2017 Group Project 1: Difference between revisions
m (Protected "2017 Group Project 1" ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
|||
| (441 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
=Cerebral Cortex= | |||
{{ANAT2341Project2017header}} | {{ANAT2341Project2017header}} | ||
<!-- Do not remove template above from the project page --> | <!-- Do not remove template above from the project page --> | ||
==Introduction== | ==Introduction== | ||
[[File:Intro section cortex.jpg|thumb|right|350px|'''Figure1. Cerebral cortex is the outermost layer of the cerebrum''' <ref>Thomas, A. (2015). [image] Available at: http://slideplayer.com/slide/6617450/ [Accessed 23 Oct. 2017].</ref> ]] | |||
<br/> | |||
The cerebral cortex is part of the brain which surrounds the cerebral hemispheres. It has a very large surface area (largest part of human brain). The cerebral cortex differs from the cerebrum because, the cerebral cortex is the outermost layer of the cerebral hemispheres (Figure 1). It is a layer of gray matter around 2-4 mm in thickness, and consists of approximately 10 billion nerve cell bodies and dendrites. It appears gray because of the cell bodies (Figure 2).<br/><br/> | |||
[[File:Brain sectin showing cortex.jpg|thumb|left|300px|'''Figure 2. Section of the human brain''' <ref>Mikayla D (2017). 23. [image] Available at: https://psych-brain-trust.wikispaces.com/Thalamus [Accessed 23 Oct. 2017].</ref> ]] | |||
The cerebral cortex | The cerebral cortex is thought to be the main control centre, playing an essential role in cognitive function, memory, sensation and association.The development of the cerebral cortex involves 3 main stages: | ||
The | *Proliferation (or growth or differentiation) | ||
*Migration of neurons | |||
*Maturation (or organisation or folding).<br/> | |||
<br/> | |||
The cortex is organised into six horizontal layers. I. Molecular layer, II. External granular layer , III. Xxternal pyramidal, IV. Internal granular layer, V. Internal pyramidal layer, VI. Multiform layer. These individual layers organise the capacity for interconnections between both input and output signals. <br/> | |||
This project will explore the various embryologic aspects of the cerebral cortex, including early development of the brain, development of the cerebral cortex, anatomy and functions of the cortex, and abnormalities associated with cortical development.<br/><ref>Moore, K., Persaud, T. and Torchia, M. (2011). The Developing Human. London: Elsevier Health Sciences, pp.The Nervous System; 379-414.</ref><ref>Van Essen, D. (2005). A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage, 28(3), pp.635-662.</ref><ref>Dartmouth.edu. (2006). Chapter 11: The Cerebral Cortex. [online] Available at: http://www.dartmouth.edu/~rswenson/NeuroSci/chapter_11.html.</ref><ref>Differencebetween.com. (2017). Difference Between Cerebrum and Cerebral Cortex. [online] Available at: http://www.differencebetween.com/difference-between-cerebrum-and-vs-cerebral-cortex/ [Accessed 26 Oct. 2017].</ref><ref name="first">Squier, W. and Jansen, A. (2010). Abnormal development of the human cerebral cortex. Journal of Anatomy, [online] 217(4), pp.312-323. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992410/ [Accessed 23 Sep. 2017]. </ref><br/> | |||
<br/> | |||
==Early Development of the Brain== | ==Early Development of the Brain== | ||
<br/> | <br/> | ||
The brain begins to develop during the third week when the neural plate and tube | The brain begins to develop during the third week of pregnancy when the neural plate and neural tube are derived from the outer most layer of embryonic cells, that is the neuroectoderm. The development of the brain is from the neural plate which folds to form the neural groove and then curls forming the neural tube, which is cranial to the fourth pair of somites, and eventually, forms the three primary brain vesicles <ref name="lecture"> Embryology.med.unsw.edu.au. (2017). Lecture - Ectoderm Development - Embryology. [online] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/Lecture_-_Ectoderm_Development. </ref>. | ||
Neuroprogenitor cells proliferate, migrate, and differentiate to form specific areas of the brain <ref name="textbook"/>. | Neuroprogenitor cells proliferate, migrate, and differentiate to form specific areas of the brain <ref name="textbook"/>. | ||
During week four fusion of the neural folds in the cranial region and closure of the rostral neuropore form three primary brain vesicles from which the brain develops <ref name="lecture"/>: | During week four fusion of the neural folds in the cranial region and closure of the rostral neuropore form three primary brain vesicles from which the brain develops <ref name="lecture"/>: | ||
[[File:Brain Development 2.png|thumb|400px|right|'''Figure 3. A longitudinal perspective of the neural tube, showing the primary brain vesicles divided into the five secondary subdivisions'''. <ref name="image">Vanderah, T., Gould, D. and Nolte, J. (2016). Nolte's The human brain. Philadelphia, PA: Elsevier, p.44. </ref>]] | |||
*Forebrain/Prosecephalon | *Forebrain/Prosecephalon | ||
*Midbrain/Mesencephalon | *Midbrain/Mesencephalon | ||
*Hindbrain/ | *Hindbrain/Rhombencephalon <br/> | ||
<br/> | |||
From the three primary vesicles, there is a further division at the anterior extremity of the medullary canal into five secondary vesicles during week five, as depicted in Figure 3. These are fundamental divisions of the adult brain and communicate freely with each other <ref name="Gray"> Gray, H. (1977). Gray's Anatomy. New York, New York: Crown Publishers, Inc. </ref>. They are <ref name ="textbook"> Moore, K., Persaud, T. and Torchia, M. (2015). The developing human. Philadelphia, PA: Elsevier. </ref>: <br/> | |||
*Telencephalon: endbrain, forms cerebral hemispheres - derived from Prosecephalon | *Telencephalon: endbrain, forms cerebral hemispheres - derived from Prosecephalon | ||
*Diencephalon: between brain, forms optic outgrowth - derived from Prosecephalon | *Diencephalon: between brain, forms optic outgrowth - derived from Prosecephalon | ||
*Mesencephalon: undivided | *Mesencephalon: undivided | ||
*Metencephalon: posterior to the brain - derived from | *Metencephalon: posterior to the brain, gives rise to the pons (bulbous expansion consisting of white matter tracts serving the cerebellum) <ref name ="ebook"/> - derived from Rhombencephalon | ||
*Myelencephalon: medulla - derived from | *Myelencephalon: gives rise to the medulla oblongata - derived from Rhombencephalon <br/> | ||
In the beginning, these five divisions are uniform in size and shape but quickly differentiate at various rates <ref name="Gray"/>. | <br/> | ||
In the beginning, these five divisions are uniform in size and shape but quickly differentiate at various rates <ref name="Gray"/>. <br/> | |||
As the telencephalon is the region that develops into the cerebral cortex, we will discuss this region specifically in more detail. The telencephalon is the utmost rostral region of the brain vesicles, and consists of: <br/> | |||
*The median region: the lamina terminalis, with the cavity forming the anterior part of the third ventricle <ref name ="discovery"> Pansky, B. (n.d.). Chapter 155. The Brain: The Telencephalon (first Vesicle) - Review of Medical Embryology Book - LifeMap Discovery. [online] Discovery.lifemapsc.com. Available at: https://discovery.lifemapsc.com/library/review-of-medical-embryology/chapter-155-the-brain-the-telencephalon-first-vesicle. </ref> | |||
*Two lateral diverticula: the cerebral hemispheres <ref name="textbook"/><br/> | |||
<br/> | |||
The cerebral hemispheres grow simultaneously in lateral, longitudinal and parietal directions <ref name="discovery"/>, and originally are in communication with the third ventricle cavity via the interventricular foramina <ref name="textbook"/>. Upon expansion, the cerebral hemispheres eventually cover the diencephalon, midbrain and hindbrain, where they will eventually congregate at the midline, resulting in the flattening of each hemispheres medial surfaces <ref name="textbook"/>. During the sixth week the corpus striatum emerges as an obvious swelling in the floor of both of the cerebral hemispheres. the floors expand at a slower rate compared to the hemispheres thin cortical walls, as it contains large crpus striatum, causing the cerebral hemispheres to become a c shape <ref name="textbook"/>. This growth and curvature of the cerebral hemispheres does have consequential effects on the shape of the lateral ventricles, which too become c-shaped and fill with cerebrospinal fluid (CSF). Each hemispheres caudal ends turn ventrally, and then rostrally resulting in the formation of the temporal lobe. | |||
The telencephalon is further subdivided into a dorsal pallium and a cenrtral subpallium. The dorsal pallium forms the large neuronal nuclei of the basal ganglia (corpus striatum, globus pallidus) <ref name="ebook"/>. These structures are essential for fulfilling commands from the cerebral hemispheres, and appear as lateral outpouchings of the pallium growing at a fast rate in order to cover the mesencephalon and diencephalon. The cortex is formed by the pallium, or vault, of each hemisphere. | |||
'''Brain Flexures''' | '''Brain Flexures''' | ||
[[File:Brain Development.png|thumb|400px|right|'''Figure 4. Lateral perspective of the neural tube, showing the three flexures, and five secondary subdivisions'''.<ref name="image"/>]] | |||
During the fifth week, the embryonic brain undergoes rapid growth, folding the neural tube, consequently resulting in three brain flexures, as depicted in Figure 4, in proximity to the five secondary vesicles. These are <ref name="lecture"/>: | |||
*Cephalic flexure - centered at the midbrain region and pushes mesencephalon upwards being the first fold to develop, ventral folding of the brain tube <ref name ="ebook"> Schoenwolf, G., Bleyl, S., Brauer, P. and Francis-West, P. (2015). Larsen's human embryology. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone, pp.197-233. </ref> | |||
*Cervical flexure - between brain stem and spinal cord at the junction of the spinal cord and hindbrain, ventral folding of the brain tube <ref name="ebook"/> | |||
*Pontine flexure - beings at the developing pons, generates the fourth ventricle; produced in the opposite direction - dorsal folding <ref name="ebook"/> - as a result of later unequal brain growth, resulting in the thinning of the roof of the hindbrain <ref name="Gray"/>. | |||
<br/> | |||
The primordial brain initially has the same basic structure as the developing spinal cord, however, consideration variations in the outline of transverse sections at different levels of the brain and in the position of the gray and white matter are produced by the brain flexures <ref name="textbook"/>. The sulcus limitans (the floor of the fourth ventricle) extends cranially to the junction of the midbrain and forebrain, and the alar and basal plates are recognisable only in the midbrain and hindbrain <ref name="textbook"/>. | |||
Through the foundation of structures and processes in during the early developmental brain, the fetus' brain is able to further develop into a much more complex organ. | |||
<br/> | |||
[[File: | ==Later Development: Development of the Cerebral Cortex== | ||
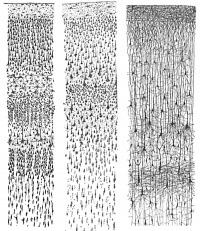
'''Main classes of neurons''' <ref><pubmed> PMC3876965 </pubmed></ref> | [[File:Neural- cortex Cajal drawing 01.jpg |thumb|right|200px|'''Figure 5. Laminar and columnar organization of the cerebral cortex (Historical drawing by Cajal)''']] | ||
<br/> | |||
Corticogenesis refers to the development of the cerebral cortex, the outer portion of the cerebrum, into its six layers. It involves a sequential process of migration and differentiation of neocortical projection neurons into a laminated structure that contains a diverse set of neuronal subtypes. The diversity in neurons contributes to the complex circuitry involved in the mammalian cortex. The large size of the cortex distinguishes humans from other mammals because it corresponds to a larger capacity to perform behavioral and cognitive tasks. <ref name="boulder"><pubmed> 18209730 </pubmed></ref> As mentioned in above in “Early Development of the Brain,” the cerebral cortex is derived from telencephalon, the rostral end of the neural tube. Origins of various neuronal subtypes come from the pallium (roof) and the subpallium (base) sections of the telencephalon which contributes to the overall laminar and columnar organization of the cortex. <ref name="migration">Marin, O., & Rubenstein, J. L. R. (2003). Cell migration in the forebrain. Annual Review of Neuroscience, 26, 441-83.</ref> | |||
<br /> | |||
'''Main classes of neurons''' <ref name="logic"><pubmed> PMC3876965 </pubmed></ref> | |||
*'''Projection neurons:''' excitatory neurons (glutamatergic) with axons that project to distant targets and are generated by progenitors in the dorsal pallium of the telencephalon. The have a typical pyramidal structure and send signals to various regions of the brain and neocortex. | *'''Projection neurons:''' excitatory neurons (glutamatergic) with axons that project to distant targets and are generated by progenitors in the dorsal pallium of the telencephalon. The have a typical pyramidal structure and send signals to various regions of the brain and neocortex. | ||
*'''Interneurons:''' inhibitory neurons (GABAergic) that have local connections within the cortex and are generated in the subpallium of the telecephalon in the ventral proliferative zone. These neurons have to migrate to the neocortex area. | *'''Interneurons:''' inhibitory neurons (GABAergic) that have local connections within the cortex and are generated in the subpallium of the telecephalon in the ventral proliferative zone. These neurons have to migrate to the neocortex area. | ||
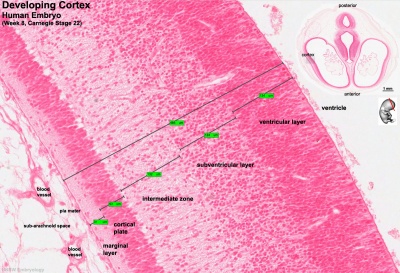
[[File:Stage 22 image 217.jpg |thumb|right|400px|super|'''Figure 6. Key developmental zones in the human cortex'''.]] | |||
<br /> | |||
'''Key developmental zones in the human cortex: ''' | '''Key developmental zones in the human cortex: ''' | ||
*Ventricular zone | *Ventricular zone | ||
| Line 63: | Line 82: | ||
*Intermediate Zone | *Intermediate Zone | ||
*Preplate: neural progenitor cells split into 2 regions | *Preplate: neural progenitor cells split into 2 regions | ||
**Marginal zone | **Marginal zone | ||
**Subplate | **Subplate | ||
*Cortical Plate: | *Cortical Plate: establishment of the 6 cortical layers form in this region between the subplate and the marginal zone | ||
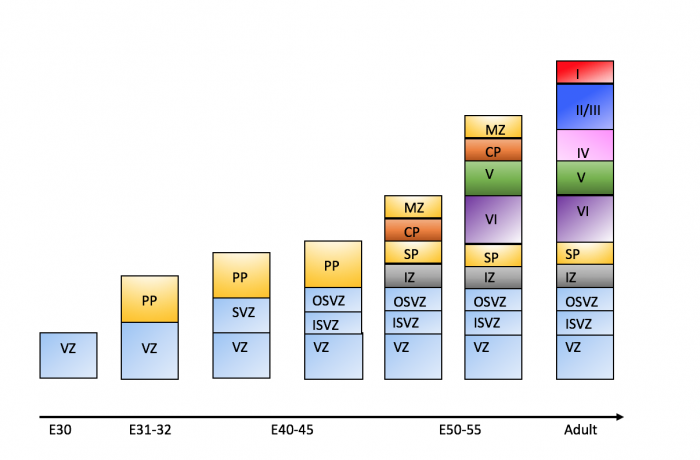
===Timeline of Corticogenesis=== | |||
The dorsolateral wall of the telencephalon is initially made up of undifferentiated neuroepithelial cells at the beginning of development. The cells are neural stem cells, capable of dividing into various neuronal subtypes. <ref name="boulder"/> The timing of birth for these neuronal subtypes determines the location (e.g fate) of the neurons so that specific connections and cortical structure are achieved. "E" relates to embryonic day in relation to corticogenesis. | |||
<br/> | |||
{| class="wikitable" | {| class="wikitable" | ||
| Line 75: | Line 96: | ||
! style="background:#f4a941" | DEVELOPMENT | ! style="background:#f4a941" | DEVELOPMENT | ||
|- | |- | ||
| E30|| Progenitors in the dorsal telencephalon divide symmetrically and give rise to the initial '''ventricular zone (VZ)''', a single layer of cells that attach their "feet" to the cerebral wall and divide. These neurons lay adjacent to the ventricular surface. <ref | | E30|| Progenitors in the dorsal telencephalon divide symmetrically and give rise to the initial '''ventricular zone (VZ)''', a single layer of cells that attach their "feet" to the cerebral wall and divide. These neurons lay adjacent to the ventricular surface. <ref name="boulder"/> | ||
|- | |- | ||
| E31-32 || Dividing cells from the VZ begin to differentiate into "pioneer" neurons to form the '''preplate.''' These neurons migrate radially and tangentially from the VZ to the pial surface in an upward fashion. These cells are often referred to as '''radial glial cells (RGCs)''' because they contain radial fibers that span the entire embryonic wall from the VZ to the pial surface. These fibers create a "scaffolding" for subsequent migrating neurons to attach to and travel along in order to expand the neocortex. These cells are multipotent cells that divide asymmetrically to produce intermediate precursor cells that can become projection neurons, astrocytes, and oligodendrocytes <ref name="cerebral"><pubmed> PMC4334136 </pubmed></ref> . | | E31-32 || Dividing cells from the VZ begin to differentiate into "pioneer" neurons to form the '''preplate.''' These neurons migrate radially and tangentially from the VZ to the pial surface in an upward fashion. These cells are often referred to as '''radial glial cells (RGCs)''' because they contain radial fibers that span the entire embryonic wall from the VZ to the pial surface. These fibers create a "scaffolding" for subsequent migrating neurons to attach to and travel along in order to expand the neocortex. These cells are multipotent cells that divide asymmetrically to produce intermediate precursor cells that can become projection neurons, astrocytes, and oligodendrocytes <ref name="cerebral"><pubmed> PMC4334136 </pubmed></ref> . In addition to RGCs, '''Cajal-Retzius (CR) cells''' are another type of early born neurons that develop at the same time as the preplate. These cells express reelin, an important signaling factor for migrating neurons to properly organize into their destined layers. They remain in the marginal zone when the preplate splits into two (see E50-55).<ref name="migration"/> The preplate is referred to as heterogeneous because it contains many developing cell types. | ||
|- | |- | ||
| E40-45 || Cells in the VZ continue to divide symmetrically and give rise to another zone known as the '''subventricular zone (SVZ)'''. This proliferative layer lies above the ventricular zone and is not attached to the ventricular surface. Radial glial cells also populate this area as well as the preplate and many of the cells in the SVZ contribute to the later cortical neurons. The SVZ also separates into the outer subventricular zone (OSVZ) and the inner subventricular zone (ISVZ). The OSVZ continues to expand as it produces more migrating immature neurons and intermediate precursor cells. <ref name="cerebral"/> | | E40-45 || Cells in the VZ continue to divide symmetrically and give rise to another zone known as the '''subventricular zone (SVZ)'''. This proliferative layer lies above the ventricular zone and is not attached to the ventricular surface. Radial glial cells also populate this area as well as the preplate and many of the cells in the SVZ contribute to the later cortical neurons. The SVZ also separates into the outer subventricular zone (OSVZ) and the inner subventricular zone (ISVZ). The OSVZ continues to expand as it produces more migrating immature neurons and intermediate precursor cells. <ref name="cerebral"/> | ||
|- | |- | ||
| E50-55|| The preplate begins to split into two subsections: the '''marginal zone''' and the '''subplate.''' An intermediate zone forms below the subplate and contains only migrating cells (migrating to the target destination) and no intermediate precursor cells. The '''cortical plate''' also begins to form in between the two regions. Newly born neurons that migrate to the cortical plate are developed '''"inside out"'''. This term "inside-out" means that earlier born cells populate the deep layers first and later born neurons populate the superficial layers of the neocortex by migrating past the deep layers. Therefore, layers 6 is populated before layer 5; layer 5 is populated before layer 4 and so on. Each layer condenses and the neurons are closely packed. As the layers form, the cortex expands. | | E50-55|| The preplate begins to split into two subsections: the '''marginal zone''' and the '''subplate.''' An intermediate zone forms below the subplate and contains only migrating cells (migrating to the target destination) and no intermediate precursor cells. The '''cortical plate''' also begins to form in between the two regions <ref name="logic"/>. Newly born neurons that migrate to the cortical plate are developed '''"inside out"'''. This term "inside-out" means that earlier born cells populate the deep layers first and later born neurons populate the superficial layers of the neocortex by migrating past the deep layers. Therefore, layers 6 is populated before layer 5; layer 5 is populated before layer 4 and so on <ref name="boulder"/>. Each layer condenses and the neurons are closely packed. As the layers form, the cortex expands. | ||
|} | |} | ||
Migration and division of all six layers of the cortex is completed during the third trimester.<ref name="boulder"/> Each layer has distinct synaptic connections and cell types that contribute to the specific functions of the cortex such as sensory and motor function. | |||
[[File:Corticogenesis.png|center|thumb|700px|super|'''Figure 7. Corticogenesis from E30 to adult human brain''']] | |||
===Signalling involved in Cerebral Cortex Development=== | |||
<br/> | |||
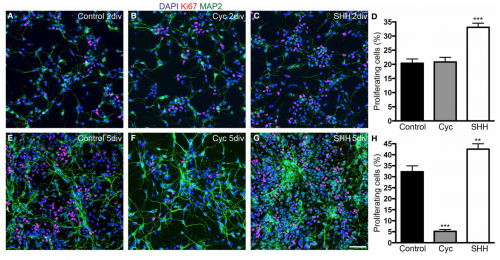
[[File:Screen Shot 2017-10-15 at 13.31.05.png |thumb|right|text-top|500px| '''Figure 8. Shh signalling increases the number of proliferating cells''']] | |||
Cell signalling is an essential part of the laminar patterning of the cerebral cortex into its 6 distinct, radially organised layers. There is a large network of interweaving signalling pathways that are responsible for the formation of this sophisticated anatomical structure and its functioning. There is still much debate about the exact pathways and signalling molecules that lead to this specific patterning, however some factors contributing to the control of cortical differentiation have been uncovered. | |||
[[File: | [[File:Cortical plate development.jpg |thumb|left|text-top|350px| '''Figure 9. Cortex development in wild-type and ''reeler'' mice''']] | ||
'''Sonic Hedgehog''' (Shh) is a morphogen involved in regulating the development of many different tissue types. During the development of the neocortex, Shh plays a role in controlling the proliferation and survival of cortical progenitor cells along with the specification of cerebral cortex GABAergic neurons. <ref><pubmed>20159447</pubmed></ref> | |||
It has been shown that blockade of Shh in murine models with cyclopamine has effects on cortical neurogenesis, with indications that Shh morphogen could be play a role in the control of cell cycle length, cell growth and the process of cell division of the dorsal telencephalon progenitors during early corticogenesis (Fig. 8) <ref><pubmed> 24653675 </pubmed></ref> | |||
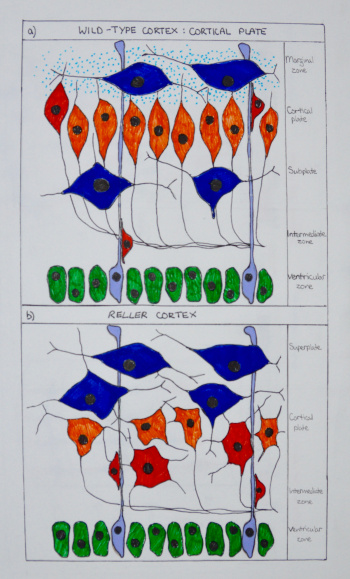
- | '''Reelin''' is one of the more well understood signalling pathways involved in corticogenesis. Cajal-Retzuis cells with are located in the marginal zone (MZ) secrete Reelin (an extracellular matrix glycoprotein). Through studies that examine the absence of Reelin in reeler mutant mice, neuronal migration is significantly altered (Fig. 9). Additionally, Reelin has been shown to have a crucial role in inducing branching and specific positioning of radial glial cells (RGC), in that the distribution of Reelin within the MZ defines the specific location of radially migrating neurons, and is essential for the characteristic ‘inside-out’ pattern of the six cortical layers. <ref><pubmed>25246510</pubmed></ref> | ||
- | '''Notch''' signalling molecular pathway is also crucial to corticogenesis. It is thought that there is an important interplay between this pathways and Reelin, as deficits of both result in impaired migration and altered morphology. <ref><pubmed>2913541</pubmed></ref> Both signalling pathways are involved in the expression of the radial glial gene, BLBP, which could be significant in the development of radial glial cells. 5. | ||
Although generally considered a developmental pathway, studies have shown the importance of Notch signalling in the adult brain, through distinguishing the balance between maintenance of neural stem cells and differentiation. These new understandings have implications for therapeutic approaches to neurodegenerative diseases. <ref><pubmed>21505516</pubmed></ref> | |||
[[File:Cortical thickness in Bmp7 knockouts.png |thumb|right|text-top|500px|'''Figure 10. Cortical thickness in the absence of Bmp7''']] | |||
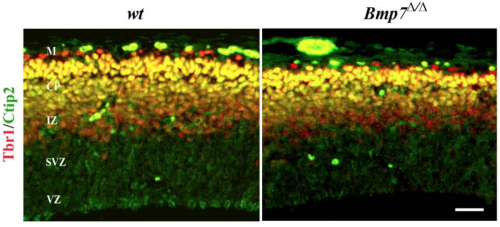
'''Bmp7''' in addition to cell intrinsic factots there are also estristic signalling factors to take into account, for example Bone Morphogenitic Protein 7 (Bmp7) with debate ongoing as to its promotion or inhibition of neurogenesis. Deletion of Bmp7 in murine models has been shown to result in reduced thickening of the cortex, likely due to the changed differentiation of progenitor cells from the subventricular zone to the upper layers, (Fig. 10) Bmp7 regulates the expression of gene Ngn2, which in Bmp7 knock out models appeared to be majorly reduced, perhaps playing a role in the development of deep layer neurons. <ref><pubmed>22461901</pubmed></ref> | |||
The signalling pathways that underlie corticogenesis are very complicated and the exact mechanisms are still under continuous investigation. Further research will only improve understanding of this network of intertwining factors and potentially lead to better appreciation of neurodevelopmental conditions and thus innovative therapeutic approaches. | |||
<br/> | |||
- | ==Anatomy of the Cerebral Cortex== | ||
<br/> | |||
The cerebral cortex is a convoluted layer of grey matter on the outer surface of the cerebrum. Grey matter is neuronal tissue containing neuronal cell bodies. In humans the cortex has a thickness of 2-3mm however it's surface area is many hundred square centimetres allowing an increased cortical surface to occupy small cranial volume. The cortex in humans contains 10 billion densely packed nerve cells (10% of the neurons in the brain). The human neocortex is the most phylogenetically developed structure of the brain compared to other species. The folding in larger mammals is important to allow addition and evolution of a greater diversity of functional areas. As the folding is highly preserved across individuals this allows the different sections sepearted by gyri and sulci to be labelled. <ref name= "cortex anatomy"><pubmed>17580069</pubmed></ref> <br/> | |||
<br/> | |||
[[File:Anatomy cerebral cortex.png|thumb|none|text-top|500px|'''Figure 11. Anatomy of the human cerebral cortex''' <ref name="drawing">Handwritten Tutorials (2012). Brain Anatomy 1- Gross Cortical Anatomy (Lateral Surface). [video] Available at: https://www.youtube.com/watch?v=woIZaLiy-eA [Accessed 5 Oct. 2017].</ref>]] <br/> | |||
The longitudinal fissure divides the cortex into the left and right hemispheres, which are connected at the midline by the longitudinal fissure. Each hemisphere is then divided into four lobes (frontal, temporal, parietal and occipital) which are labeled by their relation to bones of the skull (see figure 11). The frontal lobe is separated from the temporal lobe by the lateral sulcus also known as the Sylvian fissure. The Rolandic fissure (central sulcus) divides the frontal and parietal lobe and the parietal and occipital lobes are distinguished by the parieto-occipital sulcus. <ref><pubmed>19763105</pubmed></ref> | |||
The frontal lobe is subdivided by the superior and inferior frontal sulci into the superior, middle and inferior frontal gyri, as shown in figure 11. The frontal operculum is formed by the inferior frontal gyrus and is divided into the pars orbitalis, pars triangularis and pars opercularis. These are triangular gyri and are listed in order of anterior to posterior. <ref name="cortex anatomy"/> | |||
- | The temporal lobe can also be subdivided by the superior and inferior temporal sulci. These subdivisions include the superior, middle and inferior temporal gyri (see figure 11). Lateral to the midbrain on the inferior temporal lobe surface is the parahippocampal gryus. The occipitotemporal gyrus, also named fusiform gyrus, lies between the inferior temporal gyrus and the parahippocampal gyrus. Within the parietal lobe the angular gryrus lies above the superior temporal sulcus. Above the angular gyrus, the supramrginal gyrus lies above the lateral sulcus. Below the angular gyrus, the lateral occipital gyrus lies above the inferioir temporal sulcus. <ref name="cortex anatomy"/> | ||
<br/><br/> | |||
<html5media height="400" width="600">https://www.youtube.com/watch?v=woIZaLiy-eA</html5media> | |||
<ref name="drawing"/> | |||
<br/><br/> | |||
'''Layers of the Cortex''' | |||
- | {| class="wikitable" | ||
|- | |||
| Layer 1|| | |||
outermost layer (pial surface) | |||
molecular layer with few scattered neurons | |||
mainly extensions of pyramidal neuron apical dendrite tufts with some spiny stellate cells | |||
- | inputs to apical tufts are crucial for feedback interactions in cortex in associative learning and attention <ref><pubmed>8747184</pubmed></ref> | ||
|- | |||
| Layer 2|| | |||
external granular layer | |||
contains small pyramidal neurons and many stellate neurons | |||
pyrimidal neurons are excitatory <ref name="layers"><pubmed>17553419</pubmed></ref> | |||
|- | |||
| Layer 3|| | |||
external pyramidal layer | |||
small and medium sized pyramidal neurons | |||
non-pyramidal neurons with orientated intracortical axons | |||
layers 1,2 and 3 are the main target for interhemisphere corticocortical afferent fibres | |||
layer 3 is the main source for cortiocortical efferent fibres | |||
- | |- | ||
| Layer 4|| | |||
internal granular layer | |||
many different types of stellate and pyramidal neurons | |||
main target for thalamocortical afferents from thalamus type C neurons and intra-hemispheric corticocortical afferents <ref><pubmed>9622234</pubmed></ref> | |||
|- | |||
| Layer 5|| | |||
internal pyramidal layer | |||
large pyramidal neurons | |||
give rise to axons leaving cortex and run down to subcortical structures e.g. basal ganglia | |||
In the primary motor cortex of the frontal lobe, layer 5 contains Betz cells and their axons travel through the internal capsule, the brain stem and the spinal cord forming the corticospinal tract, which is the main pathway for voluntary motor control | |||
cortical areas with no layer 5 are named agranular and cortical areas with an undeveloped layer 5 are named dysgranular <ref name="layers"/> | |||
- | |- | ||
| Layer 6|| | |||
polymorphic/ multiform layer | |||
few large pyramidal neurons | |||
many small spindle like pyramidal and multiform neurons | |||
sends efferent fibres to thalamus and forms exact reciprocal interconnection between thalamus and cortex | |||
these connections are both inhibitory and excitatory <ref><pubmed>19447861</pubmed></ref> | |||
|} | |||
<br/> | |||
==Functions of the Cerebral Cortex== | ==Functions of the Cerebral Cortex== | ||
( | <br/> | ||
[[File:Cortical areas.png|thumb|right|text-top|450px|'''Figure 12. Cortical areas human cortex''' <ref>Guyton, A & Hall, J (2017). Functions of Specific Cortical Areas. Available at http://www.brainkart.com/article/Functions-of-Specific-Cortical-Areas_19753/. </ref>]] | |||
[[File:Homonculus Sensory and Motor Cortex .png|thumb|right|text-top|450px|'''Figure 13. Cortical Homonculus'''<ref> Bust, A & Kellogg, D. (2015). Homunculus: Somatosensory and Somatomotor Cortex. EBM Consult [online] Available at https://www.ebmconsult.com/articles/homunculus-sensory-motor-cortex#jump_ss_100125. </ref> ]] | |||
The cerebral cortex controls memory, movement, perception, cognition, consciousness, awareness and language. Majority of the connections in the cortex are from one cortex area to the other rather than with other structures. However, the cortex is connected to structures below it such as the basal ganglia and thalamus. The cortex communicates with these structures; information is sent along efferent connections and received by afferent connections. | |||
Majority of the sensory information in the brain is sent to the cortex by the thalamus and olfactory information is send to the olfactory cortex through the olfactory bulb. The cortex can be split into three cortical areas (cortices for plural) based on their function; sensory, motor and association areas, as shown in Figure 12. <ref name="overall function"><pubmed>21653723</pubmed></ref> | |||
Primary cortices have simpler functions including direct sensory input (vision, hearing and somatic sensation) or directly producing eye and limb movements. The association cortices functions are more complex and include memory, language, abstraction, creativity, judgement, emotion, attention and movement synthesis. <ref name= "overall function"/> | |||
Sensory areas receive and process information from the senses including visual, hearing and somatosensory information. The primary sensory areas receive sensory inputs from the thalamus. The sensory area in each hemisphere receives sensory information from the opposite side of the body. The sensory cortex is organised as shown in figure and provides a map of the body also known as a homunculus. A cortical homunculus is a model used to represent the area and proportions of motor and sensory functioning in the human body (see figure 13). The size of the body part represents the innervation density, for example the fingers and lips have a higher number and more complex sensory and motor connections. They require more cortical area for the processing of finer sensation. <ref><pubmed>9931268</pubmed></ref> | |||
The motor areas control voluntary movements and like the sensory areas, the motor area in the left hemisphere controls the movement for the right side of the body and vice versa. The basal ganglia receive input from the motor areas as well as the midbrain (substantia nigra) and also sends signals back to these areas aiding the control of motor movements. <ref><pubmed>29042690</pubmed></ref> | |||
The association areas produce perceptual experience and involve functions such as abstract thinking and language. The parietal, temporal and occipital lobes assimilate information stored as memories and sensory information. The association areas connect distant areas of the cortex. <ref name= "overall function"/> | |||
<br/> | |||
-visual | {| class="wikitable" | ||
! style="background:#f4a941" | Cortical Area | |||
! style="background:#f4a941" | Function | |||
|- | |||
| Primary motor cortex|| Executes voluntary movement | |||
|- | |||
| Primary visual cortex || Receives and processes visual information | |||
|- | |||
| Primary somatosensory cortex || Receives and processes somatosensory information such as heat, pain and pressure | |||
|- | |||
| Primary auditory cortex || Receives and processes auditory information | |||
|- | |||
| Motor association cortex (premotor cortex) || Selects voluntary movements | |||
|- | |||
|Auditory association area || Complex processing of auditory information | |||
|- | |||
|Sensory association area || Complex processing of multi sensory information | |||
|- | |||
|Visual association area || Complex processing of visual information | |||
|- | |||
|Prefrontal cortex || Involved in organising thoughts and actions to match internal goals. These include planning complex cognitive behaviour, making executive decisions, expressing personality, social awareness and storing short term memories. <ref><pubmed>28978697</pubmed></ref> | |||
|- | |||
|Broca's area (speech centre) || Speech production and articulation <ref name="speech"><pubmed>25218167</pubmed></ref> | |||
|- | |||
|Wernickes area || Speech comprehension <ref name="speech"/> | |||
|} | |||
<br/> | |||
<html5media height="400" width="600">https://www.youtube.com/watch?v=n6zQbTT0yoY</html5media> | |||
<ref>Learn Some (2016). Cerebral Cortex. [video] Available at: https://www.youtube.com/watch?v=n6zQbTT0yoY [Accessed 5 Oct. 2017].</ref> | |||
<br/><br/> | |||
==Abnormalities associated with Cerebral Cortex Development== | |||
[[File:Stages of development.png|thumb|right|text-top||520px|'''Figure 14. Main stages of cortical development where abnormalities may arise'''.<ref name="imaging">Mahfouz, M. (2015). Imaging of cortical formation disorders - DRE 4 - Prof. Dr Mamdouh Mahfouz. [video] Available at: https://www.youtube.com/watch?v=l_nTggR7LTE [Accessed 23 Sep. 2017].</ref>]] | |||
- | <br/> | ||
< | The embryologic development of the cerebral cortex involves highly organised and complex events. As mentioned in the 'Introduction', these events are generally divided by scientists into 3 major stages in cortical formation where crucial changes occur in neuronal cells; these stages include: <br/> | ||
< | 1. Proliferation (or Growth),<br/> | ||
2. Migration, <br/> | |||
3. Organisation (or Maturation or Differentiation). | |||
Disruptions can occur in these stages of cortical development (due to varying causes), and these disruptions give rise to malformations or irregularities in the cerebral cortex (Figure 13). <br/> | |||
( | |||
Although multitudinous and/or severe effects ensue from such disorders, epilepsy and mental retardation (of varying degrees) are almost always present with these disorders.<br/> | |||
This section will explore some abnormalities that are commonly discussed in scientific literature.<br/><ref name="first"/><ref name="second">Pang, T., Atefy, R. and Sheen, V. (2008). Malformations of Cortical Development. The Neurologist, [online] 14(3), pp.181-191. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3547618/ [Accessed 23 Sep. 2017]. </ref><ref name="third">Christopher A, C. (1999). Genetic Malformations of the Human Cerebral Cortex. Neuron, [online] 23(1), pp.19 - 29. Available at: http://www.cell.com/neuron/fulltext/S0896-6273(00)80749-7?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627300807497%3Fshowall%3Dtrue [Accessed 23 Sep. 2017].</ref><br/> | |||
=== | <br/> | ||
===Disorders due to the abnormal proliferation, growth or differentiation of neuroblasts === | |||
<br/> | <br/> | ||
<big><big>'''1) Focal cortical dysplasia (FCD)'''</big></big> | <big><big>'''1) Focal cortical dysplasia (FCD)'''</big></big> | ||
'''Focal cortical dysplasia (FCD)''' is heterogeneous developmental disorder of uncertain etiology. Focal Cortical Dysplasia is characterised by neurons arranged abnormally in the focal areas of the cerebral cortex as well as disorganisation of layers | '''Focal cortical dysplasia (FCD)''' is heterogeneous developmental disorder that results because neuroblasts are not able to successfully complete the proliferation stage of cortical development. Mechanism of development of this disorder is not well understood and FCD is categorised as of uncertain etiology. Focal Cortical Dysplasia is characterised by neurons arranged abnormally in the focal areas of the cerebral cortex as well as disorganisation of layers (lamination disorganisation). Very large dysmorphic cells called 'balloon' cells are also seen due to abnormal regulation of cell growth. <br/> FCD can affect the areas throughout the brain but occurs dominantly in the frontal and temporal lobes of the cerebral cortex. | ||
If symptoms do present, then these are associated with epilepsy ( tonic-clonic, tonic, simple partial and complex partial seizures). In all patients who present with epilepsy, FCD is the cause for about approximately 25% of them. FCD can be of two types: Type I and Type II.<br/><ref name="first"/><ref name="second"/><br/> | |||
[[File:Hemimegalencephaly2.png|thumb|left|upright=1.42|'''Figure 15. Observable radiographic features in Hemimegalencephaly'''. <ref name="imaging"/> ]] | |||
<br/> | <br/> | ||
<big><big>'''2) Hemimegalencephaly'''</big></big> | <big><big>'''2) Hemimegalencephaly'''</big></big> | ||
A rare congenital disorder that also results from the abnormal proliferation of neuroblasts is Hemimegalencephaly. <br/> | A rare congenital disorder that also results from the abnormal proliferation of neuroblasts is Hemimegalencephaly. <br/> | ||
'''Hemimegalencephaly''' is a developmental disorder which consists of | '''Hemimegalencephaly''' is a developmental disorder which consists of 'hamartomatous' overgrowth (where normal mature cells and tissues normally present in an area of the body, form tumour-like, benign malformation(s) ) of one cerebral hemisphere, part of a hemisphere or one hemisphere with partial involvement of the other hemisphere. MRI findings usually show enlargement of one hemisphere or at least one lobe and abnormal white matter (Figure 15). | ||
<br/> | Clinical symptoms of this disease may include developmental delay, mental retardation, paralysis of one side of the body and blindness over half the field of vision; but the most significant symptom is ''seizures'' as 90% of patients present with this symptom.Hemimegalencephaly accounts for 0.2% of cases of childhood epilepsy. <br/> | ||
< | <ref name="imaging"/><ref name="second"/> | ||
< | [[File:Image.png|thumb|right|upright=1.45|'''Figure 16. Possible clinical features of Microcephaly in a newborn'''. <ref>USDA & Felipe Dana/AP and Creative Commons License(CC) (2017). Understanding Zika. [online] Goinvo.com. Available at: http://www.goinvo.com/features/zika/ [Accessed 4 Oct. 2017].</ref>]]<br/> | ||
< | |||
< | |||
<br/> | |||
<big><big>'''3) Microcephaly Vera'''</big></big> | <big><big>'''3) Microcephaly Vera'''</big></big> | ||
Microcephaly or 'small brain', can be caused by abnormal cell proliferation or cell division along with the involvement of other organs. <br/> | Amongst cortical congenital disorders, Microcephaly Vera is a relatively common disorder. Microcephaly or 'small brain', can be caused by abnormal cell proliferation or cell division along with the involvement of other organs, and can be caused by genetic or non-genetic factors. <br/> | ||
'''Microcephaly Vera or Primary Microcephaly''' is genetic and does not involve other organs. It results from abnormal cortical development, specifically cell division or proliferation. In this malformation the circumference of the head (and so the brain) is much smaller than normal, while the rest of the body is of normal size. Clinically, microcephaly vera frequently presents with mental retardation and sometimes epilepsy, although some other features may also be observed (Figure 16). <br/> | |||
<ref name="imaging"/><ref name="second"/><ref name="third"/><br/> | |||
<br/> | |||
<big><big>'''4) Tuberous Sclerosis Complex'''</big></big> | <big><big>'''4) Tuberous Sclerosis Complex'''</big></big> | ||
| Line 283: | Line 309: | ||
Tuberous sclerosis complex (TSC) is a multi-organ disease resulting from mutations of certain genes, and is usually classified under defects of early cell proliferation and growth although it is also associated with defects of neuronal migration. | Tuberous sclerosis complex (TSC) is a multi-organ disease resulting from mutations of certain genes, and is usually classified under defects of early cell proliferation and growth although it is also associated with defects of neuronal migration. | ||
Tuberous sclerosis complex (TSC) | Tuberous sclerosis complex (TSC) is characterised by benign abnormal mass of cells (tumors, cysts, and other malformations including hamartomas and neoplasms), which can occur in several tissues of the body, including cerebral cortex. In the cortical gray matter, cortical 'tubers' and bizarre cells are seen because laminar disorganisation occurs (like Focal Cortical Dyplasia). TSC is thus called because initially scientists thought that the lesions they saw resembled potato tubers. <br/> | ||
No signs or symptoms are known to be observed for TSC, and diagnosis is determined after a brain scan. <br/> | |||
<ref name="imaging"/><ref name="third"/><br/> | |||
=== | ===Disorders due to abnormal neuronal migration === | ||
<br/> | <br/> | ||
<big><big>''' 5) Heterotopia'''</big></big> | <big><big>''' 5) Heterotopia'''</big></big> | ||
[[File: | [[File:Heteroptopia.png|thumb|upright=1.85|right|'''Figure 17. Observable radiographic features of subcortical band heterotopia'''. <ref name="imaging"/> ]] | ||
For neurons to successfully execute the migration stage of development, some steps have to be completed including departure from the ventricular zone, migration to the cortical plate and then arrest of movement at the appropriate layer. <br/> | |||
Migration of neurons occurs during the 8th week of development, along radial glial fibers (RGF). RGFs can be damaged due to ischemia, which can be caused by infection resulting from trauma or metabolic errors. <br/> | Migration of neurons occurs during the 8th week of development, along radial glial fibers (RGF). RGFs can be damaged due to ischemia, which can be caused by infection resulting from trauma or metabolic errors. <br/> | ||
RGF damage leads to the migration process arresting at the wrong time, resulting in abnormal or ectopic locations of neurons of the cortex. This condition is known as '''Heterotopia.''' | RGF damage leads to the migration process arresting at the wrong time, resulting in abnormal or ectopic locations of neurons of the cortex. This condition is known as '''Heterotopia.''' | ||
| Line 297: | Line 325: | ||
*'''Subcortical band heterotopia:''' <br/> | *'''Subcortical band heterotopia:''' <br/> | ||
In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-cortical regions of the cerebral cortex.<br/> | In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-cortical regions of the cerebral cortex (Figure 17).<br/> | ||
*'''Periventricular heterotopia/ sub-ependymal heterotopia:''' <br/> | *'''Periventricular heterotopia/ sub-ependymal heterotopia:''' <br/> | ||
| Line 306: | Line 334: | ||
In this type of heterotopia, abnormal location of neurons result in focal masses within deep white matter of cerebral cortex. <br/> | In this type of heterotopia, abnormal location of neurons result in focal masses within deep white matter of cerebral cortex. <br/> | ||
<ref name="imaging"/><ref name="second"/><ref name="third"/><br/><br/> | |||
<big><big>'''6) Lissencephaly'''</big></big> | <big><big>'''6) Lissencephaly'''</big></big> | ||
'''Lissencephaly''' is a congenital cortex malformation, in which gyri (and sulci) of the cerebral cortex are absent (agyria) or largely absent (pachgyria), resulting in a smooth surface of the brain. The normal lamination or layers fail to form and the cerebral cortex usually has only 4 of the typical 6 layers. Like most congenital brain disorders, the etiology of Lissencephaly is uncertain. Lissencephaly has also been associated with other abnormalities including corpus callosum hypoplasia, small brain stem and gray matter heterotopia. <br/> | |||
Lissencephaly is generally characterised by the following features: | Lissencephaly is generally characterised by the following features: | ||
| Line 323: | Line 348: | ||
*Vertically oriented Sylvian fissures of the brain, giving the brain an 'hourglass' configuration <br/> | *Vertically oriented Sylvian fissures of the brain, giving the brain an 'hourglass' configuration <br/> | ||
Lissencephaly is of different types; but these types have some common clinical symptoms, namely epilepsy and severe mental retardation. Types of Lissencephaly are the following: <br/> | |||
Lissencephaly is of different types; | |||
*'''Classical (Type I) Lissencephaly'''- results from neuronal undermigration (failed or incomplete neuronal migration) due to varying causes like mutation; additional clinical features include motor deficits. Patients often also have microcephaly [discussed later in Microlissencephaly]. <br/> | *'''Classical (Type I) Lissencephaly'''- results from neuronal undermigration (failed or incomplete neuronal migration) due to varying causes like mutation; additional clinical features include motor deficits. Patients often also have microcephaly [discussed later in Microlissencephaly]. <br/> | ||
*'''Cobblestone (Type II) lissencephaly'''- results from overmigration, where neurons migrate from the cortex into the overlying leptomeninges, causing diverse disruptions of the basement membrane; the cortex has a 'cobblestone' type of nodular appearance and additional clinical features include various eye abnormalities, congenital muscular dystrophies, hypotonia and generalized weakness in infancy. Type II Lissencephaly also includes: | |||
*'''Cobblestone (Type II) lissencephaly'''- results from overmigration, where neurons migrate from the cortex into the overlying leptomeninges, causing diverse disruptions of the basement membrane; the cortex has a 'cobblestone' type of nodular appearance and additional clinical features include various eye abnormalities, congenital muscular dystrophies, hypotonia and generalized weakness in infancy. | #Muscle-Eye-Brain Disease | ||
Type II Lissencephaly also includes: | #Walker-Warburg Syndrome | ||
#Fukuyama Syndrome <br/> | |||
#Muscle-Eye-Brain Disease | *'''Microlissencephaly''' <br/> | ||
#Walker-Warburg Syndrome | Microlissencephaly occurs when Type I Lissencephaly occurs in ''combination'' with congenital Microcephaly(discussed previously), and presents with severe symptoms including seizures, spasticity, severe developmetal delay and short life span. <br/> | ||
#Fukuyama Syndrome <br/> | <ref name="first"/><ref name="imaging"/><ref name="second"/><ref name="third"/><br/> | ||
*'''Microlissencephaly''' | |||
Microlissencephaly occurs when Type I Lissencephaly occurs in ''combination'' with congenital Microcephaly, and presents with severe symptoms including seizures, spasticity, severe developmetal delay and short life span. | |||
<br/> | |||
<br/> | <br/> | ||
<big><big>'''7) Kallmann Syndrome'''</big></big> | <big><big>'''7) Kallmann Syndrome'''</big></big> | ||
The Kallman Syndrome is syndrome | The Kallman Syndrome is a syndrome characterised by congenital Hypogonadism (diminished or failed activity of male or female gonads). Kallman Syndrome results from the failed migration of neurons that secrete Leuteinizing Hormone-Releasing Hormone(LHRH) also known as Gonadotropin Releasing Hormone(GnRH), from the olfactory placode to the hypothalamus. The hypothalamus normally secretes GnRH as this is necessary for normal gonadal function. Because of failed migration of aforementioned neurons to the hypothalamus, GnRH production is disrupted causing hypogonadism. | ||
Kallman Syndrome is | Clinical presentation of Kallman Syndrome includes archinencephaly, which is hypoplasia (underdevelopment or incomplete development) of the olfactory cortex and olfactory bulb, and deficient or absent sense of smell.<br/> | ||
<ref name="imaging"/><ref name="third"/><ref>Reference, G. (2017). Kallmann syndrome. [online] Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/condition/kallmann-syndrome [Accessed 26 Oct. 2017].</ref><br/> | |||
=== | ===Disorders due to abnormal cortical maturation and organization/folding=== | ||
<br/> | <br/> | ||
<big><big>'''8) Polymicrogyria'''</big></big> | <big><big>'''8) Polymicrogyria'''</big></big> | ||
| Line 353: | Line 373: | ||
If the neurons of the cerebral cortex successfully complete the proliferation and migration stages, but during the last organisation stage, distribute abnormally then multiple small undulating gyri can result. This condition is called '''Polymicrogyria (PMG)''', in which, the gyri become extremely small (hence the term micro) and their number is greater than normal. The cerebral cortex in this condition is flat and thickened, similar to that of pachygyria or agyria, and contains numerous small gyri. <br/> | If the neurons of the cerebral cortex successfully complete the proliferation and migration stages, but during the last organisation stage, distribute abnormally then multiple small undulating gyri can result. This condition is called '''Polymicrogyria (PMG)''', in which, the gyri become extremely small (hence the term micro) and their number is greater than normal. The cerebral cortex in this condition is flat and thickened, similar to that of pachygyria or agyria, and contains numerous small gyri. <br/> | ||
Polymicrogyria is usually focal. It is most commonly 'perisylvian' (around the Sylvian fissure) and can be | Polymicrogyria is usually focal. It is most commonly 'perisylvian' (around the Sylvian fissure) and can be bilateral or unilateral. The most common sites, in descending order, are the frontal lobe, parietal lobe, temporal lobe and then occipital lobe. <br/> | ||
Affected patients can have varying degrees of symptoms, including epilepsy and developmental delay. For example, bilateral poylmicrogyria causes developmental delay, hypertonicity, ataxia, and refractory seizures.<br/> | |||
<ref name="first"/><ref name="second"/><br/> | |||
[[File:SchizencephalicB.jpg|thumb|left|upright=1.45|'''Figure 18. Coronal and axial sections of the brain of a patient with Schizencephaly'''.<ref>PRWeb (2013). Schizencephalic Brain. [image] Available at: http://www.prweb.com/releases/2013/7/prweb3350774.htm [Accessed 5 Oct. 2017].</ref>]] | |||
<br/> | |||
<big><big>'''9) Schizencephaly'''</big></big> | |||
'''Schizencephaly''' is an organisational developmental disorder classified within the same group as polymicrogyria. It is characterised by a cleft(s) or lesion(s) on the brain surface,extending from the cerebral cortex to the ventricle and is typically filled with CSF (cerebrospinal fluid) and lined by cortex gray matter (Figure 18). These lesions or clefts are thought to be 'destructive'.<br/> | |||
''' | The clefts can be small with closed walls in '''Type I or Closed lip type''' schizencephaly; or the clefts can be large with free communication between the ventricle and subarachnoid spaces in '''Type II or Open lip type''' schizencephaly. Type I presents with partial seizures or spastic hemiparesis, while Type II presents with epilepsy or seizures, spasticity, severe developmental delay and microcephaly. | ||
Schizencephaly can be bilateral or unilateral; and commonly involves the parasylvian regions, and severity of the disease correlates with the extent of the clefts.<br/> | |||
<ref name="first"/><ref name="imaging"/><ref name="second"/><br/> | |||
<br/> | <br/> | ||
<br/> | <br/> | ||
| Line 373: | Line 394: | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
< | {| role="presentation" class="wikitable mw-collapsible mw-collapsed" | ||
< | | <big><big><big>Other disorders of varying etiology</big></big></big> | ||
< | |- | ||
< | | <big><big>''' 10) Fetal Alcohol Spectrum Disorder (FASD)'''</big></big> | ||
< | |||
<big><big>''' | |||
[[File:FASface.jpg|thumb|right| Facial features appearance in Fetal Alcohol Spetrum Disorder (FASD) <ref> MarkHill,embryology.med.unsw.edu.au (2017). Facial Appearance of Fetal Alcohol Syndrome (FAS). [image] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/File:FASface.jpg [Accessed 5 Oct. 2017].</ref> ]] | [[File:FASface.jpg|thumb|right|'''Figure 19. Facial features appearance in Fetal Alcohol Spetrum Disorder (FASD)'''.<ref> MarkHill,embryology.med.unsw.edu.au (2017). Facial Appearance of Fetal Alcohol Syndrome (FAS). [image] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/File:FASface.jpg [Accessed 5 Oct. 2017].</ref> ]] | ||
<br/> | <br/> | ||
'''Fetal Alcohol Spectrum Disorder (FASD)''' refers collectively to the malformations that occur in children due to maternal alcohol consumption during pregnancy. This results from the effects of ethanol as it crosses the placental barrier. The mechanism for these abnormalities developing is complicated and not entirely clear, but various studies show that ethanol disrupts all major processes of fetal CNS development and causes toxicity of blood (as fetal liver cannot yet detoxify ethanol well). | '''Fetal Alcohol Spectrum Disorder (FASD)''' refers collectively to the malformations that occur in children due to maternal alcohol consumption during pregnancy. This results from the effects of ethanol as it crosses the placental barrier. The mechanism for these abnormalities developing is complicated and not entirely clear, but various studies show that ethanol disrupts all major processes of fetal CNS development and causes toxicity of blood (as fetal liver cannot yet detoxify ethanol well). | ||
</ | <br/> | ||
The three major | The three major indications (that also help form the diagnoses) for FASD are facial dysmorphology, growth deficits and central nervous system defects. These conditions result in immense physiological and psychological impacts on the child. Fetal Alcohol Syndrome (FAS) is an acute form of FASD. | ||
On average, FASD is diagnosed in a child between 3-10 years. It is of utmost importance however that the diagnosis is done as early as possible so that timely interventions could be taken in order to lessen the severity of the symptoms that result from this syndrome. | On average, FASD is diagnosed in a child between 3-10 years. It is of utmost importance however that the diagnosis is done as early as possible so that timely interventions could be taken in order to lessen the severity of the symptoms that result from this syndrome.<br/> | ||
<ref>Leigland, L., Ford, M., Lerch, J. and Kroenke, C. (2013). The Influence of Fetal Ethanol Exposure on Subsequent Development of the Cerebral Cortex as Revealed by Magnetic Resonance Imaging. Alcoholism: Clinical and Experimental Research, 37(6), pp.924-932. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3670687/ [Accessed 5 Oct. 2017].</ref> | <ref>Leigland, L., Ford, M., Lerch, J. and Kroenke, C. (2013). The Influence of Fetal Ethanol Exposure on Subsequent Development of the Cerebral Cortex as Revealed by Magnetic Resonance Imaging. Alcoholism: Clinical and Experimental Research, 37(6), pp.924-932. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3670687/ [Accessed 5 Oct. 2017].</ref> | ||
<br/> | <br/> | ||
<big><big>''' | <big><big>'''11) Corpus Callosum Agenesis'''</big></big> | ||
The corpus callosum is a structure present in the brain which connects both the hemispheres of the brain. The video below briefly describes this: | |||
<html5media height=" | <html5media height="350" width="550">https://www.youtube.com/watch?v=7zOa3LLrHKc</html5media> | ||
<ref>Ezzo - Izzo, D. (2007). How the Body Works : The Corpus Callosum. [video] Available at: https://www.youtube.com/watch?v=7zOa3LLrHKc [Accessed 23 Sep. 2017].</ref> | <ref>Ezzo - Izzo, D. (2007). How the Body Works : The Corpus Callosum. [video] Available at: https://www.youtube.com/watch?v=7zOa3LLrHKc [Accessed 23 Sep. 2017].</ref> <br/> | ||
Agenesis of the corpus callosum (ACC) occurs when the corpus callosum is partially or completely absent. It is thought to be due to a disruption of brain cell migration during fetal development. | |||
<ref>Ninds.nih.gov. (2017). Agenesis of the Corpus Callosum Information Page. [online] Available at: https://www.ninds.nih.gov/Disorders/All-Disorders/Agenesis-Corpus-Callosum-Information-Page [Accessed 26 Oct. 2017].</ref> <br/> | |||
Some clinical symptoms include epilepsy or seizures, developmental delay, vision and hearing impairment, trouble in motor coordination and language skills and difficulty in muscle coordination and tone coordination. | |||
<ref>Vasudevan, C., McKechnie, L. and Levene, M. (2012). Long-term outcome of antenatally diagnosed agenesis of corpus callosum and cerebellar malformations. Seminars in Fetal and Neonatal Medicine, 17(5), pp.295-300. </ref> | |||
|} | |||
<br/> | <br/> | ||
==Models and Research== | |||
===Animal Models=== | |||
[[File:Corticogenesis of mouse and humans.jpeg|thumb|right|text-top|590px|'''Figure 20. Mouse vs Human Neurogenesis''']] | |||
The most common model to study the development of cerebral cortex is the mouse, although other animals are also used to study the development of the brain. Animal studies are necessary for gaining adequate and comprehensive understanding of pathophysiology and etiology of abnormalities/diseases, as well as for testing and formulating treatments. Apart from the mouse, other animal models used include the fruit-fly, rat, cat, dog, ferret and even worm. The zebrafish and especially primates like monkeys are also key models. <ref>Sun, T. and Hevner, R. (2014). Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nature Reviews Neuroscience, 15(4), pp.217-232.</ref><ref>Wolf, F. (2005). Course 12 Symmetry breaking and pattern selection in visual cortical development. Les Houches, pp.575-639.</ref><ref>Xu, M., Xu, H. and Qin, Z. (2015). Animal Models in Studying Cerebral Arteriovenous Malformation. BioMed Research International, 2015, pp.1-13.</ref><ref>Brainfacts.org. (2017). Why Animals Are Needed in Research 031017. [online] Available at: http://www.brainfacts.org/in-the-lab/animals-in-research/2017/why-animals-are-needed-in-research-031017 [Accessed 26 Oct. 2017].</ref> | |||
<br/>In this section we have given examples of some findings obtained using mice models. | |||
http://www. | |||
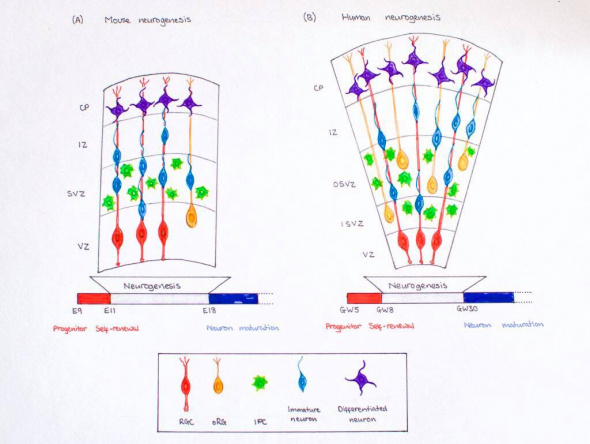
Mice have often been used to study corticogenesis in mammals. Corticogenesis lasts from E11 to E19 in mice and lasts eight days, which is far shorter than human corticogenesis. While there are many similarities between human corticogenesis, the rodent brain is much smaller than that of a human’s and therefore, there are different types of progenitor cells involved in mice and the cortex does not expand as greatly. <ref name="control">Dehay, C. and Kennedy, H. (2007). Cell-cycle control and cortical development. Nature Reviews Neuroscience, 8(6),438-450.</ref>Humans have greater numbers of intermediate precursor cells (to aid in further differentiation) and outer radial glial cells compared those of mice. <ref name="cerebral"/> In addition, the SVZ does not split into the inner and outer subventricular zone, but stays intact as one. <ref name="control"/> The importance of reelin has also been greatly studied in mice. Studies showed that mutant mice have disruptions in the migration of neurons into their subsequent layers. By injecting thymidine at various time points of gestation into the female mice, researchers were able to track the development of various populations of neurons. Later developing neurons were unable to ascend past the already early formed neurons due to disruptions in reelin signaling (in the marginal zone). Instead, superficial layer neurons resided below the older, deep layer neurons. This inverted lamination comprises sensory and motor function.<ref> Caviness, V. (1982). Neocortical histogenesis in normal and reeler mice: A developmental study based upon [3H]thymidine autoradiography. Developmental Brain Research, 4(3), 293-302.</ref> | |||
<br/> | |||
<br/> | |||
===Current Research=== | |||
[[File:3D Organoids.jpg|thumb|right|text-top|600px|'''Figure 21. Timeline and protocol of cerebral organoid development''']] | |||
In this section, we have discussed current research regarding 3D organoids. <br/> | |||
One of the limiting steps in research at present is the lack of models that can accurately recapitulate the developmental changes and pathophysiology of the human brain. Although it is recognized that certain fundamentals of brain development are conserved in mammalian brains there are distinct differences that should be considered when studying the human brain. <ref><pubmed>28845922</pubmed></ref> | |||
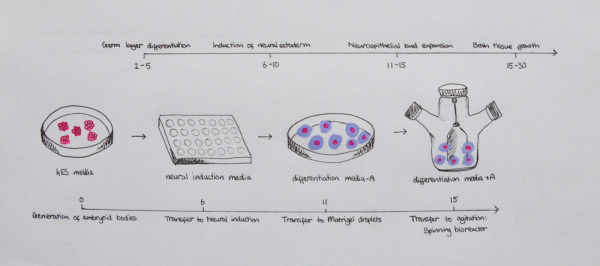
Currently there has been the development of 3 dimensional models, derived from stem cells that can be used to mimic the evolution of the human brain, and have been deemed advantageous over previous in vitro models. This method, as first described by Lancaster et al. can, in a 1-2 month period be reproduced in a tissue culture room give rise to the developing parts of the human brain, including the cerebral cortex. This is established using a protocol human pluripotent stem cells (PSCs). PSCs separate to single cells before rearranging to form embryoid bodies, which are prompted to form neuroectoderm in a medium that prevents either endoderm or mesoderm development. At day 11-15 of this protocol the tissues undergo neuroepithelial bud expansion after being transferred to Matrigel droplets. The final stage of the process involves the tissues being transferred to an agitator (either spinning bioreactor, or orbital shaking plate) which allows for more substantial growth of the brain regions due to better nutrient and oxygen supply. <ref><pubmed>25188634</pubmed></ref> | |||
The development of these brain organoids has allowed more comprehensive understanding of the self-organisational properties of the brain, and is quickly advancing, however the lack of vasculature in cultured organoids restricts their ability to be used for specific neurological disorders. Furthermore there are still challenges associated with the generation of individual, discrete brain regions, which should be addressed in the future. <ref><pubmed>28822354</pubmed></ref> | |||
<br/> | |||
==Glossary== | |||
{| class="pretty table" | |||
|-bgcolor = "DDCEF2" | |||
|'''Term''' | |||
|'''Definition''' | |||
|-bgcolor="FAF5FF" | |||
|'''Agenesis'''|| The total absence of a tissue or organ. | |||
|-bgcolor="FAF5FF" | |||
|'''Cajal-Retzius (CR) cells'''|| Cortical neurons that develop early on, at the same time as the preplate, and express an important glycoprotein known as reelin, which helps with the proper migration of neurons into their respective layers. | |||
|-bgcolor="FAF5FF" | |||
|'''Cortical Plate'''|| Forms in between the marginal zone and subplate and gives rise to the 6 layers of the cortex involved in sensory and motor function. | |||
|-bgcolor="FAF5FF" | |||
|'''Corticogenesis'''|| The development of the cerebral cortex into its six layers. It involves a sequential process of migration and differentiation of neocortical projection neurons into a laminated structure that contains a diverse set of neuronal subtypes. | |||
|-bgcolor="FAF5FF" | |||
|'''Dystrophy'''|| The wasting way of body tissues or an organ due to some disease or disorder. | |||
|-bgcolor="FAF5FF" | |||
|'''Epilepsy'''|| A neurological disorder characterised by seizures. | |||
|-bgcolor="FAF5FF" | |||
|'''Etiology'''|| The cause or origin or explaination of a disease. | |||
|-bgcolor="FAF5FF" | |||
|'''Fissures'''|| Grooves or clefts; are actually just large sulci. | |||
|-bgcolor="FAF5FF" | |||
|'''GABAergic Neurons'''|| Generate gamma aminobutyric acid (GABA) as their output, one of the two inhibitory neurotransmitters in the central nervous system (CNS). | |||
|-bgcolor="FAF5FF" | |||
|'''Gyrus '''|| Gyri (plural) are folds/ridges in the cerebral cortex. | |||
|-bgcolor="FAF5FF" | |||
|'''Heterogeneous'''|| Diverse, varied and many components of something. | |||
|-bgcolor="FAF5FF" | |||
|'''Hypoplasia'''|| Underdevelopment or incomplete development of a tissue or organ. | |||
|-bgcolor="FAF5FF" | |||
|'''Hypotonia'''|| The state of loss of muscle tone, or tension (or stretch strength) of muscles. | |||
|-bgcolor="FAF5FF" | |||
|'''Intermediate zone'''|| Forms below the subplate between E50-55 and contains only migrating cells and no intermediate precursor cells. | |||
|-bgcolor="FAF5FF" | |||
|'''Marginal Zone (MZ)'''|| A subsection of the preplate that forms around E50-55. It lies at the uppermost area of the cortex nearest the pial surface. | |||
|-bgcolor="FAF5FF" | |||
|'''Neural Plate'''|| Main developmental structure required for the development of the nervous system. | |||
|-bgcolor="FAF5FF" | |||
|'''Neural Tube|| Structure of the embryo that evolves into the spinal cord and brain. | |||
|-bgcolor="FAF5FF" | |||
|'''Olfactory'''|| Relating to nasal organs and/or the sense of smell. | |||
|-bgcolor="FAF5FF" | |||
|'''Pachygyria'''|| Gyri can be seen but are very few compared to normal brain. | |||
|-bgcolor="FAF5FF" | |||
|'''Preplate'''|| First "pioneer neurons" from dividing cells in the ventricular zone for this layer above the ventricular zone. | |||
|-bgcolor="FAF5FF" | |||
|'''Radial glial cells '''|| A class of distinct nonneuronal cells that are bipolar shaped and span the entire width of the cortex during development. | |||
|-bgcolor="FAF5FF" | |||
|'''Seizures'''|| Fits, which involve a sudden surge of electrical impulses in the brain, causing jerking of limbs and stiffness. | |||
|-bgcolor="FAF5FF" | |||
|'''Spasticity'''|| Having spasms in muscles or possibly some organs. | |||
|-bgcolor="FAF5FF" | |||
|'''Subplate'''|| A subsection of the preplate that forms around E50-55. | |||
|-bgcolor="FAF5FF" | |||
|'''Subventricular zone (SVZ)'''|| Cells in the VZ continue to divide symmetrically and give rise to another zone above the VZ known as the SVZ. This zone later splits into an inner (ISVZ) and outer ventricular zone (OSVZ). | |||
|-bgcolor="FAF5FF" | |||
|'''Sulcus'''|| Sulci (plural) are grooves in the cerebral cortex. | |||
|-bgcolor="FAF5FF" | |||
|'''Total agyria'''||Gyri and sulci absent resulting in 'smooth brain'. | |||
|-bgcolor="FAF5FF" | |||
|'''Ventricular zone (VZ)'''|| First formed single-celled layer in the cortex, arising from progenitors in the dorsal telencephalon that divide symmetrically. It lies adjacent to the ventricular surface. | |||
|-bgcolor="FAF5FF" | |||
|} | |||
==References== | ==References== | ||
Latest revision as of 08:15, 27 October 2017
Cerebral Cortex
| 2017 Student Projects | |||
|---|---|---|---|
|
Introduction
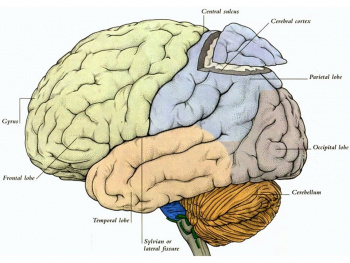
The cerebral cortex is part of the brain which surrounds the cerebral hemispheres. It has a very large surface area (largest part of human brain). The cerebral cortex differs from the cerebrum because, the cerebral cortex is the outermost layer of the cerebral hemispheres (Figure 1). It is a layer of gray matter around 2-4 mm in thickness, and consists of approximately 10 billion nerve cell bodies and dendrites. It appears gray because of the cell bodies (Figure 2).
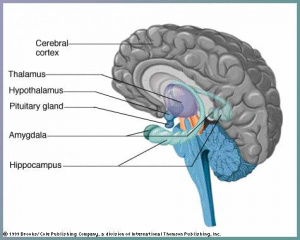
The cerebral cortex is thought to be the main control centre, playing an essential role in cognitive function, memory, sensation and association.The development of the cerebral cortex involves 3 main stages:
- Proliferation (or growth or differentiation)
- Migration of neurons
- Maturation (or organisation or folding).
The cortex is organised into six horizontal layers. I. Molecular layer, II. External granular layer , III. Xxternal pyramidal, IV. Internal granular layer, V. Internal pyramidal layer, VI. Multiform layer. These individual layers organise the capacity for interconnections between both input and output signals.
This project will explore the various embryologic aspects of the cerebral cortex, including early development of the brain, development of the cerebral cortex, anatomy and functions of the cortex, and abnormalities associated with cortical development.
[3][4][5][6][7]
Early Development of the Brain
The brain begins to develop during the third week of pregnancy when the neural plate and neural tube are derived from the outer most layer of embryonic cells, that is the neuroectoderm. The development of the brain is from the neural plate which folds to form the neural groove and then curls forming the neural tube, which is cranial to the fourth pair of somites, and eventually, forms the three primary brain vesicles [8].
Neuroprogenitor cells proliferate, migrate, and differentiate to form specific areas of the brain [9].
During week four fusion of the neural folds in the cranial region and closure of the rostral neuropore form three primary brain vesicles from which the brain develops [8]:
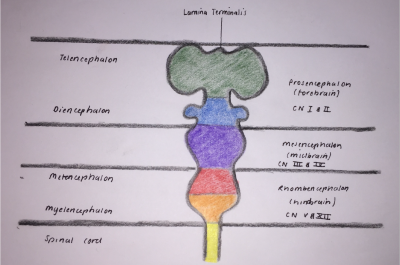
- Forebrain/Prosecephalon
- Midbrain/Mesencephalon
- Hindbrain/Rhombencephalon
From the three primary vesicles, there is a further division at the anterior extremity of the medullary canal into five secondary vesicles during week five, as depicted in Figure 3. These are fundamental divisions of the adult brain and communicate freely with each other [11]. They are [9]:
- Telencephalon: endbrain, forms cerebral hemispheres - derived from Prosecephalon
- Diencephalon: between brain, forms optic outgrowth - derived from Prosecephalon
- Mesencephalon: undivided
- Metencephalon: posterior to the brain, gives rise to the pons (bulbous expansion consisting of white matter tracts serving the cerebellum) [12] - derived from Rhombencephalon
- Myelencephalon: gives rise to the medulla oblongata - derived from Rhombencephalon
In the beginning, these five divisions are uniform in size and shape but quickly differentiate at various rates [11].
As the telencephalon is the region that develops into the cerebral cortex, we will discuss this region specifically in more detail. The telencephalon is the utmost rostral region of the brain vesicles, and consists of:
- The median region: the lamina terminalis, with the cavity forming the anterior part of the third ventricle [13]
- Two lateral diverticula: the cerebral hemispheres [9]
The cerebral hemispheres grow simultaneously in lateral, longitudinal and parietal directions [13], and originally are in communication with the third ventricle cavity via the interventricular foramina [9]. Upon expansion, the cerebral hemispheres eventually cover the diencephalon, midbrain and hindbrain, where they will eventually congregate at the midline, resulting in the flattening of each hemispheres medial surfaces [9]. During the sixth week the corpus striatum emerges as an obvious swelling in the floor of both of the cerebral hemispheres. the floors expand at a slower rate compared to the hemispheres thin cortical walls, as it contains large crpus striatum, causing the cerebral hemispheres to become a c shape [9]. This growth and curvature of the cerebral hemispheres does have consequential effects on the shape of the lateral ventricles, which too become c-shaped and fill with cerebrospinal fluid (CSF). Each hemispheres caudal ends turn ventrally, and then rostrally resulting in the formation of the temporal lobe.
The telencephalon is further subdivided into a dorsal pallium and a cenrtral subpallium. The dorsal pallium forms the large neuronal nuclei of the basal ganglia (corpus striatum, globus pallidus) [12]. These structures are essential for fulfilling commands from the cerebral hemispheres, and appear as lateral outpouchings of the pallium growing at a fast rate in order to cover the mesencephalon and diencephalon. The cortex is formed by the pallium, or vault, of each hemisphere.
Brain Flexures
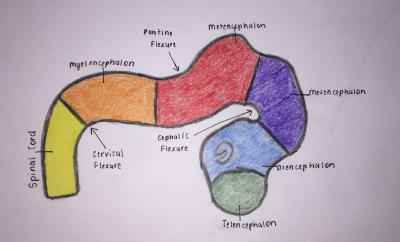
During the fifth week, the embryonic brain undergoes rapid growth, folding the neural tube, consequently resulting in three brain flexures, as depicted in Figure 4, in proximity to the five secondary vesicles. These are [8]:
- Cephalic flexure - centered at the midbrain region and pushes mesencephalon upwards being the first fold to develop, ventral folding of the brain tube [12]
- Cervical flexure - between brain stem and spinal cord at the junction of the spinal cord and hindbrain, ventral folding of the brain tube [12]
- Pontine flexure - beings at the developing pons, generates the fourth ventricle; produced in the opposite direction - dorsal folding [12] - as a result of later unequal brain growth, resulting in the thinning of the roof of the hindbrain [11].
The primordial brain initially has the same basic structure as the developing spinal cord, however, consideration variations in the outline of transverse sections at different levels of the brain and in the position of the gray and white matter are produced by the brain flexures [9]. The sulcus limitans (the floor of the fourth ventricle) extends cranially to the junction of the midbrain and forebrain, and the alar and basal plates are recognisable only in the midbrain and hindbrain [9].
Through the foundation of structures and processes in during the early developmental brain, the fetus' brain is able to further develop into a much more complex organ.
Later Development: Development of the Cerebral Cortex
Corticogenesis refers to the development of the cerebral cortex, the outer portion of the cerebrum, into its six layers. It involves a sequential process of migration and differentiation of neocortical projection neurons into a laminated structure that contains a diverse set of neuronal subtypes. The diversity in neurons contributes to the complex circuitry involved in the mammalian cortex. The large size of the cortex distinguishes humans from other mammals because it corresponds to a larger capacity to perform behavioral and cognitive tasks. [14] As mentioned in above in “Early Development of the Brain,” the cerebral cortex is derived from telencephalon, the rostral end of the neural tube. Origins of various neuronal subtypes come from the pallium (roof) and the subpallium (base) sections of the telencephalon which contributes to the overall laminar and columnar organization of the cortex. [15]
Main classes of neurons [16]
- Projection neurons: excitatory neurons (glutamatergic) with axons that project to distant targets and are generated by progenitors in the dorsal pallium of the telencephalon. The have a typical pyramidal structure and send signals to various regions of the brain and neocortex.
- Interneurons: inhibitory neurons (GABAergic) that have local connections within the cortex and are generated in the subpallium of the telecephalon in the ventral proliferative zone. These neurons have to migrate to the neocortex area.
Key developmental zones in the human cortex:
- Ventricular zone
- Subventricular zone
- Inner Subventricular Zone
- Outer Subventricular zone
- Intermediate Zone
- Preplate: neural progenitor cells split into 2 regions
- Marginal zone
- Subplate
- Cortical Plate: establishment of the 6 cortical layers form in this region between the subplate and the marginal zone
Timeline of Corticogenesis
The dorsolateral wall of the telencephalon is initially made up of undifferentiated neuroepithelial cells at the beginning of development. The cells are neural stem cells, capable of dividing into various neuronal subtypes. [14] The timing of birth for these neuronal subtypes determines the location (e.g fate) of the neurons so that specific connections and cortical structure are achieved. "E" relates to embryonic day in relation to corticogenesis.
| DAY | DEVELOPMENT |
|---|---|
| E30 | Progenitors in the dorsal telencephalon divide symmetrically and give rise to the initial ventricular zone (VZ), a single layer of cells that attach their "feet" to the cerebral wall and divide. These neurons lay adjacent to the ventricular surface. [14] |
| E31-32 | Dividing cells from the VZ begin to differentiate into "pioneer" neurons to form the preplate. These neurons migrate radially and tangentially from the VZ to the pial surface in an upward fashion. These cells are often referred to as radial glial cells (RGCs) because they contain radial fibers that span the entire embryonic wall from the VZ to the pial surface. These fibers create a "scaffolding" for subsequent migrating neurons to attach to and travel along in order to expand the neocortex. These cells are multipotent cells that divide asymmetrically to produce intermediate precursor cells that can become projection neurons, astrocytes, and oligodendrocytes [17] . In addition to RGCs, Cajal-Retzius (CR) cells are another type of early born neurons that develop at the same time as the preplate. These cells express reelin, an important signaling factor for migrating neurons to properly organize into their destined layers. They remain in the marginal zone when the preplate splits into two (see E50-55).[15] The preplate is referred to as heterogeneous because it contains many developing cell types. |
| E40-45 | Cells in the VZ continue to divide symmetrically and give rise to another zone known as the subventricular zone (SVZ). This proliferative layer lies above the ventricular zone and is not attached to the ventricular surface. Radial glial cells also populate this area as well as the preplate and many of the cells in the SVZ contribute to the later cortical neurons. The SVZ also separates into the outer subventricular zone (OSVZ) and the inner subventricular zone (ISVZ). The OSVZ continues to expand as it produces more migrating immature neurons and intermediate precursor cells. [17] |
| E50-55 | The preplate begins to split into two subsections: the marginal zone and the subplate. An intermediate zone forms below the subplate and contains only migrating cells (migrating to the target destination) and no intermediate precursor cells. The cortical plate also begins to form in between the two regions [16]. Newly born neurons that migrate to the cortical plate are developed "inside out". This term "inside-out" means that earlier born cells populate the deep layers first and later born neurons populate the superficial layers of the neocortex by migrating past the deep layers. Therefore, layers 6 is populated before layer 5; layer 5 is populated before layer 4 and so on [14]. Each layer condenses and the neurons are closely packed. As the layers form, the cortex expands. |
Migration and division of all six layers of the cortex is completed during the third trimester.[14] Each layer has distinct synaptic connections and cell types that contribute to the specific functions of the cortex such as sensory and motor function.
Signalling involved in Cerebral Cortex Development
Cell signalling is an essential part of the laminar patterning of the cerebral cortex into its 6 distinct, radially organised layers. There is a large network of interweaving signalling pathways that are responsible for the formation of this sophisticated anatomical structure and its functioning. There is still much debate about the exact pathways and signalling molecules that lead to this specific patterning, however some factors contributing to the control of cortical differentiation have been uncovered.
Sonic Hedgehog (Shh) is a morphogen involved in regulating the development of many different tissue types. During the development of the neocortex, Shh plays a role in controlling the proliferation and survival of cortical progenitor cells along with the specification of cerebral cortex GABAergic neurons. [18]
It has been shown that blockade of Shh in murine models with cyclopamine has effects on cortical neurogenesis, with indications that Shh morphogen could be play a role in the control of cell cycle length, cell growth and the process of cell division of the dorsal telencephalon progenitors during early corticogenesis (Fig. 8) [19]
Reelin is one of the more well understood signalling pathways involved in corticogenesis. Cajal-Retzuis cells with are located in the marginal zone (MZ) secrete Reelin (an extracellular matrix glycoprotein). Through studies that examine the absence of Reelin in reeler mutant mice, neuronal migration is significantly altered (Fig. 9). Additionally, Reelin has been shown to have a crucial role in inducing branching and specific positioning of radial glial cells (RGC), in that the distribution of Reelin within the MZ defines the specific location of radially migrating neurons, and is essential for the characteristic ‘inside-out’ pattern of the six cortical layers. [20]
Notch signalling molecular pathway is also crucial to corticogenesis. It is thought that there is an important interplay between this pathways and Reelin, as deficits of both result in impaired migration and altered morphology. [21] Both signalling pathways are involved in the expression of the radial glial gene, BLBP, which could be significant in the development of radial glial cells. 5. Although generally considered a developmental pathway, studies have shown the importance of Notch signalling in the adult brain, through distinguishing the balance between maintenance of neural stem cells and differentiation. These new understandings have implications for therapeutic approaches to neurodegenerative diseases. [22]
Bmp7 in addition to cell intrinsic factots there are also estristic signalling factors to take into account, for example Bone Morphogenitic Protein 7 (Bmp7) with debate ongoing as to its promotion or inhibition of neurogenesis. Deletion of Bmp7 in murine models has been shown to result in reduced thickening of the cortex, likely due to the changed differentiation of progenitor cells from the subventricular zone to the upper layers, (Fig. 10) Bmp7 regulates the expression of gene Ngn2, which in Bmp7 knock out models appeared to be majorly reduced, perhaps playing a role in the development of deep layer neurons. [23]
The signalling pathways that underlie corticogenesis are very complicated and the exact mechanisms are still under continuous investigation. Further research will only improve understanding of this network of intertwining factors and potentially lead to better appreciation of neurodevelopmental conditions and thus innovative therapeutic approaches.
Anatomy of the Cerebral Cortex
The cerebral cortex is a convoluted layer of grey matter on the outer surface of the cerebrum. Grey matter is neuronal tissue containing neuronal cell bodies. In humans the cortex has a thickness of 2-3mm however it's surface area is many hundred square centimetres allowing an increased cortical surface to occupy small cranial volume. The cortex in humans contains 10 billion densely packed nerve cells (10% of the neurons in the brain). The human neocortex is the most phylogenetically developed structure of the brain compared to other species. The folding in larger mammals is important to allow addition and evolution of a greater diversity of functional areas. As the folding is highly preserved across individuals this allows the different sections sepearted by gyri and sulci to be labelled. [24]

The longitudinal fissure divides the cortex into the left and right hemispheres, which are connected at the midline by the longitudinal fissure. Each hemisphere is then divided into four lobes (frontal, temporal, parietal and occipital) which are labeled by their relation to bones of the skull (see figure 11). The frontal lobe is separated from the temporal lobe by the lateral sulcus also known as the Sylvian fissure. The Rolandic fissure (central sulcus) divides the frontal and parietal lobe and the parietal and occipital lobes are distinguished by the parieto-occipital sulcus. [26]
The frontal lobe is subdivided by the superior and inferior frontal sulci into the superior, middle and inferior frontal gyri, as shown in figure 11. The frontal operculum is formed by the inferior frontal gyrus and is divided into the pars orbitalis, pars triangularis and pars opercularis. These are triangular gyri and are listed in order of anterior to posterior. [24]
The temporal lobe can also be subdivided by the superior and inferior temporal sulci. These subdivisions include the superior, middle and inferior temporal gyri (see figure 11). Lateral to the midbrain on the inferior temporal lobe surface is the parahippocampal gryus. The occipitotemporal gyrus, also named fusiform gyrus, lies between the inferior temporal gyrus and the parahippocampal gyrus. Within the parietal lobe the angular gryrus lies above the superior temporal sulcus. Above the angular gyrus, the supramrginal gyrus lies above the lateral sulcus. Below the angular gyrus, the lateral occipital gyrus lies above the inferioir temporal sulcus. [24]
<html5media height="400" width="600">https://www.youtube.com/watch?v=woIZaLiy-eA</html5media>
[25]
Layers of the Cortex
| Layer 1 |
outermost layer (pial surface) molecular layer with few scattered neurons mainly extensions of pyramidal neuron apical dendrite tufts with some spiny stellate cells inputs to apical tufts are crucial for feedback interactions in cortex in associative learning and attention [27] |
| Layer 2 |
external granular layer contains small pyramidal neurons and many stellate neurons pyrimidal neurons are excitatory [28] |
| Layer 3 |
external pyramidal layer small and medium sized pyramidal neurons non-pyramidal neurons with orientated intracortical axons layers 1,2 and 3 are the main target for interhemisphere corticocortical afferent fibres layer 3 is the main source for cortiocortical efferent fibres |
| Layer 4 |
internal granular layer many different types of stellate and pyramidal neurons main target for thalamocortical afferents from thalamus type C neurons and intra-hemispheric corticocortical afferents [29] |
| Layer 5 |
internal pyramidal layer large pyramidal neurons give rise to axons leaving cortex and run down to subcortical structures e.g. basal ganglia In the primary motor cortex of the frontal lobe, layer 5 contains Betz cells and their axons travel through the internal capsule, the brain stem and the spinal cord forming the corticospinal tract, which is the main pathway for voluntary motor control cortical areas with no layer 5 are named agranular and cortical areas with an undeveloped layer 5 are named dysgranular [28] |
| Layer 6 |
polymorphic/ multiform layer few large pyramidal neurons many small spindle like pyramidal and multiform neurons sends efferent fibres to thalamus and forms exact reciprocal interconnection between thalamus and cortex these connections are both inhibitory and excitatory [30]
|
Functions of the Cerebral Cortex
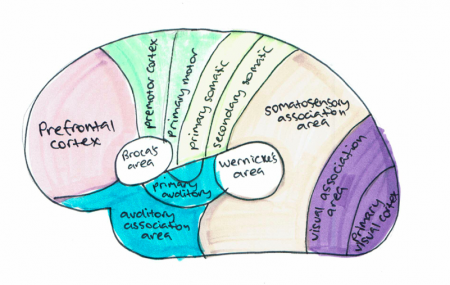
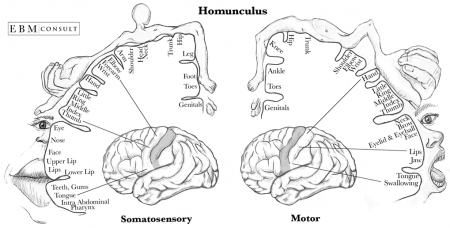
The cerebral cortex controls memory, movement, perception, cognition, consciousness, awareness and language. Majority of the connections in the cortex are from one cortex area to the other rather than with other structures. However, the cortex is connected to structures below it such as the basal ganglia and thalamus. The cortex communicates with these structures; information is sent along efferent connections and received by afferent connections. Majority of the sensory information in the brain is sent to the cortex by the thalamus and olfactory information is send to the olfactory cortex through the olfactory bulb. The cortex can be split into three cortical areas (cortices for plural) based on their function; sensory, motor and association areas, as shown in Figure 12. [33]
Primary cortices have simpler functions including direct sensory input (vision, hearing and somatic sensation) or directly producing eye and limb movements. The association cortices functions are more complex and include memory, language, abstraction, creativity, judgement, emotion, attention and movement synthesis. [33]
Sensory areas receive and process information from the senses including visual, hearing and somatosensory information. The primary sensory areas receive sensory inputs from the thalamus. The sensory area in each hemisphere receives sensory information from the opposite side of the body. The sensory cortex is organised as shown in figure and provides a map of the body also known as a homunculus. A cortical homunculus is a model used to represent the area and proportions of motor and sensory functioning in the human body (see figure 13). The size of the body part represents the innervation density, for example the fingers and lips have a higher number and more complex sensory and motor connections. They require more cortical area for the processing of finer sensation. [34]
The motor areas control voluntary movements and like the sensory areas, the motor area in the left hemisphere controls the movement for the right side of the body and vice versa. The basal ganglia receive input from the motor areas as well as the midbrain (substantia nigra) and also sends signals back to these areas aiding the control of motor movements. [35]
The association areas produce perceptual experience and involve functions such as abstract thinking and language. The parietal, temporal and occipital lobes assimilate information stored as memories and sensory information. The association areas connect distant areas of the cortex. [33]
| Cortical Area | Function |
|---|---|
| Primary motor cortex | Executes voluntary movement |
| Primary visual cortex | Receives and processes visual information |
| Primary somatosensory cortex | Receives and processes somatosensory information such as heat, pain and pressure |
| Primary auditory cortex | Receives and processes auditory information |
| Motor association cortex (premotor cortex) | Selects voluntary movements |
| Auditory association area | Complex processing of auditory information |
| Sensory association area | Complex processing of multi sensory information |
| Visual association area | Complex processing of visual information |
| Prefrontal cortex | Involved in organising thoughts and actions to match internal goals. These include planning complex cognitive behaviour, making executive decisions, expressing personality, social awareness and storing short term memories. [36] |
| Broca's area (speech centre) | Speech production and articulation [37] |
| Wernickes area | Speech comprehension [37] |
<html5media height="400" width="600">https://www.youtube.com/watch?v=n6zQbTT0yoY</html5media>
[38]
Abnormalities associated with Cerebral Cortex Development
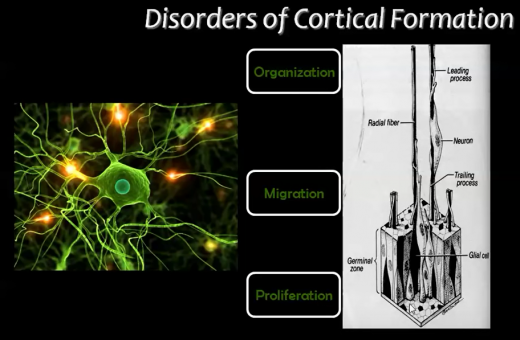
The embryologic development of the cerebral cortex involves highly organised and complex events. As mentioned in the 'Introduction', these events are generally divided by scientists into 3 major stages in cortical formation where crucial changes occur in neuronal cells; these stages include:
1. Proliferation (or Growth),
2. Migration,
3. Organisation (or Maturation or Differentiation).
Disruptions can occur in these stages of cortical development (due to varying causes), and these disruptions give rise to malformations or irregularities in the cerebral cortex (Figure 13).
Although multitudinous and/or severe effects ensue from such disorders, epilepsy and mental retardation (of varying degrees) are almost always present with these disorders.
This section will explore some abnormalities that are commonly discussed in scientific literature.
[7][40][41]
Disorders due to the abnormal proliferation, growth or differentiation of neuroblasts
1) Focal cortical dysplasia (FCD)
Focal cortical dysplasia (FCD) is heterogeneous developmental disorder that results because neuroblasts are not able to successfully complete the proliferation stage of cortical development. Mechanism of development of this disorder is not well understood and FCD is categorised as of uncertain etiology. Focal Cortical Dysplasia is characterised by neurons arranged abnormally in the focal areas of the cerebral cortex as well as disorganisation of layers (lamination disorganisation). Very large dysmorphic cells called 'balloon' cells are also seen due to abnormal regulation of cell growth.
FCD can affect the areas throughout the brain but occurs dominantly in the frontal and temporal lobes of the cerebral cortex.
If symptoms do present, then these are associated with epilepsy ( tonic-clonic, tonic, simple partial and complex partial seizures). In all patients who present with epilepsy, FCD is the cause for about approximately 25% of them. FCD can be of two types: Type I and Type II.
[7][40]
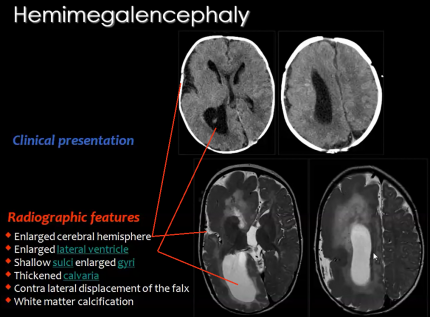
2) Hemimegalencephaly
A rare congenital disorder that also results from the abnormal proliferation of neuroblasts is Hemimegalencephaly.
Hemimegalencephaly is a developmental disorder which consists of 'hamartomatous' overgrowth (where normal mature cells and tissues normally present in an area of the body, form tumour-like, benign malformation(s) ) of one cerebral hemisphere, part of a hemisphere or one hemisphere with partial involvement of the other hemisphere. MRI findings usually show enlargement of one hemisphere or at least one lobe and abnormal white matter (Figure 15).
Clinical symptoms of this disease may include developmental delay, mental retardation, paralysis of one side of the body and blindness over half the field of vision; but the most significant symptom is seizures as 90% of patients present with this symptom.Hemimegalencephaly accounts for 0.2% of cases of childhood epilepsy.
[39][40]
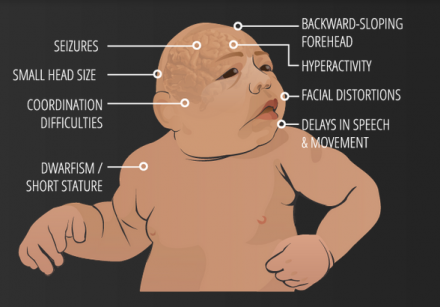
3) Microcephaly Vera
Amongst cortical congenital disorders, Microcephaly Vera is a relatively common disorder. Microcephaly or 'small brain', can be caused by abnormal cell proliferation or cell division along with the involvement of other organs, and can be caused by genetic or non-genetic factors.
Microcephaly Vera or Primary Microcephaly is genetic and does not involve other organs. It results from abnormal cortical development, specifically cell division or proliferation. In this malformation the circumference of the head (and so the brain) is much smaller than normal, while the rest of the body is of normal size. Clinically, microcephaly vera frequently presents with mental retardation and sometimes epilepsy, although some other features may also be observed (Figure 16).
[39][40][41]
4) Tuberous Sclerosis Complex
Tuberous sclerosis complex (TSC) is a multi-organ disease resulting from mutations of certain genes, and is usually classified under defects of early cell proliferation and growth although it is also associated with defects of neuronal migration.
Tuberous sclerosis complex (TSC) is characterised by benign abnormal mass of cells (tumors, cysts, and other malformations including hamartomas and neoplasms), which can occur in several tissues of the body, including cerebral cortex. In the cortical gray matter, cortical 'tubers' and bizarre cells are seen because laminar disorganisation occurs (like Focal Cortical Dyplasia). TSC is thus called because initially scientists thought that the lesions they saw resembled potato tubers.
No signs or symptoms are known to be observed for TSC, and diagnosis is determined after a brain scan.
[39][41]
Disorders due to abnormal neuronal migration
5) Heterotopia
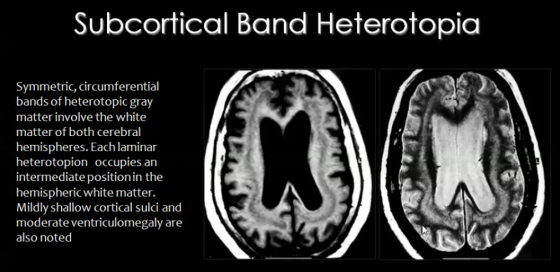
For neurons to successfully execute the migration stage of development, some steps have to be completed including departure from the ventricular zone, migration to the cortical plate and then arrest of movement at the appropriate layer.
Migration of neurons occurs during the 8th week of development, along radial glial fibers (RGF). RGFs can be damaged due to ischemia, which can be caused by infection resulting from trauma or metabolic errors.
RGF damage leads to the migration process arresting at the wrong time, resulting in abnormal or ectopic locations of neurons of the cortex. This condition is known as Heterotopia.
There are different types of heterotopia:
- Subcortical band heterotopia:
In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-cortical regions of the cerebral cortex (Figure 17).
- Periventricular heterotopia/ sub-ependymal heterotopia:
In this type of heterotopia, ectopic locations of neurons, seen as nodules, occur in the sub-ependymal or periventricular area of gray matter of cerebral cortex.
- Focal heterotopia:
In this type of heterotopia, abnormal location of neurons result in focal masses within deep white matter of cerebral cortex.
[39][40][41]
6) Lissencephaly
Lissencephaly is a congenital cortex malformation, in which gyri (and sulci) of the cerebral cortex are absent (agyria) or largely absent (pachgyria), resulting in a smooth surface of the brain. The normal lamination or layers fail to form and the cerebral cortex usually has only 4 of the typical 6 layers. Like most congenital brain disorders, the etiology of Lissencephaly is uncertain. Lissencephaly has also been associated with other abnormalities including corpus callosum hypoplasia, small brain stem and gray matter heterotopia.
Lissencephaly is generally characterised by the following features:
- Total agyria (gyri and sulci absent resulting in 'smooth brain')
- Pachygyria (gyri can be seen but are very few compared to normal brain)
- Agyric brain with areas of pachygyria
- Vertically oriented Sylvian fissures of the brain, giving the brain an 'hourglass' configuration
Lissencephaly is of different types; but these types have some common clinical symptoms, namely epilepsy and severe mental retardation. Types of Lissencephaly are the following:
- Classical (Type I) Lissencephaly- results from neuronal undermigration (failed or incomplete neuronal migration) due to varying causes like mutation; additional clinical features include motor deficits. Patients often also have microcephaly [discussed later in Microlissencephaly].
- Cobblestone (Type II) lissencephaly- results from overmigration, where neurons migrate from the cortex into the overlying leptomeninges, causing diverse disruptions of the basement membrane; the cortex has a 'cobblestone' type of nodular appearance and additional clinical features include various eye abnormalities, congenital muscular dystrophies, hypotonia and generalized weakness in infancy. Type II Lissencephaly also includes:
- Muscle-Eye-Brain Disease
- Walker-Warburg Syndrome
- Fukuyama Syndrome
- Microlissencephaly
Microlissencephaly occurs when Type I Lissencephaly occurs in combination with congenital Microcephaly(discussed previously), and presents with severe symptoms including seizures, spasticity, severe developmetal delay and short life span.
[7][39][40][41]
7) Kallmann Syndrome
The Kallman Syndrome is a syndrome characterised by congenital Hypogonadism (diminished or failed activity of male or female gonads). Kallman Syndrome results from the failed migration of neurons that secrete Leuteinizing Hormone-Releasing Hormone(LHRH) also known as Gonadotropin Releasing Hormone(GnRH), from the olfactory placode to the hypothalamus. The hypothalamus normally secretes GnRH as this is necessary for normal gonadal function. Because of failed migration of aforementioned neurons to the hypothalamus, GnRH production is disrupted causing hypogonadism.
Clinical presentation of Kallman Syndrome includes archinencephaly, which is hypoplasia (underdevelopment or incomplete development) of the olfactory cortex and olfactory bulb, and deficient or absent sense of smell.
[39][41][43]
Disorders due to abnormal cortical maturation and organization/folding
8) Polymicrogyria
If the neurons of the cerebral cortex successfully complete the proliferation and migration stages, but during the last organisation stage, distribute abnormally then multiple small undulating gyri can result. This condition is called Polymicrogyria (PMG), in which, the gyri become extremely small (hence the term micro) and their number is greater than normal. The cerebral cortex in this condition is flat and thickened, similar to that of pachygyria or agyria, and contains numerous small gyri.
Polymicrogyria is usually focal. It is most commonly 'perisylvian' (around the Sylvian fissure) and can be bilateral or unilateral. The most common sites, in descending order, are the frontal lobe, parietal lobe, temporal lobe and then occipital lobe.
Affected patients can have varying degrees of symptoms, including epilepsy and developmental delay. For example, bilateral poylmicrogyria causes developmental delay, hypertonicity, ataxia, and refractory seizures.
[7][40]
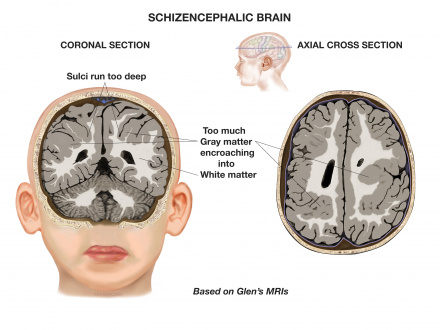
9) Schizencephaly
Schizencephaly is an organisational developmental disorder classified within the same group as polymicrogyria. It is characterised by a cleft(s) or lesion(s) on the brain surface,extending from the cerebral cortex to the ventricle and is typically filled with CSF (cerebrospinal fluid) and lined by cortex gray matter (Figure 18). These lesions or clefts are thought to be 'destructive'.
The clefts can be small with closed walls in Type I or Closed lip type schizencephaly; or the clefts can be large with free communication between the ventricle and subarachnoid spaces in Type II or Open lip type schizencephaly. Type I presents with partial seizures or spastic hemiparesis, while Type II presents with epilepsy or seizures, spasticity, severe developmental delay and microcephaly.
Schizencephaly can be bilateral or unilateral; and commonly involves the parasylvian regions, and severity of the disease correlates with the extent of the clefts.
[7][39][40]
| Other disorders of varying etiology |
10) Fetal Alcohol Spectrum Disorder (FASD)
 Figure 19. Facial features appearance in Fetal Alcohol Spetrum Disorder (FASD).[45]
The three major indications (that also help form the diagnoses) for FASD are facial dysmorphology, growth deficits and central nervous system defects. These conditions result in immense physiological and psychological impacts on the child. Fetal Alcohol Syndrome (FAS) is an acute form of FASD. On average, FASD is diagnosed in a child between 3-10 years. It is of utmost importance however that the diagnosis is done as early as possible so that timely interventions could be taken in order to lessen the severity of the symptoms that result from this syndrome. 11) Corpus Callosum Agenesis The corpus callosum is a structure present in the brain which connects both the hemispheres of the brain. The video below briefly describes this: <html5media height="350" width="550">https://www.youtube.com/watch?v=7zOa3LLrHKc</html5media>
[47] |
Models and Research
Animal Models
The most common model to study the development of cerebral cortex is the mouse, although other animals are also used to study the development of the brain. Animal studies are necessary for gaining adequate and comprehensive understanding of pathophysiology and etiology of abnormalities/diseases, as well as for testing and formulating treatments. Apart from the mouse, other animal models used include the fruit-fly, rat, cat, dog, ferret and even worm. The zebrafish and especially primates like monkeys are also key models. [50][51][52][53]
In this section we have given examples of some findings obtained using mice models.
Mice have often been used to study corticogenesis in mammals. Corticogenesis lasts from E11 to E19 in mice and lasts eight days, which is far shorter than human corticogenesis. While there are many similarities between human corticogenesis, the rodent brain is much smaller than that of a human’s and therefore, there are different types of progenitor cells involved in mice and the cortex does not expand as greatly. [54]Humans have greater numbers of intermediate precursor cells (to aid in further differentiation) and outer radial glial cells compared those of mice. [17] In addition, the SVZ does not split into the inner and outer subventricular zone, but stays intact as one. [54] The importance of reelin has also been greatly studied in mice. Studies showed that mutant mice have disruptions in the migration of neurons into their subsequent layers. By injecting thymidine at various time points of gestation into the female mice, researchers were able to track the development of various populations of neurons. Later developing neurons were unable to ascend past the already early formed neurons due to disruptions in reelin signaling (in the marginal zone). Instead, superficial layer neurons resided below the older, deep layer neurons. This inverted lamination comprises sensory and motor function.[55]
Current Research
In this section, we have discussed current research regarding 3D organoids.
One of the limiting steps in research at present is the lack of models that can accurately recapitulate the developmental changes and pathophysiology of the human brain. Although it is recognized that certain fundamentals of brain development are conserved in mammalian brains there are distinct differences that should be considered when studying the human brain. [56]
Currently there has been the development of 3 dimensional models, derived from stem cells that can be used to mimic the evolution of the human brain, and have been deemed advantageous over previous in vitro models. This method, as first described by Lancaster et al. can, in a 1-2 month period be reproduced in a tissue culture room give rise to the developing parts of the human brain, including the cerebral cortex. This is established using a protocol human pluripotent stem cells (PSCs). PSCs separate to single cells before rearranging to form embryoid bodies, which are prompted to form neuroectoderm in a medium that prevents either endoderm or mesoderm development. At day 11-15 of this protocol the tissues undergo neuroepithelial bud expansion after being transferred to Matrigel droplets. The final stage of the process involves the tissues being transferred to an agitator (either spinning bioreactor, or orbital shaking plate) which allows for more substantial growth of the brain regions due to better nutrient and oxygen supply. [57]
The development of these brain organoids has allowed more comprehensive understanding of the self-organisational properties of the brain, and is quickly advancing, however the lack of vasculature in cultured organoids restricts their ability to be used for specific neurological disorders. Furthermore there are still challenges associated with the generation of individual, discrete brain regions, which should be addressed in the future. [58]
Glossary
| Term | Definition |
| Agenesis | The total absence of a tissue or organ. |
| Cajal-Retzius (CR) cells | Cortical neurons that develop early on, at the same time as the preplate, and express an important glycoprotein known as reelin, which helps with the proper migration of neurons into their respective layers. |
| Cortical Plate | Forms in between the marginal zone and subplate and gives rise to the 6 layers of the cortex involved in sensory and motor function. |
| Corticogenesis | The development of the cerebral cortex into its six layers. It involves a sequential process of migration and differentiation of neocortical projection neurons into a laminated structure that contains a diverse set of neuronal subtypes. |
| Dystrophy | The wasting way of body tissues or an organ due to some disease or disorder. |
| Epilepsy | A neurological disorder characterised by seizures. |
| Etiology | The cause or origin or explaination of a disease. |
| Fissures | Grooves or clefts; are actually just large sulci. |
| GABAergic Neurons | Generate gamma aminobutyric acid (GABA) as their output, one of the two inhibitory neurotransmitters in the central nervous system (CNS). |
| Gyrus | Gyri (plural) are folds/ridges in the cerebral cortex. |
| Heterogeneous | Diverse, varied and many components of something. |
| Hypoplasia | Underdevelopment or incomplete development of a tissue or organ. |
| Hypotonia | The state of loss of muscle tone, or tension (or stretch strength) of muscles. |
| Intermediate zone | Forms below the subplate between E50-55 and contains only migrating cells and no intermediate precursor cells. |
| Marginal Zone (MZ) | A subsection of the preplate that forms around E50-55. It lies at the uppermost area of the cortex nearest the pial surface. |
| Neural Plate | Main developmental structure required for the development of the nervous system. |
| Neural Tube | Structure of the embryo that evolves into the spinal cord and brain. |
| Olfactory | Relating to nasal organs and/or the sense of smell. |
| Pachygyria | Gyri can be seen but are very few compared to normal brain. |
| Preplate | First "pioneer neurons" from dividing cells in the ventricular zone for this layer above the ventricular zone. |
| Radial glial cells | A class of distinct nonneuronal cells that are bipolar shaped and span the entire width of the cortex during development. |
| Seizures | Fits, which involve a sudden surge of electrical impulses in the brain, causing jerking of limbs and stiffness. |
| Spasticity | Having spasms in muscles or possibly some organs. |
| Subplate | A subsection of the preplate that forms around E50-55. |
| Subventricular zone (SVZ) | Cells in the VZ continue to divide symmetrically and give rise to another zone above the VZ known as the SVZ. This zone later splits into an inner (ISVZ) and outer ventricular zone (OSVZ). |
| Sulcus | Sulci (plural) are grooves in the cerebral cortex. |
| Total agyria | Gyri and sulci absent resulting in 'smooth brain'. |
| Ventricular zone (VZ) | First formed single-celled layer in the cortex, arising from progenitors in the dorsal telencephalon that divide symmetrically. It lies adjacent to the ventricular surface. |
References
- ↑ Thomas, A. (2015). [image] Available at: http://slideplayer.com/slide/6617450/ [Accessed 23 Oct. 2017].
- ↑ Mikayla D (2017). 23. [image] Available at: https://psych-brain-trust.wikispaces.com/Thalamus [Accessed 23 Oct. 2017].
- ↑ Moore, K., Persaud, T. and Torchia, M. (2011). The Developing Human. London: Elsevier Health Sciences, pp.The Nervous System; 379-414.
- ↑ Van Essen, D. (2005). A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage, 28(3), pp.635-662.
- ↑ Dartmouth.edu. (2006). Chapter 11: The Cerebral Cortex. [online] Available at: http://www.dartmouth.edu/~rswenson/NeuroSci/chapter_11.html.
- ↑ Differencebetween.com. (2017). Difference Between Cerebrum and Cerebral Cortex. [online] Available at: http://www.differencebetween.com/difference-between-cerebrum-and-vs-cerebral-cortex/ [Accessed 26 Oct. 2017].
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Squier, W. and Jansen, A. (2010). Abnormal development of the human cerebral cortex. Journal of Anatomy, [online] 217(4), pp.312-323. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2992410/ [Accessed 23 Sep. 2017].
- ↑ 8.0 8.1 8.2 Embryology.med.unsw.edu.au. (2017). Lecture - Ectoderm Development - Embryology. [online] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/Lecture_-_Ectoderm_Development.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Moore, K., Persaud, T. and Torchia, M. (2015). The developing human. Philadelphia, PA: Elsevier.
- ↑ 10.0 10.1 Vanderah, T., Gould, D. and Nolte, J. (2016). Nolte's The human brain. Philadelphia, PA: Elsevier, p.44.
- ↑ 11.0 11.1 11.2 Gray, H. (1977). Gray's Anatomy. New York, New York: Crown Publishers, Inc.
- ↑ 12.0 12.1 12.2 12.3 12.4 Schoenwolf, G., Bleyl, S., Brauer, P. and Francis-West, P. (2015). Larsen's human embryology. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone, pp.197-233.
- ↑ 13.0 13.1 Pansky, B. (n.d.). Chapter 155. The Brain: The Telencephalon (first Vesicle) - Review of Medical Embryology Book - LifeMap Discovery. [online] Discovery.lifemapsc.com. Available at: https://discovery.lifemapsc.com/library/review-of-medical-embryology/chapter-155-the-brain-the-telencephalon-first-vesicle.
- ↑ 14.0 14.1 14.2 14.3 14.4 <pubmed> 18209730 </pubmed>
- ↑ 15.0 15.1 Marin, O., & Rubenstein, J. L. R. (2003). Cell migration in the forebrain. Annual Review of Neuroscience, 26, 441-83.
- ↑ 16.0 16.1 <pubmed> PMC3876965 </pubmed>
- ↑ 17.0 17.1 17.2 <pubmed> PMC4334136 </pubmed>
- ↑ <pubmed>20159447</pubmed>
- ↑ <pubmed> 24653675 </pubmed>
- ↑ <pubmed>25246510</pubmed>
- ↑ <pubmed>2913541</pubmed>
- ↑ <pubmed>21505516</pubmed>
- ↑ <pubmed>22461901</pubmed>
- ↑ 24.0 24.1 24.2 <pubmed>17580069</pubmed>
- ↑ 25.0 25.1 Handwritten Tutorials (2012). Brain Anatomy 1- Gross Cortical Anatomy (Lateral Surface). [video] Available at: https://www.youtube.com/watch?v=woIZaLiy-eA [Accessed 5 Oct. 2017].
- ↑ <pubmed>19763105</pubmed>
- ↑ <pubmed>8747184</pubmed>
- ↑ 28.0 28.1 <pubmed>17553419</pubmed>
- ↑ <pubmed>9622234</pubmed>
- ↑ <pubmed>19447861</pubmed>
- ↑ Guyton, A & Hall, J (2017). Functions of Specific Cortical Areas. Available at http://www.brainkart.com/article/Functions-of-Specific-Cortical-Areas_19753/.
- ↑ Bust, A & Kellogg, D. (2015). Homunculus: Somatosensory and Somatomotor Cortex. EBM Consult [online] Available at https://www.ebmconsult.com/articles/homunculus-sensory-motor-cortex#jump_ss_100125.
- ↑ 33.0 33.1 33.2 <pubmed>21653723</pubmed>
- ↑ <pubmed>9931268</pubmed>
- ↑ <pubmed>29042690</pubmed>
- ↑ <pubmed>28978697</pubmed>
- ↑ 37.0 37.1 <pubmed>25218167</pubmed>
- ↑ Learn Some (2016). Cerebral Cortex. [video] Available at: https://www.youtube.com/watch?v=n6zQbTT0yoY [Accessed 5 Oct. 2017].
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 39.7 39.8 39.9 Mahfouz, M. (2015). Imaging of cortical formation disorders - DRE 4 - Prof. Dr Mamdouh Mahfouz. [video] Available at: https://www.youtube.com/watch?v=l_nTggR7LTE [Accessed 23 Sep. 2017].
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 Pang, T., Atefy, R. and Sheen, V. (2008). Malformations of Cortical Development. The Neurologist, [online] 14(3), pp.181-191. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3547618/ [Accessed 23 Sep. 2017].
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 Christopher A, C. (1999). Genetic Malformations of the Human Cerebral Cortex. Neuron, [online] 23(1), pp.19 - 29. Available at: http://www.cell.com/neuron/fulltext/S0896-6273(00)80749-7?_returnURL=http%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627300807497%3Fshowall%3Dtrue [Accessed 23 Sep. 2017].
- ↑ USDA & Felipe Dana/AP and Creative Commons License(CC) (2017). Understanding Zika. [online] Goinvo.com. Available at: http://www.goinvo.com/features/zika/ [Accessed 4 Oct. 2017].
- ↑ Reference, G. (2017). Kallmann syndrome. [online] Genetics Home Reference. Available at: https://ghr.nlm.nih.gov/condition/kallmann-syndrome [Accessed 26 Oct. 2017].
- ↑ PRWeb (2013). Schizencephalic Brain. [image] Available at: http://www.prweb.com/releases/2013/7/prweb3350774.htm [Accessed 5 Oct. 2017].
- ↑ MarkHill,embryology.med.unsw.edu.au (2017). Facial Appearance of Fetal Alcohol Syndrome (FAS). [image] Available at: https://embryology.med.unsw.edu.au/embryology/index.php/File:FASface.jpg [Accessed 5 Oct. 2017].
- ↑ Leigland, L., Ford, M., Lerch, J. and Kroenke, C. (2013). The Influence of Fetal Ethanol Exposure on Subsequent Development of the Cerebral Cortex as Revealed by Magnetic Resonance Imaging. Alcoholism: Clinical and Experimental Research, 37(6), pp.924-932. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3670687/ [Accessed 5 Oct. 2017].
- ↑ Ezzo - Izzo, D. (2007). How the Body Works : The Corpus Callosum. [video] Available at: https://www.youtube.com/watch?v=7zOa3LLrHKc [Accessed 23 Sep. 2017].
- ↑ Ninds.nih.gov. (2017). Agenesis of the Corpus Callosum Information Page. [online] Available at: https://www.ninds.nih.gov/Disorders/All-Disorders/Agenesis-Corpus-Callosum-Information-Page [Accessed 26 Oct. 2017].
- ↑ Vasudevan, C., McKechnie, L. and Levene, M. (2012). Long-term outcome of antenatally diagnosed agenesis of corpus callosum and cerebellar malformations. Seminars in Fetal and Neonatal Medicine, 17(5), pp.295-300.
- ↑ Sun, T. and Hevner, R. (2014). Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nature Reviews Neuroscience, 15(4), pp.217-232.
- ↑ Wolf, F. (2005). Course 12 Symmetry breaking and pattern selection in visual cortical development. Les Houches, pp.575-639.
- ↑ Xu, M., Xu, H. and Qin, Z. (2015). Animal Models in Studying Cerebral Arteriovenous Malformation. BioMed Research International, 2015, pp.1-13.
- ↑ Brainfacts.org. (2017). Why Animals Are Needed in Research 031017. [online] Available at: http://www.brainfacts.org/in-the-lab/animals-in-research/2017/why-animals-are-needed-in-research-031017 [Accessed 26 Oct. 2017].
- ↑ 54.0 54.1 Dehay, C. and Kennedy, H. (2007). Cell-cycle control and cortical development. Nature Reviews Neuroscience, 8(6),438-450.
- ↑ Caviness, V. (1982). Neocortical histogenesis in normal and reeler mice: A developmental study based upon [3H]thymidine autoradiography. Developmental Brain Research, 4(3), 293-302.
- ↑ <pubmed>28845922</pubmed>
- ↑ <pubmed>25188634</pubmed>
- ↑ <pubmed>28822354</pubmed>