2016 Group Project 6
| 2016 Student Projects | ||||
|---|---|---|---|---|
| Signalling: 1 Wnt | 2 Notch | 3 FGF Receptor | 4 Hedgehog | 5 T-box | 6 TGF-Beta | ||||
| 2016 Group Project Topic - Signaling in Development
OK you are now in a group, add a topic with your student signature to the group page. | ||||
| This page is an undergraduate science embryology student project and may contain inaccuracies in either descriptions or acknowledgements. | ||||
| Group Assessment Criteria |
|---|
 Science Student Projects Science Student Projects
|
| More Information on Assessment Criteria | Science Student Projects |
Transforming Growth Factor-Beta (TGF-β) Signalling Pathway
Introduction
The transforming growth factor beta (TGF-β) is a multifunctional and pleiotropic cytokine [1]. The TGF-β signalling pathway is crucial to the control of different biological and pathological processes, such as cellular proliferation and differentiation, angiogenesis, immune regulation/inflammation, apoptosis and cell survival. [2] TGF-β belongs to the Transforming Growth Factor superfamily - a large group of structually connected cell regulatory proteins. It consists of TGF-β 1, 2 AND 3, Growth Differentiation Factors (GDFs), Activins, Inhibins, Bone Morphogenetic Proteins (BMPs), Glial-derived Neurotrophic Factors (GDNFs) and Mullierian Inhibiting Substance (MIS). [3] Most importantly, TGF-β plays a dominant part in the development of the embryo and adult organism. [4]
This wiki aims to present a helpful overview of the TGF-β signalling pathway, but is in no means a complete resource on all information regarding the topic. We focus on ________________
History
Since the early stages of the TGF beta-signaling pathway, plenty of in-depth research and studies have been conducted that have no doubt contributed to our knowledge of the pathway today.
SMAD signaling and the three receptors for TGF-beta are two of the many fields of interest regarding the topic. In medicine and specific areas such as cancer, cardiovascular disease and inflammatory bowel disease, there are numerous alternatives for drugs that can either heighten or suppress the activity of TGF-beta.
| 1988 | The process of maturation of follicle-enclosed oocytes and cumulus-oocyte complexes was sped up by TGF beta. It was discovered that TGF beta and other growth factors are effective in vitro stimulators of oocyte maturation in the rat |
| 1989 | It was already known that TGF-beta 1 is a strong autocrine growth inhibitor of lymphocytes. Ellingsworth and colleagues found that TGF-beta 1 binds to all three cell surface-binding proteins (280-200 kD, 95-85 kD, 65 kD).
It was also found that these binding proteins are required for signal transduction. Overall, they discovered that the regulation of the expression of the TGF-beta 1 receptor is controlled by T cell mitogenic signals. [6] |
| 1989 | It was made known that the properties of R mutants classify TGF-beta type I binding protein as the receptor involved in mediating TGF-beta actions on cell adhesion and proliferation |
| 1989 | Drosophil was the only member of the TGF-beta family to be identified in invertebrates |
| 1990 | It was already known that the rapid increase in number response of mink lung epithelial cells to serum and to epidermal growth factor was inhibited by TGF beta 1. A necessary component of TGF-beta 1 mediated growth inhibition in CCL64 epithelial cells is the coupling of TGF beta 1 receptor binding to G-protein activation |
| 2000 | VegT function was found to be involved in sequence with the TGF beta pathway. Therefore, TGF beta signaling may be activated by the maternally expressed VegT to participate in endoderm determination |
| 2005 | Within the TGF beta superfamily, it was found that a limited number of type I and type II receptors worked together to produce specificity of action |
| 2010 | Deregulation of TGF beta signaling was reported in human psoriasis |
| 2015 | It was known that TGF is required in the tumorigenicity and metastasis of bone tumour. A significant event in the activation of the TGF beta signaling pathway is the binding of transcription coactivator Yes-associated protein (YAP) to Smad transcription factors |
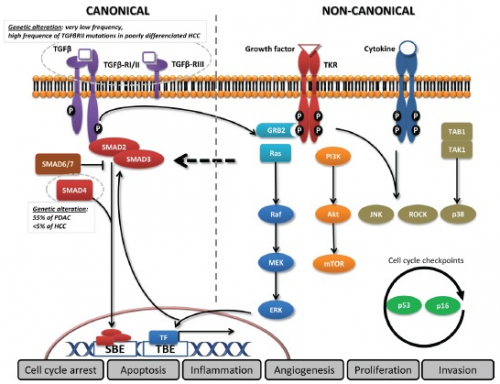
Canonical pathway
In the canonical pathway, the dormant TGF-β complex is formed when the three TGF-β ligand isoforms - TGF-B1, TGF-B2 and TGF-B3 - bind once it is synthesized as precursors. After secretion and extracellular activation, TGF-β ligands can bind to two types of receptors: the membranous TGF-β type III receptor or the TGF-β type II receptor (TGF-βRII) homodimers with high affinity. TGF-βRII binding enables dimerization with TGF-β type I receptor (TGF-βRI) homodimers, as well as activation of the TGF-βRI kinase domain and signal transduction across phosphorylation of the C-terminus of receptor-regulated SMADs, SMAD2 and SMAD3. A heterotrimeric complex is formed by the TGF-βR dimer and SMAD4, which moves and assemblies in the nucleus. TGF-β dependent signalling can operate or subdue numerous target genes through the communication of SMADs with multiple transcription factors. There are many structures in which SMAD activities are regulated, such as SMAD2/3 nucleocytoplasmic shuttling, binding to anchor proteins, phosphorylation and Smurf (SMAD-ubiquitination-regulatory factor). [15]
Non-Canonical pathway
In the non-canonical pathway, SMAD-independent pathways such as PI3K/AKT and MAPK pathways like ERK, JNK, and p38 MAPK are activated by TGF-β signalling. In addition, transversal signalling, especially at the SMAD level, allows TGF-β pathway activation to incorporate signals from integrins, Notch and Wnt dependent pathways as well as signals from cellular processes like the cell cycle or apoptosis machineries. Thus, the TGF-β signalling pathway has pleiotropic functions regulating cell growth, differentiation, apoptosis, cell motility, extracellular matrix production, angiogenesis and cellular immune response.[16]
Process of TGF-β signalling pathway
TGF-β signalling pathway is required for regulation of a large number of cellular processes such as cell proliferation, invasion and inflammation. It is also activated mitogen activated protein kinase signalling. There are two main routes in TGF-β signalling; the SMAD Dependent pathway and SMAD Independent pathway.
SMAD Dependent TGF-β signalling pathway
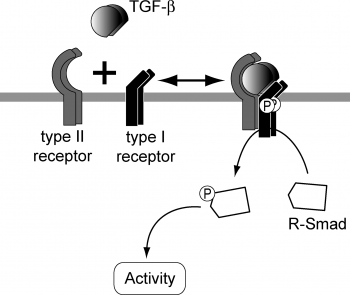
The ligands of the TGF-β superfamily form dimers that bind to heterodimeric receptor complexes composed of two type I and two type II transmembrane receptor subunits with serine/threonine kinase domains. Following ligand binding on TGF-β1, the dimerized TGF-β type II receptors phosphorylates and activates the TGF-β type I receptors. In most cell types, this leads to recruitment and phosphorylation of the receptor-regulated SMAD2 and SMAD3, presented by the SMAD anchor for receptor activation. SMAD1 and SMAD5 can be activated by the TGF-β signaling depending on the Type I receptor that is expressed. Heterologous complexes are formed by the phosphorylated receptor-regulated SMAD with the common-mediator SMAD, SMAD4, and successively move into the nucleus, where they accumulate and act as transcription factors participating in the regulation of target gene expression. [18] In addition, they recruit extra transcriptional regulators, such as DNA-binding transcription factors, co-activators and co-repressors. These control the expression of several target genes and ultimately initiates a SMAD-dependent signaling cascade that induces or represses transcriptional activity. SMADs are widely expressed in most adult tissue and cell types, indicating that the TGF-β signaling pathway is ubiquitous.
SMAD independent TGF-β signalling pathway
Rather than SMAD-mediated transciption TGF-β also has the potential to activate other signalling cascades for example the Erk, JNK and p38 MAPK kinase pathways. In some cases these pathways exhibit activation with slow kinetics which indicates SMAD-dependant mechanics, however there has also been rapid activation cases (5-15mins) suggesting independence from transcription mechanisms. Studies carried out with SMAD4 deficient cells and dominant-negative SMADS provide evidence that the MAPK pathway activation is independent from SMADS, as well as this it has be found that p38 MAPK signalling was activated in response to mutated TGF- β type 1 receptors, which were defective in SMAD activation[19].
The precise mechanisms and biological consequences of these SMAD-Independent pathways (Erk, JNK, p38 MAPK) are currently poorly characterized. Ras is implicated in TGF- β induced Erk signalling as there is rapid activation of Ras by TGF- β in epithelial cells. The JNK and p38 MAPK signalling are activated by various MAPK kinase kinases (MAPKKK) TGF- β kinase 1 (TAK 1) receptor is a MAPKKK family member. Further research and identification of various interactions between the small signalling molecules and receptor proteins will provide additional insight into the precise mechanism behind the activation of MAPK pathways by TGF- β ligands [19].
Regulation of the pathway and factors affecting it
Signalling mechanisms by TGF-β like factors are regulated in both negative and positive fashions, these are all tightly controlled through a multitude of mechanisms at extracellular, membrane, cytoplasmic and all the way to nuclear levels. Positive regulation is required to amplify signalling from TGF-β like factors, while negative regulation is important for the termination and restriction of signalling usually occurring through the mechanism of a feedback loop. There is also additional regulation of TGF-β like factors via cross-talk with other signal transduction pathways such as MAPK and JAK/STAT pathways.[20]
Positive Regulation
The positive regulation of TGF-β specifically the induction of ligands and their signalling components often is triggered by the action TGF-β-like factors themselves. For example NODAL, a secretory protein of the TGF-β superfamily which plays a role in early embryogenesis and acts through activin receptors and SMAD2 is induced by nodal signalling itself. In other types of cells TGF-β receptors as well as transcription factors which serve as targets for TGF-β like factors can be induced by ligand stimulation, as identified in case of transcription factor Runx3 which is induced by TGF-β and forms a complex with SMAD3 to be further activated by TGF-β. The mechanism of SMAD signalling is also positively modulated via the "cross-talk" (and hence the process of SMAD dependant TGF-β signalling) with other signalling pathways, SMADS may be activated by the tyrosine kinase receptor under specific circumstances and further positively regulate TGF-β like factors [20].
Negative Regulation
Signalling is regulated at the cell membrane level as well as within the cytoplasm of the cell, specifically by BAMBI, a pseudo-receptor for serine/threonine kinase receptors (in Xenopus embryos however displays a high degree of sequence similarity to human BAMBI gene). This BAMBI receptor is structurally alike to the type 1 serine/threonine kinase receptor, the only difference being that it lacks an intracellular domain. BAMBI has shown a similar expression profile to that of BMP-4 a growth factor from the TGF-β super family, and has been found to require BMP signalling for expression. BAMBI when goes on to interact with both type 1 and type 2 serine/threonine receptors and works to abolish their abilities to signal via BMPs, activins and TGF-βs, therefore it is postulated that BAMBI can be inductively expressed by BMPS to self regulate BMP signalling as well as cross-regulate signalling from other members of the TGF-β super family. [20]
Significance in Embryonic Development
TGF betas are involved in embryogenesis. During development of the embryo, members of the TGF-beta family are essential for bone and cartilage formation, mesoderm induction and patterning and dorso-ventral patterning.
Cardiovascular Development
Genetic engineering and tissue explanation studies have revealed many roles for TGF-β ligands and their signaling molecules in development. In the embryo, TGF-β appear to be involved in epithelial-mesenchymal transformations (EMT) during the formation of endocardial cushions, and in epicardial epithelial-mesenchymal transformations essential for coronary vasculature, ventricular myocardial development and compaction. It must be noted that in the normal function of the cardiovascular system in the adult, TGF-β play significant roles in cardiac hypertrophy, vascular remodeling and regulation of the renal renin-angiotensin system.
TGF-β1 is expressed in the endocardium of the developing mouse. TGF-β(-/-) mice have been found with obvious congenital cardiovascular defects, so it’s important to review its expression in the developing heart. In the blood vessels, TGF-β1 is in the intima whereas TGF-β2 and TGF-β3 are in the media and adventitia. TGF-β2 signals are found as early as embryonic day 7.25 (E7.25) [21] in the cardiogenic plate of the precardiac mesoderm and is later prominent in the myocardium of the aortic sac and outflow track regions. TGF-β2 protein is also found in the entire myocardium of the heart at the time when looping occurs (morphogenesis when heart shape is formed by looping the tube). From E8.5-9.5 when the cushion formation (cells in development that play a role in the formation of the heart septa) process occurs, there is a particularly strong TGF-β2 expression localised to the myocardium (2ABDE). After cushion formation and EMT, and before myocardialization of the endocardial cushion begins, there is also strong TGF-β2 expression in the OT myocardium and in the adjacent developing cushion mesenchym. However, as myocardialization occurs, TGF-β2 expression is reduced in the myocardium so that from E12.5 onwards, it is only expressed mainly in the mesenchyme of the cushion and OT septum. As can be seen in 2GH, TGF-β2 expression remains high in the cushion mesenchyme of the OT septum. By E15.5, TGF-β1 s now the most highly expressed isoform in the endocardial cells of the myocardium. It is seen in 2MNO, that the epidcardium TGF-β1 and TGF-β3 expression is higher than that of TGF-β2. Thus, it can be seen that all three TGF-β are expressed in the epicardium, and they are not expressed in an overlapping fashion.
Cross talk between mesoderm and underlying endoderm is needed to form the early tubular heart. This cellular and molecular induction in the primary heart forming regions is important for the specification and differentiation of myocardial and endocardial precursor cells [22]. Other endoderm-derived growth factors such as BMP2, FGF2 as well as TGFBS have been implicated in this process in the avian system (respiratory system delivering oxygen and removing carbon dioxide) [23]. TGFB2 and TGFB receptors are expressed in the precardiac mesoderm along with BMP2 [24][25][26]. Members of the TGG family can serve as inductive signals at the heart forming fields for the formation of myocardial and endocardial precursor cells. Members such as Activin, BMP, Nodal, Left and others have been found to be crucial for the establishment of embryonic asymmetry [27], and this asymmetry is in turn critical for heart development [28].
Mammary Gland Development
All three TGF-[beta] isoforms are expressed during all stages of mammary gland development except lactation (8). Mouse studies indicate key roles for TGF-[beta]s in establishing proper mammary gland architecture, regulating stem cell kinetics, maintaining the mammary epithelium in a functionally undifferentiated state, and inducing apoptosis in the involuting gland (2,9-12). The reader is referred to an earlier issue of this journal for additional comprehensive reviews (13). Importantly, TGF-[beta]s are potent inhibitors of the proliferation ofmammaryepithelial cells, both in vitro and in vivo, and the nature of the target cell may determine the type of TGF-[beta] response induced, as TGF-[beta] appears to inhibit proliferation in the ductal epithelial compartment, while inducing apoptosis without effects on proliferation in the alveolar compartment [reviewed (3)].This observation illustrates the general principle that the actions of TGF-[beta]s are very contextdependent, and are affected by cell type, environmental influences and cell history. TGF-[beta] Ligands. There are three closely-related mammalian isoforms of the TGF-[beta] ligand, and in vitro, TGF-[beta]1-3 generally elicit identical biological responses. Currently, it is thought that all three isoforms signal through the same T[beta]RII/T[beta]RI complex, though the possibility that there may be isoform selectivity in the nature and extent of post-receptor signaling has not yet been addressed. In the mammary gland, all three TGF-[beta] isoforms are expressed in the ductal epithelium at all stages of development except for lactation, but there is some isoform specificity in temporal and spatial expression patterns which may reflect isoform-specific roles [reviewed (2)]. For example, expression of epithelial TGF-[beta]2 is generally low but is upregulated during pregnancy, and TGF[beta]3 is the only isoform present in the endbud cap cells and myoepithelial cells. Uniquelyamongthe isoforms, TGF-[beta]1 is also present at high levels in the extracellular matrix that surrounds growth-quiescent ducts, consistent with a role for this isoform in the suppression of lateral budding once the ductal tree is established (2). However, it should be noted that TGF-[beta]s are synthesized as biologically latent forms, and that activation of the latent form is a critical regulatory step that must occur before the TGF-[beta]s can bind to their receptors (36). Most techniques for the localization of TGF-[beta] do not discriminate between active and latent TGF-[beta], and it is likely that the distribution of receptor-reactive TGF-[beta] is much less widespread than current techniques would imply. Indeed, using an elegant immunofluorescent technique, it has recently been shown that latent TGF-[beta] may be activated very locally on a cell-by-cell basis in the mammary gland, with activation in the nulliparous gland being confined primarily to a subpopulation of epithelial cells (4,37). Thus functional activation of the TGF-[beta] ligand/receptor signaling system should not be inferred from the mere co-localization of ligand and receptor, without additional information on ligand bioavailability.
Activin and BMP Ligands. Activins and BMPs have been less extensively studied in the mammary gland. Activin [beta]B mRNA is expressed at all stages of mammary development, and a key role for stromallyderived activin [beta]Bin promoting ductal elongation and alveolar morphogenesis can be inferred from studies with the activin [beta]B knockout mouse (38). Activin [beta]AmRNAexpression was restricted to myoepithelial cells in studies of immunoaffinity-purified cell populations from the human breast (39). BMP-2 and BMP-4 mRNAs are expressed in both epithelial and stromal compartments during mammary gland development, withBMP-2expression being constitutive through development, while BMP-4 is down-regulated in late pregnancy and involution (4). Receptors and Signal Transduction Components. Systematic analysis of the expression of TGF-[beta] family receptor and signal transduction components in the mammary gland is still at an early stage. Table II summarizes what is currently known for the receptors. Immunohistochemical studies on the human breast showed that T[beta]RII is present in the ductal and lobular epithelial cells, but not the myoepithelial cells of the lobular units (40). In mouse, both T[beta]RII and T[beta]RI (Alk5) were expressed in both the mammary epithelium and the periductal stroma at all stages of development (9). These findings are consistent with transgenic mouse experiments suggesting that endogenous TGF-[beta] can act on both epithelium and stroma (9,11). In a study using human breast cells fractionated by immunoaffinity purification, myoepithelial cells, but not luminal epithelial cells or stromal cells, were shown to express mRNA for the activin/BMP receptor ActRII (39). This observation raises the possibility that TGF-[beta] may be more important in regulating stromal and luminal epithelial responses, with activin or BMPs regulating myoepithelial cells. Intriguingly, the ActRII gene was also expressed in all breast cancer cell lines studied and in microdissected invasive carcinoma cells, suggesting that inappropriate expression of ActRII in the mammary epithelium may play a role in tumorigenesis (39). Finally for BMPS, BMPRI-A (Alk3) mRNA was expressed most highly in blood vessels in the mouse mammary gland, with some expression in the periductal stroma at all stages of development, and also in the epithelium of involuting alveoli (9). The expression in blood vessels is consistent with known roles for the BMP pathway in the developing embryonic vasculature (21). In contrast, ActRI (Alk2) was predominantly localized to the mammary epithelium (9). Since ActRI is now thought to be on the BMP response pathway (41), this finding suggests there will be specific effects ofBMPson the mammary epithelium.
So far there are no published reports on Smad expression in the normal mammary gland. However, we find mRNAs for Smads1-5 in the mouse mammary gland at all stages of development [Y. Yang and L. Wakefield, in preparation], which would support the concept that both TGF-[beta]/activin and BMP signal transduction pathways are operational in the mammary gland. Details of the distribution of the Smads between different cellular compartments in the mammary gland remain to be established. In a small study of six human breast cancer cell lines, Smads2 and 3 were found in all lines, and Smad4 in all but one (42), which suggests these three Smads are probably present in the normal epithelium.
Development of hair follicles/teeth/submandibular gland
Vascular biology and dysfunction
Maintenance of pluripotency in hESC
Role in cancer
Formation of digits from limb digits
Formation of the palate
Animal Studies
Wound healing
Animal studies have served as a useful way in providing pivotal information regarding the mechanisms of TGF-β action in wound healing. In fact, much of the current information on the action of TGF-β in wound healing has been acquired from animal studies using incisional and/or excisional wounding models and manipulation of TGF-β signalling by adding the exogenous TGF-β protein or anti-TGF-β neutralizing antibodies, or by genetic alteration in components of the TGF-β signalling pathway. This is due to the fact that animal models provide outstanding experimental methods for explaining molecular mechanisms by which TGF-β regulates wound-healing responses. Ultimately, it has led the development of therapeutic strategies focusing on how the TGF-β pathway can improve wound healing and scarring outcome.
Wound healing is an intricate physiological process distinguished by the successive overlapping stages of inflammation, proliferation and maturation. It that requires numerous growth factors, one of which includes TGF-β, which has the widest range of effects. TGF-β is a multifunctional growth factor that employs pleiotropic effects on wound healing by regulating cell differentiation, extracellular matrix production and immune modulation. The role of TGF-β signalling in wound healing was explored through examination of the development of tissue-specific expression systems for overexpression or knockout of TGF-b signalling pathway components. This study also classified that molecules might serve as molecular targets for the treatment of pathological skin conditions such as chronic wounds and excessive scarring (fibrosis).
Exogenously added TGF-β has the potential to promote wound healing by stimulating angiogenesis, immune cell infiltration, and ECM production, and that diminishing endogenous TGF-β action reduces scarring without adversely affecting wound-healing quality.
Direct modulation of TGF-β levels
Injecting TGF-β into normal skin of newborn mice led to resilient initiation of angiogenesis and fibrosis. This consisted of important new collagen synthesis combined into the matrix. As a result of these observations, people were encouraged to further study the administration of TGF-β to incisional wounds in rats. It proved that TGF-β treatment resulted in better dermal healing, as showed by prominent collagen deposition and significantly increased wound strength.
Interpreting wound-healing results obtained from the animals brought about its limitations. For instance, an underlying skin abnormality was found on many of the mouse models with genetic alterations in the TGF-β signalling pathway. Also, the pleiotropic effects of TGF-β on many different cell types throughout stages of wound healing highlighted a challenge in designing particular methods in which the TGF-β signalling pathway can assist wound healing or reduce scarring. [30]
Current Research
Abnormalities of the TGF-Beta Pathway
Mutations or deletion of the TGF-beta 1 or TGF-beta RII gene have been associated with multiple syndromes. In mice, defects have been found in haematopoiesis, vasculogenesis and endothelial differentiation of extra embryonic tissues, while knockout mice for SMAD2 or SMAD4 genes are more likely to have spontaneous tumour development and excessive inflammatory responses. In humans, various diseases have been linked to the mutation of the TGF-beta RII gene and SMAD4 mutation is genetically responsible for familial juvenile polyposis, an autosomal dominant disease characterized by predisposition to gastrointestinal polyps and cancers.
Alterations of this signalling pathway are common in cancer. Accessory proteins such as soluble or membrane-bound regulators or co-receptors can also affect TGF-beta signalling.
read articles https://www.ncbi.nlm.nih.gov/pubmed/27563484 https://www.ncbi.nlm.nih.gov/pubmed/24393789
Further Reading
Glossary
| Apoptosis | Cell death which occurs as a normal and controlled part of an organism's growth or development |
| CCL-64 | - mink lung epithelial cell |
| Cytokine | A broad and loose category of small proteins that are important in cell signalling |
| Dimer | An oligomer consisting of two structurally similar monomers joined by bonds that can be either strong or weak, covalent or intermolecular |
| Homodimers | A protein composed of two polypeptide chains that are identical in the order, number, and kind of their amino acid residues |
| Isoform | A protein that has the same function as another protein but which is encoded by a different gene and may have small differences in its sequence |
| Ligands | A molecule that binds to a larger molecule
pleiotropic |
- ↑ <pubmed>26555259</pubmed>
- ↑ <pubmed>17896911</pubmed>
- ↑ <pubmed>24270394</pubmed>
- ↑ <pubmed>23926286</pubmed>
- ↑ <pubmed>3275534</pubmed>
- ↑ <pubmed>2785999</pubmed>
- ↑ <pubmed>2536702</pubmed>
- ↑ <pubmed>2699859</pubmed>
- ↑ <pubmed>2156499</pubmed>
- ↑ <pubmed>10640706</pubmed>
- ↑ <pubmed>15483083</pubmed>
- ↑ <pubmed>19710682</pubmed>
- ↑ <pubmed>27491038</pubmed>
- ↑ <pubmed>24393789</pubmed>
- ↑ <pubmed>24393789 </pubmed>
- ↑ <pubmed>24393789 </pubmed>
- ↑ <pubmed>16446785</pubmed>
- ↑ <pubmed>21302608</pubmed>
- ↑ 19.0 19.1 <pubmed>14534577</pubmed>
- ↑ 20.0 20.1 20.2 <pubmed>10704361</pubmed>
- ↑ <pubmed>7687212</pubmed>
- ↑ <pubmed>10767078</pubmed>
- ↑ <pubmed>11322300</pubmed>
- ↑ <pubmed>7687212</pubmed>
- ↑ <pubmed>10767078</pubmed>
- ↑ <pubmed>10340759</pubmed>
- ↑ <pubmed>11836504</pubmed>
- ↑ <pubmed>11752633</pubmed>
- ↑ <pubmed>24761336</pubmed>
- ↑ <pubmed>24761336</pubmed>