Book - Contributions to Embryology Carnegie Institution No.51
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Wislocki GB. Experimental studies on fetal absorption. I. The vitally stained fetus. II. The behavior of the fetal membranes and placenta of the cat toward colloidal dyes injected into the maternal blood stream. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 274, 11: 45-60.
| Online Editor |
|---|
| This historic 1920 paper by Wislocki describes the permeability of the guinea-pig and cat placenta.
George B. Wislocki (1892 - 1956) was a pioneer in the field of histochemical anatomical studies. He conducted research on the anatomy of the endocrine system, especially the adrenal and pituitary glands. He also did comparative anatomical studies of the placenta and the blood-brain barrier. His contribution to the elucidation of the chemical processes involved in cell development lead to a better understanding of the body’s complex relationships. Wislocki graduated from Washington University in 1912 and from The Johns Hopkins University School of Medicine in 1916. He served in the U.S. Army Medical Corps as a first lieutenant during World War I. After the war he attended Harvard University as an Arthur Tracy Cabot Fellow at the Laboratory of Surgical Research. In 1920 he returned to Johns Hopkins as an associate in anatomy, and in 1923 he became an associate professor of anatomy. In 1931 he was named the Parkman Professor of Anatomy, a title he held until until 1941, when he became the James Stillman Professor of Comparative Anatomy. In 1951 he served as the first president of the Histochemical Society. (Above Text National Academy of Sciences)
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Experimental Studies on Fetal Absorption
I. The Vitally Stained Fetus
II. The Behavior of the Fetal Membranes and Placenta of the Cat toward Colloidal Dyes injected into the Maternal Blood-stream.
By George B. Wislocki,
Arthur Tracy Cabot Fellow, Laboratory of Surgical Research, Harvard Medical School.
(1920) With four plates and one text figure.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
I. The Vitally Stained Fetus
Colloidal dyes and particulate matter introduced into the blood-stream of pregnant animals fail to reach the fetus. The placenta appears to be an impenetrable barrier for inert colloids, and experiments show that even colloidal metabolites reach the fetus only after they have been split into simpler substances by enzyme action, hydrolysis, and other chemical processes. Goldmann (1909, 1912) noticed that when he injected colloidal dyes (particularly trypan-blue and pyrrholblue) into pregnant mice and rats the placentae and the fetal membranes stained very deeply with the coloring matter, but none of it reached the fetuses.
Because colloidal dyes have been of such great value in the study of problems of absorption and phagocytosis in the adult animal it has been a cause for regret that there has existed no simple means of applying these substances to the study of
similar processes in the fetus. The writer (1916) first observed that amphibian
larvae could be vitally stained by immersing them in dilute solutions of colloidal
dyes and his experiments led to further interesting observations by McClure (1918)
and Clark ( 1918) . The staining of fetuses appeared more difficult since it had been
shown that injecting the dyes into the maternal blood stream was fruitless as a
means of staining the embryo. The technical difficulties accompanying direct
introduction of the dyes into the fetal circulation are numerous, and in such procedures physiological conditions can not be maintained. It was therefore gratifying to observe that by injecting colloidal substances into the amniotic cavity they
could be introduced into the fetus with perfect ease and under physiologically
normal conditions. The present study concerns the results of injecting colloidal
dyes and other substances into the amniotic sacs of guinea-pigs. A few observations
of the same sort upon fetal cats are also described. The observations upon cats,
although incomplete, are deemed important because they confirm some of those
upon the guinea-pig and therefore the final deductions assume a broader significance.
Sterile operative technique was used in the experiments. The pregnant animal
was anesthetized and a mid-line laparotomy was done. One horn of the pregnant
uterus was brought into view through the incision. After palpating a fetus through
the uterine wall, it was grasped between thumb and index finger of the operator.
If the fetus was so young that the amniotic cavity was completey distended with
fluid, a replacement injection was performed. The technique of replacement
injections is described by Weed (1917). As applied to this study, it consists of
inserting two 24-gage needles, connected with balanced reservoirs, directly through
the uterine wall into opposite poles of the amniotic cavity and permitting the
injection fluid to enter by one needle, while the amniotic fluid escapes through the
other. In older fetuses, where the amniotic sac is incompletely distended with fluid, direct injections were made by means of a syringe. For purposes of vital staining
1 to 5 c. c. (depending on the age of the fetus) of a 1 per cent trypan-blue solution
was injected. After completing the injection the uterus was replaced in the
abdomen and the incision closed. Recovery of the animals was uneventful.
Observations upon Fetal Guinea-Pigs
The guinea-pig fetuses used in these experiments ranged from 30 mm. to fullterm. The technique was found impracticable for younger stages. The pregnant animals, injected as described above, were sacrificed at intervals varying from a few minutes up to several days, and the tissues fixed in 10 per cent formalin.
Diffusion of trypan-blue from the amniotic cavity of the guinea-pig becomes
apparent a few minutes after injection. The fetal membranes turn a uniform dark
blue (fig. 1) and within an hour the dye can be demonstrated in the fetal bloodscrum by the filter-paper test. The fetus itself assumes a slaty-blue color which gradually deepens (fig. 2) and the placenta becomes a brilliant blue (fig. 1). The stain
ceases at a definite line of demarcation between the placental labyrinth and decidua
serotina. The uterine wall everywhere about the fetus remains unstained.
Trypan-blue can not be detected in the maternal circulation, liver, kidneys, or
urine, where one would expect to find it in case it escaped, even in traces, from fetus
to mother. In a series of nearly full-term fetuses much larger quantities of trypanblue were injected into the amniotic cavities in order to determine whether the presence of a sufficiently large quantity of dye would cause it to appear in the maternal
circulation. In no instance, however, could the dye be detected in the mother.
In one animal 30 c. c. of trypan-blue was injected into the amniotic sacs without
any of it finding its way into the maternal tissues. It seems reasonable to believe,
therefore, that inert colloidal substances can not pass from fetus to mother. From
this description it is evident that trypan-blue is rapidly absorbed from the amniotic
cavity, enters the fetal circulation, and vitally stains the fetus and placenta.
Clinicians and veterinarians state that in many animals, and perhaps in man,
during the latter part of pregnancy the amniotic fluid normally diminishes. Authorities for this belief are: Kehrer (1867), who made his observations on rabbits;
Doderlein (1890), on the calf; Preyer (1895), on the avian egg; Frank (1901), on
ruminants; and Jacque (1903-4), on sheep. Since the amniotic fluid is believed to
be partially absorbed during the latter half of pregnancy, curiosity is aroused as
to the pathway of absorption. The observations of Zweifel (1875), Ahlfeld (1885),
Zuntz (1885), Doderlein (1890), Preyer (1895), and others, who found lanugo hairs,
amniotic epithelial cells, and bits of hoof and nail in the stomach and intestines of
older fetuses, show clearly that the amniotic fluid may be swallowed by the fetus.
These observations appeared to afford such a complete explanation of the partial
disappearance of the amniotic fluid that little consideration was given to other
possible paths of absorption.
One can readily conceive of a number of possible modes of absorption of fluid from the amniotic sac:
- passage into the gastrointestinal tract by movements of deglutition
- passage into the respiratory tract by movements of inspiration
- absorption through the epidermis of the fetus
- diffusion through the amniotic membrane and umbilical cord. It will be of interest to see what light our experiments upon fetal guinea-pig fetuses shed upon these possibilities.
Absorption of fluid from the amniotic sac by passage into the gastro-intestinal
tract. — The passage of fluid into the fetal stomach in the latter half of pregnancy
can be demonstrated experimentally in animals by injecting a small quantity of
any soluble dye into the amniotic cavity. Of 15 guinea-pig fetuses so injected, 11
showed heavy traces of coloring matter in the stomach one hour later. Aft era somewhat longer interval the dye appeared in the intestines. It is fair to assume that
substances in true solution may enter the fetal circulation in this way. Trypanblue probably does not, since it is in colloidal solution, and we know that in postnatal life colloidal dyes are not absorbed by the mucosa of the gastro-intestinal tract.
Absorption of fluid from the amniotic sac by passage into the respiratory tract. —
No exact observations upon the respiratory movements of the fetus exist, but
everyone who has worked upon pregnant animals is aware that movements resembling respiration occur during the latter part of pregnancy. By injecting a
colored solution into the amniotic sac it can be demonstrated that amniotic fluid is
aspirated into the trachea and lungs. In such experiments the lungs at once become
deeply stained. This phenomenon is prettily shown by performing a replacement
injection of a 1 per cent solution of potassium ferrocyanide and iron ammonium
citrate and after 30 minutes immersing the fetus in acid formalin. Prussian blue is
precipitated in the lungs (fig. 3). From these experiments the absorption of small
quantities of amniotic fluid through the lower respiratory tract is demonstrated.
The failure of previous investigators to describe lanugo hairs, etc., in the respiratory
passages does not militate against such a conclusion, since the epiglottis might
readily prevent gross material from entering the lungs.
Absorption of fluid from the amniotic site through the epidermis. — Absorption in
this manner does not appear to have occurred in any of the animals injected in these
experiments. Whenever trypan-blue was injected into the amniot ic sac the amnion
and umbilical cord stained very quickly, but the color stopped abruptly at the
junction of umbilical e] >ithelium and the skin at the umbilical ring. The skin of the
fetuses, at first uncolored, gradually turned blue as the dye accumulated in the fel al
circulation. To determine more exactly that solutions are not absorbed through
the fetal skin, in one series of animals potassium ferrocyanide and iron ammonium citrate were injected into the amniotic sac. In no instance could precipitated Prussian blue be discovered in the epidermis after such injections. The
experiments afford definite proof that passage of fluid into the fetus by this route
does not occur.
Diffusion of fluid from the amniotic sac through theamniotic membrane. — If dyestuffs are injected into the amniotic sac, the fetal membranes and umbilical cord are
quickly stained. That this is probably a diffusion phenomenon and resembles
transudation, as seen elsewhere in the body (e. g., in the peritoneal cavity), can be
demonstrated by means of the Prussian-blue reaction. In 3 experiments a 1 per cent solution of iron ammonium citrate and potassium ferrocyanide was injected into
the amniotic sacs of several fetuses. The membranes were fixed in acid formalin
at intervals varying from 5 minutes to an hour. Evidence of the diffusion of the
injected fluid was gained by finding Prussian-blue precipitate in the membranes.
Very soon after the injection this precipitate was found in the amniotic epithelial
cells, as well as between them. It also appeared, after a longer interval, in the
subepithelial mesoderm. This demonstrates the gradual penetration of the amniotic membrane by the injected solution.
To summarize these observations, it may be stated that during the latter part
of pregnancy there are three pathways of absorption from the amniotic sac. The
chief of these appears to be direct transudation through the amniotic membrane.
The other two routes are through the fetal stomach and respiratory tract,
After noting the passage of vital stains and true solutions through the amniotic
membrane, it was of interest to determine the further course which they pursue in
entering the fetal circulation. This course is necessarily determined for each
animal species by the relationship of the amniotic membrane to the chorion, allantois, and yolk-sac. The guinea-pig is very similar in development to the mouse,
which has been carefully described by Sobotta. The striking feature in both is that
amnion and embryo develop within the blastodermic vesicle instead of upon its
surface, as in other mammalia. Furthermore, the yolk-sac surrounds the internal
cell-mass and amnion; subsequently, the wall of the blastodermic vesicle and the
outer layer of the yolk-sac atrophy, leaving the fetus with a layer of endodermal
cells — the inverted yolk-sac — as its outermost covering. This endodermal membrane persists throughout fetal life, becomes richly vascularized by the omphalomesenteric vessels, and develops numerous villi whose cells are markedly phagocytic
and probably play a large part in the absorption of the embryotrophe and in the
nutrition of the fetus (Goldmann) . Due to the obliteration of the extra-embryonic
ccelom, the amnion, early in fetal life, becomes loosely adherent to this membrane
by strands of mesoderm. As a result, the amnion is brought into intimate contact
with the omphalo-mesenteric vessel (figs. 1 and 2). It can be demonstrated that
these blood-vessels are partly responsible for the absorption of solutions from the
amnion. To do tins, a solution of iron ammonium citrate and potassium ferrocyanide is injected into the amniotic sac. One hour later the membranes of the fetus
are fixed in acid formalin and the omphalo-mesenteric vessels are examined under
the microscope. Precipitated Prussian blue is encountered outside as well as inside the vessels (fig. 6) .
We now pass to a description of the fetus in the second phase of vital staining,
namely, the appearance of the vital dye in the form of granules in groups of cells,
collectively called macrophages. As we have described, trypan-blue passes from the
amniotic cavity into the circulation of the fetus. After 24 to 72 hours it appears
in the form of characteristic granules in many of the fetal tissues. It must be
pointed out that this vital staining is not comparable to that obtained after repeated
injection in the adult. As is well known, the several tissues of the adult animal do
not stain equally rapidly or heavily, and after a single injection one may fail to find any stain at all in the adrenals, testes, etc. We are aware that the staining
in these fetuses does not represent the extent to which it would occur if repeated
introduction of the dye were made. Such a procedure is technically impossible.
The appearance of the vitally stained fetus in sagittal section is of interest
(fig. 4). It is seen at a glance that the central nervous system remains unstained.
This behavior is identical with that in postnatal life. The physical conditions which
prevent the passage of foreign colloidal material from the blood-stream into the
central nervous system appear to be established relatively early in intrauterine life.
The cells within the fetus which have been observed to store trypan-blue granules
may be conveniently described under three headings: (1) endothelial cells, (2) connective-tissue cells, and (3) epithelial cells. The storage of the dye in the fetal
membranes and placenta will be described separately.
Endothelial cells. — The only endothelial cells of a fetus 30 to 40 mm. in length
which have been noted to absorb trypan-blue under the conditions described, are
those lining the sinusoid spaces of the liver. Characteristic blue granules are always
abundantly present within the cytoplasm of these cells.
Connective-tissue cells. — Large, round cells, with a single round or oval nucleus,
whose cytoplasm contains granules of vital dye, are occasionally encountered in the
connective tissue. It is noted that, as in the adult, the elastic tissue of the fetal
arteries exhibits an affinity for the dyes.
Epithelial cells. — The kidneys of a fetus 30 to 40 mm. in length are striking
because of the large quantity of dye invariably found within the epithelial cells of
the convoluted tubules (fig. 7). The dye is most abundant in the proximal portion
of the tubules, scant in the distal portion, and entirely absent in the collecting
tubules. The glomerular epithelium remains unstained. The fetal urine gives
the characteristic stain of trypan-blue on filter paper.
It may be said, therefore, that the fetal liver and kidneys play an important
part in the removal of foreign colloidal material from the fetal circulation.
Trypan-blue appears in the form of granules within cells in the umbilical cord,
amnion, yolk-sac, and placenta.
Umbilical cord. — In the umbilical cord the dye appears in the form of fine
granules in the mucoid connective tissue (fig. 8). These granules are aggregated
in the cytoplasm of the cell on both sides of the nucleus.
Amnion. — Trypan-blue occurs abundantly in the cytoplasm of the amniotic
epithelium in the form of small blue granules (fig. 9). Bondi (1905) observed similar
granules in the human amnion after staining it with neutral red. He believed that
this appearance signified a secretory activity on the part of the amniotic epithelium.
In the light of our present knowledge, the fact that the amniotic membrane stains
with neutral red and trypan-blue indicates that its cells possess cytoplasmic vacuoles
capable of absorbing and storing these substances. As to the normal role of these
cytoplasmic vacuoles very little is known. The mesothelial cells attached to the
inner surface of the amniotic epithelium are also laden with fine blue pigment (fig. 9).
Yolk-sac. — In the guinea-pig the inverted yolk-sac or vitelline membrane becomes the outermost fetal membrane and a layer of endothelial cells consequently comes in contact with the maternal tissue of the uterine wall. Goldmann showed
that in the mouse this layer of cells plays an important role, similar to that of
chorionic epithelium, in the transmission of nutritive material from mother to fetus.
The endodermal cells have an enormous capacity for storing colloidal material.
They, as well as the chorionic epithelium of the placenta, are responsible for the
failure of foreign colloids, injected into the maternal blood-stream, to enter the
fetus (figs. 5 and 10) . These same endodermal cells appear to act as a barrier to the
passage of colloids in the opposite direction, from fetus to mother, for in the vit abstained fetuses the same cells are heavily laden with dye (fig. 11).
Placenta. — Trypan-blue is absorbed and stored in a few of the endothelial
cells lining the capillaries supplying the chorionic villi. These cells are laden with
granules of dye and protrude into the lumina of the vessels.
Observations upon Fetal Cats
The development of the fetal membranes of the cat and other carnivora differs markedly from that observed in rodents, such as the mouse, rat, and guinea-pig. One of the essential differences is that in carnivora the embryo develops upon the surface of the blastodermic vesicle instead of within it, and consequently the germ layers do not undergo inversion. In the cat fetus we find as the outermost fetal covering the amniogenic chorion. A well-developed allantois separates chorion and amnion. The yolk-sac is entirely closed and becomes reduced in size as pregnancy advances. The placenta surrounds the fetus as a broad, circular band, hence called placenta zonaria. A diagram (text-figure 1) borrowed from Bonnet (1907) makes these relationships clear. Trypan-blue was injected into the amniotic cavities of a number of cat fetuses during the latter part of pregnancy. Vital staining of the fetuses always occurred. Two litters of kittens, living and vitally stained, were born 5 and 8 days respectively after a single injection of trypan-blue into the amniotic sacs.
Text-figure No. 1. — Diagrammatic representation of the fetal membranes of the dog. (After Bonnet.)
The maternal tissues remain unstained after injection of trypan-blue into the
fetuses. The reverse happens if the dye is injected into the maternal circulation.
The tissues of the mother become deeply stained, while those of the fetuses do not,
and such a mother gives birth to unstained offspring. The placentae prevent the dye
from passing into the fetal circulation, as can be easily demonstrated by examining
microscopically a section from the placental labyrinth. The fetal cells separating
the maternal blood-spaces from the fetal capillaries contained enormous numbers of
fine blue granules within their cytoplasm. The chorion, amnion, yolk-sac, and
allantois of the fetus failed to stain, either macroscopically or microscopically,
after injection of trypan-blue into the maternal circulation. In several experiments
the dye was injected into the space between uterine wall and chorion, but even then
it was not absorbed by the fetal membranes. (A detailed account of these observations is given on pages 54-59.)
Colloidal dyes were absorbed from the amniotic cavity of the cat fetus as readily
as from that of the guinea-pig. Trypan-blue was found in the stomach and small
intestine of nearly all the fetuses examined. It passed with equal readiness into the
trachea and bronchi. The amnion, soon after injection, became intensely stained
and gradually the umbilical cord, placenta, and fetus turned blue. The mesothelium between amnion and allantois is vascularized by numerous branches from
the umbilical vessels, and it was through these that absorption occurred.
It was further observed that when trypan-blue was injected into the allantoic
sac of the fetus the allantoic membrane did not stain, nor did absorption of any of
the injected dye occur. The allantoic membrane appeared to be impermeable to
the dye. It is interesting to recall that the allantoic arises as an outgrowth from
the hind-gut and also is thought to be a reservoir for fetal urine. It may also be
recalled that in the adult trypan-blue is not absorbed from the urinary bladder.
Conclusions
- Substances injected into the amniotic cavity of the guinea-pig and cat are absorbed during the latter half of pregnancy. Absorption occurs in three ways: (a) through the gastro-intestinal tract; (b) through the respiratory tract; and (c) by diffusion through the amniotic membrane.
- Amniotic fluid is normally swallowed and also inspired by the fetus during the latter half of pregnancy.
- The fetal guinea-pig may be vitally stained by injecting a colloidal dye into the amniotic sac. Vitally stained cells are abundant in the fetus and membranes. The chief of these are the endothelial cells lining the hepatic sinuses, the epithelium of the renal convoluted tubules, the amniotic epithelium, and the endothelial cells of the placental capillaries. The endodermal cells of the yolk-sac are extremely phagocytic toward vital dyes.
- Foreign colloidal material can not pass from the fetal into the maternal circulation.
My appreciation is due Dr. W. C. Quinby for suggestions which were of value in carrying out these experiments.
II. The Behavior of the Fetal Membranes and Placenta of the Cat toward Colloidal Dyes injected into the Maternal Blood-stream
The extensive researches of Gusserow, Fehling, Zuntz, Zweifel, Wiener, Benecke, Krukenberg, and Preyer have demonstrated the passage of gases and dissolved crystalline substances from the maternal into the fetal circulation. Traces of potassium iodide, potassium ferrocyanide, sodium salicylate, benzoic acid, sodium indigo sulphonate, etc., were observed in the fetal urine and in the amniotic fluid after their administration to the mother. The passage of chloroform from the maternal to the fetal circulation has been observed repeatedly. That true solutions are capable also of passing in the opposite direction, from fetus to mother, has been shown for strychnine by Savory and later by Gusserow; for nicotin and curarine by Preyer, for alcohol by Nicloux.
For many years there was less clarity concerning the passage of formed substances (such as proteins and fats) through the placenta. It is now believed, mainly from the experimental work of Ascoli, Bonnet, Hofbauer, and Goldmann, that such substances are not directly transmitted through the placenta, but must first undergo a breaking-down in the epithelium of the chorion before assimilation by the fetal blood-stream is possible. Hofbauer considers the activity of the chorionic epithelium as comparable in many respects to absorption by the intestinal mucosa. A number of observations have been made on the behavior of the fetus toward inert colloids and particulate matter introduced experimentally into the maternal blood-stream. The general conclusion drawn from them is that foreign colloidal or particulate matter fails to reach the fetal circulation, due to the fact that the placenta and fetal membranes are impenetrable to colloidal substances which the chorionic epithelium is unable to convert into an assimilable form.
Jassinsky (1867) injected a suspension of carmine into pregnant dogs and, although the animals died from this procedure in about 20 minutes, he observed that the substance did not reach the fetal circulation but was held up in the placenta. Reitz (1868) injected a suspension of cinnabar into the jugular vein of a pregnant rabbit. He claims to have subsequently found particles of the injected material in the coagulated blood of the heart and in the capillaries of the brain of the fetus. His observations appear erroneous in the light of our present knowledge. Hoffmann and Langerhans (1869) state that after injecting a nearly full-term rabbit with carmine they found no trace of the dye in the fetus or placenta.
Fehling (1876) injected india ink into the femoral vein of a pregnant rabbit and killed the animal 24 hours later. He observed that the fetus contained none of the ink, but he does not describe how the placenta prevented it from entering the fetal blood-stream.
Schlecht (1907) describes a pregnant mouse winch had been stained by the repeated injection of lithium carmine. The dye had failed to stain the fetuses but was plainly visible in the placenta and fetal membranes. Microscopically it was found throughout the placenta in the form of fine red granules in the cytoplasm of the chorionic epithelium. None of the decidual cells contained dye granules. The outermost membrane of the mouse (the inverted yolk-sac, which Schlecht wrongly terms chorion) was deeply stained but the amnion was not. He shows an illustration in which the endothelial cells of the inverted yolk-sac are seen heavily laden with dye, while the amniotic epithelium contains none.
The only complete observations on the behavior of the fetus toward injected foreign colloids are those of Goldmann (1909), who studied the mouse and rat with great care. Into a number of pregnantm ice and rats he injected colloidal solutions of the benzidine dyes, pyrrhol-blue and trypan-blue. These dyes are practically non-toxic to living tissue. The result was that in every instance the tissues of the mother became deeply stained, the dye appearing in the form of blue granules in many of the cells of the body. On opening the uterus of such a vitally stained animal the fetuses were found unstained, the dye apparently having been prevented from entering their bodies by the placenta and membranes, all of which were a dark blue. On examining sections of the placenta and membranes, Goldmann found that the dye had been absorbed by many of the fetal cells and appeared in the form of blue granules within their cytoplasm; to tins circumstance he attributed the failure of the dye to reach the fetal circulation. In the placenta he observed the dye in the fetal ectoderm, particularly in the giant cells or angioblasts, and in the entire chorionic epithelium of the labyrinth which separates the maternal bloodspaces from the fetal capillaries. The decidual cells, as well as the mesenchyme of the villi and the endothelium of the fetal capillaries, were unstained. He saw further that the dye was very abundant in the endodcrmal cells of the inverted yolksac. In Ins earlier paper Goldmann claimed that the amnioticfluid was stained blue and he believed that it consequently must be a secretion from the neighboring vitally stained endothelium of the yolk-sac and hence derived ultimately from the maternal blood-stream. In a later publication (1913) he contradicts his former assertion regarding the staining of the amniotic fluid.
Concerning the significance of vital staining in the placenta, several opinions have been advanced. Schlecht (1907) believes that the chief function of the vitally stained chorionic cells is to protect the fetus from toxic substances in the maternal blood-stream. Goldmann sees in them a group of cells which are tremendously important in the storage of nutrient material for the fetus. He was led to this view by demonstrating that these same cells are normally laden with glycogen and give staining reactions for iron and fat.
Goldmann's work upon the placenta was confined to rats and mice, whose embryology is nearly alike, and he found that these two genera behaved the same toward vital dyes. In view of the dissimilarity in the development of the placenta and fetal membranes in the different orders of mammalia, it is surprising that no further studies on the action of vital dyes in pregnant animals have been undertaken.
Turning to the laboratory animals — the mouse, rat, guinea-pig, rabbit, cat, dog, and monkey — one finds that they all belong to the Deciduata, but that they fall into three orders: Rodentia, Carnivora, and Primates. In the development of the placenta and fetal membranes these orders exhibit distinct and characteristic differences which it will be of interest to consider in the light of vital staining. The present study concerns the behavior of the placenta and fetal membranes of the cat toward trypan-blue. For comparison a number of vitally stained pregnant mice, rats, guinea-pigs, and rabbits were also examined. The dye was injected into the mother intravenously on successive days. In the cats used, pregnancy was more than half completed.
In the gross the uterus of the vitally stained cat appeared deeply stained. On opening it the placenta and unruptured membranes could be easily shelled out. The placenta, which completely surrounds the fetus as a broad band or belt, was stained a deep blue (fig. 13). The chorion (which incloses the poles of the fetus) was unstained with the exception of a zone a few millimeters in width, along the placental borders, which was a mottled blue. This zone was found to be more marked late than early in pregnancy. On rupturing the membranes the fetus, the umbilical cord, the yolk-sac, and the allantoic and amniotic membranes were found entirely unstained. There was no dye in the allantoic or the amniotic fluid.
It may be said that in the cat. when the fetuses are normal and living, staining of the amniotic fluid does not occur. In this my observation differs from that originally made by Goldmann (1909), who described the amniotic fluid of the mouse and rat as bright blue. It should be noted that in a later paper (1913) Goldmann stated briefly, without giving further experimental data, that the amniotic, fluid, as well as the embryo, is unstained. He had previously thought that his observation lent support to the theory that the amniotic fluid is in whole or in part derived from the maternal blood-stream. From the present experiments it seems probable that normally inert colloidal substances are unable to pass through the placenta or outermost fetal membrane. These experiments shed no light on the derivation of the amniotic fluid, and one can only venture the theory that the colloids normally found in the amniotic fluid are probably not of direct maternal origin. In the experiments described in the foregoing study trypan-blue was injected directly into amniotic sacs of living fetuses, in which case there was rapid absorption of the dye into the fetal blood-stream with vital staining of the embryo.
Further evidence that trypan-blue does not ordinarily reach the blood-stream of the fetus is afforded by the observation that several of the vitally stained cats in these experiments gave birth to litters of living young which were unstained.
Before describing the microscopic appearance of the vitally stained placenta of the cat, it may be of interest to recall briefly its comparative development and architecture. The placentae of mammalia have been classified by the degree of union which occurs between the fetal and maternal tissues. Thus in the most primitive type, seen in the pig, there exists merely an apposition of the chorionic epithelium to the epithelium of the uterus. Consequently the maternal vessels are widely separated from the fetal ones by a number of layers of cells. In the slightly higher developed placenta' of ruminants the uterine epithelium is nearly all absorbed by the trophoblast, so that a more intimate union of the chorion with the maternal tissues results. In carnivora, further resorption of the maternal tissues by the trophoblast occurs, so that finally a complex labyrinth of chorionic villi and maternal vessels is formed. The maternal blood is separated from the chorionic epithelium by a single layer of endothelial cells, and the distance from maternal to fetal vessels is greatly diminished. Such a placenta has been termed placenta endotheliochorialis by Grosser (1909), from the fact that endothelium and chorion form the line of juncture between the two tissues. Lastly, there is the type of placenta common to Rodentia, Insectivora, Chiroptera, and Primates, in which even the endothelium of the maternal vesselsis obliterated by thetrophoblast,sothat the maternal blood flows into spaces bounded only by the fetal cells. Therefore, there remain between the maternal and the fetal blood-streams solely the thin layer of chorionic syncytium, the delicate stroma of the villi, and the endothelium of the embryonic capillaries. This type has been termed by Grosser placenta hemochorialis. The most careful study on the development of the placenta of the cat has been made by Duval (1894), who showed that the trophoblast invades the uterine wall, absorbing the uterine epithelium and the decidua, and forming (as it advances) a mass of syncytium and giant cells which gradually invests the maternal vessels. According to this observer, the syncytium and giant cells which form the bulk of the placenta consist principally of chorionic ectoderm. One finds, therefore, in the labyrinth of the mature placenta, numerous maternal capillaries, lined with endothelium and surrounded by masses of epithelium of fetal origin, beneath which lie the stroma of the villi and the embryonic blood-vessels. Duval's view that the syncytium and the giant cells are of ectodermal origin in the cat and dog has been generally accepted, but not without several noteworthy opponents. Thus Bonnet (1903) claims that a narrow zone of decidua separates the chorionic ectoderm from the maternal vessels. He believes, however, that late in pregnancy this strip of decidua becomes intimately fused with the chorionic ectoderm and forms a lamellar or syncytial layer in which the character of the individual elements is no longer recognizable. Another view is held by Sehoenfeld (1903), who believes that decidual cells contribute to the formation of the syncytium, but that the incorporation of these cells does not prevent the chorionic epithelium trom coming into direct contact with the maternal endothelium.
A similar difference of opinion exists regarding the origin of the epithelial masses which occur at the junction of the chorion and decidua basalis ("Umlagerungs-zone" of Strahl). Duval believes that "les lames basales" and "les arcades ectodermiques," as he calls these structures, are composed of fetal ectoderm, whereas Grosser believes they are largely decidual cells and hence should be designated symplasma.
Duval has pointed out that in the cat the chorionic ectoderm is composed of cells with fairly well defined boundaries, whereas in the dog it is largely syncytial. In the cat the chorionic ectoderm forms lamella? of epithelium about the maternal capillaries, and in it two types of cells may be discerned. One finds large, oval cells (termed by Duval central or giant cells) which lie close to the capillary endothelium and which have a single nucleus or often two or three large round nuclei and a vacuolated cytoplasm. These cells become more conspicuous as the placenta matures, and Duval believes that they arise from the less differentiated epithelium beneath them. Some have thought that they are of maternal origin and have designated them decidual or serotina cells.
Surrounding the giant cells are numerous smaller cells with single, more deeply
staining nuclei. These at first possess abundant cytoplasm and distinct cell outlines, but as gestation advances their cytoplasm shrinks so that they appear as
narrow sheaths around the capillaries and the prominent giant cells.
The placental labyrinth of the cat is stained very deeply by trypan-blue. The
dye appears in both types of cells composing the chorionic ectoderm just referred to
(figs. 14 and 15). In the giant cells it is particularly striking, filling the cytoplasm
with numerous bright blue granules. In the smaller epithelial cells constituting the
lamella- it is no less abundant but tends to appear in clumps. It is also observed in
the cytoplasm of many of the endothelial cells lining the maternal capillaries, but
none of it occurs in the fetal mesoderm or in the endothelium of the embryonic bloodvessels. In the masses of chorionic ectoderm at the junction of the chorion and
spongiosa very little trypan-blue is found. One receives the impression that this
is due solely to the fact that these cells are too remote from the maternal vessels in
which the dye is circulating to participate in its absorption. No dye is seen in the
epithelium of the remains of the uterine glands or in the detritus in their lumen.
Macrophages, heavily laden with trypan-blue, are present in large numbers in the
stroma surrounding the gland and in the uterine musculature.
The only other noteworthy deposit of dye is found in the chorionic ectoderm
along the borders of the placenta. In carnivora the maternal blood extravasates
into the space between the uterine epithelium and the chorion along the margins of
the placenta; hence this region in the dog has been termed the green border and
in the cat the brown border. The chorionic villi which dip into this mass of blood
are actively engaged in its resorption, and the epithelial cells covering them are seen
under the microscope to contain numerous entire and fragmented red blood-corpuscles, besides abundant pigment. It is thought that in this way the fetus obtains
the iron necessary for its growth. In the cat the chorionic villi in this region are
covered by a single layer of high columnar epithelial cells which have club-shaped
distal ends and nuclei situated close to the basement membrane. The distal, broad
end of the cell contains numerous whole or fragmented erythrocytes, whereas
deposits of finely granular brown pigment are seen in the middle and proximal
portion of the ceil. In the vitally stained animal numerous granules of trypan-blue
are distributed throughout the central and proximal portions of these same cells
(fig. 4) . The chorionic ectoderm over the poles of the fetus is unstained.
The observations made upon pregnant cats may be briefly summarized as
follows:
1 . Trypan-blue is incapable of passing from the maternal blood-stream through the placenta and membranes into the fetus or even into the amniotic or allantoic fluids.
2. Trypan-blue, injected into the maternal blood-stream, reaches the placenta, where it is absorbed and stored, principally by the chorionic ectoderm of the labyrinth. It is stored in the cytoplasm of the chorionic cells in the form of granules.
3. The endothelium winch lines the maternal capillaries of the placental labyrinth absorbs and stores the dye in the same manner as the chorionic ectoderm.
4. Trypan-blue is also deposited in the chorionic epithelium of the brown border.
Conclusions
It appears that inert colloids are normally not transmitted through the mammalian placenta or membranes and consequently can not enter the fetal circulation or the fluids surrounding the embryo. The outermost fetal membrane, whether it be of ectodermal origin as in carnivora, or endodermal as in rodents, invariably fails to transmit such substances.
The chorionic ectoderm of the placenta possesses, in all the animals investigated,
the ability to absorb and store foreign colloids. It is interesting to note that in
the fetus vital dyes are absorbed and stored in both ectoderm and endoderm,
germ layers which in the adult rarely (choriod plexus) participate in their storage.
Cells of all three germ layers may under suitable conditions stain vitally, and it
appears that the power to ingest benzidin dyes is not limited to any one class or
group of cells.
The deposition of vital dyes is greatest in those cells of the placenta and fetal membranes in which the metabolic exchange between mother and fetus is most active. It represents an accumulation of unassimilable material along the pathways between mother and fetus.
Hofbauer (1905) has pointed out the striking similarity between the placenta
and intestines in their power of absorption and excretion. A fundamental difference would seem to exist in their behavior toward trypan-blue. Whereas the dye
becomes aggregated in large amount in the chorionic cells, none of it is absorbed by
the intestinal mucosa. Why particles the size of trypan-blue should readily enter
and be stored in the chorionic but not the intestinal epithelium is a question which
requires further study.
Bibliography
Ahlfeld, F., 1885. Ueber die Bedeutung des Fruchtwassers als Nahrungsmittel fur die Frucht. Berichte und Arbeiten, vol. 2, p. 22. Leipzig.
Bondi, J., 1905. Zur Histologic des Amnioepithels. Zentralbl. f. Gyn., vol. 29, p. 1073-1076.
Bonnet, R., 1903. Beitrage zur Embryologie des Hundes. Zweite Fortsetzung. Anat. Hefte, vol. 20, p. 323. , 1907. Lehrbuch der Entwicklungsgeschichte. Berlin.
Clark, E. L. and E. R., 1918. On the relation of certain cells in the tadpole's tail toward vital dyes. Anat. Rec, vol. i5, p. 231.
Doderlein, A., 1890. Vergleichende Untersuchungen uber Fruchtwasser und foetalen Stoffwechsel. Arch. f. Gyn., vol. 37, p. 141.
Duval, M., 1894. Le placenta des carnassiers. Jour. del'anat. etde la physiol., vol.31, pp. 189,262,649.
Fehling, H., 1876. Zur Lehre vom Stoffwechsel zwischen Mutter und Kind. Arch. f. Gyn., vol. 9, p. 523.
Frank, 1901. Handbuch der tierarztlichen Geburtshiilfe. Berlin.
Goldmann, E. E., 1909. Die aussere und innere Sekretion des gesunden und kranken Organismus im Lichte der vitalen Fiirbung. Teil 1. Beitr. z. klin. Chir., vol. 64, p. 192.
- , 1912. Same title, Teil2. Ibid., vol. 7S, p. 1.
Goldmann, E. E. 1913. Experimentelle Untersuchungen uber die Function der Plex. choroid und der Hirnhaeute. Arch. f. klin. Chir., vol. 101, p. 735.
Grosser, O., 1909. Vergleich. Anat. u. Entwicklungsgeschichte d. Eihaute und Placenta. Wien u. Leipzig.
Hofbauer, P., 1905. Grundziige einer Biologie der menschlichen Placenta. Wien u. Leipzig.
Hoffmann, F. A., and P. Langerhans, 1867. Ueber den Verbleib des in die Circulation eingefiihrten Zinnobers. Virchow's Arch. f. path. Anat., vol. 48, p. 304.
Jacque, L., 1903-4. De la genese des liquides amniotiques et allatoidiens. Mem. couron. Acad. roy. de Beige, vol. 63, p. 1.
Jassinsky, P., 1867. Zur Lehre iiber die Struktur der Placenta. Virchow's Arch. f. path. Anat., vol. 40, p. 341.
Kehrer, F. A., 1867. Vergleichende Physiologie der GeburtdesMenschen und der Saugethiere. Beitr. z. verg. u. exper. Geburtsk. Giessen, Heft 2.
McClube, C. F. W., 1918. On the behavior of Bufo and Rana toward colloidal dyes of the acid azo group (trypan-blue and dye No. 161). Amer. Anat. Memoirs, No. 8.
Preyer, W., 1895. Spezielle Physiologie des Embryos. Leipzig.
Reitz, W., 1868. Ueber die passiven Wanderungen von Zinnoberkornchen durch den thierischen Organismus. Berichte der Wiener Akad. math. naturw. Classe, vol. 57 (2), p. 8.
Schlecht, H., 1007. Experimentelle Untersuchungen liber die Resorption unci die Ausscheidung des Lithionkarmins unter physiol. u. patiiol. Bedingungen. Ziegler's Beitrage, vol. 40, p. 312.
Schoenfeld, H., 1903. Contribution a I'etude de la fixation de l'ceuf des mammiferes dans la cavity uterine, et des premiers stades de la plaeentation. Arch, de biol., vol. 19, p. 701.
Weed LH. The development of the cerebro-spinal spaces in pig and in man. (1917) Contrib. Embryol., Carnegie Inst. Wash., 5, No. 14 .
Wislocki, G. B., 1916. The staining of amphibian larvae with benzidine dyes, with especial reference to the behavior of the lymphatic epithelium. Amer. Jour. Physiol., vol. 42, p. 12.V
Zuntz, N., 1885. Ueber die Quelle und Bedeutung des Fruchtwassers. Pfliiger's Arch., vol. 6, p. 548.
Zweifel, 1875. Untersuchungen iiber das Meconium Arch. f. Gyn., vol. 7, p. 474.
Explanation of Plates
Key to Legends
|
|
Plate 1
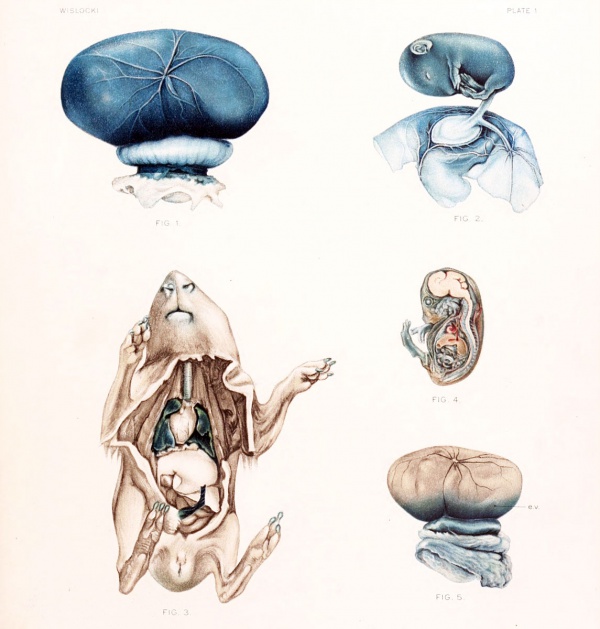
Fig. 1. Gross appearance of the placenta and fetal membranes from the uterus, 12 hours after injection of a vital stain into the amniotic cavity. Note the prominent omphalo-mesenteric vessels.
Fig. 2. Gross appearance of a guinea-pig fetus with the amnion opened 36 hours after injection of trypan-blue into the amniotic cavity. The omphalo-mesenteric vessels can be seen in the wall of the amniotic sac.
Fig. 3. Guinea-pig fetus, nearly full term, after injection of potassium ferrocyanide and iron ammonium citrate into the amniotic cavity. The fetus was killed 30 minutes after injection and immersed in acid formalin.
Notice the deep Prussian-blue stain in the lungs and trachea. The stomach contents also were blue. The kidneys are slightly stained.
Fig. 4. Sagittal section of guinea-pig, measuring 36 mm., 48 hours after the introduction of trypan-blue into the amniotic cavity. Note that the central nervous system remains unstained.
Fig. 5. Appearance of the placenta and membranes of a guinea-pig fetus after a single injection of trypan-blue into the maternal circulation. Notice the endodermal villi which are deeply stained, e. v. endodermal villi
Plate 2
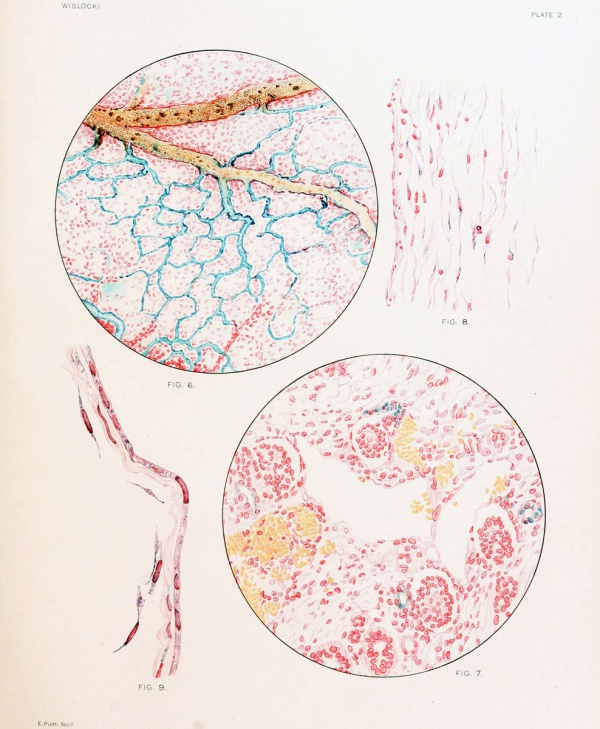
Fig. 6. Precipitation of Prussian blue in the omphalo-mesenteric vessels by immersion of the fetal membranes in acid formalin 30 minutes after the injection of 1 per cent potassium ferrocyanide and iron ammonium citrate into the amniotic cavity, demonstrating the escape of solutions from the amniotic sac into the fetal circulation.
Fig. 7. Kidney of guinea-pig fetus measuring 36 mm., showing trypan-blue in the convoluted tubules 72 hours after the injection of the dye into the amniotic cavity. Notice that the glomeruli are unstained.
Fig. 8. Section of the umbilical cord of a guinea-pig fetus measuring 42 mm., showing vitally stained mucoid connective-tissue cells, 24 hours after injection of trypan-blue into amniotic cavity. V., lumen of umbilical vein.
Fig. 9. Section of amniotic membrane of guinea-pig fetus measuring 41 mm., showing absorption of trypan-blue, 24 hours after injection of the dye into the amniotic cavity.
Plate 3
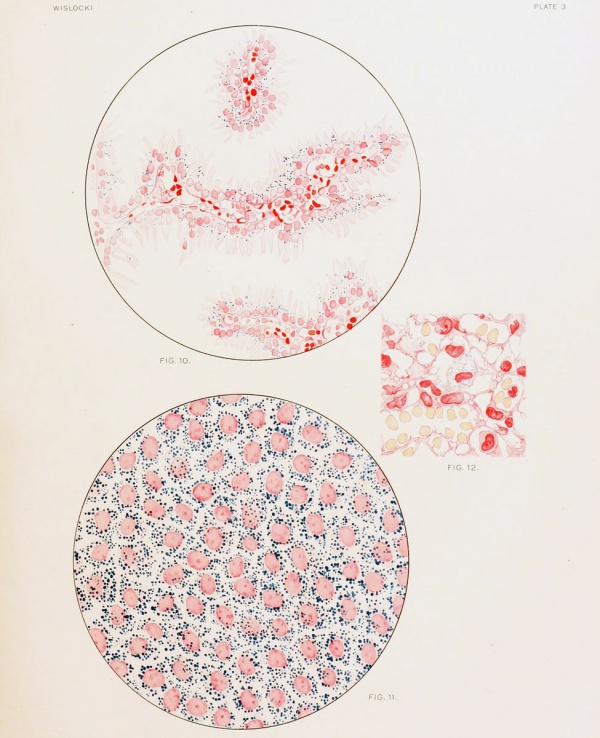
Fig. 10. Section of the endodermal villi, the modified remains of the "inverted yolk-sac," heavily laden with pigment after repeated injection of a colloidal dye into the maternal circulation.
Fig. 11. Wall of the "inverted yolk-sac," showing the endodermal cells as they appear 24 hours after an injection of trypan-blue into the amniotic cavity. Fresh membrane slightly eounterstained with alum carmine.
Fig. 12. Section through placenta of guinea-pig fetus measuring 40 mm., showing a vitally stained macrophage 26 hours after injection of trypan-blue into the amniotic cavity'.
Plate 4
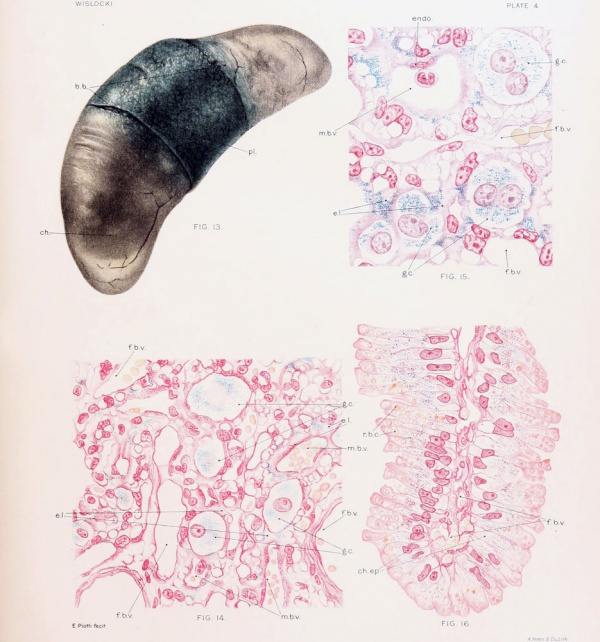
Fig. 13. Cat fetus, measuring 6.8 cm., surrounded by unruptured membranes, showing the coloration of the placenta and chorion after repeated injection of trypan-blue into the maternal circulation. Note that the chorion over the poles of the fetus is unstained. The allantoic and amniotic fluids do not contain a trace of dye and the fetus is unstained.
Fig. 14. Placenta of cat, nearly full term, after repeated injection of trypan-blue into the maternal blood stream, showing the distribution of dye in the chorionic epithelium.
Fig. 15. Placenta of a vitally stained cat, nearly full term, showing several multimiclear giant cells of the chorionic ectoderm filled with particles of trypan-blue.
Fig. 16. Section of the "brown border" of the placenta of a cat, nearly full term, showing the absorption of erythrocytes and of trypan-blue by the chorionic epithelium.
Cite this page: Hill, M.A. (2024, April 24) Embryology Book - Contributions to Embryology Carnegie Institution No.51. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.51
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G