2017 Group Project 6: Difference between revisions
No edit summary |
mNo edit summary |
||
| (44 intermediate revisions by 6 users not shown) | |||
| Line 7: | Line 7: | ||
This page will highlight the anatomy of the cerebellum, its developmental process, current research on the structure, animal models and abnormalities associated with it. The anatomy will discuss the distinguishable lobes and zones of the cerebellum. The anatomy of the cerebellum cannot be completely discussed without explaining the vasculature of the structure and hence this page will provide a brief overview of it. The development of the cerebellum discusses the neural development and where the cerebellum forms on the neural tube. The development will highlight how the circuitry of the post-natal cerebellum came to be from neurons. Hence purkinje cells, granule cells, deep nuclei cells, glia cells and cerebellar nuclei will be highlighted to discuss the developmental process. A developmental timeline of the formation of the cerebellum is also included on this page. The cerebellum is a topic of continuous research and past findings would not have been done without the use of animal models, hence key historical discoveries, current research and animal models will be discussed. Towards the end of the page there are future questions listed on future investigations involving the cerebellum and the abnormalities from an affected cerebellum are also highlighted. Terms that may be difficult to understand have also been identified and defined. | This page will highlight the anatomy of the cerebellum, its developmental process, current research on the structure, animal models and abnormalities associated with it. The anatomy will discuss the distinguishable lobes and zones of the cerebellum. The anatomy of the cerebellum cannot be completely discussed without explaining the vasculature of the structure and hence this page will provide a brief overview of it. The development of the cerebellum discusses the neural development and where the cerebellum forms on the neural tube. The development will highlight how the circuitry of the post-natal cerebellum came to be from neurons. Hence purkinje cells, granule cells, deep nuclei cells, glia cells and cerebellar nuclei will be highlighted to discuss the developmental process. A developmental timeline of the formation of the cerebellum is also included on this page. The cerebellum is a topic of continuous research and past findings would not have been done without the use of animal models, hence key historical discoveries, current research and animal models will be discussed. Towards the end of the page there are future questions listed on future investigations involving the cerebellum and the abnormalities from an affected cerebellum are also highlighted. Terms that may be difficult to understand have also been identified and defined. | ||
The following video provides a brief overview on the cerebellum which will be further discussed on | The following video provides a brief overview on the cerebellum which will be further discussed on this page: | ||
<html5media height="315" width="560">https://www.youtube.com/watch?v=Fir-v6EoZNE</html5media> <ref> 2-Minute Neuroscience on Cerebellum. (2015). Retrieved October 25, 2017, from https://www.youtube.com/watch?v=Fir-v6EoZNE </ref> | <html5media height="315" width="560">https://www.youtube.com/watch?v=Fir-v6EoZNE</html5media> <ref> 2-Minute Neuroscience on Cerebellum. (2015). Retrieved October 25, 2017, from https://www.youtube.com/watch?v=Fir-v6EoZNE </ref> | ||
| Line 13: | Line 13: | ||
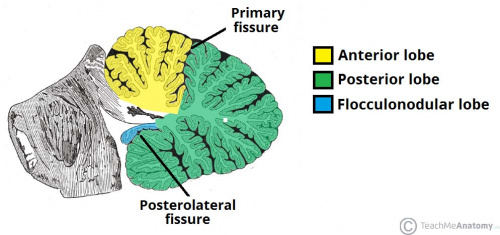
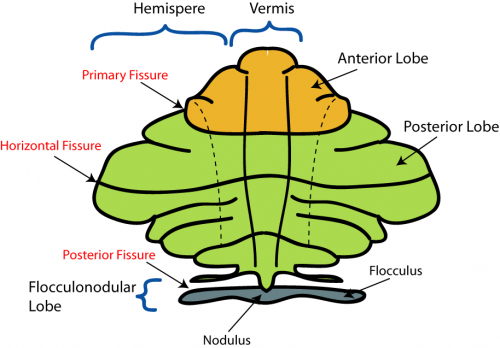
The cerebellum has 3 distinguishable lobes; flocculonodular lobe, anterior lobe and the posterior lobe. The anterior and posterior lobe can be further divided in a midline cerebellar vermis and lateral cerebellar hemispheres (Figure 1) | The cerebellum has 3 distinguishable lobes; flocculonodular lobe, anterior lobe and the posterior lobe. The anterior and posterior lobe can be further divided in a midline cerebellar vermis and lateral cerebellar hemispheres (Figure 1) | ||
{{#pmid:9735944|PMID9735944}}. In a superior cerebellar view, the cerebellum contains a vermis that runs through the middle of the organ and 2 intermediate zones located laterally from the vermis (Figure 2). | |||
[[File:Anatomical-Lobes-of-the-Cerebellum.jpg|500px]] | [[File:Anatomical-Lobes-of-the-Cerebellum.jpg|500px]] | ||
'''Figure 1:''' Anatomical lobes observed in the cerebellum; anterior lobe, posterior lobe and flocculonodular lobe, which is divided by two fissures – the primary fissure and posterolateral fissure <ref name="Venturini, S. (2017 | '''Figure 1:''' Anatomical lobes observed in the cerebellum; anterior lobe, posterior lobe and flocculonodular lobe, which is divided by two fissures – the primary fissure and posterolateral fissure <ref name="Venturini, S. (2017). The Cerebellum. Retrieved October 23, 2017, from http://teachmeanatomy.info/neuro/structures/cerebellum/">Venturini, S. (2017). The Cerebellum. Retrieved October 23, 2017, from http://teachmeanatomy.info/neuro/structures/cerebellum/</ref> | ||
[[File:Cerebellum anatomical subdivisions.png|500px]] | [[File:Cerebellum anatomical subdivisions.png|500px]] | ||
'''Figure 2:''' Superior view of the 3 cerebellar zones. The middle is the vermis. Either side of the vermis is the intermediate zone. Lateral to the intermediate zone is the lateral hemispheres. There is no difference in gross structure between the lateral hemispheres and intermediate zones. <ref>File:CerebellumDiv.png. (2016 | '''Figure 2:''' Superior view of the 3 cerebellar zones. The middle is the vermis. Either side of the vermis is the intermediate zone. Lateral to the intermediate zone is the lateral hemispheres. There is no difference in gross structure between the lateral hemispheres and intermediate zones. <ref>File:CerebellumDiv.png. (2016). Wikimedia Commons, the free media repository. Retrieved October 24, 2017, from https://commons.wikimedia.org/w/index.php?title=File:CerebellumDiv.png&oldid=226277429.</ref> | ||
[[File:Arteries of the cerebellum.jpeg|thumb|'''Figure 3:''' Diagram of the main arteries of the cerebellum <ref name="Venturini, S. (2017 | [[File:Arteries of the cerebellum.jpeg|thumb|'''Figure 3:''' Diagram of the main arteries of the cerebellum <ref name="Venturini, S. (2017). The Cerebellum. Retrieved October 23, 2017, from http://teachmeanatomy.info/neuro/structures/cerebellum/"/>]] | ||
==Vasculature== | ==Vasculature== | ||
The cerebellum contains 3 bilateral paired arteries which supplies this organ with oxygenated blood. These arteries all originate from the vertebrobasilar system | The cerebellum contains 3 bilateral paired arteries which supplies this organ with oxygenated blood. These arteries all originate from the vertebrobasilar system: Superior Cerebellar Artery (SCA), Anterior Inferior Cerebellar Artery (AICA) and Posterior inferior cerebellar artery (PICA). The SCA and AICA are branches of the basilar artery, which wraps around the anterior aspect of the pons before reaching the cerebellum. The PICA arises from the left and right vertebral artery, which form the basilar artery <ref>The Blood Supply of the Brain and Spinal Cord. (2001). In D. Purves, G. Augustine, & D. Fitzpatrick (Eds.), Neuroscience (2nd ed.). Sunderland, MA: Sinauer Associates.</ref>. The PICA and AICA combine to supply the inferior half of the cerebellum, while the SCA supplies the majority of the superior half. The PICA and SCA combine to supply the vermis{{#pmid:2535662|PMID2535662}}. Blood is then drained by superior and inferior cerebellar veins into the superior petrosal and then straight dural venous sinuses. (Figure 3){{#pmid:27766499|PMID27766499}} | ||
==Microanatomy== | ==Microanatomy== | ||
===Cortical Layers=== | ===Cortical Layers=== | ||
[[File:Screen Shot 2017-10-16 at 1.43.55 pm.png|thumb|'''Figure 4:''' (A). Cell types and where they are found in the cerebellar cortical layers. (B) Shows how the different layers of the cerebellum can be easily determined. P - the Purkinje layer; G - the granular layer; M - the molecular layer; W - the white matter. | [[File:Screen Shot 2017-10-16 at 1.43.55 pm.png|thumb|'''Figure 4:''' (A). Cell types and where they are found in the cerebellar cortical layers. (B) Shows how the different layers of the cerebellum can be easily determined. P - the Purkinje layer; G - the granular layer; M - the molecular layer; W - the white matter.{{#pmid:3527225|PMID3527225}}]] | ||
There are 3 major cortical layers of the cerebellum: the molecular layer, the purkinje cell layer, and the granule cell layer. The molecular layer contains basket cells, stellate cells and the purkinje cell and Golgi cell dendrites. The purkinje cell layer contains purkinje cell bodies and Bergmann glia. The granule cell layer contains granule cells, mossy fibers, and Golgi cell bodies. <ref>Danbolt, N. C., & Zhou, Y. (n.d.). The Cerebellar Cortex. Retrieved October 23, 2017, from http://neurotransporter.org/Cerebellum.html</ref> | There are 3 major cortical layers of the cerebellum: the molecular layer, the purkinje cell layer, and the granule cell layer. The molecular layer contains basket cells, stellate cells and the purkinje cell and Golgi cell dendrites. The purkinje cell layer contains purkinje cell bodies and Bergmann glia. The granule cell layer contains granule cells, mossy fibers, and Golgi cell bodies. <ref>Danbolt, N. C., & Zhou, Y. (n.d.). The Cerebellar Cortex. Retrieved October 23, 2017, from http://neurotransporter.org/Cerebellum.html</ref> | ||
| Line 46: | Line 42: | ||
===Deep Nuclei=== | ===Deep Nuclei=== | ||
There are four different deep nuclei of the cerebellum: the dentate, interpositus, fastigial, and vestibular nuclei. The dentate nucleus receives signals from the lateral purkinje cells, the interpositus nucleus receives signals from the intermediate purkinje cells, the fastigial nucleus receives signals from the medial purkinje cells, and the vestibular nucleus receives signals from the flocculonodular purkinje cells. The deep nuclei integrate the inhibitory signals from the purkinje cells and the excitatory signals from the mossy and climbing fibers to determine their output signals. <ref>Harting, J. K. (1997). Deep Nuclei. Retrieved October 23, 2017, from http://www.neuroanatomy.wisc.edu/cere/text/P5/intro.htm</ref> The dentate nucleus in particular is thought to be implicated in higher level cognitive processing. It is enlarged in primates and humans and not observed in non-mammalian species. | There are four different deep nuclei of the cerebellum: the dentate, interpositus, fastigial, and vestibular nuclei. The dentate nucleus receives signals from the lateral purkinje cells, the interpositus nucleus receives signals from the intermediate purkinje cells, the fastigial nucleus receives signals from the medial purkinje cells, and the vestibular nucleus receives signals from the flocculonodular purkinje cells. The deep nuclei integrate the inhibitory signals from the purkinje cells and the excitatory signals from the mossy and climbing fibers to determine their output signals. <ref>Harting, J. K. (1997). Deep Nuclei. Retrieved October 23, 2017, from http://www.neuroanatomy.wisc.edu/cere/text/P5/intro.htm</ref> The dentate nucleus in particular is thought to be implicated in higher level cognitive processing. It is enlarged in primates and humans and not observed in non-mammalian species.{{#pmid:25336734|PMID:25336734}} | ||
===Cerebellar Nuclei=== | ===Cerebellar Nuclei=== | ||
{ | {{Cerebellar Nuclei table}} | ||
===Bergmann Glia=== | |||
Glial cells of the cerebellum were described by Ramon y Cajal in 1911. He divided them into 3 main categories: the glia of the white matter, the astrocytes of the granule cell layer, and the Bergmann glia of the Purkinje cell layer. Also known as Goligi epithelial cells, Bergmann glia are unipolar astrocytes that have cell bodies located in the Purkinje cell layer and long processes projecting into the molecular layer. The Bergmann glia's processes interact with the dendrites of Purkinje cells at synapses with parallel and climbing fibers. Bergmann first characterized the long processes of cells he saw in the cerebellum of cats, dogs, and humans in 1857. Ramon y Cajal later described these cells as "epithelial cells with Bergmann fibers," giving the glia their name.{{#pmid:12418089|PMID12418089}} | |||
=Cerebellum Development= | |||
==Ectoderm== | |||
There are 3 germ layers present in the early embryo; ectoderm (most distal layer), mesoderm (middle layer) and endoderm (most proximal layer). The ectoderm differentiates into the nervous system, forming the spine, peripheral nerves, cerebrum and cerebellum. It also differentiates to form tooth enamel, epidermis, and the linings of the mouth, anus, sweat glands and nostrils.{{#pmid:14550785|PMID14550785}} | |||
|} | |||
<ref> | [[File:2 day old embryo diagram.jpeg|200px|thumb|'''Figure 5:''' Diagram of a 2 Day Old | ||
Embryo Illustrating the Beginnings of Neural Development <ref>Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Neural Development.</ref>]] | |||
== | ==Neural Development== | ||
Neural development is one of the earliest systems to begin and the last to be completed after birth due to its highly complex structure. The first step in neural development occurs at the end of week 3 and involves the neural groove fusing to form the neural tube, which then folds to form the cranial and caudal region of the embryo, and ultimately form the cerebellum. There is a high chance of neural dysfunction and defects during the fetal neural development particularly due to the long development time frame and the need of certain nutrients such as folic acid to successfully close the tube. Neural tube defects (NTDs) such as [[Talk:Spina_bifida|spina bifida]] and [[Talk:anencephaly|anencephaly]] can arise if the tube does not close effectively. | |||
==Early Brain Vesicles== | ==Early Brain Vesicles== | ||
| Line 82: | Line 70: | ||
[[File:Primary Brain.png|500px]] | [[File:Primary Brain.png|500px]] | ||
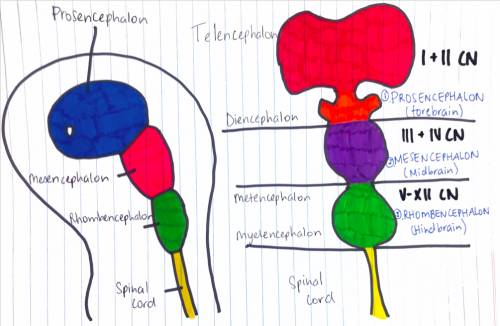
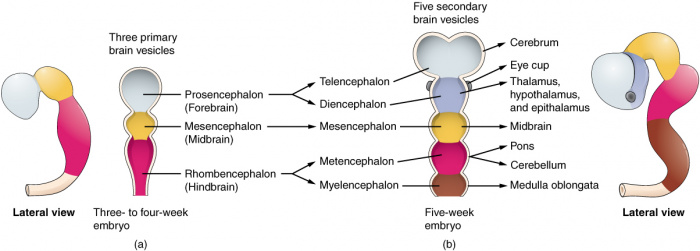
'''Figure | '''Figure 6:''' (Week 4) 3 primary brain vesicles are formed; forebrain (prosencephalon) midbrain (mesencephalon), and hindbrain (rhombencephalon){{#pmid:22237006|PMID22237006}} | ||
===Secondary=== | ===Secondary=== | ||
[[File:Secondary brain vesicle.jpeg|700px]] | [[File:Secondary brain vesicle.jpeg|700px]] | ||
'''Figure | '''Figure 7:''' (Week 5) 3 primary vesicles develop into 5 secondary vesicles <ref><pubmed>19335795</pubmed></ref>; | ||
*Prosencephalon develops into telencephalon (which includes the endbrain and cerebral hemispheres) and diencephalon (located between the brain and forms an optic outgrowth) | *Prosencephalon develops into telencephalon (which includes the endbrain and cerebral hemispheres) and diencephalon (located between the brain and forms an optic outgrowth) | ||
| Line 100: | Line 88: | ||
The dorsal surface is characterised by its highly folded folia separated by grooves termed sulci. The median area is referred to as the vermis, which eventually becomes the most superior aspect of the cerebellum. <ref>Gerardo De Iuliis PhD, Dino Pulerà MScBMC, CMI, in The Dissection of Vertebrates (Second Edition), 2011</ref> The first structure that belies the future cerebellum are the rhombic lips that appear on the metencephalon of a 5-6 week old embryo. The rhombic lips are aptly rhombus-shaped and denote the perimeter between the roof plate and the main body of the rhombencephalon. The anterior pair of lips mark the site at which the cerebellum will develop. <ref>Bruce M. Carlson MD, PhD, in Human Embryology and Developmental Biology (Fifth Edition), 2014</ref> | The dorsal surface is characterised by its highly folded folia separated by grooves termed sulci. The median area is referred to as the vermis, which eventually becomes the most superior aspect of the cerebellum. <ref>Gerardo De Iuliis PhD, Dino Pulerà MScBMC, CMI, in The Dissection of Vertebrates (Second Edition), 2011</ref> The first structure that belies the future cerebellum are the rhombic lips that appear on the metencephalon of a 5-6 week old embryo. The rhombic lips are aptly rhombus-shaped and denote the perimeter between the roof plate and the main body of the rhombencephalon. The anterior pair of lips mark the site at which the cerebellum will develop. <ref>Bruce M. Carlson MD, PhD, in Human Embryology and Developmental Biology (Fifth Edition), 2014</ref> | ||
=Cerebellum Developmental Weeks= | ==Cerebellum Developmental Weeks== | ||
===First Trimester=== | ===First Trimester=== | ||
| Line 112: | Line 100: | ||
| <center>'''Week 3'''</center> | | <center>'''Week 3'''</center> | ||
| <center> ''Neurulation'': Notochord and somites are formed under the ectoderm. The ectoderm then forms the neural plate which then forms the neural tube and then the brain and spinal chord. </center> | | <center> ''Neurulation'': Notochord and somites are formed under the ectoderm. The ectoderm then forms the neural plate which then forms the neural tube and then the brain and spinal chord. </center> | ||
| [[File:Stage9 dorsal.jpg|200px|thumb|'''Figure | | [[File:Stage9 dorsal.jpg|200px|thumb|'''Figure 8:''' Early stage of neurulation<ref>2017. '''Embryology''' ''Stage9 dorsal.jpg''. Retrieved October 15, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Stage9_dorsal.jpg</ref>]] [[File:Stage10 bf5.jpg|200px|thumb|'''Figure 9: '''Late stages of neurulation<ref>2017. '''Embryology''' ''Stage10 bf5.jpg''. Retrieved October 15, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Stage10_bf5.jpg</ref> ]] | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| <center>'''Week 4'''</center> | | <center>'''Week 4'''</center> | ||
| <center> Prosencephalon, mesencephalon and rhombencephalon is developed.</center> | | <center> Prosencephalon, mesencephalon and rhombencephalon is developed.</center> | ||
| [[File:Human Stage13 sagittal upper half01.jpg|200px|thumb|'''Figure | | [[File:Human Stage13 sagittal upper half01.jpg|200px|thumb|'''Figure 10:''' Sagittal view of superior end of embryo <ref> Hill, M.A. 2017 Embryology Human Stage13 sagittal upper half01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage13_sagittal_upper_half01.jpg</ref>]] | ||
|- | |- | ||
| <center>'''Week 5'''</center> | | <center>'''Week 5'''</center> | ||
| <center> Prosencephalon develops into telencephalon and diencephalon. The rhombencephalon differentiates into metencephalon and myelencephalon. The metencephalon will later develop the pons and cerebellum. </center> | | <center> Prosencephalon develops into telencephalon and diencephalon. The rhombencephalon differentiates into metencephalon and myelencephalon. The metencephalon will later develop the pons and cerebellum. </center> | ||
| <center> [[File:Human Stage14 neural01.jpg|200px|thumb|'''Figure | | <center> [[File:Human Stage14 neural01.jpg|200px|thumb|'''Figure 11:''' Lateral view of embryo central nervous system at 5 weeks <ref>M.A. 2017 Embryology Human Stage14 neural01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage14_neural01.jpg</ref>]] </center> | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| Line 131: | Line 119: | ||
|- | |- | ||
| <center>'''Weeks 7-9'''</center> | | <center>'''Weeks 7-9'''</center> | ||
| <center> The rhombic lip is fused medially to the midbrain. The primitive choroid plexus is fused with the cerebellar hemisphere to form the centrally located eosinophilic matrix. During this process, the inferior olive develops into a thick medulla forming a 'bulbo-pontine extension' | | <center> The rhombic lip is fused medially to the midbrain. The primitive choroid plexus is fused with the cerebellar hemisphere to form the centrally located eosinophilic matrix. During this process, the inferior olive develops into a thick medulla forming a 'bulbo-pontine extension' {{#pmid:21380713|PMID21380713}}. Development of cerebellum cell layer (future Purkinje cells) and choroid plexuses of the fourth and lateral ventricles {{#pmid:2252222|PMID2252222}}. </center> | ||
| <center> [[File:Human Stage21 neural01.jpg|200px|thumb|'''Figure | | <center> [[File:Human Stage21 neural01.jpg|200px|thumb|'''Figure 12:''' Left lateral view of embryonic CNS<ref>Hill, M.A. 2017 Embryology Human Stage21 neural01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage21_neural01.jpg</ref>]] [[File:Human Stage21 neural02.jpg|200px|thumb|'''Figure 13:''' Left medial view of lateral embryonic CNS <ref>Hill, M.A. 2017 Embryology Human Stage21 neural02.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage21_neural02.jpg</ref>]] </center> | ||
|} | |} | ||
===Second Trimester=== | ===Second Trimester=== | ||
[[File:Second Trimester Cerebellum.jpeg|200px|thumb|'''Figure | [[File:Second Trimester Cerebellum.jpeg|200px|thumb|'''Figure 14:''' Inferior image of a fetal cerebellum at second trimester<ref>Nuchal Fold Edema. Retrieved October 15, 2017, from http://www.fetalultrasound.com/online/text/2-006.HTM</ref>]] | ||
| Line 147: | Line 135: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| width="40" |<center>'''weeks 11-12'''</center> | | width="40" |<center>'''weeks 11-12'''</center> | ||
| width="65" |<center> Cerebellar hemisphere grows in size and thickness. Laminar configuration becomes present and vermis fissures start to develop. Mechanical stress such as shear and rotation can be detected by the cerebellum, causing several deep fissures to be developed | | width="65" |<center> Cerebellar hemisphere grows in size and thickness. Laminar configuration becomes present and vermis fissures start to develop. Mechanical stress such as shear and rotation can be detected by the cerebellum, causing several deep fissures to be developed{{#pmid:21380713|PMID21380713}}</center> | ||
|- | |- | ||
| Line 154: | Line 142: | ||
|} | |} | ||
[[File:Neural Circuit in the Cerebellum.jpg|thumb|'''Figure 15:''' Cerebellar neuronal circuits (A): Representation of zebrafish cerebellar neurons (B): Sagittal section of the adult zebrafish cerebellum (C): Zebrafish cerebellar structure schematic representation.{{#pmid:21309081|PMID21309081}}]] | |||
[[File:Screen Shot 2017-10-05 at 3.29.59 pm.png|thumb|'''Figure 16:''' Cerebellum cell development: A 7 day old rat cerebellum that has been sectioned to show the 3 layers of cells - external granule layer, purkinje cells and internal granular layer (a). Showing granule cell precursors proliferating to differentiate into granule neurons (b). Cell organisation (c){{#pmid:4061865|PMID4061865}}]] | |||
==Overview of Development== | |||
As the neural tube folds, the anterior portion develops the three brain vesicles: | As the neural tube folds, the anterior portion develops the three brain vesicles: | ||
*Prosencephalon | *Prosencephalon | ||
*Mesencephalon | *Mesencephalon | ||
*Rhombencephalon | *Rhombencephalon | ||
The rhombencephalon then further divides into the mesencephalic and myelincephalic vesicles on embryonic day 9. The neural tube failure to close then creates a gap along the dorsal sides and this produces a mouth-like structure as the tube bends to establish the pontine flexure. The pontine flexure further deepens bringing the mesencephalon (midbrain) closer to the primordium of the cerebellum (metencephalon); anterior aspects of the myelincephalon (brain stem) fold underneath developing the cerebellum plate | The rhombencephalon then further divides into the mesencephalic and myelincephalic vesicles on embryonic day 9. The neural tube failure to close then creates a gap along the dorsal sides and this produces a mouth-like structure as the tube bends to establish the pontine flexure. The pontine flexure further deepens bringing the mesencephalon (midbrain) closer to the primordium of the cerebellum (metencephalon); anterior aspects of the myelincephalon (brain stem) fold underneath developing the cerebellum plate{{#pmid:7605067|PMID7605067}} | ||
The cerebellar territory is defined by the expression of Hoxa2 genes (from posterior) and Otx2 (from anterior) genes and these genes are controlled by proteins Wnt and fibroblast growth factor families which regulate the expression of these genes in order to establish the cerebellar territory | The cerebellar territory is defined by the expression of Hoxa2 genes (from posterior) and Otx2 (from anterior) genes and these genes are controlled by proteins Wnt and fibroblast growth factor families which regulate the expression of these genes in order to establish the cerebellar territory{{#pmid:3213765|PMID3213765}}. Further development of the cerebellum begins between days 40 and 45 and it arises mostly from the metencephalon however the rhombic lips also contributes. The roof plate which is derived from the dorsal part of the alar plate thickens during development to become the cerebellum. The regulation of patterning involved when the primary fissure deepens by the end of the third month and thus divides the vermis, shows to be particularly important for development. The two lateral bulges are separated into the cranial anterior lob and caudal middle lobe. As the lobes divide further into lobules, fissures are formed and this continues throughout embryonic, fetal and postnatal life, thus increasing the surface area of the cerebellar cortex. The most primitive part of the cerebellum to form is the flocculonodular lobe, which is derived from separation of the first transverse fissure and this functions to keep connections with the vestibular system and it is also concerned with subconsciously controlling equilibrium. The flocculonodular lobe is separated from another crucial part of the cerebellum, corpus cerebelli, by the posterolateral fissure. | ||
==Overview of Cerebellar Cell Development== | |||
The cerebellum is connected to the brain stem via three pairs of peduncles and this allows the afferent and efferent pathways to enter and exit the cerebellum. Cerebellum afferent fibers can be grouped into two major types: mossy fibers and climbing fibres. Mossy fibres contribute to most of the afferent fibres in the cerebellum and they communicate with the cerebellar nuclei neurons and with Purkinje cells through granule cells embryonically, however postnatally they displace from Purkinje cells and synapse with their adult targets, the granule cell dendrites. Whilst mossy fibres originate from numerous sites in the nervous system, climbing fibers originate exclusively from the inferior olivary nucleus. Climbing fibers directly synapse with the cerebellar nuclei and Purkinje cells, relaying information to the cerebellum from several regions. The direction of these afferent fibres to their target neurons early in development are controlled by genes and molecules | The cerebellum is connected to the brain stem via three pairs of peduncles and this allows the afferent and efferent pathways to enter and exit the cerebellum. Cerebellum afferent fibers can be grouped into two major types: mossy fibers and climbing fibres. Mossy fibres contribute to most of the afferent fibres in the cerebellum and they communicate with the cerebellar nuclei neurons and with Purkinje cells through granule cells embryonically, however postnatally they displace from Purkinje cells and synapse with their adult targets, the granule cell dendrites. Whilst mossy fibres originate from numerous sites in the nervous system, climbing fibers originate exclusively from the inferior olivary nucleus. Climbing fibers directly synapse with the cerebellar nuclei and Purkinje cells, relaying information to the cerebellum from several regions. The direction of these afferent fibres to their target neurons early in development are controlled by genes and molecules{{#pmid:4552263|PMID4552263}}. | ||
The cerebellum has a very basic structure consisting 2 principal classes of neurons and 3 layers; layers are shown in Figure 15. | The cerebellum has a very basic structure consisting 2 principal classes of neurons and 3 layers; layers are shown in Figure 15. | ||
| Line 187: | Line 165: | ||
The nuclei from the cerebellum are formed by a complex process of neurogenesis and neuronal migration. The dorsomedial ventricular zone of the fourth ventricle gives rise to the principal neuronal output, the Purkinje cell and other neurons within the cerebellum The secondary germinal zone, coming from the adjacent rhombic lip generates the cerebellar granule cells as well as a subpopulation of neurons of cerebellar nuclei and several precerebellar nuclei <ref>Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R. and Francis-West, P.H., 2014. Larsen's Human Embryology E-Book. Elsevier Health Sciences.</ref>. | The nuclei from the cerebellum are formed by a complex process of neurogenesis and neuronal migration. The dorsomedial ventricular zone of the fourth ventricle gives rise to the principal neuronal output, the Purkinje cell and other neurons within the cerebellum The secondary germinal zone, coming from the adjacent rhombic lip generates the cerebellar granule cells as well as a subpopulation of neurons of cerebellar nuclei and several precerebellar nuclei <ref>Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R. and Francis-West, P.H., 2014. Larsen's Human Embryology E-Book. Elsevier Health Sciences.</ref>. | ||
Granule cells function in coordinating afferent input to and motor output from the cerebellum through excitatory connections with the Purkinje cell <ref name="PMID3213765" | Granule cells function in coordinating afferent input to and motor output from the cerebellum through excitatory connections with the Purkinje cell <ref name="PMID3213765"/>. There are two types of grey matter in the cerebellum, the deep cerebellar nuclei and an external cerebellar cortex. There are 4 deep nuclei formed and the output of the cerebellar cortex are relayed through these nuclei, the ventricular layer produces 4 types of neurons that migrate to the cortex. | ||
Proper cerebellum function requires well-organised neuronal connections and the integration of afferent and efferent fibres throughout the cerebellar circuit <ref name="PMID4552263"/>. The cerebellum functions in sensorimotor, balance control and vestibular ocular reflex, however recent studies have come out and shown that the cerebellum has a wide range of cognitive functions which include speech, memory and cognitive functions.<ref name="PMID3213765"/> | Proper cerebellum function requires well-organised neuronal connections and the integration of afferent and efferent fibres throughout the cerebellar circuit <ref name="PMID4552263"/>. The cerebellum functions in sensorimotor, balance control and vestibular ocular reflex, however recent studies have come out and shown that the cerebellum has a wide range of cognitive functions which include speech, memory and cognitive functions.<ref name="PMID3213765"/> | ||
| Line 193: | Line 171: | ||
===Granule Cells=== | ===Granule Cells=== | ||
Granule cells migrate tangentially from the [https://en.wikipedia.org/wiki/Rhombic_lip rhombic lip] to form the transient structure, the external germinal layer (EGL). The EGL consists of an outer and inner layer that is sandwiched between the [https://en.wikipedia.org/wiki/Pia_mater pia mater] and the purkinje cell layer. During this tangential migration, granule cells extend two horizontal processes. Granule cells move from the outer layer of the EGL to differentiate in the inner layer of the EGL. Differentiated cells continue to move radially to the granule cell layer on the inner surface of the purkinje cell layer.<ref name="PMID25336734"/> During radial migration, granule cells extend vertical processes and move through the purkinje cell layer on the radial processes of Bergmann glia. | Granule cells migrate tangentially from the [https://en.wikipedia.org/wiki/Rhombic_lip rhombic lip] to form the transient structure, the external germinal layer (EGL). The EGL consists of an outer and inner layer that is sandwiched between the [https://en.wikipedia.org/wiki/Pia_mater pia mater] and the purkinje cell layer. During this tangential migration, granule cells extend two horizontal processes. Granule cells move from the outer layer of the EGL to differentiate in the inner layer of the EGL. Differentiated cells continue to move radially to the granule cell layer on the inner surface of the purkinje cell layer.<ref name="PMID25336734"/> During radial migration, granule cells extend vertical processes and move through the purkinje cell layer on the radial processes of Bergmann glia.{{#pmid:16806506|PMID16806506}} | ||
===Purkinje Cells=== | ===Purkinje Cells=== | ||
| Line 203: | Line 181: | ||
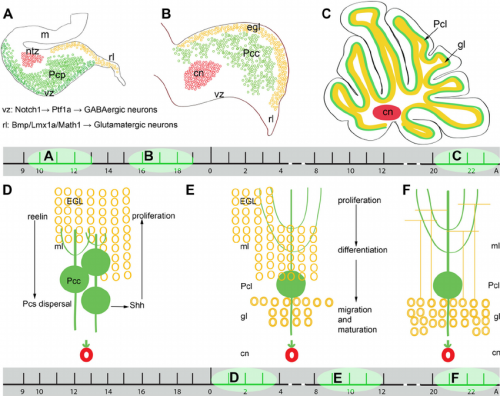
==Cell Signalling in Cerebellar Development== | ==Cell Signalling in Cerebellar Development== | ||
[[File:Cell signalling in cerebellum development.jpg|500px|thumb|'''Figure 18:''' SHH (green) acts on EGL to stimulate proliferation, on Bergmann glia (B) to induce differentiation, and has an unknown function on purkinje cells (P). Laminin and heparan sulfate have a synergistic effect on SHH and fibronectin and vitronectin have an inhibitory effect on SHH. | [[File:Cell signalling in cerebellum development.jpg|500px|thumb|'''Figure 18:''' SHH (green) acts on EGL to stimulate proliferation, on Bergmann glia (B) to induce differentiation, and has an unknown function on purkinje cells (P). Laminin and heparan sulfate have a synergistic effect on SHH and fibronectin and vitronectin have an inhibitory effect on SHH.{{#pmid:22291620|PMID22291620}}]] | ||
===Proliferation in the EGL=== | ===Proliferation in the EGL=== | ||
Many different cell signalling pathways are involved with cell migration, proliferation, and differentiation in the cerebellum. [https://en.wikipedia.org/wiki/Sonic_hedgehog '''Sonic hedgehog'''] (SHH) is particularly important in the proliferation of granule cells in the external germinal layer. The '''external germinal layer''' (EGL) is the area of transit amplification of granule cell precursors. This layer is transient and lies on the external surface of the cerebellum until the cells differentiate and migrate radially to their final destination in the internal granule cell layer. SHH is secreted by the purkinje cells and acts locally, causing the granule cell precursors to undergo mitosis and Bergmann glia to differentiate. The autocrine function of SHH on the purkinje cells is unknown. <ref><pubmed>3263706</pubmed></ref> Proliferating cells in the EGL must also be positive for '''Atoh1''', a transcription factor that represses differentiation and promotes division via SHH signalling. In a similar manner to SHH and Atoh1, the pia mater secretes '''Sdf1''' which interacts with the receptor '''Cxcr4''' to maintain proliferation in the EGL. [https://en.wikipedia.org/wiki/Notch_signaling_pathway Notch2 signalling] and its stimulation of Atoh1 and repression of Bone Morphogenetic Protein (BMP). | Many different cell signalling pathways are involved with cell migration, proliferation, and differentiation in the cerebellum. [https://en.wikipedia.org/wiki/Sonic_hedgehog '''Sonic hedgehog'''] (SHH) is particularly important in the proliferation of granule cells in the external germinal layer. The '''external germinal layer''' (EGL) is the area of transit amplification of granule cell precursors. This layer is transient and lies on the external surface of the cerebellum until the cells differentiate and migrate radially to their final destination in the internal granule cell layer. SHH is secreted by the purkinje cells and acts locally, causing the granule cell precursors to undergo mitosis and Bergmann glia to differentiate. The autocrine function of SHH on the purkinje cells is unknown. <ref><pubmed>3263706</pubmed></ref> Proliferating cells in the EGL must also be positive for '''Atoh1''', a transcription factor that represses differentiation and promotes division via SHH signalling. In a similar manner to SHH and Atoh1, the pia mater secretes '''Sdf1''' which interacts with the receptor '''Cxcr4''' to maintain proliferation in the EGL. [https://en.wikipedia.org/wiki/Notch_signaling_pathway Notch2 signalling] and its stimulation of Atoh1 and repression of Bone Morphogenetic Protein (BMP). | ||
===Exit from the EGL=== | ===Exit from the EGL=== | ||
BMPs are involved in arresting granule cell proliferation in the EGL and stimulating differentiation. | BMPs are involved in arresting granule cell proliferation in the EGL and stimulating differentiation.{{#pmid:3051031|PMID3051031}} Cells exit proliferation and move to the inner EGL where they differentiate upon expression of '''Neurod1''' and interaction with the extracellular matrix components of the inner EGL, '''vitronectin''', '''F3''', and '''contactin'''.<ref name="PMID25336734"/> Granule cell migration is stimulated by '''D-serine''' secreted by the Bergmann glia. This can be inhibited by '''DAAO''' or '''SR'''.<ref name="PMID16806506"/> | ||
[[File:Reelin signalling.jpg|300px|thumb|'''Figure 19:''' Reelin is a large signalling molecule that initiates a signal transduction pathway to induce formation of a monolayer of purkinje cells and detach purkinje cells from radial glia.<ref>Zhang, G., Assadi, A. H., McNeil, R. S., Beffert, U., Wynshaw-Boris, A., Herz, J., . . . D'Arcangelo, G. (2007). The Pafah1b Complex Interacts with the Reelin Receptor VLDLR. PLoS ONE, 2(2), 252nd ser. doi:https://doi.org/10.1371/journal.pone.0000252</ref>]] | [[File:Reelin signalling.jpg|300px|thumb|'''Figure 19:''' Reelin is a large signalling molecule that initiates a signal transduction pathway to induce formation of a monolayer of purkinje cells and detach purkinje cells from radial glia.<ref>Zhang, G., Assadi, A. H., McNeil, R. S., Beffert, U., Wynshaw-Boris, A., Herz, J., . . . D'Arcangelo, G. (2007). The Pafah1b Complex Interacts with the Reelin Receptor VLDLR. PLoS ONE, 2(2), 252nd ser. doi:https://doi.org/10.1371/journal.pone.0000252</ref>]] | ||
| Line 218: | Line 196: | ||
=Key Historical Discoveries= | |||
{| border="0" | {| border="0" | ||
| Line 233: | Line 211: | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| width="40" |<center>'''Late 1700s'''</center> | | width="40" |<center>'''Late 1700s'''</center> | ||
| width="40" | <center>Luigi Rolando, Pierre Flourens</center> | | width="40" | <center>Luigi Rolando, Pierre Flourens, David Ferrier</center> | ||
| width="80" | Provided experimental evidence for the function of the cerebellum. Luigi Rolando identified a specifically motor impact after cerebellar lesions developed, as opposed to an intellectual or sensory effect. This led to the conclusion that the cerebellum incited and managed movement. Pierre Flourens | | width="80" | [[File:Ferrier's findings of cerebellum.gif|220px|thumb|right| '''Figure 20:'''Ferrier's findings on a monkey's cerebellum with stimulation points discovered<ref>Sabbatini, R. M. (1997, March). Brain Maps The Study of Brain Function in the Nineteenth Century. Retrieved October 26, 2017, from http://www.cerebromente.org.br/n01/frenolog/frenloc.htm</ref>]] | ||
Provided experimental evidence for the function of the cerebellum. Luigi Rolando identified a specifically motor impact after cerebellar lesions developed, as opposed to an intellectual or sensory effect. This led to the conclusion that the cerebellum incited and managed movement. Pierre Flourens, Luigi Luciani and David Ferrier were able to use this albeit crude experimentation to further define that the cerebellum coordinated movement instead of creating it, and also to differentiate between the short-term and long-term effects of cerebellar lesions, respectively.{{#pmid:19272426|PMID19272426}} | |||
|- | |- | ||
| width="40" |<center>'''Early 1800s'''</center> | | width="40" |<center>'''Early 1800s'''</center> | ||
| width="40" | <center>Ernesto Lugaro</center> | | width="40" | <center>Ernesto Lugaro</center> | ||
| width="80" | First defined "plasticity" as we know it in neuroscience, and also discovered the specific cells in the cerebellum that are named after him. He also furthered research into the functions of glia and defined "nervous conduction and transmission" in its current meaning. | | width="80" | First defined "plasticity" as we know it in neuroscience, and also discovered the specific cells in the cerebellum that are named after him. He also furthered research into the functions of glia and defined "nervous conduction and transmission" in its current meaning.{{#pmid:12481483|PMID12481483}} | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| width="40" |<center>'''Early 1800s'''</center> | | width="40" |<center>'''Early 1800s'''</center> | ||
| width="40" | <center>Joseph Babinski and Gordon Holmes</center> | | width="40" | <center>Joseph Babinski and Gordon Holmes</center> | ||
| width="80" | Further defined the exact nature of the effect of cerebellar lesions on motor control, and found loss of coordination in antagonistic muscles and also basic loss of muscle control. It was also found that the side of the cerebellum the lesion developed on, affected the same side of the body. | | width="80" | Further defined the exact nature of the effect of cerebellar lesions on motor control, and found loss of coordination in antagonistic muscles and also basic loss of muscle control. It was also found that the side of the cerebellum the lesion developed on, affected the same side of the body.{{#pmid:19272426|PMID19272426}} | ||
|- | |- | ||
| width="40" |<center>'''Mid 1800s'''</center> | | width="40" |<center>'''Mid 1800s'''</center> | ||
| width="40" |<center> Ramon y Cajal</center> | | width="40" |<center> Ramon y Cajal</center> | ||
| width="80" | [[File:Screen Shot 2017-10-16 at 3.01.32 pm.png|220px|thumb|right|'''Figure | | width="80" | [[File:Screen Shot 2017-10-16 at 3.01.32 pm.png|220px|thumb|right|'''Figure 21:''' Drawing of Purkinje cells (A) and granule cells (B) from pigeon cerebellum. Drawn by Santiago Ramón y Cajal, 1899. <ref><pubmed>4308982</pubmed></ref>]] | ||
| Line 266: | Line 247: | ||
| width="40" |<center>'''1960s'''</center> | | width="40" |<center>'''1960s'''</center> | ||
| width="40" | <center>John Eccles and Janos Szentágothai</center> | | width="40" | <center>John Eccles and Janos Szentágothai</center> | ||
| width="80" | Pieced together the first complete map of the functional anatomy of the cerebellum and define the | | width="80" | [[File:Purkinje Cell Arrangement.png|220px|thumb|right|'''Figure 22:''' Modular organisation of the cerebellum purkinje fibers by Janos Szentágothai<ref><pubmed>23335884 </pubmed></ref>]] | ||
Pieced together the first complete map of the functional anatomy of the cerebellum and define the excitatory and inhibitory nature of each cell type provided by Cajal. Further discoveries into the relationships between cell synapses, cell natures, and the minutiae of their structures by Jan Voogd, Olov Oscarsson and David Armstrong around the 1970's defined the organisation of Purkinje cells into "a series of longitudinal parasagittal bands", the specificity of which explains the solely Purkinje-axon-output of the cerebellar cortex.<ref name=”PMID19272426”><pubmed>19272426</pubmed></ref> The current focus in research now leans towards connecting cerebellar function with learning, emotion and perception of time. | |||
|} | |} | ||
=Animal Models= | =Animal Studies= | ||
==Animal Models== | |||
The mouse model is a primary model organism in the study of cerebellum development, but organisms such as Drosophila (fruit fly), C. elegans (roundworm), Saccharomyces cerevisiae (Baker's yeast), cats, dogs, and cattle are also used in some types of diseases.<ref><pubmed>19669387</pubmed></ref> Looking at a variety of different of organisms' cerebellar development can elucidate the evolution of the cerebellum and further understanding of cerebellar constituents.<ref name="PMID25336734"/> | The mouse model is a primary model organism in the study of cerebellum development, but organisms such as Drosophila (fruit fly), C. elegans (roundworm), Saccharomyces cerevisiae (Baker's yeast), cats, dogs, and cattle are also used in some types of diseases.<ref><pubmed>19669387</pubmed></ref> Looking at a variety of different of organisms' cerebellar development can elucidate the evolution of the cerebellum and further understanding of cerebellar constituents.<ref name="PMID25336734"/> | ||
[[File:Isthmic Organiser.png|310px|thumb|'''Figure 23:''' The Isthmic Organizer (IsO; shown in yellow) forms at the boundary of the posterior midbrain and anterior hindbrain: the IsO secretes Fgf8 and other growth factors, and is essential for defining the regions of the neural plate that will become the posterior midbrain (shown in blue) and the cerebellum (CB; green). <ref name="PMID3870571"><pubmed>3870571</pubmed></ref>]] | |||
==Isthmic Organiser== | ==Isthmic Organiser== | ||
Throughout history there have been many investigations on the cerebellum and how it has developed through research involving chicken embryos and mice. As previously mentioned the neural plate closes to form the neural tubes which have anterior-posterior (AP) and dorsal-ventral (DV) axes. Earlier experiments involving chick-quail chimera suggested that the cerebellum was derived from both midbrain and hindbrain. However, through successive gene expression and fate mapping studies, it was discovered that the anterior-most rhombomere of the hindbrain is where the cerebellum is formed from <ref name=”PMID14567957”><pubmed> 14567957</pubmed></ref>. Once the axes are formed the isthmic organiser (IsO) is formed which plays a vital role in establishing the anterior limit of the cerebellar territory. The IsO in other words is the mid-hindbrain boundary <ref name=”PMID21309081”><pubmed>21309081</pubmed></ref>. However, the IsO does not position itself without the help of transcription factors. Studies of mouse and chicken embryos have shown that two homeo domain-containing transcription factors Otx2 and Gbx2 have an important role in positioning the isthmic organiser <ref name=”PMID21309081”/>. Surgical movement of the isthmic tissue to more anterior or posterior regions of the neural tube of 10-somite stage chick embryos led to ectopic midbrain and cerebellar structures<ref name=”PMID14567957”><pubmed> 14567957</pubmed></ref>, indicating that where the IsO is placed is an important factor in establishing where the cerebellum positions. | |||
Throughout history there have been many investigations on the cerebellum and how it has developed through research involving chicken embryos and mice. As previously mentioned the neural plate closes to form the neural tubes which have anterior-posterior (AP) and dorsal-ventral (DV) axes. Earlier experiments involving chick-quail chimera suggested that the cerebellum was derived from both midbrain and hindbrain. However, through successive gene expression and fate mapping studies, it was discovered that the anterior-most rhombomere of the hindbrain is where the cerebellum is formed from <ref name=”PMID14567957”><pubmed> 14567957</pubmed></ref>. Once the axes are formed the isthmic organiser (IsO) is formed which plays a vital role in establishing the anterior limit of the cerebellar territory. The IsO in other words is the mid-hindbrain boundary <ref name=”PMID21309081”><pubmed>21309081</pubmed></ref>. However, the IsO does not position itself without the help of transcription factors. Studies of mouse and chicken embryos have shown that two homeo domain-containing transcription factors Otx2 and Gbx2 have an important role in positioning the isthmic organiser <ref name=”PMID21309081” | [[File:Zebrafish.jpg|210px|thumb|right|'''Figure 24:''' Schematic of the cerebellar circuitry in zebrafish. PN: Purkinje neuron; E: Eurydendroid cell; G: Granule cell; S: Stellate cell. <ref name=”PMIDPMC4584246”><pubmed>PMC4584246</pubmed></ref>]] | ||
[[File:Zebrafish.jpg|210px|thumb|right|'''Figure | |||
==Cell Structures== | ==Cell Structures== | ||
The cellular structures in the cerebellum (including Purkinje cells and granule cells) have been investigated in various animal models. One animal model is the zebra fish which is a bony fish (teleosts). Like mammals, the zebrafish cerebellum contains several types of neurons which function as either excitatory or inhibitory neurons <ref name=”PMID21309081”><pubmed>21309081</pubmed></ref>. Glutamate is utilised by excitatory neurons as their major neurotransmitter. Excitatory neurons include granule cells, unipolar brush cells and eurydendroid cells. The eurydendoid cells are predicted to be corresponding to the deep cerebellar neurons in mammals <ref name=”PMID21309081” | The cellular structures in the cerebellum (including Purkinje cells and granule cells) have been investigated in various animal models. One animal model is the zebra fish which is a bony fish (teleosts). Like mammals, the zebrafish cerebellum contains several types of neurons which function as either excitatory or inhibitory neurons <ref name=”PMID21309081”><pubmed>21309081</pubmed></ref>. Glutamate is utilised by excitatory neurons as their major neurotransmitter. Excitatory neurons include granule cells, unipolar brush cells and eurydendroid cells. The eurydendoid cells are predicted to be corresponding to the deep cerebellar neurons in mammals.<ref name=”PMID21309081”/> The inhibitory neurons use y-aminobutric acid (GABA) and/or glycine (also known as GABAergic neurons) for neurotransmission. Purkinje cells, Golgi and stellate cells are inhibitory neurons. However, a difference between mammal and teleosts is the lack of basket cells. Basket cells are GABAergic neurons that contribute project their axons to the Purkinje cells <ref name=”PMID21309081”><pubmed>21309081</pubmed></ref>. This is important, because as earlier highlighted Purkinje cells (inhibitory neurons) and granule cells (excitatory cells) contribute to the three cortical layers of the cerebellum. This model is important for understanding what structures contribute to the neurotransmission process in the cerebellum. | ||
=Abnormalities= | =Abnormalities= | ||
Abnormalities found within the posterior fossa of the cranium may affect the functioning and development of the cerebellum. The abnormalities affecting the cerebellum include Dandy-Walker-Malformation, Joubert Syndrome, Tecto-Cerebllar Dysraphism and | Abnormalities found within the posterior fossa of the cranium may affect the functioning and development of the cerebellum. The abnormalities affecting the cerebellum include Dandy-Walker-Malformation, Joubert Syndrome, Tecto-Cerebllar Dysraphism, Rhombencephalosynapsis and Medulloblastoma which will be explained in the table below. | ||
{| border="0" | {| border="0" | ||
| Line 292: | Line 276: | ||
| <center>'''Dandy-Walker Malformation'''</center> | | <center>'''Dandy-Walker Malformation'''</center> | ||
| Dandy-Walker-Malformation (DWM) includes the incomplete development of the cerebellar vermis with cystic dilatation of the 4th ventricle and enlargement of the posterior fossa <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. The cerebellar vermis (found at the medial, cortico-nuclear zone of the cerebellum<ref name=”PMIDPMC3179064”><pubmed> PMC3179064</pubmed></ref>) may either be elevated or rotated upwards <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. The communication of the 4th ventricle with the midline posterior fossa cyst can be seen on MRI scans of DWM <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. Other structures that can be identified on MRI scans include upward displacement of the tentorium and anterolateral shift of cerebellar hemispheres <ref name=”PMID21093738”><pubmed> 21093738</pubmed></ref>. The causes of DWM are still being researched and hence there has not been any clear causes. However, some causes include maternal diabetes, chromosomal defects that affect foetal brain development, infections in the mother that pass to the developing foetus and exposure of the unborn baby to certain toxins.<ref name="Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation">Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation</ref> Symptoms of DWM include motor and speaking development delays, poor muscle coordination, vision and hearing impairment.<ref name="Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation"/> | | Dandy-Walker-Malformation (DWM) includes the incomplete development of the cerebellar vermis with cystic dilatation of the 4th ventricle and enlargement of the posterior fossa <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. The cerebellar vermis (found at the medial, cortico-nuclear zone of the cerebellum<ref name=”PMIDPMC3179064”><pubmed> PMC3179064</pubmed></ref>) may either be elevated or rotated upwards <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. The communication of the 4th ventricle with the midline posterior fossa cyst can be seen on MRI scans of DWM <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. Other structures that can be identified on MRI scans include upward displacement of the tentorium and anterolateral shift of cerebellar hemispheres <ref name=”PMID21093738”><pubmed> 21093738</pubmed></ref>. The causes of DWM are still being researched and hence there has not been any clear causes. However, some causes include maternal diabetes, chromosomal defects that affect foetal brain development, infections in the mother that pass to the developing foetus and exposure of the unborn baby to certain toxins.<ref name="Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation">Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation</ref> Symptoms of DWM include motor and speaking development delays, poor muscle coordination, vision and hearing impairment.<ref name="Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation"/> | ||
| [[File:DandyWalkerMalformation.jpeg|220px|thumb|center|'''Figure | | [[File:DandyWalkerMalformation.jpeg|220px|thumb|center|'''Figure 25:''' Brain MRIs. Brain imaging of patients with Dandy-Walker malformation (CCM067, CCM095) and patients with normal cerebellum and posterior fossa (CCM001). A: sagittal midline T1-weighted images. B: axial T2-weighted images. <ref name=”PMID PMC3667004”><pubmed> PMC3667004</pubmed></ref>]] | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| <center>'''Joubert Syndrome'''</center> | | <center>'''Joubert Syndrome'''</center> | ||
| Joubert syndrome (JS) is described as a rare inherited genetic disorder characterised by lack of muscle coordination, intellectual disability, respiratory disturbances and abnormal eye movement <ref name=”PMIDPMC2913941”><pubmed>PMC2913941</pubmed></ref>. On neuroimaging, the cerebellar vermis is identified to have gone through hypoplasia (underdevelopment) or dysplasia (abnormal development) <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. It is also described as the "molar tooth sign" on brain imaging, which the "molar tooth" shaped is caused by the defects in the midbrain-hindbrain development. JS is inherited as mutations in any of many genes, however, how mutations lead to JS is still being investigated. Mutations cause problems in the structure and function of cilia and hence signalling pathways may be disrupted during development <ref> Joubert syndrome. (2016). Retrieved October 22, 2017, from https://rarediseases.info.nih.gov/diseases/6802/joubert-syndrome</ref>. | | Joubert syndrome (JS) is described as a rare inherited genetic disorder characterised by lack of muscle coordination, intellectual disability, respiratory disturbances and abnormal eye movement <ref name=”PMIDPMC2913941”><pubmed>PMC2913941</pubmed></ref>. On neuroimaging, the cerebellar vermis is identified to have gone through hypoplasia (underdevelopment) or dysplasia (abnormal development) <ref name=”PMID22108217”><pubmed>22108217</pubmed></ref>. It is also described as the "molar tooth sign" on brain imaging, which the "molar tooth" shaped is caused by the defects in the midbrain-hindbrain development. JS is inherited as mutations in any of many genes, however, how mutations lead to JS is still being investigated. Mutations cause problems in the structure and function of cilia and hence signalling pathways may be disrupted during development <ref> Joubert syndrome. (2016). Retrieved October 22, 2017, from https://rarediseases.info.nih.gov/diseases/6802/joubert-syndrome</ref>. | ||
| [[File:JoubertSyndrome.jpg|220px|thumb|center|'''Figure | | [[File:JoubertSyndrome.jpg|220px|thumb|center|'''Figure 26:''' Brain Imaging of Joubert Syndrome: Cranial MRI showing “molar tooth sign” (arrows)<ref name=”PMIDPMC3896311”><pubmed>PMC3896311</pubmed></ref>]] | ||
|- | |- | ||
| <center>''' Chiari Syndrome I-III'''</center> | | <center>''' Chiari Syndrome I-III'''</center> | ||
| Chiari Syndrome I-III is when the structures within the posterior cranial fossa protrude into the spinal canal <ref name=”PMID18762395”><pubmed>18762395</pubmed></ref> which can affect the development and functioning of the cerebellum in the long term. Chiari Syndrome can be classified into four types. The downward shift of the cerebellar tonsils to beneath the foramen magnum is type 1 and the downward movement of the vermis, medulla oblongata and pons with the fourth ventricle is type 2 <ref name=”PMID18762395”><pubmed>18762395</pubmed></ref>. When majority of the cerebellum lies in the foramen magnum it is type 3 and when it is completely in the foramen magnum (and therefore cannot develop normally) it is type 4<ref name=”PMID18762395”><pubmed>18762395</pubmed></ref>. As a result of genetic mutations or maternal diet lacking vitamins or nutrients, structural defects in the brain and spinal cord can occur during foetal development leading to Chiari Syndrome <ref> Chiari Malformation Fact Sheet. (2017). Retrieved October 22, 2017, from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Chiari-Malformation-Fact-Sheet</ref> | | Chiari Syndrome I-III is when the structures within the posterior cranial fossa protrude into the spinal canal <ref name=”PMID18762395”><pubmed>18762395</pubmed></ref> which can affect the development and functioning of the cerebellum in the long term. Chiari Syndrome can be classified into four types. The downward shift of the cerebellar tonsils to beneath the foramen magnum is type 1 and the downward movement of the vermis, medulla oblongata and pons with the fourth ventricle is type 2 <ref name=”PMID18762395”><pubmed>18762395</pubmed></ref>. When majority of the cerebellum lies in the foramen magnum it is type 3 and when it is completely in the foramen magnum (and therefore cannot develop normally) it is type 4<ref name=”PMID18762395”><pubmed>18762395</pubmed></ref>. As a result of genetic mutations or maternal diet lacking vitamins or nutrients, structural defects in the brain and spinal cord can occur during foetal development leading to Chiari Syndrome <ref> Chiari Malformation Fact Sheet. (2017). Retrieved October 22, 2017, from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Chiari-Malformation-Fact-Sheet</ref> | ||
| [[File:ChiariMalformation.jpg|220px|thumb|center|'''Figure | | [[File:ChiariMalformation.jpg|220px|thumb|center|'''Figure 27:''' Cerebellar tonsils herniation on magnetic resonance imaging: Chiari malformation type I<ref name=”PMIDPMC4279813”><pubmed>PMC4279813</pubmed></ref>]] | ||
|-bgcolor="F5FAFF" | |-bgcolor="F5FAFF" | ||
| <center>'''Rhombencephalosynapsis'''</center> | | <center>'''Rhombencephalosynapsis'''</center> | ||
| Rhombencephalosynapsis is a rare cerebellar defect whereby there is dorsal fusion of the cerebral hemispheres, fusion of dentate nuclei and superior cerebellar peduncles as well as the agenesis of the cerebella vermis <ref name=”PMID18155944”><pubmed>18155944</pubmed></ref>. Rhombencephalosynapsis is predicted to result from disturbed cerebellar development at approximately 33–34 days of gestation <ref name=”PMID18409180”><pubmed>18409180</pubmed></ref>. The cause of rhombencephalosynapsis is thought to be a genetic defect in the isthmic organizer, resulting in abnormal dorsal patterning, causing the mentioned defects <ref name=”PMID18409180”><pubmed>18409180</pubmed></ref>. Like other mentioned abnormalities there is development delay because of the underdeveloped cerebellum. Rhombencephalosynapsis can be identified from a range of symptoms, from involuntary muscle coordination, severe cerebral palsy or mental retardation<ref name=”PMID25816977”><pubmed>25816977</pubmed></ref>. | | Rhombencephalosynapsis is a rare cerebellar defect whereby there is dorsal fusion of the cerebral hemispheres, fusion of dentate nuclei and superior cerebellar peduncles as well as the agenesis of the cerebella vermis <ref name=”PMID18155944”><pubmed>18155944</pubmed></ref>. Rhombencephalosynapsis is predicted to result from disturbed cerebellar development at approximately 33–34 days of gestation <ref name=”PMID18409180”><pubmed>18409180</pubmed></ref>. The cause of rhombencephalosynapsis is thought to be a genetic defect in the isthmic organizer, resulting in abnormal dorsal patterning, causing the mentioned defects <ref name=”PMID18409180”><pubmed>18409180</pubmed></ref>. Like other mentioned abnormalities there is development delay because of the underdeveloped cerebellum. Rhombencephalosynapsis can be identified from a range of symptoms, from involuntary muscle coordination, severe cerebral palsy or mental retardation<ref name=”PMID25816977”><pubmed>25816977</pubmed></ref>. | ||
| <center>[[File:Rhombencephalosynapsis.jpg|220px|thumb|center|'''Figure | | <center>[[File:Rhombencephalosynapsis.jpg|220px|thumb|center|'''Figure 28:''' Partial Rhombencephalosynapsis with fused upper parts of the cerebellum<ref name=”PMIDPMC3447431”><pubmed>PMC3447431</pubmed></ref>]]</center> | ||
|- | |||
|<center>'''Medulloblastoma'''</center> | |||
|Medulloblastoma is a type of paediatric cancer of the cerebellum that occurs due to an over-proliferation of granule cell precursors in the External Germinal Layer. These frequently occur from an activation in SHH and Wnt pathways, disrupting the normal transient proliferation in the EGL. Therapies that promote differentiation of granule cells from the granule cell precursors in the EGL such as BMPs have shown to mitigate the disease <ref name="PMID25336734"/>. Symptoms include headaches, nausea, vomit, tiredness, tilting the head to one side, difficulty in walking and balancing and problems with other motor skills. However these symptoms vary from patient to patient <ref>Medulloblastoma. (2017). Retrieved October 26, 2017, from https://www.mdanderson.org/cancer-types/medulloblastoma.html</ref>. | |||
|<center>[[File:Medulloblastoma.jpg|220px|thumb|center|'''Figure 29:''' Brain magnetic resonance imaging of pediatric medulloblastomas: ( a) sagittal post-gadolinium WNT tumor; ( b) axial T2 of a SHH tumor. Red arrows delineate the tumor/leptomeningeal disease.<ref name=”PMIDPMC5490254”><pubmed>PMC5490254</pubmed></ref>]]</center> | |||
|} | |} | ||
=Current Research= | =Current Research= | ||
==Emotional memory in depression== | ==Emotional memory in depression== | ||
[[File:Screen Shot 2017-10-26 at 10.20.18 am.png|thumb|'''Figure | [[File:Screen Shot 2017-10-26 at 10.20.18 am.png|thumb|'''Figure 30:''' In this image you can see differences between the two groups of gray matter density in the cerebellar subregions. Cool color: decreased gray matter density in depression; Warm color: increased gray matter density in depression. <ref name=”PMIDPMC5516611”><pubmed>PMC5516611</pubmed></ref>]] | ||
Past investigations have concluded that damage to the posterior lobule of the cerebellum can cause individuals to show changes in manner or emotional instability, similar to a degree of depression or psychosis, without an outward cerebellar motor syndrome<ref name="PMID16434422"><pubmed>16434422</pubmed></ref>. This is highly suggestive of the cerebellum's role in emotional memory despite its involvement with motor control. Patients with Major Depressive Disorder (MDD) display a tendency to only selectively recall aspects of scenarios that match their moods, conforming with the "mood-congruent memory (MCM)" theory <ref>Gilligan S. G., & Bower G. H. (1983). Reminding and mood‐congruent memory. Bulletin of the Psychonomic Society, 21, 431–434)</ref>. This study was undertaken with that principle in mind, and investigated the depth of cerebellar involvement in emotional memory in depression. A link between the volume and density of cerebellar gray matter with measurements of emotional memory was hypothesised. | Past investigations have concluded that damage to the posterior lobule of the cerebellum can cause individuals to show changes in manner or emotional instability, similar to a degree of depression or psychosis, without an outward cerebellar motor syndrome<ref name="PMID16434422"><pubmed>16434422</pubmed></ref>. This is highly suggestive of the cerebellum's role in emotional memory despite its involvement with motor control. Patients with Major Depressive Disorder (MDD) display a tendency to only selectively recall aspects of scenarios that match their moods, conforming with the "mood-congruent memory (MCM)" theory <ref>Gilligan S. G., & Bower G. H. (1983). Reminding and mood‐congruent memory. Bulletin of the Psychonomic Society, 21, 431–434)</ref>. This study was undertaken with that principle in mind, and investigated the depth of cerebellar involvement in emotional memory in depression. A link between the volume and density of cerebellar gray matter with measurements of emotional memory was hypothesised. | ||
| Line 319: | Line 308: | ||
==Dystonia== | ==Dystonia== | ||
[[File:Cerebellum Dystonia.png|thumb|'''Figure | [[File:Cerebellum Dystonia.png|thumb|'''Figure 31:''' Direct comparison of dystonia scores for animals with torsinA knockdown in the cerebellum alone (TorsinA CB, N = 20) compared with mice with knockdown in the cerebellum and basal ganglia (TorsinA CB+BG, N = 3) at three representative time points; 3 weeks, 11 weeks , and 13 weeks post-injection. There wasn't a significant difference in dystonia score between mice with torsinA knockdown in the cerebellum alone compared to those with knockdown in the cerebellum and basal ganglia <ref name=”PMIDPMC5340526”><pubmed>PMC5340526</pubmed></ref>]] | ||
This investigation into a potential link between dystonia -a disorder where muscles contract involuntarily- and the cerebellum attempts to delineate its pathophysiological role in the disease <ref><pubmed>5429509</pubmed></ref> . The main concern is that the etiology of dystonia appears to be extremely varied, and as such, unpredictable. There is no significant neural degeneration, but in secondary cases there may be structural lesions present in tissue which could be areas of pathophysiology in dystonia <ref><pubmed>27173653</pubmed></ref>. Dystonia may manifest itself in almost any body part, indicating that the neural area responsible most likely must not be very specific. This in combination with the involuntary nature of dystonia seems to indicate that the cerebellum is the more than likely involved in the disease. | This investigation into a potential link between dystonia -a disorder where muscles contract involuntarily- and the cerebellum attempts to delineate its pathophysiological role in the disease <ref><pubmed>5429509</pubmed></ref> . The main concern is that the etiology of dystonia appears to be extremely varied, and as such, unpredictable. There is no significant neural degeneration, but in secondary cases there may be structural lesions present in tissue which could be areas of pathophysiology in dystonia <ref><pubmed>27173653</pubmed></ref>. Dystonia may manifest itself in almost any body part, indicating that the neural area responsible most likely must not be very specific. This in combination with the involuntary nature of dystonia seems to indicate that the cerebellum is the more than likely involved in the disease. | ||
| Line 325: | Line 314: | ||
==Adaptation to Delayed Action Effects== | ==Adaptation to Delayed Action Effects== | ||
[[File:Adaptation Cerebellum.png|thumb|'''Figure | [[File:Adaptation Cerebellum.png|thumb|'''Figure 32:''' In real stimulation, a considerable increase in pre-stimulus low-frequency activity is only found in the right cerebellar hemisphere (near to the stimulation site). But in sham stimulation, pre-stimulus low-frequency activity increases in both the right cerebellar hemisphere and cerebellar vermis.<ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>.]] | ||
Sensory attenuation refers to when individuals filters unnecessary information. When there is a perturbation between actions and the following-sound, the sensory attenuation is reduced <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. | Sensory attenuation refers to when individuals filters unnecessary information. When there is a perturbation between actions and the following-sound, the sensory attenuation is reduced <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. | ||
An example of | An example of perturbation is when there is a delay when an individual presses a button with sound immediately expected, but instead the sound is delayed. Sensory attenuation can be further described by the forward model theory. The forward model theory suggests that predictions for sensory consequences (for example, sound that is heard) are made simultaneously with the motor movement<ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. Predictions and real sensory input (what really happens), after the sound delay is conducted, are compared in the model. If the prediction and real sensory input do not matchup, sensory attenuation is observed. The prediction errors are then relayed to other brain areas for further processing<ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. | ||
The process of sensory attenuation usually re-emerges after the disruption to the normal mechanism <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. The re-emerging of this is a process of either correcting previous predictions or updating initial forward models to minimise prediction errors. The cerebellum is currently being researched on how it plays an important role in updating the forward model within the brain<ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. One investigation seeks to find evidence on the involvement of the cerebellum in learning to predict the unexpected delays. This investigation has found that low-frequency activity in the cerebellum, prior to the stimulation, plays a key role in adapting to the delayed stimulus <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. | The process of sensory attenuation usually re-emerges after the disruption to the normal mechanism <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. The re-emerging of this is a process of either correcting previous predictions or updating initial forward models to minimise prediction errors. The cerebellum is currently being researched on how it plays an important role in updating the forward model within the brain<ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. One investigation seeks to find evidence on the involvement of the cerebellum in learning to predict the unexpected delays. This investigation has found that low-frequency activity in the cerebellum, prior to the stimulation, plays a key role in adapting to the delayed stimulus <ref name=”PMIDPMC5571438”><pubmed>PMC5571438</pubmed></ref>. | ||
==Addiction== | ==Addiction== | ||
[[File:Addiction Cerebellum.png|thumb|'''Figure | [[File:Addiction Cerebellum.png|thumb|'''Figure 33:''' Nissl staining showing the dopamine receptor expression within the cerebellum of a songbird <ref name=”PMIDPMC2904815”><pubmed>PMC2904815</pubmed></ref>]] | ||
The cerebellum has only recently been suspected to be involved in addictive behaviour, as some arguments provided for the initiation of research into the cerebellar role on addiction<ref><pubmed>26602022</pubmed></ref>. The cerebellum is shown to be intrinsically linked with dopamine release and reception<ref><pubmed>16451810</pubmed></ref>; with dopamine being the main hormone on which addiction is predicated, it is likely that the cerebellum could influence the response to the addictive stimuli. It has also been established that addictive drugs cause specific molecular mechanisms, changes in synapse plasticity<ref><pubmed>10224304</pubmed></ref>, and influence intracellular transduction pathways as well as gene expression in the cerebellum<ref><pubmed>25262781</pubmed></ref>. The specificity of the drugs in targeting the cerebellum could highlight the link between the affected organ and addictive behaviour. Addictive drugs such as cocaine have been demonstrated to produce a behavioral sensitivity in mice, and an associated change in cerebellar plasticity. The change of the morphology of Purkinje cells and synaptic terminals in the mice most likely contributed to the difference in reception of the drug<ref><pubmed>25619460</pubmed></ref>. However, this link has not yet been fully investigated. | The cerebellum has only recently been suspected to be involved in addictive behaviour, as some arguments provided for the initiation of research into the cerebellar role on addiction<ref><pubmed>26602022</pubmed></ref>. The cerebellum is shown to be intrinsically linked with dopamine release and reception<ref><pubmed>16451810</pubmed></ref>; with dopamine being the main hormone on which addiction is predicated, it is likely that the cerebellum could influence the response to the addictive stimuli. It has also been established that addictive drugs cause specific molecular mechanisms, changes in synapse plasticity<ref><pubmed>10224304</pubmed></ref>, and influence intracellular transduction pathways as well as gene expression in the cerebellum<ref><pubmed>25262781</pubmed></ref>. The specificity of the drugs in targeting the cerebellum could highlight the link between the affected organ and addictive behaviour. Addictive drugs such as cocaine have been demonstrated to produce a behavioral sensitivity in mice, and an associated change in cerebellar plasticity. The change of the morphology of Purkinje cells and synaptic terminals in the mice most likely contributed to the difference in reception of the drug<ref><pubmed>25619460</pubmed></ref>. However, this link has not yet been fully investigated. | ||
| Line 340: | Line 329: | ||
*Does the p53 gene function in cerebellum development? If so, does this function relate to the development of embryonic cancers? <ref name=”PMC4853753”><pubmed> 4853753</pubmed></ref> | *Does the p53 gene function in cerebellum development? If so, does this function relate to the development of embryonic cancers? <ref name=”PMC4853753”><pubmed> 4853753</pubmed></ref> | ||
*What changes in cerebellum development lead to the social impairments seen in those with Autism Spectrum Disorder?<ref><pubmed>28150911</pubmed></ref> | *What changes in cerebellum development lead to the social impairments seen in those with Autism Spectrum Disorder?<ref><pubmed>28150911</pubmed></ref> | ||
*Do we need the cerebellum to actually function and survive? | |||
=Terms= | =Terms= | ||
* | *'''ApoER2''' – Works with VLDLR and is a very important receptor in the brain affecting neuronal function and development. | ||
* '''Basket cells'''– multipolar GABAergic interneurons. They are located on different regions of the brain and cerebellum. They function in making inhibitory synapses and control the overall potential of target cells. | * '''Basket cells'''– multipolar GABAergic interneurons. They are located on different regions of the brain and cerebellum. They function in making inhibitory synapses and control the overall potential of target cells. | ||
* '''Bergmann Glia ''' – they are a type of radial astrocyte, cell bodies are in the Purkinje cell layer of the cerebellum. | * '''Bergmann Glia ''' – they are a type of radial astrocyte, cell bodies are in the Purkinje cell layer of the cerebellum. | ||
| Line 350: | Line 340: | ||
* '''Deep nuclei cells''' – Purkinje cells project to the deep nuclei cells and these are the only form of output cell in the cerebellar cortex. They also receive excitatory inputs from the mossy and climbing fibers. | * '''Deep nuclei cells''' – Purkinje cells project to the deep nuclei cells and these are the only form of output cell in the cerebellar cortex. They also receive excitatory inputs from the mossy and climbing fibers. | ||
* '''Dendrites ''' – an extension of a nerve cell, impulses travel along these extensions and they synapse at another cell body. | * '''Dendrites ''' – an extension of a nerve cell, impulses travel along these extensions and they synapse at another cell body. | ||
* | *'''Dopamine''' – neurotransmitter and a precursor of substances including adrenaline. | ||
* | *'''Eurydendoid cells''' – cerebellar efferent neurons. | ||
* '''Flocculonodular lobe ''' – lobe of the cerebellum that consists of a nodule and the flocculus. It is located on the anteroinferior surface of the cerebellum. | * '''Flocculonodular lobe ''' – lobe of the cerebellum that consists of a nodule and the flocculus. It is located on the anteroinferior surface of the cerebellum. | ||
* '''Glia cells ''' – They are nonneuronal cells and they support cells and they surround neurons and insulate them, there are many types of glia cells: oligodendrocytes, ependymal cells, astrocytes, Schwann cells etc. | * '''Glia cells ''' – They are nonneuronal cells and they support cells and they surround neurons and insulate them, there are many types of glia cells: oligodendrocytes, ependymal cells, astrocytes, Schwann cells etc. | ||
* '''Granule cells ''' – are small neurons, and they are the most numerous in the brain. They are packed in a thick layer of the cerebellar cortex and emit only 4 or 5 dendrites. They receive all input from mossy fibres. | * '''Granule cells ''' – are small neurons, and they are the most numerous in the brain. They are packed in a thick layer of the cerebellar cortex and emit only 4 or 5 dendrites. They receive all input from mossy fibres. | ||
* | *'''Hoxa2''' – a gene that encodes for a transcription factor which could be involved in the development of the positioning of the hindbrain. | ||
* '''Mossy fibres ''' – major input of the cerebellum, relays sensory information from the pons to the granule cells and then sent to the purkinje cells for processing. | * '''Mossy fibres ''' – major input of the cerebellum, relays sensory information from the pons to the granule cells and then sent to the purkinje cells for processing. | ||
* '''Neural tube ''' – a hollow prenatal structure that forms the brain and spinal cord. Formed by the folding and fusion of the opposite ectodermal folds. | * '''Neural tube ''' – a hollow prenatal structure that forms the brain and spinal cord. Formed by the folding and fusion of the opposite ectodermal folds. | ||
* | *'''Otx2''' – a protein that is involved with defining the layers and regions of the cerebral cortex and cerebellum. | ||
*'''Perturbation''' – (In the context of the Current Research) alternation to the normal mechanism that would happen in response to a stimulation | *'''Perturbation''' – (In the context of the Current Research) alternation to the normal mechanism that would happen in response to a stimulation | ||
* | *'''Plasticity''' – the brains ability to change at any age. | ||
* '''Pons ''' – a part of the brain that links the medulla oblongata and the thalamus. | * '''Pons ''' – a part of the brain that links the medulla oblongata and the thalamus. | ||
* '''Purkinje cells ''' – a class of GABAnergic neurons that are located in the cortex of the cerebellum, they are large, branched cells. | * '''Purkinje cells ''' – a class of GABAnergic neurons that are located in the cortex of the cerebellum, they are large, branched cells. | ||
* '''Stellate cells ''' – in the cerebellum, they are located in the molecular layer and they synapse onto purkinje cells. | * '''Stellate cells ''' – in the cerebellum, they are located in the molecular layer and they synapse onto purkinje cells. | ||
* | *'''Tentorium''' – fold of the dura mater forming division between cerebrum and cerebellum. | ||
*'''VLDLR''' – Very-low-density-lipoprotein receptor, it’s a transmembrane lipoprotein receptor | *'''VLDLR''' – Very-low-density-lipoprotein receptor, it’s a transmembrane lipoprotein receptor | ||
* '''Vermis ''' – rounded and elongated section of the central part of cerebellum, lies between the two hemispheres. | * '''Vermis ''' – rounded and elongated section of the central part of cerebellum, lies between the two hemispheres. | ||
Latest revision as of 14:30, 16 July 2018
| 2017 Student Projects | |||
|---|---|---|---|
|
Cerebellum
Introduction
The cerebellum is a large portion of the brain that functions in coordination, balance and control, and its development occurs both prenatally and postnatally. The cerebellum underlies the occipital and temporal lobes of the cerebral cortex and constitutes to about 10% of the brain's weight but contains around 50% of the neurons. This page will highlight the anatomy of the cerebellum, its developmental process, current research on the structure, animal models and abnormalities associated with it. The anatomy will discuss the distinguishable lobes and zones of the cerebellum. The anatomy of the cerebellum cannot be completely discussed without explaining the vasculature of the structure and hence this page will provide a brief overview of it. The development of the cerebellum discusses the neural development and where the cerebellum forms on the neural tube. The development will highlight how the circuitry of the post-natal cerebellum came to be from neurons. Hence purkinje cells, granule cells, deep nuclei cells, glia cells and cerebellar nuclei will be highlighted to discuss the developmental process. A developmental timeline of the formation of the cerebellum is also included on this page. The cerebellum is a topic of continuous research and past findings would not have been done without the use of animal models, hence key historical discoveries, current research and animal models will be discussed. Towards the end of the page there are future questions listed on future investigations involving the cerebellum and the abnormalities from an affected cerebellum are also highlighted. Terms that may be difficult to understand have also been identified and defined.
The following video provides a brief overview on the cerebellum which will be further discussed on this page: <html5media height="315" width="560">https://www.youtube.com/watch?v=Fir-v6EoZNE</html5media> [1]
Basic Anatomy of the Cerebellum
The cerebellum has 3 distinguishable lobes; flocculonodular lobe, anterior lobe and the posterior lobe. The anterior and posterior lobe can be further divided in a midline cerebellar vermis and lateral cerebellar hemispheres (Figure 1) [2]. In a superior cerebellar view, the cerebellum contains a vermis that runs through the middle of the organ and 2 intermediate zones located laterally from the vermis (Figure 2).
Figure 1: Anatomical lobes observed in the cerebellum; anterior lobe, posterior lobe and flocculonodular lobe, which is divided by two fissures – the primary fissure and posterolateral fissure [3]
Figure 2: Superior view of the 3 cerebellar zones. The middle is the vermis. Either side of the vermis is the intermediate zone. Lateral to the intermediate zone is the lateral hemispheres. There is no difference in gross structure between the lateral hemispheres and intermediate zones. [4]
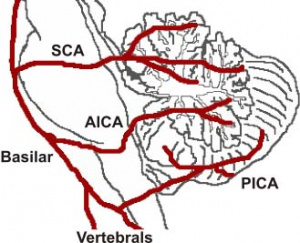
Vasculature
The cerebellum contains 3 bilateral paired arteries which supplies this organ with oxygenated blood. These arteries all originate from the vertebrobasilar system: Superior Cerebellar Artery (SCA), Anterior Inferior Cerebellar Artery (AICA) and Posterior inferior cerebellar artery (PICA). The SCA and AICA are branches of the basilar artery, which wraps around the anterior aspect of the pons before reaching the cerebellum. The PICA arises from the left and right vertebral artery, which form the basilar artery [5]. The PICA and AICA combine to supply the inferior half of the cerebellum, while the SCA supplies the majority of the superior half. The PICA and SCA combine to supply the vermis[6]. Blood is then drained by superior and inferior cerebellar veins into the superior petrosal and then straight dural venous sinuses. (Figure 3)[7]
Microanatomy
Cortical Layers
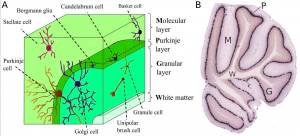
There are 3 major cortical layers of the cerebellum: the molecular layer, the purkinje cell layer, and the granule cell layer. The molecular layer contains basket cells, stellate cells and the purkinje cell and Golgi cell dendrites. The purkinje cell layer contains purkinje cell bodies and Bergmann glia. The granule cell layer contains granule cells, mossy fibers, and Golgi cell bodies. [9]
Purkinje/Pyramidal Cells
Discovered by Jan Evangelista Purkinje in 1837, purkinje cells are inhibitory neurons found in the outside layer of the cerebellum. They receive signals from the granule cell parallel fibers and the superior olive and send inhibitory signals to the deep nuclei in the white matter region via GABA signaling. Purkinje cells have a large branching network of dendrites which allows them to be identified by their morphology.
Granule Cells
Named for their small cell body, cerebellar granule cells of were discovered by Camillo Golgi and Ramon y Cajal in 1899. Cerebellar granule cells are the most numerous cell type in the human brain. They receive signals from mossy fibers of the pons and synapse on the fast network of dendrites of the pyramidal cells. Cerebellar granule cells are glutamatergic and the only excitatory neurons found in the cerebellum. [10]
Deep Nuclei
There are four different deep nuclei of the cerebellum: the dentate, interpositus, fastigial, and vestibular nuclei. The dentate nucleus receives signals from the lateral purkinje cells, the interpositus nucleus receives signals from the intermediate purkinje cells, the fastigial nucleus receives signals from the medial purkinje cells, and the vestibular nucleus receives signals from the flocculonodular purkinje cells. The deep nuclei integrate the inhibitory signals from the purkinje cells and the excitatory signals from the mossy and climbing fibers to determine their output signals. [11] The dentate nucleus in particular is thought to be implicated in higher level cognitive processing. It is enlarged in primates and humans and not observed in non-mammalian species.[12]
Cerebellar Nuclei
| Most medially located of the cerebellar nuclei. | Receives input from the vermis and cerebellar afferents that carry vestibular, proximal somatosensory, auditory and visual information. | |
| Consists of emboliform nucleus and globose nucleus. Interposed nuclei are situated laterally with respect to the fastigial nucleus. | Receives input from intermediate zone and cerebellar afferents that carry spinal, proximal somatosensory, auditory and visual information. | |
| Largest of the cerebellar nuclei. Lateral to interposed nuclei. | Receives input from lateral hemisphere and cerebellar afferents that carry information from cerebral cortex. | |
| Located outside cerebellum in the medulla. | Considered to be cerebellar nuclei as their connectivity patterns are identical to those of cerebellar nuclei. Receive input from flocculonodular lobe and from the vestibular labyrinth. | |
| Links: cerebellum |
Bergmann Glia
Glial cells of the cerebellum were described by Ramon y Cajal in 1911. He divided them into 3 main categories: the glia of the white matter, the astrocytes of the granule cell layer, and the Bergmann glia of the Purkinje cell layer. Also known as Goligi epithelial cells, Bergmann glia are unipolar astrocytes that have cell bodies located in the Purkinje cell layer and long processes projecting into the molecular layer. The Bergmann glia's processes interact with the dendrites of Purkinje cells at synapses with parallel and climbing fibers. Bergmann first characterized the long processes of cells he saw in the cerebellum of cats, dogs, and humans in 1857. Ramon y Cajal later described these cells as "epithelial cells with Bergmann fibers," giving the glia their name.[13]
Cerebellum Development
Ectoderm
There are 3 germ layers present in the early embryo; ectoderm (most distal layer), mesoderm (middle layer) and endoderm (most proximal layer). The ectoderm differentiates into the nervous system, forming the spine, peripheral nerves, cerebrum and cerebellum. It also differentiates to form tooth enamel, epidermis, and the linings of the mouth, anus, sweat glands and nostrils.[14]

Neural Development
Neural development is one of the earliest systems to begin and the last to be completed after birth due to its highly complex structure. The first step in neural development occurs at the end of week 3 and involves the neural groove fusing to form the neural tube, which then folds to form the cranial and caudal region of the embryo, and ultimately form the cerebellum. There is a high chance of neural dysfunction and defects during the fetal neural development particularly due to the long development time frame and the need of certain nutrients such as folic acid to successfully close the tube. Neural tube defects (NTDs) such as spina bifida and anencephaly can arise if the tube does not close effectively.
Early Brain Vesicles
Primary Brain Vesicles
Figure 6: (Week 4) 3 primary brain vesicles are formed; forebrain (prosencephalon) midbrain (mesencephalon), and hindbrain (rhombencephalon)[16]
Secondary
Figure 7: (Week 5) 3 primary vesicles develop into 5 secondary vesicles [17];
- Prosencephalon develops into telencephalon (which includes the endbrain and cerebral hemispheres) and diencephalon (located between the brain and forms an optic outgrowth)
- Mesencephalon does not further develop into a secondary brain vesicle
- Rhombencephalon develops into the metencephalon (behind the brain), and myelencephalon (contains medulla)
Metencephalon
The metencephalon refers to the embryonic neural structure that eventually gives rise dorsally, to the cerebellum and ventrally, to the pons. The metencephalon is the anterior part of the rhombencephalon (hindbrain) and differentiates from the posterior part of the rhombencephalon (myencephalon) at week 5 of development.
The dorsal surface is characterised by its highly folded folia separated by grooves termed sulci. The median area is referred to as the vermis, which eventually becomes the most superior aspect of the cerebellum. [18] The first structure that belies the future cerebellum are the rhombic lips that appear on the metencephalon of a 5-6 week old embryo. The rhombic lips are aptly rhombus-shaped and denote the perimeter between the roof plate and the main body of the rhombencephalon. The anterior pair of lips mark the site at which the cerebellum will develop. [19]
Cerebellum Developmental Weeks
First Trimester
 Figure 8: Early stage of neurulation[20]  Figure 9: Late stages of neurulation[21] | ||
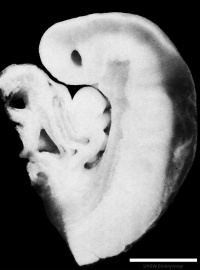 Figure 10: Sagittal view of superior end of embryo [22] | ||
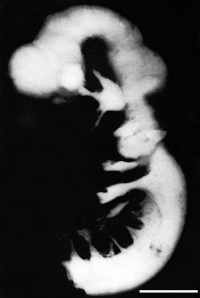 Figure 11: Lateral view of embryo central nervous system at 5 weeks [23] | ||
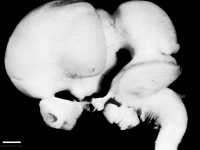 Figure 12: Left lateral view of embryonic CNS[26]  Figure 13: Left medial view of lateral embryonic CNS [27] |
Second Trimester
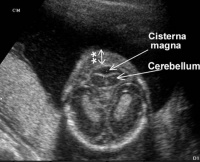
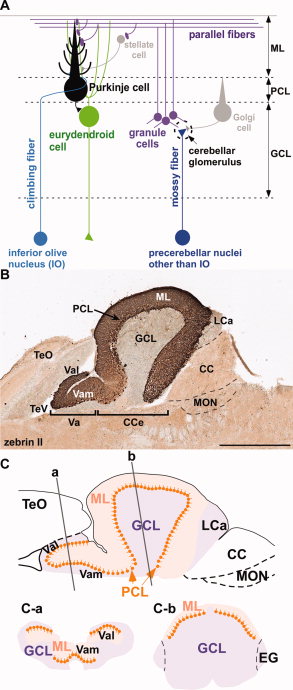
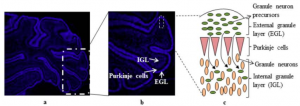
Overview of Development
As the neural tube folds, the anterior portion develops the three brain vesicles:
- Prosencephalon
- Mesencephalon
- Rhombencephalon
The rhombencephalon then further divides into the mesencephalic and myelincephalic vesicles on embryonic day 9. The neural tube failure to close then creates a gap along the dorsal sides and this produces a mouth-like structure as the tube bends to establish the pontine flexure. The pontine flexure further deepens bringing the mesencephalon (midbrain) closer to the primordium of the cerebellum (metencephalon); anterior aspects of the myelincephalon (brain stem) fold underneath developing the cerebellum plate[31]
The cerebellar territory is defined by the expression of Hoxa2 genes (from posterior) and Otx2 (from anterior) genes and these genes are controlled by proteins Wnt and fibroblast growth factor families which regulate the expression of these genes in order to establish the cerebellar territory[32]. Further development of the cerebellum begins between days 40 and 45 and it arises mostly from the metencephalon however the rhombic lips also contributes. The roof plate which is derived from the dorsal part of the alar plate thickens during development to become the cerebellum. The regulation of patterning involved when the primary fissure deepens by the end of the third month and thus divides the vermis, shows to be particularly important for development. The two lateral bulges are separated into the cranial anterior lob and caudal middle lobe. As the lobes divide further into lobules, fissures are formed and this continues throughout embryonic, fetal and postnatal life, thus increasing the surface area of the cerebellar cortex. The most primitive part of the cerebellum to form is the flocculonodular lobe, which is derived from separation of the first transverse fissure and this functions to keep connections with the vestibular system and it is also concerned with subconsciously controlling equilibrium. The flocculonodular lobe is separated from another crucial part of the cerebellum, corpus cerebelli, by the posterolateral fissure.
Overview of Cerebellar Cell Development
The cerebellum is connected to the brain stem via three pairs of peduncles and this allows the afferent and efferent pathways to enter and exit the cerebellum. Cerebellum afferent fibers can be grouped into two major types: mossy fibers and climbing fibres. Mossy fibres contribute to most of the afferent fibres in the cerebellum and they communicate with the cerebellar nuclei neurons and with Purkinje cells through granule cells embryonically, however postnatally they displace from Purkinje cells and synapse with their adult targets, the granule cell dendrites. Whilst mossy fibres originate from numerous sites in the nervous system, climbing fibers originate exclusively from the inferior olivary nucleus. Climbing fibers directly synapse with the cerebellar nuclei and Purkinje cells, relaying information to the cerebellum from several regions. The direction of these afferent fibres to their target neurons early in development are controlled by genes and molecules[33].
The cerebellum has a very basic structure consisting 2 principal classes of neurons and 3 layers; layers are shown in Figure 15.
- Molecular Layer: consists of excitatory granular cell axons, purkinje cell dendritic fibres. stellate and basket cells.
- Purkinje Cell Layer: consists of a single layer of inhibitory Purkinje cells.
- Granular Cell Layer: Dense layer of excitatory granule cells, golgi cells and unipolar brush cells.
The granule cells receive inputs from outside the cerebellum and project these inputs to purkinje cells where the majority of these inputs are further projected to a variety of cerebellar nuclei in the white matter [34]. Within the layers there are some other main neuronal cell types, the stellate and basket cells are located in the molecular layer, whilst granule, golgi and unipolar brush cells are located in the granular layer [33]. Among these principal neurons, there is a diverse set of interneurons which are responsible for coordinating the output of the Purkinje cells to the cerebellar nuclei.
The nuclei from the cerebellum are formed by a complex process of neurogenesis and neuronal migration. The dorsomedial ventricular zone of the fourth ventricle gives rise to the principal neuronal output, the Purkinje cell and other neurons within the cerebellum The secondary germinal zone, coming from the adjacent rhombic lip generates the cerebellar granule cells as well as a subpopulation of neurons of cerebellar nuclei and several precerebellar nuclei [35]. Granule cells function in coordinating afferent input to and motor output from the cerebellum through excitatory connections with the Purkinje cell [32]. There are two types of grey matter in the cerebellum, the deep cerebellar nuclei and an external cerebellar cortex. There are 4 deep nuclei formed and the output of the cerebellar cortex are relayed through these nuclei, the ventricular layer produces 4 types of neurons that migrate to the cortex. Proper cerebellum function requires well-organised neuronal connections and the integration of afferent and efferent fibres throughout the cerebellar circuit [33]. The cerebellum functions in sensorimotor, balance control and vestibular ocular reflex, however recent studies have come out and shown that the cerebellum has a wide range of cognitive functions which include speech, memory and cognitive functions.[32]
Cellular Migration
Granule Cells
Granule cells migrate tangentially from the rhombic lip to form the transient structure, the external germinal layer (EGL). The EGL consists of an outer and inner layer that is sandwiched between the pia mater and the purkinje cell layer. During this tangential migration, granule cells extend two horizontal processes. Granule cells move from the outer layer of the EGL to differentiate in the inner layer of the EGL. Differentiated cells continue to move radially to the granule cell layer on the inner surface of the purkinje cell layer.[34] During radial migration, granule cells extend vertical processes and move through the purkinje cell layer on the radial processes of Bergmann glia.[36]
Purkinje Cells
Purkinje cells originate from the ventricular neuroepithelium and migrate radially towards the pial surface. Many studies have shown that purkinje cells use radial glia processes as a scaffold in this migration. The movement results in a 3 to 4 cell thick layer of purkinje cells beneath the EGL. This layer becomes 1 cell thick 1 week after birth.[37]
Figure 17: Granule cell (yellow) and purkinje cell (green) migration timetable in mouse cerebellum. Top shows sagittal sections of entire cerebellum. Bottom shows movement of individual cells at interface of EGL and purkinje cell layer.[38]
Cell Signalling in Cerebellar Development
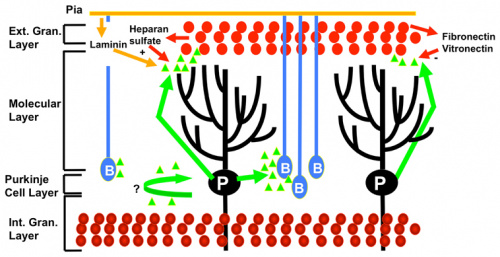
Proliferation in the EGL
Many different cell signalling pathways are involved with cell migration, proliferation, and differentiation in the cerebellum. Sonic hedgehog (SHH) is particularly important in the proliferation of granule cells in the external germinal layer. The external germinal layer (EGL) is the area of transit amplification of granule cell precursors. This layer is transient and lies on the external surface of the cerebellum until the cells differentiate and migrate radially to their final destination in the internal granule cell layer. SHH is secreted by the purkinje cells and acts locally, causing the granule cell precursors to undergo mitosis and Bergmann glia to differentiate. The autocrine function of SHH on the purkinje cells is unknown. [40] Proliferating cells in the EGL must also be positive for Atoh1, a transcription factor that represses differentiation and promotes division via SHH signalling. In a similar manner to SHH and Atoh1, the pia mater secretes Sdf1 which interacts with the receptor Cxcr4 to maintain proliferation in the EGL. Notch2 signalling and its stimulation of Atoh1 and repression of Bone Morphogenetic Protein (BMP).
Exit from the EGL
BMPs are involved in arresting granule cell proliferation in the EGL and stimulating differentiation.[41] Cells exit proliferation and move to the inner EGL where they differentiate upon expression of Neurod1 and interaction with the extracellular matrix components of the inner EGL, vitronectin, F3, and contactin.[34] Granule cell migration is stimulated by D-serine secreted by the Bergmann glia. This can be inhibited by DAAO or SR.[36]
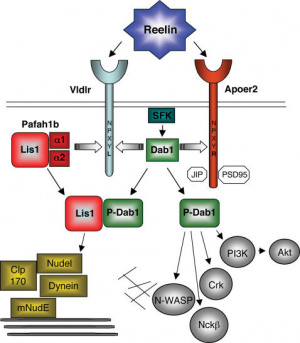
FGF and the Isthmus
Fibroblast Growth Factor (FGF) plays a role in allocating the isthmus area of the cerebellum. FGF8 is found in high concentrations in the rostral region of rhombomere 1 and decreased concentrations near the caudal region of rhombomere 1. It is necessary for the formation of the vermis of the cerebellum but the cerebellar hemispheres form independently of FGF8.[34]
Reelin and purkinje cells
Reelin is an important signalling molecule that plays a role in many different in the developing brain. Reelin is a glycoprotein secreted by cells in the EGL and rhombic lip and affects purkinje cells through a signal transduction pathway involving receptors VLDLR and ApoER2, adaptor protein Dab1, and many other further downstream intracellular signalling molecules. Reelin has shown to play a role in formation of the monolayer of purkinje cells and detachment of purkinje cells from radial glia. It may also affect other cell morphology and migration in the cerebellum.[43]
Transcription factors
The region of rhombomere 1, a segment of the metencephalon that forms the cerebellum, is bound by the expression of the transcription factors Hoxa2 and Otx2. Otx2 is expressed in the midbrain and the lack of Otx2 marks the rostral border of the cerebellar primordium. Hoxa2 is expressed in the hindbrain region and lack of Hoxa2 marks the caudal border of the cerebellar primordium.[41]
Key Historical Discoveries
| Described the macroscopic anatomy of the cerebellum | ||
 Figure 20:Ferrier's findings on a monkey's cerebellum with stimulation points discovered[44]
| ||
| First defined "plasticity" as we know it in neuroscience, and also discovered the specific cells in the cerebellum that are named after him. He also furthered research into the functions of glia and defined "nervous conduction and transmission" in its current meaning.[46] | ||
| Further defined the exact nature of the effect of cerebellar lesions on motor control, and found loss of coordination in antagonistic muscles and also basic loss of muscle control. It was also found that the side of the cerebellum the lesion developed on, affected the same side of the body.[45] | ||
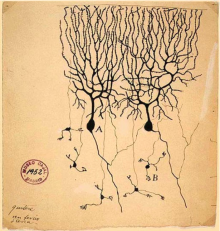 Figure 21: Drawing of Purkinje cells (A) and granule cells (B) from pigeon cerebellum. Drawn by Santiago Ramón y Cajal, 1899. [47]
Refuted Camillo Golgi's previous assertion that axons and dendrites would fuse, and also delineated the differing types of cells, most importantly of which were the mossy and climbing fibers of the cerebellum. However, this contribution could not be fully utilised until further technology was developed. | ||
| Developing a standard nomenclature for cerebellar anatomy that eventually spread world-wide, and for conducting a series of studies that illuminated our understanding of the rhombencephalon. [48] | ||
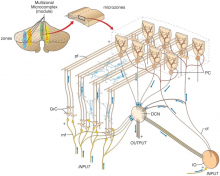 Figure 22: Modular organisation of the cerebellum purkinje fibers by Janos Szentágothai[49] Pieced together the first complete map of the functional anatomy of the cerebellum and define the excitatory and inhibitory nature of each cell type provided by Cajal. Further discoveries into the relationships between cell synapses, cell natures, and the minutiae of their structures by Jan Voogd, Olov Oscarsson and David Armstrong around the 1970's defined the organisation of Purkinje cells into "a series of longitudinal parasagittal bands", the specificity of which explains the solely Purkinje-axon-output of the cerebellar cortex.[50] The current focus in research now leans towards connecting cerebellar function with learning, emotion and perception of time. |
Animal Studies
Animal Models
The mouse model is a primary model organism in the study of cerebellum development, but organisms such as Drosophila (fruit fly), C. elegans (roundworm), Saccharomyces cerevisiae (Baker's yeast), cats, dogs, and cattle are also used in some types of diseases.[51] Looking at a variety of different of organisms' cerebellar development can elucidate the evolution of the cerebellum and further understanding of cerebellar constituents.[34]
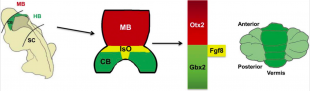
Isthmic Organiser
Throughout history there have been many investigations on the cerebellum and how it has developed through research involving chicken embryos and mice. As previously mentioned the neural plate closes to form the neural tubes which have anterior-posterior (AP) and dorsal-ventral (DV) axes. Earlier experiments involving chick-quail chimera suggested that the cerebellum was derived from both midbrain and hindbrain. However, through successive gene expression and fate mapping studies, it was discovered that the anterior-most rhombomere of the hindbrain is where the cerebellum is formed from [53]. Once the axes are formed the isthmic organiser (IsO) is formed which plays a vital role in establishing the anterior limit of the cerebellar territory. The IsO in other words is the mid-hindbrain boundary [54]. However, the IsO does not position itself without the help of transcription factors. Studies of mouse and chicken embryos have shown that two homeo domain-containing transcription factors Otx2 and Gbx2 have an important role in positioning the isthmic organiser [54]. Surgical movement of the isthmic tissue to more anterior or posterior regions of the neural tube of 10-somite stage chick embryos led to ectopic midbrain and cerebellar structures[53], indicating that where the IsO is placed is an important factor in establishing where the cerebellum positions.
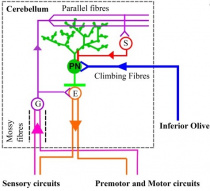
Cell Structures
The cellular structures in the cerebellum (including Purkinje cells and granule cells) have been investigated in various animal models. One animal model is the zebra fish which is a bony fish (teleosts). Like mammals, the zebrafish cerebellum contains several types of neurons which function as either excitatory or inhibitory neurons [54]. Glutamate is utilised by excitatory neurons as their major neurotransmitter. Excitatory neurons include granule cells, unipolar brush cells and eurydendroid cells. The eurydendoid cells are predicted to be corresponding to the deep cerebellar neurons in mammals.[54] The inhibitory neurons use y-aminobutric acid (GABA) and/or glycine (also known as GABAergic neurons) for neurotransmission. Purkinje cells, Golgi and stellate cells are inhibitory neurons. However, a difference between mammal and teleosts is the lack of basket cells. Basket cells are GABAergic neurons that contribute project their axons to the Purkinje cells [54]. This is important, because as earlier highlighted Purkinje cells (inhibitory neurons) and granule cells (excitatory cells) contribute to the three cortical layers of the cerebellum. This model is important for understanding what structures contribute to the neurotransmission process in the cerebellum.
Abnormalities
Abnormalities found within the posterior fossa of the cranium may affect the functioning and development of the cerebellum. The abnormalities affecting the cerebellum include Dandy-Walker-Malformation, Joubert Syndrome, Tecto-Cerebllar Dysraphism, Rhombencephalosynapsis and Medulloblastoma which will be explained in the table below.
| Dandy-Walker-Malformation (DWM) includes the incomplete development of the cerebellar vermis with cystic dilatation of the 4th ventricle and enlargement of the posterior fossa [56]. The cerebellar vermis (found at the medial, cortico-nuclear zone of the cerebellum[57]) may either be elevated or rotated upwards [56]. The communication of the 4th ventricle with the midline posterior fossa cyst can be seen on MRI scans of DWM [56]. Other structures that can be identified on MRI scans include upward displacement of the tentorium and anterolateral shift of cerebellar hemispheres [58]. The causes of DWM are still being researched and hence there has not been any clear causes. However, some causes include maternal diabetes, chromosomal defects that affect foetal brain development, infections in the mother that pass to the developing foetus and exposure of the unborn baby to certain toxins.[59] Symptoms of DWM include motor and speaking development delays, poor muscle coordination, vision and hearing impairment.[59] | 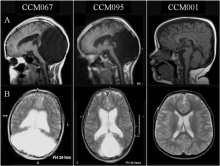 Figure 25: Brain MRIs. Brain imaging of patients with Dandy-Walker malformation (CCM067, CCM095) and patients with normal cerebellum and posterior fossa (CCM001). A: sagittal midline T1-weighted images. B: axial T2-weighted images. [60] | |
| Joubert syndrome (JS) is described as a rare inherited genetic disorder characterised by lack of muscle coordination, intellectual disability, respiratory disturbances and abnormal eye movement [61]. On neuroimaging, the cerebellar vermis is identified to have gone through hypoplasia (underdevelopment) or dysplasia (abnormal development) [56]. It is also described as the "molar tooth sign" on brain imaging, which the "molar tooth" shaped is caused by the defects in the midbrain-hindbrain development. JS is inherited as mutations in any of many genes, however, how mutations lead to JS is still being investigated. Mutations cause problems in the structure and function of cilia and hence signalling pathways may be disrupted during development [62]. | 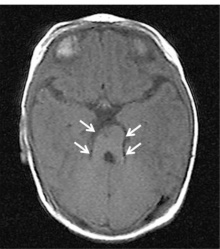 Figure 26: Brain Imaging of Joubert Syndrome: Cranial MRI showing “molar tooth sign” (arrows)[63] | |
| Chiari Syndrome I-III is when the structures within the posterior cranial fossa protrude into the spinal canal [64] which can affect the development and functioning of the cerebellum in the long term. Chiari Syndrome can be classified into four types. The downward shift of the cerebellar tonsils to beneath the foramen magnum is type 1 and the downward movement of the vermis, medulla oblongata and pons with the fourth ventricle is type 2 [64]. When majority of the cerebellum lies in the foramen magnum it is type 3 and when it is completely in the foramen magnum (and therefore cannot develop normally) it is type 4[64]. As a result of genetic mutations or maternal diet lacking vitamins or nutrients, structural defects in the brain and spinal cord can occur during foetal development leading to Chiari Syndrome [65] | 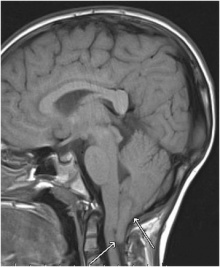 Figure 27: Cerebellar tonsils herniation on magnetic resonance imaging: Chiari malformation type I[66] | |
| Rhombencephalosynapsis is a rare cerebellar defect whereby there is dorsal fusion of the cerebral hemispheres, fusion of dentate nuclei and superior cerebellar peduncles as well as the agenesis of the cerebella vermis [67]. Rhombencephalosynapsis is predicted to result from disturbed cerebellar development at approximately 33–34 days of gestation [68]. The cause of rhombencephalosynapsis is thought to be a genetic defect in the isthmic organizer, resulting in abnormal dorsal patterning, causing the mentioned defects [68]. Like other mentioned abnormalities there is development delay because of the underdeveloped cerebellum. Rhombencephalosynapsis can be identified from a range of symptoms, from involuntary muscle coordination, severe cerebral palsy or mental retardation[69]. | 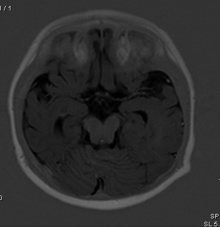 Figure 28: Partial Rhombencephalosynapsis with fused upper parts of the cerebellum[70] | |
| Medulloblastoma is a type of paediatric cancer of the cerebellum that occurs due to an over-proliferation of granule cell precursors in the External Germinal Layer. These frequently occur from an activation in SHH and Wnt pathways, disrupting the normal transient proliferation in the EGL. Therapies that promote differentiation of granule cells from the granule cell precursors in the EGL such as BMPs have shown to mitigate the disease [34]. Symptoms include headaches, nausea, vomit, tiredness, tilting the head to one side, difficulty in walking and balancing and problems with other motor skills. However these symptoms vary from patient to patient [71]. | 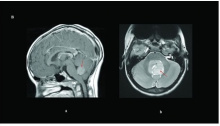 Figure 29: Brain magnetic resonance imaging of pediatric medulloblastomas: ( a) sagittal post-gadolinium WNT tumor; ( b) axial T2 of a SHH tumor. Red arrows delineate the tumor/leptomeningeal disease.[72] |
Current Research
Emotional memory in depression
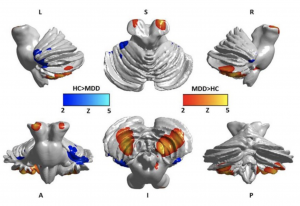
Past investigations have concluded that damage to the posterior lobule of the cerebellum can cause individuals to show changes in manner or emotional instability, similar to a degree of depression or psychosis, without an outward cerebellar motor syndrome[74]. This is highly suggestive of the cerebellum's role in emotional memory despite its involvement with motor control. Patients with Major Depressive Disorder (MDD) display a tendency to only selectively recall aspects of scenarios that match their moods, conforming with the "mood-congruent memory (MCM)" theory [75]. This study was undertaken with that principle in mind, and investigated the depth of cerebellar involvement in emotional memory in depression. A link between the volume and density of cerebellar gray matter with measurements of emotional memory was hypothesised.
The experiment was conducted between patients with Major Depressive Disorder (MDD) and healthy controls (HCs)[76]. Patients with MDD displayed an atrophy in both gray matter and white matter, most severely in the posterior lobule. There was a significant impairment in emotional memory and decreased volume of the cerebellum in both anterior and posterior lobules. There was marked abnormalities in cortical density, but only a reduction in volume was found to be associated with decreased emotional memory. The severity of depressive symptoms correlated with both volume and density reduction in the grey matter. The posterior, anterior and flocculonodular lobes of patients with MDD displayed marked structural differences from the healthy controls, and a functional connectivity between lobules VI and VII of the cerebellum and the cerebrum could indicate that the decreased density in the lobules of MDD patients contributes to alterations in this connectivity. The flocculonodular lobe is especially implicated in MDD. The lobe is associated with vestibular regulation; Soza and Aviles (2007)[77] found that patients who experienced vestibular vertigo also experienced depressive symptoms. This study also determined that patients who experienced depression also experienced bouts of dizziness. Thus, an unprecedentedly widespread area of the cerebellum is displayed to be connected with emotional memory, in particular, with positive or negative memory retention and could lead towards a cure for depression.
Dystonia
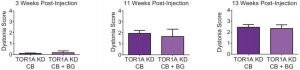
This investigation into a potential link between dystonia -a disorder where muscles contract involuntarily- and the cerebellum attempts to delineate its pathophysiological role in the disease [79] . The main concern is that the etiology of dystonia appears to be extremely varied, and as such, unpredictable. There is no significant neural degeneration, but in secondary cases there may be structural lesions present in tissue which could be areas of pathophysiology in dystonia [80]. Dystonia may manifest itself in almost any body part, indicating that the neural area responsible most likely must not be very specific. This in combination with the involuntary nature of dystonia seems to indicate that the cerebellum is the more than likely involved in the disease.
Basal ganglia abnormalities are hinted to be a causative agent in dystonia.[81]. The gap in the knowledge of the true interactions between basal ganglia and the cerebellum with regards to dystonia have led to a hypothesis; that the difference in basal ganglia malfunctioning versus abnormal interaction between the ganglia and the cerebellum could reflect the heterogenous pathophysiology of dystonia; either primary or secondary. Some experimental evidence currently available implies that cerebellar dysfunction could affect the topographic distribution of the symptoms of dystonia, and therefore further research is warranted to investigate the full depth of basal ganglia involvement in the disease.
Adaptation to Delayed Action Effects
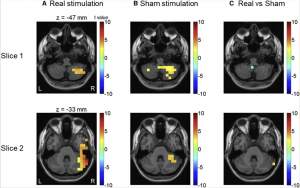
Sensory attenuation refers to when individuals filters unnecessary information. When there is a perturbation between actions and the following-sound, the sensory attenuation is reduced [82]. An example of perturbation is when there is a delay when an individual presses a button with sound immediately expected, but instead the sound is delayed. Sensory attenuation can be further described by the forward model theory. The forward model theory suggests that predictions for sensory consequences (for example, sound that is heard) are made simultaneously with the motor movement[82]. Predictions and real sensory input (what really happens), after the sound delay is conducted, are compared in the model. If the prediction and real sensory input do not matchup, sensory attenuation is observed. The prediction errors are then relayed to other brain areas for further processing[82].
The process of sensory attenuation usually re-emerges after the disruption to the normal mechanism [82]. The re-emerging of this is a process of either correcting previous predictions or updating initial forward models to minimise prediction errors. The cerebellum is currently being researched on how it plays an important role in updating the forward model within the brain[82]. One investigation seeks to find evidence on the involvement of the cerebellum in learning to predict the unexpected delays. This investigation has found that low-frequency activity in the cerebellum, prior to the stimulation, plays a key role in adapting to the delayed stimulus [82].
Addiction
The cerebellum has only recently been suspected to be involved in addictive behaviour, as some arguments provided for the initiation of research into the cerebellar role on addiction[84]. The cerebellum is shown to be intrinsically linked with dopamine release and reception[85]; with dopamine being the main hormone on which addiction is predicated, it is likely that the cerebellum could influence the response to the addictive stimuli. It has also been established that addictive drugs cause specific molecular mechanisms, changes in synapse plasticity[86], and influence intracellular transduction pathways as well as gene expression in the cerebellum[87]. The specificity of the drugs in targeting the cerebellum could highlight the link between the affected organ and addictive behaviour. Addictive drugs such as cocaine have been demonstrated to produce a behavioral sensitivity in mice, and an associated change in cerebellar plasticity. The change of the morphology of Purkinje cells and synaptic terminals in the mice most likely contributed to the difference in reception of the drug[88]. However, this link has not yet been fully investigated.
Future Questions
Although there are numerous articles and ongoing research on the cerebellum, there are still investigations yet to be performed. Some questions that could be answered in future research include:
- Is the cerebellum size related to human intelligence?
- Does the p53 gene function in cerebellum development? If so, does this function relate to the development of embryonic cancers? [89]
- What changes in cerebellum development lead to the social impairments seen in those with Autism Spectrum Disorder?[90]
- Do we need the cerebellum to actually function and survive?
Terms
- ApoER2 – Works with VLDLR and is a very important receptor in the brain affecting neuronal function and development.
- Basket cells– multipolar GABAergic interneurons. They are located on different regions of the brain and cerebellum. They function in making inhibitory synapses and control the overall potential of target cells.
- Bergmann Glia – they are a type of radial astrocyte, cell bodies are in the Purkinje cell layer of the cerebellum.
- Cerebellar nuclei – These cells receive inhibitory inputs from the purkinje cells, and excitatory inputs from mossy and climbing fibres.
- Climbing fibres – provide excitatory input to the cerebellum, they have a crucial role in motor behaviors. Axons pass through the pons, then synapse with the deep cerebellar nuclei and purkinje cells.
- Deep nuclei cells – Purkinje cells project to the deep nuclei cells and these are the only form of output cell in the cerebellar cortex. They also receive excitatory inputs from the mossy and climbing fibers.
- Dendrites – an extension of a nerve cell, impulses travel along these extensions and they synapse at another cell body.
- Dopamine – neurotransmitter and a precursor of substances including adrenaline.
- Eurydendoid cells – cerebellar efferent neurons.
- Flocculonodular lobe – lobe of the cerebellum that consists of a nodule and the flocculus. It is located on the anteroinferior surface of the cerebellum.
- Glia cells – They are nonneuronal cells and they support cells and they surround neurons and insulate them, there are many types of glia cells: oligodendrocytes, ependymal cells, astrocytes, Schwann cells etc.
- Granule cells – are small neurons, and they are the most numerous in the brain. They are packed in a thick layer of the cerebellar cortex and emit only 4 or 5 dendrites. They receive all input from mossy fibres.
- Hoxa2 – a gene that encodes for a transcription factor which could be involved in the development of the positioning of the hindbrain.
- Mossy fibres – major input of the cerebellum, relays sensory information from the pons to the granule cells and then sent to the purkinje cells for processing.
- Neural tube – a hollow prenatal structure that forms the brain and spinal cord. Formed by the folding and fusion of the opposite ectodermal folds.
- Otx2 – a protein that is involved with defining the layers and regions of the cerebral cortex and cerebellum.
- Perturbation – (In the context of the Current Research) alternation to the normal mechanism that would happen in response to a stimulation
- Plasticity – the brains ability to change at any age.
- Pons – a part of the brain that links the medulla oblongata and the thalamus.
- Purkinje cells – a class of GABAnergic neurons that are located in the cortex of the cerebellum, they are large, branched cells.
- Stellate cells – in the cerebellum, they are located in the molecular layer and they synapse onto purkinje cells.
- Tentorium – fold of the dura mater forming division between cerebrum and cerebellum.
- VLDLR – Very-low-density-lipoprotein receptor, it’s a transmembrane lipoprotein receptor
- Vermis – rounded and elongated section of the central part of cerebellum, lies between the two hemispheres.
- Vetrobrobasilar System – consists of the two vertebral arteries and one basilar artery and these are located towards the back of the brain. Provide around 20% of the intracranial bloody supply.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
References
- ↑ 2-Minute Neuroscience on Cerebellum. (2015). Retrieved October 25, 2017, from https://www.youtube.com/watch?v=Fir-v6EoZNE
- ↑ Voogd J & Glickstein M. (1998). The anatomy of the cerebellum. Trends Neurosci. , 21, 370-5. PMID: 9735944
- ↑ 3.0 3.1 Venturini, S. (2017). The Cerebellum. Retrieved October 23, 2017, from http://teachmeanatomy.info/neuro/structures/cerebellum/
- ↑ File:CerebellumDiv.png. (2016). Wikimedia Commons, the free media repository. Retrieved October 24, 2017, from https://commons.wikimedia.org/w/index.php?title=File:CerebellumDiv.png&oldid=226277429.
- ↑ The Blood Supply of the Brain and Spinal Cord. (2001). In D. Purves, G. Augustine, & D. Fitzpatrick (Eds.), Neuroscience (2nd ed.). Sunderland, MA: Sinauer Associates.
- ↑ Amarenco P & Hauw JJ. (1989). [Anatomy of the cerebellar arteries]. Rev. Neurol. (Paris) , 145, 267-76. PMID: 2535662
- ↑ Delion M, Dinomais M & Mercier P. (2017). Arteries and Veins of the Cerebellum. Cerebellum , 16, 880-912. PMID: 27766499 DOI.
- ↑ Abbasi A, Voelter W & Zaidi ZH. (1986). Isolation purification and properties of a site-specific proteolytic enzyme "valyl-proteinase" from Candida tropicalis. Biol. Chem. Hoppe-Seyler , 367, 441-5. PMID: 3527225
- ↑ Danbolt, N. C., & Zhou, Y. (n.d.). The Cerebellar Cortex. Retrieved October 23, 2017, from http://neurotransporter.org/Cerebellum.html
- ↑ D’Angelo, E. (2013). Cerebellar Granule Cell. Handbook of the Cerebellum and Cerebellar Disorders, 3, 767-791. doi: 10.1007/978-94-007-1333-8_31
- ↑ Harting, J. K. (1997). Deep Nuclei. Retrieved October 23, 2017, from http://www.neuroanatomy.wisc.edu/cere/text/P5/intro.htm
- ↑ Butts T, Green MJ & Wingate RJ. (2014). Development of the cerebellum: simple steps to make a 'little brain'. Development , 141, 4031-41. PMID: 25336734 DOI.
- ↑ Yamada K & Watanabe M. (2002). Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat Sci Int , 77, 94-108. PMID: 12418089 DOI.
- ↑ Pispa J & Thesleff I. (2003). Mechanisms of ectodermal organogenesis. Dev. Biol. , 262, 195-205. PMID: 14550785
- ↑ Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Neural Development.
- ↑ Ishikawa Y, Yamamoto N, Yoshimoto M & Ito H. (2012). The primary brain vesicles revisited: are the three primary vesicles (forebrain/midbrain/hindbrain) universal in vertebrates?. Brain Behav. Evol. , 79, 75-83. PMID: 22237006 DOI.
- ↑ <pubmed>19335795</pubmed>
- ↑ Gerardo De Iuliis PhD, Dino Pulerà MScBMC, CMI, in The Dissection of Vertebrates (Second Edition), 2011
- ↑ Bruce M. Carlson MD, PhD, in Human Embryology and Developmental Biology (Fifth Edition), 2014
- ↑ 2017. Embryology Stage9 dorsal.jpg. Retrieved October 15, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Stage9_dorsal.jpg
- ↑ 2017. Embryology Stage10 bf5.jpg. Retrieved October 15, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Stage10_bf5.jpg
- ↑ Hill, M.A. 2017 Embryology Human Stage13 sagittal upper half01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage13_sagittal_upper_half01.jpg
- ↑ M.A. 2017 Embryology Human Stage14 neural01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage14_neural01.jpg
- ↑ 24.0 24.1 24.2 Cho KH, Rodríguez-Vázquez JF, Kim JH, Abe H, Murakami G & Cho BH. (2011). Early fetal development of the human cerebellum. Surg Radiol Anat , 33, 523-30. PMID: 21380713 DOI.
- ↑ Müller F & O'Rahilly R. (1990). The human brain at stages 21-23, with particular reference to the cerebral cortical plate and to the development of the cerebellum. Anat. Embryol. , 182, 375-400. PMID: 2252222
- ↑ Hill, M.A. 2017 Embryology Human Stage21 neural01.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage21_neural01.jpg
- ↑ Hill, M.A. 2017 Embryology Human Stage21 neural02.jpg. Retrieved October 4, 2017, from https://embryology.med.unsw.edu.au/embryology/index.php/File:Human_Stage21_neural02.jpg
- ↑ Nuchal Fold Edema. Retrieved October 15, 2017, from http://www.fetalultrasound.com/online/text/2-006.HTM
- ↑ Hibi M & Shimizu T. (2012). Development of the cerebellum and cerebellar neural circuits. Dev Neurobiol , 72, 282-301. PMID: 21309081 DOI.
- ↑ Salem FB & Walash MI. (1985). Spectrophotometric determination of certain sympathomimetic amines. Analyst , 110, 1125-9. PMID: 4061865
- ↑ Hatten ME & Heintz N. (1995). Mechanisms of neural patterning and specification in the developing cerebellum. Annu. Rev. Neurosci. , 18, 385-408. PMID: 7605067 DOI.
- ↑ 32.0 32.1 32.2 Iwashige H & Maruo T. (1988). [Treatment of blepharospasm and hemifacial spasm with botulinum A toxin (Oculinum)]. Nippon Ganka Gakkai Zasshi , 92, 1637-43. PMID: 3213765
- ↑ 33.0 33.1 33.2 . (1972). State-approved schools of nursing--R.N., 1972. No. 19-1439. NLN Publ , , 1-100. PMID: 4552263
- ↑ 34.0 34.1 34.2 34.3 34.4 34.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedPMID25336734 - ↑ Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R. and Francis-West, P.H., 2014. Larsen's Human Embryology E-Book. Elsevier Health Sciences.
- ↑ 36.0 36.1 Martineau M, Baux G & Mothet JP. (2006). D-serine signalling in the brain: friend and foe. Trends Neurosci. , 29, 481-91. PMID: 16806506 DOI.
- ↑ Sotelo, C., & Rossi, F. (2013). Purkinje Cell Migration and Differentiation. Handbook of the Cerebellum and Cerebellar Disorders, 2, 147-178. doi:10.1007/978-94-007-1333-8_9
- ↑ <pubmed>26475605</pubmed>
- ↑ Manto MU & Jissendi P. (2012). Cerebellum: links between development, developmental disorders and motor learning. Front Neuroanat , 6, 1. PMID: 22291620 DOI.
- ↑ <pubmed>3263706</pubmed>
- ↑ 41.0 41.1 Waterman PG. (1988). Tannins and plant-animal interactions. Prog. Clin. Biol. Res. , 280, 77-91. PMID: 3051031
- ↑ Zhang, G., Assadi, A. H., McNeil, R. S., Beffert, U., Wynshaw-Boris, A., Herz, J., . . . D'Arcangelo, G. (2007). The Pafah1b Complex Interacts with the Reelin Receptor VLDLR. PLoS ONE, 2(2), 252nd ser. doi:https://doi.org/10.1371/journal.pone.0000252
- ↑ Hevner R. F. (2008) Reelin and the Cerebellum. In: Fatemi S. H. (eds) Reelin Glycoprotein. Springer, New York, NY
- ↑ Sabbatini, R. M. (1997, March). Brain Maps The Study of Brain Function in the Nineteenth Century. Retrieved October 26, 2017, from http://www.cerebromente.org.br/n01/frenolog/frenloc.htm
- ↑ 45.0 45.1 Glickstein M, Strata P & Voogd J. (2009). Cerebellum: history. Neuroscience , 162, 549-59. PMID: 19272426 DOI.
- ↑ Berlucchi G. (2002). The origin of the term plasticity in the neurosciences: Ernesto Lugaro and chemical synaptic transmission. J Hist Neurosci , 11, 305-9. PMID: 12481483 DOI.
- ↑ <pubmed>4308982</pubmed>
- ↑ "In Memoriam: Olof Larsell, 1886-1964." Journal of comparative neurology 123:1-4 (1964).
- ↑ <pubmed>23335884 </pubmed>
- ↑ <pubmed>19272426</pubmed>
- ↑ <pubmed>19669387</pubmed>
- ↑ <pubmed>3870571</pubmed>
- ↑ 53.0 53.1 <pubmed> 14567957</pubmed>
- ↑ 54.0 54.1 54.2 54.3 54.4 <pubmed>21309081</pubmed>
- ↑ <pubmed>PMC4584246</pubmed>
- ↑ 56.0 56.1 56.2 56.3 <pubmed>22108217</pubmed>
- ↑ <pubmed> PMC3179064</pubmed>
- ↑ <pubmed> 21093738</pubmed>
- ↑ 59.0 59.1 Pediatric Dandy-Walker Malformation. (2017). Retrieved October 23, 2017, from https://childrensnational.org/choose-childrens/conditions-and-treatments/fetal-carepregnancy/dandy-walker-malformation
- ↑ <pubmed> PMC3667004</pubmed>
- ↑ <pubmed>PMC2913941</pubmed>
- ↑ Joubert syndrome. (2016). Retrieved October 22, 2017, from https://rarediseases.info.nih.gov/diseases/6802/joubert-syndrome
- ↑ <pubmed>PMC3896311</pubmed>
- ↑ 64.0 64.1 64.2 <pubmed>18762395</pubmed>
- ↑ Chiari Malformation Fact Sheet. (2017). Retrieved October 22, 2017, from https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Chiari-Malformation-Fact-Sheet
- ↑ <pubmed>PMC4279813</pubmed>
- ↑ <pubmed>18155944</pubmed>
- ↑ 68.0 68.1 <pubmed>18409180</pubmed>
- ↑ <pubmed>25816977</pubmed>
- ↑ <pubmed>PMC3447431</pubmed>
- ↑ Medulloblastoma. (2017). Retrieved October 26, 2017, from https://www.mdanderson.org/cancer-types/medulloblastoma.html
- ↑ <pubmed>PMC5490254</pubmed>
- ↑ <pubmed>PMC5516611</pubmed>
- ↑ <pubmed>16434422</pubmed>
- ↑ Gilligan S. G., & Bower G. H. (1983). Reminding and mood‐congruent memory. Bulletin of the Psychonomic Society, 21, 431–434)
- ↑ <pubmed>5516611</pubmed>
- ↑ <pubmed>17074443</pubmed>
- ↑ <pubmed>PMC5340526</pubmed>
- ↑ <pubmed>5429509</pubmed>
- ↑ <pubmed>27173653</pubmed>
- ↑ <pubmed>9679773</pubmed>
- ↑ 82.0 82.1 82.2 82.3 82.4 82.5 82.6 <pubmed>PMC5571438</pubmed>
- ↑ <pubmed>PMC2904815</pubmed>
- ↑ <pubmed>26602022</pubmed>
- ↑ <pubmed>16451810</pubmed>
- ↑ <pubmed>10224304</pubmed>
- ↑ <pubmed>25262781</pubmed>
- ↑ <pubmed>25619460</pubmed>
- ↑ <pubmed> 4853753</pubmed>
- ↑ <pubmed>28150911</pubmed>