklung der Korperform des Menschen wahrend der fotalen Lebensstufen. Biol. Unters. N. F. 11. 1904.
ROBINSON, A., Observations upon the Development of the Segmentation Cavity, the Archenteron, the Germinal Layers, and the Amnion in Mammals. Q. J. M. S. 33. 1892.
RUBASCHKIN, W., Ueber die Reifungs- und Befruchtungs-prozesse des Meerschweincheneies. Anat. Hefte. 29. 1905. Ueber die Urgeschlechtszellen bei Saugetieren. Anat. Hefte. 39. 1909.
SCHOENFELD, H., Contribution & PEtude de la Fixation de 1'ceuf des Mammiferes dans la cavite uterine, et des premiers stades de la Placentation. Arch. Biol. 19. 1903.
SELENKA, E., Studien uber Entwickelungsgeschichte der Thiere. IV. Das Opossum. 1887. V. 1. Beutelfuchs und Kanguruhratte; zur
EntstehungsgeschichtederAmnionderKantjil (Tragulus javanicus) ;
Affen Ost-Indiens. 1891. V. 2. Keimbildung des Kalong. Dottersack und Placenta des Kalang. 1892.
SOBOTTA, J. . Die Befruchtung und Furchung des Eies der Maus. Arch. mikr. Anat. 45. 1895. (For corrections, see Kirkham.) Die Furchung -des Wirbeltiereies. Ergeb. Anat. u. Entw. 6. 1896 (1897). Die Bildung der Richtungskorper bei der Maus. Anat. Hefte. 35. 1907.
SOBOTTA, J. and BURCKHARD, G., Reifung und Befruchtung des Eies der weissen Ratte. Anat. Hefte. 42. 1910.
SPEE, F. GRAF VON, Beobachtungen an einer menschlichen Keimscheibe mit offener Medullarrinne und Canalis neurentericus. Arch. Anat. Physiol. 1889. Neue Beobachtungen liber sehr friihe Entwickelungs stufen des menschlichen Eies. Arch. Anat. Physiol. 1896. Die Implantation des Meerschweincheneies in die Uteruswand. Zeitschrift fur Morphologic und Anthropologie. 3. 1901.
STRAHL, H., See Hertwig's Handbuch, etc.
TURNER, W., Lectures on the Comparative Anatomy of the Placenta. Edinburgh. 1876.
WIDAKOWICH, V., Ueber die erste Bildung der Korperform bei Entypie des Keimes. Zeit. wiss. Zool. 94. 1910.
WINIWARTER, H. VON, Recherches sur Tovogenese et 1'organogenese de Povaire des Mammif&res (Lapin et Homme). Arch. Biol. 17. 1901
- Nachtrag zu meiner Arbeit iiber die Oogenese der Saugetiere. Anat. Anz. 21. 1902.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Kellicott WE. Outlines of Chordate Development (1913) Henry Holt and Co., New York.
Cite this page: Hill, M.A. (2024, April 20) Embryology Book - Outlines of Chordate Development 6. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Outlines_of_Chordate_Development_6
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G