2014 Group Project 4
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Genital
--Mark Hill (talk) 15:13, 26 August 2014 (EST) No sub-headings yet and I even had to add your project title! Get moving.
--Mark Hill (talk) 11:53, 6 September 2014 (EST) Just references not much else here yet.
System Development
| Weeks | M A L E | F E M A L E | |
|---|---|---|---|
| FERTILIZATION | both male and female are same at this point- only difference is presence of XY or XX chromosome | ||
| WEEKS 1-7 | GENITAL DEVELOPMENT IS UNDIFFERENTIATED. gonads derived from:
* mesothelium lining posterior abdominal wall * underlying mesenchyme * primordial germ cells. | ||
| WEEK 5 | development of indifferent gonads
| ||
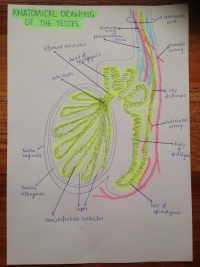
| WEEK 8 | seminiferous tubules begin to release androgens | ||
| WEEK 10 | rudimental rete ovarii forms from indifferent gonads | ||
| WEEK 12 |
| ||
| WEEK 16 | primordial follicles begin to develop |
Genital system development is an extremely interesting area of embryology as it is not until the later stages of embryogenesis (around week 4-6) that sexual differentiation occurs in the fetus, and the sexual organs actually look very similar up until this point, and the formation of the correct sex organs depend really on whether the genital ridge releases Testosterone or oestrogen
<pubmed>24240231</pubmed> <pubmed>24928207</pubmed> <pubmed>24741072</pubmed> --Z3416697 (talk) 20:06, 26 August 2014 (EST)
File:External Genitalia.jpg File:Image.jpg
Current Research, Models and Findings
Current Research
Male
• Extensive research into organogenesis of the external genitalia, mainly in males, is driven by the increasing incidence of hypospadias.
• Hypospadias are a result of the defect of fusion of the urethral folds of the lower part of the penis to fold and form the tubular penile urethra.
• The result of this in humans is the presence of an abnormal ventral urethral meatus, incomplete formation of the prepuce and an abnormal penile curvature.
• Development of the male external genitalia, which occurs in the fetal period of development, is androgen dependent and involves epithelial-mesenchymal interactions. Because of these interactions, which are very similar to limb development, research into the development of genital tubercle has utilised similar methods for both processes.
• A minority of hypospadias cases are a result of the androgenic pathways being impaired and causing this congenital defect.
• The cell-cell interactions that allow for the development of the male external genitalia are mediated by a broad range of signaling molecules and growth factors such as fibroblast growth factors (FGFs), Sonic hedgehog (SHH) and bone morphogenetic proteins (BMPs).
• Such signaling and growth factors are downstream of androgen receptor signaling and an understanding of the mechanisms that underlie normal penile development during the fetal period, will lead to a deeper understanding of the aetiology of hypospadias.
• Future research can work to decrease the incidence of hypospadias, which has more than doubled in the past 30 years.
• Current research uses mouse models and observes the development of their external genitalia, especially their penile development, which initially appears to be different to human development. However, more microscopic inspection shows that mice have very similar external genitalia and are therefore appropriate animal models for observing such fetal development. As a result, mutant mouse models can effectively be used in future research to observe molecular mechanisms underlying hypospadias and their aetiology. [1]
Female
Current Models
Current model: of the cellular and molecular mechanisms of the development of mammalian external genitalia. [2]
DEVELOPMENT OF EXTERNAL GENITALIA IN THE HUMAN
Embryonic Period – fertilisation to end of 8th week (embryonic age) = AMBISEXUAL STAGE
• The external genitalia initially begin in the perineal region as three primordia, being the genital tubercle in the midline and the bilateral genital swellings.
• These three primordia arise together with the differentiation of the cloacal part of the hindgut into the urogenital sinus, rectum and anal canal.
• The cloacal membrane extends from the perineum cranially to the root of the umbilical cord and during development, this bilayered cloacal membrane retracts into the perineum. This is due to cranial and medial migration of mesodermal cells into the ventral body wall between the ectoderm and endoderm of the cloacal membrane.
• These migrating mesodermal cells line around the membrane and accumulate, forming the three primordial swellings.
• These external features are internally related to the cloaca, which becomes divided coronally by the urorectal septum into the urogenital sinus anteriorly and the rectum and anus posteriorly. [3]
• This division of the cloaca occurs in a specific way so the allantois, Mullerian and Wolffian ducts and ureters all empty into the urogenital sinus. [4]
DRAW A PICTURE
• When the cloacal membrane becomes divided into the urogenital and anal membranes, the urogenital membrane is bound cranially by the genital tubercle in the midline and laterally by the urogenital folds and genital swellings.
• The urogenital membrane degenerates to allow communication between the urogenital sinus and amniotic cavity.
Fetal Period – from 8th week of development = SEXUAL DIFFERENTIATION
• Initially, the female and male fetuses’ external genitalia are identical and includes the genital tubercle in the midline, urogenital folds (forming the urogenital ostium) and genital swellings (laterally). [5]
Male
• In males, the genital tubercle will eventually form the penis and the genital swellings migrate caudally and a fusion event in the midline occurs, thus forming the scrotum.
• As the genital tubercle elongates to form the penis, a groove forms on the ventral surface known as the urethral groove. The urethral folds that are continuous with the urogenital folds surrounding the urogenital ostium define the urethral groove laterally.
• At first, the urethral groove and folds extend only part of the along the shaft of the elongating genital tubercle (known as the phallus at this stage).
• Distally, the urethral groove terminates at the urethral plate, consisting of epithelial cells, and then extends into the glans of the penis, forming a channel.
• As the phallus elongates, the urethral folds grow toward each other and fuse in the midline forming the midline epithelial seam, converting the urethral groove into a tubular penile urethra. [6]
DRAW A PICTURE
• The fusion of the urethral folds begins proximally in the perineal region and extends distally towards the glans of the penis.
• Hypospadias result from failure of formation or fusion of the urethral folds and this is the focus of current research.
• The elongating phallus is covered externally by ectoderm that will eventually give rise to the penile epidermis. Urethral epithelium has endodermal origins and the majority of the penis is derived from mesodermal cells.
• During development, the mesoderm separates into connective tissues and dermis.
• Dense areas of mesenchymal cells form within the shaft of the penis with the most superficial dense bodies forming the thick connective tissue capsule known as the tunica albuginae. [7]
• Mesenchyme surrounding the urethra forms smooth muscle of the urethral mucosa and submucosa. These two layers are then surrounded by erectile tissues such as the corpus spongiosum and corpus cavernosum.
• In some species, the mesenchyme of the genital tubercle also forms an os penis, comprised of bone and cartilage.
• Genital tubercle development involves an outgrowth of somatic tissue from the body surface, similar to the development of the limb.
• Development of the external genitalia is highly regulated by the endocrine system. Sexual differentiation of the external genitalia is determined by the presence or absence of androgen receptor signaling. The fetal testes produce testosterone, which travels to the genital tubercle via the bloodstream, where it is converted into 5a-dihydrotestosterone by the enzyme 5a-reductase. This formation of the highly potent 5a-dihydrotestosterone masculinizes the developing external genitalia, as binding of the 5a-dihydrotestosterone to its androgen receptor leads to the regulation of downstream signaling genes. [8]
• Sonic Hedgehog (SHH) acts as an endodermal signal that normally regulates patterning of the hindgut and is expressed in the epithelium of the cloaca, urogenital sinus and urethral plate epithelium. However has an important signaling pathway role in development of external genitalia. The SHH gene codes for a particular protein that has important roles in organogenesis as well as structures that are dependent upon mesenchymal-epithelial interactions, such as limbs, teeth and prostate. [9]
Female
• In females, the genital tubercle will eventually form the clitoris and the genital swellings remain apart and will eventually form the labia majora. The urogenital folds forming the border for the urogenital ostium will eventually form the labia minora in the female, thus forming the vestibule of the vagina. [10]
Current Findings
Male
Female
References
<pubmed>18367374</pubmed> <pubmed>15086026</pubmed> <pubmed>14641326</pubmed> <pubmed>11684660</pubmed> <pubmed>22127979</pubmed> <pubmed>24631756</pubmed> <pubmed>23192465</pubmed>
Historic Finding
Female Genital Development
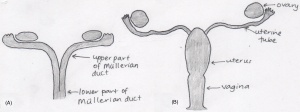
The mullerian (paramesonephric) ducts, found laterally to the wolffian ducts, are the original structures of the female reproductive system. Female sexual organs (the fallopian tubes, uterus and vagina) originate from the mullerian ducts, which differentiates within the foetal developmental phase. Initially the foetus contains two mullerian ducts, however by the ninth week, fusion of the lower portion of the ducts is complete, creating the fundamental structure of the uterus and the vagina, however the these two organs are not continuous with the vagina being solid. The non-fused upper part of the ducts emerge into the fallopian tubes. It is not until the fourth and fifth month of development that the uterus becomes continuous with the vagina, with both organs developing a hollow lumen. The muscular layers of the uterus is also present by this stage. The cervix begins to form within the fifth month in between the continuous vagina and uterus. Also within the same month, the formation of the hymen occurs. The hymen is described as a pouting vertical slit and represents the remains of the mullerian eminence. [1] [2]
Male Genital Development
The Prostate
Foetal prostate differentiation begins in the 10th week of gestation, influenced by the production of testosterone that starts within the 8th week. It is discovered that the origin of the gland is from the urogenital sinus. The prostate gland continues to grow and differentiate within the foetal period and postnatally under the influence of androgens.
Prostate foetal development has been continuously reshaping at a slow rate since the published illustrations of the male sex organs by Andreas Vesalius in 1543. Throughout this period, various anatomical classifications have been proposed via dissection procedures, hormone responses and histological methods, attributing to the current understanding of prostate development. It was not until the following century that another anatomical description of the prostate was put forth. Gerard Blasius in 1674 introduced the gland as a structure encircling the neck of the bladder. Literature in relevance to the embryonic prostate was further published in the late 19th century. The literature was reviewed and compared within the subsequent 20th century articles. Research into the structure and development of the prostate continued, however the rate of publication steeply increased in the 20th century, where each decade saw an improvement of the understanding of the development of the gland.
In 1912, Oswald S Lowsley constructed the first detailed illustration of the anatomy of the prostate, and to an extent, began the research that led to today’s understanding of the sex gland. Lowsley created his model from researching on a 13-week old foetus, 30-week old foetus, and one at full-term. From examining the samples he proposed that the prostate can be divided into five lobes consisting of differing amounts of tubules. This concept was later rendered invalid, however a discovery from Lowsley that is still accepted today is that the prostate originates from the urogenital sinus.
Lowsley’s division of the prostate into lobes was questioned by Johnson in 1920, where in his research and reconstruction of the prostate, was has unable to produce identical results as Lowsley. Upon conclusion of his work he reshaped the anatomical illustration of the prostate, however preserved the use of the term lobe in describing the prostatic divisions. It was not until 1954, 42 years post the introduction of the lobe terminology, that it was completely revoked by Franks. Franks, upon dissections, described the prostate in terms of three concentric regions. Today, it is clear that the prostate consists of zones and not lobes as introduced by Lowsley nor concentric regions as introduced by Franks. The concept of prostatic zones was established by McNeal in the 1980s and is still accepted today. [3]
Testicular descent
Testicular descent begins during the early foetal period, 8-10 weeks, and takes approximately 5 weeks for the testes to reach the inguinal region. The second phase of descent, when the testes reach the scrotum, is not complete until the 35th to 40th week. The mechanisms behind testicular descent has been debated for at least two centuries, beginning with anatomical dissections during the eighteenth and nineteenth centuries, then enhancing with endocrinological discoveries during the twentieth century.
The Scottish surgeon and anatomist, John Hunter, first documented the gubernaculum and the location of the male foetal testicles in the late 1700s. In his research, Hunter claimed that descent occurred during the 8th foetal month and was directed by the gubernaculum testis, a ligament attaching the foetal testis to the abdominal wall and the scrotum. He further proposed that the processus vaginalis closes subsequent to the decent of the testis. This is contrary to the findings of Albrecht von Haller who illustrated that foetal testis is intra-abdominal and the processus vaginalis is not closed.
Hunter described the gubernaculum as a vascular and fibrous foetal structure covered by the cremaster muscle, a muscle of unknown function. This led to more research focused on the cremaster muscle. In 1777, Palletta questioned the importance of the cremaster muscle because of its under developed state during the time of descent. This however did not stop Pancera, who in the following year, considered the muscle as the key factor in the process. Pancera’s conclusion was confirmed by Lobsetin in 1801.
The second phase of testicular descent to the scrotum has also seen many theories. Lobsetin suggested that this phase is complete by birth, influenced by respiration and the increased abdominal pressure that occurs at birth. The concept of increased abdominal pressure was reiterated by Robin in 1849, however he also introduced the theory that descent into the scrotum occurs due to the weight of the testes and muscles associated. Both Lobsetin and Robin’s work was refuted by Weber who highlighted the processus vaginalis, an embryonic pouch of peritoneum, as the main force of the migration.
In 1841, Curling detailed the structure of the gubernaculum and the cremaster muscle. Curling believed that during the foetal period, the cremaster muscle was important in descending the testis, however subsequent to the descent, the fibres of the muscle everted resulting in it’s new functions of elevating, supporting and compressing of the developed testis. The eversion of the muscle fibres were denied by Cleland, who in 1856 performed dissections on foetal specimens ranging from 5-6 gestational months old. In his experiment he found that the foetal gubernaculum did not directly attach the testicle to the scrotum and was only present in the inguinal wall. In terms of the testicular descent process, Cleland presented a similar theory as Weber, in terms that the cremaster was not the primary source of descent, second to the gubernaculum, that led the descent of the testes. In 1888, Lockwood published a completely unique theory claiming that the testes remained stationary and that it was in fact the surrounding structures that developed, resulting in the changing of the testicular location. Lockwood’s hypothesis was disagreed on by many anatomists and embryologists.
With the introduction of endocrinology and hormonal testing, the previous theories were tested on a cellular basis. Male androgen, controlled by the pituitary gland, was the first hormonal theory believed to influence testicular descent. It has been evidently proven that androgens are important in the descent however it is unclear if it is important in both stages. It is currently accepted that testosterone influences the gubernaculum during the second phase in which the testes reach the scrotum, however the exact method is currently debatable. The first phase theories are under high scrutiny, with theories ranging from the development of the gubernaculum and hormones such as the Mullerian inhibiting substance. [4]
References
4. Martyn P. L. Williams, John M. Huston The history of ideas about testicular descent. Pediatric Surgery International: 1991, 6(3):180-184 The history of ideas about testicular descent
--Z3415716 (talk) 01:10, 27 August 2014 (EST)
<pubmed>18462432</pubmed> <pubmed>17232227</pubmed> Martyn P. L. Williams, John M. Huston The history of ideas about testicular descent. Pediatric Surgery International: 1991, 6(3):180-184 The history of ideas about testicular descent
Abnormalities
We discuss both male and female genital abnormalities internally or externally, that may occur during fetal development. The abnormalities have been identified as disorders of sex differentiation(DSD), associated with congenital conditions in the atypical development of chromosomal, gonadal or phenotypical sex [1], [2]. The content will cover most common abnormalities and then also the rare cases.
FEMALE
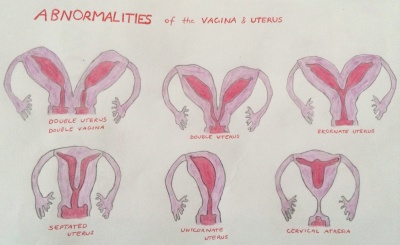
Abnormalities of the Uterus and vagina are cause by inadequate fusion or regression of Mullerian duct may result as the following;
- double uterus and double vagina
- double uterus
- bicornate uterus
- septated uterus
- unicornate uterus
- cervical atresia
Mullerian agenesis
Mullerian agenesis also known as ‘Mayer-Rokitansky-Kuster-Hauser’ syndrome, vaginal agenesis or Mullerian aplasia, is presented in the absence of the uterus or vagina or in some case even both. This is due to the unsuccessful development of the Mullerian ducts which then causes certain parts of the reproductive system to be underdeveloped. It is present in 1 of 4000-10 000 women. This condition also uses dilation therapy and following the neovaginal approach with the reconstruction of the vagina in its treatment strategies [3].
Vaginal agenesis
Vaginal agenesis is a rare condition involving the underdevelopment of the vagina. It is commonly cause by a combination of Rokitansky (Mullerian agenesis) and androgen insensitivity syndromes [4]. To ensure effectiveness in treatment, it’s advised after or during adolescence, procedures consist of vaginal dilation shown a success rate of 80% and low risks. In cases where such methods are ineffective then vaginal reconstruction is implemented as a final option for patients [5].
Turners syndrome
A chromosomal disorder occurring among women due to the absence of the whole or part of the sex chromosome (X). The condition is characterized by short stature, cardiovascular malformations, amenorrhea and estrogen insufficiency [6]. It is prevalent in 1 of 2000 live births among females [7]. Management of the syndrome depends on the extent of the condition the individual will present. Therefore treatment will vary, for short stature biosynthetic growth hormone is utalised in growth hormone. The most common cardiac malformations are bicuspid aortic valve, coarctation of the aorta and aortic stenosis that are all surgically treated. Generally patients are advised to see pediatricians, endocrinologists and many other clinicians depending on the severity of the condition, to discuss strategies to manage the syndrome [8].
Also related include;
Polycystic Ovarian Syndrome
A metabolic endocrine disorder with an immense variety of phenotypes presented. The disorder has an imbalance in female sex hormones and a resistance to insulin. Most importantly it affects the female reproductive system, with issues associated with infertility and menstrual irregularities. The treatments implemented depend on the clinical manifestations each patient develops. Insulin-sensitizing agents are among the treatments used these include Metformin, Rosiglitazone and Piglitazone all have shown to be effective [9].
MALE
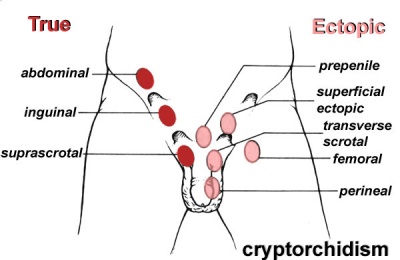
Cryptorchidism
Involves the absence of both or single testis to descend into the scrotum, the testes can be ectopic, incompletely descended, absent or atrophic. It is possible that sometimes the cryptrodism may be spontaneously corrected by 3 months of age. The abnormality can occur as a result of a number of factors including maternal, genetic or environmental [10]. The descendence of testis occur in two stages; in the first stage insulin like hormone attaches the testis to the inguinal ring this is through gubernaculum development. Following is the inguinoscrotal stage that requires testicular androgens [11].
Treatment includes human chorionic gonadotropin or gonadotroping-releasing hormones, these are not the most beneficial or advised approach. Surgical repair is intended to apply the safest and least invasive methods, focusing on repositioning the undescended testicle/s to their normal position in the scrotum. Such surgeries are recommended in early life and have proved to be most effective, with 75%+ success. The therapy used to relocate the testis into the scrotum is known as ‘Orchiopexy’, others include one-stage Fowler Stephens and two-stage FS Orchidopecy . However there are concerns with long-term effects which include infertility and testicular cancer later in life as a result of the procedure [12].
Hypospadias
In males the most common congenital malformation of the external genitalia is hypospadias, it’s also the second most common developmental disorder. It occurs due to the midline fusion of the male urethra, as a result the urethral meatus is misplaced. There are several sites where this abnormality may occur: granular, penile, penoscrotal, scrotal and perineal. [13] Its believed that genetic factors contribute to the presence of the disorder, however endocrine and environmental factors are also of significance. [14] Treatment The surgical methods currently used to treat distal hypospadias, include tabularized incised plate and meatal advancement and glansplasty intergrated repair. For proximal forms two staged procedures are employed. [15]
Klinefelter
Klinefelter is a genetic disorder caused by the addition of an X chromosome among males (47, XXY, XXY,XXXXY, XXYY), due to the inability of the extra chromosomes to detach throughout meiosis. It is believed to have an origin from either parent. The abnormality has a wide range of phenotypic variations, that typically include infertility, small testes, gynecomastia and hypergonadotropic hypogonadism [16]. An early diagnosis is important in order for treatment to be commenced right away. The treatment implemented involves Testosterone replacement therapy, which assists in easing some of the features, although infertility is still an issue. The fertility options consist of IVF, where males undergo testicular sperm extraction, cryopreservation of sperm containing semen or testicular tissue during adolescence [17].
BOTH
Congenital adrenal hyperplasia
The condition is caused by a deficiency in 21-Hydroxylase, a genetic disorder of steroidogenesis. Occurs due to mutations in genes that encode enzymes that take part in adrenal steroid synthesis therefore there is a loss of function (9). The deficiency is from mutations in CYP21A2, thus the clinical characteristics may vary. In females it results in the ambiguity of the female genitalia, fused labia majora, larger clitoris and common urogenital sinus (10). Steroid 21-OHD deficiency is examined in-utero and then prenatal treatment with dexamethasone is administered. This is a safe method used and decreases the risk of ambiguous genitalia in females (7). Among males symptoms aren’t present at birth a side from possible penile enlargement and slight hyperpigmentation (10). Generally male patients also require the administration of glucocorticoid and mineralocorticoid therapies (11).
Hydrocele
Hydrocele occurs when the space between parietal and visceral layers of tunica vaginalis accumulates an abnormal amount of serous fluid. Normally caused by an imbalance in the processes of production and reabsorption of fluid or varicocelectomy (14). To manage the condition treatments focus on ensuring draining any excess fluid and inhibiting reaccumulation. Techniques used involve sclerotherapy and hydrocelectomy (15). In females it is a very rare condition, occurs in the ‘Canal of Nuck’, a part of the inguinal canal containing a section of the processus vaginalis. A swelling is present on the labia major or inguinal ring. Techniques applied to treat the condition in females involve ligation of the processus vaginalis neck and the hydrocele is surgically resected (12).
Also related include;
Hypogonadotropic hypogonadism
Kallmann syndrome
References
- ↑ <pubmed>16882788</pubmed>
- ↑ <pubmed>25248670</pubmed>
- ↑ <pubmed>23635766</pubmed>
- ↑ <pubmed>21872517</pubmed>
- ↑ <pubmed>17995494</pubmed>
- ↑ <pubmed>16849410</pubmed>
- ↑ <pubmed>2037286</pubmed>
- ↑ <pubmed>16714725</pubmed>
- ↑ <pubmed>19405411</pubmed>
- ↑ <pubmed>24683948</pubmed>
- ↑ <pubmed>18032558</pubmed>
- ↑ <pubmed>24857650</pubmed>
- ↑ <pubmed>16006950</pubmed>
- ↑ <pubmed>24936573</pubmed>
- ↑ <pubmed>25023236</pubmed>
- ↑ <pubmed>16342850</pubmed>
- ↑ <pubmed>24563893</pubmed>
--Z3417458 (talk) 21:01, 26 August 2014 (EST)
<<pubmed>24290348</pubmed>>
<<pubmed>25064170</pubmed>>
<<pubmed>23168057</pubmed>>
A review on spermatogenesis and cyptorchidism a common in males, results in an absence of testes either one or both.
<<pubmed>24829558</pubmed>>