2012 Group Project 4: Difference between revisions
| Line 269: | Line 269: | ||
** Mental retardation <ref name="PMID16932275"/><ref name="PMID6881209"><pubmed>6881209</pubmed></ref> <ref name="PMID11531922"><pubmed>11531922</pubmed></ref> <ref name="PMID11297579"><pubmed>11297579</pubmed></ref> | ** Mental retardation <ref name="PMID16932275"/><ref name="PMID6881209"><pubmed>6881209</pubmed></ref> <ref name="PMID11531922"><pubmed>11531922</pubmed></ref> <ref name="PMID11297579"><pubmed>11297579</pubmed></ref> | ||
= | {| class="wikitable collapsible collapsed" | ||
|- | |||
|'''Diagnosis''' | |||
Due to the low incidence of Kallmann's syndrome, correct diagnosis is often delayed, despite early childhood signs such as anosmia and cryptorchidism <ref name="PMID11052640"><pubmed>11052640</pubmed></ref>. Instead, doctors often dismiss Kallmann's syndrome as constitionally delayed puberty <ref name="PMID11052640"/>. Other differential diagnoses include potential presence of hypothalamic or pituitary tumours<ref name="PMID11052640"/>. Due to the varied phenotype and genotype of Kallmann's, multiple tests are required in order to properly diagnose the syndrome. The following diagnostic tests are often employed: | Due to the low incidence of Kallmann's syndrome, correct diagnosis is often delayed, despite early childhood signs such as anosmia and cryptorchidism <ref name="PMID11052640"><pubmed>11052640</pubmed></ref>. Instead, doctors often dismiss Kallmann's syndrome as constitionally delayed puberty <ref name="PMID11052640"/>. Other differential diagnoses include potential presence of hypothalamic or pituitary tumours<ref name="PMID11052640"/>. Due to the varied phenotype and genotype of Kallmann's, multiple tests are required in order to properly diagnose the syndrome. The following diagnostic tests are often employed: | ||
* Olfactory tests | * Olfactory tests | ||
| Line 276: | Line 278: | ||
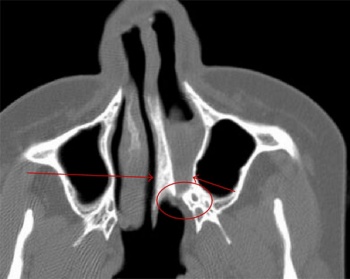
* Magnetic resonance imaging: utilised to examine the olfactory bulb as well as rule out neoplasms in the hypothalamus or pituitary gland as the cause of abnormal or reduced GnRH secretion <ref name="PMID20949504"/>. In Kallmann's syndrome, olfactory bulb is either not present or not fully developed <ref name="PMID20949504"/> | * Magnetic resonance imaging: utilised to examine the olfactory bulb as well as rule out neoplasms in the hypothalamus or pituitary gland as the cause of abnormal or reduced GnRH secretion <ref name="PMID20949504"/>. In Kallmann's syndrome, olfactory bulb is either not present or not fully developed <ref name="PMID20949504"/> | ||
* Genetic screening for mutations in genes associated with Kallmann's syndrome; however, negative result does not rule out possibility of the syndrome <ref name="PMID20949504"/>. | * Genetic screening for mutations in genes associated with Kallmann's syndrome; however, negative result does not rule out possibility of the syndrome <ref name="PMID20949504"/>. | ||
|- | |||
|'''Treatment''' | |||
* Fertility treatment | |||
* Hormone replacement therapy: testosterone injections (males), oestrogen and progesterone pills (females), GnRH injections. | |||
* Treatment to prevent osteoporosis: HRT and vitamin D supplementation <ref>http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001427/</ref>. | |||
====Treatment==== | ====Treatment==== | ||
Revision as of 11:36, 26 September 2012
Olfaction Development
Introduction
WAKE UP AND SMELL THE ROSES! The sense of smell, or otherwise known as Olfaction is the sense mediated by sensory cells located in the nasal cavity. Chemoreceptors within the naval cavity are activated by chemicals in the air which are known as odorants. Odorants produce olfactory sensation at very low concentration, and through the reaction with chemoreceptors enables the sense of smell in humans. The olfactory system are often divide into a peripheral mechanism, activated by an external stimulus and transforming it into an electric signal in neurons, and a central mechanism where all signals formed by olfactory are integrated in the central nervous system and processed to recognise odor. Over 1000 genes which make up three percent of the total human genome which encode for olfactory receptor types which can each detect a small number of related molecules and respond with different level of intensity. It has been discovered that olfactory receptor cells are highly specialized to particular odors.
This page seeks to explore the development of the olfactory system in addition to their function and physiology. This page will also examine both structural and neurological abnormalities that can arise. Within this page, current research is looked into, and analyzes the possible future research within the olfactory system.
History of Discovery
| Year | Person | Contribution |
| 1703 | Frederick Ruysch | Discovery of Vomeronasal organ [1] |
| 1856 | Maestre de San Juan | The first person to link hypogonadism to the olfactory system[2] |
| 1891 | Von Kupffer | Von Kupffer is recognised with description of the olfactory placodes as ectodermal thickenings[3]. For a time they were termed Kupffer placodes. |
| 1899 | B.H. Buxton | B. H. Buxton published a paper containing a series of photographs of a day 25 human embryo in the Journal of Anatomy and Physiology. He noted the thickened ridges of epiblast, the olfactory plates but there were at that stage no olfactory pits [4]. |
| 1900s | Julius Kollmann |  Kollmann's diagram of developing nasal placode[1]
|
| 1941 | Anthony A. Pearson | Pearson conducted a study in examining serial sections of human embryos to understand the development of the olfactory nerve. It was seen the cells migrate from the olfactory epithelium up obliquely toward the brain collecting as fibers. His research indicated that olfactory nerve fibers start to form communications with the brain six weeks into development. He also asserted that the olfactory bulb starts to form in a 17mm embryo following which the proximal end of the olfactory nerve forms a sheath of fibers over the bulb. The fibers of this sheath collect together and continue to develop to form the fila olfactoria which eventually pass through the cribriform plate[7] |
| 1944 | Frank Kallmann | Kallmann looked at three families who suffered from the now-called Kallmann's Syndrome. Frank Kallmann was a genetist and psychiatrist. By analysing these familial groups he hypothesised about the inheritance of the disease[2] |
| 1954 | De Morsier | De Morsier reported other patients suffering from similar symptoms to those reported by Kallmann (hypogonadism, anosmia, midline anatomic defect) but he termed the condition olfactogenital dysplasia suggesting a link between hypogonadism and the hypothalamus[2] |
| 2004 | Linda B. Buck
and Richard Axel |
Won the Nobel Prize in Physiology or Medicine for their work on the olfactory system[8] |
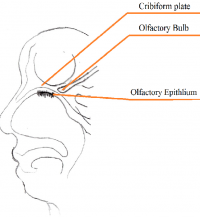
Anatomy of the Olfactory System
The nasal cavity is an important structure of the Olfactory system as within the turbinates or nasal conchae are found. These structures act to direct air inspired toward the olfactory epithelium. The epithelium is located in the upper posterior region of the nasal cavity and is approximately a couple of centimeters wide. Olfactory epithelium is a specialized epithelium which contains around 100 million receptor cells. The olfactory epithelial cells is also the origin of olfactory vesicles which are known to contain kinocilia. The Olfactory vesicles are also known to serve in the process of stimulus transduction.
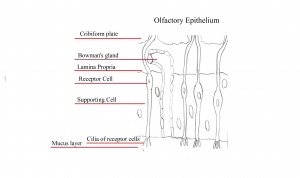
Olfactory Epithelium
Olfactory epithelium consists of pseudostratified epithelium which contain olfactory receptors along with nerve cells whose axons attach to the olfactory bulb of the brain.
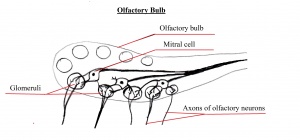
Olfactory Bulb
Normal Function
Olfactory Signal Transduction
The sensation of smell is dependent upon the dissolving of substances known as odorants in the mucus layer of the olfactory epithelium in order to bind to specific chemoreceptors. This step is necessary otherwise olfactory signal transduction is not possibly through the olfactory nerve. The olfactory epithelium, also known as the organ for smell is located at the roof of the nasal cavity.
When odorant molecules bind to receptors in olfactory epithelium, a G protein known as G(olf) is activated which then happen to activate adenylate cyclase, an enzyme which catalyses the formation of cyclic AMP. In most receptor cells, cAMP acts as a second messenger, however in the olfactory system cAMP bind to cation channels which permits sodium and calcium ions to travel through the membrane and enter the cell. The main effect of ion entry into the cell is depolarisation, and if the depolarization is great enough, an action potential is generated on the axon of the receptor cell. [9]
Timeline of developmental process
| Week/Stage | Patterning Genes | Description | Image |
| Week 4 |
Early expression of genes in the Hes5 family suggests it's role in pre-patterning of the placode ectoderm.[10] Wnt, BMP, and FGF are known to play a role in the early migration of neural crest cells to the olfactory placode however the exact mechanisms of signalling are still uncertain.[11] |
All five facial swellings form initially surrounding the stomodeum. The frontonasal prominence is the facial swelling which gives rise to olfactory placodes. It overlies the forebrain and arises from neural crest cells derived from midbrain and forebrain[12]. An interesting study of neural crest cell migration in rats revealed that the origins of neural crest cells during frononasal development change in relation to the stage of somite development[13]: - 3 to 5 somite stage: lateral edge of the prosencephalon(forebrain) produced cells which migrated to the frontonasal mass while anterior neural ridge cells in the prosencephalon contributed to the nasal placode epithelium. mesencephalic region(midbrain) produced neural crest cells which contributed to the frontonasal mass. - 5 to 10 somite stage: Anterior portion of the mesencephalon continued producing crest cells for migration to the frontonasal mass. These cranial neural crest cells follow paths determined by prepatterned Sonic Hedgehog (SHh) signalling to the ventrolateral mesenchyme of the facial primordia. It has also been evident that nonneural crest components provide important signals during craniofacial patterning of the epithelium and mesodermal mesenchyme after migration and positioning. Proliferation and differentiation into the olfactory placodes occurs after positioning. [14] Like the majority of placodes, some mesenchymal cells migrate away from the placodal epithelium and differentiate as either secretory cells or glial cells.[15] Some specialised areas in the rostrolateral regions of the head of the olfactory placode contain cells of cranial non-neural ectoderm. These cells differentiate to form the primary neurosensory cells of the future olfactory epithelium. This differentiation is a cuboidal-to-columnar transformation and so are distinguishable from the surrounding cuboidal epithelium.[16] |
image |
| Week 5 |
Genes Involved |
As the paired maxillary prominences enlarge and grow ventrally and medially, the ectodermal thickenings of the olfactory placode enlarge. The lining of the olfactory placode thickens and differentiates into three layers within the pseudo-stratified organised epithelium:[17] Inner basal layer: composed of two cell types, the horizontal and globose basal cells.[17] Intermediate layer: contains olfactory sensory neurons which ascend to the apical layer as they become more differentiated.[17] Apical layer: contains the mature olfactory sensory neurons as well as the nuclei and bodies of the supporting sustentacular cells. [17] During the fetal period, the developing olfactory epithelium concentrates its mitotically active cells in the apical layer whilst post-natally these cells migrate to the inner basal layer.[18] At the end of the 5th week, the primary neurosensory cells cells sprout axons that cross the short distance to penetrate the most cranial end of the telencephalon. The subsequent endochondral ossification of the ethmoid bone around these axons creates the perforated cribriform plate. [19] Glial cells: Appear to originate in the olfactory placode compared to most Schwann cells, that they resemble, that originate from neural crest cells. Later, they migrate to the periphery of the olfactory nerve and later into the centre of the nerve.[20] As olfactory nerve receptor neuron axons enter the olfactory bulb, the glial cells follow and distribute themselves along the edge of the olfactory nerve layer of the olfactory bulb in the central nervous system, as well as the olfactory nerve in the peripheral system.[21] Olfactory nerve glial cells ensheath bundles of many small diameter olfactory nerve axons allowing close contact between olfactory nerve axons. [22]
|
Image |
| Week 6 |
Genes |
The ectoderm at the center of each nasal placode invaginates to form an oval nasal pit, dividing the frontonasal prominence into the lateral and medial nasal processes. At the end of the 6th week, as the medial nasal processes start to merge, the dorsal region of the deepening nasal pits fuse to form a single, enlarged ectodermal nasal sac lying super posterior to the intermaxillary process. The nasal pits differentiate to form the epithelium of the nasal passages. [23] Nasolacrimal groove:This groove forms between the lateral nasal process and the adjacent maxillary prominence. The medial nasal processes migrate toward each other and fuse to form the primordium of the nasal bridge and nasal septum. Olfactory bulb growth: An outgrowth is formed where the axons of the primary neurosensory cells synapse,this is seen at the floor at each cerebral hemisphere. The synpasing cells differentiate to become the secondary sensory neurons, mitral cells, of the olfactory pathways. Olfactory nerve formation: formed due to the lengthening of the axons of the mitral cells as the proportions of the face and brain lenghthens. As a result, the CNS olfactory tracts look stalk-like. Olfactory nerve: the olfactory tract and bulb together. |
Image |
| Week 7 |
Genes |
Nasolacrimal duct and sac: The ectoderm at the floor of the nasal pit invaginates into the underlying mesenchyme. The duct becomes lined by bone during the ossfication of the maxilla After birth, it functions to drain excess tears from the conjunctiva of the eye into the nasal cavity. Intermaxillary process: The inferior tips of the medial nasal processes expand laterally and inferiorly and fuse. Separation of nasal and oral cavity: The floor and posterior wall of the nasal sac proliferate to form thickened ectoderm, Nasal fin. Oronasal Membrane: The sac enlarges as vacuoles develop within the nasal fin which fuse with the nasal sac. As a result of this, the nasal fin thins and is labelled as the oronasal membrane Primitive choana: formed as the oronasal membrane ruptures. The floor of the nasal cavity at this stage is formed by a posterior extension of the intermaxillary process called the primary palate. Palatal sheleves will later form to separate the two cavities. |
Image |
| Week 8 |
Genes |
Nasal septum and philtrum:Ectoderm and mesoderm of the frontonasal prominence and the medial nasal processes proliferate and grows down from the roof of the nasal cavity to fuse with the upper surface of the primary and secondary palates along the midline . |
image
|
EFFECT OF AMNIOTIC FLUID ON THE DEVELOPMENT OF OLFACTION IN THE FETUS still to be ncluded!
Congenital Abnormalities
Olfactory Defects
Anosmia is defined as the absence of a sense of smell. Hyposmia refers to a reduced sense of smell. These conditions, when they occur as a congenital feature, can be associated with Choanal Atresia or Kallmann's Syndrome. At present, these conditions are the most commonly recognised contributions to abnormal olfactory function.
Choanal Atresia
Introduction and Epidemiology
Choanal atresia is a congenital abnormality characterised by "narrowing of the posterior or complete obliteration of the nasal aperture" by a bony or membranous occlusion [24][25]. This anomaly occurs in 1 in every 7000 to 8000 births with a female predominance [26].
- 45% of cases are bilateral involving both choanae [25].
- Mixed bony and membranous anomalies were most common (70%) followed by pure bony atresia (30%) with no pure membranous anomalies[25].
Pathophysiology
At present, the exact cause of choanal atresia is still under debate. Ramsden [27]notes that "A number of embryological models for the development of choanal atresia have been proposed, although none of them are wholly supported by convincing clinical evidence:
- Persistence of the buccopharyngeal membrane from the foregut [28]
- Failure of perforation of the nasobuccal membrane of Hochstetter
- Abnormal persistence or location of mesoderm forming adhesions in the nasochoanal region
- Misdirection of neural crest cell migration" [29]
Kallmann's Syndrome
Introduction and Epidemiology
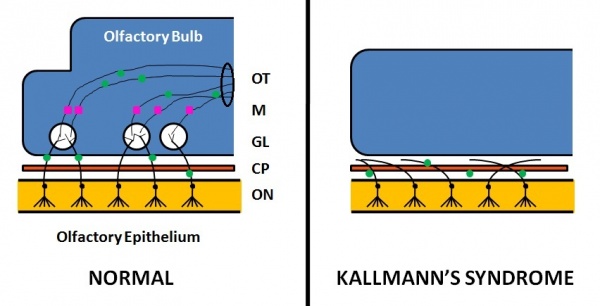
Kallmann's syndrome is a clinically and genetically heterogeneous disorder, described as a hypogonadotropic hypogonadism characterized by a diminished or absent sense of smell [30] [31]. The incidence of Kallmann's syndrome is uncertain but is estimated to occur in 1 in 10,000 to 1 in 50,000 people [2], affecting males to females in a 5:1 ratio [32]. Anosmia or hyposmia occurs as a results of impaired development of the olfactory bulbs and olfactory nerves [32]. Additionally, hypogonadism results due to the reduced production of Gonadotropin-releasing hormone (GnRH). Kallmann's syndrome can be inherited as an autosomal dominant,autosomal recessive trait, or an X-linked recessive trait [32].
Pathophysiology
The olfactory bulb is the first neuronal checkpoint for olfactory information[33]. The OB receives and processes sensory inputs from olfactory receptor neurons embedded in the olfactory epithelium and then transmits the information to the olfactory cortex[33]. During embryonic development, axons from olfactory receptor neurons exit the olfactory epithelium, grow toward the brain, and penetrate the OB where they synapse with the dendrites of mitral cells [33]. The axons of these neurons form the olfactory tract.
In Kallmann's syndrome, there are distinct abnormalities in the OB development arising due to the abnormal or lack of expression of certain proteins and genes. Kallmann's syndrome can be X-linked , autosomal dominant or autosomal recessive[32]. To date, mutations the six genes and the proteins they encode have been attributed to Kallmann's syndrome, though their functions are still being researched. However, only 30% of patients with a clinical diagnosis are found to have a mutation in these genes [34]:
- KAL1: Mutations in the KAL1 gene produce the X-linked form of Kallmann's syndrome. KAL1 gene encodes the glycoprotein anosmin 1 and is expressed in the outer neuronal layers of the developing olfactory bulb. [35]. Anosmin-1 stimulates lateral olfactory tract axon branching and outgrowth from OB towards the piriform cortex; this is through patterning of mitral and tufted cell axons to the olfactory cortex[33]. Consequently, in its absence, Kallmann's syndrome arises due to abnormal olfactory neuronal development[33]. Additionally, anosmin-1 has been shown to interact with FGFR1, explaining the digenic nature of Kallmann's syndrome[36].
- KAL2 (FGFR1): Produces the autosomal-dominant form of Kallmann's syndrome[36]. KAL2 encodes fibroblast growth factor receptor 1 involved in OB morphogenesis and GnRH neuronal development and migration[36][37].
- FGF8: Encodes the key ligand for FGFR1. FGF binds with high affinity to FGFR and induces receptor activation.
- PROKR2: Encodes the G protein-coupled receptor prokineticin receptor-2 which is known to be involved in intracellular Ca2+ signalling[38]. However, the exact role in Kallmann's syndrome has yet to be clarified[38]. In terms of mode of inheritance, monoallelic PROKR2 mutations are not sufficient to produce the disease phenotype; it is hypothesised that digenic or oligogenic inheritance of KS in patients heterozygous for PROKR2 mutations produce the disease phenotype[38].
- PROK2: Encodes the PROKR2 ligand[38].
As a result of the OB structural abnormalities and neuronal migration failures, olfactory signals from the environment cannot be transmitted to the cerebral cortex. Additionally, the failure of the GnRH neuronal migration to the hypothalamus results in a loss of a key path in the negative feedback loop for sex hormone production.
Clinical Features
Kallmann's Syndrome is a congenital hypogonadotropic hypogonadism (HH)[30]. Kallmann's Syndrome has the classical HH absence of puberty but is distinguished from other HH syndromes by an affected sense of smell. There exists additional characteristics that are not specific to Kallmann's syndrome but may aid in correct diagnosis of this particular HH [39]. The following characteristics of Kallmann's syndrome may be present or not present in different cases, often varying according to genotype [30]:
Reproductive Features
- Hypogonadotropism leading to failed or arrested puberty [40]
- Hypogonadism
- Cryptorchidism (males)
- Gynaecomastia (males)
- Absence of menstruation, amennorhoea (femaleS)
Non-Reproductive Features
- Affected sense of smell: decreased (hyponosmia) or absent (anosmia) sense of smell [40]. Anatomically, the olfactory bulbs and olfactory tracts demonstrate aplasia or hypoplasia [40].
- Eunuchoidism bone structure, defined by long limbs as a result of inadequate calcification[41]
- Unilateral renal aplasia [42]
- Cleft palate[40]
- Pes cavus [40]
- Neurological symptoms
- Synkinesia
- Abnormalities in eye movement
- Cerebellar ataxia
- Evoked horizontal nystagmus
- Sensorineural deafness
- Spatial attentional abnormalities
- Spastic paraplegia
- Mental retardation [40][43] [44] [45]
| Diagnosis
Due to the low incidence of Kallmann's syndrome, correct diagnosis is often delayed, despite early childhood signs such as anosmia and cryptorchidism [46]. Instead, doctors often dismiss Kallmann's syndrome as constitionally delayed puberty [46]. Other differential diagnoses include potential presence of hypothalamic or pituitary tumours[46]. Due to the varied phenotype and genotype of Kallmann's, multiple tests are required in order to properly diagnose the syndrome. The following diagnostic tests are often employed:
|
Treatment
Treatment
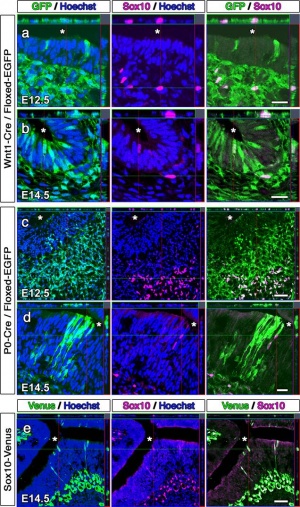
Current ResearchContribution of Neural Crest and Ectoderm to Nasal PlacodeA paperpublished last year explored the individual neural crest and ectodermal contributions to the nasal placode through the use of genetic Cre-lox tracing in two mice species. One mouse species was Wnt1Cre, a neural-crest specific line. The other species was Crect, an ectodermal specific line. The Cre-lox genetic tracing of the two species determined that olfactory ensheathing cells are neural crest in origin. Neural crest was also shown to contribute to cells of the olfactory epithelium and vomeronasal organ along with GnRH-1 neurons. The findings of this paper allowed provided an understanding of the link relating neural crest defects to diseases such as anosmia and Kallmann syndrome[49]. Contribution of Cranial Neural Crest to Olfactory SystemAnother paper also investigating the contribution of cranial neural crest cells in olfaction development used transgenic mice. The neural crest cells of these mice permanently express green fluorescent protein (GFP) which allowed them and their descendants to be traced. Analysis showed GFP-positive cells in the olfactory epithelium, olfactory ensheathing cells . Similar analysis of chick embryos demonstrated dissociated cells of the olfactory mucosa which displayed the ability to self-renew, suggesting the presence of neural crest progenitors in the olfactory mucosa. The paper concluded that the cranial neural crest contributed a larger portion than previously thought to the olfaction system and may be accountable for the olfactory epithelium’s ability to regenerate[50].
Specialisation of Olfactory Bulb and Epithelium Reliant on Specific GenesA study from August this year looked into the effect of genes Neurog1 and Neurog2 on cell specialisation in the olfactory bulb and olfactory epithelium. It was concluded that Neurog1 and Neurog2 are both necessary for the development of the olfactory system and are reliant on interactions between the olfactory bulb and olfactory epithelium. One particular part of the research looking to determine whether Neurog1 and Neurog2 were required for olfactory bulb development utilised a loss-of-function technique to compare single and double null mutants. It was concluded that Neurog1 is required for correct growth and lamination of the olfactory bulb and that Neurog1 and Neurog2 are required for overall bulb morphogenesis[51]. Migratory Path of GnRHAnother paperalso published this year examined the migratory path of Gonadtropin-releasing hormone (GnRH) neurons and how this path is modulated by members of the Slit-Robo group of ligand ligand-receptors. Gonadtropin-releasing hormone neurons originate in the nasal placode and migrate by the olfactory and vomernasal axons to the hypothalamus in the forebrain. GRH is responsible for regulation of reproduction in mammals. Deficiency in it causes hyopgonadotropic hypogonadism and Kallmann syndrome. The current study used genetically altered mouse models to demonstrate the role of Slit2 and Robo3 in GnRH migration. Mice lacking Slit2 were found to have fewer GnRH neurons compared to wild type mice with Slit2[52] SEMA3A deletion and Kallmann syndromeA recent study[53] published in the Oxford Medicine's Human Reproduction Journal sought to identify new genes responsible for Kallmann's syndrome(KS) by conducting a comparative genomic hybridization array on KS patients with no mutations in known KS genes. A family with a history of KS was involved in the study and lead to the discovery of a heterozygous deletion at locus 7q21.11. Further investigation found that this was a deletion of the gene SEMA3A. SEM3A codes for semaphorin 3A, a protein that interacts with neuropilins: transmembrane glycoprotein receptors in neurons[53]. Moreover, analysis of the pattern of KS incidence in the family in conjunction with genetic testing found the mutation to be autosomal dominant[53]. In order to consolidate the link between SEMA3A deletion and KS, the study looked to the literature. It was found that studies with semaphorin 3A-knockout mice have a KS phenotype: abnormal migration of GnRH neurons to the hypothalamus as a result of faulty signal transduction[53]. Colony Stimulation Factor-1 Receptor and Embryonic Olfactory DevelopmentA study by Erblich et al. [54] sought to study the transmembrane tyrosine kinase receptor for colony stimulating factor-1 (CSF-1R). Mice homozygous for a null mutation (-/-) in the Csflr gene as well as mice homozygous for non-mutated Csflr (+/+) were utilised to study CSF-1R function. Antibody staining for CSF-1R showed expression of CSF-1R in the microglia but not in the astrocytes, neurons or glial cells. In contrast, the -/- mice showed no CSF-1R expression. Moreover, cell counts showed that in -/- mice, the microglial numbers declined within three weeks of birth. The microglial depletion in -/- mice was accompanied by abnormal structural integrity of the brain: whilst the brain size remained normal, there was significant ventricular enlargement with reduced parenchymal volume. From these findings, it is apparent that CSF-1R has an importnt role in microglial development and normal brain architecture. In regards to the olfactory bulb, there was an apparent reduction in size for the -/- mice but no obvious change in structure. However, the olfactory bulb was hollowed out in the -/- mice as a result of enlargement of the cerebrospinal fluid compartment impinging onto the olfactory ventricle. Testing for olfactory deficits revealed that an absence of Csf1r gene is anosmic. These findings show that CSF-1 is required for the function and integrity of the olfactory system.[54] Lhx2-dependent Integration of Olfactory, Vomeronasal, and GnRH NeuronsWhen the LIM-homeodomain 2 gene (Lhx2) is normally expressed in the forebrain, the olfactory bulb, as well as in olfactory sensory neurons (OSNs) and vomeronasal sensory neurons (VSNs)[55]. When Lhx2 is not expressed, specification of olfactory sensory neurons (OSNs) becomes abnormal[55]. A study[55] published in 2012 sought to identify the exact consequences of absent Lhx2-dependent OSN specification on the development of the primary olfactory pathway. The method involved utilising transgenic mice with inactivated Lhx2 gene in OSNs but not in VSNs the olfactory bulb, or the forebrain. The study found that Lhx2-dependent OSN specification is essential for synapses between OSN and target neurons in the olfactory bulb. Moreover, the mutant phenotype showed that expansion of the olfactory bulb is dependent on innervation of the bulb by OSNs expressing Lhx2. Additionally, Lhx2-dependent maturation of OSNs is required for formation of the vomeronasal nerve and the migration of gonadotropin-releasing hormone (GnRH) cells toward the developing hypothalamus. The implications of these findings to olfactory research are a further understanding of the innervation mechanisms of the olfactory bulb during development. Moreover, the findings of the study can aid in understanding congenital olfactory defects. GlossaryAplasia: Absent development of an organ or tissue. Anosmia: Lack of smell. Cerebellar ataxia: Reduced control over muscle coordination arising from defects or damage to the cerebellum. Cryptorchidism: Failure of one or both testes to migrate into the scrotum during male foetus development. Eunuchoidism: Male hypogonadism characterised by the failure of the testes to develop and an absence of secondary sexual characteristics. Gynaecomastia: The development of abnormal mammary glands in males characterised by enlarged breasts. Hypogonadism: A state which described reduced or absence of hormone secretion by the gonads (ovaries or testes). Hypogonadotropism: Reduced or absent gonadotropin secretion, often characterised by FSH and LH deficiency leading to testicular or ovarian dysfunction. Hypoplasia:Incomplete development of an organ or tissue. Nystagmus: Refers to fast involuntary movements of the eyes that may impair vision. Can be described as a "rapid flicking side to side" movement. Olfactory bulb: The primary part of brain which processes olfactory information. Olfactory epithelium: mucous membrane superior to the nasal cavity which contain olfactory nerve cells. Olfactory nerve cell: Cells in the olfactory epithelium which detect various odors and signal the information to the CNS. Pheromone: Any molecules (scent) released by animals and affect the behavior of organisms of the same species via the olfactory system. Pes cavus: A deformity of the foot characterised by an overexaggerated arch and hyperextension of the toes. Also referred to as clawfoot. Spastic paraplegia: A hereditary paraplegia characterised by stiffness and contraction in the lower limbs as a result of neuronal dysfunction. Synkinesia: Refers to the ability to conduct voluntary movements, however, with accompanied involuntary muscular movements. Vomeronasal Organ: To do with specific reproductive olfaction e.g. the detection of pheromones References
External LinksDevelopment of the Olfactory System The Development of the Olfactory System 2 General Physiology of Olfaction Anatomy and Physiology of Olfaction
--Mark Hill 12:22, 15 August 2012 (EST) Please leave the content listed below the line at the bottom of your project page. 2012 Projects: Vision | Somatosensory | Taste | Olfaction | Abnormal Vision | Hearing |