2017 Group Project 3: Difference between revisions
m (Protected "2017 Group Project 3" ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
|||
| (508 intermediate revisions by 6 users not shown) | |||
| Line 2: | Line 2: | ||
<!-- Do not remove template above from the project page --> | <!-- Do not remove template above from the project page --> | ||
=Heart= | |||
==Introduction== | ==Introduction== | ||
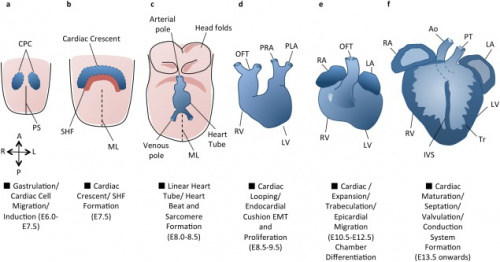
The cardiovascular system is the first system to develop and function in the human embryo, and | The cardiovascular system is the first system to develop and function in the human embryo. Rapid cardiac development is essential as the growing embryo can no longer receive oxygen and essential nutrients via diffusion alone, hence a circulatory system and a contractile heart mechanism is required to supply the embryo. | ||
We recognise the hearts normal development is vital for foetal life, and hence we have chosen to document the development of the heart from gastrulation to birth. Early cardiac development is a multifaceted procedure and is associated with other developmental processes such as: embryonic folding, coelom formation, and vascular development <ref name="PMID16479500><pubmed>16479500 </pubmed></ref>. As a result, any defects occurring during the developmental periods can lead to congenital heart abnormalities. | |||
Through researching the advances in technology, coupled with the biological use of suitable animal models our understanding of embryological cardiac development has evolved, and we are piecing together the mechanism underlying this development <ref name="PMID1767747"/>. This page will outline the embryonic development of the heart, the importance of how abnormalities arise, the treatments available and possible treatments that may be available in the future. Due to the certain knowledge gaps in current embryological heart research, we acknowledge that this will impact our assignment, and aim to address further research concepts that will improve our understanding. | |||
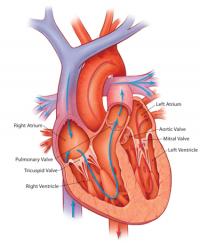
[[File:Basic anatomy of the heart.png|200px|thumb|right|'''Figure 1:''' Basic anatomy of the heart]] | |||
==Anatomy of the Heart== | |||
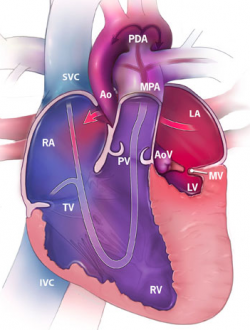
The heart is the muscular organs that pumps blood around the body via the circulatory system. It is located within the thoracic cavity, in a compartment called the mediastinum. The heart is divided into four chambers including: left and right atria and ventricles which are compartmentalised by semilunar and atrioventricular valves. Blood moves via the systemic circuit to the organs of the body and back to the heart. The pulmonary circuit is responsible for the flow of blood between the lungs and the heart. Deoxygenated blood enters the right side of the heart, while oxygenated blood returning from the lungs exits the left side. The heart’s electrical system uses electrical signals to cause the muscular walls to contract. The mechanical pumping of the heart is essential for movement of blood which exchanges gases and essential nutrients between organs of the body. <ref><pubmed> PMC1571338 </pubmed></ref>. | |||
==Developmental Origin== | ==Developmental Origin== | ||
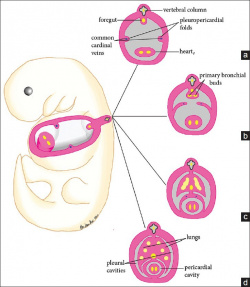
[[File:Pericardial Development 4-6 Gestation weeks .jpg|250px|thumb|right|'''Figure 2: '''Pericardial Development 4-6 Gestation weeks ]] | |||
In the developing embryo the lateral plate mesoderm splits into somatic and splanchnic layers, the latter is comprised of cardiac progenitor cells. The somatic mesoderm lines the ectoderm, the splanchnic mesoderm lines the endoderm, and in between lies an embryonic coelom <ref><pubmed> PMC2691808 </pubmed></ref> . At the end of week three, the heart develops from splanchnic mesoderm of the cardiogenic region of the embryonic plate. At the cranial end of the embryo, anterior to the developing neural tube is the initial origin of heart formation. | |||
Originally, the cardiogenic region forms laterally of the paraxial mesoderm and primitive streak of the embryonic disc. Due to the natural mesenchymal cell organisation of the mesoderm, cells ‘migrate’ and fuse at the midline of the cranial end of the embryo forming the cardiac crescent just prior cardiac tube formation and folding. Endoderm surrounding the primitive gut contracts bringing the cardiogenic precursor regions of the splanchnic region towards the midline. Early heart formation begins with angioblastic cords of the splanchnic mesoderm. The angioblastic cords develop into separate endochondral heart tubes moving closer together as the foregut pinches together and the yolk sac contracts into the embryo. Fusion of the two heart tubes is facilitated by apoptosis. The origin of the primitive heart is in the early pericardial coelom, which is later developed into the pericardial cavity, through fusion of the pleuropericardial folds to separate the pleural cavities at the later development of the lungs <ref><pubmed> PMC4374196 </pubmed></ref>. | |||
==Developmental Timeline== | ==Developmental Timeline== | ||
=== | {| class="wikitable" | ||
! style="background:#f4a941" | WEEK | |||
! style="background:#f4a941" | DEVELOPMENT | |||
! style="background:#f4a941" | HISTORIC CARDIOVASCULAR DISCOVERIES | |||
|- | |||
| 2 || Bilateral cardiogenic areas form | |||
|| In 157 AD Galen (born on 9 September AD 129 in Pergamon, Greece) discovered the pulmonary circulation. | |||
|- | |||
| 3 || Mesoderm splits, Heart tubes are brought to the midline, Heart tube fusion, Heart beat | |||
|| Realdo Columbo (1515–1559 discovered that the heart’s four valves permitted flow of blood in one direction only: from the right ventricle to the lungs, back to the left ventricle, and from there to the aorta. | |||
|- | |||
| 4 || Heart looping, Neural crest migration commences, Dorsal and ventral endocardial cushions fuse | |||
|| William Harvey (born 1 April 1578) while studying in London estimated: | |||
* the capacity of the heart was 43 g | |||
* about 6 g of blood went through the heart every time it pumped | |||
* the heart beats 1 000 times every half hour. | |||
* therefore, the heart pumps about 5 kg of blood in a half hour, or about 245 kg in a day | |||
|- | |||
| 5 || Foramen primum closed, Septum secundum srtats developing, Muscular interventricular septum develops, Bulbar ridges and trabeculation become evident | |||
|| Harvey proved blood flows in two separate loops: the pulmonary and systemic circulation <ref><pubmed>PMC3721262</pubmed></ref>. | |||
|- | |||
| 6 || Aortic and pulmonary trunks cleave | |||
|| The earliest study of the partitioning of the heart was conducted by cardiologist and anatomist Wilhelm His Jr (1863-1934) in 1886. Later in 1893 he discovered the ''bundle of His'' -the specialized tissue in the heart that transmits the electrical impulses and helps synchronize contraction <ref name="PMID16567300><pubmed>16567300 </pubmed></ref>. | |||
|- | |||
| 7 || Valves develop | |||
|| Franklin P. Mall (1862-1917) worked at the Department of Embryology at the Carnegie Institute of Washington where he studied the subdivisions of the primary heart tube, formation of the atrio-ventricular valves and bundle, and the musculature of the left ventricle. Additionally, in 1910 he demonstrated that the nascent atrium of the heart could be identified based on the close proximity of endothelium to the heart muscle <ref name="PMID17796013><pubmed>17796013 </pubmed></ref> | |||
|} | |||
====Primary Heart Field==== | |||
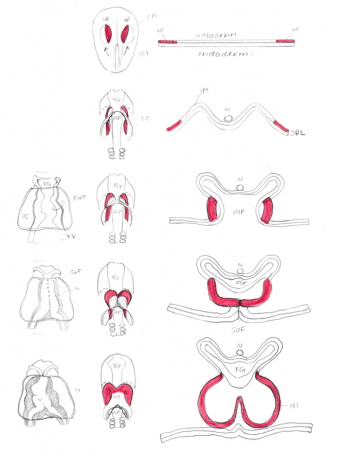
[[File:Morphology of Heart Tube Formation- Student Image .png|350px|thumb|right|'''Figure 3:''' Morphology of Heart Tube Formation- Student Image]] | |||
'''Gestational Week 4-5''' | |||
The first cells start to migrate through the primitive streak to the anterior and lateral sections of the cranial end of the embryonic disc, forming bilateral primary heart fields. These primary heart fields resemble a crescent shape. <ref name="PMID1767747><pubmed>PMC1767747</pubmed></ref>. | |||
The lateral plate mesoderm is split into two layers, namely the splanchnic mesoderm, facing the endoderm and the somatic mesoderm, facing the ectoderm. The former portion of the mesoderm gives rise to the heart. The portion between the splanchnic and somatic mesoderm is the presumptive pericardial space. Cells from the splanchnic mesoderm will merge to form 2 lateral endocardial tubes (also known as angioblastic cords) and as they form a lumen, are enveloped by myocardium. These endocardial tubes are as of now located inferior to the presumptive pericardial space. <ref name="PMID1767747"/> | |||
====Heart Tube Formation==== | |||
'''Gestational Week 5''' | |||
The embryonic disc starts to fold. This folding begins cranially and extends in a caudal direction. The endocardial tubes fuse and is now located between the pericardial space and newly formed foregut that becomes surrounded by pericardial space (also known as the pericardial coelom). At this stage, the myocardium does not completely engulf the endocardial tubes. Instead, it remains in a continuous attachment with the non-cardiac splanchnic mesoderm through a structure called the dorsal mesocardium. | |||
At this point, the primitive heart tube is bilaterally symmetrical and resembles an inverted Y shape. Starting from the inflow tract, there is the right and left sinus venouses that receives blood from the embryo, followed by the primitive atrium, primitive ventricle, bulbus cordis and then the truncus asteriosus which gives rise to the aortic and pulmonary trunk .<ref name="PMID1767747"/> | |||
<html5media height="200" width="240">File:Heart_folding_001.mp4</html5media> | |||
The video above depicts the folding and fusion of Heart tubes taken from [https://embryology.med.unsw.edu.au/embryology/index.php/Advanced_-_Heart_Tubes] | |||
====Secondary Heart Field ==== | |||
'''Gestational Week 5-6''' | |||
The secondary heart field (SHF) is a region of subpharyngeal mesodermal progenitor cells located medially and ventrally to the adjacent primary heart field (PHF) (that forms the initial heart tube). The PHF and SHF cells make up a region known as the cardiogenic field. Following heart tube fusion around 19-21 days, cells from the SHF migrate to the cranial and caudal ends of the tube and continue elongation. <ref name="PMID1767747"/> | |||
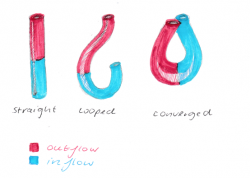
[[File:Schematic diagram of heart tube looping.png|250px|thumb|left|'''Figure 4:''' Schematic diagram of heart tube looping - Student Image]] | |||
It is understood that within the cardiac regions the progenitor cell population of the SHF is “pre-patterned”<ref>http://perspectivesinmedicine.cshlp.org/content/4/10/a015750.full</ref>, hence being termed “specified but undifferentiated”. It is the patterning of cells within the soon to become myocardium, that is responsible differentiation into chamber-specific myocytes (atrial and ventricular) and the conduction of cells. It is essential that the SHF cells remain undifferentiated and do not add prematurely to the heart tube.<ref name="PMID2794420><pubmed>2794420</pubmed></ref> | |||
The SHF cells gives rise to endocardial, myocardial and smooth muscle cells through expression of transcription factor Islet -1 <ref>http://www.sciencedirect.com/science/article/pii/S1875213613002817 </ref>. It also contributes to the right ventricle, inflow and outflow tract (OFT), and the arteries and semilunar valves that meet to form the arterial pole of the heart.<ref name="PMID2794420"/> The OFT is the point of exit from the heart via structures of early cardiac development that give rise the essential structures of the aorta and pulmonary artery. The SHF of the developing heart and its contribution the arterial pole involves complex and interconnected signalling pathways that will be covered later in further detail. | |||
[[File:Cardiac Development Overview .jpg|500px|thumb|right|'''Figure 5:''' Cardiac Development Overview]] | |||
====Cardiac Looping and Steps==== | |||
'''Gestational Week 6''' | |||
In the early embryo, the same progenitor cells that constitute the SHF are responsible for the rapid growth occurring during cardiac looping morphogenesis. Looping is essential for cardiac development as it assist further growth of the originally straight heart tube, and ensures it fits within the pericardial celom. Additionally, looping of the heart tube is known to be the first visual evidence of embryonic asymmetry. | |||
# Straight heart tube continues to elongate | |||
#Rapid growth of the bulbus cordis and the primitive ventricle causes ventral bending and right rotation = C shaped loop (convex side on the right) | |||
#The ventricular bend continues to move caudally, the inflow and outflow tracts are brought together at the atrial pole of the heart = S shape | |||
#The truncus arteriosus if formed by adding myocardial cells at the top end of the heart. This portion will form roots and the proximal portion of the aorta and the pulmonary artery <ref name="PMID16479500><pubmed>16479500 </pubmed></ref>. | |||
<html5media height="240" width="280">File:Heart looping 002.mp4</html5media> | |||
The video above depicts Cardiac Looping taken from [https://embryology.med.unsw.edu.au/embryology/index.php/Intermediate_-_Heart_Tube_Looping] | |||
=== | ====Cardiac Septation==== | ||
[ | '''Gestational Week 6-7''' | ||
This stage of heart morphogenesis refers to the development of the four main cardiac chambers from the primitive atrium and ventricle. Cardiac septation is comprised of three main events: | |||
*Division of the atrioventricular canal | |||
*Atrial septation | |||
*Ventricular septation | |||
'''Division of the atrioventricular canal''' | |||
Division of the atrioventricular canal (AVC) begins with the formation of the superior and inferior endocardial cushions, which are located on the dorsal and ventral aspects of the AVC respectively.<ref name="PMID3424040><pubmed>PMC3424040</pubmed></ref> These cushions develop as mesenchymal cells invade and proliferate within swollen regions of cardiac jelly of the AVC; this mesenchyme is derived from endothelial cells that have transdifferentiated in the process of epithelial-mesenchymal transformation (EMT).<ref name="PMID1767797><pubmed>PMC1767797</pubmed></ref> Throughout the fifth week of development, the endocardial cushions project inwards and eventually fuse to partition the AVC into the left and right atrioventricular canals; these canals will serve as the orifices in which the tricuspid and mitral valves are situated.<ref name="PMID1767797"/> | |||
'''Atrial septation''' | |||
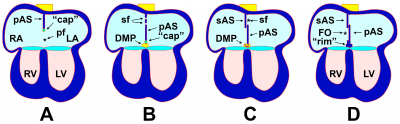
[[File:The Process of Atrial Septation.png|400px|thumb|right|'''Figure 6:''' The Process of Atrial Septation. Growth (A) and perforation (B) of septum primum. Growth of septum secundum (C). Formation of foramen ovale (D).]] | |||
As the AVC is undergoing division, a muscular outgrowth, referred to as the septum primum, extends inferiorly from the roof of the primordial atrium.<ref name="PMID24138816><pubmed>24138816</pubmed></ref> This septum partially divides the atrial chamber into left and right halves, leaving a temporary communication located between the inferior border of septum primum and the endocardial cushions known as the foramen primum.<ref name="PMID3424040"/> As the size of the foramen primum diminishes, perforations in the superior portion of the septum primum develop as a result of apoptosis, forming a second communication between the atrial chambers called the foramen secundum.<ref name="PMID1767797"/> Concurrently, an additional muscular septum, known as the septum secundum, projects inferiorly to the right of the septum primum.<ref name="PMID3424040"/> Eventually, the septum secundum will extend beyond the length of the foramen secundum, generating a partial division of the atria that forms the upper boundary of the foramen ovale.<ref name="PMID24138816"/> The development of the foramen ovale is critical as it allows oxygen-rich blood from the placenta to bypass pulmonary circulation of the embryo and directly enter systemic circulation.<ref name="PMID27188965><pubmed>27188965</pubmed></ref> Following birth, the foramen ovale is obliterated as the septum primum and septum secundum fuse, resulting in complete formation of the interatrial septum.<ref name="PMID3424040"/> | |||
'''Ventricular septation''' | |||
As with atrial septation, differentiation of the ventricles is initiated within week 4 of development.<ref name="PMID3424040"/> A muscular ridge, referred to as the interventricular septum primordium, develops and extends superiorly from the caudal aspect of the primitive ventricular chamber.<ref name="PMID1767797"/> Growth of the septum is attributed to the expansion of the ventricles, which involves the development of muscular trabeculae as cardiomyocytes proliferate within the chamber walls. <ref name="PMID24138816"/> The end result is a partial division between the left and right ventricles, which forms the muscular portion of the interventricular septum.<ref name="PMID1767797"/> A communication between the ventricles, known as the interventricular foramen, remains until approximately week 7 of development.<ref name="PMID12739611><pubmed>12739611</pubmed></ref> The foramen is obliterated by the fusion of the septum intermedium and the bulbar ridges of the bulbus cordis; this constitutes the membranous portion of the interventricular septum.<ref name="PMID3424040"/> | |||
====Cardiac Neural Crest and Outflow tract==== | |||
'''Gestational Week 8''' | |||
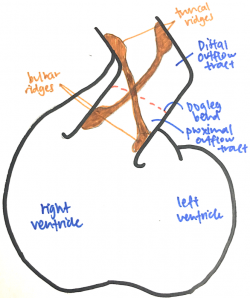
[[File:Outflow tract anatomy.png|250px|thumb|right|'''Figure 7:''' Outflow tract anatomy - Student Image]] | |||
The outflow tract is a tube that runs from the right ventricle to the aortic sac and presents with a distinctive dog-leg bend that separates the proximal (bulbus cordis) and distal (truncus arteriosus) ends of the tract. The endocardial jelly that lines the lumen of the outflow tract concentrates to form the endocardial cushion facing each other that spirals in a 180-degree twist through the length of the outflow tract. Like the outflow tract, these endocardial cushions can be divided into distal and proximal moieties. The distal endocardial cushions are also known as the truncal ridges and the proximal ones are also known as the bulbar ridges. Cells from the cardiac neural crest migrates out of the neural tube, through the pharyngeal arches and aortic sac and into the outflow tract, where it condenses in the ridges to support the septation of the outflow tract. <ref name="PMID23633400><pubmed>23633400</pubmed></ref><ref name="PMID12923046><pubmed>12923046</pubmed></ref> | |||
The fusion of the endocardial cushions starts from the distal end of the outflow tract and proceeds proximally. Fusion of the truncal endocardial cushions forms the aorticopulmonary septum that separates the truncus into an aortic and pulmonary trunk. The bulbar endocardial cushions fuse as they extend towards the interventricular septum, separating the proximal outflow tract into the prospective aortic and pulmonary trunks. As the outflow tract separates, the aortic trunk leads to the 3rd and 4th pharyngeal arch arteries and the pulmonary trunk leads to the 6th pharyngeal arch artery. <ref name="PMID23633400"/><ref name="PMID12923046"/> | |||
====Heart Valve Development==== | |||
'''Gestational Week 8''' | |||
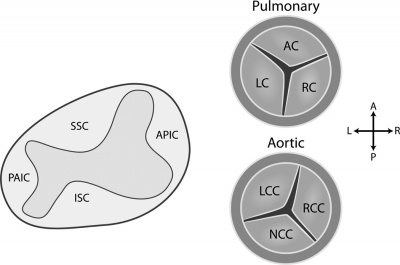
Heart valve development commences in the endocardial cushions of the atrioventricular canal (AVC) and outflow tract (OFT) during the 7th week of embryological development.<ref name="PMID3424040"/> Valve morphogenesis is comprised of four stages: | |||
[[File:Development of the Semilunar Valves.jpg|400px|thumb|right|'''Figure 8:''' Development of the Semilunar Valves.]] | |||
*Epithelial-to-mesenchymal transformation (EMT) | |||
*Growth | |||
*Remodelling | |||
*Apoptosis | |||
Development of the valves begins with the transformation of the endocardial cells into mesenchymal cells within the endocardial cushions.<ref name="PMID3424040"/> The endocardial cushions then expand through cell proliferation and synthesis of the extracellular matrix, which is primarily regulated by vascular endothelial growth factor (VEGF).<ref name="PMID24138816"/> The elongating cushions then undergo remodeling, as mesenchymal cells differentiate into collagen and other fibrous tissue.<ref name="PMID24138816"/> The valve leaflets achieve their thin, unique shape as cells near the base of the extending cushions undergo apoptosis.<ref name="PMID3424040"/> | |||
The semilunar valves differentiate from the conotruncal and intercalated endocardial cushions located at the outflow tract of the heart.<ref name="PMID3424040"/> The superior and inferior septal conotruncal cushions contribute to the left and right cusps of both the pulmonary and aortic valves.<ref name="PMID5438177><pubmed>PMC5438177</pubmed></ref> The right-posterior intercalated cushion contributes to the posterior leaflet of the aortic valve, while the left-anterior leaflet gives rise to the anterior leaflet of the pulmonary valve.<ref name="PMID5438177"/> | |||
The atrioventricular valves arise from the atrioventricular (AV) endocardial cushions. As the atrioventricular canal divides during cardiac septation, fusion and proliferation of the superior and inferior AV endocardial cushions results in the formation of the anterior mitral leaflet and the septal tricuspid leaflet.<ref name="PMID3424040"/> The right lateral AV cushion differentiates into the anterior and posterior leaflets of the tricuspid valve, while the left cushion gives rise to the posterior leaflet of the mitral valve.<ref name="PMID3424040"/> | |||
====Proepicardium and Coronary Heart Development==== | |||
Coronary vasculature arises when the fourth layer of the primary heart tube is formed. This fourth layer is known as epicardium that surrounds the myocardium which allows the development of vascular structures over the heart surface. | |||
The monolayered embryonic epicardium derives from mesothelial cells of the septum transversium and mainly from clustered of proepicardium cells. The process involves either of these two mechanisms, one is by detaching of these cells and attach to the pericardial cavity and spread over the heart surface or two is by directly attaching the proepicardium to the myocardium forming a permanent tissue bridge. The spreading of the monolayered embryonic epicardium over the bare myocardium initiates the vascularisation of coronary system. A new extracellular matrix (ECM) layer, the subepicardium forms between the epicardium and the myocardium actively promotes the development of coronary blood vessels. The coalescence of three lineages of the coronary vessel cells: smooth muscle, endothelial and connective tissue and fusion of vascular cell progenitors (angioblasts) form a vascular structure ''de novo'' via the process of vasculogenesis. They then migrate onto the primary heart tube between day 22 and day 28 of human development. <ref> Pérez-Pomares J, Pires-Gomes A, (2013) '''The Epicardium and Coronary Artery Formation''', Journal of Developmental Biology, Vol.1(3), pp.186-202, ISSN: 2221-3759, E-ISSN: 2221-3759, https://doaj.org/article/b3f5aa0ae8df409ab1664975469b95d1</ref>,<ref> Martinsen B, Lohr J, (2005) '''Cardiac Development''', Handbook of Cardiac Anatomy, Physiology and Devices Iaizzo, P.A. (Ed.), http://www.springer.com/cda/content/document/cda_downloaddocument/9781588294432-c2.pdf?SGWID=0-0-45-387660-p173728389</ref> | |||
==Developmental Signalling Processes== | ==Developmental Signalling Processes== | ||
Heart development is a very complicated and dynamic process that requires a high degree of control and regulation. This control is achieved by | Heart development is a very complicated and dynamic process that requires a high degree of control and regulation. This control is achieved by several temporally regulated signalling cascade (Fig. 9) expressed at different stages of heart development. | ||
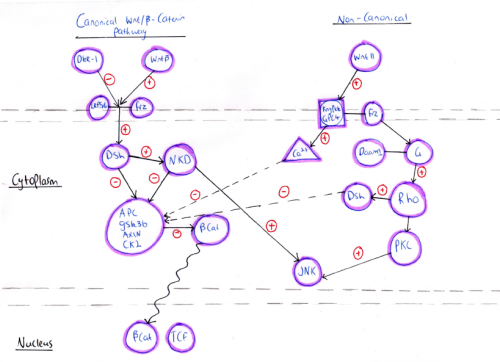
[[File:Signalling pathways in heart development.png|400px|thumb|none|'''Figure 9:''' Signalling pathways in heart development]] | |||
===Wnt signalling=== | ===Wnt signalling=== | ||
According to the primary mode of action, Wnt signalling pathways have been divided into two major classes, canonical and non-canonical Wnt signalling pathways. Both of the pathways have a role in different stages of cardiac development which could be overlapping or independent of each other. The canonical Wnt signalling pathway involves β-catenin and is activated by a number of ligands such as Wnt-1, Wnt-2, Wnt-3A, Wnt-8A, Wnt-8B, Wnt-8C, Wnt-10A, and Wnt-10B. However, the non-canonical signalling pathway is associated with planar cell polarity and Wnt/Ca2+ pathways that are activated by different ligands such as Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, and Wnt11 <ref><pubmed>25813860</pubmed></ref>. | |||
Canonical/β -catenin signaling is essential for mesoderm formation. Upon binding of the Wnt ligands to the Frizzled receptor, a seven-transmembrane receptor, or the co-receptor LRP-5/6, the Canonical signaling pathway is activated. This leads to cytoplasmic accumulation of β -catenin in one side of the embryo and its translocation to the nucleus where it drives the activation of transcription factors required to determine the site at which mesoderm and endoderm formation will occur in the embryo. | |||
Animal studies have shown that the lack of nuclear accumulation of β -catenin results in inability of axis formation and mesoderm development whereas overexpression of β -catenin leads to the formation of secondary axis and ectopic expression of mesoderm signals. Also, homozygous deletion of β -catenin in mouse models results in absence of primitive streak and mesoderm formation <ref><pubmed>16860783</pubmed></ref>. | |||
Once mesoderm formation proceeds, the β -catenin signal must be shut down by inhibiting factors otherwise cardiac mesoderm formation will be stopped. This indicates that canonical signaling act as a switch that either induces or suppresses cardiac development. One of the inhibiting factors includes dickkopf-1(DKK1) which is an extracellular Wnt inhibitor that promotes the expression of cardiac-inducing factor in the endoderm such as Hex. The latter activates paracrine factors to direct adjoining cells towards cardiac fate <ref><pubmed>4533091</pubmed></ref>. | |||
[[File:Wnt signalling pathways.png|500px||thumb|none|'''Figure 10: ''' Wnt signalling pathways - Student Image]] | |||
[[ | |||
===FGF signalling=== | |||
Fibroblast growth factors (FGF) serve a variety of functions in development, the maintainence of health and disease. FGFs are signaling proteins mostly as paracrine growth factors or endocrine hormones. There are 22 types of human FGFs, whereby paracrine FGF8, FGF9, FGF10 and FGF16 serve a major role in embryonic heart development. Additionally, FGF2, FGF9, FGF10 and FGF16 are involved in postnatal heart pathophysiology<ref><pubmed>11493531</pubmed></ref>. | |||
Communication between cardiac progenitor cells is required during embryonic heart development. During heart development, neural cress cells which migrate from the neuroectoderm of the dorsal neural tube will contribute to cushion formation and dictate the correct septation and alignment of the heart<ref><pubmed> 23799628</pubmed></ref>. In conjunction to this process FGF3, FGF8, FGF9, FGF10, FGF15, FGF19 and FGF16 function was paracrine signals in embryonic heart development. | |||
A study conducted demonstrated how a combination of both FGF2 and Bone morphogenic protein 2 (BMP2) efficiently enhances the cariomyogenic differention of embryonic stem (ES) cells at an optimal concentration. When FGF2 was inhibited, this ultimately suppressed cardiomyogenic differentiation, thus indicating that FGF signaling play a crucial role in early cardiomyogenesis. The ability for FGF2 to induce cardiac differentiation may serve a key role in treatment for heart diseases.<ref name="PMID26793421><pubmed>26793421</pubmed></ref> | |||
In addition, FGF10 also promotes cardiomyocytes differentiation from ES and induced pluripotent stem cells (iPS). In experiments, when FGF10 and ES cells were administered, this lead to the promotion of cardiomyocyte differentiation in the myocardium of the heart. This may thus serve as a treatment for those who have suffered from myocardial infarctions, where the myocardium is replaced by scar tissue. Providing new and fully functional cardiac muscle may help reduce complications. <ref><pubmed> 24307297 </pubmed></ref> | |||
The table below outlines the different types of FGFs invovled in heart development and their functions.<ref name="PMID26793421"/> | |||
{| class="pretty table" | |||
|-bgcolor = "DDCEF2" | |||
|'''Fibroblast Growth Factor''' | |||
|'''Function''' | |||
|-bgcolor="FAF5FF" | |||
| ''' FGF8''' | |||
|FGF8 is expressed in the early embryonic stages. Required for cardiac looping and migratory cardiac neural crest cell survival. FGF8 is also required for anterior heart field development. | |||
|- | |||
|''' FGF9''' | |||
|Activates FGFR1c with heparin sulfate as a co-factor in a paracrine manner. In doing so, this FGF plays a major role in stimulating the proliferation of cardiomyocytes. | |||
|-bgcolor="FAF5FF" | |||
|''' FGF10''' | |||
| Preferentially activates FGFR2b with heparin sulfate as a co-factor. Serves to regulate regional-specific cardiomyocyte proliferation in the embryonic heart in an autocrine/paracrine manner. This FGF type is also essential for the movement of cardiac fibroblasts in the compact myocardium. | |||
|- | |||
|''' FGF15/19''' | |||
| These FGF types are required for proper morphogenesis of the cardiac outflow tract. In addition these FGFs play a major role in paracrine signaling in heart development. | |||
|-bgcolor="FAF5FF" | |||
|''' FGF16''' | |||
|FGF16 serves as a major growth factor involved in stimulating the growth of embryonic cardiomyocytes. | |||
|- | |||
|-bgcolor="FAF5FF" | |||
|- | |||
|} | |||
===Transforming growth factor-β=== | |||
Transforming growth factor-β superfamily involves a large number of growth factors that are structurally related. These growth factors, including Nodal or its mimic Activin, bone morphogenic protein (BMP) and growth and differentiation factors, signal through SMAD-dependent and SMAD-independent pathways <ref><pubmed>18290874</pubmed></ref>. SMAD-dependent pathway involves the activation of SMAD proteins upon TGF-β binding to its receptor followed by SMAD transfer into the nucleus regulating gene transcription <ref><pubmed>14534577</pubmed></ref>. | |||
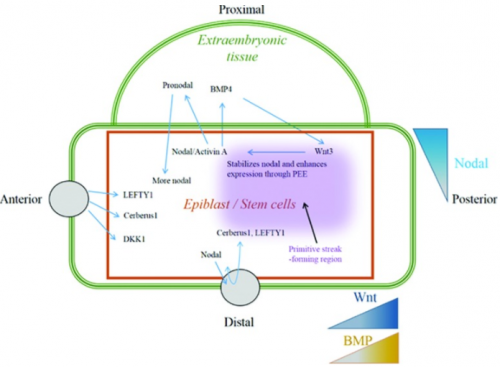
Nodal and its mimic Activin act through SMAD-2 and -3 to activate transcription. During embryo development, epiblast cells produce high levels of Nodal growth factors leading to a gradient of nodal in these cells. This gradient is important for mesoderm patterning (left-right asymmetric heart development) and lineage specification. For this reason, this gradient is maintained by Nodal antagonist secreted from the anterior visceral endoderm or by BMP-4 and Wnt-3 feedback loop in the extraembryonic tissues (Fig.11) <ref><pubmed>25813860</pubmed></ref> | |||
Nodal/Activin’s important role in heart formation is demonstrated by animal models. Presence of Activin in amphibian embryo lead to activation of heart formation whereas the absence of Nodal co-receptor ,called Cripto, in mouse embryo resulted in failure of ES cells differentiation into cardiomyocytes <ref><pubmed>20830688</pubmed></ref>. Also, knocking out copies of SMAD-2 alone or both SMAD-2 ND -3 result in inappropriate specification of axial mesoderm <ref><pubmed>12842913</pubmed></ref>. | |||
[[File:Regulation of Nodal-Activin signalling during heart formation.png|500px||thumb|none|'''Figure 11: ''' Regulation of Nodal-Activin signalling during heart formation]] | |||
===The Notch pathway=== | |||
The Notch gene is an essential gene, in that it codes for a signaling receptor that is required throughout development to regulate the processes known as spatial patterning, timing and outcomes of many different cell fate decisions. The receptor itself is a single spanning transmembrane protein which has a modular architecture including many repeats of a protein module which is reminiscent of the epidermal growth factor as well as 3 membrane-proximal Lin12/Notch/Glp-1 repeats. Notch also has an intracellular domain which is composed of four distinct regions known as the RAM domain, the Ankyrin repeats, a transcriptional activator domain as well as proline, glutamate, serine and threonine sequence. <ref><pubmed>12651094</pubmed></ref>. In the process of activation, a canonical Notch ligand binds the extracellular domain of the notch receptor. Ligand binding will thus induce the exposure of the cleavage site which will allow access by proteases responsible for cleavage under physiological conditions. This cleavage renders the remaining transmembrane-intracellular fragment a substrate for a complex known as the gamma-secretase complex. It is this complex which will catalyse the intramembrane proteolysis to release the Notch intracellular domain. Once this cleavage has occurred, the intracellular domain has the potential to enter into the nucleus and bind the DNA to induce gene transcription<ref><pubmed>27507209</pubmed></ref>. | |||
With regard to heart development Notch signaling plays a key role in the pre-patterning of the cardiac mesoderm. In gastrula-stage embryos, Notch works with estrogen receptor 9 and GATA4 transcription factor to regulate the timing of heart field specification for early cardiogenesis which is necessary for normal cardiac development. Notch receptor types 1, 2 and delta-like 1 are required for the determination of the embryonic left-right axis as well as the proper looping of the heart tube. In addition to this, Notch signaling promotes myocardial trabecular proliferation, differentiation and maturation by inducing bone morphogenetic protein-10 and EphrinB2/EphrinB4 expression. Notch signaling also modulates coronary vessel morphogenesis, in which the embryonic epicardium actively participates. Notch signaling elements are differentially expressed throughout the proepicardial-epicardial-coronary transition phases and are required for vessel wall maturation during coronary vessel development. Notch also cooperates with transforming-growth factor-beta to regulate coronary smooth muscle differentiation from epicardium-derived cells to assist in the formation of a functional coronary system. Notch signaling will also regulate cardiac conduction system function, in particular the atrioventricular node <ref><pubmed>21252157</pubmed></ref>. | |||
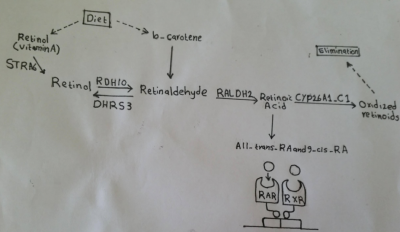
===Retinoic Acid=== | |||
[[File:Retinoic Acid activation pathway.png|400px|thumb|right|'''Figure 12:''' Retinoic Acid activation pathway - Student Image]] | |||
Retinoic acid (RA), a derivative of vitamin A, has been shown to play an important role in vertebrate embryogenesis especially heart formation. RA mediates its action by binding to two families of nuclear receptors, that is the RA and the retinoid receptors (RARs and RXRs). This binding allows RA to directly regulate gene transcription. The expression of at least one of the receptor types by almost all embryonic tissues gives these tissues responsiveness to RA. Signaling of RA is regulated by enzyme that either produce or inactivate retinoids (Fig. 12) <ref><pubmed>25813860</pubmed></ref>. | |||
At early stages, RA signaling is suggested to regulate progenitor size in that increasing RA concentrations leads to reduced heart populations in chicken and zebrafish embryos, whereas micromolecular concentrations lead to no heart formation. This regulation is though to happen by RA’s direct action on Hox gene family members such as Hoxa1 transcription factor. These transcription factors have enhancer containing RA response element (RARE). Thus, a reduction or an increase in RA signaling affects the contribution of Hoxa1-; Hoxa3- and Hoxb1-expressing progenitor cells to the heart<ref name="PMID28007475><pubmed>28007475</pubmed></ref>. At later stages of heart development, RA is involved in establishing the second heart field which forms atrial cells. Also, RA contributes to the proliferation of ventricular cells. In addition, RA has the potential to stimulate cellular differentiation of atrial and ventricular myocytes from human embryonic stem cells (hESCs). This capacity of RA has been exploited in regenerative medicine to promote atrial specification <ref><pubmed>25700171</pubmed></ref>. | |||
Embryo obtains vitamin A from maternal delivery of retinol via transplacental transfer. This transfer of Vitamin A is essential for fetal growth and development and any deficiency can lead to severe congenital defects. In fact, the high prevalence of heart malformation in developing countries can be partly explained by vitamin A insufficiency. Conversely, elevated levels of vitamin A during pregnancy have a toxic effect on organogenesis including heart development. Treatment with RA in rodents was one of the earliest teratogenic models of heart defects <ref name="PMID28007475"/>. | |||
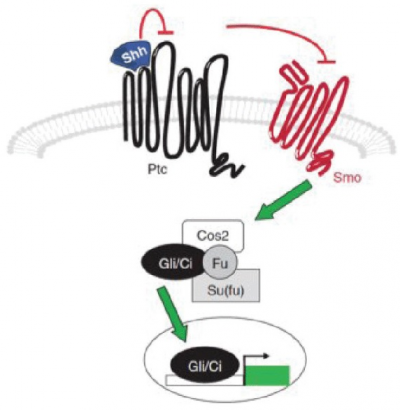
===Sonic Hedgehog=== | |||
The Sonic Hedgehog (Shh) signaling pathway is one of the major trafficking networks that is responsible for regulating the key events involved in the developmental processes. The regulatory action of Shh signaling pathway is linked to the secretion, uptake and translocation of ‘Shh protein’ an important Hedgehog ligand precursor. Activation of Shh signal requires the binding of Shh to the Ptc mediate Smoothened (Smo) (Ptc-smo) receptor complex as well as induction of downstream signaling cascade <ref><pubmed>25206052</pubmed></ref>. | |||
Shh signaling contributes to myocytes specification and a reduction in this signaling can cause a cardiomyocyte deficit whereas increased Shh signaling lead to a surplus. At early stages of cardiac development, Shh signaling induces a proper number of myocardial progenitor cells. These progenitor cells show a direct respond to SHH signals allowing it to control and determine the optimal number of cardiomyocytes <ref><pubmed>15936751</pubmed></ref>. At later stages of cardiac development, Shh plays an important role in survival and proliferation of neural crest cells (NCCs) and it maintains the proliferation of progenitor cells within the second heart field. These actions allow Shh to promote outflow tract morphogenesis. However, Shh does not act as a direct survival factor for NCCs, but rather, it acts indirectly by inducing a survival factor and inhibiting an apoptotic factor. NCCs of Shh null mice embryos show specification and migration but they are mislocalized and undergo apoptosis. Also, mice that lack Shh develop many cardiac abnormalities such as outflow tract shortening, ventricular hyperplasia and septation defects<ref><pubmed>18842815</pubmed></ref>. | |||
[[File:The Sonic Hedghehog (Shh) signalling pathway.png|400px|thumb|none|'''Figure 13:''' Sonic Hedghehog (Shh) signalling pathway]] | |||
==Current Research And Findings== | ==Current Research And Findings== | ||
===Increased regurgitant flow causes endocardial cushion defects in an avian embryonic model of congenital heart disease=== | |||
it has been discovered that an elevated regurgitant flow during the early stages of embryonic heart development eventually causes abnormalities in the endocardial cushions. However, till today it remains unclear as to how defects in the endocardial cushions during the cardiac looping stages plays a part in the septation of the heart. Thus the aim of this research was to change the blood flow within the avian embryonic heart and monitor the effects it had on the morphology of the endocardial cushions and on Congenital Heart Diseases (CHD). Optical pacing was used to provide the regurgitant flow and optical coherence tomography (OCT) was used to monitor the regurgitation and morphology. The researchers used quail hearts for this research. The quail hearts were optically paced at around 180 beats per minute at stage 13 of their embryonic development (coincides with weeks 3-4 for human development) for 5 minutes. The elevated pacing of the heart tired the heart and this caused 1 hour of regurgitant flow. The morphological changes caused by the regurgitant flow was observed using OCT imaging at stage 19 of avian embryonic development (coincides with cardiac looping stages in human development) or stage 35 (coincides with week 8 of human development). The results showed that all the paced embryos that were observed at stage 19 showed morphological changes in their endocardial cushions. It was also noted that there was an inverse relationship between the regurgitant flow and cardiac cushion size 24 hours post pacing. Out of the embryos that survived till stage 35, 17/18 of them displayed CHDs such as valve defects, ventricular defects, hypo plastic ventricles and common AV valve.<ref name="PMID28211263><pubmed>28211263</pubmed></ref> | |||
===CTCF counter-regulates cardiomyocyte development and maturation programs in the embryonic heart=== | |||
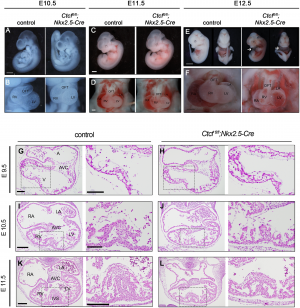
[[File:Figure 1 Morphological defects in CTCF mutant embryonic hearts.PNG|300px|thumb|right| '''Figure 14:''' Morphological defects in CTCF mutant embryonic hearts]] | |||
CTCF is a DNA binding factor that is essential in the process of genome binding and cardiogenesis. It mediates genomic interaction and maturation in the developing heart, coordinates cardiomyocyte differentiation by facilitating enhancer-promoter interactions. Although the role of CTCF in genome organisation is not fully understood. | |||
In this study by ''Gomez-Velazquez et.al (2017)'', they studied the effect of genetically deleting CTCF in differentiating cardiomyocytes at early stages of mouse development. They deleted CTCF in a population of cardiac progenitor cells which results in malformation of the heart and death of embryo (Figure 14) <ref name="PMID28846746><pubmed>28846746</pubmed></ref>. | |||
From Figure 14, they presented control and CTCF-absent mice in stage E9.5, E10.5, E11.5 and E12.5. | |||
E10.5 and E11.5 mutant embryos appeared normal (Fig 14A - 14D). Pericardial edema (Fig 14E) and non expanding cardiac chamber (Fig 14F) presented in E12.5 mutant embryo <ref name="PMID28846746><pubmed>28846746</pubmed></ref>. | |||
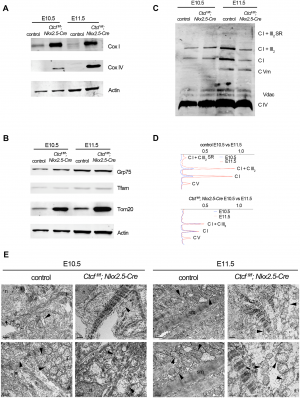
[[File:Figure 2 - defects of mitochondria in CTCF mutant hearts.PNG|300px|thumb|middle|'''Figure 15:''' Defects of mitochondria in CTCF mutant hearts]] | |||
Histological examination showed slightly disorganised interventricular septum and normal 4 chambers and atrioventricular canal in E10.5 mutant embryo (Fig 14I and 14J). Fig 14G and 14H showed no defects in E9.5 mutants hearts as compared to controls. Fig. 14K and 14L showed the thinning of myocardial wall in E11.5. | |||
They also carried out Western blot to analyse the component of oxidative phosphorylation pathway in mutant and control embryonic cardiomyocytes. They revealed that there was an increase in Complex IV subunit 1 (Cox 1, encoded in mitochondrial DNA) and Complex IV subunit IV (Cox IV) in CTCF mutants hearts at E10.5 and E11.5 (Figure 15A). Fig 15B showed an increased in Tom20 which is the major receptor of mitochondrial outer membrane translocase. Complex I and Complex V increased from E10.5 to E11.5 in control hearts, consistent with maturation of mitochondrial oxidative phosphorylation. However, they did not increase in CTCF mutant hearts and Complex V was similarly increased between E10.5 and E11.5 in both control and mutant hearts (Fig 15C and 15D). Thus, this suggests that despite increased transcription of subunit in complexes and supercomplexes at the mitochondrial inner membrane, maturation of the respiratory chain is blunted in the CTCF mutant heart. | |||
Transmission electron microscopy (TEM) analysis (Fig 15E) presented immature but normal mitochondria in CTCF mutant cardiomyocytes. At E11.5, mutant cardiomyocyte's mitochondria are swollen, and larger than controls and are disorganised at E10.5 | |||
They concluded that when CTCF is removed, genes are misregulated which leads to faulty mitochondria and incorrect expression of cardiac patterning gene. These misregulated genes control opposing genetic program incharge of development and cardiomyocyte maturation. Subsequently, embryo lethality <ref name="PMID28846746><pubmed>28846746</pubmed></ref>. | |||
===Erbb2 Is Required for Cardiac Atrial Electrical Activity during Development=== | |||
As the heart being the first organ to function during embryonic development (22 days in human, 8-8.5 days in mice) and is necessary for embryo survival. It depends on the sinoatrial node (SAN), the pacemaker of the heart’s electrical activity located in the right atrium of the heart and the conducting system, which transduces the electrical signal through the heart tissue for myocardial contraction. This system consists of atrioventricular node (AVN), atrioventricular bundle (AVB), bungle branches and Purkinje fibres, allows synchronized contraction of the atria and ventricles and a consistent heart rhythm. Thus, defects in the development of cardiac electrical function could lead to various heart disorders, such as heart block, long Q-T syndrome, atrial and ventricular fibrillation and tachycardia <ref name="PMID16643374><pubmed>16643374</pubmed></ref> | |||
Epidermal Growth Factor Receptor 2 (Erbb2) is a transmembrane protein that is essential for normal embryonic development <ref name="PMID25269082><pubmed>25269082</pubmed></ref>. | |||
It is known that at mid-gestation knockout mice lacking Erbb2 are lethal and die due to severe cardiac defects that show enlarged heart with thin ventricular myocardium, absent trabeculae and decreased atrioventricular cushions. Consequently, it results in poor circulation and irregular heartbeat <ref name="PMID7477377><pubmed>7477377</pubmed></ref>. Other studies suggested that Erbb2 participates in ventricular conduction system development and maturation. However, the role of Erbb2 in atrial conduction system development is still unknown. | |||
In a study by Tenin et. al (2014), they showed that upon mutation in Erbb2 in I11Jus8 mice resulted in specific defect in atrial electrical propagation and thus displayed cardiac hemorrhage and failure in atrial function but did not affect ventricular conduction. The role of Erbb2 in I11Jus8 mouse line reported to have significant impact on the atrial function, which allows the embryo to survive. <ref name="PMID25269082><pubmed>25269082</pubmed></ref>. Although, other studies revealed that embryonic cardiac defects are not found in mice with deletion of Erbb2, it has yet to determine whether non-cardiomyocyte tissue requires Erbb2 function for cardiac conduction system development in Tenin and his colleagues’s experiment. Further more, it has been proposed that Erbb2 functions in adult cardiomyocytes where deletions of Erbb2 causes early cardiac dysfunction and dilated cardiomyopathy <ref name="PMID12072561><pubmed>12072561</pubmed></ref>. | |||
It was also demonstrated that dilated cardiomyopathy in human and mice contributes to failure of the conduction system leading to arrhythmias and sudden death <ref name="PMID12072561><pubmed>12072561</pubmed></ref>,<ref name="PMID25269082><pubmed>25269082</pubmed></ref> | |||
===Dietary supplementation of NAD in prevention of miscarriages and birth defects=== | |||
Sydney’s Victor Chang Cardiac Research Institute is the centre point of research to prevent recurrent miscarriages and multiple types of birth defects of the heart, spine, kidney and cleft palate in newborn babies. | |||
Scientists have demonstrated a potential cure, in the form of a common dietary supplement. The research study found that a deficiency in a vital molecule, known as NAD, can prevent a baby’s organs from developing correctly in the womb. | |||
Nicotinamide adenine dinucleotide (NAD) is one of the most important molecules in all living cells. NAD synthesis is essential for energy production, DNA repair and cell communication. Environmental and genetic factors disrupt its production can cause a NAD deficiency resulting to cripple an embryo when it forms. | |||
The dietary supplement vitamin B3 is required to make NAD and is found in food such as meats and green vegetables. Research shows at least a third of women have low levels of vitamin B3 in their first trimester of pregnancy, which is the critical time in organ development. There is an indication that pregnant women may require more vitamin B3 than it is currently available in most vitamin supplements. | |||
Using a preclinical mouse model, vitamin B3 was introduced into the mother’s diet. The dietary change prevented both the miscarriages and birth defects completely. This discovery can be likened to the revolutionary breakthrough made last century that confirmed folic acid supplementation can prevent spina bifida and other neural tube defects in babies. | |||
The next step would be to develop a diagnostic test to measure NAD levels. This would enable doctors to identify the women who are at greater risk of having a baby with a birth defect, and ensure they are getting sufficient amount of vitamin B3. | |||
==Animal Models == | ==Animal Models == | ||
===Mice Model=== | |||
Understanding the importance of different molecular pathways in heart development was made possible by studies conducted in different animal models including mice models. Such studies, in particular loss of function analysis studies, have allowed for the determination of specific roles of various signaling pathways in specific stages of cardiac development. For example, one of the studies conducted in mice embryo involved studying the effect of ALK2 receptor deletion <ref><pubmed>15226263</pubmed></ref>. This receptor is expressed on cardiac neural crest cells which contribute to the formation of cardiac outflow tract (OFT). Bone morphogenetic protein (BMP), an important protein in heart signaling processes, acts as a ligand for this receptor. Thus, by binding to this receptor, BMP play a role in neural crest development and migration. Therefore, since this receptor function to determine the downstream signaling specificity, understanding its function will allow the mechanisms by which BMP regulate heart development to be uncovered. However, the receptor was not deleted as this will lead to mice lethality, but instead its function was abolished in neural crest cells. Mice with abolished ALK2 receptor function revealed various cardiac defects such as aortic arch defect and cardiac OFT defect. These defects are very similar to the common human congenital heart disease. This occurrence of these cardiac defects is indicative of impaired neural crest cell migration and it provides evidence for the importance of BMP in heart development. | |||
Since the origin of cells contributing to different parts of the valves remains controversial, another research on mice model <ref><pubmed>15297379</pubmed></ref> provides further insight into the origin of these cells. In this study, myocardial, endocardial and neural crest cells were labelled irreversibly using a genetic system during embryonic development to determine their eventual contribution to different components of the valves. At E17.5 majority of cells present in the cardiac valves were positive for β-galactosidase expression which is indicative of endocardial origin. Cells of the endocardial lineage were detected in leaflets and tendinous cords of the mitral and tricuspid valves, the atrioventricular fibrous continuity and the leaflets of the aortic and pulmonary valves. However, these components showed minimal contribution from cells of the myocardial and neural crest lineages. | |||
===Zebrafish Model=== | |||
[[File:Reduced cardiomyocyte differentiation at the venous pole in isl1 mutant embryos.png|300px|thumb|'''Figure 16: ''' Reduced cardiomyocyte differentiation at the venous pole in isl1 mutant embryos]] | |||
Growth of the heart tube into a two-chambered heart in zebrafish is regulated by two separate phases of cardiomyocyte differentiation <ref><pubmed>19395641</pubmed></ref>. These two phases are detected using a developmental timing essay and a photoconvertible marker. In the first phase, a continuous wave of cardiomyocytes differentiation appears in the ventricle initially and extend through atrium allowing the addition of cardiomyocytes to the venous pole of the heart tube. This phase is regulated by the islet1 protein and islet mutants show reduced cardiomyocyte differentiation at the venous pole (Fig. 15). However, the second phase involves Fgf signaling and it is initiated by the addition of new cardiomyocytes to the arterial pole. Therefore, these two processes are independent of each other in space, time and regulation and they are essential for heart development in Zebrafish. Modified regulation of these two phases can result in a change of heart size and morphology. | |||
===Chick Model=== | |||
Initial stages of cardiac looping involve the formation of a c-shaped tube that is curved towards the right side of the embryo. This c-shaped tube is formed as a result of ventral bending and rightward rotation. The genetic and molecular pathways involved in this process of cardiac looping have been well understand but the role mechanical forces play in this process remains poorly understood. This study on chick embryos <ref><pubmed>15282152</pubmed></ref> shows that bending and rotation are mediated by different sets of forces. While bending is controlled by intrinsic forces to the heart, rotation into a c-shaped tube is driven by external forces exerted mainly by a membrane pushing against the heart tube called splanchnopleure (SPL). Also, some of these forces come from omphalomesenteric veins (OVs) which plays a role in the directionality of left-right looping. These results were obtained from analysis of tissue stress and strain using dissection and fluorescent labelling respectively after subjecting the embryos to mechanical perturbations. | |||
Other research into chick models reveals that cardiac progenitors of the splanchnic mesoderm, cardiac neural crest and the proepicardium are the major embryonic contributors to the development of the chick heart. The contribution of these components to cardiac development occurs with precise timing and regulation during such processes as primary heart tube fusion, cardiac looping and accretion, cardiac septation and the development of the coronary vasculature. In addition to these findings, chick models revealed that one role of the anterior endoderm is to create a “cardiac field” in the overlying anterior mesoderm, and that other events will restrict this cardiac field to the region of the embryo that will become the heart later in development <ref><pubmed> 15986452</pubmed></ref>. | |||
===Xenopus Model=== | |||
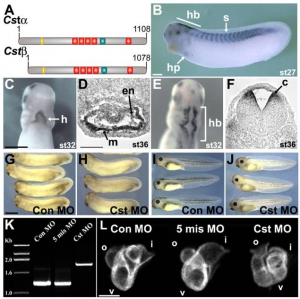
Along the molecular pathways mentioned in the heart signaling section, CASTOR (CST) protein previously identified in Drosophila for its role in maintaining stem cells competence at the dorsal midline, is now shown to play a role in cardiogenesis. A study using Xenopus as an animal model <ref><pubmed>18410736</pubmed></ref> identified CST expression in the myocardial layer of the heart in a gradient that extends from dorsal to ventral. The expression of CST in this region within a subset of cardiac progenitor cells is necessary for the initiation of cardiomyocyte differentiation at the ventral midline (Fig. 17). Tracing the fate of these cardiac tissue, it is shown that these cardiac progenitor cells at the ventral midline region give rise to a population of cells in the outer curvature of the ventricle indicating their specific cell fate. However, progenitor cells that are deficient in CST protein overproliferate and do not end up integrating into cardiac muscle. This suggests that CST within the subset of cardiac progenitor cells in vertebrates is essential for proper timing of differentiation in order for these progenitor cells to give rise to the outer curvature of the ventricle. Therefore, Xenopus animal model has helped to have a better understanding or the role of CST in vertebrate cardiac development. | |||
[[File:CST Is Required for Vertebrate Heart Development.PNG|300px|thumb|none|'''Figure 17: ''' CST Is Required for Vertebrate Heart Development]] | |||
==Abnormal Development== | ==Abnormal Development== | ||
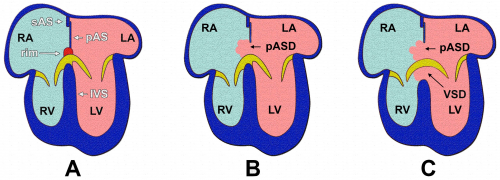
===Atrial Septal Defect=== | |||
[[File:Atrial Septal Defect (ASD).png|300px|thumb|right|'''Figure 18: ''' Atrial Septal Defect (ASD), as demonstrated by the red arrow. ]] | |||
Atrial septal defects (ASD) are one of the most prevalent congenital heart malformations, with an estimated 56 cases per 100,000 births.<ref name="PMID24725467><pubmed>24725467</pubmed></ref> This heart defect involves a communication between the left and right atria, and can be subdivided into five main types based on the nature of the defect. | |||
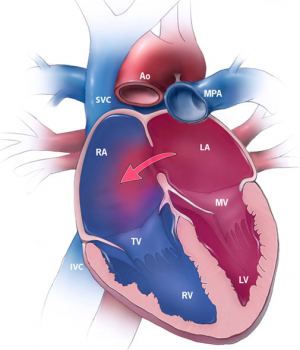
The most common ASD is a patent foramen ovale. Throughout fetal development, the foramen ovale functions to shunt oxygenated blood from the right atrium into the left atrium.<ref name="PMID4711537><pubmed>PMC4711537</pubmed></ref> After birth, the now functioning respiratory system of the newborn results in elevated pressure in the left atrium relative to that of the right.<ref name="PMID5505397><pubmed>PMC5505397</pubmed></ref> This induces fusion of the septum primum and septum secundum which closes the foramen ovale, leaving a remnant known as the fossa ovalis.<ref name="PMID4711537"/> Incomplete closure results in a patent foramen ovale. This condition is present in nearly all newborns and typically closes within the first month, however complete closure is achieved in only 70-75% of adults.<ref name="PMID24725467"/> A patent foramen ovale is often asymptomatic and thus is not usually considered a clinically significant condition, however it can be problematic when other cardiac abnormalities are present.<ref name="PMID325204><pubmed>PMC325204</pubmed></ref> | |||
A secundum ASD is the most common of the true (clinically significant) atrial septal defects. This malformation occurs within the region of the fossa ovalis and typically results from abnormal resorption of the septum primum during cardiac development.<ref name="PMID325204"/> Secundum ASDs enable left-to-right atrial shunting of blood, which increases the load on the right side of the heart and, consequently, the pulmonary circulation; this can result in the development of atrial arrhthymias and right ventricular failure.<ref name="PMID4711537"/> | |||
Sinous venosus ASD refers to a communication between one of the right pulmonary veins (typically the upper vein) and the wall of the right atrium adjacent to the superior vena cava.<ref name="PMID325204"/> This defect can occur as a result of the resorption or partial absence of the septum secundum,<ref name="PMID325204"/> and accounts for 5-10% of all atrial septal defects.<ref name="PMID4373719><pubmed>PMC4373719</pubmed></ref> | |||
A primum ASD is the result of an abnormality in the development of either the septum primum or septum secundum.<ref name="PMID325204"/> This condition is a type of atrioventricular septal defect, and will be discussed in more detail in the next section. | |||
The most extreme ASD is a common atrium. This occurs when the septum primum and septum secundum fail to develop.<ref name="PMID24725467"/> This lack of an interatrial septum is often accompanied by other cardiac abnormalities, including clefts in the leaflets of the atrioventricular valves.<ref name="PMID325204"/> | |||
===Atrioventricular Septal Defect=== | |||
[[File:Stages of Atrial Septation.png|500px|thumb|right|'''Figure 19: ''' Comparison between a normal heart (A), a heart with a primum ASD (B) and a heart with an AVSD (C). ]] | |||
An atrioventricular septal defect (AVSD) refers to a malformation involving the interatrial and/or interventricular septa, and has an estimated incidence of 1 in 2000 births.<ref name="PMID5267359><pubmed>PMC5267359</pubmed></ref> This defect typically occurs as a result of incomplete fusion of the endocardial cushions,<ref name="PMID4373719"/> although recent research has concluded that malformation of the dorsal mesenchymal protrusion (DMP) also contributes to the development of AVSDs.<ref name="PMID5267359"/> This can result in abnormal development of the interatrial septum, interventricular septum and/or the mitral and tricuspid valves.<ref name="PMID325204"/> | |||
There are two main subdivisions of AVSDs, which differ according to whether one or both of the cardiac septa are affected. In a partial AVSD, there is a defect present in the inferoanterior region of the fossa ovalis, which allows the right-to-left shunting of blood across the atria.<ref name="PMID5267359"/> In this scenario, the lack of fusion of the endocardial cushions can also result in the presence of clefts in the septal leaflets of the atrioventricular valves.<ref name="PMID325204"/> However, in a complete AVSD, a defect in the membranous portion of the interventricular septum is also present.<ref name="PMID4373719"/> This condition results in direct communications between the atria and the ventricles, and pulmonary hypertension or congestive heart failure can occur if the anomaly is severe.<ref name="PMID5267359"/> | |||
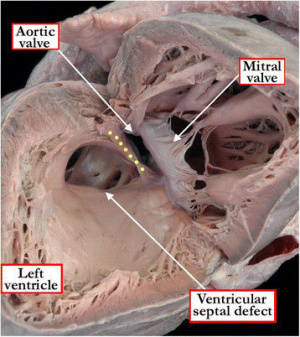
===Ventricular Septal Defect=== | ===Ventricular Septal Defect=== | ||
[ | [[File:Ventricular Septal Defect (VSD).jpeg|300px|thumb|right|'''Figure 20:''' Ventricular Septal Defect (as viewed from the left ventricle)]] | ||
Ventricular septal defects are the most prevalent of all congenital heart defects (excluding patent foramen ovales), accounting for approximately 40% of all cardiac abnormalities among newborns.<ref name="PMID21349577><pubmed>21349577</pubmed></ref> The exact definition of a VSD is still a point of contention within the scientific community, but is generally referred to as a communication between the left and right ventricles; this anomaly can manifest as a lone defect, but is often present with other abnormalities such as Tetralogy of Fallot or a complete AVSD.<ref name="PMID4316658><pubmed>PMC4316658</pubmed></ref> occur as a result of abnormal development (or absence) of any of the components forming the interventricular septum.<ref name="PMID7437181><pubmed>7437181</pubmed></ref> Hence, a VSD can occur in either the muscular or the membranous portion of the septum, with the latter being more common.<ref name="PMID7437181"/> | |||
A membranous VSD occurs when there is an incomplete occlusion of the interventricular foramen, which is due to the membranous portion of the septum failing to develop.<ref name="PMID325204"/> This is the result of a failure of fusion between the muscular portion of the interventricular septum and the endocardial cushions.<ref name="PMID325204"/> This defect is most commonly located in the outflow tract of the left ventricle.<ref name="PMID325204"/> | |||
A muscular VSD results from abnormal development of the interventricular septum primordium during cardiac septation.<ref name="PMID4316658"/> This can refer to incomplete formation of the primitive septum, or to errors in the proliferation of trabeculae within the septum.<ref name="PMID21349577"/> VSDs can form anywhere within the muscular wall of the septum, and can extend into various regions of the right ventricle including the apex, inlet or outflow tract.<ref name="PMID21349577"/> | |||
The most extreme form of VSD is a common ventricle. This very rare defect occurs when the interventricular septum primordium fails to develop.<ref name="PMID325204"/> Infants with common ventricle often die from congestive heart failure.<ref name="PMID325204"/> | |||
===Persistent Truncus Arteriosus=== | |||
Persistent truncus arteriosus (PTA) is a rare heart defect accounting for less than 1% of all congenital cardiac anomalies.<ref name="PMID21524457><pubmed>21524457</pubmed></ref> PTA occurs when the truncus arteriosus of the developing heart fails to partition into the ascending aorta and pulmonary trunk.<ref name="PMID4021394><pubmed>PMC4021394</pubmed></ref> This results in the formation of a common arterial trunk that provides a mixture of oxygenated and de-oxygenated blood to systemic, pulmonary and coronary circulations.<ref name="PMID27126954><pubmed>27126954</pubmed></ref> Although the exact aetiology of PTA is unknown, cellular ablation studies have demonstrated that aberrant migration and/or development of neural crest cells can result in abnormal aorticopulmonary septation.<ref name="PMID4021394"/> PTA has a poor prognosis without surgical intervention, with only 20% of patients surviving beyond the first year.<ref name="PMID21070356><pubmed>21070356</pubmed></ref> | |||
[[File:Truncus Arteriosus.png|250px|thumb|left|'''Figure 21: ''' Persistent Truncus Arteriosus (PTA). ]] | |||
=== | ===Tetralogy of Fallot=== | ||
[[File:Tetralogy of Fallot (TOF).png|250px|thumb|right|'''Figure 22: ''' Characteristics of Tetralogy of Fallot (TOF). Ventricular septal defect (1) Pulmonary stenosis (2) Dextroposition of aorta (3) Right ventricular hypertrophy (4). ]] | |||
Tetralogy of Fallot (TOF) is a complex congenital heart defect with an incidence of 3 cases per 10000 live births.<ref name="PMID2651859><pubmed>PMC2651859</pubmed></ref> It is also the most common cyanotic cardiac abnormality, accounting for 7-10% of all congenital heart malformations.<ref name="PMID2651859"/> The condition is characterised by four heart abnormalities:<ref name="PMID20091166><pubmed>20091166</pubmed></ref> | |||
*''Pulmonary stenosis'' – narrowing of the pulmonary trunk and associated pulmonary valve. | |||
*''Ventricular septal defect (VSD)'' – a communication between the left and right ventricles | |||
*''Dextroposition of the aorta'' – rightward displacement of the aorta over the ventricular septal defect. | |||
*''Right ventricular hypertrophy'' – enlargement of the myocardium of the right ventricle | |||
Tetralogy of Fallot typically presents as cyanosis (bluish discolouration of the skin) in the newborn.<ref name="PMID20734579><pubmed>20734579</pubmed></ref> This is due obstruction of pulmonary outflow, as well as right-to-left shunting of blood across the ventricles via the ventricular septal defect.<ref name="PMID4460219><pubmed>PMC4460219</pubmed></ref> This collectively results in poorly oxygenated blood being discharged from the left ventricle into systemic circulation.<ref name="PMID4460219"/> | |||
Newborns with TOF typically undergo corrective surgery within the first month of life.<ref name="PMID2651859"/> Pulmonary stenosis is relieved via widening of the pulmonary trunk and the ventricular septal defect is repaired, allowing the outflow of only well-oxygenated blood through the aorta.<ref name="PMID4460219"/> | |||
===Hypoplastic Left Heart Syndrome=== | |||
[[File:Hypoplastic Left Heart Syndrome (HLHS).png|250px|thumb|right|'''Figure 23: ''' Hypoplastic Left Heart Syndrome (HLHS). An atrial shunt is represented by the red arrow.]] | |||
Hypoplastic Left Heart Syndrome (HLHS) is a congenital heart defect that involves the underdevelopment of cardiac structures of the left side of the heart; structures effected include the left ventricle, ascending aorta, aortic arch and the mitral and aortic valves.<ref name="PMID1877799><pubmed>PMC1877799</pubmed></ref> Despite being one of the rarer heart defects, accounting for up to 3.8% of congenital cardiac malformations, HLHS is responsible for 23% of all cardiac-related deaths in the first week of life.<ref name="PMID5313512><pubmed>PMC5313512</pubmed></ref> Severity of the disease depends on the extent of systemic outflow obstruction, the number of heart structures effected, and the degree of hypoplasia of the left ventricle and ascending aorta.<ref name="PMID1877799"/> | |||
Initially, newborns with HLHS will appear healthy. This is because the presence of the foramen ovale and a patent ductus arteriosus allows blood to bypass the malformed left side of the heart and enter systemic circulation.<ref name="PMID5313512"/> Once these structures close, inadequate blood flow in the systemic circulatory system results in hypoxemia and cardiogenic shock; rapid surgical intervention is required to prevent death of the newborn.<ref name="PMID1877799"/> Corrective surgery for neonates with HLHS is performed in three stages, with the ultimate goal of increasing systemic circulation and bypassing the malformed side of the heart; the end result is a modified right ventricle that supports both systemic and pulmonary circulation.<ref name="PMID4460219"/> | |||
=== | The first operation, known as the Norwood operation, is performed at birth. This involves the construction of a new ascending aorta and arch that extends from the right ventricle, as well as the establishment of a communication between the right ventricle and the pulmonary trunk, which allows for both pulmonary and systemic perfusion from the right ventricle.<ref name="PMID5313512"/> Next, a Glenn shunt operation is performed at 6-8 months after birth, in which a surgical anastomosis is created between the right pulmonary artery and superior vena cava, which decreases the work on the right ventricle during venous return.<ref name="PMID1877799"/> Finally, a Fontan procedure is performed 1.5-4 years after birth, which involves establishing a communication between the now connected pulmonary artery and superior vena cava to the wall of the right atrium.<ref name="PMID1877799"/> These surgeries collectively result in the development of univentricular circulation in which oxygenated and de-oxygenated blood are unable to mix, which increases the efficiency of the neonate’s cardiovascular system.<ref name="PMID5313512"/> | ||
==Cardiac Stem Cells== | |||
Following a myocardial infarction (MI), a significant number of cardiomyocytes becomes damaged and the heart has very little regenerative functions. The loss of cardiomyocytes pose as a problem when an individual has had repeated MI or those suffering from end stage heart failure. For these individuals, heart transplantation remains as the main solution. However, there are not enough donors available. Therefore, stem cell therapy provides as an upcoming possibility in replacement for donor transplant. There are two types of cells that could be use which includes human embryonic Stem cells (hESCs) and Human Induced Pluripotent Stem Cells (hiPSCs). However, there are differences between these two cells and further testing and applications are still required to produce highly pure and mature cardiomyocytes. | |||
{| class="wikitable" | |||
! style="background:#f4a941" | FACTOR | |||
! style="background:#f4a941" | Human Embryonic Stem Cells | |||
! style="background:#f4a941" | Human Induced Pluripotent Cells | |||
|- | |||
| Ethical Issues || | |||
*More ethical issues | |||
*Need to use a large amount of embryos during derivation as the hESCs are obtained from the inner cell mass of the blastocysts<ref name="PMID9804556><pubmed>9804556</pubmed></ref> | |||
*Associated with invasive procedures | |||
|| | |||
*Lesser ethical issues | |||
*Obtained from adult cells | |||
*Need not be associated with invasive procedures as cells can be obtained from hair cells or blood cells<ref name="PMID20964482><pubmed>20964482</pubmed></ref> | |||
|- | |||
| Immune Reactions || | |||
*Cell genome not matching patient’ genome | |||
*Possible immune rejection against allogenic hESCs | |||
|| | |||
*Cell genome matching patient’s genome | |||
*Less likely immune rejection for isogenic hiPSCs | |||
|- | |||
| Availability of Cells || | |||
*Only a small amount of hESCs are able to differentiate into cardiac myocytes | |||
|| | |||
*Only a small amount of hiPSCs are able to differentiate into cardiac myocytes | |||
*hiPSCs take a very long time for differentiation. | |||
|- | |||
|} | |||
=== | {| class="wikitable" | ||
! style="background:#f4a941" | FACTOR | |||
! style="background:#f4a941" | Human Embryonic Stem Cells and Human Induced Pluripotent Stem Cells | |||
|- | |||
| Teratoma formations || Both have tendency to form teratomas. Cardiomyocytes thus have to be highly purified. A few methods have been explored to obtain a highly purified culture of stem cells to prevent teratoma formation. The following methods include: | |||
'''Mitochondrial Based Separation'''<ref name="PMID19946277><pubmed>19946277</pubmed></ref> | |||
*Non-genetic approach | |||
*Based on the fact that cardiac myocytes that are differentiated contains a high number of mitochondria | |||
*Mitochondria specific fluorescent dye used to identify differentiated cardiomyocytes from non-differentiated ones. Cells then separated using flow cytometry. | |||
*~ 99% purity | |||
*Furthur research and successful applications in stem cell experiments are required. Technological advancements and improvements are also needed for large-scale use. | |||
'''Biochemical Differences between Differentiated and Undifferentiated Cardiomyocytes'''<ref name="PMID23168164 ><pubmed>23168164 </pubmed></ref> | |||
*Non-genetic approach | |||
*Only differentiated cardiomyocytes could survive in a glucose depleted and lactate abundant culture. | |||
*Simple applications compared to the mitochondrial based separation. | |||
*~ 99% purity which did not form teratomas | |||
However, because both methods do not have a 100% purity, teratomas could still be formed. | |||
|- | |||
| Cardiac Maturation || | |||
'''Ultrastructural Analysis''' | |||
*The ultrastructural features of both hESCs and hiPSCs were both phenotypically immature, that is it had an abundant amount of lipid droplets and endoplasmic reticulum, elevated glycogen content and different degrees of myofibrillar organization <ref name="PMID21883888 ><pubmed>21883888</pubmed></ref> | |||
'''Electrophysiological Properties''' | |||
*Both hESCs and hiPSCs displayed all three action potential phenotypes: nodal, atrial and ventricular-like, generally exhibiting a phenotypically immature cardiomyocytes<ref name="PMID12791707 ><pubmed>12791707</pubmed></ref> | |||
'''Contraction Properties''' | |||
*The more mature the sarcoplasmic reticulum, the better the contractile properties of the cardiomyocytes.<ref name="PMID21614516 ><pubmed>21614516</pubmed></ref> hESCs had an immature sarcoplasmic reticulum (SR) whereas hiPSCs had a slightly more mature sarcoplasmic reticulum. <ref name="PMID16322641 ><pubmed>16322641 </pubmed></ref> | |||
Since hESCs and hiPSCs are required to replace damaged cardiomyocytes, their obvious immaturity problem would have to be resolved before it can be utilized for transplantation or therapy. | |||
|- | |||
|} | |||
==Future Questions== | ==Future Questions== | ||
==Glossary of Terms== | ===What is the aetiology of Tetralogy of Fallot?=== | ||
The aetiology of Tetralogy of Fallot is complex and still poorly understood. Current research in genetics has linked the condition with various chromosomal abnormalities, including trisomy 13, 18 and 21, as well as microdeletions within chromosome 22.<ref name="PMID2651859"/> In addition, current research has identified risk factors such as maternal diabetes and exposure to retinoic acid.<ref name="PMID2651859"/> Further research in this area is required to achieve a greater understanding of the underlying causes of Tetralogy of Fallot. | |||
===Regulation of forces involved in cardiac looping=== | |||
Studies have identified some mechanical forces involved in looping, it remains to be established how these forces are regulated spatially and temporally to produce a looped heart tube. It needs to be further investigated to determine whether the proposed actin polymerization mechanism can produce the necessary changes in tissue shape on a global scale. In addition, the possible roles played by redundant mechanisms have not been elucidated. Finally, the mechanisms of initial looping of the heart tube and s-looping have received relatively little attention. | |||
To gain a complete understanding of heart development requires finding the link between gene expression and morphomechanics, which will need to combine the expertise of developmental biologists and biomechanical engineers <ref name="PMID16479500><pubmed>16479500 </pubmed></ref>. | |||
===Signalling of the heart fields=== | |||
Multiple signalling pathways overlap to regulate SHF cell addition to the poles of the heart tube, as well as SHF proliferation and myocardial differentiation. The signalling molecules identified to highlight the continuance of SHF cells in a progenitor cell state and controls their progressive differentiation. However, the control of differentiation delay and SHF movement into the OFT remains obscure. Future research into gene regulatory networks and signalling pathways that control SHF development will identify mechanisms underlying these processes. This will provide a greater understanding of the extent to which these networks differ from those controlling differentiation of the linear heart tube. | |||
Further exploration of the mechanisms underlying SHF progenitor cell development in the early embryo is required, as it will uncover the potential of progenitor and pluripotent stem cells for cardiac repair <ref name="PMID 19390062 ><pubmed> 19390062 </pubmed></ref>. | |||
===Specific identification of cardiac precursors=== | |||
Heart precursors were identified as occupying anterior mesoderm, proximal to the embryonic-extraembryonic boundary. This identification was achieved utilising cell transplantation, labelling of live embryos and conducting embryo culture. These embryonic manipulations highlighted the plasticity of populations that contribute to the heart. This means cells obtained from a different location or developmental time point can contribute to the heart once placed in the proper location. Subsequently, such experiments <ref><pubmed>23457256 </pubmed></ref> were able to locate the cardiac precursors but they failed to specifically identify these precursors and therefore cardiac precursors have not been identified and characterised yet. Thus, future experiments will aim to address this question. | |||
===Cardiac Regeneration=== | |||
There are vast differences between the potential of cardiac regeneration in experimental models like zebrafish which is lost in adult mammals like humans. Researches speculate that it could be due to internal properties of cardiomyocytes or due to the failure of stem cell populations. They also hypothesize that it could be due to non-cardiomyocytes that could cause scarring which might hinder regeneration. In addition, more research has to be done on how regeneration begins and ends (signalling pathways involved etc). | |||
===Role of bone morphogenetic protein (BMP) in cardiac neural crest development and migration=== | |||
The role of BMP has been implicated in many aspects of organogenesis including neural crest development and migration. However, it remains unclear to whether BMP impose a direct action on neural crest cells during cardiac morphogenesis or acts indirectly by affecting gene expression <ref><pubmed>15226263</pubmed></ref>. Also, studies of BMP receptor signaling have been hard to conduct as receptor deletion lead to death of the embryo at early stages of development. It was only recently that such studies of the receptor were performed by blocking the function of the receptor in neural crest cells. Thus, future experiments must be conducted to uncover the mechanism by which BMP act to regulate neural crest development and migration. | |||
==Glossary of Terms== | |||
Definitions provided in this glossary are taken from lectures presented in the UNSW embryology course ANAT2341, as well as from the UNSW embryology wiki glossary provided online, which is linked below. | |||
{| class="pretty table" | |||
|-bgcolor = "DDCEF2" | |||
|'''Term''' | |||
|'''Definition''' | |||
|--bgcolor="FAF5FF" | |||
|''' Anastomosis''' | |||
| Term used to describe the connection between two tubes. Applied to describe the connection between peripheral blood vessels without an intervening capillary bed. | |||
|- | |||
| '''Angioblastic cords''' | |||
| Groups or ‘columns’ of embryonic precursor cells which will form the walls of both arteries and veins. | |||
|--bgcolor="FAF5FF" | |||
|''' Axial mesoderm''' | |||
| Also referred to as notochord. It is an embryonic structure lying within the midline of the trilaminar embryo. | |||
|- | |||
|''' Blastocoel''' | |||
|A fluid filled cavity formed during early embryonic development (Week 1-2) within the blastocyst. Once Morula is formed as a result of the initial cell division, further cell division followed by compaction leads to the formation of this cavity. | |||
|--bgcolor="FAF5FF" | |||
|'''Bulbar Ridge''' | |||
| Term describing a spiral region in the developing heart bulbus cordis, there is a left and right bulbar ridge, that contribute to the septation of membranous portion of ventricular septum that is continuous with the outflow tract. These regions fuse to separate the single embryonic outflow tract into the aortic and pulmonary arteries. | |||
|- | |||
|''' Bulbus cordis''' | |||
| A region of the early developing heart tube forming the common outflow tract, will differentiate to form three regions of the heart. | |||
|--bgcolor="FAF5FF" | |||
|'''Cardiac Jelly''' | |||
|Term used in early heart development to describe the initial gelatinous or sponge-like connective tissue separating the myocardium and the heart tube endothelium. | |||
|- | |||
|''' Cardiocyte''' | |||
| Mature muscle cell of the heart. These cells are characterized by striation and separated by intercalated discs. | |||
|--bgcolor="FAF5FF" | |||
|'''Cyanotic Heart Disease''' | |||
|Clinical term referring to a congenital heart abnormality (defect) resulting in lack of oxygen that causes cyanosis, a blue coloration of the skin and mucous membranes due to the presence of deoxygenated hemoglobin. | |||
|- | |||
|'''Ductus Arteriosus''' | |||
|A prenatal vascular "shunt" which connects the left pulmonary artery and the descending aorta. Postnatal neonatal patency (patent ductus arteriosus, PDA) is a relatively common congenital cardiac anomaly occurring more frequently in premature infants. | |||
|--bgcolor="FAF5FF" | |||
|'''Endocardial Cushion''' | |||
|Early heart development structures formed before atrial and ventricular septation occurs. These four heart wall in-folds (ventral, dorsal and two lateral) lie between the future atria and ventricles, later the posterior and anterior cushions fuse forming the primordial atrioventricular canals. | |||
|- | |||
|'''Endocardium''' | |||
|The epithelial membrane lining the inside surface of heart, which along with the endothelial layer forms a continuous lining of the entire cardiovascular system. | |||
|--bgcolor="FAF5FF" | |||
|''' Epicardium''' | |||
| The inner layer of the pericardium that is in contact with the surface of the heart | |||
|- | |||
|'''Heart Field''' | |||
|The term used to describe the splanchnic mesoderm cardiogenic region in the trilaminar embryo that generates most of the heart. In humans, there are two fields the primary and secondary heart fields. | |||
|--bgcolor="FAF5FF" | |||
|'''Heart Valves''' | |||
|The heart has a series of valves which regulate the directional flow of blood. The human heart has valves separating the atria from ventricles (atrioventricular, AV) and the ventricles from the outflow tract aortas. The left atrioventricular valve has two leaflets, anterior and posterior, and is the bicuspid valve or mitral valve. The right atrioventricular valve has a third leaflet (small, septal cusp) and is the tricuspid valve. | |||
|- | |||
|''' Inflow Tract''' | |||
| Entrance of blood into the heart tube; the sinus venosus portion of the tube. | |||
|--bgcolor="FAF5FF" | |||
|''' Mesothelial cells''' | |||
| Epithelial cells of mesodermal origin | |||
|- | |||
|''' Mitochondrial oxidative phosphorylation''' | |||
| A synthesis of ATP powered by the free energy of reduced compounds that are produced by other metabolic pathways, including glycolysis and the TCA cycle, occurs in the mitochondria | |||
|--bgcolor="FAF5FF" | |||
|'''Myocardium''' | |||
|Layer that forms the muscular wall of the heart, the thickest layer formed by spirally arranged cardiac muscle cells. | |||
|- | |||
|''' Omphalomesenteric vein''' | |||
| Vessels that provide the venous pole input into the heart from the yolk sac connected at the umbilicus during embryogenesis | |||
|--bgcolor="FAF5FF" | |||
|''' Outflow tract ''' | |||
| Exit of blood from the heart tube formed by the truncus arteriosus. | |||
|- | |||
|''' Pericardium''' | |||
| The membranous sac filled with serous fluid that encloses the heart, aorta and large blood vessels | |||
|--bgcolor="FAF5FF" | |||
|''' Proepicardium''' | |||
| Group of progenitor cells that forms near the venous pole of the heart gives rise to the epicardium | |||
|- | |||
|''' Septum transversium ''' | |||
| Forms from an aggregation of mesenchyme tissue that develops within the caudal part of ventral mesentery of the foregut. It is developed at day 22 in humans. | |||
|--bgcolor="FAF5FF" | |||
|''' Sinus venosus''' | |||
| An early developmental cardiovascular structure, thin walled cavity, forming the input to developing heart which has 3 venous inputs (vitelline vein, umbilical vein, common cardinal vein). Later in heart development this structure gets incorporated into the wall of the future right atrium. | |||
|- | |||
|''' Subepicardium''' | |||
| Situated or occurring beneath the epicardium or between the epicardium and myocardium. | |||
|--bgcolor="FAF5FF" | |||
|'''Truncus arteriosus''' | |||
|An embryological heart outflow structure, that forms in early endocardial tube stage and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation of the cardiac outflow pattern, where only one artery arises from the heart and forms the aorta and pulmonary artery (Persistent truncus arteriosus). | |||
|- | |||
|'''Vascular Endothelial Growth Factor (VEGF) | |||
|A secreted protein growth factor family, which stimulates the proliferation of vascular endothelial cells and therefore blood vessel growth. | |||
|--bgcolor="FAF5FF" | |||
|'''Western Blotting''' | |||
|An important technique used in cell and molecular biology. Researchers are able to identify specific proteins from a complex mixture of proteins extracted from cells with 3 techniques: (1) separation by size, (2) transfer to a solid support and (3) marking target protein using a proper primary and secondary antibody to visualise. | |||
|} | |||
''Below are links to a more extensive glossary if additional definitions are needed'' | |||
[[A]] | [[B]] | [[C]] | [[D]] | [[E]] | [[F]] | [[G]] | [[H]] | [[I]] | [[J]] | [[K]] | [[L]] | [[M]] | [[N]] | [[O]] | [[P]] | [[Q]] | [[R]] | [[S]] | [[T]] | [[U]] | [[V]] | [[W]] | [[X]] | [[Y]] | [[Z]] | |||
==References== | ==References== | ||
<references/> | |||
Latest revision as of 08:16, 27 October 2017
| 2017 Student Projects | |||
|---|---|---|---|
|
Heart
Introduction
The cardiovascular system is the first system to develop and function in the human embryo. Rapid cardiac development is essential as the growing embryo can no longer receive oxygen and essential nutrients via diffusion alone, hence a circulatory system and a contractile heart mechanism is required to supply the embryo. We recognise the hearts normal development is vital for foetal life, and hence we have chosen to document the development of the heart from gastrulation to birth. Early cardiac development is a multifaceted procedure and is associated with other developmental processes such as: embryonic folding, coelom formation, and vascular development [1]. As a result, any defects occurring during the developmental periods can lead to congenital heart abnormalities.
Through researching the advances in technology, coupled with the biological use of suitable animal models our understanding of embryological cardiac development has evolved, and we are piecing together the mechanism underlying this development [2]. This page will outline the embryonic development of the heart, the importance of how abnormalities arise, the treatments available and possible treatments that may be available in the future. Due to the certain knowledge gaps in current embryological heart research, we acknowledge that this will impact our assignment, and aim to address further research concepts that will improve our understanding.
Anatomy of the Heart
The heart is the muscular organs that pumps blood around the body via the circulatory system. It is located within the thoracic cavity, in a compartment called the mediastinum. The heart is divided into four chambers including: left and right atria and ventricles which are compartmentalised by semilunar and atrioventricular valves. Blood moves via the systemic circuit to the organs of the body and back to the heart. The pulmonary circuit is responsible for the flow of blood between the lungs and the heart. Deoxygenated blood enters the right side of the heart, while oxygenated blood returning from the lungs exits the left side. The heart’s electrical system uses electrical signals to cause the muscular walls to contract. The mechanical pumping of the heart is essential for movement of blood which exchanges gases and essential nutrients between organs of the body. [3].
Developmental Origin
In the developing embryo the lateral plate mesoderm splits into somatic and splanchnic layers, the latter is comprised of cardiac progenitor cells. The somatic mesoderm lines the ectoderm, the splanchnic mesoderm lines the endoderm, and in between lies an embryonic coelom [4] . At the end of week three, the heart develops from splanchnic mesoderm of the cardiogenic region of the embryonic plate. At the cranial end of the embryo, anterior to the developing neural tube is the initial origin of heart formation.
Originally, the cardiogenic region forms laterally of the paraxial mesoderm and primitive streak of the embryonic disc. Due to the natural mesenchymal cell organisation of the mesoderm, cells ‘migrate’ and fuse at the midline of the cranial end of the embryo forming the cardiac crescent just prior cardiac tube formation and folding. Endoderm surrounding the primitive gut contracts bringing the cardiogenic precursor regions of the splanchnic region towards the midline. Early heart formation begins with angioblastic cords of the splanchnic mesoderm. The angioblastic cords develop into separate endochondral heart tubes moving closer together as the foregut pinches together and the yolk sac contracts into the embryo. Fusion of the two heart tubes is facilitated by apoptosis. The origin of the primitive heart is in the early pericardial coelom, which is later developed into the pericardial cavity, through fusion of the pleuropericardial folds to separate the pleural cavities at the later development of the lungs [5].
Developmental Timeline
| WEEK | DEVELOPMENT | HISTORIC CARDIOVASCULAR DISCOVERIES |
|---|---|---|
| 2 | Bilateral cardiogenic areas form | In 157 AD Galen (born on 9 September AD 129 in Pergamon, Greece) discovered the pulmonary circulation. |
| 3 | Mesoderm splits, Heart tubes are brought to the midline, Heart tube fusion, Heart beat | Realdo Columbo (1515–1559 discovered that the heart’s four valves permitted flow of blood in one direction only: from the right ventricle to the lungs, back to the left ventricle, and from there to the aorta. |
| 4 | Heart looping, Neural crest migration commences, Dorsal and ventral endocardial cushions fuse | William Harvey (born 1 April 1578) while studying in London estimated:
|
| 5 | Foramen primum closed, Septum secundum srtats developing, Muscular interventricular septum develops, Bulbar ridges and trabeculation become evident | Harvey proved blood flows in two separate loops: the pulmonary and systemic circulation [6]. |
| 6 | Aortic and pulmonary trunks cleave | The earliest study of the partitioning of the heart was conducted by cardiologist and anatomist Wilhelm His Jr (1863-1934) in 1886. Later in 1893 he discovered the bundle of His -the specialized tissue in the heart that transmits the electrical impulses and helps synchronize contraction [7]. |
| 7 | Valves develop | Franklin P. Mall (1862-1917) worked at the Department of Embryology at the Carnegie Institute of Washington where he studied the subdivisions of the primary heart tube, formation of the atrio-ventricular valves and bundle, and the musculature of the left ventricle. Additionally, in 1910 he demonstrated that the nascent atrium of the heart could be identified based on the close proximity of endothelium to the heart muscle [8] |
Primary Heart Field
Gestational Week 4-5
The first cells start to migrate through the primitive streak to the anterior and lateral sections of the cranial end of the embryonic disc, forming bilateral primary heart fields. These primary heart fields resemble a crescent shape. [2].
The lateral plate mesoderm is split into two layers, namely the splanchnic mesoderm, facing the endoderm and the somatic mesoderm, facing the ectoderm. The former portion of the mesoderm gives rise to the heart. The portion between the splanchnic and somatic mesoderm is the presumptive pericardial space. Cells from the splanchnic mesoderm will merge to form 2 lateral endocardial tubes (also known as angioblastic cords) and as they form a lumen, are enveloped by myocardium. These endocardial tubes are as of now located inferior to the presumptive pericardial space. [2]
Heart Tube Formation
Gestational Week 5
The embryonic disc starts to fold. This folding begins cranially and extends in a caudal direction. The endocardial tubes fuse and is now located between the pericardial space and newly formed foregut that becomes surrounded by pericardial space (also known as the pericardial coelom). At this stage, the myocardium does not completely engulf the endocardial tubes. Instead, it remains in a continuous attachment with the non-cardiac splanchnic mesoderm through a structure called the dorsal mesocardium.
At this point, the primitive heart tube is bilaterally symmetrical and resembles an inverted Y shape. Starting from the inflow tract, there is the right and left sinus venouses that receives blood from the embryo, followed by the primitive atrium, primitive ventricle, bulbus cordis and then the truncus asteriosus which gives rise to the aortic and pulmonary trunk .[2]
<html5media height="200" width="240">File:Heart_folding_001.mp4</html5media>
The video above depicts the folding and fusion of Heart tubes taken from [1]
Secondary Heart Field
Gestational Week 5-6
The secondary heart field (SHF) is a region of subpharyngeal mesodermal progenitor cells located medially and ventrally to the adjacent primary heart field (PHF) (that forms the initial heart tube). The PHF and SHF cells make up a region known as the cardiogenic field. Following heart tube fusion around 19-21 days, cells from the SHF migrate to the cranial and caudal ends of the tube and continue elongation. [2]
It is understood that within the cardiac regions the progenitor cell population of the SHF is “pre-patterned”[9], hence being termed “specified but undifferentiated”. It is the patterning of cells within the soon to become myocardium, that is responsible differentiation into chamber-specific myocytes (atrial and ventricular) and the conduction of cells. It is essential that the SHF cells remain undifferentiated and do not add prematurely to the heart tube.[10]
The SHF cells gives rise to endocardial, myocardial and smooth muscle cells through expression of transcription factor Islet -1 [11]. It also contributes to the right ventricle, inflow and outflow tract (OFT), and the arteries and semilunar valves that meet to form the arterial pole of the heart.[10] The OFT is the point of exit from the heart via structures of early cardiac development that give rise the essential structures of the aorta and pulmonary artery. The SHF of the developing heart and its contribution the arterial pole involves complex and interconnected signalling pathways that will be covered later in further detail.
Cardiac Looping and Steps
Gestational Week 6
In the early embryo, the same progenitor cells that constitute the SHF are responsible for the rapid growth occurring during cardiac looping morphogenesis. Looping is essential for cardiac development as it assist further growth of the originally straight heart tube, and ensures it fits within the pericardial celom. Additionally, looping of the heart tube is known to be the first visual evidence of embryonic asymmetry.
- Straight heart tube continues to elongate
- Rapid growth of the bulbus cordis and the primitive ventricle causes ventral bending and right rotation = C shaped loop (convex side on the right)
- The ventricular bend continues to move caudally, the inflow and outflow tracts are brought together at the atrial pole of the heart = S shape
- The truncus arteriosus if formed by adding myocardial cells at the top end of the heart. This portion will form roots and the proximal portion of the aorta and the pulmonary artery [1].
<html5media height="240" width="280">File:Heart looping 002.mp4</html5media>
The video above depicts Cardiac Looping taken from [2]
Cardiac Septation
Gestational Week 6-7
This stage of heart morphogenesis refers to the development of the four main cardiac chambers from the primitive atrium and ventricle. Cardiac septation is comprised of three main events:
- Division of the atrioventricular canal
- Atrial septation
- Ventricular septation
Division of the atrioventricular canal
Division of the atrioventricular canal (AVC) begins with the formation of the superior and inferior endocardial cushions, which are located on the dorsal and ventral aspects of the AVC respectively.[12] These cushions develop as mesenchymal cells invade and proliferate within swollen regions of cardiac jelly of the AVC; this mesenchyme is derived from endothelial cells that have transdifferentiated in the process of epithelial-mesenchymal transformation (EMT).[13] Throughout the fifth week of development, the endocardial cushions project inwards and eventually fuse to partition the AVC into the left and right atrioventricular canals; these canals will serve as the orifices in which the tricuspid and mitral valves are situated.[13]
Atrial septation
As the AVC is undergoing division, a muscular outgrowth, referred to as the septum primum, extends inferiorly from the roof of the primordial atrium.[14] This septum partially divides the atrial chamber into left and right halves, leaving a temporary communication located between the inferior border of septum primum and the endocardial cushions known as the foramen primum.[12] As the size of the foramen primum diminishes, perforations in the superior portion of the septum primum develop as a result of apoptosis, forming a second communication between the atrial chambers called the foramen secundum.[13] Concurrently, an additional muscular septum, known as the septum secundum, projects inferiorly to the right of the septum primum.[12] Eventually, the septum secundum will extend beyond the length of the foramen secundum, generating a partial division of the atria that forms the upper boundary of the foramen ovale.[14] The development of the foramen ovale is critical as it allows oxygen-rich blood from the placenta to bypass pulmonary circulation of the embryo and directly enter systemic circulation.[15] Following birth, the foramen ovale is obliterated as the septum primum and septum secundum fuse, resulting in complete formation of the interatrial septum.[12]
Ventricular septation
As with atrial septation, differentiation of the ventricles is initiated within week 4 of development.[12] A muscular ridge, referred to as the interventricular septum primordium, develops and extends superiorly from the caudal aspect of the primitive ventricular chamber.[13] Growth of the septum is attributed to the expansion of the ventricles, which involves the development of muscular trabeculae as cardiomyocytes proliferate within the chamber walls. [14] The end result is a partial division between the left and right ventricles, which forms the muscular portion of the interventricular septum.[13] A communication between the ventricles, known as the interventricular foramen, remains until approximately week 7 of development.[16] The foramen is obliterated by the fusion of the septum intermedium and the bulbar ridges of the bulbus cordis; this constitutes the membranous portion of the interventricular septum.[12]
Cardiac Neural Crest and Outflow tract
Gestational Week 8
The outflow tract is a tube that runs from the right ventricle to the aortic sac and presents with a distinctive dog-leg bend that separates the proximal (bulbus cordis) and distal (truncus arteriosus) ends of the tract. The endocardial jelly that lines the lumen of the outflow tract concentrates to form the endocardial cushion facing each other that spirals in a 180-degree twist through the length of the outflow tract. Like the outflow tract, these endocardial cushions can be divided into distal and proximal moieties. The distal endocardial cushions are also known as the truncal ridges and the proximal ones are also known as the bulbar ridges. Cells from the cardiac neural crest migrates out of the neural tube, through the pharyngeal arches and aortic sac and into the outflow tract, where it condenses in the ridges to support the septation of the outflow tract. [17][18]
The fusion of the endocardial cushions starts from the distal end of the outflow tract and proceeds proximally. Fusion of the truncal endocardial cushions forms the aorticopulmonary septum that separates the truncus into an aortic and pulmonary trunk. The bulbar endocardial cushions fuse as they extend towards the interventricular septum, separating the proximal outflow tract into the prospective aortic and pulmonary trunks. As the outflow tract separates, the aortic trunk leads to the 3rd and 4th pharyngeal arch arteries and the pulmonary trunk leads to the 6th pharyngeal arch artery. [17][18]
Heart Valve Development
Gestational Week 8
Heart valve development commences in the endocardial cushions of the atrioventricular canal (AVC) and outflow tract (OFT) during the 7th week of embryological development.[12] Valve morphogenesis is comprised of four stages:
- Epithelial-to-mesenchymal transformation (EMT)
- Growth
- Remodelling
- Apoptosis
Development of the valves begins with the transformation of the endocardial cells into mesenchymal cells within the endocardial cushions.[12] The endocardial cushions then expand through cell proliferation and synthesis of the extracellular matrix, which is primarily regulated by vascular endothelial growth factor (VEGF).[14] The elongating cushions then undergo remodeling, as mesenchymal cells differentiate into collagen and other fibrous tissue.[14] The valve leaflets achieve their thin, unique shape as cells near the base of the extending cushions undergo apoptosis.[12]
The semilunar valves differentiate from the conotruncal and intercalated endocardial cushions located at the outflow tract of the heart.[12] The superior and inferior septal conotruncal cushions contribute to the left and right cusps of both the pulmonary and aortic valves.[19] The right-posterior intercalated cushion contributes to the posterior leaflet of the aortic valve, while the left-anterior leaflet gives rise to the anterior leaflet of the pulmonary valve.[19]
The atrioventricular valves arise from the atrioventricular (AV) endocardial cushions. As the atrioventricular canal divides during cardiac septation, fusion and proliferation of the superior and inferior AV endocardial cushions results in the formation of the anterior mitral leaflet and the septal tricuspid leaflet.[12] The right lateral AV cushion differentiates into the anterior and posterior leaflets of the tricuspid valve, while the left cushion gives rise to the posterior leaflet of the mitral valve.[12]
Proepicardium and Coronary Heart Development
Coronary vasculature arises when the fourth layer of the primary heart tube is formed. This fourth layer is known as epicardium that surrounds the myocardium which allows the development of vascular structures over the heart surface.
The monolayered embryonic epicardium derives from mesothelial cells of the septum transversium and mainly from clustered of proepicardium cells. The process involves either of these two mechanisms, one is by detaching of these cells and attach to the pericardial cavity and spread over the heart surface or two is by directly attaching the proepicardium to the myocardium forming a permanent tissue bridge. The spreading of the monolayered embryonic epicardium over the bare myocardium initiates the vascularisation of coronary system. A new extracellular matrix (ECM) layer, the subepicardium forms between the epicardium and the myocardium actively promotes the development of coronary blood vessels. The coalescence of three lineages of the coronary vessel cells: smooth muscle, endothelial and connective tissue and fusion of vascular cell progenitors (angioblasts) form a vascular structure de novo via the process of vasculogenesis. They then migrate onto the primary heart tube between day 22 and day 28 of human development. [20],[21]
Developmental Signalling Processes
Heart development is a very complicated and dynamic process that requires a high degree of control and regulation. This control is achieved by several temporally regulated signalling cascade (Fig. 9) expressed at different stages of heart development.
Wnt signalling
According to the primary mode of action, Wnt signalling pathways have been divided into two major classes, canonical and non-canonical Wnt signalling pathways. Both of the pathways have a role in different stages of cardiac development which could be overlapping or independent of each other. The canonical Wnt signalling pathway involves β-catenin and is activated by a number of ligands such as Wnt-1, Wnt-2, Wnt-3A, Wnt-8A, Wnt-8B, Wnt-8C, Wnt-10A, and Wnt-10B. However, the non-canonical signalling pathway is associated with planar cell polarity and Wnt/Ca2+ pathways that are activated by different ligands such as Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, and Wnt11 [22].
Canonical/β -catenin signaling is essential for mesoderm formation. Upon binding of the Wnt ligands to the Frizzled receptor, a seven-transmembrane receptor, or the co-receptor LRP-5/6, the Canonical signaling pathway is activated. This leads to cytoplasmic accumulation of β -catenin in one side of the embryo and its translocation to the nucleus where it drives the activation of transcription factors required to determine the site at which mesoderm and endoderm formation will occur in the embryo. Animal studies have shown that the lack of nuclear accumulation of β -catenin results in inability of axis formation and mesoderm development whereas overexpression of β -catenin leads to the formation of secondary axis and ectopic expression of mesoderm signals. Also, homozygous deletion of β -catenin in mouse models results in absence of primitive streak and mesoderm formation [23].
Once mesoderm formation proceeds, the β -catenin signal must be shut down by inhibiting factors otherwise cardiac mesoderm formation will be stopped. This indicates that canonical signaling act as a switch that either induces or suppresses cardiac development. One of the inhibiting factors includes dickkopf-1(DKK1) which is an extracellular Wnt inhibitor that promotes the expression of cardiac-inducing factor in the endoderm such as Hex. The latter activates paracrine factors to direct adjoining cells towards cardiac fate [24].
FGF signalling
Fibroblast growth factors (FGF) serve a variety of functions in development, the maintainence of health and disease. FGFs are signaling proteins mostly as paracrine growth factors or endocrine hormones. There are 22 types of human FGFs, whereby paracrine FGF8, FGF9, FGF10 and FGF16 serve a major role in embryonic heart development. Additionally, FGF2, FGF9, FGF10 and FGF16 are involved in postnatal heart pathophysiology[25].
Communication between cardiac progenitor cells is required during embryonic heart development. During heart development, neural cress cells which migrate from the neuroectoderm of the dorsal neural tube will contribute to cushion formation and dictate the correct septation and alignment of the heart[26]. In conjunction to this process FGF3, FGF8, FGF9, FGF10, FGF15, FGF19 and FGF16 function was paracrine signals in embryonic heart development.
A study conducted demonstrated how a combination of both FGF2 and Bone morphogenic protein 2 (BMP2) efficiently enhances the cariomyogenic differention of embryonic stem (ES) cells at an optimal concentration. When FGF2 was inhibited, this ultimately suppressed cardiomyogenic differentiation, thus indicating that FGF signaling play a crucial role in early cardiomyogenesis. The ability for FGF2 to induce cardiac differentiation may serve a key role in treatment for heart diseases.[27] In addition, FGF10 also promotes cardiomyocytes differentiation from ES and induced pluripotent stem cells (iPS). In experiments, when FGF10 and ES cells were administered, this lead to the promotion of cardiomyocyte differentiation in the myocardium of the heart. This may thus serve as a treatment for those who have suffered from myocardial infarctions, where the myocardium is replaced by scar tissue. Providing new and fully functional cardiac muscle may help reduce complications. [28]
The table below outlines the different types of FGFs invovled in heart development and their functions.[27]
| Fibroblast Growth Factor | Function |
| FGF8 | FGF8 is expressed in the early embryonic stages. Required for cardiac looping and migratory cardiac neural crest cell survival. FGF8 is also required for anterior heart field development. |
| FGF9 | Activates FGFR1c with heparin sulfate as a co-factor in a paracrine manner. In doing so, this FGF plays a major role in stimulating the proliferation of cardiomyocytes. |
| FGF10 | Preferentially activates FGFR2b with heparin sulfate as a co-factor. Serves to regulate regional-specific cardiomyocyte proliferation in the embryonic heart in an autocrine/paracrine manner. This FGF type is also essential for the movement of cardiac fibroblasts in the compact myocardium. |
| FGF15/19 | These FGF types are required for proper morphogenesis of the cardiac outflow tract. In addition these FGFs play a major role in paracrine signaling in heart development. |
| FGF16 | FGF16 serves as a major growth factor involved in stimulating the growth of embryonic cardiomyocytes. |
Transforming growth factor-β
Transforming growth factor-β superfamily involves a large number of growth factors that are structurally related. These growth factors, including Nodal or its mimic Activin, bone morphogenic protein (BMP) and growth and differentiation factors, signal through SMAD-dependent and SMAD-independent pathways [29]. SMAD-dependent pathway involves the activation of SMAD proteins upon TGF-β binding to its receptor followed by SMAD transfer into the nucleus regulating gene transcription [30]. Nodal and its mimic Activin act through SMAD-2 and -3 to activate transcription. During embryo development, epiblast cells produce high levels of Nodal growth factors leading to a gradient of nodal in these cells. This gradient is important for mesoderm patterning (left-right asymmetric heart development) and lineage specification. For this reason, this gradient is maintained by Nodal antagonist secreted from the anterior visceral endoderm or by BMP-4 and Wnt-3 feedback loop in the extraembryonic tissues (Fig.11) [31] Nodal/Activin’s important role in heart formation is demonstrated by animal models. Presence of Activin in amphibian embryo lead to activation of heart formation whereas the absence of Nodal co-receptor ,called Cripto, in mouse embryo resulted in failure of ES cells differentiation into cardiomyocytes [32]. Also, knocking out copies of SMAD-2 alone or both SMAD-2 ND -3 result in inappropriate specification of axial mesoderm [33].
The Notch pathway
The Notch gene is an essential gene, in that it codes for a signaling receptor that is required throughout development to regulate the processes known as spatial patterning, timing and outcomes of many different cell fate decisions. The receptor itself is a single spanning transmembrane protein which has a modular architecture including many repeats of a protein module which is reminiscent of the epidermal growth factor as well as 3 membrane-proximal Lin12/Notch/Glp-1 repeats. Notch also has an intracellular domain which is composed of four distinct regions known as the RAM domain, the Ankyrin repeats, a transcriptional activator domain as well as proline, glutamate, serine and threonine sequence. [34]. In the process of activation, a canonical Notch ligand binds the extracellular domain of the notch receptor. Ligand binding will thus induce the exposure of the cleavage site which will allow access by proteases responsible for cleavage under physiological conditions. This cleavage renders the remaining transmembrane-intracellular fragment a substrate for a complex known as the gamma-secretase complex. It is this complex which will catalyse the intramembrane proteolysis to release the Notch intracellular domain. Once this cleavage has occurred, the intracellular domain has the potential to enter into the nucleus and bind the DNA to induce gene transcription[35].
With regard to heart development Notch signaling plays a key role in the pre-patterning of the cardiac mesoderm. In gastrula-stage embryos, Notch works with estrogen receptor 9 and GATA4 transcription factor to regulate the timing of heart field specification for early cardiogenesis which is necessary for normal cardiac development. Notch receptor types 1, 2 and delta-like 1 are required for the determination of the embryonic left-right axis as well as the proper looping of the heart tube. In addition to this, Notch signaling promotes myocardial trabecular proliferation, differentiation and maturation by inducing bone morphogenetic protein-10 and EphrinB2/EphrinB4 expression. Notch signaling also modulates coronary vessel morphogenesis, in which the embryonic epicardium actively participates. Notch signaling elements are differentially expressed throughout the proepicardial-epicardial-coronary transition phases and are required for vessel wall maturation during coronary vessel development. Notch also cooperates with transforming-growth factor-beta to regulate coronary smooth muscle differentiation from epicardium-derived cells to assist in the formation of a functional coronary system. Notch signaling will also regulate cardiac conduction system function, in particular the atrioventricular node [36].
Retinoic Acid
Retinoic acid (RA), a derivative of vitamin A, has been shown to play an important role in vertebrate embryogenesis especially heart formation. RA mediates its action by binding to two families of nuclear receptors, that is the RA and the retinoid receptors (RARs and RXRs). This binding allows RA to directly regulate gene transcription. The expression of at least one of the receptor types by almost all embryonic tissues gives these tissues responsiveness to RA. Signaling of RA is regulated by enzyme that either produce or inactivate retinoids (Fig. 12) [37].
At early stages, RA signaling is suggested to regulate progenitor size in that increasing RA concentrations leads to reduced heart populations in chicken and zebrafish embryos, whereas micromolecular concentrations lead to no heart formation. This regulation is though to happen by RA’s direct action on Hox gene family members such as Hoxa1 transcription factor. These transcription factors have enhancer containing RA response element (RARE). Thus, a reduction or an increase in RA signaling affects the contribution of Hoxa1-; Hoxa3- and Hoxb1-expressing progenitor cells to the heart[38]. At later stages of heart development, RA is involved in establishing the second heart field which forms atrial cells. Also, RA contributes to the proliferation of ventricular cells. In addition, RA has the potential to stimulate cellular differentiation of atrial and ventricular myocytes from human embryonic stem cells (hESCs). This capacity of RA has been exploited in regenerative medicine to promote atrial specification [39].
Embryo obtains vitamin A from maternal delivery of retinol via transplacental transfer. This transfer of Vitamin A is essential for fetal growth and development and any deficiency can lead to severe congenital defects. In fact, the high prevalence of heart malformation in developing countries can be partly explained by vitamin A insufficiency. Conversely, elevated levels of vitamin A during pregnancy have a toxic effect on organogenesis including heart development. Treatment with RA in rodents was one of the earliest teratogenic models of heart defects [38].
Sonic Hedgehog
The Sonic Hedgehog (Shh) signaling pathway is one of the major trafficking networks that is responsible for regulating the key events involved in the developmental processes. The regulatory action of Shh signaling pathway is linked to the secretion, uptake and translocation of ‘Shh protein’ an important Hedgehog ligand precursor. Activation of Shh signal requires the binding of Shh to the Ptc mediate Smoothened (Smo) (Ptc-smo) receptor complex as well as induction of downstream signaling cascade [40].
Shh signaling contributes to myocytes specification and a reduction in this signaling can cause a cardiomyocyte deficit whereas increased Shh signaling lead to a surplus. At early stages of cardiac development, Shh signaling induces a proper number of myocardial progenitor cells. These progenitor cells show a direct respond to SHH signals allowing it to control and determine the optimal number of cardiomyocytes [41]. At later stages of cardiac development, Shh plays an important role in survival and proliferation of neural crest cells (NCCs) and it maintains the proliferation of progenitor cells within the second heart field. These actions allow Shh to promote outflow tract morphogenesis. However, Shh does not act as a direct survival factor for NCCs, but rather, it acts indirectly by inducing a survival factor and inhibiting an apoptotic factor. NCCs of Shh null mice embryos show specification and migration but they are mislocalized and undergo apoptosis. Also, mice that lack Shh develop many cardiac abnormalities such as outflow tract shortening, ventricular hyperplasia and septation defects[42].
Current Research And Findings
Increased regurgitant flow causes endocardial cushion defects in an avian embryonic model of congenital heart disease
it has been discovered that an elevated regurgitant flow during the early stages of embryonic heart development eventually causes abnormalities in the endocardial cushions. However, till today it remains unclear as to how defects in the endocardial cushions during the cardiac looping stages plays a part in the septation of the heart. Thus the aim of this research was to change the blood flow within the avian embryonic heart and monitor the effects it had on the morphology of the endocardial cushions and on Congenital Heart Diseases (CHD). Optical pacing was used to provide the regurgitant flow and optical coherence tomography (OCT) was used to monitor the regurgitation and morphology. The researchers used quail hearts for this research. The quail hearts were optically paced at around 180 beats per minute at stage 13 of their embryonic development (coincides with weeks 3-4 for human development) for 5 minutes. The elevated pacing of the heart tired the heart and this caused 1 hour of regurgitant flow. The morphological changes caused by the regurgitant flow was observed using OCT imaging at stage 19 of avian embryonic development (coincides with cardiac looping stages in human development) or stage 35 (coincides with week 8 of human development). The results showed that all the paced embryos that were observed at stage 19 showed morphological changes in their endocardial cushions. It was also noted that there was an inverse relationship between the regurgitant flow and cardiac cushion size 24 hours post pacing. Out of the embryos that survived till stage 35, 17/18 of them displayed CHDs such as valve defects, ventricular defects, hypo plastic ventricles and common AV valve.[43]
CTCF counter-regulates cardiomyocyte development and maturation programs in the embryonic heart
CTCF is a DNA binding factor that is essential in the process of genome binding and cardiogenesis. It mediates genomic interaction and maturation in the developing heart, coordinates cardiomyocyte differentiation by facilitating enhancer-promoter interactions. Although the role of CTCF in genome organisation is not fully understood. In this study by Gomez-Velazquez et.al (2017), they studied the effect of genetically deleting CTCF in differentiating cardiomyocytes at early stages of mouse development. They deleted CTCF in a population of cardiac progenitor cells which results in malformation of the heart and death of embryo (Figure 14) [44].
From Figure 14, they presented control and CTCF-absent mice in stage E9.5, E10.5, E11.5 and E12.5. E10.5 and E11.5 mutant embryos appeared normal (Fig 14A - 14D). Pericardial edema (Fig 14E) and non expanding cardiac chamber (Fig 14F) presented in E12.5 mutant embryo [44].
Histological examination showed slightly disorganised interventricular septum and normal 4 chambers and atrioventricular canal in E10.5 mutant embryo (Fig 14I and 14J). Fig 14G and 14H showed no defects in E9.5 mutants hearts as compared to controls. Fig. 14K and 14L showed the thinning of myocardial wall in E11.5.
They also carried out Western blot to analyse the component of oxidative phosphorylation pathway in mutant and control embryonic cardiomyocytes. They revealed that there was an increase in Complex IV subunit 1 (Cox 1, encoded in mitochondrial DNA) and Complex IV subunit IV (Cox IV) in CTCF mutants hearts at E10.5 and E11.5 (Figure 15A). Fig 15B showed an increased in Tom20 which is the major receptor of mitochondrial outer membrane translocase. Complex I and Complex V increased from E10.5 to E11.5 in control hearts, consistent with maturation of mitochondrial oxidative phosphorylation. However, they did not increase in CTCF mutant hearts and Complex V was similarly increased between E10.5 and E11.5 in both control and mutant hearts (Fig 15C and 15D). Thus, this suggests that despite increased transcription of subunit in complexes and supercomplexes at the mitochondrial inner membrane, maturation of the respiratory chain is blunted in the CTCF mutant heart.
Transmission electron microscopy (TEM) analysis (Fig 15E) presented immature but normal mitochondria in CTCF mutant cardiomyocytes. At E11.5, mutant cardiomyocyte's mitochondria are swollen, and larger than controls and are disorganised at E10.5
They concluded that when CTCF is removed, genes are misregulated which leads to faulty mitochondria and incorrect expression of cardiac patterning gene. These misregulated genes control opposing genetic program incharge of development and cardiomyocyte maturation. Subsequently, embryo lethality [44].
Erbb2 Is Required for Cardiac Atrial Electrical Activity during Development
As the heart being the first organ to function during embryonic development (22 days in human, 8-8.5 days in mice) and is necessary for embryo survival. It depends on the sinoatrial node (SAN), the pacemaker of the heart’s electrical activity located in the right atrium of the heart and the conducting system, which transduces the electrical signal through the heart tissue for myocardial contraction. This system consists of atrioventricular node (AVN), atrioventricular bundle (AVB), bungle branches and Purkinje fibres, allows synchronized contraction of the atria and ventricles and a consistent heart rhythm. Thus, defects in the development of cardiac electrical function could lead to various heart disorders, such as heart block, long Q-T syndrome, atrial and ventricular fibrillation and tachycardia [45]
Epidermal Growth Factor Receptor 2 (Erbb2) is a transmembrane protein that is essential for normal embryonic development [46]. It is known that at mid-gestation knockout mice lacking Erbb2 are lethal and die due to severe cardiac defects that show enlarged heart with thin ventricular myocardium, absent trabeculae and decreased atrioventricular cushions. Consequently, it results in poor circulation and irregular heartbeat [47]. Other studies suggested that Erbb2 participates in ventricular conduction system development and maturation. However, the role of Erbb2 in atrial conduction system development is still unknown. In a study by Tenin et. al (2014), they showed that upon mutation in Erbb2 in I11Jus8 mice resulted in specific defect in atrial electrical propagation and thus displayed cardiac hemorrhage and failure in atrial function but did not affect ventricular conduction. The role of Erbb2 in I11Jus8 mouse line reported to have significant impact on the atrial function, which allows the embryo to survive. [46]. Although, other studies revealed that embryonic cardiac defects are not found in mice with deletion of Erbb2, it has yet to determine whether non-cardiomyocyte tissue requires Erbb2 function for cardiac conduction system development in Tenin and his colleagues’s experiment. Further more, it has been proposed that Erbb2 functions in adult cardiomyocytes where deletions of Erbb2 causes early cardiac dysfunction and dilated cardiomyopathy [48]. It was also demonstrated that dilated cardiomyopathy in human and mice contributes to failure of the conduction system leading to arrhythmias and sudden death [48],[46]
Dietary supplementation of NAD in prevention of miscarriages and birth defects
Sydney’s Victor Chang Cardiac Research Institute is the centre point of research to prevent recurrent miscarriages and multiple types of birth defects of the heart, spine, kidney and cleft palate in newborn babies.
Scientists have demonstrated a potential cure, in the form of a common dietary supplement. The research study found that a deficiency in a vital molecule, known as NAD, can prevent a baby’s organs from developing correctly in the womb. Nicotinamide adenine dinucleotide (NAD) is one of the most important molecules in all living cells. NAD synthesis is essential for energy production, DNA repair and cell communication. Environmental and genetic factors disrupt its production can cause a NAD deficiency resulting to cripple an embryo when it forms. The dietary supplement vitamin B3 is required to make NAD and is found in food such as meats and green vegetables. Research shows at least a third of women have low levels of vitamin B3 in their first trimester of pregnancy, which is the critical time in organ development. There is an indication that pregnant women may require more vitamin B3 than it is currently available in most vitamin supplements. Using a preclinical mouse model, vitamin B3 was introduced into the mother’s diet. The dietary change prevented both the miscarriages and birth defects completely. This discovery can be likened to the revolutionary breakthrough made last century that confirmed folic acid supplementation can prevent spina bifida and other neural tube defects in babies.
The next step would be to develop a diagnostic test to measure NAD levels. This would enable doctors to identify the women who are at greater risk of having a baby with a birth defect, and ensure they are getting sufficient amount of vitamin B3.
Animal Models
Mice Model
Understanding the importance of different molecular pathways in heart development was made possible by studies conducted in different animal models including mice models. Such studies, in particular loss of function analysis studies, have allowed for the determination of specific roles of various signaling pathways in specific stages of cardiac development. For example, one of the studies conducted in mice embryo involved studying the effect of ALK2 receptor deletion [49]. This receptor is expressed on cardiac neural crest cells which contribute to the formation of cardiac outflow tract (OFT). Bone morphogenetic protein (BMP), an important protein in heart signaling processes, acts as a ligand for this receptor. Thus, by binding to this receptor, BMP play a role in neural crest development and migration. Therefore, since this receptor function to determine the downstream signaling specificity, understanding its function will allow the mechanisms by which BMP regulate heart development to be uncovered. However, the receptor was not deleted as this will lead to mice lethality, but instead its function was abolished in neural crest cells. Mice with abolished ALK2 receptor function revealed various cardiac defects such as aortic arch defect and cardiac OFT defect. These defects are very similar to the common human congenital heart disease. This occurrence of these cardiac defects is indicative of impaired neural crest cell migration and it provides evidence for the importance of BMP in heart development.
Since the origin of cells contributing to different parts of the valves remains controversial, another research on mice model [50] provides further insight into the origin of these cells. In this study, myocardial, endocardial and neural crest cells were labelled irreversibly using a genetic system during embryonic development to determine their eventual contribution to different components of the valves. At E17.5 majority of cells present in the cardiac valves were positive for β-galactosidase expression which is indicative of endocardial origin. Cells of the endocardial lineage were detected in leaflets and tendinous cords of the mitral and tricuspid valves, the atrioventricular fibrous continuity and the leaflets of the aortic and pulmonary valves. However, these components showed minimal contribution from cells of the myocardial and neural crest lineages.
Zebrafish Model
Growth of the heart tube into a two-chambered heart in zebrafish is regulated by two separate phases of cardiomyocyte differentiation [51]. These two phases are detected using a developmental timing essay and a photoconvertible marker. In the first phase, a continuous wave of cardiomyocytes differentiation appears in the ventricle initially and extend through atrium allowing the addition of cardiomyocytes to the venous pole of the heart tube. This phase is regulated by the islet1 protein and islet mutants show reduced cardiomyocyte differentiation at the venous pole (Fig. 15). However, the second phase involves Fgf signaling and it is initiated by the addition of new cardiomyocytes to the arterial pole. Therefore, these two processes are independent of each other in space, time and regulation and they are essential for heart development in Zebrafish. Modified regulation of these two phases can result in a change of heart size and morphology.
Chick Model
Initial stages of cardiac looping involve the formation of a c-shaped tube that is curved towards the right side of the embryo. This c-shaped tube is formed as a result of ventral bending and rightward rotation. The genetic and molecular pathways involved in this process of cardiac looping have been well understand but the role mechanical forces play in this process remains poorly understood. This study on chick embryos [52] shows that bending and rotation are mediated by different sets of forces. While bending is controlled by intrinsic forces to the heart, rotation into a c-shaped tube is driven by external forces exerted mainly by a membrane pushing against the heart tube called splanchnopleure (SPL). Also, some of these forces come from omphalomesenteric veins (OVs) which plays a role in the directionality of left-right looping. These results were obtained from analysis of tissue stress and strain using dissection and fluorescent labelling respectively after subjecting the embryos to mechanical perturbations.
Other research into chick models reveals that cardiac progenitors of the splanchnic mesoderm, cardiac neural crest and the proepicardium are the major embryonic contributors to the development of the chick heart. The contribution of these components to cardiac development occurs with precise timing and regulation during such processes as primary heart tube fusion, cardiac looping and accretion, cardiac septation and the development of the coronary vasculature. In addition to these findings, chick models revealed that one role of the anterior endoderm is to create a “cardiac field” in the overlying anterior mesoderm, and that other events will restrict this cardiac field to the region of the embryo that will become the heart later in development [53].
Xenopus Model
Along the molecular pathways mentioned in the heart signaling section, CASTOR (CST) protein previously identified in Drosophila for its role in maintaining stem cells competence at the dorsal midline, is now shown to play a role in cardiogenesis. A study using Xenopus as an animal model [54] identified CST expression in the myocardial layer of the heart in a gradient that extends from dorsal to ventral. The expression of CST in this region within a subset of cardiac progenitor cells is necessary for the initiation of cardiomyocyte differentiation at the ventral midline (Fig. 17). Tracing the fate of these cardiac tissue, it is shown that these cardiac progenitor cells at the ventral midline region give rise to a population of cells in the outer curvature of the ventricle indicating their specific cell fate. However, progenitor cells that are deficient in CST protein overproliferate and do not end up integrating into cardiac muscle. This suggests that CST within the subset of cardiac progenitor cells in vertebrates is essential for proper timing of differentiation in order for these progenitor cells to give rise to the outer curvature of the ventricle. Therefore, Xenopus animal model has helped to have a better understanding or the role of CST in vertebrate cardiac development.
Abnormal Development
Atrial Septal Defect
Atrial septal defects (ASD) are one of the most prevalent congenital heart malformations, with an estimated 56 cases per 100,000 births.[55] This heart defect involves a communication between the left and right atria, and can be subdivided into five main types based on the nature of the defect.
The most common ASD is a patent foramen ovale. Throughout fetal development, the foramen ovale functions to shunt oxygenated blood from the right atrium into the left atrium.[56] After birth, the now functioning respiratory system of the newborn results in elevated pressure in the left atrium relative to that of the right.[57] This induces fusion of the septum primum and septum secundum which closes the foramen ovale, leaving a remnant known as the fossa ovalis.[56] Incomplete closure results in a patent foramen ovale. This condition is present in nearly all newborns and typically closes within the first month, however complete closure is achieved in only 70-75% of adults.[55] A patent foramen ovale is often asymptomatic and thus is not usually considered a clinically significant condition, however it can be problematic when other cardiac abnormalities are present.[58]
A secundum ASD is the most common of the true (clinically significant) atrial septal defects. This malformation occurs within the region of the fossa ovalis and typically results from abnormal resorption of the septum primum during cardiac development.[58] Secundum ASDs enable left-to-right atrial shunting of blood, which increases the load on the right side of the heart and, consequently, the pulmonary circulation; this can result in the development of atrial arrhthymias and right ventricular failure.[56]
Sinous venosus ASD refers to a communication between one of the right pulmonary veins (typically the upper vein) and the wall of the right atrium adjacent to the superior vena cava.[58] This defect can occur as a result of the resorption or partial absence of the septum secundum,[58] and accounts for 5-10% of all atrial septal defects.[59]
A primum ASD is the result of an abnormality in the development of either the septum primum or septum secundum.[58] This condition is a type of atrioventricular septal defect, and will be discussed in more detail in the next section.
The most extreme ASD is a common atrium. This occurs when the septum primum and septum secundum fail to develop.[55] This lack of an interatrial septum is often accompanied by other cardiac abnormalities, including clefts in the leaflets of the atrioventricular valves.[58]
Atrioventricular Septal Defect
An atrioventricular septal defect (AVSD) refers to a malformation involving the interatrial and/or interventricular septa, and has an estimated incidence of 1 in 2000 births.[60] This defect typically occurs as a result of incomplete fusion of the endocardial cushions,[59] although recent research has concluded that malformation of the dorsal mesenchymal protrusion (DMP) also contributes to the development of AVSDs.[60] This can result in abnormal development of the interatrial septum, interventricular septum and/or the mitral and tricuspid valves.[58]
There are two main subdivisions of AVSDs, which differ according to whether one or both of the cardiac septa are affected. In a partial AVSD, there is a defect present in the inferoanterior region of the fossa ovalis, which allows the right-to-left shunting of blood across the atria.[60] In this scenario, the lack of fusion of the endocardial cushions can also result in the presence of clefts in the septal leaflets of the atrioventricular valves.[58] However, in a complete AVSD, a defect in the membranous portion of the interventricular septum is also present.[59] This condition results in direct communications between the atria and the ventricles, and pulmonary hypertension or congestive heart failure can occur if the anomaly is severe.[60]
Ventricular Septal Defect
Ventricular septal defects are the most prevalent of all congenital heart defects (excluding patent foramen ovales), accounting for approximately 40% of all cardiac abnormalities among newborns.[61] The exact definition of a VSD is still a point of contention within the scientific community, but is generally referred to as a communication between the left and right ventricles; this anomaly can manifest as a lone defect, but is often present with other abnormalities such as Tetralogy of Fallot or a complete AVSD.[62] occur as a result of abnormal development (or absence) of any of the components forming the interventricular septum.[63] Hence, a VSD can occur in either the muscular or the membranous portion of the septum, with the latter being more common.[63]
A membranous VSD occurs when there is an incomplete occlusion of the interventricular foramen, which is due to the membranous portion of the septum failing to develop.[58] This is the result of a failure of fusion between the muscular portion of the interventricular septum and the endocardial cushions.[58] This defect is most commonly located in the outflow tract of the left ventricle.[58]
A muscular VSD results from abnormal development of the interventricular septum primordium during cardiac septation.[62] This can refer to incomplete formation of the primitive septum, or to errors in the proliferation of trabeculae within the septum.[61] VSDs can form anywhere within the muscular wall of the septum, and can extend into various regions of the right ventricle including the apex, inlet or outflow tract.[61]
The most extreme form of VSD is a common ventricle. This very rare defect occurs when the interventricular septum primordium fails to develop.[58] Infants with common ventricle often die from congestive heart failure.[58]
Persistent Truncus Arteriosus
Persistent truncus arteriosus (PTA) is a rare heart defect accounting for less than 1% of all congenital cardiac anomalies.[64] PTA occurs when the truncus arteriosus of the developing heart fails to partition into the ascending aorta and pulmonary trunk.[65] This results in the formation of a common arterial trunk that provides a mixture of oxygenated and de-oxygenated blood to systemic, pulmonary and coronary circulations.[66] Although the exact aetiology of PTA is unknown, cellular ablation studies have demonstrated that aberrant migration and/or development of neural crest cells can result in abnormal aorticopulmonary septation.[65] PTA has a poor prognosis without surgical intervention, with only 20% of patients surviving beyond the first year.[67]
Tetralogy of Fallot
Tetralogy of Fallot (TOF) is a complex congenital heart defect with an incidence of 3 cases per 10000 live births.[68] It is also the most common cyanotic cardiac abnormality, accounting for 7-10% of all congenital heart malformations.[68] The condition is characterised by four heart abnormalities:[69]
- Pulmonary stenosis – narrowing of the pulmonary trunk and associated pulmonary valve.
- Ventricular septal defect (VSD) – a communication between the left and right ventricles
- Dextroposition of the aorta – rightward displacement of the aorta over the ventricular septal defect.
- Right ventricular hypertrophy – enlargement of the myocardium of the right ventricle
Tetralogy of Fallot typically presents as cyanosis (bluish discolouration of the skin) in the newborn.[70] This is due obstruction of pulmonary outflow, as well as right-to-left shunting of blood across the ventricles via the ventricular septal defect.[71] This collectively results in poorly oxygenated blood being discharged from the left ventricle into systemic circulation.[71]
Newborns with TOF typically undergo corrective surgery within the first month of life.[68] Pulmonary stenosis is relieved via widening of the pulmonary trunk and the ventricular septal defect is repaired, allowing the outflow of only well-oxygenated blood through the aorta.[71]
Hypoplastic Left Heart Syndrome
Hypoplastic Left Heart Syndrome (HLHS) is a congenital heart defect that involves the underdevelopment of cardiac structures of the left side of the heart; structures effected include the left ventricle, ascending aorta, aortic arch and the mitral and aortic valves.[72] Despite being one of the rarer heart defects, accounting for up to 3.8% of congenital cardiac malformations, HLHS is responsible for 23% of all cardiac-related deaths in the first week of life.[73] Severity of the disease depends on the extent of systemic outflow obstruction, the number of heart structures effected, and the degree of hypoplasia of the left ventricle and ascending aorta.[72]
Initially, newborns with HLHS will appear healthy. This is because the presence of the foramen ovale and a patent ductus arteriosus allows blood to bypass the malformed left side of the heart and enter systemic circulation.[73] Once these structures close, inadequate blood flow in the systemic circulatory system results in hypoxemia and cardiogenic shock; rapid surgical intervention is required to prevent death of the newborn.[72] Corrective surgery for neonates with HLHS is performed in three stages, with the ultimate goal of increasing systemic circulation and bypassing the malformed side of the heart; the end result is a modified right ventricle that supports both systemic and pulmonary circulation.[71]
The first operation, known as the Norwood operation, is performed at birth. This involves the construction of a new ascending aorta and arch that extends from the right ventricle, as well as the establishment of a communication between the right ventricle and the pulmonary trunk, which allows for both pulmonary and systemic perfusion from the right ventricle.[73] Next, a Glenn shunt operation is performed at 6-8 months after birth, in which a surgical anastomosis is created between the right pulmonary artery and superior vena cava, which decreases the work on the right ventricle during venous return.[72] Finally, a Fontan procedure is performed 1.5-4 years after birth, which involves establishing a communication between the now connected pulmonary artery and superior vena cava to the wall of the right atrium.[72] These surgeries collectively result in the development of univentricular circulation in which oxygenated and de-oxygenated blood are unable to mix, which increases the efficiency of the neonate’s cardiovascular system.[73]
Cardiac Stem Cells
Following a myocardial infarction (MI), a significant number of cardiomyocytes becomes damaged and the heart has very little regenerative functions. The loss of cardiomyocytes pose as a problem when an individual has had repeated MI or those suffering from end stage heart failure. For these individuals, heart transplantation remains as the main solution. However, there are not enough donors available. Therefore, stem cell therapy provides as an upcoming possibility in replacement for donor transplant. There are two types of cells that could be use which includes human embryonic Stem cells (hESCs) and Human Induced Pluripotent Stem Cells (hiPSCs). However, there are differences between these two cells and further testing and applications are still required to produce highly pure and mature cardiomyocytes.
| FACTOR | Human Embryonic Stem Cells | Human Induced Pluripotent Cells |
|---|---|---|
| Ethical Issues |
|
|
| Immune Reactions |
|
|
| Availability of Cells |
|
|
| FACTOR | Human Embryonic Stem Cells and Human Induced Pluripotent Stem Cells |
|---|---|
| Teratoma formations | Both have tendency to form teratomas. Cardiomyocytes thus have to be highly purified. A few methods have been explored to obtain a highly purified culture of stem cells to prevent teratoma formation. The following methods include:
Mitochondrial Based Separation[76]
Biochemical Differences between Differentiated and Undifferentiated Cardiomyocytes[77]
However, because both methods do not have a 100% purity, teratomas could still be formed. |
| Cardiac Maturation |
Ultrastructural Analysis
Electrophysiological Properties
Contraction Properties
Since hESCs and hiPSCs are required to replace damaged cardiomyocytes, their obvious immaturity problem would have to be resolved before it can be utilized for transplantation or therapy. |
Future Questions
What is the aetiology of Tetralogy of Fallot?
The aetiology of Tetralogy of Fallot is complex and still poorly understood. Current research in genetics has linked the condition with various chromosomal abnormalities, including trisomy 13, 18 and 21, as well as microdeletions within chromosome 22.[68] In addition, current research has identified risk factors such as maternal diabetes and exposure to retinoic acid.[68] Further research in this area is required to achieve a greater understanding of the underlying causes of Tetralogy of Fallot.
Regulation of forces involved in cardiac looping
Studies have identified some mechanical forces involved in looping, it remains to be established how these forces are regulated spatially and temporally to produce a looped heart tube. It needs to be further investigated to determine whether the proposed actin polymerization mechanism can produce the necessary changes in tissue shape on a global scale. In addition, the possible roles played by redundant mechanisms have not been elucidated. Finally, the mechanisms of initial looping of the heart tube and s-looping have received relatively little attention. To gain a complete understanding of heart development requires finding the link between gene expression and morphomechanics, which will need to combine the expertise of developmental biologists and biomechanical engineers [1].
Signalling of the heart fields
Multiple signalling pathways overlap to regulate SHF cell addition to the poles of the heart tube, as well as SHF proliferation and myocardial differentiation. The signalling molecules identified to highlight the continuance of SHF cells in a progenitor cell state and controls their progressive differentiation. However, the control of differentiation delay and SHF movement into the OFT remains obscure. Future research into gene regulatory networks and signalling pathways that control SHF development will identify mechanisms underlying these processes. This will provide a greater understanding of the extent to which these networks differ from those controlling differentiation of the linear heart tube. Further exploration of the mechanisms underlying SHF progenitor cell development in the early embryo is required, as it will uncover the potential of progenitor and pluripotent stem cells for cardiac repair [82].
Specific identification of cardiac precursors
Heart precursors were identified as occupying anterior mesoderm, proximal to the embryonic-extraembryonic boundary. This identification was achieved utilising cell transplantation, labelling of live embryos and conducting embryo culture. These embryonic manipulations highlighted the plasticity of populations that contribute to the heart. This means cells obtained from a different location or developmental time point can contribute to the heart once placed in the proper location. Subsequently, such experiments [83] were able to locate the cardiac precursors but they failed to specifically identify these precursors and therefore cardiac precursors have not been identified and characterised yet. Thus, future experiments will aim to address this question.
Cardiac Regeneration
There are vast differences between the potential of cardiac regeneration in experimental models like zebrafish which is lost in adult mammals like humans. Researches speculate that it could be due to internal properties of cardiomyocytes or due to the failure of stem cell populations. They also hypothesize that it could be due to non-cardiomyocytes that could cause scarring which might hinder regeneration. In addition, more research has to be done on how regeneration begins and ends (signalling pathways involved etc).
Role of bone morphogenetic protein (BMP) in cardiac neural crest development and migration
The role of BMP has been implicated in many aspects of organogenesis including neural crest development and migration. However, it remains unclear to whether BMP impose a direct action on neural crest cells during cardiac morphogenesis or acts indirectly by affecting gene expression [84]. Also, studies of BMP receptor signaling have been hard to conduct as receptor deletion lead to death of the embryo at early stages of development. It was only recently that such studies of the receptor were performed by blocking the function of the receptor in neural crest cells. Thus, future experiments must be conducted to uncover the mechanism by which BMP act to regulate neural crest development and migration.
Glossary of Terms
Definitions provided in this glossary are taken from lectures presented in the UNSW embryology course ANAT2341, as well as from the UNSW embryology wiki glossary provided online, which is linked below.
| Term | Definition |
| Anastomosis | Term used to describe the connection between two tubes. Applied to describe the connection between peripheral blood vessels without an intervening capillary bed. |
| Angioblastic cords | Groups or ‘columns’ of embryonic precursor cells which will form the walls of both arteries and veins. |
| Axial mesoderm | Also referred to as notochord. It is an embryonic structure lying within the midline of the trilaminar embryo. |
| Blastocoel | A fluid filled cavity formed during early embryonic development (Week 1-2) within the blastocyst. Once Morula is formed as a result of the initial cell division, further cell division followed by compaction leads to the formation of this cavity. |
| Bulbar Ridge | Term describing a spiral region in the developing heart bulbus cordis, there is a left and right bulbar ridge, that contribute to the septation of membranous portion of ventricular septum that is continuous with the outflow tract. These regions fuse to separate the single embryonic outflow tract into the aortic and pulmonary arteries. |
| Bulbus cordis | A region of the early developing heart tube forming the common outflow tract, will differentiate to form three regions of the heart. |
| Cardiac Jelly | Term used in early heart development to describe the initial gelatinous or sponge-like connective tissue separating the myocardium and the heart tube endothelium. |
| Cardiocyte | Mature muscle cell of the heart. These cells are characterized by striation and separated by intercalated discs. |
| Cyanotic Heart Disease | Clinical term referring to a congenital heart abnormality (defect) resulting in lack of oxygen that causes cyanosis, a blue coloration of the skin and mucous membranes due to the presence of deoxygenated hemoglobin. |
| Ductus Arteriosus | A prenatal vascular "shunt" which connects the left pulmonary artery and the descending aorta. Postnatal neonatal patency (patent ductus arteriosus, PDA) is a relatively common congenital cardiac anomaly occurring more frequently in premature infants. |
| Endocardial Cushion | Early heart development structures formed before atrial and ventricular septation occurs. These four heart wall in-folds (ventral, dorsal and two lateral) lie between the future atria and ventricles, later the posterior and anterior cushions fuse forming the primordial atrioventricular canals. |
| Endocardium | The epithelial membrane lining the inside surface of heart, which along with the endothelial layer forms a continuous lining of the entire cardiovascular system. |
| Epicardium | The inner layer of the pericardium that is in contact with the surface of the heart |
| Heart Field | The term used to describe the splanchnic mesoderm cardiogenic region in the trilaminar embryo that generates most of the heart. In humans, there are two fields the primary and secondary heart fields. |
| Heart Valves | The heart has a series of valves which regulate the directional flow of blood. The human heart has valves separating the atria from ventricles (atrioventricular, AV) and the ventricles from the outflow tract aortas. The left atrioventricular valve has two leaflets, anterior and posterior, and is the bicuspid valve or mitral valve. The right atrioventricular valve has a third leaflet (small, septal cusp) and is the tricuspid valve. |
| Inflow Tract | Entrance of blood into the heart tube; the sinus venosus portion of the tube. |
| Mesothelial cells | Epithelial cells of mesodermal origin |
| Mitochondrial oxidative phosphorylation | A synthesis of ATP powered by the free energy of reduced compounds that are produced by other metabolic pathways, including glycolysis and the TCA cycle, occurs in the mitochondria |
| Myocardium | Layer that forms the muscular wall of the heart, the thickest layer formed by spirally arranged cardiac muscle cells. |
| Omphalomesenteric vein | Vessels that provide the venous pole input into the heart from the yolk sac connected at the umbilicus during embryogenesis |
| Outflow tract | Exit of blood from the heart tube formed by the truncus arteriosus. |
| Pericardium | The membranous sac filled with serous fluid that encloses the heart, aorta and large blood vessels |
| Proepicardium | Group of progenitor cells that forms near the venous pole of the heart gives rise to the epicardium |
| Septum transversium | Forms from an aggregation of mesenchyme tissue that develops within the caudal part of ventral mesentery of the foregut. It is developed at day 22 in humans. |
| Sinus venosus | An early developmental cardiovascular structure, thin walled cavity, forming the input to developing heart which has 3 venous inputs (vitelline vein, umbilical vein, common cardinal vein). Later in heart development this structure gets incorporated into the wall of the future right atrium. |
| Subepicardium | Situated or occurring beneath the epicardium or between the epicardium and myocardium. |
| Truncus arteriosus | An embryological heart outflow structure, that forms in early endocardial tube stage and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation of the cardiac outflow pattern, where only one artery arises from the heart and forms the aorta and pulmonary artery (Persistent truncus arteriosus). |
| Vascular Endothelial Growth Factor (VEGF) | A secreted protein growth factor family, which stimulates the proliferation of vascular endothelial cells and therefore blood vessel growth. |
| Western Blotting | An important technique used in cell and molecular biology. Researchers are able to identify specific proteins from a complex mixture of proteins extracted from cells with 3 techniques: (1) separation by size, (2) transfer to a solid support and (3) marking target protein using a proper primary and secondary antibody to visualise. |
Below are links to a more extensive glossary if additional definitions are needed
A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z
References
- ↑ 1.0 1.1 1.2 <pubmed>16479500 </pubmed>
- ↑ 2.0 2.1 2.2 2.3 2.4 <pubmed>PMC1767747</pubmed>
- ↑ <pubmed> PMC1571338 </pubmed>
- ↑ <pubmed> PMC2691808 </pubmed>
- ↑ <pubmed> PMC4374196 </pubmed>
- ↑ <pubmed>PMC3721262</pubmed>
- ↑ <pubmed>16567300 </pubmed>
- ↑ <pubmed>17796013 </pubmed>
- ↑ http://perspectivesinmedicine.cshlp.org/content/4/10/a015750.full
- ↑ 10.0 10.1 <pubmed>2794420</pubmed>
- ↑ http://www.sciencedirect.com/science/article/pii/S1875213613002817
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 <pubmed>PMC3424040</pubmed>
- ↑ 13.0 13.1 13.2 13.3 13.4 <pubmed>PMC1767797</pubmed>
- ↑ 14.0 14.1 14.2 14.3 14.4 <pubmed>24138816</pubmed>
- ↑ <pubmed>27188965</pubmed>
- ↑ <pubmed>12739611</pubmed>
- ↑ 17.0 17.1 <pubmed>23633400</pubmed>
- ↑ 18.0 18.1 <pubmed>12923046</pubmed>
- ↑ 19.0 19.1 <pubmed>PMC5438177</pubmed>
- ↑ Pérez-Pomares J, Pires-Gomes A, (2013) The Epicardium and Coronary Artery Formation, Journal of Developmental Biology, Vol.1(3), pp.186-202, ISSN: 2221-3759, E-ISSN: 2221-3759, https://doaj.org/article/b3f5aa0ae8df409ab1664975469b95d1
- ↑ Martinsen B, Lohr J, (2005) Cardiac Development, Handbook of Cardiac Anatomy, Physiology and Devices Iaizzo, P.A. (Ed.), http://www.springer.com/cda/content/document/cda_downloaddocument/9781588294432-c2.pdf?SGWID=0-0-45-387660-p173728389
- ↑ <pubmed>25813860</pubmed>
- ↑ <pubmed>16860783</pubmed>
- ↑ <pubmed>4533091</pubmed>
- ↑ <pubmed>11493531</pubmed>
- ↑ <pubmed> 23799628</pubmed>
- ↑ 27.0 27.1 <pubmed>26793421</pubmed>
- ↑ <pubmed> 24307297 </pubmed>
- ↑ <pubmed>18290874</pubmed>
- ↑ <pubmed>14534577</pubmed>
- ↑ <pubmed>25813860</pubmed>
- ↑ <pubmed>20830688</pubmed>
- ↑ <pubmed>12842913</pubmed>
- ↑ <pubmed>12651094</pubmed>
- ↑ <pubmed>27507209</pubmed>
- ↑ <pubmed>21252157</pubmed>
- ↑ <pubmed>25813860</pubmed>
- ↑ 38.0 38.1 <pubmed>28007475</pubmed>
- ↑ <pubmed>25700171</pubmed>
- ↑ <pubmed>25206052</pubmed>
- ↑ <pubmed>15936751</pubmed>
- ↑ <pubmed>18842815</pubmed>
- ↑ <pubmed>28211263</pubmed>
- ↑ 44.0 44.1 44.2 <pubmed>28846746</pubmed>
- ↑ <pubmed>16643374</pubmed>
- ↑ 46.0 46.1 46.2 <pubmed>25269082</pubmed>
- ↑ <pubmed>7477377</pubmed>
- ↑ 48.0 48.1 <pubmed>12072561</pubmed>
- ↑ <pubmed>15226263</pubmed>
- ↑ <pubmed>15297379</pubmed>
- ↑ <pubmed>19395641</pubmed>
- ↑ <pubmed>15282152</pubmed>
- ↑ <pubmed> 15986452</pubmed>
- ↑ <pubmed>18410736</pubmed>
- ↑ 55.0 55.1 55.2 <pubmed>24725467</pubmed>
- ↑ 56.0 56.1 56.2 <pubmed>PMC4711537</pubmed>
- ↑ <pubmed>PMC5505397</pubmed>
- ↑ 58.00 58.01 58.02 58.03 58.04 58.05 58.06 58.07 58.08 58.09 58.10 58.11 58.12 <pubmed>PMC325204</pubmed>
- ↑ 59.0 59.1 59.2 <pubmed>PMC4373719</pubmed>
- ↑ 60.0 60.1 60.2 60.3 <pubmed>PMC5267359</pubmed>
- ↑ 61.0 61.1 61.2 <pubmed>21349577</pubmed>
- ↑ 62.0 62.1 <pubmed>PMC4316658</pubmed>
- ↑ 63.0 63.1 <pubmed>7437181</pubmed>
- ↑ <pubmed>21524457</pubmed>
- ↑ 65.0 65.1 <pubmed>PMC4021394</pubmed>
- ↑ <pubmed>27126954</pubmed>
- ↑ <pubmed>21070356</pubmed>
- ↑ 68.0 68.1 68.2 68.3 68.4 <pubmed>PMC2651859</pubmed>
- ↑ <pubmed>20091166</pubmed>
- ↑ <pubmed>20734579</pubmed>
- ↑ 71.0 71.1 71.2 71.3 <pubmed>PMC4460219</pubmed>
- ↑ 72.0 72.1 72.2 72.3 72.4 <pubmed>PMC1877799</pubmed>
- ↑ 73.0 73.1 73.2 73.3 <pubmed>PMC5313512</pubmed>
- ↑ <pubmed>9804556</pubmed>
- ↑ <pubmed>20964482</pubmed>
- ↑ <pubmed>19946277</pubmed>
- ↑ <pubmed>23168164 </pubmed>
- ↑ <pubmed>21883888</pubmed>
- ↑ <pubmed>12791707</pubmed>
- ↑ <pubmed>21614516</pubmed>
- ↑ <pubmed>16322641 </pubmed>
- ↑ <pubmed> 19390062 </pubmed>
- ↑ <pubmed>23457256 </pubmed>
- ↑ <pubmed>15226263</pubmed>