2017 Group Project 1
| 2017 Student Projects | |||
|---|---|---|---|
|
Cerebral Cortex
Mark Hill (talk) 15:59, 14 September 2017 (AEST) Feedback
- lots of sub-headings, but no actual text.
- no defined structure to the sequence of sub-headings.
- no research images related to what is an extensively researched topic.
- historic background of key research findings, put our understanding today in perspective.
- Lon list of abnormalities, with no description.
Introduction
What is it?
The cerebral cortex surrounds the cerebrum, and is the largest part of the human brain. It is thought to be the main control centre, playing an essential role in cognitive function, memory, sensation and association.
The cerebral cortex is actually the outermost layer of the cerebral hemispheres; it is around 2-4 mm in thickness, consists of the layer of grey matter and has ~ 10 billion nerve cell bodies and dendrites.
The development of the cerebral cortex involves 3 main stages: proliferation/growth/differentiation, migration of neurons, and maturation/organisation/folding. The cortex is organised into six horizontal layers. I. Molecular layer, II. External granular layer , III. external pyramidal, IV. internal granular layer, V. internal pyramidal layer, VI. multiform layer. These individual layers organise the capacity for interconnections between both input and output signals.
Early Development of the Brain
( z5059949 )
The brain begins to develop during the third week when the neural plate and tube derive from the outer most layer of embryonic cells, the neuroectoderm. The development of the brain is from the neural plate which folds to form the neural groove and then curls forming the neural tube, which is cranial to the fourth pair of somites, and eventually, forms the three primary brain vesicles [1].
Neuroprogenitor cells proliferate, migrate, and differentiate to form specific areas of the brain [2].
During week four fusion of the neural folds in the cranial region and closure of the rostral neuropore form three primary brain vesicles from which the brain develops [1]:
- Forebrain/Prosecephalon
- Midbrain/Mesencephalon
- Hindbrain/Rhombencephalone
From there three primary vesicles, there is a further division at the anterior extremity of the medullary canal into five secondary vesicles during week five. These are fundamental divisions of the adult brain and communicate freely with each other [3]. They are [2]:
- Telencephalon: endbrain, forms cerebral hemispheres - derived from Prosecephalon
- Diencephalon: between brain, forms optic outgrowth - derived from Prosecephalon
- Mesencephalon: undivided
- Metencephalon: posterior to the brain - derived from Rhomabencephalon
- Myelencephalon: medulla - derived from Rhomabencephalon
In the beginning, these five divisions are uniform in size and shape but quickly differentiate at various rates [3].
Brain Flexures
During the fifth week, the embryonic brain undergoes rapid growth folding the neural tube and consequently resulting in three brain flexures. These are [1]:
- Cephalic flexure - pushes mesencephalon upwards
- Cervical flexure - between brain stem and spinal cord at the junction of the spinal cord and hindbrain
- Pontine flexure - generates fourth ventricle; produced in the opposite direction as a result of later unequal brain growth, resulting in the thinning of the roof of the hindbrain [3]
The primordial brain initially has the same basic structure as the developing spinal cord, however consideration variations in the outline of transverse sections at different levels of the brain and in the position of the gray and white matter are produced by the brain flexures [2]. The sulcus limitans (the floor of the fourth ventricle) extends cranially to the junction of the midbrain and forebrain, and the alar and basal plates are recognisable only in the midbrain and hindbrain [2].
Development of Cerebral Cortex
( z5177691 )( z5178570 )
Main classes of neurons [4]
- Projection neurons: excitatory neurons (glutamatergic) with axons that project to distant targets and are generated by progenitors in the dorsal pallium of the telencephalon. The have a typical pyramidal structure and send signals to various regions of the brain and neocortex.
- Interneurons: inhibitory neurons (GABAergic) that have local connections within the cortex and are generated in the subpallium of the telecephalon in the ventral proliferative zone. These neurons have to migrate to the neocortex area.
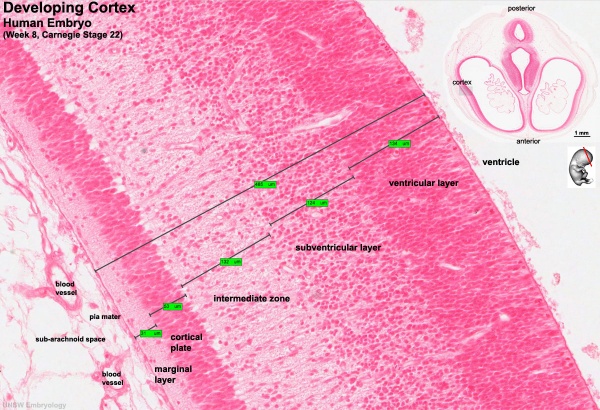
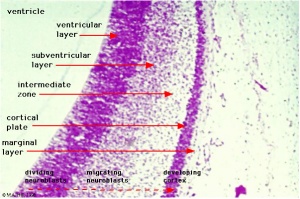
Key developmental zones in the human cortex:
- Ventricular zone
- Subventricular zone
- Inner Subventricular Zone
- Outer Subventricular zone
- Intermediate Zone
- Preplate: neural progenitor cells split into 2 regions
- Marginal zone:
- Subplate:
- Cortical Plate: where the 6 layers of the cortex form, exists in between the subplate and the marginal zone
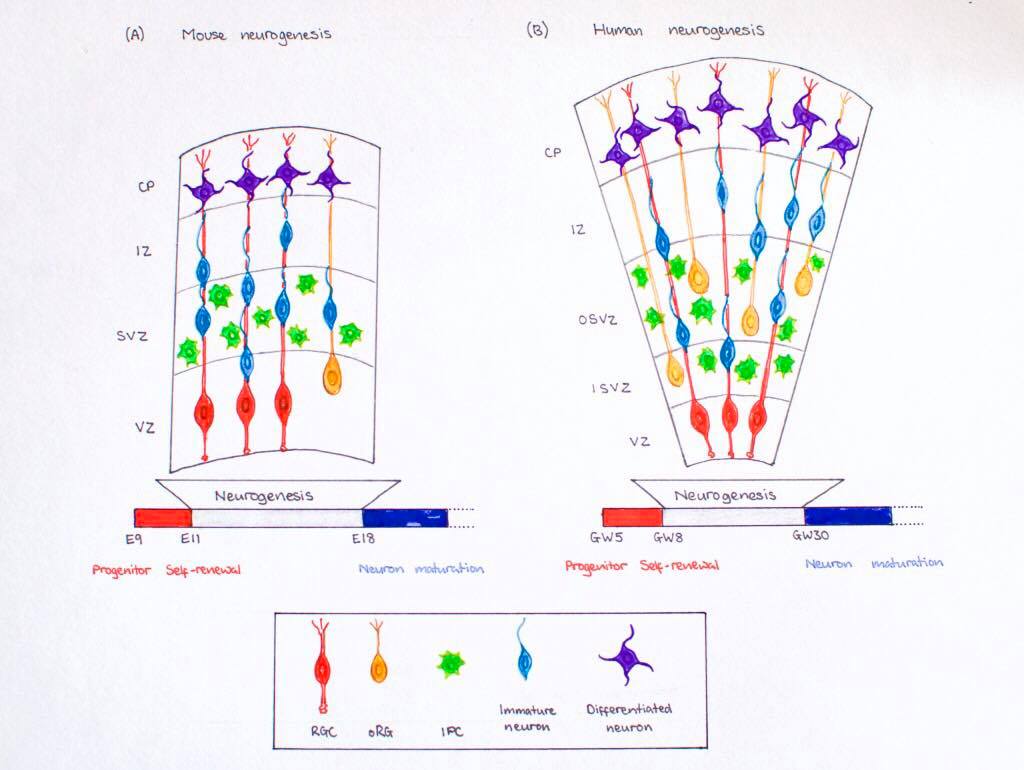
Timeline of Corticogenesis
The dorsolateral wall of the telencephalon is initially made up of undifferentiated neuroepithelial cells at the beginning of development. The cells are neural stem cells, capable of dividing into various neuronal subtypes. The timing of birth for these neuronal subtypes determines the location (e.g fate) of the neurons so that specific connections and cortical structure are achieved. "E" relates to embryonic day in relation to corticogenesis.
| DAY | DEVELOPMENT |
|---|---|
| E30 | Progenitors in the dorsal telencephalon divide symmetrically and give rise to the initial ventricular zone (VZ), a single layer of cells that attach their "feet" to the cerebral wall and divide. These neurons lay adjacent to the ventricular surface. [5] |
| E31-32 | Dividing cells from the VZ begin to differentiate into "pioneer" neurons to form the preplate. These neurons migrate radially and tangentially from the VZ to the pial surface in an upward fashion. These cells are often referred to as radial glial cells (RGCs) because they contain radial fibers that span the entire embryonic wall from the VZ to the pial surface. These fibers create a "scaffolding" for subsequent migrating neurons to attach to and travel along in order to expand the neocortex. These cells are multipotent cells that divide asymmetrically to produce intermediate precursor cells that can become projection neurons, astrocytes, and oligodendrocytes [6] . The preplate is referred to as heterogeneous because it contains many developing cell types. |
| E40-45 | Cells in the VZ continue to divide symmetrically and give rise to another zone known as the subventricular zone (SVZ). This proliferative layer lies above the ventricular zone and is not attached to the ventricular surface. Radial glial cells also populate this area as well as the preplate and many of the cells in the SVZ contribute to the later cortical neurons. The SVZ also separates into the outer subventricular zone (OSVZ) and the inner subventricular zone (ISVZ). The OSVZ continues to expand as it produces more migrating immature neurons and intermediate precursor cells. [6] |
| E50-55 | The preplate begins to split into two subsections: the marginal zone and the subplate. An intermediate zone forms below the subplate and contains only migrating cells (migrating to the target destination) and no intermediate precursor cells. The cortical plate also begins to form in between the two regions. Newly born neurons that migrate to the cortical plate are developed "inside out". This term "inside-out" means that earlier born cells populate the deep layers first and later born neurons populate the superficial layers of the neocortex by migrating past the deep layers. Therefore, layers 6 is populated before layer 5; layer 5 is populated before layer 4 and so on. Each layer condenses and the neurons are closely packed. As the layers form, the cortex expands. |
Migration and division of all six layers of the cortex is completed during the third trimester. Each layer has distinct synaptic connections and cell types that contribute to the specific functions of the cortex.
Anatomy of the Cerebral Cortex
( z5059696)
-layer grey matter outer surface of cerebrum
-2-4mm thickness
-most anterior (rostral) brain region
-outer zone of neuronal tissue (grey matter) containing neuronal cell bodies
-densely packed in humans with over 10 billion nerve cells (about 10% of all the neurons in the brain)
-where much of the neural activities of the cerebrum takes place
-divided left and right hemispheres by longitudinal fissure
-two hemispheres joined by corpus callosum at midline
-divided into functional areas that serve various sensory, motor and cognitive functions
-subdivisions of layers organizing input and output connectivity of resident neurons
-is folded in larger mammals to increase surface area, important allows addition and evolution of a greater diversity functional areas
-gyrus (gyri)= folds/ ridges
-sulcus (sulci)= groove
Layers
https://en.wikipedia.org/wiki/File:Gray754.png
Layer 1
-outer layer (pial surface)
-molecular layer, few scattered neurons
-mainly extensions of pyramidal neuron apical dentrite tufts
-some spiny stellate cells
-inputs to apical tufts crucial for feedback interactions in cortex in associative learning and attention
Layer 2
-external granular layer
-small pyramidal neurons
-many stellate neurons
Layer 3
-external pyramidal layer
-small and medium sized pyramidal neurons
-non-pyramidal neurons with vertically orientated intracortical axons
-layers 1, 2, 3 main target of interhemisphere corticocortical afferent fibres
-layer 3 main source of cortiocortical efferent fibres
Layer 4
-internal granular layer
-different types stellate and pyramidal neyrons
-main target thalamocortical afferents from thalamus type C neurons and intra-hemipsheric corticocortical afferents
Layer 5
-internal pyramidal layer
-large pyramidal neurons
-give rise to axons leaving cortex and run down to subcortical structures e.g. basal ganglia
-In primary motor cortex of the frontal lobe, this layer contains Betz cells and their axons travel through the internal capsule, the brain stem and the spinal cord forming the corticospinal tract
-which is the main pathway for voluntary motor control
Layer 6
-polymorphic/ multiform layer
-few large pyramidal neurons
-many small spindle like pyramidal and multiform neurons
-sends efferent fibers to thalamus forms exact reciprocal interconnection between thalamus and cortex
-connections are both inhibitory and excitatory
Other info to add
three large surfaces: superolateral surface, medial surface, inferior surface
-surfaces characterised by sulci and gyri
three borders: superomedial border, inferomedial border, inferolateral border
lobes defined by large sulci (fissures)
named according to their relation to bones of skull
1) frontal lobe
2) parietal lobe
3) temporal lobe
4) occipital lobe
Blood Supply
Functions of the Cerebral Cortex
( z5059696)
Functional Areas
-Motor area (primary motor cortex)
-premotor area (motor association cortex)
-sensory area (pimary somatosensory cortex)
-auditory (acoustic) area
-olfactory area
-visual areas
-occipital eye field
-prefrontal areas (prefrontal cortex)
<html5media height="400" width="600">https://www.youtube.com/watch?v=n6zQbTT0yoY</html5media> [7]
Abnormalities associated with Cerebral Cortex Development
( z5093005 )
References used to write: [8] [9] [10] [11]
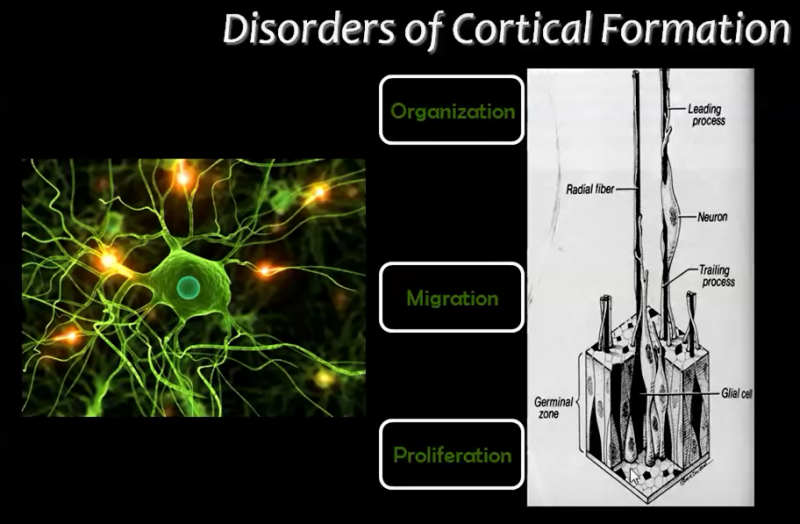
A) Disorders due to abnormal proliferation, growth or differentiation of neuroblasts
1) Focal cortical dysplasia (FCD)
Focal cortical dysplasia (FCD) is heterogeneous developmental disorder of uncertain etiology. Focal Cortical Dysplasia is characterised by neurons arranged abnormally in the focal areas of the cerebral cortex as well as disorganisation of layers and very large cells called 'balloon' cells.
FCD can affect the areas throughout the brain but occurs dominantly in the frontal and temporal lobes of the cerebral cortex.
In all patients who present with epilepsy, FCD is the cause for about approximately 25% of them.
FCD is of two types: Type I and Type II.
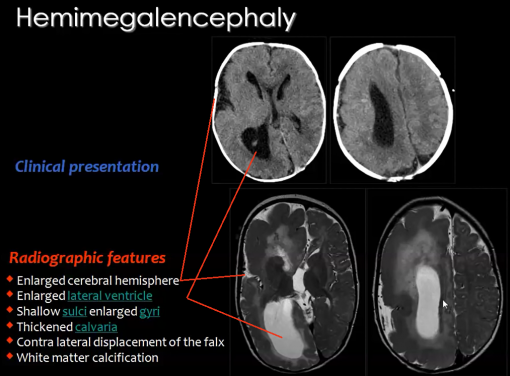
2) Hemimegalencephaly
A rare congenital disorder that also results from the abnormal proliferation of neuroblasts is Hemimegalencephaly.
Hemimegalencephaly is a developmental disorder which consists of a 'hamartomatous' overgrowth (where normal mature cells and tissues normally present in an area of the body, form a tumour-like, benign malformation) on part or all of the cerebral hemisphere.
Hemimegalencephaly accounts for 0.2% of cases of childhood epilepsy. Clinical symptoms of this disease may include developmental delay, paralysis of one side of the body and blindness over half the field of vision; but the most significant symptom is seizures as 90% of patients present with this symptom.
3) Microcephaly Vera
Microcephaly or 'small brain', can be caused by abnormal cell proliferation or cell division along with the involvement of other organs.
Primary Microcephaly or Microcephaly Vera results only due to abnormal cortical development, specifically cell division or proliferation. It is a congenital malformation in which the circumference of the head is quite less than normal and is accompanied by mental retardation and sometimes epilepsy.
4) Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is a multi-organ disease resulting from mutations of certain genes, and is usually classified under defects of early cell proliferation and growth although it is also associated with defects of neuronal migration.
Tuberous sclerosis complex (TSC) is thus called due to lesions seen that resembled potato tubers; and is characterised by benign abnormal mass of cells (tumors, cysts, and other malformations including hamartomas and neoplasms) in the cortex.
" Beneath the cortex, the brain in tuberous sclerosis also shows nodular collections of small cells along the surface of the lateral ventricle that resemble ventricular cells and are called subependymal nodules...or, more descriptively, “candle drippings.” These nodules often contain numerous balloon cells and can in some cases become transformed into glial tumors referred to as subependymal giant cell astrocytomas..."
B) Disorders due to abnormal neuronal migration
5) Heterotopia
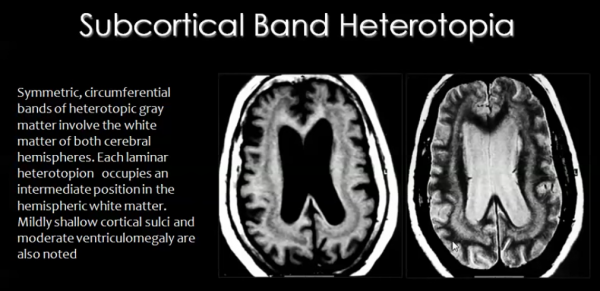
Migration of neurons occurs during the 8th week of development, along radial glial fibers (RGF). RGFs can be damaged due to ischemia, which can be caused by infection resulting from trauma or metabolic errors.
RGF damage leads to the migration process arresting at the wrong time, resulting in abnormal or ectopic locations of neurons of the cortex. This condition is known as Heterotopia.
There are different types of heterotopia:
- Subcortical band heterotopia:
In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-cortical regions of the cerebral cortex.
- Periventricular heterotopia/ sub-ependymal heterotopia:
In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-ependymal or periventricular area of gray matter of cerebral cortex.
- Focal heterotopia:
In this type of heterotopia, abnormal location of neurons result in focal masses within deep white matter of cerebral cortex.
6) Lissencephaly
Lissencephaly is a congenital cortex malformation, in which gyri (and sulci) of the cerebral cortex are absent (agyria) or largely absent (pachgyria), resulting in a smooth surface of the brain. The normal lamination or layers fail to form and the cerebral cortex usually has only 4 of the typical 6 layers. Like most congenital brain disorders, the etiology of Lissencephaly is uncertain.
Lissencephaly has also been associated with other abnormalities including corpus callosum hypoplasia, small brain stem and gray matter heterotopia.
Lissencephaly is generally characterised by the following features:
- Total agyria (gyri and sulci absent resulting in 'smooth brain')
- Pachygyria (gyri can be seen but are very few compared to normal brain)
- Agyric brain with areas of pachygyria
- Vertically oriented Sylvian fissures of the brain, giving the brain an 'hourglass' configuration
Lissencephaly is of different types; even so common clinical symptoms are 'epilepsy' and 'severe mental retardation'. Types of Lissencephaly are the following:
- Classical (Type I) Lissencephaly- results from neuronal undermigration (failed or incomplete neuronal migration) due to varying causes like mutation; additional clinical features include motor deficits. Patients often also have microcephaly [discussed later in Microlissencephaly].
- Cobblestone (Type II) lissencephaly- results from overmigration, where neurons migrate from the cortex into the overlying leptomeninges, causing diverse disruptions of the basement membrane; the cortex has a 'cobblestone' type of nodular appearance and additional clinical features include various eye abnormalities, congenital muscular dystrophies, hypotonia and generalized weakness in infancy.
Type II Lissencephaly also includes:
- Muscle-Eye-Brain Disease
- Walker-Warburg Syndrome
- Fukuyama Syndrome
- Microlissencephaly
Microlissencephaly occurs when Type I Lissencephaly occurs in combination with congenital Microcephaly, and presents with severe symptoms including seizures, spasticity, severe developmetal delay and short life span.
7) Kallmann Syndrome
The Kallman Syndrome is syndrome which results from the migration of neurons that secrete leuteinizing hormone-releasing hormone (LHRH) from the olfactory placode to the hypothalamus, causing congenital hypogonadism (since LHRH is necessary for normal gonadal function).
Kallman Syndrome is associated with hypoplasia of the olfactory bulbs and olfactory cortex, called arhinencephaly, and lack of the sense of smell. [not yet changed into own words]
C) Disorders due to abnormal cortical maturation and organization/folding
8) Polymicrogyria
If the neurons of the cerebral cortex successfully complete the proliferation and migration stages, but during the last organisation stage, distribute abnormally then multiple small undulating gyri can result. This condition is called Polymicrogyria (PMG), in which, the gyri become extremely small (hence the term micro) and their number is greater than normal. The cerebral cortex in this condition is flat and thickened, similar to that of pachygyria or agyria, and contains numerous small gyri.
Polymicrogyria is usually focal. It is most commonly 'perisylvian' (around the Sylvian fissure) and can be 'bilateral' or 'unilateral'. The most common sites, in descending order, are the frontal lobe, parietal lobe, temporal lobe and then occipital lobe.
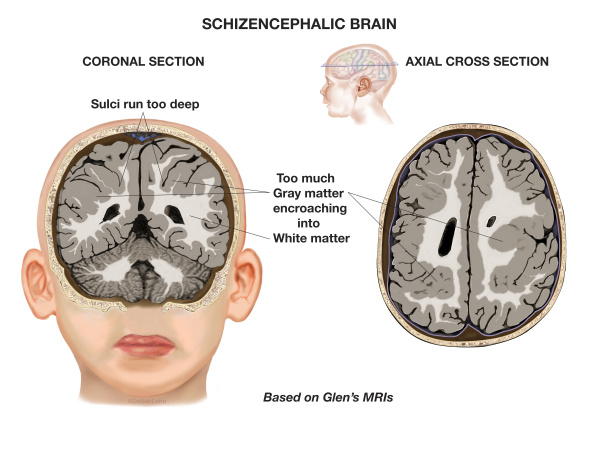
9) Schizencephaly
Schizencephaly is an organisational developmental disorder characterised by a cleft(s) or lesion(s) on the brain surface, which is filled with CSF and lined by gray matter. Schizencephaly can be bilateral or unilateral.
The clefts can be small with closed walls in Type I or Closed lip type schizencephaly; or the clefts can be large with free communication between the ventricle and subarachnoid spaces in Type II or Open lip type schizencephaly.
Schizencephaly commonly involves the parasylvian regions, and severity of the disease correlates with the extent of the clefts.
D) Others
12) Fetal Alcohol Spectrum Disorder (FASD)

Fetal Alcohol Syndrome (FAS) refers to the effects that are seen on a child if there was prenatal alcohol exposure. Various studies have showed that developmental cerebral cortical abnormalities are caused from prenatal ethanol exposure. Further studies have shown that abnormalities in the cerebral cortices of human children and are also shown. The main three impacts that are seen and which are also the diagnoses for FAS are facial dysmorphology, growth deficits and central nervous system problems (Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis; National Center on Birth Defects and Developmental Disabilities, 2004). These conditions result in immense physiological and psychological impacts on the child. On average, FAS is diagnosed in a child between 3-10 years. It is of utmost importance however that the diagnosis is done as early as possible so that timely interventions could be taken in order to lessen the severity of the symptoms that result from this syndrome. [15]
13) Corpus Callosum Agenesis
<html5media height="400" width="500">https://www.youtube.com/watch?v=7zOa3LLrHKc</html5media>
[16]
INFO/ Research Links (temporary heading)
https://www.youtube.com/watch?v=n6zQbTT0yoY
https://www.youtube.com/watch?v=X-m0JDCw6TE
https://www.youtube.com/watch?v=luXDQrmMoUU
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992410/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3547618/
http://www.cell.com/neuron/fulltext/S0896-6273(00)80749-7?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627300807497%3Fshowall%3Dtrue
https://academic.oup.com/jnen/article/61/1/1/2916251
https://pdfs.semanticscholar.org/67ea/2ce13f57f4ddbfb7033b6bb1328a5dff7742.pdf
https://www.youtube.com/watch?v=l_nTggR7LTE
For concept:
https://www.youtube.com/watch?v=dNngOlsLuGI
You might find this helpful (although you need to request copyright permission):
"Difference Between Cerebrum and Cerebral Cortex." DifferenceBetween.Com. August 7, 2012. < http://www.differencebetween.com/difference-between-cerebrum-and-vs-cerebral-cortex/ >
PubMed Searches: Cerebral Cortex Development | Cerebrum Development
BMC Dev Biol Search: Cerebral Cortex Development
Recent papers
<pubmed limit=5>Cerebral+Cortex+Development</pubmed>
References
- ↑ 1.0 1.1 1.2 Embryology.med.unsw.edu.au. (2017). Lecture - Ectoderm Development - Embryology. [online] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/Lecture_-_Ectoderm_Development.
- ↑ 2.0 2.1 2.2 2.3 Moore, K., Persaud, T. and Torchia, M. (2015). The developing human. Philadelphia, PA: Elsevier.
- ↑ 3.0 3.1 3.2 Gray, H. (1977). Gray's Anatomy. New York, New York: Crown Publishers, Inc.
- ↑ <pubmed> PMC3876965 </pubmed>
- ↑ <pubmed> 18209730 </pubmed>
- ↑ 6.0 6.1 <pubmed> PMC4334136 </pubmed>
- ↑ Learn Some (2016). Cerebral Cortex. [video] Available at: https://www.youtube.com/watch?v=n6zQbTT0yoY [Accessed 5 Oct. 2017].
- ↑ 8.0 8.1 8.2 8.3 Mahfouz, M. (2015). Imaging of cortical formation disorders - DRE 4 - Prof. Dr Mamdouh Mahfouz. [video] Available at: https://www.youtube.com/watch?v=l_nTggR7LTE [Accessed 23 Sep. 2017].
- ↑ Squier, W. and Jansen, A. (2010). Abnormal development of the human cerebral cortex. Journal of Anatomy, [online] 217(4), pp.312-323. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992410/ [Accessed 23 Sep. 2017].
- ↑ Pang, T., Atefy, R. and Sheen, V. (2008). Malformations of Cortical Development. The Neurologist, [online] 14(3), pp.181-191. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3547618/ [Accessed 23 Sep. 2017].
- ↑ Christopher A, C. (2017). Genetic Malformations of the Human Cerebral Cortex. Neuron, [online] 23(1), pp.19 - 29. Available at: http://www.cell.com/neuron/fulltext/S0896-6273(00)80749-7?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627300807497%3Fshowall%3Dtrue [Accessed 23 Sep. 2017].
- ↑ USDA & Felipe Dana/AP and Creative Commons License(CC) (2017). Understanding Zika. [online] Goinvo.com. Available at: http://www.goinvo.com/features/zika/ [Accessed 4 Oct. 2017].
- ↑ PRWeb (2013). Schizencephalic Brain. [image] Available at: http://www.prweb.com/releases/2013/7/prweb3350774.htm [Accessed 5 Oct. 2017].
- ↑ MarkHill,embryology.med.unsw.edu.au (2017). Facial Appearance of Fetal Alcohol Syndrome (FAS). [image] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/File:FASface.jpg [Accessed 5 Oct. 2017].
- ↑ Leigland, L., Ford, M., Lerch, J. and Kroenke, C. (2013). The Influence of Fetal Ethanol Exposure on Subsequent Development of the Cerebral Cortex as Revealed by Magnetic Resonance Imaging. Alcoholism: Clinical and Experimental Research, 37(6), pp.924-932. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3670687/ [Accessed 5 Oct. 2017].
- ↑ Ezzo - Izzo, D. (2007). How the Body Works : The Corpus Callosum. [video] Available at: https://www.youtube.com/watch?v=7zOa3LLrHKc [Accessed 23 Sep. 2017].