Book - Contributions to Embryology Carnegie Institution No.14: Difference between revisions
(Created page with " http://www.archive.org/details/contributionstoe04carn CONTRIBUTIONS TO EMBRYOLOGY Volume IV, Xos. 10, 11. 12, 13 Published by the Carnegie Ixstitltion of Washingt...") |
mNo edit summary |
||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Header}} | |||
{{Ref-Weed1917}} | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! Online Editor | |||
|- | |||
| [[File:Mark_Hill.jpg|90px|left]] This historic 1917 paper by Weed describes the development of the cerebro-spinal spaces. | |||
<br> | |||
<br> | |||
'''Modern Notes:''' | |||
<br> | |||
{{Neural Links}} | |||
|} | |||
{{Historic Disclaimer}} | |||
=The Development of the Cerebro-Spinal Spaces in Pig and in Man= | |||
[[Book_-_Contributions_to_Embryology|Contributions to Embryology]] | |||
By Lewis H. Weed | |||
Volume V, No. 14 | |||
==I. Introductory== | |||
Probably no field in embryology has been less explored than that relating to the meninges. Our knowledge of the transformation of the perimedulla | |||
ry mesenchyme into the three fully developed membranes about the cerebro-spinal axis has been largely of a crude sort, with gross generalities based on inexact or incomplete evidence. The present work was undertaken in the hope that by a study of the various stages in the development of the cerebro-spinal spaces there might be gained some knowledge which would afford a basis for a conception of this dynamic metamorphosis. | |||
Many of the problems centering around the development of the meningeal spaces have recently been expounded by Cushing^^) . * Not only do we lack knowledge as to the method of differentiation of the primitive mesenchyme, but we know little about the establishment of the circulation of the cerebro-spinal fluid. When do the chorioid plexuses begin to secrete? When does the venous absorption of the fluid take place? When does the perivascular system begin to remove waste products from the cerebral tissue? And also, what factors play a part in the formation of the subarachnoid and subdural spaces? | |||
These questions, some of which it is hoped the present study will answer, relate to the field of physiological anatomy. Consideration of the subject, however, serves to convince one that they must be investigated coincidently with the stages of morphological differentiation; for it may readily be conceived that the physiological use of the meningeal spaces may precede any morphological differentiation of the three membranes, nor indeed is it unlikely that one of the active causative factors in the metamorphosis concerns this filling of the mesenchyme about the nervous system with fluid. | |||
This study, therefore, has been anatomical, but with a broader scope than purely morphological studies would have afforded. Not only has it dealt with the morphological differentiations about the nervous system, but throughout the investigation the relationship of these structures to the possible presence of cerebrospinal fluid has been considered. As the problem developed it was projected more and more into the difficult realm of "tissue spaces." Interest in these spaces largely concerned their physiology, but many points of correspondence between structure and function were found. | |||
In some measure this work is a development of an earher study of some of the anatomical and physiological problems of the cerebro-spinal fluid, carried out in the laboratory of Dr. Harvej^ Cushing at the Harvard Medical School. | |||
* The figures in parentheses refer to the bibliography at the end of this paper. | |||
==II. Review of Literature== | |||
In order fully to understand the problems which confront one in the study of the embr3'onic cerebro-spinal spaces, a comprehension of the stage to which investigations have brought our knowledge of these fluid-pathways in the adult is necessar}'. It is with this purpose that the adult relationships are here considered. The inclusion of this material may be pardoned, for it will be seen that unanimity of opinion has bj' no means existed in regard to any of the problems concerned in the circulation of the cerebro-spinal fluid. | |||
Modern anatomical knowledge of the meninges dates from the work of Axel Key and Gustav Retzius'29). These Swedish investigators, in their excellent monograph published in 1875, first conclusively demonstrated the anatomical continuity of the spinal and cerebral subarachnoid spaces. But for years after their publication appeared, a physiological continuity between the subdural and subarachnoid spaces was argued for bj' many observers, notably by HilK^*). Gradually, however, workers in this field have reached the opinion that the subarachnoid spaces (the interrupted but continuous channels between arachnoidea and pia) are functionally the channels for the cerebro-spinal fluid. Between the intra-leptomeningeal and the subdural spaces no anatomical connection exists; physiologically there may be some mode of fluid-passage. Thus Hill' 24) states that either by filtration or through actual foramina fluid passes readilj- from one space to the other. Quincke '*^, from observations on animals, somewhat similarly premised a connection between the two spaces, but only in the direction from subdural to subarachnoid. His experiments, based on the results of the injection of cinnabar granules, are open to criticism as indicating a normal passage-way for the fluid; for, as he has recorded, an intense phagocytosis of practically all of his granules occurred. More modern conceptions of the subdural space treat it as a space anatomically closed, lined externally by a polygonal mesothelium. Less error is introduced if it be regarded as analogous in many respects to well-known serous cavities rather than as an essential portion of the pathway for the cerebro-spinal fluid. | |||
The question of the absorption or escape of cerebro-spinal fluid from the subarachnoid space has claimed the attention of many workers. Since the original conception that the meningeal coverings were actually serous cavities, anatomical investigations have furnished many new views. Key and Retzius, by spinal subarachnoid injection of gelatine masses colored with Berlin blue, demonstrated an apparent passage of the injection fluid into the great cerebral venous sinuses through the Pacchionian granulations (die Arachnoidzotten). Their observations were made on a cadaver and the injections carried out under fairly low pressures (about 60 mm. of mercury). A lesser drainage of the fluid into the lymphatics was also shown. | |||
Since the view advanced by Key and Retzius of the absorption of cerebrospinal fluid, the general trend has been away from the idea of an absorption into the venous sinuses. Quincke's observations, made on lower animals after the subarachnoid introduction of cinnabar granules, really offer some substantiation of this theory, but the failure to find the great Pacchionian granulations in infants and in the lower animals caused many workers to reject utterly the conception of the Pacchionian granulations as the functionally active mechanism for the fluid escape. | |||
Physiological evidence, however, advanced by Hill^24) fj-Q^i intraspinous injection of methylene blue, indicated that the major escape of the cerebro-spinal fluid was into the venous sinuses of the dura, while a slow and minor absorption took place along the lymphatic channels. Ziegler(57j^ with potassium ferrocyanide introduced into the cerebro-spinal space, Ukewise found that the venous absorption was much greater and more rapid than the lymphatic. Reiner and Schnitzler with the same agent detected the ferrocyanide in the jugular blood-stream after injection. With oUve oil these investigators found a similar venous absorption, but with a slowing of the venous blood-stream. Lewandowsky also using ferrocyanide. found this salt in the urine within 30 minutes after its subarachnoid injection. Spina 52'^ from observations on freshly killed and hving animals, presented somewhat similar evidence of a major venous and lesser lymphatic absorption. Gushing suggested a valve-Uke mechanism of escape of the fluid, his hypothesis being based on the findings after the introduction of mercurj' into the meningeal spaces. | |||
Several theories concerning the absorption of cerebro-spinal fluid into the bloodvascular system have more recenth' been offered. Mott '**', from a studj' of dilated perivascular and permeuronal spaces, has advanced the idea of fluid-escape by way of the perivascular system into the cerebral capillaries. Dandy and Blackfan^'"^, from an analysis of their evidence, consider that the chief drainage of the fluid is into the capillaries of the pia-arachnoid. Opposed to this conception of a major drainage of cerebro-spinal fluid into the l)lood-vascular system is the view championed by Cathelin'^', that the Ij'mphatic drainage is the chief method of fluid-escape. Cathelin's contention of a veritable circulation of the fluid has not received support from other workers. | |||
Thus it will be seen that since the work of Key and Retzius the trend of opinion has been away from the view that the Pacchionian granulations carry the cerebrospmal fluid into the venous sinuses. | |||
In the earlier investigation carried out in the Harvard Medical School the problems of this fluid absorption were attacked in a somewhat different manner than by previous workers. True solutions of potassium ferrocyanide and ironammonium citrate, such as have been used in the present investigation, were injected into the spinal subarachnoid space under pressures but shghtly above the normal. The animals (dogs, cats, and monkej-s) were kept imder anesthesia during the period of injection, which was usually continued for several hours. Complete filling of the subarachnoid channels was secured by this technique, provided the injections were continued for a sufficient length of time. At the conclusion of the experiment the foreign solution was precipitated in situ and blocks were carried through for histological purposes. | |||
Many of the anatomical findings in this work carried out as outlined are of interest in the present problem. The complete correspondence of the spinal and cerebral subarachnoid spaces as demonstrated by Key and Retzius was amply verified. The normal return of the cerebro-spinal fluid to the general circulation by way of the arachnoidal villi into the great dural sinuses was demonstrated. These viUi are projections of the arachnoidea through the dural wall, prolonged directly beneath the vascular endothelium of the venous sinuses. Furthermore, columns of arachnoid cells were found, normally affording fluid channels in the dura. In addition to the major escape of cerebro-spinal fluid into the sinuses a lesser drainage was alsi) demonstrated, slower than the primary drainage, out along certain of the emergent nerves into the lymjihatic system. No evidence whatsoever was obtained in support of any of the theories of a drainage of cerebro-spinal fluid into either the leptomeningeal or cerebral capillaries, nor could an anatomical valve-like mechanism along the great sagittal sinus be demonstrated. The process of escape of cerebrospinal fluid from the arachnoid villus unto the great sinus appeared to be a simple one of filtration or of diffusion. | |||
Another of the problems concerning cerebro-spinal fluid, which has been of interest to anatomists and phj'siologists, is the source of the fluid. Haller^^D and Magendie'^^ to whom the greatest credit for work on this subject must be given, believed it to be the product of the leptomeninges. Faivre^^^} {^ 1853 and Luschka^''*) in 1855 were the first to suggest the chorioid plexuses as the elaborators of this circumambient medium. Since then the view has been generall}- accepted that these villous structures do give origin to the fluid, but the early evidence was based wholly on the glandular character of the plexus. Cappelletti^^) and Pettit and Girardi) offered more definite proof of this relationship by the introduction of pharmacological agents which affected the rate of production of the fluid. These latter authors recorded definite histological changes in the cells of the plexus when influenced by these drugs, indicating, in conjunction with the changed rate of production of the fluid, an undoubted relationship of the chorioid plexus to the fluid elaboration. Since these early investigations many observers — Findlay, Meek(37)^ Mott^'*^', Pellizzi''*2), Hworostuchin'26)^ and others — have studied the histology of the chorioid plexus with reference to its function as an elaborator of the cerebro-spinal fluid. | |||
In addition to the elaboration of the fluid by the chorioid plexuses, increments are furnished bj' the nervous tissue itself. This elimination from the nervous system occurs bj' waj' of the j^erivascular spaces. In the previous work referred to'55j it was found possible to inject the entire perivascular system by continuing a physiological injection of the spinal subarachnoid space, and subsequently causing an extreme cerebral anemia. By this procedure an injection of the system to its termination about the cerebral capillaries and nerve-cells could be secured. From this and other evidence the view was advanced that the cerebro-spinal fluid was derived from a dual source — in part from the perivascular system and in greater part from the chorioid plexuses. This view had already been advanced, but on rather insufficient grounds, by Mestrezat'^s* and by Plaut, Rehm, and Schottmuller (, Recently Frazier) has signified his acceptance of this conception of the source of the fluid. | |||
Such, then, is the basis for our present understanding of the meninges, in regard to their characteristic morphology and particularly their functional relationship to the cerebro-spinal fluid. Without a consideration of the circumambient fluid morphological studies of these membranes would be incomplete, for in order to understand the meninges knowledge concerning the cerebro-spinal fluid is necessary. | |||
===The Comparative Anatomy of the Meninges=== | |||
Sterzi's has pubUshed a comprehensive report of the comparative anatomy of the spinal meninges. From his studies he has advanced hypotheses, supported by observations on a Hmited number of fetuses, regarding the development of the human meninges. On account of the interest of this subject in relation to the present discussion a brief summarj' of Sterzi's work will be here included. | |||
e | |||
In the acrania there is no special envelope of the central nervous system, but rather a fibrous sheath corresponding to the meninges of higher forms. This fibrous sheath is largely made up of circular fibers, except in the median ventral line, where there occurs a ventral hgament of longitudinal fibers. In cyclostomes, however, there is found a single "primitive meninx" — vascular and composed of white and elastic fibrils coursing in a longitudinal direction. Some of these fibrils traverse the perimeningeal spaces (filled with star-like cells, with some fatty tissue) and are attached to the inner surface of the vertebrae. This same general plan of a single "primitive meninx" is hkewise found in fishes (elasmobranchs, teleosts, etc.); the membrane here is often pigmented and follows closely the external architecture of the spinal cord. The perimeningeal space is filled by mucus in elasmobranchs, but in teleosts this is replaced by fat. For the most part there are found dorsal and ventral ligaments and two lateral ligaments. | |||
The next stage in the development of a more complete form of spinal covering is found in the urodele amphibia. A "primitive meninx," formed of two layers, often artificially separated from each other, replaces the simpler meninx of C5'clostomes and fishes. Of the two layers in this membrane the external is thin and free from pigment; the inner, strongly pigmented, adheres to the spinal cord. The meninx is perforated by the denticulate Ugaments. | |||
In amphibia (Anura) Sterzi found the first evidence of a "secondary meninx," corresponding to the pia-arachnoid. Surrounding this membrane, but separated from it, is the dura, thin and transparent; between the two meninges is the intradural (subdural) space. The dura lies in the peridural space. The spinal prolongations of the endohonphatic canals he in the dorsal part of the peridural space. Both the dura and the "secondarj^ menmx" continue outward along the roots of the spinal nerves and along the filum terminale. Embryologically the perimedullary mesenchyme is differentiated into these two meninges in the Anura. | |||
This arrangement of the two meninges in Anura is followed out in reptiles. The dura, thin as in the amphibia, is covered by endothehum and is vascular. The "secondary meninx" possesses laterally the denticulated hgaments and ventrally the ventral hgament. Both the peridural and intradural spaces are very small. | |||
Likewise in birds Sterzi was able to differentiate only two meninges — the dura and the "secondary meuinx." These membranes are quite similar to those of reptiles. The " secondary meninx " has acquired three layers — an outer endothelial covering, a middle vascular layer, and an inner membrane closely adhering to the cord. This is a distinct approach to the three meninges of mammals. An intradural (subdural) space covered by endothelium can be easily made out. The development of these avian meninges concerns a differentiation of the perimedullary mesenchyme. | |||
The arachnoid, according to Sterzi, first appears as a definite membrane in mammals (marsupials and placentals). In marsupials this arachnoid has become well differentiated and the pia mater possesses denticulated and ventral Ugaments. A transformation of the extradural portion of the denticulated ligaments unites the dura to the endorachis. In perissodactyla the differentiation of the three meninges (particularly of the arachnoid) is incomplete. The arachnoid is separated from the pia mater by a pecuUar tissue wliich contains numerous lymphatic lakes, forming the intra-arachnoid spaces. No intradural (subdural) space is apparent, due to the approximation of dura and arachnoid. The subdural space is clothed by endothelial cells; these can not be made out in the intra-arachnoid spaces. The dura is surrounded by a fatty pad. | |||
According to Sterzi the augmentation of the intra-arachnoid (subarachnoid) space is the distinguishing characteristic of the meninges of carnivora. This increase takes place at the expense of the peridural space. | |||
As Sterzi developed the knowledge of the comparative anatomy of the lower forms — of the transition from the primitive meninx of the cyclostomes to the three membranes of mammals — the possible correlation of this analogj'^ to the embryological development in mammals became apparent. He extended his observations to human beings and to human fetuses. His findings will be detailed in the following section. | |||
Farrar'i, in a short discussion of the development of the meninges of the chick, finds in early stages three laminse about the spinal cord, "the middle one of which alone still presents the primitive features of the mesoblastic-sheath." The inner layer, close to the medullary tissue, is highly vascular; in the outer zone "the connective-tissue elements arc assuming elongated forms and crowding together with long axes parallel, giving a very close mesh with long but extremely narrow spaces, in contradistinction to the loose irregular reticulum of the pia-arachnoid." The outer lamina becomes dura mater, while the inner two zones are considered together as the embryonic pia-arachnoid. Farrar defines the pia-arachnoid as developmentally a single membrane consisting of a loose reticulum, at the outer and inner borders of which limiting membranes are formed. | |||
===Literature On The Development Of The Mammalian Meningeal Spaces=== | |||
The development of the meningeal spaces in mammals has not been studied extensivel}', and the literature in regard to it is quite meager. Only a very few workers have touched upon the subject except casually. Reford"*^', working in the Anatomical Laboratory of the Johns Hopkins University, studied the development of these spaces bj^ the method of injection with india ink. His work unfortunatelj' has never been published, but it has been rather extensively referred to bj' Sabin *3' in 1912 and by Cushing'^^ in 1914. Their sununaries of this work are here included. Miss Sabin thus speaks of it: | |||
"In a study of the arachnoid made bj' the injection method in the Anatomical Laboratory of the Johns Hopkins University by L. L. Reford, and as j^et unpublished, it has been shown that the thinning out of the mesenchjine around the central nervous sj-stem is not haphazard, but that injections of the same stage give the same pattern, and that the form of the arachnoid space changes as the brain develops. That is to say, the arachnoid space has as definite a form as the coelom, and it never connects with the IjTnphatics." | |||
Cushing() gives the following summary: | |||
:"It was thought that an investigation of the cerebro-spinal spaces in the embryo would most hkely shed Ught on the subject, and some unpublished studies in this direction were undertaken in 1904 and 1905 by Lewis L. Reford in ^Mall's laboratory' in Baltimore. In living pig embryos of various stages low spiaal india-ink injections were made either into the wide central canal or into the subarachnoid space, and the embryos were subsequently cleared. It appeared from the course taken by the injection mass that the full development of the spinal arachnoid preceded that of the intracranial spaces, the impression being gained that the separation of the primitive meninx into its layers occurred later over the cerebral vertex than in the basilar portion of the chamber. Still, I never felt quit« convinced that the failure of injection of the meninges over the surface of the hemispheres in many of Reford's specimens was not due to the floating up of the brain against its envelopes by the introduction of the injection mass from below. Howe\'er this may be, it was nevertheless apparent that a venous injection of the body of the embryo was often produced, and the impression was gained that a communication existed between the basal subarachnoid spaces and the precursors of the sinusoidal veins of the cranial chamber which empty into the jugulars. If due to an artifact from a vascular ruptm-e, at all events the conununication always occurred at the same point. Reford, moreover, in agreement with Cruveilhier, Reichert, and KolUker, came to doubt the existence of the foraminal opening described by Magendie, beUeving that the opening was an artifact and that the fluid escaped by seepage through a persistent membrane." | |||
It is regrettable that Reford's study has not been pubUshed, as it represents the only attempt to solve the problenas of the development of the cerebro-spinal space by the method of injection. As stated in subsequent sections of this communication, his apparent failure to control pressures of injection and to use only granular suspensions is unfortunate. | |||
In a study of the development of the blood-vessels of the human brain, (Mall) noted the ease with which an extravasation into the embryonic arachnoid spaces could be brought about by increasing the pressure in a venous injection. In a specimen of 46 mm. an arterial injection with aqueous prussian-blue resulted in a complete subarachnoid spread, due to rupture of the vessels as they perforated the nervous tissue. In general, it was found that this rule held: an arterial extravasation always took place from the perforating capillaries, while a similar venous rupture occurred in the veins themselves. | |||
Mall made similar observations on living pig embryos from 30 to 80 mm. in length, with analogous results. But when, in these embryos, the arachnoid spaces were completely filled by an intraventricular injection of india ink, no passage of the granular injection into the veins or sinuses occurred. The ventricular injection flowed into the extraventricular spaces "through the medial opening of the fourth ventricle." From the spinal cord the ink extended for a short distance along the main trunks of the spinal nerves. In the larger embryos (above 50 mm.) the ink usually gushed from the mouth, reaching it by way of the Eustachian tube. Using, in the pig embryo, the heart as the mechanism for injecting the ink, extravasation from the cerebral vessels in the arachnoid spaces occurred. | |||
In one human specimen of 90 mm., Mall found both the arachnoid spaces and the cerebral ventricles filled with india ink after an arterial injection of that suspension. He states: "The injection passes through the medial opening into the fourth ventricle (Magendie), and apparently the ventricles are injected through this opening from the arachnoid." | |||
" | To His'25) and to Kolliker belongs the credit of first having established on a firm basis the development of all the meninges in man from mesenchyme. This perimedullay' layer of mesenchyme Salvi'^o^ called the "primitive meninx" — a term now used extensively in comparative anatomy. The primitive meninx divides into two layers, the outer forming the dura and the inner the pia-arachnoid. Sterzi(53), working on the development of the human spinal meninges, advanced a view similar to that of KoUiker. The perimedullary mesenchjTne (the "primitive meninx") divides into two portions, one hugging the inner surfaces of the vertebra? and the other adhering to the cord. This inner layer of the perimedullary mesenchyme, according to Sterzi, should properly be termed the "primitive meninx," as it divides subsequently into dura and the "secondary meninx," which in turn forms both arachnoid and pia. The denticulate hgaments develop in the "primitive meninx." The dura and arachnoid in human embryos are modeled up to a certain point on the cord; then, with the augmentation of the subarachnoid space, they follow the outline of the vertebral canal. | ||
His'25) has given information regarding the development of the meninges, with particular reference to the formation of the subarachnoid space. He affirms the mesenchymal origin of all of the cerebro-spinal membranes. His describes the first differentiation of mesenchyme to form the meninges as consisting of two zones of condensation, the outer being closely associated with the developing perichondrium of the vertebral column and the inner facing upon the cord. Between these two zones of condensation the subarachnoid space develops, posterior and anterior spaces first appearing, with later fusion laterally. These appearances were met with in chicks of 10 to 12 days' incubation. Quite soon after this process of spacedevelopment a separation occurs which gives rise to a complete subarachnoid space. Later the splitting-off of dura from the vertebral periosteum takes place. | |||
==III. Methods Of Investigation== | |||
In the study of any problem dealing with the development of fluid-spaces within the body, the method of investigation must of necessity be such as to offer exceptional opportunities for control. In the present work several well-known and generally accepted anatomical procedures were naturally suggested, such as injection of the spaces about the central nervous system, reconstruction from serial sections, or merely study of the various stages by means of serial sections. | |||
It was ascertained early in the investigation that by injection and serial sections without reconstruction the necessary stages in the process of meningeal differentiation could be estabUshed. In regard actually to the physiological aspects of the problem more reUance was placed on the results of injection than on any histological differentiation, for, as explained above, considerations of the pathway and of the flow of the cerebro-spinal fluid were deemed most important. No method of injection, however, holds out much promise in such a problem unless it can be applied, under conditions approximating the normal, wnthin the spaces about the nervous system. The greatest objection to reliance upon injections in this problem is in relation to pressures. From the very nature of the case it wall be reahzed that any ordinary injection into the embryonic central canal or perispinal space must result in an extraordinary increase in the normal tension of the fluid. This objection applies to any method employed, whether that of a simple syringe and needle, the glass tube and bulb devised by Knower, or a glass capUlary-tube contrivance. | |||
The erroneous conclusions drawTi by investigators from the emploj^ment of excessive pressures of injection are nowhere more strikingly illustrated than in studies of the circulation of the cerebro-spinal fluid. Many such examples were recently brought forward in a critical review ''5' published in connection with a study of the fluid. In the embryo, with structures and membranes still of very little tensile strength, the consequences of a disregard for the pressures of injection are even more disastrous. | |||
A second criterion for the study of fluid-pathways in the body is necessarily the type of injection mass. Not only should attention be paid to the pressures involved, but the peculiarities of the particular body-fluid concerned must be considered. Adopting for this work on the embryo the same standards followed in the previous investigation on the adult, true solutions were used in place of the customary granular suspensions. Emulsions and viscous solutions were not emploj'ed because of their obvious disadvantages in studying the passage through membranes. India ink and process black (in which carbon granules are the particulate matter) were also used, but only for comparison with the standard true solution, as the likelihood of the insoluble granules being phagocyted wathin the period of experimentation or of being caught mechanicalh'^ in tissue meshes appeared a priori to be too great. | |||
In any study of fluid-pathways in the body, not only must the injection fluid be a true solution, but it must also be one which is not attracted to particular cells (as with many stains). Likewise, colloid stains (such as the benzidene group) could not be employed, because of the fact that certain cells (macrophages, as described by Evans’s phagocyte the small colloidal particles. In addition, the true solution must be readily precipitated as an insoluble salt, capable of remainhig unchanged in histological technique. After trying many salts in long-continued injections into the adult cerebro-spmal spaces, it was found that solutions of potassium ferrocyanide and iron-ammonium citrate in equal parts were admirably adaj^ted to the purposes of the experiment. By the addition of a mineral acid (preferably hydrochloric) ferric ferrocyanide could be precipitated. This prussian-blue is insoluble in the routine technique and is readily identified in sections. After mounting in damar or balsam the blue granules can be observed unchanged for several months, but after a year there is some deterioration in the specimen, due to a conversion of the blue into indefinite greens. | |||
Text-figure 1. — .Schematic sketch of mechanism used (or replacing ventricular and spinal fluid of an embno with a foreien solution or suspension. The system is here shown in balance, the difference in fluid-level in reservoirs and needles representing the hydrostatic pressure necessary' to overcome the capillary resistance of tubes and needles. The stands holding the injecting needles may be moved about without altering the balance of the system. As one reserv'oir is raised, the other is lowered in a corresponding degree. | |||
In regard to these two major factors in the employment of injections (pressure and true solution) it was found necessary to devise a method of experimentation which would satisfy the requirements of the problem. Solutions of the ferrocyanide and of the citrate were non-toxic within the central nervous sy.stem and afforded an excellent histological means for following the fluid-pathways. It was hoped at first that a simple "replacement" type of injection might be employed, as in the adult animals. In this procedure a given amount of fluid was withdrawn from the subarachnoid spaces and immediately replaced by an equal quantity of the injection fluid. The method was successfully tried on fetal cats of consideralile size, but was impracticable on small embryos. After such a replacement the animals were allowed to Uve for varying periods of time (up to 3 hours) and then killed. | |||
It was soon ascertained that the essential circulation of the cerebro-spinal fluid was established in pig cml^ryos of less than 30 mm. in crown-rump measurement. Hence the ordinary method of replacement had to be discarded for some more delicate system. With the realization that a simultaneous withdrawal and introduction in a living embryo would be far preferable to a two-stage procedure, the extremely simple apparatus pictured in text-figures 1 and 2 was employed. This device consists of two glass tubes of uniform and like bores, suspended from above by a string running over a pulley. To the tapering lower ends of these reservoirs are attached rubber tubes which connect the reservoirs to two needles. These needles are held at the same level by two metal brackets which can be moved at will on a level glass plate. | |||
Text-figure 2. — Diagrammatic representation of the method of rcplacinR the cerebro-spinal fluid in a living embryo. The spinal needle is inserted into the central canal of the spinal cord, while the cerebral needle is introduced into one of the cerebral ventricles. The canal of the spinal cord and the cerebral ventricles are represented by the interrupted lines. The foreign fluids are introduced by the spinal needle and withdrawn by the cranial. | |||
The apparatus is employed as follows : Both tubular reservoirs are filled up to the point where the fluid is just ready to fall from the needle in a drop. This point is easily obtained by fiUing the reservoirs slightly in excess and allowing this excess fluid to run off from the needle. With the system thus in balance the needles he in the same horizontal plane and can be moved without altering the balance of the solutions. The injection is made by inserting one needle into the central canal of the spinal cord and the other into one of the lateral ventricles; then as the reservoir connecting with the spinal needle is raised the other is lowered, so that an amount of fluid equal to that introduced into the spinal canal is withdrawn from the cerebral ventricles. In this way the whole contents of the cerebral ventricles and central canal of the spinal cord can be slowlj' withdrawn without increasing the pressure in the central nervous sj^stem. The initial pressure necessary to secure this flow is only that required to overcome the capillary resistance of the medullarj'-canal system. In practically all cases this can be accomplished by using a positive pressure of less than 60 mm. of water (associated with a negative pressure of the same degree) . | |||
In the present study the above procedure was the routine method of injection employed. Pig embryos, brought from the abattoir, contained in the uterus, were found to be wholly satisfactory material. If not permitted to cool excessively in transit the embryos lived for at least two hours in a 38° incubator. On being received at the laboratory a section of the uterine wall contaming the placenta was excised, with the embryo left connected by the umbilical structures. As soon as the technical preparations for injection were completed the amnion was opened and the embryo placed upon a padded block at the proper level. The first needle was then inserted into the easily discernible central canal of the spinal cord and the second into the left cerebral ventricle or into the mesencephalic ventricle. By elevation of the reservoir connected with the first needle the cerebro-spmal fluid was replaced by the injection solution. As soon as the replacement was complete the needles were withdrawal and the embryo and its uterine portion replaced in the incubator. The heart of the embryo could be easily obser\^ed in the smaller forms and served as the index of a continued circulation. | |||
The incubation of the embryos was continued for varying periods of time, but it was soon ascertained that a period of over 30 minutes generally resulted in a complete spread of the injection solution. For comparison the period of incubation was lengthened and shortened, but the best results were usually obtained with a 45-minute incubation after the replacement. | |||
Injections of the necessary true solutions were made, in the routine experiment, with a 1 per cent concentration of potassium ferrocyanide and iron-ammonium citrate in distilled water. By a 1 per cent solution is meant a salt concentration of this amount (potassium ferrocyanide, 0.5 gm.; iron-ammonium citrate, 0.5 gm.; water, 100 c.c). The resultant true solution should be practically isotonic with the body-fluids. In this way any injurious consequences due to hypertonic or hypotonic solutions were apparently overcome. The factors of osmosis and diffusion also had to be considered in this connection. | |||
Other concentrations of the so-called "ferrocyanide mixture" were used, but only for the sake of comparison or for the purpose of investigating some particular phase of the problem. The results obtained by the use of these concentrations were not relied upon as affording standards for the normal pathway of the cerebro-spinal fluid. | |||
In addition to the replacement type of injection, many observations were carried out on pig embryos, with a simple syringe-injection of the ferrocyanide solution into the central canal of the spinal cord or into the cerebral ventricles. It proved to be a very simple matter to regulate the pressures by this method, and three arbitrary standards (mild, moderate, and strong) were found to be of value in a comparison of the extent of the spread obtained by replacement and by injection. | |||
The prussian-blue reaction (formation of ferric ferrocyanide) was obtained in these experiments by fixing the whole embryo in an agent containing hydrochloric acid. For histological study the best results were obtained by immersing the specimen from 1 to 10 minutes in a 10 per cent formaldehyde solution containing 1 per cent hydrochloric acid. After this primary procedure, during which the ferrocyanide was precipitated, the embryo was transferred to Bouin's fluid (saturated aqueous picric acid, 75; formaldehyde 40 per cent, 20; glacial acetic acid, 5). The specimens were allowed to fix over night and were then dehydrated in graded alcohols. From 30 per cent alcohol, use was made of 4 per cent changes up to 60 per cent; and from this point to absolute the changes were by 2 per cent gradations. | |||
In addition to the technique outlined above, Carnoy's solution and 10 per cent formol were employed. The Carnoy fluid, containing acid (absolute alcohol, 60; chloroform, 30; glacial acetic acid, 10; hydrochloric acid, 1) proved to be of particular service in the study of specimens cleared by the Spalteholz method; histologically, however, it has not been as valuable as Bouin's fluid. | |||
of | |||
Besides the ferrocyanide solution, two other injection masses were constantly employed. Solutions of silver nitrate in concentrations of 0.5 per cent were injected into the central canal of the spinal cord and into the cerebral ventricles. This method, with reduction of the silver salt in the sunlight, gives very pleasing preparations. It is, however, subject to obvious limitations. The intraspinous toxicity of the silver, together with its action as a precipitant of albuminous substances, renders its use unsatisfactory in replacement experiments. Furthermore, it reacts apparently with any protein tissue, irrespective of the true function of that tissue (as, for example, its coagulation of the lining ependyma of the ventricles). | |||
India ink, the other substance employed, is of extreme value in anatomical studies. Because of the suspension of carbon granules it possesses the disadvantages already commented upon for the study of any true pathway of fluid. It has been of service, however, in the present work in showing marked differences in spread from that of true solutions and in furnishing information in regard to fluid passage through a membrane. | |||
This investigation has been carried out on the basic idea of correlating the physiological spread of the embryonic cerebro-spinal fluid with the gradual transformation of the perimedullary mesenchyme into the three fully formed meninges. This has necessitated a histological study of the embryo. Pigs for the most part were the animals used, but the findings have all been verified by a study of the same regions in the human embryos in possession of the [[Carnegie Collection|Carnegie Institution of Washington]]. In addition, certain structural characters have likewise been identified in sections of chick, rabbit, and cat embryos. | |||
It was early apparent that the material to be of value must be free from any great shrinkage about the central nervous system. Comparative freedom from this artifact was obtained by fixing the embryo alive in Bouin's fluid and dehydrating by 2 and 4 per cent gradations of alcohol. The material was chiefly cut in paraffin after being embedded by means of xjdol. | |||
The methods of investigation outhned in the foregoing paragraphs have been followed throughout the major portion of the work. In many minor instances other procedures not commented upon have been employed ; these wiU be detailed in appropriate subdivisions of this paper. | |||
==IV. Injections And Replacements In The Cerebro-Spinal System== | |||
Results Of Replacements In The Ventricular System Of True Solutions. | |||
The results of experiments carried out on embryo pigs by the technical procedures outlined in the previous section will be detailed here. The study was made on this animal because of the facility with which it could be obtained living and in good condition and also because it exhibits the characteristic meningeal anatomy of all mammals. The chick could not be used in this investigation on account of the dissimilarity between the avian and the mammalian menmges. | |||
The chief problem concerned here was the actual physiological extent of the cerebro-spinal spaces. This apparently could be ascertained by the replacement of cerebro-spinal fluid by the ferrocyanide mass. But there was also to be considered the passage of fluid from the ventricles out into the periaxial* spaces, corresponding exactly to a similar passage in the adult. | |||
of | |||
If into the central canal of the spinal cord of a hving pig embryo of 9 mm., crown to rump measurement, an injection of the ferrocyanide solution be made under very mild syringe-pressure, the ventricles can be fairly well filled without rupture of any element. Incubation of this experimental embryo with its circulation continuing almost unabated for an hour should cause a further spread of the fluid throughout the normal canals. If at the end of this time the whole embryo is fixed in an acid medium the ferrocyanide will be precipitated in situ. | |||
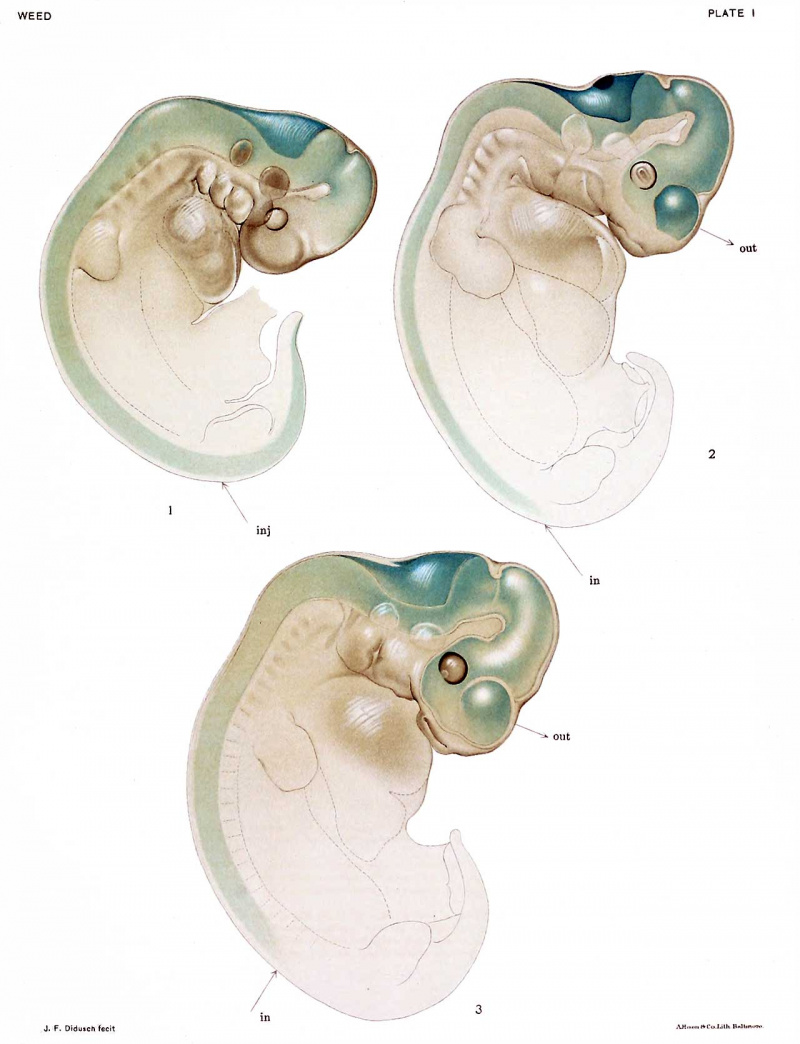
Such a specimen, subsequently cleared by the Spalteholz method, is represented in figure l.f In this drawing the spread of the injection solution is clearly shown. Running upward from the point of introduction, wholly within the central canal of the spinal cord, it reaches the bulbar region and extends outward into the large fourth ventricle, appearing as a dense collection of the prussian-blue. Cephalad from this region it spreads in diminishing intensity until it is finally lacking in the diencephalon. | |||
The injected solution, then, in spite of the unavoidable increase in the normal intramedullary pressure, is contained only within the medullary-canal system (central canal of spinal cord and cerebral ventricles). There is no evidence of any spread outwards, either from the third or fourth ventricle. | |||
In the next stage of meningeal development the replacement method can be used, as the embryo is no longer too small for its employment. In figure 2 is represented an embryo of 13 mm., in which the circulation continued for 90 minutes after the replacement. The same general picture shown in figure 1 results. The whole medullary-canal system is filled with the precipitated prussian-blue, which is densest in the region of the fourth ventricle. The roof of the ventricle, however, shows a striking difference from that of the ventricle in the embryo of 9 mm. Just posterior to the cerebellar lip is a regular oval, which is covered from within by a dense collection of prussian-blue granules, causing it to stand out in clear contrast to the thinner and more evenly distributed blue lining of the remainder of the roof. This oval area is comparatively large and comprises a portion of the superior or anterior half of the ventricular roof. This area, differentiated from the remainder of the rhombencephalic roof, is clearly shown in figure 2, a drawing of a cleared specimen of this stage. | |||
* Throughout this paper the term "periaxial " has been used in the seii90 of "around the central nervous system " or "around the ccrebro-spinal axis." | |||
* Throughout this work the reference "figure " 1, etc., refers to plate illustrations; the word "text-figure" refers to the illustrations inserted in the text. | |||
With the exception of this strikingly dense area in the rhombic roof, the injection spread in an embryo of 13 mm., subjected to replacement of the cerebro-spinal fluid by the ferrocyanide, differs in no way from that in the embryo of 9 mm. Careful inspection of figure 2 is convincing that the spread still remains within the medullary canals, with no extension of the fluid into the spaces outside of the cerebrospinal axis. It seems justifiable, then, to speak of the cerebro-spinal spaces at this stage of development as being only mtramedullary in type, with no indication as yet of a meningeal fluid cushion (corresponding to the adult subaraclmoid space). | |||
mm. | With the use of larger embryos, however, for the medullary replacement with ferrocyanide and citrate, the picture gradually changes. The first indication of a more advanced stage of development is obtained in embryos whose length exceeds 14 mm. Figure 3, of a pig embryo of 14.5 mm., is included here as representing this further extension of the injection fluid. The cerebro-spinal fluid of this specimen was replaced, by the compensating mechanism, by a solution of potassium ferrocyanide and iron-ammonium citrate. The embryo was then kept alive (as judged by the heart-beat) for a period of one hour. At the end of this time it was fixed in an acid medium and subsequently cleared in oil of wintergreen after careful dehydration. | ||
The essential differences between an embryo of this stage and one of the stage represented in figure 2 concerns the spread of the injection fluid from the roof of the fourth ventricle. Both specimens show a complete filling of the intramedullary system (cerebral ventricles and central canal of the spmal cord) with the precipitated prussian-blue granules. The specimen of 13 mm. (fig. 2) is characterized bj' a dense oval collection of the prussian-blue on the upper and inner surface of the rhombic roof. In the specimen shown in figure 3, in contradistinction to this localized aggregation of granular matter, there is a deUcate extension of the injection fluid caudalwards from the roof of the fourth ventricle. This fusiform projection is here readily made out, lying beneath the skin over the ventricular roof and separated quite distinctly from the easily discernible line of the roof. This outward extension of the fluid has a fairly wide and deep origin from the upper portion of the roof, but tapers caudally to a sharp point with considerable rapidity. | |||
At the stage of 14 mm. the roof of the fourth ventricle shows the small depression which marks the formation of the chorioid plexuses. With this depression occurrmg transversely the relation of the external surface of the embryo to the ventricular roof necessarily alters somewhat in this region. The chorioidal depression of the roof graduallj' becomes separated from the skin; and it is into this area between the skin and the ventricular ependyma that the first spread from the cerebral ventricles occurs. At this stage, illustrated in figure 3, the injection is intramedullary in type, with but sUght extension into the pericerebral tissues. | |||
The pericerebral spread may be made out in nearly all replacements in embryos of 14 mm., but in a few cases the injection has remained intramedullary in type. In embryos of 16 mm. the spread into the pericerebral tissues is invariably found. Often, with this extension of the replacement solution outside the ventricles, the oval area noted in the stage of 13 mm. persists. (This phenomenon is especially well shown in a simple injection of silver nitrate, illustrated in figure 11.) | |||
The next stage of importance in the development of the cerebro-spinal spaces is represented in figure 4, a drawing of a pig embryo of 18 mm. in which a typical intramedullary replacement of the cerebro-spinal fluid with a solution of potassium ferrocyanide and iron-ammonium citrate had been made. Here, with the exception of the region of the roof of the fourth ventricle, the replaced fluid is contained solely within the central canal of the spinal cord and within the cerebral ventricles. The roof region, however, exhibits a new phenomenon, which distinguishes it from the stage shown in figure 3. The chorioid plexus invagination has become strongly developed, dividing the roof into two parts. These roof divisions have been termed superior and inferior, the former lying anteriorly and orally from the chorioid fold. The general surface outline is but little changed, due to the mesenchyme filling up the area between roof and skin. From two areas in the entire roof of the fourth ventricle the foreign fluid has escaped into the pericerebral tissue. These points of fluid passage he in the two divisions of the ventricular roof. The superior area of escape corresponds to the oval outlined by the prussian-blue in figure 2 and to the point of emergence of fluid shown in figure 3. The lower area of fluid escape is in the inferior half of the ventricular roof, where the ependymal lining and its supporting tissue are developing into a well-marked dorsal distension. This area corresponds to Blake's'3' caudal protrusion, though, as Heuser'23) has pointed out, the shape of the structure in the pig in no way resembles the "finger of a glove." | |||
The extraventricular spread of the injection fluid in this specimen is considerably greater than in the pig embryo of 14 mm. (fig. 3). On the whole, however, the distribution of the replaced fluid is not extensive as compared with the adult relationship, where the central nervous axis is entirely surrounded by its subarachnoid cushion of cerebro-spinal fluid. From the superior area of fluid passage the replaced solution (as shown by the resultant precipitation of the prussian-blue) has passed both superiorly and inferiorly. In the median line, and extending laterally but slightly, a projection of the blue may be seen occu])ying a large portion of the extraventricular area formed from the chorioidal invagination. This area of fluid passage occupies at this stage about one-third of the total transverse diameter of the ventricular roof. From it the blue tapers caudally, diminishing in all directions. Above, the precipitate may be made out extending superiorly over the cerebellar lip. Its extension into the pericerebellar tissue is not marked; here again it tapers from the area of fluid passage, its midline prolongation stretching farthest anteriorly. This relationship is easily made out in figure 4, a frank lateral view of such an experimental rei)lacement. The granules which result from the introduced ferrocyanide solution are found only in the central canal of the spinal cord and not in any perispinal arrangement. | |||
In the pig embryo of 18 mm., shown in figure 4, the replaced solution has been carried somewhat farther than in the embryo of 14 mm. (fig. 3). The chief point of differentiation lies in the fact that in the latter stages two areas have apparently become permeable to the intraventricular fluid, so that a larger periaxial spread has resulted. Then, too, the extension of the ferrocyanide solution from the superior area is considerablj^ greater, overlapping the cerebellar Up and filling in some degree the pericerebral tissue in the chorioidal invagination. | |||
With a definite periaxial spread established for the cerebro-spinal fluid in pig embryos of 14 to 18 mm., it seemed not unreasonable to expect a gradual increase in the extent of the future subarachnoid distribution in more advanced stages. The earliest extension of the fluid into the peribulbar tissues occurred with the inception of the infolding of the ventricular roof to form the chorioid plexuses of the fourth ventricle. Its further extension, particularly its passages through a second area, occurred with the greater development of the chorioidal invagination (i. e., 18 mm. stage). A still more extensive pericerebral flow of the ferrocyanide and citrate is illustrated in figure 5. Here the cerebro-spinal fluid in a hving pig embryo of 19 mm. was replaced by the ferrocyanide solution. The embryo was kept alive for about an hour after the replacement and was then fixed in toto in an acid fixing medium, which caused the precipitation of the prussian-blue. On clearing subsequently by the Spalteholz method the spread of the solution was found to be somewhat more extensive than in the stage of 18 mm. (cf. figs. 4 and 5). In figure 5 the whole periaxial area over the roof of the fourth ventricle is shown to be completely filled by a dense aggregation of the prussian-blue granules. The separation of the two areas of fluid passage can not be made out in such a specimen. This dense periaxial extension ahnost completely covers the cerebellar Up, not onlj^ in the medial region but laterally to the limit of the ventricular roof. The injection precipitate lies directly beneath the skin in this area, but more posteriorly its separation from the skin becomes more marked. Tracing this dense periaxial injection posteriorly, it is seen (fig. 5) to end somewhat abruptly in the region of the cephaUc flexure. The Une of termination of the denser mass, to the ventral surface of the medulla, tapers somewhat anteriorly. This extraventricular spread is medial to the otic vesicle, but extends peripheraUy along the caudal cerebral nerves, reaching outward as far as the peripheral gangUa. The periaxial spread also closely covers the ventral surface of the medulla and extends in this plane around the pontine flexure for a short distance upwards along the basilar surface of the mid-brain. | |||
Examined from its dorsal aspects, the superior portion of the spinal cord is found to be covered (in a perispinal relation) by a fine deposit of the prussian-blue. This is shown in figure 5. Caudally from the higher cervical region there is no exidence indicating a further spread in the perispinal tissues. Such a spread from above downward is wholly at variance wdth Reford's^^' conception of a development of the spinal meningeal spaces before the cerebral. The complete filling here of the central canal of the spinal cord and of the cerebral ventricles with the replaced fluid, with no evidence of a periaxial spread except in the region of the fourth ventricle, indicates that in the pig cinbrj'o the adult human relationship between the cerebral ventricles and the subarachnoid spaces endures. There is apparently in this embryo no evidence of the foramina of Bichat and of Mierzejewsky, a findmg in accord with the observations of Dandy and Blackfan'i*''. | |||
In the slightly larger embryos the further extension of the embryonic extraventricular spaces progresses rapidly. Figure 6 represents such an extension in a pig embrj-o of 21 mm., in which the normal cerebro-spinal fluid was rei)laced by a dilute solution of potassium ferrocyanide and iron-ammonium citrate. In this specimen the central canal of the spinal cord and the cerebral ventricles are completely filled with the precipitated prussian-blue. But m addition there is almost a total filling of the periaxial spaces. Viewed laterally the densest aggregation of the blue granules is again in the region of the roof of the fourth ventricle. As in the embryo of 19 mm. (fig. 5), the whole extraventricular tissue posterior to this ventricular roof is filled with the granules precipitated from the foreign solution. The spread from this region is similar to that in the previous specimen, except in its far greater extent. The granules may be traced caudalwards in the perispinal spaces to the point of injection. The arrangement of the precipitated material, both withm the central canal of the spinal cord and surrounding it in the perispinal relationship, is well shown in figure 7, a frank dorsal view of the same specimen represented m figure 6. The greater density of the perispinal granules in the upper region of the cord, as contrasted with the granules in the thoracic region, is probably of importance in indicating the direction of the flow from above dowaiwards. The increased amount of the injection fluid in the region about the pomt of insertion of the spinal needle.is in all likelihood due to a local spread from the needle, such as frequently occurs in a very limited area. The phenomenon may, however, be due to an actual increase in the size of the potential perispinal space, though observations upon other embiyos of the same stage of development argue against this view. The segmental outlming of the caudal portion of the perispinal space is to be noted in this figure. | |||
The cephalic regions in the specimen of 21 mm. show a quite extensive spread (fig. 6), and there is the same general distribution of the granules about the medulla, as in the specimen shown in figure 5. The rhombencephalon is completely surrounded by the blue, the ventral sheet inclosing it tightly. Laterally the prussianblue is shown in a dense mass, in intimate relation to the cranial nerves as they join the brain-stem. The cerebellum is practically completely covered by the precipitate; from the ventral portion of the pericerebellar granules the replaced solution (as evidenced by the granules of prussian-blue) spreads forward and surrounds a portion of the mid-brain. Only the ventral surface of the posterior half of the mid-brain is circumscribed by the granules; anteriorly it is wholly surrounded by the i)eriaxial injection; more anteriorly tlic extension is iimited to the mesial structures, leaving unsurrounded the cerebral hemispheres, althougli creejnng between the hemispheres and the mid-brain. | |||
The pecuhar avoidance by the replacement fluid of the extreme dorsal half of the mid-brain is also to be made out in the dorsal view of the specimen (fig. 7). | |||
The two lateral extensions from the ventral sheet of the injection granules approach on either side this mesencephalic eminence. The peculiar appearance of the injection spread caused by the chorioidal invagination of the roof of the fourth ventricle is also here illustrated. | |||
In this specimen, then, of a pig embryo 21 mm. the periaxial spread is almost complete, the only areas not entirely surrounded being the aiiterior mesencephalon and the cerebral hemispheres. In an embryo but a few milhmeters larger this periaxial exten.sion of the solution is complete. The mesencephalon first becomes entireW covered by the jjrussian-blue precipitate, with later extension over the hemispheres. This complete periaxial injection occurs usually in replacements in embryos varying in length from 24 to 28 mm. | |||
A specimen exhibiting a complete extension of the replaced solution around the central nervous system is shown in figure 8. This specimen was prepared by replacing the cerebro-spinal fluid in a Uving embryo of 26 mm. and then keeping the embryo alive for an hour. After fixation in an acid medium, dehydration, and clearing, the uijection was found to occupy the whole medullarj-canal system and also to surround completely the cerebro-spinal axis, as shown in the lateral view. The striking features of this stage are similar to those observed in the younger specimens — the dense accumulation of granular material in the region of the roof of the fourth ventricle, the surrounding of the central portion of the caudal cranial nerves, and the thin pericerebral covering by the replacement mass. In addition the specimen exhibits in the thoracic region an extension of the granular material laterally along each spinal nerve. An observation of this peculiarity reveals the prussian-blue extending outwards only as far as the ganglia on the posterior roots. | |||
The relationships, then, observed in an embryo pig of 26 mm. are those which exist in the adult; the cerebro-spinal axis contains cerebro-spinal fluid within its cerebral ventricles and within the central canal of the spinal cord, while in turn it is cornpletely surrounded by cerebro-spinal fluid within the subarachnoid space. Communication between the ventricles or intra-medullary sj'stem and the perispinal spaces occurs only in the region of the fourth ventricle. Here again the adult human relationship holds. The evidence, therefore, from a study of the fluid spread in a replacement experiment with the use of true solutions, indicates that in pig embryos of about 26 ram. an adult distribution of cerebro-spmal fluid occurs. | |||
===The Results of Injections of True Solutions=== | |||
In the preceding section there have been detailed the results of experiments on living pig embrj-os in which the cerebro-spinal fluid of both the central canal of the spmal cord and the cerebral ventricles has been replaced by a dilute solution of potassium ferrocyanide and iron-ammonium citrate. After the replacement, carried out so as to avoid any increase in the normal tension, the embryos were incubated for varj^ing periods of time so that the normal current of the fluid might cause an extension of the loreign solution. In the experiments which will be recorded in this section the same true solution was injected from an ordinary syringe and the salts immediately precipitated as prussian-blue. The purpose of these observations was solely to ascertain the effect of injections at pressures above the normal tension, so that the conclusions drawn from the replacement method might be more fully substantiated. | |||
It was soon ascertained that the pressures caused by injections with a simple syringe could be fairly well controlled and that several degrees of tension might be employed. Thus it was found to be simple and serviceable to designate the injections as those made with mild, moderate, or strong syringe-pressure. Most of these injections were made into the central canal of the spinal cord, but occasionally into the perispinal spaces or cerebral ventricles. Injections under equivalent pressures in the central canal of the spinal cord or into the cerebral ventricles always gave corresponding results. It is necessary to record that the injections, even under strong pressure, were not carried to the point of macroscopic rupture. | |||
The so-called mild syrmge-pressure, making use of solutions of potassium ferrocyanide and iron-ammonium citrate, resulted in extensions of the prussian-blue wholly similar to those obtained in the replacement experiments which were carried on for 30 minutes and over. This similarity indicates a complete filhng of the available cerebro-spinal system in the replacement method, for certainly (even in the mildest syringe injections) the intraventricular pressure must be excessively increased. Figure 1 shows a specimen under such conditions, with a marked thinning of the injection mass in the region of the fore-brain. This finding is customarily present in the injections under mild pressure, due to the pushing upwards of an existent ventricular fluid. | |||
"When moderate pressures are employed with the syringe the picture gradually changes. The essential difference in the results obtained by moderate syringe injection and by the replacement method lies in the greater extension of the foreign solution in the smaller embryos. Thus in figure 9 the spread of the injection precipitate in a pig embryo 16 mm. is shown to be about as extensive as that obtained by the replacement method in an embryo of 19 mm. (fig. 5). The extra ventricular distribution of the injected solution around the medulla, the extension (even more marked here) along the central roots of the caudal cranial nerves, and the localized perispinal spread are easily made out in this specimen of 16 mm. | |||
This general rule applies to all of the results obtained with the use of syringepressures above the mildest. Dependent upon the degree of syringe-tension, the spread extends in simple ratio. Thus, by the use of moderate pressures of injection into the central canal of the spinal cord, a complete intramedullary and periaxial spread was secured in a pig embryo of 22 mm. somewhat earlier than the equivalent stage was obtained by the use of the replacement method. | |||
With the highest syringe-pressures (insufficient, however, to cause macroscopic rupture) the same general type of injoctiou spread was obtained, bringing the more complete stages down into smaller and smaller embryos. Most of these embryos, however, on microscopic section showed obvious rupture of some part of the central nervous system. | |||
The most important feature of these findings in the embryo pig injected with true sohitions under moderate pressures from a syringe concerns the fact that the extension of the injection coincides, except as to the size of the embryo, in every instance with that obtained by the replacement method. Thus similar and analogous spaces are filled by injections under syringe-pressures in small embryos and by the solution under normal tension in larger embryos. It must be assumed, then, that the pressure of injection is sufficient to dilate potential cerebro-spinal spaces which normally would not be concerned in the pathway taken bj' the cerebro-spinal fluid. No evidence of new or abnormal pathways for the fluid is afforded by the observations made with the increased pressure; these phenomena indicate great potential strength in the tissues which limit the immature cerebro-spinal spaces. | |||
Injections with a simple syringe may he made with such a degree of pressure that gross rupture of the tissues becomes apparent. In such an injection into the central canal of the spinal cord the infundibular region ordinarily ruptures in the smaller embryos (under 15 mm.), while in larger embryos rupture usually occurs into the subcutaneous tissues of the back of the neck over the fourth ventricle. | |||
In discussing the effects of the introduction of solutions of ferrocyanide under pressures higher than normal into the central canal of the spinal cord, it may be appropriate to record observations made in the attempt to inject the cerebro-spinal spaces from the perispinal space. In embryos under 15 mm. in length it is quite difficult to make a perispinal injection. As the embryos exceed this measurement the injection becomes increasinglj' easy, but not until a length of 20 mm. is attained can it be made under the mild pressure advisable. These observations tend to substantiate the findings alreadj' recorded in both the intramedullary replacements and the injections under mild pressure. | |||
===Results Of Injections Of Nitrate Of Silver=== | |||
In a number of experiments a dilute solution (0.5 per cent) of nitrate of silver was injected into the central canal of the spinal cord and the salt then reduced in the sunhght. This solution, although a true one, is wholh- unsuited for the replacement type of injection, on account of its great toxicity and its power to coagulate protein. It was employed here onh' for the simple type of injection. | |||
The results obtained by this intraspinous injection of solutions of nitrate of silver were of but little value in the determination of a pathway for the cerebrospinal fluid, but they vividly present certain aspects of the problem. Thus, in figure 11, a drawing of a specimen (pig) of 16 mm., the area through which fluid passes in the superior portion of the roof of the fourth ventricle is clearly outlined by a denser deposition of the silver. This specimen was prepared by introducing the solution of nitrate of silver into the central canal of the spinal cord under the so-called moderate sj-ringe-pressure. The drawing shows a shght, cone-shaped extraventricular spread of the injection fluid. This spread takes place solely from the superior area of fluid passage, a result in accord with the finding that the solution of potassium ferrocyanide and iron-ammonium citrate passed first through the superior area. Of course it is realized that the precipitant action of the silver may have exerted a more potent action on the structures constituting the lower area of fluid passage. | |||
Another interesting phenomenon of the injections of silver nitrate is shown in figure 12. The embryo of 13 mm. here represented was injected under strong syringe-pressure with a sohition of silver nitrate into the central canal of the spinal cord. On subsequent reduction and clearing it was found that the excessive pressure had resulted in a comjilete intramedullary injection with a localized pedunculate spread into the tissues from the roof of the fourth ventricle. This bulbous extravasation into the extravcntricular tissue has not been observed in any specimens except those into which the solution of silver nitrate was injected. Such a spread is probably to be accounted for by an immediate coagulation of the surrounding tissue. | |||
The extensive use of solutions of sUver nitrate as a means of demonstrating vascular channels naturally suggests a careful comparison of the results obtained from its use and those obtained from the employment of other available true solutions, in regard to the evidence afforded by the two methods of intraspinous injections. The chief objection to the use of silver nitrate, as has aheady been mentioned, is its power to coagulate protein. This is illustrated by many features of the specimen shown in figure 11 — by the sharp outhning of the area of fluid passage, the markings on the caudal process of the fourth ventricle, and the delimitation of the cerebellar hp. But much more marked are the evidences of this coagulative power as shown in figure 12, the pedunculated extraventricular spread, the transverse corrugation of the cerebellar hp (amountmg to circumscribed indentations), and the peculiar outlining of the roof attachment to the bulb. These phenomena obtained by the intraspinous injection of solutions of silver nitrate must be classed as artifacts. The different degrees of this corrosive action of the silver probably result from the varj^ing rates of reduction of the salt to the metal, a factor which is not easily regulated. The findings, therefore, with this method are worthless unless controlled. | |||
Many embryos of varying sizes were injected with the silver nitrate. In the main these observations followed the course of development of the cerebro-spinal spaces as evidenced by the replacement experiments with the ferroc3'^anide. The injections required moderate pressure in the syringe in order to secure more than a local extension from the roof of the fourth ventricle, and to secure the same extent of spread it was generally necessary to use embryos a few millimeters larger than those required in the replacement experiments; but this is to be expected, m view of the i^robability of a constant precipitation of the albuminous tissues by the injection fluid. | |||
Specimens prepared by the intraspinous injection of silver nitrate, then, afford but little reliable evidence in this j)roblem except of a substantiative sort. The findings by this method indicate that the perispinal and pericerebral spaces, in pig embryos of 25 mm. and upward, could be filled by an injection of silver nitrate under moderate pressures into the central canal of the spinal cord. The point of passage of the fluid from the intramedullary to the periaxial system was in the region of the roof of the fourth ventricle. | |||
===The Injection Of India Ink=== | |||
The objections to the use of any fluid of insoluble particles in suspension have already been discussed in considering the methods of injection which were possible for use in this study; but for comjiarisou Avith results obtained by more promising methods and to ascertain to what extent injections with India ink are reliable they will be further considered here. No granular substance other than India ink (carbon granules) was employed in this investigation. In every way this suspension possesses advantages over other possible masses — in its ease of preparation, in the small size of the granules, and the insolubihty in the reagents used for microscopic technique. | |||
Suspensions of nidia ink (diluted from 4 to 10 times) were introduced first into the medullary-canal system of living pig embryos by the replacement method. In no case, however, even though the circulation of the embryo may have continued for 90 minutes, was there any evidence of an extension of the replaced mass outward into the periaxial spaces. The carbon granules remained wholly within the ventricles, a striking difference from the results obtained by the ferrocyanide replacements. It would appear, then, without the further evidence afforded by microscopic section of the specimens, that there is an existing mechanism which prevents the passage of the carbon granules from the fourth ventricle into the periaxial spaces. This finding was found to be constant ui all the living embryos subjected to the cerebro-spinal replacement. | |||
Quite similar to these results by the replacement method are those from the injection of a suspension of india ink under mild syringe-pressure. In no instance, provided the pressure was maintained at a low enough degree, was there any passage of the granular material into the periaxial tissue. In embryos of over 30 mm., however, even with the lowest pressure, it becomes increasingly difficult to prevent a sudden spread into the periaxial spaces. The type of spread indicates a sudden release of some restraining agent and suggests a rupture of a membrane. This spread is usually local and takes place from the roof of the fourth ventricle. | |||
With moderate and strong syringe-pressures, however, it is possible to secure a periaxial spread, but this is quite different from the distribution of the uijections by the use of ferrocyanide solutions. Figure 10 illustrates a specimen of a pig embryo of 21 mm. into whose central spinal canal india ink was injected under strong syringe-pressure. The resultant spread of the injection is easily discerned; the cerebral ventricles are quite filled with the carbon, while from the superior portion of the roof of the fourth ventricle a dense but localized periaxial spread is made out. This extraventricular extension of the ink is well defined; it stretches caudalwards for a slight distance, curving about the bulbous caudal portion of the ventricle and extending lateralwards but a short distance. The median portion of the cerebellar lip is covered by the granules. E\adeuces of the excessive pressure at which the injection was made are shown by the lines of mvasion of the spinal cord and mid-brain. A comparison of the spread of this injection mass with the extension of a ferrocyanide replacement in an embryo of the same size (21 mm.) is afforded by figures 10 and 6. With such a divergence in the results obtained by the two methods of approach it is not surprising that observations such as Reford's) fail to coincide with these findings. The unsuitabiHty of suspensions of granular material in the investigation of the cerebro-spinal sjiaces has been many times verified in this work. | |||
In the further study of the course of the spread with injections of india mk it was found that, in pig embryos of approximately 22 mm. and over, a partial periaxial injection could be secured by plunging the syringe-needle into the perispinal spaces. The carbon granules could subsequently be seen filling the perispinal spaces and also mounting upwards in jjartial pericerebral relationships, particularly around the medulla. This result was obtained by the use of strong syringe-pressures. Apparently the resistance to the spread of the ink in injections or replacements in the medullary-canal system occurs in the passage of the fluid from the roof of the fourth ventricle into the periaxial spaces. So far as is known, Reford^"*^) did not control his injection pressures. These results with the injection of india mk under strong pressures coincide with the idea of his observations afforded by the abstracts given by 8abin(*3) and Cushing^^'. Suspensions of india ink. then, injected under mild syringe-pressure or by the replacement method, offer no evidence, in the pig embryo, of a passage of the cerebro-spinal fluid into the j^eriaxial spaces. Only by employing pressures much above the normal tension can such evidence be obtained. | |||
==V. Undescribed Structures in Roof of the Fourth Ventricle== | |||
2. | The results of the replacement of the existing cerebro-spinal fluid by a true solution of potassium ferrocyanide and iron-ammonium citrate in a living pig embryo indicated, as detailed in the foregoing section, that the fluid passed from the ventricular system into the periaxial tissues in the region of the roof of the fourth ventricle. This important transit of the fluid, agreeing with the established conception of the relationship in the adult, was first observed in an embryo pig of 14 mm. (fig. 3). At this stage the exudation of the replaced fluid occurred in one defined area, seemingly corresponding to the dense oval in a smaller embryo shown in figure 2. | ||
Such a passage of fluid from ventricle to i)eriaxial tissue is necessarily a ])hysiological phenomenon, and it was in the hope of finding an anatomic basis for this phenomenon that the roof of the fourth ventricle was studied histologically. It was reahzed that failure to demonstrate anatomically differentiated structures would not vitiate the physiological observations, but that a correspondence between function and structure was most desirable. Hence observations were undertaken to determine, if pn.ssible, an area of histological differentiation in the roof of the fourth ventricle which might be concerned in the primary passage of fluid from the cerebral ventricles into the periaxial tissues. The investigation concerned first the examination of this region in pig embryos of 14 to 15 mm., at which stages the fluid passes from a single area. Subsequently, similar studies were undertaken in regard to the second, more inferior area (shown in figure 4). The results of these studies will be given here. | |||
AN UNDESCRIBED AREA IN THE SUPERIOR PORTION OF THE ROOF OF THE FOURTH VENTRICLE. | |||
===The Area Membranacea Superior In The Pig Embryo=== | |||
Examination of the roof of the fourth ventricle in a pig embryo of 14 mm. revealed a peculiarly differentiated area in the superior portion. The general topography of this area is show7i in the rectangular area marked off in figure 32 — a median sagittal section from a pig embryo of this critical stage. In figure 33 this rectangular area is enlarged to show the morphologA' in greater detail. | |||
In this figure the densely staining ependj'ma lining the fourth ventricle approaches from both sides. The superior portion of the ependyma ends abruptly, while the inferior Hne of the layer tapor.s more slowly. Between these two jjoints is an area having none of the characteristics of the ventricular lining at all other points. The comparatively smooth contour of the ependymal cells is replaced by an irregular cell-border. The pyknotic nuclei of the cells have been replaced by less densely staining, elongated, spindle-like nuclear bodies. The cell-layer lining the ventricle is here really only of a single cell in thickness, although blood-capillaries closely applied to it suggest a greater thickness. The mesenchj-nie lietween this layer and the peripheral epidermis is quite thin, but resembles in everj' way the mesenchyme in the immediate neighborhood. | |||
There is, therefore, as pictured hi figiues 32 and 33, an area in the roof of the fourth ventricle which is morphologically dissimilar to the characteristic ependyma lining the cavity. Is this the result of some distortion in fixation or in the routine histological technique? Is it a constant finding and, if so, what is its historj'? Does it arise at a definite period and persist throughout intra-uterine life onlj' or through adult life also? | |||
The question of the actual existence of this area, or of its being caused by technical manipulations, is one which must be answered. That this differentiated portion of the roof of the fourth ventricle is not an artifact is verified bj* the general history of its formation, by its invariable occurrence (not onh' in the pig but in other animals), and by its general histological appearance. Moreover, the physiological importance of this area undoubtedh' incUnes one completely from the possible explanation that it is due to an artifact. Xo single finding wholly excludes such a possibility; rather is one convinced, by many features, of its actual occurrence. | |||
Considering the fact, then, that this differentiated structure in the roof of the fourth ventricle may be found in all embryo pigs at the stage of 14 mm., it becomes necessary to ascertain at what time in the development of the embryo it first appears and how it is formed. Obviously the most satisfactory' method is to trace the area through the lower stages and also through the older embryos. For the sake of greater clearness, however, a description of the area \s-ili be given from its first differentiation through its maximum transformation to its final disappearance — for the structure is only temporary. | |||
In pig embryos of 8 mm. and less in crown-rump measurement, the roof of the fourth ventricle is fcfrmed of cells morphologically and tinctorially different from those lining other parts of the ventricular cavities. These cells are quite unlike the deeply staining ependjTiial cells, which can be so readily identified as the lining cells in older embryos. In this yoiuiger stage of 8 mm., the entire ventricular roof is composed of several layers of cells with round or somewhat oval nuclei and fairly abundant cytoplasm. The cell-boundaries are not well defined. The nuclei are not deeplj' tinged with hematoxylin. The chromatin material is sparse and irregularly distributed. Nucleoli are prominent. The cytoplasmic border hning the ventricular cavity is rough and ragged at times, often blending with the coaguUvted albumen of the cerebro-spinal fluid. Altogether, these lining cells bear a much greater resemblance to the epithelial cells than to the ependymal. | |||
These characteristics of the lining cells of the roof of the fourth ventricle are shown in figures 24 and 25, from a pig embryo of 8 mm. The close association of the roof cells to the surface epithelium is easily made out in figure 25, as well as the general character of the lining cells. | |||
At the stages of 8 mm. and under, in the pig embryo, the roof of the fourth ventricle is relatively'' quite large. In its whole extent it is formed of the peculiar lining cells described above. With the growth of the embryonic nervous sj^stem, the roof of the fourth ventricle is subjected to alterations in form and position; to some extent these changes influence the cells which line the cavity in the early stages. | |||
In pig embryos between 8 and 12 mm. in length the roof of the fourth ventricle undergoes a change. The ependyma, which from comparison with later stages is regarded as typical, begins to encroach upon the epithelial-like cells which are so numerous in the 8 mm. stage (fig. 25). The area occupied by these cells diminishes, not only relatively but absolutely. It becomes smaller and the cells gradually change their character. These changes are shown in figures 26 and 27, from a pig embryo of 11 mm. Figure 26 gives the location, in a sagittal section near the midline of the area in figure 27, taken at a higher magnification. | |||
In figure 27 the densely staining lips of ependymal and nerve cells are seen approaching each other. For a considerable space in the central portion of the photograph there is an area similar to that shown in figure 33. But considered in connection with figure 25 this area represents the epithelial-like cells of the roof of the fourth ventricle. This relationship is more clearly shown in figures 28 and 29, taken in a more lateral plane from the same embryo (11 mm.). Examination, however, of th(; area in figure 29 shows the epithelial-like cells again a])i)arent in the roof of the fourth ventricle. | |||
The i^rocess of transformation, then, as shown in these photographs from an embryo i)ig of 11 mm., concerns a gradual encroachment upon the area of epithelial-like cells by the more densely staining and more closely packed ependymal cells. Gradually the epithelial-like cells in the central portion of the area lose their former character (fig. 27), while around the periphery, especially on the lateral sides, the epith('lial-lik(! ajjpearance persists (fig. 29). | |||
On the lateral side of this area, just as the tyincal ependymal lining is al)out to become isolated (fig. 29), the epithelial-like lining cells form a several-celled layer. | |||
The nuclei are poor in chromatin material and the cytoplasm somewhat small in amount. The inner cytoplasmic border hning the ventricle is in contrast, by its ragged outline, with adjacent smoother ependj^ma on both sides. At this stage of the pig embryo the characteristics of the epithehal-like cells are still to be made out, but a gradual transformation is becoming evident. | |||
The metamorphosis becomes much more marked in the central portion of the area, as shown in figures 26 and 27. In these figures the whole central area seems to have lost some of its former character as an intact cell-laj'er. Closer examination, however, under higher power demonstrates that it still possesses an intact surface as a hning for the ventricle. Delicate cytoplasmic strands stretch in a continuous line across the whole area between the Ups of denser tj-pical ependjina. The nuclei in this difi'erentiated area are seeminglj' altered from their rounded form and have elongated almost into spindles. The inner cytoplasmic border is characteristically rough, with, small amounts of coagulated albumen adhering to the processes. The area, then, in its central portion, at the stage of 11 mm., has assumed the character of the stage of 14 mm. (fig. 32). On the periphery, however, the cells stiU resemble those of smaller stages (8 mm.). | |||
From the pictures presented by the intermediate stages (figs. 27, 28, and 29) the differentiation goes on verj' rapidly, so that in the pig embryo of 13 mm. there is rarely any evidence of the epithelial-like cells. Figures 30 and 31 are photomicrographs of a sagittal section of an embryo pig of 13 mm.; here there are no evidences of the epitheUal-like cells. The whole area, pictured in figure 31 as sharply delimited from the tongues of tjijical ependyma above and below, has become well differentiated. The cell-character observed in figures 27 and 33 (elongated nuclei and scanty strands of protoplasm) has become very obvious. The ragged and roughened intraventricular border, the coagulated albumen, and the abrupt transition from the neighboring tj-pical ependyma are well shown in the photomicrographs of this specimen. | |||
The differentiation of this area in the roof of the fourth ventricle of the pig embryo proceeds at a very rapid rate, so that within the growth of a few millimeters (from 8 to 13 or 14) a great histological change occurs. Figures 32 and 33, already described, show the extent of this metamorphosis in a pig embrj^o of 14 mm. The process, however, continues, modified possibh' bj- the changing of the roof of the fourth ventricle. For this roof structure is subjected to marked alteration in stages of 14 mm. and upwards, both by the lateral development of the chorioid ple.xuses and b}^ the readjustment of the cervical and pontine flexures. Its maximal differentiation may be said to appear at a stage of 18 mm.; this is maintained through several millimeters, until undergoing final retrogression. | |||
This maximal change in the roof of the fourth ventricle is shown in figures 34, 35, 36, and 37. Several points of interest are brought out in these photomicrographs. Figure 35 represents an enlargement of the rectangular area in figure 34, taken from transverse sections of an embryo pig of 18 mm. The area is particularly well shown in this figure, in which, from the right, the typical ependyma, in a fairly smooth single-cell layer, approaches the differentiated cells in the central portion. On the left, too, similar typical ependyma is shown. In the central area, which has been repeatedly described, the elongated nuclei, the strands of protoplasm, and the ragged, iiTegular intraventricular surface are well presented. The photomicrograph has been reproduced to show the relation of this differentiated area to the various blood-channels in the supporting mesenchyme. Apparently the whole ventricular roof is, at this stage, a site for an extensive capillary plexus; from both sides, as shown in figure 35, vessels (one of great caliber) approach the central area of differentiation. Directly beneath this area smaller capillary channels can be made out, from which, apparently, a sUght extravasation of red blood-cells has occurred. Here, as in the greater part of the basilar pericerebral region, extravasation of the blood-cells is very frequent. This phenomenon has already been pointed out by INIaU'^"". | |||
The large extent and the great differentiation of this pecuUar area in the roof of the fourth ventricle are well shown in figures 36 and 37, taken from a transverse section of a pig embryo of 18 mm. In the photomicrograph of higher magnification the two sharp tongues of typical ependyma are quite striking. Their abrupt termination in the wide, differentiated area has nowhere been more convincingly shown. The resemblance of these lining cells in the central area to the mesenchymal elements adjoining is here also seen. The most interesting of all the phenomena exhibited in this reproduction, however, is the attachment, apparently by precipitation, of the coagulated albumen of the cerebro-spinal fluid. This coagulation, in this specimen, deUmits the differentiated area in the roof of the fourth ventricle. The phenomenon is seemingly only an ampUfication of a similar attachment of small fragments of the albuminous precipitate shown in other figures. | |||
Beyond the stage of 18 mm., which may be termed the maximal stage, the differentiated area in the roof of the fourth ventricle undergoes a regression. This is apparently due to the morphological alterations in this rhombic roof. The chorioid plexuses in embryos over 18 mm. long deeply invaginate the fourth ventricle, possibly drawing some of the true roof with them, but surely encroaching upon the mid-hne with their lateral tuftings. This growth tends to decrease the available extent of the differentiated area, but an even more potent factor is the rapid development of the cerebellum. The caudal growth of the cerebellar lip soon largely occupies or replaces the superior half of the roof. These two factors, the cerebellar growth and the enlargement of the chorioid plexuses, render the persistence of the differentiated area impossible, so that a regression or disappearance is to be expected. | |||
With these considerations before us, the study of sectioned pig embryos of a greater length than 18 mm. becomes important. The process of disappearance, however, does not occur at once. Thus, in an embryo pig of 19 mm. (figs. 42 and 43) the differentiated area is as large and as characteristic as in the stage of 18 mm. This same aijpoarance and maintenance of size may be observed through the next several millimeters' growth, but in pig embryos of 23 mm. the chorioid plexus has usually developed to such an extent that a continuation of the former size becomes impossible. This is shown in figures 44 and 45. Figure 45, the enlarged squared area from figure 44, is a photomicrograph from a pig embryo of 23 mm. The differentiated area, duo to the factors favoring its regression, now appears in close proximit}^ to the chorioid plexus. It has more the appearance of a degenerating area at this stage than in any of the younger embryos, but it still shows a characteristic delimitation of both edges — on the one from the typical ventricular ependyma, and on the other from the differentiated ependj'ma of the chorioid plexus. The cytoplasmic strands of the area which forms the ventricular border do not show to advantage in the photomicrograph, but the .same ragged character with the covering of coagulum may be made out. The process of regression, mechanical as it perhaps is, has begun at this stage in the pig, and in the course of the next few millimeters' growth will become even more active. | |||
With the encroaclmaent of the chorioid plexuses and the downward growth of the cerebellar lip, the superior portion of the ventricular roof soon disappears, and is practically non-existent in embryos of 30 mm. and more in length. The differentiated area thus encroached upon from the sides and above becomes a mere vestige of its former size. Thus in a pig embryo of 32 mm. (figs. 46 and 47) it appears as a very small break in the lining continuity of the ventricular ependyma. Without the intermediate stages such a picture would undoubtedly be considered as an artificial erosion of the ependjinal lining of the ventricle, but when studied in connection with figure 45 the true vestigial character of the area becomes established. | |||
The final fate of this differentiated area in the roof of the fourth ventricle is a complete disappearance, with the occupation of the region by chorioidal epithelium and cerebellum. In this study it was impossible to find traces of the differentiated areas in pig embryos of over 33 mm. in length; vestiges maj' persist, but so small as to present difficulties of decision. The persistence of such a differentiated vestige in rare instances would not be surprising; the transitory character of the area and the method of disappearance make this seem not unlikely. | |||
This transitory area of differentiation in the roof of the fourth ventricle of the pig has not, so far as can be determined, been noted or described bj- any p^e^•ious author. His'25\ in a retouched photomicrograph of a sagittal section of a human embryo of 17 mm., reproduced the area as differentiated from the roof, but he has made no comment upon it. I have called this differentiated area in the superior portion of the rhombic roof ventricle the "area membranacoa superior ventriculi quarti." This terminology is based on the anatomical character of the area as a continuous membrane, but chiefly on its physiological significance. For, as will be shown in the succeeding section of this paper, the transit of embryonic cerebrospinal fluid from ventricle to periaxial tissue occurs in this area, which functions apparenth- as a physiological membrane. With such a physiological conception of the area, the ttim "area membranacea" seems most suitable, inasmuch as it also meets the anatomical requirements. | |||
===The Area Membranacea Superior in the Human Embryo=== | |||
The finding of the diflferentiated area in the superior portion of the roof of the fourth ventricle in the embryo pig suggested the value of a study of the same region in the human embryo in the further solution of the problems underlying its occurrence. Hence this region in the roof of the fourth ventricle has been examined in the sectioned human embryos of the Department of Embryology of the Carnegie Institution of Washington. It was found that a similar area occurred in the human embryo of approximately the same age. | |||
The study of the roof of the fourth ventricle is usually more difficult in the human embrj^o than in the pig. This is due to the fact that the roof of the fourth ventricle quickly suffers from poor fixation and dehydration — collapse or inversion of the whole structure being commonly met with. It is rarely possible, in the younger embryos, to secure the most satisfactory fixation, whereas in the pig these factors may be controlled as desired. Furthermore, the undue pressures to which the human OAaim is frequently subjected in abortion may cause crushing of the more delicate parts of the nervous sj^stem. | |||
It is probably best, in the human embryo as in the pig, to trace the formation of the area membranacea superior ventriculi quarti from its beginning, through the various differentiations. | |||
In a human embryo of 4 mm. (No. {{CE836}} of the Collection of the Carnegie Institution of Washington) the entire roof of the fourth ventricle is composed of cells with round or slightly oval nuclei and palely staining cytoplasm. The nuclei of the cells are poor in chromatin material as contrasted with the pyknotic character of the typical ependymal cells. The lining tissue is of the thickness of several cells. The ventricular cytoplasmic border is fairly smooth at this stage. This characteristic ventricular lining is shown in figures 40 and 41, both taken from embryo No. 836. The whole picture is similar to that exhibited by the pig embryo of 8 mm. (figs. 24 and 25). | |||
A similar accumulation of epithelial-like cells is found in a human embryo of 7 mm. (No. {{CE617}} of the Carnegie collection). This is pictured in figures 48 and 49. The photomicrograph of higher magnification shows these poorly staining cells heaped up in a rather localized part of the ventricle, fairly sharply dolimitod from the adjoining ventricular lining. This accumulation of cells in the roof of the ventricle invariably occurs, and it must not be considered as being due to the distortion of the ventricular roof. The reason for the asymmetry of the rhombic roof shown in these figures hes in the fact that in this embryo, as in practically all the embryos of similar stages in this collection, some degree of distortion of the roof of the fourth ventricle is present. Photomicrographs (figs. 50 and 51) taken more posteriorly (from embryo No. {{CE617}}) give strong evidence of this distortion. They are reproduced not only to show the possible distortion, but also to give a further picture of the lining of the ventricle, with its epithelial-like cells in several layers (fig. 51). | |||
Similar accumulations of these epithelial-like cells are to be found in human embryos of 9 mm. Reproductions of a much fragmented specimen of this size (No. {{CE721}}) are given in figures 52 and 53. In the latter figure the complete occupation of the ventricular roof by these cells is well illustrated. Moreover, the specimen shows the many-layered stage to a degree but seldom found. It is unfortunate that such a degree of fragmentation and distortion is found throughout this specimen. | |||
Thus far, in human embryos up to and including 9 mm. in length, the roof of the fourth ventricle has shown the same architecture as appears in the pig. As will be recalled, the first evidence of a further differentiation of these cells in the pig embryo was found at a stage of 11 mm. (figs. 26 and 27). In one human embryo of this stage (No. 544) a distinct break in the roof of the fourth ventricle can be made out. This is shown in two photomicrographs (figs. 54 and 55). The picture in this cas3 is somewhat obscured bj' the shrinkage and distortion of the ventricular roof, but a distinct differentiation of the lining epithelium can be made out. On the caudal side of figure 55 considerable nervous tissue is seen. Just superior to this (toward the left) the lining tissue is almost lacking, a few nuclei, only, preserving the contour of the ventricle. Above this area appears again the ventricular lining of many layers of cells. It has been quite difficult to interpret these findings. The area under discussion shows a rather typical adherence to the coagulated albumen ; there is evidence of its extension also into the adjacent mesenchyme, a finding observed in no other similar stage. The caudal position of the opening, the character of the tissue approximating the ventricular cavity, and the presence of the albumen in large amount in the adjacent mesenchyme — all indicate that in great measure the pictures presented in this specimen are largely artifacts. It seems most likely, though, that some differentiation of the tissue in this area has occurred. | |||
In a human embryo of 14 mm., as in the pig of the same stage, the area membranacea superior has attained a great degree of differentiation. This is particularly well shown in figures 56 and 57, the latter being an enlargement of the squared area in the former. These photomicrographs are from embryo No. {{CE144}} of the collection of the Carnegie Institution of Washington. Figure 57 shows a characteristic which distinguishes the area membranacea from that of the pig, although in the later stages of the pig embryo (figs. 45 and 47) this feature is present. This concerns the marked decrease of cellular tissue in the membranous area. In figure 57 the deeply stainmg typical ependyma is shown approaching from below. These cells end abruptly at the border of the area membranacea; the ventricle in this area is lined by cells possessing small elongated nuclei and long cytoplasmic processes, which unite to form a ventricular lining. The oval nuclei along the ventricular border become more closely massed together in the superior portion of the area, but nowhere is there the same architecture as in the equivalent stage in the pig (fig. 33). A feature of the histological appearance of the membranous area in the pig embryo is also shown in figure 57; this is the marked adherence of the coagulated albumen of the cerebro-spinal fluid to the area membranacea superior. | |||
The roof of the fourth ventricle in the human embryo is subjected to the same factors causing changes in the form and relationships which were commented upon in the pig; but these play little part until the chorioid plexuses become of sufficient size to divide the ventricle into a superior and inferior portion. In the human embryo, as in the pig, the superior half of the ventricular roof is sacrificed to the greater growth of the cerebellum. | |||
* Measured on the slide after mounting. | |||
In human embryos of 17 mm., however, these factors have not begun to influence the membranous area. This is shown in figures 58 and 59, photomicrographs from embryo No. 57G. The section is somewhat to the side of the midline, but in the superior portion of the roof of the fourth ventricle the differentiated membranous area can be made out. The sharp delimitation of this area from the denser t3T5ical ependyma on both sides is quite apparent. The ragged character of the ventricular border, with its few elongated spindles, seems wholly in keeping with the transverse view of this area afforded by figure 37. | |||
Embryo No. 576 exhibits one characteristic of the area membranacea superior very frequently seen in human embryos, but almost invariably absent in these stages in the pig. Along the lateral margins of the superior membranous area are dense borders of the many-layered epithelial-like cells which lined the ventricular roof in younger stages. This feature is well shown in figures 60 and 61, the latter figure being a higher magnification of the former. The cellular border of the superior area reaches transversely only through a few 15-micron sections, but it extends throughout the whole cephalo-caudal diameter of the area. It seems likely that this represents purely a survival of the epithelial-like cells in the younger embryos. In rarer instances the whole area membranacea superior may be surrounded bj^ such a border of many-layered cells, but even in these cases the superior and inferior margins are quite thin. | |||
No apparent agencies favoring the disappearance of the superior membranous area m the roof of the fourth ventricle of the human embryo are apparent in stages up to the fetus. Thus, in human embryos of 18 mm. this differentiated area in the roof has reached its maximal differentiation. A section from an embryo of this size (embryo No. 409) is reproduced to show the distortion and its influence upon the topography of the area membranacea. The two photomicrographs (figs. 62 and 63) show the extreme collapse and distortion of the roof of the fourth ventricle. In the figure of higher power (No. 63) the membranous area appears facing posteriorly, due to the shrinkage; the proper leader runs to this area. It shows the differentiation from the adjoining tj'pical ei)endyma which is characteristic of the full}' developed area membranacea superior. | |||
In a beautifully preserved and sectioned human embryo of 21 mm. (No. {{CE460}}) in the collection of the Carnegie Institution of Washington the area membranacea superior appears as a sharply delimited area (figs. 64 and 65). These figures give a very good idea of the definiteness of this area when the fixation and dehydration approach the perfect. The tissue of this membranous area lining the ventricle here appears to be wholly lacking in an epithelial covering; the mesenchyme seems to serve as the ependymal lining. Study of this area, however, through different stages argues most strongly against such a view. | |||
he process of regression of the area memhranacea sujjerior in the human embryo differs somewhat from that described in the pig. This alteration in the mode of disappearance is largely due to the fact that in the period of growth from 20 to 35 mm. the superior portion of the roof of the fourth ventricle in the human embryo is not sacrificed to the cerebellar lips; for in the human the cerebellum grows largely into the fourth ventricle, enlarging beneath the superior part of its roof. Thus, the attachment of this part of the roof is not greatly interfered with by the rapid development of the cerebellum. The total extent, then, of the superior portion of the roof is hardly altered in these stages in the human, while in the pig embryo the roof is shortened by its attachment to the inferior portion of the cerebellar hp, which retains its earUer characters. These differences in the relationship of the superior portion of the ventricular roof in human and pig embryos may be seen by comparison of figures 74 and 89. | |||
Another factor which renders the mode of disappearance different in the two embryos concerns the greater tufting and development of the chorioid plexuses of the fourth ventricle in the pig. This greater size and complexity of the plexus causes an encroachment upon the roof structures which, in the pig embryo, seems of considerable importance in the t'nal closure. | |||
In the human embryo, however, it has been found very difficult to explain the final disappearance of the superior membranous area on the same mechanical factors w^hich seemed so well to account for its transitory characters in the pig; but at approximately the same stage of growth the process of regression occurs in the human fetus. The area maintains a fair size in stages up to a length of 23 mm. Thus, in figures 89 and 90 (No. {{CE453}} of the Carnegie collection) a sagittal section from a human fetus of this size is illustrated. In the higher power (fig. 90) the superior membranous area is short, rather sharply delimited on its superior border by the typical, dense ventricular ependyma. Below, its edge is irregularly formed by the deeply staining ependyma over the invagination of the chorioid plexus. The cell-character of this area resembles that shown in the photomicrographs from the specimen of 21 mm. (figs. 64 and 65). There is left in the area no indication of the cellular architecture which characterized the original ventricular ependj-ma; the cells with their elongated cj'toplasmic processes here have the oval nuclei which are found almost invariabh' in this membranous area. | |||
In the human fetus of 26 mm. (No. {{CE1008}} of the collection of the Carnegie Institution of Washington) there is but sHght evidence of a superior membranous area in the upper portion of the roof of the fourth ventricle. The evidence present in this specimen consists in a localized thickening of the lining cells of the ventricle in the situation of the area in other stages. This thickening is illustrated in figures 91 and 92; it consists of several layers of epithelial-hke cells, similar in all respects to the many-layered border shown in figure 83. The picture is somewhat obscured by the vascular pl-^xus directly beneath the ventricular lining. | |||
There is difficulty in determining exactly when the last evidences of the superior membranous area in the roof of the fourth ventricle may be found. This is due to the likelihood of artifacts disturbing the character of the ventricular lining in human material, where the freshness and fixation of the specimen may not be ideal. In the larger specimens in the collection of the Carnegie Institution, which are well fixed and sectioned, the existence of the area membranacea superior could not be wholly verified. Thus, in specimen 405 (26 mm.) the presence of the area seemed probable though not definite. In another embiyo of this same size (So. 782) the existence of this area was still more questionable. In a larger embryo (30 mm. No. {{CE75}}) the presence or absence of the area could not be assured; many indications suggested its existence, but the resemblance to an artificially separated ependyma was strong. In all specimens of human embryos of over 30 mm. examined, no evidence of the area membranacea superior could be found. It appears likely, then, that the final disappearance of this differentiated area in the roof of the fourth ventricle occurs at a slightly earlier stage in the human embryo than in the pig. | |||
The final disappearance of the area membranacea superior in the human embryo is not accompanied by the same ingrowth of typical ependyma that characterizes the process in the pig. There is a great tendency, in the human, as indicated in figure 92, for a replacement of the area by the same type of epithelial-like cell which comprised the whole ventricular roof in the earlier stages (fig. 41) and later formed lateral borders for the superior membranous area (fig. 83). Thus, in a human embryo of 24 mm. (No. G32 of the Carnegie collection) there is evidence of a very small membranous area surrounded by a border of epithelial-like cells. In a slightly larger specimen (No. {{CE840}}, 24.8 mm.) the whole membranous area is occupied by the epithelial-like cells. The frequent association of these cells with the area indicates that in disappearing the area membranacea is probably replaced first by these cells, which in turn disappear, so that the whole roof is finally composed of the typical, densely staining ependyma. | |||
===The Area Membranacea Superior in Other Animals=== | |||
In order to ascertain whether the area membranacea superior existed in other animals examinations of serial sections of the rabbit, cat, sheep, and chick of suitable stages were made. All of these animals were found to possess a differentiated area in the roof of the fourth ventricle. | |||
Opportunity was afforded for the study of serial sections of the head of a chick* of 121 hours' incubation. The head was carefully dehydrated and embedded by Dr. E. R. Clark, and was subsequently sectioned bj' Dr. C. R. Essick. The material was beautifully fixed and dehydrated, showing practically no evidence of shrinkage. Typical portions of the superior membranous area are reproduced in figures 66, 67, 68, and 69. Figure 67, taken near the crown of the embr3-o and representing the squared area in figure 66, shows the two dense masses of ependyma separated by the more lightly staining area membranacea. The cellular character of this differentiated zone resembles more the histological features of the similar afea in the pig than those of the human embryo. This resemblance is also to be seen in figure 69, taken more posteriorly than the two preceding figures. The dense ependynia approaching on both sides is sharply delimited at the edge of the hroad membranous area. This is composed of cells having elongated, chromarin-poor nuclei, and long cytoplasmic processes, which form the ventricular roof. The adlierence of the albuminous coagulum occurs here also. | |||
* The chick measured 14 mm. in 40 percent alcohol. | |||
In the rabbit the occurrence of the superior membranous area was verified as in the other species studied. In a rabbit embryo of 13 mm. (series x in the embryological collection of this laboratory the area was well differentiated from the surrounding typical ependyma. The cells of the area resembled those of the adjacent mesenchyme. The ventricular surface was roughened by the projection of numerous protoplasmic processes. An albuminous coagulum was attached to the cells of the membranous zone. | |||
One sheep embryo from the collection of this laboratory was also studied. The sections, although labeled as an embryo of 10 mm., resembled in every way a pig embryo of 18 mm. The area membranacea was easily identified in the roof of the fourth ventricle; it is similar in every respect to the same area in the pig and the human embrjo. | |||
In a cat embryo of 10 mm. a smiJl but highly differentiated area membranacea superior was made out. The most striking feature in this specimen is the great adherence of the coagulated albumen to the cells of the area and the resemblance of these cells to the mesenchymal elements adjacent. The edges of this differentiated area are sharp and clear-cut. | |||
No attempt was made to identify the area membranacea superior in other animals — as further suitable material was not immediateh- available. The chief study has been made on pig embryos and on human embryos. The occurrence of the area in the cat, sheep, and rabbit probably indicates its existence in aU mammals. The finding of such an area in the chick is also suggestive. | |||
===General Consideration of the Area Membranacea Superior=== | |||
The occurrence of a definite area of differentiation in the superior portion of the roof of the fourth ventricle has been pointed out in preceding subdivisions of this paper. It has been described in detail in the pig embryo and in the human embryo; it has been identified also in cat, sheep, rabbit, and chick embryos. It remains here to discuss the general characteristics of this area. | |||
No description of such an area of differentiation in the ventricular roof has been found in the literature. It may be that the distortion of this structure in the course of the usual embryological technique has rendered its discovery Iess likely. His'-='', m his description of the ventricular roof, has not commented upon the occurrence of this membranous area, even though in a retouched photomicrograph of his fetus C-1 (a human specimen, of the beginnmg of the third month) the area membranacea superior can be made out. Likewise in his description of the pUca chorioidea he faJs to mention any differentiated areas in the roof, although plate I, in his "Die Entwickelung des menschUchen Rautenhims, von Ende des ersten bis zum Beginn des dritten Monats," shows a slight irregularity in the roof. Practically all of the contributions to the anatomy of the roof of the fourth ventricle deal with the lower half of the structure, with particular reference to the occurrence of the foramen of Magendie. | |||
The general biological process involved in the formation of the area membranacea superior concerns a dilTerentiation of the epidermal elements which Une the ventricular cavity. This differentiation, both in human and in pig embryos, first begins with the occurrence in the ventricular roof of an area of epithelial-hke cells. These, in the course of enlargement of the roof, become more or less isolated in the superior portion of the structure, and then undergo a metamorphosis into the typical cells of the membranous area. They are characterized by oval or elongated nuclei (rather poor in chromatin as compared with the nuclei of the typical ependjonal elements) and by cytoplasmic strands (in which the cell-boundaries are very poorly marked) which compose the ventricular border. The ventricular surface in the area membranacea is more ragged and u-regular than where lined by typical ependjana. In many instances, as in figure 57, from a human embryo of 14 mm., this transformation has proceeded to such an extent that the epithehal character of the lining cells is almost wholly lost, and the ventricle seems, in this area, to be lined by mesenchyme. Study of the membranous area in many stages convinces one that such an hypothesis is untenable; in every case the ventricle must be considered as being lined by epidermal elements, no matter to what extent the process of differentiation has proceeded. There is no real evidence to support the view that the ependymal lining of the ventricle has been replaced by mesenchymal elements to form the area membranacea superior. | |||
In general the area membranacea superior is a rounded oval. Its measurement is quite difficult except when fixation and dehydration have been excellent, because of the highly abnormal distortion of the ventricular roof which frequently occurs in the technically poor specimens. Measurements have been made in a considerable number of favorable specimens, both of human and pig embryos. With the history of this area in mind, it will be realized that the size of the structure necessarily varies with the length of the embryo, attaining its greatest dimensions at about the length of 18 or 20 mm. Herewith is a short table of the measurements taken. | |||
Dimensions of area membranacea superior. | |||
Species. | |||
No. of specimen. | |||
Length of embryo. | |||
Width of area. | |||
Length of area. | |||
Species. | |||
No. of specimen. | |||
Length of embryo | |||
Width of area. | |||
Length of area. | |||
Pig | |||
98 107 144 119 106 | |||
mm. | |||
12 | |||
13 | |||
14* | |||
14 | |||
14 | |||
mm. 0.37 0.95 1.25 0.45 0.65 | |||
7iim. 0.5 0.4 1.1 0.6 0.85 | |||
Pig | |||
1 mm. | |||
121 1 16 | |||
576 1 17 | |||
108 18 (?) | |||
45 18 | |||
84 1 22 | |||
mm. 0.6 1.5 0.8 0.9 0.8 | |||
mm. | |||
0.48 | |||
0.9 | |||
0.8 | |||
0.4 | |||
0.7 | |||
Rabbit | |||
Human | |||
Pig | |||
Human | |||
Sheep | |||
Pig | |||
Chick | |||
Pig | |||
In a rough way, then, we may consider the area membranacea as an oval; in some cases the longitudinal diameter exceeds the lateral, and in others the reverse holds. The measurements given above were taken from mounted sections and are probably somewhat disturbed by the histological tcchnifjue which was followed. | |||
* MuisurecI on alidc uftpr iiiouDting. | |||
The borders of this oval area membranacea are usually fairly regular and smooth, but in some instances they are irregular, due to the fact that small extensions of the area run into the bordering ependyma. These extensions are more commonly met with at the stage when the area has reached its maximum size, as in figures 38 and 39, photomicrographs from an embryo pig of 19 mm. The higher power of these two photographs shows two areas in the smoother ependymal wall. These are extensions of the area membranacea, and within a section or two directly connect with the differentiated area. Both of these small spots on the circumference resemble technical errors; their ragged appearance, the relative excavation of their surface, and the intact ependymal borders w'ould seem to encourage such a view; but when considered in connection with the character of the whole area membranacea they assume a definite relationship in this regard. Other similar areas, rather rare in occurrence, are found separated entirely from the main area membranacea. These isolated areas are of the same size as those shown in figure 39. In significance and character they are probably identical with the larger area membranacea superior. | |||
Most of the general features of the area membranacea superior have been commented upon in descriptions of the various stages of differentiation in both pig and human embryos. The characteristics most commonly observed concern the differentiated character of the cells of the area, the sharp borders of the typical ependyma, the ragged ventricular surface throughout the whole extent, and the peculiar adhesion of the albuminous coagulum from the embryonic cerebro-spinal fluid to the lining cells. The area membranacea superior should be considered, then, as a transitory focus of differentiation of the typical ependymal hning of the roof of the fourth ventricle. | |||
===An Undescribed Area In The Inferior Portion Of The Roof Of The Fourth Ventricle=== | |||
With success attending the effort to find in the superior portion of the rhombic roof an anatomically differentiated area which would furnish a morphological basis for the jihysiological phenomenon of the extraventricular passage of the cerebrospinal fluid, attention was necessarily directed to the inferior portion of this roof (considering the whole roof structure to be divided by the chorioid plexuses). The spread of the replaced injection fluid (fig. 4) into the periaxial tissues through two points in the roof of the ventricle suggested a study of this stage (pig embryo of 18 mm.) as the basis of the investigation. As a histologically differentiated area in this inferior portion of the roof is easily made out, the complete history of the area will be given chronologically. It has been termed the "area membranacea inferior ventricuU quarti," the terminology being based on the same physiological and anatomical features which led to its adoption in the case of the analogous area in the upper portion of the roof. | |||
===The Area Membranacea Inferior In The Pig Embryo=== | |||
The inferior portion of the fourth ventricle shows no evidence of a differentiation from the typical lining ependyma until the length of 15 mm. is reached. In this development consideration must be given to the factors concerned in the process. It will be recalled that in the younger embryos, both pig and human, up to and including a length of 9 mm. the whole roof of the ventricle is occupied by the epithehal-like cells. With rapid growth of the medulla and corresponding enlargement of the fourth ventricle the roof becomes elongated and widened. This process results in the isolation of the area composed originally of the ejiithelial-like cells and the subsequent formation of the superior membranous area. The epithehal-like cells remain in the superior portion of the enlarged ventricular roof, while the whole inferior half is composed of the densely staining, typical ependyma. The division of the roof by the laterally developing chorioid plexuses becomes evident in pig embryos of 14 mm. At this stage the whole inferior portion shows a ventricular lining composed of the typical ependyma. | |||
The first indication of a differentiation in this inferior half of the roof was found in a pig embryo of 15 mm. This is illustrated in figures 70 and 71. The sagittal section from which these photomicrographs were taken is near the mid-line of the embryo, as is indicated by the partial section of the central canal of the spinal cord (fig. 70). The division of the ventricular roof into two parts is also indicated in figure 70 by the invagination of the chorioid plexus. The squared area in the lower half is reproduced in figure 71 under higher magnification; here the first evidence of an ependjonal differentiation is observed. The dense Une of the typical ependjona appears from both sides, but in the center of this ventricular lining a small area of differentiation is seen. This area, isolated by the abruptly terminating pyknotic ependymal elements, is composed of two or three layers of less deeply staining cells. The nuclei are round, rather larger than those of the adjacent mesenchyme, and contain httle chromatin. The cytoplasm stains fairly well with eosin and is not scanty in amount. The cells resemble those epithelial-hke elements which so largely make up the ventricular roof in the earlier stages. No albumen is found near this point of differentiation, although the whole ventricular cavity is filled with the normal amount. In figure 70 the marked zone of the area membranacea superior may easily be seen. | |||
After this initial indication of a differentiation in pig embryos, the further differentiation of the tissue proceeds but slowly until the length of 18 mm. is attained. Thus, in a similar specimen from an embryo pig of 18 mm. the area of differentiation is not greatly hicreased in size. This is shown in figures 72 and 73. In the higherpower figure (fig. 73) both the superior and inferior membranous areas can be made out by the attachment to these areas of the protein coagulum of the ventricular cerebro-spinal fluid. | |||
In the higher-power figure (fig. 73) of the squared area from figure 72, the area membranacea inferior shows the same character as exhibited by the specimen of 15 mm. (fig. 71). The opening maintains the same approximation to the lateral lip of the medulla, but the area is larger and the histological character more nearly approaches the permanent feature of the tissue. The nuclei in this zone are paler than those of the adjoining ependymal elements and contain less chromatin. The cytoplasm is not scanty, nor is it very abundant in amount. The area is also characterized by the occurrence of the cells in a layer, two or three cells in thickness. | |||
In view of the very slow differentiation of the area membranacea inferior in the growth of the embryo from 15 to 18 mm., the enormous enlargement of the region within the next few millimeters growth is very astonishing. This period, as has been jjointed out, is a critical one in the extension of the embryonic cerebro-spinal fluid from a ventricular to a periaxial relationship. Apjjarently, in the course of the embryo's growth during these next few millimeters the whole inferior roof of the ventricle undergoes a transformation and enlargement, so that the differentiated area membranacea comes to occupy practically the whole inferior half of the roof. This portion of the roof, persisting, enlarging, and suffering no extension of nervous t'ssue upon it, becomes the tela chorioidea inferior. | |||
The rapid differentiation of the whole iiiferior half of the roof of the fourth ventricle is a very interesting process. Apparently the typical ependymal elements, visible on both sides of the membranous area in figure 73, undergo a very rapid alteration, so that in the course of a few millimeters' growth the cubical lining of the ventricle is replaced by a low- type cell, with round or oval nuclei, staining much less densely than do the ependymal elements. The whole area membranacea rapidly becomes a membrane in the true sense of the word; it is a continuous, intact laj'^er of cells, generally only one cell in thickness, closing in the fourth ventricle from the chorioid plexus above and the bulbar lips on the sides. | |||
The general characteristics of this transformation are seen in figures 74 and 75. These photomicrographs are taken from a sagittal section of a pig embryo of 23 mm. On one side of the sharply deUmited membrane shown in figure 75 is a tongue of nervous tissue of the medulla; on the other is the differentiated ependjina of the chorioid plexus; between these two structures stretches uninterruptedly the area membranacea inferior. The flattened cells of the membrane, with their oval nuclei and almost continuous cytoplasm, effectually close the whole ventricle. The photomicrograph also shows an interesting characteristic of this membranous area which is universally present in the larger forms; this is the relatively unsupported character of the membrane. The highly vascular mesenchyme posterior to the area has gradually developed, during growth, larger and larger interstices between the cytoplasmic processes. The phenomenon is not due to shrinkage, but is intimately connected with the formation of the future cisterna cerebello-medullaris. This phase of the mesenchymal differentiation will be more fully considered in an appropriate section of this paper. It will suffice here merely to record the lack of support of the membrane. | |||
Another phenomenon of unportauce in the cerebro-spinal fluid relationships of this stage is shown in figure 75. In the mesenchjanal spaces directly beneath the membranous area there is a large amount of albuminous coagulum. This phenomenon does not occur to any appreciable extent in earlier stages or in other parts of the mesenchyme, except about the nervous system. The c1o.se association of the coagulum from the ventricular cerebro-spinal fluid with the inner border of the area membranacea (shown in figure 75 as a slight roughening of the border) is of very great significance in this connection. In one i)oint in the membranous area (fig. 75) the albumen can be traced almost without interruption from the ventricle into the wide spaces of the mesenchyme (cf. fig. 8). This observation strongly suggests that the embryonic cerebro-spinal fluid, which is rich in protein material, is passing, in this stage of embryonic growth, from the ventricle into the periaxial mesenchyme; and such an interpretation becomes established by the comparative findings in the embryo of the same stage in which a replacement of the cerebro-spinal fluid by the ferrocyanide solution had been effected. These comparable findings are surelj^ of the utmost importance for the final solution of the problems centering about the embryonic cerebro-spinal fluid. | |||
In the later stages of development of the area membranacea inferior in the pig embryo the same structural relationships persist that are shown in figure 75. Figures 76 and 77 are photomicrographs taken from a sagittal section of a specimen of 32 mm. In the enlargement of the squared area, from the first of these figures, the continuity and completeness of the membrane are well established. The photograph shows well the flattened character of the cells comprising the membrane and its sharp differentiation from the nervous tissue and ependyma below and from the ependj'ma and chorioid plexus above. Most important in this case is the distribution of the albuminous coagulum. Within the ventricular cavity this appears in considerable amount, and in several places it is in close adhesion to the lining area membranacea. This albuminous precipitate may likewise be traced in some places apparently through the cellular membrane into the periaxial spaces. For here, as indicated in figure 75, the clotted albumen from the cerebro-spinal fluid apjjarently exists in large amounts in the space just posterior to the membrane — the future cisterna cerebello-medullaris. Delicate strands of mesenchyme are still observed running through the wide space, but in general the whole tissue has returned to the line of the future arachnoid. The relative lack of substantial support of the membrane is well brought out in figure 77. A characteristic feature of this membrane, which Blake'3^ has championed, and which is indicated in figures 76 and 77, is the posterior bulging of the roof — "the caudal process like the finger of a glove." | |||
Another section from the same pig embryo, taken more laterally, is represented in figures 78 and 79. In the photomicrograph of higher power the flattened character of the lining cells, the intactness of the membrane in isolating the ventricular cavity, the unsupported freedom of the membrane, and the relation to the albumen coagulum on both sides are of |)articular interest. | |||
The ultimate fate of the area membranacea inferior will not be more fully entered into until the early history of the similar area in the human embryo has been detailed. For in this connection the occurrence of the foramen of Magendie requires discussion, and it seems best to delay the further consideration of the present topic until the whole question can be reviewed. | |||
THE AREA MEMBRANACEA INFERIOR IN THE HUHMN EMBRYO. | |||
The same process in the formation of an area of differentiation in the inferior portion of the roof of the fourth ventricle may also be followed in the human embryo. Unfortunately, however, human omliryological material can rarely be subjected to the immediate fixation and preservation which j'ield excellent histological results in the more plentiful specimens. It does not seem strange, therefore, that the determination of the exact stage at which an area of differentiation can be made out in the ventricular roof should be practicall}' impossible; for, in poor technical procedures, the roof of the fourth ventricle suffers almost more than does any other portion of the specimen. | |||
In a human embryo of 13 mm. (No. {{CE695}} in the collection of the Carnegie Institution of Washington) there is slight evidence of a differentiation in the lower portion of the rhombic roof. The changing character of cells in this specimen is not marked, but as the central portion of this inferior roof is reached the ependymal cells seem to assume gradually a more cubical morphology. Associated with this change in shape, there is also a slight loss of the deeply staining character of their nuclei. The whole differentiation, however, is slight and would be commented upon only from the conception of this area in the pig embryo. | |||
The first definite evidence of differentiation in the inferior portion of the ventricular roof was found (specimen {{CE390}} in the Carnegie collection) in a human embryo of 15.5 mm. This initial differentiation occurs, then, in the human embryo of approximately the same length as in the pig. The specimen showed the same change in character of the hning ependj-ma as was found in the pig. The deeply staining ependymal elements are replaced in a limited central area in the inferior portion of the roof by cells with more elongated nuclei, poorer in chromatin, and resembling somewhat the epithehal-like cells which early filled the ventricular roof. These cells tend to compose a layer of more than one cell in thickness — a feature particularly noticeable in the peripheral portions. | |||
The size of the area membranacea inferior observed in specimen {{CE390}} suggested that the earUest evidence was probabh' to be observed in somewhat smaller specimens. This could not, with the material at my disposal, be verified, but it is probablj^ safe to assume that the first signs of an ei)end\Tnal differentiation will be found in human embryos of about 15 mm. This time of appearance of the area in the human would coincide with its time of primarj* differentiation in the pig embryo. In this limitation of the first appearance of the area membranacea inferior, the standard has been an unmistakable differentiation of ependyma and not an isolated change of a lining-cell or two which might have been the result of the technical procedure. Such a criterion was necessitated by the verj' marked changes in the ventricular borders observed in specimens in which distortion of the chorioidal roof had occurred. | |||
The area membranacea inferior very rapidly increases in extent after the onset of the process of ependymal differentiation. This was hkewise observed in the pig embryo, although perhaps more stages could be made out. In a human embryo of 16 mm. (No. {{CE406}} of the collection of the Carnegie Institution) the area nierabranacea inferior is quite extensive, as is shown in figures 80 and 81. In the photomicrograph under higher power (fig. 81) the denselj' stained ependyma approaches the membranous area (ami) as tongue-hke processes from above and below. These tips gradually lose their dense character and are prolonged as a delicate membrane, lining, in this localized area, the ventricular cavity. The nuclei of the cells here are not heavily laden with chromatin; they are oval and somewhat larger than the more densely packed nuclei of the typical ependj-mal element. Unfortunately, the middle portions of the membranous area in this specimen are surrounded by extravasated red blood-cells obscuring somewhat the structure (fig. 81). The process, though, of the differentiation of these ependymal elements into paler and larger epithelial-like ceUs is quite apparent. | |||
As in the pig, the tendency of the differentiated ependymal cells forming the area membranacea inferior to lose in some degree their distinctive appearance and to approach in character the undifferentiated mesenchymal element is apparent in the human embryo very shortly after the original steps in the process of differentiation have occurred. Photomicrographs from two human embryos of 17 mm. have been included to show this phenomenon. Thus, in figure 88, an enlargement of the blocked area from figure 58, the area membranacea inferior (ami) is well defined. The sagittal section from which this photomicrograph was taken is from embryo No. {{CE576}}, in the Carnegie collection. Above and below the dense fine of ependj-ma may be made out; this tapers quite abrupt!}', to be succeeded bj' the cells of the area membranacea inferior. These cells, products of ependymal differentiation, have lost much of their epithelial-like appearance; they now show rather small, oval or rounded nuclei, poor in chromatin. The cytoplasm of the cells is small in amount, but not disproportionate for the size of the nucleus. The ventricular border of these cells (fig. 88) exhibits a rather characteristic phenomenon, the adherence of a shght albuminous coaguluni. The fine processes of this coagulum fuse^ith the cytoplasmic borders of the cells and render these borders vague and indefinite. Beneath the cells of this inferior area small vascular channels may be made out. These tend to make the membrane appear denser than its cellular character warrants. | |||
In another section from this same embryo (No. {{CE576}}) the inferior membranous area is shown in relation to the tufted chorioid plexuses (figs. 82 and 83). In the reproduction under higher magnification (fig. 83) the ependymal lining may be traced caudalwards to a gradual fusion into the area membranacea inferior. From the rather high cubical cells in the immediate proximity to the plexuses the ependymal elements become reduced in size and in height, and then rather abruptly the pyknotic character of the ventricular lining is lost. This loss of the deeply staining character coincides with the superior border of the area membranacea inferior {ami). The membrane of this area shows the same cell-character as already desorilied for this embryo. On the superior side of the plexuses (fig. 83) the lateral border of the area membranacea superior (ams) is shown composed of epithelial-like cells. | |||
The apparent tendency of the cells composing the inferior membranous area to lose the epithelial-like character, as shown in the figures from embryo Xo. 576, is not an invariable phenomenon. Rather is an aggregation of epithelial-like cells met with in human embryos very commonly in this area, not onlj- in embryos of small size, but also in small fetuses. This phenomenon is illustrated in figures 84 and 85, reproductions of photomicrographs from a human embryo of 18 mm. (No. {{CE409}} in the collection of the Carnegie Institution). In figure 85 the total transverse extent of the area membranacea inferior (ami) is illustrated, with the villous chorioid plexuses appearing to the left. Although this membranous portion of the embryo has been distorted somewhat by the technical procedures to which the specimen was subjected, the cellular character of the membranous area is well indicated. The most striking feature, apart from the characteristic tinctorial differentiation from the typical ependymal elements, consists in the marked clumping of the cells in certain parts of the membrane. On one lateral extent the membrane is thickened into a bulbous swelling several cells in thickness. These cells have palely staining nuclei, poor in chromatin, with an oval or round form. In other places in the membrane smaller but no less characteristic clumps of similar cells maj' be made out. Between these cellular aggregations the membrane stretches in a continuous line with but few nuclei. | |||
Analogous clumps of cells, with pale, rounded or oval nuclei, may be made out in figures 86 and 87, taken from a human embryo of 19 mm., No. {{CE431}} in the collection of the Carnegie Institution. Only a small portion of the membrane is reproduced in the figure under higher magnification, but a characteristic clump of epithelial-like cells (epc) is shown. These cells of the differentiated ependyma here again have oval and rounded nuclei, poor in chromatin, similar to those which have been pointed out manj^ times in the foregoing pages. A second broadened area in the inferior membrane is also showm in figure 87. | |||
The further development of the area membranacea inferior proceeds in the human embryo in a manner very similar to that described for the pig. In the stages but shghtly above those already described the differentiation goes on slowly, but withui a few millimeters the cellular pictures resemble those given for the embryo of 17 mm. (figs. 82, S3, and 88). The cellular clumps which appeared quite frequently in the embryos under 20 mm. have not been found in the larger forms. Thus, in an embryo of 23 mm. (No. {{CE453}} in the collection of the Carnegie Institution) the inferior membranous area {ami) appears as an extensive membrane comprising almost wholly the inferior portion of the chorioidal roof. The membrane is here of a single cell in thickness; these cells are rather small, with oval nuclei, simulating in some measure those of the surrounding mesenchj-me. The most mteresting phase of the membranous area at this stage of 23 mm. concerns its completed cellular differentiation and its rather slow increase in size. | |||
Wholly similar pictures of the inferior membranous area of the roof of the fourth ventricle are afforded by a human fetus of 26 mm. (figs. 91 and 92). These photomicrographs were taken from embryo No. {{CE1008}} in the collection of the Carnegie Institution. In this specimen (fig. 92) the fourth ventricle seems almost to lack a lining of oj^endymal (epidermal) elements in the area membranacea inferior (ami). The cells of this area are small, inconspicuous ua their distinctions from the underlying mesenchjme. The whole character resembles that of the superior area membranacea shown in figure 57. | |||
The appearances exhibited by the inferior membranous area in the stages above 26 mm. are modified in great part by the development of the great cisterna cerebellomedullaris. As in the pig, the breaking-down of mesenchyme to form this cistern results finally in the almost total isolation of the inferior membranous area. The cistern is fairly rapidly formed when once the process begins, and so in an embryo of 35 mm. (No. {{CE199}} in the Carnegie collection) the isolated character of the area membranacea inferior (a?ni) may be easily made out. This is shown in figure 94, an enlargement of the blocked area in figure 93. The general architecture of the membrane, particularly its intact character, appears in this photomicrograph, but its finer structure is obscured by the albuminous coagula which adhere on both surfaces. The cell structure of the area membranacea resembles closely that described in the embryos already pictured. | |||
Discussion of the final disposition of the area membranacea inferior will be undertaken in the following subdivision of this paper, in order that the findings in the pig and in the human embryo may be correlated. | |||
===General Consideration of the Area Membranacea Inferior=== | |||
The ependymal lining of the caudal portion of the roof of the fourth ventricle undergoes a process of differentiation which results in the formation of the area membranacea inferior. This transformation has been observed in pig and human embryo | |||
s; in both, the first definite evidence of the cellular change has been observed in specimens of 15 mm. The essential phases of the process are identical in the two embryos. The tendency of the deeply staining typical ependymal elements is to lose their highly pyknotic character; the nuclei become poorer in chromatin and the cytoplasm somewhat more abundant. In the first stages of the metamorphosis the lining cells come to assume epithelial-like appearances, but in the final change the nuclei become small oval bodies, poor in chromatin, resembling to some degree the nuclei of the adjoining undiff'erentiated mesenchyme. In the human embryo, a tendency for the epitheUal-like characters to persist in isolated cellular aggregations is apparent. | |||
After the initial process of differentiation has begun, the area membranacea inferior increases rapidly in extent and the differentiated cells which characterize it come to occupy the greater portion of the caudal part of the chorioidal roof. In the somewhat later stages the area membranacea is almost wholly unsupported by other tissues, due to the development of the cisterna cerebello-medullaris. As soon as the cistern forms, the area membranacea serves as practically the sole dividing membrane between the ventricular system and the future subarachnoid spaces. | |||
The ultimate fate of this area membranacea inferior is necessarily involved in the distribution of the tela chorioidea inferior. Likewise it necessitates a discussion of the possible formation of the so-called foramen of Magendic and its mode of origin from the "caudal process" of Blake. It is proposed to discuss briefly some of these questions in the hope that some phases of the problem may be brought forth. | |||
It must be clearly understood that the questions of the ultimate fate of this area membranacea inferior probably differ considerably in the different species of mammals. In the horse and in the pig the absence of the medial foramen (]\Iagendie) is fairly well established, but in man its existence .seems to rest on equally firm grounds. While, ))rimarily, this investigation has not been concerned with the possible existence of the foramen of IMagendie, the question has been presented manj' times in regard to the pig and human embryos examined. | |||
As far as can be determined, no descriptive study of the development and differentiation of the inferior portion of the rhombic roof has been published. Heuser's'23' studies on the form of the cerebral ventricles of the pig have afforded a very good conception of the gradually changing relationships in this region. Hess(22j has devoted attention to the histological appearances of the inferior roof in the embryo. One of his interesting obseivations concerns the caudal portion of the rhombic roof in a fetal cat of 10 cm., where he noticed a very sudden interruption in the epithelial lining of the ventricle, with a complete closing by a fibrous net. This description by Hess is the only comment upon the histological appearance of the ventricular roof that has been found. His^^s^ pictures, without comment, in a retouched jihotomicrograj^h, a differentiated area in the proper situation in his fetus C-1 (beginning of the third month) . | |||
The many writers in embryology have commented upon the roof of the fourth ventricle. Minot, in 1892, stated regarding it: | |||
:"Several writers have thought that the membrane was broken through at several points, but it probably is really continuous throughout life. The fourth ventricle is to be regarded, then, as an expansion of the central canal permanently bounded by the original medullary walls." | |||
Kollman (32) on the other hand, advances the view that during the third month the rhombic roof is broken down to form the foramen of Magendie and the two foramina of Luschka. Streeter^S'*), in his chapter on the development of the nervous system in the Keibel-i\Iall Handbook of Embryology, advances a similar view. The majority of investigators to-daj' incline to the beUef that the roof of the fourth ventricle in man is perforated to form the median foramen of ]Magendie. | |||
jjpgg (22) has advanced a conception of the foramen of Magendie that is supported by numerous observations. To test KolUker's statement that the fourth ventricle remained closed during human embryonic life. Hess sectioned the region in human fetuses, new-born infants, and in adults. The lengths of the fetuses cut were as follows: 7, 12.5, 15, 16, and 17 cm. In the 47 cases the roof showed a medial opening (IMagendie), except in one case, in which it was closed by a "thin pial membrane." Hess's conception of the process of formation of this membrane was that in earh' embn'ological life the rhombic roof was bordered by a regular, meshed tissue. Later the small meshes in this tissue fused to form the larger foramen of Magendie. | |||
Blake's (2) hypothesis of the formation of the medial foramen has been quite extensively quoted in the more recent publications on this subject. In a study of the chorioidal roof Blake found a caudal bulging of the inferior velum; this outpouching became more and more extensive in the older embryos.. In man this pouch became sheared off at its neck, leaving the foramen of IMagendie. | |||
In addition to the few studies referred to above, there have been in the past 25 years a great mmiber of articles (notablj' those of Wilder^^s) and Cannieu^^^) offering evidence that this median foramen of the fourth ventricle is an existent, functional opening. Into this literature it is not proposed to go in the present communication; it may be stated that in the larger part the views presented have been in favor of the consideration of the true occurrence of the foramen of IMagendie. | |||
The material on which this study is based has been purely embryological in type, so that no relialile data regarding the foramen of IMagendie could be obtained. But even in the largest fetuses examined, there was no evidence which indicated a breaking-down or a shearing-off of the inferior roof of the fourth ventricle. In the largest human fetus at my disposal, in which the histological material was good enough to permit an accurate examination of the chorioidal roof (embryo No. 448, 52 mm. in the Carnegie collection) the area membranacea inferior appeared as an intact membrane supported only be a few pial cells. In the pig the material at hand has been such that accurate study of the roof could be made in specimens up to 20 cm. ; in all of these later fetal pigs the roof has been wholly without foramina. If, however, in these larger stages the histological procedures have not been of the best, ruptures and other artificial separations are very frequently found. | |||
The area membranacea inferior, then, may be regarded as a region of ependymal differentiation. Whether it persists as an intact membrane or undergoes, in certain animals, a perforation to form a foramen of Magendie can not be here answered; this study has been concerned solely with the embrj^ology of the cerebro-spinal spaces, and it affords no evidence in favor of or against the existence of such a foramen. Nor has any study been made of the two foramina of Luschka, the two openings from the lateral recesses of the fourth ventricle into the subarachnoid spaces. It can Ije stated, however, that these foramina arc not in existence at the time of estal)lishment of the circulation of the ccrebro-si)inal fluid. This phenomenon, as recorded in the previous section, occurs in pig embryos of 26 mm.; at this time the lateral reces.ses are anatomically and physiologically closed. | |||
of | |||
==VI. Passage Of Fluid Through Roof Of The Fourth Ventricle== | |||
On pages 20 to 'SO is a description of the passage of a true solution, substituted without increase in pressure for the embryonic cerebro-spinal fluid, through the roof of the fourth ventricle into the extravcntricular or periaxial spaces. This extension of fluid occurred in two localized areas, one in the superior half and the other in the inferior half of the rhombic roof. Histological study of these regions revealed a localized differentiation of the ependyraa, both in the upper and lower halves of the ventricular roof. It becomes necessary, then, to correlate, if possible, the areas of this fluid-passage to the anatomical differentiations pointed out. | |||
===The Accumulation of Injection-Masses in the Superior Membranous Area=== | |||
It has already been recorded that the first evidence of a change in the reaction to a replacement injection occurred in an embryo about 13 mm. long (fig. 2). This stage was characterized by a dense collection of the precipitated granules in a definite area in the roof of the fourth ventricle. At this stage also the area membranacea superior is well differentiated (fig. 31). That the site of the granular accumulation is this membranous area is easily proved by an inspection of figure 117, which represents an enlargement of the squared area in figure 116. In the low power photomicrograph the prussian-blue granules are not represented, but are found scattered through the ventricles, with a definite collection in the posterior region of the fourth ventricle. Under a higher magnification (fig. 117) the blue can be traced in but small quantity along the normal ependj'mal lining (shown to the left in the figure), but as soon as the differentiated area (area membranacea superior) is reached the granular material is heaped up in a dense mass, which extends as a thickened pad into the ventricle. | |||
The same phenomenon of the accumulation of the injection fluid in the superior membranous area is shown in figures 112 and 113, the second photomicrograph representing the area outlined in the first, but reproduced under much higher magnification. In this specimen (an embryo pig) a dilute solution of silver nitrate was injected into the central canal of the spinal cord. On histological examination the accumulation of the silver also shown in figure 11 was found. Thus, in figure 113 the ventricular epithelium can be made out in the upper right-hand corner, while below (in the area membranacea superior) the silver is densely accumulated. | |||
The explanation of this phenomenon of accumulation in the superior membranous area is not whoUj' clear. It occurs only in stages in which the histological differentiation of the ventricular roof has proceeded to some degree and in stages where the fluid-passage into the periaxial tissues is not wholly unobstructed. This aggregation of the precipitated granules of prussian-blue and of the reduced silver in a locaUzed area certainly suggests a phj'sical exi^lanation, as in these cases the physical laws of precipitation and reduction must hold. The many figures of the superior membranous area of the ventricular roof show that in the stage under consideration the cell-outlines projecting into the ventricles are rough and ragged as contrasted with the smoother and more regular surface of the adjoining ependyma. | |||
Could not these roughened, irregular cell-surfaces become the site of the first and most extreme precipitation of the prussian-bluc and of the reduction of the silver? Certainly they would serve much more efficiently as the foreign substances about which precipitation would occur in greatest amount. This phj^sical explanation finds man}' arguments for its suj^ijort in these studies. | |||
Another explanation of the phenomenon concerns the normal flow of the fluid and the relation of the direction of this flow to the roof of the fourth ventricle. As has already been emphasized, it is difficult to assume that there is any marked production of cerebro-spinal fluid before the periaxial spread occurs. Such an assumption would argue against the development of any special current toward the roof of the fourth ventricle in any stage smaller than that represented in figure 3, and would vitiate the explanation of the occurrence of the granular accumulation shown in figure 2 (a pig embryo of 13 mm.). In the later stages (16 mm., cj. fig. 11) this explanation would probably suffice for the phenomenon exhibited. | |||
===The Sites of Fluid Passage Through the Roof of the Fourth Ventricle=== | |||
With consideration of the evidence presented as to the accumulation of the precipitates of the injected fluid about the area membranacea superior during certain stages in the development of the cerebro-spinal spaces, it would seem that the same area must be concerned in the passage of fluid from the ventricular cavities into the periaxial tissues. This view receives support from the reproduction of a cleared specimen (fig. 11) in which an injection of silver nitrate had been made into the central canal of the spinal cord. The pressure employed was great enough to force the fluid into the periaxial spaces, but the resultant picture clearly showed the oval outline of the area membranacea superior. | |||
The study of the passage of fluid from the ventricular to the extraventricular spaces can best be made by simple histological serial sections. In these observations pig embryos in which the cerebro-spinal fluid had been replaced by the compensating device, supplying a true solution of potassium ferrocyanide and iron-ammonium citrate, were sectioned and examined with reference to the sites of fluid passage. The results of these studies are given here in order that the whole question of the connection of the cerebral ventricles with the subarachnoid spaces may be discussed. | |||
In the stage represented by figure 3 (in which fluid passes from one area in the roof of the fourth ventricle into the extraventricular tissues) histological sections show that the point of fluid ])assage is localized and concerns solely the area membranacea superior. The replaced fluid (as demonstrated by the subsequent precipitation of the prussian-blue) passes through this entire membranous area into the adjoining mesenchyme. The process is wholly confined to this area; the adjoining ependyma is entirely imj)ervious to the ferrocyanide. This phenomenon of passage of the replaced fluid through the sujjerior membranous urea is well shown in figures 14, 18, and 23. | |||
. | The distriltution of the minute granules of ])russian-l)lue in the cells of the superior membranous area is of iini)ortance in any discussion of the passage of fluid through a membrane; for this area (in the superior portion of the roof of the embryonic fourth ventricle) must be considered as a memljrane permeable in certain degrees to the fluids bathing it. That the area mcml:)ranacea is intact and does not contain stomata or other minute foramina has been demonstrated histologically. Further evidence of the entire lack of intercellular stomata is afforded by the distribution of the prussian-blue granules precipitated in situ after the replacement of the cerebro-spinal fluid by the ferrocyanide solution. | ||
Figure 14 is a reproduction of the superior area from a transverse section of a pig embrj^o in which the routine replacement had been made. The position of the area is shown by the squared outline in figure 13. On both sides the impermeable ependyma is seen, with granules of the blue adhering to the ventricular border of the cells, but not penetrating them at all. To the left of the drawing the few ependymal cells possess, beneath their central border, a chain of the granules which have entered from the abrupt edge of the area membranacea. In the cellular border between the two hps of the ependyma, the area membranacea superior, the passage of the replaced fluid is easily made out by the resultant blue granules. The area is roughly deUmited by a ventricular collection of the blue granules. Examination of these cells shows that the prussian-blue is present within the cytoplasm, avoiding the nuclei with perfect precision. Some of the cells are rounded and almost free from the granules; others, particularly those whose cj'^toplasm is elongated, are completely filled with the granules, the nuclei standing out in a blue granular cytoplasm. | |||
The question of the passage of the fluid between the cells must also be answered by the histological evidence. In the same drawing (fig. 14) in one or two places there are indications of a slight stream of granules between the cells of the area membranacea superior. This apparent transit of the fluid through intercellular passages is particularly clear in the small areas where the cellular cytoplasm is relatively free from the granular deposits. But upon careful examination of these areas under oil immersion it is always apparent that the adjoining cytoplasm is also mvolved in the granular precipitation, indicating that the cells, although almost free from the deposit, are also engaged in the process of the fluid passage. Compared to the whole area of fluid transit, the points indicative of a passage through possible intercellular stigmata are almost negUgible. It seems not unlikely that the outlining of canals between cells may be a physical phenomenon, as in most cases no cellular borders (as demonstrated bj' the precipitated granules) can be made out. These pecuUarities of fluid passage may be seen in figures 14, 18, and 23. | |||
Consideration of all the evidence afforded by histological examinations of the essential character of the area membranacea superior and of the passage of fluid through it incUnes one inevitably to the belief that this area functionates as a cellular membrane. The fluid passes through it as through any permeable liv-ing membrane. Histologically the passage is for the most part through the cj-toplasm of the cells, but occasionally an intercellular course is suggested. Both processes are wholly compatible with the accepted view of a cellular membrane de\dsed for the passage of fluid through it. | |||
mm. | The same phenomenon of the passage of fluid from the fourth ventricle into the periaxial spaces is beautifullj' illustrated in figure 23. This drawing is from a transverse section of a pig embryo (23 mm. in length) in a stage when the superior membranous area is rapidly being encroached upon by the developing cerebellum and by the caudal chorioid plexuses. Between the deeplj' staining epondj-mal cells on cither side the membranous area is densely outlined by the deposition of the granules of prussian-blue in the cytoplasm of the cells of the area membranacea superior. The avoidance of the nuclei of these cells by the ferrocyanide is well demonstrated in this reproduction, as is also the impenetrability of the ependymal cells. In a specimen of this nature the question of the passage of the injection fluid through possible intercellular foramina loses its significance; for the drawing shows clearly the importance of considering the entire area membranacea as a functioning whole — a permeable, Uvmg, cellular membrane. | ||
It has been shown in a foregoing section of this memoir that histologically the area membranacea superior decreases to an almost neghgible remains in specimens of embryo pigs over 30 mm. long. This same rule apparently holds for its functional importance, as determined by the relative and absolute amount of prussian-blue granules deposited in the cells of the superior membrane. This decrease in the functional imi;)ortance may be inferred from figure 47, a photomicrograph from a pig embryo of 32 mm. Apparently the size of the membrane determines in large measure the amount of the replaced fluid which passes through it. | |||
Thus far we have been concerned solely with the passage of fluid through the area membranacea superior. In the earUer stages of from 14 to 23 mm. the importance of the superior membrane functionally is great, but in the later stages the inferior membrane assumes far greater significance. This is demonstrated not only by the structural history of the two areas, but by the functional index afforded by the replacement of the cerebro-spinal fluid by a foreign solution. | |||
In the foregoing section the first evidence of any histological differentiation in the inferior portion of the roof of the fourth ventricle was shown to occur in pig embryos of 15 mm. in length. From this stage upwards (figs. 4, 5, etc.) a portion of the inferior roof allows fluid to pass through it. The exact point of fluid iiassage is the localized ependymal differentiation forming the area membranacea inferior. This relationship is easily verified by reference to figure 18. In this drawing of a median sagittal section of a pig embryo the two localized pomts of fluid passage into the periaxial tissue are readily identified; they are quite Umited in comparison to the extent of the periaxial spread. | |||
Figure IG represents the inferior membranous area of the roof of the fourth ventricle from a i)ig embryo of .similar size (18 mm.). The histological character of the inferior area is well shown in this drawing. It will be seen that, except in small areas, the histological differentiation of the ependyma has not proceeded to any great extent; the fluid from the ventricular cavity (as traced by the precipitated granules) closely follows the points of greatest cellular differentiation. There is no possibility of an intfri)r('tation of the findings concerned with the existence of intercellular stomata; the passage of fluid is here again to be looked upon as a transit through a cellular membrane. | |||
The same general i)heiioinena of the pa.ssagc of fluid through a localized area (the area membranacea inferior, hi the caudal portion of the roof of the fourth ventricle) that have been observed in the superior portion of the roof are shown in figure 18. Cliief among these phenomena is the careful avoidance bj- the precipitated granules of the ependymal lining of the ventricles and the adherence of the granules to the Uning walls at the points of fluid passage. The ependymal lining, except in the two areas of differentiation, is everywhere impenetrable to the solution of the ferrocyanide. | |||
As the size of the embryo increases the functional importance of this more caudal area becomes much greater (c/. figs. 3, 4, 5, and 76). The whole caudal half of the fourth ventricle becomes an area of ependj-mal differentiation and of fluid passage. It serves everywhere as a complete diff'using membrane, un))roken by the occurrence of stomata. Through this whole membrane the replaced solutions of potassium ferrocyanide and iron-ammonium citrate pass with apparent ease, as demonstrated by the precipitated granules of prussian-blue (fig. 18). From stages of 24 mm. and over, the lower membranous area is the onlj' one of significance in the total fluid passage. | |||
The areas, therefore, through which the replaced solution of potassium ferrocyanide and iron-ammonium citrate jiassed, in the experimental pig embryos, are the two areas of histological differentiation in the roof of the fourth ventricle — the areae membranacese superior et inferior. There is no evidence whatsoever of any other point of escape of the fluid from the ventricular system into the periaxial spaces. The precipitated prussian-blue does not penetrate any of the lining cells of the ventricle except in the two areas under consideration. Nor is any evidence afforded by histological study of the escape of ventricular fluid through the described foramina of Bichat and of ^Mierzejewsky. | |||
===Factors Concerned in the Experimental Fluid Passage=== | |||
It becomes necessary to discuss the question of the passage of the replaced fluid through the two cellular membranes in order to ascertain to what extent the results obtamed by the method may be relied upon. Naturally in such questions the factors concerned in the normal transit of body-fluid through such structures must be considered. | |||
Probably the most essential element in obtaining rehable results in any injection is the control of the pressure at which the foreign fluid or mass is introduced. This matter has been fully discussed in the resume of the methods employed; it is suflRcient to reaffirm here that, in these observations, the normal cerebro-spinal tension has not been disturbed because of the use of a compensatory replacement. Other experiments, carried out under increasing pressures of injection, have been made, in order to compare the results with those furnished by the replacementmethod. | |||
Consideration must next be given to the factors of diffusion, filtration, and osmosis in the passage of fluid through the roof of the fourth ventricle. The third factor, however, may be largely excluded, owing to the fact that the solutions of potassium ferrocyanide and iron-ammonium citrate employed were for the most part practically isotonic with the body-fluids. Furthermore, the use of hypertonic solutions apparently gave no difi"crent results (except in the increased density of the resultant precipitate) from those obtained by the employment of the isotonic solutions. Finally, it was found to be of service to use hypotonic replacement solutions in order to obtain very sUght precipitates; in these experiments also the spread of the replaced ferrocyanide solution was similar to the standard result afforded by the isotonic solution. These observations with varying concentrations of the foreign solutions replacing the cerebro-spinal fluid serve to indicate that osmosis plays but little part in the passage of fluid through the roof structures of the fourth ventricle. Undoubtedly the factor of osmosis can not be ignored in any consideration of the passage of fluid through a cellular membrane, but it seems unhkely that with solutions of practically the same salt-content it should be of great importance. | |||
The influence of diffusion in this passage of the solution of the ferrocyanide and citrate from the cerebral ventricles into the extraventricular space is probably great. The whole plan of the experiment concerns the introduction of salts foreign to the body-fluids, even though in analogous concentrations. It seems not unhkely that as soon as the replacement of the existent cerebro-spinal fluid is effected the ferrocyanide and citrate must immediately begin to diffuse out into the periaxial tissues and the normal salts return to the ventricles. Probably, however, this same phenomenon plays a normal role in the human body. Jacobson's^^?) extensive and important studies on the chemistry of cerebro-spinal fluid have shown that the ventricular cerebro-spinal fluid is not identical with the subarachnoid fluid. The differences in the two fluids are probably to be accounted for by the fact that the ventricular fluid represents the pure elaboration of the chorioid plexuses, whereas the lumbar subarachnoid fluid is composed not only of the products of the chorioid plexuses but also of the fluids from the perivascular system. In this transference of the ventricular fluid to the subarachnoid space diffusion may play some part, the relative importance of which can hardly be estimated. | |||
But will diffusion alone accoimt for the passage of the experimental fluid in the ventricle through two well-defined areas into the periaxial tissues? Will diffusion account for the varying extent of the injection in different stages of embryonic develojjment? There are several arguments against according diffusion a maximal imjjortance in the process. In the first i)lace, an injection of the solution of the ferrocyanide under mild syringe-pressure will give a spread similar in every re.spect to thos(; obtained by the replacement method. This indicates that the course taken by the two solutions is not necessarily the result of diffusion, but rather of the capabilities of the tissues for fluid-spread; and similarly the jiassage of this true solution through the roof areas need not be solely a diffusion process, but may be accounted for by the true flow of the fluid in this direction. Again, in the stages represented in figure 2 one would expect as extensive a spread of the replacing solution into the periaxial tissue were diffusion the active force in the movement of the fluid. Instead of such a periaxial spread the injection fluid remains wholly within the ventricular system, indicating that other forces than that of diffusion play an active role, in the more advanced stages, in the movement of the fluid. Finally, if diffusion is to be considered the sole agent in the distribution of the replacing fluid, why does not the ferrocyanide penetrate all the cellular structures lining the ventricular cavity? Surely it would he expected that diffusion between the body-fluids and the ferrocyanide solution would occur in each ependymal cell — a phenomenon observed only in the cells comprising the ventricular surfaces of the membranous areas of the rhombic roof. | |||
While acknowledging that diffusion and osmosis may play important parts in the process of the passage of fluid from the fourth ventricle into the periaxial tissues, it seems apparent that some other factor or factors must be the determining agent or agents. It is not unlikely that the formation of cerebro-spinal fluid by the cells of the chorioid j)lexus may cause, in the replacement experiments, further passage of fluid into the extraventricular regions. Such an elaboration of fluid, with the ventricles filled with the experimental solution, would result in an increase in the normal ventricular tension. If this be the real explanation, the passage of the fluid into the extraventricular spaces would result in part from the increase in pressure on one (the ventricular) side of the membrane. The process, then, would be one of filtration through the membrane from the point of higher to that of lower pressure. This explanation best seems to cover the results obtained by the replacement method, and is supported by the histological examination of the developing chorioid plexuses and by many other features which are dealt with in other sections of the paper. This view is also strongly supported by the results of injections under mild syringe-pressure . | |||
On the basis that the passage of fluid from the fourth ventricle into the periaxial tissues is in large measure a process of membrane filtration, the phenomenon of the fluid transit of the replaced solutions may be taken as a real index of the circulation and distribution of the cerebro-spinal fluid. It may be assumed, therefore, that the resulting distribution of the prussian-blue granules represents the course and extent of the fluid channels of the embryonic cerebro-spinal fluid. | |||
The discussion of the fluid passage outward from the cerebral ventricles into the subarachnoid spaces has thus far been concerned with the processes involved for the transit of the true solutions of the salts. There is, however, an undoubted passage outward, as has already been indicated in a foregoing section, of the protein content of the normal cerebro-spinal fluid. This occurs in specimens in which a trul}- definitive membrane, intact throughout, can be seen inclosing the chorioidal roof. The explanations which suffice for the passage outward of the true solutions will not serve for this phenomenon. | |||
The cells of the body probably are equipped to handle colloidal solutions in several ways, but two methods seem possible as explanatory of the problem at hand. | |||
In the first place, it is conceivable that the cells in the differentiated areae membranaceae could phagocyte the colloidal albuminous particles of the ventricular fluid and excrete them into the subarachnoid spaces on the other side of the membrane; but it does not seem probable that this explanation is correct. Much more likely is it that the colloidal masses may follow the same laws of fluid-passage as the true solutions. But in such a passage through a cellular membrane the rate of passage will be much slower with the colloid. | |||
These two theories regarding the passage of the albumen of the ventricular cerebro-spinal fluid into the subarachnoid spaces are not based on any findings presented in this article, but are ventured as being in keeping with current physiological explanations of such phenomena. On the basis of the second hypothesis, the failure of granular material to pass through the cellular membrane of the chorioidal roof must be explained as being due to the inability of the cells to handle the foreign material except in sizes which could be absorbed. The fact that the original unit was not phagocyted or passed through the membrane probably depended on the size of the molecule and the specific character of the lining-cells. | |||
THE PASSAGE OF SILVER NITRATE AND INDIA INK THROUGH THE MEMBRANOUS AREAS IN THE ROOF OF THE FOURTH VENTRICLE. | |||
Thus far in the discussion of the passage of the experimental fluids through the ventricular roof, true solutions of potassium ferrocyanide and iron-ammonium citrate only have been considered. This solution, as has been pointed out in this and in a previous article^^^)^ jg non-to.xic and is not taken up by the cells. With the dilute solutions (0.25 to 0.5 per cent) of silver nitrate, a far different problem is presented. Replacement experiments with this salt are rendered impossible bj- its intraspinous toxicity and by its precipitating action upon protein; but beautiful preparations may be made by this method by the simple injection with a syringe into the central canal of the spinal cord. | |||
With mild syringe-pressure the result of such an injection with silver nitrate is in all cases a simple ventricular spread, with no extension into the ])eriaxial tissues. This general rule holds in all stages in which the central canal can be definitely entered without causing a spread into the j^erispinal tissues. This failure of the spread to extend into the periaxial tissues under mild pressure is undoubtedly due to the coagulating effect of the silver, which renders further passage of the fluid impo.ssible. The reduced silver collects about the superior membranous area in the roof of the fourth ventricle, outlining it distinctly. This phenomenon is illustrated in figure 115 (a transverse section of a pig emljryo of 19 mm.). At this stage the rejjlacement of cerebro-spinal fluid by a ferrocyanide solution results in a quite extensive spread {cf. fig. 5). | |||
With increa.sed pressures of injection the silver may be pushed into the periaxial tissue through the roof structures of the fourth ventricle. The transit of the injection-mjuss occurs in the area meml^ranacea sujjcrior in practically all cases (c/. fig. 12). The inferior membranous ar(>a, in the earlier stages, is almost invariably impermeable to the silver (unless the injection-jiressure i.s extreme). When the superior area is examined after such an injection under high pressure the silver is found deposited throughout the cells of the area, extending only a short distance into the adjacent tissue. This feature of the injection is pictured in figure 113. In these injections the high pressure undoubtedlj' suffices to force the silver through the coagulated area membranacea. Its coagulating effect on the ependjina is almost equally marked, but the point of least resistance is apparently in the membranous area, allowing the fluid to pass through it. | |||
Replacements of the cerebro-spinal fluid with diluted solutions of mdia ink within the medullary-canal system of small pig embryos never result in any extension of the granules into the periaxial tissues, for under the normal tension in the ventricles of the pig the arese membranaceaj are impermeable to the passage of granular material. After such a replacement the carbon masses maj' be found everywhere throughout the ventricles, but not in the periaxial tissues. However, india ink may be forced into the periaxial tissues by the use of high pressures of injection, as shown in figure 10. In this s])ecimen of a pig embryo (21 mm. m length) the periaxial spread occurred solely from the superior membranous area. This is analogous to the results obtained with silver nitrate, shown in figure 12. Without doubt in the earlier stages the superior area is much more permeable than the inferior. Histological examination of these specimens after an injection of india ink under high pressure reveals that the carbon granules gain the extraventricular space onh' through the area membranacea superior; some cells in this area are crowded with the granules, but for the most part extensive intercellular stomata have been made. The whole process must be viewed as a result of the excessive pressure of injection. | |||
In the more advanced stages of the pig embryo (30 mm. and upwards) the pressure necessary to occasion an extraventricular spread of the india ink after intraspinous injection decreases somewhat, so that \\ith mild syringe-pressure a local periaxial sjn-ead from the fourth ventricle may be obtained from an injection into the central canal of the spinal cord. This is in accordance with the observation of MalU'^), who found that the injection flowed "through the medial opening of the fourth ventricle." The opening in these cases is in the area membranacea inferior, and in many instances subsequent examination showed rupture of the membrane with escape of the ink, even though the injection-pressure was moderate. | |||
Taken as a whole, then, the findings are against the passage of solutions of silver nitrate or suspensions of india ink from the ventricles into the periaxial tissues, except when injected under pressures far above the normal intraventricular tension. | |||
RELATION OF THE EPENDYMAL DIFFERENTIATION TO THE PASSAGE OF FLUID. | |||
Under this heading it is jjroposed to discuss the relationship, if any, existing between the stages of differentiation of the ependjona of the roof of the fourth ventricle and the prssage of fluid through the two membranous areas. The discussion must necessarily be of a temporal character, with an attempt to consider possible factors in the process. | |||
The most important question in this connection is whether the ependymal differentiation is necessary for the passage of fluid through it. In tlie \ng embryo of 13 mm. the area membranacea superior has reached a stage of marked differentiation (fig. 31), but at this same stage (fig. 2) there is no evidence of any pas.sage of fluid through the roof of the fourth ventricle into the periaxial tissue, only an outlining of the oval membranous area. Here, then, the histological differentiation has definitely preceded the assumption of function on the part of the area membranacea superior. The passage of fluid through the lower area occurs at a relatively earlier stage than it does through the superior opening. The first evidence of differentiation of the inferior roof of the fourth ventricle was observed in pig embryos of 15 mm. in length. At 18 mm., even though the process of differentiation was far from complete, some of the replaced fluid was able to pass through the lower area (figs. 4, 16, and 18). | |||
A | A consideration of these observations leads to the assumption that some histological differentiation of the ependj^ma is necessary for the extraventricular passage of the replaced fluid. In the case of the superior area the differentiation occurs at a considerable developmental interval before fluid passes through it; in regard to the inferior area the assumption of function occurs at a somewhat earlier period in its differentiation. This slight difference between the two areas may possiblj' be explained on the basis that as soon as the stage of 14 mm. is attained (by the pig embryo) a greater amount of cerebro-spinal fluid is produced than can be cared for by the more slowly enlarging ventricular cavities. As soon as this disproportion occurs the excess of fluid is poured into the periaxial tissues through the already differentiated area membranacea superior; therefore, when the inferior area first shows evidence of formation there is still this excess of fluid in the ventricles. The fluid apparently avails itself almost at once of the new opening and its functional existence becomes immediate. It is apparent, moreover, that the capacity of the membranous areas for the passage of fluid is considerably in excess of the demands made upon them, and furthermore, that the provision for the passage of increasing amounts of fluid is completed before the demand arises. | ||
In the passage of fluid from the ventricles into the mesenchyme, there is one other factor which has not as yet been considered. This concerns the iKitentiality of the adjacent mesenchyme to afford channels for the fluid poured into it. Were resistance offered to the flow of solutions through the mesenchymal tissue spaces, fluid could escape from the ventricles in only very small amounts, if at all ; as soon, however, as easily traversed fluid channels became established, the cerebro-spinal fluid could readily escape through the two membranous areas. The question as to what part the embr\'onic cerebro-spinal fluid i)lays in the further development of the meningeal si)aees also arises in this connection. It is at i)resent impossible to assign to any one of these factors a specific role in the passage of fluid from the fourth ventricle into the jieriaxial spaces, but it is important to consider them as possible determining agents. The evidence all indicates that the rate of production of the embryonic cerebro-spinal fluid is the most important factor, by far, in the extraventricular escape of the fluid. | |||
==VII. General Histological Differentiation Of The Cerebro-Spinal Spaces== | |||
The general problems concerned in the formation of the meninges and of the spaces inclosed within them deal with the gradual adaptation of a primitive undifferentiated mesenchyme to the anatomical and physiological requirements of the adult. Originally the meninges were held to be derived from the same epidermal infolding which gave origin to the central nervous system; then, with increasing knowledge of the structure, the dura alone was said to be a product of the middle germ-laj'er; and finally, by the researches of His^^s) and of K6lliker,'3i) the mesenchymal origin of the three meninges was established. The general process of the differentiation and the stages in this transformation have not been reported in great detail; here, too, the investigations must have an outlook for physiological anatomy as well as for pure morphology. | |||
It may be well to comment briefly on the relationships of the three meninges found in adult mammals. The dura is well estabUshed as the fibrous-tissue envelope of the leptomeninges and the central nervous system. But there is a tendency to regard the arachnoid and pia mater as constituting one structure — the leptomeninges or "pia-arachnoid," in the terminology of Middlemass and Robertson'^"). This difference of opinion in regard to the two inner meninges is due to their structural and intimate relationships. The arachnoid may well be a.ssumed to be a single membrane, worthy of being regarded as a single structure if one considers only its outer continuous membrane as the essential structure. But the inner surface of this membrane sends processes inward to fuse with the pia mater, which is so closely applied to the ner\-ous tissue. These processes divide the subarachnoid space (mcluded between arachnoid and pia) into the well-known meshes in which the cerebro-spinal fluid circulates. From the standpoint of these channels (the subarachnoid spaces) the arachnoid constitutes the parietal and the pia the visceral layer. Thus the intimate structural unitj' of the two membranes seems, in the opinion of many investigators, to warrant their designation as a single membrane. This view, however, has been strongly opposed by Poirier and Charpy^*^', who considered the distinction of three meninges very essential. Hence, in considering the transformation of tissues in the embryo, regard must be had for the dura as a well-differentiated structure, and for the leptomeninges as units, but certainly to be regarded from the standpoint of the subarachnoid spaces. In tliis connection Sterzi's'^^') observations on the comparative anatomy of the meninges are of interest. It will be recalled that the dura in lower forms becomes well estabhshed before the leptomeninges emerge from a primitive mesenchyme. | |||
THE PERIAXIAL MESENCHYME. | |||
Surrounding the central nervous system in young embryos is a rather thick cushion of undifferentiated mesenchyme, similar m all respects to the undifferentiated tissue in other parts of the embiyo. But verj' soon in the course of development the nuclei in this mesenchyme increase along the clear marginal zone of the spinal cord ami ha.silar structures, forming the initial indication of the pia mater. This phenomenon is indicated somewhat in figure 40, a photomicrograph taken from a human embryo (Xo. 836) of 4 mm., the earliest stage here illustrated. | |||
The next essential change in the great differentiation of the meninges concerns a blastemal condensation of this same mesenchymal tissue to form ultimately the bony covering of the central nervous sj'stem and a portion of the dura; but between these two zones of differentiation the mesenchyme remains for a time almost unaltered. A portion of this tissue will go to form the arachnoid membrane and the trabeculaD which mark off the subarachnoid spaces. This process in the formation of the arachnoid will be discussed here; the formation of the pia mater and dura will be detailed in succeeding divisions of the paper. The differentiation will be discussed as a general process, in regard to both human and pig embryos, for in no respect has any essential difference between the two been observed. | |||
The general character of the periaxial mesenchyme may be commented upon here. The tissue is of a verj' loose and typical structure, forming a syncytial network of rather small mesh, but fragile. The nuclei of the cells are oval, with a definite chromatin content; the cytoj^lasm is largely devoted to the maintenance of long processes which connect with adjacent cells. Adhering to the cj^toplasmic processes are very tiny albuminous coagula, of such small amount as to be hardly noticeable; also in the meshes of the mesenclwme very small quantities of this albumen may be identified. These albuminous coagula undoubtedly represent the protein of the tissue fluids in the undifferentiated stages. | |||
THE FORMATION OF THE ARACHNOIDEA. | |||
A general consideration of the problems here involved will surely shed light on some of the various factors concerned. It must be noted that in its development this membrane proceeds from an undifferentiated but small-meshed mesenchyme into the adult structure which contains the relatively large cerebro-spinal channels. Then, too, the enlargement of the tissue meshes in certain places — as the future cistcrnae — must be enormous. Besides this necessary dilatation of the spaces in the periaxial m(>senchymc, the outer portion of the tissue must separate from the future dura and form the outer surface of the arachnoid membrane. Here the process must be one of tissue condensation and proliferation. A similar agencj' is involved in the growth of the mesothelial cells which cover the outer surface of the arachnoid and also the inner suliarachnoid spaces. | |||
The g(>n<'ral process, then, in the formation of the arachnoid membrane concerns a thiiuiing and readjustment of the primitive mesenchyme in certain areas, while in others the process is reversed, the membrane reaching the adult form through proliferative and condi-nsing i)henonieiia. Such alterative processes must naturally result from the apj)lication of certain mechanical or vital agents in the growth of th(? embryo. Is the mere growth of the central nervous system sufficient to furnish these alterative agents, or must we likewise trace the corres])onding development of the bony coverings of the brain and spinal cord? X{>ith(>r factor seems relatively of great importance when comi)ared to the possible influence of the presence and circulation of cerchro-spinal fluid on this periaxial tissue. This seems to be the most important factor, an internally-modifying influence to which the periaxial mesenchyme is subjected in the formation of an arachnoid and its subarachnoid spaces. It will therefore be from this standpoint that the development of the spaces will be discussed; for, as has already been pointed out, the periaxial mesenchyme becomes a functionally active tissue for the circulation of the cerebrospinal fluid at a stage when difi"erentiation has not begim. On this basis, the lack of differentiation shown in the |)eriaxial mesenchyme in the stages before the ventricular cerebro-spinal fluid is poured into the mesenchyme in the neighborhood of the roof of the fourth ventricle is not surprising. The character of the periaxial mesenchyme in the early stages is reproduced in numerous photomicrographs (figs. 25, 49, 51 , and 53). The mesenchj'me is here characterized by a rather dense meshwork of cytoplasmic processes, interspersed b^' a considerable number of oval nuclei. The content of the interstices in albumen, as judged by the persisting coagula, is very small. This picture of the periaxial mesenchyme persists untU cerebro-spinal fluid is poured from the ventricle through the area membranacea superior. | |||
As will be seen in figure 3, the first indication of an extraventricular spread of the replaced fluid in the ventricles occurred in a pig embryo of 14 mm. At this stage the membranous area in the superior portion of the roof of the fourth ventricle has already' become well differentiated. The fluid from the ventricles, however, does not reach any considerable spread until after a length of 18 mm. is attained; the periaxial spread during this period of growth is wholh^ confined to the peribulbar tissues. It is quite important in this connection that the first obvious differentiation of the mesenchyme for the formation of the arachnoid .should appear during this period and should involve the peribulbar tissues. | |||
The first change to be noted in the transformation of primitive mesenchjone into the future arachnoid is an obvious thinning of the structure with a decrease in the number of nuclei per unit-volume. This is made out in a photomicrograph (fig. 57) of a section from a human embryo 14 mm.* long, when contrasted with a similar mesenchymal area posterior to the ventricular roof (fig. 53). In the pig embryo this thinning of the mesenchyme is as obvious at this early stage. | |||
The process of dilatation of the mesenchymal spaces at this stage hardly seems to concern a direct disruption of the syncj'tial strands, but resembles more the spreading of the cell-bodies by the introduction of more fluid into the tissue spaces. This process would certainly result in an ap])earance similar in everj' way to that represented by figures 35 and 57. It probably also concerns other factors, as, possibly, the growth of the whole embryo without a corresponding degree of mesenchymal proliferation. | |||
In a human embryo of 17 mm. (Xo. 576) evidences are apparent of such a thinning of the mesenchyme about the medulla. Thus, in figures 58 and 59, from this specimen, the cellular decrease can be made out both in the region of the roof of the fourth ventricle and around the basilar surface of the medulla. It wdll be noted that the differentiation (i. e., the thinning) about the roof has proceeded more rapidly than along the anterior bulbar surface. This is perhaps to be expected in view of the initial pouring-out of the cerebro-spinal fluid into the mesenchyme just posterior to the roof. | |||
*This embryo measured 14 mm. on the slide. | |||
In this mesenchymal differentiation a slightly increased amount of albuminous coagulum may be noticed. The truth of this is made obvious by an examination of figure 61, a photomicrograph from a human embryo of 17 mm. The almost entire freedom of the mesenchyme from albuminous detritus is most noticeable at earlier stages. | |||
As was pointed out in the description of the results of replacing the cerebrospinal fluid, a marked change in the rate of development of the cerebro-spinal spaces in the pig-embryo ensues just after attaining the length of 18 mm. Within the growth of 2 mm. the injection spreads completely down the spinal cord and about the basilar structures of the cerebral cavity. This rapid extension finds its analogous process in the equally rapid changes which may be traced in the periaxial mesenclnTTie. Thus, in figure 72, a photomicrograph from a sagittal section of a pig embryo of 18 mm., the whole nervous tissue appears surrounded by a very thin, Ughtly staining tissue; this is the periaxial mesenchyme, which is undergoing its rapid metamorphosis. It will be noticed in this figure that the posterior structures (rhombencephalon) are surrounded by a much less dense mesenchyme than are the anterior (mesencephalon). This relative differentiation between the bulbar tissue and that around the mid-brain is only of temporal character; the mesenchyme about the medulla, as has already been pointed out, begins to differentiate first, the differentiation of the mesenchyme about the other nervous structures following somewhat later. | |||
Figure 73 is a photomicrograph of higher power, taken from the squared area in figure 72. It shows to what a surprising degree the mesenchymal differentiation has proceeded during a few millimeters' growth. Two striking features of the process are brought out in this reproduction. In the first place, many of the mesenchymal trabecular have apparently been broken down, sacrificed to a few larger remaining strands. The cells connected with the destroyed trabeculie appear to recede until one of the heavier surviving strands is met with, when they adhere and apparently aid in the future development of a permanent arachnoid trabecula. The second feature of importance in figure 73 concerns the large amount of allnimen seen in the periaxial space. There is here a much greater amount of albumen than is ever found in the periaxial mesenchyme before the differentia ting process which results in the future subarachnoid space has become definite. The occurrence of this large amount of albuminous coagulum is apparently related directly to the outflow of the cnibrj'onic cerebro-spinal fluid, for the embryonic fluid is very rich in protein material, a.s can be readily seen by the partial filling of the embryonic cerebral ventricles with the clotted albumen. | |||
This process of the l)reaking-down of the mesenchymal spaces to form fewer and hirger spaces goes on very rapidly in pig embryos as the\' exceed the length of 18 mm. Thus, figure 75 (from a pig embryo of 2.3 mm.) shows a marked decrease in the mesenchymal elements al)out the medulla; the strands are becoming fewer in number, and the albumen-filled spaces are increasing rapidlj' in size, but decreasing in number. About the mcsencej)halon, however, the process has only just begun (also shown by fig. 74). In this photomicrograjih (fig. 75) the mesenchj^mal elements have broken down somewhat; the spaces are Ijecoming enlarged, and a fine albuminous coagulum fills the interstices between the mesenchymal processes. The whole picture conveys an excellent idea of the forces which convert the many-spaced mesenchyme into the much fewer cerebro-spinal channels. | |||
This general plan of the formation of the larger subarachnoid canals reaches its maximum in the formation of the various cisternae for cerebro-spinal fluid. The process is probably best illustrated in the case of the cisterna magna, which persists in the posterior cerebello-bulbar angle. Figures 74 and 75, taken from an embryo pig 23 mm. long, give an idea of the initial formation of the cisterna cerebellomedullaris. The mesenchymal strands, as shown in figure 75, are already broken down in part, and are profusely covered with albuminous coagula. The process has not proceeded to any extent in this specimen of 23 mm., but in the course of the next 10 millimeters' growth extensive changes occur, as arc shown in figures 76 and 77, photomicrographs from an embryo of 32 mm. In the space outside the inferior membranous area the mesenchymal trabecula? have almost disappeared; the space — or cistern, as it should now properly be called — is almost completely filled with the clotted albumen. The mesenchjTne is seen running through this embryonic cistern as a few isolated strands, but most of the tissue appears now as a fairly definite membrane on the outer side of the space. This membrane will go to form the inner surface of the dura and the continuous outer layer of the arachnoidea, as it furnishes a visceral layer for the subdural space. | |||
More laterally in this same specimen the formation of the cistern has progressed to an even greater extent. In figures 78 and 79 the total freedom of the lower portion of the cistern from trabecular strands is seen; above, the mesenchjTne still sweeps down as a supporting structure for the chorioid plexus. A definite differential hne of mesenchymal condensation indicates the future outer border of the arachnoid as it incloses the cisterna cerebello-medullaris. This general process of mesench^Tnal breaking-down, altering the original small spaces into the larger arachnoid channels, holds as the embryo develops into larger forms. | |||
In addition to this formation of the subarachnoid spaces in the adult through the enlargement of the embrA-onic mesenchjTnal spaces, the perimedullary mesench\Tne undergoes in these same localities condensations which result ultimately in the formation of the arachnoid membrane and the trabeculse diAnding up the cavum subarachnoidealo. Mention has already been made of the adhesion of the cellbodies of the disrupted mesenchymal elements to the persistmg strands — the initial step apparenth- in the ultimate differentiation of the mesothehal cells which line these spaces. Gradually ^vith the increasing growth of the embryo these cells seemingly become arranged in definite columns covering the persisting arachnoidal trabeculae. At the same time a differentiation of these primitive mesenchj-mal elements occurs, the cells ultimately being transformed into the very low cuboidal mesothelium of the subarachnoid spaces. This differentiation begins first in the basilar portions of the cranium and spreads upward, in a way similar to the course of development of the cranium and of the enlargement of the pericerebral spaces. | |||
While such a general process as outUned accounts for the formation of the arachnoidal trabeculae and the subarachnoid spaces, it has but little bearing on the development of the outer intact membrane of the arachnoidea. This portion of the arachnoidea (which might be termed the arachnoid membrane as distinguished from the arachnoid trabecule) first appears as a distinct line of mesenchymal condensation separating the mesenchyme into the primitive arachnoid and dura mater, as in figures 76 and 77, dmc. This rather thin zone of cellular density in reality represents not only the outer surface of the arachnoidea, but also the inner surface of the dura mater. At first these develop in close fusion with a later separation of the two membranes. With this cleavage of the two surfaces, the arachnoid membrane rapidly differentiates, forming an intact layer over the subarachnoid spaces. The cells covering the surface membrane seem to change gradually into the low cuboidal type, similar to those covering the arachnoidal trabeculae. The details of these processes may be most easily studied in the region of the cerebral hemispheres; in this situation the transformation of the tissues occurs at a later period than in the basilar regions, for the differentiation of this mesenchyme follows the general plan of development of the cartilaginous and bony cranium. | |||
The greatest problem in connection with the development of an external arachnoid membrane naturally concerns the separation of this leptomeningeal tissue from the pachymeninx. In the solution of this particular problem gross dissections have been found of benefit. For this purpose, pig embryos of larger size were used, and attempts were made to ascertain at what stage of development a true anatomical separation of the two membranes occurred. It was found that in embryo pigs of about 40 mm. the dura over the calvarium could be well separated from the arachnoid, but areas of unseparated tissue still persisted at this stage. This was also found to be true in pig embryos of 50 mm. ; on the inner surface of the dura at this stage a mesothelial cell pattern could be demonstrated, although areas of attachment to the arachnoid existed. However, the differentiation of the periaxial mesenchjTne into the adult arachnoid does not occur coincidently with the possibility of a forceful separation of the dura from the surface of the brain; ])ut before this separation of the pachymeninx can l)e made the mesechyme which will go to form the arachnoid must undergo some differentiation. This process invohcs a condensation or accumulation of mesenchymal elements directly in the secondary dural thickening; the cells, with oval nuclei, soon form a continuous membrane of two or three cells in thickness. Apparently soon after the cellular accumulation has been accom])lished, a separation of the dnni from the arachnoid may be made. In certain areas, varying greatly in size, there is still an intimate connection between dura and arachnoid. These connections are particularly prominent over the developing cerebral hemispheres, and it is with this differentiation in the formation of the arachnoid spaces that we will now deal. | |||
In a human fetus of 76 mm. (No. 1134) the arachnoid was found to constitute, in the region about the great sagittal sinus, a cellular layer which adhered quite closely to the dura, even though a Une of difTerentiation between the two meninges could be made out. This adhesion could undoubtedly be separated, even bj' gross dissection, although the tendency to adhesion was stronger than the attachment of the pia to the cortex. From its cell-character and general histology' the arachnoid at this stage must be considered as a formed membrane, but in a primitive state. | |||
A somewhat similar but more advanced stage in the formation of the arachnoid membrane is seen in a human fetus of 100 mm. (No. 928-E) and in a fetal pig of 114 mm. In both the arachnoid membrane is verj' cellular, adhering to the dura only along the superior longitudinal sinus and in certain isolated areas. The cells comprising the arachnoidea possess oval, rather large nuclei which stain palelj- with hematoxyhn. No typical arachnoidal trabeculae could be made out in specimens in this cortical region. | |||
The cellular character of the arachnoid persists in the larger embryos and fetuses as a layer, several cells in thickness, constituting the outer arachnoid membrane. In a fetal pig 190 mm. in length the membrane was practically differentiated, its outer wall being covered by me.sotheUal cells with large nuclei lying about a small fibrous-tissue base. The arachnoid trabeculae were developed only in the larger sulci, where they appeared as typical cellular cords about a core of fibrous tissue. At this stage, too, the vessels traversing the arachnoid spaces were found covered with similar cells. These may now be justly termed the mesotheUal cells. | |||
Quite similar stages of arachnoidal differentiation occur in human fetuses of 200 (No. 870) and of 240 mm. (No. 1131). The arachnoid has everj-where practically become adult in character, except for a further decrea.se in the number of the peripheral layers of mesothelial cells. The fibrous tissue underlying this covering membrane possesses, as in the adult, almost a minimum of support. | |||
In certain areas, however, the differentiation of the mesenchA-me into the adult arachnoidea does not keep pace with the general process. In the present study this phenomenon of unequal develoj^ment was especially well shown in fetal pigs of 150 mm. and upwards. It concerns the development of arachnoid trabeculae in the cerebral sulci. As is well known, the arachnoid membrane bridges the cerebral fissures, wliile the pia follows the cerebral contour. In the fetal pigs of the stages specified above, certain furrows showed a typical adult relationship with the covering arachnoid membrane and lining pia, the intervening space being traversed bj' definite arachnoid trabeculae. Other of the sulci were filled with an almost emb^^•onic tj-pe of mesench}Tne — a loose meshwork of cytoplasmic processes containing rather small oval nuclei. The explanation of this embryonic type of tissue seems to be that it occurs in the newly developing sulci and that some time must elapse in this formation before the tissue fully differentiates into the adult arachnoid membrane. Strangely enough, a similar collection of an embryonic type of tissue is sometimes met with, in these stages, between the two hemispheres. | |||
The general process, then, of the formation of the arachnoidea involves both a breaking-down (or thinning-out) of the mesenchymal spaces and a condensation of the cells. The first of these processes results in the transformation of the interstices of the periaxial mesenchyme into the larger subarachnoid spaces, divided off by arachnoid trabeculae; the second finds its final accomplishment in the development of the outer arachnoid membrane which, covered with mesothelial cells, forms the inner surface of the subdural space. The transformation begins in the basilar regions of the cranium and spreads upward over the hemispheres. | |||
THE CIRCULATION OF FLUID THROUGH THE SUBARACHNOID SPACES. | |||
In view of the processes of differentiation involved in the formation of the arachnoidea and the subarachnoid spaces, the circulation of fluid through this pecuhar membrane must be considered. It seems important to ascertain, if jjossible, the relationships between the beginning of the passage of the cerebro-spinal fluid and the onset of the histological changes. | |||
The conceptions of the development of the circulation of the cerebro-spinal fluid which are presented in this conmiunication are dependent, in large measure, upon the results of the replacement of the fluid, in living embryos, by the ferrocyanide solution. Additional evidence was obtained from the identification of albuminous coagula in the periaxial tissues. The correlation of these findings with the development of the chorioid plexuses and with the results of injections under low I^ressures, from a syringe and so forth, gave evidence of their correctness. | |||
The differentiation of the mesenchyme into arachnoid membrane may be said to keep pace with the establishment of the periaxial channels for the cerebro-spinal fluid. In the main, the passage of this fluid into the undifferentiated mesenchyme about the nervous system precedes the process of histological change. This phenomenon is shown in figure 14, from a pig embryo of 18 mm. The replaced fluid is seen passing out into the mesenchyni(^ through the two membranous areas in the roof of the fourth ventricle. The mesenchyme at this stage has already differentiated somewhat, but hardly in proportion to the length of time during which the fluid has been passing into the space. | |||
There are several features of interest in the course of the fluid through the periaxial spaces. In sections of embryos in which the cerebro-spinal fluid has been replaced by a foreign solution the granules of the precipitated salts may be identified in the periaxial mesenchyme in situations corresponding exactly to the extent of th(! spread shown in the cleared specimens (figs. 1 to 9). The exact location of the prussian-blue granules is of importance in this connection, as the exact form and distribution of the periaxial spaces and their relation to the adult subarachnoid spaces may thus be determined. | |||
Kxamiiiation of serial sections from an embryo in which the embryonic ventricular fluid has been replaced by the ferrocyanide will reveal, if the embryo exceeded 14 mm. in length, granules of prussian-blue in the peribulbar mesenchj'me (fig. 14). The granule.s are not found in any cell-bodies in this tissue; they are made out, in large measure, adhering to the mesenchymal cell-processes or lying free in the mesenchymal interstices. The granules do not penetrate the pia mater or the dura mater, a finding which will be discussed more fully in the sections dealing with these membranes. Everywhere the transit of fluid into the nervous tissue seems to be prohibited by the pia; in some areas, however, the outer condensation of mesenchyme to form the dura-periosteum has not j'et occurred. This is shown particularly well in the region of the roof of the fourth ventricle (fig. 18), where the epidermis offers the only barrier to the passage of fluid from the pericerebral spaces. | |||
In the earlier stages in which the phenomenon of fluid passage about the central nervous system may be observed, the outer layer of the arachnoid is not at all differentiated. Here the barrier to the fluid is the blastemal condensation of mesenchyme (fig. 16). In the later stages, when the outer layer of the arachnoid is beginning to appear as a mesenchymal thickening, the fluid (as indicated by the precipitated prussian-blue) is confined strictly within the immature arachnoid membrane. | |||
The course, then, of the fluid which has replaced the cerebro-spinal fluid in the embryo follows that of the aduh cerebro-spinal fluid (as shown by the resultant blue granules). It is everywhere contained within spaces which topographicaUj' and embryologically correspond to the subarachnoid spaces in the adult. The spread of the replaced solution from the embryonic ventricle into the peribulbar tissue is analogous in every way to the passage of cerebro-spinal fluid from the fourth ventricle of the adult into subarachnoid spaces. | |||
==VIII. A Consideration Of The Embryonic Pia Mater== | |||
Our present conceptions of the embryolog}' of the pia mater are largely due to the work of His^^s) and of Kolliker^^D. who first firmly established the idea that this inner leptomeninx was mesodermal in origin. While generally accepted (Farrar'i^*). this view has not been widely referred to in the literature; but the absence from all embryologies of any information concerning the development of the meninges is quite striking and it does not seem strange, therefore, that our information regarding the pia mater has not advanced in keeping wnth our knowledge of the embryology of other structures of the body. In the present section of this conmiunication it is purposed to present merely a general consideration of the process by which the pia mater is formed and to point out some of its functional characteristics, especially in regard to the fluid channels. | |||
The term pia mater is accepted throughout this article as designating solely the cellular membrane which adheres closely to the outer surface of the nervous sj-stem, but it is in direct connection with the arachnoidal trabecular which traverse the subarachnoid space Whether the two membranes should be considered together as the pia-arachnoid or as the leptomeninx is a question in regard to which there is some disagreement ; it will suffice to consider the pia as a separate membrane. | |||
THE GENERAL HISTOLOGY OF THE PIA MATER. | |||
The findings in this mvestigation are wholly in accord with the conclusions of His(25), of Kolliker('i), and of Farrar^^^), that the pia mater is derived from the middle germ-layer. In the earliest stages the mesenchymal elements may be made out adhering to the outer i)ortion of the primitive nervous system. In the course of growth these cells are grouped about the mantle zone of the spinal cord in a rather dense laj'er, two or possibly three cells in thickness, with the tyjjical oval nuclei of the mesench5Tnal elements. Certain stages of this process may be made out in the figures in this paper. Thus, in a human embryo of 4 mm. (No. 836 of the Carnegie collection) the mesenchymal elements form a definite layer around the neural axis (fig. 41). The nuclei are oval in shape, possessing a moderate amount of chromatin, and are found in a layer two cells in thickness. This membrane, with its fairly scant cytoplasm, is sharply differentiated by its existence between two layers, in one of which nuclei are wanting, and in the other somewhat widely separated — the mantle zone of the spinal cord and the periaxial mesenchyme. | |||
This typical arrangement of the mesenchj'mal elements about the cerebro-spinal axis holds in almost unchanged form throughout the whole embryonic growth. Thus, about the nervous tissue in figures 48 and 52 (from human embryos of 7 and 9 mm., respectively) the same condensation of the mesenchymal elements to form the pia mater are made out. This ajipearance is so familiar that further description in the later stages seems needless, but certain characters of this embryonic arrangement seem to require comment. | |||
The general appearance of the pial layer is greatly altered by the early formation of the capillary blood plexus about the nervous sj'stem. This plexus tends to render the pial tissue more cellular, on first microscopic examination, as the endothehal channels branch greatly outside of the nervous tissue in this mesenchymal pia. The general character of the pial layer, however, as a membrane with prominent nuclei and scanty protoplasm, is not altered at all by the vascular plexuses. | |||
The ultimate fate of these undifferentiated mesenchjinal elements forming this initial \nti\ condensation is a gradual transformation of the cells into ver}' low cuboidal mesothelial elements constituting the adult pia. The transformation concerns not only the differentiation of the cells but also a rearrangement so that the original layer of two or more cells in thickness becomes finally of but a single cell in thickness. The jjrocess, in a way similar to the development of the subarachnoid spaces, begins in the basilar portions and spreads upward; the process, hence, may often be studied in a single suitable sjiecimen. | |||
More imjjortant, for our consideration, is the i)eculiar relationship of the pia mater to the roof of the foiutli ventricle, and in particular to the two area^ membranaceje. In this situation, in place of the slight mesenchymal condensation which characterizes the jjia, and which ^Minot''*"^ pictures in his figure 114, the mesenchyme seems altered. The condensation to form the pia, which takes place in other situations about the true nervous tissue, has not here occurred. This absence of the typical pial arnuigenit-nt may be noted even in very small embryos — those in which the roof of the fourth ventricle is composed of the many-layered, epitheUal-like cells. This is well shown in a photomicrograjih (fi}^. 53) from an injected human embryo of 9 mm. (Xo. 721) of the Carnegie collection. Likewi.sc, in this region in a [)ig embryo of 8 mm. (fig. 25), the same absence of a real pial condensation may be made out. But this peculiarity of the pia is most striking at the period of maximal differentiation of the superior membranous area in the rhombic roof. In figures 37 and 43, photomicrographs from pig embryos of this stage, the mesenchymal condensation, augmented by some vascular endothelium, is shown in adhesion to the ependyma on both sides of the membranous area; but directly behind the differentiated cells of the area membranacea evidence of a condensation of mesenchyme is whollj' lacking, even though both specimens show vascular channels in close appro.ximation. Similarly, in a human embryo of 14 mm. (No. 144, Carnegie collection) a total lack of the true pial thickening is to be observed (fig. 57). | |||
Quite similar is the failure of a pial thickening about the inferior membranous area. This can be made out in figures 83 and 87, from human embryos in which the process of differentiation of the area is proceeding. In later stages of the formation of the area membranacea inferior, the marked absence of a true pial condensation in the mesenchyme in this region is noted in figure 75 (a specimen from a fetal pig of 23 mm.) But this apparent failure to form the typical mesenchymal condensation of the pia mater in certain areas in the roof of the fourth ventricle must not be construed as indicating an absence of pia mater. Such does not seem to be the case here, for in the later stages of the formation of the cisterna cerebellomedullaris the area membranacea inferior is found entirely unsupported, except for a layer of mesenchymal cells. This is shown in figures 77 and 79, both taken from fetal pigs of 32 mm. This mesenchymal laj'er must be considered as pia mater apparently modified for a specific purpose. | |||
The general process, then, of formation of the pia mater concerns a condensation of mesenchymal elements to form an embryonic membrane about the central nervous system. From its earliest beginning very slight modification is needed to reduce it finally to the histological character of the adult membrane. The general process holds, except in the regions of the area? membranaceic in the roof of the fourth ventricle; here, apparently, a modification of the pia for a specific purpose, involving an absence of the primary pial condensation, takes place. | |||
THE RELATION OF THE PIA MATER TO THE FLUID CHANNELS. | |||
The cerebro-spinal fluid in its normal pathways comes everywhere into contact with the pia mater, which serves as the inner retainer for the subarachnoid space; therefore the functional relation of this membrane to the fluid which bathes it becomes of interest. To some degree the results of the experiments recorded in the earlier portions of this paper throw light upon the relation of the pia mater to the circulating fluid. The most important question in this connection is naturally that dealing with the possible penetration of the normal fluid through this embryonic membrane. In this regard the findings in replacement experiments with ferrocyanide solution serve to elucidate the problem. These observations give no evidence of any penetration of the pia mater by the fluid. This is well brought out in figures 14 and 18. In every respect (as demonstrated by numerous experiments of this type in pig embryos of varying lengths) the pia mater is wholly impenetrable to true solutions of foreign salts when injected so that the normal tension is not altered. The whole subarachnoid sjiace may, in such an experiment, be filled with the prussianblue, but none of these granules are found within the cells of the pia mater or in any layer between these cells and the nervous system. Evidence that the fluid has bathed the outer pial cells is afforded by the adhesion of granules of prussian-blue to the outer cytoplasmic borders of the cells. | |||
Likewise the cells comprising the embryonic pia have been found to be impenetrable to true solutions (ferrocj'anide) when injected under varying pressures from a syringe. In these cases, rupture of the roof of the fourth ventricle or of the infundibulum may be produced by great pressure, without causing any of the fluid to penetrate the intact layer of the pia mater. The same result is obtained when india ink is substituted for the true solution. | |||
The pia mater, then, even in its embryonic form, serves as an efficient fluidbarrier. This is demonstrated, in regard to the adult pia mater, in the report^^^) of the observations made on adult cats, dogs, and monkej's. But the barrier which the pia offers to the entrance of fluid from without exists also for fluid coming in the reverse direction. This is shown by the well-known phenomenon of the so-called subpial extravasation, which occurs in blood vascular injections when the injections are continued for too long a time at too high a pressure. The perforating vessels in such cases rupture as they enter the nervous system, and the injection mass spreads extensivelj' beneath the pia, stripping it away from the nervous tissue. Of interest in this discussion is the fact that the injection mass in these extravasations does not rupture the pia, which seemingly is an equallj' efficient fluid barrier to pressure exerted on it from within. Similar subpial spreads of the injection fluids have been observed in the course of this work. These extravasations resulted from the rupture of the whole nervous tissue from within, particularly in the region of the infundibulum, when the inj(>ction was made into the ventricular system under excessive pressure. In this respect, too, the i)ia seems to be wholly efficient as a retainer for true solutions or for granular suspensions. It is realized that the embryonic pia mater will not resist the passage of fluids through it under the highest pressures afforded by the syringe, but the membrane serves as an efficient barrier for all pressures such as are employed in careful anatomic injections. | |||
With this conception of the impenetrabihty of the pia mater to fluids under ordinary pressures, it does not seem strange that there is a variation in the process of formation of the pia mater in the region of the roof of the fourth ventricle. It has been shown in the foregoing paragraj^hs that the phenomenon of mesenchymal condensation which results in the formation of pia elsewhere does not occur in the region of the two area? membranacefe. In view of the passage of cerebro-spinal fluid through these two membranous areas, the pia mater must necessarily be altered in these places. For were it not adapted to the jjurpose of affording fluid passage the cerebro-spinal fluid would, in its course from the ventricle to the subarachnoid space, form a subpial extravasation. It would seem that this modification of the pia is designed to meet the particular need and function of this region. | |||
==The Adhesion of the Pia Mater to the Cerebral Tissue== | |||
It is a well-known fact in embryology that tlie pia mater and the peria.\ial mesenchyme in poorly dehydrated specimens split away from nervous tissue, but in adult preparations, if the meninges and brain are dehydrated in a block, the separation of the tissues occurs between the dura and the arachnoid, or (in more exceptional instances) the dura and arachnoid come away, leaving the pial layer closely applied to the cortical tissue. It is quite difficult in any adult mammal to separate the pia from the brain tissue. Realization of these peculiarities in the degree of adhesion of the pia led to an attempt to ascertain what structures were involved in the attachment of this mesodermal layer to the epidermal nervous system. The results of this attempt add nothing to the ultimate solution of the problem, but are perhaps of sufficient interest to justify brief presentation. | |||
. | Two theories in explanation of this adhesion of the pia immediately suggested themselves. One of these concernod a possible growth into the pia of neuroglial elements, causing an intimate association between the pia and the cerebral cortex. Our findings in reference to the neuroglial outgrowth in fetal pigs gave no reUable basis for the assumption. The second theory dealt with a diminution in the elasticity of the walls of the perforating blood-vessels which supply the nervous system. The early embryonic vessels, with walls comjjosed solelj- of endothelium, when subjected to the distortions of poor dehydration, might possibly offer less resistance to the separation, so that the pia would come awaj' from the nervous tissue. In the later stages, however, the thicker-walled perforating vessels w^ould naturally oppose such a cleavage, so that the pia would remain adhering to the cortical tissue. This theory is also purely an hj^Dothesis, although it does not seem unlikely, especially if one takes into account a possible connection of the pia with the perivascular system. In examining blocks of the meninges and brain tissue taken together it was found that the pia mater separated cleanly from the nervous tissue in fetal pigs 15 cm. in length. Beyond this stage the arachnoid might remain in adhesion to the dura, but in such cases there was always found a layer of cells on the outer side of the cortical tissue, constituting a true pia mater. | ||
==IX. The Development Of The Cranial Dura Mater== | |||
The dura mater, like the two other meninges, is derived from the mesenchj-me about the central nervous system. The researches of Sterzi'"' on the comparative anatomj" of the meninges furnish additional evidence for this conception in the higher mammals. The origin of the pachymeninx from the middle germ-layer is now well established. But there is lacking in the literature a comprehensive account of the formation of this fibrous envelope. The gross generaUties of the process are given in pari, liut there is an almost total absence of the more intimate details of the transformation. One of the most essential points in the process concerns the relationship of the dura to the bony coverings of the cerebro-spinal axis. Does the adult dura serve as the periosteum of the bony skull? In the standard text-books of anatomy the adult human dura is described as being composed of two layers. In the skull these layers split, to comprise the walls of the great venous smuses. The outer layer of the dura serves as the periosteum for the bony skull, but below the foramen magnum the two layers separate to inclose the epidural space. The outer dural layer in this spinal region adheres to the inner surface of the bony vertebral column, where it functions as the periosteum; the inner layer here becomes the spinal dura. | |||
In this account of the adult dura mater there is indicated a very suggestive periosteal relationship which implies an embryological basis for the disposition of the two laj-ers of the membrane. It must be granted, however, that this division of the cranial pachymeninx into two layers is quite arbitrary; there is nothing in the general histology of the fibrous covering to suggest such a halving except its division about the sinuses and its spinal relationships. | |||
THE GENERAL PROCESS OF THE FORMATION OF CRANIAL DURA. | |||
The first evidence of the development of the pachymeninx is found in the basilar region of the skull, where the mesenchyme thickens, to form eventually the bony covering of the brain. This thickening of the mesenchymal elements results not only in the formation of the chondro-cranium, but also in the final formation of the bony skull and possibly its internal periosteum and dura. In the process of differentiation the condensation of mesenchyme in the early stages gives no index of the varied character of the resultant tissues, so that, in the first place, the study of the process was necessarily related to the more adult stages. In this paper, however, the whole history of the dura will be detailed chronologically, beginning with the earliest stages. | |||
Bardeen(2) has given data on the first appearance of the mesenchymal condensations which go to form the blastemal phenomena in both the cranial and spinal regions. The blastemal vertebra? become fairly well differentiated in human embryos during the first month of intra-uterine growth. At the end of the first month, in the occijiital region, three fairly well-marked occii)ital myotomes may be made out; these afterwards disappear. "During the early part of the second month the membranous anlage of the skull becomes extensively developed. The roof of the cranial cavity is formed by a dense membranous layer, which fu'st becomes marked at the side of the head in embryos from 9 to 11 millimeters in length" (Bardeen). | |||
of | |||
These evidences of a primary mesenchymal condensation about the central nervous system are concerned in the problem of the differentiation of the dura only in so far as they indicate the onset of the process which will give rise to the bone and possibly the periosteum — a part of the dura about the cerebro-sjjinal axis. Gaupp^'^) has already pointed out that this cranial l)lastemal condensation gives rise to these adjacent Init wholly different structures. These cranial mesenchymal condensations persist in simple form until after the cerebro-spinal fluid begins to fill its extraventricular bed; then, within a short time, the tissue becomes transformed by the development within it of cartilage, so that in the human embrj^o the caudal half of the chondro-cranium forms a ring of cartilage about the posterior portion of the brain. On the inner side of this ring of cartilage the mesenchyme later shows a marked condensation in the midst of the rarefied perimedullary tissues. In this layer the nuclei soon become fewer in number and the cytoplasmic structures fibrillar, the whole resulting ulthnately in the formation of the fibrous adult dura. The mesenchymal condensations in the regions of the skull, where membranous bone formation holds, go directly into a membrane of fibrous tissue, in the outer portions of which bone is laid do\\Ti. The details of these processes will now be taken up. | |||
In figures 30 and 32, photomicrographs from pig embryos of 13 and 14 mm., respectively, the well-established vertebral differentiations and the now poorly differentiated base of the skull are shown. From this stage upward the mesenchymal condensation in the head region proceeds rapidly. Thus at a stage of 17 mm. in the human embryo (fig. 60) the ventral portion of the vertebral canal has become cartilaginous, while the base of the skull has also undergone the chondrogenous transformation in its mere posterior portions. But of especial interest in our problem is the line of mesenchjTnal condensation, which may now be traced whollj^ around the brain-stem and hemispheres (fig. 60). The nature of this condensation is well shown in figure 61, an enlargement of the squared area of figure 60. The mesenchymal nuclei have become closely packed and rather sharply differentiated from the looser mesenchj^me which in part goes to form the arachnoidea. Figure 59 similarlj' shows this condensation proceeding upward to the vault. | |||
Examined in another plane, the process of mesenchymal condensation seems to proceed much more rapidly in the posterior than in the anterior region. This is brought out in a transverse section of a human embryo of 18 mm. (fig. 62). Here the condensation is much more extreme about the medulla and roof of the fourth ventricle than in the more anterior parts of the mesencephalon. The same general appearance, typical of this stage, may be made out in figures 56 and 57 from a human embryo of 14 mm.* (No. 144, Carnegie collection). In the slightly larger stages the process of mesenchj^mal condensation about the nervous system becomes rapidly more marked. This increase in the number of cells comprising the denser membrane is shown in figures 64 and 65, photomicrographs of embryo No. 460 (21 mm.). | |||
The degree of condensation of the mesenchyme in the various stages of the human embryo is followed quite closely in the pig embryo. The comparative degree of differentiation coincides within a millimeter or two. Thus, in a section from a pig embryo of 19 mm. (fig. 38), the degree of condensation about the roof of the fourth ventricle is practically similar to that in human embryos of the same length. | |||
The phenomena just commented upon represent the stages concerned in the formation merely of a cranial blastema and are related to the formation of the dura only so far as it is out of this mesenchymal condensation that the periosteal portion of the pachymeninx may be derived. The degree of condensation referred to in the figure.s has been solely of the blastemal type, but in some of the specimens this simple condensation is seen only in the more cephalic portions of the cranium. Thus, in the figures (64 and 60) taken from embryo 460, the mesenchymal condensation is still of the simjile undifferentiated type, whereas in this same embryo the more caudal sections show a chondro-cranium which is well develojied. The i)rocess of formation of the cranial dura, then, is one which begins in the basilar j^ortions of the cranium and proceeds from these points into the region of the calvarium. In general, all of the phases of this transformation into dura may be found in one specimen of sufficient and suitable size, the basilar differentiation re]:)resenting the advanced stages, while the steps in the differentiation are found in the areas nearer the vertex. | |||
* Measured on slide after sectioning. | |||
It is quite difficult to decide exactly what importance the primary condensation of mesenchyme maintains in the formation of the dura, because, coincident with the chondrification of the blastema, there occurs another condensation which forms the line of division between the inner surface of the dura mater and the outer arachnoid membrane. The first evidence of this secondary perimedullary condensation is found in pig embryos of about 17 mm. In these specimens, in the narrow space formed by the mesencephalic flexure, mesenchymal cells collect together in the form of a fairly definite membrane. After its primary beginning in this area, the narrow line of its thickening may be traced to the basis cranii in embryos a little larger. In slightly older stages this secondary fine of condensation is found to be fairly extensive throughout the area between the middle and posterior cranial chambers. | |||
At a stage of 20 to 21 mm. the whole basilar portion of the cranium shows evidence of this secondary line of condensation lying between the pia mater and the cartilaginous skull. The condensation occurs in the outer portions of the loose tissue which, as shown in a foregoing section (No. vii) becomes the subarachnoid trabeculae. The line of condensation is not broad on section; it comprises a cell-layer from three to six cells in thickness. Between this cellular border and the cartilaginous skull the tissue rapidly differentiates (a process seemingly sj^nchronous with the develojiment of this membrane). This tissue, which maintains dural relationships, is far more cellular and compact than the original perimedullary mesenchyme. Even without the rather dense line of division in the mesenchymal ti.ssue, the dural structure can be easily outlined by its characteristic apjiearanco. | |||
The original dural condensation between the two wings of nervous tissue which unite in the me8encei)halic flexure can be traced in slightly later stages around into the tentorium cerebelli. This structure develops as a wholly similar mesenchymal thickening in the midst of the jK-rimeduliary mesenchyme. The tentorium consists in these embryos of 20 to 2") nun. of two thin lateral plates which widen at their cranial attaclunents into prismatic areas. These areas, which finally lodge, in the two layers f)f dura, the sinus transversus, arc characterized by the same dense tyjie of mesenchyme. The jx-ripheral edges of the prismatic jwrtion of the tentorium sjjreads caudalwards as a definite line of condensation. In the earlier stages this line becomes indefinite as it extends from its teiitori:il attachment, but finnllv a similar line of condensation about the whole posterior chamber may be made out. This lies within the area of the cartilaginous skull and bounds the subarachnoid spaces. | |||
This same process of formation of dura holds for the formation of the basilar dura in the more anterior portions of the cranium. The appearance of the .secondary zone, narrow and rather dense, may be made out inclosing the more cellular mesenchyme w^hich extends to the cartilaginous skull. The same process also endures for the formation of the dura of the calvarium, but here the addition of tissue from the undifferentiated mesenchyme is undoubtedly very small in amount. This will be discussed in a later paragraph. The various stages in the formation of this secondary condensation which goes to form the major portion of the dura may be fairly well studied in any one embryo of suitable stage, because the process, as pointed out above, begins in the basilar portion of the cranium and extends upward. Likewise, the condensations directly beneath the region of the dorsal membrane are delayed as compared to those of the lateral regions. | |||
Some of the phenomena shown in the formation of the dura mater are illustrated in figures 46, 76, 77, 78, 79, and 94. Throughout these figures the letters dmc refer to the Une of the secondary mesenchymal condensation, which borders internallj^ the dura and which goes to form the outer membrane of the arachnoidea. | |||
In figure 46, a photomicrograph of a pig embryo of 32 mm., the dura mater (dmc) is shown as a somewhat condensed tissue separated a sUght distance from the chondro-cranium. On the basilar surface, the inner line of dural tissue is quite remote from the inner surface of the basioccipital. Tracing this line of condensation forward, it is soon seen to merge more closely with the perichondrium* of the basioccipital. More anteriorly it again leaves the occij^ital plate and after a brief interval it fuses with the temporal perichondrium. Continuing slightlj^ more anteriorly the dural process toward the mesencephalic angle maj' be made out; this appears as a doubled membrane at its basal attachment. In its further prolongations the dural surface is at times a distinct structure; at other times it is completely fused with the perichondrium. | |||
Posteriorly, in this figure 46, the line of dural condensation (incorporated also with the outer arachnoidea) may be traced upward around the cisterna cerebeUomedulla | |||
ris. This hne of condensation is seen to lose its definitive character as it curves inward toward the chorioid plexus of the fourth ventricle — a phenomenon shown particularly well in figures 77 and 79, taken from the same pig embryo of 32 mm. The dura in this termination maj' be said to be in its formative stage; but dorsally, over the mesencephalon, the inner surface of the dura again becomes a definite membrane, as shown in figures 76 and 78. In the latter figure it is shown inclosing a wide mesh of dural vessels, between the arachnoidal surface and the membranous skull. Anteriorly, again, it seems to lose its definite hne of condensation. | |||
•The term "perichondi.L n" is used tliroiighout this paper to designate only the ver>' dense cellular line delimiting the edge of the cartilngo. This dense zone is composed of the nuclei of the cartilage, crowded together, and represents probably some phenomenon of the growth or resorption of the cartilage. In a much broader sense, the whole dural tissue, lying between the line of secondarj- condensation and the cartilaginous border, could be termed "perichondritmi," as it probably represents the sole internal membrane which could be stripped from the cartilage. | |||
Quite similar pictures are obtained regarding the dura mater in the human embryo. The relationships of the dura to the cisterna cerebello-medulla | |||
ris are shown in figure 94, a photomicrograph of a human fetus of 35 mm. (No. 199 in the collection of the Carnegie Institution). In this rejiroduction the line of secondary mesenchymal condensation (representing the outer membrane of the arachnoidea and the inner surface of the dura) becomes widely separated from the occipitale superius in its superior portion. | |||
In a fetal pig of 8 cm. the same general arrangements of the dura mater could be made out. The inner surface of the dura was in places still fused with, the outer arachnoid membrane, but in other places the areas of attachment were lacking, so that a true separation of arachnoid from dura had taken place. Along the ])eripheral points of the tentorium the dura and arachnoid were still closelj' a{)plied to each other. The dura itself was of the same cellular, rather loose tissue, with a dense inner surface. In i)laces, as described in the younger stages, the dural tissue was incorporated with the definitive perichondrium over certain cartilages or even over parts of the same structure. In other places a definitive perichondrium may be wholly lacking; in these areas the indefinite cartilaginous border gradually merges into the dura. In still other situations an intermediate arrangement of dura and perichondrium exists, where the cartilage is bounded bj' a somewhat condensed but not fully developed perichondrium which is continuous with the dura. Everywhere in the membranous sutures between the cranial cartilages or bones, the dura bridges the gap as a loose, cellular tissue. Over the calvarium the dura appears solely as a dense, rather fibrous membrane which is incorporated with and serves as the inner periosteum. This dura over the hemispheres is continuous with the fibrous sutures of the cranial vault. | |||
The findings in a fetal pig of 98 mm. were not dissimilar to those just recorded. The dura was everywhere quite well developed, a rather loose cellular tissue except over the hemispheres, where it showed a more fibrous character. In the region of the occipito-atlantoid ligament the dura was fused with the ligamentous tissue, while above (over the occipitale superius) the dura became a distinct, thick cellular layer. The structure of the tentorium was wholly similar to the occijiital dura. In the basis cranii there are areas in which the dura is wholly fused with the periosteum or jierichondrium; in other areas it bridges the sutures or exists as a definite membrane on the inner surface of a definite perichondrium. | |||
The dura mater in a fetal pig of 15 cm. did not vary greatly from those larger stages already tlescribed. The tissue, however, had become somewhat more fibroiis. The prismatic attachment of the tentorium was no longer as large proportionately, but the dura lining the occipitale superius remained a thick bulbous swelling on the dorsal surface. Hut most striking of all the features in the specimen was the very dense fusion of the dura of the calvarium with the fibrous sutures of the cranium. No line of demarcation betwecm dura and fibrous suture could be made out; the two fibrous layers are anatomically one structure. | |||
The falx cerebri forms in the pig and human embrj-o by a process similar to that of the inner portion of the dura mater. In the sulcus between the two cerebral hemispheres the mesenchyme remains undifferentiated until quite late; then there appears in the posterior portion a narrow zone of condensation which soon presents two lateral surfaces separated by a layer of rather loose cellular tissue, similar in all regards to the dural tissue already described. This zone of condensation spreads forward to comprise the whole falx. The double surfaces of this membrane finally separate into two parts, forming the outer surface of the arachnoidea and the inner surface of the falx. At the cranial attachment of the fabc, the loose tissue forms a prismatic base, containing the suj)erior sagittal sinus and spreading laterally over the denser dura of the calvarium. The whole appearance of this region, which will again be referred to, is that the falx has been added onto the dura of the vertex. Its time of initial appearance is later than that of the rest of the cranial dura and there is apparently no additional acceleration of development. Hence the dural tissue in the fabc cerebri presents, in appropriate stages, a more immature type of differentiation than does the adjoining dura. | |||
The process of the formation of the dura is not wholly a simple one due to the relation of the adult dura to, or its function as, the inner periosteum of the skull. In the figures already referred to, the almost complete fusion in some areas of the inner Une of dural condensation with the perichondrium has been commented upon. In other situations definite separations of the inner dural surface from the perichondrium occurred; in still other regions no perichondrium could be made out as a definite membrane. These differences in relationships of the dural tissue to the line of the perichondrium can not at present be wholly explained, but some indication of the meaning of the process can be given. | |||
Out of the original cranial blastema, as described by Gaupp'^i^\ there develops the cartilaginous and bony skull, the periosteum, and the dura. But the observ^ations recorded above indicate that by far the major portion of the dura is formed by a secondarj^ mesenchymal condensation, which was indicated bj' a thin zone of more condensed cells on its inner border. This inner zone ultimately separated to form the inner surface of the dura and the outer membrane of the arachnoidea. The tissue included between this inner line of condensation and the cranial wall gradually differentiated into a more condensed but still a loose cellular tissue and finally became a fibrous dura. | |||
In all cases the dural tissue extends from the inner line of condensation to the cranial blastema, to the perichondrium, or to the cartilage of the skull. The presence of a definitive perichondrium can not at present be explained, but apparently the perichondrium is manufactured by the cells of the original cranial blastema and not by the dural tissue which lies in approximation to it. When a definite perichondrium is found, it seems quite uninfluenced by the dura; at other times a fusion of an indefinite perichondrium with the dura seems to occur. The fusion of the perichondrium with the dural tissue derived from the secondary mesenchjinal condensation may occur, so that the small outer portion of the dura may be derived from this laj^er. The findings, however, in this investigation, are against any addition of perichondrium to the dural tissue; histologically, a definitive perichondrium is a membrane entirely apart from the dural condensation. | |||
Over the cerebral hemispheres the dura of the cranial vault offers more difficulties of study than does that of the basilar regions. With the formation of a blastcmal condensation over the whole vertex — an extension of the dorsal membrane to form the membranous skull — there occurs very quickly a condensation to form the dura. This condensation may be first detected as a continuation anteriorl)'of the leaflet of the tentorium cerebelli, which stretches forward from the prismatic zone of the tentorial attachment. This zone of condensation is wholly similar to the narrow line of the mesenchymal thickening which was found in the more basilar regions of the skull. This zone of condensation occurs just wathin the cranial blastema and may be traced upward over the mesencephalon and laterally around the rapidly enlarging hemispheres. As the distance from the cerebellar attachment increases, the zone tends to approach the blastema, except in those regions in which the precursors of the dural veins occur. In such a situation this inner dural zone swings inward to encompass the vessels. Between this inner line of the dura (representing also the outer surface of the arachnoid) the same rather loose cellular tissue exists. | |||
From the fabc cerebri a zone of dural condensation in the mesenchyme spreads laterally also; this gradually may be traced anteriorly and laterally until fusion with the similar lines of condensation from the basis cranii and the prismatic zone of the tentorium are reached. The condensation connected with the falx cerebri, however, is not an extensive process, the greater part of the hemispheres being covered by the development from the basis cranii and from the tentorium. It must be understood, however, that there is no active migration of this line of condensation, for the whole process is a development in situ. The appearance of an active extension is derived solely from the study of various stages and the increased area of condensation appears as an increment which has developed at the terminal points of the previous condensation. | |||
The amount of dural tissue delimited in the mesenchyme by the secondary zone of condensation is not great in the region of the vertex. It is a thin layer which fuses to the inner surface of the cranial blastema. At the stage of this fusion the blastema has become somewhat fibrous and it constitutes the membranous skull. In this fibrous tissue (the union of the blastema and the dura) bone is deposited, but only in the outer layers. The phenomenon is easily studied in any suitable stage, for the sutures between the flat cranial bones remain incorporated with the inner memliranf!— the dura which includes the periosteum. Hence, over the cranial vault, the dura and periosteum become incorporated as a single membrane; this serves as the membranous .skull, into the outer layer of which bone is deposited. | |||
In the basis cranii, as soon as ossification of the cartilaginous skull takes place, tin; dura becomes inc()r|)orated as the periosteum in a manner similar to that which takes i)lace in the cranial vault. While no definite relationship of dura to the peri-chondrium could bo made out in the earlier stages, the later function of the dura as the inner cranial periosteum is Cjuite obvious. Thus the adult relationships of the dura are obtained. But it is quite difficult to decide to what extent the dura (or internal cranial periosteum) is derived from the primary cranial blastema. It seems probable that this blastemal condensation, in its final resolution into bone, may contribute, in the form of a periosteal element, somewhat to the formation of the dura. Such an addition is verj' difficult of verification; certainly the greater part of the dura is derived by the secondary condensation from the perimedullary mesenchyme. | |||
Before giving details of the fibrosis of the dura, it may perhaps be interesting to point out a peculiarity of the primarj' cranial blastema, which does not seem to be connected directly with the formation of the dura. This concerns the tendency of the membranous skull to form more than one layer in its original zone of condensation. In certain areas, as in figure 64, from a human embrj^o of 21 mm., the dorsal membrane is .shown split into two layers. Somewhat similar to this is the occurrence of two zones in the cranial blastema of a pig embryo of 23 mm. (figs. 22 and 101). Inthis latter figure a less cellular outer layer and a more cellular inner layer are seen. Neither of these have particular significance in the formation of the meninges, although the inner layer in early stages actively functions as a fluid retainer. | |||
The question of the development of fibrous tissue in the dura mater in the course of its development requires consideration here. This phase of the problem concerning the formation of the pachymeninx has been followed, in this stud}', in the dura of the vertex about the sinus sagittalis superior. The tissue was removed in blocks, including the meninges and cortex cerebri, and was then sectioned in the coronal plane. For the most part the deposition of fibrous tissue was studied in sections stained with hematoxyhn and eosin; the findings were controlled by treating other sections from the same blocks with IMallory's connective-tissue stain. In this way the general histogenesis of the dural tissue could be well investigated. | |||
Sections from such a block from a human fetus of 76 mm. (Xo. 1134, Carnegie collection) showed the dura to be composed of fibrous tissue everywhere except in the region of the great sagittal sinus. About this sinus an immature, almost embryonic, tj'pe of loose mj-xomatous tissue was observed. The fibrous tissue comprising the dura elsewhere is of a quite cellular, somewhat immature type of white connective tissue, with a considerable number of true fibrils. A wholly similar picture is found in a section, stained by Mallory's method, of a block from a fetal pig of SO mm. (fig. 104). Unfortunately the cellular character of the fibrous dura is not brought out, but the photomicrograph shows well the avoidance of the lateral walls of the sinus by the process of fibrosis. The more embryonic type of tissue in the region between the hemispheres is also well presented. | |||
The dura mater of a human fetus of 100 mm. (No. 928-E, Carnegie collection) possesses fewer nuclei in a given area than does the dura from the specimen of 76 mm. (No. 1134). The tissue is fibrous, except in the immediate region of the sinus sagittaUs superior; but interspersed among the connective-tissue fibrils are many stellate or spindle-like nuclei, greatly exceeding in number the nuclei found in the dense dura of the adult. Bone is being laid down in the outer portion of this dura where it merges \\dth the membranous skull. The lateral walls of the great sinus are still free from fibrillar depositions. A somewhat analogous picture is afforded b}' a photomicrograph of a specimen stained after IMallory's method, from a fetal pig of the same length (fig. 105). In this specimen the outer portion of the dura, incorporated into a part of the membranous skull, is quite dense with the fibrous tissue; about the superior sinus, however, the decrease in the amount of fibrous tissue is very striking. The falx is beginning to exhibit a fair degree of fibrillar structure; it forms a definite division between the two hemispheres. | |||
In the larger fetuses, above 100 mm. in length, the process of formation in the dura of denser and denser connective tissue proceeds rather slowly. It is realized, however, that this fibrous transformation in fetuses of 10 cm. is veiy extensive, the region about the sinuses alone remaining comparatively free from the development of the fibrils. The chief difference between the dura of this stage and the dura of the adult is a greater number of cell-nuclei in the fetal membrane. It is well, then, to consider the cellular character of the fibrous membrane and the region about the sinuses in the larger stages. | |||
In a human fetus of 125 mm. (No. 900-H) the dura is quite fibrous, but still contains an increased number of the stellate and spindle forms of nuclei; likewise, about the superior sinus the tissue is an immature m30iomatous structure, fairly free from connective-tissue fibrils. This increased number of nuclei in the dural tissue holds also for human fetuses of 165 mm. (as in No. 745), but seems slightly decreased as compared with the smaller specimens. The lateral wall of the great sagittal sinus in this stage possesses distinct bands of white fibrils, but the tissue is much looser and more cellular than the fibrous dura over the hemispheres. These phenomena may be made out in similar stages of the fetal pig, as shown in figure 106, a photomicrograph from a specimen of 17 cm. In this specimen, treated by Mallory's stain, the superior longitudinal sinus is shown surrounded b}' a clear zone in which the deeply staining fibrils are comparatively few in number. On each lateral wall of the venous channels distinct fibrous bands may be made out, lying in the looser, more immature tissue. The lower portion of the falx has assumed quite an adult character. | |||
Gradually the conversion of the tissue about the cerebral sinuses into the adult structure progresses. Thus, in both human and pig fetuses of 20 cm. length, the dura mater has acquired practically all of its adult features. Everywhere over the cereljral cortex the dura is characterized ])y dense layers of interlacing strands of white fibrous tissue, but the number of nuclei in the.se bundles may still be slightly greater than in the adult structure. In the more posterior regions, at this stage of 20 cm., the lateral walls of the sinus sagittalis superior are found to be completely occupied by the white fibrous tissue; in the anterior portion of the sinus much thinner tissue, resembling myxomatous structure, appears, as shown in figure 107. But in this specimen the invasion of the area about the great venous channel by fibrils has begun; isolated bundles may be made out everywhere in the lateral walls of the sinus. This freedom from connective-tissue formation does not persist, however, and the area is gradually invaded by the continued growth of the fibrils. The avoidance of the region about the sinuses by the connective-tissue resolution will be further commented on in the following subdivision of this paper. | |||
The dura, then, develops probably first in connection with the mesenchjinal condensation which ultimately forms the bony skull and a portion of the dura (the cranial periosteum). It first becomes apparent, as a structural unit, as a more cellular layer differentiated, by a secondary condensation, out of the peria.xial mesenchyme. As the chondrogenous stage is approached it becomes differentiated as a distinct layer, maintaining varying relationships with the inner perichondrium of certain of the cranial bones. At a stage of 40 mm. m the fetal pig, the dura of the vertex may be dissected out as a distinct, somewhat fibrous laj'er. The process of fibrous-tissue transformation, however, is slow; the dura until late in fetal life shows an increased number of nuclei, as does any young connective tissue. The invasion of the region about the superior longitudinal sinus by connective-tissue fibrils is much more tardj' than is the transformation over the hemispheres. | |||
THE SUBDURAL SPACE AND THE MESOTHELIAL LINING OF THE DURA. | |||
The subdural space (cavum subdurale) has been the subject of controversy in regard to its role in the pathway of the cerebro-spinal fluid. Before the work of Key and Retzius^^s) ^\^q yj^^^y ^.f^g ]^q.](\ ^)jat the cerebro-spinal fluid occupied the subarachnoid space in the spinal cord, but that in the cranium the subdural space afforded an analogous pathway. This conception was largely due to the fact that, in dissection on fresh material, the dura and arachnoid in the spinal region are found to be in approximation; in the cranium the greater adhesion, bj' trabeculse, of the arachnoidea to the pia renders the freeing of the dura from the leptomeninges the simplest line of cleavage. This view was entirely disproved by the beautiful injections of Key and Retzius, who demonstrated the anatomical and physiological continuity of the subarachnoid spaces. | |||
With the introduction of this latter view by Key and Retzius the conception of the subdural space naturally changed. These Swedish investigators demonstrated a typical mesothehal cell-lining on the inner surface of the dura, as shown by the method of silver reductions. Without an intimate connection with the true cerebro-spinal fluid, the subdural space has come to be looked upon as somewhat analogous to the serous cavities of the body. Quincke^-*^', after a subdural injection of cinnabar granules, ascertained that communications existed between the subdural and subarachnoid spaces, but only in the direction from subdural to subarachnoid. Leonard HilK^^), from the results of physiological experiments, assumed that fluid passed from the subdural to the subarachnoid space, and in the reverse direction, with great ease. The more recent investigations, however, lend evidence to the view that in the normal animal with undisturbed intracranial pressure relations the two spaces are physiologically as well as anatomically separate. The current impression that the subdural space is in manj' respects a serous cavity will probably finally have greatest support; intimate connections with the lymphatic system are, however, entirely lacking in the dura. | |||
The development of the subdural space must necessarily follow the develoj)ment of the dura. It has been mentioned that in fetal pigs of 50 mm. the dura can be freed from the arachnoid by gross dissection, but that at this stage many areas of adhesion between the two membranes exist. Such an observation has considerable bearing on the subdural space. For in the development of this space two processes must proceed far enough to permit the separation of the dura and arachnoidea by the capillary layer of fluid. The first of these processes, in order of probable importance, concerns the condensation of mesenchj'mal cells to form the outer membrane of the arachnoidea. This thickening and resolution into a true membrane takes place in close approximation to the inner surface of the dura. The second factor concerns the covering of this inner surface of the dura with mesothelial cells. | |||
The lining of the subdural space by mesothelial cells can be readily demonstrated on the inner surface of the dura by silver reductions, but the outer membrane of the arachnoid does not permit of a similar technique. This technical failure in regard to the outer arachnoid surface is probably to be accounted for by the dissimilarity in cell-structure in the two situations. Similar difficulties have been encountered by other observers. | |||
In order, then, to ascertain, if possible, at what stage a really adult subdural space could be demonstrated, the inner surface of the dura from fetal pigs of various lengths was subjected to treatment with silver nitrate. After the reduction had taken place to a sufficient degree, the whole dura was washed with distilled water, stained with hematoxylin, and cleared in glycerin. The i)ictures afforded by this method were quite satisfactory, and the technical procedure was so simple and reliable that considerable faith could be placed in the absence of the intercellular reduction lines. | |||
The smallest fetal pig in which a typical mesothelial cell-j)attern could lie demonstrated on the inner surface of the cranial dura was one of 50 mm. In this specimen the inner surface of the dura was not uniformly covered with the mesothelial cells; certain ragged areas seemed to represent the points of adhesion of the arachnoid to the dura. Figure 108 is a reproduction of a drawing made from one of the areas in this specimen where a mesothelial cell pattern could bo seen. The drawing shows many of the characteristics of mesothelial ci'll-i)ut terns of other parts of the body. The irregularities in the cell-borders, the frefiuent accumulations of the reduced silver in the cellular angles, and thegeneral cellular pattern are quite typical ; but the variation in the size of the cells, as shown in figure 108, is also somewhat different from the usual finding in the adult, where there is considerable constancy in the size of the cells. About half the cells in this fetal pig of 50 mm. are diminutive in size; the smallest are hardly a third the size of the largest. Transitions between the smallest and largest cells are al.so shown in this figure. It i.s difficult to a.scertain whether these smaller cells represent young elements which have not yet reached their maximal growth; no evidence of cellular division, as evidenced by mitotic figures, has been observed, although in this connection it must be granted that the cleared si^ecimens are hardly the most favorable. Und()ul)tedly this explanation of the smaller cells would seem to be the true one, but there is little proof for the view, except their absence from the adult dura and their disappearance in larger specimens. | |||
This disappearance of the smaller mesothelial cells is not rapid, but is seemingly delayed over into the larger fetuses; thus, in figure 109, a similar preparation from a fetal pig of 75 mm., corresponding smaller cells arc outhned. On account of the absence from the field of the drawing of the larger elements, these cells do not appear relatively as great in number as in the preceding figure. Likewise, in figure 110 every gradation in cell-size is shown, in a specimen made in the same manner from a dura of a fetal pig of 90 mm. | |||
Very slowly in the course of growth of the fetus the cells Uning the inner surface of the dura reach their standard size and compose the mesothehal surface, with very little variation in size. The process, however, is apparently very tard}', even though the fetus at 16 cm. shows an inner surface to the dura which is largely composed of standard cells (fig. HI); but even in this figure, from a relatively large fetus, the standard size of the cells has not been attained, for a few cells of small size appear in the drawing. In other respects the whole pattern, in general appearance, resembles closely the adult. | |||
It seems most fair to assume that the occurrence of a true mesothelial cellpattern on the inner surface of the dura represents the initial estabUshment of a subdural space. On this basis the subdural space may be said to occur in fetal pigs 50 mm. in length; in the present investigation it has been found impossible to demonstrate the existence of the mesothehal cell-pattern in fetuses smaller than 50 mm. The separation of the dura, possible bj'^ gross dissection in pig fetuses of 40 mm., suggests that the space may be found at a sUghtly earUer stage than that in which the mesothehal cells have been demonstrated. | |||
Anatomically the subdural space in pig fetuses resembles m everj' particular the adult space in cats and dogs; this was described in a paper^^S) pubhshed in 1914. In the large pig fetuses injections of solutions of potassium ferrocyanide and ironammonium citrate were made into the spinal subarachnoid space. After precipitating the foreign salts as prussian-blue, the injection is found to be wholly within the subarachnoid spaces, both in the spinal and cranial regions; the subdural space is absolutely free from any evidence of connection with the subarachnoid space. These findings wholly accord with the opinion concerning the adult subdural space which has been repeatedly expressed. | |||
THE COMPETENCY OF THE EARLY DURA AS A CELLULAR MEMBRANE. | |||
During the stage when the condensation of mesenchyme to form the cranial blastema is prono'inced the spread of the cerebro-spinal fluid becomes more and more extensive. In these stages, when the pig embryo measures from 16 to 25 m m. approximately, the outer membrane of the arachnoid is not 3'et formed, the arachnoid spaces extending from pial to blastemal condensation. WTien in these embryos the cerebro-spinal fluid is replaced by the ferrocyanide solution and the embryo kept alive for some time, the course of the injection may be traced to varying extents throughout the periaxial tissue. To this spread of the injection fluid (a true solution, during the progress of the experiment), however, the blastemal condensation of mesenchjme opposes an absolute barrier. This pecuUarity of the early condensation may be readily seen in figures 16 and 18. At this stage in development the blastemal thickening may be said to play the role of the outer membrane of the arachnoidea or of the inner surface of the dura. | |||
This feature of the blastema as an impenetrable membrane — an absolute barrier to the passage of fluid — is found also to endure during injections of the ferrocyanide solution under pressures sufficient to rupture other parts of the central nervous system. Similarly, the early blastemal condensation resists the inflow of the other injections used (india ink and silver nitrate) under similar pressure conditions. In later stages the injection solutions, from ventricular or subarachnoid mtroduction, do not reach the dura. This is due to the development of an outer membrane of the arachnoidea and the formation of the subdural space. The arachnoid membrane when formed does not permit fluid to pass outward into the subdural space; but the competency of the early blastemal condensation in the mesenchyme affords a very good example of the perfect function of a tissue as a fluid barrier. | |||
An interesting phase of the competency of the secondary mesenchymal condensation (forming dura and outer membrane of arachnoid) may be seen in the region of the cisterna cerebello-medullaris. Here, as shown in figure 77, the zone of secondary condensation, while complete below, does not remain definitive above as the mesenchyme sweeps inward to the chorioid plexuses. At such a stage of 32 mm. in the pig, a replacement experiment would show no penetration of this secondary dural condensation by the foreign solution, where the condensation made a definitive membrane; above, however, in the region of the plexuses, a limited penetration by the introduced fluid could be made out. | |||
X. THE RETURN OF CEREBRO SPINAL FLUID TO THE VENOUS SYSTEM. | |||
The question of the exact mode of return of the cerebro-spinal fluid to the general circulation has interested many investigators. It has occasioned a large amount of work, with the presentation of several hypotheses. Key and Iletzius<2»), from the results of injections of colored gelatin into the spinal subarachnoid space, held that the cerebro-spinal fluid returned through Paccliionian granulations into the great dural sinuses. Other workers, following Key and Retzius, were dissatisfied with this theory, because of the apparent lack of these granulations in infants and in the lower animals. Cathclin(6), with but little evidence, hypothecated an absorption of the fluid by way of the perineural sheaths into the lymphatic system, although the physiological findings of Ziegler(57j^ Reiner and Schnitzler(<«), Leonard 11111*2^), and others made it necessary to consider a direct absorption into the blood system. Cushing(9) premised the drainage of fluid into the great sinuses through a valve-Hke mechanism. Dandy and Blackfan'"' .-suggested its absorption l)y the capillaries of the pia-arachnoid — an untenable hypothesis in view of the work of Kadyi'^Sj, Shroeder van der Kolk(5i), Ekker(i*>, Adamkiewicz^*), and others. Still another conception of the process has been advanced by Mott('**), namely, that the absorption of cerebro-spinal fluid isone of the functionsof the cerebral capillaries. Ina previous investigation'^), making use of a method similar to the one here employed in the rej)lacement experiments, evidence was presented indicating the drainage of cerebro-spinal directly into the great dural sinuses through arachnoid villi. These structures represent an invasion of arachnoid tissue through the lateral wall of the sinuses. | |||
In view of the findings in adult laboratory animals, interest naturally turned, during the course of this work, to the process of drainage of the embryonic cerebrospmal fluid. The evidence afforded by the replacement experiments with the ferrocyanide solution indicated that in pig embryos of over 20 mm. cerebro-spinal fluid circulated throughout most of the periaxial tissue, and that in embryos of about 26 mm. the periaxial distribution was complete, the relations of the fluid at this stage becoming adult. With this evidence before us, the question of the drainage of the fluid became important. as the absorption process similar to the normal adult procedure, or was it entirely lacking, the production of the fluid being balanced by the growth of the nervous system and its meningeal spaces? | |||
The question of the absorption of cerebro-spinal fluid was approached in the embryo in a similar manner to that employed in the adult animal. The problems incurred by the use of abnormal intracranial pressureswere eliminated by the method of replacing, without disturbing the normal tension, the embryonic cerebro-spinal fluid with the ferrocyanide solution. The embryo was then kept aUve and was finally fixed in a preservative which would precipitate the replaced fluid as prussianblue. This procedure was carried out in many embryos of varying lengths and the specimens were subsequently stained in serial sections. | |||
The smallest embryo in which any evidence of absorption of the fluid from the periaxial tissue was obtained was a pig embryo, 23 mm. in length. In this specimen granules of prussian-blue could be traced through the mesenchymal spaces (arachnoidal) to the inner wall of the sinus transversus. The sinus is well differentiated at this stage in the human embryo of 21 mm., as demonstrated by Streeter^**). The wall of the sinus in this pig embryo was quite thin, the mesenchvTne lending the endothelium but Uttle support. The prussian-blue granules could be traced directly through the endothehnl wall of the sinus, and a few were identified lying free in the lumen. The conditions of the observations, permitting a flow of venous blood through the sinus, undoubtedly accounted for the fact that but few of the granules were found Ijing free in the sinus. This passage of the replaced fluid into the lateral sinus is portrayed in figure 21, taken from the pig embrj-o of 27 mm. | |||
The same process of drainage of cerebro-spinal fluid may be obser^-ed in pig embryos more than 23 mm. in length. In all but one particular it corresponds exactly to the process observed in adult laboratory animals. There is the same lack of absorption on the part of the cerebral veins and embryonic capillary plexuses. In the adult, however, the process is not diffuse, but is confined to the arachnoidal villi, while in the embryo a considerable extent of the inner wall of the sinus lying in the mesenchymal tissue, which is breaking down to form the arachnoidal spaces, serves as a site for the fluid passage. In these earlier stages the sinus transversus functions as the chief sinus of absorption. This is probably to be explained by the primary basilar spread of the replaced cerebro-spinal fluid and also by the fact that the true sinus sagittalis superior is a much later addendum. In the human embryo, according to Streeterf^^), it is found in stages of over 50 mm. | |||
The absorption of cerebro-spinal fluid in the embryo seems to follow the directing agencies which operate in the adult. Increase in the pressure employed in the injection of true solutions results in the drainage of more of the fluid, as determined by subsequent microscopical examination. This suggests that the process is determined by factors other than that of difTusion; it seems most likely that here, too, the process is one of filtration, with a possible distension of the cellular membrane, so that intercellular spaces are opened. The histological picture of the sinus waU, however, undoubtedlj^ gives the impression that the fluid has passed almost solely through the cytoplasm of the endoth(>lial cells and likewise through the layer of supporting mesenchyme. These findings are in accord with observations on the adult. | |||
With dilute suspensions of india ink as the injection mass, the results are quite different in regard to the passage of the material into the sinus. Replacement experiments making use of this suspension of particulate matter yield no evidence, as the carbon granules do not leave the ventricular system. Likewise, simple injections of the suspension into either the central canal of the spinal cord or into the perispinal spaces furnish no information unless the syringe-pressure be high. In this case the carbon granules may be traced into the sinus transversus, which is apparently the point of least resistance. Because of the obscuring of the picture by the carbon it can not be determined histologically whether the granules pass into the sinus in the same manner as does a true solution, or whether the passage is effected by numerous small ruptures of the tissue. The impression gained from our study would incline one toward the latter view. | |||
If the injection of india ink be made under very great pressure from a syringe, the segmental veins may be filled with the carbon. This filling is always subsequent to its flow into the sinus transversus. But in no case was an evidence of a flow into lymphatic channels observed. | |||
The process of drainage of the cerebro-s])inal fluid into the venous system of fetuses will not l)e (U^tailed here. This undoubtcdl}' concerns a study of the formation of arachnoidal villi and of the differentiation of the lateral walls of the superior sagittal sinus, the best site for this study. The material at hand is not suited for this investigation, so that postponement is necessary. | |||
==XI. The Chorioid Plexuses and the Elaboration of Cerebro-Spinal Fluid== | |||
With the realization that at a definite period in embryonic Ufe, cerebro-spinal fluid passes from the cerebral ventricles into the periaxial spaces, it seemed desirable to ascertain what relationship existed between the developing chorioid jilexuses and the elaboration of the fluid; for with the extension of the fluid into the periaxial tissue it becomes obvious that the balance between the development of the intraventricular fluid and the volume of the ventricles is destroyed and that more fluid is being elaborated than can be contained within the medullary-canal sjstem. This relationship between the ventricular volume and the production of cerebro-spinal fluid has been described at some length in a preceding section of this communication. | |||
The determination, then, of the exact role plaj-ed by the chorioid plexuses in the further extension of the fluid into the periaxial tissue appeared to be of importance, for it could be conceived that the embryonic ependymal cells might be capable of elaborating the excess of fluid. With this purpose in mind the chorioid plexuses were investigated from morphological and cytological standpoints, in the hope that some index might be afforded as to the assumption of function on the part of the developing chorioid plexuses. These methods of study were apphed solely to the chorioid plexuses of pig embryos, for it is from them alone that evidence of the period of extraventricular extension of the cerebro-spinal fluid has been obtained. | |||
THE DEVELOPMENT OF THE CHORIOID PLEXUSES. | |||
The development of the chorioid plexuses is so well understood that only a ven,brief outline will be given here. The general scheme of origin of these glandular structures concerns a gradual histological differentiation in certain localities of the ventricular ependyma. The ependyma of the roof of the fourth ventricle thickens along the transverse invagination (phca chorioidea) and then gradually becomes tufted in villous projections into the ventricle, following the ingrowth of a capillar^' plexus and supporting mesenchyme. This general process of differentiation occurs at first along the lateral portions of the phca; the central portion of the ependj-ma remains unaffected by the villi even when the tufts have become quite well differentiated (fig. 23). | |||
Quite similar to this process of development of the plexus chorioideus of the fourth ventricle is the differentiation of the other plexuses. The plexus of the third ventricle develops as an infolding of the tela chorioidea of the roof. In every case the process holds of ependymal invagination and subsequent vascularization and suspension by mesenchymal ingrowth. | |||
The histological differentiation of the ependjTnal cells into the glandular tj-pe of plexus, as first determined by Luschka''^^ and Faivre^^^)^ jg hardlj- satisfactory as an index of the production of fluid, as the secretory phenomena of the adult cells have not as yet bten completely established. The researches of Pettit and Girard(^\ dealing with the correlation of histological changes in the chorioidal cells and their functional state, first furnished reliable evidence that these cells give rise to cerebrospinal fluid. Since the publication of their investigations in 1900 many workers — Meek(37), Findlay("), Pellizzi(''2^, Mott^^D, Hworostuchin(26), Engel(>2), and othershave been concerned with this problem and have established on fairly definite bases the relationship of the plexuses to the production of the fluid. The histological appearances of the secretory cells, however, does not rest on incontrovertible ground, as has been stated in a previous paper^^^). | |||
The process of diff'erentiation of the ependymal cells which form the glandular elements of the chorioid plexuses occurs with the invagmation and tufting of these structures. The various stages of transformation from the low type of cubical epithelium constituting the ependymal layer are shown in various figures in this paper. The nuclei of these cells assume basilar positions and the outer zones of the C3-toplasm become granular with their greater height. The process is rather a slow one, as might be expected from the fact that the whole villus is gradually enlarging and becoming more and more tufted. | |||
The histological differentiation of the plexuses need hardly concern us here, except as an index of the assumption of function. The final completion of this change into the adult morphology occurs at a much later stage of development than our evidence indicates for the establishment of a cerebro-spinal circulation. It becomes obvious, then, that the final liistological changes are not necessary for the process of elaboration of the fluid. This assumption seems warranted also.b}'' the fact that the embryonic fluid contains much more albuminous material than does the adult fluid. | |||
The time of appearance of the chorioid plexuses in relation to the extraventricular spread of the fluid would surely seem to offer undoubted evidence in regard to the first elaboration of the fluid by the plexuses. It has been shown that in pig embryos over 14 mm. in length the replaced solution in the cerebro-spinal system spreads from the roof of the fourth ventricle into the periaxial tissues. This extraventricular extension occurs practically simultaneously with the first indications, in the pig embryo, of the formation of the chorioid plexuses of the fourth ventricle. Thus, in a pig embryo of 14 mm., the primitive thickening and tufting of the ependyma of the roof of the fourth ventricle may be observed (fig. 32). In earlier stages no definite evidence of this developmental process is found. | |||
From the first indication of a developing chorioid plexus in a pig embryo of 14 mm., the growth of the tufts progresses rapidly, so that at 18 nmi. the process is well advanced. In embryos of 20 mm. and over the tufts of the plexuses in the fourth ventricle are quite marked, as shown in figures 22, 44, 40, and 92. | |||
The chorioid plexuses of the third and lateral ventricles api)ear at a somewhat later stage than do those in the more caudal v(>ntricle. Thus the first indication of their ai)i)earance in pig eml)ryos is found in si)ecimens measuring 19 mm. in length. This coincides well with the further extension of the replaced fluid in specimens of 19 mm. and over. The definite differentiation of these plexuses, however, does not actually take place until the embryo reaches a length of 23 mm.— a fact suggestive of some relationshif) to the complete periaxial spread found in embryos of this measurement. | |||
Considered, then, as a whole, there seems to be a very definite relationship between the developing chorioid plexuses and the periaxial spread of the embryonic cerebro-spinal fluid; for immediately after the first appearance of chorioidal tufting in the roof of the fourth ventricle (at 14 mm.) the replaced injection sjjread appears in the periaxial tissue (fig. 3). This extraventricular spread does not become marked until a length of 19 mm. is attained (fig. 5) — a factor in accord with the elaboration of the villi in the chorioid plexus of the fourth ventricle. The periaxial spread remains localized in the rhombencephalic region until the 20 mm. stage is attained, when it rapidly becomes pericerebral and perispinal (figs. 6 and 7). This coincides with the first indications of the chorioid plexuses in the more cephalic ventricles. But the further spread is here delayed (as in the stages between 14 and 19 mm.) until a length of at least 24 mm. is reached — which is perhaps of importance in the further development of the cerebral plexuses and the greater elaboration of the cerebro-spinal fluid. Thus it seems possible to conclude that coincident with the first appearance of the chorioid plexuses a more rapid production of cerebro-spinal fluid occurs, necessitating the passage of the fluid into the periaxial tissues. | |||
THE GLYCOGEN CONTENT OF THE CHORIOID PLEXUSES. | |||
In the hope that some cytological method might afford direct and incontrovertible evidence of the time of the assumption of function by the chorioid plexuses, stains demonstrating the intracellular presence of glycogen were applied to these structures. The quantitj' of the starch in the chorioid plexuses of rat and mouse embryos, as shown bj' Goldmann, suggested that this substance might be associated with the early elaboration of the cerebro-spinal fluid. Furthermore, the presence in the adult fluid of a definite reducing bodj', demonstrated by XawTatschi to be dextrose, added some weight to the hope that a definite conclusion might thus be afforded. | |||
Several important studies concerning the presence of glycogen in the cells of the embryonic and fetal chorioid plexuses have been made. Creighton W found that the glycogen of the chorioid plexus was verj^ abundant about the middle of embryonic life, while von Loeper concluded that the great content in the cells of the fetal plexus was characteristic. Goldmann^^o) found large quantities of gh'cogen in the plexus in rats and mice, not only in embryonic life but also in animals from two to three weeks old. In the adult plexuses the cells contained no trace of glycogen. | |||
The observations here included were made after fixing the chorioid plexuses of various pig embryos in absolute alcohol and staining the sections (cut either from celloidin or paraffin blocks) by Best's carmine method. This technique is similar to that employed by Goldmann. The staining reaction is such that a very striking differentiation of the glycogen occurs, but the shrinkage of the embryonic tissue in the fixation in absolute alcohol is a disadvantage. In these obsers'^ations the plexuses from the fourth and lateral ventricles were used. | |||
As shown in the table on page 94, glycogen could be identified in the cells of the chorioid plexuses in pig embryos varying in length from 28 to 155 mm. | |||
Below the first measurement no glycogen was demonstrated bj' the method employed; above the higher limit in only one instance (series No. 41) was glyocgen found. This finding of a limited period in the embryonic hfe of a pig during which glycogen occurs in the cells of the chorioid plexuses does not coincide with Goldmann's observations on the rat and mouse. Furthermore, it was found here that in stages up to 100 mm. the glycogen was practically generally distributed throughout all the cells of the chorioid jilexus, occurring with gn^it regularity in every villus and cell. This general distribution was not found in the plexuses of embiyos over 110 mm. in length; in these more advanced stages the cells containing starch occurred in clumps, giving a localized distribution. In the stages under 100 mm. the glycogen was present in very large amount, as estimated histologically. As the stages advanced the quantity of glycogen decreased rapidly. This great amount of starch was present in the same stages in wliich the general distribution of the cells occurred. | |||
Occurrence of glycogen in the chorioid plexuses of embryo pigs. | |||
C.P. | |||
series, | |||
No. | |||
C.R. | |||
naeasure, | |||
mm. | |||
Glycogen. | |||
Globular forms of glycogen. | |||
Plaques of glycogen. | |||
Amount of glycogen. | |||
Distribution | |||
of glycogen | |||
throughout | |||
plexus. | |||
Intracellular distribution of glycogen. | |||
16 | |||
3 | |||
13 | |||
12 | |||
14 | |||
6 | |||
9 | |||
8 | |||
4 | |||
1 | |||
42 | |||
17 | |||
15 | |||
20 | |||
10 | |||
18 | |||
27 | |||
39 | |||
25 | |||
32 | |||
40 | |||
41 | |||
24 | |||
19 | |||
23 | |||
11 | |||
21 | |||
22 | |||
26 | |||
5 | |||
18 | |||
23 | |||
28 | |||
33 | |||
36 | |||
39 | |||
40 | |||
55 | |||
66 | |||
80 | |||
90 | |||
100 | |||
105 | |||
118 | |||
132 | |||
155 | |||
155 | |||
155 | |||
158 | |||
160 | |||
163 | |||
170 | |||
173 | |||
185 | |||
195 | |||
209 | |||
213 | |||
223 | |||
244 | |||
260 | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present . . | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Absent | |||
Present | |||
Present | |||
Present | |||
Absent | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Present | |||
Absent | |||
Present | |||
Present | |||
Absent | |||
Present | |||
Absent | |||
Absent | |||
Absent | |||
Absent | |||
Absent | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Great | |||
Small | |||
Small | |||
SmaU | |||
SmaU | |||
Small | |||
SmaU | |||
General | |||
Localized | |||
General | |||
General | |||
General | |||
General | |||
General | |||
General | |||
General | |||
Genearl | |||
General | |||
Localized | |||
General | |||
LocaUzed | |||
Localized | |||
Localized | |||
Basilar. Basilar. Basilar. Basilar. Basilar. Basilar. General. General. General. General. Basilar. General. General. General. General. General. | |||
Absent | |||
Present | |||
Absent | |||
Present | |||
Absent | |||
Small | |||
Localized | |||
General. | |||
Absent | |||
Absent | |||
Absent | |||
Absent | |||
Absent | |||
| Line 1,393: | Line 1,596: | ||
Cioldmann'20) pictures the glycogen as occurring throughout the cells of the chorioid i)lexus in the form of globules of larger or smaller size. Some of these globules may be .seen even in the s\n'rounding cerebro-sjiinaj fluiil. This general intracellular disposition was observed in this series in specimens measuring 6G mm. and over (fig. 95). Below this measurement the glycogen occurred practically entirely in the basilar portion of the cell, central to the nucleus. Furthermore, in the stages between 30 and 00 mm. the glj'cogen globules were present in but small numbers and the glycogen was found in crescentic plaques (fig. 96). This formation of definite plaques is ai)parently to be ascribed to the fusion of the globules when the amount of glycogen becomes extreme. As far as is known tliis plaque formation with glycogen has not previously been noted; in one of Goldmann's figures the fusion of some of the globules has apparently taken place. | |||
The table on page 94 records the findings in these observations. | |||
The | The occurrence of glycogen in the cells of the chorioid plexus only during a certain portion of embryonic life is, as shown l)y the foregoing table, a fairly definite phenomenon, but there is surelj' no indication that this temporary presence of the animal starch bears any relation to the assumption of function on the part of the chorioid plexuses. The evidence afforded by the extraventricular flow of the replaced fluid, with the apparent relationship of the developing chorioid plexuses to the periaxial extension of the fluid, argues strongly against such an assumption. | ||
XII. PERIVASCULAR SPACES IN THE EMBRYO. | |||
In 1865 His' using a puncture injection, found that each nerve-cell existed in a so-called space. These pericellular spaces connected, as demonstrated by the flow of the injection mass, with an extensive perivascular network, more complex in its gray matter than in the white. In all of His's cases continuation of the injection led to a peripheral spread toward the pia, both in the spinal medulla and in the brain. | |||
]Mott('*^\ working on the brains of animals in which an experimental cerebral anemia had been produced by ligation of the head arteries, found the perivascular spaces enormously dilated and the perineuronal spaces Likewise verj' evident. Direct connections between the perivascular and perineuronal spaces are pictured in Mott's communication. | |||
The deduction which ^Nlott made from his findings, regarding the possible absorption of cerebro-spinal fluid by the cerebral capillary bed from this perivascular and perineuronal system, was discussed by the present author in a paper two years ago(55). It was there shown that, with the use of true solutions as the injection (potassium ferrocyanide and iron-ammonium citrate), the whole perivascular sj'stem could be filled. This injection of the spaces, however, occurred only when the pressure conditions within the cranial cavity were such that the subarachnoid pressure exceeded the vascular tension. This reversion of the pressure relations was accomplished by maintaining at normal the subarachnoid pressure with the injection fluid, and occasioning a simultaneous and complete vascular anemia. Under the routine conditions of injection (with undisturbed pressure relations) no injection of the perivascular system from the subarachnoid space resulted. It was found impossible to mject the perivascular system, using granular suspensions as the injection mass, without employing pressures far above the normal. | |||
From these results here recorded briefly, the belief was expressed in tliis former paper that each nerve-cell was surrounded by a capillary space which drained along the perivascular channels into the subarachnoid spaces. Probably this sj'^stem represents a mechanism for accessory tissue drainage comparable ph3^siologically to the lymphatic channels of the other parts of the body. | |||
In view of these findings in the adult mammal it seemed desirable to ascertain at what period of intra-uterine life such function was acquired. It also seemed not unhkely that information of interest might be acquired from the embryonic intramedullary circulation which would amplify our knowledge of this sj^stem in the adult. It was thought that there might be a correlation between the production of the perivascular fluid and the enlargement of the subarachnoid channels, similar to the evident connection between the chorioidal invagination and the extraventricular spread of the fluid. | |||
Experiments to demonstrate possible perivascular and perineiu-onal spaces were first attempted on rather large fetuses (pig), as follows: The spinal meninges were exposed in a fetus in which the heart was still beating vigorously. Into the spinal subarachnoid space was introduced a needle connected with a small reservoir, containing the injection solution (potassium ferrocyanide, 0.5 gm.; iron-ammonium citrate, 0.5 gm. ; water, 100 c.c). The reservoir was then adjusted so that a pressure of 160 mm. of water was maintained in the subarachnoid sjiace. The arteries and veins in the neck of the fetus were then severed, and the subarachnoid pressure maintained at its former level. At the end of 20 minutes the head was placed in a fixative containing 1 per cent hj^drochloric acid. | |||
This procedure, as outlined above, in the adult laboratory mammal, usually resulted in a complete injection of the perivascular system. In the embryo, however, the procedure was uniformly unsuccessful. The injection solution, as shown subsequently by the precipitated prussian-blue, rarely ascended over a centimeter above the point of injection. This indicated that the existent cerebro-spinal fluid was not replaced by the injection solution, and that the failure to demonstrate the perivascular system was to be explained on this basis, if the system were functional at this stage. Attempts were then made to replace the subarachnoid fluid with the injection solution before the cerebral anemia occurred. These attempts likewise m^t with failure, because of the impossibility of keeping the heart beating for any length of time in the larger pig fetuses. Other attem]its were also made to demonstrate these channels, in larger pig embryos, by means of a procedure which in the adult gave at times good injections of these intracortical canals. This method differed from the method first employed only in the maintenance of a high pressure (100 mm. Hg) in the spinal subarachnoid si)aces. It likewise met with failure, due ai)|)arently to the same causes which occasioned its failure in the adult: the high sul)arachnoid pressure o})erated chiefly to compress the cerebral and spinal tissues, rendering the injection of the i)erivascular spaces impossible. | |||
The same procedures were attempted in smaller pig embryos (15 to GO mm.). The method usually successful in demonstrating the spaces (subarachnoid pressure slightly above normal, with subsequent cerel)ral anemia) failed, ajjparently because the cranial cavity at these stages is in no sense a rigid closed box. as in the adult. | |||
Any method of service in the adult — which must have in consideration the physical character of the skull as a closed box — was here necessarih' doomed to failure. | |||
Together with these technical failures to demonstrate a perivascular system, it must be borne in mind that these are merely failures to demonstrate the existence of the perivascular system in the pig embryo. The system wall probably be demonstrated as soon as a suitable technique is devised. The spaces are very likely present soon after the capillary plexus invades the nervous system, but the observation in many histological preparations of the spaces around the cerebral vessels must not be considered as offering proof of their existence, because of the likehhood of shrinkage influencing the picture. It is interesting, how^ever, to note that elasticity of the cerebral tissues seems greatest along the course of the blood-vessels, for here the phenomenon of shrinkage is most frequently observed. The existence of the perivascular and perineuronal spaces, probably of only capillary thickness, must remain — in the embryo as in the adult — a subject of physiological demonstration; histological e\adence, except with proper physiological regard, is of no value. | |||
The early development and function of such a system as the perivascular and perineuronal canals afford seems most likely from the standpoint of pure speculation. It is not improbable that fluid is poured from this system into the embryonic subarachnoid space at a period soon after the capillary plexus invades the cerebrum. There is no evidence, however, from the observations recorded in foregoing paragraphs, that adequate subarachnoid channels are afforded until the pig embryo reaches a length of about 25 mm. The hypothesis of Essick^^^) regarding the damming of the perivascular fluid as the cause of the two cava corporis striati is of extreme interest in this connection. It remains, however, for future work to afford real evidence in regard to the embryonic perivascular system. | |||
XIII. THE PERINEURAL SPACES IN THE PIG EMBRYO. | |||
The question of the existence of potential or actively functional spaces around the peripheral nerves is of great interest, partly because of the possible relation of these spaces to the developing lymphatic system, and also on account of the anatomical evidence of the possible existence of such spaces. | |||
It is realized that before much dependence can be placed on any theory regarding these potential spaces around the cerebro-spinal nerves, the possibility of their being purely artifacts must be dealt with. The methods of demonstration, in the adult, in the hands of the earliest workers were such as to favor the production of artifacts. As far as can be ascertained, Cotugno('), dealing with the ner\-us ischiadicus, was the first to conceive of these possible spaces. His method of demonstration consisted in filling the spinal subarachnoid space with mercurj' (in a cadaver placed in the erect posture) . Globules of the mercurj^ were subsequently found about the sciatic nerve in what then became the perineural spaces. | |||
Modern anatomical interest in these spaces was aroused by the remarkable injections of Key and Retzius^^s). These investigators, by means of gelatin injections into the spinal subarachnoid space, were able to demonstrate perineural spaces around the cranial nerves, especially around the optic pair. Their results, however, are open to criticism, because of the excessive pressures employed ("not over 60 millimeters of mercury") and because the injections were made in fresh cadavers kept warm for periods of 10 or more hours. | |||
" | |||
In | Some of the difficulties concerned in the problems of the perineural spaces were cleared up in a studj'^^^) of the cerebro-spinal circulation published in 1914. In this work injections of true solutions (similar to those used in the present study) were introduced into the spinal subarachnoid space in living cats and dogs, under pressures but sUghtly exceeding the normal intraspinal tension. These injections were continued for several hours, and the course of the injection fluid was then estabhshed bj'' precipitating the solution in situ. By means of this procedure, which it was beUeved approached the physiological, the perineural spaces around the cranial nerves could be demonstrated. In these adult laboratory mammals the cerebral nerves without exception showed prussian-blue granules in a perineural relation, extending outward along the nerves bej^ond the termination of the dural cuff. This extension of the injection mass outward was more striking around the first two cranial nerves than about any of the others. Thus, the olfactorj'^ nerves uniformlj'^ showed perineural deposits beyond the cribriform plate, extending downwards into the nasal epitheUura, while the optic nerves were surrounded by the granules in the inf ravaginal sheath, which spreads out over the posterior surface of the eyeball. The caudal cranial nerves were likewise characterized by extensive perineural injections. | ||
These findings were interpreted as e\'idencing a true perineural space, probably of only capillary thickness, which could be injected by filling the cerebro-spinal spaces with a demonstrable true solution. As far as could be made out under the microscope, they had no appreciable existence except when fiUed with the precipitated true solution. These spaces were not filled in the early moments of the injections under low pressures, and could be demonstrated only when the injection had been continued for several hours. | |||
The perineural spaces are quite different from the spaces surrounding the spinal ganglia and the gangUa of the cranial nerves. These ganglia he in the true subarachnoid space, wath the dura investing the arachnoid membrane. Distal to the ganglion the dura ends upon each nerve. In the injection under low pressure with the ferrocyanide the cranial and spinal ganglia were all surrounded bj^ the precipitated salts; the cranial nerves .showed extensive perineural injections, whereas the spinal nerves rarely showed a true perineural injection, and then only of hmited extent. | |||
The existence of perineural spaces in the embryo, however, has been under dispute. The larger nerv^es in sectioned embryos almost invariably show spaces about them, either a complete separation of the surrounding mesenchyme or a partial dilatation of the mesenchymal interstices. Sabint'*^), in 1902, noted that in pcrispinal injections with inflia ink the spinal nerves could be outlined by the carbon granules, but in no case did such an injection run into true lymphatic channels. No evidence was afforded by her work of any lymphatic channels arising from these apparent perineural channels. | |||
In the course of this investigation of the cerebro-spinal spaces interest naturally turned to the perineural spaces. In the typical experiments (a replacement of the embryonic cerebro-spinal fluid with a demonstrable true solution in the living embryo), there was evidence of a spread of the replaced solution around the cranial nerves. Because of the procedure u.sed (merely a filling of the ventricles and central canal of the spinal cord) no evidence of a perineural spread occurred until the foreign solution passed into the periaxial tissues. Here the spread chiefly involved the caudal cranial nerves curving around the lateral surface of the medulla in fanshaped processes (figs. 5, 6, 8, and 9). The spread, however, was not extensive. In figure 8 a similar slight spread along the spinal nerves is to be made out. Closer study of these cleared specimens, and examination of the same and of similarly injected embryos after serial sectioning, convinces one that the apparent perineural spread in these cases extends around the sensory ganglia and not further toward the periphery. In no case, cither in the caudal portion of the cranial or in the spinal region, has the replaced injection fluid passed the blastemal condensation of mesenchyme. This finding is well shown by the distribution of the injection fluid in figures 9, 16, and 18. | |||
The optic nerves, however, jjossessing gangha in the retina, usually show, in the typical replacements in the living embryo, a partial or complete surrounding of the nerves by the prccij)itated pru.ssian-bluc. An incomplete example of this — more typical, according to these observations, than a total circumvention — is given in figures 19 and 20. The higher-power reproduction of this field is very interesting. It shows in the central portion the fiber bundles comprising the optic nerve, surrounded by mesenchyme and the developing ocular muscles. In the region between the ners'e and the muscles is an undiflferentiated mesenchyme which is characterized by a crescent of the precipitated granules of prussian-blue. The non-penetration of the surrounding tissue by the ferrocyanide is verj- well brought out in this drawing. The prussian-blue has reached its position about the nerve by extension from the pericerebral spaces; actually it has still the same distribution as noted in figure 8 above. The adult dura will completelj- surround the optic nerve in its whole extent; the subarachnoid space will likewise extend unbroken to the posterior surface of the eyeball. Hence it must be assumed that in this case the perineural space does not extend beyond the peripheral ganglion. With regard to the olfactory nerves, no evidence of a perineural spread was obtained in specimens of pig embryos up to 45 mm. in length. | |||
It seems obvious, then, that in the embryo pig true solutions, when substituted for the cerebro-spinal fluid, do not extend peripherally along the nerves any further than does the dura in the adult. The replaced fluid (if, as appears most likely, it indicates the true circulation of the cerebro-spinal fluid) extends only through the future subarachnoid space. Such a conclusion is best supported bj' the observations. The only discreparcv between the findings in the pig embryo and those in the adult with the same method lies in the fact that in the adult the cranial nerves showed a much more extensive permeural injection. This seeming discrepancy may be accounted for in two ways. In the first place, the experimental replacement in the embryo pigs lasted at most one hour (due to the fact that the embryo's heart frequently ceased beating at the end of this time), while in the adult cat or dog they were continued for several hours; and it was only in the long-continued experiments in the adult that the extensive perineural injections were obtained. On this basis it seems more than likely that the communications between peripheral perineural spaces and the subarachnoid space are very small and that diffusion must account for the slow filhng of the peripheral system. The second explanation seems undoubtedly to concern the time of development of these perineural spaces in the embryo. It may be that the spaces are morphologically non-existent until late in fetal life; in that case, of course, it is not strange that they have not been filled wdth the injection fluid. | |||
From the observations recorded above it is quite apparent that in the typical experiment in which the normal cerebro-spinal tension is not increased no evidence of the perineural space, as injected by Miss Sabin, has been adduced. However, the possibility of mjecting these spinal spaces as was done by Miss Sabin is easily demonstrated. The injections may be made with ease, either with . granular suspensions or with true solutions. Success invariably attends such an injection into the perispinal tissues. The injection solutions easily run out around each nerve, more readily, apparently, in the younger embryo than in the older. It is not clear whether this difference is due to the fact that in younger embryos the resistance is greater to the perispinal flow and less peripherally, or merely to the fact that a greater amount of fluid must be introduced in order to attain the same result. Careful repetition of these observations has led to the conclusion that such a demonstration of the spinal perineural spaces results from excessive pressures of injection. WTienever the pressure exerted by the injection is but slightly above the normal, or does not exceed the normal (as in replacements), the perineural spaces are not injected around the spinal nerves. Miss Sabm's conclusions from her results, that no connection exists between the spaces and the lymphatic system, seem to be wholly substantiated by these observations. | |||
The apparent perineural spaces around the embryonic nerv^es must be looked upon as artifacts. In tissue carefully fixed, dehydrated, and embedded, there is no real evidence of these spaces. Theh- size apparently varies with the care observed in the histological technique. | |||
==XIV. General Summary== | |||
In the foregoing sections of this communication some of the problems concerned with the embryology of the cerebro-spinal spaces have been discussed and observ^ations have been presented in the hope that a better conception of the processes might obtain. It is purposed to present here briefly the results of these obser^^ations and to attempt to correlate the findings so far as is possible; and in this, as in the detailed reports in the preceding pages, the relationship of the jihysiological processes concerned will be referred to the morphological changes in the developing embryo. | |||
As a means of studying the physiological extent of the embryonic cerebro-spinal spaces, a method of replacing the medullary fluid with a foreign solution was devised. The procedure consisted in substituting, in the hving embryo, a solution of potassium ferrocyanide and iron-ammonium citrate for the cerebro-spinal fluid. The embryos were then kept alive, for periods of about an hour, by placing them with the attached placenta; in an incubator at 38°. At the end of this time, which varied in the many experiments, the whole embryo was fixed in a medium containing hydrochloric acid, thereby precipitating an insoluble prussian-blue. Specimens prepared in this manner were studied after sectioning or after clearing by the Spalteholz method. | |||
Pig embryos, subjected to such experimental replacements, exhibited only an intraventricular retention of the foreign solution until after a stage of 14 mm. was attained. In the earliest specimens, embryos of about 9 mm., there was no characteristic distribution of the foreign solution, except that it remained within the medullary-canal system. In stages of about 13 mm. the replaced fluid also was retained within the cerebral ventricles, but in these specimens a dense accumulation of the precipitated prussian-blue may be made out in a distinct oval in the superior portion of the rhombic roof. This granular aggregation occurs against a histological differentiated area in the roof of the fourth ventricle — an area which represents apparently the more epithelial-Uke elements of the earher roof-plate. This area must be considered solely as a differentiation of the epidermal lining of the medullary-canal system. | |||
In hving pig embryos of 14 mm. and over, the result of the routine replacement of the ventricular cerebro-spinal fluid was a sUght extraventricular spread into the tissues posterior to the rhombic roof. The passage of this foreign solution outward occurred through the same area of ependjTnal differentiation, outlined bj' the collection of granules against its inner surface in the previous stage. The extraventricular spread remains definitely locahzed to a ver}' small conical area which does not rapidly increase in size. | |||
The factors which cause this initial flow into the pericerebral spaces are of interest. It follows that in the growth of the embryo the production of the intraventricular and intraspinal cerebro-spinal fluid must necessarily keep pace with the increasing size of the cerebral ventricles. It is also necessarj' for the occurrence of an extraventricular spread of the fluid that the production of the fluid within the ventricles must exceed the amount required to keep the medullary-canal sj'stem filled. From our knowledge of the elaboration of the adult cerebro-spinal fluid, it is impossible to conceive of the production of a true cerebro-spinal fluid in the perimcdullary mesenchyme. Such a view would be a reversion to the old hypothesis of Haller, who regarded the leptomeninges as the elaborators of the fluid. Likewise, the passage of the replaced foreign solution into the extraventricular spaces would render such a hjiDothesis untenable. | |||
Hence, it becomes incumbent to regard such an extraventricular spread of the experimental solution as an mdication that the production of the cerebro-spinal fluid within the cerebral ventricles exceeds the capacity of the ventricles to care for the fluid. This argues strongly that the process of elaboration of the fluid in these pig embryos of 14 mm. is no longer sluggish, but that an active production, sufficient to cause a sUght extraventricular flow during the observation, is now taking place. This acceleration of the flow is not great, but it represents a marked change in the relationship of the process of fluid elaboration to the increasing volume of the ventricles. | |||
It seemed desirable to endeavor to correlate this extraventricular spread of the experimental fluid with the morphology of some intraventricular structure at this critical stage of 14 mm. m the pig embryo. The first evidences of villous tufting in the chorioid plexus of the fourth ventricles were found to occur at this stage in the pig. Other studies of this plexus, particularly those which concerned the occurrence of glycogen in these glandular cells, were found to offer no additional evidence of value in regard to the onset of function in these structures. The correspondence between the initial tufting of the ependyma to form the rhombic chorioid plexuses and the initial extraventricular spread must be regarded as of the utmost importance. It would appear most Ukely that as soon as the chorioid tufts occurred an increased production of cerebro-spinal fluid took place, necessitating an extraventricular expulsion of the excess of fluid. Such a view receives the utmost support from these recorded observations; it is in keeping with the best conceptions of the processes of production of cerebro-spinal fluid in adult mammals. | |||
With the initial pericerebral extension of the experimental fluid occurrmg in pig embryos of about 14 nmti., the further extension of this spread did not occur until after a length of 18 mm. was attained. At this stage the replaced foreign solution passed from the fourth ventricle through two areas in the roof-plate. The chorioid plexuses now have divided the roof into two portions; from each, fluid escaped. The superior area of fluid passage is the same which was concerned in the mitial outpouring of the ventricular fluid. The inferior area, like the superior, is an area of ependymal differentiation, of which the first evidence may be made out in pig and human embryos of 15 mm. This differentiation consists in the transformation of the densely staining ependymal elements into cells with larger nuclei, poor in chromatin, and with more abundant cytoplasm. | |||
After the functional employment of the two membranous areas is established at about 18 mm. in the pig, the further pericerebral spri.'ad of the replaced solution occurs very rapidly. The peribulbar tissues are filled with the fluid and from this region extensions occur downward into perispinal spaces and upward into the more basilar pericerebral spaces. Thus, the spinal spaces must be considered as develop>ing physiologically from above, and not from below upward, as Reford found. The complete filling of these perispinal spaces is found in pig embryos of 21 mm. At this stage the pericerebral spaces are filled, with the exception of those around the superior portion of the midbrain and about the cerebral hemispheres. | |||
The final filling of all the periaxial spaces occurred in pig embryos of about 26 mm. This phenomenon may be taken to indicate the estabUshment of the true cerebro-spinal relationships of the adult, for in this case there is an intraventricular production of the fluid and an extraventricular spread. Likewise, the fluid returns to the venous system in embryos of over 23 mm., and this escape of the fluid from its periaxial bed is, as in the adult, directly into the venous sinuses of the dura mater. | |||
The rapidity of the further extension of the replaced solution after the stage of 18 mm. is passed is apparently due to a second marked acceleration in the rate of production of the ventricular cerebro-spinal fluid. As in the first instance, this increased elaboration seems connected intimately with the formation of the chorioid plexuses of the third and lateral ventricles. As soon as these tufts develop, the cerebro-spinal fluid is produced in amounts which far exceed the quantities for which the more slowlj' enlarging ventricles can provide. | |||
The histories of the two areae membranaceae of the fourth ventricle are dissimilar. Both are areas apparently differentiated from the normal lining ependyma for a specific functional purpose — the passage of fluid from the ventricles into the future subarachnoid spaces. The superior membranous area reaches its maximum functional importance in the stages of 18 to 20 mm. in the pig and also in the human embryo and from these stages on it slowly regresses. The final obUteration of the area, if it do not persist as an occasional small remnant, is due to the increasing growth of the cerebellum and the enlargement of the chorioid plexuses of the fourth ventricle. On the other hand, the inferior membranous area continues to increase both in size and functional importance after its initial differentiation from the ependyma; it finally occupies the greater portion of the velum chorioidea inferior. These observations can not solve the interesting question of a perforation of the inferior velum to form the foramen of Magendie. | |||
Of the factors which influence the passage of fluid outward into the periaxial spaces, it must be reahzed that probably there is difference in this regard between the true solutions of the salts and the colloidal suspensions. For the true solutions (as in the experimental replacements) diffusion probably plays some role; but that this is not the sole factor is shown by the failure of the fluid to pass through the membrane in the stages under 14 mm. The findings of the granules of prussian-blue within the cytoplasm of the cells of this membrane mdicates that the fluid passage is similar in every way to that through a true membrane. There is also a possible site of fluid passage between the cells of this membrane. But, surely, the most important factor in this process is one of filtration of the fluid from the point of higher pressure to one of lower. This is mdicated by all of the findings : that the mcreased production of the fluid or the increased mtra ventricular pressure (whether due to normal or experimental agencies) causes a marked extraventricular spread seems firmly established. For the colloidal suspensions (particularly the protein of the normal ventricular fluid) a slower process of diffusion and filtration seems the probable agencj^ for passing the ventricular colloids into the subarachnoid spaces. | |||
That the results obtained by the method of replacement were not solely due to diffusion, but represent a fiUing of the physiological extent of the cerebro-spinal spaces, has been shown in many ways, but probably the chief argument against such a view is that whollj^ similar extensions of the foreign solution may be obtained by injections under mild pressures from a syrmge; with increasing pressures these injections show the same type of spread, but always in a smaller embryo than the replacement method demonstrates as the standard for a given stage of the extension. The results recorded in the foregoing pages indicate also that suspensions (India ink) and true solutions (when powerful precipitants) are valuable only for affording comparisons in problems concerning the normal processes of absorption. | |||
Of interest in any discussion of the results of injections into the perispinal spaces or into the spinal central canal are the findings in regard to the perineural spaces. It is possible to inject such spaces around each of the segmental nerves, but only when the pressures of injections are extreme. In no case, however, were such injections found to enter the lymphatic system — a finding in accord with the observations of Reford and Sabm. The physiological importance of these spaces in the adult is probably great, but the same methods of demonstration (with carefully controlled pressures) which suffice in the adult are unavailing in the embryo. | |||
The origin of the three meninges from the perimedullary mesenchjTue is well established. His, Kolliker, Sterzi, Farrar, and others have placed this conception on a very firm basis. Most of the investigators have been concerned with the differentiation of the spmal meninges, while the observations here reported have been concerned solely with the cranial portion of these membranes. In general, the same phenomena in the transformation of the primitive periaxial mesenchyme as recorded by these earlier workers may be found in the cranium. The division of the primitive mesenchyme by a secondary condensation, a view advanced chiefly by Salvi, seems well supported. The findings in the cranium are in accord with this concej^tion; the outer portion of this primitive meninx becomes the dura mater, the inner forms both the pia and arachnoid. The processes in the formation of the arachnoid are, however, quite diversified and concern both the formation of the subarachnoid spaces and the outer membrane of the arachnoid. | |||
Out of the rather loose-meshed periaxial mesenchyme, the subarachnoid spaces develop. The process concerns the transformation of the small " tissue spaces " of this mesenchyme into the larger subarachnoid channels, which are interrupted by the well-known arachnoid trabecule. Well-marked stages in this metamorphosis, which begins in the basis cranii, can be made out. The first appearance of a differentiation is seen in the gradual increase in the size of the mesenchj'mal mesh. This is closely as.sociated with an increased amount of an albuminous coagulum which in a measure fills the larger interstices. Following this initial dilatation of the spaces occurs a breaking-down of some of the syncytial strands; these ruptured mesenchymal processes then retract and adhere to the persisting trabeculue. The process continues with the formation of larger channels in this mesodermal tissue, with also the formation of the permanent arachnoidal trabeculae. Throughout these larger spaces, in the smaller fetuses, the coagula of protein material are everyT\-here found, the remains apparently of the albuminous portion of the circumambient fluid. | |||
In the formation of the various cisternse, particularly the great cistema cerebellomedullaris, the process of the dilatation and confluence of the original mesenchymal spaces reaches its maximum. Here the breaking-down of the original sjTicytial strands proceeds to such an extent that very few of the strands remain to persist through life. | |||
Such a process of the enlargement of mesenchj^ual spaces to form the larger subarachnoid spaces, as described in some measure by His for the spinal meninges, is apparently intimately connected with the circulation through these spaces of the embryonic cerebro-spinal fluid. The fluid flows everj- where through the spaces, as evidenced by the replacement experiments and by the increased content in albumen, before the process of enlargement of the mesenchymal spaces begins. It seems most likely that this circulation of the fluid acts as the causative agent in initiating and probably also in completing the enlargement of the "tissue spaces." The great content of albumen in the embryonic cerebro-spinal fluid has greatly facihtated the investigation, as the presence of the coagula from this protein has permitted the absolute exclusion of artifacts in the process of the tissue-dilatation. | |||
This mechanism of enlargement of the tissue spaces finds its analogue in the formation of the anterior chamber of the eye and in the perilymphatic spaces of the ear (Streeter). In both these situations, as in the meningeal spaces, there are special body-fluids, more or less characteristic in their physical and chemical characters, obviouslj' subserving specialized functions. In both the eye and cranium, the absorption of the fluids is by way of special organs, directly into venous sinuses; in both, the origin of the specialized fluid is from epidermal organs; this fluid is at fijst poured into epidermal spaces and then subsequently into mesodermal spaces (subarachnoid space and anterior chamber of the eye). Thus, m these situations, the characteristic fluids have certain definite channels through rather larger spaces, connected finally with the venous system, and only indirectly with the Ij-mphatic system. | |||
In no sense must the cerebro-spinal circulation be taken as a portion of the lymphatic system. Increasing knowledge of the cerebro-spinal fluid, of its physiology and chemistry, and of its pathway, have separated it permanently from any connection with the lymph of the lymphatic system, variable though that be. No longer may the meningeal spaces be compared to serous cavities, except possibly in the case of the subdural space, and this space is really a space apart from the true cerebro-spinal or subarachnoid spaces. Quite s i milarly, in place of the many varjdng channels in the dura and to a lesser extent in the leptomeninges, which older writers considered lyonphatic in nature, our increasing knowledge has caused the introduction of specialized arachnoidal cell-chains running throughout the pachymeninx. Unquestionably, the cerebro-spinal fluid i)ossesses its own peculiar and characteristic pathway, analogous in no way to the lymphatic vessels of other tissues. | |||
The outer continuous membrane of the arachnoidea forms as a mesenchymal condensation, at first in common with the inner surface of the dura mater, but soon separated from it by the subdural space. The very low cubical mesothelium which covers the arachnoid membrane on both surfaces and also invests the arachnoid trabeculae differentiates apparently from the original mesenchymal elements in the periaxial tissues. | |||
One of the most interesting features of this study has been the relation of the various mesenchymal condensations to the foreign true solution which was introduced into the medullary-canal system. This fluid circulated throughout the periaxial spaces which enlarge to form the subarachnoid channels, but it never penetrated the primary blastema which served as a primitive dura, nor did it ever invade the pial cells which so closelj^ adhere to the nervous tissue; hkewise, as soon as the secondary mesenchymal condensation dividing the dura from the arachnoid spaces appeared, this condensation was impervious to the true solution. No evidence of any penetration, as might be expected as due to diffusion, could be made out. | |||
This summary has been included in order that some correlation between the topics discussed separately in the foregoing sections might be made. No attempt has been made here to present the findings in abstract form; these have been summarized at the end of each division of this communication. | |||
==XV. Conclusions== | |||
Based on the observations recorded in the foregoing sections, the following conclusions seem warranted: | |||
# During the earlj' part of the growth of the pig embryo there is no extraventricular spread of the cerebro-spinal fluid. The first extension of the ventricular fluid into the periaxial tissues occurs in pig embryos of 14 mm.; the adult relationship of the ventricular and meningeal cerebro-spinal fluid is established in pig embryos of about 26 mm. | |||
# The ventricular cerebro-spinal fluid escapes into the periaxial tissues through two areas of ependjmal differentiation in the roof of the fourth ventricle. Both of these areas differentiate at a shghtly earlier period than that at which they function actively. The area membranacea superior undergoes a gradual regression and obliteration due to the changing form of the rhombic roof; the area membranacea inferior gradually occupies the major portion of the velum chorioidea inferior. | |||
# The embryonic cerebro-spinal fluid, as evidenced by the replacement with a true solution, spreads from the ventricles into the mesenchymal tissue about the central nervous system. It docs not penetrate the cranial or vertebral blastemal condensations, nor does it invade the pial cellular layer. | |||
# The subarachnoid spaces arise by a process of breaking-down of the perimedullary mesenchj'mal sj'ncytium and a dilatation of the existent mesench3Tnal spaces. This phenomenon of the enlargement of the mesenchymal spaces is associated with the presence in the spaces of an increased amount of albumen. The process occurs at a period shghtly later than that at which the initial flow of the cerebro-spinal fluid into the spaces is recorded. | |||
# The dura mater, arachnoid, and pia mater develop out of the perimedullary mesenchj'me. The arachnoid trabeculae are left by the breaking-down of the original mesenchymal strands, while the outer arachnoid membrane is formed, together with the inner surface of the dura, by a separate mesenchymal condensation. The dura develops between this secondary Une of condensation and the embryonic skull. | |||
# There is indicated a very close relationship between the tufting of the chorioid plexuses of the fourth ventricle and the first extraventricular spread of the cerebro-spinal fluid. | |||
# By means of the method of replacement it is possible to demonstrate perineural spaces as far out along the nerve trunks as the peripheral gangUa. The extensive injections of the perineural spaces along the segmental nerves are not obtained by the method of replacement. | |||
The work, of which this paper forms the report, was done in the Anatomical Laboratory- of the Johns Hopkins Medical School. It was largely due to aid received from the Department of embryology of the Carnegie Institution of Washington that the completion and scope of this paper were possible. The wTiter gladly acknowledges his indebtedness to the Carnegie Institution. January, 1916. | |||
==Bibliography== | |||
Ad AMKiEwicz, Albert. Die Blutgef &S8e des menschlichen Rilckenmarkes. I Theil: Die Gefassc der RQckenmarks-Substanz. II Theil: Die Gefassc der Rlickcnmarksobcrflache. Sitjungshcrichte der k. Akad. d. Wiss. in Wien. Math.-naturw. CI., 1H81, Lxxxrv, pt. 3. 469; 1882, Lxxxv, pt. 3, 101. | |||
{{Ref-Bardeen1910}} | |||
Bardeen, C. R. The development of the skeleton and of the connective tissues. In Kcibel and Mall, Manual Fluman Embryology, Philadelphia and Ix>ndon, 1910, I, 292. | |||
BuAKE, J. A. The roof and lateral recesses of the fourth ventricle considered morphologically and embryologically. Jour. Comp. Neurol., Granville, Ohio, 1900, X, 79. | |||
Canniec, a. Contribution & I'dtude de la voOve du quatrifeme ventriculc chez Ics mammifires; le trou de Magendie. Jour, de Mfed. de Bordeaux, 1897, xxvii, 547. | |||
Cappelletti, L. L'^coulemcnt de liquide cerebrospinal par la fistule c^phalo-rachidienne en conditions normales ct sous I'influence de quelques m6dicaments. Arch. Ital. de Biol., Turin, 1900, xxxv, pt. 2. 463. | |||
/ | .L'efflusso del liquido cerebro-spinale dalla fistola cefalo-rachidiana in condizioni normali e sotto I'influenza di alcuni farmaei. .Vtti dell' / ccademia d. Scienze Med. e Nat. di Fcrrara, 1900, ^xxiv, 85. | ||
Cathelin, F. La circulation du liquide ctphalorachidien. Paris, J. B. Bailli^re, 1912. | |||
CoTL'GNO [CoTUNNius], DoMENico. De ischiade nervosa commentarius. Viennae, R. GriifTcr, 1770. | |||
Creighton, C. Microscopic researches on the formative properties of glycogen. London, A. & C. Black, 1896-99. | |||
CusniNO, H. Physiologische und anatomische Beobachtungen iiber den Einfluss von Hirnkompres-sion auf den intracraniellen Kreislauf und ilber einigc hiermit verwandtc Erscheinungcn. Mittcilungen a. d. Grenzgeb. d. Med. und Chir., Jena, 1902, ix, 773. | |||
. Some experimental and clinical observations concerning states of increased intracranial tension. Am. Jour. Med. Sciences, Philadelphia and New York, 1902, cxxi\-, 375. | |||
. Studies on cerebro-spinal fluid: No. 1. Introductory. Jour. Med. Research, Boston, 1914, xxxi (n. s. XXVI), 1. | |||
Dandy, W. E., and K. D. Buckfan. An experimental and clinical study of internal hydrocephalus. Jour. Am. Mod. Assoc, Chicago, 1913, Lxi, 2216. | |||
. Internal hydrocephalus: An experimental, clinical and pathological study. Aiii. Joiir. Dis. Children, Chicago, 1914, viii, 406. | |||
Ekker, H. De cerebri et meduUte spinalis systemate vasorura capillari. Trajecti ad Rhenum, 1853. | |||
Kngel, E. A. Uelx^r die Secretionscrscheinungen in den Zellen der plexus chorioidei des Mcnschen. Arch. f. Zellforschung, Leipzig, 1909, ii. 191. | |||
Essick, C. R. Transitory cavities in the corpus striatum of the human embryo. Contributions to Embryology, 1915, II, No. 6, 95. (Carnegie Institution of Washington, Pub. No. 222.) | |||
14. Evans, H. McL. The macrophages of mammals, Am. Jour. Physiol., Baltimore, 1915, xxxvii, 1. | |||
15. Faivre, J.-J.-A.-E. Des granulationes miningiennes. Thfee de Paris, 1853. (No. 142, vol. 540.) | |||
16. Farrar, C. B. The embryonic pia. .\m. Jour. Insanity, Baltimore, 190& 7 LXiii, 295. | |||
17. FiNDLAT, J. W. The choroid plexuses of the lateral ventricles of the brain — their histolog}-, normal and pathological. Brain, London and New York, 1899, xxu, 161. | |||
18. Frazier, C. H. The cerebro-spinal fluid in health and disease. Jour. Am. Med. Assoc, Chicago, 1915, LXIV, 1119. | |||
19. Gaupp, E. Die Entwickelung des Kopfskelettes. In Hertwig's Lehrbuch der Entwickelungsgeschichte d. Menschcn u. d. W'irbcltierc. Jena, 1906, K od., 692. | |||
20. Goldmann, E. E. V'italfarbung am Zentralnervensystcm. Berlin, G. Reimcr, 1913. | |||
21. Haller, a. Elementa physiologix corp^jris humanis. Lausanne, 1757-78. | |||
22. Hess, Carl. Das Foramen Magendii und die Oeffnungen an den Reces.iu.s laterales des \*ierten Ventrikels. Morphol. Jahrbuch, Leipzig, 1885, x, 578. | |||
23. Hecser, C. H. The development of the cerebral ventricles in the pig. Am. Jour. Anat., Philadelphia, 1913-14, XV, 215. | |||
24. Hill, L. Physiology- and pathology of the cerebral circulation. London, J. & A. Churchill, 1896. | |||
25. His, W. Ueber ein perivasculiires canalsystem in den nervoseu Ccntralorganen. Leipzig, Engelmann, 1865. . Die Hfiute und Hdhlen des Korpers; ein academisches Programm. Basel, Schweighauser, 1865. | |||
. Die Entwickelung des mcnschlichcn Rautenhirns, von Ende des ersten bis zum Beginn des dritten Monats. Leipzig, Hirzel, 1890. | |||
\ | . Die Hiiute und Hohlen des Korpers. Arch. f. Anatomie u. Physiol., Anat. .\bth., 1903, 368. | ||
. Die Entwickelung des menschlichen Gesims. Leipzig, Hirzel, 1904. | |||
26. HwoROSTUCHiN, W. Zur Frage uber den Bau des Plexus chorioideus. Arch. f. mikr. Anat., Bonn, 1911, Lxxvii, 232. | |||
27. Jacobson, C. Unpublished. | |||
28. Kadvi, Heinrich. Ueber die Blutgefisse des menschlichen RQckenmarkes. Lemberg, Gubrj-nowicz 4 Schmidt, 1889. | |||
29. Key, E. A. H., and G. Retzius. Anatomie des Nervensystems und des Bindegewebcs. Stockholm, Samson & Wallin, 1875-76. | |||
30. Knower, H. McE. a new and sensitive method of injecting the vessels of small embrjos, etc., under the microscope. Anat. Record, Philadelphia, 1908, ii, 207. | |||
31. KoLLlKER, A. Entwiekelungsgeschichte des Mcnschen und der hoheren Thiere. 2ed. Leipzig, Engelmann, 1879. | |||
. Gnmdriss der Entwiekelungsgeschichte des Mcnschen. I^eipzig, Engelmann, 18S0. | |||
32. KoLLMANN, J. Lehrbuch der Entwiekelungsgeschichte des Menschen. Jena, Fischer, 1898. | |||
33. Lewandowskt, M. Zur Lehre von der CerebrospinalflQssigkcit. Zeitachr. f. klin. Med., Berlin, 1900, XL, 480. | |||
34. vov LrscHKA, H. Die .\dergeflechte des menschlichen Gehimes. Berlin, G. Reimer, 1855. | |||
35. Magen-die, Francois. Recherches sur le liquide csfephalorachidien. Paris, 1825. | |||
. Recherches philosophiqucs et cUniques sur le liquide c^phalo-rachidien ou c6r6bro-spinal. Paris, 1842. | |||
{{Ref-Mall1904}} | |||
36. Mall, F. P. On the development of the blood-vessels of the brain in the human embryo. Am. Jour. Anat., Baltimore, 1904, rv, 1. | |||
37. Meek, Walter J. A study of the choroid plexus. Jour. Comp. Neurol, and Psychol., 1907, xvii. No. 3, 286. | |||
38. Mestrez.\t, W. Le liquide c^phalo-rachidien. Paris, Maloine, 1912. Thise de Montpellier, 1911, No. 17. . | |||
39. MiDDLEM.tss, James, and W. F. Robertson. Pathologyof the nervous system in relation to mental diseases. Part III. Morbid conditions of the dura mater. Edinburgh Med. Jour. 1895, xl, pt. 2, 704. | |||
MiNOT, C. S. Human Embryology. New York, W. | |||
Wood A Co.. 1892. MoTT. F. W. The Oliver-Sharpey lectures on the cerebro-spinal fluid. Liincct. London. 1910, ii, 1 ; 79. Peluzzi. B. Experinientelle histologische Untersuchungcn ilbcr die Plexus chorioidei (Adcrgeflechte). | |||
Foli.i neuro-biologica, Haarlem, 1911. v, No. 4, 305. Pf.ttit. a., and J. Giiuhd. Sur la fonetion .s4or6toire ct la morphologie des plexus chorioldes des ventrilulcs latfraux du Syst^nie ncrveux central. Arch. | |||
d'anat. micr., Paris, 1902, v. 214. PuLT, F., O. Kehm, and H Schott.mOller. Leitfadcn zur Untcrsuchung der ZerebrospinalflOssigkeit. | |||
Jena, Fischer, 1913. PoiBiER, P., and A. Charpy. Traits d'antomie huniaine. | |||
Pari,'*, BattaiUe, 1892-1902. Qci-vcKE, H. Zur Physiologie der Cerebrospinalflussigkeit. Arch, f . Anat., Physiol, u. wissensch. Med. (Du Bois-Reymond), Leipzig, 1872, 153. Refobd, L. L. Unpublished. Work referred to by Sabin (49) and Gushing (9). Reiner, M., and J. Schnitzler. Ueber die Abflu.sswege des Liquor cerebrospinalis. Centralblatt f. Physiol., Leipzig and Wien, 1894, viii, 6H4. . Zur Lehre vom Hirndruck. Wiener klin. Wochenschr.. 1895, viii, 371. | |||
Sabin, F. R. Development of lymphatic systcm. In Keibel and Mall's Manual Human Embryology, London 4 Philadelphia, 1912, ii, 709. Salvi, G. Histogenise et structure des meninges. | |||
Thise de Pari.", 1898. . Quoted in Poiricr et Charpy. Traitt d'Anatomie humaine. Paris, 1901, tome iii, 113. ScHROEDER VAN DER KoLK, J. L. C. Bau und Functionen der Medulla spinalis und oblongata. Aus dem Holliindischen iihertragen von Dr. Fr. W. | |||
Theile. Braunschweig, Vieweg & Sohn, 1859. Spin.i, a. Expcrimenteller Beitrag zur Kenntniss der Hyperamic des Gehirns. Wiener med. Blatter, 1898, XXI, 17; 247. . Experimentelle Untcrsuchungen iiber die Bildung des Liquor cerebrospinalis. Arch. f. d. gesamte Physiol., Bonn, 1899, Lxxvi, 204. | |||
52. Spina. A. Uebcr den Einfluss des hohen Blutdrucks auf die Neubildung des CerebrospinalflQssigkeit. Arch, f. d. gcsamtc Physiol., Bonn, 1900, i.xxx, 370. | |||
. Untersuchungen uber die Resorption des Liquor bei normulcm und crhohtem intracraniellem Drucke. .Vrch. f. d. gesamte Physiol., Bonn, 1900-1901, Lxxxiii, 120; 415. | |||
53. .'^TERZi, G. Recherches sur I'anatomie comparfee et sur rontogent-se des m6ninges. Arch. Ital. de Biol., 1902, XXXVII, 257. | |||
. Ricerche inforno all' anatomia comparata ed air ontogenesi dcllc mcningi, e considerazioni suUa filogenesi. Atti del R. Instituto Veneto di scienze, letterc ed .\rti, 1900-1901, LX, parte ii, 1101. | |||
54. Streeter, G. L. Development of the nervous system. In Keibel and Mall, Manual Human Embryology, London & Philadelphia, 1912, ii, 1. | |||
. The development of the venous sinuses of the dura mater in the human embryo. Am. Jour, of Anat., Philadelphia, 1915, xviii, 145. | |||
55. Weed. L. H. Studies on cerebro-spinal fluid. No. II: The theories of drainage of cerebro-spinal fluid with an analysis of the methods of investigation. Jour.Med. Research, Boston, 1914, xxxi (n. s. xxvi), 21. . Studies on cerebro-spinal fluid. No. Ill: The pathways of escape from the subarachnoid spaces, with particular reference to the arachnoid Wlli. Jour. Med. Research, Boston, 1914, xxxi (n. a. XX VI), 51. . Studies on cerebro-spinal fluid. No. IV: The dual source of cerebro-spinal fluid. Jour. Med. Research. Boston, 1914. xxxi (n. s. xxvi). 93. | |||
56. Wilder, B. G. Notes on the Foramina of Mageudie in man and the eat. Jour. Nerv. and Ment. Dis., New York, 1886, xiii, 206. . The metapore (foramen of Magendie) in man and an orang. Med. News, Philadelphia, 1893, LXiii 439. . Meninges. Ref. Handbuch Med. Sciences, New York, 1893, ix (suppl.), 606. | |||
57. ZiEOLER, P. Ueber die Mechanik des normalen und pathologischen Hirndruckes. Arch. f. klin. Chirurgie, Berlin, 1896, Liii, 75. | |||
==Explanation of Plates== | |||
KEY FOR FIGURE-LEGENDS. | |||
ami, area membranacea inferior. dmc, dura mater cerebri (inner surface, in pme, pia mater cerebri. | |||
amt, area membranacea .superior. approximation with arachnoid). j»p6, precipitated prussian-blue. | |||
cbl, cranial blastema. epe, epithelial-like cells lining ventricle. pun, reduced silver nitrate. | |||
cent, cisterna cerebello-mcduUari.i. epe, ependyma. s<u, subarachnoid spaces. | |||
chp, plexus chorioiduus. 4"^, vcntriculus quarlus. tir, sinus trans\ersu3. | |||
===Plate I.=== | |||
[[File:Weed1917 plate01.jpg|800px]] | |||
'''Fig. 1.''' Drawing of a pig embryo of 9 mm, into the spinal central canal of which an injection of 0.5 per cent solution of potassium ferrocyanide and iron-ammonium citrate was made under very mild syringe-pressure. The embryo was fixed in Camoy's fluid to which 1 per cent hydrochloric acid had been added. The specimen was carefully dehydrated and cleared by the Spalteholz method. The resultant precipitate of prussian-blue is found w holly within the central canal of the spinal cord and within the cerebral ventricles. Enlargement, 11 diameters. | |||
. | '''Fig. 2.''' Drawing of a pig embryo of 13 mm, in which the cerebro-spinal fluid was replacc<l by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate. The embryo was kept alive for 90 minutes after this replacement and was then fixed in 10 per cent formol containing 1 per cent hydrochloric acid, .\fter dehydration the specimen was cleared by the Spalteholz method. The occiurence of a definite oval, outlined by the denser mass of the granules, in the roof of the fourth ventricle, is characteristic of this stage. Enlargement, 9 diameters. | ||
'''Fig. 3.''' Drawing of a pig embryo of 14.5 mm, in which the cerebro-spinal fluid was likewise replaced by the ferrocyanide solution, After the replacement, the embryo was kept alive for 60 minutes; it was fixed in Camoy's fluid (with 1 per cent hydrochloric acid added) and after dehydration it was cleared by the SpaltehoU method. The earliest indications of a peria.idal spread of the replaced fluid from the roof of the fourth ventricle is here shown. Enlargement, 8 diameters. | |||
===Plate II.=== | |||
[[File:Weed1917 plate02.jpg|800px]] | |||
'''Fig. 4.''' Drawing of a pig embryo of 18 mm., in which a typical replacement of the spinal fluid had been made. The animal was kept alive for 45 minute." and was then fixed, dehydrated, and cleared in the usual manner. The extra ventricular spread of the replaced fluid from two are;is in the roof of the fourth ventricle is well illustrated. Enlargement, 9 diameters. | |||
'''Fig. 5.''' Drawing of a pig embryo of 19 mm., in which likewise a typical replacement of the cerebro-spinal fluid by the ferrocyanide solution had been made. After this procedure, the embryo was kept aUve for 55 minutes and was then carried through the routine technique for the Spalteholz method. The ftirther pericerebral spread of the replaced fluid is recorded. Enlargement, 8 diameters. | |||
===Plate III.=== | |||
[[File:Weed1917 plate03.jpg|800px]] | |||
'''Fig. 6.''' A frank lateral drawing of a pig embryo of 21 mm. The typical replacement of the embryonic cerebro-spinal fluid by the ferrocyanide solution was effected in this embryo and it was then kept alive for 45 minutes. At the end of this time the embryo was fi.xed in an acid fluid, dehydrated, ana cleared. The almost complete periaxial spread of the replaced fluid is indicated by the precipitated granules. Enlargement, 7.6 diameters. | |||
'''Fig. 7.''' A dorsal view of the embryo illustrated in fig. 6. The perispinal spread of the replaced fluid is well shown. Enlargement, 7.8 diameters. | |||
===Plate IV.=== | |||
[[File:Weed1917 plate04.jpg|800px]] | |||
Fig. 8. Drawing of a pig embryo of 26 mm. in which the typical replacement of the cerebro-spinal fluid has been made. After the introduction of the ferrocyanide solution the embryo was kept alive for one hour; at the end of this time it was fixed in an acid solution, subsequently dehydrated, and cleared in oil of wintergreen. The specimen shows a complete periaxial spread of the replaced fluid, as evidenced by the precipitated granules, in addition to a total filling of the intramedullary system. Enlargement, 6.5 diameters. | |||
Fig. 9. Drawing of a pig embryo of 16 m.m., in which the central canal of the spinal cord was injected with the ferrocyanide so.u'ion under moderate syringe-pressure. After fixation in an acid mediiun the embn,-owaa dehydrated and cleared by the Spalteholz method. The extraventricular snread in the peribulbar region Ls easily made out. Enlargement, 9 diameters. | |||
===Plate V.=== | |||
[[File:Weed1917 plate05.jpg|800px]] | |||
''Fig. 10.'' Drawing of a pig embryo of 21 mm., in which an injection of diluted india ink was made into the central canal of the spinal cord. The preasure employed was the highest obtainable from the syringe, yet below the tension causing rupture. The specimen, after injection, was fixed, dehydrated, and cleared. The slight extent of the periaxial spread of the carbon granules can be easily seen. Enlargement, 7 diameters. | |||
'''Fig. 11.''' Drawing of a pig embryo of 16 mm., in which an injection (under moderate syringe-pressure) of 0.5 per cent solution of silver nitrate was made into the central canal of the spinal cord. The silver was reduced in the sunlight, the embryo then fixed. After dehydration, the embryo was cleared in benzol and oil of wintergreen. Enlargement, 7. .5 diameters. | |||
'''Fig. 12.''' Drawing of a pig embryo of 13 mm.; into the central canal of the spinal cord a dilute solution of nitrate of silver was injected under strong syringe-pressure. Reduction of the silver was accomplished by exposure to sunlight; the embryo was then fixed, dehydrated, and cleared. Enlargement, diameters. | |||
===Plate VI.=== | |||
[[File:Weed1917 plate06.jpg|800px]] | |||
''Fig. 13.'' Photomicrograph of transverse section of a pig embryo of 15 mm. Specimen obtained from an embryo in which the cerebro-spinal fluid was replaced by a 1 per cent solution of potassium ferrocyanide and ironammonium citrate. After this replacement the embryo was kept aUve for 65 minutes. The resultant priL'Ssian-blue precipitate is not included in this photomicrograph. Enlargement, 13 diameters. | |||
'''Fig. 14.''' Drawing of blocked area in fig. 13, under higher magnification and including the resultant precipitate of prussian-blue. The typical ependymal cells (epc) lining the fourth ventricle are shown on either side; between them occurs the area membranacea superior {ams). The transit of the replacement fluid through the membranous area and the spread through the adjacent mesenchyme are illustrated. Enlargement, 245 diameters. | |||
'''Fig. 15''' Photomicrograph of transverse section from embryo pig illustrated in fig. 13. Section taken from more caudal plane than that given in the former figure. The prussian-blue spread is not illustrated. Enlargement, 10 diameters. | |||
'''Fig. 16.''' Drawing, under higher magnification, of the rectangular area in fig. 15. The passage of the replaced solution, as shown by the resultant precipitate of prussian-blue, through tlie area membranacea inferior (ami) is here illustrated. The extension of the replaced fluid through the adjacent mesenchyme and the nonpenetration of the solution into the condensed mesenchyme are shown. Enlargement, 140 diameters. | |||
'''Flg. 17.''' Photomicrograph of sagittal section of a pig embryo of 18 mm. Specimen obtained from an embrj-o in which the cerebro-spinal fluid was replaced by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate. After this replacement the animal was kept alive for 45 minutes. Fixed for 5 minutes in 10 per cent formol containing 1 per cent hydrochloric acid; then over night in modified Boiiin's solution (saturated aqueous solution of picric acid 75, formaldi-hyde 10, glacial acetic arid 10). Dehydrated by 2 and 4 per cent grades of alcohol; embedded in xylol-paraffin. Serial sections, stained by hematoxylin and eosin. The resultant precipitate of prussian-blue has not been reproduced in the photomicrograph. Enlargement, 8 diameters. | |||
'''Fig. 18.''' Drawing of blocked area in fig. 17 under higher magnification. The granules of prussian-blue are here represented by the blue stenciling. The transit of the fluid, as shown by the granules, into the periaxial mesenchyme through the two membranous areas {arm and ami) in the roof of the fourth ventricle are well shown. Enlargement, 35 diameters. | |||
===Plate VII.=== | |||
[[File:Weed1917 plate01.jpg|800px]] | |||
'''Fig. 19.''' Photomicrograph from a sagittal section of a fetal pig of 27 mm. The cerebro-spinal fluid in this specimen was replaced by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate; the fetus was kept alive for 40 minutes; fixed in 10 per cent formol containing 1 per cent hydrochloric acid for 15 minutes; then over night in modified Benin's solution; dehydrated by 2 and 4 per cent grades of alcohol; embedded in xylol-paraffin. The prussian-blue granules are not represented in this photomicrograph. Enlargement, 8 diameters. | |||
'''Fig. 20.''' Drawing of squared area in fig. 19. The center of the field is occupied by the optic nerrve; around it the developing extrinsic optic muscles are shown. The precipitate of prussian-blue occurs in the perineural mesenchyme. Enlargement, 190 diameters. | |||
'''Fig. 21.''' Photomicrograph of rectangular area in fig. 19. The passage of the ferrocyanide solution into the sinus transvcrsus {sir) is represented by the precipitated blue granules. Enlargement, 133 diameters. | |||
'''Fig. 22.''' Photomicrograph of a transverse section of a pig enibrj-o of 23 mm. The cerebro-spinal fluid was replaced in this embr>'o with a 1 per cent solution of potassium fenocyanide and iron-ammonium citrate. The embryo was kept alive for .50 minutes and was then fixed over night in 10 per cent formol containing 1 [ler cent hydrochloric acid. The granules of pnissian-blue are not shown in this reproduction. Enlargement, 13 diameters. | |||
'''Fig. 23.''' Drawing of squared area in fig. 22. The area membranacea superior (rima) is shown, sunounded on either side by tufts of the chorioid plexus (rhp) .ind the typical ventricular ependyma. The transit of the solution is shown, as represented by the resultant granules, through the areji, with the subsequent spreail into the periaxial mesenchyme. Enlargement, 125 diameters. | |||
===Plate VIII.=== | |||
[[File:Weed1917 plate08.jpg|800px]] | |||
'''Fig. 24.''' Photomicrograph of a transverse section of a pig embryo of 8 mm. Fixed in modified Bouin's solution over night, dehydrated by 2 and 4 per cent grades of alcohol, embedded in xylol-parafiin. Enlargement, 30 diameters. | |||
'''Fig. 25.''' Photomicrograph, retouched, of the blocked area in fig. 24. The character of the cells (epc) composing the roof of the fourth ventricle Uve) is showTi in this reproduction. Enlargement, 16.5 diameters. | |||
'''Fig. 26.''' Photomicrograph of a sagittal section from a pig embryo of 11 mm. Fi.xed in modified Bouin's solution over night, dehydrated by 2 and 4 per cent gracles of alcohol, embedded in xylol-parafhn. Enlargement, 11 diameters. | |||
'''Fig. 27.''' Photomicrograph of the blocked area in fig. 26. The area membranacea superior (ams) in the roof of the fourth ventricle is shown sharply delimited from the two processes of typical ependyma {epe). Enlargement, 67 diameters. | |||
'''Fig. 28.''' Photomicrograph of a more lateral section of the pig embryo of 11 mm. given in fig. 26. Enlargement, 11 diameters. | |||
'''Fig. 29.''' Photomicrograph, under higher magnification, of the blocked area in fig. 28. The lateral border of the area membranacea superior {ams) of the roof of the fourth ventricle is given. Enlargement, 50 diameters. | |||
'''Fig. 30.''' Photomicrograph of a sagittal section from a pig embryo of 13 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 8 diameters. | |||
'''Fig. 31.''' Photomicrograph, under higher magnification, of the squared area in fig. 30. The reproduction comprises a sagittal section of the area membranacea superior (ams) of the roof of the fourth ventricle. Enlargement, 67 diameters. | |||
'''Fig. 32.''' Photomicrograph of a sagittal section of a pig embryo of 14 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades, and embedded in xylol-paraffin. Enlargement, 11 diameters. | |||
'''Fig. 33.''' Photomicrograph of the blocked area in fig. 32 under higher magnification. The area membranacea superior (ami) in the roof of the fourth ventricle is reproduced. Enlargement, 75 diameters. | |||
===Plate IX.=== | |||
[[File:Weed1917 plate09.jpg|800px]] | |||
'''Fig. 34.''' Photomicrograph of a transverse section of a pig embryo of 18 mm. Fixed in Camoy's fluid (6:3:1), deh3'drated by 2 and 4 per cent changes of alcohol, and embedded in xylol-paraffin. Enlargement, 13 diameters. | |||
'''Fig. 35.''' Photomicrograph, under higher magnification, of the blocked area in fig. 34. The area membranacea superior (atns) is here given, flanked on either side by typical ependyma (ept). Enlargement, 170 diameters. | |||
'''Fig. 36.''' Photomicrograph of a transverse section of a pig embrj-o of 18 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Enlargement, 13 diameters. | |||
in | |||
'''Fig. 37.''' Photomicrograph of rectangular area outlined in fig. 36. The extent of the area membranacea superior (ams), with its adherent coagulum of albuminous material, is well differentiated from the adjacent typical ventricular ependyma (epe). Enlargement, 100 di.imeters. | |||
( | |||
'''Fig. 38.''' Photomicrograph of a transverse section of a pig embryo of 19 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades, and embedded in xylol-paraffin. Enlargement, 13 diameters. | |||
'''Fig. 39.''' Photomicrograph, under higher power, of the rectangular area in fig. 38. A small break in the integrity of the lining ependyma of the roof of the fourth ventricle, representing the irregular boundary of the area membranacea superior (ams), is given. Enlargement, 290 diameters. | |||
'''Fig. 40.''' Photomicrograph of a transverse section of a human embryo of 4 mm. (No. {{CE836}} of collection of Carnegie Institution of Washington). Enlargement, 33 diameters. | |||
''Fig. 41.'' Photomicrograph, retouched, of the blocked area in fig. 40. The epithelial-like cells (epe) composing the roof of the fourth ventricle (4^) are here shown separated from the denser nervous tissue. Enlargement , 100 diameters. | |||
===Plate X.=== | |||
[[File:Weed1917 plate10.jpg|800px]] | |||
'''Fig. 42.''' Photomicrograph of transverse section of pig embryo of 19 mm. Fixed over night in modified Bouin's solution, dehydrated by 2 and 4 per cent changes of alcohol, and embedded in x>-lol-paraffin. Enlargement, 13 diameters. | |||
'''Fig. 43.''' Photomicrograph of squared area in figure 42, under higher magnification. The area membranacea superior (ams) with the attached coagulum of albumen is reproduced. Enlargement, 115 diameters. | |||
'''Fig. 44.''' Photomicrograph of sagittal section of pig embryo of 23 mm. Fuced in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Enlargement, 5 diameters. | |||
'''Fig. 45.''' Photomicrograph, under higher magnification, of squared area in fig. 44. The area membranacea superior (atns) is here shown, delimited by the cells of the chorioid plexus (chp) on one side and by the further ependymal prolongation (epc) of the cerebellar lip. Enlargement, 88 diameters. | |||
'''Fig. 46.''' Photomicrograph of sagittal section of pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Certain portions of the dura mater (dmc) are indicated. Enlargement, 5 diameters. | |||
'''Fig. 47.''' Photomicrograph of blocked area in fig. 46, under higher magnification. The small remaining area membranacea superior (ams) is quite surrounded by encroaching ependyma in the chorioidal folds. Enlargement, 88 diametere. | |||
'''Fig. 48.''' Photomicrograph of transverse section of human embryo of 7 nun. (No. {{CE617}} of the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters. | |||
'''Fig. 49.''' Photomicrograph of squared area in fig. 48, under higher magnification. The epithelial-like cells (epc) composing the roof of the fourth ventricle at this stage are well shown. Enlargement, 100 diameters. | |||
'''Fig. 50.''' Photomicrograph of transverse section of human embryo of 7 mm. (No. 617 in the Caniegie Institution of Washington). Enlargement, 10 diameters. Fig. | |||
'''Fig. 51.''' Photomicrograph of blocked area in fig. 50. The marked invagination of the roof of the fourth ventricle (4if ) with the lining of epithelial-like cells (epc) is given. Enlargement, 33 diameters. | |||
'''Fig. 52.''' Photomicrograph of transverse section of human embryo of 9 mm. (No. {{CE721}} in the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters. | |||
'''Fig. 53.''' Photomicrograph of squared area outlined in fig. 52. The pale, large cells {epc) comprising the roof of the fourth ventricle characterize the reproduction. Enlargement, 50 diameters. | |||
'''Fig. 54.''' Photomicrograph of sagittal section of human embryo of 11 mm. (No. {{CE544}} in the collection of the Caniegie Institution of Washington). Enlargement, 6 diameters. | |||
'''Fig. 55.''' Photomicrograph of blocked area in fig. 54. The apparent break in the continuity of the roof of the fourth ventricle with exudation of the ventricular albumen into the mesenchyme is brought out. Enlargement, .50 diameters. | |||
===Plate XI.=== | |||
[[File:Weed1917 plate11.jpg|800px]] | |||
'''Fig. 56.''' Photomicrograph of sagittal section of human embryo of 14 mm. measured on the slide (No. {{CE144}} of the collection of the Carnegie Institution of Washington). Enlargement, 8 diameters. | |||
'''Fig. 57.''' Photomicrograph, under higher magnification, of blocked area in fig. 50. The greater part of the ventricular wall shown is composed of the area membranacea superior (ams), bounded below by typical ventricular ependyma {epe). Enlargement, 67 diameters. | |||
'''Fig. 58.''' Photomicrograph of sagittal section of human embryo of 17 ram. (No. {{CE576}} of the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters. | |||
'''Fig. 59.''' Photomicrograph of rectangular area in fig. 58, showing the area membranacea superior {ams) of the roof of the fourth ventricle. Enlargement, 50 diametere. | |||
'''Fig. 60.''' Photomicrograph of sagittal section of human embryo of 17 mm. (No. {{CE576}} of the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters. | |||
'''Fig. 61.''' Photomicrograph of tlie blocked area in fig. 60 under higher magnification. The aggregation of epithehal-like cells (epc) on the lateral border of the area membranacea superior is here portrayed. Enlargement, 67 diameters. | |||
'''Fig. 62.''' Photomicrograph of transverse section of human embryo of 18 mm. (No. 409 of the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters. | |||
'''Fig. 63.''' Photomicrograph, under liigher power, of squared field in fig. 62. The peculiar inversion of the roof of the fourth ventricle (/fVe) indicated in fig. 62, has resulted in a marked dislocation of the area membranacea superior (nm«), shown in this figure. Enlargement, 75 diameters. | |||
===Plate XII.=== | |||
[[File:Weed1917 plate12.jpg|800px]] | |||
'''Fig. 64.''' Photomicrograph, retouched, of a transverse section of a human embryo of 21 mm. (No. {{CE460}} of the collection of the Carnegie Institution of Washington). The field taken consists of a portion of the fourth ventricle with the lining of typical ependyma (cjk) on either side. The area membranacea superior {ams) is shown between the two lips of ependyma. Enlargement, 33 diameters. | |||
'''Fig. 65.''' Photomicrograph, retouched, of a similar section to that given in fig. 64, but taken from a more anterior plane from the same embryo. The field shown is analogous in everj' way to that in the preceding figure. | |||
'''Fig. 66.''' Photomicrograph of a transverse section of an embryo chick of 121 hours' incubation. Fixed in Bouin's solution. Enlargement, 15 diameters. | |||
'''Fig. 67.''' Itetouched photomicrograph, under higher magnification, of the blocked area in fig. 66. The area memhranacoH superior (ams) is here given, delimited sharply from the lips of ependyma {cpc) which line the rtmf of the fourth ventricle. Enlargement, 133 diameters. | |||
'''Fig. 68.''' Photomicrograph of a more caudal section from the same embryo as portrayed in fig. 66. Enlargement, 15 diameters. | |||
'''Fig. 69.''' Retouched photomicrograph, under higher magnification, of the blocked area in fig. 68. The area membranacea superior {am») is shown at the point of its greatest transverse diameter. Enlargement, 88 diameters. | |||
'''Fig. 70.''' Photomicrograph of a migittul section of a pig embryo of 15 mm. Fixed in modlfied Bouin's solution, dehydratiil by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement , S diameters. | |||
'''Fig. 71.''' Phutomicrograph, under higher magnification, of blocked area in fig. 70. The earliest evidence of the area membranacea inferior (ami) in the roof of the fourth ventricle is here shown. Enlargement, 12.'i diametere. | |||
'''Fig. 72.''' Photomicrograph of sagittal section of a pig embryo of 18 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 11 diameters. | |||
'''Fig. 73.''' Photomicrograph, under higher power, of the rectangular area outlined in fig. 72. The enlarging area membranucea inferior (ami) is shown in the midst of the typical lining ependyma of the roof. Enlargement, 100 diameters. | |||
'''Fig. 74.''' Photomicrograph of sagittal section of a pig embryo of 23 mm. Fixed in modified Bouin's solution, dehydrated by 2 :\iid 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 6 diameters. | |||
'''Fig. 75.''' Photomicrograph of blocked area in fig. 74. The area membranacea inferior {ami) is, at this stage, quite extensive, as shown in the reproduction; the early stages in the development of the cistema cerebellomedullaria may also be seen. Enlargement, 75 diameters. | |||
'''Fig. 76.''' Photomicrograph of sagittal section of a pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 7 diameters. | |||
'''Fig. 77.''' Photomicrograph, under higher magnification, of blocked area in fig. 76. The unsupported character of the area menibranarca inferior and the formation of the cistema cerebello-medullaris is here reproduced. Enlargement, 67 diameters. | |||
. | ===Plate XIV.=== | ||
[[File:Weed1917 plate14.jpg|800px]] | |||
'''Fig. 78.''' Photomicrograph of a sagittal section of a pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xj'lol-parafiin. Enlargement, 7 diametera. | |||
'''Fig. 79.''' Photomicrograph of the blocked area in fig. 78, under higher magnification. The intact area membranacea inferior (ami), unsupported by any mass of tissue, is shown separating the ventricular cavity from the developing cisterna ccrebello-medullaris. Enlargement, 67 diameters. | |||
'''Fig. 80.''' Photomicrograph of a sagittal section of a human embryo of 16 mm. (No. {{CE406}} in the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters. | |||
'''Fig. 81.''' Photomicrograph of the area outlined in fig. 80, but under liigher magnification. An early stage in the differentiation of the area membranacea inferior (ami) is given. Enlargement, 50 diameters. | |||
'''Fig. 82.''' Photomicrograph of a sagittal section of a human embryo of 17 mm. (No. {{CE576}} in the collection of the Carnegie Institution of Washington). Enlargement, 6 diameters. | |||
'''Fig. 83.''' Photomicrograph, under higher power, of the area blocked in fig. 82. The chorioid plexuses of the fourth ventricle Ue in the central portion of the field; above is the thick cell-layer on the lateral side of the area membranacea suf)erior (ams), while below the upper limit of the area membranacea inferior (ami) appears. Enlargement, 67 diameters. | |||
'''Fig. 84.''' Photomicrograph of a transverse section of a human embryo of 18 mm. (No. {{CE409}} in the collection of the Carnegie Institution of Washington). Enlargement, 5 diameters. | |||
'''Fig. 85.''' Photomicrograph of the blocked area in fig. 84. The cellular character, and especially the clumping of cells, of the area membranacea inferior (ami) is shown. Enlargement, 25 diameters. | |||
'''Fig. 86.''' Photomicrograph of a sagittal section of a human embryo of 19 mm. (No. {{CE431}} in the collection of the Camegie Institution of Washington). Enlargement, 5 diameters. | |||
'''Fig. 87.''' Photomicrograph of the blocked area outUned in fig. 86. The area membranacea inferior (ami) appears separating the fourth ventricle from the developing cistema cerebello-medullaris. Enlargement, 25 diameters. | |||
===Plate XV.=== | |||
[[File:Weed1917 plate15.jpg|800px]] | |||
'''Fig. 88.''' Photomicrograph from a sagittal section of a human embryo of 17 mm. (No. {{CE576}} of the collection of the Carnegie Institution of Washington), representmg an enlargement of the second blocked area in fig. 58. The area membranacea inferior (ami) appears sharply delimited from the adjoining tj^pical ependjina. Enlargement, 67 diameters. | |||
'''Fig. 89.''' Photomicrograph of a sagittal section of a human embryo of 23 mm. (No. {{CE453}} of the collection of the Carnegie Institution of Washington). Enlargement, 6 diameters. | |||
'''Fig. 90.''' Photomicrograph of the blocked area in fig. 89. The area membranacea superior (ams) appears in the stage of closure, while the area membranacea inferior (ami) is becoming well differentiated from the typical ependyma lining the other portions of the fourth ventricle. Enlargement, 26 diameters. | |||
'''Fig. 91.''' Photomicrograph of a sagittal section of a human embryo of 26 mm. (No. {{CE1008}} of the collection of the Carnegie Institution of Washington). Enlargement, 4.5 diameters. | |||
'''Fig. 92.''' Photomicrograph, under higher magnification, of the blocked area in fig. 91 . The area membranacea superior has been almost completely closed by the dense ependyma of the superior half of the roof of the fourth ventricle, while the inferior area (ami) has become a membrane lacking wholly the character of ependyma. Enlargement, 23 diameters. | |||
'''Fig. 93.''' Photomicrograph of a sagittal section of a human embryo of 35 mm. (No. {{CE199}} of the collection of the Carnegie Institution of Washington). Enlargement, 3 diameters. | |||
'''Fig. 94.''' Photomicrograph, under higher powers, of the blocked areas in fig. 93. The formation of the cistema cerebello-medullaris is shown in relation to the ventricular roof. Enlargement, 23 diameters. | |||
'''Fig. 95.''' Drawing of cells of the chorioid plexus from the lateral ventricles of a fetal pig of 132 mm. The specimen waa fixed in absolute alcohol, and stained by Best's carmine stain for glycogen. The glycogen occurs in the form of globules within the epithelial cells. Enlargement, 950 diameters. | |||
''Fig. 96.'' Drawings of the cells of the chorioid plexus from the lateral ventricles of a fetal pig of 36 mm. The specimen was fixed in absolute alcohol and stained by Best's carmine method. The glycogen appears in the epitheUal eclls in the form of basilar plaques. Enlargement, 950 diameters. | |||
===Plate XVI.=== | |||
[[File:Weed1917 plate16.jpg|800px]] | |||
'''Fig. 97.''' Photomicrograph of a transverse section of a pig embryo of 10 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent f;radcs of alcohol, and embedded in xylol-paraffin. Enlargement, 10 diameters. | |||
'''Fig. 98.''' Photomicrograph, under higher magnification, of the blocked area in fig. 97. The double condensations of mesenchyme to form pia mater (pmc) and cerebral blastema (cbl) appear separated by a region of mesenchyme which is breaking down. This central area of mesenchyme, with the marked albumen-content, is to become the arachnoid spaces. Enlargement, 133 diameters. | |||
'''Fig. 99.''' Photomicrograph of a transverse section of a pig embryo of 20 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes of alcohol, and embedded in xylol-paraffm. Enlargement, 10 diameters. | |||
'''Fig. 100.''' Photomicrograph, under higlior powers, of the blocked areas in fig. 99. The relations of the pial condpn.sation ipmc) of mesenchyme to tlie nervous system, as well as the infiltration of tlie arachnoid mesenchyme (sas) with albumen, is reproduced. Enlargement, 133 diameters. | |||
'''Fig. 101.''' Photomicrograph, under higher magnification, of the blocked area in fig. 22. The reproduction is included here to show the double condensjition {cht) of mesenchyme which goes to form ultimately bone and possibly a portion of the dura. Enlargement, 132 diameters. | |||
'''Fig. 102.''' Photomicrograph of a transverse section of a pig embryo of 18 mm. The embryo was one in which the cerebro-spinal fluid was replaced by the ferrocyanide solution. Subsequently the embryo was fixed in 10 per cent formol containing 1 per cent hydrochloric acid for a few minutes to precipitate the prussianblue. It was then transferred to modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. The granules of prussian-blue are not represented in this figure. Enlargement, 10 diameters. | |||
'''Fig. 103.''' Photomicrograph of the squared area in fig. 102. The relation of the thinning mesenchyme in the arachnoid areas to the caudal cranial nerves is shown. The granules of prussian-blue, scattered through the area of thin mesenchyme (sas), are not reproduced. Enlargement, 40 diameters. | |||
'''Fig. 104.''' Photomicrograph of a coronal section of a tissue block which includes the meninges and cerebral cortex in the region of the sinus sagittalis superior. The block was obtained from a fetal pig of 80 mm., fixed in Zenker's fluid, and stained, after embedding in celloidin, by Mallory's technique for coimective tissue. Enlargement, 27 diameters. | |||
===Plate XVII.=== | |||
[[File:Weed1917 plate17.jpg|800px]] | |||
'''Fig. 105.''' Photomicrograph of a coronal section of a tissue block including cerebral cortex and meninges in the region of the sinus sagittalis superior. The block was obtained from a fetal pig of 10 cm., fixed in Zenker's fluid, and stained by Mallory's technique for connective tissue. Enlargement, 13 diameters. | |||
'''Fig. 106.''' Photomicrograph of a coronal section, similar to that in figs. 104 and 105, except in that it was obtained from a fetal pig of 17 cm. The same technical procedures employed in the other specimens were used in this. Enlargement, 27 diameters. | |||
'''Fig. 107.''' Photomicrograph of a similar section to those of the foregoing figures. The specimen was obtained from a fetal pig of 20 cm. and was treated in the manner outlined above. Enlargement, 20 diameters. | |||
'''Fig. 108.''' Drawing of the cell pattern from the inner surface of the dura mater of a fetal pig of .5 cm. The specimen was prepared by the reduction of a dilute solution of silver nitrate in sunlight. The preparation was subsequently stained by hematoxylin. Enlargement, 190 diameters. | |||
'''Fig. 109.''' Drawing of a preparation, similar to that of fig. 108, but obtained from the inner surface of the dura mater of a fetal pig of 7.5 mm. Enlargement, 28.5 diameters. | |||
'''Fig. 110.''' Drawing of a preparation, similar to those of figs. 108 and 109, obtained from the inner surface of the dura mater of a fetal pig of 90 mm. Enlargement, 28.5 diameters. | |||
'''Fig. 111.''' Drawing of a preparation from the inner surface of the dura mater of a fetal pig of 16 cm. The specimen was made in the same manner as outlined in fig. 108. Enlargement, 285 diameters. | |||
'''Fig. 112.''' Photomicrograph of a sagittal section of a pig embryo of 17 mm. An injection of an 0.5 per cent solution of nitrate of silver was made into the central canal of the spinal cord; the silver was reduced in sunlight and the embrjo fixed in formalin. Enlargement, 13 diameters. | |||
'''Fig. 113.''' Photomicrograph, under higher powers, of the blocked areas in fig. 112. The accumulation of the reduced silver (p»n) again.st the area membranacea superior is represented in black. Enlargement, 117 diameters. | |||
'''Fig. 114.''' Photomicrograph of a transverse section of a pig embryo of 19 mm. An injection of 0.5 per cent solution of silver nitrate was made into the central canal of this embryo and the silver immediately reduced in sunlight. The embryo waa fixed in formalin, carefully dehydrated, and embedded in xylol-peiraflBn. Enlargement, 10 diameters. | |||
of | '''Fig. 115.''' Photomicrograph, under higher magnification, of the blocked area in fig. 114. The collection of reduced silver {psn) against the cells at the inferior end of the area membranacea superior is illustrated. Enlargement, 100 diameters. | ||
'''Fig. 116.''' Photomicrograph of a tranverse section of a pig embryo of 16 mm. The central canal of the spinal cord of this embryo was injected with a 1 per cent ferrocyanide and citrate solution under mild syringe-pressure; the embryo was then fixed in 10 per cent formol containing 1 i>er cent hydrochloric acid. Enlargement, | |||
10 diameters. | |||
'''Fig. 117.''' Photoniicrogrnph of the blocked area in fig. 116, under higher magnification. The accumulation of the | |||
precipitated injection fluid against the area membranacea superior is represented in black. A slight | |||
extravcntriculai Bprciul of the fluid, which is found in this as in all embryos of this stage, can not be | |||
miule out in the reproduction. Enlargement, 07 diamuters. | |||
{{Footer}} | |||
[[Category:Draft]] | |||
Latest revision as of 16:45, 5 December 2019
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Weed LH. The development of the cerebro-spinal spaces in pig and in man. (1917) Contrib. Embryol., Carnegie Inst. Wash., 5, No. 14 .
| Online Editor |
|---|
| This historic 1917 paper by Weed describes the development of the cerebro-spinal spaces.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Development of the Cerebro-Spinal Spaces in Pig and in Man
By Lewis H. Weed
Volume V, No. 14
I. Introductory
Probably no field in embryology has been less explored than that relating to the meninges. Our knowledge of the transformation of the perimedulla
ry mesenchyme into the three fully developed membranes about the cerebro-spinal axis has been largely of a crude sort, with gross generalities based on inexact or incomplete evidence. The present work was undertaken in the hope that by a study of the various stages in the development of the cerebro-spinal spaces there might be gained some knowledge which would afford a basis for a conception of this dynamic metamorphosis.
Many of the problems centering around the development of the meningeal spaces have recently been expounded by Cushing^^) . * Not only do we lack knowledge as to the method of differentiation of the primitive mesenchyme, but we know little about the establishment of the circulation of the cerebro-spinal fluid. When do the chorioid plexuses begin to secrete? When does the venous absorption of the fluid take place? When does the perivascular system begin to remove waste products from the cerebral tissue? And also, what factors play a part in the formation of the subarachnoid and subdural spaces?
These questions, some of which it is hoped the present study will answer, relate to the field of physiological anatomy. Consideration of the subject, however, serves to convince one that they must be investigated coincidently with the stages of morphological differentiation; for it may readily be conceived that the physiological use of the meningeal spaces may precede any morphological differentiation of the three membranes, nor indeed is it unlikely that one of the active causative factors in the metamorphosis concerns this filling of the mesenchyme about the nervous system with fluid.
This study, therefore, has been anatomical, but with a broader scope than purely morphological studies would have afforded. Not only has it dealt with the morphological differentiations about the nervous system, but throughout the investigation the relationship of these structures to the possible presence of cerebrospinal fluid has been considered. As the problem developed it was projected more and more into the difficult realm of "tissue spaces." Interest in these spaces largely concerned their physiology, but many points of correspondence between structure and function were found.
In some measure this work is a development of an earher study of some of the anatomical and physiological problems of the cerebro-spinal fluid, carried out in the laboratory of Dr. Harvej^ Cushing at the Harvard Medical School.
- The figures in parentheses refer to the bibliography at the end of this paper.
II. Review of Literature
In order fully to understand the problems which confront one in the study of the embr3'onic cerebro-spinal spaces, a comprehension of the stage to which investigations have brought our knowledge of these fluid-pathways in the adult is necessar}'. It is with this purpose that the adult relationships are here considered. The inclusion of this material may be pardoned, for it will be seen that unanimity of opinion has bj' no means existed in regard to any of the problems concerned in the circulation of the cerebro-spinal fluid.
Modern anatomical knowledge of the meninges dates from the work of Axel Key and Gustav Retzius'29). These Swedish investigators, in their excellent monograph published in 1875, first conclusively demonstrated the anatomical continuity of the spinal and cerebral subarachnoid spaces. But for years after their publication appeared, a physiological continuity between the subdural and subarachnoid spaces was argued for bj' many observers, notably by HilK^*). Gradually, however, workers in this field have reached the opinion that the subarachnoid spaces (the interrupted but continuous channels between arachnoidea and pia) are functionally the channels for the cerebro-spinal fluid. Between the intra-leptomeningeal and the subdural spaces no anatomical connection exists; physiologically there may be some mode of fluid-passage. Thus Hill' 24) states that either by filtration or through actual foramina fluid passes readilj- from one space to the other. Quincke '*^, from observations on animals, somewhat similarly premised a connection between the two spaces, but only in the direction from subdural to subarachnoid. His experiments, based on the results of the injection of cinnabar granules, are open to criticism as indicating a normal passage-way for the fluid; for, as he has recorded, an intense phagocytosis of practically all of his granules occurred. More modern conceptions of the subdural space treat it as a space anatomically closed, lined externally by a polygonal mesothelium. Less error is introduced if it be regarded as analogous in many respects to well-known serous cavities rather than as an essential portion of the pathway for the cerebro-spinal fluid.
The question of the absorption or escape of cerebro-spinal fluid from the subarachnoid space has claimed the attention of many workers. Since the original conception that the meningeal coverings were actually serous cavities, anatomical investigations have furnished many new views. Key and Retzius, by spinal subarachnoid injection of gelatine masses colored with Berlin blue, demonstrated an apparent passage of the injection fluid into the great cerebral venous sinuses through the Pacchionian granulations (die Arachnoidzotten). Their observations were made on a cadaver and the injections carried out under fairly low pressures (about 60 mm. of mercury). A lesser drainage of the fluid into the lymphatics was also shown.
Since the view advanced by Key and Retzius of the absorption of cerebrospinal fluid, the general trend has been away from the idea of an absorption into the venous sinuses. Quincke's observations, made on lower animals after the subarachnoid introduction of cinnabar granules, really offer some substantiation of this theory, but the failure to find the great Pacchionian granulations in infants and in the lower animals caused many workers to reject utterly the conception of the Pacchionian granulations as the functionally active mechanism for the fluid escape.
Physiological evidence, however, advanced by Hill^24) fj-Q^i intraspinous injection of methylene blue, indicated that the major escape of the cerebro-spinal fluid was into the venous sinuses of the dura, while a slow and minor absorption took place along the lymphatic channels. Ziegler(57j^ with potassium ferrocyanide introduced into the cerebro-spinal space, Ukewise found that the venous absorption was much greater and more rapid than the lymphatic. Reiner and Schnitzler with the same agent detected the ferrocyanide in the jugular blood-stream after injection. With oUve oil these investigators found a similar venous absorption, but with a slowing of the venous blood-stream. Lewandowsky also using ferrocyanide. found this salt in the urine within 30 minutes after its subarachnoid injection. Spina 52'^ from observations on freshly killed and hving animals, presented somewhat similar evidence of a major venous and lesser lymphatic absorption. Gushing suggested a valve-Uke mechanism of escape of the fluid, his hypothesis being based on the findings after the introduction of mercurj' into the meningeal spaces.
Several theories concerning the absorption of cerebro-spinal fluid into the bloodvascular system have more recenth' been offered. Mott '**', from a studj' of dilated perivascular and permeuronal spaces, has advanced the idea of fluid-escape by way of the perivascular system into the cerebral capillaries. Dandy and Blackfan^'"^, from an analysis of their evidence, consider that the chief drainage of the fluid is into the capillaries of the pia-arachnoid. Opposed to this conception of a major drainage of cerebro-spinal fluid into the l)lood-vascular system is the view championed by Cathelin'^', that the Ij'mphatic drainage is the chief method of fluid-escape. Cathelin's contention of a veritable circulation of the fluid has not received support from other workers.
Thus it will be seen that since the work of Key and Retzius the trend of opinion has been away from the view that the Pacchionian granulations carry the cerebrospmal fluid into the venous sinuses.
In the earlier investigation carried out in the Harvard Medical School the problems of this fluid absorption were attacked in a somewhat different manner than by previous workers. True solutions of potassium ferrocyanide and ironammonium citrate, such as have been used in the present investigation, were injected into the spinal subarachnoid space under pressures but shghtly above the normal. The animals (dogs, cats, and monkej-s) were kept imder anesthesia during the period of injection, which was usually continued for several hours. Complete filling of the subarachnoid channels was secured by this technique, provided the injections were continued for a sufficient length of time. At the conclusion of the experiment the foreign solution was precipitated in situ and blocks were carried through for histological purposes.
Many of the anatomical findings in this work carried out as outlined are of interest in the present problem. The complete correspondence of the spinal and cerebral subarachnoid spaces as demonstrated by Key and Retzius was amply verified. The normal return of the cerebro-spinal fluid to the general circulation by way of the arachnoidal villi into the great dural sinuses was demonstrated. These viUi are projections of the arachnoidea through the dural wall, prolonged directly beneath the vascular endothelium of the venous sinuses. Furthermore, columns of arachnoid cells were found, normally affording fluid channels in the dura. In addition to the major escape of cerebro-spinal fluid into the sinuses a lesser drainage was alsi) demonstrated, slower than the primary drainage, out along certain of the emergent nerves into the lymjihatic system. No evidence whatsoever was obtained in support of any of the theories of a drainage of cerebro-spinal fluid into either the leptomeningeal or cerebral capillaries, nor could an anatomical valve-like mechanism along the great sagittal sinus be demonstrated. The process of escape of cerebrospinal fluid from the arachnoid villus unto the great sinus appeared to be a simple one of filtration or of diffusion.
Another of the problems concerning cerebro-spinal fluid, which has been of interest to anatomists and phj'siologists, is the source of the fluid. Haller^^D and Magendie'^^ to whom the greatest credit for work on this subject must be given, believed it to be the product of the leptomeninges. Faivre^^^} {^ 1853 and Luschka^*) in 1855 were the first to suggest the chorioid plexuses as the elaborators of this circumambient medium. Since then the view has been generall}- accepted that these villous structures do give origin to the fluid, but the early evidence was based wholly on the glandular character of the plexus. Cappelletti^^) and Pettit and Girardi) offered more definite proof of this relationship by the introduction of pharmacological agents which affected the rate of production of the fluid. These latter authors recorded definite histological changes in the cells of the plexus when influenced by these drugs, indicating, in conjunction with the changed rate of production of the fluid, an undoubted relationship of the chorioid plexus to the fluid elaboration. Since these early investigations many observers — Findlay, Meek(37)^ Mott^'*^', Pellizzi*2), Hworostuchin'26)^ and others — have studied the histology of the chorioid plexus with reference to its function as an elaborator of the cerebro-spinal fluid.
In addition to the elaboration of the fluid by the chorioid plexuses, increments are furnished bj' the nervous tissue itself. This elimination from the nervous system occurs bj' waj' of the j^erivascular spaces. In the previous work referred to'55j it was found possible to inject the entire perivascular system by continuing a physiological injection of the spinal subarachnoid space, and subsequently causing an extreme cerebral anemia. By this procedure an injection of the system to its termination about the cerebral capillaries and nerve-cells could be secured. From this and other evidence the view was advanced that the cerebro-spinal fluid was derived from a dual source — in part from the perivascular system and in greater part from the chorioid plexuses. This view had already been advanced, but on rather insufficient grounds, by Mestrezat'^s* and by Plaut, Rehm, and Schottmuller (, Recently Frazier) has signified his acceptance of this conception of the source of the fluid.
Such, then, is the basis for our present understanding of the meninges, in regard to their characteristic morphology and particularly their functional relationship to the cerebro-spinal fluid. Without a consideration of the circumambient fluid morphological studies of these membranes would be incomplete, for in order to understand the meninges knowledge concerning the cerebro-spinal fluid is necessary.
The Comparative Anatomy of the Meninges
Sterzi's has pubUshed a comprehensive report of the comparative anatomy of the spinal meninges. From his studies he has advanced hypotheses, supported by observations on a Hmited number of fetuses, regarding the development of the human meninges. On account of the interest of this subject in relation to the present discussion a brief summarj' of Sterzi's work will be here included.
e In the acrania there is no special envelope of the central nervous system, but rather a fibrous sheath corresponding to the meninges of higher forms. This fibrous sheath is largely made up of circular fibers, except in the median ventral line, where there occurs a ventral hgament of longitudinal fibers. In cyclostomes, however, there is found a single "primitive meninx" — vascular and composed of white and elastic fibrils coursing in a longitudinal direction. Some of these fibrils traverse the perimeningeal spaces (filled with star-like cells, with some fatty tissue) and are attached to the inner surface of the vertebrae. This same general plan of a single "primitive meninx" is hkewise found in fishes (elasmobranchs, teleosts, etc.); the membrane here is often pigmented and follows closely the external architecture of the spinal cord. The perimeningeal space is filled by mucus in elasmobranchs, but in teleosts this is replaced by fat. For the most part there are found dorsal and ventral ligaments and two lateral ligaments.
The next stage in the development of a more complete form of spinal covering is found in the urodele amphibia. A "primitive meninx," formed of two layers, often artificially separated from each other, replaces the simpler meninx of C5'clostomes and fishes. Of the two layers in this membrane the external is thin and free from pigment; the inner, strongly pigmented, adheres to the spinal cord. The meninx is perforated by the denticulate Ugaments.
In amphibia (Anura) Sterzi found the first evidence of a "secondary meninx," corresponding to the pia-arachnoid. Surrounding this membrane, but separated from it, is the dura, thin and transparent; between the two meninges is the intradural (subdural) space. The dura lies in the peridural space. The spinal prolongations of the endohonphatic canals he in the dorsal part of the peridural space. Both the dura and the "secondarj^ menmx" continue outward along the roots of the spinal nerves and along the filum terminale. Embryologically the perimedullary mesenchyme is differentiated into these two meninges in the Anura.
This arrangement of the two meninges in Anura is followed out in reptiles. The dura, thin as in the amphibia, is covered by endothehum and is vascular. The "secondary meninx" possesses laterally the denticulated hgaments and ventrally the ventral hgament. Both the peridural and intradural spaces are very small.
Likewise in birds Sterzi was able to differentiate only two meninges — the dura and the "secondary meuinx." These membranes are quite similar to those of reptiles. The " secondary meninx " has acquired three layers — an outer endothelial covering, a middle vascular layer, and an inner membrane closely adhering to the cord. This is a distinct approach to the three meninges of mammals. An intradural (subdural) space covered by endothelium can be easily made out. The development of these avian meninges concerns a differentiation of the perimedullary mesenchyme.
The arachnoid, according to Sterzi, first appears as a definite membrane in mammals (marsupials and placentals). In marsupials this arachnoid has become well differentiated and the pia mater possesses denticulated and ventral Ugaments. A transformation of the extradural portion of the denticulated ligaments unites the dura to the endorachis. In perissodactyla the differentiation of the three meninges (particularly of the arachnoid) is incomplete. The arachnoid is separated from the pia mater by a pecuUar tissue wliich contains numerous lymphatic lakes, forming the intra-arachnoid spaces. No intradural (subdural) space is apparent, due to the approximation of dura and arachnoid. The subdural space is clothed by endothelial cells; these can not be made out in the intra-arachnoid spaces. The dura is surrounded by a fatty pad.
According to Sterzi the augmentation of the intra-arachnoid (subarachnoid) space is the distinguishing characteristic of the meninges of carnivora. This increase takes place at the expense of the peridural space.
As Sterzi developed the knowledge of the comparative anatomy of the lower forms — of the transition from the primitive meninx of the cyclostomes to the three membranes of mammals — the possible correlation of this analogj'^ to the embryological development in mammals became apparent. He extended his observations to human beings and to human fetuses. His findings will be detailed in the following section.
Farrar'i, in a short discussion of the development of the meninges of the chick, finds in early stages three laminse about the spinal cord, "the middle one of which alone still presents the primitive features of the mesoblastic-sheath." The inner layer, close to the medullary tissue, is highly vascular; in the outer zone "the connective-tissue elements arc assuming elongated forms and crowding together with long axes parallel, giving a very close mesh with long but extremely narrow spaces, in contradistinction to the loose irregular reticulum of the pia-arachnoid." The outer lamina becomes dura mater, while the inner two zones are considered together as the embryonic pia-arachnoid. Farrar defines the pia-arachnoid as developmentally a single membrane consisting of a loose reticulum, at the outer and inner borders of which limiting membranes are formed.
Literature On The Development Of The Mammalian Meningeal Spaces
The development of the meningeal spaces in mammals has not been studied extensivel}', and the literature in regard to it is quite meager. Only a very few workers have touched upon the subject except casually. Reford"*^', working in the Anatomical Laboratory of the Johns Hopkins University, studied the development of these spaces bj^ the method of injection with india ink. His work unfortunatelj' has never been published, but it has been rather extensively referred to bj' Sabin *3' in 1912 and by Cushing'^^ in 1914. Their sununaries of this work are here included. Miss Sabin thus speaks of it: "In a study of the arachnoid made bj' the injection method in the Anatomical Laboratory of the Johns Hopkins University by L. L. Reford, and as j^et unpublished, it has been shown that the thinning out of the mesenchjine around the central nervous sj-stem is not haphazard, but that injections of the same stage give the same pattern, and that the form of the arachnoid space changes as the brain develops. That is to say, the arachnoid space has as definite a form as the coelom, and it never connects with the IjTnphatics."
Cushing() gives the following summary:
- "It was thought that an investigation of the cerebro-spinal spaces in the embryo would most hkely shed Ught on the subject, and some unpublished studies in this direction were undertaken in 1904 and 1905 by Lewis L. Reford in ^Mall's laboratory' in Baltimore. In living pig embryos of various stages low spiaal india-ink injections were made either into the wide central canal or into the subarachnoid space, and the embryos were subsequently cleared. It appeared from the course taken by the injection mass that the full development of the spinal arachnoid preceded that of the intracranial spaces, the impression being gained that the separation of the primitive meninx into its layers occurred later over the cerebral vertex than in the basilar portion of the chamber. Still, I never felt quit« convinced that the failure of injection of the meninges over the surface of the hemispheres in many of Reford's specimens was not due to the floating up of the brain against its envelopes by the introduction of the injection mass from below. Howe\'er this may be, it was nevertheless apparent that a venous injection of the body of the embryo was often produced, and the impression was gained that a communication existed between the basal subarachnoid spaces and the precursors of the sinusoidal veins of the cranial chamber which empty into the jugulars. If due to an artifact from a vascular ruptm-e, at all events the conununication always occurred at the same point. Reford, moreover, in agreement with Cruveilhier, Reichert, and KolUker, came to doubt the existence of the foraminal opening described by Magendie, beUeving that the opening was an artifact and that the fluid escaped by seepage through a persistent membrane."
It is regrettable that Reford's study has not been pubUshed, as it represents the only attempt to solve the problenas of the development of the cerebro-spinal space by the method of injection. As stated in subsequent sections of this communication, his apparent failure to control pressures of injection and to use only granular suspensions is unfortunate.
In a study of the development of the blood-vessels of the human brain, (Mall) noted the ease with which an extravasation into the embryonic arachnoid spaces could be brought about by increasing the pressure in a venous injection. In a specimen of 46 mm. an arterial injection with aqueous prussian-blue resulted in a complete subarachnoid spread, due to rupture of the vessels as they perforated the nervous tissue. In general, it was found that this rule held: an arterial extravasation always took place from the perforating capillaries, while a similar venous rupture occurred in the veins themselves.
Mall made similar observations on living pig embryos from 30 to 80 mm. in length, with analogous results. But when, in these embryos, the arachnoid spaces were completely filled by an intraventricular injection of india ink, no passage of the granular injection into the veins or sinuses occurred. The ventricular injection flowed into the extraventricular spaces "through the medial opening of the fourth ventricle." From the spinal cord the ink extended for a short distance along the main trunks of the spinal nerves. In the larger embryos (above 50 mm.) the ink usually gushed from the mouth, reaching it by way of the Eustachian tube. Using, in the pig embryo, the heart as the mechanism for injecting the ink, extravasation from the cerebral vessels in the arachnoid spaces occurred.
In one human specimen of 90 mm., Mall found both the arachnoid spaces and the cerebral ventricles filled with india ink after an arterial injection of that suspension. He states: "The injection passes through the medial opening into the fourth ventricle (Magendie), and apparently the ventricles are injected through this opening from the arachnoid."
To His'25) and to Kolliker belongs the credit of first having established on a firm basis the development of all the meninges in man from mesenchyme. This perimedullay' layer of mesenchyme Salvi'^o^ called the "primitive meninx" — a term now used extensively in comparative anatomy. The primitive meninx divides into two layers, the outer forming the dura and the inner the pia-arachnoid. Sterzi(53), working on the development of the human spinal meninges, advanced a view similar to that of KoUiker. The perimedullary mesenchjTne (the "primitive meninx") divides into two portions, one hugging the inner surfaces of the vertebra? and the other adhering to the cord. This inner layer of the perimedullary mesenchyme, according to Sterzi, should properly be termed the "primitive meninx," as it divides subsequently into dura and the "secondary meninx," which in turn forms both arachnoid and pia. The denticulate hgaments develop in the "primitive meninx." The dura and arachnoid in human embryos are modeled up to a certain point on the cord; then, with the augmentation of the subarachnoid space, they follow the outline of the vertebral canal.
His'25) has given information regarding the development of the meninges, with particular reference to the formation of the subarachnoid space. He affirms the mesenchymal origin of all of the cerebro-spinal membranes. His describes the first differentiation of mesenchyme to form the meninges as consisting of two zones of condensation, the outer being closely associated with the developing perichondrium of the vertebral column and the inner facing upon the cord. Between these two zones of condensation the subarachnoid space develops, posterior and anterior spaces first appearing, with later fusion laterally. These appearances were met with in chicks of 10 to 12 days' incubation. Quite soon after this process of spacedevelopment a separation occurs which gives rise to a complete subarachnoid space. Later the splitting-off of dura from the vertebral periosteum takes place.
III. Methods Of Investigation
In the study of any problem dealing with the development of fluid-spaces within the body, the method of investigation must of necessity be such as to offer exceptional opportunities for control. In the present work several well-known and generally accepted anatomical procedures were naturally suggested, such as injection of the spaces about the central nervous system, reconstruction from serial sections, or merely study of the various stages by means of serial sections.
It was ascertained early in the investigation that by injection and serial sections without reconstruction the necessary stages in the process of meningeal differentiation could be estabUshed. In regard actually to the physiological aspects of the problem more reUance was placed on the results of injection than on any histological differentiation, for, as explained above, considerations of the pathway and of the flow of the cerebro-spinal fluid were deemed most important. No method of injection, however, holds out much promise in such a problem unless it can be applied, under conditions approximating the normal, wnthin the spaces about the nervous system. The greatest objection to reliance upon injections in this problem is in relation to pressures. From the very nature of the case it wall be reahzed that any ordinary injection into the embryonic central canal or perispinal space must result in an extraordinary increase in the normal tension of the fluid. This objection applies to any method employed, whether that of a simple syringe and needle, the glass tube and bulb devised by Knower, or a glass capUlary-tube contrivance.
The erroneous conclusions drawTi by investigators from the emploj^ment of excessive pressures of injection are nowhere more strikingly illustrated than in studies of the circulation of the cerebro-spinal fluid. Many such examples were recently brought forward in a critical review 5' published in connection with a study of the fluid. In the embryo, with structures and membranes still of very little tensile strength, the consequences of a disregard for the pressures of injection are even more disastrous.
A second criterion for the study of fluid-pathways in the body is necessarily the type of injection mass. Not only should attention be paid to the pressures involved, but the peculiarities of the particular body-fluid concerned must be considered. Adopting for this work on the embryo the same standards followed in the previous investigation on the adult, true solutions were used in place of the customary granular suspensions. Emulsions and viscous solutions were not emploj'ed because of their obvious disadvantages in studying the passage through membranes. India ink and process black (in which carbon granules are the particulate matter) were also used, but only for comparison with the standard true solution, as the likelihood of the insoluble granules being phagocyted wathin the period of experimentation or of being caught mechanicalh'^ in tissue meshes appeared a priori to be too great.
In any study of fluid-pathways in the body, not only must the injection fluid be a true solution, but it must also be one which is not attracted to particular cells (as with many stains). Likewise, colloid stains (such as the benzidene group) could not be employed, because of the fact that certain cells (macrophages, as described by Evans’s phagocyte the small colloidal particles. In addition, the true solution must be readily precipitated as an insoluble salt, capable of remainhig unchanged in histological technique. After trying many salts in long-continued injections into the adult cerebro-spmal spaces, it was found that solutions of potassium ferrocyanide and iron-ammonium citrate in equal parts were admirably adaj^ted to the purposes of the experiment. By the addition of a mineral acid (preferably hydrochloric) ferric ferrocyanide could be precipitated. This prussian-blue is insoluble in the routine technique and is readily identified in sections. After mounting in damar or balsam the blue granules can be observed unchanged for several months, but after a year there is some deterioration in the specimen, due to a conversion of the blue into indefinite greens.
Text-figure 1. — .Schematic sketch of mechanism used (or replacing ventricular and spinal fluid of an embno with a foreien solution or suspension. The system is here shown in balance, the difference in fluid-level in reservoirs and needles representing the hydrostatic pressure necessary' to overcome the capillary resistance of tubes and needles. The stands holding the injecting needles may be moved about without altering the balance of the system. As one reserv'oir is raised, the other is lowered in a corresponding degree.
In regard to these two major factors in the employment of injections (pressure and true solution) it was found necessary to devise a method of experimentation which would satisfy the requirements of the problem. Solutions of the ferrocyanide and of the citrate were non-toxic within the central nervous sy.stem and afforded an excellent histological means for following the fluid-pathways. It was hoped at first that a simple "replacement" type of injection might be employed, as in the adult animals. In this procedure a given amount of fluid was withdrawn from the subarachnoid spaces and immediately replaced by an equal quantity of the injection fluid. The method was successfully tried on fetal cats of consideralile size, but was impracticable on small embryos. After such a replacement the animals were allowed to Uve for varying periods of time (up to 3 hours) and then killed.
It was soon ascertained that the essential circulation of the cerebro-spinal fluid was established in pig cml^ryos of less than 30 mm. in crown-rump measurement. Hence the ordinary method of replacement had to be discarded for some more delicate system. With the realization that a simultaneous withdrawal and introduction in a living embryo would be far preferable to a two-stage procedure, the extremely simple apparatus pictured in text-figures 1 and 2 was employed. This device consists of two glass tubes of uniform and like bores, suspended from above by a string running over a pulley. To the tapering lower ends of these reservoirs are attached rubber tubes which connect the reservoirs to two needles. These needles are held at the same level by two metal brackets which can be moved at will on a level glass plate.
Text-figure 2. — Diagrammatic representation of the method of rcplacinR the cerebro-spinal fluid in a living embryo. The spinal needle is inserted into the central canal of the spinal cord, while the cerebral needle is introduced into one of the cerebral ventricles. The canal of the spinal cord and the cerebral ventricles are represented by the interrupted lines. The foreign fluids are introduced by the spinal needle and withdrawn by the cranial.
The apparatus is employed as follows : Both tubular reservoirs are filled up to the point where the fluid is just ready to fall from the needle in a drop. This point is easily obtained by fiUing the reservoirs slightly in excess and allowing this excess fluid to run off from the needle. With the system thus in balance the needles he in the same horizontal plane and can be moved without altering the balance of the solutions. The injection is made by inserting one needle into the central canal of the spinal cord and the other into one of the lateral ventricles; then as the reservoir connecting with the spinal needle is raised the other is lowered, so that an amount of fluid equal to that introduced into the spinal canal is withdrawn from the cerebral ventricles. In this way the whole contents of the cerebral ventricles and central canal of the spinal cord can be slowlj' withdrawn without increasing the pressure in the central nervous sj^stem. The initial pressure necessary to secure this flow is only that required to overcome the capillary resistance of the medullarj'-canal system. In practically all cases this can be accomplished by using a positive pressure of less than 60 mm. of water (associated with a negative pressure of the same degree) .
In the present study the above procedure was the routine method of injection employed. Pig embryos, brought from the abattoir, contained in the uterus, were found to be wholly satisfactory material. If not permitted to cool excessively in transit the embryos lived for at least two hours in a 38° incubator. On being received at the laboratory a section of the uterine wall contaming the placenta was excised, with the embryo left connected by the umbilical structures. As soon as the technical preparations for injection were completed the amnion was opened and the embryo placed upon a padded block at the proper level. The first needle was then inserted into the easily discernible central canal of the spinal cord and the second into the left cerebral ventricle or into the mesencephalic ventricle. By elevation of the reservoir connected with the first needle the cerebro-spmal fluid was replaced by the injection solution. As soon as the replacement was complete the needles were withdrawal and the embryo and its uterine portion replaced in the incubator. The heart of the embryo could be easily obser\^ed in the smaller forms and served as the index of a continued circulation.
The incubation of the embryos was continued for varying periods of time, but it was soon ascertained that a period of over 30 minutes generally resulted in a complete spread of the injection solution. For comparison the period of incubation was lengthened and shortened, but the best results were usually obtained with a 45-minute incubation after the replacement.
Injections of the necessary true solutions were made, in the routine experiment, with a 1 per cent concentration of potassium ferrocyanide and iron-ammonium citrate in distilled water. By a 1 per cent solution is meant a salt concentration of this amount (potassium ferrocyanide, 0.5 gm.; iron-ammonium citrate, 0.5 gm.; water, 100 c.c). The resultant true solution should be practically isotonic with the body-fluids. In this way any injurious consequences due to hypertonic or hypotonic solutions were apparently overcome. The factors of osmosis and diffusion also had to be considered in this connection.
Other concentrations of the so-called "ferrocyanide mixture" were used, but only for the sake of comparison or for the purpose of investigating some particular phase of the problem. The results obtained by the use of these concentrations were not relied upon as affording standards for the normal pathway of the cerebro-spinal fluid.
In addition to the replacement type of injection, many observations were carried out on pig embryos, with a simple syringe-injection of the ferrocyanide solution into the central canal of the spinal cord or into the cerebral ventricles. It proved to be a very simple matter to regulate the pressures by this method, and three arbitrary standards (mild, moderate, and strong) were found to be of value in a comparison of the extent of the spread obtained by replacement and by injection.
The prussian-blue reaction (formation of ferric ferrocyanide) was obtained in these experiments by fixing the whole embryo in an agent containing hydrochloric acid. For histological study the best results were obtained by immersing the specimen from 1 to 10 minutes in a 10 per cent formaldehyde solution containing 1 per cent hydrochloric acid. After this primary procedure, during which the ferrocyanide was precipitated, the embryo was transferred to Bouin's fluid (saturated aqueous picric acid, 75; formaldehyde 40 per cent, 20; glacial acetic acid, 5). The specimens were allowed to fix over night and were then dehydrated in graded alcohols. From 30 per cent alcohol, use was made of 4 per cent changes up to 60 per cent; and from this point to absolute the changes were by 2 per cent gradations.
In addition to the technique outlined above, Carnoy's solution and 10 per cent formol were employed. The Carnoy fluid, containing acid (absolute alcohol, 60; chloroform, 30; glacial acetic acid, 10; hydrochloric acid, 1) proved to be of particular service in the study of specimens cleared by the Spalteholz method; histologically, however, it has not been as valuable as Bouin's fluid.
Besides the ferrocyanide solution, two other injection masses were constantly employed. Solutions of silver nitrate in concentrations of 0.5 per cent were injected into the central canal of the spinal cord and into the cerebral ventricles. This method, with reduction of the silver salt in the sunlight, gives very pleasing preparations. It is, however, subject to obvious limitations. The intraspinous toxicity of the silver, together with its action as a precipitant of albuminous substances, renders its use unsatisfactory in replacement experiments. Furthermore, it reacts apparently with any protein tissue, irrespective of the true function of that tissue (as, for example, its coagulation of the lining ependyma of the ventricles).
India ink, the other substance employed, is of extreme value in anatomical studies. Because of the suspension of carbon granules it possesses the disadvantages already commented upon for the study of any true pathway of fluid. It has been of service, however, in the present work in showing marked differences in spread from that of true solutions and in furnishing information in regard to fluid passage through a membrane.
This investigation has been carried out on the basic idea of correlating the physiological spread of the embryonic cerebro-spinal fluid with the gradual transformation of the perimedullary mesenchyme into the three fully formed meninges. This has necessitated a histological study of the embryo. Pigs for the most part were the animals used, but the findings have all been verified by a study of the same regions in the human embryos in possession of the Carnegie Institution of Washington. In addition, certain structural characters have likewise been identified in sections of chick, rabbit, and cat embryos.
It was early apparent that the material to be of value must be free from any great shrinkage about the central nervous system. Comparative freedom from this artifact was obtained by fixing the embryo alive in Bouin's fluid and dehydrating by 2 and 4 per cent gradations of alcohol. The material was chiefly cut in paraffin after being embedded by means of xjdol.
The methods of investigation outhned in the foregoing paragraphs have been followed throughout the major portion of the work. In many minor instances other procedures not commented upon have been employed ; these wiU be detailed in appropriate subdivisions of this paper.
IV. Injections And Replacements In The Cerebro-Spinal System
Results Of Replacements In The Ventricular System Of True Solutions.
The results of experiments carried out on embryo pigs by the technical procedures outlined in the previous section will be detailed here. The study was made on this animal because of the facility with which it could be obtained living and in good condition and also because it exhibits the characteristic meningeal anatomy of all mammals. The chick could not be used in this investigation on account of the dissimilarity between the avian and the mammalian menmges.
The chief problem concerned here was the actual physiological extent of the cerebro-spinal spaces. This apparently could be ascertained by the replacement of cerebro-spinal fluid by the ferrocyanide mass. But there was also to be considered the passage of fluid from the ventricles out into the periaxial* spaces, corresponding exactly to a similar passage in the adult.
If into the central canal of the spinal cord of a hving pig embryo of 9 mm., crown to rump measurement, an injection of the ferrocyanide solution be made under very mild syringe-pressure, the ventricles can be fairly well filled without rupture of any element. Incubation of this experimental embryo with its circulation continuing almost unabated for an hour should cause a further spread of the fluid throughout the normal canals. If at the end of this time the whole embryo is fixed in an acid medium the ferrocyanide will be precipitated in situ.
Such a specimen, subsequently cleared by the Spalteholz method, is represented in figure l.f In this drawing the spread of the injection solution is clearly shown. Running upward from the point of introduction, wholly within the central canal of the spinal cord, it reaches the bulbar region and extends outward into the large fourth ventricle, appearing as a dense collection of the prussian-blue. Cephalad from this region it spreads in diminishing intensity until it is finally lacking in the diencephalon.
The injected solution, then, in spite of the unavoidable increase in the normal intramedullary pressure, is contained only within the medullary-canal system (central canal of spinal cord and cerebral ventricles). There is no evidence of any spread outwards, either from the third or fourth ventricle.
In the next stage of meningeal development the replacement method can be used, as the embryo is no longer too small for its employment. In figure 2 is represented an embryo of 13 mm., in which the circulation continued for 90 minutes after the replacement. The same general picture shown in figure 1 results. The whole medullary-canal system is filled with the precipitated prussian-blue, which is densest in the region of the fourth ventricle. The roof of the ventricle, however, shows a striking difference from that of the ventricle in the embryo of 9 mm. Just posterior to the cerebellar lip is a regular oval, which is covered from within by a dense collection of prussian-blue granules, causing it to stand out in clear contrast to the thinner and more evenly distributed blue lining of the remainder of the roof. This oval area is comparatively large and comprises a portion of the superior or anterior half of the ventricular roof. This area, differentiated from the remainder of the rhombencephalic roof, is clearly shown in figure 2, a drawing of a cleared specimen of this stage.
- Throughout this paper the term "periaxial " has been used in the seii90 of "around the central nervous system " or "around the ccrebro-spinal axis."
- Throughout this work the reference "figure " 1, etc., refers to plate illustrations; the word "text-figure" refers to the illustrations inserted in the text.
With the exception of this strikingly dense area in the rhombic roof, the injection spread in an embryo of 13 mm., subjected to replacement of the cerebro-spinal fluid by the ferrocyanide, differs in no way from that in the embryo of 9 mm. Careful inspection of figure 2 is convincing that the spread still remains within the medullary canals, with no extension of the fluid into the spaces outside of the cerebrospinal axis. It seems justifiable, then, to speak of the cerebro-spinal spaces at this stage of development as being only mtramedullary in type, with no indication as yet of a meningeal fluid cushion (corresponding to the adult subaraclmoid space).
With the use of larger embryos, however, for the medullary replacement with ferrocyanide and citrate, the picture gradually changes. The first indication of a more advanced stage of development is obtained in embryos whose length exceeds 14 mm. Figure 3, of a pig embryo of 14.5 mm., is included here as representing this further extension of the injection fluid. The cerebro-spinal fluid of this specimen was replaced, by the compensating mechanism, by a solution of potassium ferrocyanide and iron-ammonium citrate. The embryo was then kept alive (as judged by the heart-beat) for a period of one hour. At the end of this time it was fixed in an acid medium and subsequently cleared in oil of wintergreen after careful dehydration.
The essential differences between an embryo of this stage and one of the stage represented in figure 2 concerns the spread of the injection fluid from the roof of the fourth ventricle. Both specimens show a complete filling of the intramedullary system (cerebral ventricles and central canal of the spmal cord) with the precipitated prussian-blue granules. The specimen of 13 mm. (fig. 2) is characterized bj' a dense oval collection of the prussian-blue on the upper and inner surface of the rhombic roof. In the specimen shown in figure 3, in contradistinction to this localized aggregation of granular matter, there is a deUcate extension of the injection fluid caudalwards from the roof of the fourth ventricle. This fusiform projection is here readily made out, lying beneath the skin over the ventricular roof and separated quite distinctly from the easily discernible line of the roof. This outward extension of the fluid has a fairly wide and deep origin from the upper portion of the roof, but tapers caudally to a sharp point with considerable rapidity.
At the stage of 14 mm. the roof of the fourth ventricle shows the small depression which marks the formation of the chorioid plexuses. With this depression occurrmg transversely the relation of the external surface of the embryo to the ventricular roof necessarily alters somewhat in this region. The chorioidal depression of the roof graduallj' becomes separated from the skin; and it is into this area between the skin and the ventricular ependyma that the first spread from the cerebral ventricles occurs. At this stage, illustrated in figure 3, the injection is intramedullary in type, with but sUght extension into the pericerebral tissues.
The pericerebral spread may be made out in nearly all replacements in embryos of 14 mm., but in a few cases the injection has remained intramedullary in type. In embryos of 16 mm. the spread into the pericerebral tissues is invariably found. Often, with this extension of the replacement solution outside the ventricles, the oval area noted in the stage of 13 mm. persists. (This phenomenon is especially well shown in a simple injection of silver nitrate, illustrated in figure 11.)
The next stage of importance in the development of the cerebro-spinal spaces is represented in figure 4, a drawing of a pig embryo of 18 mm. in which a typical intramedullary replacement of the cerebro-spinal fluid with a solution of potassium ferrocyanide and iron-ammonium citrate had been made. Here, with the exception of the region of the roof of the fourth ventricle, the replaced fluid is contained solely within the central canal of the spinal cord and within the cerebral ventricles. The roof region, however, exhibits a new phenomenon, which distinguishes it from the stage shown in figure 3. The chorioid plexus invagination has become strongly developed, dividing the roof into two parts. These roof divisions have been termed superior and inferior, the former lying anteriorly and orally from the chorioid fold. The general surface outline is but little changed, due to the mesenchyme filling up the area between roof and skin. From two areas in the entire roof of the fourth ventricle the foreign fluid has escaped into the pericerebral tissue. These points of fluid passage he in the two divisions of the ventricular roof. The superior area of escape corresponds to the oval outlined by the prussian-blue in figure 2 and to the point of emergence of fluid shown in figure 3. The lower area of fluid escape is in the inferior half of the ventricular roof, where the ependymal lining and its supporting tissue are developing into a well-marked dorsal distension. This area corresponds to Blake's'3' caudal protrusion, though, as Heuser'23) has pointed out, the shape of the structure in the pig in no way resembles the "finger of a glove."
The extraventricular spread of the injection fluid in this specimen is considerably greater than in the pig embryo of 14 mm. (fig. 3). On the whole, however, the distribution of the replaced fluid is not extensive as compared with the adult relationship, where the central nervous axis is entirely surrounded by its subarachnoid cushion of cerebro-spinal fluid. From the superior area of fluid passage the replaced solution (as shown by the resultant precipitation of the prussian-blue) has passed both superiorly and inferiorly. In the median line, and extending laterally but slightly, a projection of the blue may be seen occu])ying a large portion of the extraventricular area formed from the chorioidal invagination. This area of fluid passage occupies at this stage about one-third of the total transverse diameter of the ventricular roof. From it the blue tapers caudally, diminishing in all directions. Above, the precipitate may be made out extending superiorly over the cerebellar lip. Its extension into the pericerebellar tissue is not marked; here again it tapers from the area of fluid passage, its midline prolongation stretching farthest anteriorly. This relationship is easily made out in figure 4, a frank lateral view of such an experimental rei)lacement. The granules which result from the introduced ferrocyanide solution are found only in the central canal of the spinal cord and not in any perispinal arrangement.
In the pig embryo of 18 mm., shown in figure 4, the replaced solution has been carried somewhat farther than in the embryo of 14 mm. (fig. 3). The chief point of differentiation lies in the fact that in the latter stages two areas have apparently become permeable to the intraventricular fluid, so that a larger periaxial spread has resulted. Then, too, the extension of the ferrocyanide solution from the superior area is considerablj^ greater, overlapping the cerebellar Up and filling in some degree the pericerebral tissue in the chorioidal invagination.
With a definite periaxial spread established for the cerebro-spinal fluid in pig embryos of 14 to 18 mm., it seemed not unreasonable to expect a gradual increase in the extent of the future subarachnoid distribution in more advanced stages. The earliest extension of the fluid into the peribulbar tissues occurred with the inception of the infolding of the ventricular roof to form the chorioid plexuses of the fourth ventricle. Its further extension, particularly its passages through a second area, occurred with the greater development of the chorioidal invagination (i. e., 18 mm. stage). A still more extensive pericerebral flow of the ferrocyanide and citrate is illustrated in figure 5. Here the cerebro-spinal fluid in a hving pig embryo of 19 mm. was replaced by the ferrocyanide solution. The embryo was kept alive for about an hour after the replacement and was then fixed in toto in an acid fixing medium, which caused the precipitation of the prussian-blue. On clearing subsequently by the Spalteholz method the spread of the solution was found to be somewhat more extensive than in the stage of 18 mm. (cf. figs. 4 and 5). In figure 5 the whole periaxial area over the roof of the fourth ventricle is shown to be completely filled by a dense aggregation of the prussian-blue granules. The separation of the two areas of fluid passage can not be made out in such a specimen. This dense periaxial extension ahnost completely covers the cerebellar Up, not onlj^ in the medial region but laterally to the limit of the ventricular roof. The injection precipitate lies directly beneath the skin in this area, but more posteriorly its separation from the skin becomes more marked. Tracing this dense periaxial injection posteriorly, it is seen (fig. 5) to end somewhat abruptly in the region of the cephaUc flexure. The Une of termination of the denser mass, to the ventral surface of the medulla, tapers somewhat anteriorly. This extraventricular spread is medial to the otic vesicle, but extends peripheraUy along the caudal cerebral nerves, reaching outward as far as the peripheral gangUa. The periaxial spread also closely covers the ventral surface of the medulla and extends in this plane around the pontine flexure for a short distance upwards along the basilar surface of the mid-brain.
Examined from its dorsal aspects, the superior portion of the spinal cord is found to be covered (in a perispinal relation) by a fine deposit of the prussian-blue. This is shown in figure 5. Caudally from the higher cervical region there is no exidence indicating a further spread in the perispinal tissues. Such a spread from above downward is wholly at variance wdth Reford's^^' conception of a development of the spinal meningeal spaces before the cerebral. The complete filling here of the central canal of the spinal cord and of the cerebral ventricles with the replaced fluid, with no evidence of a periaxial spread except in the region of the fourth ventricle, indicates that in the pig cinbrj'o the adult human relationship between the cerebral ventricles and the subarachnoid spaces endures. There is apparently in this embryo no evidence of the foramina of Bichat and of Mierzejewsky, a findmg in accord with the observations of Dandy and Blackfan'i*.
In the slightly larger embryos the further extension of the embryonic extraventricular spaces progresses rapidly. Figure 6 represents such an extension in a pig embrj-o of 21 mm., in which the normal cerebro-spinal fluid was rei)laced by a dilute solution of potassium ferrocyanide and iron-ammonium citrate. In this specimen the central canal of the spinal cord and the cerebral ventricles are completely filled with the precipitated prussian-blue. But m addition there is almost a total filling of the periaxial spaces. Viewed laterally the densest aggregation of the blue granules is again in the region of the roof of the fourth ventricle. As in the embryo of 19 mm. (fig. 5), the whole extraventricular tissue posterior to this ventricular roof is filled with the granules precipitated from the foreign solution. The spread from this region is similar to that in the previous specimen, except in its far greater extent. The granules may be traced caudalwards in the perispinal spaces to the point of injection. The arrangement of the precipitated material, both withm the central canal of the spinal cord and surrounding it in the perispinal relationship, is well shown in figure 7, a frank dorsal view of the same specimen represented m figure 6. The greater density of the perispinal granules in the upper region of the cord, as contrasted with the granules in the thoracic region, is probably of importance in indicating the direction of the flow from above dowaiwards. The increased amount of the injection fluid in the region about the pomt of insertion of the spinal needle.is in all likelihood due to a local spread from the needle, such as frequently occurs in a very limited area. The phenomenon may, however, be due to an actual increase in the size of the potential perispinal space, though observations upon other embiyos of the same stage of development argue against this view. The segmental outlming of the caudal portion of the perispinal space is to be noted in this figure.
The cephalic regions in the specimen of 21 mm. show a quite extensive spread (fig. 6), and there is the same general distribution of the granules about the medulla, as in the specimen shown in figure 5. The rhombencephalon is completely surrounded by the blue, the ventral sheet inclosing it tightly. Laterally the prussianblue is shown in a dense mass, in intimate relation to the cranial nerves as they join the brain-stem. The cerebellum is practically completely covered by the precipitate; from the ventral portion of the pericerebellar granules the replaced solution (as evidenced by the granules of prussian-blue) spreads forward and surrounds a portion of the mid-brain. Only the ventral surface of the posterior half of the mid-brain is circumscribed by the granules; anteriorly it is wholly surrounded by the i)eriaxial injection; more anteriorly tlic extension is iimited to the mesial structures, leaving unsurrounded the cerebral hemispheres, althougli creejnng between the hemispheres and the mid-brain.
The pecuhar avoidance by the replacement fluid of the extreme dorsal half of the mid-brain is also to be made out in the dorsal view of the specimen (fig. 7).
The two lateral extensions from the ventral sheet of the injection granules approach on either side this mesencephalic eminence. The peculiar appearance of the injection spread caused by the chorioidal invagination of the roof of the fourth ventricle is also here illustrated.
In this specimen, then, of a pig embryo 21 mm. the periaxial spread is almost complete, the only areas not entirely surrounded being the aiiterior mesencephalon and the cerebral hemispheres. In an embryo but a few milhmeters larger this periaxial exten.sion of the solution is complete. The mesencephalon first becomes entireW covered by the jjrussian-blue precipitate, with later extension over the hemispheres. This complete periaxial injection occurs usually in replacements in embryos varying in length from 24 to 28 mm.
A specimen exhibiting a complete extension of the replaced solution around the central nervous system is shown in figure 8. This specimen was prepared by replacing the cerebro-spinal fluid in a Uving embryo of 26 mm. and then keeping the embryo alive for an hour. After fixation in an acid medium, dehydration, and clearing, the uijection was found to occupy the whole medullarj-canal system and also to surround completely the cerebro-spinal axis, as shown in the lateral view. The striking features of this stage are similar to those observed in the younger specimens — the dense accumulation of granular material in the region of the roof of the fourth ventricle, the surrounding of the central portion of the caudal cranial nerves, and the thin pericerebral covering by the replacement mass. In addition the specimen exhibits in the thoracic region an extension of the granular material laterally along each spinal nerve. An observation of this peculiarity reveals the prussian-blue extending outwards only as far as the ganglia on the posterior roots.
The relationships, then, observed in an embryo pig of 26 mm. are those which exist in the adult; the cerebro-spinal axis contains cerebro-spinal fluid within its cerebral ventricles and within the central canal of the spinal cord, while in turn it is cornpletely surrounded by cerebro-spinal fluid within the subarachnoid space. Communication between the ventricles or intra-medullary sj'stem and the perispinal spaces occurs only in the region of the fourth ventricle. Here again the adult human relationship holds. The evidence, therefore, from a study of the fluid spread in a replacement experiment with the use of true solutions, indicates that in pig embryos of about 26 ram. an adult distribution of cerebro-spmal fluid occurs.
The Results of Injections of True Solutions
In the preceding section there have been detailed the results of experiments on living pig embrj-os in which the cerebro-spinal fluid of both the central canal of the spmal cord and the cerebral ventricles has been replaced by a dilute solution of potassium ferrocyanide and iron-ammonium citrate. After the replacement, carried out so as to avoid any increase in the normal tension, the embryos were incubated for varj^ing periods of time so that the normal current of the fluid might cause an extension of the loreign solution. In the experiments which will be recorded in this section the same true solution was injected from an ordinary syringe and the salts immediately precipitated as prussian-blue. The purpose of these observations was solely to ascertain the effect of injections at pressures above the normal tension, so that the conclusions drawn from the replacement method might be more fully substantiated.
It was soon ascertained that the pressures caused by injections with a simple syringe could be fairly well controlled and that several degrees of tension might be employed. Thus it was found to be simple and serviceable to designate the injections as those made with mild, moderate, or strong syringe-pressure. Most of these injections were made into the central canal of the spinal cord, but occasionally into the perispinal spaces or cerebral ventricles. Injections under equivalent pressures in the central canal of the spinal cord or into the cerebral ventricles always gave corresponding results. It is necessary to record that the injections, even under strong pressure, were not carried to the point of macroscopic rupture.
The so-called mild syrmge-pressure, making use of solutions of potassium ferrocyanide and iron-ammonium citrate, resulted in extensions of the prussian-blue wholly similar to those obtained in the replacement experiments which were carried on for 30 minutes and over. This similarity indicates a complete filhng of the available cerebro-spinal system in the replacement method, for certainly (even in the mildest syringe injections) the intraventricular pressure must be excessively increased. Figure 1 shows a specimen under such conditions, with a marked thinning of the injection mass in the region of the fore-brain. This finding is customarily present in the injections under mild pressure, due to the pushing upwards of an existent ventricular fluid.
"When moderate pressures are employed with the syringe the picture gradually changes. The essential difference in the results obtained by moderate syringe injection and by the replacement method lies in the greater extension of the foreign solution in the smaller embryos. Thus in figure 9 the spread of the injection precipitate in a pig embryo 16 mm. is shown to be about as extensive as that obtained by the replacement method in an embryo of 19 mm. (fig. 5). The extra ventricular distribution of the injected solution around the medulla, the extension (even more marked here) along the central roots of the caudal cranial nerves, and the localized perispinal spread are easily made out in this specimen of 16 mm.
This general rule applies to all of the results obtained with the use of syringepressures above the mildest. Dependent upon the degree of syringe-tension, the spread extends in simple ratio. Thus, by the use of moderate pressures of injection into the central canal of the spinal cord, a complete intramedullary and periaxial spread was secured in a pig embryo of 22 mm. somewhat earlier than the equivalent stage was obtained by the use of the replacement method.
With the highest syringe-pressures (insufficient, however, to cause macroscopic rupture) the same general type of injoctiou spread was obtained, bringing the more complete stages down into smaller and smaller embryos. Most of these embryos, however, on microscopic section showed obvious rupture of some part of the central nervous system.
The most important feature of these findings in the embryo pig injected with true sohitions under moderate pressures from a syringe concerns the fact that the extension of the injection coincides, except as to the size of the embryo, in every instance with that obtained by the replacement method. Thus similar and analogous spaces are filled by injections under syringe-pressures in small embryos and by the solution under normal tension in larger embryos. It must be assumed, then, that the pressure of injection is sufficient to dilate potential cerebro-spinal spaces which normally would not be concerned in the pathway taken bj' the cerebro-spinal fluid. No evidence of new or abnormal pathways for the fluid is afforded by the observations made with the increased pressure; these phenomena indicate great potential strength in the tissues which limit the immature cerebro-spinal spaces.
Injections with a simple syringe may he made with such a degree of pressure that gross rupture of the tissues becomes apparent. In such an injection into the central canal of the spinal cord the infundibular region ordinarily ruptures in the smaller embryos (under 15 mm.), while in larger embryos rupture usually occurs into the subcutaneous tissues of the back of the neck over the fourth ventricle.
In discussing the effects of the introduction of solutions of ferrocyanide under pressures higher than normal into the central canal of the spinal cord, it may be appropriate to record observations made in the attempt to inject the cerebro-spinal spaces from the perispinal space. In embryos under 15 mm. in length it is quite difficult to make a perispinal injection. As the embryos exceed this measurement the injection becomes increasinglj' easy, but not until a length of 20 mm. is attained can it be made under the mild pressure advisable. These observations tend to substantiate the findings alreadj' recorded in both the intramedullary replacements and the injections under mild pressure.
Results Of Injections Of Nitrate Of Silver
In a number of experiments a dilute solution (0.5 per cent) of nitrate of silver was injected into the central canal of the spinal cord and the salt then reduced in the sunhght. This solution, although a true one, is wholh- unsuited for the replacement type of injection, on account of its great toxicity and its power to coagulate protein. It was employed here onh' for the simple type of injection.
The results obtained by this intraspinous injection of solutions of nitrate of silver were of but little value in the determination of a pathway for the cerebrospinal fluid, but they vividly present certain aspects of the problem. Thus, in figure 11, a drawing of a specimen (pig) of 16 mm., the area through which fluid passes in the superior portion of the roof of the fourth ventricle is clearly outlined by a denser deposition of the silver. This specimen was prepared by introducing the solution of nitrate of silver into the central canal of the spinal cord under the so-called moderate sj-ringe-pressure. The drawing shows a shght, cone-shaped extraventricular spread of the injection fluid. This spread takes place solely from the superior area of fluid passage, a result in accord with the finding that the solution of potassium ferrocyanide and iron-ammonium citrate passed first through the superior area. Of course it is realized that the precipitant action of the silver may have exerted a more potent action on the structures constituting the lower area of fluid passage.
Another interesting phenomenon of the injections of silver nitrate is shown in figure 12. The embryo of 13 mm. here represented was injected under strong syringe-pressure with a sohition of silver nitrate into the central canal of the spinal cord. On subsequent reduction and clearing it was found that the excessive pressure had resulted in a comjilete intramedullary injection with a localized pedunculate spread into the tissues from the roof of the fourth ventricle. This bulbous extravasation into the extravcntricular tissue has not been observed in any specimens except those into which the solution of silver nitrate was injected. Such a spread is probably to be accounted for by an immediate coagulation of the surrounding tissue.
The extensive use of solutions of sUver nitrate as a means of demonstrating vascular channels naturally suggests a careful comparison of the results obtained from its use and those obtained from the employment of other available true solutions, in regard to the evidence afforded by the two methods of intraspinous injections. The chief objection to the use of silver nitrate, as has aheady been mentioned, is its power to coagulate protein. This is illustrated by many features of the specimen shown in figure 11 — by the sharp outhning of the area of fluid passage, the markings on the caudal process of the fourth ventricle, and the delimitation of the cerebellar hp. But much more marked are the evidences of this coagulative power as shown in figure 12, the pedunculated extraventricular spread, the transverse corrugation of the cerebellar hp (amountmg to circumscribed indentations), and the peculiar outlining of the roof attachment to the bulb. These phenomena obtained by the intraspinous injection of solutions of silver nitrate must be classed as artifacts. The different degrees of this corrosive action of the silver probably result from the varj^ing rates of reduction of the salt to the metal, a factor which is not easily regulated. The findings, therefore, with this method are worthless unless controlled.
Many embryos of varying sizes were injected with the silver nitrate. In the main these observations followed the course of development of the cerebro-spinal spaces as evidenced by the replacement experiments with the ferroc3'^anide. The injections required moderate pressure in the syringe in order to secure more than a local extension from the roof of the fourth ventricle, and to secure the same extent of spread it was generally necessary to use embryos a few millimeters larger than those required in the replacement experiments; but this is to be expected, m view of the i^robability of a constant precipitation of the albuminous tissues by the injection fluid.
Specimens prepared by the intraspinous injection of silver nitrate, then, afford but little reliable evidence in this j)roblem except of a substantiative sort. The findings by this method indicate that the perispinal and pericerebral spaces, in pig embryos of 25 mm. and upward, could be filled by an injection of silver nitrate under moderate pressures into the central canal of the spinal cord. The point of passage of the fluid from the intramedullary to the periaxial system was in the region of the roof of the fourth ventricle.
The Injection Of India Ink
The objections to the use of any fluid of insoluble particles in suspension have already been discussed in considering the methods of injection which were possible for use in this study; but for comjiarisou Avith results obtained by more promising methods and to ascertain to what extent injections with India ink are reliable they will be further considered here. No granular substance other than India ink (carbon granules) was employed in this investigation. In every way this suspension possesses advantages over other possible masses — in its ease of preparation, in the small size of the granules, and the insolubihty in the reagents used for microscopic technique.
Suspensions of nidia ink (diluted from 4 to 10 times) were introduced first into the medullary-canal system of living pig embryos by the replacement method. In no case, however, even though the circulation of the embryo may have continued for 90 minutes, was there any evidence of an extension of the replaced mass outward into the periaxial spaces. The carbon granules remained wholly within the ventricles, a striking difference from the results obtained by the ferrocyanide replacements. It would appear, then, without the further evidence afforded by microscopic section of the specimens, that there is an existing mechanism which prevents the passage of the carbon granules from the fourth ventricle into the periaxial spaces. This finding was found to be constant ui all the living embryos subjected to the cerebro-spinal replacement.
Quite similar to these results by the replacement method are those from the injection of a suspension of india ink under mild syringe-pressure. In no instance, provided the pressure was maintained at a low enough degree, was there any passage of the granular material into the periaxial tissue. In embryos of over 30 mm., however, even with the lowest pressure, it becomes increasingly difficult to prevent a sudden spread into the periaxial spaces. The type of spread indicates a sudden release of some restraining agent and suggests a rupture of a membrane. This spread is usually local and takes place from the roof of the fourth ventricle.
With moderate and strong syringe-pressures, however, it is possible to secure a periaxial spread, but this is quite different from the distribution of the uijections by the use of ferrocyanide solutions. Figure 10 illustrates a specimen of a pig embryo of 21 mm. into whose central spinal canal india ink was injected under strong syringe-pressure. The resultant spread of the injection is easily discerned; the cerebral ventricles are quite filled with the carbon, while from the superior portion of the roof of the fourth ventricle a dense but localized periaxial spread is made out. This extraventricular extension of the ink is well defined; it stretches caudalwards for a slight distance, curving about the bulbous caudal portion of the ventricle and extending lateralwards but a short distance. The median portion of the cerebellar lip is covered by the granules. E\adeuces of the excessive pressure at which the injection was made are shown by the lines of mvasion of the spinal cord and mid-brain. A comparison of the spread of this injection mass with the extension of a ferrocyanide replacement in an embryo of the same size (21 mm.) is afforded by figures 10 and 6. With such a divergence in the results obtained by the two methods of approach it is not surprising that observations such as Reford's) fail to coincide with these findings. The unsuitabiHty of suspensions of granular material in the investigation of the cerebro-spinal sjiaces has been many times verified in this work.
In the further study of the course of the spread with injections of india mk it was found that, in pig embryos of approximately 22 mm. and over, a partial periaxial injection could be secured by plunging the syringe-needle into the perispinal spaces. The carbon granules could subsequently be seen filling the perispinal spaces and also mounting upwards in jjartial pericerebral relationships, particularly around the medulla. This result was obtained by the use of strong syringe-pressures. Apparently the resistance to the spread of the ink in injections or replacements in the medullary-canal system occurs in the passage of the fluid from the roof of the fourth ventricle into the periaxial spaces. So far as is known, Reford^"*^) did not control his injection pressures. These results with the injection of india mk under strong pressures coincide with the idea of his observations afforded by the abstracts given by 8abin(*3) and Cushing^^'. Suspensions of india ink. then, injected under mild syringe-pressure or by the replacement method, offer no evidence, in the pig embryo, of a passage of the cerebro-spinal fluid into the j^eriaxial spaces. Only by employing pressures much above the normal tension can such evidence be obtained.
V. Undescribed Structures in Roof of the Fourth Ventricle
The results of the replacement of the existing cerebro-spinal fluid by a true solution of potassium ferrocyanide and iron-ammonium citrate in a living pig embryo indicated, as detailed in the foregoing section, that the fluid passed from the ventricular system into the periaxial tissues in the region of the roof of the fourth ventricle. This important transit of the fluid, agreeing with the established conception of the relationship in the adult, was first observed in an embryo pig of 14 mm. (fig. 3). At this stage the exudation of the replaced fluid occurred in one defined area, seemingly corresponding to the dense oval in a smaller embryo shown in figure 2.
Such a passage of fluid from ventricle to i)eriaxial tissue is necessarily a ])hysiological phenomenon, and it was in the hope of finding an anatomic basis for this phenomenon that the roof of the fourth ventricle was studied histologically. It was reahzed that failure to demonstrate anatomically differentiated structures would not vitiate the physiological observations, but that a correspondence between function and structure was most desirable. Hence observations were undertaken to determine, if pn.ssible, an area of histological differentiation in the roof of the fourth ventricle which might be concerned in the primary passage of fluid from the cerebral ventricles into the periaxial tissues. The investigation concerned first the examination of this region in pig embryos of 14 to 15 mm., at which stages the fluid passes from a single area. Subsequently, similar studies were undertaken in regard to the second, more inferior area (shown in figure 4). The results of these studies will be given here.
AN UNDESCRIBED AREA IN THE SUPERIOR PORTION OF THE ROOF OF THE FOURTH VENTRICLE.
The Area Membranacea Superior In The Pig Embryo
Examination of the roof of the fourth ventricle in a pig embryo of 14 mm. revealed a peculiarly differentiated area in the superior portion. The general topography of this area is show7i in the rectangular area marked off in figure 32 — a median sagittal section from a pig embryo of this critical stage. In figure 33 this rectangular area is enlarged to show the morphologA' in greater detail.
In this figure the densely staining ependj'ma lining the fourth ventricle approaches from both sides. The superior portion of the ependyma ends abruptly, while the inferior Hne of the layer tapor.s more slowly. Between these two jjoints is an area having none of the characteristics of the ventricular lining at all other points. The comparatively smooth contour of the ependymal cells is replaced by an irregular cell-border. The pyknotic nuclei of the cells have been replaced by less densely staining, elongated, spindle-like nuclear bodies. The cell-layer lining the ventricle is here really only of a single cell in thickness, although blood-capillaries closely applied to it suggest a greater thickness. The mesenchj-nie lietween this layer and the peripheral epidermis is quite thin, but resembles in everj' way the mesenchyme in the immediate neighborhood.
There is, therefore, as pictured hi figiues 32 and 33, an area in the roof of the fourth ventricle which is morphologically dissimilar to the characteristic ependyma lining the cavity. Is this the result of some distortion in fixation or in the routine histological technique? Is it a constant finding and, if so, what is its historj'? Does it arise at a definite period and persist throughout intra-uterine life onlj' or through adult life also?
The question of the actual existence of this area, or of its being caused by technical manipulations, is one which must be answered. That this differentiated portion of the roof of the fourth ventricle is not an artifact is verified bj* the general history of its formation, by its invariable occurrence (not onh' in the pig but in other animals), and by its general histological appearance. Moreover, the physiological importance of this area undoubtedh' incUnes one completely from the possible explanation that it is due to an artifact. Xo single finding wholly excludes such a possibility; rather is one convinced, by many features, of its actual occurrence.
Considering the fact, then, that this differentiated structure in the roof of the fourth ventricle may be found in all embryo pigs at the stage of 14 mm., it becomes necessary to ascertain at what time in the development of the embryo it first appears and how it is formed. Obviously the most satisfactory' method is to trace the area through the lower stages and also through the older embryos. For the sake of greater clearness, however, a description of the area \s-ili be given from its first differentiation through its maximum transformation to its final disappearance — for the structure is only temporary.
In pig embryos of 8 mm. and less in crown-rump measurement, the roof of the fourth ventricle is fcfrmed of cells morphologically and tinctorially different from those lining other parts of the ventricular cavities. These cells are quite unlike the deeply staining ependjTiial cells, which can be so readily identified as the lining cells in older embryos. In this yoiuiger stage of 8 mm., the entire ventricular roof is composed of several layers of cells with round or somewhat oval nuclei and fairly abundant cytoplasm. The cell-boundaries are not well defined. The nuclei are not deeplj' tinged with hematoxylin. The chromatin material is sparse and irregularly distributed. Nucleoli are prominent. The cytoplasmic border hning the ventricular cavity is rough and ragged at times, often blending with the coaguUvted albumen of the cerebro-spinal fluid. Altogether, these lining cells bear a much greater resemblance to the epithelial cells than to the ependymal.
These characteristics of the lining cells of the roof of the fourth ventricle are shown in figures 24 and 25, from a pig embryo of 8 mm. The close association of the roof cells to the surface epithelium is easily made out in figure 25, as well as the general character of the lining cells.
At the stages of 8 mm. and under, in the pig embryo, the roof of the fourth ventricle is relatively quite large. In its whole extent it is formed of the peculiar lining cells described above. With the growth of the embryonic nervous sj^stem, the roof of the fourth ventricle is subjected to alterations in form and position; to some extent these changes influence the cells which line the cavity in the early stages.
In pig embryos between 8 and 12 mm. in length the roof of the fourth ventricle undergoes a change. The ependyma, which from comparison with later stages is regarded as typical, begins to encroach upon the epithelial-like cells which are so numerous in the 8 mm. stage (fig. 25). The area occupied by these cells diminishes, not only relatively but absolutely. It becomes smaller and the cells gradually change their character. These changes are shown in figures 26 and 27, from a pig embryo of 11 mm. Figure 26 gives the location, in a sagittal section near the midline of the area in figure 27, taken at a higher magnification.
In figure 27 the densely staining lips of ependymal and nerve cells are seen approaching each other. For a considerable space in the central portion of the photograph there is an area similar to that shown in figure 33. But considered in connection with figure 25 this area represents the epithelial-like cells of the roof of the fourth ventricle. This relationship is more clearly shown in figures 28 and 29, taken in a more lateral plane from the same embryo (11 mm.). Examination, however, of th(; area in figure 29 shows the epithelial-like cells again a])i)arent in the roof of the fourth ventricle.
The i^rocess of transformation, then, as shown in these photographs from an embryo i)ig of 11 mm., concerns a gradual encroachment upon the area of epithelial-like cells by the more densely staining and more closely packed ependymal cells. Gradually the epithelial-like cells in the central portion of the area lose their former character (fig. 27), while around the periphery, especially on the lateral sides, the epith('lial-lik(! ajjpearance persists (fig. 29).
On the lateral side of this area, just as the tyincal ependymal lining is al)out to become isolated (fig. 29), the epithelial-like lining cells form a several-celled layer.
The nuclei are poor in chromatin material and the cytoplasm somewhat small in amount. The inner cytoplasmic border hning the ventricle is in contrast, by its ragged outline, with adjacent smoother ependj^ma on both sides. At this stage of the pig embryo the characteristics of the epithehal-like cells are still to be made out, but a gradual transformation is becoming evident.
The metamorphosis becomes much more marked in the central portion of the area, as shown in figures 26 and 27. In these figures the whole central area seems to have lost some of its former character as an intact cell-laj'er. Closer examination, however, under higher power demonstrates that it still possesses an intact surface as a hning for the ventricle. Delicate cytoplasmic strands stretch in a continuous line across the whole area between the Ups of denser tj-pical ependjina. The nuclei in this difi'erentiated area are seeminglj' altered from their rounded form and have elongated almost into spindles. The inner cytoplasmic border is characteristically rough, with, small amounts of coagulated albumen adhering to the processes. The area, then, in its central portion, at the stage of 11 mm., has assumed the character of the stage of 14 mm. (fig. 32). On the periphery, however, the cells stiU resemble those of smaller stages (8 mm.).
From the pictures presented by the intermediate stages (figs. 27, 28, and 29) the differentiation goes on verj' rapidly, so that in the pig embryo of 13 mm. there is rarely any evidence of the epithelial-like cells. Figures 30 and 31 are photomicrographs of a sagittal section of an embryo pig of 13 mm.; here there are no evidences of the epitheUal-like cells. The whole area, pictured in figure 31 as sharply delimited from the tongues of tjijical ependyma above and below, has become well differentiated. The cell-character observed in figures 27 and 33 (elongated nuclei and scanty strands of protoplasm) has become very obvious. The ragged and roughened intraventricular border, the coagulated albumen, and the abrupt transition from the neighboring tj-pical ependyma are well shown in the photomicrographs of this specimen.
The differentiation of this area in the roof of the fourth ventricle of the pig embryo proceeds at a very rapid rate, so that within the growth of a few millimeters (from 8 to 13 or 14) a great histological change occurs. Figures 32 and 33, already described, show the extent of this metamorphosis in a pig embrj^o of 14 mm. The process, however, continues, modified possibh' bj- the changing of the roof of the fourth ventricle. For this roof structure is subjected to marked alteration in stages of 14 mm. and upwards, both by the lateral development of the chorioid ple.xuses and b}^ the readjustment of the cervical and pontine flexures. Its maximal differentiation may be said to appear at a stage of 18 mm.; this is maintained through several millimeters, until undergoing final retrogression.
This maximal change in the roof of the fourth ventricle is shown in figures 34, 35, 36, and 37. Several points of interest are brought out in these photomicrographs. Figure 35 represents an enlargement of the rectangular area in figure 34, taken from transverse sections of an embryo pig of 18 mm. The area is particularly well shown in this figure, in which, from the right, the typical ependyma, in a fairly smooth single-cell layer, approaches the differentiated cells in the central portion. On the left, too, similar typical ependyma is shown. In the central area, which has been repeatedly described, the elongated nuclei, the strands of protoplasm, and the ragged, iiTegular intraventricular surface are well presented. The photomicrograph has been reproduced to show the relation of this differentiated area to the various blood-channels in the supporting mesenchyme. Apparently the whole ventricular roof is, at this stage, a site for an extensive capillary plexus; from both sides, as shown in figure 35, vessels (one of great caliber) approach the central area of differentiation. Directly beneath this area smaller capillary channels can be made out, from which, apparently, a sUght extravasation of red blood-cells has occurred. Here, as in the greater part of the basilar pericerebral region, extravasation of the blood-cells is very frequent. This phenomenon has already been pointed out by INIaU'^"".
The large extent and the great differentiation of this pecuUar area in the roof of the fourth ventricle are well shown in figures 36 and 37, taken from a transverse section of a pig embryo of 18 mm. In the photomicrograph of higher magnification the two sharp tongues of typical ependyma are quite striking. Their abrupt termination in the wide, differentiated area has nowhere been more convincingly shown. The resemblance of these lining cells in the central area to the mesenchymal elements adjoining is here also seen. The most interesting of all the phenomena exhibited in this reproduction, however, is the attachment, apparently by precipitation, of the coagulated albumen of the cerebro-spinal fluid. This coagulation, in this specimen, deUmits the differentiated area in the roof of the fourth ventricle. The phenomenon is seemingly only an ampUfication of a similar attachment of small fragments of the albuminous precipitate shown in other figures.
Beyond the stage of 18 mm., which may be termed the maximal stage, the differentiated area in the roof of the fourth ventricle undergoes a regression. This is apparently due to the morphological alterations in this rhombic roof. The chorioid plexuses in embryos over 18 mm. long deeply invaginate the fourth ventricle, possibly drawing some of the true roof with them, but surely encroaching upon the mid-hne with their lateral tuftings. This growth tends to decrease the available extent of the differentiated area, but an even more potent factor is the rapid development of the cerebellum. The caudal growth of the cerebellar lip soon largely occupies or replaces the superior half of the roof. These two factors, the cerebellar growth and the enlargement of the chorioid plexuses, render the persistence of the differentiated area impossible, so that a regression or disappearance is to be expected.
With these considerations before us, the study of sectioned pig embryos of a greater length than 18 mm. becomes important. The process of disappearance, however, does not occur at once. Thus, in an embryo pig of 19 mm. (figs. 42 and 43) the differentiated area is as large and as characteristic as in the stage of 18 mm. This same aijpoarance and maintenance of size may be observed through the next several millimeters' growth, but in pig embryos of 23 mm. the chorioid plexus has usually developed to such an extent that a continuation of the former size becomes impossible. This is shown in figures 44 and 45. Figure 45, the enlarged squared area from figure 44, is a photomicrograph from a pig embryo of 23 mm. The differentiated area, duo to the factors favoring its regression, now appears in close proximit}^ to the chorioid plexus. It has more the appearance of a degenerating area at this stage than in any of the younger embryos, but it still shows a characteristic delimitation of both edges — on the one from the typical ventricular ependyma, and on the other from the differentiated ependj'ma of the chorioid plexus. The cytoplasmic strands of the area which forms the ventricular border do not show to advantage in the photomicrograph, but the .same ragged character with the covering of coagulum may be made out. The process of regression, mechanical as it perhaps is, has begun at this stage in the pig, and in the course of the next few millimeters' growth will become even more active.
With the encroaclmaent of the chorioid plexuses and the downward growth of the cerebellar lip, the superior portion of the ventricular roof soon disappears, and is practically non-existent in embryos of 30 mm. and more in length. The differentiated area thus encroached upon from the sides and above becomes a mere vestige of its former size. Thus in a pig embryo of 32 mm. (figs. 46 and 47) it appears as a very small break in the lining continuity of the ventricular ependyma. Without the intermediate stages such a picture would undoubtedly be considered as an artificial erosion of the ependjinal lining of the ventricle, but when studied in connection with figure 45 the true vestigial character of the area becomes established.
The final fate of this differentiated area in the roof of the fourth ventricle is a complete disappearance, with the occupation of the region by chorioidal epithelium and cerebellum. In this study it was impossible to find traces of the differentiated areas in pig embryos of over 33 mm. in length; vestiges maj' persist, but so small as to present difficulties of decision. The persistence of such a differentiated vestige in rare instances would not be surprising; the transitory character of the area and the method of disappearance make this seem not unlikely.
This transitory area of differentiation in the roof of the fourth ventricle of the pig has not, so far as can be determined, been noted or described bj- any p^e^•ious author. His'25\ in a retouched photomicrograph of a sagittal section of a human embryo of 17 mm., reproduced the area as differentiated from the roof, but he has made no comment upon it. I have called this differentiated area in the superior portion of the rhombic roof ventricle the "area membranacoa superior ventriculi quarti." This terminology is based on the anatomical character of the area as a continuous membrane, but chiefly on its physiological significance. For, as will be shown in the succeeding section of this paper, the transit of embryonic cerebrospinal fluid from ventricle to periaxial tissue occurs in this area, which functions apparenth- as a physiological membrane. With such a physiological conception of the area, the ttim "area membranacea" seems most suitable, inasmuch as it also meets the anatomical requirements.
The Area Membranacea Superior in the Human Embryo
The finding of the diflferentiated area in the superior portion of the roof of the fourth ventricle in the embryo pig suggested the value of a study of the same region in the human embryo in the further solution of the problems underlying its occurrence. Hence this region in the roof of the fourth ventricle has been examined in the sectioned human embryos of the Department of Embryology of the Carnegie Institution of Washington. It was found that a similar area occurred in the human embryo of approximately the same age.
The study of the roof of the fourth ventricle is usually more difficult in the human embrj^o than in the pig. This is due to the fact that the roof of the fourth ventricle quickly suffers from poor fixation and dehydration — collapse or inversion of the whole structure being commonly met with. It is rarely possible, in the younger embryos, to secure the most satisfactory fixation, whereas in the pig these factors may be controlled as desired. Furthermore, the undue pressures to which the human OAaim is frequently subjected in abortion may cause crushing of the more delicate parts of the nervous sj^stem.
It is probably best, in the human embryo as in the pig, to trace the formation of the area membranacea superior ventriculi quarti from its beginning, through the various differentiations.
In a human embryo of 4 mm. (No. 836 of the Collection of the Carnegie Institution of Washington) the entire roof of the fourth ventricle is composed of cells with round or slightly oval nuclei and palely staining cytoplasm. The nuclei of the cells are poor in chromatin material as contrasted with the pyknotic character of the typical ependymal cells. The lining tissue is of the thickness of several cells. The ventricular cytoplasmic border is fairly smooth at this stage. This characteristic ventricular lining is shown in figures 40 and 41, both taken from embryo No. 836. The whole picture is similar to that exhibited by the pig embryo of 8 mm. (figs. 24 and 25).
A similar accumulation of epithelial-like cells is found in a human embryo of 7 mm. (No. 617 of the Carnegie collection). This is pictured in figures 48 and 49. The photomicrograph of higher magnification shows these poorly staining cells heaped up in a rather localized part of the ventricle, fairly sharply dolimitod from the adjoining ventricular lining. This accumulation of cells in the roof of the ventricle invariably occurs, and it must not be considered as being due to the distortion of the ventricular roof. The reason for the asymmetry of the rhombic roof shown in these figures hes in the fact that in this embryo, as in practically all the embryos of similar stages in this collection, some degree of distortion of the roof of the fourth ventricle is present. Photomicrographs (figs. 50 and 51) taken more posteriorly (from embryo No. 617) give strong evidence of this distortion. They are reproduced not only to show the possible distortion, but also to give a further picture of the lining of the ventricle, with its epithelial-like cells in several layers (fig. 51).
Similar accumulations of these epithelial-like cells are to be found in human embryos of 9 mm. Reproductions of a much fragmented specimen of this size (No. 721) are given in figures 52 and 53. In the latter figure the complete occupation of the ventricular roof by these cells is well illustrated. Moreover, the specimen shows the many-layered stage to a degree but seldom found. It is unfortunate that such a degree of fragmentation and distortion is found throughout this specimen.
Thus far, in human embryos up to and including 9 mm. in length, the roof of the fourth ventricle has shown the same architecture as appears in the pig. As will be recalled, the first evidence of a further differentiation of these cells in the pig embryo was found at a stage of 11 mm. (figs. 26 and 27). In one human embryo of this stage (No. 544) a distinct break in the roof of the fourth ventricle can be made out. This is shown in two photomicrographs (figs. 54 and 55). The picture in this cas3 is somewhat obscured bj' the shrinkage and distortion of the ventricular roof, but a distinct differentiation of the lining epithelium can be made out. On the caudal side of figure 55 considerable nervous tissue is seen. Just superior to this (toward the left) the lining tissue is almost lacking, a few nuclei, only, preserving the contour of the ventricle. Above this area appears again the ventricular lining of many layers of cells. It has been quite difficult to interpret these findings. The area under discussion shows a rather typical adherence to the coagulated albumen ; there is evidence of its extension also into the adjacent mesenchyme, a finding observed in no other similar stage. The caudal position of the opening, the character of the tissue approximating the ventricular cavity, and the presence of the albumen in large amount in the adjacent mesenchyme — all indicate that in great measure the pictures presented in this specimen are largely artifacts. It seems most likely, though, that some differentiation of the tissue in this area has occurred.
In a human embryo of 14 mm., as in the pig of the same stage, the area membranacea superior has attained a great degree of differentiation. This is particularly well shown in figures 56 and 57, the latter being an enlargement of the squared area in the former. These photomicrographs are from embryo No. 144 of the collection of the Carnegie Institution of Washington. Figure 57 shows a characteristic which distinguishes the area membranacea from that of the pig, although in the later stages of the pig embryo (figs. 45 and 47) this feature is present. This concerns the marked decrease of cellular tissue in the membranous area. In figure 57 the deeply stainmg typical ependyma is shown approaching from below. These cells end abruptly at the border of the area membranacea; the ventricle in this area is lined by cells possessing small elongated nuclei and long cytoplasmic processes, which unite to form a ventricular lining. The oval nuclei along the ventricular border become more closely massed together in the superior portion of the area, but nowhere is there the same architecture as in the equivalent stage in the pig (fig. 33). A feature of the histological appearance of the membranous area in the pig embryo is also shown in figure 57; this is the marked adherence of the coagulated albumen of the cerebro-spinal fluid to the area membranacea superior.
The roof of the fourth ventricle in the human embryo is subjected to the same factors causing changes in the form and relationships which were commented upon in the pig; but these play little part until the chorioid plexuses become of sufficient size to divide the ventricle into a superior and inferior portion. In the human embryo, as in the pig, the superior half of the ventricular roof is sacrificed to the greater growth of the cerebellum.
- Measured on the slide after mounting.
In human embryos of 17 mm., however, these factors have not begun to influence the membranous area. This is shown in figures 58 and 59, photomicrographs from embryo No. 57G. The section is somewhat to the side of the midline, but in the superior portion of the roof of the fourth ventricle the differentiated membranous area can be made out. The sharp delimitation of this area from the denser t3T5ical ependyma on both sides is quite apparent. The ragged character of the ventricular border, with its few elongated spindles, seems wholly in keeping with the transverse view of this area afforded by figure 37.
Embryo No. 576 exhibits one characteristic of the area membranacea superior very frequently seen in human embryos, but almost invariably absent in these stages in the pig. Along the lateral margins of the superior membranous area are dense borders of the many-layered epithelial-like cells which lined the ventricular roof in younger stages. This feature is well shown in figures 60 and 61, the latter figure being a higher magnification of the former. The cellular border of the superior area reaches transversely only through a few 15-micron sections, but it extends throughout the whole cephalo-caudal diameter of the area. It seems likely that this represents purely a survival of the epithelial-like cells in the younger embryos. In rarer instances the whole area membranacea superior may be surrounded bj^ such a border of many-layered cells, but even in these cases the superior and inferior margins are quite thin.
No apparent agencies favoring the disappearance of the superior membranous area m the roof of the fourth ventricle of the human embryo are apparent in stages up to the fetus. Thus, in human embryos of 18 mm. this differentiated area in the roof has reached its maximal differentiation. A section from an embryo of this size (embryo No. 409) is reproduced to show the distortion and its influence upon the topography of the area membranacea. The two photomicrographs (figs. 62 and 63) show the extreme collapse and distortion of the roof of the fourth ventricle. In the figure of higher power (No. 63) the membranous area appears facing posteriorly, due to the shrinkage; the proper leader runs to this area. It shows the differentiation from the adjoining tj'pical ei)endyma which is characteristic of the full}' developed area membranacea superior.
In a beautifully preserved and sectioned human embryo of 21 mm. (No. 460) in the collection of the Carnegie Institution of Washington the area membranacea superior appears as a sharply delimited area (figs. 64 and 65). These figures give a very good idea of the definiteness of this area when the fixation and dehydration approach the perfect. The tissue of this membranous area lining the ventricle here appears to be wholly lacking in an epithelial covering; the mesenchyme seems to serve as the ependymal lining. Study of this area, however, through different stages argues most strongly against such a view.
he process of regression of the area memhranacea sujjerior in the human embryo differs somewhat from that described in the pig. This alteration in the mode of disappearance is largely due to the fact that in the period of growth from 20 to 35 mm. the superior portion of the roof of the fourth ventricle in the human embryo is not sacrificed to the cerebellar lips; for in the human the cerebellum grows largely into the fourth ventricle, enlarging beneath the superior part of its roof. Thus, the attachment of this part of the roof is not greatly interfered with by the rapid development of the cerebellum. The total extent, then, of the superior portion of the roof is hardly altered in these stages in the human, while in the pig embryo the roof is shortened by its attachment to the inferior portion of the cerebellar hp, which retains its earUer characters. These differences in the relationship of the superior portion of the ventricular roof in human and pig embryos may be seen by comparison of figures 74 and 89.
Another factor which renders the mode of disappearance different in the two embryos concerns the greater tufting and development of the chorioid plexuses of the fourth ventricle in the pig. This greater size and complexity of the plexus causes an encroachment upon the roof structures which, in the pig embryo, seems of considerable importance in the t'nal closure.
In the human embryo, however, it has been found very difficult to explain the final disappearance of the superior membranous area on the same mechanical factors w^hich seemed so well to account for its transitory characters in the pig; but at approximately the same stage of growth the process of regression occurs in the human fetus. The area maintains a fair size in stages up to a length of 23 mm. Thus, in figures 89 and 90 (No. 453 of the Carnegie collection) a sagittal section from a human fetus of this size is illustrated. In the higher power (fig. 90) the superior membranous area is short, rather sharply delimited on its superior border by the typical, dense ventricular ependyma. Below, its edge is irregularly formed by the deeply staining ependyma over the invagination of the chorioid plexus. The cell-character of this area resembles that shown in the photomicrographs from the specimen of 21 mm. (figs. 64 and 65). There is left in the area no indication of the cellular architecture which characterized the original ventricular ependj-ma; the cells with their elongated cj'toplasmic processes here have the oval nuclei which are found almost invariabh' in this membranous area.
In the human fetus of 26 mm. (No. 1008 of the collection of the Carnegie Institution of Washington) there is but sHght evidence of a superior membranous area in the upper portion of the roof of the fourth ventricle. The evidence present in this specimen consists in a localized thickening of the lining cells of the ventricle in the situation of the area in other stages. This thickening is illustrated in figures 91 and 92; it consists of several layers of epithelial-hke cells, similar in all respects to the many-layered border shown in figure 83. The picture is somewhat obscured by the vascular pl-^xus directly beneath the ventricular lining.
There is difficulty in determining exactly when the last evidences of the superior membranous area in the roof of the fourth ventricle may be found. This is due to the likelihood of artifacts disturbing the character of the ventricular lining in human material, where the freshness and fixation of the specimen may not be ideal. In the larger specimens in the collection of the Carnegie Institution, which are well fixed and sectioned, the existence of the area membranacea superior could not be wholly verified. Thus, in specimen 405 (26 mm.) the presence of the area seemed probable though not definite. In another embiyo of this same size (So. 782) the existence of this area was still more questionable. In a larger embryo (30 mm. No. 75) the presence or absence of the area could not be assured; many indications suggested its existence, but the resemblance to an artificially separated ependyma was strong. In all specimens of human embryos of over 30 mm. examined, no evidence of the area membranacea superior could be found. It appears likely, then, that the final disappearance of this differentiated area in the roof of the fourth ventricle occurs at a slightly earlier stage in the human embryo than in the pig.
The final disappearance of the area membranacea superior in the human embryo is not accompanied by the same ingrowth of typical ependyma that characterizes the process in the pig. There is a great tendency, in the human, as indicated in figure 92, for a replacement of the area by the same type of epithelial-like cell which comprised the whole ventricular roof in the earlier stages (fig. 41) and later formed lateral borders for the superior membranous area (fig. 83). Thus, in a human embryo of 24 mm. (No. G32 of the Carnegie collection) there is evidence of a very small membranous area surrounded by a border of epithelial-like cells. In a slightly larger specimen (No. 840, 24.8 mm.) the whole membranous area is occupied by the epithelial-like cells. The frequent association of these cells with the area indicates that in disappearing the area membranacea is probably replaced first by these cells, which in turn disappear, so that the whole roof is finally composed of the typical, densely staining ependyma.
The Area Membranacea Superior in Other Animals
In order to ascertain whether the area membranacea superior existed in other animals examinations of serial sections of the rabbit, cat, sheep, and chick of suitable stages were made. All of these animals were found to possess a differentiated area in the roof of the fourth ventricle.
Opportunity was afforded for the study of serial sections of the head of a chick* of 121 hours' incubation. The head was carefully dehydrated and embedded by Dr. E. R. Clark, and was subsequently sectioned bj' Dr. C. R. Essick. The material was beautifully fixed and dehydrated, showing practically no evidence of shrinkage. Typical portions of the superior membranous area are reproduced in figures 66, 67, 68, and 69. Figure 67, taken near the crown of the embr3-o and representing the squared area in figure 66, shows the two dense masses of ependyma separated by the more lightly staining area membranacea. The cellular character of this differentiated zone resembles more the histological features of the similar afea in the pig than those of the human embryo. This resemblance is also to be seen in figure 69, taken more posteriorly than the two preceding figures. The dense ependynia approaching on both sides is sharply delimited at the edge of the hroad membranous area. This is composed of cells having elongated, chromarin-poor nuclei, and long cytoplasmic processes, which form the ventricular roof. The adlierence of the albuminous coagulum occurs here also.
- The chick measured 14 mm. in 40 percent alcohol.
In the rabbit the occurrence of the superior membranous area was verified as in the other species studied. In a rabbit embryo of 13 mm. (series x in the embryological collection of this laboratory the area was well differentiated from the surrounding typical ependyma. The cells of the area resembled those of the adjacent mesenchyme. The ventricular surface was roughened by the projection of numerous protoplasmic processes. An albuminous coagulum was attached to the cells of the membranous zone.
One sheep embryo from the collection of this laboratory was also studied. The sections, although labeled as an embryo of 10 mm., resembled in every way a pig embryo of 18 mm. The area membranacea was easily identified in the roof of the fourth ventricle; it is similar in every respect to the same area in the pig and the human embrjo.
In a cat embryo of 10 mm. a smiJl but highly differentiated area membranacea superior was made out. The most striking feature in this specimen is the great adherence of the coagulated albumen to the cells of the area and the resemblance of these cells to the mesenchymal elements adjacent. The edges of this differentiated area are sharp and clear-cut.
No attempt was made to identify the area membranacea superior in other animals — as further suitable material was not immediateh- available. The chief study has been made on pig embryos and on human embryos. The occurrence of the area in the cat, sheep, and rabbit probably indicates its existence in aU mammals. The finding of such an area in the chick is also suggestive.
General Consideration of the Area Membranacea Superior
The occurrence of a definite area of differentiation in the superior portion of the roof of the fourth ventricle has been pointed out in preceding subdivisions of this paper. It has been described in detail in the pig embryo and in the human embryo; it has been identified also in cat, sheep, rabbit, and chick embryos. It remains here to discuss the general characteristics of this area.
No description of such an area of differentiation in the ventricular roof has been found in the literature. It may be that the distortion of this structure in the course of the usual embryological technique has rendered its discovery Iess likely. His'-=, m his description of the ventricular roof, has not commented upon the occurrence of this membranous area, even though in a retouched photomicrograph of his fetus C-1 (a human specimen, of the beginnmg of the third month) the area membranacea superior can be made out. Likewise in his description of the pUca chorioidea he faJs to mention any differentiated areas in the roof, although plate I, in his "Die Entwickelung des menschUchen Rautenhims, von Ende des ersten bis zum Beginn des dritten Monats," shows a slight irregularity in the roof. Practically all of the contributions to the anatomy of the roof of the fourth ventricle deal with the lower half of the structure, with particular reference to the occurrence of the foramen of Magendie.
The general biological process involved in the formation of the area membranacea superior concerns a dilTerentiation of the epidermal elements which Une the ventricular cavity. This differentiation, both in human and in pig embryos, first begins with the occurrence in the ventricular roof of an area of epithelial-hke cells. These, in the course of enlargement of the roof, become more or less isolated in the superior portion of the structure, and then undergo a metamorphosis into the typical cells of the membranous area. They are characterized by oval or elongated nuclei (rather poor in chromatin as compared with the nuclei of the typical ependjonal elements) and by cytoplasmic strands (in which the cell-boundaries are very poorly marked) which compose the ventricular border. The ventricular surface in the area membranacea is more ragged and u-regular than where lined by typical ependjana. In many instances, as in figure 57, from a human embryo of 14 mm., this transformation has proceeded to such an extent that the epithehal character of the lining cells is almost wholly lost, and the ventricle seems, in this area, to be lined by mesenchyme. Study of the membranous area in many stages convinces one that such an hypothesis is untenable; in every case the ventricle must be considered as being lined by epidermal elements, no matter to what extent the process of differentiation has proceeded. There is no real evidence to support the view that the ependymal lining of the ventricle has been replaced by mesenchymal elements to form the area membranacea superior.
In general the area membranacea superior is a rounded oval. Its measurement is quite difficult except when fixation and dehydration have been excellent, because of the highly abnormal distortion of the ventricular roof which frequently occurs in the technically poor specimens. Measurements have been made in a considerable number of favorable specimens, both of human and pig embryos. With the history of this area in mind, it will be realized that the size of the structure necessarily varies with the length of the embryo, attaining its greatest dimensions at about the length of 18 or 20 mm. Herewith is a short table of the measurements taken.
Dimensions of area membranacea superior.
Species.
No. of specimen.
Length of embryo.
Width of area.
Length of area.
Species.
No. of specimen.
Length of embryo
Width of area.
Length of area.
Pig
98 107 144 119 106
mm.
12
13
14*
14
14
mm. 0.37 0.95 1.25 0.45 0.65
7iim. 0.5 0.4 1.1 0.6 0.85
Pig
1 mm.
121 1 16
576 1 17
108 18 (?)
45 18
84 1 22
mm. 0.6 1.5 0.8 0.9 0.8
mm.
0.48
0.9
0.8
0.4
0.7
Rabbit
Human
Pig
Human
Sheep
Pig
Chick
Pig
In a rough way, then, we may consider the area membranacea as an oval; in some cases the longitudinal diameter exceeds the lateral, and in others the reverse holds. The measurements given above were taken from mounted sections and are probably somewhat disturbed by the histological tcchnifjue which was followed.
- MuisurecI on alidc uftpr iiiouDting.
The borders of this oval area membranacea are usually fairly regular and smooth, but in some instances they are irregular, due to the fact that small extensions of the area run into the bordering ependyma. These extensions are more commonly met with at the stage when the area has reached its maximum size, as in figures 38 and 39, photomicrographs from an embryo pig of 19 mm. The higher power of these two photographs shows two areas in the smoother ependymal wall. These are extensions of the area membranacea, and within a section or two directly connect with the differentiated area. Both of these small spots on the circumference resemble technical errors; their ragged appearance, the relative excavation of their surface, and the intact ependymal borders w'ould seem to encourage such a view; but when considered in connection with the character of the whole area membranacea they assume a definite relationship in this regard. Other similar areas, rather rare in occurrence, are found separated entirely from the main area membranacea. These isolated areas are of the same size as those shown in figure 39. In significance and character they are probably identical with the larger area membranacea superior.
Most of the general features of the area membranacea superior have been commented upon in descriptions of the various stages of differentiation in both pig and human embryos. The characteristics most commonly observed concern the differentiated character of the cells of the area, the sharp borders of the typical ependyma, the ragged ventricular surface throughout the whole extent, and the peculiar adhesion of the albuminous coagulum from the embryonic cerebro-spinal fluid to the lining cells. The area membranacea superior should be considered, then, as a transitory focus of differentiation of the typical ependymal hning of the roof of the fourth ventricle.
An Undescribed Area In The Inferior Portion Of The Roof Of The Fourth Ventricle
With success attending the effort to find in the superior portion of the rhombic roof an anatomically differentiated area which would furnish a morphological basis for the jihysiological phenomenon of the extraventricular passage of the cerebrospinal fluid, attention was necessarily directed to the inferior portion of this roof (considering the whole roof structure to be divided by the chorioid plexuses). The spread of the replaced injection fluid (fig. 4) into the periaxial tissues through two points in the roof of the ventricle suggested a study of this stage (pig embryo of 18 mm.) as the basis of the investigation. As a histologically differentiated area in this inferior portion of the roof is easily made out, the complete history of the area will be given chronologically. It has been termed the "area membranacea inferior ventricuU quarti," the terminology being based on the same physiological and anatomical features which led to its adoption in the case of the analogous area in the upper portion of the roof.
The Area Membranacea Inferior In The Pig Embryo
The inferior portion of the fourth ventricle shows no evidence of a differentiation from the typical lining ependyma until the length of 15 mm. is reached. In this development consideration must be given to the factors concerned in the process. It will be recalled that in the younger embryos, both pig and human, up to and including a length of 9 mm. the whole roof of the ventricle is occupied by the epithehal-like cells. With rapid growth of the medulla and corresponding enlargement of the fourth ventricle the roof becomes elongated and widened. This process results in the isolation of the area composed originally of the ejiithelial-like cells and the subsequent formation of the superior membranous area. The epithehal-like cells remain in the superior portion of the enlarged ventricular roof, while the whole inferior half is composed of the densely staining, typical ependyma. The division of the roof by the laterally developing chorioid plexuses becomes evident in pig embryos of 14 mm. At this stage the whole inferior portion shows a ventricular lining composed of the typical ependyma.
The first indication of a differentiation in this inferior half of the roof was found in a pig embryo of 15 mm. This is illustrated in figures 70 and 71. The sagittal section from which these photomicrographs were taken is near the mid-line of the embryo, as is indicated by the partial section of the central canal of the spinal cord (fig. 70). The division of the ventricular roof into two parts is also indicated in figure 70 by the invagination of the chorioid plexus. The squared area in the lower half is reproduced in figure 71 under higher magnification; here the first evidence of an ependjonal differentiation is observed. The dense Une of the typical ependjona appears from both sides, but in the center of this ventricular lining a small area of differentiation is seen. This area, isolated by the abruptly terminating pyknotic ependymal elements, is composed of two or three layers of less deeply staining cells. The nuclei are round, rather larger than those of the adjacent mesenchyme, and contain httle chromatin. The cytoplasm stains fairly well with eosin and is not scanty in amount. The cells resemble those epithelial-hke elements which so largely make up the ventricular roof in the earlier stages. No albumen is found near this point of differentiation, although the whole ventricular cavity is filled with the normal amount. In figure 70 the marked zone of the area membranacea superior may easily be seen.
After this initial indication of a differentiation in pig embryos, the further differentiation of the tissue proceeds but slowly until the length of 18 mm. is attained. Thus, in a similar specimen from an embryo pig of 18 mm. the area of differentiation is not greatly hicreased in size. This is shown in figures 72 and 73. In the higherpower figure (fig. 73) both the superior and inferior membranous areas can be made out by the attachment to these areas of the protein coagulum of the ventricular cerebro-spinal fluid.
In the higher-power figure (fig. 73) of the squared area from figure 72, the area membranacea inferior shows the same character as exhibited by the specimen of 15 mm. (fig. 71). The opening maintains the same approximation to the lateral lip of the medulla, but the area is larger and the histological character more nearly approaches the permanent feature of the tissue. The nuclei in this zone are paler than those of the adjoining ependymal elements and contain less chromatin. The cytoplasm is not scanty, nor is it very abundant in amount. The area is also characterized by the occurrence of the cells in a layer, two or three cells in thickness.
In view of the very slow differentiation of the area membranacea inferior in the growth of the embryo from 15 to 18 mm., the enormous enlargement of the region within the next few millimeters growth is very astonishing. This period, as has been jjointed out, is a critical one in the extension of the embryonic cerebro-spinal fluid from a ventricular to a periaxial relationship. Apjjarently, in the course of the embryo's growth during these next few millimeters the whole inferior roof of the ventricle undergoes a transformation and enlargement, so that the differentiated area membranacea comes to occupy practically the whole inferior half of the roof. This portion of the roof, persisting, enlarging, and suffering no extension of nervous t'ssue upon it, becomes the tela chorioidea inferior.
The rapid differentiation of the whole iiiferior half of the roof of the fourth ventricle is a very interesting process. Apparently the typical ependymal elements, visible on both sides of the membranous area in figure 73, undergo a very rapid alteration, so that in the course of a few millimeters' growth the cubical lining of the ventricle is replaced by a low- type cell, with round or oval nuclei, staining much less densely than do the ependymal elements. The whole area membranacea rapidly becomes a membrane in the true sense of the word; it is a continuous, intact laj'^er of cells, generally only one cell in thickness, closing in the fourth ventricle from the chorioid plexus above and the bulbar lips on the sides.
The general characteristics of this transformation are seen in figures 74 and 75. These photomicrographs are taken from a sagittal section of a pig embryo of 23 mm. On one side of the sharply deUmited membrane shown in figure 75 is a tongue of nervous tissue of the medulla; on the other is the differentiated ependjina of the chorioid plexus; between these two structures stretches uninterruptedly the area membranacea inferior. The flattened cells of the membrane, with their oval nuclei and almost continuous cytoplasm, effectually close the whole ventricle. The photomicrograph also shows an interesting characteristic of this membranous area which is universally present in the larger forms; this is the relatively unsupported character of the membrane. The highly vascular mesenchyme posterior to the area has gradually developed, during growth, larger and larger interstices between the cytoplasmic processes. The phenomenon is not due to shrinkage, but is intimately connected with the formation of the future cisterna cerebello-medullaris. This phase of the mesenchymal differentiation will be more fully considered in an appropriate section of this paper. It will suffice here merely to record the lack of support of the membrane.
Another phenomenon of unportauce in the cerebro-spinal fluid relationships of this stage is shown in figure 75. In the mesenchjanal spaces directly beneath the membranous area there is a large amount of albuminous coagulum. This phenomenon does not occur to any appreciable extent in earlier stages or in other parts of the mesenchyme, except about the nervous system. The c1o.se association of the coagulum from the ventricular cerebro-spinal fluid with the inner border of the area membranacea (shown in figure 75 as a slight roughening of the border) is of very great significance in this connection. In one i)oint in the membranous area (fig. 75) the albumen can be traced almost without interruption from the ventricle into the wide spaces of the mesenchyme (cf. fig. 8). This observation strongly suggests that the embryonic cerebro-spinal fluid, which is rich in protein material, is passing, in this stage of embryonic growth, from the ventricle into the periaxial mesenchyme; and such an interpretation becomes established by the comparative findings in the embryo of the same stage in which a replacement of the cerebro-spinal fluid by the ferrocyanide solution had been effected. These comparable findings are surelj^ of the utmost importance for the final solution of the problems centering about the embryonic cerebro-spinal fluid.
In the later stages of development of the area membranacea inferior in the pig embryo the same structural relationships persist that are shown in figure 75. Figures 76 and 77 are photomicrographs taken from a sagittal section of a specimen of 32 mm. In the enlargement of the squared area, from the first of these figures, the continuity and completeness of the membrane are well established. The photograph shows well the flattened character of the cells comprising the membrane and its sharp differentiation from the nervous tissue and ependyma below and from the ependj'ma and chorioid plexus above. Most important in this case is the distribution of the albuminous coagulum. Within the ventricular cavity this appears in considerable amount, and in several places it is in close adhesion to the lining area membranacea. This albuminous precipitate may likewise be traced in some places apparently through the cellular membrane into the periaxial spaces. For here, as indicated in figure 75, the clotted albumen from the cerebro-spinal fluid apjjarently exists in large amounts in the space just posterior to the membrane — the future cisterna cerebello-medullaris. Delicate strands of mesenchyme are still observed running through the wide space, but in general the whole tissue has returned to the line of the future arachnoid. The relative lack of substantial support of the membrane is well brought out in figure 77. A characteristic feature of this membrane, which Blake'3^ has championed, and which is indicated in figures 76 and 77, is the posterior bulging of the roof — "the caudal process like the finger of a glove."
Another section from the same pig embryo, taken more laterally, is represented in figures 78 and 79. In the photomicrograph of higher power the flattened character of the lining cells, the intactness of the membrane in isolating the ventricular cavity, the unsupported freedom of the membrane, and the relation to the albumen coagulum on both sides are of |)articular interest.
The ultimate fate of the area membranacea inferior will not be more fully entered into until the early history of the similar area in the human embryo has been detailed. For in this connection the occurrence of the foramen of Magendie requires discussion, and it seems best to delay the further consideration of the present topic until the whole question can be reviewed.
THE AREA MEMBRANACEA INFERIOR IN THE HUHMN EMBRYO.
The same process in the formation of an area of differentiation in the inferior portion of the roof of the fourth ventricle may also be followed in the human embryo. Unfortunately, however, human omliryological material can rarely be subjected to the immediate fixation and preservation which j'ield excellent histological results in the more plentiful specimens. It does not seem strange, therefore, that the determination of the exact stage at which an area of differentiation can be made out in the ventricular roof should be practicall}' impossible; for, in poor technical procedures, the roof of the fourth ventricle suffers almost more than does any other portion of the specimen.
In a human embryo of 13 mm. (No. 695 in the collection of the Carnegie Institution of Washington) there is slight evidence of a differentiation in the lower portion of the rhombic roof. The changing character of cells in this specimen is not marked, but as the central portion of this inferior roof is reached the ependymal cells seem to assume gradually a more cubical morphology. Associated with this change in shape, there is also a slight loss of the deeply staining character of their nuclei. The whole differentiation, however, is slight and would be commented upon only from the conception of this area in the pig embryo.
The first definite evidence of differentiation in the inferior portion of the ventricular roof was found (specimen 390 in the Carnegie collection) in a human embryo of 15.5 mm. This initial differentiation occurs, then, in the human embryo of approximately the same length as in the pig. The specimen showed the same change in character of the hning ependj-ma as was found in the pig. The deeply staining ependymal elements are replaced in a limited central area in the inferior portion of the roof by cells with more elongated nuclei, poorer in chromatin, and resembling somewhat the epithehal-like cells which early filled the ventricular roof. These cells tend to compose a layer of more than one cell in thickness — a feature particularly noticeable in the peripheral portions.
The size of the area membranacea inferior observed in specimen 390 suggested that the earUest evidence was probabh' to be observed in somewhat smaller specimens. This could not, with the material at my disposal, be verified, but it is probablj^ safe to assume that the first signs of an ei)end\Tnal differentiation will be found in human embryos of about 15 mm. This time of appearance of the area in the human would coincide with its time of primarj* differentiation in the pig embryo. In this limitation of the first appearance of the area membranacea inferior, the standard has been an unmistakable differentiation of ependyma and not an isolated change of a lining-cell or two which might have been the result of the technical procedure. Such a criterion was necessitated by the verj' marked changes in the ventricular borders observed in specimens in which distortion of the chorioidal roof had occurred.
The area membranacea inferior very rapidly increases in extent after the onset of the process of ependymal differentiation. This was hkewise observed in the pig embryo, although perhaps more stages could be made out. In a human embryo of 16 mm. (No. 406 of the collection of the Carnegie Institution) the area nierabranacea inferior is quite extensive, as is shown in figures 80 and 81. In the photomicrograph under higher power (fig. 81) the denselj' stained ependyma approaches the membranous area (ami) as tongue-hke processes from above and below. These tips gradually lose their dense character and are prolonged as a delicate membrane, lining, in this localized area, the ventricular cavity. The nuclei of the cells here are not heavily laden with chromatin; they are oval and somewhat larger than the more densely packed nuclei of the typical ependj-mal element. Unfortunately, the middle portions of the membranous area in this specimen are surrounded by extravasated red blood-cells obscuring somewhat the structure (fig. 81). The process, though, of the differentiation of these ependymal elements into paler and larger epithelial-like ceUs is quite apparent.
As in the pig, the tendency of the differentiated ependymal cells forming the area membranacea inferior to lose in some degree their distinctive appearance and to approach in character the undifferentiated mesenchymal element is apparent in the human embryo very shortly after the original steps in the process of differentiation have occurred. Photomicrographs from two human embryos of 17 mm. have been included to show this phenomenon. Thus, in figure 88, an enlargement of the blocked area from figure 58, the area membranacea inferior (ami) is well defined. The sagittal section from which this photomicrograph was taken is from embryo No. 576, in the Carnegie collection. Above and below the dense fine of ependj-ma may be made out; this tapers quite abrupt!}', to be succeeded bj' the cells of the area membranacea inferior. These cells, products of ependymal differentiation, have lost much of their epithelial-like appearance; they now show rather small, oval or rounded nuclei, poor in chromatin. The cytoplasm of the cells is small in amount, but not disproportionate for the size of the nucleus. The ventricular border of these cells (fig. 88) exhibits a rather characteristic phenomenon, the adherence of a shght albuminous coaguluni. The fine processes of this coagulum fuse^ith the cytoplasmic borders of the cells and render these borders vague and indefinite. Beneath the cells of this inferior area small vascular channels may be made out. These tend to make the membrane appear denser than its cellular character warrants.
In another section from this same embryo (No. 576) the inferior membranous area is shown in relation to the tufted chorioid plexuses (figs. 82 and 83). In the reproduction under higher magnification (fig. 83) the ependymal lining may be traced caudalwards to a gradual fusion into the area membranacea inferior. From the rather high cubical cells in the immediate proximity to the plexuses the ependymal elements become reduced in size and in height, and then rather abruptly the pyknotic character of the ventricular lining is lost. This loss of the deeply staining character coincides with the superior border of the area membranacea inferior {ami). The membrane of this area shows the same cell-character as already desorilied for this embryo. On the superior side of the plexuses (fig. 83) the lateral border of the area membranacea superior (ams) is shown composed of epithelial-like cells.
The apparent tendency of the cells composing the inferior membranous area to lose the epithelial-like character, as shown in the figures from embryo Xo. 576, is not an invariable phenomenon. Rather is an aggregation of epithelial-like cells met with in human embryos very commonly in this area, not onlj- in embryos of small size, but also in small fetuses. This phenomenon is illustrated in figures 84 and 85, reproductions of photomicrographs from a human embryo of 18 mm. (No. 409 in the collection of the Carnegie Institution). In figure 85 the total transverse extent of the area membranacea inferior (ami) is illustrated, with the villous chorioid plexuses appearing to the left. Although this membranous portion of the embryo has been distorted somewhat by the technical procedures to which the specimen was subjected, the cellular character of the membranous area is well indicated. The most striking feature, apart from the characteristic tinctorial differentiation from the typical ependymal elements, consists in the marked clumping of the cells in certain parts of the membrane. On one lateral extent the membrane is thickened into a bulbous swelling several cells in thickness. These cells have palely staining nuclei, poor in chromatin, with an oval or round form. In other places in the membrane smaller but no less characteristic clumps of similar cells maj' be made out. Between these cellular aggregations the membrane stretches in a continuous line with but few nuclei.
Analogous clumps of cells, with pale, rounded or oval nuclei, may be made out in figures 86 and 87, taken from a human embryo of 19 mm., No. 431 in the collection of the Carnegie Institution. Only a small portion of the membrane is reproduced in the figure under higher magnification, but a characteristic clump of epithelial-like cells (epc) is shown. These cells of the differentiated ependyma here again have oval and rounded nuclei, poor in chromatin, similar to those which have been pointed out manj^ times in the foregoing pages. A second broadened area in the inferior membrane is also showm in figure 87.
The further development of the area membranacea inferior proceeds in the human embryo in a manner very similar to that described for the pig. In the stages but shghtly above those already described the differentiation goes on slowly, but withui a few millimeters the cellular pictures resemble those given for the embryo of 17 mm. (figs. 82, S3, and 88). The cellular clumps which appeared quite frequently in the embryos under 20 mm. have not been found in the larger forms. Thus, in an embryo of 23 mm. (No. 453 in the collection of the Carnegie Institution) the inferior membranous area {ami) appears as an extensive membrane comprising almost wholly the inferior portion of the chorioidal roof. The membrane is here of a single cell in thickness; these cells are rather small, with oval nuclei, simulating in some measure those of the surrounding mesenchj-me. The most mteresting phase of the membranous area at this stage of 23 mm. concerns its completed cellular differentiation and its rather slow increase in size.
Wholly similar pictures of the inferior membranous area of the roof of the fourth ventricle are afforded by a human fetus of 26 mm. (figs. 91 and 92). These photomicrographs were taken from embryo No. 1008 in the collection of the Carnegie Institution. In this specimen (fig. 92) the fourth ventricle seems almost to lack a lining of oj^endymal (epidermal) elements in the area membranacea inferior (ami). The cells of this area are small, inconspicuous ua their distinctions from the underlying mesenchjme. The whole character resembles that of the superior area membranacea shown in figure 57.
The appearances exhibited by the inferior membranous area in the stages above 26 mm. are modified in great part by the development of the great cisterna cerebellomedullaris. As in the pig, the breaking-down of mesenchyme to form this cistern results finally in the almost total isolation of the inferior membranous area. The cistern is fairly rapidly formed when once the process begins, and so in an embryo of 35 mm. (No. 199 in the Carnegie collection) the isolated character of the area membranacea inferior (a?ni) may be easily made out. This is shown in figure 94, an enlargement of the blocked area in figure 93. The general architecture of the membrane, particularly its intact character, appears in this photomicrograph, but its finer structure is obscured by the albuminous coagula which adhere on both surfaces. The cell structure of the area membranacea resembles closely that described in the embryos already pictured.
Discussion of the final disposition of the area membranacea inferior will be undertaken in the following subdivision of this paper, in order that the findings in the pig and in the human embryo may be correlated.
General Consideration of the Area Membranacea Inferior
The ependymal lining of the caudal portion of the roof of the fourth ventricle undergoes a process of differentiation which results in the formation of the area membranacea inferior. This transformation has been observed in pig and human embryo
s; in both, the first definite evidence of the cellular change has been observed in specimens of 15 mm. The essential phases of the process are identical in the two embryos. The tendency of the deeply staining typical ependymal elements is to lose their highly pyknotic character; the nuclei become poorer in chromatin and the cytoplasm somewhat more abundant. In the first stages of the metamorphosis the lining cells come to assume epithelial-like appearances, but in the final change the nuclei become small oval bodies, poor in chromatin, resembling to some degree the nuclei of the adjoining undiff'erentiated mesenchyme. In the human embryo, a tendency for the epitheUal-like characters to persist in isolated cellular aggregations is apparent.
After the initial process of differentiation has begun, the area membranacea inferior increases rapidly in extent and the differentiated cells which characterize it come to occupy the greater portion of the caudal part of the chorioidal roof. In the somewhat later stages the area membranacea is almost wholly unsupported by other tissues, due to the development of the cisterna cerebello-medullaris. As soon as the cistern forms, the area membranacea serves as practically the sole dividing membrane between the ventricular system and the future subarachnoid spaces.
The ultimate fate of this area membranacea inferior is necessarily involved in the distribution of the tela chorioidea inferior. Likewise it necessitates a discussion of the possible formation of the so-called foramen of Magendic and its mode of origin from the "caudal process" of Blake. It is proposed to discuss briefly some of these questions in the hope that some phases of the problem may be brought forth.
It must be clearly understood that the questions of the ultimate fate of this area membranacea inferior probably differ considerably in the different species of mammals. In the horse and in the pig the absence of the medial foramen (]\Iagendie) is fairly well established, but in man its existence .seems to rest on equally firm grounds. While, ))rimarily, this investigation has not been concerned with the possible existence of the foramen of IMagendie, the question has been presented manj' times in regard to the pig and human embryos examined.
As far as can be determined, no descriptive study of the development and differentiation of the inferior portion of the rhombic roof has been published. Heuser's'23' studies on the form of the cerebral ventricles of the pig have afforded a very good conception of the gradually changing relationships in this region. Hess(22j has devoted attention to the histological appearances of the inferior roof in the embryo. One of his interesting obseivations concerns the caudal portion of the rhombic roof in a fetal cat of 10 cm., where he noticed a very sudden interruption in the epithelial lining of the ventricle, with a complete closing by a fibrous net. This description by Hess is the only comment upon the histological appearance of the ventricular roof that has been found. His^^s^ pictures, without comment, in a retouched jihotomicrograj^h, a differentiated area in the proper situation in his fetus C-1 (beginning of the third month) .
The many writers in embryology have commented upon the roof of the fourth ventricle. Minot, in 1892, stated regarding it:
- "Several writers have thought that the membrane was broken through at several points, but it probably is really continuous throughout life. The fourth ventricle is to be regarded, then, as an expansion of the central canal permanently bounded by the original medullary walls."
Kollman (32) on the other hand, advances the view that during the third month the rhombic roof is broken down to form the foramen of Magendie and the two foramina of Luschka. Streeter^S'*), in his chapter on the development of the nervous system in the Keibel-i\Iall Handbook of Embryology, advances a similar view. The majority of investigators to-daj' incline to the beUef that the roof of the fourth ventricle in man is perforated to form the median foramen of ]Magendie.
jjpgg (22) has advanced a conception of the foramen of Magendie that is supported by numerous observations. To test KolUker's statement that the fourth ventricle remained closed during human embryonic life. Hess sectioned the region in human fetuses, new-born infants, and in adults. The lengths of the fetuses cut were as follows: 7, 12.5, 15, 16, and 17 cm. In the 47 cases the roof showed a medial opening (IMagendie), except in one case, in which it was closed by a "thin pial membrane." Hess's conception of the process of formation of this membrane was that in earh' embn'ological life the rhombic roof was bordered by a regular, meshed tissue. Later the small meshes in this tissue fused to form the larger foramen of Magendie.
Blake's (2) hypothesis of the formation of the medial foramen has been quite extensively quoted in the more recent publications on this subject. In a study of the chorioidal roof Blake found a caudal bulging of the inferior velum; this outpouching became more and more extensive in the older embryos.. In man this pouch became sheared off at its neck, leaving the foramen of IMagendie.
In addition to the few studies referred to above, there have been in the past 25 years a great mmiber of articles (notablj' those of Wilder^^s) and Cannieu^^^) offering evidence that this median foramen of the fourth ventricle is an existent, functional opening. Into this literature it is not proposed to go in the present communication; it may be stated that in the larger part the views presented have been in favor of the consideration of the true occurrence of the foramen of IMagendie.
The material on which this study is based has been purely embryological in type, so that no relialile data regarding the foramen of IMagendie could be obtained. But even in the largest fetuses examined, there was no evidence which indicated a breaking-down or a shearing-off of the inferior roof of the fourth ventricle. In the largest human fetus at my disposal, in which the histological material was good enough to permit an accurate examination of the chorioidal roof (embryo No. 448, 52 mm. in the Carnegie collection) the area membranacea inferior appeared as an intact membrane supported only be a few pial cells. In the pig the material at hand has been such that accurate study of the roof could be made in specimens up to 20 cm. ; in all of these later fetal pigs the roof has been wholly without foramina. If, however, in these larger stages the histological procedures have not been of the best, ruptures and other artificial separations are very frequently found.
The area membranacea inferior, then, may be regarded as a region of ependymal differentiation. Whether it persists as an intact membrane or undergoes, in certain animals, a perforation to form a foramen of Magendie can not be here answered; this study has been concerned solely with the embrj^ology of the cerebro-spinal spaces, and it affords no evidence in favor of or against the existence of such a foramen. Nor has any study been made of the two foramina of Luschka, the two openings from the lateral recesses of the fourth ventricle into the subarachnoid spaces. It can Ije stated, however, that these foramina arc not in existence at the time of estal)lishment of the circulation of the ccrebro-si)inal fluid. This phenomenon, as recorded in the previous section, occurs in pig embryos of 26 mm.; at this time the lateral reces.ses are anatomically and physiologically closed.
VI. Passage Of Fluid Through Roof Of The Fourth Ventricle
On pages 20 to 'SO is a description of the passage of a true solution, substituted without increase in pressure for the embryonic cerebro-spinal fluid, through the roof of the fourth ventricle into the extravcntricular or periaxial spaces. This extension of fluid occurred in two localized areas, one in the superior half and the other in the inferior half of the rhombic roof. Histological study of these regions revealed a localized differentiation of the ependyraa, both in the upper and lower halves of the ventricular roof. It becomes necessary, then, to correlate, if possible, the areas of this fluid-passage to the anatomical differentiations pointed out.
The Accumulation of Injection-Masses in the Superior Membranous Area
It has already been recorded that the first evidence of a change in the reaction to a replacement injection occurred in an embryo about 13 mm. long (fig. 2). This stage was characterized by a dense collection of the precipitated granules in a definite area in the roof of the fourth ventricle. At this stage also the area membranacea superior is well differentiated (fig. 31). That the site of the granular accumulation is this membranous area is easily proved by an inspection of figure 117, which represents an enlargement of the squared area in figure 116. In the low power photomicrograph the prussian-blue granules are not represented, but are found scattered through the ventricles, with a definite collection in the posterior region of the fourth ventricle. Under a higher magnification (fig. 117) the blue can be traced in but small quantity along the normal ependj'mal lining (shown to the left in the figure), but as soon as the differentiated area (area membranacea superior) is reached the granular material is heaped up in a dense mass, which extends as a thickened pad into the ventricle.
The same phenomenon of the accumulation of the injection fluid in the superior membranous area is shown in figures 112 and 113, the second photomicrograph representing the area outlined in the first, but reproduced under much higher magnification. In this specimen (an embryo pig) a dilute solution of silver nitrate was injected into the central canal of the spinal cord. On histological examination the accumulation of the silver also shown in figure 11 was found. Thus, in figure 113 the ventricular epithelium can be made out in the upper right-hand corner, while below (in the area membranacea superior) the silver is densely accumulated.
The explanation of this phenomenon of accumulation in the superior membranous area is not whoUj' clear. It occurs only in stages in which the histological differentiation of the ventricular roof has proceeded to some degree and in stages where the fluid-passage into the periaxial tissues is not wholly unobstructed. This aggregation of the precipitated granules of prussian-blue and of the reduced silver in a locaUzed area certainly suggests a phj'sical exi^lanation, as in these cases the physical laws of precipitation and reduction must hold. The many figures of the superior membranous area of the ventricular roof show that in the stage under consideration the cell-outlines projecting into the ventricles are rough and ragged as contrasted with the smoother and more regular surface of the adjoining ependyma.
Could not these roughened, irregular cell-surfaces become the site of the first and most extreme precipitation of the prussian-bluc and of the reduction of the silver? Certainly they would serve much more efficiently as the foreign substances about which precipitation would occur in greatest amount. This phj^sical explanation finds man}' arguments for its suj^ijort in these studies.
Another explanation of the phenomenon concerns the normal flow of the fluid and the relation of the direction of this flow to the roof of the fourth ventricle. As has already been emphasized, it is difficult to assume that there is any marked production of cerebro-spinal fluid before the periaxial spread occurs. Such an assumption would argue against the development of any special current toward the roof of the fourth ventricle in any stage smaller than that represented in figure 3, and would vitiate the explanation of the occurrence of the granular accumulation shown in figure 2 (a pig embryo of 13 mm.). In the later stages (16 mm., cj. fig. 11) this explanation would probably suffice for the phenomenon exhibited.
The Sites of Fluid Passage Through the Roof of the Fourth Ventricle
With consideration of the evidence presented as to the accumulation of the precipitates of the injected fluid about the area membranacea superior during certain stages in the development of the cerebro-spinal spaces, it would seem that the same area must be concerned in the passage of fluid from the ventricular cavities into the periaxial tissues. This view receives support from the reproduction of a cleared specimen (fig. 11) in which an injection of silver nitrate had been made into the central canal of the spinal cord. The pressure employed was great enough to force the fluid into the periaxial spaces, but the resultant picture clearly showed the oval outline of the area membranacea superior.
The study of the passage of fluid from the ventricular to the extraventricular spaces can best be made by simple histological serial sections. In these observations pig embryos in which the cerebro-spinal fluid had been replaced by the compensating device, supplying a true solution of potassium ferrocyanide and iron-ammonium citrate, were sectioned and examined with reference to the sites of fluid passage. The results of these studies are given here in order that the whole question of the connection of the cerebral ventricles with the subarachnoid spaces may be discussed.
In the stage represented by figure 3 (in which fluid passes from one area in the roof of the fourth ventricle into the extraventricular tissues) histological sections show that the point of fluid ])assage is localized and concerns solely the area membranacea superior. The replaced fluid (as demonstrated by the subsequent precipitation of the prussian-blue) passes through this entire membranous area into the adjoining mesenchyme. The process is wholly confined to this area; the adjoining ependyma is entirely imj)ervious to the ferrocyanide. This phenomenon of passage of the replaced fluid through the sujjerior membranous urea is well shown in figures 14, 18, and 23.
The distriltution of the minute granules of ])russian-l)lue in the cells of the superior membranous area is of iini)ortance in any discussion of the passage of fluid through a membrane; for this area (in the superior portion of the roof of the embryonic fourth ventricle) must be considered as a memljrane permeable in certain degrees to the fluids bathing it. That the area mcml:)ranacea is intact and does not contain stomata or other minute foramina has been demonstrated histologically. Further evidence of the entire lack of intercellular stomata is afforded by the distribution of the prussian-blue granules precipitated in situ after the replacement of the cerebro-spinal fluid by the ferrocyanide solution.
Figure 14 is a reproduction of the superior area from a transverse section of a pig embrj^o in which the routine replacement had been made. The position of the area is shown by the squared outline in figure 13. On both sides the impermeable ependyma is seen, with granules of the blue adhering to the ventricular border of the cells, but not penetrating them at all. To the left of the drawing the few ependymal cells possess, beneath their central border, a chain of the granules which have entered from the abrupt edge of the area membranacea. In the cellular border between the two hps of the ependyma, the area membranacea superior, the passage of the replaced fluid is easily made out by the resultant blue granules. The area is roughly deUmited by a ventricular collection of the blue granules. Examination of these cells shows that the prussian-blue is present within the cytoplasm, avoiding the nuclei with perfect precision. Some of the cells are rounded and almost free from the granules; others, particularly those whose cj'^toplasm is elongated, are completely filled with the granules, the nuclei standing out in a blue granular cytoplasm.
The question of the passage of the fluid between the cells must also be answered by the histological evidence. In the same drawing (fig. 14) in one or two places there are indications of a slight stream of granules between the cells of the area membranacea superior. This apparent transit of the fluid through intercellular passages is particularly clear in the small areas where the cellular cytoplasm is relatively free from the granular deposits. But upon careful examination of these areas under oil immersion it is always apparent that the adjoining cytoplasm is also mvolved in the granular precipitation, indicating that the cells, although almost free from the deposit, are also engaged in the process of the fluid passage. Compared to the whole area of fluid transit, the points indicative of a passage through possible intercellular stigmata are almost negUgible. It seems not unlikely that the outlining of canals between cells may be a physical phenomenon, as in most cases no cellular borders (as demonstrated bj' the precipitated granules) can be made out. These pecuUarities of fluid passage may be seen in figures 14, 18, and 23.
Consideration of all the evidence afforded by histological examinations of the essential character of the area membranacea superior and of the passage of fluid through it incUnes one inevitably to the belief that this area functionates as a cellular membrane. The fluid passes through it as through any permeable liv-ing membrane. Histologically the passage is for the most part through the cj-toplasm of the cells, but occasionally an intercellular course is suggested. Both processes are wholly compatible with the accepted view of a cellular membrane de\dsed for the passage of fluid through it.
The same phenomenon of the passage of fluid from the fourth ventricle into the periaxial spaces is beautifullj' illustrated in figure 23. This drawing is from a transverse section of a pig embryo (23 mm. in length) in a stage when the superior membranous area is rapidly being encroached upon by the developing cerebellum and by the caudal chorioid plexuses. Between the deeplj' staining epondj-mal cells on cither side the membranous area is densely outlined by the deposition of the granules of prussian-blue in the cytoplasm of the cells of the area membranacea superior. The avoidance of the nuclei of these cells by the ferrocyanide is well demonstrated in this reproduction, as is also the impenetrability of the ependymal cells. In a specimen of this nature the question of the passage of the injection fluid through possible intercellular foramina loses its significance; for the drawing shows clearly the importance of considering the entire area membranacea as a functioning whole — a permeable, Uvmg, cellular membrane.
It has been shown in a foregoing section of this memoir that histologically the area membranacea superior decreases to an almost neghgible remains in specimens of embryo pigs over 30 mm. long. This same rule apparently holds for its functional importance, as determined by the relative and absolute amount of prussian-blue granules deposited in the cells of the superior membrane. This decrease in the functional imi;)ortance may be inferred from figure 47, a photomicrograph from a pig embryo of 32 mm. Apparently the size of the membrane determines in large measure the amount of the replaced fluid which passes through it.
Thus far we have been concerned solely with the passage of fluid through the area membranacea superior. In the earUer stages of from 14 to 23 mm. the importance of the superior membrane functionally is great, but in the later stages the inferior membrane assumes far greater significance. This is demonstrated not only by the structural history of the two areas, but by the functional index afforded by the replacement of the cerebro-spinal fluid by a foreign solution.
In the foregoing section the first evidence of any histological differentiation in the inferior portion of the roof of the fourth ventricle was shown to occur in pig embryos of 15 mm. in length. From this stage upwards (figs. 4, 5, etc.) a portion of the inferior roof allows fluid to pass through it. The exact point of fluid iiassage is the localized ependymal differentiation forming the area membranacea inferior. This relationship is easily verified by reference to figure 18. In this drawing of a median sagittal section of a pig embryo the two localized pomts of fluid passage into the periaxial tissue are readily identified; they are quite Umited in comparison to the extent of the periaxial spread.
Figure IG represents the inferior membranous area of the roof of the fourth ventricle from a i)ig embryo of .similar size (18 mm.). The histological character of the inferior area is well shown in this drawing. It will be seen that, except in small areas, the histological differentiation of the ependyma has not proceeded to any great extent; the fluid from the ventricular cavity (as traced by the precipitated granules) closely follows the points of greatest cellular differentiation. There is no possibility of an intfri)r('tation of the findings concerned with the existence of intercellular stomata; the passage of fluid is here again to be looked upon as a transit through a cellular membrane.
The same general i)heiioinena of the pa.ssagc of fluid through a localized area (the area membranacea inferior, hi the caudal portion of the roof of the fourth ventricle) that have been observed in the superior portion of the roof are shown in figure 18. Cliief among these phenomena is the careful avoidance bj- the precipitated granules of the ependymal lining of the ventricles and the adherence of the granules to the Uning walls at the points of fluid passage. The ependymal lining, except in the two areas of differentiation, is everywhere impenetrable to the solution of the ferrocyanide.
As the size of the embryo increases the functional importance of this more caudal area becomes much greater (c/. figs. 3, 4, 5, and 76). The whole caudal half of the fourth ventricle becomes an area of ependj-mal differentiation and of fluid passage. It serves everywhere as a complete diff'using membrane, un))roken by the occurrence of stomata. Through this whole membrane the replaced solutions of potassium ferrocyanide and iron-ammonium citrate pass with apparent ease, as demonstrated by the precipitated granules of prussian-blue (fig. 18). From stages of 24 mm. and over, the lower membranous area is the onlj' one of significance in the total fluid passage.
The areas, therefore, through which the replaced solution of potassium ferrocyanide and iron-ammonium citrate jiassed, in the experimental pig embryos, are the two areas of histological differentiation in the roof of the fourth ventricle — the areae membranacese superior et inferior. There is no evidence whatsoever of any other point of escape of the fluid from the ventricular system into the periaxial spaces. The precipitated prussian-blue does not penetrate any of the lining cells of the ventricle except in the two areas under consideration. Nor is any evidence afforded by histological study of the escape of ventricular fluid through the described foramina of Bichat and of ^Mierzejewsky.
Factors Concerned in the Experimental Fluid Passage
It becomes necessary to discuss the question of the passage of the replaced fluid through the two cellular membranes in order to ascertain to what extent the results obtamed by the method may be relied upon. Naturally in such questions the factors concerned in the normal transit of body-fluid through such structures must be considered.
Probably the most essential element in obtaining rehable results in any injection is the control of the pressure at which the foreign fluid or mass is introduced. This matter has been fully discussed in the resume of the methods employed; it is suflRcient to reaffirm here that, in these observations, the normal cerebro-spinal tension has not been disturbed because of the use of a compensatory replacement. Other experiments, carried out under increasing pressures of injection, have been made, in order to compare the results with those furnished by the replacementmethod.
Consideration must next be given to the factors of diffusion, filtration, and osmosis in the passage of fluid through the roof of the fourth ventricle. The third factor, however, may be largely excluded, owing to the fact that the solutions of potassium ferrocyanide and iron-ammonium citrate employed were for the most part practically isotonic with the body-fluids. Furthermore, the use of hypertonic solutions apparently gave no difi"crent results (except in the increased density of the resultant precipitate) from those obtained by the employment of the isotonic solutions. Finally, it was found to be of service to use hypotonic replacement solutions in order to obtain very sUght precipitates; in these experiments also the spread of the replaced ferrocyanide solution was similar to the standard result afforded by the isotonic solution. These observations with varying concentrations of the foreign solutions replacing the cerebro-spinal fluid serve to indicate that osmosis plays but little part in the passage of fluid through the roof structures of the fourth ventricle. Undoubtedly the factor of osmosis can not be ignored in any consideration of the passage of fluid through a cellular membrane, but it seems unhkely that with solutions of practically the same salt-content it should be of great importance.
The influence of diffusion in this passage of the solution of the ferrocyanide and citrate from the cerebral ventricles into the extraventricular space is probably great. The whole plan of the experiment concerns the introduction of salts foreign to the body-fluids, even though in analogous concentrations. It seems not unhkely that as soon as the replacement of the existent cerebro-spinal fluid is effected the ferrocyanide and citrate must immediately begin to diffuse out into the periaxial tissues and the normal salts return to the ventricles. Probably, however, this same phenomenon plays a normal role in the human body. Jacobson's^^?) extensive and important studies on the chemistry of cerebro-spinal fluid have shown that the ventricular cerebro-spinal fluid is not identical with the subarachnoid fluid. The differences in the two fluids are probably to be accounted for by the fact that the ventricular fluid represents the pure elaboration of the chorioid plexuses, whereas the lumbar subarachnoid fluid is composed not only of the products of the chorioid plexuses but also of the fluids from the perivascular system. In this transference of the ventricular fluid to the subarachnoid space diffusion may play some part, the relative importance of which can hardly be estimated.
But will diffusion alone accoimt for the passage of the experimental fluid in the ventricle through two well-defined areas into the periaxial tissues? Will diffusion account for the varying extent of the injection in different stages of embryonic develojjment? There are several arguments against according diffusion a maximal imjjortance in the process. In the first i)lace, an injection of the solution of the ferrocyanide under mild syringe-pressure will give a spread similar in every re.spect to thos(; obtained by the replacement method. This indicates that the course taken by the two solutions is not necessarily the result of diffusion, but rather of the capabilities of the tissues for fluid-spread; and similarly the jiassage of this true solution through the roof areas need not be solely a diffusion process, but may be accounted for by the true flow of the fluid in this direction. Again, in the stages represented in figure 2 one would expect as extensive a spread of the replacing solution into the periaxial tissue were diffusion the active force in the movement of the fluid. Instead of such a periaxial spread the injection fluid remains wholly within the ventricular system, indicating that other forces than that of diffusion play an active role, in the more advanced stages, in the movement of the fluid. Finally, if diffusion is to be considered the sole agent in the distribution of the replacing fluid, why does not the ferrocyanide penetrate all the cellular structures lining the ventricular cavity? Surely it would he expected that diffusion between the body-fluids and the ferrocyanide solution would occur in each ependymal cell — a phenomenon observed only in the cells comprising the ventricular surfaces of the membranous areas of the rhombic roof.
While acknowledging that diffusion and osmosis may play important parts in the process of the passage of fluid from the fourth ventricle into the periaxial tissues, it seems apparent that some other factor or factors must be the determining agent or agents. It is not unlikely that the formation of cerebro-spinal fluid by the cells of the chorioid j)lexus may cause, in the replacement experiments, further passage of fluid into the extraventricular regions. Such an elaboration of fluid, with the ventricles filled with the experimental solution, would result in an increase in the normal ventricular tension. If this be the real explanation, the passage of the fluid into the extraventricular spaces would result in part from the increase in pressure on one (the ventricular) side of the membrane. The process, then, would be one of filtration through the membrane from the point of higher to that of lower pressure. This explanation best seems to cover the results obtained by the replacement method, and is supported by the histological examination of the developing chorioid plexuses and by many other features which are dealt with in other sections of the paper. This view is also strongly supported by the results of injections under mild syringe-pressure .
On the basis that the passage of fluid from the fourth ventricle into the periaxial tissues is in large measure a process of membrane filtration, the phenomenon of the fluid transit of the replaced solutions may be taken as a real index of the circulation and distribution of the cerebro-spinal fluid. It may be assumed, therefore, that the resulting distribution of the prussian-blue granules represents the course and extent of the fluid channels of the embryonic cerebro-spinal fluid.
The discussion of the fluid passage outward from the cerebral ventricles into the subarachnoid spaces has thus far been concerned with the processes involved for the transit of the true solutions of the salts. There is, however, an undoubted passage outward, as has already been indicated in a foregoing section, of the protein content of the normal cerebro-spinal fluid. This occurs in specimens in which a trul}- definitive membrane, intact throughout, can be seen inclosing the chorioidal roof. The explanations which suffice for the passage outward of the true solutions will not serve for this phenomenon.
The cells of the body probably are equipped to handle colloidal solutions in several ways, but two methods seem possible as explanatory of the problem at hand.
In the first place, it is conceivable that the cells in the differentiated areae membranaceae could phagocyte the colloidal albuminous particles of the ventricular fluid and excrete them into the subarachnoid spaces on the other side of the membrane; but it does not seem probable that this explanation is correct. Much more likely is it that the colloidal masses may follow the same laws of fluid-passage as the true solutions. But in such a passage through a cellular membrane the rate of passage will be much slower with the colloid.
These two theories regarding the passage of the albumen of the ventricular cerebro-spinal fluid into the subarachnoid spaces are not based on any findings presented in this article, but are ventured as being in keeping with current physiological explanations of such phenomena. On the basis of the second hypothesis, the failure of granular material to pass through the cellular membrane of the chorioidal roof must be explained as being due to the inability of the cells to handle the foreign material except in sizes which could be absorbed. The fact that the original unit was not phagocyted or passed through the membrane probably depended on the size of the molecule and the specific character of the lining-cells.
THE PASSAGE OF SILVER NITRATE AND INDIA INK THROUGH THE MEMBRANOUS AREAS IN THE ROOF OF THE FOURTH VENTRICLE.
Thus far in the discussion of the passage of the experimental fluids through the ventricular roof, true solutions of potassium ferrocyanide and iron-ammonium citrate only have been considered. This solution, as has been pointed out in this and in a previous article^^^)^ jg non-to.xic and is not taken up by the cells. With the dilute solutions (0.25 to 0.5 per cent) of silver nitrate, a far different problem is presented. Replacement experiments with this salt are rendered impossible bj- its intraspinous toxicity and by its precipitating action upon protein; but beautiful preparations may be made by this method by the simple injection with a syringe into the central canal of the spinal cord.
With mild syringe-pressure the result of such an injection with silver nitrate is in all cases a simple ventricular spread, with no extension into the ])eriaxial tissues. This general rule holds in all stages in which the central canal can be definitely entered without causing a spread into the j^erispinal tissues. This failure of the spread to extend into the periaxial tissues under mild pressure is undoubtedly due to the coagulating effect of the silver, which renders further passage of the fluid impo.ssible. The reduced silver collects about the superior membranous area in the roof of the fourth ventricle, outlining it distinctly. This phenomenon is illustrated in figure 115 (a transverse section of a pig emljryo of 19 mm.). At this stage the rejjlacement of cerebro-spinal fluid by a ferrocyanide solution results in a quite extensive spread {cf. fig. 5).
With increa.sed pressures of injection the silver may be pushed into the periaxial tissue through the roof structures of the fourth ventricle. The transit of the injection-mjuss occurs in the area meml^ranacea sujjcrior in practically all cases (c/. fig. 12). The inferior membranous ar(>a, in the earlier stages, is almost invariably impermeable to the silver (unless the injection-jiressure i.s extreme). When the superior area is examined after such an injection under high pressure the silver is found deposited throughout the cells of the area, extending only a short distance into the adjacent tissue. This feature of the injection is pictured in figure 113. In these injections the high pressure undoubtedlj' suffices to force the silver through the coagulated area membranacea. Its coagulating effect on the ependjina is almost equally marked, but the point of least resistance is apparently in the membranous area, allowing the fluid to pass through it.
Replacements of the cerebro-spinal fluid with diluted solutions of mdia ink within the medullary-canal system of small pig embryos never result in any extension of the granules into the periaxial tissues, for under the normal tension in the ventricles of the pig the arese membranaceaj are impermeable to the passage of granular material. After such a replacement the carbon masses maj' be found everywhere throughout the ventricles, but not in the periaxial tissues. However, india ink may be forced into the periaxial tissues by the use of high pressures of injection, as shown in figure 10. In this s])ecimen of a pig embryo (21 mm. m length) the periaxial spread occurred solely from the superior membranous area. This is analogous to the results obtained with silver nitrate, shown in figure 12. Without doubt in the earlier stages the superior area is much more permeable than the inferior. Histological examination of these specimens after an injection of india ink under high pressure reveals that the carbon granules gain the extraventricular space onh' through the area membranacea superior; some cells in this area are crowded with the granules, but for the most part extensive intercellular stomata have been made. The whole process must be viewed as a result of the excessive pressure of injection.
In the more advanced stages of the pig embryo (30 mm. and upwards) the pressure necessary to occasion an extraventricular spread of the india ink after intraspinous injection decreases somewhat, so that \\ith mild syringe-pressure a local periaxial sjn-ead from the fourth ventricle may be obtained from an injection into the central canal of the spinal cord. This is in accordance with the observation of MalU'^), who found that the injection flowed "through the medial opening of the fourth ventricle." The opening in these cases is in the area membranacea inferior, and in many instances subsequent examination showed rupture of the membrane with escape of the ink, even though the injection-pressure was moderate.
Taken as a whole, then, the findings are against the passage of solutions of silver nitrate or suspensions of india ink from the ventricles into the periaxial tissues, except when injected under pressures far above the normal intraventricular tension.
RELATION OF THE EPENDYMAL DIFFERENTIATION TO THE PASSAGE OF FLUID.
Under this heading it is jjroposed to discuss the relationship, if any, existing between the stages of differentiation of the ependjona of the roof of the fourth ventricle and the prssage of fluid through the two membranous areas. The discussion must necessarily be of a temporal character, with an attempt to consider possible factors in the process.
The most important question in this connection is whether the ependymal differentiation is necessary for the passage of fluid through it. In tlie \ng embryo of 13 mm. the area membranacea superior has reached a stage of marked differentiation (fig. 31), but at this same stage (fig. 2) there is no evidence of any pas.sage of fluid through the roof of the fourth ventricle into the periaxial tissue, only an outlining of the oval membranous area. Here, then, the histological differentiation has definitely preceded the assumption of function on the part of the area membranacea superior. The passage of fluid through the lower area occurs at a relatively earlier stage than it does through the superior opening. The first evidence of differentiation of the inferior roof of the fourth ventricle was observed in pig embryos of 15 mm. in length. At 18 mm., even though the process of differentiation was far from complete, some of the replaced fluid was able to pass through the lower area (figs. 4, 16, and 18).
A consideration of these observations leads to the assumption that some histological differentiation of the ependj^ma is necessary for the extraventricular passage of the replaced fluid. In the case of the superior area the differentiation occurs at a considerable developmental interval before fluid passes through it; in regard to the inferior area the assumption of function occurs at a somewhat earlier period in its differentiation. This slight difference between the two areas may possiblj' be explained on the basis that as soon as the stage of 14 mm. is attained (by the pig embryo) a greater amount of cerebro-spinal fluid is produced than can be cared for by the more slowly enlarging ventricular cavities. As soon as this disproportion occurs the excess of fluid is poured into the periaxial tissues through the already differentiated area membranacea superior; therefore, when the inferior area first shows evidence of formation there is still this excess of fluid in the ventricles. The fluid apparently avails itself almost at once of the new opening and its functional existence becomes immediate. It is apparent, moreover, that the capacity of the membranous areas for the passage of fluid is considerably in excess of the demands made upon them, and furthermore, that the provision for the passage of increasing amounts of fluid is completed before the demand arises.
In the passage of fluid from the ventricles into the mesenchyme, there is one other factor which has not as yet been considered. This concerns the iKitentiality of the adjacent mesenchyme to afford channels for the fluid poured into it. Were resistance offered to the flow of solutions through the mesenchymal tissue spaces, fluid could escape from the ventricles in only very small amounts, if at all ; as soon, however, as easily traversed fluid channels became established, the cerebro-spinal fluid could readily escape through the two membranous areas. The question as to what part the embr\'onic cerebro-spinal fluid i)lays in the further development of the meningeal si)aees also arises in this connection. It is at i)resent impossible to assign to any one of these factors a specific role in the passage of fluid from the fourth ventricle into the jieriaxial spaces, but it is important to consider them as possible determining agents. The evidence all indicates that the rate of production of the embryonic cerebro-spinal fluid is the most important factor, by far, in the extraventricular escape of the fluid.
VII. General Histological Differentiation Of The Cerebro-Spinal Spaces
The general problems concerned in the formation of the meninges and of the spaces inclosed within them deal with the gradual adaptation of a primitive undifferentiated mesenchyme to the anatomical and physiological requirements of the adult. Originally the meninges were held to be derived from the same epidermal infolding which gave origin to the central nervous system; then, with increasing knowledge of the structure, the dura alone was said to be a product of the middle germ-laj'er; and finally, by the researches of His^^s) and of K6lliker,'3i) the mesenchymal origin of the three meninges was established. The general process of the differentiation and the stages in this transformation have not been reported in great detail; here, too, the investigations must have an outlook for physiological anatomy as well as for pure morphology.
It may be well to comment briefly on the relationships of the three meninges found in adult mammals. The dura is well estabUshed as the fibrous-tissue envelope of the leptomeninges and the central nervous system. But there is a tendency to regard the arachnoid and pia mater as constituting one structure — the leptomeninges or "pia-arachnoid," in the terminology of Middlemass and Robertson'^"). This difference of opinion in regard to the two inner meninges is due to their structural and intimate relationships. The arachnoid may well be a.ssumed to be a single membrane, worthy of being regarded as a single structure if one considers only its outer continuous membrane as the essential structure. But the inner surface of this membrane sends processes inward to fuse with the pia mater, which is so closely applied to the ner\-ous tissue. These processes divide the subarachnoid space (mcluded between arachnoid and pia) into the well-known meshes in which the cerebro-spinal fluid circulates. From the standpoint of these channels (the subarachnoid spaces) the arachnoid constitutes the parietal and the pia the visceral layer. Thus the intimate structural unitj' of the two membranes seems, in the opinion of many investigators, to warrant their designation as a single membrane. This view, however, has been strongly opposed by Poirier and Charpy^*^', who considered the distinction of three meninges very essential. Hence, in considering the transformation of tissues in the embryo, regard must be had for the dura as a well-differentiated structure, and for the leptomeninges as units, but certainly to be regarded from the standpoint of the subarachnoid spaces. In tliis connection Sterzi's'^^') observations on the comparative anatomy of the meninges are of interest. It will be recalled that the dura in lower forms becomes well estabhshed before the leptomeninges emerge from a primitive mesenchyme.
THE PERIAXIAL MESENCHYME.
Surrounding the central nervous system in young embryos is a rather thick cushion of undifferentiated mesenchyme, similar m all respects to the undifferentiated tissue in other parts of the embiyo. But verj' soon in the course of development the nuclei in this mesenchyme increase along the clear marginal zone of the spinal cord ami ha.silar structures, forming the initial indication of the pia mater. This phenomenon is indicated somewhat in figure 40, a photomicrograph taken from a human embryo (Xo. 836) of 4 mm., the earliest stage here illustrated.
The next essential change in the great differentiation of the meninges concerns a blastemal condensation of this same mesenchymal tissue to form ultimately the bony covering of the central nervous sj'stem and a portion of the dura; but between these two zones of differentiation the mesenchyme remains for a time almost unaltered. A portion of this tissue will go to form the arachnoid membrane and the trabeculaD which mark off the subarachnoid spaces. This process in the formation of the arachnoid will be discussed here; the formation of the pia mater and dura will be detailed in succeeding divisions of the paper. The differentiation will be discussed as a general process, in regard to both human and pig embryos, for in no respect has any essential difference between the two been observed.
The general character of the periaxial mesenchyme may be commented upon here. The tissue is of a verj' loose and typical structure, forming a syncytial network of rather small mesh, but fragile. The nuclei of the cells are oval, with a definite chromatin content; the cytoj^lasm is largely devoted to the maintenance of long processes which connect with adjacent cells. Adhering to the cj^toplasmic processes are very tiny albuminous coagula, of such small amount as to be hardly noticeable; also in the meshes of the mesenclwme very small quantities of this albumen may be identified. These albuminous coagula undoubtedly represent the protein of the tissue fluids in the undifferentiated stages.
THE FORMATION OF THE ARACHNOIDEA.
A general consideration of the problems here involved will surely shed light on some of the various factors concerned. It must be noted that in its development this membrane proceeds from an undifferentiated but small-meshed mesenchyme into the adult structure which contains the relatively large cerebro-spinal channels. Then, too, the enlargement of the tissue meshes in certain places — as the future cistcrnae — must be enormous. Besides this necessary dilatation of the spaces in the periaxial m(>senchymc, the outer portion of the tissue must separate from the future dura and form the outer surface of the arachnoid membrane. Here the process must be one of tissue condensation and proliferation. A similar agencj' is involved in the growth of the mesothelial cells which cover the outer surface of the arachnoid and also the inner suliarachnoid spaces.
The g(>n<'ral process, then, in the formation of the arachnoid membrane concerns a thiiuiing and readjustment of the primitive mesenchyme in certain areas, while in others the process is reversed, the membrane reaching the adult form through proliferative and condi-nsing i)henonieiia. Such alterative processes must naturally result from the apj)lication of certain mechanical or vital agents in the growth of th(? embryo. Is the mere growth of the central nervous system sufficient to furnish these alterative agents, or must we likewise trace the corres])onding development of the bony coverings of the brain and spinal cord? X{>ith(>r factor seems relatively of great importance when comi)ared to the possible influence of the presence and circulation of cerchro-spinal fluid on this periaxial tissue. This seems to be the most important factor, an internally-modifying influence to which the periaxial mesenchyme is subjected in the formation of an arachnoid and its subarachnoid spaces. It will therefore be from this standpoint that the development of the spaces will be discussed; for, as has already been pointed out, the periaxial mesenchyme becomes a functionally active tissue for the circulation of the cerebrospinal fluid at a stage when difi"erentiation has not begim. On this basis, the lack of differentiation shown in the |)eriaxial mesenchyme in the stages before the ventricular cerebro-spinal fluid is poured into the mesenchyme in the neighborhood of the roof of the fourth ventricle is not surprising. The character of the periaxial mesenchyme in the early stages is reproduced in numerous photomicrographs (figs. 25, 49, 51 , and 53). The mesenchj'me is here characterized by a rather dense meshwork of cytoplasmic processes, interspersed b^' a considerable number of oval nuclei. The content of the interstices in albumen, as judged by the persisting coagula, is very small. This picture of the periaxial mesenchyme persists untU cerebro-spinal fluid is poured from the ventricle through the area membranacea superior.
As will be seen in figure 3, the first indication of an extraventricular spread of the replaced fluid in the ventricles occurred in a pig embryo of 14 mm. At this stage the membranous area in the superior portion of the roof of the fourth ventricle has already' become well differentiated. The fluid from the ventricles, however, does not reach any considerable spread until after a length of 18 mm. is attained; the periaxial spread during this period of growth is wholh^ confined to the peribulbar tissues. It is quite important in this connection that the first obvious differentiation of the mesenchyme for the formation of the arachnoid .should appear during this period and should involve the peribulbar tissues.
The first change to be noted in the transformation of primitive mesenchjone into the future arachnoid is an obvious thinning of the structure with a decrease in the number of nuclei per unit-volume. This is made out in a photomicrograph (fig. 57) of a section from a human embryo 14 mm.* long, when contrasted with a similar mesenchymal area posterior to the ventricular roof (fig. 53). In the pig embryo this thinning of the mesenchyme is as obvious at this early stage.
The process of dilatation of the mesenchymal spaces at this stage hardly seems to concern a direct disruption of the syncj'tial strands, but resembles more the spreading of the cell-bodies by the introduction of more fluid into the tissue spaces. This process would certainly result in an ap])earance similar in everj' way to that represented by figures 35 and 57. It probably also concerns other factors, as, possibly, the growth of the whole embryo without a corresponding degree of mesenchymal proliferation.
In a human embryo of 17 mm. (Xo. 576) evidences are apparent of such a thinning of the mesenchyme about the medulla. Thus, in figures 58 and 59, from this specimen, the cellular decrease can be made out both in the region of the roof of the fourth ventricle and around the basilar surface of the medulla. It wdll be noted that the differentiation (i. e., the thinning) about the roof has proceeded more rapidly than along the anterior bulbar surface. This is perhaps to be expected in view of the initial pouring-out of the cerebro-spinal fluid into the mesenchyme just posterior to the roof.
- This embryo measured 14 mm. on the slide.
In this mesenchymal differentiation a slightly increased amount of albuminous coagulum may be noticed. The truth of this is made obvious by an examination of figure 61, a photomicrograph from a human embryo of 17 mm. The almost entire freedom of the mesenchyme from albuminous detritus is most noticeable at earlier stages.
As was pointed out in the description of the results of replacing the cerebrospinal fluid, a marked change in the rate of development of the cerebro-spinal spaces in the pig-embryo ensues just after attaining the length of 18 mm. Within the growth of 2 mm. the injection spreads completely down the spinal cord and about the basilar structures of the cerebral cavity. This rapid extension finds its analogous process in the equally rapid changes which may be traced in the periaxial mesenclnTTie. Thus, in figure 72, a photomicrograph from a sagittal section of a pig embryo of 18 mm., the whole nervous tissue appears surrounded by a very thin, Ughtly staining tissue; this is the periaxial mesenchyme, which is undergoing its rapid metamorphosis. It will be noticed in this figure that the posterior structures (rhombencephalon) are surrounded by a much less dense mesenchyme than are the anterior (mesencephalon). This relative differentiation between the bulbar tissue and that around the mid-brain is only of temporal character; the mesenchyme about the medulla, as has already been pointed out, begins to differentiate first, the differentiation of the mesenchyme about the other nervous structures following somewhat later.
Figure 73 is a photomicrograph of higher power, taken from the squared area in figure 72. It shows to what a surprising degree the mesenchymal differentiation has proceeded during a few millimeters' growth. Two striking features of the process are brought out in this reproduction. In the first place, many of the mesenchymal trabecular have apparently been broken down, sacrificed to a few larger remaining strands. The cells connected with the destroyed trabeculie appear to recede until one of the heavier surviving strands is met with, when they adhere and apparently aid in the future development of a permanent arachnoid trabecula. The second feature of importance in figure 73 concerns the large amount of allnimen seen in the periaxial space. There is here a much greater amount of albumen than is ever found in the periaxial mesenchyme before the differentia ting process which results in the future subarachnoid space has become definite. The occurrence of this large amount of albuminous coagulum is apparently related directly to the outflow of the cnibrj'onic cerebro-spinal fluid, for the embryonic fluid is very rich in protein material, a.s can be readily seen by the partial filling of the embryonic cerebral ventricles with the clotted albumen.
This process of the l)reaking-down of the mesenchymal spaces to form fewer and hirger spaces goes on very rapidly in pig embryos as the\' exceed the length of 18 mm. Thus, figure 75 (from a pig embryo of 2.3 mm.) shows a marked decrease in the mesenchymal elements al)out the medulla; the strands are becoming fewer in number, and the albumen-filled spaces are increasing rapidlj' in size, but decreasing in number. About the mcsencej)halon, however, the process has only just begun (also shown by fig. 74). In this photomicrograjih (fig. 75) the mesenchj^mal elements have broken down somewhat; the spaces are Ijecoming enlarged, and a fine albuminous coagulum fills the interstices between the mesenchymal processes. The whole picture conveys an excellent idea of the forces which convert the many-spaced mesenchyme into the much fewer cerebro-spinal channels.
This general plan of the formation of the larger subarachnoid canals reaches its maximum in the formation of the various cisternae for cerebro-spinal fluid. The process is probably best illustrated in the case of the cisterna magna, which persists in the posterior cerebello-bulbar angle. Figures 74 and 75, taken from an embryo pig 23 mm. long, give an idea of the initial formation of the cisterna cerebellomedullaris. The mesenchymal strands, as shown in figure 75, are already broken down in part, and are profusely covered with albuminous coagula. The process has not proceeded to any extent in this specimen of 23 mm., but in the course of the next 10 millimeters' growth extensive changes occur, as arc shown in figures 76 and 77, photomicrographs from an embryo of 32 mm. In the space outside the inferior membranous area the mesenchymal trabecula? have almost disappeared; the space — or cistern, as it should now properly be called — is almost completely filled with the clotted albumen. The mesenchjTne is seen running through this embryonic cistern as a few isolated strands, but most of the tissue appears now as a fairly definite membrane on the outer side of the space. This membrane will go to form the inner surface of the dura and the continuous outer layer of the arachnoidea, as it furnishes a visceral layer for the subdural space.
More laterally in this same specimen the formation of the cistern has progressed to an even greater extent. In figures 78 and 79 the total freedom of the lower portion of the cistern from trabecular strands is seen; above, the mesenchjTne still sweeps down as a supporting structure for the chorioid plexus. A definite differential hne of mesenchymal condensation indicates the future outer border of the arachnoid as it incloses the cisterna cerebello-medullaris. This general process of mesench^Tnal breaking-down, altering the original small spaces into the larger arachnoid channels, holds as the embryo develops into larger forms.
In addition to this formation of the subarachnoid spaces in the adult through the enlargement of the embrA-onic mesenchjTnal spaces, the perimedullary mesench\Tne undergoes in these same localities condensations which result ultimately in the formation of the arachnoid membrane and the trabeculse diAnding up the cavum subarachnoidealo. Mention has already been made of the adhesion of the cellbodies of the disrupted mesenchymal elements to the persistmg strands — the initial step apparenth- in the ultimate differentiation of the mesothehal cells which line these spaces. Gradually ^vith the increasing growth of the embryo these cells seemingly become arranged in definite columns covering the persisting arachnoidal trabeculae. At the same time a differentiation of these primitive mesenchj-mal elements occurs, the cells ultimately being transformed into the very low cuboidal mesothelium of the subarachnoid spaces. This differentiation begins first in the basilar portions of the cranium and spreads upward, in a way similar to the course of development of the cranium and of the enlargement of the pericerebral spaces.
While such a general process as outUned accounts for the formation of the arachnoidal trabeculae and the subarachnoid spaces, it has but little bearing on the development of the outer intact membrane of the arachnoidea. This portion of the arachnoidea (which might be termed the arachnoid membrane as distinguished from the arachnoid trabecule) first appears as a distinct line of mesenchymal condensation separating the mesenchyme into the primitive arachnoid and dura mater, as in figures 76 and 77, dmc. This rather thin zone of cellular density in reality represents not only the outer surface of the arachnoidea, but also the inner surface of the dura mater. At first these develop in close fusion with a later separation of the two membranes. With this cleavage of the two surfaces, the arachnoid membrane rapidly differentiates, forming an intact layer over the subarachnoid spaces. The cells covering the surface membrane seem to change gradually into the low cuboidal type, similar to those covering the arachnoidal trabeculae. The details of these processes may be most easily studied in the region of the cerebral hemispheres; in this situation the transformation of the tissues occurs at a later period than in the basilar regions, for the differentiation of this mesenchyme follows the general plan of development of the cartilaginous and bony cranium.
The greatest problem in connection with the development of an external arachnoid membrane naturally concerns the separation of this leptomeningeal tissue from the pachymeninx. In the solution of this particular problem gross dissections have been found of benefit. For this purpose, pig embryos of larger size were used, and attempts were made to ascertain at what stage of development a true anatomical separation of the two membranes occurred. It was found that in embryo pigs of about 40 mm. the dura over the calvarium could be well separated from the arachnoid, but areas of unseparated tissue still persisted at this stage. This was also found to be true in pig embryos of 50 mm. ; on the inner surface of the dura at this stage a mesothelial cell pattern could be demonstrated, although areas of attachment to the arachnoid existed. However, the differentiation of the periaxial mesenchjTne into the adult arachnoid does not occur coincidently with the possibility of a forceful separation of the dura from the surface of the brain; ])ut before this separation of the pachymeninx can l)e made the mesechyme which will go to form the arachnoid must undergo some differentiation. This process invohcs a condensation or accumulation of mesenchymal elements directly in the secondary dural thickening; the cells, with oval nuclei, soon form a continuous membrane of two or three cells in thickness. Apparently soon after the cellular accumulation has been accom])lished, a separation of the dnni from the arachnoid may be made. In certain areas, varying greatly in size, there is still an intimate connection between dura and arachnoid. These connections are particularly prominent over the developing cerebral hemispheres, and it is with this differentiation in the formation of the arachnoid spaces that we will now deal.
In a human fetus of 76 mm. (No. 1134) the arachnoid was found to constitute, in the region about the great sagittal sinus, a cellular layer which adhered quite closely to the dura, even though a Une of difTerentiation between the two meninges could be made out. This adhesion could undoubtedly be separated, even bj' gross dissection, although the tendency to adhesion was stronger than the attachment of the pia to the cortex. From its cell-character and general histology' the arachnoid at this stage must be considered as a formed membrane, but in a primitive state.
A somewhat similar but more advanced stage in the formation of the arachnoid membrane is seen in a human fetus of 100 mm. (No. 928-E) and in a fetal pig of 114 mm. In both the arachnoid membrane is verj' cellular, adhering to the dura only along the superior longitudinal sinus and in certain isolated areas. The cells comprising the arachnoidea possess oval, rather large nuclei which stain palelj- with hematoxyhn. No typical arachnoidal trabeculae could be made out in specimens in this cortical region.
The cellular character of the arachnoid persists in the larger embryos and fetuses as a layer, several cells in thickness, constituting the outer arachnoid membrane. In a fetal pig 190 mm. in length the membrane was practically differentiated, its outer wall being covered by me.sotheUal cells with large nuclei lying about a small fibrous-tissue base. The arachnoid trabeculae were developed only in the larger sulci, where they appeared as typical cellular cords about a core of fibrous tissue. At this stage, too, the vessels traversing the arachnoid spaces were found covered with similar cells. These may now be justly termed the mesotheUal cells.
Quite similar stages of arachnoidal differentiation occur in human fetuses of 200 (No. 870) and of 240 mm. (No. 1131). The arachnoid has everj-where practically become adult in character, except for a further decrea.se in the number of the peripheral layers of mesothelial cells. The fibrous tissue underlying this covering membrane possesses, as in the adult, almost a minimum of support.
In certain areas, however, the differentiation of the mesenchA-me into the adult arachnoidea does not keep pace with the general process. In the present study this phenomenon of unequal develoj^ment was especially well shown in fetal pigs of 150 mm. and upwards. It concerns the development of arachnoid trabeculae in the cerebral sulci. As is well known, the arachnoid membrane bridges the cerebral fissures, wliile the pia follows the cerebral contour. In the fetal pigs of the stages specified above, certain furrows showed a typical adult relationship with the covering arachnoid membrane and lining pia, the intervening space being traversed bj' definite arachnoid trabeculae. Other of the sulci were filled with an almost emb^^•onic tj-pe of mesench}Tne — a loose meshwork of cytoplasmic processes containing rather small oval nuclei. The explanation of this embryonic type of tissue seems to be that it occurs in the newly developing sulci and that some time must elapse in this formation before the tissue fully differentiates into the adult arachnoid membrane. Strangely enough, a similar collection of an embryonic type of tissue is sometimes met with, in these stages, between the two hemispheres.
The general process, then, of the formation of the arachnoidea involves both a breaking-down (or thinning-out) of the mesenchymal spaces and a condensation of the cells. The first of these processes results in the transformation of the interstices of the periaxial mesenchyme into the larger subarachnoid spaces, divided off by arachnoid trabeculae; the second finds its final accomplishment in the development of the outer arachnoid membrane which, covered with mesothelial cells, forms the inner surface of the subdural space. The transformation begins in the basilar regions of the cranium and spreads upward over the hemispheres.
THE CIRCULATION OF FLUID THROUGH THE SUBARACHNOID SPACES.
In view of the processes of differentiation involved in the formation of the arachnoidea and the subarachnoid spaces, the circulation of fluid through this pecuhar membrane must be considered. It seems important to ascertain, if jjossible, the relationships between the beginning of the passage of the cerebro-spinal fluid and the onset of the histological changes.
The conceptions of the development of the circulation of the cerebro-spinal fluid which are presented in this conmiunication are dependent, in large measure, upon the results of the replacement of the fluid, in living embryos, by the ferrocyanide solution. Additional evidence was obtained from the identification of albuminous coagula in the periaxial tissues. The correlation of these findings with the development of the chorioid plexuses and with the results of injections under low I^ressures, from a syringe and so forth, gave evidence of their correctness.
The differentiation of the mesenchyme into arachnoid membrane may be said to keep pace with the establishment of the periaxial channels for the cerebro-spinal fluid. In the main, the passage of this fluid into the undifferentiated mesenchyme about the nervous system precedes the process of histological change. This phenomenon is shown in figure 14, from a pig embryo of 18 mm. The replaced fluid is seen passing out into the mesenchyni(^ through the two membranous areas in the roof of the fourth ventricle. The mesenchyme at this stage has already differentiated somewhat, but hardly in proportion to the length of time during which the fluid has been passing into the space.
There are several features of interest in the course of the fluid through the periaxial spaces. In sections of embryos in which the cerebro-spinal fluid has been replaced by a foreign solution the granules of the precipitated salts may be identified in the periaxial mesenchyme in situations corresponding exactly to the extent of th(! spread shown in the cleared specimens (figs. 1 to 9). The exact location of the prussian-blue granules is of importance in this connection, as the exact form and distribution of the periaxial spaces and their relation to the adult subarachnoid spaces may thus be determined.
Kxamiiiation of serial sections from an embryo in which the embryonic ventricular fluid has been replaced by the ferrocyanide will reveal, if the embryo exceeded 14 mm. in length, granules of prussian-blue in the peribulbar mesenchj'me (fig. 14). The granule.s are not found in any cell-bodies in this tissue; they are made out, in large measure, adhering to the mesenchymal cell-processes or lying free in the mesenchymal interstices. The granules do not penetrate the pia mater or the dura mater, a finding which will be discussed more fully in the sections dealing with these membranes. Everywhere the transit of fluid into the nervous tissue seems to be prohibited by the pia; in some areas, however, the outer condensation of mesenchyme to form the dura-periosteum has not j'et occurred. This is shown particularly well in the region of the roof of the fourth ventricle (fig. 18), where the epidermis offers the only barrier to the passage of fluid from the pericerebral spaces.
In the earlier stages in which the phenomenon of fluid passage about the central nervous system may be observed, the outer layer of the arachnoid is not at all differentiated. Here the barrier to the fluid is the blastemal condensation of mesenchyme (fig. 16). In the later stages, when the outer layer of the arachnoid is beginning to appear as a mesenchymal thickening, the fluid (as indicated by the precipitated prussian-blue) is confined strictly within the immature arachnoid membrane.
The course, then, of the fluid which has replaced the cerebro-spinal fluid in the embryo follows that of the aduh cerebro-spinal fluid (as shown by the resultant blue granules). It is everywhere contained within spaces which topographicaUj' and embryologically correspond to the subarachnoid spaces in the adult. The spread of the replaced solution from the embryonic ventricle into the peribulbar tissue is analogous in every way to the passage of cerebro-spinal fluid from the fourth ventricle of the adult into subarachnoid spaces.
VIII. A Consideration Of The Embryonic Pia Mater
Our present conceptions of the embryolog}' of the pia mater are largely due to the work of His^^s) and of Kolliker^^D. who first firmly established the idea that this inner leptomeninx was mesodermal in origin. While generally accepted (Farrar'i^*). this view has not been widely referred to in the literature; but the absence from all embryologies of any information concerning the development of the meninges is quite striking and it does not seem strange, therefore, that our information regarding the pia mater has not advanced in keeping wnth our knowledge of the embryology of other structures of the body. In the present section of this conmiunication it is purposed to present merely a general consideration of the process by which the pia mater is formed and to point out some of its functional characteristics, especially in regard to the fluid channels.
The term pia mater is accepted throughout this article as designating solely the cellular membrane which adheres closely to the outer surface of the nervous sj-stem, but it is in direct connection with the arachnoidal trabecular which traverse the subarachnoid space Whether the two membranes should be considered together as the pia-arachnoid or as the leptomeninx is a question in regard to which there is some disagreement ; it will suffice to consider the pia as a separate membrane.
THE GENERAL HISTOLOGY OF THE PIA MATER.
The findings in this mvestigation are wholly in accord with the conclusions of His(25), of Kolliker('i), and of Farrar^^^), that the pia mater is derived from the middle germ-layer. In the earliest stages the mesenchymal elements may be made out adhering to the outer i)ortion of the primitive nervous system. In the course of growth these cells are grouped about the mantle zone of the spinal cord in a rather dense laj'er, two or possibly three cells in thickness, with the tyjjical oval nuclei of the mesench5Tnal elements. Certain stages of this process may be made out in the figures in this paper. Thus, in a human embryo of 4 mm. (No. 836 of the Carnegie collection) the mesenchymal elements form a definite layer around the neural axis (fig. 41). The nuclei are oval in shape, possessing a moderate amount of chromatin, and are found in a layer two cells in thickness. This membrane, with its fairly scant cytoplasm, is sharply differentiated by its existence between two layers, in one of which nuclei are wanting, and in the other somewhat widely separated — the mantle zone of the spinal cord and the periaxial mesenchyme.
This typical arrangement of the mesenchj'mal elements about the cerebro-spinal axis holds in almost unchanged form throughout the whole embryonic growth. Thus, about the nervous tissue in figures 48 and 52 (from human embryos of 7 and 9 mm., respectively) the same condensation of the mesenchymal elements to form the pia mater are made out. This ajipearance is so familiar that further description in the later stages seems needless, but certain characters of this embryonic arrangement seem to require comment.
The general appearance of the pial layer is greatly altered by the early formation of the capillary blood plexus about the nervous sj'stem. This plexus tends to render the pial tissue more cellular, on first microscopic examination, as the endothehal channels branch greatly outside of the nervous tissue in this mesenchymal pia. The general character of the pial layer, however, as a membrane with prominent nuclei and scanty protoplasm, is not altered at all by the vascular plexuses.
The ultimate fate of these undifferentiated mesenchjinal elements forming this initial \nti\ condensation is a gradual transformation of the cells into ver}' low cuboidal mesothelial elements constituting the adult pia. The transformation concerns not only the differentiation of the cells but also a rearrangement so that the original layer of two or more cells in thickness becomes finally of but a single cell in thickness. The jjrocess, in a way similar to the development of the subarachnoid spaces, begins in the basilar portions and spreads upward; the process, hence, may often be studied in a single suitable sjiecimen.
More imjjortant, for our consideration, is the i)eculiar relationship of the pia mater to the roof of the foiutli ventricle, and in particular to the two area^ membranaceje. In this situation, in place of the slight mesenchymal condensation which characterizes the jjia, and which ^Minot*"^ pictures in his figure 114, the mesenchyme seems altered. The condensation to form the pia, which takes place in other situations about the true nervous tissue, has not here occurred. This absence of the typical pial arnuigenit-nt may be noted even in very small embryos — those in which the roof of the fourth ventricle is composed of the many-layered, epitheUal-like cells. This is well shown in a photomicrograjih (fi}^. 53) from an injected human embryo of 9 mm. (Xo. 721) of the Carnegie collection. Likewi.sc, in this region in a [)ig embryo of 8 mm. (fig. 25), the same absence of a real pial condensation may be made out. But this peculiarity of the pia is most striking at the period of maximal differentiation of the superior membranous area in the rhombic roof. In figures 37 and 43, photomicrographs from pig embryos of this stage, the mesenchymal condensation, augmented by some vascular endothelium, is shown in adhesion to the ependyma on both sides of the membranous area; but directly behind the differentiated cells of the area membranacea evidence of a condensation of mesenchyme is whollj' lacking, even though both specimens show vascular channels in close appro.ximation. Similarly, in a human embryo of 14 mm. (No. 144, Carnegie collection) a total lack of the true pial thickening is to be observed (fig. 57).
Quite similar is the failure of a pial thickening about the inferior membranous area. This can be made out in figures 83 and 87, from human embryos in which the process of differentiation of the area is proceeding. In later stages of the formation of the area membranacea inferior, the marked absence of a true pial condensation in the mesenchyme in this region is noted in figure 75 (a specimen from a fetal pig of 23 mm.) But this apparent failure to form the typical mesenchymal condensation of the pia mater in certain areas in the roof of the fourth ventricle must not be construed as indicating an absence of pia mater. Such does not seem to be the case here, for in the later stages of the formation of the cisterna cerebellomedullaris the area membranacea inferior is found entirely unsupported, except for a layer of mesenchymal cells. This is shown in figures 77 and 79, both taken from fetal pigs of 32 mm. This mesenchymal laj'er must be considered as pia mater apparently modified for a specific purpose.
The general process, then, of formation of the pia mater concerns a condensation of mesenchymal elements to form an embryonic membrane about the central nervous system. From its earliest beginning very slight modification is needed to reduce it finally to the histological character of the adult membrane. The general process holds, except in the regions of the area? membranaceic in the roof of the fourth ventricle; here, apparently, a modification of the pia for a specific purpose, involving an absence of the primary pial condensation, takes place.
THE RELATION OF THE PIA MATER TO THE FLUID CHANNELS.
The cerebro-spinal fluid in its normal pathways comes everywhere into contact with the pia mater, which serves as the inner retainer for the subarachnoid space; therefore the functional relation of this membrane to the fluid which bathes it becomes of interest. To some degree the results of the experiments recorded in the earlier portions of this paper throw light upon the relation of the pia mater to the circulating fluid. The most important question in this connection is naturally that dealing with the possible penetration of the normal fluid through this embryonic membrane. In this regard the findings in replacement experiments with ferrocyanide solution serve to elucidate the problem. These observations give no evidence of any penetration of the pia mater by the fluid. This is well brought out in figures 14 and 18. In every respect (as demonstrated by numerous experiments of this type in pig embryos of varying lengths) the pia mater is wholly impenetrable to true solutions of foreign salts when injected so that the normal tension is not altered. The whole subarachnoid sjiace may, in such an experiment, be filled with the prussianblue, but none of these granules are found within the cells of the pia mater or in any layer between these cells and the nervous system. Evidence that the fluid has bathed the outer pial cells is afforded by the adhesion of granules of prussian-blue to the outer cytoplasmic borders of the cells.
Likewise the cells comprising the embryonic pia have been found to be impenetrable to true solutions (ferrocj'anide) when injected under varying pressures from a syringe. In these cases, rupture of the roof of the fourth ventricle or of the infundibulum may be produced by great pressure, without causing any of the fluid to penetrate the intact layer of the pia mater. The same result is obtained when india ink is substituted for the true solution.
The pia mater, then, even in its embryonic form, serves as an efficient fluidbarrier. This is demonstrated, in regard to the adult pia mater, in the report^^^) of the observations made on adult cats, dogs, and monkej's. But the barrier which the pia offers to the entrance of fluid from without exists also for fluid coming in the reverse direction. This is shown by the well-known phenomenon of the so-called subpial extravasation, which occurs in blood vascular injections when the injections are continued for too long a time at too high a pressure. The perforating vessels in such cases rupture as they enter the nervous system, and the injection mass spreads extensivelj' beneath the pia, stripping it away from the nervous tissue. Of interest in this discussion is the fact that the injection mass in these extravasations does not rupture the pia, which seemingly is an equallj' efficient fluid barrier to pressure exerted on it from within. Similar subpial spreads of the injection fluids have been observed in the course of this work. These extravasations resulted from the rupture of the whole nervous tissue from within, particularly in the region of the infundibulum, when the inj(>ction was made into the ventricular system under excessive pressure. In this respect, too, the i)ia seems to be wholly efficient as a retainer for true solutions or for granular suspensions. It is realized that the embryonic pia mater will not resist the passage of fluids through it under the highest pressures afforded by the syringe, but the membrane serves as an efficient barrier for all pressures such as are employed in careful anatomic injections.
With this conception of the impenetrabihty of the pia mater to fluids under ordinary pressures, it does not seem strange that there is a variation in the process of formation of the pia mater in the region of the roof of the fourth ventricle. It has been shown in the foregoing paragraj^hs that the phenomenon of mesenchymal condensation which results in the formation of pia elsewhere does not occur in the region of the two area? membranacefe. In view of the passage of cerebro-spinal fluid through these two membranous areas, the pia mater must necessarily be altered in these places. For were it not adapted to the jjurpose of affording fluid passage the cerebro-spinal fluid would, in its course from the ventricle to the subarachnoid space, form a subpial extravasation. It would seem that this modification of the pia is designed to meet the particular need and function of this region.
The Adhesion of the Pia Mater to the Cerebral Tissue
It is a well-known fact in embryology that tlie pia mater and the peria.\ial mesenchyme in poorly dehydrated specimens split away from nervous tissue, but in adult preparations, if the meninges and brain are dehydrated in a block, the separation of the tissues occurs between the dura and the arachnoid, or (in more exceptional instances) the dura and arachnoid come away, leaving the pial layer closely applied to the cortical tissue. It is quite difficult in any adult mammal to separate the pia from the brain tissue. Realization of these peculiarities in the degree of adhesion of the pia led to an attempt to ascertain what structures were involved in the attachment of this mesodermal layer to the epidermal nervous system. The results of this attempt add nothing to the ultimate solution of the problem, but are perhaps of sufficient interest to justify brief presentation.
Two theories in explanation of this adhesion of the pia immediately suggested themselves. One of these concernod a possible growth into the pia of neuroglial elements, causing an intimate association between the pia and the cerebral cortex. Our findings in reference to the neuroglial outgrowth in fetal pigs gave no reUable basis for the assumption. The second theory dealt with a diminution in the elasticity of the walls of the perforating blood-vessels which supply the nervous system. The early embryonic vessels, with walls comjjosed solelj- of endothelium, when subjected to the distortions of poor dehydration, might possibly offer less resistance to the separation, so that the pia would come awaj' from the nervous tissue. In the later stages, however, the thicker-walled perforating vessels w^ould naturally oppose such a cleavage, so that the pia would remain adhering to the cortical tissue. This theory is also purely an hj^Dothesis, although it does not seem unlikely, especially if one takes into account a possible connection of the pia with the perivascular system. In examining blocks of the meninges and brain tissue taken together it was found that the pia mater separated cleanly from the nervous tissue in fetal pigs 15 cm. in length. Beyond this stage the arachnoid might remain in adhesion to the dura, but in such cases there was always found a layer of cells on the outer side of the cortical tissue, constituting a true pia mater.
IX. The Development Of The Cranial Dura Mater
The dura mater, like the two other meninges, is derived from the mesenchj-me about the central nervous system. The researches of Sterzi'"' on the comparative anatomj" of the meninges furnish additional evidence for this conception in the higher mammals. The origin of the pachymeninx from the middle germ-layer is now well established. But there is lacking in the literature a comprehensive account of the formation of this fibrous envelope. The gross generaUties of the process are given in pari, liut there is an almost total absence of the more intimate details of the transformation. One of the most essential points in the process concerns the relationship of the dura to the bony coverings of the cerebro-spinal axis. Does the adult dura serve as the periosteum of the bony skull? In the standard text-books of anatomy the adult human dura is described as being composed of two layers. In the skull these layers split, to comprise the walls of the great venous smuses. The outer layer of the dura serves as the periosteum for the bony skull, but below the foramen magnum the two layers separate to inclose the epidural space. The outer dural layer in this spinal region adheres to the inner surface of the bony vertebral column, where it functions as the periosteum; the inner layer here becomes the spinal dura.
In this account of the adult dura mater there is indicated a very suggestive periosteal relationship which implies an embryological basis for the disposition of the two laj-ers of the membrane. It must be granted, however, that this division of the cranial pachymeninx into two layers is quite arbitrary; there is nothing in the general histology of the fibrous covering to suggest such a halving except its division about the sinuses and its spinal relationships.
THE GENERAL PROCESS OF THE FORMATION OF CRANIAL DURA.
The first evidence of the development of the pachymeninx is found in the basilar region of the skull, where the mesenchyme thickens, to form eventually the bony covering of the brain. This thickening of the mesenchymal elements results not only in the formation of the chondro-cranium, but also in the final formation of the bony skull and possibly its internal periosteum and dura. In the process of differentiation the condensation of mesenchyme in the early stages gives no index of the varied character of the resultant tissues, so that, in the first place, the study of the process was necessarily related to the more adult stages. In this paper, however, the whole history of the dura will be detailed chronologically, beginning with the earliest stages.
Bardeen(2) has given data on the first appearance of the mesenchymal condensations which go to form the blastemal phenomena in both the cranial and spinal regions. The blastemal vertebra? become fairly well differentiated in human embryos during the first month of intra-uterine growth. At the end of the first month, in the occijiital region, three fairly well-marked occii)ital myotomes may be made out; these afterwards disappear. "During the early part of the second month the membranous anlage of the skull becomes extensively developed. The roof of the cranial cavity is formed by a dense membranous layer, which fu'st becomes marked at the side of the head in embryos from 9 to 11 millimeters in length" (Bardeen).
These evidences of a primary mesenchymal condensation about the central nervous system are concerned in the problem of the differentiation of the dura only in so far as they indicate the onset of the process which will give rise to the bone and possibly the periosteum — a part of the dura about the cerebro-sjjinal axis. Gaupp^'^) has already pointed out that this cranial l)lastemal condensation gives rise to these adjacent Init wholly different structures. These cranial mesenchymal condensations persist in simple form until after the cerebro-spinal fluid begins to fill its extraventricular bed; then, within a short time, the tissue becomes transformed by the development within it of cartilage, so that in the human embrj^o the caudal half of the chondro-cranium forms a ring of cartilage about the posterior portion of the brain. On the inner side of this ring of cartilage the mesenchyme later shows a marked condensation in the midst of the rarefied perimedullary tissues. In this layer the nuclei soon become fewer in number and the cytoplasmic structures fibrillar, the whole resulting ulthnately in the formation of the fibrous adult dura. The mesenchymal condensations in the regions of the skull, where membranous bone formation holds, go directly into a membrane of fibrous tissue, in the outer portions of which bone is laid do\\Ti. The details of these processes will now be taken up.
In figures 30 and 32, photomicrographs from pig embryos of 13 and 14 mm., respectively, the well-established vertebral differentiations and the now poorly differentiated base of the skull are shown. From this stage upward the mesenchymal condensation in the head region proceeds rapidly. Thus at a stage of 17 mm. in the human embryo (fig. 60) the ventral portion of the vertebral canal has become cartilaginous, while the base of the skull has also undergone the chondrogenous transformation in its mere posterior portions. But of especial interest in our problem is the line of mesenchjTnal condensation, which may now be traced whollj^ around the brain-stem and hemispheres (fig. 60). The nature of this condensation is well shown in figure 61, an enlargement of the squared area of figure 60. The mesenchymal nuclei have become closely packed and rather sharply differentiated from the looser mesenchj^me which in part goes to form the arachnoidea. Figure 59 similarlj' shows this condensation proceeding upward to the vault.
Examined in another plane, the process of mesenchymal condensation seems to proceed much more rapidly in the posterior than in the anterior region. This is brought out in a transverse section of a human embryo of 18 mm. (fig. 62). Here the condensation is much more extreme about the medulla and roof of the fourth ventricle than in the more anterior parts of the mesencephalon. The same general appearance, typical of this stage, may be made out in figures 56 and 57 from a human embryo of 14 mm.* (No. 144, Carnegie collection). In the slightly larger stages the process of mesenchj^mal condensation about the nervous system becomes rapidly more marked. This increase in the number of cells comprising the denser membrane is shown in figures 64 and 65, photomicrographs of embryo No. 460 (21 mm.).
The degree of condensation of the mesenchyme in the various stages of the human embryo is followed quite closely in the pig embryo. The comparative degree of differentiation coincides within a millimeter or two. Thus, in a section from a pig embryo of 19 mm. (fig. 38), the degree of condensation about the roof of the fourth ventricle is practically similar to that in human embryos of the same length.
The phenomena just commented upon represent the stages concerned in the formation merely of a cranial blastema and are related to the formation of the dura only so far as it is out of this mesenchymal condensation that the periosteal portion of the pachymeninx may be derived. The degree of condensation referred to in the figure.s has been solely of the blastemal type, but in some of the specimens this simple condensation is seen only in the more cephalic portions of the cranium. Thus, in the figures (64 and 60) taken from embryo 460, the mesenchymal condensation is still of the simjile undifferentiated type, whereas in this same embryo the more caudal sections show a chondro-cranium which is well develojied. The i)rocess of formation of the cranial dura, then, is one which begins in the basilar j^ortions of the cranium and proceeds from these points into the region of the calvarium. In general, all of the phases of this transformation into dura may be found in one specimen of sufficient and suitable size, the basilar differentiation re]:)resenting the advanced stages, while the steps in the differentiation are found in the areas nearer the vertex.
- Measured on slide after sectioning.
It is quite difficult to decide exactly what importance the primary condensation of mesenchyme maintains in the formation of the dura, because, coincident with the chondrification of the blastema, there occurs another condensation which forms the line of division between the inner surface of the dura mater and the outer arachnoid membrane. The first evidence of this secondary perimedullary condensation is found in pig embryos of about 17 mm. In these specimens, in the narrow space formed by the mesencephalic flexure, mesenchymal cells collect together in the form of a fairly definite membrane. After its primary beginning in this area, the narrow line of its thickening may be traced to the basis cranii in embryos a little larger. In slightly older stages this secondary fine of condensation is found to be fairly extensive throughout the area between the middle and posterior cranial chambers.
At a stage of 20 to 21 mm. the whole basilar portion of the cranium shows evidence of this secondary line of condensation lying between the pia mater and the cartilaginous skull. The condensation occurs in the outer portions of the loose tissue which, as shown in a foregoing section (No. vii) becomes the subarachnoid trabeculae. The line of condensation is not broad on section; it comprises a cell-layer from three to six cells in thickness. Between this cellular border and the cartilaginous skull the tissue rapidly differentiates (a process seemingly sj^nchronous with the develojiment of this membrane). This tissue, which maintains dural relationships, is far more cellular and compact than the original perimedullary mesenchyme. Even without the rather dense line of division in the mesenchymal ti.ssue, the dural structure can be easily outlined by its characteristic apjiearanco.
The original dural condensation between the two wings of nervous tissue which unite in the me8encei)halic flexure can be traced in slightly later stages around into the tentorium cerebelli. This structure develops as a wholly similar mesenchymal thickening in the midst of the jK-rimeduliary mesenchyme. The tentorium consists in these embryos of 20 to 2") nun. of two thin lateral plates which widen at their cranial attaclunents into prismatic areas. These areas, which finally lodge, in the two layers f)f dura, the sinus transversus, arc characterized by the same dense tyjie of mesenchyme. The jx-ripheral edges of the prismatic jwrtion of the tentorium sjjreads caudalwards as a definite line of condensation. In the earlier stages this line becomes indefinite as it extends from its teiitori:il attachment, but finnllv a similar line of condensation about the whole posterior chamber may be made out. This lies within the area of the cartilaginous skull and bounds the subarachnoid spaces.
This same process of formation of dura holds for the formation of the basilar dura in the more anterior portions of the cranium. The appearance of the .secondary zone, narrow and rather dense, may be made out inclosing the more cellular mesenchyme w^hich extends to the cartilaginous skull. The same process also endures for the formation of the dura of the calvarium, but here the addition of tissue from the undifferentiated mesenchyme is undoubtedly very small in amount. This will be discussed in a later paragraph. The various stages in the formation of this secondary condensation which goes to form the major portion of the dura may be fairly well studied in any one embryo of suitable stage, because the process, as pointed out above, begins in the basilar portion of the cranium and extends upward. Likewise, the condensations directly beneath the region of the dorsal membrane are delayed as compared to those of the lateral regions.
Some of the phenomena shown in the formation of the dura mater are illustrated in figures 46, 76, 77, 78, 79, and 94. Throughout these figures the letters dmc refer to the Une of the secondary mesenchymal condensation, which borders internallj^ the dura and which goes to form the outer membrane of the arachnoidea.
In figure 46, a photomicrograph of a pig embryo of 32 mm., the dura mater (dmc) is shown as a somewhat condensed tissue separated a sUght distance from the chondro-cranium. On the basilar surface, the inner line of dural tissue is quite remote from the inner surface of the basioccipital. Tracing this line of condensation forward, it is soon seen to merge more closely with the perichondrium* of the basioccipital. More anteriorly it again leaves the occij^ital plate and after a brief interval it fuses with the temporal perichondrium. Continuing slightlj^ more anteriorly the dural process toward the mesencephalic angle maj' be made out; this appears as a doubled membrane at its basal attachment. In its further prolongations the dural surface is at times a distinct structure; at other times it is completely fused with the perichondrium.
Posteriorly, in this figure 46, the line of dural condensation (incorporated also with the outer arachnoidea) may be traced upward around the cisterna cerebeUomedulla
ris. This hne of condensation is seen to lose its definitive character as it curves inward toward the chorioid plexus of the fourth ventricle — a phenomenon shown particularly well in figures 77 and 79, taken from the same pig embryo of 32 mm. The dura in this termination maj' be said to be in its formative stage; but dorsally, over the mesencephalon, the inner surface of the dura again becomes a definite membrane, as shown in figures 76 and 78. In the latter figure it is shown inclosing a wide mesh of dural vessels, between the arachnoidal surface and the membranous skull. Anteriorly, again, it seems to lose its definite hne of condensation.
•The term "perichondi.L n" is used tliroiighout this paper to designate only the ver>' dense cellular line delimiting the edge of the cartilngo. This dense zone is composed of the nuclei of the cartilage, crowded together, and represents probably some phenomenon of the growth or resorption of the cartilage. In a much broader sense, the whole dural tissue, lying between the line of secondarj- condensation and the cartilaginous border, could be termed "perichondritmi," as it probably represents the sole internal membrane which could be stripped from the cartilage.
Quite similar pictures are obtained regarding the dura mater in the human embryo. The relationships of the dura to the cisterna cerebello-medulla
ris are shown in figure 94, a photomicrograph of a human fetus of 35 mm. (No. 199 in the collection of the Carnegie Institution). In this rejiroduction the line of secondary mesenchymal condensation (representing the outer membrane of the arachnoidea and the inner surface of the dura) becomes widely separated from the occipitale superius in its superior portion.
In a fetal pig of 8 cm. the same general arrangements of the dura mater could be made out. The inner surface of the dura was in places still fused with, the outer arachnoid membrane, but in other places the areas of attachment were lacking, so that a true separation of arachnoid from dura had taken place. Along the ])eripheral points of the tentorium the dura and arachnoid were still closelj' a{)plied to each other. The dura itself was of the same cellular, rather loose tissue, with a dense inner surface. In i)laces, as described in the younger stages, the dural tissue was incorporated with the definitive perichondrium over certain cartilages or even over parts of the same structure. In other places a definitive perichondrium may be wholly lacking; in these areas the indefinite cartilaginous border gradually merges into the dura. In still other situations an intermediate arrangement of dura and perichondrium exists, where the cartilage is bounded bj' a somewhat condensed but not fully developed perichondrium which is continuous with the dura. Everywhere in the membranous sutures between the cranial cartilages or bones, the dura bridges the gap as a loose, cellular tissue. Over the calvarium the dura appears solely as a dense, rather fibrous membrane which is incorporated with and serves as the inner periosteum. This dura over the hemispheres is continuous with the fibrous sutures of the cranial vault.
The findings in a fetal pig of 98 mm. were not dissimilar to those just recorded. The dura was everywhere quite well developed, a rather loose cellular tissue except over the hemispheres, where it showed a more fibrous character. In the region of the occipito-atlantoid ligament the dura was fused with the ligamentous tissue, while above (over the occipitale superius) the dura became a distinct, thick cellular layer. The structure of the tentorium was wholly similar to the occijiital dura. In the basis cranii there are areas in which the dura is wholly fused with the periosteum or jierichondrium; in other areas it bridges the sutures or exists as a definite membrane on the inner surface of a definite perichondrium.
The dura mater in a fetal pig of 15 cm. did not vary greatly from those larger stages already tlescribed. The tissue, however, had become somewhat more fibroiis. The prismatic attachment of the tentorium was no longer as large proportionately, but the dura lining the occipitale superius remained a thick bulbous swelling on the dorsal surface. Hut most striking of all the features in the specimen was the very dense fusion of the dura of the calvarium with the fibrous sutures of the cranium. No line of demarcation betwecm dura and fibrous suture could be made out; the two fibrous layers are anatomically one structure.
The falx cerebri forms in the pig and human embrj-o by a process similar to that of the inner portion of the dura mater. In the sulcus between the two cerebral hemispheres the mesenchyme remains undifferentiated until quite late; then there appears in the posterior portion a narrow zone of condensation which soon presents two lateral surfaces separated by a layer of rather loose cellular tissue, similar in all regards to the dural tissue already described. This zone of condensation spreads forward to comprise the whole falx. The double surfaces of this membrane finally separate into two parts, forming the outer surface of the arachnoidea and the inner surface of the falx. At the cranial attachment of the fabc, the loose tissue forms a prismatic base, containing the suj)erior sagittal sinus and spreading laterally over the denser dura of the calvarium. The whole appearance of this region, which will again be referred to, is that the falx has been added onto the dura of the vertex. Its time of initial appearance is later than that of the rest of the cranial dura and there is apparently no additional acceleration of development. Hence the dural tissue in the fabc cerebri presents, in appropriate stages, a more immature type of differentiation than does the adjoining dura.
The process of the formation of the dura is not wholly a simple one due to the relation of the adult dura to, or its function as, the inner periosteum of the skull. In the figures already referred to, the almost complete fusion in some areas of the inner Une of dural condensation with the perichondrium has been commented upon. In other situations definite separations of the inner dural surface from the perichondrium occurred; in still other regions no perichondrium could be made out as a definite membrane. These differences in relationships of the dural tissue to the line of the perichondrium can not at present be wholly explained, but some indication of the meaning of the process can be given.
Out of the original cranial blastema, as described by Gaupp'^i^\ there develops the cartilaginous and bony skull, the periosteum, and the dura. But the observ^ations recorded above indicate that by far the major portion of the dura is formed by a secondarj^ mesenchymal condensation, which was indicated bj' a thin zone of more condensed cells on its inner border. This inner zone ultimately separated to form the inner surface of the dura and the outer membrane of the arachnoidea. The tissue included between this inner line of condensation and the cranial wall gradually differentiated into a more condensed but still a loose cellular tissue and finally became a fibrous dura.
In all cases the dural tissue extends from the inner line of condensation to the cranial blastema, to the perichondrium, or to the cartilage of the skull. The presence of a definitive perichondrium can not at present be explained, but apparently the perichondrium is manufactured by the cells of the original cranial blastema and not by the dural tissue which lies in approximation to it. When a definite perichondrium is found, it seems quite uninfluenced by the dura; at other times a fusion of an indefinite perichondrium with the dura seems to occur. The fusion of the perichondrium with the dural tissue derived from the secondary mesenchjinal condensation may occur, so that the small outer portion of the dura may be derived from this laj^er. The findings, however, in this investigation, are against any addition of perichondrium to the dural tissue; histologically, a definitive perichondrium is a membrane entirely apart from the dural condensation.
Over the cerebral hemispheres the dura of the cranial vault offers more difficulties of study than does that of the basilar regions. With the formation of a blastcmal condensation over the whole vertex — an extension of the dorsal membrane to form the membranous skull — there occurs very quickly a condensation to form the dura. This condensation may be first detected as a continuation anteriorl)'of the leaflet of the tentorium cerebelli, which stretches forward from the prismatic zone of the tentorial attachment. This zone of condensation is wholly similar to the narrow line of the mesenchymal thickening which was found in the more basilar regions of the skull. This zone of condensation occurs just wathin the cranial blastema and may be traced upward over the mesencephalon and laterally around the rapidly enlarging hemispheres. As the distance from the cerebellar attachment increases, the zone tends to approach the blastema, except in those regions in which the precursors of the dural veins occur. In such a situation this inner dural zone swings inward to encompass the vessels. Between this inner line of the dura (representing also the outer surface of the arachnoid) the same rather loose cellular tissue exists.
From the fabc cerebri a zone of dural condensation in the mesenchyme spreads laterally also; this gradually may be traced anteriorly and laterally until fusion with the similar lines of condensation from the basis cranii and the prismatic zone of the tentorium are reached. The condensation connected with the falx cerebri, however, is not an extensive process, the greater part of the hemispheres being covered by the development from the basis cranii and from the tentorium. It must be understood, however, that there is no active migration of this line of condensation, for the whole process is a development in situ. The appearance of an active extension is derived solely from the study of various stages and the increased area of condensation appears as an increment which has developed at the terminal points of the previous condensation.
The amount of dural tissue delimited in the mesenchyme by the secondary zone of condensation is not great in the region of the vertex. It is a thin layer which fuses to the inner surface of the cranial blastema. At the stage of this fusion the blastema has become somewhat fibrous and it constitutes the membranous skull. In this fibrous tissue (the union of the blastema and the dura) bone is deposited, but only in the outer layers. The phenomenon is easily studied in any suitable stage, for the sutures between the flat cranial bones remain incorporated with the inner memliranf!— the dura which includes the periosteum. Hence, over the cranial vault, the dura and periosteum become incorporated as a single membrane; this serves as the membranous .skull, into the outer layer of which bone is deposited.
In the basis cranii, as soon as ossification of the cartilaginous skull takes place, tin; dura becomes inc()r|)orated as the periosteum in a manner similar to that which takes i)lace in the cranial vault. While no definite relationship of dura to the peri-chondrium could bo made out in the earlier stages, the later function of the dura as the inner cranial periosteum is Cjuite obvious. Thus the adult relationships of the dura are obtained. But it is quite difficult to decide to what extent the dura (or internal cranial periosteum) is derived from the primary cranial blastema. It seems probable that this blastemal condensation, in its final resolution into bone, may contribute, in the form of a periosteal element, somewhat to the formation of the dura. Such an addition is verj' difficult of verification; certainly the greater part of the dura is derived by the secondary condensation from the perimedullary mesenchyme.
Before giving details of the fibrosis of the dura, it may perhaps be interesting to point out a peculiarity of the primarj' cranial blastema, which does not seem to be connected directly with the formation of the dura. This concerns the tendency of the membranous skull to form more than one layer in its original zone of condensation. In certain areas, as in figure 64, from a human embrj^o of 21 mm., the dorsal membrane is .shown split into two layers. Somewhat similar to this is the occurrence of two zones in the cranial blastema of a pig embryo of 23 mm. (figs. 22 and 101). Inthis latter figure a less cellular outer layer and a more cellular inner layer are seen. Neither of these have particular significance in the formation of the meninges, although the inner layer in early stages actively functions as a fluid retainer.
The question of the development of fibrous tissue in the dura mater in the course of its development requires consideration here. This phase of the problem concerning the formation of the pachymeninx has been followed, in this stud}', in the dura of the vertex about the sinus sagittalis superior. The tissue was removed in blocks, including the meninges and cortex cerebri, and was then sectioned in the coronal plane. For the most part the deposition of fibrous tissue was studied in sections stained with hematoxyhn and eosin; the findings were controlled by treating other sections from the same blocks with IMallory's connective-tissue stain. In this way the general histogenesis of the dural tissue could be well investigated.
Sections from such a block from a human fetus of 76 mm. (Xo. 1134, Carnegie collection) showed the dura to be composed of fibrous tissue everywhere except in the region of the great sagittal sinus. About this sinus an immature, almost embryonic, tj'pe of loose mj-xomatous tissue was observed. The fibrous tissue comprising the dura elsewhere is of a quite cellular, somewhat immature type of white connective tissue, with a considerable number of true fibrils. A wholly similar picture is found in a section, stained by Mallory's method, of a block from a fetal pig of SO mm. (fig. 104). Unfortunately the cellular character of the fibrous dura is not brought out, but the photomicrograph shows well the avoidance of the lateral walls of the sinus by the process of fibrosis. The more embryonic type of tissue in the region between the hemispheres is also well presented.
The dura mater of a human fetus of 100 mm. (No. 928-E, Carnegie collection) possesses fewer nuclei in a given area than does the dura from the specimen of 76 mm. (No. 1134). The tissue is fibrous, except in the immediate region of the sinus sagittaUs superior; but interspersed among the connective-tissue fibrils are many stellate or spindle-like nuclei, greatly exceeding in number the nuclei found in the dense dura of the adult. Bone is being laid down in the outer portion of this dura where it merges \\dth the membranous skull. The lateral walls of the great sinus are still free from fibrillar depositions. A somewhat analogous picture is afforded b}' a photomicrograph of a specimen stained after IMallory's method, from a fetal pig of the same length (fig. 105). In this specimen the outer portion of the dura, incorporated into a part of the membranous skull, is quite dense with the fibrous tissue; about the superior sinus, however, the decrease in the amount of fibrous tissue is very striking. The falx is beginning to exhibit a fair degree of fibrillar structure; it forms a definite division between the two hemispheres.
In the larger fetuses, above 100 mm. in length, the process of formation in the dura of denser and denser connective tissue proceeds rather slowly. It is realized, however, that this fibrous transformation in fetuses of 10 cm. is veiy extensive, the region about the sinuses alone remaining comparatively free from the development of the fibrils. The chief difference between the dura of this stage and the dura of the adult is a greater number of cell-nuclei in the fetal membrane. It is well, then, to consider the cellular character of the fibrous membrane and the region about the sinuses in the larger stages.
In a human fetus of 125 mm. (No. 900-H) the dura is quite fibrous, but still contains an increased number of the stellate and spindle forms of nuclei; likewise, about the superior sinus the tissue is an immature m30iomatous structure, fairly free from connective-tissue fibrils. This increased number of nuclei in the dural tissue holds also for human fetuses of 165 mm. (as in No. 745), but seems slightly decreased as compared with the smaller specimens. The lateral wall of the great sagittal sinus in this stage possesses distinct bands of white fibrils, but the tissue is much looser and more cellular than the fibrous dura over the hemispheres. These phenomena may be made out in similar stages of the fetal pig, as shown in figure 106, a photomicrograph from a specimen of 17 cm. In this specimen, treated by Mallory's stain, the superior longitudinal sinus is shown surrounded b}' a clear zone in which the deeply staining fibrils are comparatively few in number. On each lateral wall of the venous channels distinct fibrous bands may be made out, lying in the looser, more immature tissue. The lower portion of the falx has assumed quite an adult character.
Gradually the conversion of the tissue about the cerebral sinuses into the adult structure progresses. Thus, in both human and pig fetuses of 20 cm. length, the dura mater has acquired practically all of its adult features. Everywhere over the cereljral cortex the dura is characterized ])y dense layers of interlacing strands of white fibrous tissue, but the number of nuclei in the.se bundles may still be slightly greater than in the adult structure. In the more posterior regions, at this stage of 20 cm., the lateral walls of the sinus sagittalis superior are found to be completely occupied by the white fibrous tissue; in the anterior portion of the sinus much thinner tissue, resembling myxomatous structure, appears, as shown in figure 107. But in this specimen the invasion of the area about the great venous channel by fibrils has begun; isolated bundles may be made out everywhere in the lateral walls of the sinus. This freedom from connective-tissue formation does not persist, however, and the area is gradually invaded by the continued growth of the fibrils. The avoidance of the region about the sinuses by the connective-tissue resolution will be further commented on in the following subdivision of this paper.
The dura, then, develops probably first in connection with the mesenchjinal condensation which ultimately forms the bony skull and a portion of the dura (the cranial periosteum). It first becomes apparent, as a structural unit, as a more cellular layer differentiated, by a secondary condensation, out of the peria.xial mesenchyme. As the chondrogenous stage is approached it becomes differentiated as a distinct layer, maintaining varying relationships with the inner perichondrium of certain of the cranial bones. At a stage of 40 mm. m the fetal pig, the dura of the vertex may be dissected out as a distinct, somewhat fibrous laj'er. The process of fibrous-tissue transformation, however, is slow; the dura until late in fetal life shows an increased number of nuclei, as does any young connective tissue. The invasion of the region about the superior longitudinal sinus by connective-tissue fibrils is much more tardj' than is the transformation over the hemispheres.
THE SUBDURAL SPACE AND THE MESOTHELIAL LINING OF THE DURA.
The subdural space (cavum subdurale) has been the subject of controversy in regard to its role in the pathway of the cerebro-spinal fluid. Before the work of Key and Retzius^^s) ^\^q yj^^^y ^.f^g ]^q.](\ ^)jat the cerebro-spinal fluid occupied the subarachnoid space in the spinal cord, but that in the cranium the subdural space afforded an analogous pathway. This conception was largely due to the fact that, in dissection on fresh material, the dura and arachnoid in the spinal region are found to be in approximation; in the cranium the greater adhesion, bj' trabeculse, of the arachnoidea to the pia renders the freeing of the dura from the leptomeninges the simplest line of cleavage. This view was entirely disproved by the beautiful injections of Key and Retzius, who demonstrated the anatomical and physiological continuity of the subarachnoid spaces.
With the introduction of this latter view by Key and Retzius the conception of the subdural space naturally changed. These Swedish investigators demonstrated a typical mesothehal cell-lining on the inner surface of the dura, as shown by the method of silver reductions. Without an intimate connection with the true cerebro-spinal fluid, the subdural space has come to be looked upon as somewhat analogous to the serous cavities of the body. Quincke^-*^', after a subdural injection of cinnabar granules, ascertained that communications existed between the subdural and subarachnoid spaces, but only in the direction from subdural to subarachnoid. Leonard HilK^^), from the results of physiological experiments, assumed that fluid passed from the subdural to the subarachnoid space, and in the reverse direction, with great ease. The more recent investigations, however, lend evidence to the view that in the normal animal with undisturbed intracranial pressure relations the two spaces are physiologically as well as anatomically separate. The current impression that the subdural space is in manj' respects a serous cavity will probably finally have greatest support; intimate connections with the lymphatic system are, however, entirely lacking in the dura.
The development of the subdural space must necessarily follow the develoj)ment of the dura. It has been mentioned that in fetal pigs of 50 mm. the dura can be freed from the arachnoid by gross dissection, but that at this stage many areas of adhesion between the two membranes exist. Such an observation has considerable bearing on the subdural space. For in the development of this space two processes must proceed far enough to permit the separation of the dura and arachnoidea by the capillary layer of fluid. The first of these processes, in order of probable importance, concerns the condensation of mesenchj'mal cells to form the outer membrane of the arachnoidea. This thickening and resolution into a true membrane takes place in close approximation to the inner surface of the dura. The second factor concerns the covering of this inner surface of the dura with mesothelial cells.
The lining of the subdural space by mesothelial cells can be readily demonstrated on the inner surface of the dura by silver reductions, but the outer membrane of the arachnoid does not permit of a similar technique. This technical failure in regard to the outer arachnoid surface is probably to be accounted for by the dissimilarity in cell-structure in the two situations. Similar difficulties have been encountered by other observers.
In order, then, to ascertain, if possible, at what stage a really adult subdural space could be demonstrated, the inner surface of the dura from fetal pigs of various lengths was subjected to treatment with silver nitrate. After the reduction had taken place to a sufficient degree, the whole dura was washed with distilled water, stained with hematoxylin, and cleared in glycerin. The i)ictures afforded by this method were quite satisfactory, and the technical procedure was so simple and reliable that considerable faith could be placed in the absence of the intercellular reduction lines.
The smallest fetal pig in which a typical mesothelial cell-j)attern could lie demonstrated on the inner surface of the cranial dura was one of 50 mm. In this specimen the inner surface of the dura was not uniformly covered with the mesothelial cells; certain ragged areas seemed to represent the points of adhesion of the arachnoid to the dura. Figure 108 is a reproduction of a drawing made from one of the areas in this specimen where a mesothelial cell pattern could bo seen. The drawing shows many of the characteristics of mesothelial ci'll-i)ut terns of other parts of the body. The irregularities in the cell-borders, the frefiuent accumulations of the reduced silver in the cellular angles, and thegeneral cellular pattern are quite typical ; but the variation in the size of the cells, as shown in figure 108, is also somewhat different from the usual finding in the adult, where there is considerable constancy in the size of the cells. About half the cells in this fetal pig of 50 mm. are diminutive in size; the smallest are hardly a third the size of the largest. Transitions between the smallest and largest cells are al.so shown in this figure. It i.s difficult to a.scertain whether these smaller cells represent young elements which have not yet reached their maximal growth; no evidence of cellular division, as evidenced by mitotic figures, has been observed, although in this connection it must be granted that the cleared si^ecimens are hardly the most favorable. Und()ul)tedly this explanation of the smaller cells would seem to be the true one, but there is little proof for the view, except their absence from the adult dura and their disappearance in larger specimens.
This disappearance of the smaller mesothelial cells is not rapid, but is seemingly delayed over into the larger fetuses; thus, in figure 109, a similar preparation from a fetal pig of 75 mm., corresponding smaller cells arc outhned. On account of the absence from the field of the drawing of the larger elements, these cells do not appear relatively as great in number as in the preceding figure. Likewise, in figure 110 every gradation in cell-size is shown, in a specimen made in the same manner from a dura of a fetal pig of 90 mm.
Very slowly in the course of growth of the fetus the cells Uning the inner surface of the dura reach their standard size and compose the mesothehal surface, with very little variation in size. The process, however, is apparently very tard}', even though the fetus at 16 cm. shows an inner surface to the dura which is largely composed of standard cells (fig. HI); but even in this figure, from a relatively large fetus, the standard size of the cells has not been attained, for a few cells of small size appear in the drawing. In other respects the whole pattern, in general appearance, resembles closely the adult.
It seems most fair to assume that the occurrence of a true mesothelial cellpattern on the inner surface of the dura represents the initial estabUshment of a subdural space. On this basis the subdural space may be said to occur in fetal pigs 50 mm. in length; in the present investigation it has been found impossible to demonstrate the existence of the mesothehal cell-pattern in fetuses smaller than 50 mm. The separation of the dura, possible bj'^ gross dissection in pig fetuses of 40 mm., suggests that the space may be found at a sUghtly earUer stage than that in which the mesothehal cells have been demonstrated.
Anatomically the subdural space in pig fetuses resembles m everj' particular the adult space in cats and dogs; this was described in a paper^^S) pubhshed in 1914. In the large pig fetuses injections of solutions of potassium ferrocyanide and ironammonium citrate were made into the spinal subarachnoid space. After precipitating the foreign salts as prussian-blue, the injection is found to be wholly within the subarachnoid spaces, both in the spinal and cranial regions; the subdural space is absolutely free from any evidence of connection with the subarachnoid space. These findings wholly accord with the opinion concerning the adult subdural space which has been repeatedly expressed.
THE COMPETENCY OF THE EARLY DURA AS A CELLULAR MEMBRANE.
During the stage when the condensation of mesenchyme to form the cranial blastema is prono'inced the spread of the cerebro-spinal fluid becomes more and more extensive. In these stages, when the pig embryo measures from 16 to 25 m m. approximately, the outer membrane of the arachnoid is not 3'et formed, the arachnoid spaces extending from pial to blastemal condensation. WTien in these embryos the cerebro-spinal fluid is replaced by the ferrocyanide solution and the embryo kept alive for some time, the course of the injection may be traced to varying extents throughout the periaxial tissue. To this spread of the injection fluid (a true solution, during the progress of the experiment), however, the blastemal condensation of mesenchjme opposes an absolute barrier. This pecuUarity of the early condensation may be readily seen in figures 16 and 18. At this stage in development the blastemal thickening may be said to play the role of the outer membrane of the arachnoidea or of the inner surface of the dura.
This feature of the blastema as an impenetrable membrane — an absolute barrier to the passage of fluid — is found also to endure during injections of the ferrocyanide solution under pressures sufficient to rupture other parts of the central nervous system. Similarly, the early blastemal condensation resists the inflow of the other injections used (india ink and silver nitrate) under similar pressure conditions. In later stages the injection solutions, from ventricular or subarachnoid mtroduction, do not reach the dura. This is due to the development of an outer membrane of the arachnoidea and the formation of the subdural space. The arachnoid membrane when formed does not permit fluid to pass outward into the subdural space; but the competency of the early blastemal condensation in the mesenchyme affords a very good example of the perfect function of a tissue as a fluid barrier.
An interesting phase of the competency of the secondary mesenchymal condensation (forming dura and outer membrane of arachnoid) may be seen in the region of the cisterna cerebello-medullaris. Here, as shown in figure 77, the zone of secondary condensation, while complete below, does not remain definitive above as the mesenchyme sweeps inward to the chorioid plexuses. At such a stage of 32 mm. in the pig, a replacement experiment would show no penetration of this secondary dural condensation by the foreign solution, where the condensation made a definitive membrane; above, however, in the region of the plexuses, a limited penetration by the introduced fluid could be made out.
X. THE RETURN OF CEREBRO SPINAL FLUID TO THE VENOUS SYSTEM.
The question of the exact mode of return of the cerebro-spinal fluid to the general circulation has interested many investigators. It has occasioned a large amount of work, with the presentation of several hypotheses. Key and Iletzius<2»), from the results of injections of colored gelatin into the spinal subarachnoid space, held that the cerebro-spinal fluid returned through Paccliionian granulations into the great dural sinuses. Other workers, following Key and Retzius, were dissatisfied with this theory, because of the apparent lack of these granulations in infants and in the lower animals. Cathclin(6), with but little evidence, hypothecated an absorption of the fluid by way of the perineural sheaths into the lymphatic system, although the physiological findings of Ziegler(57j^ Reiner and Schnitzler(<«), Leonard 11111*2^), and others made it necessary to consider a direct absorption into the blood system. Cushing(9) premised the drainage of fluid into the great sinuses through a valve-Hke mechanism. Dandy and Blackfan'"' .-suggested its absorption l)y the capillaries of the pia-arachnoid — an untenable hypothesis in view of the work of Kadyi'^Sj, Shroeder van der Kolk(5i), Ekker(i*>, Adamkiewicz^*), and others. Still another conception of the process has been advanced by Mott('**), namely, that the absorption of cerebro-spinal fluid isone of the functionsof the cerebral capillaries. Ina previous investigation'^), making use of a method similar to the one here employed in the rej)lacement experiments, evidence was presented indicating the drainage of cerebro-spinal directly into the great dural sinuses through arachnoid villi. These structures represent an invasion of arachnoid tissue through the lateral wall of the sinuses.
In view of the findings in adult laboratory animals, interest naturally turned, during the course of this work, to the process of drainage of the embryonic cerebrospmal fluid. The evidence afforded by the replacement experiments with the ferrocyanide solution indicated that in pig embryos of over 20 mm. cerebro-spinal fluid circulated throughout most of the periaxial tissue, and that in embryos of about 26 mm. the periaxial distribution was complete, the relations of the fluid at this stage becoming adult. With this evidence before us, the question of the drainage of the fluid became important. as the absorption process similar to the normal adult procedure, or was it entirely lacking, the production of the fluid being balanced by the growth of the nervous system and its meningeal spaces?
The question of the absorption of cerebro-spinal fluid was approached in the embryo in a similar manner to that employed in the adult animal. The problems incurred by the use of abnormal intracranial pressureswere eliminated by the method of replacing, without disturbing the normal tension, the embryonic cerebro-spinal fluid with the ferrocyanide solution. The embryo was then kept aUve and was finally fixed in a preservative which would precipitate the replaced fluid as prussianblue. This procedure was carried out in many embryos of varying lengths and the specimens were subsequently stained in serial sections.
The smallest embryo in which any evidence of absorption of the fluid from the periaxial tissue was obtained was a pig embryo, 23 mm. in length. In this specimen granules of prussian-blue could be traced through the mesenchymal spaces (arachnoidal) to the inner wall of the sinus transversus. The sinus is well differentiated at this stage in the human embryo of 21 mm., as demonstrated by Streeter^**). The wall of the sinus in this pig embryo was quite thin, the mesenchvTne lending the endothelium but Uttle support. The prussian-blue granules could be traced directly through the endothehnl wall of the sinus, and a few were identified lying free in the lumen. The conditions of the observations, permitting a flow of venous blood through the sinus, undoubtedly accounted for the fact that but few of the granules were found Ijing free in the sinus. This passage of the replaced fluid into the lateral sinus is portrayed in figure 21, taken from the pig embrj-o of 27 mm.
The same process of drainage of cerebro-spinal fluid may be obser^-ed in pig embryos more than 23 mm. in length. In all but one particular it corresponds exactly to the process observed in adult laboratory animals. There is the same lack of absorption on the part of the cerebral veins and embryonic capillary plexuses. In the adult, however, the process is not diffuse, but is confined to the arachnoidal villi, while in the embryo a considerable extent of the inner wall of the sinus lying in the mesenchymal tissue, which is breaking down to form the arachnoidal spaces, serves as a site for the fluid passage. In these earlier stages the sinus transversus functions as the chief sinus of absorption. This is probably to be explained by the primary basilar spread of the replaced cerebro-spinal fluid and also by the fact that the true sinus sagittalis superior is a much later addendum. In the human embryo, according to Streeterf^^), it is found in stages of over 50 mm.
The absorption of cerebro-spinal fluid in the embryo seems to follow the directing agencies which operate in the adult. Increase in the pressure employed in the injection of true solutions results in the drainage of more of the fluid, as determined by subsequent microscopical examination. This suggests that the process is determined by factors other than that of difTusion; it seems most likely that here, too, the process is one of filtration, with a possible distension of the cellular membrane, so that intercellular spaces are opened. The histological picture of the sinus waU, however, undoubtedlj^ gives the impression that the fluid has passed almost solely through the cytoplasm of the endoth(>lial cells and likewise through the layer of supporting mesenchyme. These findings are in accord with observations on the adult.
With dilute suspensions of india ink as the injection mass, the results are quite different in regard to the passage of the material into the sinus. Replacement experiments making use of this suspension of particulate matter yield no evidence, as the carbon granules do not leave the ventricular system. Likewise, simple injections of the suspension into either the central canal of the spinal cord or into the perispinal spaces furnish no information unless the syringe-pressure be high. In this case the carbon granules may be traced into the sinus transversus, which is apparently the point of least resistance. Because of the obscuring of the picture by the carbon it can not be determined histologically whether the granules pass into the sinus in the same manner as does a true solution, or whether the passage is effected by numerous small ruptures of the tissue. The impression gained from our study would incline one toward the latter view.
If the injection of india ink be made under very great pressure from a syringe, the segmental veins may be filled with the carbon. This filling is always subsequent to its flow into the sinus transversus. But in no case was an evidence of a flow into lymphatic channels observed.
The process of drainage of the cerebro-s])inal fluid into the venous system of fetuses will not l)e (U^tailed here. This undoubtcdl}' concerns a study of the formation of arachnoidal villi and of the differentiation of the lateral walls of the superior sagittal sinus, the best site for this study. The material at hand is not suited for this investigation, so that postponement is necessary.
XI. The Chorioid Plexuses and the Elaboration of Cerebro-Spinal Fluid
With the realization that at a definite period in embryonic Ufe, cerebro-spinal fluid passes from the cerebral ventricles into the periaxial spaces, it seemed desirable to ascertain what relationship existed between the developing chorioid jilexuses and the elaboration of the fluid; for with the extension of the fluid into the periaxial tissue it becomes obvious that the balance between the development of the intraventricular fluid and the volume of the ventricles is destroyed and that more fluid is being elaborated than can be contained within the medullary-canal sjstem. This relationship between the ventricular volume and the production of cerebro-spinal fluid has been described at some length in a preceding section of this communication.
The determination, then, of the exact role plaj-ed by the chorioid plexuses in the further extension of the fluid into the periaxial tissue appeared to be of importance, for it could be conceived that the embryonic ependymal cells might be capable of elaborating the excess of fluid. With this purpose in mind the chorioid plexuses were investigated from morphological and cytological standpoints, in the hope that some index might be afforded as to the assumption of function on the part of the developing chorioid plexuses. These methods of study were apphed solely to the chorioid plexuses of pig embryos, for it is from them alone that evidence of the period of extraventricular extension of the cerebro-spinal fluid has been obtained.
THE DEVELOPMENT OF THE CHORIOID PLEXUSES.
The development of the chorioid plexuses is so well understood that only a ven,brief outline will be given here. The general scheme of origin of these glandular structures concerns a gradual histological differentiation in certain localities of the ventricular ependyma. The ependyma of the roof of the fourth ventricle thickens along the transverse invagination (phca chorioidea) and then gradually becomes tufted in villous projections into the ventricle, following the ingrowth of a capillar^' plexus and supporting mesenchyme. This general process of differentiation occurs at first along the lateral portions of the phca; the central portion of the ependj-ma remains unaffected by the villi even when the tufts have become quite well differentiated (fig. 23).
Quite similar to this process of development of the plexus chorioideus of the fourth ventricle is the differentiation of the other plexuses. The plexus of the third ventricle develops as an infolding of the tela chorioidea of the roof. In every case the process holds of ependymal invagination and subsequent vascularization and suspension by mesenchymal ingrowth.
The histological differentiation of the ependjTnal cells into the glandular tj-pe of plexus, as first determined by Luschka^^ and Faivre^^^)^ jg hardlj- satisfactory as an index of the production of fluid, as the secretory phenomena of the adult cells have not as yet bten completely established. The researches of Pettit and Girard(^\ dealing with the correlation of histological changes in the chorioidal cells and their functional state, first furnished reliable evidence that these cells give rise to cerebrospinal fluid. Since the publication of their investigations in 1900 many workers — Meek(37), Findlay("), Pellizzi(2^, Mott^^D, Hworostuchin(26), Engel(>2), and othershave been concerned with this problem and have established on fairly definite bases the relationship of the plexuses to the production of the fluid. The histological appearances of the secretory cells, however, does not rest on incontrovertible ground, as has been stated in a previous paper^^^).
The process of diff'erentiation of the ependymal cells which form the glandular elements of the chorioid plexuses occurs with the invagmation and tufting of these structures. The various stages of transformation from the low type of cubical epithelium constituting the ependymal layer are shown in various figures in this paper. The nuclei of these cells assume basilar positions and the outer zones of the C3-toplasm become granular with their greater height. The process is rather a slow one, as might be expected from the fact that the whole villus is gradually enlarging and becoming more and more tufted.
The histological differentiation of the plexuses need hardly concern us here, except as an index of the assumption of function. The final completion of this change into the adult morphology occurs at a much later stage of development than our evidence indicates for the establishment of a cerebro-spinal circulation. It becomes obvious, then, that the final liistological changes are not necessary for the process of elaboration of the fluid. This assumption seems warranted also.b} the fact that the embryonic fluid contains much more albuminous material than does the adult fluid.
The time of appearance of the chorioid plexuses in relation to the extraventricular spread of the fluid would surely seem to offer undoubted evidence in regard to the first elaboration of the fluid by the plexuses. It has been shown that in pig embryos over 14 mm. in length the replaced solution in the cerebro-spinal system spreads from the roof of the fourth ventricle into the periaxial tissues. This extraventricular extension occurs practically simultaneously with the first indications, in the pig embryo, of the formation of the chorioid plexuses of the fourth ventricle. Thus, in a pig embryo of 14 mm., the primitive thickening and tufting of the ependyma of the roof of the fourth ventricle may be observed (fig. 32). In earlier stages no definite evidence of this developmental process is found.
From the first indication of a developing chorioid plexus in a pig embryo of 14 mm., the growth of the tufts progresses rapidly, so that at 18 nmi. the process is well advanced. In embryos of 20 mm. and over the tufts of the plexuses in the fourth ventricle are quite marked, as shown in figures 22, 44, 40, and 92.
The chorioid plexuses of the third and lateral ventricles api)ear at a somewhat later stage than do those in the more caudal v(>ntricle. Thus the first indication of their ai)i)earance in pig eml)ryos is found in si)ecimens measuring 19 mm. in length. This coincides well with the further extension of the replaced fluid in specimens of 19 mm. and over. The definite differentiation of these plexuses, however, does not actually take place until the embryo reaches a length of 23 mm.— a fact suggestive of some relationshif) to the complete periaxial spread found in embryos of this measurement.
Considered, then, as a whole, there seems to be a very definite relationship between the developing chorioid plexuses and the periaxial spread of the embryonic cerebro-spinal fluid; for immediately after the first appearance of chorioidal tufting in the roof of the fourth ventricle (at 14 mm.) the replaced injection sjjread appears in the periaxial tissue (fig. 3). This extraventricular spread does not become marked until a length of 19 mm. is attained (fig. 5) — a factor in accord with the elaboration of the villi in the chorioid plexus of the fourth ventricle. The periaxial spread remains localized in the rhombencephalic region until the 20 mm. stage is attained, when it rapidly becomes pericerebral and perispinal (figs. 6 and 7). This coincides with the first indications of the chorioid plexuses in the more cephalic ventricles. But the further spread is here delayed (as in the stages between 14 and 19 mm.) until a length of at least 24 mm. is reached — which is perhaps of importance in the further development of the cerebral plexuses and the greater elaboration of the cerebro-spinal fluid. Thus it seems possible to conclude that coincident with the first appearance of the chorioid plexuses a more rapid production of cerebro-spinal fluid occurs, necessitating the passage of the fluid into the periaxial tissues.
THE GLYCOGEN CONTENT OF THE CHORIOID PLEXUSES.
In the hope that some cytological method might afford direct and incontrovertible evidence of the time of the assumption of function by the chorioid plexuses, stains demonstrating the intracellular presence of glycogen were applied to these structures. The quantitj' of the starch in the chorioid plexuses of rat and mouse embryos, as shown bj' Goldmann, suggested that this substance might be associated with the early elaboration of the cerebro-spinal fluid. Furthermore, the presence in the adult fluid of a definite reducing bodj', demonstrated by XawTatschi to be dextrose, added some weight to the hope that a definite conclusion might thus be afforded.
Several important studies concerning the presence of glycogen in the cells of the embryonic and fetal chorioid plexuses have been made. Creighton W found that the glycogen of the chorioid plexus was verj^ abundant about the middle of embryonic life, while von Loeper concluded that the great content in the cells of the fetal plexus was characteristic. Goldmann^^o) found large quantities of gh'cogen in the plexus in rats and mice, not only in embryonic life but also in animals from two to three weeks old. In the adult plexuses the cells contained no trace of glycogen.
The observations here included were made after fixing the chorioid plexuses of various pig embryos in absolute alcohol and staining the sections (cut either from celloidin or paraffin blocks) by Best's carmine method. This technique is similar to that employed by Goldmann. The staining reaction is such that a very striking differentiation of the glycogen occurs, but the shrinkage of the embryonic tissue in the fixation in absolute alcohol is a disadvantage. In these obsers'^ations the plexuses from the fourth and lateral ventricles were used.
As shown in the table on page 94, glycogen could be identified in the cells of the chorioid plexuses in pig embryos varying in length from 28 to 155 mm.
Below the first measurement no glycogen was demonstrated bj' the method employed; above the higher limit in only one instance (series No. 41) was glyocgen found. This finding of a limited period in the embryonic hfe of a pig during which glycogen occurs in the cells of the chorioid plexuses does not coincide with Goldmann's observations on the rat and mouse. Furthermore, it was found here that in stages up to 100 mm. the glycogen was practically generally distributed throughout all the cells of the chorioid jilexus, occurring with gn^it regularity in every villus and cell. This general distribution was not found in the plexuses of embiyos over 110 mm. in length; in these more advanced stages the cells containing starch occurred in clumps, giving a localized distribution. In the stages under 100 mm. the glycogen was present in very large amount, as estimated histologically. As the stages advanced the quantity of glycogen decreased rapidly. This great amount of starch was present in the same stages in wliich the general distribution of the cells occurred.
Occurrence of glycogen in the chorioid plexuses of embryo pigs.
C.P.
series,
No.
C.R.
naeasure,
mm.
Glycogen.
Globular forms of glycogen.
Plaques of glycogen.
Amount of glycogen.
Distribution of glycogen throughout plexus.
Intracellular distribution of glycogen.
16 3 13 12 14 6 9 8 4 1 42 17 15 20 10 18 27 39 25 32 40 41 24 19 23 11 21 22 26 5
18
23
28
33
36
39
40
55
66
80
90
100
105
118
132
155
155
155
158
160
163
170
173
185
195
209
213
223
244
260
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present . .
Present
Present
Present
Present
Present
Absent
Present
Present
Present
Absent
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Present
Absent
Present
Present
Absent
Present
Absent
Absent
Absent
Absent
Absent
Great
Great
Great
Great
Great
Great
Great
Great
Great
Great
Small
Small
SmaU
SmaU
Small
SmaU
General
Localized
General
General
General
General
General
General
General
Genearl
General
Localized
General
LocaUzed
Localized
Localized
Basilar. Basilar. Basilar. Basilar. Basilar. Basilar. General. General. General. General. Basilar. General. General. General. General. General.
Absent
Present
Absent
Present
Absent
Small
Localized
General.
Absent
Absent
Absent
Absent
Absent
Cioldmann'20) pictures the glycogen as occurring throughout the cells of the chorioid i)lexus in the form of globules of larger or smaller size. Some of these globules may be .seen even in the s\n'rounding cerebro-sjiinaj fluiil. This general intracellular disposition was observed in this series in specimens measuring 6G mm. and over (fig. 95). Below this measurement the glycogen occurred practically entirely in the basilar portion of the cell, central to the nucleus. Furthermore, in the stages between 30 and 00 mm. the glj'cogen globules were present in but small numbers and the glycogen was found in crescentic plaques (fig. 96). This formation of definite plaques is ai)parently to be ascribed to the fusion of the globules when the amount of glycogen becomes extreme. As far as is known tliis plaque formation with glycogen has not previously been noted; in one of Goldmann's figures the fusion of some of the globules has apparently taken place.
The table on page 94 records the findings in these observations.
The occurrence of glycogen in the cells of the chorioid plexus only during a certain portion of embryonic life is, as shown l)y the foregoing table, a fairly definite phenomenon, but there is surelj' no indication that this temporary presence of the animal starch bears any relation to the assumption of function on the part of the chorioid plexuses. The evidence afforded by the extraventricular flow of the replaced fluid, with the apparent relationship of the developing chorioid plexuses to the periaxial extension of the fluid, argues strongly against such an assumption.
XII. PERIVASCULAR SPACES IN THE EMBRYO.
In 1865 His' using a puncture injection, found that each nerve-cell existed in a so-called space. These pericellular spaces connected, as demonstrated by the flow of the injection mass, with an extensive perivascular network, more complex in its gray matter than in the white. In all of His's cases continuation of the injection led to a peripheral spread toward the pia, both in the spinal medulla and in the brain.
]Mott('*^\ working on the brains of animals in which an experimental cerebral anemia had been produced by ligation of the head arteries, found the perivascular spaces enormously dilated and the perineuronal spaces Likewise verj' evident. Direct connections between the perivascular and perineuronal spaces are pictured in Mott's communication.
The deduction which ^Nlott made from his findings, regarding the possible absorption of cerebro-spinal fluid by the cerebral capillary bed from this perivascular and perineuronal system, was discussed by the present author in a paper two years ago(55). It was there shown that, with the use of true solutions as the injection (potassium ferrocyanide and iron-ammonium citrate), the whole perivascular sj'stem could be filled. This injection of the spaces, however, occurred only when the pressure conditions within the cranial cavity were such that the subarachnoid pressure exceeded the vascular tension. This reversion of the pressure relations was accomplished by maintaining at normal the subarachnoid pressure with the injection fluid, and occasioning a simultaneous and complete vascular anemia. Under the routine conditions of injection (with undisturbed pressure relations) no injection of the perivascular system from the subarachnoid space resulted. It was found impossible to mject the perivascular system, using granular suspensions as the injection mass, without employing pressures far above the normal.
From these results here recorded briefly, the belief was expressed in tliis former paper that each nerve-cell was surrounded by a capillary space which drained along the perivascular channels into the subarachnoid spaces. Probably this sj'^stem represents a mechanism for accessory tissue drainage comparable ph3^siologically to the lymphatic channels of the other parts of the body.
In view of these findings in the adult mammal it seemed desirable to ascertain at what period of intra-uterine life such function was acquired. It also seemed not unhkely that information of interest might be acquired from the embryonic intramedullary circulation which would amplify our knowledge of this sj^stem in the adult. It was thought that there might be a correlation between the production of the perivascular fluid and the enlargement of the subarachnoid channels, similar to the evident connection between the chorioidal invagination and the extraventricular spread of the fluid.
Experiments to demonstrate possible perivascular and perineiu-onal spaces were first attempted on rather large fetuses (pig), as follows: The spinal meninges were exposed in a fetus in which the heart was still beating vigorously. Into the spinal subarachnoid space was introduced a needle connected with a small reservoir, containing the injection solution (potassium ferrocyanide, 0.5 gm.; iron-ammonium citrate, 0.5 gm. ; water, 100 c.c). The reservoir was then adjusted so that a pressure of 160 mm. of water was maintained in the subarachnoid sjiace. The arteries and veins in the neck of the fetus were then severed, and the subarachnoid pressure maintained at its former level. At the end of 20 minutes the head was placed in a fixative containing 1 per cent hj^drochloric acid.
This procedure, as outlined above, in the adult laboratory mammal, usually resulted in a complete injection of the perivascular system. In the embryo, however, the procedure was uniformly unsuccessful. The injection solution, as shown subsequently by the precipitated prussian-blue, rarely ascended over a centimeter above the point of injection. This indicated that the existent cerebro-spinal fluid was not replaced by the injection solution, and that the failure to demonstrate the perivascular system was to be explained on this basis, if the system were functional at this stage. Attempts were then made to replace the subarachnoid fluid with the injection solution before the cerebral anemia occurred. These attempts likewise m^t with failure, because of the impossibility of keeping the heart beating for any length of time in the larger pig fetuses. Other attem]its were also made to demonstrate these channels, in larger pig embryos, by means of a procedure which in the adult gave at times good injections of these intracortical canals. This method differed from the method first employed only in the maintenance of a high pressure (100 mm. Hg) in the spinal subarachnoid si)aces. It likewise met with failure, due ai)|)arently to the same causes which occasioned its failure in the adult: the high sul)arachnoid pressure o})erated chiefly to compress the cerebral and spinal tissues, rendering the injection of the i)erivascular spaces impossible.
The same procedures were attempted in smaller pig embryos (15 to GO mm.). The method usually successful in demonstrating the spaces (subarachnoid pressure slightly above normal, with subsequent cerel)ral anemia) failed, ajjparently because the cranial cavity at these stages is in no sense a rigid closed box. as in the adult.
Any method of service in the adult — which must have in consideration the physical character of the skull as a closed box — was here necessarih' doomed to failure.
Together with these technical failures to demonstrate a perivascular system, it must be borne in mind that these are merely failures to demonstrate the existence of the perivascular system in the pig embryo. The system wall probably be demonstrated as soon as a suitable technique is devised. The spaces are very likely present soon after the capillary plexus invades the nervous system, but the observation in many histological preparations of the spaces around the cerebral vessels must not be considered as offering proof of their existence, because of the likehhood of shrinkage influencing the picture. It is interesting, how^ever, to note that elasticity of the cerebral tissues seems greatest along the course of the blood-vessels, for here the phenomenon of shrinkage is most frequently observed. The existence of the perivascular and perineuronal spaces, probably of only capillary thickness, must remain — in the embryo as in the adult — a subject of physiological demonstration; histological e\adence, except with proper physiological regard, is of no value.
The early development and function of such a system as the perivascular and perineuronal canals afford seems most likely from the standpoint of pure speculation. It is not improbable that fluid is poured from this system into the embryonic subarachnoid space at a period soon after the capillary plexus invades the cerebrum. There is no evidence, however, from the observations recorded in foregoing paragraphs, that adequate subarachnoid channels are afforded until the pig embryo reaches a length of about 25 mm. The hypothesis of Essick^^^) regarding the damming of the perivascular fluid as the cause of the two cava corporis striati is of extreme interest in this connection. It remains, however, for future work to afford real evidence in regard to the embryonic perivascular system.
XIII. THE PERINEURAL SPACES IN THE PIG EMBRYO.
The question of the existence of potential or actively functional spaces around the peripheral nerves is of great interest, partly because of the possible relation of these spaces to the developing lymphatic system, and also on account of the anatomical evidence of the possible existence of such spaces.
It is realized that before much dependence can be placed on any theory regarding these potential spaces around the cerebro-spinal nerves, the possibility of their being purely artifacts must be dealt with. The methods of demonstration, in the adult, in the hands of the earliest workers were such as to favor the production of artifacts. As far as can be ascertained, Cotugno('), dealing with the ner\-us ischiadicus, was the first to conceive of these possible spaces. His method of demonstration consisted in filling the spinal subarachnoid space with mercurj' (in a cadaver placed in the erect posture) . Globules of the mercurj^ were subsequently found about the sciatic nerve in what then became the perineural spaces.
Modern anatomical interest in these spaces was aroused by the remarkable injections of Key and Retzius^^s). These investigators, by means of gelatin injections into the spinal subarachnoid space, were able to demonstrate perineural spaces around the cranial nerves, especially around the optic pair. Their results, however, are open to criticism, because of the excessive pressures employed ("not over 60 millimeters of mercury") and because the injections were made in fresh cadavers kept warm for periods of 10 or more hours.
Some of the difficulties concerned in the problems of the perineural spaces were cleared up in a studj'^^^) of the cerebro-spinal circulation published in 1914. In this work injections of true solutions (similar to those used in the present study) were introduced into the spinal subarachnoid space in living cats and dogs, under pressures but sUghtly exceeding the normal intraspinal tension. These injections were continued for several hours, and the course of the injection fluid was then estabhshed bj precipitating the solution in situ. By means of this procedure, which it was beUeved approached the physiological, the perineural spaces around the cranial nerves could be demonstrated. In these adult laboratory mammals the cerebral nerves without exception showed prussian-blue granules in a perineural relation, extending outward along the nerves bej^ond the termination of the dural cuff. This extension of the injection mass outward was more striking around the first two cranial nerves than about any of the others. Thus, the olfactorj'^ nerves uniformlj'^ showed perineural deposits beyond the cribriform plate, extending downwards into the nasal epitheUura, while the optic nerves were surrounded by the granules in the inf ravaginal sheath, which spreads out over the posterior surface of the eyeball. The caudal cranial nerves were likewise characterized by extensive perineural injections.
These findings were interpreted as e\'idencing a true perineural space, probably of only capillary thickness, which could be injected by filling the cerebro-spinal spaces with a demonstrable true solution. As far as could be made out under the microscope, they had no appreciable existence except when fiUed with the precipitated true solution. These spaces were not filled in the early moments of the injections under low pressures, and could be demonstrated only when the injection had been continued for several hours.
The perineural spaces are quite different from the spaces surrounding the spinal ganglia and the gangUa of the cranial nerves. These ganglia he in the true subarachnoid space, wath the dura investing the arachnoid membrane. Distal to the ganglion the dura ends upon each nerve. In the injection under low pressure with the ferrocyanide the cranial and spinal ganglia were all surrounded bj^ the precipitated salts; the cranial nerves .showed extensive perineural injections, whereas the spinal nerves rarely showed a true perineural injection, and then only of hmited extent.
The existence of perineural spaces in the embryo, however, has been under dispute. The larger nerv^es in sectioned embryos almost invariably show spaces about them, either a complete separation of the surrounding mesenchyme or a partial dilatation of the mesenchymal interstices. Sabint'*^), in 1902, noted that in pcrispinal injections with inflia ink the spinal nerves could be outlined by the carbon granules, but in no case did such an injection run into true lymphatic channels. No evidence was afforded by her work of any lymphatic channels arising from these apparent perineural channels.
In the course of this investigation of the cerebro-spinal spaces interest naturally turned to the perineural spaces. In the typical experiments (a replacement of the embryonic cerebro-spinal fluid with a demonstrable true solution in the living embryo), there was evidence of a spread of the replaced solution around the cranial nerves. Because of the procedure u.sed (merely a filling of the ventricles and central canal of the spinal cord) no evidence of a perineural spread occurred until the foreign solution passed into the periaxial tissues. Here the spread chiefly involved the caudal cranial nerves curving around the lateral surface of the medulla in fanshaped processes (figs. 5, 6, 8, and 9). The spread, however, was not extensive. In figure 8 a similar slight spread along the spinal nerves is to be made out. Closer study of these cleared specimens, and examination of the same and of similarly injected embryos after serial sectioning, convinces one that the apparent perineural spread in these cases extends around the sensory ganglia and not further toward the periphery. In no case, cither in the caudal portion of the cranial or in the spinal region, has the replaced injection fluid passed the blastemal condensation of mesenchyme. This finding is well shown by the distribution of the injection fluid in figures 9, 16, and 18.
The optic nerves, however, jjossessing gangha in the retina, usually show, in the typical replacements in the living embryo, a partial or complete surrounding of the nerves by the prccij)itated pru.ssian-bluc. An incomplete example of this — more typical, according to these observations, than a total circumvention — is given in figures 19 and 20. The higher-power reproduction of this field is very interesting. It shows in the central portion the fiber bundles comprising the optic nerve, surrounded by mesenchyme and the developing ocular muscles. In the region between the ners'e and the muscles is an undiflferentiated mesenchyme which is characterized by a crescent of the precipitated granules of prussian-blue. The non-penetration of the surrounding tissue by the ferrocyanide is verj- well brought out in this drawing. The prussian-blue has reached its position about the nerve by extension from the pericerebral spaces; actually it has still the same distribution as noted in figure 8 above. The adult dura will completelj- surround the optic nerve in its whole extent; the subarachnoid space will likewise extend unbroken to the posterior surface of the eyeball. Hence it must be assumed that in this case the perineural space does not extend beyond the peripheral ganglion. With regard to the olfactory nerves, no evidence of a perineural spread was obtained in specimens of pig embryos up to 45 mm. in length.
It seems obvious, then, that in the embryo pig true solutions, when substituted for the cerebro-spinal fluid, do not extend peripherally along the nerves any further than does the dura in the adult. The replaced fluid (if, as appears most likely, it indicates the true circulation of the cerebro-spinal fluid) extends only through the future subarachnoid space. Such a conclusion is best supported bj' the observations. The only discreparcv between the findings in the pig embryo and those in the adult with the same method lies in the fact that in the adult the cranial nerves showed a much more extensive permeural injection. This seeming discrepancy may be accounted for in two ways. In the first place, the experimental replacement in the embryo pigs lasted at most one hour (due to the fact that the embryo's heart frequently ceased beating at the end of this time), while in the adult cat or dog they were continued for several hours; and it was only in the long-continued experiments in the adult that the extensive perineural injections were obtained. On this basis it seems more than likely that the communications between peripheral perineural spaces and the subarachnoid space are very small and that diffusion must account for the slow filhng of the peripheral system. The second explanation seems undoubtedly to concern the time of development of these perineural spaces in the embryo. It may be that the spaces are morphologically non-existent until late in fetal life; in that case, of course, it is not strange that they have not been filled wdth the injection fluid.
From the observations recorded above it is quite apparent that in the typical experiment in which the normal cerebro-spinal tension is not increased no evidence of the perineural space, as injected by Miss Sabin, has been adduced. However, the possibility of mjecting these spinal spaces as was done by Miss Sabin is easily demonstrated. The injections may be made with ease, either with . granular suspensions or with true solutions. Success invariably attends such an injection into the perispinal tissues. The injection solutions easily run out around each nerve, more readily, apparently, in the younger embryo than in the older. It is not clear whether this difference is due to the fact that in younger embryos the resistance is greater to the perispinal flow and less peripherally, or merely to the fact that a greater amount of fluid must be introduced in order to attain the same result. Careful repetition of these observations has led to the conclusion that such a demonstration of the spinal perineural spaces results from excessive pressures of injection. WTienever the pressure exerted by the injection is but slightly above the normal, or does not exceed the normal (as in replacements), the perineural spaces are not injected around the spinal nerves. Miss Sabm's conclusions from her results, that no connection exists between the spaces and the lymphatic system, seem to be wholly substantiated by these observations.
The apparent perineural spaces around the embryonic nerv^es must be looked upon as artifacts. In tissue carefully fixed, dehydrated, and embedded, there is no real evidence of these spaces. Theh- size apparently varies with the care observed in the histological technique.
XIV. General Summary
In the foregoing sections of this communication some of the problems concerned with the embryology of the cerebro-spinal spaces have been discussed and observ^ations have been presented in the hope that a better conception of the processes might obtain. It is purposed to present here briefly the results of these obser^^ations and to attempt to correlate the findings so far as is possible; and in this, as in the detailed reports in the preceding pages, the relationship of the jihysiological processes concerned will be referred to the morphological changes in the developing embryo.
As a means of studying the physiological extent of the embryonic cerebro-spinal spaces, a method of replacing the medullary fluid with a foreign solution was devised. The procedure consisted in substituting, in the hving embryo, a solution of potassium ferrocyanide and iron-ammonium citrate for the cerebro-spinal fluid. The embryos were then kept alive, for periods of about an hour, by placing them with the attached placenta; in an incubator at 38°. At the end of this time, which varied in the many experiments, the whole embryo was fixed in a medium containing hydrochloric acid, thereby precipitating an insoluble prussian-blue. Specimens prepared in this manner were studied after sectioning or after clearing by the Spalteholz method.
Pig embryos, subjected to such experimental replacements, exhibited only an intraventricular retention of the foreign solution until after a stage of 14 mm. was attained. In the earliest specimens, embryos of about 9 mm., there was no characteristic distribution of the foreign solution, except that it remained within the medullary-canal system. In stages of about 13 mm. the replaced fluid also was retained within the cerebral ventricles, but in these specimens a dense accumulation of the precipitated prussian-blue may be made out in a distinct oval in the superior portion of the rhombic roof. This granular aggregation occurs against a histological differentiated area in the roof of the fourth ventricle — an area which represents apparently the more epithelial-Uke elements of the earher roof-plate. This area must be considered solely as a differentiation of the epidermal lining of the medullary-canal system.
In hving pig embryos of 14 mm. and over, the result of the routine replacement of the ventricular cerebro-spinal fluid was a sUght extraventricular spread into the tissues posterior to the rhombic roof. The passage of this foreign solution outward occurred through the same area of ependjTnal differentiation, outlined bj' the collection of granules against its inner surface in the previous stage. The extraventricular spread remains definitely locahzed to a ver}' small conical area which does not rapidly increase in size.
The factors which cause this initial flow into the pericerebral spaces are of interest. It follows that in the growth of the embryo the production of the intraventricular and intraspinal cerebro-spinal fluid must necessarily keep pace with the increasing size of the cerebral ventricles. It is also necessarj' for the occurrence of an extraventricular spread of the fluid that the production of the fluid within the ventricles must exceed the amount required to keep the medullary-canal sj'stem filled. From our knowledge of the elaboration of the adult cerebro-spinal fluid, it is impossible to conceive of the production of a true cerebro-spinal fluid in the perimcdullary mesenchyme. Such a view would be a reversion to the old hypothesis of Haller, who regarded the leptomeninges as the elaborators of the fluid. Likewise, the passage of the replaced foreign solution into the extraventricular spaces would render such a hjiDothesis untenable.
Hence, it becomes incumbent to regard such an extraventricular spread of the experimental solution as an mdication that the production of the cerebro-spinal fluid within the cerebral ventricles exceeds the capacity of the ventricles to care for the fluid. This argues strongly that the process of elaboration of the fluid in these pig embryos of 14 mm. is no longer sluggish, but that an active production, sufficient to cause a sUght extraventricular flow during the observation, is now taking place. This acceleration of the flow is not great, but it represents a marked change in the relationship of the process of fluid elaboration to the increasing volume of the ventricles.
It seemed desirable to endeavor to correlate this extraventricular spread of the experimental fluid with the morphology of some intraventricular structure at this critical stage of 14 mm. m the pig embryo. The first evidences of villous tufting in the chorioid plexus of the fourth ventricles were found to occur at this stage in the pig. Other studies of this plexus, particularly those which concerned the occurrence of glycogen in these glandular cells, were found to offer no additional evidence of value in regard to the onset of function in these structures. The correspondence between the initial tufting of the ependyma to form the rhombic chorioid plexuses and the initial extraventricular spread must be regarded as of the utmost importance. It would appear most Ukely that as soon as the chorioid tufts occurred an increased production of cerebro-spinal fluid took place, necessitating an extraventricular expulsion of the excess of fluid. Such a view receives the utmost support from these recorded observations; it is in keeping with the best conceptions of the processes of production of cerebro-spinal fluid in adult mammals.
With the initial pericerebral extension of the experimental fluid occurrmg in pig embryos of about 14 nmti., the further extension of this spread did not occur until after a length of 18 mm. was attained. At this stage the replaced foreign solution passed from the fourth ventricle through two areas in the roof-plate. The chorioid plexuses now have divided the roof into two portions; from each, fluid escaped. The superior area of fluid passage is the same which was concerned in the mitial outpouring of the ventricular fluid. The inferior area, like the superior, is an area of ependymal differentiation, of which the first evidence may be made out in pig and human embryos of 15 mm. This differentiation consists in the transformation of the densely staining ependymal elements into cells with larger nuclei, poor in chromatin, and with more abundant cytoplasm.
After the functional employment of the two membranous areas is established at about 18 mm. in the pig, the further pericerebral spri.'ad of the replaced solution occurs very rapidly. The peribulbar tissues are filled with the fluid and from this region extensions occur downward into perispinal spaces and upward into the more basilar pericerebral spaces. Thus, the spinal spaces must be considered as develop>ing physiologically from above, and not from below upward, as Reford found. The complete filling of these perispinal spaces is found in pig embryos of 21 mm. At this stage the pericerebral spaces are filled, with the exception of those around the superior portion of the midbrain and about the cerebral hemispheres.
The final filling of all the periaxial spaces occurred in pig embryos of about 26 mm. This phenomenon may be taken to indicate the estabUshment of the true cerebro-spinal relationships of the adult, for in this case there is an intraventricular production of the fluid and an extraventricular spread. Likewise, the fluid returns to the venous system in embryos of over 23 mm., and this escape of the fluid from its periaxial bed is, as in the adult, directly into the venous sinuses of the dura mater.
The rapidity of the further extension of the replaced solution after the stage of 18 mm. is passed is apparently due to a second marked acceleration in the rate of production of the ventricular cerebro-spinal fluid. As in the first instance, this increased elaboration seems connected intimately with the formation of the chorioid plexuses of the third and lateral ventricles. As soon as these tufts develop, the cerebro-spinal fluid is produced in amounts which far exceed the quantities for which the more slowlj' enlarging ventricles can provide.
The histories of the two areae membranaceae of the fourth ventricle are dissimilar. Both are areas apparently differentiated from the normal lining ependyma for a specific functional purpose — the passage of fluid from the ventricles into the future subarachnoid spaces. The superior membranous area reaches its maximum functional importance in the stages of 18 to 20 mm. in the pig and also in the human embryo and from these stages on it slowly regresses. The final obUteration of the area, if it do not persist as an occasional small remnant, is due to the increasing growth of the cerebellum and the enlargement of the chorioid plexuses of the fourth ventricle. On the other hand, the inferior membranous area continues to increase both in size and functional importance after its initial differentiation from the ependyma; it finally occupies the greater portion of the velum chorioidea inferior. These observations can not solve the interesting question of a perforation of the inferior velum to form the foramen of Magendie.
Of the factors which influence the passage of fluid outward into the periaxial spaces, it must be reahzed that probably there is difference in this regard between the true solutions of the salts and the colloidal suspensions. For the true solutions (as in the experimental replacements) diffusion probably plays some role; but that this is not the sole factor is shown by the failure of the fluid to pass through the membrane in the stages under 14 mm. The findings of the granules of prussian-blue within the cytoplasm of the cells of this membrane mdicates that the fluid passage is similar in every way to that through a true membrane. There is also a possible site of fluid passage between the cells of this membrane. But, surely, the most important factor in this process is one of filtration of the fluid from the point of higher pressure to one of lower. This is mdicated by all of the findings : that the mcreased production of the fluid or the increased mtra ventricular pressure (whether due to normal or experimental agencies) causes a marked extraventricular spread seems firmly established. For the colloidal suspensions (particularly the protein of the normal ventricular fluid) a slower process of diffusion and filtration seems the probable agencj^ for passing the ventricular colloids into the subarachnoid spaces.
That the results obtained by the method of replacement were not solely due to diffusion, but represent a fiUing of the physiological extent of the cerebro-spinal spaces, has been shown in many ways, but probably the chief argument against such a view is that whollj^ similar extensions of the foreign solution may be obtained by injections under mild pressures from a syrmge; with increasing pressures these injections show the same type of spread, but always in a smaller embryo than the replacement method demonstrates as the standard for a given stage of the extension. The results recorded in the foregoing pages indicate also that suspensions (India ink) and true solutions (when powerful precipitants) are valuable only for affording comparisons in problems concerning the normal processes of absorption.
Of interest in any discussion of the results of injections into the perispinal spaces or into the spinal central canal are the findings in regard to the perineural spaces. It is possible to inject such spaces around each of the segmental nerves, but only when the pressures of injections are extreme. In no case, however, were such injections found to enter the lymphatic system — a finding in accord with the observations of Reford and Sabm. The physiological importance of these spaces in the adult is probably great, but the same methods of demonstration (with carefully controlled pressures) which suffice in the adult are unavailing in the embryo.
The origin of the three meninges from the perimedullary mesenchjTue is well established. His, Kolliker, Sterzi, Farrar, and others have placed this conception on a very firm basis. Most of the investigators have been concerned with the differentiation of the spmal meninges, while the observations here reported have been concerned solely with the cranial portion of these membranes. In general, the same phenomena in the transformation of the primitive periaxial mesenchyme as recorded by these earlier workers may be found in the cranium. The division of the primitive mesenchyme by a secondary condensation, a view advanced chiefly by Salvi, seems well supported. The findings in the cranium are in accord with this concej^tion; the outer portion of this primitive meninx becomes the dura mater, the inner forms both the pia and arachnoid. The processes in the formation of the arachnoid are, however, quite diversified and concern both the formation of the subarachnoid spaces and the outer membrane of the arachnoid.
Out of the rather loose-meshed periaxial mesenchyme, the subarachnoid spaces develop. The process concerns the transformation of the small " tissue spaces " of this mesenchyme into the larger subarachnoid channels, which are interrupted by the well-known arachnoid trabecule. Well-marked stages in this metamorphosis, which begins in the basis cranii, can be made out. The first appearance of a differentiation is seen in the gradual increase in the size of the mesenchj'mal mesh. This is closely as.sociated with an increased amount of an albuminous coagulum which in a measure fills the larger interstices. Following this initial dilatation of the spaces occurs a breaking-down of some of the syncytial strands; these ruptured mesenchymal processes then retract and adhere to the persisting trabeculue. The process continues with the formation of larger channels in this mesodermal tissue, with also the formation of the permanent arachnoidal trabeculae. Throughout these larger spaces, in the smaller fetuses, the coagula of protein material are everyT\-here found, the remains apparently of the albuminous portion of the circumambient fluid.
In the formation of the various cisternse, particularly the great cistema cerebellomedullaris, the process of the dilatation and confluence of the original mesenchymal spaces reaches its maximum. Here the breaking-down of the original sjTicytial strands proceeds to such an extent that very few of the strands remain to persist through life.
Such a process of the enlargement of mesenchj^ual spaces to form the larger subarachnoid spaces, as described in some measure by His for the spinal meninges, is apparently intimately connected with the circulation through these spaces of the embryonic cerebro-spinal fluid. The fluid flows everj- where through the spaces, as evidenced by the replacement experiments and by the increased content in albumen, before the process of enlargement of the mesenchymal spaces begins. It seems most likely that this circulation of the fluid acts as the causative agent in initiating and probably also in completing the enlargement of the "tissue spaces." The great content of albumen in the embryonic cerebro-spinal fluid has greatly facihtated the investigation, as the presence of the coagula from this protein has permitted the absolute exclusion of artifacts in the process of the tissue-dilatation.
This mechanism of enlargement of the tissue spaces finds its analogue in the formation of the anterior chamber of the eye and in the perilymphatic spaces of the ear (Streeter). In both these situations, as in the meningeal spaces, there are special body-fluids, more or less characteristic in their physical and chemical characters, obviouslj' subserving specialized functions. In both the eye and cranium, the absorption of the fluids is by way of special organs, directly into venous sinuses; in both, the origin of the specialized fluid is from epidermal organs; this fluid is at fijst poured into epidermal spaces and then subsequently into mesodermal spaces (subarachnoid space and anterior chamber of the eye). Thus, m these situations, the characteristic fluids have certain definite channels through rather larger spaces, connected finally with the venous system, and only indirectly with the Ij-mphatic system.
In no sense must the cerebro-spinal circulation be taken as a portion of the lymphatic system. Increasing knowledge of the cerebro-spinal fluid, of its physiology and chemistry, and of its pathway, have separated it permanently from any connection with the lymph of the lymphatic system, variable though that be. No longer may the meningeal spaces be compared to serous cavities, except possibly in the case of the subdural space, and this space is really a space apart from the true cerebro-spinal or subarachnoid spaces. Quite s i milarly, in place of the many varjdng channels in the dura and to a lesser extent in the leptomeninges, which older writers considered lyonphatic in nature, our increasing knowledge has caused the introduction of specialized arachnoidal cell-chains running throughout the pachymeninx. Unquestionably, the cerebro-spinal fluid i)ossesses its own peculiar and characteristic pathway, analogous in no way to the lymphatic vessels of other tissues.
The outer continuous membrane of the arachnoidea forms as a mesenchymal condensation, at first in common with the inner surface of the dura mater, but soon separated from it by the subdural space. The very low cubical mesothelium which covers the arachnoid membrane on both surfaces and also invests the arachnoid trabeculae differentiates apparently from the original mesenchymal elements in the periaxial tissues.
One of the most interesting features of this study has been the relation of the various mesenchymal condensations to the foreign true solution which was introduced into the medullary-canal system. This fluid circulated throughout the periaxial spaces which enlarge to form the subarachnoid channels, but it never penetrated the primary blastema which served as a primitive dura, nor did it ever invade the pial cells which so closelj^ adhere to the nervous tissue; hkewise, as soon as the secondary mesenchymal condensation dividing the dura from the arachnoid spaces appeared, this condensation was impervious to the true solution. No evidence of any penetration, as might be expected as due to diffusion, could be made out.
This summary has been included in order that some correlation between the topics discussed separately in the foregoing sections might be made. No attempt has been made here to present the findings in abstract form; these have been summarized at the end of each division of this communication.
XV. Conclusions
Based on the observations recorded in the foregoing sections, the following conclusions seem warranted:
- During the earlj' part of the growth of the pig embryo there is no extraventricular spread of the cerebro-spinal fluid. The first extension of the ventricular fluid into the periaxial tissues occurs in pig embryos of 14 mm.; the adult relationship of the ventricular and meningeal cerebro-spinal fluid is established in pig embryos of about 26 mm.
- The ventricular cerebro-spinal fluid escapes into the periaxial tissues through two areas of ependjmal differentiation in the roof of the fourth ventricle. Both of these areas differentiate at a shghtly earlier period than that at which they function actively. The area membranacea superior undergoes a gradual regression and obliteration due to the changing form of the rhombic roof; the area membranacea inferior gradually occupies the major portion of the velum chorioidea inferior.
- The embryonic cerebro-spinal fluid, as evidenced by the replacement with a true solution, spreads from the ventricles into the mesenchymal tissue about the central nervous system. It docs not penetrate the cranial or vertebral blastemal condensations, nor does it invade the pial cellular layer.
- The subarachnoid spaces arise by a process of breaking-down of the perimedullary mesenchj'mal sj'ncytium and a dilatation of the existent mesench3Tnal spaces. This phenomenon of the enlargement of the mesenchymal spaces is associated with the presence in the spaces of an increased amount of albumen. The process occurs at a period shghtly later than that at which the initial flow of the cerebro-spinal fluid into the spaces is recorded.
- The dura mater, arachnoid, and pia mater develop out of the perimedullary mesenchj'me. The arachnoid trabeculae are left by the breaking-down of the original mesenchymal strands, while the outer arachnoid membrane is formed, together with the inner surface of the dura, by a separate mesenchymal condensation. The dura develops between this secondary Une of condensation and the embryonic skull.
- There is indicated a very close relationship between the tufting of the chorioid plexuses of the fourth ventricle and the first extraventricular spread of the cerebro-spinal fluid.
- By means of the method of replacement it is possible to demonstrate perineural spaces as far out along the nerve trunks as the peripheral gangUa. The extensive injections of the perineural spaces along the segmental nerves are not obtained by the method of replacement.
The work, of which this paper forms the report, was done in the Anatomical Laboratory- of the Johns Hopkins Medical School. It was largely due to aid received from the Department of embryology of the Carnegie Institution of Washington that the completion and scope of this paper were possible. The wTiter gladly acknowledges his indebtedness to the Carnegie Institution. January, 1916.
Bibliography
Ad AMKiEwicz, Albert. Die Blutgef &S8e des menschlichen Rilckenmarkes. I Theil: Die Gefassc der RQckenmarks-Substanz. II Theil: Die Gefassc der Rlickcnmarksobcrflache. Sitjungshcrichte der k. Akad. d. Wiss. in Wien. Math.-naturw. CI., 1H81, Lxxxrv, pt. 3. 469; 1882, Lxxxv, pt. 3, 101.
Bardeen CR. XI. Development of the Skeleton and of the Connective Tissues in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
Bardeen, C. R. The development of the skeleton and of the connective tissues. In Kcibel and Mall, Manual Fluman Embryology, Philadelphia and Ix>ndon, 1910, I, 292.
BuAKE, J. A. The roof and lateral recesses of the fourth ventricle considered morphologically and embryologically. Jour. Comp. Neurol., Granville, Ohio, 1900, X, 79.
Canniec, a. Contribution & I'dtude de la voOve du quatrifeme ventriculc chez Ics mammifires; le trou de Magendie. Jour, de Mfed. de Bordeaux, 1897, xxvii, 547.
Cappelletti, L. L'^coulemcnt de liquide cerebrospinal par la fistule c^phalo-rachidienne en conditions normales ct sous I'influence de quelques m6dicaments. Arch. Ital. de Biol., Turin, 1900, xxxv, pt. 2. 463.
.L'efflusso del liquido cerebro-spinale dalla fistola cefalo-rachidiana in condizioni normali e sotto I'influenza di alcuni farmaei. .Vtti dell' / ccademia d. Scienze Med. e Nat. di Fcrrara, 1900, ^xxiv, 85.
Cathelin, F. La circulation du liquide ctphalorachidien. Paris, J. B. Bailli^re, 1912.
CoTL'GNO [CoTUNNius], DoMENico. De ischiade nervosa commentarius. Viennae, R. GriifTcr, 1770.
Creighton, C. Microscopic researches on the formative properties of glycogen. London, A. & C. Black, 1896-99.
CusniNO, H. Physiologische und anatomische Beobachtungen iiber den Einfluss von Hirnkompres-sion auf den intracraniellen Kreislauf und ilber einigc hiermit verwandtc Erscheinungcn. Mittcilungen a. d. Grenzgeb. d. Med. und Chir., Jena, 1902, ix, 773.
. Some experimental and clinical observations concerning states of increased intracranial tension. Am. Jour. Med. Sciences, Philadelphia and New York, 1902, cxxi\-, 375.
. Studies on cerebro-spinal fluid: No. 1. Introductory. Jour. Med. Research, Boston, 1914, xxxi (n. s. XXVI), 1.
Dandy, W. E., and K. D. Buckfan. An experimental and clinical study of internal hydrocephalus. Jour. Am. Mod. Assoc, Chicago, 1913, Lxi, 2216.
. Internal hydrocephalus: An experimental, clinical and pathological study. Aiii. Joiir. Dis. Children, Chicago, 1914, viii, 406.
Ekker, H. De cerebri et meduUte spinalis systemate vasorura capillari. Trajecti ad Rhenum, 1853.
Kngel, E. A. Uelx^r die Secretionscrscheinungen in den Zellen der plexus chorioidei des Mcnschen. Arch. f. Zellforschung, Leipzig, 1909, ii. 191.
Essick, C. R. Transitory cavities in the corpus striatum of the human embryo. Contributions to Embryology, 1915, II, No. 6, 95. (Carnegie Institution of Washington, Pub. No. 222.)
14. Evans, H. McL. The macrophages of mammals, Am. Jour. Physiol., Baltimore, 1915, xxxvii, 1.
15. Faivre, J.-J.-A.-E. Des granulationes miningiennes. Thfee de Paris, 1853. (No. 142, vol. 540.)
16. Farrar, C. B. The embryonic pia. .\m. Jour. Insanity, Baltimore, 190& 7 LXiii, 295.
17. FiNDLAT, J. W. The choroid plexuses of the lateral ventricles of the brain — their histolog}-, normal and pathological. Brain, London and New York, 1899, xxu, 161.
18. Frazier, C. H. The cerebro-spinal fluid in health and disease. Jour. Am. Med. Assoc, Chicago, 1915, LXIV, 1119.
19. Gaupp, E. Die Entwickelung des Kopfskelettes. In Hertwig's Lehrbuch der Entwickelungsgeschichte d. Menschcn u. d. W'irbcltierc. Jena, 1906, K od., 692.
20. Goldmann, E. E. V'italfarbung am Zentralnervensystcm. Berlin, G. Reimcr, 1913.
21. Haller, a. Elementa physiologix corp^jris humanis. Lausanne, 1757-78.
22. Hess, Carl. Das Foramen Magendii und die Oeffnungen an den Reces.iu.s laterales des \*ierten Ventrikels. Morphol. Jahrbuch, Leipzig, 1885, x, 578.
23. Hecser, C. H. The development of the cerebral ventricles in the pig. Am. Jour. Anat., Philadelphia, 1913-14, XV, 215.
24. Hill, L. Physiology- and pathology of the cerebral circulation. London, J. & A. Churchill, 1896.
25. His, W. Ueber ein perivasculiires canalsystem in den nervoseu Ccntralorganen. Leipzig, Engelmann, 1865. . Die Hfiute und Hdhlen des Korpers; ein academisches Programm. Basel, Schweighauser, 1865.
. Die Entwickelung des mcnschlichcn Rautenhirns, von Ende des ersten bis zum Beginn des dritten Monats. Leipzig, Hirzel, 1890.
. Die Hiiute und Hohlen des Korpers. Arch. f. Anatomie u. Physiol., Anat. .\bth., 1903, 368.
. Die Entwickelung des menschlichen Gesims. Leipzig, Hirzel, 1904.
26. HwoROSTUCHiN, W. Zur Frage uber den Bau des Plexus chorioideus. Arch. f. mikr. Anat., Bonn, 1911, Lxxvii, 232.
27. Jacobson, C. Unpublished.
28. Kadvi, Heinrich. Ueber die Blutgefisse des menschlichen RQckenmarkes. Lemberg, Gubrj-nowicz 4 Schmidt, 1889.
29. Key, E. A. H., and G. Retzius. Anatomie des Nervensystems und des Bindegewebcs. Stockholm, Samson & Wallin, 1875-76.
30. Knower, H. McE. a new and sensitive method of injecting the vessels of small embrjos, etc., under the microscope. Anat. Record, Philadelphia, 1908, ii, 207.
31. KoLLlKER, A. Entwiekelungsgeschichte des Mcnschen und der hoheren Thiere. 2ed. Leipzig, Engelmann, 1879.
. Gnmdriss der Entwiekelungsgeschichte des Mcnschen. I^eipzig, Engelmann, 18S0.
32. KoLLMANN, J. Lehrbuch der Entwiekelungsgeschichte des Menschen. Jena, Fischer, 1898.
33. Lewandowskt, M. Zur Lehre von der CerebrospinalflQssigkcit. Zeitachr. f. klin. Med., Berlin, 1900, XL, 480.
34. vov LrscHKA, H. Die .\dergeflechte des menschlichen Gehimes. Berlin, G. Reimer, 1855.
35. Magen-die, Francois. Recherches sur le liquide csfephalorachidien. Paris, 1825.
. Recherches philosophiqucs et cUniques sur le liquide c^phalo-rachidien ou c6r6bro-spinal. Paris, 1842.
Mall FP. Hosp. Bull., XIV, 1903. Catalogue of the collection of human embryos in the Anatomical Laboratory of the Johns Hopkins University. Johns Hopkins Hopkins University. Baltimore, 1904.
36. Mall, F. P. On the development of the blood-vessels of the brain in the human embryo. Am. Jour. Anat., Baltimore, 1904, rv, 1.
37. Meek, Walter J. A study of the choroid plexus. Jour. Comp. Neurol, and Psychol., 1907, xvii. No. 3, 286.
38. Mestrez.\t, W. Le liquide c^phalo-rachidien. Paris, Maloine, 1912. Thise de Montpellier, 1911, No. 17. .
39. MiDDLEM.tss, James, and W. F. Robertson. Pathologyof the nervous system in relation to mental diseases. Part III. Morbid conditions of the dura mater. Edinburgh Med. Jour. 1895, xl, pt. 2, 704.
MiNOT, C. S. Human Embryology. New York, W.
Wood A Co.. 1892. MoTT. F. W. The Oliver-Sharpey lectures on the cerebro-spinal fluid. Liincct. London. 1910, ii, 1 ; 79. Peluzzi. B. Experinientelle histologische Untersuchungcn ilbcr die Plexus chorioidei (Adcrgeflechte).
Foli.i neuro-biologica, Haarlem, 1911. v, No. 4, 305. Pf.ttit. a., and J. Giiuhd. Sur la fonetion .s4or6toire ct la morphologie des plexus chorioldes des ventrilulcs latfraux du Syst^nie ncrveux central. Arch.
d'anat. micr., Paris, 1902, v. 214. PuLT, F., O. Kehm, and H Schott.mOller. Leitfadcn zur Untcrsuchung der ZerebrospinalflOssigkeit.
Jena, Fischer, 1913. PoiBiER, P., and A. Charpy. Traits d'antomie huniaine.
Pari,'*, BattaiUe, 1892-1902. Qci-vcKE, H. Zur Physiologie der Cerebrospinalflussigkeit. Arch, f . Anat., Physiol, u. wissensch. Med. (Du Bois-Reymond), Leipzig, 1872, 153. Refobd, L. L. Unpublished. Work referred to by Sabin (49) and Gushing (9). Reiner, M., and J. Schnitzler. Ueber die Abflu.sswege des Liquor cerebrospinalis. Centralblatt f. Physiol., Leipzig and Wien, 1894, viii, 6H4. . Zur Lehre vom Hirndruck. Wiener klin. Wochenschr.. 1895, viii, 371.
Sabin, F. R. Development of lymphatic systcm. In Keibel and Mall's Manual Human Embryology, London 4 Philadelphia, 1912, ii, 709. Salvi, G. Histogenise et structure des meninges.
Thise de Pari.", 1898. . Quoted in Poiricr et Charpy. Traitt d'Anatomie humaine. Paris, 1901, tome iii, 113. ScHROEDER VAN DER KoLK, J. L. C. Bau und Functionen der Medulla spinalis und oblongata. Aus dem Holliindischen iihertragen von Dr. Fr. W.
Theile. Braunschweig, Vieweg & Sohn, 1859. Spin.i, a. Expcrimenteller Beitrag zur Kenntniss der Hyperamic des Gehirns. Wiener med. Blatter, 1898, XXI, 17; 247. . Experimentelle Untcrsuchungen iiber die Bildung des Liquor cerebrospinalis. Arch. f. d. gesamte Physiol., Bonn, 1899, Lxxvi, 204.
52. Spina. A. Uebcr den Einfluss des hohen Blutdrucks auf die Neubildung des CerebrospinalflQssigkeit. Arch, f. d. gcsamtc Physiol., Bonn, 1900, i.xxx, 370.
. Untersuchungen uber die Resorption des Liquor bei normulcm und crhohtem intracraniellem Drucke. .Vrch. f. d. gesamte Physiol., Bonn, 1900-1901, Lxxxiii, 120; 415.
53. .'^TERZi, G. Recherches sur I'anatomie comparfee et sur rontogent-se des m6ninges. Arch. Ital. de Biol., 1902, XXXVII, 257.
. Ricerche inforno all' anatomia comparata ed air ontogenesi dcllc mcningi, e considerazioni suUa filogenesi. Atti del R. Instituto Veneto di scienze, letterc ed .\rti, 1900-1901, LX, parte ii, 1101.
54. Streeter, G. L. Development of the nervous system. In Keibel and Mall, Manual Human Embryology, London & Philadelphia, 1912, ii, 1.
. The development of the venous sinuses of the dura mater in the human embryo. Am. Jour, of Anat., Philadelphia, 1915, xviii, 145.
55. Weed. L. H. Studies on cerebro-spinal fluid. No. II: The theories of drainage of cerebro-spinal fluid with an analysis of the methods of investigation. Jour.Med. Research, Boston, 1914, xxxi (n. s. xxvi), 21. . Studies on cerebro-spinal fluid. No. Ill: The pathways of escape from the subarachnoid spaces, with particular reference to the arachnoid Wlli. Jour. Med. Research, Boston, 1914, xxxi (n. a. XX VI), 51. . Studies on cerebro-spinal fluid. No. IV: The dual source of cerebro-spinal fluid. Jour. Med. Research. Boston, 1914. xxxi (n. s. xxvi). 93.
56. Wilder, B. G. Notes on the Foramina of Mageudie in man and the eat. Jour. Nerv. and Ment. Dis., New York, 1886, xiii, 206. . The metapore (foramen of Magendie) in man and an orang. Med. News, Philadelphia, 1893, LXiii 439. . Meninges. Ref. Handbuch Med. Sciences, New York, 1893, ix (suppl.), 606.
57. ZiEOLER, P. Ueber die Mechanik des normalen und pathologischen Hirndruckes. Arch. f. klin. Chirurgie, Berlin, 1896, Liii, 75.
Explanation of Plates
KEY FOR FIGURE-LEGENDS.
ami, area membranacea inferior. dmc, dura mater cerebri (inner surface, in pme, pia mater cerebri.
amt, area membranacea .superior. approximation with arachnoid). j»p6, precipitated prussian-blue.
cbl, cranial blastema. epe, epithelial-like cells lining ventricle. pun, reduced silver nitrate.
cent, cisterna cerebello-mcduUari.i. epe, ependyma. s<u, subarachnoid spaces.
chp, plexus chorioiduus. 4"^, vcntriculus quarlus. tir, sinus trans\ersu3.
Plate I.
Fig. 1. Drawing of a pig embryo of 9 mm, into the spinal central canal of which an injection of 0.5 per cent solution of potassium ferrocyanide and iron-ammonium citrate was made under very mild syringe-pressure. The embryo was fixed in Camoy's fluid to which 1 per cent hydrochloric acid had been added. The specimen was carefully dehydrated and cleared by the Spalteholz method. The resultant precipitate of prussian-blue is found w holly within the central canal of the spinal cord and within the cerebral ventricles. Enlargement, 11 diameters.
Fig. 2. Drawing of a pig embryo of 13 mm, in which the cerebro-spinal fluid was replacc<l by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate. The embryo was kept alive for 90 minutes after this replacement and was then fixed in 10 per cent formol containing 1 per cent hydrochloric acid, .\fter dehydration the specimen was cleared by the Spalteholz method. The occiurence of a definite oval, outlined by the denser mass of the granules, in the roof of the fourth ventricle, is characteristic of this stage. Enlargement, 9 diameters.
Fig. 3. Drawing of a pig embryo of 14.5 mm, in which the cerebro-spinal fluid was likewise replaced by the ferrocyanide solution, After the replacement, the embryo was kept alive for 60 minutes; it was fixed in Camoy's fluid (with 1 per cent hydrochloric acid added) and after dehydration it was cleared by the SpaltehoU method. The earliest indications of a peria.idal spread of the replaced fluid from the roof of the fourth ventricle is here shown. Enlargement, 8 diameters.
Plate II.
Fig. 4. Drawing of a pig embryo of 18 mm., in which a typical replacement of the spinal fluid had been made. The animal was kept alive for 45 minute." and was then fixed, dehydrated, and cleared in the usual manner. The extra ventricular spread of the replaced fluid from two are;is in the roof of the fourth ventricle is well illustrated. Enlargement, 9 diameters.
Fig. 5. Drawing of a pig embryo of 19 mm., in which likewise a typical replacement of the cerebro-spinal fluid by the ferrocyanide solution had been made. After this procedure, the embryo was kept aUve for 55 minutes and was then carried through the routine technique for the Spalteholz method. The ftirther pericerebral spread of the replaced fluid is recorded. Enlargement, 8 diameters.
Plate III.
Fig. 6. A frank lateral drawing of a pig embryo of 21 mm. The typical replacement of the embryonic cerebro-spinal fluid by the ferrocyanide solution was effected in this embryo and it was then kept alive for 45 minutes. At the end of this time the embryo was fi.xed in an acid fluid, dehydrated, ana cleared. The almost complete periaxial spread of the replaced fluid is indicated by the precipitated granules. Enlargement, 7.6 diameters.
Fig. 7. A dorsal view of the embryo illustrated in fig. 6. The perispinal spread of the replaced fluid is well shown. Enlargement, 7.8 diameters.
Plate IV.
Fig. 8. Drawing of a pig embryo of 26 mm. in which the typical replacement of the cerebro-spinal fluid has been made. After the introduction of the ferrocyanide solution the embryo was kept alive for one hour; at the end of this time it was fixed in an acid solution, subsequently dehydrated, and cleared in oil of wintergreen. The specimen shows a complete periaxial spread of the replaced fluid, as evidenced by the precipitated granules, in addition to a total filling of the intramedullary system. Enlargement, 6.5 diameters.
Fig. 9. Drawing of a pig embryo of 16 m.m., in which the central canal of the spinal cord was injected with the ferrocyanide so.u'ion under moderate syringe-pressure. After fixation in an acid mediiun the embn,-owaa dehydrated and cleared by the Spalteholz method. The extraventricular snread in the peribulbar region Ls easily made out. Enlargement, 9 diameters.
Plate V.
Fig. 10. Drawing of a pig embryo of 21 mm., in which an injection of diluted india ink was made into the central canal of the spinal cord. The preasure employed was the highest obtainable from the syringe, yet below the tension causing rupture. The specimen, after injection, was fixed, dehydrated, and cleared. The slight extent of the periaxial spread of the carbon granules can be easily seen. Enlargement, 7 diameters.
Fig. 11. Drawing of a pig embryo of 16 mm., in which an injection (under moderate syringe-pressure) of 0.5 per cent solution of silver nitrate was made into the central canal of the spinal cord. The silver was reduced in the sunlight, the embryo then fixed. After dehydration, the embryo was cleared in benzol and oil of wintergreen. Enlargement, 7. .5 diameters.
Fig. 12. Drawing of a pig embryo of 13 mm.; into the central canal of the spinal cord a dilute solution of nitrate of silver was injected under strong syringe-pressure. Reduction of the silver was accomplished by exposure to sunlight; the embryo was then fixed, dehydrated, and cleared. Enlargement, diameters.
Plate VI.
Fig. 13. Photomicrograph of transverse section of a pig embryo of 15 mm. Specimen obtained from an embryo in which the cerebro-spinal fluid was replaced by a 1 per cent solution of potassium ferrocyanide and ironammonium citrate. After this replacement the embryo was kept aUve for 65 minutes. The resultant priL'Ssian-blue precipitate is not included in this photomicrograph. Enlargement, 13 diameters.
Fig. 14. Drawing of blocked area in fig. 13, under higher magnification and including the resultant precipitate of prussian-blue. The typical ependymal cells (epc) lining the fourth ventricle are shown on either side; between them occurs the area membranacea superior {ams). The transit of the replacement fluid through the membranous area and the spread through the adjacent mesenchyme are illustrated. Enlargement, 245 diameters.
Fig. 15 Photomicrograph of transverse section from embryo pig illustrated in fig. 13. Section taken from more caudal plane than that given in the former figure. The prussian-blue spread is not illustrated. Enlargement, 10 diameters.
Fig. 16. Drawing, under higher magnification, of the rectangular area in fig. 15. The passage of the replaced solution, as shown by the resultant precipitate of prussian-blue, through tlie area membranacea inferior (ami) is here illustrated. The extension of the replaced fluid through the adjacent mesenchyme and the nonpenetration of the solution into the condensed mesenchyme are shown. Enlargement, 140 diameters.
Flg. 17. Photomicrograph of sagittal section of a pig embryo of 18 mm. Specimen obtained from an embrj-o in which the cerebro-spinal fluid was replaced by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate. After this replacement the animal was kept alive for 45 minutes. Fixed for 5 minutes in 10 per cent formol containing 1 per cent hydrochloric acid; then over night in modified Boiiin's solution (saturated aqueous solution of picric acid 75, formaldi-hyde 10, glacial acetic arid 10). Dehydrated by 2 and 4 per cent grades of alcohol; embedded in xylol-paraffin. Serial sections, stained by hematoxylin and eosin. The resultant precipitate of prussian-blue has not been reproduced in the photomicrograph. Enlargement, 8 diameters.
Fig. 18. Drawing of blocked area in fig. 17 under higher magnification. The granules of prussian-blue are here represented by the blue stenciling. The transit of the fluid, as shown by the granules, into the periaxial mesenchyme through the two membranous areas {arm and ami) in the roof of the fourth ventricle are well shown. Enlargement, 35 diameters.
Plate VII.
Fig. 19. Photomicrograph from a sagittal section of a fetal pig of 27 mm. The cerebro-spinal fluid in this specimen was replaced by a 1 per cent solution of potassium ferrocyanide and iron-ammonium citrate; the fetus was kept alive for 40 minutes; fixed in 10 per cent formol containing 1 per cent hydrochloric acid for 15 minutes; then over night in modified Benin's solution; dehydrated by 2 and 4 per cent grades of alcohol; embedded in xylol-paraffin. The prussian-blue granules are not represented in this photomicrograph. Enlargement, 8 diameters.
Fig. 20. Drawing of squared area in fig. 19. The center of the field is occupied by the optic nerrve; around it the developing extrinsic optic muscles are shown. The precipitate of prussian-blue occurs in the perineural mesenchyme. Enlargement, 190 diameters.
Fig. 21. Photomicrograph of rectangular area in fig. 19. The passage of the ferrocyanide solution into the sinus transvcrsus {sir) is represented by the precipitated blue granules. Enlargement, 133 diameters.
Fig. 22. Photomicrograph of a transverse section of a pig enibrj-o of 23 mm. The cerebro-spinal fluid was replaced in this embr>'o with a 1 per cent solution of potassium fenocyanide and iron-ammonium citrate. The embryo was kept alive for .50 minutes and was then fixed over night in 10 per cent formol containing 1 [ler cent hydrochloric acid. The granules of pnissian-blue are not shown in this reproduction. Enlargement, 13 diameters.
Fig. 23. Drawing of squared area in fig. 22. The area membranacea superior (rima) is shown, sunounded on either side by tufts of the chorioid plexus (rhp) .ind the typical ventricular ependyma. The transit of the solution is shown, as represented by the resultant granules, through the areji, with the subsequent spreail into the periaxial mesenchyme. Enlargement, 125 diameters.
Plate VIII.
Fig. 24. Photomicrograph of a transverse section of a pig embryo of 8 mm. Fixed in modified Bouin's solution over night, dehydrated by 2 and 4 per cent grades of alcohol, embedded in xylol-parafiin. Enlargement, 30 diameters.
Fig. 25. Photomicrograph, retouched, of the blocked area in fig. 24. The character of the cells (epc) composing the roof of the fourth ventricle Uve) is showTi in this reproduction. Enlargement, 16.5 diameters.
Fig. 26. Photomicrograph of a sagittal section from a pig embryo of 11 mm. Fi.xed in modified Bouin's solution over night, dehydrated by 2 and 4 per cent gracles of alcohol, embedded in xylol-parafhn. Enlargement, 11 diameters.
Fig. 27. Photomicrograph of the blocked area in fig. 26. The area membranacea superior (ams) in the roof of the fourth ventricle is shown sharply delimited from the two processes of typical ependyma {epe). Enlargement, 67 diameters.
Fig. 28. Photomicrograph of a more lateral section of the pig embryo of 11 mm. given in fig. 26. Enlargement, 11 diameters.
Fig. 29. Photomicrograph, under higher magnification, of the blocked area in fig. 28. The lateral border of the area membranacea superior {ams) of the roof of the fourth ventricle is given. Enlargement, 50 diameters.
Fig. 30. Photomicrograph of a sagittal section from a pig embryo of 13 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 8 diameters.
Fig. 31. Photomicrograph, under higher magnification, of the squared area in fig. 30. The reproduction comprises a sagittal section of the area membranacea superior (ams) of the roof of the fourth ventricle. Enlargement, 67 diameters.
Fig. 32. Photomicrograph of a sagittal section of a pig embryo of 14 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades, and embedded in xylol-paraffin. Enlargement, 11 diameters.
Fig. 33. Photomicrograph of the blocked area in fig. 32 under higher magnification. The area membranacea superior (ami) in the roof of the fourth ventricle is reproduced. Enlargement, 75 diameters.
Plate IX.
Fig. 34. Photomicrograph of a transverse section of a pig embryo of 18 mm. Fixed in Camoy's fluid (6:3:1), deh3'drated by 2 and 4 per cent changes of alcohol, and embedded in xylol-paraffin. Enlargement, 13 diameters.
Fig. 35. Photomicrograph, under higher magnification, of the blocked area in fig. 34. The area membranacea superior (atns) is here given, flanked on either side by typical ependyma (ept). Enlargement, 170 diameters.
Fig. 36. Photomicrograph of a transverse section of a pig embrj-o of 18 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Enlargement, 13 diameters.
Fig. 37. Photomicrograph of rectangular area outlined in fig. 36. The extent of the area membranacea superior (ams), with its adherent coagulum of albuminous material, is well differentiated from the adjacent typical ventricular ependyma (epe). Enlargement, 100 di.imeters.
Fig. 38. Photomicrograph of a transverse section of a pig embryo of 19 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades, and embedded in xylol-paraffin. Enlargement, 13 diameters.
Fig. 39. Photomicrograph, under higher power, of the rectangular area in fig. 38. A small break in the integrity of the lining ependyma of the roof of the fourth ventricle, representing the irregular boundary of the area membranacea superior (ams), is given. Enlargement, 290 diameters.
Fig. 40. Photomicrograph of a transverse section of a human embryo of 4 mm. (No. 836 of collection of Carnegie Institution of Washington). Enlargement, 33 diameters.
Fig. 41. Photomicrograph, retouched, of the blocked area in fig. 40. The epithelial-like cells (epe) composing the roof of the fourth ventricle (4^) are here shown separated from the denser nervous tissue. Enlargement , 100 diameters.
Plate X.
Fig. 42. Photomicrograph of transverse section of pig embryo of 19 mm. Fixed over night in modified Bouin's solution, dehydrated by 2 and 4 per cent changes of alcohol, and embedded in x>-lol-paraffin. Enlargement, 13 diameters.
Fig. 43. Photomicrograph of squared area in figure 42, under higher magnification. The area membranacea superior (ams) with the attached coagulum of albumen is reproduced. Enlargement, 115 diameters.
Fig. 44. Photomicrograph of sagittal section of pig embryo of 23 mm. Fuced in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Enlargement, 5 diameters.
Fig. 45. Photomicrograph, under higher magnification, of squared area in fig. 44. The area membranacea superior (atns) is here shown, delimited by the cells of the chorioid plexus (chp) on one side and by the further ependymal prolongation (epc) of the cerebellar lip. Enlargement, 88 diameters.
Fig. 46. Photomicrograph of sagittal section of pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent changes, and embedded in xylol-paraffin. Certain portions of the dura mater (dmc) are indicated. Enlargement, 5 diameters.
Fig. 47. Photomicrograph of blocked area in fig. 46, under higher magnification. The small remaining area membranacea superior (ams) is quite surrounded by encroaching ependyma in the chorioidal folds. Enlargement, 88 diametere.
Fig. 48. Photomicrograph of transverse section of human embryo of 7 nun. (No. 617 of the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters.
Fig. 49. Photomicrograph of squared area in fig. 48, under higher magnification. The epithelial-like cells (epc) composing the roof of the fourth ventricle at this stage are well shown. Enlargement, 100 diameters.
Fig. 50. Photomicrograph of transverse section of human embryo of 7 mm. (No. 617 in the Caniegie Institution of Washington). Enlargement, 10 diameters. Fig.
Fig. 51. Photomicrograph of blocked area in fig. 50. The marked invagination of the roof of the fourth ventricle (4if ) with the lining of epithelial-like cells (epc) is given. Enlargement, 33 diameters.
Fig. 52. Photomicrograph of transverse section of human embryo of 9 mm. (No. 721 in the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters.
Fig. 53. Photomicrograph of squared area outlined in fig. 52. The pale, large cells {epc) comprising the roof of the fourth ventricle characterize the reproduction. Enlargement, 50 diameters.
Fig. 54. Photomicrograph of sagittal section of human embryo of 11 mm. (No. 544 in the collection of the Caniegie Institution of Washington). Enlargement, 6 diameters.
Fig. 55. Photomicrograph of blocked area in fig. 54. The apparent break in the continuity of the roof of the fourth ventricle with exudation of the ventricular albumen into the mesenchyme is brought out. Enlargement, .50 diameters.
Plate XI.
Fig. 56. Photomicrograph of sagittal section of human embryo of 14 mm. measured on the slide (No. 144 of the collection of the Carnegie Institution of Washington). Enlargement, 8 diameters.
Fig. 57. Photomicrograph, under higher magnification, of blocked area in fig. 50. The greater part of the ventricular wall shown is composed of the area membranacea superior (ams), bounded below by typical ventricular ependyma {epe). Enlargement, 67 diameters.
Fig. 58. Photomicrograph of sagittal section of human embryo of 17 ram. (No. 576 of the collection of the Carnegie Institution of Washington). Enlargement, 10 diameters.
Fig. 59. Photomicrograph of rectangular area in fig. 58, showing the area membranacea superior {ams) of the roof of the fourth ventricle. Enlargement, 50 diametere.
Fig. 60. Photomicrograph of sagittal section of human embryo of 17 mm. (No. 576 of the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters.
Fig. 61. Photomicrograph of tlie blocked area in fig. 60 under higher magnification. The aggregation of epithehal-like cells (epc) on the lateral border of the area membranacea superior is here portrayed. Enlargement, 67 diameters.
Fig. 62. Photomicrograph of transverse section of human embryo of 18 mm. (No. 409 of the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters.
Fig. 63. Photomicrograph, under liigher power, of squared field in fig. 62. The peculiar inversion of the roof of the fourth ventricle (/fVe) indicated in fig. 62, has resulted in a marked dislocation of the area membranacea superior (nm«), shown in this figure. Enlargement, 75 diameters.
Plate XII.
Fig. 64. Photomicrograph, retouched, of a transverse section of a human embryo of 21 mm. (No. 460 of the collection of the Carnegie Institution of Washington). The field taken consists of a portion of the fourth ventricle with the lining of typical ependyma (cjk) on either side. The area membranacea superior {ams) is shown between the two lips of ependyma. Enlargement, 33 diameters.
Fig. 65. Photomicrograph, retouched, of a similar section to that given in fig. 64, but taken from a more anterior plane from the same embryo. The field shown is analogous in everj' way to that in the preceding figure.
Fig. 66. Photomicrograph of a transverse section of an embryo chick of 121 hours' incubation. Fixed in Bouin's solution. Enlargement, 15 diameters.
Fig. 67. Itetouched photomicrograph, under higher magnification, of the blocked area in fig. 66. The area memhranacoH superior (ams) is here given, delimited sharply from the lips of ependyma {cpc) which line the rtmf of the fourth ventricle. Enlargement, 133 diameters.
Fig. 68. Photomicrograph of a more caudal section from the same embryo as portrayed in fig. 66. Enlargement, 15 diameters.
Fig. 69. Retouched photomicrograph, under higher magnification, of the blocked area in fig. 68. The area membranacea superior {am») is shown at the point of its greatest transverse diameter. Enlargement, 88 diameters.
Fig. 70. Photomicrograph of a migittul section of a pig embryo of 15 mm. Fixed in modlfied Bouin's solution, dehydratiil by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement , S diameters.
Fig. 71. Phutomicrograph, under higher magnification, of blocked area in fig. 70. The earliest evidence of the area membranacea inferior (ami) in the roof of the fourth ventricle is here shown. Enlargement, 12.'i diametere.
Fig. 72. Photomicrograph of sagittal section of a pig embryo of 18 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 11 diameters.
Fig. 73. Photomicrograph, under higher power, of the rectangular area outlined in fig. 72. The enlarging area membranucea inferior (ami) is shown in the midst of the typical lining ependyma of the roof. Enlargement, 100 diameters.
Fig. 74. Photomicrograph of sagittal section of a pig embryo of 23 mm. Fixed in modified Bouin's solution, dehydrated by 2 :\iid 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 6 diameters.
Fig. 75. Photomicrograph of blocked area in fig. 74. The area membranacea inferior {ami) is, at this stage, quite extensive, as shown in the reproduction; the early stages in the development of the cistema cerebellomedullaria may also be seen. Enlargement, 75 diameters.
Fig. 76. Photomicrograph of sagittal section of a pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. Enlargement, 7 diameters.
Fig. 77. Photomicrograph, under higher magnification, of blocked area in fig. 76. The unsupported character of the area menibranarca inferior and the formation of the cistema cerebello-medullaris is here reproduced. Enlargement, 67 diameters.
Plate XIV.
Fig. 78. Photomicrograph of a sagittal section of a pig embryo of 32 mm. Fixed in modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xj'lol-parafiin. Enlargement, 7 diametera.
Fig. 79. Photomicrograph of the blocked area in fig. 78, under higher magnification. The intact area membranacea inferior (ami), unsupported by any mass of tissue, is shown separating the ventricular cavity from the developing cisterna ccrebello-medullaris. Enlargement, 67 diameters.
Fig. 80. Photomicrograph of a sagittal section of a human embryo of 16 mm. (No. 406 in the collection of the Carnegie Institution of Washington). Enlargement, 7 diameters.
Fig. 81. Photomicrograph of the area outlined in fig. 80, but under liigher magnification. An early stage in the differentiation of the area membranacea inferior (ami) is given. Enlargement, 50 diameters.
Fig. 82. Photomicrograph of a sagittal section of a human embryo of 17 mm. (No. 576 in the collection of the Carnegie Institution of Washington). Enlargement, 6 diameters.
Fig. 83. Photomicrograph, under higher power, of the area blocked in fig. 82. The chorioid plexuses of the fourth ventricle Ue in the central portion of the field; above is the thick cell-layer on the lateral side of the area membranacea suf)erior (ams), while below the upper limit of the area membranacea inferior (ami) appears. Enlargement, 67 diameters.
Fig. 84. Photomicrograph of a transverse section of a human embryo of 18 mm. (No. 409 in the collection of the Carnegie Institution of Washington). Enlargement, 5 diameters.
Fig. 85. Photomicrograph of the blocked area in fig. 84. The cellular character, and especially the clumping of cells, of the area membranacea inferior (ami) is shown. Enlargement, 25 diameters.
Fig. 86. Photomicrograph of a sagittal section of a human embryo of 19 mm. (No. 431 in the collection of the Camegie Institution of Washington). Enlargement, 5 diameters.
Fig. 87. Photomicrograph of the blocked area outUned in fig. 86. The area membranacea inferior (ami) appears separating the fourth ventricle from the developing cistema cerebello-medullaris. Enlargement, 25 diameters.
Plate XV.
Fig. 88. Photomicrograph from a sagittal section of a human embryo of 17 mm. (No. 576 of the collection of the Carnegie Institution of Washington), representmg an enlargement of the second blocked area in fig. 58. The area membranacea inferior (ami) appears sharply delimited from the adjoining tj^pical ependjina. Enlargement, 67 diameters.
Fig. 89. Photomicrograph of a sagittal section of a human embryo of 23 mm. (No. 453 of the collection of the Carnegie Institution of Washington). Enlargement, 6 diameters.
Fig. 90. Photomicrograph of the blocked area in fig. 89. The area membranacea superior (ams) appears in the stage of closure, while the area membranacea inferior (ami) is becoming well differentiated from the typical ependyma lining the other portions of the fourth ventricle. Enlargement, 26 diameters.
Fig. 91. Photomicrograph of a sagittal section of a human embryo of 26 mm. (No. 1008 of the collection of the Carnegie Institution of Washington). Enlargement, 4.5 diameters.
Fig. 92. Photomicrograph, under higher magnification, of the blocked area in fig. 91 . The area membranacea superior has been almost completely closed by the dense ependyma of the superior half of the roof of the fourth ventricle, while the inferior area (ami) has become a membrane lacking wholly the character of ependyma. Enlargement, 23 diameters.
Fig. 93. Photomicrograph of a sagittal section of a human embryo of 35 mm. (No. 199 of the collection of the Carnegie Institution of Washington). Enlargement, 3 diameters.
Fig. 94. Photomicrograph, under higher powers, of the blocked areas in fig. 93. The formation of the cistema cerebello-medullaris is shown in relation to the ventricular roof. Enlargement, 23 diameters.
Fig. 95. Drawing of cells of the chorioid plexus from the lateral ventricles of a fetal pig of 132 mm. The specimen waa fixed in absolute alcohol, and stained by Best's carmine stain for glycogen. The glycogen occurs in the form of globules within the epithelial cells. Enlargement, 950 diameters.
Fig. 96. Drawings of the cells of the chorioid plexus from the lateral ventricles of a fetal pig of 36 mm. The specimen was fixed in absolute alcohol and stained by Best's carmine method. The glycogen appears in the epitheUal eclls in the form of basilar plaques. Enlargement, 950 diameters.
Plate XVI.
Fig. 97. Photomicrograph of a transverse section of a pig embryo of 10 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent f;radcs of alcohol, and embedded in xylol-paraffin. Enlargement, 10 diameters.
Fig. 98. Photomicrograph, under higher magnification, of the blocked area in fig. 97. The double condensations of mesenchyme to form pia mater (pmc) and cerebral blastema (cbl) appear separated by a region of mesenchyme which is breaking down. This central area of mesenchyme, with the marked albumen-content, is to become the arachnoid spaces. Enlargement, 133 diameters.
Fig. 99. Photomicrograph of a transverse section of a pig embryo of 20 mm. Fixed in modified Bouin's fluid, dehydrated by 2 and 4 per cent changes of alcohol, and embedded in xylol-paraffm. Enlargement, 10 diameters.
Fig. 100. Photomicrograph, under higlior powers, of the blocked areas in fig. 99. The relations of the pial condpn.sation ipmc) of mesenchyme to tlie nervous system, as well as the infiltration of tlie arachnoid mesenchyme (sas) with albumen, is reproduced. Enlargement, 133 diameters.
Fig. 101. Photomicrograph, under higher magnification, of the blocked area in fig. 22. The reproduction is included here to show the double condensjition {cht) of mesenchyme which goes to form ultimately bone and possibly a portion of the dura. Enlargement, 132 diameters.
Fig. 102. Photomicrograph of a transverse section of a pig embryo of 18 mm. The embryo was one in which the cerebro-spinal fluid was replaced by the ferrocyanide solution. Subsequently the embryo was fixed in 10 per cent formol containing 1 per cent hydrochloric acid for a few minutes to precipitate the prussianblue. It was then transferred to modified Bouin's solution, dehydrated by 2 and 4 per cent grades of alcohol, and embedded in xylol-paraffin. The granules of prussian-blue are not represented in this figure. Enlargement, 10 diameters.
Fig. 103. Photomicrograph of the squared area in fig. 102. The relation of the thinning mesenchyme in the arachnoid areas to the caudal cranial nerves is shown. The granules of prussian-blue, scattered through the area of thin mesenchyme (sas), are not reproduced. Enlargement, 40 diameters.
Fig. 104. Photomicrograph of a coronal section of a tissue block which includes the meninges and cerebral cortex in the region of the sinus sagittalis superior. The block was obtained from a fetal pig of 80 mm., fixed in Zenker's fluid, and stained, after embedding in celloidin, by Mallory's technique for coimective tissue. Enlargement, 27 diameters.
Plate XVII.
Fig. 105. Photomicrograph of a coronal section of a tissue block including cerebral cortex and meninges in the region of the sinus sagittalis superior. The block was obtained from a fetal pig of 10 cm., fixed in Zenker's fluid, and stained by Mallory's technique for connective tissue. Enlargement, 13 diameters.
Fig. 106. Photomicrograph of a coronal section, similar to that in figs. 104 and 105, except in that it was obtained from a fetal pig of 17 cm. The same technical procedures employed in the other specimens were used in this. Enlargement, 27 diameters.
Fig. 107. Photomicrograph of a similar section to those of the foregoing figures. The specimen was obtained from a fetal pig of 20 cm. and was treated in the manner outlined above. Enlargement, 20 diameters.
Fig. 108. Drawing of the cell pattern from the inner surface of the dura mater of a fetal pig of .5 cm. The specimen was prepared by the reduction of a dilute solution of silver nitrate in sunlight. The preparation was subsequently stained by hematoxylin. Enlargement, 190 diameters.
Fig. 109. Drawing of a preparation, similar to that of fig. 108, but obtained from the inner surface of the dura mater of a fetal pig of 7.5 mm. Enlargement, 28.5 diameters.
Fig. 110. Drawing of a preparation, similar to those of figs. 108 and 109, obtained from the inner surface of the dura mater of a fetal pig of 90 mm. Enlargement, 28.5 diameters.
Fig. 111. Drawing of a preparation from the inner surface of the dura mater of a fetal pig of 16 cm. The specimen was made in the same manner as outlined in fig. 108. Enlargement, 285 diameters.
Fig. 112. Photomicrograph of a sagittal section of a pig embryo of 17 mm. An injection of an 0.5 per cent solution of nitrate of silver was made into the central canal of the spinal cord; the silver was reduced in sunlight and the embrjo fixed in formalin. Enlargement, 13 diameters.
Fig. 113. Photomicrograph, under higher powers, of the blocked areas in fig. 112. The accumulation of the reduced silver (p»n) again.st the area membranacea superior is represented in black. Enlargement, 117 diameters.
Fig. 114. Photomicrograph of a transverse section of a pig embryo of 19 mm. An injection of 0.5 per cent solution of silver nitrate was made into the central canal of this embryo and the silver immediately reduced in sunlight. The embryo waa fixed in formalin, carefully dehydrated, and embedded in xylol-peiraflBn. Enlargement, 10 diameters.
Fig. 115. Photomicrograph, under higher magnification, of the blocked area in fig. 114. The collection of reduced silver {psn) against the cells at the inferior end of the area membranacea superior is illustrated. Enlargement, 100 diameters.
Fig. 116. Photomicrograph of a tranverse section of a pig embryo of 16 mm. The central canal of the spinal cord of this embryo was injected with a 1 per cent ferrocyanide and citrate solution under mild syringe-pressure; the embryo was then fixed in 10 per cent formol containing 1 i>er cent hydrochloric acid. Enlargement,
10 diameters.
Fig. 117. Photoniicrogrnph of the blocked area in fig. 116, under higher magnification. The accumulation of the
precipitated injection fluid against the area membranacea superior is represented in black. A slight
extravcntriculai Bprciul of the fluid, which is found in this as in all embryos of this stage, can not be
miule out in the reproduction. Enlargement, 07 diamuters.
Cite this page: Hill, M.A. (2024, April 24) Embryology Book - Contributions to Embryology Carnegie Institution No.14. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.14
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G