Book - Brain and behavioural development 1
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Dickerson JWT. and McGurk H. Brain And Behavioural Development. (1982) Blackie & Son Ltd., Glasgow.
Brain and Behavioural Development - 1982: 1 Neural Development | 2 Comparative Neural | 3 Malnutrition | 4 Hormones and Growth Factors | 5 Cortical Activity | 6 Functional Asymmetry | 7 Plasticity | 8 Sex Differences
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter One - The Development Of The Human Nervous System
Martin Berry
Introduction
A massive escalation in the volume of research effort in developmental neuroscience has occurred over the past ten years. Unfortunately, during this period investigation of the ontogeny of the human nervous system has attracted very little attention and it is difficult to understand the cause. Constraints like a lack of either material or technique no longer apply, since changes in the abortion laws in many countries have made fresh human embryos available to most laboratories in which modern neurobiological staining and tracing techniques are practised. Perhaps the brake applied to a new surge of research is the belief that developmental neuroscience has not matured to the point where it can tackle the apparent complexities of human brain development. Paradoxically, as Sidman and Rakic (1973) have pointed out, the long time-course of the period of human brain development allows temporal resolution of events that in other mammals are compressed often to the extent that sequential stages are indiscernible. Moreover, the large size of the human brain relative to that of most other mammals facilitates the morphological study of individual centres along with their chemical and behavioural correlates. In any event, we need to know more about how our brains develop, not merely for purely academic reasons but also to understand the aetiology and sequelae of congenital brain disease—particularly since this is at present a rapidly expanding area of medicine aided by advances in foetal diagnosis.
Within the brain, a series of distinct but overlapping phases of development characterize regional growth. These may be listed chronologically as neurogenesis, neuroblast migration and neuronal differentiation. Gliogenesis, glio- blast migration and glial differentiation probably take place pari passu with the former but they are less precisely documented. The phases of neurogenesis and neuroblast migration extend into the latter part of the first year of life, and perhaps beyond in certain areas. In most cases neuronal differentiation begins during migration with the elaboration of an axon. Dendritic growth usually commences when migration has ceased. Synaptogenesis occurs as dendrites grow and, indeed, it has been suggested that adhesive interaction between growing axons and dendrites determines all the physical characteristics of the dendritic field (Berry et al ., 1980a). Glial neuronal interaction is apparent from the beginning, since it is glial processes that provide the substrate for migration. Myelination is under way in the second trimester, and the blood brain barrier matures in the last trimester (Pappas and Purpura, 1964). Gliogenesis continues into adult life. The stem cell source is from dormant glioblasts dispersed within the neuropil and the subependymal layer.
Of course, each brain region develops differently, and much regional ontogeny is already documented in existing text books of human embryology. This review concentrates on those areas of the human brain which have received the most attention over the past ten years or so. These regions include the cerebral and cerebellar cortices, the basal ganglia, the brain stem and the spinal cord.
Neurogenesis
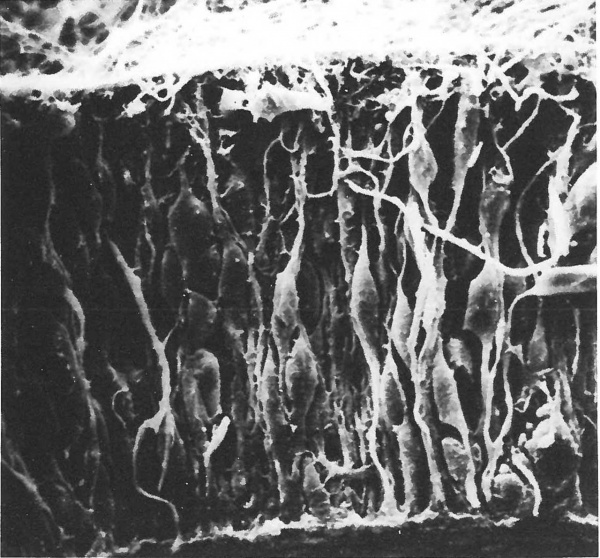
All neurones originate from germinal cells located within a layer which cuffs the neural tube and ventricles of the primitive nervous system. Some of the dividing cells become relocated elsewhere within the developing neuropil to form secondary germinal centres, but the major part of the nervous system is formed from matrix cells in the ventricular and subventricular zones (Fig. 1.1). Within this population three species of cell are probably produced—radial glia, neuroblasts and germinal cells. The cell lineage of each type is poorly understood. There is some measure of agreement that activity in the germinal epithelium can be divided into two phases—a proliferative and a migratory phase (Berry, 1974)—and that definitive neuroblasts are formed only throughout the latter. During the proliferative phase the structure of the epithelium is that of a typical pseudostratified columnar type with the basal processes attached to the subpial basement membrane and the apical processes anchored to each other by terminal bars at the lumen. Proliferation within the epithelium is associated with a peculiar interkinetic movement of the germinal cell nucleus (Seymour and Berry, 1975, 1979). Division occurs at the luminal edge, haploid daughter nuclei migrate to the subpial margin where they synthesize DNA, and diploid nuclei then return to the luminal border to complete mitosis. Many of the changes in cell shape associated with interkinetic nuclear migration can be seen in a typical scanning electron micrograph of the neuroepithelium (Fig. 1.2). Although these changes were first described in the neuroepithelium of the cerebral vesicle of the rat (Seymour and Berry, 1975, 1979) and mouse (Meller and Tetzlaff, 1975) parallel studies on the human subventricular zone (Fujita, 1973a, 1975; Fujita et al ., 1975; Hattori and Fujita, 1974, 1976a, b) suggest that the process may be similar in man—see Fig. 1.15. During the proliferative phase, glia and neural germinal cells may be produced but their morphological differentiation is not apparent until the marginal layer appears.
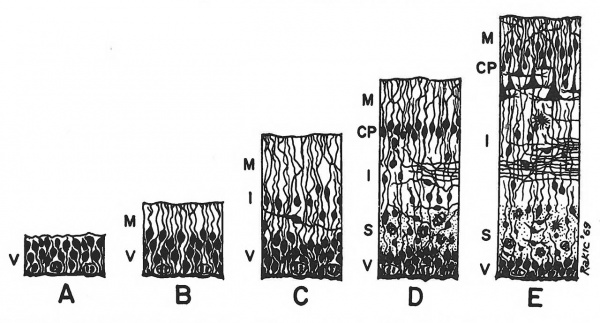
Figure 1.1 Semidiagrammatic drawing of the development of the basic embryonic zones of the cortical plate. Terminology from Boulder Committee (1970): CP, cortical plate; I, intermediate zone; M, marginal zone; S, subventricular zone; V, ventricular zone. Stages A, B and C are common to the primary germinal epithelium in all parts of the CNS. D and E are specific to the cerebral neocortex but the migration of cells to form the cortical plate is a phenomenon not fundamentally different from the establishment of many other neural centres.
As proliferation continues in the subventricular zone, a fundamental change occurs in the organization of the neural epithelium which culminates in the appearance of the marginal zone. One can only speculate about the possible changes in structure of the germinal epithelium which could represent the first signs of differentiation of the constituent cells into radial glia and neuronal matrix cells. Thus, radial glia may maintain their attachment at the inner and outer limiting membranes as mural thickness increases. Presumptive neural matrix cells, however, may detach their basal processes which come to lie within the subventricular zone (Fig. 1.1). Thus, the marginal zone may be occupied only by radial glia process at this early stage. Thereafter, interkinetic nuclear migration is confined to the ventricular and subventricular zones and continued growth separates the outer margin of the subventricular zone from the pial surface widening the marginal zone. The stage is now set for neuroblast production and migration.
During the migratory phase of neurogenesis, germinal cells produce definitive neuroblasts and probably more radial glia. The daughter cells which differentiate into neuroblasts leave the subventricular zone and migrate to other regions in the CNS, whilst daughter cells retaining germinal cell status continue interkinetic nuclear migration within the ventricular/subventricular complex. Throughout the migratory phase of neurogenesis the germinal epithelium undergoes intense mitotic activity. Most neuroblasts leaving the subventricular zone never divide again and differentiate into neurones. Some, however, do establish secondary germinal centres elsewhere in the CNS, such as in the ganglionic eminence in the forebrain (Rakic and Sidman, 1969), in the dentate gyrus (Angevine, 1965; Schlessinger et al, 1978; Stanfield and Cowan, 1979; Kaplan and Hinds, 1977); and the external granular layer of the cerebellum (Rakic and Sidman, 1970; Zecevic and Rakic, 1976) and the olfactory lobe (Kaplan and Hinds, 1977). In general both primary and secondary neurogenesis cease at the end of the migratory period except in the dentate gyrus and olfactory lobe where neurone production may continue for some considerable time beyond birth in rodents. The factors controlling the rate and duration of mitosis in the germinal centres in the CNS are completely unknown.
Figure 1.2 Scanning electron micrograph of the wall of the telencephalic vesicle of a foetal rat aged 14 days post-conception. Note the pseudostratified nature of the epithelium (pial surface is uppermost)— x 1050.
Elgure 1.3 Three-dimensional reconstruction of migrating neurones, based on electron micrographs of semi-serial sections. This reconstruction was made at the level of the intermediate zone indicated by the rectangle and asterisk in Fig. 1.14. The lower portion of the diagram contains uniform, parallel fibres of the optic radiation (OR) and the remainder is occupied by more variable and irregularly disposed fibre systems. The relationships of radial glial fibres (RF, 1-6) with migrating cells (A, B and C) and with other vertical processes is seen. The soma of migrating cell A, with its nucleus (N) and voluminous leading process (LP) are also shown. Cross-sections of cell A in relation to several vertical fibres are drawn at levels a-d. LE, lamellate expansion; PS, pseudopodia (from Rakic, 1972, with permission).
Migration
In most cases, neuroblasts move out from their primary germinal centres in the ventricular and subventricular zone along the radial processes of glia (Sidman and Rakic, 1973). Migrating cells are bipolar, with a leading and trailing process, and use the radially orientated glial fibres as guides. They become detached from the radial glia when they reach their definitive destinations. The morphological characteristics of migrating neuroblasts and their intimate relationship with the radial glia are shown in Fig. 1.3. The way in which the migratory neuroblasts actually move along the glial process, and the mechanisms of obtaining the correct addresses for individual neuroblasts terminating migration at different distances along the process are not known, although analysis of the reeler mouse may help elucidate this problem (Caviness and Sidman, 1972, 1973a,b, 1976). It is likely that the trailing process differentiates into the axon during the course of migration, but the commencement of dendritic growth occurs later, usually after the neuroblast has reached its destination.
In some instances, migration is not guided by radial glia. For example, the establishment of the external granular layer in the cerebellum involves the migration of germinal cells from the subventricular zone in the most caudo- lateral parts of the roof of the fourth ventricle. In this region the neuroblasts accumulate in the presumptive marginal layer and then move tangentially and rostro-medially within this layer to populate the entire surface of the cerebellum (Fig. 1.4) (Rakic and Sidman, 1970; Sidman and Rakic, 1973c). There appear to be no cellular guides to direct this migration and, since all cells over the external surface are replicating, it is possible that cellular movements are achieved as new cells jostle for space within the confines of the marginal layer (Miale and Sidman, 1961). Similar activity is seSn to occur at the secondary rhombic lip of the human embryo as the migratory population of the corpus pontobulbare is established (Fig. 1.4). These cells ultimately form the inferior olivary nuclei and pontine grey matter (Essick, 1907, 1912). Another massive secondary germinal centre, the ganglionic eminence, is established in the forebrain (Rakic and Sidman, 1968, 1969, 1973b) and this provides the neuroblasts destined to form the caudate, putamen, amygdala and other forebrain and diencephalic structures. The mode of migration from the ganglionic eminence is unknown but masses of bipolar neuroblasts streaming into the thalamus, for example, form a continuous cellular band between the two structures called the corpus gangliothalamicus (Fig. 1.5) (Rakic and Sidman, 1969).
Figure 1.4 Model (A) and transverse section (B) of the human rhombencephalon at the end of the third lunar month of gestation. The arrows indicate the migration pathways of the external granular layer (EG) and the corpus pontobulbare (CPB). AO, accessory olive; AR, arcuate nucleus; BIC, brachium of the inferior colliculus; CH, cerebellar hemisphere; CP, choroid plexus; FN, facial nerve’ IC, inferior colliculus, IO, inferior olive; LL, lateral lemniscus, P, pineal gland; RL, rhombic lip; SC, superior colliculus; TN, trigeminal nerve; V, vermis; 4V, fourth ventricle (from Sidman and Rakic, 1973, with permission).
Migratory failure leads to ectopia (Clarke and Cowan, 1975). Movement may be arrested at variable distances along the migratory path or migration may never commence, in which case neuroblasts never leave their germinal centres. Curtailment of migration occurs in many sites in the human nervous system (Rakic, 19756). Granule cell nests may remain in the region of the embryonic external granular layer in the cerebella of normal individuals (Brustowicz and Kernohan, 1952), and subventricular cell ectopia also seems to be common and could constitute potential sites of periventricular neoplasm (Hrabowska, 1978). Ectopia is also a feature of many disease states. In the telencephalon, cells may become arrested anywhere along their path of migration to form radial columns spanning the white matter (Jacob, 1936; Norman, 1966; Volpe and Adams, 1972). In other diseases, cells become arrested to form ectopic laminae (Hanaway et al , 1968; Stewart et al , 1975; Rickman et al , 1973, 1974, 1975). In the cerebellum, arrest of migration of granule cells in cases of neuroblastoma (Kadin et al ., 1970) may occur throughout the depths of the molecular layer. Ectopias associated with migratory failure in the corpus pontobulbare and the ganglionic eminence are relatively common in a variety of brain defects (Rakic, 19756).
Differentiation of neuronal processes
It has already been mentioned that the growth of the axon precedes that of the dendrites. Indeed, in many instances the axon may have contacted its target before migration has terminated. It is through such junctions that trophic and/or inductive chemicals may pass from target cell into afferent terminal and thence by retrograde axonal transport, to the source neurone. In fact, there is good evidence that such a trophic mechanism is operating to control the magnitude of neuronal populations (Hamburger, 1975). Thus, proliferation within primary and secondary germinal centres overproduces neurones. In practice, this redundancy is a safety factor which ensures that adequate numbers of source neurones connect with their targets. Death ensues if the target is not contacted, either because postsynaptic sites are already saturated or because axons are guided to inappropriate destinations. Good examples of this phenomenon are provided by the murine mutants weaver and staggerer (Rakic and Sidman, 1973a, b; Sidman et al , 1965; Sidman, 1968) where, for different reasons, granule cells fail to contact Purkinje cells and die.
Figure 1.5 Migration pathways to the posterior portion of the human thalamus. A and B, semidiagrammatic drawings of the thalamus and adjacent structures sectioned in the horizontal plane. The arrows indicate the main paths of cell migration during formation of the thalamus. Section A is from a 10-week foetus (mag. x 8), section B is from a 24-week foetus (mag. x 4). The arrows near the circle marked 1 indicate that the ventricular zone in the wall of the Illrd ventricle serves as the source of thalamic neurones during early development. The arrows extending from circle 2 indicate the path of migration of cells from their origin in the ganglionic eminence (GE) to the lateral thalamus. The arrows from circle 3 indicate the migratory path through the corpus gangliothalamicus (CGT) into the thalamus. Migrations 2 and 3 occur at a time when the ventricular zone of the Illrd ventricle is spent. C, Golgi impregnated posterior pole of the dorsal thalamus, including the medial portion of the ganglionic eminence (GE). The figure shows the bipolar migratory cells and the gradient of their increasingly more complex shapes as they enter the deeper zones of the thalamus. The 0.1 mm scale at the bottom indicates the magnification of the Golgi preparation. Other abbreviations; C, caudate nucleus; Cl, internal capsule; CM, nucleus centrum medianum; GP, globus pallidus; H, hippocampal formation; LV, lateral ventricle; 3V, the Illrd ventricle (from Rakic and Sidman, 1969, with permission).
It is possible also that trophic cues originating within the target could initiate the dendritic growth of source neurones, and there is some evidence for this in the developing basal lamina of the spinal cord where motor neurones may only develop dendrites when their axons have contacted muscle (Barron, 1943). In the adult, severance of motor nerves results in a collapse of the dendritic tree which regrows when the muscle is reinnervated (Sumner and Watson, 1971). Dendritic growth could also perhaps be initiated by afferent fibres ramifying about the postmigratory neuroblasts. For example, Pinto Lord and Caviness (1979) have suggested that fibres in the plexiform layer of the primitive cerebral cortex could be responsible for initiating apical dendritic growth of neocortical pyramidal cells. Monoaminergic fibres could be major contenders for a role in primary dendritic induction (Berry et al , 1980b,c; Sievers et al ., 1981a) since this neuronal system is one of the first to mature and quickly innervates all areas of the developing CNS (Sievers et al, 1981a).
The pattern established by the initial dendritic outgrowth is often quite different from that of the adult tree. For example, Purkinje cells in the cerebellum (Rakic and Sidman, 1970; Zecevic and Rakic, 1976) at first have multiple perisomatic processes but later they become resorbed as the cell achieves polarity and the typical dendritic tree develops. The factors which bring about such changes are poorly understood but might be mediated by specific groups of afferent fibres (Berry et al, 1978). In all regions of the CNS the period of most active growth of dendritic fields coincides with the arrival of major afferent systems (Morest, 1969a, b). There is now experimental evidence to support the idea that axons and dendrites interact during development to the extent that most of the parameters of dendritic fields appear to be determined by such interactions (Berry et al, 1979a, b; 1980 a,b,c). Selective adhesion between dendritic growth cone filopodia and specific axon groups might be the basis of the interaction for Purkinje cells in the cerebellum, but in many other dendritic systems the attenuated segment lengths and paucity of branch points indicates that modes of interaction differ quantitatively and qualitatively from region to region in the CNS.
Dendritic growth probably continues for some considerable time after neurogenesis is complete; moreover, the potential for growth appears to be prolonged into adulthood and probably accounts for some aspects of plasticity. It is not known how the growth of axonal and dendritic fields is modulated to achieve perfect matching of pre- and postsynaptic elements. There is some evidence that the density of postsynaptic sites may be determined genetically. Certainly, postsynaptic spines form on Purkinje cell dendrites independently of any presynaptic influence (Berry et al ., 1978) and this may be true for other neurones. Axons tend to become segregated topologically before entering a nucleus or cortical area (e.g. Goldman-Rakic, 1980) and such order may simply result from the sequential addition of axons to a projection tract. Segregation also occurs within a centre. The palisading of eye dominance columns in the visual cortex may be achieved by interaction across the geniculo-striate synapse such that appropriately functioning synapses are reinforced, but inappropriate contacts are lost (Stent, 1973). This functional stabilization of connections probably occurs in most central and peripheral sites. Changeux and Danchin (1976) consider that the first formed connections are spatially dispersed and poorly specified. As centres interact, connections are fined down until only those with functional integrity stabilize and survive.
Foliation
The cortices of the cerebral hemispheres and cerebellum tend to fold during growth to form intricate convolutional patterns which are characteristic for each structure (Figs. 1.6,1.7). In the cerebellum, foliation occurs over the period from the 11th foetal week until the 7th postnatal month and reflects the massive increase in volume achieved (Rakic and Sidman, 1970; Zecevic and Rakic, 1976). Thus, the surface area increases by about 2000 times over the period from the 13th foetal week till term. This growth and the accompanying foliation is largely attributable to the proliferative activity of the external granular layer (Altman and Anderson, 1969; Rakic and Sidman, 19736; Lauder et al ., 1974). A mature fissure arrangement is present by the second postnatal month. The pattern is very stereotyped and each lobe can be identified accurately from individual to individual (Loeser et al ., 1972; Larsell, 1947). Inouye and Sen-Ichi (1980) studied the morphological variations in folial pattern in the cerebella of several strains of mice. They found the patterns to be strain specific and concluded that, if regional differences in the rate of proliferation of the external granular layer account for foliation, the former may be under genetic control.
The development of the cerebral hemisphere is also characterized by foliation but the patterns are quite different from those exhibited by the cerebellum. Since there are no secondary germinal centres within the neocortex, the enlargement of the hemisphere surface can only be achieved by an expansion of the neuropil (Dobbing and Sands, 1970, 1973; Dobbing, 1970). By three and a half months (100 days) the major division of the cortex into four lobes has taken place and by five months the first evidence of the lateral fissure is discernible as a V-shaped groove delineating the boundaries of the insular cortex. By seven months central and other primary sulci are formed. At term, all primary sulci are present as well as most secondary convolutions, but the insular cortex is still exposed through a gaping lateral sulcus (Fig. 1.7). Tertiary convolutions begin to appear in the third trimester and only become fully demarcated postnatally (Sidman and Rakic, 1975).
Figure 1.6 Outline of the cerebellar surface in the midsagittal plane in specimens ranging in age from 11 foetal weeks (W) to seven postnatal months (PNM), all at the same magnification as indicated by the 5 mm scale at the bottom of the figure. The asterisks indicate the primary fissure (from Rakic and Sidman,.1970, with permission).
The mechanism of foliation/fissuration is not understood but probably involves the interaction of pial mesenchyme and basal lamina with the glial end- feet (Sievers et al , 1981b; Allen et al ., 1981). The experimental work of Barron (1950) on sheep neocortex focused attention on differential growth within the
Figure 1.7 The developing human brain showing the development of the cerebral hemispheres and their convolutional patterns (from Cowan, 1979, with permission).
cortex as a possible cause of folding. Studies of the human conditions of microgyria and lissencephaly (Rickman et al , 1973, 1974, 1975; Stewart et al , 1975) have been undertaken because these represent the upper and lower limit respectively of the tertiary convolutional spectrum. The lissencephalic brain has no tertiary gyri and supragranular layers are either absent or hypoplastic. The microgyric brain has a large number of small tertiary sulci but the granular and infragranular laminae are either absent or hypoplastic. It was suggested that the differences in cortical folding seen in these two conditions might reflect respective differences in the growth rates of superficial and deep cortical laminae. The results of computer modelling experiments bore out these predictions, strongly indicating that in normal brains random tertiary folding may be brought about by exuberant growth of superficial relative to deep laminae. The problem of how primary and secondary convolutions are formed remains, however. The constancy of the locations of the sulci suggests the mediation of genetic instruction but the nature of the substrate which is encoded remains a mystery.
Development of the cerebellum
The cerebellar anlagen first appear at the 5.7-7.3mm (GL—greatest length) stage and fuse in the mid-line at 24-27 mm (CRL-crown rump length) stage (Bartelmez and Dekaban, 1962). The subventricular zone in the roof of the fourth ventricle forms successively the neurones of the basal nuclei, Purkinje cells and Golgi cells. Matrix cells proliferating in the caudo-lateral margin of the rhombic lip migrate to the surface of the anlage. Their continued mitotic activity causes a sheet of cells, the external granular layer, to populate the entire cerebellar surface (Rakic and Sidman, 1970). During the initial proliferative phase (3-8 foetal weeks) the cerebellum is made up of ventricular, subventricular and marginal layers (see Fig. 1.9). The external granular layer is established over the 10-11th foetal weeks. Mitotic activity declines in the subventricular zone by the 12—13th foetal week and all deep cerebellar nuclei, Purkinje cells and Golgi cells are formed by the 13th foetal week. At this stage the mitotic epithelium is replaced by differentiated ependymal cells (Rakic and Sidman, 1968). An exception to this pattern is the floccular-nodular lobe region of the subventricular/ventricular zone which retains its thickness and continues to proliferate until after birth (Rakic and Sidman, 1970). The external granular layer covers the external surface of the cerebellum by 11-13 foetal weeks (Rakic and Sidman, 1970). Supravital thymidine autoradiography shows that the layer achieves a labelling index of 30 % (Rakic and Sidman, 1968).
Figure 1.8 Composite drawing of the cellular components from a 27-week-old human foetus impregnated according to the Golgi method, a; Bergmann glial cell; b, immature Purkinje cell; c, Golgi cell; d, basket or stellate cell; e, f, granule cells in the horizontal, bipolar stage; g, ‘T’-shaped granule cell; h, immature astrocyte; EG, external granular layer; ML, molecular layer; PL, Purkinje cell layer (from Zecevic and Rakic, 1976, with permission).
The external granular layer increases in thickness over the period of the 19-20th foetal week and at 21 weeks granule cells begin to migrate through the molecular layer to form the granular layer. Granule cell migration occurs along radially directed Bergmann glial processes (Rakic and Sidman, 1970). Their cell bodies lie in the deep cellular layer, and their superficially directed processes course in a quasi-parallel alignment through the molecular layer and the external granular layer, terminating in end-feet which abut against basement membrane forming the glia limitans externa of the cerebellum (Choi and Lapham, 1980; Antanitus et al , 1976; Fig. 1.8).
Granule cells become bipolar in the lower margin of the external granule layer (Zecevic and Rakic, 1976). From each pole a transversely orientated axon grows for some distance before the granule cell commences migration. As migration gets under way the cells transform into a tripolar morphology as the descending leading process glides over the vertically orientated Bergmann glia process. As the cell moves through the molecular layer it leaves the transversely running axon attached, at a ‘T’ junction, to a vertical ascending axon which is paid out behind the migrating granule cell (Fig. 1.8).
A clear lamina appears below the layer of cells which form the lower border of the molecular layer. This is the lamina dissecans (Rakic and Sidman, 1970) which is a transient acellular area present only in the human cerebellum from the 21st-40th foetal week (Fig. 1.9). By 30-32 foetal weeks the cells in the layer above the lamina dissecans are recognizable as definitive Purkinje cells. The granule layer develops below. Electron microscope studies of the lamina dissecans between the 12th and 14th week reveal many randomly organized dendritic and axonal profiles with many presumptive synaptic appositions (Rakic and Sidman, 1970; Zecevic and Rakic, 1976). It is possible that the axons within the middle and deep aspects of this layer are mossy fibres, whilst those in the upper region about Purkinje cell somata are granule and basket cell axons and climbing fibres. The latter first arrive in the cortex between 12 and 14 weeks. No definitive synapses are seen over this period. Within the molecular layer, adjacent to the Purkinje cell layer, a plexus of axons develops at a stage just before granule cells migrate. The origin of these fibres is unknown but they could be monoaminergic and mediate important trophic/inductive cues at about this time.
Figure 1.9 Semidiagrammatic drawing to summarize the main events of histogenesis in the human cerebellar cortex from the ninth foetal week to the seventh postnatal month, at 4, 9, 30 and 40 foetal weeks (wks) and the 7th postnatal month (p.n:m.). The arrows point in the direction of the main stream of cell migrations (E, edendyma; EG, external granular layer; G, granular layer; I, intermediate layer; LD, lamina dissecans; M, molecular layer; P, Purkinje layer; V, ventricular zone; W, white matter (from Rakic and Sidman, 1970, with permission).
Over the period from 12 to 16 weeks, Purkinje cells are bipolar and occupy a lamina in the cortex several rows deep (Fig. 1.10). Between 16 and 24 weeks Purkinje cells become arranged into a single row above the lamina dissecans, and their somata increase dramatically in size, develop spines and synapses appear on the latter (Zecevic and Rakic, 1976). The apical dendritic system develops and invades the molecular layer, but never invades the granular layer. The dendrites ramify randomly within the molecular layer, but over the period from 24 to 28 weeks the system becomes planar, oriented at right angles to the long axes of folia. After 28 weeks, somatic spines disappear and the tree develops secondary and tertiary spiny branchlets.
Figure 1.10 Composite drawings of Purkinje cell images in the human cerebellar cortex at various foetal and postnatal ages (A-J). All cells were selected from sections that were cut in the plane transverse to the folium, close to the midline. Cell profiles in the external granular layer are also outlined. Ages in foetal weeks (W) and postnatal months (PNM) are given on each drawing (Scale 100 ^m, from Zecevic and Rakic, 1976, with permission).
The time-course of development of the Purkinje cell tree is coincident with the period of parallel fibre deposition in the molecular layer (Figs. 1.9, 1.10). It has been suggested that parameters like planar organization, segment lengths, number of branches, direction of growth, etc., are all determined by parallel fibre/Purkinje cell dendritic growth cone filipodial interaction and there is good experimental evidence corroborating this proposition (Berry et al , 1978, 1980a, b, c).
Synapses first appear at 16-18 weeks in the region of the perisomatic plexus in the upper part of the lamina dissecans. Most junctions are asymmetrical and are probably transient contacts of climbing fibres on Purkinje cell somatic spines. At 15 weeks Purkinje cells first develop long finger-like spines on their dendrites without presynaptic attachments or postsynaptic specializations. By 7-8 months spines have become shorter and developed terminal enlargements and synaptic junctions.
The termination of cerebellar development has been nicely documented by Gadsdon and Emery (1976). Postnatally the cell concentration in the external granular layer rapidly decreases over the 6-8 month period. No cells remain after 12-18 months (Fig. 1.11). The number of cells in the internal granular layer is maximal by 22-24 months, at 61 000-62 000 cells/mm 2 . This value is maintained until 6 years, when the number of granule cells begins to decline steadily (Fig. 1.12). The number of cells in the molecular layer reflects the period of granule cell migration which is complete by about 14 months post partum (Fig. 1.13). DNA optical density studies showed that mitotic activity is over by 9 months. Gadsdon and Emery (1976) showed that Purkinje cells were diploid at all stages of development, an observation in agreement with Mann and Yates (1973) and Fujita (1974). These findings refute the work of others who have found Purkinje cells in man to be tetraploid (Lapham, 1968; Lentz and Lapham, 1970).
Several workers have studied the human foetal cerebellum in organ culture. Markesbery and Lapham (1972, 1974) maintained 10-19 week foetal cerebellar cortex in culture for 3-4 months. Cells exhibited migration, differentiated, and retained good organization. Many neurones migrated out from the confines of the tissue over astrocytic processes (Lapham and Markesbery, 1971; Lapham and Williams, 1973). The differentiation of the cerebellar cortex in vitro is remarkably similar to that described in vivo by Rakic and Sidman (1970) (Markesbery and Lapham, 1974).
Figure 1.11 Number of cells per surface millimetre of cortex in the external granular layer of the lateral lobe, related to postnatal age. Each spot represents the mean reading for an individual cerebellum. (Age scale expanded for the first 2 years—from Gadsdon and Emery, 1976, with permission).
Figure 1.12 The cell concentration per mm 2 in the internal granular layer of the lateral lobe related to postnatal age. (Age scale expanded over the first 2 years—from Gadsdon and Emery, 1976, with permission).
Figure 1.13 The cell concentration per mm 2 in the molecular layer of the lateral lobe related to postnatal age. (Age scale is expanded for the first 2 years—from Gadsdon and Emery, 1976, with permission).
Cerebral neocortex
The cerebral vesicle first appears in the embryo at 5.5-7.3 mm (GL) stage (Bartelmez and Dekaban, 1962). Proliferation in the ventricular/subventricular zone leads to migration of neuroblasts along radial glia to establish a lamina, called the cortical plate, below the marginal layer over the 7-10 foetal weeks (see Figs. 1.1, 1.14 and 1.15). The radial glia differentiate early and contain glial fibrillar protein by at least 7 weeks (Choi and Lapham, 1978; Antanitus et al, 1976). The pattern of migration of neuroblasts was first described by Angevine and Sidman (1961) and Berry and Eayrs (1962) as an inside-out progressive establishment of the cortical laminae and it appears that the same sequence occurs in man (Sidman and Rakic, 1973; Rakic, 1975h; Marin-Padilla, 1970a, b).
The work of Poliakov (1961; and cited by Sidman and Rakic, 1973) suggests that the cerebral cortex may be formed by phasic proliferative and migratory activity. The first wave, probably comprising layer Y and VI, commences at about 7-8 weeks and is over by the 10th week when the second wave begins. The duration of this latter wave is long, probably extending into the neonatal period, but most of the cells arrive in the first six weeks. The number of cells migrating in the second is much greater than during the first wave, and this wave establishes layer IV, III and II. Most migratory cells move through the cortical plate to the lower border of the marginal zone where their migration ceases.
Some cells, however, are deposited at all levels of the plate throughout development and this may particularly apply to stellate neurones. Radial glia in the monkey are mitotically active during the initial and later periods of gestation but are mitotically inactive in the midgestational period (Schmechel and Rakic, 1979). This dormant period could allow radial glia to maintain their pial- ventricular attachments as the cortex expands during foliation. This would be important if one function of the radial guides is to accumulate radially orientated columns of cells in the cortex. Thus, the cells in a given column will have originated from the same germinal population, i.e., they are of similar lineage despite changes in the surface area of the cortex relative to that of the ventricle (Rakic, 1978). The exact timing of the sequence of establishment of the different layers of the human cortex is unknown because it is impossible to study this using the 3 H-thymidine labelling technique. Nevertheless, subsequent differentiation of the laminae leaves no doubt that layers VI, V, IV, III and II are sequentially deposited in that order during migration. There are, however, some peculiarities in that some of the first cells to migrate subsequently become trapped in the cortical white matter as interstitial cells (Kostovic and Rakic, 1980) whilst others, probably originating in the lateral wall of the hemisphere, migrate tangentially within the marginal layer to form a lamina of cells, called the subpial granular layer (Brun, 1965). This latter structure first appears at about the 12- 13th foetal week in allocortex and covers the entire cortex by about the 18th week. It is a transitory zone, having disappeared by term, and probably gives rise to glia and neurones. The latter may migrate into the cortical plate or remain in the marginal zone as Cajal-Retzius cells (Rickman et al , 1977). Glioneural heterotopia may arise from persistent cell nests of subpial granular layer origin (Brun, 1965).
Figure 1.14 Camera lucida drawings of a coronal section of the Golgi-impregnated telencephalon of a 97-day monkey foetus. A: coronal section through the parieto-occipital lobe. The area delineated by the white strip between arrowheads is drawn in B at higher magnification. B: enlargement of the portion of cerebral wall indicated by the white strip in A, the middle 2500 ^m of the intermediate zone is omitted (C, cortical plate; D, deep cortical cells; I, intermediate zone; M, marginal layer; MN, migratory cell; RF, radial fibre; S, superficial cortical cells; SV, sub ventricular zone; V, ventricular zone—from Rakic, 1972, with permission).
The period of development of the cortical plate has been divided into five stages (Poliakov, cited by Sidman and Rakic, 1973). Stage I begins at the 7-10th foetal week with the formation of the plate, as the first cellular migration gets under way. The plate divides the wall of the cerebral vesicle into several strata called, sequentially, from the luminal surface outwards, the ventricular zone, the subventricular zone, the intermediate zone, the cortical plate and the marginal zone (Figs. 1.1,1.14). Stage II occupies the period of the 10-llth foetal week in which definitive fibres invade the cortex and form a plexus above and below the plate but never in it (Molliver et al , 1973; Kostovic and Molliver, 1974). Symmetrical axo-dendritic synapses are present above and below the plate with a sharp peak in synaptic density in the plexus deep in the marginal layer. Supra- and infra-plate plexi are probably made up of thalamocortical afferents and monoaminergic fibres (Molliver et al , 1973; Poliakov, 1961; Marin-Padilla, 1970a,h, 1969). During stage III (11—13th foetal week) the. first migratory phase wanes and the first arrivals begin to differentiate and occupy successively deeper positions within the plate as migrating cells take up more superficial positions (Fig. 1.15). The neocortical cells differentiating at this time are probably layer VI and layer V neurones. Stage IV (13—15th foetal week) marks the commencement of the second wave of migration which probably establishes the granular and supragranular layer (Sidman and Rakic, 1973). Stage V marks the decline of the secondary phase of migration in mid-gestation and this last stage includes the entire period of cortical maturation which continues in the neonate and is characterized by a massive expansion in cortical neuropil. Growth during this period mostly takes place in dendritic and axonal systems and is accompanied by maturation of glia.
As post-mitotic neuroblasts migrate out from the subventricular zone, their trailing processes probably develop into definitive axons. Many grow centri- petally to establish connections with sub-cortical targets. Meanwhile, migrating neuroblasts reach the lower border of the marginal zone where their upward movement terminates (see Fig. 1.16). In the case of pyramidal cells the leading
Figure 1.15 The cerebral wall of a 15-week-old human foetus. The four layers which comprise the cerebral wall at this stage of development are the periventricular layer (PV) made up of ventricular and subventricular zones, migratory cells (migr) in the intermediate zone, the cortical plate (CP) made up of closely packed columns of young neurones and the marginal zone (marg) (from Hattori and Fujita, 1974, with permission; x 93).
process, juxtaposed to the marginal layer, develops into the definitive apical dendrite. It was mentioned above that post-migratory neurones occupy successively deeper positions within the cortical plate as cohorts of younger migrating cells attain positions above them. Basal dendrites are elaborated by neurones deep in the cortical plate where axons of the sub-plate plexus ramify (Marin- Padilla, 1970b). Neuroblasts terminating migration in the deep cortical plate may be those which ultimately differentiate into stellate cells. The induction of dendritic growth thus appears to be mediated by fibres residing in the marginal zone and in the sub-plate region. The evidence for these propositions comes from the work of Pinto Lord and Caviness (1979) on the reeler mouse cortex, where apical dendrites bud out from the somal surface of post-migratory neuroblasts apposed to the fibres of the aberrant intermediate plexiform (marginal) layer. Neuroblasts lying above these fibres develop apical dendrites from their deep surface which course centripetally, whilst those below develop apical dendrites from their superficial surface which are directed centrifugally. Thus, the leading process of the migratory neuroblast is not the presumptive apical dendrite. Post-migratory neuroblasts sandwiched between fascicles of fibres develop a bipolar morphology with both principal dendrites having invaded fascicles above and below them. Since the fibres coursing in the cortex at this time are largely, if not entirely monoaminergic (Schlumpf et al , 1980), it is possible that this primary dendritic induction is mediated by this fibre group. Moreover, since in the normal developing cortex, these fibres form a plexus above and below the cortical plate (respectively in the marginal and sub-plate layers) apical dendrites could be induced by fibres in the former and the basal dendrites of pyramidal cells and stellate cell dendrites could be induced by fibres in the latter (Pinto Lord and Caviness, 1979). An additional factor which has largely been ignored, but which may be of importance in dendritic initiation, is the influence of trophic cues passing antidromically from target sites to cortical neurones. Such tropic substances could prime the cells before monoaminergic induction.
The subsequent fate of apical and basal dendritic systems and the dendrites of stellate cells is different. Apical dendrites normally course radially through the cortex and terminate in the marginal layer. The apical shaft thus marks the course and extent of centripetal soma displacement as cortical thickness increases. Elongation would be brought about by interstitial growth. Oblique or lateral branches appear on the apical shaft in regions of cortex where axon strata are concentrated (Kristt, 1978; Wise et al , 1979) and subsequently grow terminally and branch randomly (Hollingworth and Berry, 1975). Inverted pyramidal cells also possess radially directed apical dendrites which terminate in the cortical depths (Van der Loos, 1965; Caviness, 1976). This observation suggests that all apical dendrites might be constrained to grow radially by guidance along similarly orientated elements, like radial glia, but Van der Loos’s (1965) observation on other malorientated pyramidal cells indicates that apical dendrites follow a course orthogonal to the basal axis of the cell with little environmental constraint.
Basal dendrites in contrast grow very slowly through the cortical neuropil (10-80 /rni/day). The distribution of branching angles (Smit and Uylings, 1975) indicates that they are orientated randomly in rat visual cortex but this may not be universal for all areas of cortex (Glaser et al , 1979). Branching has been shown to be random and to occur almost exclusively on terminal segments (Hollingworth and Berry, 1975). Averaged over the period of development in the rat, for example, branching occurs at a frequency of one branch per day but, in reality, is probably non-linear over this period being highest at the time thalamocortical afferents invade the cortex (Kristt, 1978; Wise et al , 1979; Richter, 1980) and zero in later stages when very long, unbranched terminal segments are produced. Basal dendrites possess prominent terminal growth cones and some of the varicosities on their segments may also be growth cones (Morest, 1969a, b; Peters and Feldman, 1973). These sitings probably account for both terminal and intersegmental elongations of dendrites.
It seems doubtful that thalamocortical fibres determine branching patterns according to the filopodial adhesive hypothesis (Berry and Bradley, 1976) since the low frequency of dichotomy is not compatible with the presence of large numbers of axons in the cortex during dendritic growth. Thalamocortical and commissural afferents may trophically support growth (Wise and Jones, 1978) and may constrain dendritic fields within defined domains.
Stellate cell dendrites probably obey rules similar to those for basal dendrites but their domains are probably more tightly controlled by cortical afferents. Like basal and oblique pyramidal cell dendrites their growth spurt coincides with the arrival of afferents (Parnavelas et al, 1978; Mathers, 1979; Wise and Jones, 1978; Kristt, 1978; Richter, 1980; Geisert and Guillery, 1979).
All dendritic systems in the neocortex are modified by either ablating the afferent input or exposing animals to altered environments. In general reducing input causes dendritic fields to either grow less or become retracted (Jones and Thomas, 1962; Coleman and Riesen, 1968; Berry and Hollingworth, 1973), whilst increasing environmental stimulation results in more extensive and more branched fields (Rutledge et al, 1974; Greenough and Volkmar, 1973; Greenough et al, 1973, 1979). In both instances numbers of primary dendrites are not affected and from the results of experiments on the effects of starvation on dendritic development, branching patterns may also be universally unchanged (McConnel and Uylings, in preparation). Plasticity may be attributed to changes in segment lengths and the numbers of branches. Uylings et al (1978) have presented evidence that such plastic changes also occur in mature animals, and this has been confirmed by Greenough et al (1979). Both groups demonstrated that increases in length and branching take place principally over terminal segments. In particular, branching occurred by collateral sprouting some distance from the tip.
If the environment controls the degree of axonal terminal arborization in cortical afferents (possibly by Hebb synaptic modification—Stent, 1973), and if these axons inject quanta of trophic chemical into cortical cells, which mediates dendritic growth, then incrementing numbers of presynaptic elements would mediate an elevated trophic effect. By this means the extent of dendritic fields may be related to the density of ramifying cortical afferents. Preterminal branching of cortical dendrites may occur only when dendrites advance above a threshold rate precipitating microtubule instability in the terminal segment and the production of paraterminal growth cones (Bray et al , 1978). Thus, if branching is related only to the rate of growth, it is easy to see why branching frequency falls off during development (Juraska and Fifkova, 1979) as afferent ingrowth ceases, and how branching might be stimulated as axonal terminal arbors increase in size under the influence of increased traffic of impulses. According to this hypothesis there is no critical period in dendritic development. Thus, dendrites can equate the extent of their postsynaptic membranes with the number of potential presynaptic elements throughout the life of the animal.
Stellate cells exhibit a property, in their response to environmental manipulation, which may be lacking in pyramidal cells in that they can reorientate their fields (Valverde, 1968; Borges and Berry, 1978; Harris and Woolsey, 1979). This response suggests that stellate cells may be trophically influenced by particular afferents occupying defined regions of cortex. Redirection of growth appears to be brought about by suppression of growth of some dendrites and augmentation in others (Borges and Berry, 1978).
Dendritic field growth in the human neocortex (Fig. 1.16) is very well illustrated in the papers by Poliakov (1961, 1966), Rabinowicz (1964), Marin- Padilla (1970a,5, 1969), Purpura (1975a,5, 1977) and Takashima et al (1980). In general primary motor and sensory cortex differentiates before association cortex and in all areas layers Y and VI are always more advanced than the granular and supra-granular layers, except for Cajal-Retzius cells in the marginal layer which develop earlier than any other neurone type in the cortex (Poliakov, 1961; Marin-Padilla, 1970b).
Axon systems develop pari passu with dendrites. Over the mid-gestational period efferent axons develop their collateral systems and prominent afferent projections form horizontal plexi within the cortex. Before mid-term, axon plexi are found in the marginal zone and within the intermediate zone (Marin- Padilla, 19705; Poliakov, 1961). The latter actually consists of an inner and outer plexus of which the outer is the denser. These fibres traverse the internal capsule probably originating in the basal ganglia and thalamus. Some are callosal fibres. This commissure appears in the fourth foetal month and grows very rapidly in the preterm infant (Rakic and Yakovlev, 1968). By 5 months the cells in layers VI and V have achieved their characteristic morphology and by 7 months their dendrites have become studded with spines (Marin-Padilla, 1970; Poliakov, 1966). Layer IV has appeared and the internal and external axonal plexi of Baillarger are present at 7 months, heralding an advanced state of development of intracortical afferent terminal axon arbors (Marin-Padilla, 19705). By 7.5 months miniature stellate cells are recognizable in layer IV and by birth these interneurones have populated all layers. Their axon collaterals terminate in pericellular baskets about pyramidal cells in all layers although deep baskets mature earlier than those placed more superficially. The appearance of pericellular baskets indicates that interneurone inhibitory pathways are established at about birth. In motor cortex layer IV is no longer distinguishable after 2.5 months post partum as pyramidal cells from layers III and V encroach into the layer. Stellate cells establish a slab-shaped vertical axonal field domain extending through the whole depth of the cortex (Marin-Padilla, 1969, 19706, 1972; Marin-Padilla and Stibitz, 1974). Interestingly, their dendritic fields occupy a much smaller area than do their axon fields. The pattern of vertical slab domains adopted by stellate axonal and dendritic fields is mirrored by the afferent fibres engaging them, and probably represents a forerunner of the striped distribution of thalamocortical afferents within cortex (Rakic, 1977; Hubei et al, 1977).
Figure 1.16 Camera lucida representations from rapid Golgi method specimens of brains at different ages, a—14 weeks; b—20 weeks; c—24 weeks; d—28 weeks; e—30 weeks; f — 3 5 weeks; g— 40 weeks; h—6 months (redrawn from Takashima et ai, 1980).
Spines are sparse on apical and basal dendrites before the mid-term period. Thereafter spine density increases dramatically (Takashima et al , 1980). At first spines are long and their number is greater over proximal dendrites. Basal dendritic spines appear to mature before apical spines. A common correlate of mental retardation in human infants is spine dysgenesis (Purpura, 1974).
Hippocampus
The anlage of the hippocampus first appears in the medial wall of the cerebral vesicle at about 6 weeks (Humphrey, 1966) when cells migrate from the sub-
ventricular zone into the cell-free marginal zone. Some time after, cells in the germinal epithelium of the vesicle wall laterodorsal to the dentate field develop into the primordium of Ammon’s horn. As already mentioned, cells destined to form the dentate gyrus continue to divide after migration (Cowan et al , 1980). By 10 weeks the pyramidal layer of Ammon’s horn is beginning to differentiate and the fimbria is present. The cells of the dentate gyrus remain undifferentiated and form dense clumps of cells. At 11.5 weeks the double pyramids of Ammon’s horn are distinguishable. By 13.5 weeks all layers of Ammon’s horn and the dentate gyrus are represented (Fig. 1.17) (Humphrey, 1966) and the alveus is present throughout. In the 18-20-week foetus' pyramidal cells are already developing apical dendrites. At 24-28 weeks the apical dendritic systems of pyramidal cells in Ammon’s horn are better developed than the basilar system, and pedunculated knobs and fine spines are present on the former. Infra- and suprapyramidal bundles of the perforant pathway are present but poorly developed (Purpura, 1973a, b, 19746, 1975a,6, 1977). At 28-32 weeks the branching patterns of the basilar dendrites of pyramidal cells are more mature.
Figure 1.17 Stages of development of the hippocampus and hippocampal fissure correlated with CRL and gestational age (from Lemire et al ., 1975).
Apical dendritic growth is extensive and fibre contacts are present. In the immediate postnatal period typical ‘baskets’ develop about pyramidal cell somata. Spine density is mature by about 6 months post partum (Purpura 1973a, b, 1975a, b). Dendritic growth in the dentate gyrus lags behind that in Ammon’s horn and shows a progressive superior-inferior gradient, being most advanced in the suprapyramidal and least in the infrapyramidal limbs (Purpura, 1975a,ft). The period of maximal axonal and dendritic growth in the hippocampus is between 18 'and 33 weeks (Green, 1964; Purpura, 1974b, 1975a, b, 1977). Over this period the branching angles of pyramidal dendrites (Paldino and Purpura, 1979a, b) become reduced, suggesting dynamic compression of the fields. The angle reduction increases at incrementing distances from the soma. Dendritic development is complete by about the 5th-6th month.
Diencephalon
Over the period from the 4-6th week the diencephalic wall is delineated into epithalamus, dorsal thalamus, ventral thalamus and hypothalamus by dorsal, middle and ventral longitudinal sulci (Richter, 1966; Reinoso-Suarez, 1966). In the mouse, neurones formed first in the subventricular zone populate the ventrolateral-caudal parts of the thalamus; those generated successively later form progressively more dorso-medial-rostral regions (Angevine, 1970). In man, the ventricular/subventricular complex produces neurones from the 8th through to the 13th—15th week (Rakic, 1974). By the end of the 3rd month fibre systems demarcate most of the ventral and posteroventral thalamic nuclei (see Fig. 1.5). However, the posterior portion of the thalamus begins to increase in size after the 15th week, as the number of neurones in the pulvinar increases significantly during the mid-trimester when other thalamic nuclei have their full complement of neurones (Rakic and Sidman, 1969). The source of these new neurones is not the subventricular zone in the diencephalic wall, which produces no new neurones after foetal week 18-22 (Rakic and Sidman, 1968,1969). Late-arriving neurones populating the pulvinar originate in the ganglionic eminence in the telencephalon and migrate to the pulvinar through the corpus gangliothala- micus (Fig. 1.5) despite being separated from the thalamus by an ever-deepening sulcus terminalis—Fig. 1.18 (Rakic and Sidman, 1969; Rakic, 1974, 1975b; Sidman and Rakic, 1973). The late development of the pulvinar correlates with the late development of association cortex relative to that of other cortical areas (Poliakov, 1961, 1966). The ganglionic eminence also produces neurones which migrate ventrally to produce the basal ganglia (Fig. 1.18).
Figure 1.18 Coronal section at approximately the same level of the forebrain in 10 to 14 week human foetuses to illustrate the relationship of the ganglionic eminence (GE) to the basal ganglia and thalamus (T). (MG, medial globus pallidus; FTD, telencephalic-diencephalic fissure; A, amygdala; AC, archicortex; C, caudate nucleus; Cl, internal capsule; CL, claustrum; LG, lateral globus pallidus; P, putamen; RF, rhinal fissure;.SB, subthalamic nucleus (from Sidman and Rakic, 1973, with permission).
Brain stem and spinal cord
Neurones containing catecholamine (CA) and indolamine (IA) first appear at about the 7th foetal week and most systems are present by 13 weeks—Fig. 1.19 (Olson et al ., 1973; Choi et al ., 1975). Proliferation and differentiation over the 7th-23rd weeks establishes a pattern very similar to that laid down in the mammals. By the 3-4th month the system is very well developed (Nobin and Bjorklund, 1973). The nigro-striatal dopaminergic (DA) system is first seen as a condensation of positive CA fluorescent cell bodies in the substantia nigra, from which axons later project to the putamen and later still to the caudate nucleus. CA fluorescent fibres first appear in the hypothalamus during the 10th foetal week and within the median eminence over the 13th week (Hyyppa, 1972). The locus coeruleus and its noradrenergic (NA) axons and the 5-hydroxytryptamine (5-HT) raphe complex are well established by the 13th week.
The remarkable similarity between other mammals and man in the timing, duration and pattern of generation, migration and differentiation of neural systems in the brain stem and spinal cord is also exemplified by the development of the interpeduncular nucleus (Halfron and Lenn, 1976; Lenn et al ., 1978).
The possible role of the monoaminergic (MA) neurone system in development has been emphasized by many workers (Ahmad and Zamenhof, 1978; McMahon, 1974; Bloom, 1975; Sievers et al , 1979, 1980, 1981a) and supported by the findings that MA cell groups are among the first to be generated (Lauder and Bloom, 1974; Nicholson et al , 1973; Taber Pierce, 1973). They quickly metabolize neurotransmitters (Olson and Seiger, 1972; Seiger and Olson, 1973) and connect with their targets, establishing innervation densities similar to those of the adult (Coyle and Molliver, 1977; Schmidt and Bhatnagar, 1979; Zecevic and Molliver, 1978). At the same time ^-adrenergic receptors are elaborated by the target neurones (Walton et al , 1979).
However, the MA inductive/trophic hypothesis has recently been tested (Lauder and Krebs, 1976, 1978; Maeda et al ., 1974; Pettigrew and Kasamatsu, 1978; Wendlandt et al ., 1977) with inconclusive results. Recent experiments by Sievers et al. (1979, 1980, 1981a) have demonstrated that the NA system originating from the locus coeruleus has no effect on the course of postnatal development of the rat cerebellum. Thus, the monoaminergic system may have an important inductive or trophic role in central neural development but probably for early rather than late developmental events (Sievers et al ., 1981a).
In the spinal cord, proliferation in the subventricular/ventricular zones establishes the alar and basal plates (Fujita, 1973b; Wozniak et al ., 1980). Work has tended to concentrate on the development of the basal plate because this region offers the opportunity of correlating the acquisition of cutaneous spinal reflexes with the development of the motor neuropil. In general, the first synapses to appear in the embryo are located in the marginal zone of the basal plate (Wozniak et al , 1980; Gamble, 1969). They are always asymmetrical and axodendritic. The presynaptic element contains rounded vesicles. Synapto- genesis proceeds in a cranio-caudal direction from the cervical cord. Axons often have a peculiar relationship with radial glia, in that they become invested in glial cytoplasm (Gamble, 1969). This observation may have an important bearing on the problem of axonal guidance. Myelination of axons begins at about 10-11 weeks (Okado et al, 1980) beginning in the dorsal ventral and peripheral lateral tracts but the cortico-spinal tracts myelinate relatively late (Meier, 1976). Glial production and differentiation continues through the first trimester (Fujita, 1973b; Malinsky et al, 1968; Malinsky, 1972).
Figure 1.19 Schematic drawing of the brain of a 7 cm human foetus. A horizontal projection of major monoamine cell groups as well as of the CA fluorescence of the putamen (large shaded area) and the nucleus accumbens septi area (smaller shaded area) was reconstructed from drawings of about 170 serial sagittal sections. CA—level of anterior retroflexus, P—principal locus coeruleus. The small CA cell groups in the hypothalamus are probably at least partly of the DA type. Similarly, the large CA cell complex of the mesencephalon in all probability represents the developing DA neurones of the substantia nigra. In the pons a large complex of CA cell bodies, probably of the NA type, can be found centred around the dense arrangement of CA cells of the principal locus coeruleus. The projection of CA cell bodies in the medulla oblongata belongs to at least two groups, one ventral and one dorsal. Yellow fluorescent neurones, probably 5-HT-containing, occupy several partly confluent areas along the brain stem from the medulla oblongata to the mesencephalon. (Approx, x 2.5 mag. CA nerve cell groups, □. 5-HT nerve cell groups, 0. Dense diffuse CA innervation gg. Cut surface of the neopallium, ★. From Olson et al., 1973, with permission).
Okado et al (1979, 1980) have studied synaptogenesis in the cord in more detail. They find the first synapses at 34-36 days within the motor neuropil and at 40-42 days outside this neuropil. This period is the time of acquisition of reflex activity. The first synapses to appear over the 7-8 week period are axodendritic. Later axosomatic synapses are seen between 10.5 and 13 weeks. All vesicles are round, never flattened.
Synaptogenesis may continue beyond the 19th week. The first appearance of reflex activity at 5.5 weeks is correlated with synapse formation in the neuropil (Okado et al, 1979; Okado, 1980). At 7.5—8.5 weeks the elicitation of definitive local cutaneous reflexes corresponds to a massive increase in density of axodendritic synapses. By 11.5-12.5 weeks the emergence of responses elicited by simultaneously applied double stimuli may correspond to the production of axosomatic synapses which increase the integrative capacity of the neuropil. Thus, in the spinal cord of man, the motoneurone system matures before the afferent input to the cord as it does in other animals (Humphrey, 1964; Vaughan and Grieshaber, 1973) supporting the concept that cutaneous reflexes mature retrogradely with respect to the flow of afferent nerve impulses (Okado et al, 1979; Okado, 1980).
Conclusions
It is clear that the major advances made towards our understanding of human brain development over the past decade are attributable to the revival in the use of the Golgi technique, the application of methods for the visualization of the monoaminergic system, and the use of the electron microscope. The dynamics of developmental events have, however, so far been inferred from animal studies in which experiments have indicated that the same mechanisms may be responsible for similar processes in all mammals. Although, in the past, it seemed impossible to study the dynamics of neural ontogeny in humans because experimentation in vivo is ethically unacceptable, tissue culture in vitro, organ culture and brain slice techniques could be more universally applied to investigate cell kinetics, migration and connectivity using modern labelling methods. Moreover, the greater availability of fresh tissue also means that better fixation would enable standard light and electron microscope methods to unfold more qualitative and quantitative details about synaptogenesis, differentiation and the growth of neural processes.
References
Ahmad, G. and Zamenhof, S. (1978) Serotonin as a growth factor for chick embryo brain. Life Scl, 22, 963-970.
Allen, C., Sievers, J. and Berry, M. (1981) Experimental studies on cerebellar foliation. II. Quantitative analysis of cerebellar foliation defects after treatment with 6-OHDA. J. Comp. Neurol, in press.
Altman, J. and Anderson, W. J. (1969) Early effects of x-irradiation of the cerebellum in infant rats. Decimation and reconstruction of the external granular layer. Exper. Neurol, 24, 196-216.
Angevine, J. B. Jnr. (1965) Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp. Neurol, 13, suppl. 2, 1-70.
Angevine, J. B. Jnr. (1970) The time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J. Comp. Neurol, 139, 329-188.
Angevine, J. B. Jnr. and Sidman, R. L. (1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature, 192, 766-768.
Antanitus, D. S., Choi, B. H. and Lapham, L. W. (1976) The demonstration of glial fibrillary acidic protein in the cerebellum of the human foetus by indirect immunofluorescence. Brain Res., 103, 613-616.
Barron, D. H. (1943) The early development of the motor cells and columns in the spinal cord of the sheep. J. Comp. Neurol, 78, 1-26.
Barron, D. H. (1950) An experimental analysis of some factors involved in the development of the fissure pattern of the cerebral cortex. J. Exp. Zool, 113, 553-573.
Bartelmez, G. W. and Dekaban, A. S. (1962) The early development of the human brain. Contr. Embryol. Carneg. Inst., 37, 13-32.
Berry, M. (1974) ‘Development of the cerebral neocortex of the rat’ in Aspects of Neurogenesis, Vol. 2, Studies on the development of behaviour and the nervous system, (ed. G. Gottlieb), Academic Press N.Y., 7-67.
Berry, M. and Bradley, P. (1976) The application of network analysis to the study of branching patterns of large dendritic fields. Brain Res., 109, 111-132.
Berry, M. and Eayrs, J. T. (1963) Histogenesis of the cerebral cortex. Nature, 197, 984-985.
Berry, M., Bradley, P. and Borges, S. (1978) ‘Environmental and genetic determinants of connectivity in the central nervous system. An approach through dendritic field analysis’, in Maturation of the nervous system, Progr. Brain Res., Vol.48, (eds. M. A. Corner, R. E. Baker, N. E. van de Pol, D. F. Swaab and H. B. M. Uylings), Elsevier/North Holland Biomedical Press, 133-148.
Berry, M. and Hollingworth, T. (1973) Development of isolated cortex. Experientia, 29, 204-207.
Berry, M., McConnel, P. and Sievers, J. (1980a) ‘Dendritic growth and the control of neuronal form’ in Current Topics in Developmental Biology, Vol. 15, Neural Development, Part I, Emergence of Specificity in Neural Histogenesis, Academic Press, N.Y., 67-101.
Berry, M., Sievers, J. and Baumgarten, H. G. (1980b) ‘Adaptation of the cerebellum to deafferenta- tion’, in Adaptive Capabilities of the Nervous System, Progr. Brain Res. Vol. 52, (eds. P. S. McConnell, G. J. Boer, H. J. Romijn, N. E. van der Pol and M. A. Corner), Elsevier/North Holland, 65-92.
Berry, M., Sievers, J. and Baumgarten, H. G. (1980c) ‘The influence of afferent fibres in the development of the cerebellum’, in Developments in Neuroscience, Vol. 9, Multidisciplinary Approach to Brain Development, (eds. C. Di Benedetta, R. Batags, G. Ginibos and G. Procellati), Elsevier/ North Holland, 91 106.
Bloom, F. E. (1975) The role of cyclic nucleotides in central synaptic function. Rev. Physiol. Biochem. Pharmacol, 74, 1-103.
Boulder Committee (1970) Embryonic vertebrate central nervous system: revised terminology. Anat. Rec., 257 -261.
Borges, S. and Berry, M. (1978) The effects of dark rearing on the development of the visual cortex of the rat. J. Comp. Neurol, 180, 277-300.
Bray, D., Thomas, C. and Shaw, G. (1978) Growth cone formation in cultures of sensory neurons. Proc. Natl Acad. Sci. U.S.A., 75, 5226-5229.
Brun, A. (1965) The subpial granular layer of the foetal cerebral cortex in man. Acta Path. Microbiol. Scand. Suppl, 179, 1-98.
Brustowicz, R. J. and Kernohan, J. W. (1952) Cell nests in the region of the 4th ventricle. Arch. Neurol. Psychiat., 67, 592.
Caviness, V. S. Jnr. and Sidman, R. L. (1972) Olfactory structures of the forebrain in the reeler mutant mouse. J. Comp. Neurol, 145, 85-104.
Caviness, V. S. Jnr. and Sidman, R. L. (1973a) Retrohippocampal, hippocampal, and related structures of the forebrain in the reeler mutant mouse. J. Comp. Neurol ., 147, 235-254.
Caviness, V. S. Jnr. and Sidman, R. L. (1973b) Time of origin of corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. J. Comp. Neurol, 148, 141-151.
Caviness, V. S. Jnr. (1976) Reeler mutant mice and laminar distribution of afferents in the neocortex. Exp. Brain Res., Suppl. 1, 267-273.
Changeux, J. P. and Danchin, A. (1976) Selective stabilisation of developing synapses, a mechanism for the specification of neural networks. Nature, 264, 705-712.
Choi, B. H., Antanitus, D. S. and Lapham, L. W. (1975) Fluorescence histochemistry and ultra- structural studies of locus coeruleus of human foetal brain. J. Neuropath. Exp. Neurol, 34, 507-516.
Choi, B. H. and Lapham, L. W. (1978) Radial glia in the human foetal cerebellum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res., 148, 295-311.
Choi, B. H. and Lapham, L. W. (1980) Evolution of Bergmann glia in developing human foetal cerebellum: a Golgi, electron microscopic and immunofluorescent study. Brain Res., 190, 369-383.
Clarke, P. H. and Cowan, W. M. (1975) Ectopic neurons and aberrant connections during neural development. Proc. Natl. Acad. Sci. U.S.A., 72, 4455-4458.
Coleman, P. D. and Riesen, A. H. (1968) Environmental effects on cortical dendritic fields. I. Rearing in the dark. J. Anat., 102, 363-374.
Cowan, W. M. (1973) ‘Neuronal death as a regulative mechanism in the control of cell number in the nervous system’, in Development and Aging in the Nervous System, (ed. M. Rockstein), Academic Press, New York, 19-41.
Cowan, W. M. (1979) ‘The development of the brain’, in The Brain, Scientific American Inc., U.S.A., 57-67.
Cowan, W. M., Stanfield, B. B. and Kishi, K. (1980) ‘The development of the dentate gyrus’, in Current Topics in Developmental Biology , Vol. 15, Neural Development, Part /, Emergence of Specificity in Neural Histogenesis, (ed. R. K. Hunt), Academic Press, New York, 103-157.
Coyle, J. T. and Molliver, M. E. (1977) Major innervation of newborn rat cortex by monoamine neurons. Science, 196, 444-447.
Cragg, B. G. (1972) The development of synapses in cat visual cortex. Invest. Path., 11, 337-385.
Dobbing, J. (1970) Undernutrition and the developing brain. Amer. J. Dis. Child, 120, 411-415.
Dobbing, J. and Sands, J. (1970) Timing of neuroblast multiplication in developing human brain. Nature, 226, 637-640.
Dobbing, J. and Sands, J. (1973) Quantitative growth and development of human brain. Arch. Dis. Child., 48, 757-767.
Essick, C. R. (1907) The corpus ponto-bulbare, a hitherto undescribed nuclear mass in the human hind brain. Amer. J. Anat., 7,119-135.
Essick, C. R. (1912) The development of the nuclei pontis and the nucleus arcuatus in man. Amer. J. Anat., 13, 25-54.
Fujita, S. (1973a) Development and differentiation of neurons (in Japanese). Kagaku (Tokyo), 43, 530-5 40 .
Fujita, S. (1973b) Genesis of glioblasts in the human spinal cord as revealed by Feulgen cytophotometry. J. Comp. Neurol, 151, 25-34.
Fujita, S. (1974) DNA constancy in neurons of the human cerebellum and spinal cord as revealed by Feulgen cytophotometry and cytofluorometry. J. Comp. Neurol., 155, 195-202.
Fujita, S. (1975) A genetic basis of neuron differentiation (in Japanese). Symp. Cell Biol , 57, 97-105.
Fujita, S., Hattori, T. and Kitamura, T. (1975) Neurogenesis as studied by SEM-fractography (in Japanese). Saibo (Tokyo), 7, 19-20.
Gadsdon, D. R. and Emery, J. L. (1976) Some quantitative morphological aspects of post-natal human cerebellar growth. J. Neurol. Sci., 29, 137-148.
Gamble, H. J. (1969) Electron microscope observations on the human foetal and embryonic spinal cord. J. Anat., 104, 435-453.
Giesert, E. E. Jnr. and Guillery, R. W. (1979) The horizontal organisation of stellate cell dendrites in layer IV of the visual cortex of Tree Shrews. Neuroscience , 4, 889-896.
Glaser, E. M., Van der Loos, H. and Gissler, M. (1979) Tangential orientation and spatial order in dendrites of cat auditory cortex: a computer microscope study of Golgi-impregnated material. Exp. Brain Res., 36, 411-431.
Goldman-Rakic, P. S. (1980) ‘Morphological consequences of prenatal injury of the primate brain’, in Prog. Brain Research, Vol. 53, Adaptive Capabilities of the Nervous System, (eds. P. S. McConnell, G. J. Boer, H. J. Romyn, N. E. Van de Pol and M. A. Corner), Elsevier/North Holland, 3-19.
Green, J. D. (1964) The hippocampus. Physiol. Rev., 44, 561-608.
Greenough, W. T. and Volkmar, F. R. (1973) Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp. Neurol., 40, 491-504.
Greenough, W. T., Volkmar, F. R. and Juraska, J. M. (1973) Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp. Neurol, 41, 371-378.
Greenough, W. T., Juraska, J. M. and Volkmar, F. R. (1979) Maze training effects on dendritic branching in occipital cortex of adult rats. Behav. Neurol. Biol, 26, 287-297.
Halfon, N. and Lenn, N. J. (1976) Prenatal developmental of the human interpeduncular nucleus. N euro sci. Abs., 2, 214.
Hamburger, V. (1975) Cell death in the development of the lateral motor column of the chick embryo. J. Comp. Neurol, 160, 535-546.
Hanaway, J., Lee, S. I. and Nestley, M. G. (1968) Pachygyria: relation of findings to modern embryo- logical concepts. Neurology, 18, 791-799.
Harris, R. M. and Woolsey, T. A. (1979) Morphology of Golgi-impregnated neurons in mouse cortical barrels following vibrissae damage at different postnatal ages. Brain Res., 161, 143- 149.
Hattori, T. and Fujita, S. (1974) Scanning electron microscopic studies on morphology of matrix cells and on development and migration of neuroblasts in human and chick embryos. J. Elec. Micros., (Tokyo), 23, 269-276.
Hattori, T. and Fujita, S. (1976a) The primitive genesis of the central nervous system (Part 1) (in Japanese). Brain Nerve (Tokyo), 28, 6-11.
Hattori, T. and Fujita, S. (19766) Early development of the central nervous system. II. Stage II of cytogenesis (in Japanese). Brain Nerve (Tokyo), 28, 122-127.
Hollingworth, T. and Berry, M. (1975) Network analysis of dendritic fields of pyramidal cells in the neocortex and Purkinje cells in the cerebellum of the rat. Phil Trans. Roy. Soc. B., 270, 227-264.
Hrabowska, M. (1978) Morphogenesis of cerebral matrix ectopias in human foetus and newborn. J. Hirnforsch, 19, 485-495.
Hubei, D. H., Wiesel, T. N. and Le Vay, S. (1977) Plasticity in ocular dominance columns in monkey striate cortex. Phil. Trans. Roy. Soc. B., 278, 377-410.
Humphrey, T. (1964) ‘Some correlations between the appearance of human foetal reflexes and the development of the nervous system’ in Growth and maturation of the brain, Progr. Brain Research, Vol. 4, (eds. D. P. Purpura and J. P. Schade), Elsevier, Amsterdam, 93-135.
Humphrey, T. (1966) ‘The development of the human hippocampus formation correlated with some aspects of its phylogenetic history’, in Evolution of the Forebrain (eds. R. Hassler and H. Stephan ), Georg Thieme Verlag, Stuttgart, 104-116.
Huttenlocher, P. R. (1974) Dendritic development in neocortex of children with mental defects and infantile spasms. Neurology, 24, 203-210.
Huttenlocher, P. R. (1975) ‘Synaptic and dendritic development and mental defect’, in Brain Mechanisms in Mental Retardation (eds. N. A. Buchwald and M. A. Brazier), UCCA Forum in Medical Sciences (No. 18), Academic Press, New York, 123-140.
Hyyppa, M. (1972) Hypothalamic monoamines in human foetuses. Neuroendocrinol, 9, 257-266.
Inouye, M. and Sen-Ichi, O. (1980) Strain specific variations in the folial pattern of the mouse cerebellum. J. Comp. Neurol, 190, 357-362.
Jacob, H. (1936) Faktoren bei der Entstehung der normalen und der entwicklungsgestorten Hirmunde. Z. Ges. Neurol. Psychiat., 155, 1-39.
Jammes, J. L., Yakovlev, P. L. and Gilles, F. H. (1973) Measures of development of matrix and of isocortex and allocortex in the human foetus. Neurology, 23, 401-402.
Jones, W. H. and Thomas, D. B. (1962) Changes in the dendritic organisation of neurons in the cerebral cortex following deafferentation. J. Anat., 96, 375-381.
Juraska, J. M. and Fifkova, E. (1979) A Golgi study of the early postnatal development of the visual cortex of the hooded rat. J. Comp. Neurol, 183, 247-256.
Kadin, M. E., Rubinstein, L. J. and Nelson, J. S. (1970) Neonatal cerebellar medulloblastoma originating from the foetal external granular layer. J. Neuropath. Exper. 'Neurol, 29, 583-600.
Kaplan, M. S. and Hinds, J. W. (1977) Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science, 197, 1092-1094.
Kostovic, I. and Molliver, M. E. (1974) A new interpretation of the laminar development of the cerebral cortex: synaptogenesis in different layers of neopallium in the human foetus. Anat. Res., 178, 395.
Kostovic, J., Krunpotic-Nemanic, J. and Kelocic, Z. (1975) The early development of glia in human neocortex. Yugoslav. Academy of Sciences, 371, 155-160.
Kostovic, I. and Rakic, P. (1980) Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J. Neurocytol, 9, 219-242.
Kristt, D. A. (1978) Neuronal differentiation in somatosensory cortex of the rat. I. Relationship of synaptogenesis in the first postnatal week. Brain Res., 150, 467-486.
Lapham, L. W. (1968) Tetraploid DNA content of Purkinje neurons of human cerebellar cortex. Science, 159, 310-322.
Lapham, L. W. and Markesbery, W. R. (1971) Human foetal cerebellar cortex: organisation and maturation in vitro. Science , 173, 829-832.
Lapham, L. W. and Williams, R. (1973) Relationship between migrating neurites and astrocytes in early growth in tissue cultures from human cerebellar and cerebral cortex. J. Neuropath., Exper. Neurol, 32,156.
Larsell, O. (1947) The development of the cerebellum in man in relation to its comparative anatomy. J. Comp. Neurol, 87, 85-129.
Lauder, J. M., Altman, J. and Krebs, H. (1974) Some mechanisms of cerebellar foliation. Effects of early hypo- and hyperthyroidism. Brain Res., 76, 33-40.
Lauder, J. M. and Bloom, F. E. (1974) Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J. Comp. Neurol, 155, 469- 482.
Lauder, J. M. and Bloom, F. E. (1975) Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. II. Synaptogenesis. J. Comp. Neurol ., 163, 251-264.
Lauder, J. M. and Krebs, H. (1976) Effects of p-chlorophenylalanine on time of neuronal origin during embryogenesis in the rat. Brain Res., 107, 638-644.
Lauder, J. M. and Krebs, H. (1978) Serotonin as a differentiation signal in early neurogenesis. Dev. Neurosci., 1, 15-30.
Lemire, R. J., Leosser, J. D., Leech, R. S. and Alvord, E. C., Jnr. (eds.) (1975) Normal and Abnormal Development of the Human Nervous System, Harper and Row, Hagerstown, Md., U.S.A.
Lindsey, R. D. and Scheibel, A. B. (1976) Quantitative analysis of dendritic branching patterns of granular cells from human dentate gyrus. Exper. Neurol, 52, 295-310.
Lenn, N. J., Halfon, N. and Rakic, P. (1978) Development of the interpeduncular nucleus in the midbrain of rhesus monkey and human. Anat. Embryol. , 152, 273-289.
Lentz, R. D. and Lapham, L. W. (1970) Post-natal development of tetraploid DNA content in rat Purkinje cells. J. Neuropath., Exper. Neurol, 29, 43-58.
Loeser, J. D., Lenure, R. J. and Alvord, E. C. (1972) The development of the folia in the human cerebellar vermis. Anat. Record., 173, 109-114.
McMahon, D. (1974) Chemical messengers in development: a hypothesis. Science, 185 ,1012-1021.
Maeda, T., Tohyama, M. and Schimizu, N (1974) Modification of postnatal development of neocortex in rat brain with experimental deprivation of locus coeruleus. Brain Res., 70 , 515-520.
Malinsky, J. (1972) Ultrastructural changes of cell elements during the differentiation of neuroblasts and glioblasts in human spinal cord. Acta Univ. Palach. Olom. Fac. Med., 64 , 183-194.
Malinsky, J. and Krajeci, D. (1968) ‘Electron microscopy of developing neurons in the spinal cord of human embryos’, in Macromolecules and the Function of the Neuron (eds. Z. Lodin and S. P. R. Rose), Excerpta Medica Foundation, Amsterdam, 91-100.
Mann, D. M. A. and Yates, P. O. (1973) Polyploidy in the human nervous system. Part I. The DNA content of neurons and glia of the cerebellum. J. Neurol. Sci., 18 , 183-196.
Marin-Padilla, M. (1969) Origin of the pericellular baskets of the pyramidal cells of the human motor cortex: A Golgi study. Brain Res., 14, 633-646.
Marin-Padilla, M. (1970a) Prenatal and early postnatal ontogenesis of the human motor cortex: A Golgi study. The sequential development of the cortical layers. Brain Res., 23 , 167-183.
Marin-Padilla, M. (19705) Prenatal and early postnatal ontogenesis of the human motor cortex: A Golgi study. II. The basket-pyramidal system. Brain Res., 23 ,185-191.
Marin-Padilla, M. (1972) Double origin of the pericellular baskets of the pyramidal cells of the human motor cortex: A Golgi study. Brain Res., 38, 1-12.
Marin-Padilla, M. and Stibitz, G. R. (1974) Three-dimensional reconstruction of the basket cell of the human motor cortex. Brain Res., 70 , 511-514.
Markesbery, W. R. and Lapham, L. W. (1972) In vitro developmental studies of human foetal cerebellar cortex. J. Neuropath., Exper. Neurol, 31 , 192-193.
Markesbery, W. R. and Lapham, L. W. (1974) A correlated light and electron microscopic study of the early phase of growth in vitro of human foetal cerebral cortex. J. Neuropath., Exper. Neurol., 33,113-127.
Mathers, L. H., Jnr. (1979) Postnatal dendritic development in the rabbit visual cortex. Brain Res., 168 , 21-29.
Meier, C. (1976) Some observations on early myelination in the human spinal cord. Light and electron microscopic study. Brain Res., 104 , 21-32.
Meller, K. and Teztlaff, W. (1975) Neuronal migration during the early development of the cerebral cortex. A scanning electron microscopic study. Cell Tis. Res., 163 , 313-325.
Miale, I. L. and Sidman, R. L. (1961) An autoradiographic analysis of histogenesis in the mouse cerebellum. Exper. Neurol, 4, 277-296.
Molliver, M. E. and Van der Loos, H. (1970) The ontogenesis of cortical circuitry: the spatial distribution of synapses in somaesthetic cortex of newborn dog. Ergebn. Anat. Entwickl-Gesch., 42 , 1-54.
Molliver, M. E„ Kostovic, I. and Van der Loos, H. (1973) The development of synapses in cerebral cortex of human foetus. Brain Res., 501 , 403-407.
Morest, D. K. (1969a) The differentiation of cerebral dendrites: A study of the post-migratory neuroblasts in the medial nucleus of the trapezoid body. Z. Anat. Entwickl-Gesch., 128 , 271-289.
Morest, D. K. (19695) Growth of dendrites in the mammalian brain. Z. Anat. Entwickl-Gesch., 128 , 290-317.
Nicholson, J., Lauder, J. and Bloom, F. E. (1973) Cell differentiation and synaptogenesis in the locus coeruleus, raphe nuclei, and substantia nigra of the rat. Anat. Rec., 175 , 398-399.
Nobin, A. and Bjorklund, A. L. (1973) Topography of the monoamine neuron system in the human brain as revealed in foetuses. Acta Physiol. Scand. Suppl, 388 ,1-40.
Norman, R. M. (1966) Neuropathological findings in trisomies 13-15 and 17-18 with special reference to the cerebellum. Dev. Med. Child Neurol, 8 ,170-177.
Okado, N. (1980) Development of the human cervical spinal cord with reference to synapse formation in the motor nucleus. J. Comp. Neurol, 191 , 495-513.
Okado, N., Kakumi, S. and Kojuna, T. (1979) Synaptogenesis in the cervical cord of the human embryo: sequence of synapse formation in a spinal reflex pathway. J. Comp. Neurol, 184 , 491-518.
Olson, L. and Seiger, A. (1972) Early prenatal ontogeny of central monoamine neurons in the rat: Fluorescence histochemical observations. Z. Anat. Entwickl-Gesch., 137 , 301-316.
Olson, L., Boreus, L. O. and Seiger, A. (1973) Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human foetal brain. Z. Anat. Entwickl-Gesch., 139 , 259-282.
Paldino, A. M. and Purpura, D. P. (1979a) Quantitative analysis of the spatial distribution of axonal and dendritic terminals of hippocampal pyramidal neurons in immature human brain. Exper. Neurol, 64 , 604-619.
Paldino, A. M. and Purpura, D. P. (1979b) Branching patterns of hippocampal neurons of human foetus during dendritic differentiation. Exper. Neurol, 64 , 620-631.
Pappas, G. D. and Purpura, D. P. (1964) ‘Electron microscopy of immature human and feline neocortex’, in Progr. Brain Res., Vol. 4, (eds. D. P. Purpura and J. P. Schade), Elsevier, Amsterdam, 176-186.
Parnavelas, J. G., Bradford, R., Mounty, E. J. and Lieberman, A. R. (1978) The development of non- pyramidal neurons in the visual cortex of the rat. Anat. Embryo]., 155 , 1-14.
Peters, A. and Feldman, M. (1973) The cortical plate and molecular layer of the late rat foetus. Z. Anat. Entwickl-Gesch., 141 , 3-37.
Pettigrew, J. D. and Kasamatsu, T. (1978) Local perfusion of noradrenaline maintains visual cortical plasticity. Nature, 271 , 761-763.
Pinto Lord, M. C. and Caviness, V. S. (1979) Determinants of cell shape and orientation: a comparative Golgi analysis of cell-axon interrelationships in the developing neocortex of normal and reeler mice. J. Comp. Neurol, 187 , 49-70.
Poliakov, G. I. (1961) Some results of research into the development of the neuronal structure of the cortical ends of the analysers in man. J. Comp. Neurol, 117 , 197-212.
Poliakov, G. I. (1966) ‘Embryonal and post-embryonal development of neurons of the human cerebral cortex’, in Evolution of the Forebrain, Phylogenesis, and Ontogenesis of the Forebrain (eds. R. Hassler and H. Stephen), Plenum, New York, 249-258.
Purpura, D. P. (1973a) ‘Analysis of morphological developmental process in mammalian brain’, in Biological and Environmental Determinants of Early Development (ed. J. I. Nurnberger). Association for Research in Nervous and Mental Disease Research Publications, Vol. 51, Raven Press, New York, 79-110.
Purpura, D. P. (1973b) Normal and aberrant development of synaptic-pathways in human hippocampus. Trans. Amer. Neurol. Ass., 98 , 224-226.
Purpura, D. P. (1974a) Dendritic spine dysgenesis and mental retardation. Science, 186 , 1126-1128.
Purpura, D. P. (1974b) ‘Neuronal migration and dendritic differentiation; Normal and aberrant development of human cerebral cortex’, in Biological and Clinical Aspects of Brain Development. Mead Johnson Symposium on Perinatal and Developmental Medicine, No. 6, Marco Island, Fla. 12/74,13-27.
Purpura, D. P. (1975a) ‘Dendritic differentiation in human cerebral cortex. Normal and aberrant developmental patterns’, in Advances in Neurology, Vol. 12, (ed. G. W. Kreutzberg), Raven Press, New York, 91-116.
Purpura, D. P. (1975b) ‘Normal and aberrant neuronal development in the cerebral cortex of human foetus and young infant’. Brain Mechanisms in Mental Retardation (ed. M. A. Brazier), Academic Press, New York, 141-169.
Purpura, D. P. (1977) RG627 ‘Developmental pathobiology of cortical neurons in immature human brain’, in Intrauterine Asphyxia and the Developing Brain (ed. L. Gluck), Year Book Medical Publisher Inc., Chicago, 349-373.
Rabinowicz, T. (1964) ‘The cerebral cortex of the premature infant of the 8th month’, in Growth and Maturation of the Brain, Progr. Brain Research (eds. D. P. Purpura and J. P. Schade), Elsevier, Amsterdam, 39-86.
Rakic, P. (1972) Mode of cell migration to the superficial layers of foetal monkey neocortex. J. Comp. Neurol, 145 , 61-84.
Rakic, P. (1974) ‘Embryonic development of the LP-pulvinar complex in man’, in Pulvinar LP Complex (eds. I. S. Cooper, M. Ricklin and P. Rakic), Thomas, Springfield, Ill., 3-25.
Rakic, P. (1975a) ‘Timing of major mitogenic events in the visual cortex of the rhesus monkey’, in Brain Mechanisms in Mental Retardation (eds. N. A. Buchwald and M. Brazier), California University Press, 3-40.
Rakic, P. (1975b) ‘Cell migration and neuronal ectopias in the brain’, in Birth Defects: Original Article Series, Vol. XI, No. 7. The National Foundation, 95-129.
Rakic, P. (1977) Prenatal development of the visual system in rhesus monkey. Phil Trans. Roy. Soc. B., 278 , 245-260.
Rakic, P. (1978) Neuronal migration and contact guidance in the primate telencephalon. Post Grad. Med. J., 54 , Suppl. 1, 25-37.
Rakic, P. and Sidman, R. L. (1968) Supravital DNA synthesis in the developing human and mouse brain. J. Neuropath ., Exper. Neurol ., 27 , 246-276.
Rakic, P. and Sidman, R. L. (1969) Telencephalic origin of pulvinar neurons in the foetal human brain. Z. Anat. Entwickl-Gesch., 129 , 53-82.
Rakic, P. and Sidman, R. L. (1970) Histogenesis of cortical layers in human cerebellum particularly the lamina dissecans. J. Comp. Neurol, 139 , 473-500.
Rakic, P. and Sidman, R. L. (1973a) Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J. Comp. Neurol, 152 , 103-132.
Rakic, P. and Sidman, R. L. (19736) Organisation of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J. Comp. Neurol, 152 , 133-162.
Rakic, P. and Yakovlev, P. I. (1968) Development of the corpus callosum and cavum septi in man. J. Comp. Neurol, 132 , 45-72.
Reinoso-Suarez, F. (1966) ‘Development of the human diencephalon’, in Evolution of the Forebrain (eds. R. Hassler and H. Stephan), Georg Thieme, Verlag, Stuttgart, 296-305.
Rickman, D. P., Stewart, R. M. and Caviness, V. S., Jnr. (1973) Microgyria lissencephaly and neuron migration to the cerebral cortex: an architectonic approach. Neurology, 23 , 413.
Rickman, D. P., Stewart, R. M. and Caviness, V. S., Jnr. (1974) Cerebral microgyria in a 27-week foetus: an architectonic and topographic analysis. J. Neuropath., Exper. Neurol, 33 , 374-384.
Rickman, D. P., Stewart, R. M., Hutchinson, J. W. and Caviness, V. S., Jnr. (1975) Mechanical model of brain convolutional development. Science, 189 , 18-21.
Rickman, M., Chronwall, B. M. and Wolff, J. R. (1977) The development of non-pyramidal neurons and axons outside the cortical plate: the early marginal zone is a pallial anlage. Anat. Embryol, 151 , 285-307.
Richter, V. M. E. (1966) ‘Uber die Entwicklung des globus pallidus und des corpus subthalamicum beim Menschen’, in Evolution of the Forebrain (eds. R. Hassler and H. Stephan), Georg Thieme, Stuttgart, 285-295.
Richter, W. (1980) Neurohistologische und morphometrische Untersuchungen der Ontogenese der Regio cingularis mesoneocorticalis der Ratte. J. Hirnforsch, 21 , 53-87.
Rutledge, L. T., Wright, C. and Duncan, J. (1974) Morphological changes in pyramidal cells of mammalian neocortex associated with increased use. Exper. Neurol, 44 , 209-228.
Schlessinger, A. R., Cowan, W. M. and Swanson, L. W. (1978) The time of origin of neurons in Ammon’s horn and the associated retrohippocampal fields. Anat. Embryol, 154 , 153-173.
Schlumpf, M., Shoemaker, W. J. and Bloom, F. E. (1980) Innervation of embryonic rat cerebral cortex by catecholamine-containing fibres. J. Comp. Neurol, 192 , 361-376.
Schmechel, D. E. and Rakic, P. (1979) Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature, 277, 303-305.
Schmidt, R. H. and Bhatnagar, R. K. (1979) Regional development of norepinephrine, dopamine-/?- hydroxylase and tyrosine hydroxylase in the rat brain subsequent to neonatal treatment with subcutaneous 6-hydroxydopamine. Brain Res., 166 , 293-308.
Seiger, A. and Olson, L. (1973) Late prenatal ontogeny of central monoamine neurons in the rat. Fluorescence histochemical observations. Z. Anat. Entwickl-Gesch., 140 , 281-318.
Seymour, R. M. and Berry, M. (1975) Scanning and transmission electron microcsope studies of interkinetic nuclear migration in the cerebral vesicles of the rat. J. Comp. Neurol, 160 , 105-126.
Seymour, R. M. and Berry, M. (1979) The nervous system’, in Biomedical Applications of Scanning Electron Microscopy (eds. M. Hodges and R. C. Hallowes), Academic Press, London, 127-176.
Sidman, R. L. (1968) ‘Development and biochemical aspects of nervous integration’, in Physiological and Biochemical Aspects of Integration, (ed. F. D. Carlson), Prentice-Hall, New York 163-193.
Sidman, R. L., Green, M. C. and Appel, S. H. (1965) Catalog of Neurological Mutants of the Mouse, Harvard Univ. Press, Cambridge, Mass.
Sidman, R. L. and Rakic, P. (1973) Neuronal migration, with special reference to developing human brain: a review. Brain Res., 62,1-35.
Sidman, R. L. and Rakic, P. (1975) ‘Development of the human central nervous system’, in Cytology and Cellular Neuropathology (eds. R. D. Adams and W. Haymaker), Thomas, Springfield, Ill., 2nd edition.
Sievers, J., Baumgarten, H. G., Berry, M., Klemm, H. P. and Jenner, S. (1978) The locus coeruleus neuroblasts: Plasticity and function in development’, in Catecholamines: Basic and Clinical Frontiers, Vol. 1, Pergamon Press, N.Y., 848-850.
Sievers, J., Klemm, H. P., Jenner, S., Baumgarten, H. G. and Berry, M. (1980) Neuronal and extraneuronal effects of intracisternally administered 6-OHDA on the developing rat brain. J. Neurochem., 34, 765-771.
Sievers, J., Berry, M. and Baumgarten, H. (1981a) The role of noradrenergic fibres in the control of post-natal cerebellar development. Brain Res., 207 , 200-208.
Sievers, J., Mangold, H., Berry, M., Allen, C. and Schlossberger, H. G. (19816) Experimental studies in cerebellar foliation. I. Qualitative analysis of cerebellar foliation defects after treatment with 6-OHDA. J. Comp. Neurol, in press.
Smit, J. S. and Uylings, H. B. M. (1975) The morphometry of the branching pattern in dendrites of the visual cortex pyramidal cells. Brain Res., 87 , 41-53.
Stanfield, B. B. and Cowan, W. M. (1979) The development of the hippocampus and dentate gyrus in normal and reeler mice. J. Comp. Neurol, 185 , 423-460.
Stent, G. S. (1973) A physiological mechanism for Hebb’s postulate of learning. Proc. Nat. Acad. Sci., U.S.A., 70 , 997-1001.
Stewart, R. M., Rickman, D. P. and Caviness, V. S., Jnr. (1975) Lissencephaly and pachygyria. An architectonic and topographical analysis. Acta Neuropath., 31 , 1-12.
Sumner, B. E. H. and Watson, W. E. (1971) Retraction and expansion of the dendritic tree of motor neurons of adult rats produced in vivo. Nature , 233 , 273-275.
Sturrock, R. R. (1975) A light and electron microscopic study of proliferation and maturation of fibrous astrocytes in the optic nerve of the human embryo. J. Anat. (.Lond .), 119 , 223-234.
Taber Pierce, E. (1973) Time of origin of neurons in the brain stem of the mouse. Progr. Brain Res., 40 , 64-65.
Takashima, S., Chan, F., Becker, L. E. and Armstrong, D. L. (1980) Morphology of the developing visual cortex of the human infant. A quantitative and qualitative Golgi study. J. Neuropath., Exp. Neur., 39, 487-501.
Uylings, H. B. M., Kuypers, K., Diamond, M. C. and Veltman, W. A. M. (1978) Effects of differential environments on plasticity of dendrites of cortical pyramidal neurons in adult rats. Exp. Neurol, 62 , 658- 677.
Valverde, F. (1968) Structural changes in the area striata of the mouse after enucleation. Exp. Brain Res., 5 , 274-292.
Van der Loos, H. (1965) The ‘improperly’ oriented pyramidal cell in the cerebral cortex and its possible bearing on problems of neuronal growth and cell orientation. Bull. Johns Hopkins Hosp., 117,228-250.
Vaughan, J. E. and Greeshaber, J. A. (1973) A morphological investigation of an early reflex pathway in developing rat spinal cord. J. Comp. Neurol, 148, 177-210.
Volpe, J. J. and Adams, R. D. (1972) Cerebro-hepato-renal syndrome of Zellwager: an inherited disorder of neuronal migration. Acta Neuropathol, 20 , 175-198.
Walton, K. G., Miller, E. and Baldessarini, R. J. (1979) Prenatal and early postnatal jS-adrenergic receptor-mediated increase of cyclic AMP in slices of rat brain. Brain Res., Ill, 515-522.
Wendlant, S., Crow, T. J. and Stirling, R. V. (1977) The involvement of the noradrenergic system arising from the locus coeruleus in the postnatal development of the cortex in rat brain. Brain Res., 125 , 1-9.
Wendle, W. F. and Fitzgerald, J. E. (1937) Development of spinal reflex mechanisms in human embryos. J. Comp. Neurol, 67, 493-509.
Wise, S. P., Fleshman, J. W. and Jones, E. G. (1979) Maturation of pyramidal cell form in relation to developing afferent and efferent connections of rat somatic sensory cortex. Neuroscience, 4, 1275-1297.
Wise, S. P. and Jones, E. G. (1976) The organisation and postnatal development of the commissural projection of the rat somatic sensory cortex. J. Comp. Neurol, 168 , 313-344.
Wise, S. P. and Jones, E. G. (1978) Developmental studies of thalamocortical and commissural connections in the rat somatic sensory cortex. J. Comp. Neurol, 178 , 187-208.
Wozniak, W., O’Rahilly, R. and Olszewska, B. (1980) The fine structure of the spinal cord in human embryos and early foetuses. J. Hirnforsch, 21 , 101-124.
Zecevic, N. R. and Molliver, M. E. (1978) The origin of the monoaminergic innervation of immature rat neocortex: an ultrastructural analysis following lesions. Brain Res., 150 , 387-397.
Zecevic, N. and Rakic, P. (1976) Differentiation of Purkinje cells and their relationship to other components of the developing cerebellar cortex in man. J. Comp. Neurol, 167 , 27-48.
Dickerson JWT. and McGurk H. Brain And Behavioural Development. (1982) Blackie & Son Ltd., Glasgow.
Brain and Behavioural Development - 1982: 1 Neural Development | 2 Comparative Neural | 3 Malnutrition | 4 Hormones and Growth Factors | 5 Cortical Activity | 6 Functional Asymmetry | 7 Plasticity | 8 Sex Differences
Cite this page: Hill, M.A. (2024, April 24) Embryology Book - Brain and behavioural development 1. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Brain_and_behavioural_development_1
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G