2014 Group Project 9: Difference between revisions
No edit summary |
m (Protected "2014 Group Project 9" ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (211 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
=Genital= | =Genital= | ||
This page is the second page of the [https://embryology.med.unsw.edu.au/embryology/index.php/2014_Group_Project_4 Group 4 Project] | |||
==Historic Finding== | |||
The development of the genital system has been a focus of scientific research and literature for many centuries, with anatomists publishing articles on both the male and female reproductive system. Historical findings are based mainly on dissections and observation of foetal and deceased neonates in comparison to the current emphasis on molecular research. The different research methods and findings have been published in worldwide journals over hundreds of years, with articles considering the system as a whole or specifically analysing one organ. It is through the previous work of these academics that embryology has developed to the complex, abundant study it is today. | |||
{{Historic Disclaimer}} | |||
===<font color=deeppink>Female Genital Development</font>=== | |||
<pubmed> | Female genital system development has been a subject of many historical literatures dating to the 17th century. Certain research articles aimed to focus on the female genital system as a whole, whereas others delved into specific areas such as the epithelium or specific organs such as the vagina. With the development of technology and research skills over the years, the understanding of the female genital system has improved substantially from the understanding of origin, the structure of the organs and even the nomenclature of the system. <ref name=PMID13475148><pubmed>13475148</pubmed></ref> <ref name=PMID17232984><pubmed>17232984</pubmed></ref> | ||
< | |||
<pubmed> | |||
Majority of the findings led to a proposal of a theory of that organ or the system, with some of these theories still accepted today while others disproven. The research themes and theories found in historical literature can be divided into three groups. <ref name=PMID13475148><pubmed>13475148</pubmed></ref> | |||
# Those who researched the origin of the vagina and inner genital organs, concluding the origin to be the Mullerian ducts. | |||
# Those who focused on the vaginal epithelium, indicating that it arises from part of the Wolffian ducts. | |||
# Those who emphasised the importance of the epithelium of the urogenital sinus in contributing to the vagina. | |||
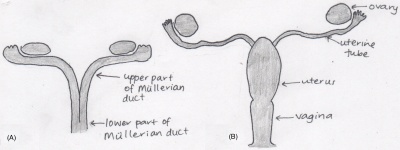
[[File:Mullerian ducts development.jpeg|400px|right|thumb|Development of Mullerian Ducts into mature female organs]] | |||
Prior to the discovery of the importance of the Mullerian ducts, the origin of the vagina was considered to be the urogenital sinus. It was not until later that century, roughly in 1864 that the Mullerian ducts and their fusion pattern and foetal development was introduced. This realisation was later supported by many academics in their published work, particularly in the early 1900s (1912, 1927, 1930, and 1939). <ref name=PMID13475148><pubmed>13475148</pubmed></ref> | |||
According to the works of the early embryologists, Thiersch, Banks, Felix, Bloomfield & Frazer, Hunter and von Lippmann, all who published within the time frame of 1868 to 1939, concluded that the Mullerian (paramesonephric) ducts, found laterally to the Wolffian ducts, are the original structures of the female reproductive organs (the fallopian tubes, uterus and vagina). Initially the foetus contains two Mullerian ducts, however by the ninth week, fusion of the lower portion of the ducts is complete, creating the fundamental structure of the uterus and the vagina, and the non-fused upper part of the ducts emerge into the fallopian tubes. At the time it was also known that it was not until the fourth and fifth month of development that the uterus becomes continuous with the vagina, with both organs developing a hollow lumen. The muscular layers of the uterus is also present by this stage. The cervix begins to form within the fifth month in between the continuous vagina and uterus. <ref name=PMID17232227><pubmed>17232227</pubmed></ref> <ref name=PMID13230915><pubmed>13230915</pubmed></ref> | |||
====The hymen==== | |||
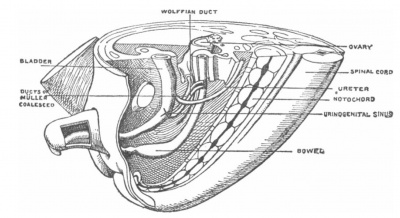
[[File:Keibel's model of urogenital organs.jpg|400px|right|thumb|Model depicting the development of the urogenital organs in an eight week old foetus, illustrated by Franz Keibel in 1896.]] | |||
= | Throughout history the categorisation of the hymen has been debated, with academics either considering this feature as part of or separate to the vagina <ref name=PMID17232227><pubmed>17232227</pubmed></ref>. Studies carried out in the 19th century concluded that a hymen was always present in a foetus at full term, even in those with genital disorders such as atresia vaginae, absence or incomplete development of the vagina, indicating that the development of the hymen is a process irrespective of vagina development <ref name=PMID17232227><pubmed>17232227</pubmed></ref>. | ||
== | The origin of the hymen has been proposed since the 1800s, with model illustrations from anatomists and embryologists such as Franz Keibel <ref name=PMID17232227><pubmed>17232227</pubmed></ref>. The origin is said to be from the junction of the lower part of the Mullerian duct and the superior portion of the urogenital sinus, an area referred to as the Mullerian eminence <ref name=PMID13475148><pubmed>13475148</pubmed></ref>. The developed hymen has been mentioned in numerous historical published articles, describing the membranous structure as a protruding vertical slit, circular or oblong, and composed of connective tissue and vaginal epithelium <ref name=PMID13475148><pubmed>13475148</pubmed></ref> <ref name=PMID17232227><pubmed>17232227</pubmed></ref> <ref name=PMID17232036><pubmed>17232036</pubmed></ref>. | ||
==== | ====The clitoris==== | ||
The clitoris, a female organ found anterior to the urethral opening at the junction of the labia minora, is the most sensitive sexual organ of a female and has been researched for many centuries. In 1896 Berry Hart examined the foetal development of the gland, describing the gland clitorides in the early foetus as bulbous and entire <ref name=PMID17232227><pubmed>17232227</pubmed></ref>. He proposed that the cells of this gland are epithelial in nature and that a horse-shoe like structure is found surrounding the gland, the prepuce. The prepuce and the clitoris is said to separate at the third month of development when the epithelial cells begin desquamating <ref name=PMID17232227><pubmed>17232227</pubmed></ref>. | |||
The nerve supplying the clitoris, dorsalis clitoridis or dorsal nerve of clitoris, was studied by Yamada in 1950. The nerve was described as containing thick sensory fibres <ref name=PMID14884176><pubmed>14884176</pubmed></ref>. Pacinian corpuscles were also found along the nerve and in the clitoris, however they were simply branched or unbranched. Furthermore, it was discovered that degeneration of the nerve fibre occurs during the foetal period as the intraepithelial nerve fibres in the 10th month foetus was poorer in comparison to the 7th month foetus. <ref name=PMID14884176><pubmed>14884176</pubmed></ref> | |||
===<font color=dodgerblue>Male Genital Development</font>=== | |||
====The Prostate==== | |||
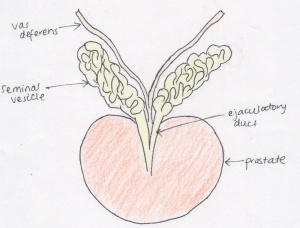
[[File:Prostate.jpeg|300px|right|thumb|The prostate with the adjoining seminal vesicles and vas deferens]] | |||
The | The mechanism behind prostate foetal development and modern understanding has been continuously reshaping since the 16th century. Throughout this period, various anatomical classifications have been proposed via dissection procedures, hormone responses and histological methods, attributing to the current understanding of prostate development. The rate of research into the structure and development of the prostate steeply increased in the 20th century, where each decade saw an improvement in the understanding of the development of the gland. <ref><pubmed>18462432</pubmed></ref> <ref><pubmed>13948442</pubmed></ref> <ref><pubmed>18942121</pubmed></ref> | ||
The | {| | ||
|- | |||
|bgcolor="lightskyblue"| '''Date''' ||bgcolor="lightskyblue"| '''Description''' | |||
|- | |||
|bgcolor="FCFCFC"| 1543 ||bgcolor="FCFCFC"| Andreas Vesalius published the first illustration of the prostate gland. | |||
|- | |||
|bgcolor="F5FAFF"| 1611 ||bgcolor="F5FAFF"| Caspar Batholin, described the prostate as a spongy double organ that is continuous with the urethra, secreting protective substances. | |||
|- | |||
|bgcolor="FCFCFC"| 1674 ||bgcolor="FCFCFC"| Gerard Blasius introduced the gland as a structure encircling the neck of the bladder. | |||
|- | |||
|bgcolor="F5FAFF"| 1678 ||bgcolor="F5FAFF"| The first illustration of the prostate with the seminal vesicles and seminal ducts attached. This diagram was published by a Dutch anatomist Reinier De Graaf. | |||
|- | |||
|bgcolor="FCFCFC"| 1792 ||bgcolor="FCFCFC"| William Cheselden, an English anatomists and surgeon, challenged the theory that the prostate was two organs, stating that it may in fact one gland. | |||
|- | |||
|bgcolor="F5FAFF"| 1800 ||bgcolor="F5FAFF"| The double gland idea was disproven with the discovery that the perceived two glands had identical morphology and thus were one gland. | |||
|- | |||
|bgcolor="FCFCFC"| 1901 ||bgcolor="FCFCFC"| Pallin thoroughly investigated the prostate gland and its origin. | |||
|- | |||
|bgcolor="F5FAFF"| 1912 ||bgcolor="F5FAFF"| Oswald S Lowsley constructed the first detailed drawing of the anatomy of the prostate by dissecting and researching on a 13-week old foetus, 30-week old foetus, and one at full-term. He proposed the concept of separating the gland into five lobes, and that the prostate originates from the urogenital sinus. | |||
|- | |||
|bgcolor="FCFCFC"| 1920 ||bgcolor="FCFCFC"| Johnson reshaped the anatomical illustration after being unable to replicate Lowsley’s results. He preserved the use of the term ‘lobe’ in describing the prostatic divisions. | |||
|- | |||
|bgcolor="F5FAFF"| 1954 ||bgcolor="F5FAFF"| Three concentric regions became the accepted categorising model of the prostate, as proposed by Franks. | |||
|- | |||
|bgcolor="FCFCFC"| 1983 ||bgcolor="FCFCFC"| McNeal organised the gland into prostatic zones, rejecting the lobe and concentric regions theory. | |||
|} | |||
====Testicular descent==== | |||
[[File:Keith1902 fig103.jpg|400px|right|thumb|Representation of the lower foetal abdominal area at 6 months, highlighting the location of the testis prior to descent and the large gubernaculum, in comparison to the testis, attaching the gonads to the scrotum in preparation for testicular descent]] | |||
Testicular | Testicular descent, which beings during the early foetal period, has been an area of research from the 1700s, when anatomists such as John Hunter began to notice the origin and development of the testicles and their location <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref> <ref name=PMID17104926><pubmed>17104926</pubmed></ref> <ref name=PMID4380018><pubmed>4380018</pubmed></ref> <ref name=PMID14172018><pubmed>14172018</pubmed></ref>. The mechanisms behind testicular descent has been debated for at least two centuries, beginning with anatomical dissections on both human and animal foetuses during the eighteenth and nineteenth centuries <ref name=PMID4380018><pubmed>4380018</pubmed></ref> <ref name=PMID4379058><pubmed>4379058</pubmed></ref>, then enhancing with endocrinological discoveries during the twentieth century <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref> <ref name=PMID17104926><pubmed>17104926</pubmed></ref>. | ||
Many theories were proposed and revoked since the discovery of testicular descent. One of the earliest debates was between John Hunter and Albretch von Haller, who concluded that the foetal testis is intra-abdominal and the processus vaginalis remains opened, contrary to the results published by Hunter <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref> <ref name=PMID14172018><pubmed>14172018</pubmed></ref>. | |||
Hunter’s description of the gubernaculum, as a vascular and fibrous foetal structure, and the covering cremaster muscle led to further research and numerous theories <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref> <ref name=PMID14172018><pubmed>14172018</pubmed></ref>. Over the years academics mainly disputed the importance of the gubernaculum and the cremaster muscle in both the first and second phase of testicular descent <ref name=PMID14172018><pubmed>14172018</pubmed></ref>. | |||
With the introduction of endocrinology and hormonal testing, the previous historical theories were tested on a cellular basis <ref name=PMID17104926><pubmed>17104926</pubmed></ref>. It has been evidently proven, mainly utilising animal populations, that androgens are important in the descent <ref name=PMID6652187><pubmed>6652187</pubmed></ref>, however it is unclear if it is important in both stages <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref>. It is currently accepted that testosterone influences the gubernaculum during the second phase in which the testes reach the scrotum, however the exact method is currently debatable. The first phase theories are under high scrutiny, with theories ranging from the development of the gubernaculum and hormones such as the Mullerian inhibiting substance <ref name=Martyn1991>Martyn P. L. Williams, John M. Huston '''The history of ideas about testicular descent'''. Pediatric Surgery International: 1991, 6(3):180-184</ref>. | |||
==== | {| | ||
|- | |||
|bgcolor="lightskyblue"| '''Date''' ||bgcolor="lightskyblue"| '''Description''' | |||
|- | |||
|bgcolor="FCFCFC"| Late 1700s ||bgcolor="FCFCFC"| Scottish surgeon and anatomist, John Hunter, first documented the gubernaculum and the location of the male foetal testicles. | |||
|- | |||
|bgcolor="F5FAFF"| 1770 ||bgcolor="F5FAFF"| Palleta emphasised that the cremaster muscle was under developed during the time of descent and thus is not an important factor in the process. | |||
|- | |||
|bgcolor="FCFCFC"| 1771 ||bgcolor="FCFCFC"| Pancera considered the cremaster muscle as the key factor in testicular descent. | |||
|- | |||
|bgcolor="F5FAFF"| 1801 ||bgcolor="F5FAFF"| Lobsetin confirmed the findings of Pancera, further highlighting the cremaster muscle. Also suggested that the second phase of testicular descent is complete by birth, influenced by respiration and the increased abdominal pressure that occurs at birth. | |||
|- | |||
|bgcolor="FCFCFC"| 1841 ||bgcolor="FCFCFC"| Curling published his work on the structure of the gubernaculum and the cremaster muscle, concluding that that muscle was important in descending the testis, and subsequent to the descent, the fibres of the muscle everted resulting in it’s new functions of elevating, supporting and compressing of the developed testis. | |||
|- | |||
|bgcolor="F5FAFF"| 1847 ||bgcolor="F5FAFF"| Weber highlighted the processus vaginalis, an embryonic pouch of peritoneum, as the main force of the migration. | |||
|- | |||
|bgcolor="FCFCFC"| 1849 ||bgcolor="FCFCFC"| The theory that descent into the scrotum occurs due to the weight of the testes and muscle associated was introduced. | |||
|- | |||
|bgcolor="F5FAFF"| 1856 ||bgcolor="F5FAFF"| Cleland performed dissections on foetal specimens finding that the foetal gubernaculum did not directly attach the testicle to the scrotum and was only present in the inguinal wall. He presented the theory that the cremaster was not the primary source of descent, second to the gubernaculum. | |||
|- | |||
|bgcolor="FCFCFC"| 1888 ||bgcolor="FCFCFC"| Lockwood proposed a completely unique theory claiming that the testes remained stationary and that it was in fact the surrounding structures that developed, resulting in the changing of the testicular location. | |||
|- | |||
|bgcolor="F5FAFF"| Early 20th Century ||bgcolor="F5FAFF"| Introduction of endocrinology and hormonal testing leading to the theory that the male androgen, controlled by the pituitary gland, influence testicular descent. | |||
|} | |||
====The prepuce==== | |||
The development of the prepuce is another genital process that takes place during the foetal phase. It was through medical cases, such as diphtheria <ref name=PMID19971144><pubmed>19971144</pubmed></ref>, presence of adhesions <ref name=PMID20764862><pubmed>20764862</pubmed></ref>, and the most common, congenital phimosis <ref name=PMID20764862><pubmed>20764862</pubmed></ref>, that this penile feature become of interest in medical literature. | |||
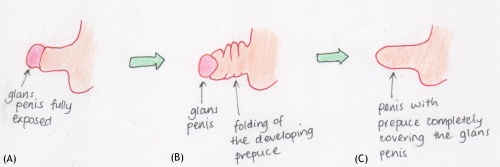
Studies from the late 19th century to early 20th century began to focus on the prepuce and discovered the stages of growth and differentiation <ref name=PMID20764862><pubmed>20764862</pubmed></ref> <ref name=PMID17104576><pubmed>17104576</pubmed></ref>. Conflicting results were obtained throughout this period, with some experiments concluding that this epithelial superficial layer was present and with foetal development, it began degeneration and separation from the enlarging glans, whereas others forwarded the theory that it was the prepuce that eventually grew over a previously exposed glans penis, a theory that was more widely supported <ref name=PMID17104576><pubmed>17104576</pubmed></ref>. | |||
The initial tests carried on the development of the prepuce were able to divide the progress into stages <ref name=PMID20764862><pubmed>20764862</pubmed></ref> <ref name=PMID17104576><pubmed>17104576</pubmed></ref>. The stages were ultimately based on the gestational age of the foetus, however this was expressed in terms of the caudal-rostral length of the foetus <ref name=PMID17104576><pubmed>17104576</pubmed></ref>. The construction of stages became accepted as various academics, without collaboration, obtained similar results organised in a similar method <ref name=PMID20764862><pubmed>20764862</pubmed></ref>. | |||
<pubmed> | This step-theory explained that each stage correlated to further growth of the prepuce until the glans was completely covered. It also described its origin and highlighted the desquamating and degenerating process of the ectodermal tissue, resulting in the prepuce not being entirely attached to the glans and allowing retraction <ref name=PMID17104576><pubmed>17104576</pubmed></ref>, a process absent in males with adherent prepuce <ref name=PMID20764862><pubmed>20764862</pubmed></ref>. | ||
[[File:Prepuce.jpeg|500px|centre|thumb|The steps of the developing prepuce in the male foetus condensed into three overall events]] | |||
{|class="wikitable mw-collapsible mw-collapsed" | |||
! '''Further information on the historical teachings of the genital system''' | |||
|- bgcolor="A3BFB1" | |||
| | |||
* [https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Human_Embryology_and_Morphology_9 The Urogenital System (1902)] | |||
* [https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Manual_of_Human_Embryology_19 The Development of the Urinogenital Organs (1912)] | |||
* [https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Text-Book_of_Embryology_15 The Development of the Urogenital System (1921)] | |||
* [https://embryology.med.unsw.edu.au/embryology/index.php/Book_-_Contributions_to_Embryology_Carnegie_Institution_No.61 The Development of the External Genitalia in the Human Embryo (1921)] | |||
|} | |||
==Abnormalities== | |||
We discuss both male and female genital abnormalities internally or externally, that may occur during fetal development. The abnormalities have been identified as disorders of sex differentiation(DSD), associated with congenital conditions in the atypical development of chromosomal, gonadal or phenotypical sex <ref><pubmed>16882788</pubmed></ref>, <ref><pubmed>25248670</pubmed></ref>. | |||
The content will cover most common abnormalities and then also the rare cases. | |||
Most genital abnormalities have a high risk in affecting fertility of both sexes. | |||
Currently there are a variety of methods applied to ensure that infertility can be treated and this will be mentioned. All abnormalities may have a specific approach to deal with each case, however management strategies are combined with the addition of psychological treatment. It is implemented to allow parents and patients to deal with any distress or issues that these disorders may cause throughout the child's life. | |||
===<font color=magenta>FEMALE</font>=== | |||
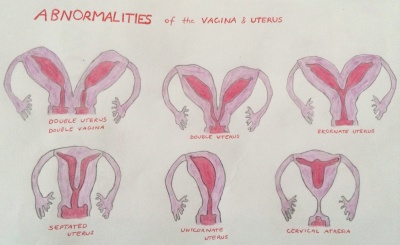
[[File:Uterus and Vagina Abnormalities.jpg|400px|right|border|thumb|Abnormalities of the Uterus and Vagina]] | |||
Abnormalities of the Uterus and vagina are cause by inadequate fusion or regression of Mullerian duct may result as the following; | |||
- | *double uterus and double vagina | ||
*double uterus | |||
*bicornate uterus | |||
*septated uterus | |||
*unicornate uterus | |||
*cervical atresia <ref>Schoenwolf, GC., Bleyl, S.B., Brauer, P.R., Francis-West, P.H., (2008). Larsen's Human Embryology, 4th ed. Chapter 15 Development of the Urogenital System. New York; Edinburgh: Churchill Livingstone, an imprint of Elsevier.</ref> | |||
====<font color=violet>Mullerian agenesis</font>==== | |||
Mullerian agenesis also known as ‘Mayer-Rokitansky-Kuster-Hauser’ syndrome, vaginal agenesis or Mullerian aplasia, is presented in the absence of the uterus or vagina or in some case even both. This is due to the unsuccessful development of the Mullerian ducts which then causes certain parts of the reproductive system to be underdeveloped. It is present in 1 of 4000-10 000 women. This condition also uses dilation therapy and following the neovaginal approach with the reconstruction of the vagina in its treatment strategies <ref><pubmed>23635766</pubmed></ref>. | |||
< | ====<font color=violet>Vaginal agenesis</font>==== | ||
Vaginal agenesis is a rare condition involving the underdevelopment of the vagina. It is commonly cause by a combination of Rokitansky (Mullerian agenesis) and androgen insensitivity syndromes <ref><pubmed>21872517</pubmed></ref>. The congenital disorder affects 1 in 5,000 females <ref>http://www.urologyhealth.org/urology/index.cfm?article=50</ref>. | |||
To ensure effectiveness in treatment, it’s advised after or during adolescence, procedures consist of vaginal dilation shown a success rate of 80% and low risks. In cases where such methods are ineffective then vaginal reconstruction is implemented as a final option for patients <ref><pubmed>17995494</pubmed></ref>. | |||
====<font color=violet>Turners Syndrome</font>==== | |||
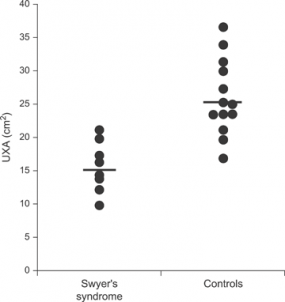
The | A chromosomal disorder occurring among women due to the absence of the whole or part of the sex chromosome (X). The condition is characterized by short stature, cardiovascular malformations, amenorrhea and estrogen insufficiency <ref><pubmed>16849410</pubmed></ref>. It is prevalent in 1 of 2000 live births among females <ref><pubmed>2037286</pubmed></ref>. Management of the syndrome depends on the extent of the condition the individual will present. Therefore treatment will vary, for short stature biosynthetic growth hormone is utalised in growth hormone. The most common cardiac malformations are bicuspid aortic valve, coarctation of the aorta and aortic stenosis that are all surgically treated. Generally patients are advised to see pediatricians, endocrinologists and many other clinicians depending on the severity of the condition, to discuss strategies to manage the syndrome <ref><pubmed>16714725</pubmed></ref>. [[File:WomenwithSwyerSyndrome.png|285px|thumb|border|right|Comparison of measures among women with Swyer Syndrome and women without it. The measures are uterine cross sectional areas (UXA).]] | ||
====<font color=violet>Swyer Syndrome</font>==== | |||
Swyer syndrome (46 XY, gonadal dysgenesis) is a type of hypogonadism disorder in which an individual from birth is phenotypically female with unambiguous genital form and normal mullerian structures. The condition is usually observed during adolescence since the gonads have no hormonal or reproductive function amenorrhea occurs and puberty is delayed <ref><pubmed>3182960</pubmed></ref>. The syndrome affects 1 in 30,000 people <ref>http://ghr.nlm.nih.gov/condition/swyer-syndrome</ref>. It has been found that 10-20% of women with this condition have a deletion of the SRY gene in the DNA-binding site. | |||
In other cases the SRY gene is normal however mutations may present in different determining factors. Managing the syndrome consists of hormone replacement therapy (including estrogen and progesterone), to ensure bone mineral density is maintained and uterine size and shape is improved <ref><pubmed>18410658</pubmed></ref>. | |||
-- | {| class="wikitable mw-collapsible mw-collapsed" | ||
! <font color=magenta>Also related include;</font> | |||
|- | |||
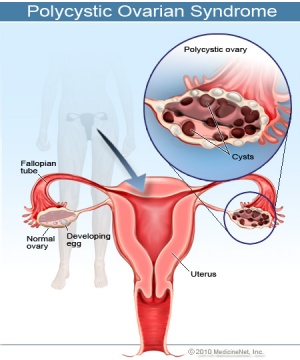
''' | | | <font color=violet>'''Polycystic Ovarian Syndrome'''</font> | ||
[[File:PolycysticOvarianSyndrome.jpg|300px|right|thumb|The Female reproductive system showing a normal ovary compared to one that is affected by Polycystic Ovarian Syndrome]] | |||
A metabolic endocrine disorder with an immense variety of phenotypes presented. The disorder shows an imbalance in female sex hormones and a resistance to insulin. | |||
Most importantly it affects the female reproductive system, with issues associated with infertility and menstrual irregularities. It is most common in 5-10% of women in their reproductive age <ref>http://www.myvmc.com/diseases/polycystic-ovarian-syndrome-pcos/</ref>. The treatments implemented depend on the clinical manifestations each patient develops. | |||
Insulin-sensitizing agents are among the treatments used these include Metformin, Rosiglitazone and Piglitazone all have shown to be effective <ref><pubmed>23435473</pubmed></ref>. | |||
|} | |||
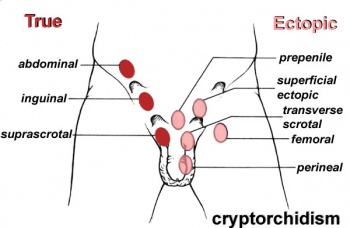
[[File:Cryptorchidism.jpg|350px|right|thumb|The sites where Cryptorchidism may occur]] | |||
===<font color=dodgerblue>MALE</font>=== | |||
====<font color=lightskyblue>Cryptorchidism</font>==== | |||
Involves the absence of both or single testis to descend into the scrotum, the testes can be ectopic, | |||
incompletely descended, absent or atrophic. It is possible that sometimes the cryptrodism may be spontaneously corrected by 3 months of age. The abnormality can occur as a result of a number of factors including maternal, genetic or environmental <ref><pubmed>24683948</pubmed></ref>. The condition is prevalent in 2-4% of infant males <ref>http://livehealthy.chron.com/cryptorchidism-infertility-1080.html</ref>. The descendence of testis occur in two stages; in the first stage insulin like hormone attaches the testis to the inguinal ring this is through gubernaculum development. Following is the inguinoscrotal stage that requires testicular androgens <ref><pubmed>18032558</pubmed></ref>. | |||
Treatment includes human chorionic gonadotropin or gonadotroping-releasing hormones, these are not the most beneficial or advised approach. | |||
Surgical repair is intended to apply the safest and least invasive methods, focusing on repositioning the undescended testicle/s to their normal position in the scrotum. Such surgeries are recommended in early life and have proved to be most effective, with 75%+ success. The therapy used to relocate the testis into the scrotum is known as ‘Orchiopexy’, others include one-stage Fowler Stephens and two-stage FS Orchidopecy. However there are concerns with long-term effects which include infertility and testicular cancer later in life as a result of the procedure <ref><pubmed>24857650</pubmed></ref>. [[File:Hypospadia classifications.jpg|280px|right|thumb|Different locations of the Meatus in Hypospadia]] | |||
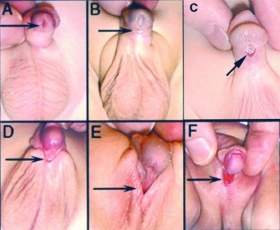
====<font color=lightskyblue>Hypospadias</font>==== | |||
--- | In males the most common congenital malformation of the external genitalia is hypospadias, it’s also the second most common developmental disorder. It occurs due to the midline fusion of the male urethra, as a result the urethral meatus is misplaced. There are several sites where this abnormality may occur: granular, penile, penoscrotal, scrotal and perineal. <ref><pubmed>16006950</pubmed></ref> Its believed that genetic factors contribute to the presence of the disorder, however endocrine and environmental factors are also of significance | ||
<ref><pubmed>24936573</pubmed></ref>. Generally occurs in 1 of 125-300 male births <ref>http://www.hypospadiasuk.co.uk/statistics-about-hypospadias/</ref>. | |||
The surgical methods currently used to treat distal hypospadias, include tabularized incised plate and meatal advancement and glansplasty intergrated repair. | |||
For proximal forms two staged procedures are employed. <ref><pubmed>25023236</pubmed></ref> | |||
[[File:Klinefelter.jpg|300px|right|thumb|Characteristics presented among men with Klinefelter Syndrome]] | |||
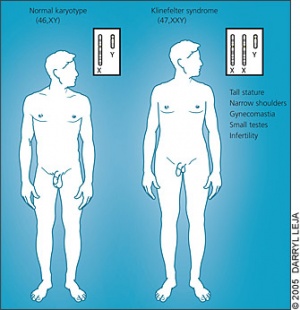
====<font color=lightskyblue>Klinefelter</font>==== | |||
Klinefelter is a genetic disorder caused by the addition of an X chromosome among males (47, XXY, XXY,XXXXY, XXYY), due to the inability of the extra chromosomes to detach throughout meiosis. It is believed to have an origin from either parent. The abnormality has a wide range of phenotypic variations, that typically include infertility, small testes, gynecomastia and hypergonadotropic hypogonadism <ref><pubmed>16342850</pubmed></ref>. The disorder occurs among 1 in 500-1,000 male births <ref>http://ghr.nlm.nih.gov/condition/klinefelter-syndrome</ref>. An early diagnosis is important in order for treatment to be commenced right away. | |||
The treatment implemented involves Testosterone replacement therapy, which assists in easing some of the features, although infertility is still an issue. | |||
The fertility options consist of IVF, where males undergo testicular sperm extraction, cryopreservation of sperm containing semen or testicular tissue during adolescence <ref><pubmed>24563893</pubmed></ref>. | |||
=== | ====<font color=lightskyblue>Peyronie's Disease</font>==== | ||
The acquired disease occurs due to fibrotic plaque formations in the tunica albuginea of the penis. This leads to sexual dysfunction, a loss in penile flexibility, shortening and penile malformations <ref><pubmed>20497306</pubmed></ref>. The penis is curved upward as a result of the plaque structure. Adult males are at risk of the condition where about 3.2-8.9% are affected among the population <ref><pubmed>3826933</pubmed></ref>. Strategies applied vary in the extent of the deformities; some procedures involve grafting in the lengthening of the penis, plaque removal and prosthesis implantation in erectile dysfunction <ref><pubmed>23435473</pubmed></ref>. | |||
{| class="wikitable mw-collapsible mw-collapsed" | |||
! <font color=dodgerblue>Also related include;</font> | |||
|- | |||
| <font color=lightskyblue>''Chordee'''</font> | |||
Chordee is an abnormality in the development of the penis, it may occur in conjunction with hypospadias. The disorder results in the curvature of the penis and erectile dysfunction | |||
<ref>http://www.webmd.com/men/guide/chordee-repair-treatment</ref>. The causes are evident among males with hypospadias, however in cases where hypospadia is absent the causes are not known and haven’t thus far been identified. The condition occurs in about 4-10% of males. There are a variety of methods used to treat the curvatur these include; skin bridge and frenular release, skin release transfer, pitation technique, dermal grafts, corporal rotation and penile disassembly and finally penile torsion. Any surgical techniques are recommended at the first year of life. Through the treatment surgeons focus on ensuring the urethral plate and neurovascular structures are preserved <ref><pubmed>21805016</pubmed></ref>. | |||
{|class="wikitable mw-collapsible mw-collapsed" | |||
! | |||
|- | |||
| | |||
|} | |} | ||
===<font color=blueviolet>BOTH</font>=== | |||
====<font color=mediumslateblue>Congenital adrenal hyperplasia</font>==== | |||
- | The condition is caused by a deficiency in 21-Hydroxylase, a genetic disorder of steroidogenesis. Occurs due to mutations in genes that encode enzymes that take part in adrenal steroid synthesis therefore there is a loss of function <ref><pubmed>18844712</pubmed></ref>. The deficiency is from mutations in CYP21A2, thus the clinical characteristics may vary. In females it results in the ambiguity of the female genitalia, fused labia majora, larger clitoris and common urogenital sinus <ref><pubmed>15964450</pubmed></ref>. Steroid 21-OHD deficiency is examined in-utero and then prenatal treatment with dexamethasone is administered. It occur is about 1 in 15,000 live births <ref>http://www.patient.co.uk/health/congenital-adrenal-hyperplasia-leaflet</ref>. This is a safe method used and decreases the risk of ambiguous genitalia in females <ref><pubmed>20392211</pubmed></ref>. Among males symptoms aren’t present at birth a side from possible penile enlargement and slight hyperpigmentation <ref><pubmed>15964450</pubmed></ref>. Generally male patients also require the administration of glucocorticoid and mineralocorticoid therapies <ref><pubmed>18446680</pubmed></ref>. | ||
[[File:Hydrocele.jpg|300px|thumb|right|A fetal ultrasound showing Hydrocele surrounding the testis]] | |||
| | |||
| | |||
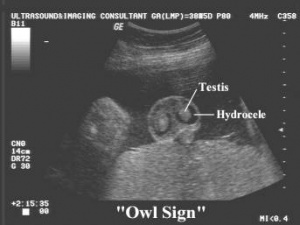
====<font color=mediumslateblue>Hydrocele</font>==== | |||
Hydrocele occurs when the space between parietal and visceral layers of tunica vaginalis accumulates an abnormal amount of serous fluid. Normally caused by an imbalance in the processes of production and reabsorption of fluid or varicocelectomy. The condition is prevalent in about 1-3% of births <ref><pubmed>http://bestpractice.bmj.com/best-practice/monograph/1104/basics/epidemiology.html</ref>. To manage the condition treatments focus on ensuring draining any excess fluid and inhibiting reaccumulation. | |||
Techniques used involve sclerotherapy and hydrocelectomy <ref><pubmed>20548330</pubmed></ref>. In females it is a very rare condition, occurs in the ‘Canal of Nuck’, a part of the inguinal canal containing a section of the processus vaginalis. A swelling is present on the labia major or inguinal ring. Techniques applied to treat the condition in females involve ligation of the processus vaginalis neck and the hydrocele is surgically resected <ref><pubmed>16416273</pubmed></ref>. | |||
[[File:Ovotestes.jpg|180px|thumb|left|Tissue in True Hermaphroditism disorder, showing the Ovotestes that comprises of primary follicles and seminiferous tubules.]] | |||
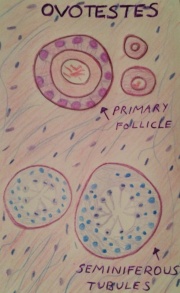
==== | ====<font color=mediumslateblue>True Hermaphroditism</font>==== | ||
True hermaphroditism also known as ovo-testicular disorder of sexual differentiation or ‘true gonadal intersex’, is a rare intersex abnormality in which an individual has both male and female genitalia. The gonads are asymmetrical with ovarian and testicular differentiation combines as ovo-testis or separately on either side <ref><pubmed>3418019</pubmed></ref>. The individual may have XX, XY or both chromosome types. The disorder occur in 1 of 1,500-2,000 births <ref>http://www.isna.org/faq/frequency</ref>. | |||
Treatment involves reconstructive surgery upon each individual case having to choose a gender, this decision has short and long-term consequences. | |||
Each case is determined differently as there are many factors to consider when choosing the gender identity. | |||
This comprises a long process providing parents with support and guidance in making their decision <ref>http://www.nlm.nih.gov/medlineplus/ency/article/001669.htm</ref>. | |||
== | {| class="wikitable mw-collapsible mw-collapsed" | ||
! <font color=blueviolet>Also related include;</font> | |||
|- | |||
| <font color=mediumslateblue>'''Kallmann syndrome'''</font> | |||
<ref | Kallmann’s syndrome is a heterogenous disease expressed during puberty due to a combination of hypogonadotropic hypogonadism and anosmia. The genetic disease is responsible for infertility and the inability to smell. Seems to have affects on 1 in 10 000 males and 1 in 50 000 females <ref><pubmed>16952059</pubmed></ref>. It occurs during embryonic development at a time in which hypothalamic neurons (gonadotropin-releasing hormones) are unable to migrate into the hypothalamus. Currently there are no available treatments for the olfactory deficit, however among males hormone replacement therapy is implemented with human chorionic gonadotropin, human menopause gonnadotropin and testosterone undecanoate <ref><pubmed>24432625</pubmed></ref>. In females treatment focuses on maintaining and inducing secondary sex characteristics <ref><pubmed>23368665</pubmed></ref>. | ||
< | |||
< | |||
== | ====<font color=mediumslateblue>Hypogonadotropic hypogonadism</font>==== | ||
== | |||
The condition results in a failure to secrete gonadotropin such as luteinizing (LH) and follicular stimulating hormones (FSH), which then reduce the gonadotropin levels <ref>http://www.ncbi.nlm.nih.gov/books/NBK1334/</ref> . This indicates possible issues with the hypothalamus or the pituitary gland. It may occur in conjunction with Kallmann’s syndrome or a decreased gonadotropin-releasing hormone (GnRH). The incidence is about 1 in 2,000 births <ref>http://www.rightdiagnosis.com/h/hypogonadotropic_hypogonadism_syndactyly/prevalence.htm</ref>. | |||
In males treatment methods depend on how the condition is presented and whether it’s associated with another abnormality. Generally the therapies may require testosterone in cases with micropenis and to generate spermatogenesis gonadotropin replacement is utalised. Hypogonadotropic Hypogonadism is rare among females, however it may be presented thus similar treatment options are available. Treatments consist of gonadotropins administration of FSH and LH, to ensure successful occyte formation <ref><pubmed>17260221</pubmed></ref>. | |||
|} | |} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Latest revision as of 23:21, 24 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Genital
This page is the second page of the Group 4 Project
Historic Finding
The development of the genital system has been a focus of scientific research and literature for many centuries, with anatomists publishing articles on both the male and female reproductive system. Historical findings are based mainly on dissections and observation of foetal and deceased neonates in comparison to the current emphasis on molecular research. The different research methods and findings have been published in worldwide journals over hundreds of years, with articles considering the system as a whole or specifically analysing one organ. It is through the previous work of these academics that embryology has developed to the complex, abundant study it is today.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Female Genital Development
Female genital system development has been a subject of many historical literatures dating to the 17th century. Certain research articles aimed to focus on the female genital system as a whole, whereas others delved into specific areas such as the epithelium or specific organs such as the vagina. With the development of technology and research skills over the years, the understanding of the female genital system has improved substantially from the understanding of origin, the structure of the organs and even the nomenclature of the system. [1] [2]
Majority of the findings led to a proposal of a theory of that organ or the system, with some of these theories still accepted today while others disproven. The research themes and theories found in historical literature can be divided into three groups. [1]
- Those who researched the origin of the vagina and inner genital organs, concluding the origin to be the Mullerian ducts.
- Those who focused on the vaginal epithelium, indicating that it arises from part of the Wolffian ducts.
- Those who emphasised the importance of the epithelium of the urogenital sinus in contributing to the vagina.
Prior to the discovery of the importance of the Mullerian ducts, the origin of the vagina was considered to be the urogenital sinus. It was not until later that century, roughly in 1864 that the Mullerian ducts and their fusion pattern and foetal development was introduced. This realisation was later supported by many academics in their published work, particularly in the early 1900s (1912, 1927, 1930, and 1939). [1]
According to the works of the early embryologists, Thiersch, Banks, Felix, Bloomfield & Frazer, Hunter and von Lippmann, all who published within the time frame of 1868 to 1939, concluded that the Mullerian (paramesonephric) ducts, found laterally to the Wolffian ducts, are the original structures of the female reproductive organs (the fallopian tubes, uterus and vagina). Initially the foetus contains two Mullerian ducts, however by the ninth week, fusion of the lower portion of the ducts is complete, creating the fundamental structure of the uterus and the vagina, and the non-fused upper part of the ducts emerge into the fallopian tubes. At the time it was also known that it was not until the fourth and fifth month of development that the uterus becomes continuous with the vagina, with both organs developing a hollow lumen. The muscular layers of the uterus is also present by this stage. The cervix begins to form within the fifth month in between the continuous vagina and uterus. [3] [4]
The hymen
Throughout history the categorisation of the hymen has been debated, with academics either considering this feature as part of or separate to the vagina [3]. Studies carried out in the 19th century concluded that a hymen was always present in a foetus at full term, even in those with genital disorders such as atresia vaginae, absence or incomplete development of the vagina, indicating that the development of the hymen is a process irrespective of vagina development [3].
The origin of the hymen has been proposed since the 1800s, with model illustrations from anatomists and embryologists such as Franz Keibel [3]. The origin is said to be from the junction of the lower part of the Mullerian duct and the superior portion of the urogenital sinus, an area referred to as the Mullerian eminence [1]. The developed hymen has been mentioned in numerous historical published articles, describing the membranous structure as a protruding vertical slit, circular or oblong, and composed of connective tissue and vaginal epithelium [1] [3] [5].
The clitoris
The clitoris, a female organ found anterior to the urethral opening at the junction of the labia minora, is the most sensitive sexual organ of a female and has been researched for many centuries. In 1896 Berry Hart examined the foetal development of the gland, describing the gland clitorides in the early foetus as bulbous and entire [3]. He proposed that the cells of this gland are epithelial in nature and that a horse-shoe like structure is found surrounding the gland, the prepuce. The prepuce and the clitoris is said to separate at the third month of development when the epithelial cells begin desquamating [3].
The nerve supplying the clitoris, dorsalis clitoridis or dorsal nerve of clitoris, was studied by Yamada in 1950. The nerve was described as containing thick sensory fibres [6]. Pacinian corpuscles were also found along the nerve and in the clitoris, however they were simply branched or unbranched. Furthermore, it was discovered that degeneration of the nerve fibre occurs during the foetal period as the intraepithelial nerve fibres in the 10th month foetus was poorer in comparison to the 7th month foetus. [6]
Male Genital Development
The Prostate
The mechanism behind prostate foetal development and modern understanding has been continuously reshaping since the 16th century. Throughout this period, various anatomical classifications have been proposed via dissection procedures, hormone responses and histological methods, attributing to the current understanding of prostate development. The rate of research into the structure and development of the prostate steeply increased in the 20th century, where each decade saw an improvement in the understanding of the development of the gland. [7] [8] [9]
| Date | Description |
| 1543 | Andreas Vesalius published the first illustration of the prostate gland. |
| 1611 | Caspar Batholin, described the prostate as a spongy double organ that is continuous with the urethra, secreting protective substances. |
| 1674 | Gerard Blasius introduced the gland as a structure encircling the neck of the bladder. |
| 1678 | The first illustration of the prostate with the seminal vesicles and seminal ducts attached. This diagram was published by a Dutch anatomist Reinier De Graaf. |
| 1792 | William Cheselden, an English anatomists and surgeon, challenged the theory that the prostate was two organs, stating that it may in fact one gland. |
| 1800 | The double gland idea was disproven with the discovery that the perceived two glands had identical morphology and thus were one gland. |
| 1901 | Pallin thoroughly investigated the prostate gland and its origin. |
| 1912 | Oswald S Lowsley constructed the first detailed drawing of the anatomy of the prostate by dissecting and researching on a 13-week old foetus, 30-week old foetus, and one at full-term. He proposed the concept of separating the gland into five lobes, and that the prostate originates from the urogenital sinus. |
| 1920 | Johnson reshaped the anatomical illustration after being unable to replicate Lowsley’s results. He preserved the use of the term ‘lobe’ in describing the prostatic divisions. |
| 1954 | Three concentric regions became the accepted categorising model of the prostate, as proposed by Franks. |
| 1983 | McNeal organised the gland into prostatic zones, rejecting the lobe and concentric regions theory. |
Testicular descent
Testicular descent, which beings during the early foetal period, has been an area of research from the 1700s, when anatomists such as John Hunter began to notice the origin and development of the testicles and their location [10] [11] [12] [13]. The mechanisms behind testicular descent has been debated for at least two centuries, beginning with anatomical dissections on both human and animal foetuses during the eighteenth and nineteenth centuries [12] [14], then enhancing with endocrinological discoveries during the twentieth century [10] [11].
Many theories were proposed and revoked since the discovery of testicular descent. One of the earliest debates was between John Hunter and Albretch von Haller, who concluded that the foetal testis is intra-abdominal and the processus vaginalis remains opened, contrary to the results published by Hunter [10] [13].
Hunter’s description of the gubernaculum, as a vascular and fibrous foetal structure, and the covering cremaster muscle led to further research and numerous theories [10] [13]. Over the years academics mainly disputed the importance of the gubernaculum and the cremaster muscle in both the first and second phase of testicular descent [13].
With the introduction of endocrinology and hormonal testing, the previous historical theories were tested on a cellular basis [11]. It has been evidently proven, mainly utilising animal populations, that androgens are important in the descent [15], however it is unclear if it is important in both stages [10]. It is currently accepted that testosterone influences the gubernaculum during the second phase in which the testes reach the scrotum, however the exact method is currently debatable. The first phase theories are under high scrutiny, with theories ranging from the development of the gubernaculum and hormones such as the Mullerian inhibiting substance [10].
| Date | Description |
| Late 1700s | Scottish surgeon and anatomist, John Hunter, first documented the gubernaculum and the location of the male foetal testicles. |
| 1770 | Palleta emphasised that the cremaster muscle was under developed during the time of descent and thus is not an important factor in the process. |
| 1771 | Pancera considered the cremaster muscle as the key factor in testicular descent. |
| 1801 | Lobsetin confirmed the findings of Pancera, further highlighting the cremaster muscle. Also suggested that the second phase of testicular descent is complete by birth, influenced by respiration and the increased abdominal pressure that occurs at birth. |
| 1841 | Curling published his work on the structure of the gubernaculum and the cremaster muscle, concluding that that muscle was important in descending the testis, and subsequent to the descent, the fibres of the muscle everted resulting in it’s new functions of elevating, supporting and compressing of the developed testis. |
| 1847 | Weber highlighted the processus vaginalis, an embryonic pouch of peritoneum, as the main force of the migration. |
| 1849 | The theory that descent into the scrotum occurs due to the weight of the testes and muscle associated was introduced. |
| 1856 | Cleland performed dissections on foetal specimens finding that the foetal gubernaculum did not directly attach the testicle to the scrotum and was only present in the inguinal wall. He presented the theory that the cremaster was not the primary source of descent, second to the gubernaculum. |
| 1888 | Lockwood proposed a completely unique theory claiming that the testes remained stationary and that it was in fact the surrounding structures that developed, resulting in the changing of the testicular location. |
| Early 20th Century | Introduction of endocrinology and hormonal testing leading to the theory that the male androgen, controlled by the pituitary gland, influence testicular descent. |
The prepuce
The development of the prepuce is another genital process that takes place during the foetal phase. It was through medical cases, such as diphtheria [16], presence of adhesions [17], and the most common, congenital phimosis [17], that this penile feature become of interest in medical literature.
Studies from the late 19th century to early 20th century began to focus on the prepuce and discovered the stages of growth and differentiation [17] [18]. Conflicting results were obtained throughout this period, with some experiments concluding that this epithelial superficial layer was present and with foetal development, it began degeneration and separation from the enlarging glans, whereas others forwarded the theory that it was the prepuce that eventually grew over a previously exposed glans penis, a theory that was more widely supported [18].
The initial tests carried on the development of the prepuce were able to divide the progress into stages [17] [18]. The stages were ultimately based on the gestational age of the foetus, however this was expressed in terms of the caudal-rostral length of the foetus [18]. The construction of stages became accepted as various academics, without collaboration, obtained similar results organised in a similar method [17].
This step-theory explained that each stage correlated to further growth of the prepuce until the glans was completely covered. It also described its origin and highlighted the desquamating and degenerating process of the ectodermal tissue, resulting in the prepuce not being entirely attached to the glans and allowing retraction [18], a process absent in males with adherent prepuce [17].
| Further information on the historical teachings of the genital system |
|---|
Abnormalities
We discuss both male and female genital abnormalities internally or externally, that may occur during fetal development. The abnormalities have been identified as disorders of sex differentiation(DSD), associated with congenital conditions in the atypical development of chromosomal, gonadal or phenotypical sex [19], [20]. The content will cover most common abnormalities and then also the rare cases. Most genital abnormalities have a high risk in affecting fertility of both sexes. Currently there are a variety of methods applied to ensure that infertility can be treated and this will be mentioned. All abnormalities may have a specific approach to deal with each case, however management strategies are combined with the addition of psychological treatment. It is implemented to allow parents and patients to deal with any distress or issues that these disorders may cause throughout the child's life.
FEMALE
Abnormalities of the Uterus and vagina are cause by inadequate fusion or regression of Mullerian duct may result as the following;
- double uterus and double vagina
- double uterus
- bicornate uterus
- septated uterus
- unicornate uterus
- cervical atresia [21]
Mullerian agenesis
Mullerian agenesis also known as ‘Mayer-Rokitansky-Kuster-Hauser’ syndrome, vaginal agenesis or Mullerian aplasia, is presented in the absence of the uterus or vagina or in some case even both. This is due to the unsuccessful development of the Mullerian ducts which then causes certain parts of the reproductive system to be underdeveloped. It is present in 1 of 4000-10 000 women. This condition also uses dilation therapy and following the neovaginal approach with the reconstruction of the vagina in its treatment strategies [22].
Vaginal agenesis
Vaginal agenesis is a rare condition involving the underdevelopment of the vagina. It is commonly cause by a combination of Rokitansky (Mullerian agenesis) and androgen insensitivity syndromes [23]. The congenital disorder affects 1 in 5,000 females [24]. To ensure effectiveness in treatment, it’s advised after or during adolescence, procedures consist of vaginal dilation shown a success rate of 80% and low risks. In cases where such methods are ineffective then vaginal reconstruction is implemented as a final option for patients [25].
Turners Syndrome
A chromosomal disorder occurring among women due to the absence of the whole or part of the sex chromosome (X). The condition is characterized by short stature, cardiovascular malformations, amenorrhea and estrogen insufficiency [26]. It is prevalent in 1 of 2000 live births among females [27]. Management of the syndrome depends on the extent of the condition the individual will present. Therefore treatment will vary, for short stature biosynthetic growth hormone is utalised in growth hormone. The most common cardiac malformations are bicuspid aortic valve, coarctation of the aorta and aortic stenosis that are all surgically treated. Generally patients are advised to see pediatricians, endocrinologists and many other clinicians depending on the severity of the condition, to discuss strategies to manage the syndrome [28].
Swyer Syndrome
Swyer syndrome (46 XY, gonadal dysgenesis) is a type of hypogonadism disorder in which an individual from birth is phenotypically female with unambiguous genital form and normal mullerian structures. The condition is usually observed during adolescence since the gonads have no hormonal or reproductive function amenorrhea occurs and puberty is delayed [29]. The syndrome affects 1 in 30,000 people [30]. It has been found that 10-20% of women with this condition have a deletion of the SRY gene in the DNA-binding site. In other cases the SRY gene is normal however mutations may present in different determining factors. Managing the syndrome consists of hormone replacement therapy (including estrogen and progesterone), to ensure bone mineral density is maintained and uterine size and shape is improved [31].
| Also related include; |
|---|
| Polycystic Ovarian Syndrome
A metabolic endocrine disorder with an immense variety of phenotypes presented. The disorder shows an imbalance in female sex hormones and a resistance to insulin. Most importantly it affects the female reproductive system, with issues associated with infertility and menstrual irregularities. It is most common in 5-10% of women in their reproductive age [32]. The treatments implemented depend on the clinical manifestations each patient develops. Insulin-sensitizing agents are among the treatments used these include Metformin, Rosiglitazone and Piglitazone all have shown to be effective [33]. |
MALE
Cryptorchidism
Involves the absence of both or single testis to descend into the scrotum, the testes can be ectopic, incompletely descended, absent or atrophic. It is possible that sometimes the cryptrodism may be spontaneously corrected by 3 months of age. The abnormality can occur as a result of a number of factors including maternal, genetic or environmental [34]. The condition is prevalent in 2-4% of infant males [35]. The descendence of testis occur in two stages; in the first stage insulin like hormone attaches the testis to the inguinal ring this is through gubernaculum development. Following is the inguinoscrotal stage that requires testicular androgens [36].
Treatment includes human chorionic gonadotropin or gonadotroping-releasing hormones, these are not the most beneficial or advised approach.
Surgical repair is intended to apply the safest and least invasive methods, focusing on repositioning the undescended testicle/s to their normal position in the scrotum. Such surgeries are recommended in early life and have proved to be most effective, with 75%+ success. The therapy used to relocate the testis into the scrotum is known as ‘Orchiopexy’, others include one-stage Fowler Stephens and two-stage FS Orchidopecy. However there are concerns with long-term effects which include infertility and testicular cancer later in life as a result of the procedure [37].
Hypospadias
In males the most common congenital malformation of the external genitalia is hypospadias, it’s also the second most common developmental disorder. It occurs due to the midline fusion of the male urethra, as a result the urethral meatus is misplaced. There are several sites where this abnormality may occur: granular, penile, penoscrotal, scrotal and perineal. [38] Its believed that genetic factors contribute to the presence of the disorder, however endocrine and environmental factors are also of significance [39]. Generally occurs in 1 of 125-300 male births [40]. The surgical methods currently used to treat distal hypospadias, include tabularized incised plate and meatal advancement and glansplasty intergrated repair. For proximal forms two staged procedures are employed. [41]
Klinefelter
Klinefelter is a genetic disorder caused by the addition of an X chromosome among males (47, XXY, XXY,XXXXY, XXYY), due to the inability of the extra chromosomes to detach throughout meiosis. It is believed to have an origin from either parent. The abnormality has a wide range of phenotypic variations, that typically include infertility, small testes, gynecomastia and hypergonadotropic hypogonadism [42]. The disorder occurs among 1 in 500-1,000 male births [43]. An early diagnosis is important in order for treatment to be commenced right away. The treatment implemented involves Testosterone replacement therapy, which assists in easing some of the features, although infertility is still an issue. The fertility options consist of IVF, where males undergo testicular sperm extraction, cryopreservation of sperm containing semen or testicular tissue during adolescence [44].
Peyronie's Disease
The acquired disease occurs due to fibrotic plaque formations in the tunica albuginea of the penis. This leads to sexual dysfunction, a loss in penile flexibility, shortening and penile malformations [45]. The penis is curved upward as a result of the plaque structure. Adult males are at risk of the condition where about 3.2-8.9% are affected among the population [46]. Strategies applied vary in the extent of the deformities; some procedures involve grafting in the lengthening of the penis, plaque removal and prosthesis implantation in erectile dysfunction [47].
| Also related include; |
|---|
| Chordee'
Chordee is an abnormality in the development of the penis, it may occur in conjunction with hypospadias. The disorder results in the curvature of the penis and erectile dysfunction [48]. The causes are evident among males with hypospadias, however in cases where hypospadia is absent the causes are not known and haven’t thus far been identified. The condition occurs in about 4-10% of males. There are a variety of methods used to treat the curvatur these include; skin bridge and frenular release, skin release transfer, pitation technique, dermal grafts, corporal rotation and penile disassembly and finally penile torsion. Any surgical techniques are recommended at the first year of life. Through the treatment surgeons focus on ensuring the urethral plate and neurovascular structures are preserved [49]. |
BOTH
Congenital adrenal hyperplasia
The condition is caused by a deficiency in 21-Hydroxylase, a genetic disorder of steroidogenesis. Occurs due to mutations in genes that encode enzymes that take part in adrenal steroid synthesis therefore there is a loss of function [50]. The deficiency is from mutations in CYP21A2, thus the clinical characteristics may vary. In females it results in the ambiguity of the female genitalia, fused labia majora, larger clitoris and common urogenital sinus [51]. Steroid 21-OHD deficiency is examined in-utero and then prenatal treatment with dexamethasone is administered. It occur is about 1 in 15,000 live births [52]. This is a safe method used and decreases the risk of ambiguous genitalia in females [53]. Among males symptoms aren’t present at birth a side from possible penile enlargement and slight hyperpigmentation [54]. Generally male patients also require the administration of glucocorticoid and mineralocorticoid therapies [55].
Hydrocele
Hydrocele occurs when the space between parietal and visceral layers of tunica vaginalis accumulates an abnormal amount of serous fluid. Normally caused by an imbalance in the processes of production and reabsorption of fluid or varicocelectomy. The condition is prevalent in about 1-3% of births [56]. To manage the condition treatments focus on ensuring draining any excess fluid and inhibiting reaccumulation. Techniques used involve sclerotherapy and hydrocelectomy [57]. In females it is a very rare condition, occurs in the ‘Canal of Nuck’, a part of the inguinal canal containing a section of the processus vaginalis. A swelling is present on the labia major or inguinal ring. Techniques applied to treat the condition in females involve ligation of the processus vaginalis neck and the hydrocele is surgically resected [58].
True Hermaphroditism
True hermaphroditism also known as ovo-testicular disorder of sexual differentiation or ‘true gonadal intersex’, is a rare intersex abnormality in which an individual has both male and female genitalia. The gonads are asymmetrical with ovarian and testicular differentiation combines as ovo-testis or separately on either side [59]. The individual may have XX, XY or both chromosome types. The disorder occur in 1 of 1,500-2,000 births [60]. Treatment involves reconstructive surgery upon each individual case having to choose a gender, this decision has short and long-term consequences. Each case is determined differently as there are many factors to consider when choosing the gender identity. This comprises a long process providing parents with support and guidance in making their decision [61].
| Also related include; |
|---|
| Kallmann syndrome
Kallmann’s syndrome is a heterogenous disease expressed during puberty due to a combination of hypogonadotropic hypogonadism and anosmia. The genetic disease is responsible for infertility and the inability to smell. Seems to have affects on 1 in 10 000 males and 1 in 50 000 females [62]. It occurs during embryonic development at a time in which hypothalamic neurons (gonadotropin-releasing hormones) are unable to migrate into the hypothalamus. Currently there are no available treatments for the olfactory deficit, however among males hormone replacement therapy is implemented with human chorionic gonadotropin, human menopause gonnadotropin and testosterone undecanoate [63]. In females treatment focuses on maintaining and inducing secondary sex characteristics [64]. Hypogonadotropic hypogonadismThe condition results in a failure to secrete gonadotropin such as luteinizing (LH) and follicular stimulating hormones (FSH), which then reduce the gonadotropin levels [65] . This indicates possible issues with the hypothalamus or the pituitary gland. It may occur in conjunction with Kallmann’s syndrome or a decreased gonadotropin-releasing hormone (GnRH). The incidence is about 1 in 2,000 births [66]. In males treatment methods depend on how the condition is presented and whether it’s associated with another abnormality. Generally the therapies may require testosterone in cases with micropenis and to generate spermatogenesis gonadotropin replacement is utalised. Hypogonadotropic Hypogonadism is rare among females, however it may be presented thus similar treatment options are available. Treatments consist of gonadotropins administration of FSH and LH, to ensure successful occyte formation [67]. |
References
- ↑ 1.0 1.1 1.2 1.3 1.4 <pubmed>13475148</pubmed>
- ↑ <pubmed>17232984</pubmed>
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 <pubmed>17232227</pubmed>
- ↑ <pubmed>13230915</pubmed>
- ↑ <pubmed>17232036</pubmed>
- ↑ 6.0 6.1 <pubmed>14884176</pubmed>
- ↑ <pubmed>18462432</pubmed>
- ↑ <pubmed>13948442</pubmed>
- ↑ <pubmed>18942121</pubmed>
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Martyn P. L. Williams, John M. Huston The history of ideas about testicular descent. Pediatric Surgery International: 1991, 6(3):180-184
- ↑ 11.0 11.1 11.2 <pubmed>17104926</pubmed>
- ↑ 12.0 12.1 <pubmed>4380018</pubmed>
- ↑ 13.0 13.1 13.2 13.3 <pubmed>14172018</pubmed>
- ↑ <pubmed>4379058</pubmed>
- ↑ <pubmed>6652187</pubmed>
- ↑ <pubmed>19971144</pubmed>
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 <pubmed>20764862</pubmed>
- ↑ 18.0 18.1 18.2 18.3 18.4 <pubmed>17104576</pubmed>
- ↑ <pubmed>16882788</pubmed>
- ↑ <pubmed>25248670</pubmed>
- ↑ Schoenwolf, GC., Bleyl, S.B., Brauer, P.R., Francis-West, P.H., (2008). Larsen's Human Embryology, 4th ed. Chapter 15 Development of the Urogenital System. New York; Edinburgh: Churchill Livingstone, an imprint of Elsevier.
- ↑ <pubmed>23635766</pubmed>
- ↑ <pubmed>21872517</pubmed>
- ↑ http://www.urologyhealth.org/urology/index.cfm?article=50
- ↑ <pubmed>17995494</pubmed>
- ↑ <pubmed>16849410</pubmed>
- ↑ <pubmed>2037286</pubmed>
- ↑ <pubmed>16714725</pubmed>
- ↑ <pubmed>3182960</pubmed>
- ↑ http://ghr.nlm.nih.gov/condition/swyer-syndrome
- ↑ <pubmed>18410658</pubmed>
- ↑ http://www.myvmc.com/diseases/polycystic-ovarian-syndrome-pcos/
- ↑ <pubmed>23435473</pubmed>
- ↑ <pubmed>24683948</pubmed>
- ↑ http://livehealthy.chron.com/cryptorchidism-infertility-1080.html
- ↑ <pubmed>18032558</pubmed>
- ↑ <pubmed>24857650</pubmed>
- ↑ <pubmed>16006950</pubmed>
- ↑ <pubmed>24936573</pubmed>
- ↑ http://www.hypospadiasuk.co.uk/statistics-about-hypospadias/
- ↑ <pubmed>25023236</pubmed>
- ↑ <pubmed>16342850</pubmed>
- ↑ http://ghr.nlm.nih.gov/condition/klinefelter-syndrome
- ↑ <pubmed>24563893</pubmed>
- ↑ <pubmed>20497306</pubmed>
- ↑ <pubmed>3826933</pubmed>
- ↑ <pubmed>23435473</pubmed>
- ↑ http://www.webmd.com/men/guide/chordee-repair-treatment
- ↑ <pubmed>21805016</pubmed>
- ↑ <pubmed>18844712</pubmed>
- ↑ <pubmed>15964450</pubmed>
- ↑ http://www.patient.co.uk/health/congenital-adrenal-hyperplasia-leaflet
- ↑ <pubmed>20392211</pubmed>
- ↑ <pubmed>15964450</pubmed>
- ↑ <pubmed>18446680</pubmed>
- ↑ <pubmed>http://bestpractice.bmj.com/best-practice/monograph/1104/basics/epidemiology.html
- ↑ <pubmed>20548330</pubmed>
- ↑ <pubmed>16416273</pubmed>
- ↑ <pubmed>3418019</pubmed>
- ↑ http://www.isna.org/faq/frequency
- ↑ http://www.nlm.nih.gov/medlineplus/ency/article/001669.htm
- ↑ <pubmed>16952059</pubmed>
- ↑ <pubmed>24432625</pubmed>
- ↑ <pubmed>23368665</pubmed>
- ↑ http://www.ncbi.nlm.nih.gov/books/NBK1334/
- ↑ http://www.rightdiagnosis.com/h/hypogonadotropic_hypogonadism_syndactyly/prevalence.htm
- ↑ <pubmed>17260221</pubmed>