Book - A Laboratory Text-Book of Embryology 8 (1903): Difference between revisions
mNo edit summary |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 123: | Line 123: | ||
===2. Tellyesnicky's Fluid=== | ===2. Tellyesnicky's Fluid=== | ||
Formula: Bichromate of potassium, 3 gm. | Formula: Bichromate of potassium, 3 gm. | ||
Water, ioo c.c. | Water, ioo c.c. | ||
Immediately before using add 5 c.c. glacial acetic acid. | Immediately before using add 5 c.c. glacial acetic acid. | ||
| Line 162: | Line 159: | ||
Formula:* 70 per cent, alcohol, 100 c.c. | Formula:* 70 per cent, alcohol, 100 c.c. | ||
Formaldehyde, I c.c. | Formaldehyde, I c.c. | ||
| Line 172: | Line 168: | ||
===6. Flemming's Fluid=== | ===6. Flemming's Fluid=== | ||
Formula: 1 per cent, solution of chromic acid, 50C.C. | Formula: 1 per cent, solution of chromic acid, 50C.C. | ||
2 percent, solution of osmic acid, 12 c.e. | 2 percent, solution of osmic acid, 12 c.e. | ||
Glacial acetic acid, 3 c.c. | Glacial acetic acid, 3 c.c. | ||
| Line 187: | Line 180: | ||
===7. Hermann's Fluid=== | ===7. Hermann's Fluid=== | ||
Formula : I per cent, platinum chloride in distilled water 60 c.c. | Formula : I per cent, platinum chloride in distilled water 60 c.c. | ||
2 per cent, osmic acid in distilled water, 8 c.c. | 2 per cent, osmic acid in distilled water, 8 c.c. | ||
Glacial acetic acid, . 4 c.c. | Glacial acetic acid, . 4 c.c. | ||
Used in the same manner and with the same precautions as No. 6. | Used in the same manner and with the same precautions as No. 6. | ||
| Line 262: | Line 251: | ||
===1. Alum Cochineal=== | ===1. Alum Cochineal=== | ||
Formula : Powdered cochineal, 6 gm. | Formula : Powdered cochineal, 6 gm. | ||
Potassic alum, 6 gm. | Potassic alum, 6 gm. | ||
Water, So c.c. | Water, So c.c. | ||
| Line 277: | Line 263: | ||
For in toto staining place the specimen in water until it sinks ; then transfer it to the cochineal for twenty-four hours, or for large specimens longer; the depth of the stain will depend upon the strength of the solution; transfer to clean water for fifteen to twenty minutes to extract the alum, which otherwise will crystallize in the tissues when the specimen is placed in alcohol ; the object must not be left too long in water, because it extracts the color also ; put in 50 per cent, alcohol for one hour, then successively in 70 per cent., 80 per cent., and 95 per cent., when the specimen will be ready for imbedding. | For in toto staining place the specimen in water until it sinks ; then transfer it to the cochineal for twenty-four hours, or for large specimens longer; the depth of the stain will depend upon the strength of the solution; transfer to clean water for fifteen to twenty minutes to extract the alum, which otherwise will crystallize in the tissues when the specimen is placed in alcohol ; the object must not be left too long in water, because it extracts the color also ; put in 50 per cent, alcohol for one hour, then successively in 70 per cent., 80 per cent., and 95 per cent., when the specimen will be ready for imbedding. | ||
===2. Borax Carmine=== | ===2. Borax Carmine=== | ||
Formula : Best carmine, •> g m . | Formula : Best carmine, •> g m . | ||
Borax, 2 gm. | Borax, 2 gm. | ||
Water 50 c.c. | Water 50 c.c. | ||
| Line 317: | Line 299: | ||
Eosin Formula : 2 per cent, in 95 per cent, alcohol. | Eosin Formula : 2 per cent, in 95 per cent, alcohol. | ||
Orange G Formula: I per cent, in 95 per cent, alcohol. Lyons blue Formula : I per cent, in 95 per cent, alcohol. | Orange G Formula: I per cent, in 95 per cent, alcohol. Lyons blue Formula : I per cent, in 95 per cent, alcohol. | ||
===4. Heidenhain's Iron Hematoxylin=== | ===4. Heidenhain's Iron Hematoxylin=== | ||
Formula I : Iron alum, 2 gm. | Formula I : Iron alum, 2 gm. | ||
Distilled water 100 c.c. | Distilled water 100 c.c. | ||
II : Hematoxylin crystals, I gm. | II : Hematoxylin crystals, I gm. | ||
95 per cent, alcohol, 10 c.c. | 95 per cent, alcohol, 10 c.c. | ||
Distilled water, 90 c.c. | Distilled water, 90 c.c. | ||
| Line 353: | Line 328: | ||
===5. Beale's Carmine=== | ===5. Beale's Carmine=== | ||
Formula : Best carmine I gm. | Formula : Best carmine I gm. | ||
Ammonia, 3 c.c. | Ammonia, 3 c.c. | ||
Pure Glycerin, 96 c.c. | Pure Glycerin, 96 c.c. | ||
Distilled water, 96 c.c. | Distilled water, 96 c.c. | ||
Alcohol, 95 per cent., .- 24 c.c. | Alcohol, 95 per cent., .- 24 c.c. | ||
| Line 410: | Line 380: | ||
II : Phosphomolybdic acid l-° g m Distilled water, 1°°° c - c III : Aniline blue, soluble in water, °-5 g m Orange G 2.0 gm. | II : Phosphomolybdic acid l-° g m Distilled water, 1°°° c - c III : Aniline blue, soluble in water, °-5 g m Orange G 2.0 gm. | ||
Oxalic acid, 2.0 gm. | Oxalic acid, 2.0 gm. | ||
Distilled water, 1 00.0 c.c. | Distilled water, 1 00.0 c.c. | ||
| Line 429: | Line 397: | ||
This method gives a perfect differential stain of connective-tissue fibrils, and it is to be used whenever the fibrils are to be especially studied. | This method gives a perfect differential stain of connective-tissue fibrils, and it is to be used whenever the fibrils are to be especially studied. | ||
{{Minot1903 footer}} | {{Minot1903 footer}} | ||
[[Category:Histology]] | |||
Latest revision as of 23:18, 19 June 2019
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Minot CS. A Laboratory Text-Book Of Embryology. (1903) Philadelphia:P. Blakiston's Son & Co.
| Online Editor |
|---|
| This historic 1903 embryology textbook by Minot describes human development.
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Chapter VIII. Methods
Measuring Length of Embryos
Owing to the many changes during development in the curvature of the longitudinal axis of the mammalian embryo, it is impracticable to measure that axis or to employ any one system of measurements to obtain comparable results for all ages. For this reason the best practice is to measure in all cases the greatest length of the embryo in its natural attitude along a straight line. The limbs are not to be included in such measurements. This greatest length in young stages will not include the head (compare, for example, Fig. 80), but in most stages the head would be included. Many German authors employ the measurements introduced by His, which he calls the Nackenlange, which corresponds to the distance in a straight line from the neck-bend to the caudal bend. As it is impossible to measure this distance in later stages, it seems best not to use it at all. The length of an embryo, as given by German authors, is often indicated by the abbreviation NL., and is, of course, often different from the measures used in this work.
Methods of Reconstruction
It is often important to obtain definite plastic conceptions of the anatomy of embryos or parts of embryos too small for dissection. To secure these in the best form, it is necessary to reconstruct either drawings or models from sections. The methods employed for these two forms of reconstruction, being different, must be described separately.
Reconstruction of Drawings from Sections
To make these reconstructions satisfactory, it is indispensable to have an accurate outline of the embryo representing it in the point of view to be used for the reconstruction and enlarged to the precise scale upon which the reconstruction is to be made. This drawing must, of course, be made before the embryo is imbedded and sectioned. It is further necessary to know accurately the plane of the sections and their thickness, and, finally, the total number of sections in the series must be counted. A convenient scale for the reconstruction of the anatomy of mammalian embryos is a magnification of from 16 to 20 diameters.
Let us suppose that a pig of 1 2 mm. has been drawn in a side view magnified 20 diameters ; that the embryo has been cut into 900 transverse sections and the approximate plane of the sections is known. It may be more exactly determined by the study of the sections themselves; for instance, it may be determined what section is the last to pass through the surface of the head in the region of the fore-brain and the last to pass through the border of the anterior limb. Then it can be further ascertained through which dorsal segments these two sections pass. By these data the plane of the two sections can be accurately fixed. Over the outline of the embryo is now drawn a series of lines which represent the position of the sections. It is generally sufficient to put in lines which represent only every second, third, or even fourth section. If at any point where the structure is complicated more details are needed, lines for the additional sections can be interpolated. In our supposed case, our lines representing every fourth section, there would be 225 parallel lines, and these should be numbered to correspond to the sections which they represent.
The outlines of the actual sections corresponding to the numbered lines in the diagram must now be made with the camera lucida. In regard to these great care is necessary, especially if, as is likely to be the case, the sections are formed from embryos imbedded in paraffin, because when an embryo is so imbedded it always shrinks, and after imbedding is smaller than before. The shrinkage seems to be uniform throughout and not to disturb the topographical relations even of the finest structures. Unfortunately the shrinkage is not constant, but varies from specimen to specimen, hence a camera drawing made from the sections and magnified 20 diameters will not be of the right size to fit in the diagram, and these drawings must, therefore, be corrected. This may be done either, as is best, by making the original camera lucida drawings of the right magnification for direct use in reconstruction, or they may be made nearly the right magnification and when they are measured off the necessary correction may be introduced by measuring them with proportional dividers.
From the camera lucida drawings of the single sections the measurements are taken to fix the position of the parts in the reconstruction.
For a given section the exact position in the reconstruction is given by the line on the outline drawing of the embryo corresponding to the number of the section. On the drawing of the section the distance of the organ to be recontructed from the point in the section corresponding to the outline of the embryo is measured off, and then marked upon the proper line of the reconstruction diagram. A similar measurement is then taken from the next section and transferred to the diagram in the same manner, and so on with successive sections until a series of dots is obtained which mark the outline of the organ. These dots are then connected by a continuous line, which will indicate the form and correct position of the organ. Simple reconstructions may be easily made by these means. When, however, more complicated reconstructions are attempted, much judgment and skill are necessary in the selecting of parts which may be successfully represented in a single drawing, bearing in mind always the point of view which is assumed for the reconstruction, so that organs may be correctly represented in their relative positions, nearer or further from the observer as he looks at the drawing. After the outlines are completed the shading of the parts must be added, and this often requires a special degree of skill and a considerable faculty of plastic imagination. As examples of complicated reconstructions the student is referred to figures ioo and 104, pages 163 and 165.
Oftentimes simpler reconstructions are very helpful in which only a few sections are combined, as, for example, to show the course and branches of the spinal nerves in young embryos. In such a case the outline of the middle section of the series proposed to be combined may be selected to give the outline of the reconstructed drawing. Camera lucida drawings of this and the neighboring sections to be included should be made of the desired magnification. The reconstruction itself may be made upon tracing paper, which is laid successively over the drawings of the sections and the parts required from each can be added upon the tracing paper, which will thus combine in a single drawing the parts intended to be represented. Reconstructions of this kind are easily made by students and are often very instructive.
Reconstruction with Wax Plates by Bom's Method
The basis of this method is to make in wax a magnified reproduction of the single sections, representing in the wax such portions of the section as it is desired to reproduce in plastic reconstruction. To this end wax plates must be made which represent a definite magnification of the thickness of a section. For working by this method it is usually advantageous to employ rather thick sections, say, of 20 it. If the magnification chosen is fifty times, which is practically often convenient, then the wax plates should be made fifty times 20 /i in thickness, or 1 mm. The most convenient plates to work with are those from 1 to 2 mm. thick. Upon a wax plate of the requisite thickness a camera lucida drawing is made. This may be done with a lithographic crayon or with a fine steel point. The drawings in 11st be of exactly the right magnification; in the illustration chosen, 50 diameters. Next, the wax plate is put upon a glass or a metal surface where it lies perfectly flat, and with a sharp thin-bladed knife or scalpel the outline of the organs which it is intended to reconstruct is cut out as may be desired. Our bit of wax then represents a model of the parts selected from the section, and equally magnified in the three dimensions of space. Wax plates made from successive sections are then piled up, one on top of the other, in the proper order,. If they are rightly superimposed, an operation which often requires skill and judgment, and always requires the utmost care, then the pile of plates will correctly represent the form of the parts included in the reconstruction. To fasten the plates together it is only necessary to pass a warm metal instrument over the edges of the plates, enough to melt the wax a little. With proper care this may readily be accomplished without destroying the surface modelling of the reconstruction.
The simplest method of making wax plates is to have a large tin pan with vertical sides. This is filled with hot water and melted beeswax is poured on the surface of the water and allowed to cool. Plates of sufficiently exact and even thickness may be cast in this way, provided the operation is carried out in a quiet place so that the surface of the water is not disturbed while the wax is hardening. It will be found convenient to have a large plate of iron, not less than \ of an inch in thickness, which may be placed upon supporters. The tin pan should be set upon this plate and the plate heated by lamps below in order to keep the water hot enough to allow the wax to spread evenly over the surface of the water. The water must be freed from air before the wax is poured in, but must not be allowed to boil after the wax has been added. If bubbles appear in the wax plate, they may be removed while the wax is still hot by directing the blue flame from a Bunsen burner down upon them. If the pan is" heated directly without the iron plate, it is sure to warp and become unfit for use. Thin iron plates are also liable to be warped.
To determine the thickness of the plates cast as described we proceed empirically. A weighed quantity of wax is melted and poured into the pan. After the plate has solidified it is removed by cutting it free from the edges of the pan, and the thickness of the plate is then measured at various points by micrometer callipers. From these data it is easy to calculate exactly what thickness of plate one gram of beeswax represents. To get accurate results it is advisable to cast several plates of varying thickness and determine the average for one gram in that way. Having determined what one gram represents in thickness, it becomes thereafter only necessary to weigh out the proper number of grams in order to obtain any desired thickness of wax plate. It will be found advantageous to filter the wax before using it. This may easily be done by a double hotwater filter. Such a filter may be made of copper. It is desirable to connect it with a Mariotti's flask to maintain a constant water level.
Directions for Orienting Serial Sections of Embryos. (Note: The lower edge of the ribbon is the one to the left, when the observer has the object between himself and the knife.)
i. Transverse Series
Normal thickness: 10 p.
Dorsal surface to be toward the lower edge of the ribbon.
Series to begin with the head.
In cutting, the left side of the embryo must strike the knife first.
2. Sagittal Series
Normal thickness: Small embryos, 10 ft.
Medium " 15 /i.
Large 20 ft.
The head of the embryo to be toward the lower edge of the ribbon. Series to begin with the right side. In cutting, the ventral side of the embryo must strike the knife first.
3. Frontal Sections
Normal thickness: Small embryos, 10 //. Medium " 15 ft. Large " 20 p..
The head of the embryo is to be toward the lower edge of the ribbon. The series is to begin with the ventral side. In cutting, the left side of the embryo must strike the knife first. In mounting leave space for the label at the left-hand end of the slide. Keep the sections in the order cut. Arrange them on the slides in the sequence of ordinary written lines.
Microtomes
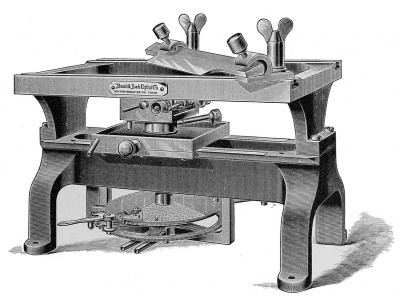
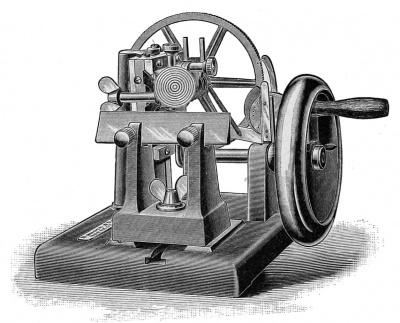
There are many forms of microtome which may be used with good results and which will work very satisfactorily for making sections of small objects. The cutting of larger objects, such as pig embryos of from 1 5 to 20 mm. , and of pieces of the uterus or other organs, is more difficult, and microtomes which work satisfactorily with small objects often fail to give good even sections of more difficult objects. For embryological work a microtome ought, therefore, to be selected which will give perfectly regular sections in long series of any desired thickness from 1 up to 25 mikrons. It is also desirable for economy of time to have a microtome which works automatically. These considerations lead the author to recommend for embryological use especially two forms of microtome made by Messrs. Bausch & Lomb, of Rochester, N. Y., and designated by them as the "precision" and "rotary" microtomes.
The precision microtome (Fig. 217) consists, first, of an upper square form upon which the knife may be clamped in any desired position; second, of two horizontal ways upon which moves the carriage which bears the object-holder; and, third, of a micrometer screw with an automatic feeding contrivance on the under side of the movable carriage. The construction is very solid and great rigidity of the parts is secured. The microtome may be used for either paraffin or celloidin cutting. According to the author's experience, this microtome considerably surpasses all other types in the accuracy of the work which may be done with it. The rotary microtome was originally made in Germany, and various patterns have been put upon the market by German, French, English, and American manufacturers. The new pattern recently introduced by Messrs. Bausch & Lomb embodies a considerable number of improvements, which render the instrument (Fig. 218) very desirable for general laboratory use. It works with accuracy, is very easy to manipulate, and cuts sections with extreme rapidity. It is adapted only for paraffin work. For the general use of students, in elementary courses especially, this microtome is to be preferred to the " precision," as it requires less care and works more rapidly. A single rotary microtome will be found sufficient for a class of from twenty to thirty students in embryology.
Fig. 217. The Precision Microtome.
The microtome is an instrument of precision, which implies that it must be treated with extreme delicacy and kept most scrupulously clean. It will be found usually, when complaint is made against the microtome, that the complaint is misdirected, and ought to be not against the machine, but against the owner. The modern microtome necessarily lias several adjustments, every one of which must be exact and secure. If any one of them is imperfect and insecure, if any of the movable parts are allowed to become corroded, or gummed up with oil, or loose, or clogged with dust or dirt of any kind, the microtome will not and cannot work as an instrument of precision. The knife used for cutting ought to be regarded as an integral part of the microtome and as its most delicate and easily injured part. A perfect knife-edge is the greatest treasure of the microtomist. To sharpen the knife satisfactorily for fine section cutting is a really serious difficulty. A skilful person, however, may get a good edge by using the very finest grade of oil-stone. No oil should be used, but instead a mixture of equal parts of glycerin and water. Before the knife is honed it must be made as clean as possible. The oil-stone itself also must be cleaned with equal care, and the mixture of glycerin and water should, if necessary, be filtered before using to keep it free from dirt. A single particle of dirt may be the cause of making many microscopic notches in the edge of a knife. A knife is well sharpened when its edge appears smooth and straight under a magnifying power of twentyfive diameters.
Fig. 218. The Automatic Rotary Microtome.
The microtome knife should be as unlike a razor as possible. It must have a very thick back and be as heavy and rigid as practicable, so that the actual cutting-edge may be as steady and inflexible as it can be made. Knives of suitably heavy construction are now furnished with all the best microtomes.
Methods of Hardening and Preserving
The two most generally useful methods for preserving embryos are with Zenker's and Tellyesnicky's fluids. Good results may be had with the other reagents. Specimens preserved with picro-sulphuric acid have the advantage of staining readily. To study the medullary sheaths of nerve-fibers, as is necessary to follow the development of the fiber tracts in later stages, the specimens must be preserved in Midler's fluid. Flemming's and Hermann's fluid are valuable, especially for cytological study, but are applicable only to small pieces.
1. Zenker's Fluid
Formula : Corrosive sublimate, 5 gm.
Potassium bichromate, I g m .
Sodium sulphate I g m .
Water, 100 c.c.
Add 5 c.c. of glacial acetic acid to the fluid immediately before using.
The fluid does not have great penetrating power, but may be used for embryos of 25 mm. The amount of fluid should be from ten to twenty times the volume of the specimen, and better results are obtained if the fluid is changed after a few hours. Chicks of the first and second days are hardened in two to four hours ; embryos of 6 to 8 mm. in eight to ten hours; embryos of 12 mm. in twenty-four hours ; larger embryos in thirty to forty hours. After the proper interval in Zenker's fluid the specimens must be removed and washed in running water for twelve to twenty-four hours. Transfer to 50 per cent, alcohol for one to three hours, then to 60 per cent., 70 per cent., and 80 per cent. It is indispensable to remove now the excess of corrosive sublimate by adding sufficient tincture of iodine to give the alcohol the color of port wine; if the iodine disappears, it must be renewed. After from one to three days, according to the size of the specimen, transfer it to fresh 80 per cent, alcohol, which must be changed until it no longer extracts any iodine from the specimen.
2. Tellyesnicky's Fluid
Formula: Bichromate of potassium, 3 gm.
Water, ioo c.c.
Immediately before using add 5 c.c. glacial acetic acid.
This reagent is employed in the same manner as Zenker's fluid, except that the treatment with iodine is omitted.
3. Plcro-Sulphuric Acid
Formula : Picric acid, I gm.
Sulphuric acid 6 c.c.
Water, '000 c.c.
Specimens are kept in the fluid from four to twenty-four hours, not longer, according to their size; transfer to 30 per cent, alcohol for one hour, to 50 per cent, alcohol for one to two hours, to 60 per cent, alcohol for twelve hours, and finally to 70 per cent, alcohol, which must be changed daily until it no longer shows even a trace of yellow discoloration by picric acid.
4. Muller's Fluid
Formula: Bichromate of potassium, 20 gm.
Sulphate of sodium 10 gm.
Water, 1000 c.c.
Muller's fluid is a valuable reagent, and for the study of the later stages of the nervous system indispensable. The objections to its use are that it requires a long time to act, that it renders the specimens brittle, and makes them somewhat difficult to stain. It must be used in large quantities and be frequently changed, and allowed to act on the specimens from three to eight weeks according to their size. The appearance of a film or scum indicates that the fluid needs to be changed.
5. Parker's Fluid
Formula:* 70 per cent, alcohol, 100 c.c.
Formaldehyde, I c.c.
Very convenient when a simple and expeditious preservative is necessary. The specimens are placed in the fluid, which ought to be renewed in a few hours. They may be kept permanently in the fluid, but it is desirable, before using them for study, to remove the formaldehyde by treating them with fresh 70 per cent, alcohol.
- Differs slightly from the original formula.
6. Flemming's Fluid
Formula: 1 per cent, solution of chromic acid, 50C.C.
2 percent, solution of osmic acid, 12 c.e.
Glacial acetic acid, 3 c.c.
The solution must be used freshly made, and must not be kept in the dark. The specimens must be of small size and as fresh as possible. They are kept in the fluid from twenty-four to forty-eight hours, then washed in running water from four to twenty-four hours, then transferred to alcohols of gradually increasing strength. The fluid is useful chiefly for cytological work.
7. Hermann's Fluid
Formula : I per cent, platinum chloride in distilled water 60 c.c.
2 per cent, osmic acid in distilled water, 8 c.c.
Glacial acetic acid, . 4 c.c.
Used in the same manner and with the same precautions as No. 6.
Preservation in Alcohol
When a specimen is first put into alcohol, it should be transferred gradually, being put first in 30 or 50 per cent, alcohol for an hour or more, then into 60 per cent, for several hours, 70 per cent, for twelve to twenty-four hours, and finally into 80 per cent., in which it should be kept until required for use. If the specimen is to be sectioned, it must be placed in 95 per cent, alcohol, which must be renewed at least once, and be allowed to act for twenty-four hours or more, unless the specimen is very small, when a somewhat shorter time may suffice.
Directions for Imbedding Specimens to be Microtomed
A. To Imbed in Paraffin:
- Stain intoto. (See pages 367 and 368.)
- Dehydrate in alcohol from three to twenty-four hours.
- Place in oil of cloves and turpentine (equal parts), one to twenty-four hours.
- Place in fresh cloves and turpentine for one to twenty-four hours.
- Place in soft paraffin at 54 C. for thirty to ninety minutes.
- Place in hard paraffin at 54 C. for thirty to ninety minutes.
- Warm a glass plate to about 70 C; place on it a paper tray or metal imbedding frame ; fill the box with hard paraffin at 54 C. Warm a spatula and with it remove the specimen to the tray or frame, and arrange it in a proper position. As soon as the paraffin has set, chill it rapidly with cold water, otherwise the paraffin is likely to crystallize and therefore to cut badly.
B. To Imbed in Celloidin:
- Dehydrate the mass thoroughly in 95 per cent, alcohol, four to twentyfour hours.
- Place mass for twenty-four hours in alcohol and ether, equal parts.
- Place mass in thin syrupy solution of celloidin in equal parts of ether and alcohol for twenty-four hours.
- Place mass in thick viscid solution of celloidin in equal parts of ether and alcohol for twenty-four hours.
- Set mass on block of vulcanite, compressed fiber, or maple-wood, and as soon as a film has formed over the surface of the celloidin (two to five minutes) —
- Immerse in 80 per cent, alcohol for twenty-four hours.
- Cut.
Method of Mounting Paraffin Sections
The student is advised to use slides 40 x 76 mm. and from 1.75 to 2 mm. thick. The thick slides are much better than the thin ones recommended by dealers. The cover-glasses ought to be 35 x 50 mm. and 0.17 to 0.18 mm.; they may be readily obtained from German makers, but are difficult to secure from American manufacturers except at an exorbitant price.
The serial order of the sections should be preserved with the utmost care, and time spent in arranging the sections in straight rows will be found to be time saved.
The albumen-glycerin methods of fastening the sections to the slide will be found satisfactory.
Formula : Take the white of one fresh egg, beat slightly until equally fluid, filter it (it will take about twenty-four hours) and add an equal amount of glycerin. To this fluid add a small piece of camphor.
- Clean the slide thoroughly.
- Put on a small drop of albumen solution.
- Spread it out very thin with the finger.
- Add five or six drops of distilled water, which must flow evenly over the coating of albumen.
- Place the sections on slide in regular rows.
- Warm the slides gently over an alcohol flame, to allow the sections to flatten. The paraffin must not melt.
- Place the slides in oven for twelve to twenty-four hours to evaporate the water completely.
- Dissolve off the paraffin in turpentine.
- Put in absolute alcohol for three to five minutes.
- Clear in turpentine or chloroform.
- Mount in dammar varnish. (Canada balsam is undesirable, because it becomes much discolored with age.)
Methods of Staining
In embryologieal work the specimens are usually stained in toto before imbedding, either with alum cochineal or with borax carmine, the former being the more generally useful stain. Staining on the slide is also much used either to secure a counterstain after the in toto coloration or to secure some special result. For counterstains eosin, Lyons blue, and orange G are particularlv recommended. A few of the most important special stains are given below.
1. Alum Cochineal
Formula : Powdered cochineal, 6 gm.
Potassic alum, 6 gm.
Water, So c.c.
Boil vigorously for twenty minutes; allow the fluid to settle; decant the clear fluid ; add more water and boil again ; filter the entire solution and evaporate the filtrate to So c.c. ; add a small piece of thymol or camphor to prevent the growth of fungi. Alum cochineal is, on the whole, the best reagent for in toto staining, as it will penetrate quite large objects and color them uniformly throughout, and gives a good differentiation of the tissues.
For in toto staining place the specimen in water until it sinks ; then transfer it to the cochineal for twenty-four hours, or for large specimens longer; the depth of the stain will depend upon the strength of the solution; transfer to clean water for fifteen to twenty minutes to extract the alum, which otherwise will crystallize in the tissues when the specimen is placed in alcohol ; the object must not be left too long in water, because it extracts the color also ; put in 50 per cent, alcohol for one hour, then successively in 70 per cent., 80 per cent., and 95 per cent., when the specimen will be ready for imbedding.
2. Borax Carmine
Formula : Best carmine, •> g m .
Borax, 2 gm.
Water 50 c.c.
Boil for twenty minutes ; allow the solution to cool ; add water enough to restore that lost by evaporation, then add 50 c.c. of 70 per cent, alcohol, let the solution stand twenty-four hours ; filter. Borax carmine gives a good nuclear stain and may be advantageously supplemented by counterstains.
For in toio staining place the specimen in water until it sinks ; transfer to the carmine for twenty-four hours, or longer for large specimens ; wash in water for five minutes; then place it in 70 per cent, alcohol, to every 100 c.c. of which 2 c.c. of hydrochloric acid have been added; after one hour transfer to fresh 70 per cent, alcohol, which must be renewed in an hour or two, and finally transfer to 80 per cent, and 95 per cent, alcohol, and the specimen will be ready to imbed.
3. Counterstains
Counterstains are used either with celloidin sections treated singly, or with paraffin sections after they have been fastened on the slide. The three here recommended are alcoholic solutions, and the method of using is the same for all.
For staining paraffin sections on the slide it is convenient to have eight jars or dishes large enough to hold a slide. The slide is transferred from jar to jar in the order below, being allowed to remain in each jar a few minutes. The very most scrupulous care is necessary to keep all the fluids clean, and it is indispensable to filter them frequently; the sections on the slide catch and hold the particles floating in the reagents when they are not clean.
Order of jars:
- Xylol.
- Xylol.
- Xylol and absolute alcohol, equal parts.
- Absolute alcohol.
- Counterstain.
- Alcohol of 95 per cent.
- Absolute alcohol.
- Xylol.
Eosin Formula : 2 per cent, in 95 per cent, alcohol.
Orange G Formula: I per cent, in 95 per cent, alcohol. Lyons blue Formula : I per cent, in 95 per cent, alcohol.
4. Heidenhain's Iron Hematoxylin
Formula I : Iron alum, 2 gm.
Distilled water 100 c.c.
II : Hematoxylin crystals, I gm.
95 per cent, alcohol, 10 c.c.
Distilled water, 90 c.c.
- Place sections in the iron solution for thirty to sixty minutes. (Specimens hardened with Flemming's or Hermann's fluid require longer than specimens from Zenker's or Tellycsnicky's fluid.)
- Wash quickly in water.
- Transfer to the hematoxylin solution for five to ten minutes.
- Wash in tap-water.
- Decolorize in the iron solution.
- Wash thoroughly in tap-water.
- Dehydrate and mount.
This stain is useful for cytologieal work, the study of cell division, etc. The preparations are often improved by counterstaining with orange G.
5. Beale's Carmine
Formula : Best carmine I gm.
Ammonia, 3 c.c.
Pure Glycerin, 96 c.c.
Distilled water, 96 c.c.
Alcohol, 95 per cent., .- 24 c.c.
Dissolve in ammonia plus part of the water, add the rest of the water, and allow the solution to stand in an open dish until the ammonia is nearly all driven off. Then add the alcohol and glycerin. For use dilute with an equal part of glycerin. Stain for twenty-four hours in an open dish, which, together with a second open dish containing acetic acid, is placed under a bell-jar; wash the sections thoroughly in water and then in very weak hydrochloric acid (1 c.c. to 500 c.c. water), and again in water.
Beale's carmine is especially valuable for the study of the central nervous system and of the placenta.
6. Weigert's Copper Hematoxylin
Formula I : Copper solution : Acetate of copper in saturated aqueous solution. II : Hematoxylin solution : Hematoxylin crystals, 2 gm.
95 per cent, alcohol, 20 c.c.
Distilled water, 80 c.c.
This stain is indispensable for the study of the nervous system after the medullary sheaths have begun to develop ; the specimens must be preserved in M tiller's fluid. The method is also valuable for the study of the placenta and uterus.
- Place the sections in water.
- Place the sections in the copper solution for twenty- four hours.
- Wash quickly in water.
- Put them in the hematoxylin solution for five to ten minutes. The sections should turn a deep blue-black.
- Wash thoroughly in water.
- Decolorize in the iron solution; the section must be gently moved about to r secure an even decolorization. When part of the section shows a brown color, it'should be removed and examined.
- Wash thoroughly in water to remove the iron solution, no trace of which can be left without ruining the specimen.
- Dehydrate with alcohol and mount at once in dammar.
- Mallory's Triple Connective-tissue Stain.
Formula I : Acid fuchsine, I.o gra.
Distilled water 1000.0 c.c.
II : Phosphomolybdic acid l-° g m Distilled water, 1°°° c - c III : Aniline blue, soluble in water, °-5 g m Orange G 2.0 gm.
Oxalic acid, 2.0 gm.
Distilled water, 1 00.0 c.c.
- Preserve in corrosive sublimate or Zenker's fluid.
- Stain the sections in the fuchsine solution one to three minutes.
- Wash in water.
- Place in the phosphomolybdic solution one minute.
- Wash in two changes of water.
- Stain in blue and orange solution two to twenty minutes.
- Wash in water.
- Dehydrate in 95 per cent, alcohol and mount in dammar.
This method gives a perfect differential stain of connective-tissue fibrils, and it is to be used whenever the fibrils are to be especially studied.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 19) Embryology Book - A Laboratory Text-Book of Embryology 8 (1903). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_A_Laboratory_Text-Book_of_Embryology_8_(1903)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G