Abnormal Development - Teratogens: Difference between revisions
| Line 28: | Line 28: | ||
Birth Defects Res B Dev Reprod Toxicol. 2011 Feb;92(1):69-76. doi: 10.1002/bdrb.20283. Epub 2011 Jan 19. | Birth Defects Res B Dev Reprod Toxicol. 2011 Feb;92(1):69-76. doi: 10.1002/bdrb.20283. Epub 2011 Jan 19. | ||
* '''Maternal exposure to multi-wall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats.''' | * '''Maternal exposure to multi-wall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats.''' <ref>Lim JH, Kim SH, Shin IS, Park NH, Moon C, Kang SS, Kim SH, Park SC, Kim JC. '''Maternal exposure to multi-wall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats.''' Birth Defects Res (Part B) 92:69-76, 2011. [http://www.ncbi.nlm.nih.gov/pubmed/21254368 PMID:21254368]</ref> "The results show that repeated oral doses of multi-wall CNTs (MWCNTs) during pregnancy induces minimal maternal toxicity and no embryo-fetal toxicity at 1,000 mg/kg/day in rats. The no-observed-adverse-effect level of MWCNTs is considered to be 200 mg/kg/day for dams and 1,000 mg/kg/day for embryo-fetal development. In this study, the dosing formulation was not analyzed to determine the degree of reaggregation (or not), nor were blood levels of CNT's measured in the dosed animals to verify or characterize absorption." | ||
Lim JH, Kim SH, Shin IS, Park NH, Moon C, Kang SS, Kim SH, Park SC, Kim JC. | |||
==Critical Periods of Development== | ==Critical Periods of Development== | ||
Revision as of 09:56, 27 February 2011
Introduction
How and why do things go wrong in development? Embryological development is a robust biological system able to cope with many stresses without long-term consequences. When development does go wrong there are generally 3 major types groups: Genetic (inherited), Environmental (maternal) derived and Unknown (not determined or known) abnormalities. Also often not considered, is that pregnancy itself can also expose abnormalities in the mother (congenital heart disease, diabetes, reproductive disorders) that until the pregnancy had gone undetected.
- Infections, collectively grouped under the acronym TORCH for Toxoplasmosis, Other organisms (parvovirus, HIV, Epstein-Barr, herpes 6 and 8, varicella, syphilis, enterovirus) , Rubella, Cytomegalovirus and Hepatitis. See also the related topics on maternal hyperthermia and bacterial infections.
- Maternal diet the best characterised is the role of low folic acid and Neural Tube Defects (NTDs) see also abnormal neural development and Neural Tube Defects (NTDs). More recently the focus has been on dietary iodine levels and the role they also play on neural development.
- Maternal drugs effects either prescription drugs (therapeutic chemicals/agents, thalidomide limb development), non-prescription drugs (smoking), and illegal drugs (Cannabis/Marijuana, Methamphetamine/Amphetamine, Cocaine, Heroin, Lysergic Acid Diethylamide)
- Environment (smoking, chemicals, heavy metals, radiation) and maternal endocrine function (maternal diabetes, thyroid development) and maternal stress.
- Teratogen synergism, different environmental effects can act individually or in combination on the same developing system. For example, neural development can be impacted upon by alcohol (fetal alcohol syndrome), viral infection (rubella) and/or inadequate dietry folate intake (neural tube defects). These effects may also not be seen as a direct effect on a system or systems but result in a reduced birth weight and the potential postnatal developmental effects. Consider also this in relation to the increasing support to the fetal origins hypothesis.
Use the page links below to explore specific teratogens.
Some Recent Findings
|
Birth Defects Res B Dev Reprod Toxicol. 2011 Feb;92(1):69-76. doi: 10.1002/bdrb.20283. Epub 2011 Jan 19.
- Maternal exposure to multi-wall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats. [2] "The results show that repeated oral doses of multi-wall CNTs (MWCNTs) during pregnancy induces minimal maternal toxicity and no embryo-fetal toxicity at 1,000 mg/kg/day in rats. The no-observed-adverse-effect level of MWCNTs is considered to be 200 mg/kg/day for dams and 1,000 mg/kg/day for embryo-fetal development. In this study, the dosing formulation was not analyzed to determine the degree of reaggregation (or not), nor were blood levels of CNT's measured in the dosed animals to verify or characterize absorption."
Critical Periods of Development
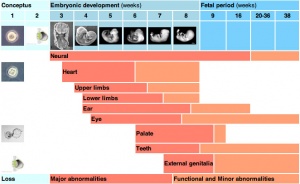
When studying this topic remember the concept of "critical periods of development" that will affect the overall impact of the above listed factors, as outlined in the table below. This can be extended to the potential differences between prenatal and postnatal effects, for example with infections and outcomes.
| Conceptus | Embryonic development (weeks) | Fetal period (weeks) | |||||||||||||||||
|
|||||||||||||||||||
| Neural | |||||||||||||||||||
| Heart | |||||||||||||||||||
| Upper limbs | |||||||||||||||||||
| Lower limbs | |||||||||||||||||||
| Ear | |||||||||||||||||||
| Eye | |||||||||||||||||||
| Palate | |||||||||||||||||||
| Teeth | |||||||||||||||||||
| External genitalia | |||||||||||||||||||
| Loss | Major abnormalities | Functional and Minor abnormalities | |||||||||||||||||
'
References
- ↑ <pubmed>20561110</pubmed>
- ↑ Lim JH, Kim SH, Shin IS, Park NH, Moon C, Kang SS, Kim SH, Park SC, Kim JC. Maternal exposure to multi-wall carbon nanotubes does not induce embryo-fetal developmental toxicity in rats. Birth Defects Res (Part B) 92:69-76, 2011. PMID:21254368
Journals
- Birth Defects Research Part A: Clinical and Molecular Teratology
- Birth Defects Research Part B: Developmental and Reproductive Toxicology
- Part C: Embryo Today: Reviews
Reviews
Articles
Search Pubmed
Search Pubmed: teratogen | teratogenesis
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 18) Embryology Abnormal Development - Teratogens. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Abnormal_Development_-_Teratogens
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G