2018 Group Project 3: Difference between revisions
(→Hair) |
|||
| Line 177: | Line 177: | ||
====Uveal Melanoma==== | ====Uveal Melanoma==== | ||
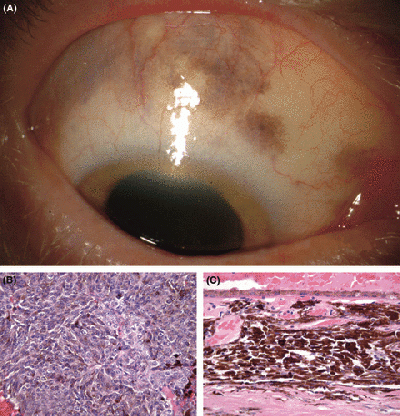
Melanoma that occurs in the eye is known as uveal melanoma. This is an uncommon form of cancer that only accounts for about 3% of all melanomas. The risk factors of this cancer are light skin colour, red or blonde hair and blue or light irises. {{#pmid:24508696|PMID24508696}}. About 95% of uveal melanomas occur in the posterior eye (the ciliary body and choroid). Even though this is a rare from of cancer, it still contributes to a large percent of deaths and has a high chance of leading to distant metastases despite successful initial therapies in the local tumour or tumours of the eye. {{#pmid:21305041|PMID21305041}} | Melanoma that occurs in the eye is known as uveal melanoma. This is an uncommon form of cancer that only accounts for about 3% of all melanomas. The risk factors of this cancer are light skin colour, red or blonde hair and blue or light irises. {{#pmid:24508696|PMID24508696}}. About 95% of uveal melanomas occur in the posterior eye (the ciliary body and choroid). Even though this is a rare from of cancer, it still contributes to a large percent of deaths and has a high chance of leading to distant metastases despite successful initial therapies in the local tumour or tumours of the eye. {{#pmid:21305041|PMID21305041}} | ||
[[File:Uveal Melanoma.gif|400px|thumb|center|Figure 8. Ciliochoroidal melanoma in an eye with melanocytosis.]] | |||
===Heart=== | ===Heart=== | ||
Revision as of 17:14, 12 October 2018
| Projects 2018: 1 Adrenal Medulla | 3 Melanocytes | 4 Cardiac | 5 Dorsal Root Ganglion |
Project Pages are currently being updated (notice removed when completed)
Melanocytes
Introduction
Melanocytes are a type of neural crest-derived cells in the body [1], most commonly found in the stratum basale, the bottom layer of the epidermis of the skin, eyes [2], and to a lesser known degree, the meninges (membrane around the brain), the heart [3] and the inner ear [4].
Each melanocyte possesses a main cell body, where most of the cellular functions, including the production of melanin take place. They also possess finger-like projections that extend into the surrounding tissue, similar to neurons, called dendrites. Melanin produced within the cell is packaged into granules, before being transported to the surrounding cells via dendrites, which reach in between the surrounding tissue.
Their most commonly known function is the production of melanin, melanogenesis, of which there are two types; eumelanin (black) and pheomelanin (reddish yellow) [5], and are responsible for the pigmentation of various parts of the human body including the skin, hair and irises, etc. Melanogenesis has different levels - basal and activated. The basal level of melanogenesis determines an individual's skin colour and is determined by genetics, i.e. a light-skinned individual has low levels of basal melanogenesis. Activated levels of melanogenesis are usually due to external factors, such as exposure to UV-B radiation, resulting in increased levels of melanogenesis [6], which can be commonly seen as sunburn or tan after long exposure to sunlight. Other cells in the body are able to produce pigment, such as epithelium of the retina, some neurons and adipocytes, but melanocytes are the only cell of neural crest origin that can perform this function.
History
Melanocytes were first described and named ‘chromatophores’ by Giosué Sangiovanni in 1819, who identified them in squid [7] . The location of pigmentation in the skin was examined by various scientists from the 1600s, and was found to reside in the epidermis. It wasn't until the early 20th century that the dark granules responsible for this pigmentation, nowadays termed melanosomes, were first identified using light microscopy. The origin of melanosome synthesis within the cell was discovered later in the century using electron microscopy: in 1961, Moyer found that stage 1 melanosomes (those containing matrix material, but no melanin), in pigmented retina, originate from the endoplasmic reticulum of epithelial cells. These findings were later confirmed by Turner, who studied melanosomes in goldfish [8] [9].
The idea that melanocytes originate from neural crest cells was first suggested by Ross Granville Harrison in 1910, when writing a paper on the outgrowth of the nerve fibre [10]. This was later proven by DuShane in 1935: He removed sections of neural fold in stage 15 Amphibia punctatum and tigrinum embryos, and observed a lack of pigment in their trunk region. He then placed the neural fold tissue explants in Holtfreter’s solution (a solution designed to facilitate amphibian development), and observed their differentiation into melanocytes [11]. In an article published in 1963, James Weston describes using tritiated thymidine labelling to track chick embryo neural crest migration in vivo. He identified a dorsolateral stream of neural crest derived melanoblasts (precursors to melanocytes), migrating to enter the ectoderm [12].
Embryonic Origins
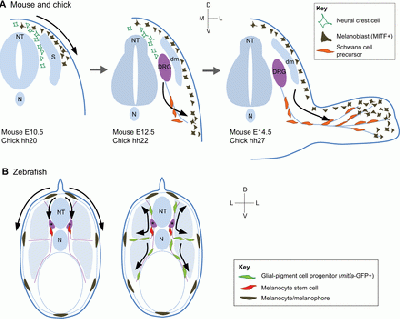
As mentioned earlier, melanocytes originate from the pluripotent cells in the neural crest [1]. After formation of the neural tube, neural crest cells eventually form four different functional domains, namely the cranial neural crest, trunk neural crest, vagal and sacral neural crest, and the cardiac neural crest [13]. Melanocytes are descended from melanoblasts, which in turn are formed from trunk neural crest cells migrating along the dorsolateral pathway into the ectoderm via minute holes in the basal lamina [14]. These eventually form part of the ears, eyes, skin and leptomeninges, therefore explaining the presence of melanin in these parts as a result of the development of melanoblast subpopulations during early migration [15].
Cochlear melanocytes of the inner ear
These melanocytes have recently been found to originate from neural crest cells delaminating from the rhombomere 6 region of the hindbrain, where the glossopharyngeal nerve and third pharyngeal arch are located [16].
Tissue Organ Structure and Function
Skin
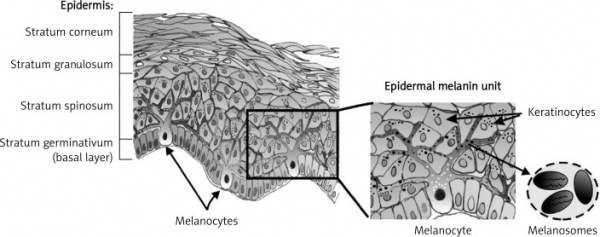
Melanocytes are located in the basal layer of the skin epidermis, amongst keratinocytes. Via dendrites, one melanocyte can communicate with around 30-40 keratinocytes. The role of melanocytes is to produce melanosomes - granules containing the pigment melanin, which are then transferred to keratinocytes [17]. Melanocytes synthesise two types of melanin: pheomelanin, which is red/yellow; and eumelanin, which is brown/black. These are produced in varying proportions depending on the individual. Paler skinned individuals synthesise more pheomelanin, whilst darker skinned individuals synthesise more eumelanin [5].
Mature melanosomes travel from the cell body to the dendrites of the melanocyte, where they are transported to neighbouring keratinocytes. A recent study has shown that melanosome transfer occurs under the following process:
- Melanocyte dendrites release pigment globules that contain clusters of melanosomes into the extracellular space.
- The pigment globules are then phagocytosed by keratinocytes.
- The globule membrane degrades in the keratinocyte cytosol and the melanosomes are released to populate the perinuclear space [18].
In the skin, melanin provides photoprotection to keratinocyte nuclear DNA: By aggregating above the nuclei of keratinocytes, it absorbs ultra violet radiation, which would otherwise damage DNA [19].
We all have around the same number of melanocytes in our skin epidermis, but the quantity of melanosomes taken up into keratinocytes varies between individuals - this, as well as the pheomelanin-eumelanin ratio, is what causes variation in skin colour. The darkening of skin can occur due to two causes: Ultraviolet irradiation and locally produced α-Melanocyte-stimulating hormone (α-MSH), both of which stimulate melanogenesis [20][21][5].
Hair
Though the hair follicle is initially formed from epithelial-mesenchymal interaction, the migration of neural crest derived melanocytes contributes to its completion [23]. Melanocytes migrate from the epidermis into the developing hair follicles, populating the hair matrix at the lower half of the hair bulb. Like melanocytes of the skin, they secrete mature melanosomes to keratinocytes, also populating the hair bulb. These melanin-containing keratinocytes form the cortex of the hair shaft[24]. Hair melanocytes differ from those in the skin, in that each melanocyte interacts with fewer keratinocytes. They also tend to be larger and have longer dendritic processes [25].
Ears
Melanocytes are mainly present in the cochlea, vestibular organ and endolymphatic sac.
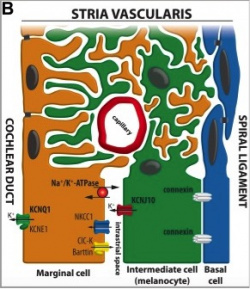
The cochlea of the inner ear functions as a transducer, by converting sound vibrations to electrical potentials in the auditory nerve via hair cells [26]. In the cochlea of humans, melanocytes are found in the vascularised epithelial tissue in the intermediate layer of the stria vascularis and the modiolus, between the marginal and basal cell layers [27](Figure 1). During development, marginal layer cuboidal epithelium develops processes which interdigitate with the intermediate melanocytes and basal cells [28].Based on studies performed on mice models, it is thought that melanocytes are important for the development of the endocochlear potential (EP), produced by strial cells. Intermediate melanocytes conduct K+, which plays an important role in sound conductance, as it flows from the endolymph into the ciliated epithelial cells of the ear via mechano-electrical channels. This influx of K+ is driven by a combination of the membrane potential of these ciliated epithelial cells, and the EP. A study on guinea pig models showed that blocking the K+ channels of melanocytes produces a lower EP [29]. This study agrees with evidence showing that mice deficient in cochlea melanocytes have a lower EP, requiring a greater sound stimulus to produce an action potential [30].
Eyes
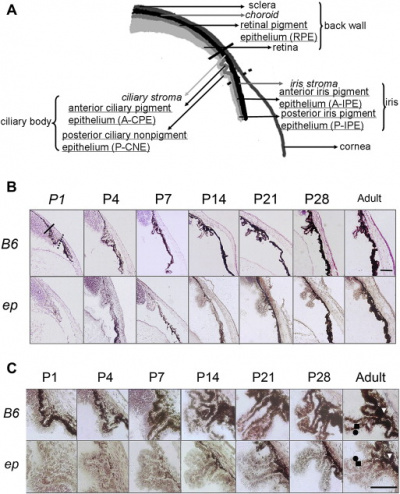
Uveal melanocytes contain both eumelanin and pheomelanin. It has been speculated that eumelanin is photoreceptive, whereas pheomelanin is phototoxic. Uveal melanocytes produce growth factors, such as vascular endothelial growth factor that controls blood circulation around the eye, as well as extracellular matrix degrading enzymes. It was found that both of these roles may play a key part in the toxicity of uveal melanocytes. [31]The uveal tract (iris, ciliary body and choroid) and conjunctiva normally contains melanocytes derived from the neuro-ectodermal neural crest. Peri-orbital soft tissue is also derived from the embryonic neural crest.[32] On the other hand, pigmented melanocytes (such as those found on the retina) were derived from the neuroepithelium or from different layers of the optic cup. [33] Uveal melanocytes (like all other melanocytes) differentiate into melanin-producing cells, which in this case determines a person's eye colour, protects the eye from UV radiation [34] and can mutate to form many ocular diseases that can cause blindness, including age-related macular degeneration. [35] Melanocytes found in the eye are not able to participate in regeneration unlike epidermal melanocytes. [36]
Heart
The term ‘cardiac melanocytes’ is used to describe the population of cells found in the human heart that express the melanin-synthesis enzymes. These cardiac melanocytes also resemble the morphology of the melanocytes found at the cutaneous level [37].
Central Nervous System
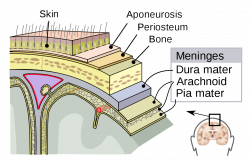
Melanocytes can also be found within the Central Nervous System, primarily in the leptomeninges [38], which consists of the inner two layers membranes of the meninges, the pia mater and arachnoid mater, which encapsulate the brain and the spinal cord. While its function within the meninges is unknown, they are essential to the health of the meninges, with their removal increasing the risk of aseptic meningitis [39]. Furthermore, due to its receptivity to the same signalling molecules as neurons, scientists can study diseases afflicting the central nervous system using it as a model [40].
Development Time Course
Melanocytes originate from neural crest cells, where they migrate to and localise in many different parts of the embryo during the first trimester of embryogenesis (in particular between weeks 4 to 18). Skin melanocytes follow hair follicles closely and localise around them, but little is known about melanocytes in volar skin (skin of the palms and soles), but it is suggested that they localise around eccrine sweat glands. [41]
Week 4= Visible neural plate where neural crest cells derive from.
Weeks 6-8= Melanoblast (pre-cursor cell of melanocytes) migration along dorsolateral trunk. Melanoblast proliferation also takes place along pathway, as the cells reach their areas of localisation, such as the eyes and the meninges. Melanoblasts have to migrate over incredibly long distances within the embryo, while at the same time proliferating and maintaining their chances of survival, so their development is a very dynamic process.
Weeks 9-12= Beginning of melanoblast migration to developing hair buds.
Weeks 12-13= A majority of melanoblasts are located in the epidermis and hair follicles. All melanoblasts differentiate into melanocytes.
Week 18= Hair breaks through skin surface containing melanocytes. [22]
Molecular Mechanisms/ Factors/ Genes
- unedited **
melanocytes in the skin-
transcription factors* microphthalmia-associated transcription factor (MITF)
intrinsic factors: - Hmx1 - Krox20
extrinsic factors modulating Hmx1 and Krox20: - Neuregulin-1 - IGF - PDGF
derivation pathways: - differentiate from ncc via dorsolateral path - from schwann cell precursors via ventral pathway
[36].
Animal Models
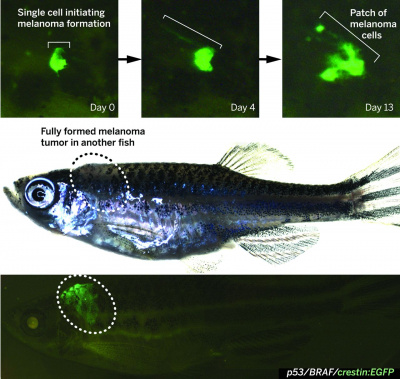
Zebrafish Melanoma Model Reveals Emergence of Neural Crest Identity During Melanoma Initation
Melanoma is mainly driven by mutations in BRAF (particularly BRAFV600E) or RAS genes. Melanoma is considered to be easily treatable when it is localised and has not broken through the tissue boundary, this is known as in situ cancer. When the mutated cells begin to invade the underlying tissue, the cancer is now metastatic and cannot be treated effectively, even with newly developed kinase and immune checkpoint targeted therapies. BRAFV600E gene was placed under the influence of melanocyte-specific mitfa-promoter in transgenic zebrafish. When the fish were crossed with a p53 mutant loss-of-function background, they developed nevi, and after a few months the nevi became invasive cancer. The p53 gene is the main tumour suppressor gene that stops cell division when the cell undergoes damage. Tumours with mutations in the p53 gene are more aggressive, metastatic and have a poorer patient prognosis. It was found that the fish were prone to developing one to three melanoma tumours after several months, illustrating that molecular alterations are important for tumour initiation. PubmedParser error: The PubmedParser extension received invalid XML data. ()
Genetically Engineered Mice (GEM) Models For Melanocyte Development
The development of melanocytes in mice and humans are very similar, except they take longer to form in humans, so mice can be used to study melanocytes as they would occur in the human body. SCF (melanocyte growth factor) is essential for the maintenance and survival of melanocytes throughout life. The receptor for SCF is c-kit, and if it mutates, it can lead to sterility, anaemia and pigmentation disorders. If the receptor is completely lost, it results in loss of melanocytes, primordial germ cells and haematopoietic stem cells completely. In embryonic development however, ETRB (endothelin receptor) is more important to melanocyte developed compared to c-kit, as it regulates the number of precursor melanoblasts that will differentiate into melanocytes. [42]
Genetically Engineered Mice (GEM) Models For UV-induced Melanoma
UV-induced melanoma is explored in GEM as the skin of a mouse is similar to the skin of a human except that mice are covered in hair that undergo frequent cycles for follicular development, which in turn causes mature melanocytes to be shed. There is also a distinct difference in the levels of eumelanin and pheomalanin between mice and humans. These mice models allowed scientists to study the part UVR plays in melanomagenesis, in particular those that over-expresses HGF (hepatocyte growth factor). [43]
Current Research
Generation of Melanocytes from Induced Pluripotent Stem Cells (iPSCs)
Melanocytes can be derived from autologous iPSCs, meaning the cells come from the one person. In this case the likelihood of the melanocytes being rejected by the immune system of the person they are being implanted in, is incredibly low. A benefit of autologous iPSCs from a patient with a pigmented disorder (such as albinism), is that the pathogenesis can be studied in order to find a suitable treatment for the disease, and possibly a cure. [44]
Altered Chromosome Expression of Uveal Melanoma in the Setting of Melanocytosis
Uveal melanoma seems to effect chromosomes 3p, 3q, 1p, 8p and 8q in particular as they show the most significant areas of DNA loss or amplification. However, the most important DNA alteration is monosomy on chromosome 3. In melanocytosis tissue, there is normalcy on chromosome 1, 3, 6 and 8, but there is exhibited disomy on chromosome 3. Ocular melanocytosis is predicted to impart a 1/400 lifetime risk of uveal melanoma in Caucasian individuals (which is 400x higher than the general population). It has been found that alterations in chromosome 3 does not always lead to the development of uveal melanoma, but it does take place sometime during the tumour development. [45]
Abnormalities
<html5media height="300" width="400">https://www.youtube.com/watch?v=aiARvdeXdKM</html5media> [46]
Skin
Abnormalities associated with melanocytes are often caused by disruptions to or loss of function of the pigmentation and its related factors. These pigmentary disorders are classified as hyperpigmentation disorder, hypopigmentation disorder, mixed hyperpigmentation disorder and mixed hypopigmentation disorder which are further subclassified into acquired and congenital [36].
Hyperpigmentation disorders refer to an array of usually benign skin conditions characterised by the darkening in colour of patches of skin. The darkening in colour is caused by the deposition of an excess of melanin in the skin, thus ‘hyper’ pigmentation [47].
LEOPARD Syndrome
LEOPARD syndrome is a congenital hyperpigmentation disorder that causes abnormalities to the appearance and functioning of the skin, heart, genitalia and inner ears. The name LEOPARD is an acronym which describes the characteristics of the condition;
L for Lentigines. These are pigmented macules (spots) on the skin. E for Electrocardiographic conduction defects. Aberration in the electrical activity of the heart due to factors including arrhythmia, intraventricular conduction delay etc leading to cardiac abnormalities. O for Ocular hypertelorism. An increased distance between the orbits within which they eyes lie resulting in widely spaced eyes. P for Pulmonary stenosis. Abnormalities of blood circulation caused by an obstruction to the pathway of blood outflow from the right ventricle to the heart. A for Abnormal genitalia. R for Retardation of growth. Slowed growth leading to short stature. D for Deafness. Sensorineural deafness (discussed below)
LEOPARD syndrome is autosomal dominant and can be inheritable. It can be caused by mutations to 3 different genes; protein tyrosine phosphatase, non-receptor type 11 (PTPN11), Raf-1 proto-oncogene (RAF1) or B-Raf proto-oncogene (BRAF) [48]
The lentigines in LEOPARD syndrome are caused by the development of additional matured melanosomes in the melanocytes and keratinocytes. An experiment conducted by the Department of Dermatology at Gunma University Graduate school of Medicine in 2015 used in vitro assays to study the melanocytes of patients with LEOPARD syndrome. The in vitro assay of a patient with LEOPARD syndrome caused by a mutation in the PTPN11 gene showed that melanin synthesis in the patient’s melanoma cells were higher than those of unaffected individuals due to the melanoma cells’ expression of SHP-2. SHP-2 is the protein tyrosine phosphatase encoded by the PTPN11 gene. This discovery suggested that the mutations to SHP-2 when associated with LEOPARD syndrome cause an increase in melanin synthesis by the melanocytes, thus resulting in the large pigmented spots [49].
Melasma
Melasma is an acquired hyperpigmentation disorder. It is a pigmentary condition occurring primarily on the face, characterised by brown patches usually found in three identified patterns; centrofacial , malar and mandibular. The most common pattern is centrofacial where pigmentation occurs on the forehead, nose, cheeks, chin and the upper lip. Malar pattern refers to the presence of melasma of the malar cheeks (cheek bones) on the face whilst the mandibular pattern features melasma on the mandible (jawline) and chin. Melasma occurring outside of the face in regions like the neck, sternum, forearms and other upper extremities is referred to as being of the extra-facial melasma pattern [50].
Ears
Waardenburg Syndrome
There are varying forms of Waardenburg Syndrome, but they all result in deafness due to loss of melanocytes in the inner ears. As explained in the above section "Tissue Organ Structure and Function", melanocytes are necessary for sound transduction. The mutations that cause Waardenburg Syndrome, result in a lack of Melanogenesis Associated Transcription Factor (MITF) expression. MITF is a protein which works along side the transcription factor LEF-1 to regulate melanocyte gene expression [51]. These mutations may be in Sox10 and Pax3 genes, as these both regulate the expression of the MITF protein [52][53]. Hence, Waardenburg Syndrome can have varying genetic causes, but with the same outcome.
Sensorineural hearing loss (SNHL)
Eyes
Uveal Melanoma
Melanoma that occurs in the eye is known as uveal melanoma. This is an uncommon form of cancer that only accounts for about 3% of all melanomas. The risk factors of this cancer are light skin colour, red or blonde hair and blue or light irises. [32]. About 95% of uveal melanomas occur in the posterior eye (the ciliary body and choroid). Even though this is a rare from of cancer, it still contributes to a large percent of deaths and has a high chance of leading to distant metastases despite successful initial therapies in the local tumour or tumours of the eye. [54]
Heart
Central Nervous System
Glossary
Autologous- cells or tissues obtained from the same individual
Lentigine: pigmented spots on the skin
Macule: discoloured patch of skin.
Melanoblasts- melanocyte pre-cursor cells
Melanocytosis- the presence of an excessive number of melanocytes
Melanoma- a tumour of melanin-forming cells
Melanogenesis - the production of melanin
Nevus- chronic lesion of skin or mucosa
Proto-oncogene: a normal gene that can become an oncogene contributing to cancer if it gets altered by a mutation
Volar Skin- skin of the palms of the hands and the soled of the feet (thick skin)
References
- ↑ 1.0 1.1 RAWLES ME. (1947). Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. , 20, 248-66. PMID: 20256541
- ↑ Westerhof W. (2006). The discovery of the human melanocyte. Pigment Cell Res. , 19, 183-93. PMID: 16704452 DOI.
- ↑ Theriault LL & Hurley LS. (1970). Ultrastructure of developing melanosomes in C57 black and pallid mice. Dev. Biol. , 23, 261-75. PMID: 5476812
- ↑ Markert CL & Silvers WK. (1956). The Effects of Genotype and Cell Environment on Melanoblast Differentiation in the House Mouse. Genetics , 41, 429-50. PMID: 17247639
- ↑ 5.0 5.1 5.2 Thody AJ, Higgins EM, Wakamatsu K, Ito S, Burchill SA & Marks JM. (1991). Pheomelanin as well as eumelanin is present in human epidermis. J. Invest. Dermatol. , 97, 340-4. PMID: 2071942
- ↑ Agar N & Young AR. (2005). Melanogenesis: a photoprotective response to DNA damage?. Mutat. Res. , 571, 121-32. PMID: 15748643 DOI.
- ↑ Sangiovanni, G. (1819) Descrizione di un particolare sistema di organi cromoforo espansivo-dermoideo e dei fenomeni che esso produce, scoperto nei molluschi cefaloso. G. Enciclopedico Napoli, 9, 1-13
- ↑ Moyer, F. H. Electron microscope observations on the origin, development and genetic control of melanin granules in the mouse eye. In: The Structure of the Eye, G. K. Smelser (ed.). New York: Academic Press, 1961, pp. 469–486
- ↑ Turner WA, Taylor JD & Tchen TT. (1975). Melanosome formation in the goldfish: the role of multivesicular bodies. J. Ultrastruct. Res. , 51, 16-31. PMID: 805261
- ↑ Granville Harrison, R. Archiv für Entwicklungsmechanik der Organismen (1910) 30: 15. https://doi.org/10.1007/BF02263801
- ↑ DuShane, Graham P. "An Experimental Study of the Origin of Pigment Cells in Amphibia." Journal of Experimental Zoology 72, no. 1 (1935): 1-31. https://onlinelibrary-wiley-com.wwwproxy1.library.unsw.edu.au/doi/epdf/10.1002/jez.1400720102
- ↑ WESTON JA. (1963). A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. , 6, 279-310. PMID: 14000137
- ↑ Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000. The Neural Crest. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10065/
- ↑ Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000. The Neural Crest. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10065/
- ↑ Tolleson WH. (2005). Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev , 23, 105-61. PMID: 16291526 DOI.
- ↑ 16.0 16.1 Locher H, de Groot JC, van Iperen L, Huisman MA, Frijns JH & Chuva de Sousa Lopes SM. (2015). Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol , 75, 1219-40. PMID: 25663387 DOI.
- ↑ Nordlund, J. J., Boissy, R. E., Hearing, V. A., King, R. S., Oetting, W. P., & Ortonne, J. (2007). The Pigmentary System: Physiology and Pathophysiology: Second Edition. Blackwell Publishing: https://onlinelibrary-wiley-com.ezproxy.is.ed.ac.uk/doi/pdf/10.1002/9780470987100
- ↑ Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB & Ichihashi M. (2012). Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Invest. Dermatol. , 132, 1222-9. PMID: 22189785 DOI.
- ↑ Lin JY & Fisher DE. (2007). Melanocyte biology and skin pigmentation. Nature , 445, 843-50. PMID: 17314970 DOI.
- ↑ Tsatmali M, Ancans J, Yukitake J & Thody AJ. (2000). Skin POMC peptides: their actions at the human MC-1 receptor and roles in the tanning response. Pigment Cell Res. , 13 Suppl 8, 125-9. PMID: 11041369
- ↑ Eller MS, Yaar M & Gilchrest BA. (1994). DNA damage and melanogenesis. Nature , 372, 413-4. PMID: 7984233 DOI.
- ↑ 22.0 22.1 Cichorek M, Wachulska M, Stasiewicz A & Tymińska A. (2013). Skin melanocytes: biology and development. Postepy Dermatol Alergol , 30, 30-41. PMID: 24278043 DOI.
- ↑ Barsh & Cotsarelis. 2007 How Hair Gets Its Pigment. Cell. 130(5), pp.779–781. https://www.sciencedirect.com/science/article/pii/S0092867407010951#bbib6
- ↑ Weiner L, Han R, Scicchitano BM, Li J, Hasegawa K, Grossi M, Lee D & Brissette JL. (2007). Dedicated epithelial recipient cells determine pigmentation patterns. Cell , 130, 932-42. PMID: 17803914 DOI.
- ↑ Hirobe, T., 2014. Keratinocytes regulate the function of melanocytes. Dermatologica Sinica, 32(4), pp.200–204. https://www.sciencedirect.com/science/article/pii/S1027811714000238
- ↑ Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. The Inner Ear. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10946/
- ↑ Meyer zum Gottesberge AM. (1988). Physiology and pathophysiology of inner ear melanin. Pigment Cell Res. , 1, 238-49. PMID: 3070525
- ↑ Steel KP & Barkway C. (1989). Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development , 107, 453-63. PMID: 2612372
- ↑ Takeuchi S, Kakigi A, Takeda T, Saito H & Irimajiri A. (1996). Intravascularly applied K(+)-channel blockers suppress differently the positive endocochlear potential maintained by vascular perfusion. Hear. Res. , 101, 181-5. PMID: 8951443
- ↑ 30.0 30.1 Cable J, Huszar D, Jaenisch R & Steel KP. (1994). Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse. Pigment Cell Res. , 7, 17-32. PMID: 7521050
- ↑ Hu DN, Savage HE & Roberts JE. (2002). Uveal melanocytes, ocular pigment epithelium, and Müller cells in culture: in vitro toxicology. Int. J. Toxicol. , 21, 465-72. PMID: 12537643 DOI.
- ↑ 32.0 32.1 Schoenfield L. (2014). Uveal melanoma: A pathologist's perspective and review of translational developments. Adv Anat Pathol , 21, 138-43. PMID: 24508696 DOI.
- ↑ Baderca F, Solovan C & Boghian L. (2013). Epidemiological and morphological data of ocular melanocytic lesions. Rom J Morphol Embryol , 54, 77-83. PMID: 23529312
- ↑ Hou L & Pavan WJ. (2008). Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf?. Cell Res. , 18, 1163-76. PMID: 19002157 DOI.
- ↑ Hu DN, Simon JD & Sarna T. (2008). Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. , 84, 639-44. PMID: 18346089 DOI.
- ↑ 36.0 36.1 36.2 Yamaguchi Y & Hearing VJ. (2014). Melanocytes and their diseases. Cold Spring Harb Perspect Med , 4, . PMID: 24789876 DOI.
- ↑ 37.0 37.1 Hwang H, Liu F, Levin MD & Patel VV. (2014). Isolating primary melanocyte-like cells from the mouse heart. J Vis Exp , , 4357. PMID: 25285608 DOI.
- ↑ Gudjohnsen SA, Atacho DA, Gesbert F, Raposo G, Hurbain I, Larue L, Steingrimsson E & Petersen PH. (2015). Meningeal Melanocytes in the Mouse: Distribution and Dependence on Mitf. Front Neuroanat , 9, 149. PMID: 26635543 DOI.
- ↑ Goldgeier MH, Klein LE, Klein-Angerer S, Moellmann G & Nordlund JJ. (1984). The distribution of melanocytes in the leptomeninges of the human brain. J. Invest. Dermatol. , 82, 235-8. PMID: 6699426
- ↑ Yaar M & Park HY. (2012). Melanocytes: a window into the nervous system. J. Invest. Dermatol. , 132, 835-45. PMID: 22158549 DOI.
- ↑ Nakamura M, Fukunaga-Kalabis M, Yamaguchi Y, Furuhashi T, Nishida E, Kato H, Mizuno T, Sugiura M & Morita A. (2015). Site-specific migration of human fetal melanocytes in volar skin. J. Dermatol. Sci. , 78, 143-8. PMID: 25818865 DOI.
- ↑ Herlyn M, Berking C, Li G & Satyamoorthy K. (2000). Lessons from melanocyte development for understanding the biological events in naevus and melanoma formation. Melanoma Res. , 10, 303-12. PMID: 10985664
- ↑ Day CP, Marchalik R, Merlino G & Michael H. (2017). Mouse models of UV-induced melanoma: genetics, pathology, and clinical relevance. Lab. Invest. , 97, 698-705. PMID: 28092363 DOI.
- ↑ Ohta S, Imaizumi Y, Akamatsu W, Okano H & Kawakami Y. (2013). Generation of human melanocytes from induced pluripotent stem cells. Methods Mol. Biol. , 989, 193-215. PMID: 23483397 DOI.
- ↑ Horgan N, Shields CL, Swanson L, Teixeira LF, Eagle RC, Ganguly A & Shields JA. (2009). Altered chromosome expression of uveal melanoma in the setting of melanocytosis. Acta Ophthalmol , 87, 578-80. PMID: 18547285 DOI.
- ↑ MelaFind MELASciences (2012, March 10) From Melanocyte to Melanoma [Video file]. Retrieved from https://www.youtube.com/watch?v=aiARvdeXdKM
- ↑ Aocd.org. Hyperpigmentation - American Osteopathic College of Dermatology (AOCD). [online] Available at: https://www.aocd.org/page/Hyperpigmentation
- ↑ Genetic and Rare Diseases Information Center. (2016). LEOPARD syndrome. [online] Available at: https://rarediseases.info.nih.gov/diseases/1100/leopard-syndrome
- ↑ Motegi S, Yokoyama Y, Ogino S, Yamada K, Uchiyama A, Perera B, Takeuchi Y, Ohnishi H & Ishikawa O. (2015). Pathogenesis of multiple lentigines in LEOPARD syndrome with PTPN11 gene mutation. Acta Derm. Venereol. , 95, 978-84. PMID: 25917897 DOI.
- ↑ Ogbechie-Godec OA & Elbuluk N. (2017). Melasma: an Up-to-Date Comprehensive Review. Dermatol Ther (Heidelb) , 7, 305-318. PMID: 28726212 DOI.
- ↑ Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K & Shibahara S. (2002). Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. , 21, 2703-14. PMID: 12032083 DOI.
- ↑ Lee M, Goodall J, Verastegui C, Ballotti R & Goding CR. (2000). Direct regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J. Biol. Chem. , 275, 37978-83. PMID: 10973953 DOI.
- ↑ Potterf SB, Furumura M, Dunn KJ, Arnheiter H & Pavan WJ. (2000). Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum. Genet. , 107, 1-6. PMID: 10982026
- ↑ An J, Wan H, Zhou X, Hu DN, Wang L, Hao L, Yan D, Shi F, Zhou Z, Wang J, Hu S, Yu J & Qu J. (2011). A comparative transcriptomic analysis of uveal melanoma and normal uveal melanocyte. PLoS ONE , 6, e16516. PMID: 21305041 DOI.