2018 Group Project 3
| Projects 2018: 1 Adrenal Medulla | 3 Melanocytes | 4 Cardiac | 5 Dorsal Root Ganglion |
Project Pages are currently being updated (notice removed when completed)
Melanocytes
Introduction
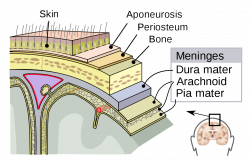
Melanocytes are a type of neural crest-derived cells in the body [1], most commonly found in the stratum basale, the bottom layer of the epidermis of the skin, eyes [2], and to a lesser known degree, the meninges (membrane around the brain), the heart [3] and the inner ear [4].
Each melanocyte possesses a main cell body, where most of the cellular functions, including the production of melanin take place. They also possess finger-like projections that extend into the surrounding tissue, similar to neurons, called dendrites.
Their most commonly known function is the production of melanin, melanogenesis, of which there are two types; eumelanin (black) and pheomelanin (reddish yellow) [5], and are responsible for the pigmentation of various parts of the human body including the skin, hair and irises, etc. Melanogenesis has different levels, basal and activated. The basal level of melanogenesis determines an individual's skin colour and is determined by genetics, i.e. a light-skinned individual has low levels of basal melanogenesis. Activated levels of melanogenesis are usually due to external factors, such as exposure to UV-B radiation, resulting in increased levels of melanogenesis [6], which can be commonly seen as sunburn or tan after long exposure to sunlight.
Melanin produced with the cell is packaged into granules, before being transported to the surrounding cells via dendrites which reach in between the surrounding tissue.
History
The history of the discovery of the melanocyte spans about 4000 years, from when a pigmentation disorder of skin was first documented in 2200 bc, to when the melanocyte was confirmed as a pigment synthesising cell in 1917[2]. Melanocytes were first described and named ‘chromatophores’ by Giosué Sangiovanni in 1819, who identified them in squid [7] . Later, in 1837, Friedrich Henle identified melanocytes to be in the human skin epidermis and eye[2]. In 1910, Ross Granville Harrison, an American anatomist, proposed that melanocytes originated from the neural crest [8]. But it wasn’t until 1917, that Bruno Bloch identified and confirmed the enzyme tyrosinase within melanocytes to be responsible for producing melanin pigment [9].
Tissue Organ Structure and Function
Skin
Ears
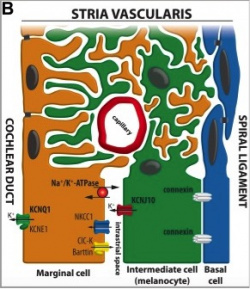
Melanocytes are mainly present in the cochlea, vestibular organ and endolymphatic sac:
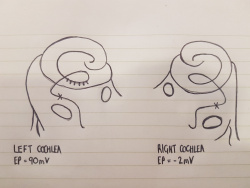
The cochlea of the inner ear functions as a transducer, by converting sound vibrations to electrical potentials in the auditory nerve via hair cells [11]. In the cochlea of humans, melanocytes are found in the vascularised epithelial tissue in the intermediate layer of the stria vascularis and the modiolus, between the marginal and basal cell layers [12](Figure 1). During development, marginal layer cuboidal epithelium develops processes which interdigitate with the intermediate melanocytes and basal cells [13].Based on studies performed on mice models, it is thought that melanocytes are important for the development of the endocochlear potential (EP), produced by strial cells. Intermediate melanocytes conduct K+, which plays an important role in sound conductance, as it flows from the endolymph into the ciliated epithelial cells of the ear via mechano-electrical channels. This influx of K+ is driven by a combination of the membrane potential of these ciliated epithelial cells, and the EP. A study on guinea pig models showed that blocking the K+ channels of melanocytes produces a lower EP [14]. This study agrees with evidence showing that mice deficient in cochlea melanocytes have a lower EP, requiring a greater sound stimulus to produce an action potential [15].
Eyes
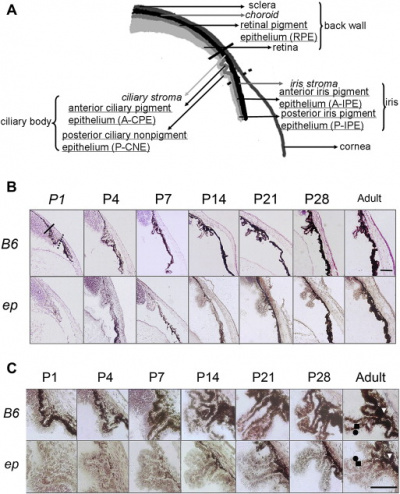
Uveal melanocytes contain both eumelanin and pheomelanin. It has been speculated that eumelanin is photoreceptive, whereas pheomelanin is phototoxic. Uveal melanocytes produce growth factors, such as vascular endothelial growth factor that controls blood circulation around the eye, as well as extracellular matrix degrading enzymes. It was found that both of these roles may play a key part in the toxicity of uveal melanocytes. [17]The uveal tract (iris, ciliary body and choroid) and conjunctiva normally contains melanocytes derived from the neuro-ectodermal neural crest. Peri-orbital soft tissue is also derived from the embryonic neural crest.[18] On the other hand, pigmented melanocytes (such as those found on the retina) were derived from the neuroepithelium or from different layers of the optic cup. [19] Uveal melanocytes (like all other melanocytes) differentiate into melanin-producing cells, which in this case determines a person's eye colour, protects the eye from UV radiation [20] and can mutate to form many ocular diseases that can cause blindness, including age-related macular degeneration. [21] Melanocytes found in the eye are not able to participate in regeneration unlike epidermal melanocytes. [22]
Heart
Central Nervous System
Melanocytes can also be found within the Central Nervous System, primarily in the leptomeninges [24], which consists of the inner two layers membranes of the meninges, the pia mater and arachnoid, which encapsulate the brain and the spinal cord. While its function within the meninges is unknown, they are essential to the health of the meninges, with their removal increases the risk of aseptic meningitis [25]. Furthermore, due to its receptivity to the same signalling molecules as neurons, scientists can study diseases afflicting the central nervous system using it as a model [26].
Embryonic Origins
Development Time Course
Molecular Mechanisms/ Factors/ Genes
Animal Models
Genetically Engineered Mice (GEM) Models For Melanocyte Development
The development of melanocytes in mice and humans are very similar, except they take longer to form in humans, so mice can be used to study melanocytes as they would occur in the human body. SCF (melanocyte growth factor) is essential for the maintenance and survival of melanocytes throughout life. The receptor for SCF is c-kit, and if it mutates, it can lead to sterility, anaemia and pigmentation disorders. If the receptor is completely lost, it results in loss of melanocytes, primordial germ cells and haematopoietic stem cells completely. In embryonic development however, ETRB (endothelin receptor) is more important to melanocyte developed compared to c-kit, as it regulates the number of precursor melanoblasts that will differentiate into melanocytes. [27]
Genetically Engineered Mice (GEM) Models For UV-induced Melanoma
UV-induced melanoma is explored in GEM as the skin of a mouse is similar to the skin of a human except that mice are covered in hair that undergo frequent cycles for follicular development, which in turn causes mature melanocytes to be shed. There is also a distinct difference in the levels of eumelanin and pheomalanin between mice and humans. These mice models allowed scientists to study the part UVR plays in melanomagenesis, in particular those that over-expresses HGF (hepatocyte growth factor). [28]
Current Research
Generation of Melanocytes from Induced Pluripotent Stem Cells (iPSCs)
Melanocytes can be derived from autologous iPSCs, meaning the cells come from the one person. In this case the likelihood of the melanocytes being rejected by the immune system of the person they are being implanted in, is incredibly low. A benefit of autologous iPSCs from a patient with a pigmented disorder (such as albinism), is that the pathogenesis can be studied in order to find a suitable treatment for the disease, and possibly a cure. [29]
Altered Chromosome Expression of Uveal Melanoma in the Setting of Melanocytosis
Uveal melanoma seems to effect chromosomes 3p, 3q, 1p, 8p and 8q in particular as they show the most significant areas of DNA loss or amplification. However, the most important DNA alteration is monosomy on chromosome 3. In melanocytosis tissue, there is normalcy on chromosome 1, 3, 6 and 8, but there is exhibited disomy on chromosome 3. Ocular melanocytosis is predicted to impart a 1/400 lifetime risk of uveal melanoma in Caucasian individuals (which is 400x higher than the general population). It has been found that alterations in chromosome 3 does not always lead to the development of uveal melanoma, but it does take place sometime during the tumour development. [30]
Abnormalities
Skin
Ears
Waardenburg Syndrome
There are varying forms of Waardenburg Syndrome, but they all result in deafness due to loss of melanocytes in the inner ears. As explained in the above section "Tissue Organ Structure and Function", melanocytes are necessary for sound transduction. The mutations that cause Waardenburg Syndrome, result in a lack of Melanogenesis Associated Transcription Factor (MITF) expression. MITF is a protein which works along side the transcription factor LEF-1 to regulate melanocyte gene expression [31]. These mutations may be in Sox10 and Pax3 genes, as these both regulate the expression of the MITF protein [32][33]. Hence, Waardenburg Syndrome can have varying genetic causes, but with the same outcome.
Eyes
Uveal Melanoma
Melanoma that occurs in the eye is known as uveal melanoma. This is an uncommon form of cancer that only accounts for about 3% of all melanomas. The risk factors of this cancer are light skin colour, red or blonde hair and blue or light irises. [18]. About 95% of uveal melanomas occur in the posterior eye (the ciliary body and choroid). Even though this is a rare from of cancer, it still contributes to a large percent of deaths and has a high chance of leading to distant metastases despite successful initial therapies in the local tumour or tumours of the eye. [34]
Heart
Central Nervous System
Glossary
Autologous- cells or tissues obtained from the same individual
Melanocytosis- the presence of an excessive number of melanocytes
References
- ↑ RAWLES ME. (1947). Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. , 20, 248-66. PMID: 20256541
- ↑ 2.0 2.1 2.2 Westerhof W. (2006). The discovery of the human melanocyte. Pigment Cell Res. , 19, 183-93. PMID: 16704452 DOI.
- ↑ Theriault LL & Hurley LS. (1970). Ultrastructure of developing melanosomes in C57 black and pallid mice. Dev. Biol. , 23, 261-75. PMID: 5476812
- ↑ Markert CL & Silvers WK. (1956). The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics , 41, 429-50. PMID: 17247639
- ↑ Thody AJ, Higgins EM, Wakamatsu K, Ito S, Burchill SA & Marks JM. (1991). Pheomelanin as well as eumelanin is present in human epidermis. J. Invest. Dermatol. , 97, 340-4. PMID: 2071942
- ↑ Agar N & Young AR. (2005). Melanogenesis: a photoprotective response to DNA damage?. Mutat. Res. , 571, 121-32. PMID: 15748643 DOI.
- ↑ Sangiovanni, G. (1819) Descrizione di un particolare sistema di organi cromoforo espansivo-dermoideo e dei fenomeni che esso produce, scoperto nei molluschi cefaloso. G. Enciclopedico Napoli, 9, 1-13
- ↑ Granville Harrison, R. Archiv für Entwicklungsmechanik der Organismen (1910) 30: 15. https://doi.org/10.1007/BF02263801
- ↑ Boissy RF. Histopathology of vitiliginous skin. In: Hann SK, Nordlund JJ, editors. Vitiligo. Oxford: Blackwell Science Ltd; 2000. pp. 23–34: https://doi.org/10.1002/9780470760116.ch5
- ↑ Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH & Chuva de Sousa Lopes SM. (2015). Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol , 75, 1219-40. PMID: 25663387 DOI.
- ↑ Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. The Inner Ear. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10946/
- ↑ Meyer zum Gottesberge AM. (1988). Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. , 1, 238-49. PMID: 3070525
- ↑ Steel KP & Barkway C. (1989). Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development , 107, 453-63. PMID: 2612372
- ↑ Takeuchi S, Kakigi A, Takeda T, Saito H & Irimajiri A. (1996). Intravascularly applied K(+)-channel blockers suppress differently the positive endocochlear potential maintained by vascular perfusion. Hear. Res. , 101, 181-5. PMID: 8951443
- ↑ Cable J, Huszar D, Jaenisch R & Steel KP. (1994). Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse. Pigment Cell Res. , 7, 17-32. PMID: 7521050
- ↑ DENIER A. (1951). [Microwaves of 12 cm; their biological and therapeutic effect]. J Radiol Electrol Arch Electr Medicale , 32, 664-5. PMID: 14909014
- ↑ Hu DN, Savage HE & Roberts JE. (2002). Uveal melanocytes, ocular pigment epithelium, and Müller cells in culture: in vitro toxicology. Int. J. Toxicol. , 21, 465-72. PMID: 12537643 DOI.
- ↑ 18.0 18.1 Schoenfield L. (2014). Uveal melanoma: A pathologist's perspective and review of translational developments. Adv Anat Pathol , 21, 138-43. PMID: 24508696 DOI.
- ↑ Baderca F, Solovan C & Boghian L. (2013). Epidemiological and morphological data of ocular melanocytic lesions. Rom J Morphol Embryol , 54, 77-83. PMID: 23529312
- ↑ Hou L & Pavan WJ. (2008). Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf?. Cell Res. , 18, 1163-76. PMID: 19002157 DOI.
- ↑ Hu DN, Simon JD & Sarna T. (2008). Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. , 84, 639-44. PMID: 18346089 DOI.
- ↑ Yamaguchi Y & Hearing VJ. (2014). Melanocytes and their diseases. Cold Spring Harb Perspect Med , 4, . PMID: 24789876 DOI.
- ↑ Hwang H, Liu F, Levin MD & Patel VV. (2014). Isolating primary melanocyte-like cells from the mouse heart. J Vis Exp , , 4357. PMID: 25285608 DOI.
- ↑ Gudjohnsen SA, Atacho DA, Gesbert F, Raposo G, Hurbain I, Larue L, Steingrimsson E & Petersen PH. (2015). Meningeal Melanocytes in the Mouse: Distribution and Dependence on Mitf. Front Neuroanat , 9, 149. PMID: 26635543 DOI.
- ↑ Goldgeier MH, Klein LE, Klein-Angerer S, Moellmann G & Nordlund JJ. (1984). The distribution of melanocytes in the leptomeninges of the human brain. J. Invest. Dermatol. , 82, 235-8. PMID: 6699426
- ↑ Yaar M & Park HY. (2012). Melanocytes: a window into the nervous system. J. Invest. Dermatol. , 132, 835-45. PMID: 22158549 DOI.
- ↑ Herlyn M, Berking C, Li G & Satyamoorthy K. (2000). Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. , 10, 303-12. PMID: 10985664
- ↑ Day CP, Marchalik R, Merlino G & Michael H. (2017). Mouse models of UV-induced melanoma: genetics, pathology, and clinical relevance. Lab. Invest. , 97, 698-705. PMID: 28092363 DOI.
- ↑ Ohta S, Imaizumi Y, Akamatsu W, Okano H & Kawakami Y. (2013). Generation of human melanocytes from induced pluripotent stem cells. Methods Mol. Biol. , 989, 193-215. PMID: 23483397 DOI.
- ↑ Horgan N, Shields CL, Swanson L, Teixeira LF, Eagle RC, Ganguly A & Shields JA. (2009). Altered chromosome expression of uveal melanoma in the setting of melanocytosis. Acta Ophthalmol , 87, 578-80. PMID: 18547285 DOI.
- ↑ Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K & Shibahara S. (2002). Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. , 21, 2703-14. PMID: 12032083 DOI.
- ↑ Lee M, Goodall J, Verastegui C, Ballotti R & Goding CR. (2000). Direct regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J. Biol. Chem. , 275, 37978-83. PMID: 10973953 DOI.
- ↑ Potterf SB, Furumura M, Dunn KJ, Arnheiter H & Pavan WJ. (2000). Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum. Genet. , 107, 1-6. PMID: 10982026
- ↑ An J, Wan H, Zhou X, Hu DN, Wang L, Hao L, Yan D, Shi F, Zhou Z, Wang J, Hu S, Yu J & Qu J. (2011). A comparative transcriptomic analysis of uveal melanoma and normal uveal melanocyte. PLoS ONE , 6, e16516. PMID: 21305041 DOI.