2014 Group Project 2: Difference between revisions
No edit summary |
|||
| Line 152: | Line 152: | ||
===Polycystic Kidney Disease=== | ===Polycystic Kidney Disease=== | ||
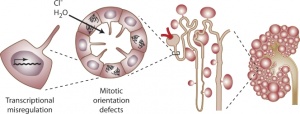
[[File:PKD.jpg|thumb|Cyst formation at the level of the cell, nephron, and kidney]] | [[File:PKD.jpg|thumb|Cyst formation at the level of the cell, nephron, and kidney]] | ||
Revision as of 08:13, 6 October 2014
| 2014 Student Projects | ||||
|---|---|---|---|---|
| 2014 Student Projects: Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | ||||
| The Group assessment for 2014 will be an online project on Fetal Development of a specific System.
This page is an undergraduate science embryology student and may contain inaccuracies in either description or acknowledgements. | ||||
Renal
--Mark Hill (talk) 15:11, 26 August 2014 (EST) No subheadings yet and I even had to add your project title! Get moving.
--Mark Hill (talk) 16:00, 6 September 2014 (EST) OK some sub-headings and a few refs. No content yet expelling the feral component or how the references you have selected relate to the topic.
Introduction
The renal system's main function is the production, storage and elimination of urine, and to maintain the balance of chemicals and water of the body. Kidneys are the primary organ of the renal system, and consist of smaller units known as nephrons - which filter the blood to remove urea and other wastes, and reabsorb or excrete excess water according to the needs of the body as directed by hormones released by the pituitary glands. Nephrons are made up of glomeruli to filter the blood, tubules to reabsorb any solutes or fluids, and more tubule networks to carry the urine to the bladder and outside the body. Small amounts of urine is released from the kidneys every 1 ~ 15 seconds into the ureter, which carry the urine to the bladder.[1] The bladder is a hollow organ which has the ability to change its epithelium according to how full the bladder is of urine. "The bladder's walls relax and expand to store urine, and contract and flatten to empty urine through the urethra. The typical healthy adult bladder can store up to two cups of urine for two to five hours.”[2] Two sphincter muscles are present at the base of the bladder, and two more at the end of the urethra (internal & external) to voluntarily control the excretion of urine.
Development of these components begin during the embryonic phase, and continue to develop and mature throughout the fetal stages. During the fetal stages, some abnormalities may form. During the embryonic period and fetal periods, the mother's placenta work to remove wastes from the fetus.
Abnormalities may arise during the embryonic and fetal stages of development of the renal system, such as Hereditary renal adysplasia, or polycystic kidney disease. Hereditary renal adysplasia is an inherited condition, where there is malformations in organs derived of the embryonic mesoderm.[3] Polycystic kidney disease is a fairly common genetic disorder in which fluid-filled cysts displace normal renal tubules.[4]
References
Historic findings
Developmental Timeline
Week 3 – nephrogenesis begins, pronephri form
Week ~4 - embryonic development of ureter begins from the ureteric bud
Week 5 – metanephros forms
Week 8 – mature kidney is formed
Week ~10 - Kidneys begin to produce urine
Week 36 – nephrogenesis is complete
Postnatal – maturation of neonatal glomerular filtration
Current research models
Animals are good models to use when researching the development of the renal system as there are fewer ethical issues surrounding animals compared with humans and their generation time is much shorter so mutations can be identified much faster.
One research paper that has recently come out that used mice in order to determine the impact of maternal cigarette smoke exposure on the development of the renal system, in particular kidneys. It was proposed that smoke exposure would lead to a change in the expression of growth and transcription factors which would lead to kidney disease later on in life. The experiment found that some fibroblast growth factors were up-regulated whilst others were down-regulated and this led to delayed nephron development and fewer nephrons present at birth. The study showed by using an animal model that cigarette smoke exposure during pregnancy and lactation period leads to underdeveloped renal system which can result in chronic kidney disorders in adulthood.
<pubmed>25058584</pubmed>
Kidney
Early Development
The kidneys first develop in the embryo by a process called nephrogenesis, in which self-renewing mesenchymal renal stem cells produce nephrons, the main functional unit, and form a simple embryonic kidney called the pronephros[1]. This process of nephron formation is stimulated by the signaling between the ureteric buds and these stem cells, named progenitor cells and located at the tips of the ureteric buds, causing nephrons to develop and the ureteric buds to branch[2].
While it is not well known the mechanisms by which nephron number is determined, the causes of several disorders and diseases, such as renal disease and hypertension, have been attributed to a low nephron count[2]. It has been determined that a decrease in the number of progenitor cells, a possible result of genetic abnormalities, toxic insults, and nutritional deficiencies[3], can result in fewer branching of the ureteric buds, leading to impaired kidney growth[2]. Therefore, nephron number is important as it can show the success/extent of nephrogenesis, and thus be used to determine if any and what genes and environmental factors may aid this process[4].
When sufficient development has occurred during week 3 of gestation, two pronephri are produced and nephrotomes, a series of tubules, begin to fuse together with the pronepheric duct. As the pronephri continue to develop, they elongate and induce the nearby mesoderm to form mesonephri, and the pronepheric duct to become the mesonephric (Woffian) duct. Towards the bottom of this duct, close to where it connects to the cloaca, is the ureteric bud connected by the ureter. Surrounding this bud is a mass of metanephric mesoderm (blastema), the two of which react together to form the metanephros which goes on to form the mature kidney. The cells of the ureteric bud differentiate to form the major and minor calyces as well as the collecting tubules, while the cells of the metanephrogenic blastema develop into the renal tubules and glomeruli. This process begins from as early as week 3 and continues until week 8 of gestation. The development of the nephrons however, continue through to week 32-36 of gestation.[5]
A drawing of the following picture will be made and uploaded as a direct copy is prohibited under copyright. It shows the formation of the kidney during the embryonic development:http://renalsystem.weebly.com/uploads/1/4/9/9/14997296/9282501_orig.jpg?1
Fetal Development
There are a number of factors that occur in regards to the kidneys during the fetal period of development, the most important of which is the continued generation of nephrons. There are specific genes expressed as well as hormones released for their generation, with vasculature created to supply the newly formed kidneys. There are certain events that occur for the kidneys to achieve their correct anatomical position before they are fully formed, as well as further growth post-natally before the kidneys are fully matured.
An embryonic gene named gremlin (GREM1) has been found to play a key role in the formation of the kidneys and nephrogenesis in general. When fully formed, the expression of this gene is relatively low in an adult. However, it is thought that many renal diseases and their progressions are linked to an overexpression of this gremlin gene.
<pubmed>25036148</pubmed>
At birth, although the infant’s kidneys are developed enough to maintain homeostasis and are sufficient for growth and development, their functional capabilities are decreased. This is a result of the transition from depending on the placenta to maintain homeostasis of fluid and electrolyte balance while in-utero, to maturation of the neonatal glomeruli once born.
<pubmed>24781774</pubmed>
<pubmed>24623338</pubmed>
<pubmed>24011574</pubmed>
<pubmed>19726549</pubmed>
References
Urethra
The urethra develops from the cloaca during fetal development. The cloaca can be divided into the anorectal canal (dorsally) and the urogential sinus (ventrally). The bladder develops from the superior portion of this urogenital sinus, and the inferior portion develops into the urethra. The endoderm of the urogenital sinus derives the urethral epithelium, and the splanchnic mesenchyme develop into the connective tissue and smooth muscle components of the urethra.[1] External urethral sphincters are thought to develop within week 10 of development. [2]
References
Urine Formation during the fetal period
The amniotic fluid is mainly composed of nutrients that will supply the fetus, and its components fluctuate during pregnancy according to the fetal development. [5] As the fetus develops, it excretes fetal urine into the amniotic sac.
Polyhydramnios and oligohydramnios
amniotic fluid: not just urine anymore [5]
Sebe. P., Schwentner. C., Oswald. J., Radmayr. C., Bartsch. G., Fritsch. H. Fetal development of striated and smooth muscle sphincters of the male urethra from a common primordium and modifications due to the development of the prostate: an anatomic and histologic study. Prostate (2005) [1]
Werff. V. D., Nievelstein. R.A, Brands. E., Luijsterburg. A.J., Vermeij-Keers. C. Normal development of the male anterior urethra. Teratology (2005) [2]
Sebe. P., Fritsch. H., Oswald. J., Schwentner. C., Lunacek. A., Bartsch. G., Radmayr. C. Fetal development of the female external urinary sphincter complex: an anatomical and histological study. J urol (2005) [3]
Ludwikowski B, Hayward OI, Brenner E, Fritsch H. The development of the external urethral sphincter in humans. BJU Int. 2001 Apr;87(6):565-8. [4]
Kidney Anatomical Position
Kidneys initially begin to develop proximally to the pelvis, located ventrally to the sacrum. As the fetus develops, the kidneys must move to attain their adult anatomical positions which is towards the dorsal sides of the body at around T12~L3 levels. This process is usually completed by week 9 of fetal development, and result mainly from the kidneys coming in contact to the supra-adrenal glands, and also from the growth in size of the embryo’s body and the abdominal cavity. Medial rotation of the kidneys by up to ninety degrees, and the blood supply to the kidneys from more superior parts of the abdominal aorta (branches begin initially near the common iliac arteries, and receive blood from new branches higher up from the aorta) both contribute to the ascending of the fetal kidneys. [1]
[1] Moore: the developing human 9th edition. Saunders 2011. An imprint of Elsevier
Ureter
The development of the ureter typically begins during week 4 of gestation. The process begins from the ureteric bud, which arises from the caudal region of the mesonephric ducts (also known as Wolffian ducts) that run along the edge of the intermediate mesoderm and grows into the adjacent metanephric mesenchyme.
<pubmed>23123402</pubmed> <pubmed>23408557</pubmed>
Duplicated Ureter
<pubmed>24469670</pubmed> <pubmed>25010444</pubmed>
Bladder
The role of the urinary balder in the renal system is to store urine produced by the kidneys and transported in the ureter before it is excreted via the urethra. A flexible epithelium is essential for the bladder as it changes volume by contracting and relaxing depending on the volume of urine in the body. A fusion event occurs between the common urogenital sinus and the mesonephric duct, this divides the rectal components from the urine components and it allows the balder to develop.
The urinary bladder develops in the first 12 weeks of gestation from the urogenital sinus and the surrounding splanchnic mesenchyme, these development events are controlled by by complex epithelial–mesenchymal signals. The vesical part of the urogenital sinus is attached to the allantois and goes on to form the bladder. (23371862) The lamina propria, the muscle coat and the adventitia all develop from the splanchmic mesoderm whilst the epithelial lining is derived from the endoderm of the urogenital sinus. (Newman)
During foetal development the bladder is supplied by an increasing number of nerves in the detrusor muscle, as the gestational period continues different peptide containing nerves are observed.
<pubmed>23371862</pubmed>
During development the bladder only produces immature reflexes rather than the voluntary bladder control that is only seen once the infant is toilet trained. It is suggested that the switch between involuntary reflexes and voluntary contractions is due to the development of the central and peripheral neural pathways that control the contraction of the bladder.
<pubmed>22535797</pubmed>
Initially the epithelium lining of the urinary bladder is made from two distinct cell layers, the superficial layer and the basal layer. Up to week 11 the rest of the balder wall consists of mesenchyme that gradually matures into lose connective tissue. At the 13th week collagen begins to appear, by the 14th week it is abundant in the lamina propria and by week 15 it has extended into the superficial muscle bundles. Smooth muscle cells begin to appear in the connective tissue during week 12, they initially appear in the proximal part of the organ but they spread distally over time. At 21 weeks the epithelium is 3-4 layers thick, the superficial layer, the intermediate layer, lamina propria and the basal lamina. This epithelium is specialised and contains features that are characteristic of urothelial differentiation so that urine is unable to pass through the bladder wall. The muscular coat of the bladder does not develop until after the kidneys have begun to produce urine but as the urine is released into the amniotic cavity this is not a problem for the foetus. (Newman)
<pubmed>2621133</pubmed>
<pubmed>23408557</pubmed>
Abnormalities
Renal agenesis
Renal agenesis is a congenital abnormality referring to the failure of the development of the kidneys and ureter produced by a lack of interaction between the ureteric bud and the metanephric mesenchyme. Renal agenesis can occur in two forms, infants can be born with either bilateral or unilateral renal agenesis. Infants born with bilateral renal agenesis are incompatible with life and are born usually stillborn, or die within a few days after birth [1].
- ↑ <pubmed>23169372</pubmed>
<pubmed>20798957</pubmed> <pubmed>24439109</pubmed> <pubmed>18252215</pubmed>
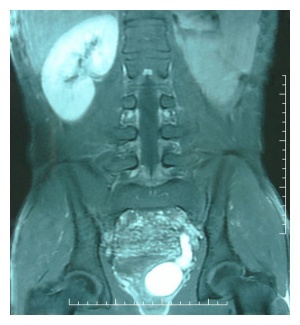
Polycystic Kidney Disease
Polycystic kidney disease (PKD) is a common genetic disorder characterized by the formation of fluid filled cysts in the kidneys, which displace normal renal tubules. There are two types of PKD, autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD).
PKD affects approximately 1 in 1000 individuals, with ADPKD accounting for the majority of the cases reported[1] [2]. ADPKD, also known as adult-onset polycystic kidney disease is commonly reported to present in adulthood in association with hypertension and renal failure [3]. The disease is linked to the mutations in the genes encoding polycystin-1 (PC1) and polycystin-2 (PC2), which is characterized by perturbations of renal epithelial cell growth control, fluid transport, and morphogenesis [1]. These mutations ultimately affect multiple signaling pathways, which cause aberrant gene transcription, cell proliferation, and ion secretion, which in turn result in the formation of benign fluid-filled cysts. As cysts balloon out from individual nephrons, their collective effect leads to the displacement of the normal renal parenchyma and the formation of a cyst-filled kidney with reduced functional capacity [1]. In ADPKD, the growth of renal cysts produces a progressive increase in renal volume and destruction of the parenchyma, leading to terminal chronic renal failure in adulthood [2].
ARPKD, however is the rarer form of PKD, affecting approximately 15% of cases. It commonly presents during the second or third trimester of fetal development. Ultrasound images show kidneys that are usually 'bright' or echogenic and are often associated with progressive oligohydramnios [3].
References
<pubmed>18728845</pubmed> <pubmed>25263802</pubmed> Mutations in SALL4, a transcription factor important in renal development, can result in renal malformations. <pubmed>21258884</pubmed> <pubmed>25211294</pubmed> <pubmed>16462154</pubmed> <pubmed>11458035</pubmed>
Horseshoe Kidney
<pubmed>18059107</pubmed> <pubmed>17593682</pubmed> <pubmed>10862660</pubmed>
References
<pubmed>18631884</pubmed> <pubmed>20807610</pubmed> <pubmed>20388228</pubmed> <pubmed>11746154</pubmed> <pubmed>25036148</pubmed>
The overexpression of the gremlin gene (GREM1) has been found to be a cause of renal disease.
<pubmed>24500691</pubmed>