2012 Group Project 3: Difference between revisions
| Line 237: | Line 237: | ||
{| | {| | ||
| [[File:Figure 1 Spry1-2.jpeg|160px|]] | | [[File:Figure 1 Spry1-2.jpeg|160px|thumb|]] | ||
| [[File:CVP of WT(top) and DKO(bottom) mice with H&E and SEM.png]] | | [[File:CVP of WT(top) and DKO(bottom) mice with H&E and SEM.png|thumb|]] | ||
|- | |- | ||
| <center>[[Location and Number of CVP]]</center> | | <center>[[Location and Number of CVP]]</center> | ||
| <center>[[CVP of WT(top) and DKO(bottom) mice with H&E and SEM | | <center>[[CVP of WT(top) and DKO(bottom) mice with H&E and SEM]]</center> | ||
|} | |} | ||
Revision as of 13:25, 5 October 2012
Taste Development
You are out to dinner and your meal arrives in front of you, the aromas and the presentation of your food strikes you immediately. Half way through, the waiter returns and asks “How does your meal taste?” The answer is not as simple as one might think. Why does the meal taste pleasant? How do we experience different tastes? In the following page, we aim to discern how exactly one would answer these questions by looking at the embryological development of the taste system from week to week.
Taste is a complex sensory system and its precise workings are to this day not fully understood. What makes it so complicated is the interaction between the structure of tongue and taste buds and how this contributes to the representation of taste qualities (salty, bitter, sweet, sour and umami) in the brain. We will also look at some current research on animal models being undertaken to evaluate the role of specific genes in the development of sensory structures. By looking at the taste system’s genetic basis, we now have a greater understanding of the abnormalities that may occur during development and a direction to pursue in future research to attempt to fill in the gaps of scientific knowledge.
History of Discoveries
| Date | Significant Discovery |
| 350BC | Aristotle writes about the basic tastes, sweet and bitter. He also notes that it can be modified by salty and acidic. [1] |
| 1901 | D. Hanig publishes a paper describing taste sensitivity in different regions of the tongue.
Interesting fact: The modern concept of a 'tongue map' is a misinterpretation of this study. [1] |
| 1908 | Kikunae Ikeda, a professor of the Tokyo Imperial University, discovered and identified the fifth basic taste: umami (savouriness), made palatable by glutamate. [2] |
| 1931-32 | A chemist named Arthur Fox and his college noted that they had different sensitivities to the bitter tasting Phenylthiocarbamide (PTC).[3] Geneticists later confirm these findings, and discover that non-tasting is a recessive genetic trait. [4] |
| 1965 | Farbman's study of the developing taste but in rat fungiform papilla was significant in increasing our understanding of taste bud development. [5]
Electron microscope study of the developing taste bud in rat fungiform papilla. |
| 1992 | McLaughlin SK et al. discovery a taste cell-specific G-protein within the taste buds called Gustucon. This protein is later used to mark bitter, umami and sweet cells. [6]
Gustducin is a taste-cell-specific G protein closely related to the transducins |
| 1995 | Barlow et al. experimented with Axolotl salamanders and concluded that taste buds from this species arise exclusively from epithelial tissue, "oropharyngeal epithelium". [7] |
| 1996 | Witt M and Reutter K of the Technical University Dresden in Germany, carried out a transmission electron microscopy study to investigate the embryonic and fetal development of Human taste buds. Their results suggest an "at least dual function of embryonic/fetal taste buds", including non-gustatory, paracrine functions prior to the 14th week and gustatory after the 14th week. [8] |
| 2000 | Chandrashekar, J. et al. discover the first taste sonsors, the T2R bitter taste receptors. [9] |
| 2001 | Nelson, G. et al. discover the sweet receptor: a combination of T1R2 and T1R3. [10] |
| 2002 | Nelson G. et al. discover the amino acid (umami) taste receptor: a combination of T1R1 and T1R3 identified. [11] |
| 2005 | Dyer, J. et al. discover sweet taste receptors in the GI tract. [12] |
| 2006 | Huang, A. L et al. discover cells for sour taste, identified by PKD2L1 (a polycystic kidney disease-like ion channel). [13] |
| 2007 | Harlow DE, Barlow LA of the University of Colorado Denver Health Sciences Center provide evidence of the "embryonic origin of gustatory cranial sensory neurons". [7] |
| 2009 | A study by Hevezi P et al, presents "the first comprehensive characterization of gene expression in primate taste buds", as opposed to previous studies which focused on rodents. [14]
Genome-wide analysis of gene expression in primate taste buds reveals links to diverse processes |
| 2010 | Chandrashekar, J. et al. identify epithelial sodium channel (ENaC) as the sodium-salt taste receptor. [15]
The cells and peripheral representation of sodium taste in mice |
Adult Tongue and Taste Buds – Structure and Function
Structure
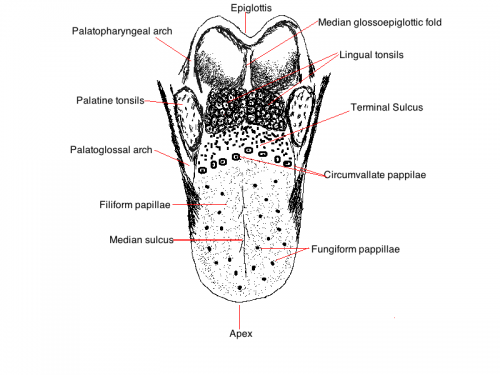
The tongue is located on the floor of the oral cavity, It is a muscular structure with sensory cells found on the surface. The tongue is divided into an anterior two thirds and a posterior one third. These regions are divided by a V-shaped groove at the back of the tongue (sulcus terminalis). The anterior two thirds of the tongue is covered by stratified squamous epithelium, It contains a roughened surface and has projections called papillae.
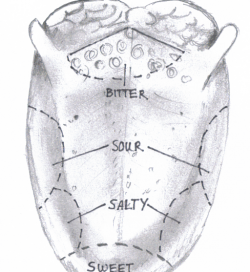
'The image below is a simplistic diagram of the surface of the tongue showing the locations of the different papillae and other important features
|
Papillae There are 4 types of papillae on the tongue. The most numerous papillae are the filiform papillae, which function to provide a surface that aids in holding food on the tongue during chewing but do not contain taste buds. The larger, less numerous fungiform papillae which contain taste buds, as do foliate papillae. Circumvallate papillae form a wide V at the sulcus terminalis also containing taste bus. There are no papillae or taste buds located on the posterior third of the tongue, having mucosal folds and the lingual tonsils instead.[2]
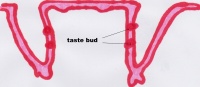
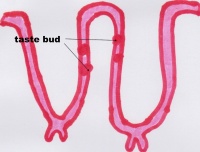
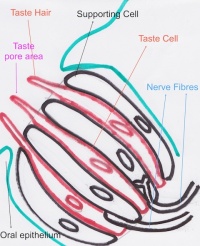
Function The Tongue has muscular and sensory functions, muscular functions include swallowing and speech where as sensory function involves taste. This section will focus on the sensory functions of the tongue. The functional unit of the taste bud is a taste cell, there are between 50 and 100 taste cells in each taste bud these taste cells represent all 5 different tastes. Historically is was believed that different areas of the tongue were responsible for different taste sensations although this has since been disregarded.[3] The papillae contain taste buds which are connected to the oral cavity via a taste pore, the function of the taste bud is to transmit a chemical signal from the oral cavity to a taste cell, this chemical signal is the converted into an electrical impulse and delivered to the brain via nerve fibres for interpretation. [4]
The pathways of smell and taste are very similar (chemosensation). Pathways both involve the conversion of a dissolved chemical stimulus transformed into a electrical impulse for interpretation by the brain. Taste and smell overlap when humans experience flavour, flavour involves an interaction of taste, smell, texture and temperature, where taste is only a portion of this interaction. when the brain interprets a flavour, contribution from other senses has a significant role in identification. In conclusion Taste and Smell in combination with other senses help develop a sensory interpretation called flavour.[5] Human Weekly Development - Two Prominent StudiesMartin Witt and Klaus Reutter of the University of Tubingen in Germany published two prominent studies regarding developing taste buds in humans. Their first study in 1996 was a transmission electron microscopical (TEM) study of the taste bud primordium and its morphological changes during the 8th-15th postovulatory week. Their next study in 1997 built on their previous findings by using Scanning Electron Microscopy (SEM) to observe the development of gustatory papillae during postovulatory weeks 6-15.
Timeline of Developmental Processes of Human Taste Buds
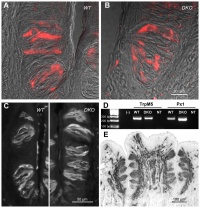
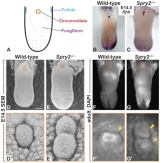
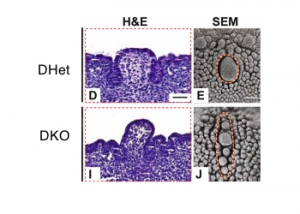
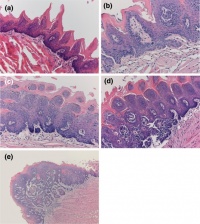
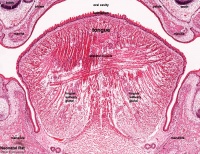
AbnormalitiesIt should be mentioned that gustatory abnormalities have not been widely researched. Most research conducted in this field has been conducted through animal testing on mice and this is the basis of information provided below. Knocking out P2X ReceptorsThe sensation of taste is made through neurotransmitters. But what happens when there is a disruption in this 'transmission', either through not releasing neurotransmitter or by a reduction in receptor number? Huang, 2008 [17] use the the premise that P2X receptors are essential in taste transduction and that their absence would lead to the inability to taste. This team used to the neurotransmitter ATP, as a quantitative measure of gustatory sensation and taste. ATP is only one neurotransmitter that is involved in taste transduction among others including "acetylcholine, glutamate, norepinephrine (NE), serotonin (5-HT), γ-aminobutyric acid (GABA) and a number of peptides." This was done by using a comparison of wild type (WT) and double knockout (DKO) mice. P2X receptors, namely P2X2 and P2X3, were knocked out in the DKO mice. The premise of this article was that knocking out P2X receptors reduces transmitter secretion of ATP in taste buds, therefore they cannot taste. A tastant was administered to mucosal lingual epithelium the tongue of the nice and collected. The release of ATP was measured using luciferase and IHC (Immunohisto Chemistry). It should be noted that the taste buds in DKO are functional, but are not stimulated by the administration of tastants. The transmission of release of ATP is secreted through gap junction hemichannels (pannexin 1 gap junction). When both P2X2 and P2X3 are knocked out, no taste is elicited. However they found that if either P2X2 OR P2X3 was knocked out there was a taste response. So the inference made from this is that if one of the two receptors from the P2X family was knocked out there still can have taste response. The WT mice showed significant stimulation by tastants whereas DKO had little to no stimulation of ATP release.[18] FGF signalling and genesSprouty, or Srpy, genes have been related to regulating the development of circumvallate papillae (CVP). The CVP are large dome shaped papillae, which form a 'V' just in front of the terminal sulcus (see image under 'Structure of the tongue'). By knocking out Spry genes using mice which had both Spry1 and Spry2 knocked out showed that the number of CVP doubled. However, when Fibroblast Growth Factor gene ("Fgf10") was absent, the number of CVP was significantly reduced, if not completely absent. The correlation between Spry1/2 and Fgf10 is that Spry1/2 antagonizes "Fgf10" to limit the size of the CVP progenitor placode. Exclusive expression of Fgf10 in the mesenchyme is necessary for the formation of CVP.
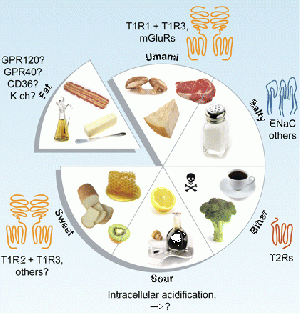
Taste buds develop from the mesenchyme but require local signalling to properly differentiate. Some signalling factors for proper development of taste buds besides FGF are: Sonic Hedgehog (SHH), Bone Morphogenetic Proteins (BMPs), Epidermal Growth Factor (EGF). Additionally, this proves that the anterior and posterior developments of the tongue are derived from embryonic tissues, where the anterior tongue is derived from the ectoderm and the posterior tongue is derived from the endoderm. Taste buds develop from the mesenchyme but require local signalling to properly differentiate. Some signalling factors for proper development of taste buds besides FGF are: Sonic Hedgehog (SHH), Bone Morphogenetic Proteins (BMPs), Epidermal Growth Factor (EGF). As mentioned above, this article shows that the anterior and posterior developments of the tongue are derived from embryonic tissues, where the anterior tongue is derived front the ectoderm and the posterior tongue is derived from the endoderm. Secondly, FGF is required to regulate the growth of taste buds, while Spry genes limit the number of CVP. Fgf10& Spry 1&2work antagonistically through receptor tyrosine kinase (RTK) signalling. However, the deletion of Spry2led to the increase of CVPs it should be noted that they found that a significant decrease in number of fungiform papillae. In contrast, the absence of Fgf10, while leading to the absence of CVPs lead to an increase in number and size of fungiform papillae. Therefore these genes have different effects on the anterior and posterior developing tongue and taste buds.[19] Gustatory SystemTaste or more appropriately gustation, is a fundamental survival tool in animals as it directs the consumption of essential nutrients. The five tastes that exist within the human gustatory system: salty, sweet, sour, bitter and umami, all signify basic physiological requirements. Salty tastes denote the presence of Na+, an important ion involved in the transportation and retention of water across cell membranes. Sweetness is the recognition of carbohydrates, essential for maintaining optimal brain function and providing the basis for energy production in muscle tissue via ATP hydrolysis. Similarly umami codes for the presence of L-amino acids, especially L-glutamate which is an integral component of protein synthesis.
Cell BiologyThe five taste qualities are not all detected by the same type of receptor cell located within the taste bud. Like rods and cones in the eye which detect different wavelengths of light, there are specific types of receptors for different tastes. For example, sweet, umami and bitter are recognized by Type II G-protein coupled receptors, whereas sour is related to Type III presynaptic cells. The cell type involved in salty taste transduction is unknown however it is known that sodium ions can enter the receptor cell membrane via ion channel permeation. Type II receptorsWhen a bitter, sweet or umami ligand binds to a type II G-coupled receptor, a cascade of chemical reactions causes the release of Ca2+ which in turn mediates exocystosis of ATP. The function of this ATP is threefold:
The role of serotonin is believed to be in the form of lateral inhibition i.e. when a bitter quality is recognized, adjacent receptors for sweetness are deactivated and thus the two tastes may be clearly differentiated. It also has a negative feedback effect on receptor cells, inhibiting umami, bitter and sweet taste transduction. Taste MapThe idea of a tongue map has disseminated through society for many years. This concept purports that different areas of the tongue are specialized to detect either sweet, salty, sour and bitter tastes. Recent research however has completely nullified such claims and suggests instead that different forms of taste are recognized all over the tongue as well as via the palate. Thus the term taste map has come to take on a new meaning and that is, the precise areas of taste modalities processing in areas of cortex and its subsequent neural inputs. Neural PathwaysFirst order neuron - From the receptors located in the taste buds, gustatory nerve afferents project to the ipsilateral rostral 1/3 of the nucleus tractus solitarius (NTS), located in the medulla. This rostral 1/3 is commonly referred to as the gustatory nucleus. The gustatory nucleus receives input from cranial nerves VII (Facial n.), IX (Hypglossal n.), and X (Vagus n.) via special visceral afferent (SVA) nerve fibers. A summary of their functions is as follows:
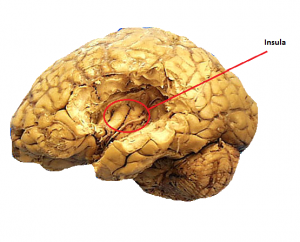
Copious scientific conjecture surrounds how each taste modality is transmitted to the brain. The ‘labelled-line’ hypothesis suggests that there are taste specific neurons which exclusively carry that taste modality to cortical areas. In analogous terms it can be viewed like the pipelines leading toward a house. Each pipeline carries its own utility, there is one for gas, another for water and finally for electricity with each terminating in slightly different areas of the house. In the same way, on the tongues there are different receptors for each taste which concurrently have individual nerve tracts leading to the primary gustatory cortex. Contrapuntally there is electrophysiological evidence that single nerve afferents carry multiple modalities. These studies show one nerve afferent may have both a strong and a weak activation in response to a multiple taste stimuli. Cortical AreasThe location of the taste perception centers has been observed via functional magnetic resonance imaging (fMRI) studies. The primary taste cortex has been identified as being located in the anterior insula/frontal operculum (I/fO)[22], with the secondary taste cortex in the caudolateral orbitofrontal cortex.[23] Within the insula there are further subdivisions related to each taste modality. Two photon calcium imaging research has outlined certain ’hot-spots’ of activation which are clearly delineated in relation to each taste. For example, the bitter modality is represented on the insula cortex approximately 1mm posterior to the middle cerebral artery whereas the sweet modality is represented 2.5mm rostrodorsal to the bitter field with no apparent overlap.[21] These findings lend weight to the idea that there is only one receptor for each taste quality.
Neuronal DevelopmentThe gustatory system must not be solely thought of as being comprised of simply the tongue and palate. The types of neurons which carry taste information to the brain are also pivotal in constructing the expression of taste qualities. The two main gustatory neurons which develop are the geniculate ganglion and the petrosal ganglion. These are the visceral sensory ganglion of cranial nerves VII (Facial) and IX (Hypoglossal) respectively which carry taste information from the tongue to the nucleus tractus solitarius. They arise at the posterior placodal region from the epibranchial placodes, a thickening of ectoderm. The first epibranchial placode gives rise to the geniculate ganglion and the second to the petrosal ganglion. The molecular basis for gustatory neuron formation is not completely understood though there are a number of transcription factors which are thought to play an important role in firstly, placode formation and secondly, neuron differentiation.
The neurons involved in the gustatory system are overproduced and thus in the embryonic stage, undergo programmed cell death (apoptosis). The total amount of neurons in the geniculate ganglion, appears to be quite stable over the embryonic stages. This suggests, unlike the petrosal ganglion, the levels of neuroblast proliferation and apoptosis are fairly similar. The factors which regulate these processes remains unclear. It is posited that neurotrophins BDNF, NT4/5 and NT3 may regulate neuronal survival whilst also playing a part in axon growth from the sensory ganglion. More research is required to discern the exact individual functions of each of the neurotrophins. Current Research
In an animal study using mice by Suzuki Y, Ikeda K, Kawakami K.(2011) Firstly nominating "Six Genes" as a major component in gustatory development, stating that deficiencies in certain Six genes (specifically Six1 & Six4) leads to poor development. Their research also highlights evidence of cooperative relationships between Six genes for normal advance. This experiment involved breeding mice containing exclusively Six1 and Six4 genes and examining the expression of these genes in papillae under high powered microscope observation. Understanding the role of certain genes along with the intrinsic relationships they hold is crucial for the ability to identify possible causes and correction of any abnormalities [24]
Neural Crest responsibilities Another Animal Study involving mice explores a new idea of Neural crest (NC) contribution in taste development, specifically the development of papillae and taste buds. Liu HX, Komatsu Y, Mishina Y, Mistretta CM. (2012) suggest that Neural crest cells travel to the location of the tongue in early embryonic stages, gain "epithelium" phenotypes, multiply and then differentiate to eventually form taste papillae. The experiment involved the comparison of 2 different types of Cre line mice, which both express Cre gene in neural crest protocol, the different distribution patterns where observed in specific regions that NC is responsible for.[25]
In an animal study conducted by Rothova M, Thompson H, Lickert H, Tucker AS.(2012) exploring the historically debated issue of "endoderm" contribution to tongue development showed promising evidence that position of taste buds are patterned by the border of "ectoderm" and endoderm derivative epithelium. This study was accomplished via microscopic examination of previously stained specimens. Concluding endoderm has direct influence on gustatory development [26]
Changes in Taste Cells over time Research by Ozdener H, Spielman AI, Rawson NE.(2012) developing a culture which allows taste cells to survive for up to 12 months, empowers researchers to study the processes of proliferation, differentiation and function. This experiment will provide a precedent for future study of taste cells, as these cells are able to operate and grow normally. [27]
A tamoxifen treatment which suppresses "Sonic hedgehog" (Shh) secretion in mice proving to reduce the number of cells visible within papillae, in contrast mice not treated with tamoxifen showed a mark increase of cells within papillae, these cells are assumed to become taste cells in later development. Specific mice were bred to trace the destination of taste placode cells, the study concluded that Shh expressing placodes are taste bud proginators which in turn become taste cells within taste buds although do not have any precursors relationship with papillae. The results were obtain by examination of mice embryos using Bright-field or multichannel fluorescent images through the use of an "Axiocam CCD camera and Axioplan fluorescence microscope with Axiovision software" a study by Harlow, Yang, Williams, Barlow (2011)[28]
WNT family exhibit various roles
This method was used to show that cell populations which contained only blue or red cells could have progenitor cells migrate in. If the progenitor cell had the same colour, then it would be from the same origin. If not, a different progenitor origin. They found that the cells are likely to originate from local epithelium due to local tissue interactions. However, it was also found that lingual epithelium which had the potential for form taste buds were not necessarily from ectodermal or endodermal origins. Glossary
References
External LinksExternal Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation. 2.http://embryology.med.unsw.edu.au/embryology/index.php?title=2012_Group_Project_4 3.http://www.webmd.com/oral-health/picture-of-the-tongue 5.http://www.cf.ac.uk/biosi/staffinfo/jacob/teaching/sensory/taste.html --Mark Hill 00:19, 5 October 2012 (EST) There should be text with these links.
2012 Projects: Vision | Somatosensory | Taste | Olfaction | Abnormal Vision | Hearing |