2014 Group Project 6: Difference between revisions
No edit summary |
|||
| Line 251: | Line 251: | ||
'''Fetal Stage of pancreas Development - from week 8 of gestation onwards:''' | '''Fetal Stage of pancreas Development - from week 8 of gestation onwards:''' | ||
* Week 7 to 20 – Maternal insulin increases exponentially as fetus grows. | |||
* Week 10 – The first cells to differentiate are glucagon (alpha) cells followed by somatostatin (delta), and insulin (beta) cells. Fetus begins to secrete insulin | |||
* Week 15 – Levels of glucagon become noticeable in fetal plasma | |||
Hormones: | Hormones: | ||
Revision as of 22:29, 8 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
The Endocrine System
--Mark Hill (talk) 15:18, 26 August 2014 (EST) OK you have 2 sub-headings after 2 weeks work, and I even had to add the title for your project. How about some content, references, sources for each section. See Lab 3 Assessment.
--Mark Hill (talk) 11:41, 6 September 2014 (EST) Still just references and some of these are not relevant. How about trying to get some timeline info in your project and some fetal endocrine images.
Introduction
--Z3414648 (talk) 15:23, 26 August 2014 (EST)
Pineal gland
The pineal gland is part of the epithalamus, located in the diencephalon. Like the other neurosecretory glands, it is formed by the neuroectoderm of the neural plate. It’s primary function is to regulate circadian cycles postnatally through its secretion of melatonin, however it also has a role in reproductive development. Recent findings have suggested that the pineal gland does play an important role during foetal development through the production of it's primary hormone, melatonin. Pineal and placental melatonin have a regulatory effect on maintaining homeostasis in the uterus as well as fetal maturation and reproductive development. The suprachiasmatic nuclei in the hypothalamus acts as the central pacemaker for melatonin production. As the two circuits are already interconnected in the mammilian fetus, intrinsic circadian rhythms are already established prior to birth. However it is not until after birth that the infant produces significant levels of melatonin to sustain cyclic rhythms associated with day/night and light exposure.
Table 1. Summarises the hormones released by the human pineal gland and their role in embryonic and foetal development
| Hormone | Produced by | Function |
|---|---|---|
| Melatonin | pinealocytes | Contribute to development of gametes, maintaining uterine homeostasis |
Timeline:
- The pineal gland primarily begins its development after the diencephalon is differentiated from the prosenecephalon in week 5 of gestation.
- On the caudal roof of the hollow diverticulum, cells begin proliferation and differentiate into pinealoctyes to form the solid epiphysis
- By the second trimester, small amounts of melatonin production by the fetus pineal gland has begun.
Abornmalities
- Pineal tumors
- Pineal hypoplasia
Recent Findings
References
<pubmed>9852259</pubmed>
M. Hulsemann, 1971, Development of the Innervation in the Human Pineal Organ, Light and Electron Microscopic Investigations, 115: 396-415
<pubmed>16021838</pubmed>
Hypothalamus
The hypothalamus is part of the diencephalon and plays an important role in the maintenance of homeostasis and the driving of motivated behaviours. Distinct nuclei in the hypothalamus secrete specific hormones that function to regulate thirst, hunger, thermoregulation, circadian rhythms, reproduction and defensive behaviour.
Table 1. Summarises the hormones released by the human hypothalamus and their role in embryonic and foetal development
| Hormone | Abbreviation | Produced by | Function |
|---|---|---|---|
| Vasopressin | ADH | Paraventricular and Magnocellullar neurosecretory neurons | Example |
| Oxytocin | Example | Example | |
| Example | Example | Example | Example |
| Example | Example | Example | Example |
| Example | Example | Example | Example |
| Example | Example | Example | Example |
Figure 1. illustrates the location of nuclei in the hypothalamus
[image]
The sexually dimorphic nucleus (SDN, intermediate nucleus) is twice as large in young male adults as in young females. Immediately after birth, only 20% of the SDN cell number is present. During the post-natal period up till two to four years of age cell numbers continue to increase rapidly and equally in both sexes. Past this age, cell numbers start to decrease in girls and this is the point of physiological differentiation in sex.
Neurosecretory cells of the supraoptic (SON) and paraventricular nucleus (PVN) project to the neurohypophysis, where they release vasopressin and oxytocin into the blood circulation. These hormones play an important role in foetal development up till and including the birth process. Foetal oxytocin may initiate or accelerate the course of labor whereas foetal vasopressin plays a role in the adaptation to stress caused by the birth process, by redistribution of the foetal blood flow.
Abnormalities
Complications in development of these nuclei regions lead to disorders characteristic to those regions affected.
Timeline
- Week 5: The development of the CNS has reached the five vesicle stage, where the prosencephalon divides into the diencephalon which is more caudal and in which the hypothalamus is formed, and the telencephalon located more rostrally.
- Week 6: During pre-foetal phase when the head folds begin to take shape, a thickening called the hypophyseal placode forms at the midline of the rostral ectoderm, adjacent to the area where the hypothalamus will form on the neural fold.
- Week 9: The hypophyseal placode changes shape as it is pulled upwards, towards the overlying neuroepithelium, to form Rathke's pouch.
- Week 18: By mid-gestation, this simple epithelial invagination separates from the underlying ectoderm to form the definitive Rathke's pouch. Subsequent cell proliferation and differentiation of the intermediate zone allows for the formation of the primordial hypothalamus. The posterior lobe and the pituitary stalk connects the gland to the hypothalamus.
- Week 28: Sexual differentiation of hypothalamus is complete
http://www.nature.com/nature/journal/v480/n7375/box/480044a_BX1.html
Recent Findings
A. Peruffoa, M. Giacomellob, S. Montellia, M. Panina, B. Cozzia, 2013, Expression profile of the pore-forming subunits α1A and α1D in the foetal bovine hypothalamus: A mammal with a long gestation. Neuroscience Letters. Vol. 556, pp 124–128http://www.sciencedirect.com/science/article/pii/S0304394013009300
E. Muraa, M. Sumana, S. Montellia, A. Peruffoa, B. Cozzia, V. Farinab, 2013, Characterization of an established endothelial cell line from primary cultures of fetal sheep hypothalamus. Research in Veterinary Science. Vol. 94:3, pp 388–393http://www.sciencedirect.com/science/article/pii/S0034528812003256
References
Rizzoti, K. & Lovell-Badge, R. Development of the pituitary and hypothalamus, Regenerative Medicine: Organ recital in a dish. Nature Vol. 480, pp 44–46http://www.nature.com/nature/journal/v480/n7375/box/480044a_BX1.html
<pubmed>11954031</pubmed>
<pubmed>7643957</pubmed>
Y. Koutcherov, J.K, Mai, G. Paxinos Hypothalamus of the human fetus, Journal of Chemical Neuroanatomy, 26:4, pp 253–270
Pituitary gland
<pubmed>10.1016/j.acthis.2014.04.003</pubmed>
<pubmed>10.1371/journal.pone.0004815</pubmed>
<pubmed>10.1371/journal.pone.0004513</pubmed>
Timeline
Pre-fetal stage of pituitary gland development:
- Formation of Rathke's Pouch by week 4-5 of gestation
- At the point of the oropharynx in the primitive gut there is an invagination of the ectoderm and this is the origin of the anterior pituitary lobe
- Eventually Rathke's pouch is pinched off and separates from the oral cavity. All the Rathke cells need to migrate down to sit in the sphenoid bone of the skull. Any cells left behind can becomes tumours.
- The posterior pituitary is formed from the downward outgrowth of the third ventricle forming a median eminence
- Together with cells from the mammillary body, a neural stalk forms giving the neurohypophysis [1]
- ↑ Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Oxford: BIOS Scientific Publishers; 2001. Chapter 7, The pituitary gland
Thyroid
<pubmed>10.1371/journal.pone.0080801</pubmed>
<pubmed>10.1530/JOE-14-0025</pubmed>
<pubmed>10.1371/journal.pone.0016752</pubmed>
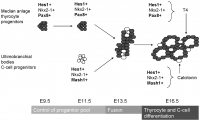
Thyroid Development
The functional unit of the thyroid gland is the follicle hence it is necessary to understand how the follicle develops in order to understand the important function of this gland. There are progenitor cells in the anterior endoderm that are specified thyroid progenitor cells and contribute to thyroid organogenesis. The proliferation of these cells results in the formation of a placode along the midline of the pharyngeal floor, just below the future tongue. The left and right lobes of the thyroid gland start off as single structures: buds of endoderm surrounded by mesoderm.
The mammalian thyroid gland is unique in the sense that there is a second endocrine cell called the parafollicular C cell. The progenitor cell for this enters the gland at the stage where there is a fusion between the thyroid progenitor proper and the ultimobranchial bodies. The ultimobranchial bodies arise bilaterally in the most inferior pharyngeal arches and are important in the final organ.
Eventually the midline primordium stretches laterally to reach the ultimobranchial bodies and there is a bilobation event resulting in the recognisable bilobed thyroid gland. [1]
Timeline
How far has the thyroid gland developed by week 8 of gestation?
- Formation of medial anlage (foregut endoderm origin) and 2 lateral anlages (neuroectodermal origin and derivatives of the 4th pharyngeal pouch)
- Budding of ventral pharynx to form thryoid primoridum
- Fusion event of the median anlage with the lateral angales (ultimobranchial bodies) followed by migration of median anlage to it's final pretracheal location
- Sonic hedgehog plays a role in directing correct lobulation of the median anlage into two lobes connected by an isthmus
Fetal Stage of Thyroid Development - from week 8 of gestation onwards:
- Terminal differentiation of thyroid gland occurs from week 7 to 8 of embryo gestation and involves the onset of the gland function
- Pre-colloid stage is week 7-9 and is where the thyroid gland contains strands of compact unpolarised Thyroid Follicular Cell (TFC) precursors
- The beginning colloid stage is week 10-11 and involves the polarisation of the TFC precursors.
- This gives the first appearance of small thyroid follicles (the eventual functional unit of the gland)
- Progressive follicular growth occurs in week 12
- At this point the fetal thyroid gland gains the ability to accumulate iodine and begin thyroid hormone synthesis [2]
Parathyroid gland
The parathyroid gland is an important endocrine organ that plays an essential role in regulating extracellular calcium homeostasis and hence serves many physiological processes that involve muscle contraction, blood coagulation, and synaptic activity. They detect changes fluctuations of calcium levels in blood which is detected by the calcium-sensing receptor (CasR). This process then stimulates the secretion of parathyroid hormone (PTH) which releases calcium from internal stores such as bone in order to counterbalance any extremities.
Timeline
How far has the parathyroid gland developed by week 8 of gestation?
- Week 5 - the Parathyroid glands arise from the endodermal third and fourth pharyngeal pouches in cranial portions.
- Cranial third pharyngeal pouches form inferior parathyroids and cranial fourth pharyngeal pouches forms superior parathyroids.
- Pouches are bilateral and hence form four parathyroids
- Parathyroid gland development cannot occur without the transcription factor encoded by Gcm-2.
- Week 6 - diverticulum extends from the pouch which is hollow at first and then solidifies with dorsal cell proliferation
Fetal Stage of parathyroid Development - from week 8 of gestation onwards:
- Active transport regulates high fetal calcium concentrations levels (11-12 mg/dl) from maternal serum via an ATP-dependent calcium pump situated across the syncytiotrophoblast.
- The middle portion of the parathormone related peptide (PTHrP) is secreted via the fetal parathyroid and activates the placental calcium pump.
- Sections 1-34 of the Parathormone (PTH) or PTHrP stimulate PTH/PTHrP receptors causing a fetal skeletal calcium flux. This subsequently leads to the excretion of calcium via the fetal renal 1, 25 (OH) 2 D production also occurs which serves to increase the calcium transport occurring in carrying mothers. Calcium reabsorption from amniotic fluid also takes place through this action.
<pubmed>22808183</pubmed>
<pubmed>22649358</pubmed>
<pubmed>21881196</pubmed>
<pubmed>21904825</pubmed>
Thymus
The thymus gland is an organ that belongs to two systems of the human body which are the endocrine and immune system. It consists of two distinct but identical lobes which are both encased by a tough and fibrous capsule. Within each lobe are two layers which is the cortex that is superficial to the deep medullary layer in the tissue. Epithelial tissues and lymphatic tissues including macrophages make up majority of the thymus. In terms of its role in the endocrine system, it is responsible for the development of hormone called thymosin. This hormone is needed to tranform white blood cells (lymphocytes) that pass through the thymus gland into T cells, thereby forming the link to aid the immune system. This important gland is located in the upper anterior chest straight behind the sternum and in between the lungs. Other associated hormones of the thymus gland include thymopoietin hormones, thymic humoral factors , thymostimulin and Factor thymic serum.
Timeline
How far has the thymus gland developed by week 8 of gestation?
- It originates primarily from the third pharyngeal pouch.
- The primordia is initially divided into the thymic and parathyroid domains which are both encased in a neural crest-derived mesenchymal capsule.
- Week 7 - mid week 8, the thymic part of the primordium migrates ventrally and attach at the pericardium
Fetal Stage of Thymus Development - from week 8 of gestation onwards:
- Week 8 - the thymic primordium contains undifferentiated epithelial cells
- Week 8-9, intrathymic cell types such as mesenchymal, vascular and lymphoid cells begin to develop
- Weeks 8-16 - Medullary development occurs from week 8 and distinct cortical and medullary compartments are formed by week 16
- Weeks 14- 16, mature lymphocytes begin to migrate from the thymus to seed the peripheral immune system
<pubmed>21733645</pubmed>
<pubmed>20836742</pubmed>
<pubmed>21263742</pubmed>
<pubmed>512270</pubmed>
Pancreas
Timeline
How far has the pancreas developed by week 8 of gestation?
- Week 4 – Pancreatic development begin at the septum transversum as dorsal and ventral endodermic buds forms. Dorsal and ventral mesentery are formed by splanchnic mesoderm.
- Dorsal buds normally develop first and form majority of the pancreas whereas the ventral bud only forms a portion of the head and uncinated process of the pancreas.
- Week 6-8 – These buds migrate and fuse from duodenum growth and rotation. In order to make space for the pancreas, the duodenum rotates in to C-shaped conformation. The ventral bud also situates itself dorsally behind the dorsal bud.
- Pancreatic bud endoderm in particularly the ventral bud duct and distal part of dorsal bud differentiates into islet cell clusters which form acini and exocrine ducts needed for exocrine function. At the periphery of these exocrine clusters form the pancreatic islets which serve endocrine function.
Fetal Stage of pancreas Development - from week 8 of gestation onwards:
- Week 7 to 20 – Maternal insulin increases exponentially as fetus grows.
- Week 10 – The first cells to differentiate are glucagon (alpha) cells followed by somatostatin (delta), and insulin (beta) cells. Fetus begins to secrete insulin
- Week 15 – Levels of glucagon become noticeable in fetal plasma
Hormones:
| Hormone | Produced by | Function |
|---|---|---|
| Glucagon | Alpha cells of the islets of Langerhans | Elevates blood sugar levels when blood sugar levels are low. |
| Insulin | Beta cells of the islets of Langerhans | Reduces blood sugar levels when blood sugar levels are too high. It also converts glucose into glycogen to store in the liver for future source of energy. |
| Somatostatin | Delta cells of pancreas | Inhibits the secretion of other pancreatic hormones such as insulin and glucagon. |
| Pancreatic Polypeptide | Pancreatic polypeptide cells | Prevents secretion of somatostatin from the pancreas. |
<pubmed>22761699</pubmed> <pubmed>22893718</pubmed> <pubmed>24496309</pubmed> <pubmed>24595965</pubmed> <pubmed>22968764</pubmed>
Adrenal gland
The characteristic zonation of the adult adrenal gland is absent in the fetal gland which is instead arranged in an inner fetal zone and an outer definitive (adult) zone. The inner zone atrophies following birth and contains steroid-secreting cell characteristics while the adult zone contains cells that resemble those present in the adult zona glomerulosa. The adrenal medulla is not a distinct, recogniseable zone during gestation, except for scattered chromaffin cells present throughout the cortex in small clusters.
'Development overview:
- Week 6: Adrenal gland is present at the cranial side of the mesonephric kidney as a condensed mass of coelomic epithelium, appearing as large cells like those of older fetus fetal zones.
- Week 8: The definitive or adult zone is formed by a second round of epithelial cell proliferation, where a cap is formed by a narrow rim of cells over the fetal zone. Around the central part of the gland can be seen clumps of medullary cells and neural elements infiltrate it through the vascular pole.
- Weeks 10-20: Rapid growth of the adrenal gland by increased size of the fetal and definitive zones from about 100mg in week 10 to 2g at week 20. Appearance of vasculature and sinusoidal plexuses and increase of medullary cells.
- Weeks 20-30: Gland size doubles with adult-type zonation appearing in the definitive zone around week 30. The zona glomerulosa is delineated by connective tissue stroma and cells appear arranged in a columnar fashion in the developing zona fasciculata.
- Week 30-term: Weight of fetal adrenal gland doubles and 80% of the gland’s volume is made up of the fetal zone.
The zona reticularis develops post-natally in year 3 of development, unlike the two other cortical zones.
Table of hormones produced by the adrenal gland:
| Hormone | Type | Produced in | Function |
|---|---|---|---|
| Aldosterone | Mineralocorticoid | Zona glomerulosa | Works on the kidneys, sweat and salivary glands to maintain normal extracellular concentrations of Na+ and K+ and so extracellular volume |
| Cortisol | Glucocorticoid | Zona fasciculata | Restoration of homeostasis following stress; suppresses immune system, increases blood sugar by gluconeogenesis, helps metabolise protein, carbohydrates and fat, activates the CNS. In the foetus/neonate, causes organ development and maturation e.g. lungs. There are high levels of cortisol at childbirth |
| Adrenaline and noradrenaline | Catecholamine | Adrenal medulla | Increase; heart rate contractility, vasoconstriction, ventilation, lipolysis, glycogenolysis and decrease gut motility. Work with the sympathetic nervous system to regulate 'flight or fight' response. |
<pubmed>7011178</pubmed>
<pubmed>24116052</pubmed>
<pubmed>PMC3365797</pubmed>
<pubmed>15635500</pubmed>
Gonad development
Ovary
Development overview:
- Weeks 8 and 9:
- Week 8- Change to the gonad’s internal structure; it can be identified as an ovary now. Cortical differentiation occurs from the cranial pole to the lower pole. The dense central core spans the mesovarian into the mesonephric organ in a caudal direction, resembling the ‘rete blastema’ which differentiates.
- Inner ovary is composed of the indifferent gonad’s disintegrating blastema, surrounded by a thick blastemal layer, giving the ovary a non-uniform crenated surface
- Ovarian tissue has a cortical region and a central medullary region with an irregular demarcation. The cortex encloses primordial germ cells (PGCs) between somatic cells and medulla has a reticulum of somatic cells
- Weeks 10 to 12:
- Growth and lobulation of the cortex. Supporting cells grow peripherally and segment the cortex into irregular globules containing rapidly multiplying germ cells and light and dark somatic supporting cells, interspersed with connective tissue.
- Week 12- Cortex is penetrated by dark supporting cells, giving the superficial epithelium a ‘dark’ appearance, amongst the original ‘light’ cells of the coelomic epithelium.
- Oogonia appear in clusters, primordial cells still dominate and oocytes in the premeiotic period exist in small groups.
- Medulla contains less densely-packed globules, mainly oogonia
- Weeks 14 to 28:
- In mid-gestation- Depletion of the germ cells by apoptosis, highest from weeks 14-28 and decreasing closer to birth
- Week 16- Cortical cords break up into primordial follicles (cell clusters) housing an oogonium each from a PGC. Follicles enclosed by monolayer of flat follicular cells from surface epithelium
- Primordial follicle formation is the result of active mitosis of oogonia
References
<pubmed>17237341</pubmed> <pubmed>7623307</pubmed> <pubmed>7158813</pubmed> <pubmed>22106406</pubmed>
Testis
Testis migration
The human testes early in fetal development begins at the abdominal cavity and migrates progressively towards the scrotum. This migration is caused by both mechanical determinants (genitofemroal nerve development, cremasteric muscle and epipdydmis development and gubernaculum development) and hormonal regulators (influences of gonadotropin and androgens such as testosterone).
- Gestational week 17- Migration begins
- By Week 23- Approximately 90% of testes still remain in the abdomen, with migration accelerating in weeks 24-26.
- Weeks 26-28: Arrival of testes in the inguinal canal within a couple of days through the deep inguinal ring, helped by the gubernaculum
- Week 28- Passing of testes through superficial inguinal ring to scrotum. Is usually completed in 3-4 weeks but can occasionally take up to 12 weeks post-natally.
By 22 weeks, 10% of testes are descending and this changes to 50% by 25 weeks, 75% by 26 weeks and 80% by 32 weeks.
Testes development
By week 8, masculine differentiation is induced in the mesonephric duct and external genitalis. This is caused by the interstitial cells (Leydig cells) in the mesenchymal tissue surrounding seminiferous tubules beginning secretion of the androgens androstenedione and testosterone, which is stimulated by human chorionic gonadotropin, peaking in weeks 8-12.
Antimulleran hormone, AMH (or mullerian-inhibiting substance, MIS), a glycoprotein, is produced by sustentacular (Sertoli) cells. This causes mesonephric duct suppression to prevent formation of the falltopian tubes and uterus in the developing male.
The majority of the seminiferous epithelium of the fetal testes is composed of Sertoli cells and this epithelium later flattens forming external mesothelium. 15-20 mesonephric tubules are continuous with the rete testes later form efferent ductules, connected to the mesonephric duct to form the epididymis duct.
References
<pubmed>PMC1260417</pubmed> <pubmed>6846859</pubmed> <pubmed>8292535</pubmed> <pubmed>10510117</pubmed> Moore: The Developing Human, 9th ed. Chapter 12
Placenta
<pubmed>10419690</pubmed>
<pubmed>7673080</pubmed>
Associated Abnormalities
| Disease | Description |
|---|---|
| Diabetes Mellitus | |
| Congenital Hypothyroidism | |
| Example | |
| Example | |
| Example | |
| Example |
<pubmed>22808198</pubmed>