2011 Group Project 9: Difference between revisions
No edit summary |
|||
| (354 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{2011ProjectsMH}} | {{2011ProjectsMH}} | ||
=Williams-Beuren Syndrome= | |||
==Introduction== | |||
[[File:Facial features of four individuals with Willams Syndrome.gif|400px|thumb|'''Figure 1: Facial Characteristics of four individuals with Williams Syndrome''']] | |||
--- | Williams-Beuren Syndrome, more commonly known as Williams Syndrome, is a [[#Glossary | '''congenital abnormality''']] caused by the deletion of genetic material on chromosome 7q11.23, which encompasses 28 neighbouring genes.<ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> <ref>[http://omim.org/entry/194050?search=williams%20syndrome&highlight=william%20syndrome OMIM Entry #194050-Williams-Beuren Syndrome]</ref> | ||
This multisystem developmental genetic disorder implicates psychological, behavioural and medical defects, including diverse phenotypic characteristics such as distinctive facial deformities, cardiovascular abnormalities,intellectual disabilities/mental retardation, growth abnormalities, endocrine abnormalities and a unique personality and cognitive profile. <ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> <ref name="PMID19568270"><pubmed>19568270 </pubmed></ref> Some or all of these features may be present in varying degrees, with the condition becoming apparent at the onset of birth or in early infancy. <ref name="PMID21107555"><pubmed>21107555 </pubmed></ref> | |||
- | Williams Syndrome, in a majority of cases, is not inherited. It can manifest in families with no history of the disorder, this is because Williams Syndrome is the result of a random event that occurs at gamete formation in the parent of a Williams Syndrome patient. Only a single copy of the altered chromosome 7 in each cell is required to cause this syndrome, therefore it is considered to be an autosomal dominant condition. There is only a small percentage of cases where people inherit the chromosomal deletion associated with Williams Syndrome from a parent with the condition. <ref>Genetics Home Reference 2011, Williams Syndrome, U.S. National Library of Medicine, viewed 10 October 2011, <http://ghr.nlm.nih.gov/condition/williams-syndrome></ref> | ||
Ever since it was discovered that the cause of this genetic abnormality was a [[#Glossary | '''microdeletion''']] in chromosome 7, one of the main focuses of research has been attempting to identify the different responsibilities each gene of the deletion has for the function and development of the brain as well as their role in physical characteristics. These research efforts have the common aim to provide knowledge of how cognitive, behavioural and physical features arise as a result of the gene absence and their interplay with the environment. | |||
Current research into the links between genes absent in Williams Syndrome and the brain, behaviour and structural and functional abnormalities have been confirmed and are continuously being investigated through the use of mouse models. <ref name="PMID16272111"><pubmed>16272111 </pubmed></ref> The exact links between the missing genes and the [[#Glossary | '''phenotype''']] of Williams Syndrome is yet to be fully explored. | |||
=Williams-Beuren Syndrome | '''Links:''' [[Williams Syndrome]] | [http://omim.org/entry/194050?search=williams%20syndrome&highlight=william%20syndrome OMIM Entry #194050-Williams-Beuren Syndrome] | ||
== | ==History of the disease== | ||
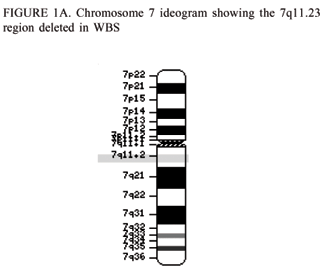
[[File: Chromosome 7, indicating 7q11.23 region of Williams Syndrome.gif|500px|thumb|'''Figure 2: Chromosome 7 indicating the region,7q11.23, that contains the genes which are deleted in Williams Syndrome''']] | |||
William-Beuren Syndrome is named after John C.P. Williams, a cardiologist from New Zealand, and Alois J Beuren, a German physician and cardiac researcher. | |||
Initial investigation into Williams-Beuren Syndrome came from two apparently different disorders, [[#Glossary | '''idiopathic infantile hypercalcemia''']] and [[#Glossary | '''Supravalvular Aortic Stenosis(SVAS)''']] . With further research, these abnormalities were identified as being aspects of this same syndrome. <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> | |||
The first cases related to Williams Syndrome involved idiopathic infantile Hypercalcemia. From as early as 1952, research into infantile hypercalcemia, by Falconi et.al, found that children with this disorder had common clinical characteristics such as a short stature and a variety of [[#Glossary | '''congenital malformations''']]. <ref name="PMID12980492"><pubmed>12980492 </pubmed></ref> In the years that followed many other studies were conducted in this area revealing additional correlations. For example in 1957 Stapleton and colleagues studied the effects of hypercalcemia in a number of infants and noted several consistencies between them including abnormal facial features, failure to thrive, developmental delay, and [[#Glossary | '''systolic murmurs''']] of the heart, all of which have now been associated with Williams Syndrome. <ref name="PMID13469755"><pubmed>13469755 </pubmed></ref> <ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> | |||
J.C.P Williams was one of the first to recognise some of the clinical factors associated with this syndrome. In a study conducted in 1961 of SVAS, an obstruction occurring in the [[#Glossary | '''left ventricular outflow tract (LVOT)''']], Williams and his colleagues made the observation that patients suffering from this heart condition had strikingly similar unusual facial features that included broad foreheads, eyes set wider apart than normal, wide mouths with pouting lips and [[#Glossary | '''malocclusion''']] of teeth, pointy chin and prominent pointed ears. As well as this they discovered their subjects also presented with mental retardation and a low IQ. Williams and his colleagues suggested that their findings might be ''"indicative of a previously unrecognised syndrome."'' <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> <ref name="PMID14007182"><pubmed>14007182 </pubmed></ref> | |||
In this same year Rashkind and colleagues published a paper detailing the relationship between idiopathic childhood hypercalcemia and cardiac abnormalities <ref name="PMID13739645"><pubmed>13739645 </pubmed></ref> <ref name="PMID18941598"><pubmed>18941598 </pubmed></ref> | |||
In 1962 AJ Beuren and associates also studied the correlations between | In 1962 AJ Beuren and associates also studied the correlations between SVAS, mental retardation and distinctive facial features of a number of subjects and made similar observations to Williams, particularly pointing out the characteristic “elfin” features of Williams syndrome patients. <ref name="PMID13967885"><pubmed>13967885 </pubmed></ref> <ref name="PMID18941598"><pubmed>18941598 </pubmed></ref> Beuren also noted the behavioural traits of his subjects suffering from SVAS, describing them as all having a “friendly nature”, something which would later be recognised as one of the unique personality traits of people diagnosed with Williams-Beuren Syndrome. <ref name="PMID13967885"><pubmed>13967885 </pubmed></ref> <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> | ||
In further studies conducted in 1964, Beuren and his colleagues detailed the possible association of Peripheral Pulmonary Stenosis and complex dental malformations with | In further studies conducted in 1964, Beuren and his colleagues detailed the possible association of [[#Glossary | '''Peripheral Pulmonary Stenosis (PPS)''']] and complex dental malformations with SVAS, mental retardation and certain facial appearance which they examined previously. They too came to the conclusion that these complications were representative of a new syndrome. <ref name="PMID14136289"><pubmed>14136289 </pubmed></ref> <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> | ||
===Timeline=== | ===Timeline=== | ||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
| bgcolor="springgreen" | '''Year''' | |||
| bgcolor="springgreen" | '''Events''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen" |'''1950s''' | |||
|Increasing awareness and research into infantile idiopathic hypercalcemia <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen" |'''1952-1957''' | |||
|Studies conducted by several researchers on infantile hypercalcemia revealed correlations between it and several defects known to be characteristic of Williams Syndrome today. These included abnormal facies, developmental delay, mental retardation and heart palpitations <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> <ref name="PMID12980492"><pubmed>12980492 </pubmed></ref> <ref name="PMID13469755"><pubmed>13469755 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen" |'''1961''' | |||
|J.C.P Williams and colleagues were the first to suggest that SVAS may be an element of a previously unidentified syndrome which results in mental retardation and an abnormal facial structure. <ref>Hagen, J.M. van,2007''Williams Syndrome: from genes to clinical features,''Erasmus University Rotterdam,Rotterdam</ref> <ref name="PMID14007182"><pubmed>14007182 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1962''' | |||
|A.J Beuren and peers made same observations as Williams detailing links between SVAS, mental retardation and abnormal faces, specifically describing them as “elfin-like.” They were also the first to highlight the “over-friendly nature” of their subjects. <ref name="PMID13967885"><pubmed>13967885 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1963''' | |||
|Black and Bonham-Carter, from England, drew attention to the fact that those individuals who had been affected by Infantile Hypercalcemia had very similar facial features to those patients with SVAS <ref name="PMID14055045"><pubmed>14055045 </pubmed></ref> <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1964''' | |||
|Beuren and colleagues made associations between Peripheral Pulmonary [[#Glossary | '''Stenosis''']] and complex dental malformations with SVAS. Like Williams, they too believed these were signs of a common undiscovered syndrome. <ref name="PMID14136289"><pubmed>14136289 </pubmed></ref> | |||
In this same year a further advancement into research that links Idiopathic Hypercalcemia with SVAS was made by R.Garcia and associates. They presented a case which proved that these two conditions were related and proposed both were symptoms of “a newly recognized syndrome.” <ref name="PMID14148234"><pubmed>14148234 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1970''' | |||
|The above reports all contributed to a large series of phenotypes that now became known as 'Williams Syndrome' or 'Williams elfin facies syndrome'. Further research into specific cases continued to reinforce this newly identified syndrome. <ref name="PMID1133652"><pubmed>1133652 </pubmed></ref> <ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1972''' | |||
|Beuren presented compelling evidence that “Williams syndrome” and infantile hypercalcemia are the same disorder. <ref>Hagen, J.M. van,2007''Williams Syndrome: from genes to clinical features,''Erasmus University Rotterdam,Rotterdam</ref> | |||
|- | |||
| bgcolor="springgreen"|'''1980s''' | |||
|Research continued and revealed further disabilities associated with Williams Syndrome, including physical characteristics such as, growth problems, joint limitations, genitourinary problems and other developmental disabilities, <ref name="PMID2456379"><pubmed>2456379 </pubmed></ref> giving it the title of a multisystem disorder. <ref name="PMID8475063"><pubmed>8475063 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''1993''' | |||
|A major discovery was made by Morris et.al. Genetic research conducted on large and small families affected by Williams Syndrome revealed that the SVAS phenotype was closely linked with several DNA markers, including elastin, along the long arm of Chromosome 7. <ref name="PMID8475063"><pubmed>8475063 </pubmed></ref> | |||
Further research into this concluded that the mutation of the Elastin gene on chromosome 7 was the cause of SVAS. <ref name="PMID8096434"><pubmed>8096434 </pubmed></ref> <ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> This was rapidly confirmed in many other studies and led to the first laboratory test of the disorder. <ref name="PMID20425781"><pubmed>20425781 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"|'''2006''' | |||
|In recent years, studies conducted on Williams Syndrome patients with the use of functional [[#Glossary | '''MRI''']] of the brain, demonstrated changes linked to the behavioural characteristics of this condition, for example difficulties in [[#Glossary |'''visualspatial construction''']]. <ref name="PMID21120465"><pubmed>21120465 </pubmed></ref> <ref name="PMID16760918"><pubmed>16760918 </pubmed></ref> | |||
|} | |||
'''Links:''' [http://www.wsf.org/family/news/perspective.pdf John C. P. Williams of Williams-Beuren syndrome] | |||
==Genetic factors and Etiology== | ==Genetic factors and Etiology== | ||
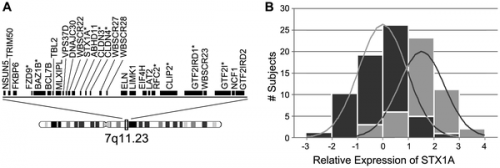
Williams Syndrome is a multi-system genomic disorder that occurs due to a hemizygous deletion/nonallelic homologous recombination (NAHR). The sizes of deletion commonly range from 1.55 to 1.84 mega base pairs (Mb) on chromosome 7q11.23 which encompasses 28 genes.<ref name="PMID20425781"><pubmed>2042578 </pubmed></ref> <ref name="PMID19568270"><pubmed>19568270 </pubmed></ref> | [[File: Distribution of quantitative transcription of genes deleted in WS.png|500px|thumb|'''Figure 3: Distribution of quantitative transcription of genes deleted in WS. a: Map of genes commonly deleted in WS. b: Z-scores (relative to the WS mean) of STX1A expression levels''']] | ||
Williams Syndrome is a multi-system genomic disorder that occurs due to a [[#Glossary | '''hemizygous''']] deletion/nonallelic homologous recombination [[#Glossary | '''(NAHR)''']] . The sizes of deletion commonly range from 1.55 to 1.84 mega base pairs [[#Glossary | '''(Mb)''']] on chromosome 7q11.23 which encompasses 28 genes.<ref name="PMID20425781"><pubmed>2042578</pubmed></ref> <ref name="PMID19568270"><pubmed>19568270 </pubmed></ref> | |||
The region associated with Williams syndrome contains a single copy gene region with repetitive sequences or Low Copy Repeats [[#Glossary | '''(LCR)''']] . The deletions that cause Williams syndrome are due to a misalignment of these repetitive sequences or gametes within the Williams-Beuren syndrome critical region. <ref>Morris CA. Williams Syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews (Internet). Seattle (WA): University of Washington, Seattle; 1993-. | |||
1999 Apr 09 (updated 2006 Apr 21). [http://www.ncbi.nlm.nih.gov/pubmed/20301427 PMID: 20301427]</ref> This occurs during the process of [[#Glossary | '''meiosis''']] and follows an unequal crossing over that is due to a high similarity of LCRs. | |||
A genotype-phenotype correlation has been found for some of the genes within the deletion region. The most well known of these being elastin gene (ELN), whereby elastin [[#Glossary | '''haploinsufficiency''']] is responsible for a number of abnormalities characteristic of Williams Syndrome, particularly connective tissue abnormalities and cardiovascular disease including arterial stenosis. <ref name="PMID20425789"><pubmed>20425789</pubmed></ref> CLIP2, GTF2I, GTF2IRD1 and LIMK1 are some of the other genes that are most commonly deleted in individuals with Williams syndrome. Researchers have suggested that the deletion of these genes could help in the explanation of some of the characteristic signs of Williams syndrome including the unique behavioural characteristics and also some of the related cognitive difficulties. | |||
In most cases, Williams syndrome occurs sporadically and the deletions can occur with no reference to the parental origin of the chromosome that transmits the disease. There have been, however, a very small number of cases where [[#Glossary | '''autosomal dominant inheritance''']] of Williams syndrome has been reported. | |||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
| bgcolor="springgreen" | '''Gene''' | |||
| bgcolor="springgreen" | '''Normal Function''' | |||
| bgcolor="springgreen" | '''Relation to Williams Syndrome''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen"| '''Elastin (ELN)''' | |||
| Elastin is a main component of elastic fibres. It also contributes to the structure of connective tissue, particularly its flexibility and strength. The expression of the ELN gene is largely limited to the third trimester of fetal development and early postnatal years.<ref name="PMID9819363"><pubmed>9819363 </pubmed></ref> | |||
| The loss of one copy of this gene reduces the normal production of elastin by half. | |||
Elastin haploinsufficiency is responsible for a number of abnormalities characteristic of Williams Syndrome, particularly connective tissue abnormalities and cardiovascular disease including arterial stenosis. <ref name="PMID20425789"><pubmed>20425789 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"| '''LIM domain kinase 1 (LIMK1)''' | |||
| Studies, such as Wang et al in 1998 <ref name="PMID9685409"><pubmed>9685409 </pubmed></ref> suggest that LIMK1 is involved in the area of the brain that is in control of the visualisation of an object as a set of parts. | |||
It has also been implicated in other visual tasks such as drawing, making models and writing. | |||
| Studies differ in regards to the contribution of LIMK1 deletions to the phenotype expressed in Williams syndrome. Some studies have suggested that the loss of this gene leads to the problems with visuo-spatial tasks that are common in Williams syndrome. Other studies suggest that it is involved with the characteristic progressive loss of hearing, <ref name="PMID21655442"><pubmed>21655442 </pubmed></ref> while other studies have not found these connections. | |||
|- | |||
| bgcolor="springgreen"| '''General transcription factor IIi (GTF2I)''' | |||
| This gene is involved in the production of the following two proteins: | |||
*BAP-135: involved in the normal function of the immune system | |||
*TFII-I: helps in the regulation of other genes activity and is therefore active in many tissues of the body, particularly in the brain. | |||
| It has been suggested that the loss of one copy of this gene may be responsible for the intellectual disability seen in Williams syndrome. It may also be involved in the social characteristics of those with Williams syndrome. <ref name="PMID19109438"><pubmed>19109438 </pubmed></ref> | |||
|- | |||
|bgcolor="springgreen"| '''General Transcription Factor II-I Repeat Domain-containing Protein 1 (GTF2IRD1)''' | |||
| It was shown in 2005 by Tassabehji et al. <ref name="PMID16293761"><pubmed>16293761 </pubmed></ref> that the GTF2IRD1 gene is involved in the development of both craniofacial features and cognitive development in mammals. | |||
It was also shown by Yan et al in 2000 that GTF2IRD1 is involved with the function of the RB1 protein both in vitro and in vivo. <ref name="PMID10642537"><pubmed>10642537 </pubmed></ref> | |||
| The deletion of a copy of this gene has been implicated in some of the phenotypic features seen in Williams syndrome through the use of data from human genetic mapping. These features include the hypersociabilty and visuaspatial deficits, but the main implication are the craniofacial abnormalities. <ref name="PMID19109438"><pubmed>19109438 </pubmed></ref> | |||
|} | |||
'''Links:''' | |||
[http://omim.org/entry/130160?search=elastin&highlight=elastin OMIM Entry #130160 – Elastin (ELN)] | [http://omim.org/entry/601329 OMIM Entry #601329 - LIM domain kinase 1 (LIMK1)] | [http://omim.org/entry/601679?search=General%20transcription%20factor%20IIi%20%28GTF2I%29&highlight=general%20transcription%20gtf2i%20iii%20factor OMIM Entry #601679 - General transcription factor IIi (GTF2I)] |[http://www.omim.org/entry/604318?search=General%20Transcription%20Factor%20II-I%20Repeat%20Domain-containing%20Protein%201%20%28GTF2IRD1%29&highlight=repeat%20transcription%20gtf2ird1%20general%201%20domaincontaining%20factor%20protein%20iii General Transcription Factor II-I Repeat Domain-containing Protein 1 (GTF2IRD1)] | |||
==Diagnosis== | ==Diagnosis== | ||
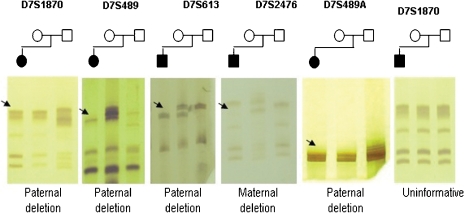
[[File: Genotyping_of_the_five_microsatellites_markers_in_WBS_families.jpg|500px|thumb|'''Figure 4: Genotyping of the five microsatellites markers in WBS families. DNA fragments of those affected are always the first column of each gel followed by DNA from the mother and the last column the DNA of the father. Black arrows indicate allelic loss''']] | |||
There are ways to clinically diagnose Williams Syndrome even though the phenotype varies between individuals. There is not a particular clinical feature that is able to ascertain the diagnosis alone, but there are particular features that individuals with Williams Syndrome may present with. These features include conditions and abnormalities discussed in the associated medical conditions sections, but most commonly include: | There are ways to clinically diagnose Williams Syndrome even though the phenotype varies between individuals. There is not a particular clinical feature that is able to ascertain the diagnosis alone, but there are particular features that individuals with Williams Syndrome may present with. These features include conditions and abnormalities discussed in the associated medical conditions sections, but most commonly include: | ||
*Distinctive facial features | |||
*Unique personality | |||
*Intellectual disability | |||
*Growth abnormalities | |||
*Cardiovascular Disease | |||
*Endocrine Abnormalities | |||
Although these clinical criteria for diagnosis are available, the detection of the contiguous gene deletion responsible for Williams Syndrome is the main form of diagnosis. It has been found that over 99% of individuals that meet the clinical diagnosis criteria for Williams Syndrome also have this contiguous gene deletion. This gene deletion is detected through the use of fluorescent in situ hybridisation (FISH) or through targeted mutation analysis. <ref name="PMID7693128"><pubmed>7693128 </pubmed></ref> | Although these clinical criteria for diagnosis are available, the detection of the contiguous gene deletion responsible for Williams Syndrome is the main form of diagnosis. It has been found that over 99% of individuals that meet the clinical diagnosis criteria for Williams Syndrome also have this contiguous gene deletion. This gene deletion is detected through the use of fluorescent in situ hybridisation (FISH) or through targeted mutation analysis. <ref name="PMID7693128"><pubmed>7693128 </pubmed></ref> | ||
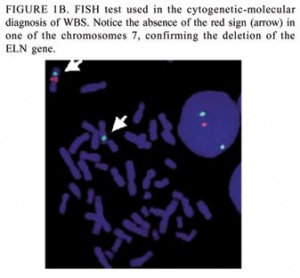
[[File: FISH test used to confirm the deletion of the ELN gene.jpg|300px|thumb|'''Figure 5: FISH test used to confirm the deletion of the EN gene''']] | |||
'''Fluorescent in situ hybridisation [[#Glossary | '''(FISH)''']] :''' FISH uses elastin probes for a specialised form of chromosome analysis. This method is commercially available and commonly used to determine if an individual showing the clinical signs of William’s syndrome does have the deletion of the genes in the Williams-Beuren syndrome critical region. If the individual only has one copy of the elastin gene, then a diagnosis of William’s syndrome is confirmed. <ref name="PMID17180802 | |||
"><pubmed>17180802 </pubmed></ref> | |||
'''Targeted Mutation Analysis:''' these are non FISH methods that are also used to detect the contiguous gene deletion in order to determine whether or not an individual has Williams Syndrome. | '''Targeted Mutation Analysis:''' these are non FISH methods that are also used to detect the contiguous gene deletion in order to determine whether or not an individual has Williams Syndrome. | ||
*Real-time quantitative PCR: is used to establish the number of copies of three of the genes contained within the Williams-Beuren syndrome critical region. A deletion in this area would mean that only a single copy of one of these genes would be found. | |||
*Genomic microarray analysis: makes use of array genomic hybridisation techniques which involved examining multiple genes simultaneously and comparing them in order to determine whether there are any abnormalities in the amount of chromosomal material. | |||
*Heterozygosity testing: is mostly used to identify the size of the deletions, and involves the testing of short tandem repeats [[#Glossary | '''(STRs)''']] from the Williams-Beuren syndrome critical region. If there is no deletion, than the STR sizes should be different at each of the markers determining heterozygosity. But if only one STR size is found at a marker, than this may indicate that there is a deletion, but may also indicate homozygosity. Quantitive PCR is then needed to determine whether or not the single STR size is dur to a deletion in the critical area or a non-abnormal finding of homozygosity. | |||
==Epidemiology== | |||
===Rate of Incidence=== | |||
Williams-Beuren Syndrome occurs in 1 in 7,500-20,000 births.<ref name="PMID18489677 "><pubmed>18489677 </pubmed></ref> Many of the clinical manifestations associated with Williams syndrome are globally consistent, however some studies have shown that some symptoms are less common in certain countries. A study conducted in the Hong Kong Chinese population showed that SVAS, the most common defect associated with Williams syndrome in the West, is less common than peripheral pulmonary stenosis in the study area.<ref name="PMID14967851"><pubmed>14967851 </pubmed></ref> Another study showed that the prevalence of cardiovascular defects in a group of Williams syndrome patients in Greece was lower than that documented in the majority studies performed (8/50 rather than half or more of the study group).<ref name="PMID15774842"><pubmed>15774842 </pubmed></ref> With regard to sex, it has been noted that the severity of SVAS and total cardiovascular disease is significantly greater in males than in females with WS, proving that the severity of elastin arteriopathy is indeed affected by sex. This difference, as hypothesised, may be related to prenatal hormone effects.<ref name="PMID11743512"><pubmed>11743512 </pubmed></ref> | |||
Genetically, 90%–95% of patients clinically diagnosed with the syndrome have an approximate 1.55-Mb deletion associated with the loss of 26–28 genes on the 7q11.23 chromosome; 5%–8% of those diagnosed have a slightly larger deletion of approximately 1.84-Mb pair deletion associated with the loss of 28 genes.<ref name="PMID18452001"><pubmed>18452001 </pubmed></ref> | |||
==Facial Characteristics== | |||
Most physical musculoskeletal abnormalities result from the absence of elastin due to the elastin gene deletion. The facial phenotype also changes with time as the child develops. This makes it harder to diagnose Williams Syndrome based on facial characteristics in adults. <ref name="PMID20425781"><pubmed>20425781</pubmed></ref> | |||
<ref>Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> | |||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
| bgcolor="springgreen" | | |||
| bgcolor="springgreen" | '''Features''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen" |'''Mouth and Nose''' | |||
| | |||
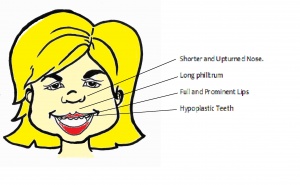
*Lips are full and prominent. Often wide and held open.[[File:WS girl labelled.jpg|thumb|300px|'''Figure 6: Shorter and upturned nose, long philtrum, full and prominent lips and hypoplastic teeth commonly seen in the Williams syndrome phenotype''']] | |||
*Teeth are often missing and/or hypoplastic, has thin enamel. Teeth present are misshaped, often with a screwdriver shape and are smaller that normal teeth. Malocclusion, the misalignment of teeth is caused by the abnormal shape and size of the teeth. | |||
*A long philltrum is also present in the majority of cases. | |||
*Flat nasal bridge with a shorter, upturned nose and anterverted nares. | |||
|- | |||
| bgcolor="springgreen" |'''Eyes and Ears''' | |||
| | |||
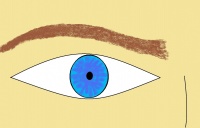
*Eyes often have a stellate arrangement of the iris.[[File:Williams Syndrome eye.jpg|thumb|200px|'''Figure 7: Stellate Iris. This image shows a typical eye of an individual with Williams Syndrome showing the stellate iris.''']] | |||
*Eyebrows flare medially. | |||
*Hyperopia, vertical strabismuth or esotropia, inward strabismuth is present in most cases of Williams syndrome. | |||
= | *Ears are lower than the normal height on the face and have prominent ear lobes. | ||
|- | |||
| bgcolor="springgreen" |'''Craniofacial Skeleton''' | |||
| | |||
*Cranial base is shorter in length on both the anterior and posterior halves but maintain the same cranial base angle as a normally developing individual. | |||
*The saggital length of the maxillary is shorter and more inclined anteriorly. | |||
= | *A retrusive mandible is present as the mandible is at a higher plane angle and the chin is deficit. <ref>Krishnan, A 2011, ''Pediatric Supravalvar Aortic Stenosis: Background'', Webscape Reference, viewed 11 October 2011, <http://www.lds.no/stream_file.asp?iEntityId=13482></ref> | ||
|} | |||
'''Links:''' [[Lecture - Head Development]] | |||
==Cardiac Conditions== | ==Cardiac Conditions== | ||
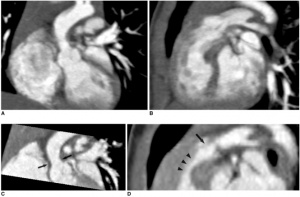
===Stenosis=== | [[File: Scans of Supravalvular Aortic Stenosis and Pulmonary Stenosis.jpg|300px|thumb|'''Figure 8: Scans indicating Supravalvular aortic stenosis (arrows on C) and combined valvar (arrow on D) and subvalvar (arrowheads on D) pulmonary stenoses in 9-month-old boy with Williams syndrome''']] | ||
One of the major manifestations of Williams Syndrome is cardiac abnormalities, 80% of those diagnosed with this syndrome show some form of heart condition. <ref name="PMID11701637"><pubmed>11701637 </pubmed></ref> | |||
Most heart defects result from the mutation or deletion of the Elastin (ELN) gene on chromosome 7. Damage or loss of this gene causes its subsequent loss in function, no longer providing the necessary elasticity in tissues like the lungs, the dermis and larger blood vessels, with one of the major results being elastin arteriopathy. <ref name="PMID11701637"><pubmed>11701637</pubmed></ref> <ref name="PMID7726172"><pubmed>7726172</pubmed></ref> This is present in about 75% of affected individuals and may be present in any artery, the most common of them being in the aorta causing a condition known as Supravalvular Aortic Stenosis (SVAS). <ref name="Morris">Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> <ref name="PMID11701637"><pubmed>11701637</pubmed></ref> | |||
Other cardiac anomalies include Hypertension, Peripheral Pulmonic Stenosis (PPS), [[#Glossary | '''Mitral Valve Disease''']], [[#Glossary | '''Atrial Septal Defect''']] and [[#Glossary | '''Ventricular Septal Defect''']]. These conditions and their severity varies from patient to patient. <ref name="PMID11701637"><pubmed>11701637</pubmed></ref> | |||
It is reported that males diagnosed with Williams syndrome are more likely to suffer from cardiovascular disease than females who are diagnosed with this same disorder. <ref name="PMID11743512"><pubmed>11743512</pubmed></ref> | |||
===Stenoses=== | |||
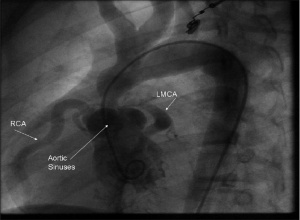
[[File: Angiography image indicating Supravalvular aortic stenosis.jpg|300px|thumb|'''Figure 9: Left Ventricular Cine angiography picture demonstrating Supravalvular aortic stenosis in a 12 year old Williams Syndrome patient''']] | |||
Stenoses are the typical types of cardiovascular disorders in Williams Syndrome. This manifestation is characterised by the narrowing of arteries due to the thickening of these blood vessels caused by smooth-muscle overgrowth. Stenoses may be isolated or may occur simultaneously in numerous places, in both medium and large arteries such as the aorta (in SVAS) and pulmonary artery (in PPS) <ref name="PMID20089974"><pubmed>20089974 </pubmed></ref> | |||
The most prevalent classes of stenoses are SVAS and PPS, with 70-75% and 39% of people with Williams syndrome reporting to suffer from SVAS and PPS respectively. <ref name="PMID20808633"><pubmed>20808633 </pubmed></ref> Other stenoses occur in peripheral vessels such as renal, carotid, coronary, subclavian and mesenteric arteries.<ref name="PMID20089974"><pubmed>20089974</pubmed></ref> | |||
It has been found that PPS is more common in infancy and tends to improve over time. On the other hand, SVAS affects Williams Syndrome sufferers of any age and is most likely to worsen over time if not treated. <ref name="Morris">Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> | |||
*'''Supravalvular Aortic Stenosis (SVAS)''' | |||
SVAS is the most common condition in Williams Syndrome, and was one of the first to be associated with this anomaly. It is characterised by elastin arteriopathy in the ascending aorta causing its narrowing. <ref name="PMID8475063"><pubmed>8475063</pubmed></ref> This condition is caused by the loss of function of the Elastin (ELN) gene as a result of a mutation or deletions within this gene. <ref name="PMID11701637"><pubmed>11701637</pubmed></ref> Histological analysis of the arterial walls of Williams Syndrome patients with SVAS show a disorganised structure with fragmented elastic fibers and an increase in the size of smooth muscle cells (muscle hypertrophy). <ref name="PMID20926892"><pubmed>20926892</pubmed></ref> | |||
SVAS is the dominant cause of morbidity and mortality for this syndrome’s patients <ref name="PMID20425781"><pubmed>20425781</pubmed></ref> because when left uncorrected, it may lead to increased [[#Glossary | '''intracardiac pressure''']], [[#Glossary | '''myocardial hypertrophy''']], heart failure, and eventually death. <ref name="PMID8475063"><pubmed>8475063</pubmed></ref> | |||
SVAS can be diagnosed through [[#Glossary | '''2-dimensional echocardiography''']] that allows for multiple views of the heart to be examined. <ref>Krishnan, A 2011, ''Pediatric Supravalvar Aortic Stenosis: Background'', Webscape Reference, viewed 11 October 2011, <http://emedicine.medscape.com/article/892252-overview></ref> | |||
*'''Peripheral Pulmonary Stenosis (PPS)''' | |||
PPS is the second most common cardiac abnormality associated with Williams Syndrome. It is a narrowing at the level of the main pulmonary artery, occurring in multiple places along this artery and can be related to hypoplasia or incomplete development of the pulmonary arterial bed. <ref name="PMID11331257"><pubmed>11331257</pubmed></ref> <ref>Xiushui, M 2011, ''Pulmonic Stenosis: Pathophysiology'', Medscape Reference, viewed 11 October 2011, <http://emedicine.medscape.com/article/157737-overview#a0104></ref> PPS is also the cause of vessel arteriopathy, an outcome of deletion or mutation of the ELN gene. <ref name="PMID18452001"><pubmed>18452001</pubmed></ref> | |||
===Other problems=== | ===Other problems=== | ||
*'''Hypertension''' | |||
Hypertension or high blood pressure is another common cardiac condition that can result from Williams Syndrome. Studies have shown that an increased risk of high blood pressure in Williams Syndrome sufferers is the result of the re-organisation of lamellar structures in large vessel walls and the degeneration of elastic fibers caused by the loss of the ELN allele. <ref name="PMID20926892"><pubmed>20926892</pubmed></ref> In some cases it can arise as a result of the narrowing of the renal artery (Renal artery stenosis)<ref name="Morris">Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> | |||
Hypertension in Williams syndrome can develop during childhood, however it is more commonly found in adults, with more than half of them developing high blood pressures.<ref name="PMID18452001"><pubmed>18452001</pubmed></ref> <ref name="PMID20089974"><pubmed>20089974</pubmed></ref> Consequences of this condition can be an increased risk of [[#Glossary | '''myocardial infarction''']] and stroke. | |||
*'''Mitral valve disease''' | |||
The Mitral valve, or bicuspid valve, between the left atrium and ventricle is another structure of the heart that can be affected in Williams Syndrome. The most common abnormality associated with this syndrome is mitral valve prolapse(MVP)which results in multiple, unnecessary [[#Glossary | '''mitral valve leaflets''']], more elongated [[#Glossary | '''chordae tendineae''']] and [[#Glossary | '''myxomatous degeneration''']] of the leaflet tips. These characteristics lead to the leaflets of the valves bulging into the left atrium during systole and the inability of the leaflet tips to join together. The mitral valve isn't able to function in closing the left ventricle off from the right atrium once [[#Glossary | '''diastole''']] has finished and so [[#Glossary | '''mitral regurgitation of blood''']] back into the atrium occurs when blood is trying to be pumped out. <ref name="PMID21545515"><pubmed>21545515</pubmed></ref> | |||
==Genitourinary== | |||
Table showing "the prevalence of CV abnormalities in 423 patients from nine selected international series published in the last two decades" <ref name="PMID18452001"><pubmed>18452001</pubmed></ref> | |||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
| bgcolor="springgreen" | '''CV abnormality''' | |||
| bgcolor="springgreen" | '''Prevalence (%)''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen" |'''All CV diseases''' | |||
| 84 | |||
|- | |||
| bgcolor="springgreen" |'''SVAS''' | |||
|69 | |||
|- | |||
| bgcolor="springgreen" |'''PPS''' | |||
|34 | |||
|- | |||
| bgcolor="springgreen" |'''Hypertension''' | |||
|17 | |||
|- | |||
| bgcolor="springgreen" |'''Mitral valve disease''' | |||
|15 | |||
|- | |||
| bgcolor="springgreen" |'''Pulmonary Valve disease''' | |||
|5 | |||
|- | |||
| bgcolor="springgreen" |'''Aortic valve disease''' | |||
|3 | |||
|} | |||
'''Links:''' [[Lecture - Heart Development]] | |||
==Genitourinary Conditions== | |||
===Renal Tract Abnormalities=== | ===Renal Tract Abnormalities=== | ||
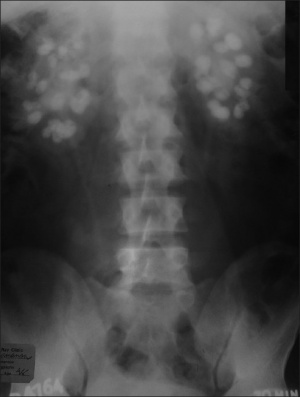
[[File:X-ray Abdomen bilateral nephrocalcinosis.jpg|300px|thumb|'''Figure 10: Abdominal X-ray showing extensive bilateral nephrocalcinosis''']] | |||
In a study of 40 patients with Williams Syndrome, some form of renal abnormality was detected in 7 of the patients from the study group. This study provided the foundation for the statistic stating that 18% of people with Williams Syndrome have some form of renal tract abnormality.<ref name="PUBMED8488870"><pubmed>8488870 </pubmed></ref> Some of these abnormalities include: | |||
*'''Renal Agenesis''' | |||
Renal agenesis is a disorder involving the absence of one or both of the kidneys, categorised into unilateral or bilateral respectively. In this case, renal agenesis is a secondary condition to the developmental genetic disorder that is Williams Syndrome. | |||
Bilateral renal agenesis is fatal and most infants die within the first four hours following birth. This is due to the noticeable deficiency of amniotic fluid (oligohydramnios) after 12-13 weeks causing pulmonary hypoplasia. | |||
It is diagnosed firstly by the absence of amniotic fluid followed by the absence of the bladder and kidneys. <ref name="PMID8066039"><pubmed>8066039 </pubmed></ref> | |||
Following diagnostic tests such as CT scans and [[#Glossary | '''ultrasonography''']], [[#Glossary | '''Colour Doppler''']] has been found to be useful in detecting and identifying vascular anomalies related to renal agenesis. <ref name="PMID8610763"><pubmed>8610763 </pubmed></ref> | |||
Unilateral renal agenesis results in mostly normal development. Patients must be examined periodically as there is an increased incidence of urinary tract infections amongst those affected. Elevated blood pressure may result in kidney damage. Patients are advised to not participate in contact sports in order to protect the sole remaining kidney. | |||
Unilateral/Bilateral renal agenesis results from a lack of induction of the metanephric blastema by the ureteral bud. <ref name="PMID9763817"><pubmed>9763817 </pubmed></ref> | |||
*'''Duplicated kidneys''' | |||
Also known as duplex kidneys, duplicated collecting systems and duplex collecting systems. This is defined as the doubling of the renal units; either containing 2 pyelocaliceal systems in relation to a single ureter or with double ureters. The 2 ureters may empty separately into the bladder or fuse to form a single ureteral orifice. As with renal agenesis, duplicated kidneys may be classified as unilateral or bilateral. In general, patients usually have complete ureteric duplication in which results in a greater tendency for the ureters to develop obstructions, reflux, and infections. <ref name="PMID11110565"><pubmed>11110565 </pubmed></ref> | |||
A duplex kidney with either a bifid renal pelvis or bifid ureter results when a single ureteral bud bifurcates before the ampulla bifurcates. <ref name="PMID2117358"><pubmed>2117358 </pubmed></ref> | |||
*'''Vesicourinary reflux''' | |||
Vesicourinary (vesicoureteral) reflux is the reverse flow of urine from the bladder to the ureters or even the kidneys. It results as a failure of the ureteric openings which store and void urine. | |||
The International Reflux Grading system classifies VUR into 5 grades, depending on the degree of retrograde filling and dilatation of the renal collecting system. | |||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
''' | | bgcolor="springgreen" | '''Grading''' | ||
| bgcolor="springgreen" | '''Characteristics''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen"| '''Grade I''' | |||
| Urine backs up into the ureter only, and the renal pelvis appears healthy, with sharp calyces. | |||
|- | |||
| bgcolor="springgreen"| '''Grade II''' | |||
| Urine backs up into the ureter, renal pelvis, and calyces. The renal pelvis appears healthy and has sharp calyces. | |||
|- | |||
| bgcolor="springgreen"| '''Grade III''' | |||
| Urine backs up into the ureter and collecting system. The ureter and pelvis appear mildly dilated, and the calyces are mildly blunted. | |||
|- | |||
| bgcolor="springgreen"| '''Grade IV''' | |||
| Urine backs up into the ureter and collecting system. The ureter and pelvis appear moderately dilated, and the calyces are moderately blunted. | |||
|- | |||
| bgcolor="springgreen"| '''Grade V''' | |||
| Urine backs up into the ureter and collecting system. The pelvis is severely dilated, the ureter appears tortuous, and the calyces are severely blunted. <ref name="PMID3975102"><pubmed>3975102 </pubmed></ref> | |||
|} | |||
In most cases, vesicourinary reflux presents with no symptoms. When symptoms are present, the most common is a urinary tract infection (UTI). Bacteria thrive in this suitable habitat as the urine remains in the child’s urinary tract, thus causing the urinary tract infection. Studies estimate that 30 percent of children and up to 70 percent of infants that present with a urinary tract infection have vesicourinary reflux. <ref name="PMID6333147"><pubmed>6333147 </pubmed></ref> | |||
''' | *'''Nephrocalcinosis''' | ||
Nephrocalcinosis occurs in less than 5% of patients with WS. Although relatively rare, it is one symptom which presents in infant, child and adult life. It is the deposition of excess calcium in the kidneys which may lead to kidney failure, kidney stones or obstructive uropathy. Nephrocalcinosis may be diagnosed by abdominal CT scans, ultrasounds of the kidney and [[#Glossary | '''urinalysis''']]. <ref name="PMID8723124"><pubmed>8723124 </pubmed></ref> | |||
'''Links:''' [[Lecture - Renal Development]] | |||
==Endocrine== | ==Endocrine Conditions== | ||
===Hypercalcemia=== | ===Hypercalcemia=== | ||
Hypercalcemia refers to elevated levels of calcium in the bloodstream. It is not always observed in individuals with Williams syndrome but it is more common among children with infantile hypercalcemia being reported in approximately 15% of infants diagnosed with Williams syndrome. Many individuals diagnosed with Williams syndrome show the symptoms associated with hypercalcemia. For instance, in infants, these symptoms are most commonly irritability, vomiting and constipation, whereas in adults there are more commonly urinary infections and petic ulcer disease. Most of these cases are reported within the first four years of age, but cases of recurrence in puberty have also been reported. Typically, the infantile hypercalcemia is transient and found only in mind forms, but in rare cases it can also be life-threateningly severe. <ref name="PMID15466114"><pubmed>15466114 </pubmed></ref> | |||
===Diabetes Mellitus=== | ===Diabetes Mellitus=== | ||
It is reported that approximately 75% of adult individuals with Williams syndrome suffer from a form of pre-diabetes, such as impaired glucose tolerance, or diabetes mellitus. <ref name="PMID20425788"><pubmed>20425788 </pubmed></ref> In the Williams-Beuren syndrome critical region, one of the genes is syntaxin-1A (STX-1A). This gene codes for a protein that is involved in exocytosis of insulin granules in pancreatic beta-cells. Lam et al in 2005 <ref name="PMID16123365"><pubmed>16123365 </pubmed></ref> used a mouse model and an intraperitoneal glucose challenge to show that an overproduction of this syntaxin-1A gene resulted in hyperglycemia (described above) as well as a lower secretary level of insulin. | |||
===Thyroid=== | ===Thyroid=== | ||
The prevalence of thyroid abnormalities is increased in individuals diagnosed with Williams syndrome. Some of these abnormalities include thyroid-gland hypoplasia, [[#Glossary | '''hypothyroidism''']], and morpho-volumetric abnormalities in the thyroid gland. Thyroid-gland hypoplasia, which is present in 75% of Williams syndrome individuals has been impicated as a cause of the congenital hypothyroidism, a form of thyroid dydgenesis, seen in 35% of these individuals. Also, there is a 67.5% prevalence of morpho-volumetric abnormalities in the thyroid gland associated with Williams syndrome. <ref name="Morris">Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> | |||
'''Links:''' [[Lecture - Endocrine Development]] | |||
==Other Associated Medical Conditions== | ==Other Associated Medical Conditions== | ||
'''Joint Abnormalities''' : | '''Other Abnormalities''' | ||
There are a number of other abnormalities associated with Williams Syndrome including a hoarse voice, inguinal hernias and joint abnormalities. These abnormalities vary in severity between different individuals and elastin haploinsufficiency is responsible for a number of these abnormalities characteristic of Williams Syndrome.<ref name="PMID20425789"><pubmed>20425789 </pubmed></ref> | |||
{|style="background:white" border="1px" cellspacing="1" align="centre" cellpadding="4" | |||
| bgcolor="springgreen" | '''Abnormality''' | |||
| bgcolor="springgreen" | '''Cause''' | |||
| bgcolor="springgreen" | '''Effect and Relation to Williams Syndrome''' | |||
|- style="background:springgreen | |||
| bgcolor="springgreen"| '''Joint Abnormalities''' | |||
| The elastin deficiency seen in Williams syndrome is the cause of the lax joints and other joint abnormalities. | |||
| Individuals with Williams syndrome typically have loose joints during infancy which then get worse with age and towards later childhood, they may develop joint contractures. | |||
The lax joints that young children with Williams syndrome suffer from often lead to compensatory measures of posture, resulting in mild [[#Glossary | '''lordosis''']] and [[#Glossary | '''kyphosis''']] . <ref name="PMID20425789"><pubmed>20425789 </pubmed></ref> | |||
These joint abnormalities occur in the lower limbs more often than anywhere else in the body. | |||
|- | |||
| bgcolor="springgreen"| '''Ingiunal Hernias''' | |||
| Although further research needs to be done, it is suggested that mutations in the SERPINA1 gene, could play a role in the prevalence of direct inguinal hernias in individuals with Williams syndrome. This is due to the fact that this particular gene, encodes for the elastase inhibitor - alpha-1-antitrypsin (AAT). . | |||
| Inguinal hernias are more common in individuals with Williams syndrome. They occur in approximately 40% of individuals with Williams syndrome, but only in approximately 5% of individuals that are not diagnosed with Williams syndrome. These inguinal hernias are usually diagnosed when the individual is an infant and they are more common in males then females, with an approximate ratio of 10:1. <ref name="PMID18267160"><pubmed>18267160 </pubmed></ref> <ref name="PMID20425789"><pubmed>20425789 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"| '''Auditory Abnormalities''' | |||
| Chronic otitis media or chronic ear infections occur more commonly in children with Williams syndrome. Children in the average population have an average occurrence rate of 41% while those with Williams have an occurrence rate of 50%. <ref name="PMID12949276"><pubmed>12949276 </pubmed></ref> | |||
| Hearing loss is widely seen in adults with Williams syndrome and although not as extensive, it is also commonly seen in school-aged children with Williams syndrome. There is previous research that suggests that the hearing loss that is associated with Williams syndrome begins early and is likely to be progressive with age. <ref name="PMID20425785"><pubmed>20425785 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"| '''Hoarse Voice''' | |||
| Due to a connective tissue abnormality, where the [[#Glossary | '''lamina propria''']] in the vocal folds has a decreased amount of elastic fibres. <ref name="PMID12784297"><pubmed>12784297 </pubmed></ref> | |||
| The hoarse voice is present in 98% of people with Williams Syndrome. | |||
|- | |||
| bgcolor="springgreen"| '''Hallux Valgus''' | |||
| Hallux Valgus is the result of the musculoskeletal deviation seen in Williams syndrome. | |||
| This results in the projection of the [[#Glossary | '''metasarsophalangeal joint''']] inward to the inner foot. It is reported that in approximately 78% of individuals diagnosed with Williams syndrome have a big toe which is displaced under or over their other toes. <ref>[http://omim.org/entry/194050?search=williams%20syndrome&highlight=william%20syndrome OMIM Entry #194050-Williams-Beuren Syndrome]</ref> | |||
|- | |||
| bgcolor="springgreen"| '''Developmental delay in height and weight''' | |||
| This delay, as well as many of the other abnormalities seen in Williams syndrome has been associated with the lack of connective tissue, due to the deletion of the ELN gene. | |||
| Individuals with Williams syndrome often present smaller than others without Williams syndrome at the same gestational age, and grow to have a short stature. A study by Pankau et al in 1992, found intrauterine growth retardation in 35% of females with Williams Syndrome and 22% in males. <ref name="PMID1425797"><pubmed>1425797 </pubmed></ref> | |||
|- | |||
| bgcolor="springgreen"| '''Early onset of puberty''' | |||
| It has been suggested that the early onset of puberty could be related to a disruption in hormonal secretions associated with Williams syndrome. There has also been evidence to implicate the hypothalamic-pituitary mediated activation. <ref name="PMID10319200"><pubmed>10319200 </pubmed></ref> | |||
| It has been found that individuals with Williams syndrome are more likely to have an earlier onset of puberty. With females beginning their menstrual cycle and males showing Tanner III pubic hair development under the age of 12. <ref name="PMID10319200"><pubmed>10319200 </pubmed></ref> | |||
|} | |||
==Structural Differences in the Brain== | |||
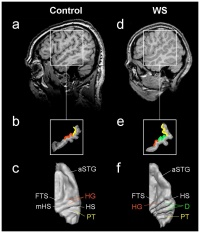
[[File:Auditory Cortex Location - Comparison Between Control Subject and WS Subject.jpg|200px|thumb|'''Figure 11: Auditory Cortex Location - Comparison Between Control Subject and WS Subject.''']] | |||
Williams syndrome has been characterized by the smaller brain mass, but relative enlargement of particular areas of the brain. The [[#Glossary | '''neocellebellar vermis''']] has been shown to be larger in Williams syndrome individuals and a differential development of the [[#Glossary | '''paleocerebellum''']] and other areas of the brain was found. | |||
===Language=== | |||
''' | The abnormal language expressed in Williams syndrome has been related to the abnormal functioning of the brain during processing. The normal left-anterior asymmetry in language processing has been lost and a more diverse arrangement has been found. Event-related potential [[#Glossary | '''(ERP)''']] studies have shown the functioning of a different pathway to that of a normal person in language processing. This abnormal organization of the neural network is what has allowed for the relative sparing of the language processing in Williams syndrome. | ||
''' | ===Cognitive Abilities=== | ||
A reduction in the size of the parietal occipital region has been found in the majority of Williams syndrome patients. This reduction in the posterior cerebral area has been predicted to contribute to the visuospatial processing difficulty found in the syndrome. The isthmus and splenium are the major white matter tracts which connect the visual processing and associated processing areas of the brain. This would result in the impaired visospatial processing of visual information in the brains of Williams syndrome individuals. The areas of language and affect have been shown to be relatively preserved as the frontal lobe is correctly proportioned in William syndrome individuals. The hypersociability of Williams syndrome individuals may be attributed to the enlargement of the neocerebellar vermis. The social and emotional functions of the posterior cerebellum has been the area thought to be responsible for these phenotypes. | |||
There has been a consistently long central sulcus found in William syndrome individuals. There is an unusual configuration of the central sulcus which is associated with abnormal behavior. There is also an overall unusual configuration of the other gyri on the medial surface and temporal lobes. There is brain asymmetry and [[#Glossary | '''cingulate cortex''']] dysfunctioning has been attributed to the abnormal social engagement expressed by William syndrome individuals. <ref name="Bellugi"> Bellugi, U. et al, 2001, ''Journey From Cognition to Brain to Gene: Perspectives From Williams Syndrome,'' Massachusetts Institute of Technology,the United States of America </ref> | |||
===Auditory=== | |||
''' | The amygdala has been related to the lateral nucleus and contributes to the inhibited fear response to new faces. | ||
The auditory centres are known to be the thalamus and cortex, however, the visual cortex has been shown to activate in Williams syndrome individuals when stimulated by music. At a cortival level, There seems to be a hyperactivity when a musical stimuli is present. This is the result of a more excitable auditory response formed by hyperexcitable neurons which form irregular neural systems with other functional areas of the brain. [[#Glossary | '''Heschl’s gyrus''']] has been found to be duplicated in William syndrome individuals who present with 2-4 pairs of Heschl’s gyrus. The auditory cortex of these individuals has been shown to be relatively larger than an average human, 2.2 times in the left hemisphere and 1.2 in the right. <ref name="PMID20808792"><pubmed>20808792 </pubmed></ref> | |||
''' | '''Links:''' [[Lecture - Neural Development]] | ||
==Cognitive, Behavioural and Neurological | ==Cognitive, Behavioural and Neurological Phenotype== | ||
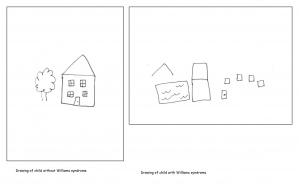
[[File:House drawings Williams.jpg|300px|thumb|'''Figure 12: Drawings of a house by a child with Williams syndrome and a child without Williams syndrome''']] | |||
Williams syndrome patients have been described as having a cognitive variety of relative strengths and weaknesses. Relative strengths include the social use of language, facial recognition and attraction to music. However there are deficits in visuospatial learning, use of vocabulary, onset of words in infancy and arithmetic. There is also a distinct behavioural characteristic of Williams syndrome, hyper sociability. These individuals are overly enthusiastic and socially interactive to an abnormal extent. Over 90% of Williams Syndrome cases also present with some form of anxiety or an anxiety disorder. Ironically, phobias often present themselves despite the affiliation for other people. Individuals with Williams syndrome also typically have poor coordination as well as a low IQ. Their average IQ is 55, ranging from 40-90, whereas average IQ is 90 and above. This means that these individuals will show a failure to thrive during infancy, especially in relation to language and motor development. | |||
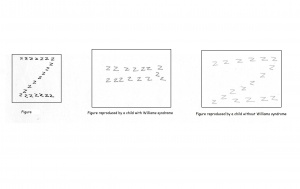
=== | ===Spatial cognition=== | ||
=== | Individuals with Williams syndrome typically have a deficiency in spatial cognition. An adult with Williams will function at a 5 year olds level of spatial cognition. This is often seen as the result of the attention paid to detail at the expense of the whole. There is often a bias present to carefully note the local aspects of the visual stimuli thus overlooking the global picture. An example of this is the replication of the letter Z constructed by a number of letter z’s. A Williams syndrome individual will draw a number of the smaller, local z as opposed to the complete structure. When asked to draw freehand images of a house, William syndrome adolescents were unable to present a logical arrangement of the building. Compared to a normal control of the same age, Williams syndrome individuals are highly impaired in spatial cognitive functions. Spatial cognition follows a specific path throughout development which remains impaired as the individual matures.[[File:Z diagram Williams.jpg|300px|thumb|'''Figure 13: A replication of the "Z" figure by a Williams syndrome individual and a control.''']] | ||
These spatial insufficiencies also extend to influence speech negatively. An example of this would be describing the tree relative to the house as behind the house when the tree is in fact next to the house. There is an obstruction of language expressed to the poor spacial skills exhibited in Williams syndrome. Williams Syndrome individuals often have difficulty with spatial representations and this effectively impairs other cognitive functions which require such spacial skills. <ref name="PMID20425784"><pubmed>20425784 </pubmed></ref> | |||
===Language Representations=== | |||
Many studies have been performed on the effects of Williams syndrome on individuals language abilities as it is one of the relative strengths in cognitive functioning. One area of interest is the expressive language used by individuals with Williams syndrome. A key feature of Williams syndrome is the incorrect use of sophisticated words in expressive language. The choice of these words is the result of selecting the right semantic field however failing to select for the appropriate context. This is possibly due to the presence of a different wiring mechanism of neurons in [[#Glossary | '''semantic processing''']]. | |||
Adolescents and adults are often articulate and very talkative which prevents other individuals engaged in conversation with them to recognise any cognitive abnormalities. | |||
During infancy, there is a stunted development of language as the onset of the first word is later than normal infants. All aspects of the behavioural phenotype of Williams syndrome is delayed heavily during the earlier years, this includes language. Although the onset of words is delayed, it is followed by a rapid acquisition words. However, the meaning of words may not be fully understood by the developing child saying them. For example, a 4 year old child could repeat long and often complex words such as encyclopaedia, proficiency and accomplishment without understanding the meaning of the word. The development of strong grammar during childhood years is followed by the rapid development of language. It is this rapid development of grammar that allows Williams syndrome to be distinguished from other forms of mental retardation. The acquisition of proper grammar allows adolescents and adults with Williams syndrome to form complex grammatical forms thus having greater language production compared to others with various forms of mental retardation. <ref name="Bellugi"> Bellugi, U. et al, 2001, ''Journey From Cognition to Brain to Gene: Perspectives From Williams Syndrome,'' Massachusetts Institute of Technology,the United States of America </ref> | |||
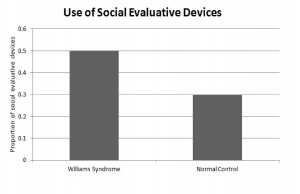
[[File:Use of social evaluation cues for WS and non WS individuals.jpg|300px|thumb|'''Figure 14: Use of social evaluation cues for WS and non WS individuals.''']] | |||
===Sociability=== | ===Sociability=== | ||
Williams Syndroms is a distinct disorder which is characterised by the individuals hypersociability and need for social interactions. Unlike most other disorders, individuals are remarkably social, empathetic and friendly. Williams syndrome individuals perceive others as friends rather than strangers. Across various studies, it has been found that individuals with Williams syndrome rate unfamiliar faces more approachable than most other normal controls. This finding is consistent with their interest in approaching strangers and their social phenotype. An early emergence of this social behaviour is present in infants with Williams syndrome. Whilst conducting tests on infants, an obstacle to retrieving accurate results is the tendency for the child to pay more attention to the people present than the task at hand. One theory is that the social behaviour is a mechanism by which the child can avoid the difficult task at hand. <ref name="PMID18211726"><pubmed>18211726 </pubmed></ref> | |||
Individuals with Williams Syndrome use language to effectively communicate with other on a social level. They not only engage in conversation with others, but also maintain the conversation by the extensive use of linguistic devices. There is an over use of lexical evaluative devices and vocal prosody to construct coherent and complex stories. The hypersocial phenotype of Williams syndrome is characterised by the individuals use of expressive devices over various linguistic settings to engage and maintain their audiences attention during conversation. This skill is developed throughout childhood and perfected during adolescents once grammar is established firmly in the individual. <ref name="PMID18924169"><pubmed>18924169 </pubmed></ref> | |||
===Musicality=== | |||
There is relatively less known about the musicality of Williams syndrome, however it has been marked as a relatively spared cognitive function. This could be explained by the multisensory processing which occurs when listening to music. Williams syndrome patients exhibit increased sensitivity towards auditory stimuli. There is known hypercusis, [[#Glossary | '''phonophobia''']] and auditory fascination. 80% of Williams syndrome patients shows an aversion toward everyday noises, as the individuals find such stimuli as painfully strong. Phonophobia has been diagnosed in 91% of the Williams syndrome individuals. This fear of normal sounds and aversion to everyday noises have been shown to be the result of an abnormalities in the limbic system aswell as the autonomic system. This avoidance and anxiety towards everyday noises and normal sounds are seen in younger children peaking at about 5-8 years and slowly reduces. This initial fear toward sounds however, is thought to be the start of auditory fascination. | |||
William Syndrome individuals are captivated by music and their strong emotional response to it. There is uniform and holistic sound perception found in the majority of Williams syndrome individuals. There is a high rate of rhythmic creativity found and a particular liking for rhythmic and percussion instruments. <ref name="PMID20733255"><pubmed>20733255 </pubmed></ref> | |||
===Anxiety and Phobias=== | |||
It has been found that when compared to the general population, children with Williams syndrome have a significantly higher rate of anxiety related disorders. They particularly showed a higher occurrence of generalised anxiety disorder and specific phobia disorder. | |||
Children with Williams syndrome often present with various anxiety disorders and phobias that carry-on through to adulthood. A study using the diagnostic and statistical manual of psychiatric disorders[[#Glossary | '''(DSM-IV)''']] showed that 5% of these children expressed sever psychological disorders. 80.7% of the individuals were shown to have been diagnosed with at least one of the diagnosed disorders in the DSM-IV. Of the many disorders, Attention Deficit/Hyperactivity Disorder and specific phobias were the highest occurrences. AD/HD was the most common disorder affecting almost 65% of the Williams syndrome children. Specific phobias, most commonly a phobia towards loud noises, was present in 54% of the individuals. A few other notable disorders were separation anxiety (7%), obsessive compulcive disorder (3%), social phobias (2%), post traumatic stress disorder (1%) and panic disorders (1%). <ref name="PMID20161441"><pubmed>20161441 </pubmed></ref> | |||
===Facial processing=== | |||
Despite deficits in spacial cognition, facial representations are an area of sparing in Williams syndrome. There is a remarkable ability to remember, discriminate between and recognise between both familiar and unfamiliar faces. This strength of facial recognition extends to the perception of faces in various contexts. Changing the lighting, orientation and background does not affect the identification of faces in Williams syndrome. Williams syndrome individuals are just as skilled as normal individuals of the same age in facial perception if not, better. Younger individuals with Williams syndrome are shown to have superior facial recognition abilities compared to age-matched individuals. <ref name="PMID19047076"><pubmed>19047076 </pubmed></ref>[[File:Cognitive performance in WS subjects (n = 67) versus normal controls.png|450px|thumb|'''Figure 15: Cognitive performance in Williams syndrome subjects (n = 67) versus normal controls.png''']] | |||
===Other cognitive functions=== | |||
William syndrome individuals show moderate to mild forms of mental retardation with an IQ average of 55 for the population. The IQ ranges from 40-90, which means some individuals have the IQ of a normal person. By adulthood, there is a failure to successfully perform piagetian seriation and conservation tasks normally achieved by 8 years of age. The cognitive impairment of Williams syndrome has been shown to be similar to that of Down syndrome. There is little difference shown between the verbal and performance IQ scores of the two groups. Arithmatics is shown to be the greatest challenge for the syndrome and language to be a relative strength. A severe impairment with the use of arithmetics and its implications to daily life has been shown to be expressed in Williams syndrome individuals. This can be shown by their preference for a hundred 5c as opposed to a $5 note. Some individuals however, are known to have adequately learn addition, subtraction and even division. Adults are also shown to have difficulty estimating quantities a normal developing child can do. For example, the length of a bus can be estimated to be as small as 1cm or as large as 100m when a normal child would say about 11m. | |||
Reading is a more variable function as some find it difficult to reading whilst others read particular topics of interests. Williams syndrome presents an uneven cognitive profile with specific strengths and various deficits unlike most forms of mental retardation where there is a general deficiency in all aspects of cognitive function. | |||
<ref name="Bellugi"> Bellugi, U. et al, 2001, ''Journey From Cognition to Brain to Gene: Perspectives From Williams Syndrome,'' Massachusetts Institute of Technology,the United States of America </ref> | |||
==Management== | |||
Currently, there is no cure for Williams-Beuren Syndrome as it is a complex multisystem medical condition. As symptoms of Williams-Beuren Syndrome involve multiple disciplines, treatment of those symptoms requires a large clinical team of doctors and nurses. With this team, a number of periodical assessments and systemic evaluations must be made if WS is diagnosed. This includes: | |||
*Complete physical and neurological examination | |||
*Growth parameters plotted on WS growth charts (see: [http://www.williams-syndrome.org/growth-charts/growth-charts]) | |||
*Cardiological evaluation | |||
*Genitourinary system evaluation | |||
*[[#Glossary | '''Ophthalmologic''']] evaluation | |||
*Multidisciplinary developmental evaluation (if patient is over 2 years of age) | |||
*Ultrasonography of bladder and kidneys | |||
*Urinalysis | |||
*Calcium determinations | |||
*Thyroid function tests | |||
*FISH to determine ELN deletion <ref>AMERICAN ACADEMY OF PEDIATRICS Committee on Genetics (2001) Health Care Supervision for Children With Williams Syndrome Pediatrics Vol. 107 No. 5 May 1, 2001 pp. 1192 -1204</ref> | |||
==Treatment== | |||
Currently, there is no cure for Williams Syndrome as it is a complex multisystem medical condition. The treatment of conditions brought about by Williams-Beuren Syndrome can be difficult depending on the number of recognised conditions. | |||
===Cardiac Treatment=== | |||
== | Surgery is generally required to repair supravalvular aortic stenosis if the severity of the condition is notable. <ref name="PMID11167112 "><pubmed>11167112 </pubmed></ref> Other procedures such as [[#Glossary | '''angioplasty''']] and [[#Glossary | '''stent insertion''']] are also practiced, but are highly susceptible to [[#Glossary | '''aneurysm''']], rupture or restenosis. <ref name="PMID11331257 "><pubmed>11331257 </pubmed></ref> <ref name="PMID15691415 "><pubmed>15691415 </pubmed></ref> | ||
Pulmonary artery lesions in patients with WS who aren’t diagnosed with SVAS can be monitored, as many patients with lesions in this area show gradual improvement with the lesions resolving over time. | |||
===Genitourinary Treatment=== | |||
The treatment of hypercalcemia involves constant monitoring of the patients’ blood calcium levels including before the administration of any anesthetic or sedative agent and prior to any invasive procedure. Food intake should also be regulated and monitored. <ref name="PMID15466114 "><pubmed>15466114 </pubmed></ref> 15% of patients with Williams-Beuren Syndrome show signs of hypercalcemia and it is important to note as the condition contributes to the presence of extreme irritability, vomiting, constipation, and muscle cramps. Treatment options for hypercalcemia include restricted dietary calcium intake and bisphosphonate therapy. In any case, the WS patient should be referred to a [[#Glossary | '''nephrologist''']] in order to treat renal abnormalities as they arise. <ref name="Morris">Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle</ref> | |||
Treatment for hypertension in WS patients is individualised and should be treated when identified. | |||
[[#Glossary | '''Osteopenia''']] can be difficult to treat in patients with Williams-Beuren syndrome as its treatment could have reverse effects on, and increase the severity of hypercalcemia. | |||
===Endocrine Treatment=== | |||
= | The treatment of endocrine abnormalities affiliated with Williams syndrome such as hypothyroidism requires regular monitoring and the prescribing of thyroid hormone related medicine should be done so if absolutely necessary. As glucose intolerance amongst WS patients is invariably high, adult patients should be screened regularly. As early puberty may bring an abundance of further challenges and complications to the management of WS, especially in females, it is also possible to delay [[#Glossary | '''menarche''']] with the use of a gonadotropin-releasing hormone such as [[#Glossary | '''leuprolide''']]. <ref name="PMID12219071 "><pubmed>12219071 </pubmed></ref> | ||
===Behavioural Treatment=== | |||
In terms of the emotional and psychiatric tendencies associated with Williams syndrome, these go some way in determining the quality of life for adult patients. Approximately half of adolescents and adults are being treated or have been treated with an [[#Glossary | '''antianxiety agent''']]. Types of drugs prescribed include selective serotonin-reuptake inhibitors such as [[#Glossary | '''Zoloft''']] and [[#Glossary | '''Prozac''']]. | |||
Counselling benefits those patients with relatively strong verbal skills. Patients may practice relaxation techniques and utilise strategies to deal with potentially anxiety-provoking situations. | |||
==Specialised Facilities and Supportive Associations== | ==Specialised Facilities and Supportive Associations== | ||
===Australia=== | |||
In Australia, there are a number of supportive associations and groups helping both individuals and families affected by Williams syndrome. These include: | |||
'''Williams Syndrome Family Support Group (Victoria)''' | |||
This group is described as a “community of families with Williams syndrome members, based in Victoria, Australia.” Its aims include: | |||
*To provide help and support for families with a child or adult with Williams syndrome | |||
*To provide resources for parents, teachers, students, employers and the medical profession | |||
*To provide information on current research | |||
*To empower those with WS to reach their full potential | |||
*To increase awareness and understanding of WS among the medical and teaching professions and the general public | |||
This group may be contacted using the following details: | |||
Honorary Secretary: Catherine Stenford | |||
PO Box 389, Balnarring, Victoria 3926 | |||
The website of the Williams Syndrome Family Support Group (Victoria) may be accessed here: [http://www.vicnet.net.au/~wsfsg] | |||
'''Williams Syndrome Family Support Group of South East Queensland''' | |||
This group may be contacted using the following details: | |||
Rebecca Carter | |||
15 Azalea Place, Currimundi, Queensland 4551, Australia | |||
Tel: (07) 5493 1185 | |||
'''Williams Syndrome Family Support Group of Western Australia (Inc)''' | |||
Secretary: Mr Rob Hendry | |||
Tel: (08) 9459 3716 | |||
'''Williams Syndrome Association of South Australia''' | |||
Regional Director: Gerry Mitchell | |||
Contact: Amanda Strybos or Mandy Turner | |||
PO Box 247, Salisbury, Adelaide 5108, Australia | |||
Tel: 8258 3867 | |||
The website of the Williams Syndrome Association of South Australia may be accessed here: | |||
[http://www.wsasa.org.au/index.html] | |||
'''Williams Syndrome (IHC) Association of New South Wales, part of the Association of Genetic Support of Australasia (Inc)''' | |||
Dianne Petrie | |||
c/o Association of Genetic Support of Australasia Inc | |||
66 Albion Street, Surry Hills, New South Wales 2021 | |||
Australia | |||
Tel: +61 2 9211 1462 | |||
'''Online Australian Williams Syndrome Forum''' | |||
===International Supportive Associations=== | |||
Outside of Australia, the most notable Williams syndrome supportive association is found in the United States of America with the Williams Syndrome Association (WSA). According to its mission statement the WSA “is a non-profit organisation that strives to enrich the lives of individuals and families affected by Williams syndrome and similar conditions through support, research and education. The WSA also provides the contact details and locations of various Williams syndrome clinics established throughout the U.S. These clinics are specialty clinics where those with Williams syndrome may be examined by a team of WS specialists and referred onwards to other services which may help. | |||
The website of the WSA can be accessed here: [http://www.williams-syndrome.org/] | |||
==Current research and developments== | ==Current research and developments== | ||
'''Induced chromosome deletion in a Williams-Beuren syndrome mouse model causes cardiovascular abnormalities''' - published in 2011, this study involved the deletion of the elastin gene ELN and what effect it had on cardiovascular abnormalities found in Williams syndrome. Results of this deletion were measured through ELN transcript levels, blood pressure, histological sectioning and M-mode ultrasound to determine circumferential cyclic strain. The results showed that ELN transcript levels were reduced by 38-41% in WS mice who had only one copy of the ELN gene instead of the normal two. These same mice showed an increase in mean blood pressure and also a reduced circumferential cyclic strain. The histological sections showed disorganised and fragmented elastic sheets. The paper concluded that the deletion of ELN in mice with Williams syndrome results in lower gene expression, hypertension, reduced cyclic strain and fragmented elastin sheets. Due to no change in medial lamella units in mice with Williams syndrome, the study suggests other genes may be involved in vascular development. | |||
'''Linking LIMK1 deficiency to hyperacusis and progressive hearing loss in individuals with Williams syndrome''' - published in 2011, this article suggests that a reduced expression of the LIM kinase1 gene involved in the regulation of the motile responses of cochlear outer hair cells and cochlear amplification results in and is the cause of hyperacusis and progressing hearing loss as observed in patients with Williams syndrome. | |||
'''Negative autoregulation of GTF2IRD1 in Williams-Beuren syndrome via a novel DNA binding mechanism''' - published in 2010, this study shows “the existence of a negative autoregulatory mechanism that controls the level of GTF2IRD1 transcription via direct binding of the GTF2IRD1 protein to a highly conserved region of the GTF2IRD1 promoter containing an array of three binding sites. This protein-DNA interaction is dependent upon multiple interactions between separate domains of the protein and at least two of the DNA binding sites. This mechanism leads to dosage compensation of GTF2IRD1 transcription”, resulting in the craniofacial dysmorphology, hypersociability, and visuospatial deficits displayed in Williams syndrome patients. | |||
'''Partial 7q11.23 deletions further implicate GTF2I and GTF2IRD1 as the main genes responsible for the Williams-Beuren syndrome neurocognitive profile''' - published in 2010, this study concludes that after revealing low expression levels of all the typical and atypical genes through lymphoblastoid cell lines, the “functional hemizygosity of both GTF2I and GTF2IRD1 genes is the main cause of the neurocognitive profile” seen in WS patients. | |||
'''Transcriptome profile in Williams-Beuren syndrome lymphoblast cells reveals gene pathways implicated in glucose intolerance and visuospatial construction deficits''' - published in 2010, this article states that through comparing the transcriptome profile of lymphoblastoid cell lines from various patients, 47 genes had been deregulated in WS patients. The pathways that were affected the most included glycolysis and neuronal migration. Genes involved in microtubule formation were also deregulated in patients with the common deletion. This abnormal regulation of gene pathways may be related to the cognitive, visuospatial and metabolic disturbances as seen in WS patients. | |||
'''Research Projects funded by the Williams Syndrome Foundation''' | |||
Mayada Elsabbagh, Mazal Cohen & Annette Karmiloff-Smith, '''Hearing and Hypersensitivity to Sound''', Institute of Child Health London started Autumn 2004. | |||
Professors Janette Atkinson & Oliver Braddick, '''Long Term Research into how Visual & Visuo-cognitive abilities develop in the eyes and brain in people with Williams Syndrome''' | |||
Visual Development Unit, Dept of Psychology, UCL London. | |||
More research projects funded by the Williams Syndrome Foundation can be found here:[http://www.williams-syndrome.org.uk/resources/research/research.html] | |||
'''Current research projects in affiliation with the Williams Syndrome Association''' | |||
*Dr. Carolyn Mervis, University of Louisville | |||
"Conducting clinical research studies primarily devoted to the cognitive processes in WS. Her team is conducting longitudinal studies of Language and Cognition in WS, as well as studies of language in very young children and the relationships between language, cognition and adaptive behaviour in Williams syndrome." | |||
*Dr. Helen Tager-Flusberg, Boston University | |||
"Conducting clinical research studies of the sociability of individuals with Williams syndrome. Dr. Flusberg's primary studies in this area are with teens and adults with WS." | |||
More research projects funded by the Williams Syndrome Association can be found here: [http://williams-syndrome.org/researcher/current-studies] | |||
===Mouse Models=== | |||
In recent years research has been focused on investigating the genetic origins of the physical, behavioural and psychological characteristics of Williams Syndrome, this is mainly being achieved through the use of mouse models. Mice share many genes with humans therefore [[#Glossary | '''"Knock-out mouse"''']] models can be developed to examine the results of gene deletion. The genes on chromosome 7 believed to be the cause of a particular manifestation in Williams Syndrome can be inactivated or "knocked-out" so that the implications of this action can be observed in the mouses' phenotype, including its appearance, behaviour and other observable physical and biochemical characteristics. This technique provides valuable clues about what these genes normally do and how their deletion contributes to Williams Syndrome. <ref>National Human Genome Research Institute 2010, ''Knockout Mice'', The National Human Genome Research Institute, viewed 11 October 2011, <http://www.genome.gov/12514551></ref> | |||
The following is an example of a research study conducted to explore the genotype-phenotype relationship in Williams Syndrome using mouse models: | |||
Enkhmandakh, B et.al, 2009, conducted a mouse model based study on the “essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development.” <ref name="PMID19109438"><pubmed>19109438</pubmed></ref> They investigated the little known contributions of the TFII-I transcription factors in embryonic development. | |||
Through the use of ‘knock-out’ mouse models, this study showed that the homozygous loss in function of either Gtf2ird1 or Gtf2i genes, which code for TFII-I, results in multiple abnormalities in the phenotype of the ‘knock-out’ mouse, including death of the embryo, brain haemorrhage and defects relating to blood vessels, neural tube and craniofacial regions of the embryo. Additional analysis demonstrated that embryo death could be caused by defects in the yolk sac and a subset of the two heterozygous genes presented retarded growth and skeletal and craniofacial defects. This therefore showed that an insufficient production of the TFII-I proteins, resulting from the ‘knock-out’ of Gtf2ird1 and Gtf2i genes in the 7q11.23 critical region of chromosome 7, causes some of the developmental manifestations associated with Williams Syndrome. | |||
'''Links:''' [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2629217/?tool=pubmed Article] | [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2629217/figure/F2/ Phenotypes of Gtf2ird1 and Gtf2i mutant mouse embryos] | [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2629217/figure/F3/ Morphological analysis of the head in mutant embryos and expression of Gtf2i and Gtf2ird1] | [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2629217/figure/F4/ Reduced growth, craniofacial and pigmentation defects in heterozygous Gtf2ird1 and Gtf2i animals] | | |||
==Acknowledgement== | |||
The creators of this page would like to sincerely thank Dr. Stephen Palmer of the School of Medical Sciences at the University of New South Wales for his assistance and guidance over the duration of this project. His willingness to help and teaching were greatly appreciated by all members of the group. | |||
== Glossary == | == Glossary == | ||
'''Congenital | '''2-dimensional echocardiography:''' Cross-sectional echocardiography, ultrasound-based diagnostic method. | ||
'''Aneurysm:''' Blood-filled dilation of a blood vessel, caused by a weakening of the vessel wall. | |||
'''Angioplasty:''' A medical procedure where a small balloon is inserted into the narrowed or obstructed blood vessel, inflated, and then removed once the blood flow through the vessel is normal. | |||
'''Antianxiety agent:''' Drugs used for the treatment of anxiety, and its related psychological and physical symptoms (also referred to as Anxiolytics). | |||
'''Atrial Septal Defect:''' A congenital heart defect in which the wall that separates the upper heart chambers (atria) does not close completely. | |||
'''Autosomal inheritance:''' Some hereditary diseases are described as autosomal which means that the disease is due to a DNA error in one of the 22 pairs that are not sex chromosomes. Both boys and girls can then inherit this error. If the error is in a sex chromosome, the inheritance is said to be sex-linked. | |||
'''Chordae Tendineae:''' Cord-like tendons that connect the papillary muscles to the tricuspid valve and the mitral valve in the heart . | |||
'''Cingulate Cortex:'''The cingulate cortex is a part of the brain situated in the medial aspect of the cortex, which includes the cortex of the cingulate gyrus. | |||
'''Colour Doppler:''' Ultrasound technique allowing simultaneous grey scale imaging and a dynamic colour flow vascular image. | |||
'''Congenital anomalies/malformations:''' Developmental abnormalities detected before or present at birth. There are no internationally agreed definitions for congenital anomalies and there are a range of classifications including the International Classification of Diseases (ICD), developed by the World Health Organization (WHO) to enable comparability for mortality statistics. | |||
'''Diastole:''' The period of time when the heart fills with blood during relaxation after systole. | |||
'''DSM-IV:''' The Diagnostic and Statistical Manual of Mental Disorders provides a criteria for the diagnosis and classifaction of mental disorders. It is published by the American Psychiatric Association. | |||
'''Elastin ateriopathy:''' Broken and disorganized elastin fibers caused by the mutation of the Elatin gene on chromosome 7. | |||
'''ERP:''' Event-related potential is a measured brain response that is directly the result of a thought or perception. | |||
'''FISH:''' Fluorescence in situ hybridisation; a technique used to detect the presence or absence of targeted DNA sequences on chromosomes. | |||
'''Haploinsufficiency:'''The incomplete or partial dominance, as a heterozygote (with one mutant and one normal allele) displays a phenotypic effect. | |||
'''Hemizygous:''' Only having a single copy of a gene as opposed to the normal two copies. | |||
'''Heschl's gyrus:''' The transverse temporal gyrus which is found in the area of the primary auditory cortex. | |||
'''Hypothyroidism:''' A condition in which the thyroid gland does not make enough thyroid hormone. | |||
'''Idiopathic infantile hypercalcemia:''' Abnormally high levels of calcium in the blood at childhood arising from an unknown cause. | |||
'''Intracardiac pressure:''' Pressure within the heart. | |||
'''Joint contractures:''' Abnormal stiffness of the joints causing the muscle to be shorter and unable to extend. | |||
'''"Knock-out" mouse:'''a transgenic mouse that has been genetically engineered to inactivate a particular existing gene by disrupting it or replacing it with an artificial piece of DNA. | |||
'''Kyphosis:'''An abnormal curvature of the spine, specifically at the upper thoracic segments creating a "hunch". | |||
'''Lamina Propria:''' A thin vascular layer of connective tissue which lies beneath the epithelium of the organs of the body. | |||
'''Leuprolide:''' An injectable, man-made hormone that is used for treating prostate cancer, endometriosis, central precocious puberty, and fibroids. It is similar to but stronger than human gonadotropin releasing hormone (GnRH). | |||
'''Lordosis:''' An abnormal curvature of the spine, usually at the lower half of the back. | |||
'''LCR:''' Low Copy Reapeats, a single copy gene region with repetitive sequences. | |||
'''LVOT:''' Left ventricular outflow tract, portion of the left ventricle of the heart through which blood passes to get pumped out by the aorta. | |||
'''Malocclusion:''' The abnormal positioning of the teeth when the jaw is closed. | |||
'''Mb:''' Mega base pairs. | |||
'''Menarche:''' The first menstrual cycle in female human beings. | |||
'''Metasarsophalangeal joint:''' The joints between the metatarsal bones of the foot and the more proximal bones. | |||
'''Microdeletion:''' The absence of a gene or part of a gene usually caused by inheritance. | |||
'''Mitral regurgitation of blood:''' A disorder of the heart in which the mitral valve does not close properly when the heart pumps out blood. It is the abnormal leaking of blood from the left ventricle, through the mitral valve, and into the left atrium, when the left ventricle contracts. | |||
'''Mitral Valve Disease:''' A degeneration of the heart's mitral valve resulting in backflow. | |||
'''Mitral Valve Leaflets:''' Leaflets of the mitral valve composed of tissue whose sole purpose is to open and close tightly in order to ensure the correct flow of blood through the heart. | |||
'''MRI:''' Magnetic resistance imaging, a diagnostic technique that uses a magnetic field and radio waves to provide computerized images of internal body tissues. | |||
'''Myocardial hypertrophy:''' Increase in size of myocardial muscle due to increase in size of individual myocardial cells, usually in response to an added afterload | |||
'''Myocardial infarction:''' An interruption in the blood supply to the heart because of narrowed or blocked blood vessels. Also referred to as a heart attack. | |||
'''Myxomatous degeneration:''' A pathological weakening of connective tissue. | |||
'''Neocerebellar:''' A part of the lower, posterior half of the brain which plays an important role in motor control. | |||
'''Nephrologist:''' Someone who studies the function and diseases of the kidney. | |||
'''NAHR:''' Nonallelic homologous recombination is a form of homologous recombination occuring between two lengths of DNA which are not alleles but have high sequence homology. | |||
'''Ophthalmology:''' The branch of medicine that deals with the anatomy, functions, pathology, and treatment of the eye. | |||
'''Osteopenia:''' A condition where bone mineral density is lower than normal. | |||
'''Paleocerebellum:'''The anterior lobe of the cerebellum. | |||
'''Phonophobia:''' A morbid fear of sounds including your own voice and particularly loud noises. | |||
'''PPS:''' Peripheral Pulmonary Stenosis, an obstruction anywhere in the pulmonary artery caused by vessel wall thickening as a result of elastin abnormality. | |||
'''Prozac:''' A selective serotonin reuptake inhibitor used to treat major depressive disorder, bulimia nervosa (an eating disorder) obsessive-compulsive disorder, panic disorder, and premenstrual dysphoric disorder (PMDD). | |||
'''Semantic processing:''' Coding information into or decoding information from communication patterns. | |||
'''Stenosis:''' An abnormal narrowing in a blood vessel or other tubular organ or structure. | |||
'''Stent Insertion:''' The insertion of an artificial cylinder into an artery in order to prevent flow constriction. | |||
'''STRs:''' short tandem repeats. | |||
'''SVAS:''' Supravalvular Aortic Stenosis; an obstruction occurring in the left ventricular outflow tract (LVOT)caused by vessel wall thickening as a result of elastin abnormality. | |||
'''Systolic murmurs:''' Murmur or sound occurring during the contraction of the heart and pumping of blood out of the ventricle. | |||
'''Ultrasonography:''' An ultrasound-based diagnostic imaging technique used for visualizing subcutaneous body structures including tendons, muscles, joints, vessels and internal organs for possible pathology or lesions. | |||
'''Urinalysis:''' An array of tests performed on urine. | |||
''' | '''Ventricular Septal Defect:''' One or more holes in the wall that separates the right and left ventricles of the heart. | ||
''' | '''Visualspatial construction:''' the ability of a person to perceive, analyze, and understand visual information taken in from the world around them. | ||
''' | '''WS:''' Williams Syndrome. | ||
''' | '''Zoloft:''' An antidepressant drug used to treat depression, obsessive-compulsive disorder, panic disorder, anxiety disorders, post-traumatic stress disorder (PTSD), and premenstrual dysphoric disorder (PMDD). | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Latest revision as of 11:01, 19 October 2011
| Note - This page is an undergraduate science embryology student group project 2011. |
Williams-Beuren Syndrome
Introduction
Williams-Beuren Syndrome, more commonly known as Williams Syndrome, is a congenital abnormality caused by the deletion of genetic material on chromosome 7q11.23, which encompasses 28 neighbouring genes.[1] [2]
This multisystem developmental genetic disorder implicates psychological, behavioural and medical defects, including diverse phenotypic characteristics such as distinctive facial deformities, cardiovascular abnormalities,intellectual disabilities/mental retardation, growth abnormalities, endocrine abnormalities and a unique personality and cognitive profile. [1] [3] Some or all of these features may be present in varying degrees, with the condition becoming apparent at the onset of birth or in early infancy. [4]
Williams Syndrome, in a majority of cases, is not inherited. It can manifest in families with no history of the disorder, this is because Williams Syndrome is the result of a random event that occurs at gamete formation in the parent of a Williams Syndrome patient. Only a single copy of the altered chromosome 7 in each cell is required to cause this syndrome, therefore it is considered to be an autosomal dominant condition. There is only a small percentage of cases where people inherit the chromosomal deletion associated with Williams Syndrome from a parent with the condition. [5]
Ever since it was discovered that the cause of this genetic abnormality was a microdeletion in chromosome 7, one of the main focuses of research has been attempting to identify the different responsibilities each gene of the deletion has for the function and development of the brain as well as their role in physical characteristics. These research efforts have the common aim to provide knowledge of how cognitive, behavioural and physical features arise as a result of the gene absence and their interplay with the environment. Current research into the links between genes absent in Williams Syndrome and the brain, behaviour and structural and functional abnormalities have been confirmed and are continuously being investigated through the use of mouse models. [6] The exact links between the missing genes and the phenotype of Williams Syndrome is yet to be fully explored.
Links: Williams Syndrome | OMIM Entry #194050-Williams-Beuren Syndrome
History of the disease
William-Beuren Syndrome is named after John C.P. Williams, a cardiologist from New Zealand, and Alois J Beuren, a German physician and cardiac researcher.
Initial investigation into Williams-Beuren Syndrome came from two apparently different disorders, idiopathic infantile hypercalcemia and Supravalvular Aortic Stenosis(SVAS) . With further research, these abnormalities were identified as being aspects of this same syndrome. [7]
The first cases related to Williams Syndrome involved idiopathic infantile Hypercalcemia. From as early as 1952, research into infantile hypercalcemia, by Falconi et.al, found that children with this disorder had common clinical characteristics such as a short stature and a variety of congenital malformations. [8] In the years that followed many other studies were conducted in this area revealing additional correlations. For example in 1957 Stapleton and colleagues studied the effects of hypercalcemia in a number of infants and noted several consistencies between them including abnormal facial features, failure to thrive, developmental delay, and systolic murmurs of the heart, all of which have now been associated with Williams Syndrome. [9] [1]
J.C.P Williams was one of the first to recognise some of the clinical factors associated with this syndrome. In a study conducted in 1961 of SVAS, an obstruction occurring in the left ventricular outflow tract (LVOT), Williams and his colleagues made the observation that patients suffering from this heart condition had strikingly similar unusual facial features that included broad foreheads, eyes set wider apart than normal, wide mouths with pouting lips and malocclusion of teeth, pointy chin and prominent pointed ears. As well as this they discovered their subjects also presented with mental retardation and a low IQ. Williams and his colleagues suggested that their findings might be "indicative of a previously unrecognised syndrome." [7] [10]
In this same year Rashkind and colleagues published a paper detailing the relationship between idiopathic childhood hypercalcemia and cardiac abnormalities [11] [12]
In 1962 AJ Beuren and associates also studied the correlations between SVAS, mental retardation and distinctive facial features of a number of subjects and made similar observations to Williams, particularly pointing out the characteristic “elfin” features of Williams syndrome patients. [13] [12] Beuren also noted the behavioural traits of his subjects suffering from SVAS, describing them as all having a “friendly nature”, something which would later be recognised as one of the unique personality traits of people diagnosed with Williams-Beuren Syndrome. [13] [7]
In further studies conducted in 1964, Beuren and his colleagues detailed the possible association of Peripheral Pulmonary Stenosis (PPS) and complex dental malformations with SVAS, mental retardation and certain facial appearance which they examined previously. They too came to the conclusion that these complications were representative of a new syndrome. [14] [7]
Timeline
| Year | Events |
| 1950s | Increasing awareness and research into infantile idiopathic hypercalcemia [7] |
| 1952-1957 | Studies conducted by several researchers on infantile hypercalcemia revealed correlations between it and several defects known to be characteristic of Williams Syndrome today. These included abnormal facies, developmental delay, mental retardation and heart palpitations [7] [8] [9] |
| 1961 | J.C.P Williams and colleagues were the first to suggest that SVAS may be an element of a previously unidentified syndrome which results in mental retardation and an abnormal facial structure. [15] [10] |
| 1962 | A.J Beuren and peers made same observations as Williams detailing links between SVAS, mental retardation and abnormal faces, specifically describing them as “elfin-like.” They were also the first to highlight the “over-friendly nature” of their subjects. [13] |
| 1963 | Black and Bonham-Carter, from England, drew attention to the fact that those individuals who had been affected by Infantile Hypercalcemia had very similar facial features to those patients with SVAS [16] [7] |
| 1964 | Beuren and colleagues made associations between Peripheral Pulmonary Stenosis and complex dental malformations with SVAS. Like Williams, they too believed these were signs of a common undiscovered syndrome. [14]
In this same year a further advancement into research that links Idiopathic Hypercalcemia with SVAS was made by R.Garcia and associates. They presented a case which proved that these two conditions were related and proposed both were symptoms of “a newly recognized syndrome.” [17] |
| 1970 | The above reports all contributed to a large series of phenotypes that now became known as 'Williams Syndrome' or 'Williams elfin facies syndrome'. Further research into specific cases continued to reinforce this newly identified syndrome. [18] [1] |
| 1972 | Beuren presented compelling evidence that “Williams syndrome” and infantile hypercalcemia are the same disorder. [19] |
| 1980s | Research continued and revealed further disabilities associated with Williams Syndrome, including physical characteristics such as, growth problems, joint limitations, genitourinary problems and other developmental disabilities, [20] giving it the title of a multisystem disorder. [21] |
| 1993 | A major discovery was made by Morris et.al. Genetic research conducted on large and small families affected by Williams Syndrome revealed that the SVAS phenotype was closely linked with several DNA markers, including elastin, along the long arm of Chromosome 7. [21]
Further research into this concluded that the mutation of the Elastin gene on chromosome 7 was the cause of SVAS. [22] [1] This was rapidly confirmed in many other studies and led to the first laboratory test of the disorder. [1] |
| 2006 | In recent years, studies conducted on Williams Syndrome patients with the use of functional MRI of the brain, demonstrated changes linked to the behavioural characteristics of this condition, for example difficulties in visualspatial construction. [7] [23] |
Links: John C. P. Williams of Williams-Beuren syndrome
Genetic factors and Etiology
Williams Syndrome is a multi-system genomic disorder that occurs due to a hemizygous deletion/nonallelic homologous recombination (NAHR) . The sizes of deletion commonly range from 1.55 to 1.84 mega base pairs (Mb) on chromosome 7q11.23 which encompasses 28 genes.[1] [3]
The region associated with Williams syndrome contains a single copy gene region with repetitive sequences or Low Copy Repeats (LCR) . The deletions that cause Williams syndrome are due to a misalignment of these repetitive sequences or gametes within the Williams-Beuren syndrome critical region. [24] This occurs during the process of meiosis and follows an unequal crossing over that is due to a high similarity of LCRs.
A genotype-phenotype correlation has been found for some of the genes within the deletion region. The most well known of these being elastin gene (ELN), whereby elastin haploinsufficiency is responsible for a number of abnormalities characteristic of Williams Syndrome, particularly connective tissue abnormalities and cardiovascular disease including arterial stenosis. [25] CLIP2, GTF2I, GTF2IRD1 and LIMK1 are some of the other genes that are most commonly deleted in individuals with Williams syndrome. Researchers have suggested that the deletion of these genes could help in the explanation of some of the characteristic signs of Williams syndrome including the unique behavioural characteristics and also some of the related cognitive difficulties.
In most cases, Williams syndrome occurs sporadically and the deletions can occur with no reference to the parental origin of the chromosome that transmits the disease. There have been, however, a very small number of cases where autosomal dominant inheritance of Williams syndrome has been reported.
| Gene | Normal Function | Relation to Williams Syndrome |
| Elastin (ELN) | Elastin is a main component of elastic fibres. It also contributes to the structure of connective tissue, particularly its flexibility and strength. The expression of the ELN gene is largely limited to the third trimester of fetal development and early postnatal years.[26] | The loss of one copy of this gene reduces the normal production of elastin by half.
Elastin haploinsufficiency is responsible for a number of abnormalities characteristic of Williams Syndrome, particularly connective tissue abnormalities and cardiovascular disease including arterial stenosis. [25] |
| LIM domain kinase 1 (LIMK1) | Studies, such as Wang et al in 1998 [27] suggest that LIMK1 is involved in the area of the brain that is in control of the visualisation of an object as a set of parts.
It has also been implicated in other visual tasks such as drawing, making models and writing. |
Studies differ in regards to the contribution of LIMK1 deletions to the phenotype expressed in Williams syndrome. Some studies have suggested that the loss of this gene leads to the problems with visuo-spatial tasks that are common in Williams syndrome. Other studies suggest that it is involved with the characteristic progressive loss of hearing, [28] while other studies have not found these connections. |
| General transcription factor IIi (GTF2I) | This gene is involved in the production of the following two proteins:
|
It has been suggested that the loss of one copy of this gene may be responsible for the intellectual disability seen in Williams syndrome. It may also be involved in the social characteristics of those with Williams syndrome. [29] |
| General Transcription Factor II-I Repeat Domain-containing Protein 1 (GTF2IRD1) | It was shown in 2005 by Tassabehji et al. [30] that the GTF2IRD1 gene is involved in the development of both craniofacial features and cognitive development in mammals.
It was also shown by Yan et al in 2000 that GTF2IRD1 is involved with the function of the RB1 protein both in vitro and in vivo. [31] |
The deletion of a copy of this gene has been implicated in some of the phenotypic features seen in Williams syndrome through the use of data from human genetic mapping. These features include the hypersociabilty and visuaspatial deficits, but the main implication are the craniofacial abnormalities. [29] |
Links:
OMIM Entry #130160 – Elastin (ELN) | OMIM Entry #601329 - LIM domain kinase 1 (LIMK1) | OMIM Entry #601679 - General transcription factor IIi (GTF2I) |General Transcription Factor II-I Repeat Domain-containing Protein 1 (GTF2IRD1)
Diagnosis
There are ways to clinically diagnose Williams Syndrome even though the phenotype varies between individuals. There is not a particular clinical feature that is able to ascertain the diagnosis alone, but there are particular features that individuals with Williams Syndrome may present with. These features include conditions and abnormalities discussed in the associated medical conditions sections, but most commonly include:
- Distinctive facial features
- Unique personality
- Intellectual disability
- Growth abnormalities
- Cardiovascular Disease
- Endocrine Abnormalities
Although these clinical criteria for diagnosis are available, the detection of the contiguous gene deletion responsible for Williams Syndrome is the main form of diagnosis. It has been found that over 99% of individuals that meet the clinical diagnosis criteria for Williams Syndrome also have this contiguous gene deletion. This gene deletion is detected through the use of fluorescent in situ hybridisation (FISH) or through targeted mutation analysis. [32]
Fluorescent in situ hybridisation (FISH) : FISH uses elastin probes for a specialised form of chromosome analysis. This method is commercially available and commonly used to determine if an individual showing the clinical signs of William’s syndrome does have the deletion of the genes in the Williams-Beuren syndrome critical region. If the individual only has one copy of the elastin gene, then a diagnosis of William’s syndrome is confirmed. [33]
Targeted Mutation Analysis: these are non FISH methods that are also used to detect the contiguous gene deletion in order to determine whether or not an individual has Williams Syndrome.
- Real-time quantitative PCR: is used to establish the number of copies of three of the genes contained within the Williams-Beuren syndrome critical region. A deletion in this area would mean that only a single copy of one of these genes would be found.
- Genomic microarray analysis: makes use of array genomic hybridisation techniques which involved examining multiple genes simultaneously and comparing them in order to determine whether there are any abnormalities in the amount of chromosomal material.
- Heterozygosity testing: is mostly used to identify the size of the deletions, and involves the testing of short tandem repeats (STRs) from the Williams-Beuren syndrome critical region. If there is no deletion, than the STR sizes should be different at each of the markers determining heterozygosity. But if only one STR size is found at a marker, than this may indicate that there is a deletion, but may also indicate homozygosity. Quantitive PCR is then needed to determine whether or not the single STR size is dur to a deletion in the critical area or a non-abnormal finding of homozygosity.
Epidemiology
Rate of Incidence
Williams-Beuren Syndrome occurs in 1 in 7,500-20,000 births.[34] Many of the clinical manifestations associated with Williams syndrome are globally consistent, however some studies have shown that some symptoms are less common in certain countries. A study conducted in the Hong Kong Chinese population showed that SVAS, the most common defect associated with Williams syndrome in the West, is less common than peripheral pulmonary stenosis in the study area.[35] Another study showed that the prevalence of cardiovascular defects in a group of Williams syndrome patients in Greece was lower than that documented in the majority studies performed (8/50 rather than half or more of the study group).[36] With regard to sex, it has been noted that the severity of SVAS and total cardiovascular disease is significantly greater in males than in females with WS, proving that the severity of elastin arteriopathy is indeed affected by sex. This difference, as hypothesised, may be related to prenatal hormone effects.[37]
Genetically, 90%–95% of patients clinically diagnosed with the syndrome have an approximate 1.55-Mb deletion associated with the loss of 26–28 genes on the 7q11.23 chromosome; 5%–8% of those diagnosed have a slightly larger deletion of approximately 1.84-Mb pair deletion associated with the loss of 28 genes.[38]
Facial Characteristics
Most physical musculoskeletal abnormalities result from the absence of elastin due to the elastin gene deletion. The facial phenotype also changes with time as the child develops. This makes it harder to diagnose Williams Syndrome based on facial characteristics in adults. [1] [39]
| Features | |
| Mouth and Nose |
|
| Eyes and Ears |
|
| Craniofacial Skeleton |
|
Links: Lecture - Head Development
Cardiac Conditions
One of the major manifestations of Williams Syndrome is cardiac abnormalities, 80% of those diagnosed with this syndrome show some form of heart condition. [41] Most heart defects result from the mutation or deletion of the Elastin (ELN) gene on chromosome 7. Damage or loss of this gene causes its subsequent loss in function, no longer providing the necessary elasticity in tissues like the lungs, the dermis and larger blood vessels, with one of the major results being elastin arteriopathy. [41] [42] This is present in about 75% of affected individuals and may be present in any artery, the most common of them being in the aorta causing a condition known as Supravalvular Aortic Stenosis (SVAS). [43] [41] Other cardiac anomalies include Hypertension, Peripheral Pulmonic Stenosis (PPS), Mitral Valve Disease, Atrial Septal Defect and Ventricular Septal Defect. These conditions and their severity varies from patient to patient. [41] It is reported that males diagnosed with Williams syndrome are more likely to suffer from cardiovascular disease than females who are diagnosed with this same disorder. [37]
Stenoses
Stenoses are the typical types of cardiovascular disorders in Williams Syndrome. This manifestation is characterised by the narrowing of arteries due to the thickening of these blood vessels caused by smooth-muscle overgrowth. Stenoses may be isolated or may occur simultaneously in numerous places, in both medium and large arteries such as the aorta (in SVAS) and pulmonary artery (in PPS) [44] The most prevalent classes of stenoses are SVAS and PPS, with 70-75% and 39% of people with Williams syndrome reporting to suffer from SVAS and PPS respectively. [45] Other stenoses occur in peripheral vessels such as renal, carotid, coronary, subclavian and mesenteric arteries.[44]
It has been found that PPS is more common in infancy and tends to improve over time. On the other hand, SVAS affects Williams Syndrome sufferers of any age and is most likely to worsen over time if not treated. [43]
- Supravalvular Aortic Stenosis (SVAS)
SVAS is the most common condition in Williams Syndrome, and was one of the first to be associated with this anomaly. It is characterised by elastin arteriopathy in the ascending aorta causing its narrowing. [21] This condition is caused by the loss of function of the Elastin (ELN) gene as a result of a mutation or deletions within this gene. [41] Histological analysis of the arterial walls of Williams Syndrome patients with SVAS show a disorganised structure with fragmented elastic fibers and an increase in the size of smooth muscle cells (muscle hypertrophy). [46] SVAS is the dominant cause of morbidity and mortality for this syndrome’s patients [1] because when left uncorrected, it may lead to increased intracardiac pressure, myocardial hypertrophy, heart failure, and eventually death. [21] SVAS can be diagnosed through 2-dimensional echocardiography that allows for multiple views of the heart to be examined. [47]
- Peripheral Pulmonary Stenosis (PPS)
PPS is the second most common cardiac abnormality associated with Williams Syndrome. It is a narrowing at the level of the main pulmonary artery, occurring in multiple places along this artery and can be related to hypoplasia or incomplete development of the pulmonary arterial bed. [48] [49] PPS is also the cause of vessel arteriopathy, an outcome of deletion or mutation of the ELN gene. [38]
Other problems
- Hypertension
Hypertension or high blood pressure is another common cardiac condition that can result from Williams Syndrome. Studies have shown that an increased risk of high blood pressure in Williams Syndrome sufferers is the result of the re-organisation of lamellar structures in large vessel walls and the degeneration of elastic fibers caused by the loss of the ELN allele. [46] In some cases it can arise as a result of the narrowing of the renal artery (Renal artery stenosis)[43] Hypertension in Williams syndrome can develop during childhood, however it is more commonly found in adults, with more than half of them developing high blood pressures.[38] [44] Consequences of this condition can be an increased risk of myocardial infarction and stroke.
- Mitral valve disease
The Mitral valve, or bicuspid valve, between the left atrium and ventricle is another structure of the heart that can be affected in Williams Syndrome. The most common abnormality associated with this syndrome is mitral valve prolapse(MVP)which results in multiple, unnecessary mitral valve leaflets, more elongated chordae tendineae and myxomatous degeneration of the leaflet tips. These characteristics lead to the leaflets of the valves bulging into the left atrium during systole and the inability of the leaflet tips to join together. The mitral valve isn't able to function in closing the left ventricle off from the right atrium once diastole has finished and so mitral regurgitation of blood back into the atrium occurs when blood is trying to be pumped out. [50]
Table showing "the prevalence of CV abnormalities in 423 patients from nine selected international series published in the last two decades" [38]
| CV abnormality | Prevalence (%) |
| All CV diseases | 84 |
| SVAS | 69 |
| PPS | 34 |
| Hypertension | 17 |
| Mitral valve disease | 15 |
| Pulmonary Valve disease | 5 |
| Aortic valve disease | 3 |
Links: Lecture - Heart Development
Genitourinary Conditions
Renal Tract Abnormalities
In a study of 40 patients with Williams Syndrome, some form of renal abnormality was detected in 7 of the patients from the study group. This study provided the foundation for the statistic stating that 18% of people with Williams Syndrome have some form of renal tract abnormality.[51] Some of these abnormalities include:
- Renal Agenesis
Renal agenesis is a disorder involving the absence of one or both of the kidneys, categorised into unilateral or bilateral respectively. In this case, renal agenesis is a secondary condition to the developmental genetic disorder that is Williams Syndrome.
Bilateral renal agenesis is fatal and most infants die within the first four hours following birth. This is due to the noticeable deficiency of amniotic fluid (oligohydramnios) after 12-13 weeks causing pulmonary hypoplasia.
It is diagnosed firstly by the absence of amniotic fluid followed by the absence of the bladder and kidneys. [52]
Following diagnostic tests such as CT scans and ultrasonography, Colour Doppler has been found to be useful in detecting and identifying vascular anomalies related to renal agenesis. [53]
Unilateral renal agenesis results in mostly normal development. Patients must be examined periodically as there is an increased incidence of urinary tract infections amongst those affected. Elevated blood pressure may result in kidney damage. Patients are advised to not participate in contact sports in order to protect the sole remaining kidney. Unilateral/Bilateral renal agenesis results from a lack of induction of the metanephric blastema by the ureteral bud. [54]
- Duplicated kidneys
Also known as duplex kidneys, duplicated collecting systems and duplex collecting systems. This is defined as the doubling of the renal units; either containing 2 pyelocaliceal systems in relation to a single ureter or with double ureters. The 2 ureters may empty separately into the bladder or fuse to form a single ureteral orifice. As with renal agenesis, duplicated kidneys may be classified as unilateral or bilateral. In general, patients usually have complete ureteric duplication in which results in a greater tendency for the ureters to develop obstructions, reflux, and infections. [55]
A duplex kidney with either a bifid renal pelvis or bifid ureter results when a single ureteral bud bifurcates before the ampulla bifurcates. [56]
- Vesicourinary reflux
Vesicourinary (vesicoureteral) reflux is the reverse flow of urine from the bladder to the ureters or even the kidneys. It results as a failure of the ureteric openings which store and void urine.
The International Reflux Grading system classifies VUR into 5 grades, depending on the degree of retrograde filling and dilatation of the renal collecting system.
| Grading | Characteristics |
| Grade I | Urine backs up into the ureter only, and the renal pelvis appears healthy, with sharp calyces. |
| Grade II | Urine backs up into the ureter, renal pelvis, and calyces. The renal pelvis appears healthy and has sharp calyces. |
| Grade III | Urine backs up into the ureter and collecting system. The ureter and pelvis appear mildly dilated, and the calyces are mildly blunted. |
| Grade IV | Urine backs up into the ureter and collecting system. The ureter and pelvis appear moderately dilated, and the calyces are moderately blunted. |
| Grade V | Urine backs up into the ureter and collecting system. The pelvis is severely dilated, the ureter appears tortuous, and the calyces are severely blunted. [57] |
In most cases, vesicourinary reflux presents with no symptoms. When symptoms are present, the most common is a urinary tract infection (UTI). Bacteria thrive in this suitable habitat as the urine remains in the child’s urinary tract, thus causing the urinary tract infection. Studies estimate that 30 percent of children and up to 70 percent of infants that present with a urinary tract infection have vesicourinary reflux. [58]
- Nephrocalcinosis
Nephrocalcinosis occurs in less than 5% of patients with WS. Although relatively rare, it is one symptom which presents in infant, child and adult life. It is the deposition of excess calcium in the kidneys which may lead to kidney failure, kidney stones or obstructive uropathy. Nephrocalcinosis may be diagnosed by abdominal CT scans, ultrasounds of the kidney and urinalysis. [59]
Links: Lecture - Renal Development
Endocrine Conditions
Hypercalcemia
Hypercalcemia refers to elevated levels of calcium in the bloodstream. It is not always observed in individuals with Williams syndrome but it is more common among children with infantile hypercalcemia being reported in approximately 15% of infants diagnosed with Williams syndrome. Many individuals diagnosed with Williams syndrome show the symptoms associated with hypercalcemia. For instance, in infants, these symptoms are most commonly irritability, vomiting and constipation, whereas in adults there are more commonly urinary infections and petic ulcer disease. Most of these cases are reported within the first four years of age, but cases of recurrence in puberty have also been reported. Typically, the infantile hypercalcemia is transient and found only in mind forms, but in rare cases it can also be life-threateningly severe. [60]
Diabetes Mellitus
It is reported that approximately 75% of adult individuals with Williams syndrome suffer from a form of pre-diabetes, such as impaired glucose tolerance, or diabetes mellitus. [61] In the Williams-Beuren syndrome critical region, one of the genes is syntaxin-1A (STX-1A). This gene codes for a protein that is involved in exocytosis of insulin granules in pancreatic beta-cells. Lam et al in 2005 [62] used a mouse model and an intraperitoneal glucose challenge to show that an overproduction of this syntaxin-1A gene resulted in hyperglycemia (described above) as well as a lower secretary level of insulin.
Thyroid
The prevalence of thyroid abnormalities is increased in individuals diagnosed with Williams syndrome. Some of these abnormalities include thyroid-gland hypoplasia, hypothyroidism, and morpho-volumetric abnormalities in the thyroid gland. Thyroid-gland hypoplasia, which is present in 75% of Williams syndrome individuals has been impicated as a cause of the congenital hypothyroidism, a form of thyroid dydgenesis, seen in 35% of these individuals. Also, there is a 67.5% prevalence of morpho-volumetric abnormalities in the thyroid gland associated with Williams syndrome. [43]
Links: Lecture - Endocrine Development
Other Associated Medical Conditions
Other Abnormalities
There are a number of other abnormalities associated with Williams Syndrome including a hoarse voice, inguinal hernias and joint abnormalities. These abnormalities vary in severity between different individuals and elastin haploinsufficiency is responsible for a number of these abnormalities characteristic of Williams Syndrome.[25]
| Abnormality | Cause | Effect and Relation to Williams Syndrome |
| Joint Abnormalities | The elastin deficiency seen in Williams syndrome is the cause of the lax joints and other joint abnormalities. | Individuals with Williams syndrome typically have loose joints during infancy which then get worse with age and towards later childhood, they may develop joint contractures.
The lax joints that young children with Williams syndrome suffer from often lead to compensatory measures of posture, resulting in mild lordosis and kyphosis . [25] These joint abnormalities occur in the lower limbs more often than anywhere else in the body. |
| Ingiunal Hernias | Although further research needs to be done, it is suggested that mutations in the SERPINA1 gene, could play a role in the prevalence of direct inguinal hernias in individuals with Williams syndrome. This is due to the fact that this particular gene, encodes for the elastase inhibitor - alpha-1-antitrypsin (AAT). . | Inguinal hernias are more common in individuals with Williams syndrome. They occur in approximately 40% of individuals with Williams syndrome, but only in approximately 5% of individuals that are not diagnosed with Williams syndrome. These inguinal hernias are usually diagnosed when the individual is an infant and they are more common in males then females, with an approximate ratio of 10:1. [63] [25] |
| Auditory Abnormalities | Chronic otitis media or chronic ear infections occur more commonly in children with Williams syndrome. Children in the average population have an average occurrence rate of 41% while those with Williams have an occurrence rate of 50%. [64] | Hearing loss is widely seen in adults with Williams syndrome and although not as extensive, it is also commonly seen in school-aged children with Williams syndrome. There is previous research that suggests that the hearing loss that is associated with Williams syndrome begins early and is likely to be progressive with age. [65] |
| Hoarse Voice | Due to a connective tissue abnormality, where the lamina propria in the vocal folds has a decreased amount of elastic fibres. [66] | The hoarse voice is present in 98% of people with Williams Syndrome. |
| Hallux Valgus | Hallux Valgus is the result of the musculoskeletal deviation seen in Williams syndrome. | This results in the projection of the metasarsophalangeal joint inward to the inner foot. It is reported that in approximately 78% of individuals diagnosed with Williams syndrome have a big toe which is displaced under or over their other toes. [67] |
| Developmental delay in height and weight | This delay, as well as many of the other abnormalities seen in Williams syndrome has been associated with the lack of connective tissue, due to the deletion of the ELN gene. | Individuals with Williams syndrome often present smaller than others without Williams syndrome at the same gestational age, and grow to have a short stature. A study by Pankau et al in 1992, found intrauterine growth retardation in 35% of females with Williams Syndrome and 22% in males. [68] |
| Early onset of puberty | It has been suggested that the early onset of puberty could be related to a disruption in hormonal secretions associated with Williams syndrome. There has also been evidence to implicate the hypothalamic-pituitary mediated activation. [69] | It has been found that individuals with Williams syndrome are more likely to have an earlier onset of puberty. With females beginning their menstrual cycle and males showing Tanner III pubic hair development under the age of 12. [69] |
Structural Differences in the Brain
Williams syndrome has been characterized by the smaller brain mass, but relative enlargement of particular areas of the brain. The neocellebellar vermis has been shown to be larger in Williams syndrome individuals and a differential development of the paleocerebellum and other areas of the brain was found.
Language
The abnormal language expressed in Williams syndrome has been related to the abnormal functioning of the brain during processing. The normal left-anterior asymmetry in language processing has been lost and a more diverse arrangement has been found. Event-related potential (ERP) studies have shown the functioning of a different pathway to that of a normal person in language processing. This abnormal organization of the neural network is what has allowed for the relative sparing of the language processing in Williams syndrome.
Cognitive Abilities
A reduction in the size of the parietal occipital region has been found in the majority of Williams syndrome patients. This reduction in the posterior cerebral area has been predicted to contribute to the visuospatial processing difficulty found in the syndrome. The isthmus and splenium are the major white matter tracts which connect the visual processing and associated processing areas of the brain. This would result in the impaired visospatial processing of visual information in the brains of Williams syndrome individuals. The areas of language and affect have been shown to be relatively preserved as the frontal lobe is correctly proportioned in William syndrome individuals. The hypersociability of Williams syndrome individuals may be attributed to the enlargement of the neocerebellar vermis. The social and emotional functions of the posterior cerebellum has been the area thought to be responsible for these phenotypes. There has been a consistently long central sulcus found in William syndrome individuals. There is an unusual configuration of the central sulcus which is associated with abnormal behavior. There is also an overall unusual configuration of the other gyri on the medial surface and temporal lobes. There is brain asymmetry and cingulate cortex dysfunctioning has been attributed to the abnormal social engagement expressed by William syndrome individuals. [70]
Auditory
The amygdala has been related to the lateral nucleus and contributes to the inhibited fear response to new faces. The auditory centres are known to be the thalamus and cortex, however, the visual cortex has been shown to activate in Williams syndrome individuals when stimulated by music. At a cortival level, There seems to be a hyperactivity when a musical stimuli is present. This is the result of a more excitable auditory response formed by hyperexcitable neurons which form irregular neural systems with other functional areas of the brain. Heschl’s gyrus has been found to be duplicated in William syndrome individuals who present with 2-4 pairs of Heschl’s gyrus. The auditory cortex of these individuals has been shown to be relatively larger than an average human, 2.2 times in the left hemisphere and 1.2 in the right. [71]
Links: Lecture - Neural Development
Cognitive, Behavioural and Neurological Phenotype
Williams syndrome patients have been described as having a cognitive variety of relative strengths and weaknesses. Relative strengths include the social use of language, facial recognition and attraction to music. However there are deficits in visuospatial learning, use of vocabulary, onset of words in infancy and arithmetic. There is also a distinct behavioural characteristic of Williams syndrome, hyper sociability. These individuals are overly enthusiastic and socially interactive to an abnormal extent. Over 90% of Williams Syndrome cases also present with some form of anxiety or an anxiety disorder. Ironically, phobias often present themselves despite the affiliation for other people. Individuals with Williams syndrome also typically have poor coordination as well as a low IQ. Their average IQ is 55, ranging from 40-90, whereas average IQ is 90 and above. This means that these individuals will show a failure to thrive during infancy, especially in relation to language and motor development.
Spatial cognition
Individuals with Williams syndrome typically have a deficiency in spatial cognition. An adult with Williams will function at a 5 year olds level of spatial cognition. This is often seen as the result of the attention paid to detail at the expense of the whole. There is often a bias present to carefully note the local aspects of the visual stimuli thus overlooking the global picture. An example of this is the replication of the letter Z constructed by a number of letter z’s. A Williams syndrome individual will draw a number of the smaller, local z as opposed to the complete structure. When asked to draw freehand images of a house, William syndrome adolescents were unable to present a logical arrangement of the building. Compared to a normal control of the same age, Williams syndrome individuals are highly impaired in spatial cognitive functions. Spatial cognition follows a specific path throughout development which remains impaired as the individual matures.
These spatial insufficiencies also extend to influence speech negatively. An example of this would be describing the tree relative to the house as behind the house when the tree is in fact next to the house. There is an obstruction of language expressed to the poor spacial skills exhibited in Williams syndrome. Williams Syndrome individuals often have difficulty with spatial representations and this effectively impairs other cognitive functions which require such spacial skills. [72]
Language Representations
Many studies have been performed on the effects of Williams syndrome on individuals language abilities as it is one of the relative strengths in cognitive functioning. One area of interest is the expressive language used by individuals with Williams syndrome. A key feature of Williams syndrome is the incorrect use of sophisticated words in expressive language. The choice of these words is the result of selecting the right semantic field however failing to select for the appropriate context. This is possibly due to the presence of a different wiring mechanism of neurons in semantic processing. Adolescents and adults are often articulate and very talkative which prevents other individuals engaged in conversation with them to recognise any cognitive abnormalities.
During infancy, there is a stunted development of language as the onset of the first word is later than normal infants. All aspects of the behavioural phenotype of Williams syndrome is delayed heavily during the earlier years, this includes language. Although the onset of words is delayed, it is followed by a rapid acquisition words. However, the meaning of words may not be fully understood by the developing child saying them. For example, a 4 year old child could repeat long and often complex words such as encyclopaedia, proficiency and accomplishment without understanding the meaning of the word. The development of strong grammar during childhood years is followed by the rapid development of language. It is this rapid development of grammar that allows Williams syndrome to be distinguished from other forms of mental retardation. The acquisition of proper grammar allows adolescents and adults with Williams syndrome to form complex grammatical forms thus having greater language production compared to others with various forms of mental retardation. [70]
Sociability
Williams Syndroms is a distinct disorder which is characterised by the individuals hypersociability and need for social interactions. Unlike most other disorders, individuals are remarkably social, empathetic and friendly. Williams syndrome individuals perceive others as friends rather than strangers. Across various studies, it has been found that individuals with Williams syndrome rate unfamiliar faces more approachable than most other normal controls. This finding is consistent with their interest in approaching strangers and their social phenotype. An early emergence of this social behaviour is present in infants with Williams syndrome. Whilst conducting tests on infants, an obstacle to retrieving accurate results is the tendency for the child to pay more attention to the people present than the task at hand. One theory is that the social behaviour is a mechanism by which the child can avoid the difficult task at hand. [73]
Individuals with Williams Syndrome use language to effectively communicate with other on a social level. They not only engage in conversation with others, but also maintain the conversation by the extensive use of linguistic devices. There is an over use of lexical evaluative devices and vocal prosody to construct coherent and complex stories. The hypersocial phenotype of Williams syndrome is characterised by the individuals use of expressive devices over various linguistic settings to engage and maintain their audiences attention during conversation. This skill is developed throughout childhood and perfected during adolescents once grammar is established firmly in the individual. [74]
Musicality
There is relatively less known about the musicality of Williams syndrome, however it has been marked as a relatively spared cognitive function. This could be explained by the multisensory processing which occurs when listening to music. Williams syndrome patients exhibit increased sensitivity towards auditory stimuli. There is known hypercusis, phonophobia and auditory fascination. 80% of Williams syndrome patients shows an aversion toward everyday noises, as the individuals find such stimuli as painfully strong. Phonophobia has been diagnosed in 91% of the Williams syndrome individuals. This fear of normal sounds and aversion to everyday noises have been shown to be the result of an abnormalities in the limbic system aswell as the autonomic system. This avoidance and anxiety towards everyday noises and normal sounds are seen in younger children peaking at about 5-8 years and slowly reduces. This initial fear toward sounds however, is thought to be the start of auditory fascination. William Syndrome individuals are captivated by music and their strong emotional response to it. There is uniform and holistic sound perception found in the majority of Williams syndrome individuals. There is a high rate of rhythmic creativity found and a particular liking for rhythmic and percussion instruments. [75]
Anxiety and Phobias
It has been found that when compared to the general population, children with Williams syndrome have a significantly higher rate of anxiety related disorders. They particularly showed a higher occurrence of generalised anxiety disorder and specific phobia disorder. Children with Williams syndrome often present with various anxiety disorders and phobias that carry-on through to adulthood. A study using the diagnostic and statistical manual of psychiatric disorders (DSM-IV) showed that 5% of these children expressed sever psychological disorders. 80.7% of the individuals were shown to have been diagnosed with at least one of the diagnosed disorders in the DSM-IV. Of the many disorders, Attention Deficit/Hyperactivity Disorder and specific phobias were the highest occurrences. AD/HD was the most common disorder affecting almost 65% of the Williams syndrome children. Specific phobias, most commonly a phobia towards loud noises, was present in 54% of the individuals. A few other notable disorders were separation anxiety (7%), obsessive compulcive disorder (3%), social phobias (2%), post traumatic stress disorder (1%) and panic disorders (1%). [76]
Facial processing
Despite deficits in spacial cognition, facial representations are an area of sparing in Williams syndrome. There is a remarkable ability to remember, discriminate between and recognise between both familiar and unfamiliar faces. This strength of facial recognition extends to the perception of faces in various contexts. Changing the lighting, orientation and background does not affect the identification of faces in Williams syndrome. Williams syndrome individuals are just as skilled as normal individuals of the same age in facial perception if not, better. Younger individuals with Williams syndrome are shown to have superior facial recognition abilities compared to age-matched individuals. [77]
Other cognitive functions
William syndrome individuals show moderate to mild forms of mental retardation with an IQ average of 55 for the population. The IQ ranges from 40-90, which means some individuals have the IQ of a normal person. By adulthood, there is a failure to successfully perform piagetian seriation and conservation tasks normally achieved by 8 years of age. The cognitive impairment of Williams syndrome has been shown to be similar to that of Down syndrome. There is little difference shown between the verbal and performance IQ scores of the two groups. Arithmatics is shown to be the greatest challenge for the syndrome and language to be a relative strength. A severe impairment with the use of arithmetics and its implications to daily life has been shown to be expressed in Williams syndrome individuals. This can be shown by their preference for a hundred 5c as opposed to a $5 note. Some individuals however, are known to have adequately learn addition, subtraction and even division. Adults are also shown to have difficulty estimating quantities a normal developing child can do. For example, the length of a bus can be estimated to be as small as 1cm or as large as 100m when a normal child would say about 11m. Reading is a more variable function as some find it difficult to reading whilst others read particular topics of interests. Williams syndrome presents an uneven cognitive profile with specific strengths and various deficits unlike most forms of mental retardation where there is a general deficiency in all aspects of cognitive function. [70]
Management
Currently, there is no cure for Williams-Beuren Syndrome as it is a complex multisystem medical condition. As symptoms of Williams-Beuren Syndrome involve multiple disciplines, treatment of those symptoms requires a large clinical team of doctors and nurses. With this team, a number of periodical assessments and systemic evaluations must be made if WS is diagnosed. This includes:
- Complete physical and neurological examination
- Growth parameters plotted on WS growth charts (see: [1])
- Cardiological evaluation
- Genitourinary system evaluation
- Ophthalmologic evaluation
- Multidisciplinary developmental evaluation (if patient is over 2 years of age)
- Ultrasonography of bladder and kidneys
- Urinalysis
- Calcium determinations
- Thyroid function tests
- FISH to determine ELN deletion [78]
Treatment
Currently, there is no cure for Williams Syndrome as it is a complex multisystem medical condition. The treatment of conditions brought about by Williams-Beuren Syndrome can be difficult depending on the number of recognised conditions.
Cardiac Treatment
Surgery is generally required to repair supravalvular aortic stenosis if the severity of the condition is notable. [79] Other procedures such as angioplasty and stent insertion are also practiced, but are highly susceptible to aneurysm, rupture or restenosis. [48] [80]
Pulmonary artery lesions in patients with WS who aren’t diagnosed with SVAS can be monitored, as many patients with lesions in this area show gradual improvement with the lesions resolving over time.
Genitourinary Treatment
The treatment of hypercalcemia involves constant monitoring of the patients’ blood calcium levels including before the administration of any anesthetic or sedative agent and prior to any invasive procedure. Food intake should also be regulated and monitored. [60] 15% of patients with Williams-Beuren Syndrome show signs of hypercalcemia and it is important to note as the condition contributes to the presence of extreme irritability, vomiting, constipation, and muscle cramps. Treatment options for hypercalcemia include restricted dietary calcium intake and bisphosphonate therapy. In any case, the WS patient should be referred to a nephrologist in order to treat renal abnormalities as they arise. [43]
Treatment for hypertension in WS patients is individualised and should be treated when identified.
Osteopenia can be difficult to treat in patients with Williams-Beuren syndrome as its treatment could have reverse effects on, and increase the severity of hypercalcemia.
Endocrine Treatment
The treatment of endocrine abnormalities affiliated with Williams syndrome such as hypothyroidism requires regular monitoring and the prescribing of thyroid hormone related medicine should be done so if absolutely necessary. As glucose intolerance amongst WS patients is invariably high, adult patients should be screened regularly. As early puberty may bring an abundance of further challenges and complications to the management of WS, especially in females, it is also possible to delay menarche with the use of a gonadotropin-releasing hormone such as leuprolide. [81]
Behavioural Treatment
In terms of the emotional and psychiatric tendencies associated with Williams syndrome, these go some way in determining the quality of life for adult patients. Approximately half of adolescents and adults are being treated or have been treated with an antianxiety agent. Types of drugs prescribed include selective serotonin-reuptake inhibitors such as Zoloft and Prozac.
Counselling benefits those patients with relatively strong verbal skills. Patients may practice relaxation techniques and utilise strategies to deal with potentially anxiety-provoking situations.
Specialised Facilities and Supportive Associations
Australia
In Australia, there are a number of supportive associations and groups helping both individuals and families affected by Williams syndrome. These include:
Williams Syndrome Family Support Group (Victoria)
This group is described as a “community of families with Williams syndrome members, based in Victoria, Australia.” Its aims include:
- To provide help and support for families with a child or adult with Williams syndrome
- To provide resources for parents, teachers, students, employers and the medical profession
- To provide information on current research
- To empower those with WS to reach their full potential
- To increase awareness and understanding of WS among the medical and teaching professions and the general public
This group may be contacted using the following details:
Honorary Secretary: Catherine Stenford
PO Box 389, Balnarring, Victoria 3926
The website of the Williams Syndrome Family Support Group (Victoria) may be accessed here: [2]
Williams Syndrome Family Support Group of South East Queensland
This group may be contacted using the following details:
Rebecca Carter
15 Azalea Place, Currimundi, Queensland 4551, Australia
Tel: (07) 5493 1185
Williams Syndrome Family Support Group of Western Australia (Inc)
Secretary: Mr Rob Hendry
Tel: (08) 9459 3716
Williams Syndrome Association of South Australia
Regional Director: Gerry Mitchell
Contact: Amanda Strybos or Mandy Turner
PO Box 247, Salisbury, Adelaide 5108, Australia
Tel: 8258 3867
The website of the Williams Syndrome Association of South Australia may be accessed here: [3]
Williams Syndrome (IHC) Association of New South Wales, part of the Association of Genetic Support of Australasia (Inc)
Dianne Petrie c/o Association of Genetic Support of Australasia Inc
66 Albion Street, Surry Hills, New South Wales 2021
Australia
Tel: +61 2 9211 1462
Online Australian Williams Syndrome Forum
International Supportive Associations
Outside of Australia, the most notable Williams syndrome supportive association is found in the United States of America with the Williams Syndrome Association (WSA). According to its mission statement the WSA “is a non-profit organisation that strives to enrich the lives of individuals and families affected by Williams syndrome and similar conditions through support, research and education. The WSA also provides the contact details and locations of various Williams syndrome clinics established throughout the U.S. These clinics are specialty clinics where those with Williams syndrome may be examined by a team of WS specialists and referred onwards to other services which may help.
The website of the WSA can be accessed here: [4]
Current research and developments
Induced chromosome deletion in a Williams-Beuren syndrome mouse model causes cardiovascular abnormalities - published in 2011, this study involved the deletion of the elastin gene ELN and what effect it had on cardiovascular abnormalities found in Williams syndrome. Results of this deletion were measured through ELN transcript levels, blood pressure, histological sectioning and M-mode ultrasound to determine circumferential cyclic strain. The results showed that ELN transcript levels were reduced by 38-41% in WS mice who had only one copy of the ELN gene instead of the normal two. These same mice showed an increase in mean blood pressure and also a reduced circumferential cyclic strain. The histological sections showed disorganised and fragmented elastic sheets. The paper concluded that the deletion of ELN in mice with Williams syndrome results in lower gene expression, hypertension, reduced cyclic strain and fragmented elastin sheets. Due to no change in medial lamella units in mice with Williams syndrome, the study suggests other genes may be involved in vascular development.
Linking LIMK1 deficiency to hyperacusis and progressive hearing loss in individuals with Williams syndrome - published in 2011, this article suggests that a reduced expression of the LIM kinase1 gene involved in the regulation of the motile responses of cochlear outer hair cells and cochlear amplification results in and is the cause of hyperacusis and progressing hearing loss as observed in patients with Williams syndrome.
Negative autoregulation of GTF2IRD1 in Williams-Beuren syndrome via a novel DNA binding mechanism - published in 2010, this study shows “the existence of a negative autoregulatory mechanism that controls the level of GTF2IRD1 transcription via direct binding of the GTF2IRD1 protein to a highly conserved region of the GTF2IRD1 promoter containing an array of three binding sites. This protein-DNA interaction is dependent upon multiple interactions between separate domains of the protein and at least two of the DNA binding sites. This mechanism leads to dosage compensation of GTF2IRD1 transcription”, resulting in the craniofacial dysmorphology, hypersociability, and visuospatial deficits displayed in Williams syndrome patients.
Partial 7q11.23 deletions further implicate GTF2I and GTF2IRD1 as the main genes responsible for the Williams-Beuren syndrome neurocognitive profile - published in 2010, this study concludes that after revealing low expression levels of all the typical and atypical genes through lymphoblastoid cell lines, the “functional hemizygosity of both GTF2I and GTF2IRD1 genes is the main cause of the neurocognitive profile” seen in WS patients.
Transcriptome profile in Williams-Beuren syndrome lymphoblast cells reveals gene pathways implicated in glucose intolerance and visuospatial construction deficits - published in 2010, this article states that through comparing the transcriptome profile of lymphoblastoid cell lines from various patients, 47 genes had been deregulated in WS patients. The pathways that were affected the most included glycolysis and neuronal migration. Genes involved in microtubule formation were also deregulated in patients with the common deletion. This abnormal regulation of gene pathways may be related to the cognitive, visuospatial and metabolic disturbances as seen in WS patients.
Research Projects funded by the Williams Syndrome Foundation
Mayada Elsabbagh, Mazal Cohen & Annette Karmiloff-Smith, Hearing and Hypersensitivity to Sound, Institute of Child Health London started Autumn 2004.
Professors Janette Atkinson & Oliver Braddick, Long Term Research into how Visual & Visuo-cognitive abilities develop in the eyes and brain in people with Williams Syndrome Visual Development Unit, Dept of Psychology, UCL London.
More research projects funded by the Williams Syndrome Foundation can be found here:[5]
Current research projects in affiliation with the Williams Syndrome Association
- Dr. Carolyn Mervis, University of Louisville
"Conducting clinical research studies primarily devoted to the cognitive processes in WS. Her team is conducting longitudinal studies of Language and Cognition in WS, as well as studies of language in very young children and the relationships between language, cognition and adaptive behaviour in Williams syndrome."
- Dr. Helen Tager-Flusberg, Boston University
"Conducting clinical research studies of the sociability of individuals with Williams syndrome. Dr. Flusberg's primary studies in this area are with teens and adults with WS."
More research projects funded by the Williams Syndrome Association can be found here: [6]
Mouse Models
In recent years research has been focused on investigating the genetic origins of the physical, behavioural and psychological characteristics of Williams Syndrome, this is mainly being achieved through the use of mouse models. Mice share many genes with humans therefore "Knock-out mouse" models can be developed to examine the results of gene deletion. The genes on chromosome 7 believed to be the cause of a particular manifestation in Williams Syndrome can be inactivated or "knocked-out" so that the implications of this action can be observed in the mouses' phenotype, including its appearance, behaviour and other observable physical and biochemical characteristics. This technique provides valuable clues about what these genes normally do and how their deletion contributes to Williams Syndrome. [82]
The following is an example of a research study conducted to explore the genotype-phenotype relationship in Williams Syndrome using mouse models:
Enkhmandakh, B et.al, 2009, conducted a mouse model based study on the “essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development.” [29] They investigated the little known contributions of the TFII-I transcription factors in embryonic development. Through the use of ‘knock-out’ mouse models, this study showed that the homozygous loss in function of either Gtf2ird1 or Gtf2i genes, which code for TFII-I, results in multiple abnormalities in the phenotype of the ‘knock-out’ mouse, including death of the embryo, brain haemorrhage and defects relating to blood vessels, neural tube and craniofacial regions of the embryo. Additional analysis demonstrated that embryo death could be caused by defects in the yolk sac and a subset of the two heterozygous genes presented retarded growth and skeletal and craniofacial defects. This therefore showed that an insufficient production of the TFII-I proteins, resulting from the ‘knock-out’ of Gtf2ird1 and Gtf2i genes in the 7q11.23 critical region of chromosome 7, causes some of the developmental manifestations associated with Williams Syndrome.
Links: Article | Phenotypes of Gtf2ird1 and Gtf2i mutant mouse embryos | Morphological analysis of the head in mutant embryos and expression of Gtf2i and Gtf2ird1 | Reduced growth, craniofacial and pigmentation defects in heterozygous Gtf2ird1 and Gtf2i animals |
Acknowledgement
The creators of this page would like to sincerely thank Dr. Stephen Palmer of the School of Medical Sciences at the University of New South Wales for his assistance and guidance over the duration of this project. His willingness to help and teaching were greatly appreciated by all members of the group.
Glossary
2-dimensional echocardiography: Cross-sectional echocardiography, ultrasound-based diagnostic method.
Aneurysm: Blood-filled dilation of a blood vessel, caused by a weakening of the vessel wall.
Angioplasty: A medical procedure where a small balloon is inserted into the narrowed or obstructed blood vessel, inflated, and then removed once the blood flow through the vessel is normal.
Antianxiety agent: Drugs used for the treatment of anxiety, and its related psychological and physical symptoms (also referred to as Anxiolytics).
Atrial Septal Defect: A congenital heart defect in which the wall that separates the upper heart chambers (atria) does not close completely.
Autosomal inheritance: Some hereditary diseases are described as autosomal which means that the disease is due to a DNA error in one of the 22 pairs that are not sex chromosomes. Both boys and girls can then inherit this error. If the error is in a sex chromosome, the inheritance is said to be sex-linked.
Chordae Tendineae: Cord-like tendons that connect the papillary muscles to the tricuspid valve and the mitral valve in the heart .
Cingulate Cortex:The cingulate cortex is a part of the brain situated in the medial aspect of the cortex, which includes the cortex of the cingulate gyrus.
Colour Doppler: Ultrasound technique allowing simultaneous grey scale imaging and a dynamic colour flow vascular image.
Congenital anomalies/malformations: Developmental abnormalities detected before or present at birth. There are no internationally agreed definitions for congenital anomalies and there are a range of classifications including the International Classification of Diseases (ICD), developed by the World Health Organization (WHO) to enable comparability for mortality statistics.
Diastole: The period of time when the heart fills with blood during relaxation after systole.
DSM-IV: The Diagnostic and Statistical Manual of Mental Disorders provides a criteria for the diagnosis and classifaction of mental disorders. It is published by the American Psychiatric Association.
Elastin ateriopathy: Broken and disorganized elastin fibers caused by the mutation of the Elatin gene on chromosome 7.
ERP: Event-related potential is a measured brain response that is directly the result of a thought or perception.
FISH: Fluorescence in situ hybridisation; a technique used to detect the presence or absence of targeted DNA sequences on chromosomes.
Haploinsufficiency:The incomplete or partial dominance, as a heterozygote (with one mutant and one normal allele) displays a phenotypic effect.
Hemizygous: Only having a single copy of a gene as opposed to the normal two copies.
Heschl's gyrus: The transverse temporal gyrus which is found in the area of the primary auditory cortex.
Hypothyroidism: A condition in which the thyroid gland does not make enough thyroid hormone.
Idiopathic infantile hypercalcemia: Abnormally high levels of calcium in the blood at childhood arising from an unknown cause.
Intracardiac pressure: Pressure within the heart.
Joint contractures: Abnormal stiffness of the joints causing the muscle to be shorter and unable to extend.
"Knock-out" mouse:a transgenic mouse that has been genetically engineered to inactivate a particular existing gene by disrupting it or replacing it with an artificial piece of DNA.
Kyphosis:An abnormal curvature of the spine, specifically at the upper thoracic segments creating a "hunch".
Lamina Propria: A thin vascular layer of connective tissue which lies beneath the epithelium of the organs of the body.
Leuprolide: An injectable, man-made hormone that is used for treating prostate cancer, endometriosis, central precocious puberty, and fibroids. It is similar to but stronger than human gonadotropin releasing hormone (GnRH).
Lordosis: An abnormal curvature of the spine, usually at the lower half of the back.
LCR: Low Copy Reapeats, a single copy gene region with repetitive sequences.
LVOT: Left ventricular outflow tract, portion of the left ventricle of the heart through which blood passes to get pumped out by the aorta.
Malocclusion: The abnormal positioning of the teeth when the jaw is closed.
Mb: Mega base pairs.
Menarche: The first menstrual cycle in female human beings.
Metasarsophalangeal joint: The joints between the metatarsal bones of the foot and the more proximal bones.
Microdeletion: The absence of a gene or part of a gene usually caused by inheritance.
Mitral regurgitation of blood: A disorder of the heart in which the mitral valve does not close properly when the heart pumps out blood. It is the abnormal leaking of blood from the left ventricle, through the mitral valve, and into the left atrium, when the left ventricle contracts.
Mitral Valve Disease: A degeneration of the heart's mitral valve resulting in backflow.
Mitral Valve Leaflets: Leaflets of the mitral valve composed of tissue whose sole purpose is to open and close tightly in order to ensure the correct flow of blood through the heart.
MRI: Magnetic resistance imaging, a diagnostic technique that uses a magnetic field and radio waves to provide computerized images of internal body tissues.
Myocardial hypertrophy: Increase in size of myocardial muscle due to increase in size of individual myocardial cells, usually in response to an added afterload
Myocardial infarction: An interruption in the blood supply to the heart because of narrowed or blocked blood vessels. Also referred to as a heart attack.
Myxomatous degeneration: A pathological weakening of connective tissue.
Neocerebellar: A part of the lower, posterior half of the brain which plays an important role in motor control.
Nephrologist: Someone who studies the function and diseases of the kidney.
NAHR: Nonallelic homologous recombination is a form of homologous recombination occuring between two lengths of DNA which are not alleles but have high sequence homology.
Ophthalmology: The branch of medicine that deals with the anatomy, functions, pathology, and treatment of the eye.
Osteopenia: A condition where bone mineral density is lower than normal.
Paleocerebellum:The anterior lobe of the cerebellum.
Phonophobia: A morbid fear of sounds including your own voice and particularly loud noises.
PPS: Peripheral Pulmonary Stenosis, an obstruction anywhere in the pulmonary artery caused by vessel wall thickening as a result of elastin abnormality.
Prozac: A selective serotonin reuptake inhibitor used to treat major depressive disorder, bulimia nervosa (an eating disorder) obsessive-compulsive disorder, panic disorder, and premenstrual dysphoric disorder (PMDD).
Semantic processing: Coding information into or decoding information from communication patterns.
Stenosis: An abnormal narrowing in a blood vessel or other tubular organ or structure.
Stent Insertion: The insertion of an artificial cylinder into an artery in order to prevent flow constriction.
STRs: short tandem repeats.
SVAS: Supravalvular Aortic Stenosis; an obstruction occurring in the left ventricular outflow tract (LVOT)caused by vessel wall thickening as a result of elastin abnormality.
Systolic murmurs: Murmur or sound occurring during the contraction of the heart and pumping of blood out of the ventricle.
Ultrasonography: An ultrasound-based diagnostic imaging technique used for visualizing subcutaneous body structures including tendons, muscles, joints, vessels and internal organs for possible pathology or lesions.
Urinalysis: An array of tests performed on urine.
Ventricular Septal Defect: One or more holes in the wall that separates the right and left ventricles of the heart.
Visualspatial construction: the ability of a person to perceive, analyze, and understand visual information taken in from the world around them.
WS: Williams Syndrome.
Zoloft: An antidepressant drug used to treat depression, obsessive-compulsive disorder, panic disorder, anxiety disorders, post-traumatic stress disorder (PTSD), and premenstrual dysphoric disorder (PMDD).
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 <pubmed>20425781 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID20425781' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20425781' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20425781' defined multiple times with different content - ↑ OMIM Entry #194050-Williams-Beuren Syndrome
- ↑ 3.0 3.1 <pubmed>19568270 </pubmed>
- ↑ <pubmed>21107555 </pubmed>
- ↑ Genetics Home Reference 2011, Williams Syndrome, U.S. National Library of Medicine, viewed 10 October 2011, <http://ghr.nlm.nih.gov/condition/williams-syndrome>
- ↑ <pubmed>16272111 </pubmed>
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 <pubmed>21120465 </pubmed>
- ↑ 8.0 8.1 <pubmed>12980492 </pubmed>
- ↑ 9.0 9.1 <pubmed>13469755 </pubmed>
- ↑ 10.0 10.1 <pubmed>14007182 </pubmed>
- ↑ <pubmed>13739645 </pubmed>
- ↑ 12.0 12.1 <pubmed>18941598 </pubmed>
- ↑ 13.0 13.1 13.2 <pubmed>13967885 </pubmed>
- ↑ 14.0 14.1 <pubmed>14136289 </pubmed>
- ↑ Hagen, J.M. van,2007Williams Syndrome: from genes to clinical features,Erasmus University Rotterdam,Rotterdam
- ↑ <pubmed>14055045 </pubmed>
- ↑ <pubmed>14148234 </pubmed>
- ↑ <pubmed>1133652 </pubmed>
- ↑ Hagen, J.M. van,2007Williams Syndrome: from genes to clinical features,Erasmus University Rotterdam,Rotterdam
- ↑ <pubmed>2456379 </pubmed>
- ↑ 21.0 21.1 21.2 21.3 <pubmed>8475063 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID8475063' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID8475063' defined multiple times with different content - ↑ <pubmed>8096434 </pubmed>
- ↑ <pubmed>16760918 </pubmed>
- ↑ Morris CA. Williams Syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews (Internet). Seattle (WA): University of Washington, Seattle; 1993-. 1999 Apr 09 (updated 2006 Apr 21). PMID: 20301427
- ↑ 25.0 25.1 25.2 25.3 25.4 <pubmed>20425789</pubmed> Cite error: Invalid
<ref>tag; name 'PMID20425789' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20425789' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20425789' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20425789' defined multiple times with different content - ↑ <pubmed>9819363 </pubmed>
- ↑ <pubmed>9685409 </pubmed>
- ↑ <pubmed>21655442 </pubmed>
- ↑ 29.0 29.1 29.2 <pubmed>19109438 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID19109438' defined multiple times with different content - ↑ <pubmed>16293761 </pubmed>
- ↑ <pubmed>10642537 </pubmed>
- ↑ <pubmed>7693128 </pubmed>
- ↑ <pubmed>17180802 </pubmed>
- ↑ <pubmed>18489677 </pubmed>
- ↑ <pubmed>14967851 </pubmed>
- ↑ <pubmed>15774842 </pubmed>
- ↑ 37.0 37.1 <pubmed>11743512 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID11743512' defined multiple times with different content - ↑ 38.0 38.1 38.2 38.3 <pubmed>18452001 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID18452001' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID18452001' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID18452001' defined multiple times with different content - ↑ Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle
- ↑ Krishnan, A 2011, Pediatric Supravalvar Aortic Stenosis: Background, Webscape Reference, viewed 11 October 2011, <http://www.lds.no/stream_file.asp?iEntityId=13482>
- ↑ 41.0 41.1 41.2 41.3 41.4 <pubmed>11701637 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID11701637' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID11701637' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID11701637' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID11701637' defined multiple times with different content - ↑ <pubmed>7726172</pubmed>
- ↑ 43.0 43.1 43.2 43.3 43.4 Morris, C 2006, ‘Williams Syndrome’, in RA Pagon et.al (ed.), Gene Reviews, University of Washington, Seattle
- ↑ 44.0 44.1 44.2 <pubmed>20089974 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID20089974' defined multiple times with different content Cite error: Invalid<ref>tag; name 'PMID20089974' defined multiple times with different content - ↑ <pubmed>20808633 </pubmed>
- ↑ 46.0 46.1 <pubmed>20926892</pubmed>
- ↑ Krishnan, A 2011, Pediatric Supravalvar Aortic Stenosis: Background, Webscape Reference, viewed 11 October 2011, <http://emedicine.medscape.com/article/892252-overview>
- ↑ 48.0 48.1 <pubmed>11331257</pubmed> Cite error: Invalid
<ref>tag; name 'PMID11331257' defined multiple times with different content - ↑ Xiushui, M 2011, Pulmonic Stenosis: Pathophysiology, Medscape Reference, viewed 11 October 2011, <http://emedicine.medscape.com/article/157737-overview#a0104>
- ↑ <pubmed>21545515</pubmed>
- ↑ <pubmed>8488870 </pubmed>
- ↑ <pubmed>8066039 </pubmed>
- ↑ <pubmed>8610763 </pubmed>
- ↑ <pubmed>9763817 </pubmed>
- ↑ <pubmed>11110565 </pubmed>
- ↑ <pubmed>2117358 </pubmed>
- ↑ <pubmed>3975102 </pubmed>
- ↑ <pubmed>6333147 </pubmed>
- ↑ <pubmed>8723124 </pubmed>
- ↑ 60.0 60.1 <pubmed>15466114 </pubmed> Cite error: Invalid
<ref>tag; name 'PMID15466114' defined multiple times with different content - ↑ <pubmed>20425788 </pubmed>
- ↑ <pubmed>16123365 </pubmed>
- ↑ <pubmed>18267160 </pubmed>
- ↑ <pubmed>12949276 </pubmed>
- ↑ <pubmed>20425785 </pubmed>
- ↑ <pubmed>12784297 </pubmed>
- ↑ OMIM Entry #194050-Williams-Beuren Syndrome
- ↑ <pubmed>1425797 </pubmed>
- ↑ 69.0 69.1 <pubmed>10319200 </pubmed>
- ↑ 70.0 70.1 70.2 Bellugi, U. et al, 2001, Journey From Cognition to Brain to Gene: Perspectives From Williams Syndrome, Massachusetts Institute of Technology,the United States of America
- ↑ <pubmed>20808792 </pubmed>
- ↑ <pubmed>20425784 </pubmed>
- ↑ <pubmed>18211726 </pubmed>
- ↑ <pubmed>18924169 </pubmed>
- ↑ <pubmed>20733255 </pubmed>
- ↑ <pubmed>20161441 </pubmed>
- ↑ <pubmed>19047076 </pubmed>
- ↑ AMERICAN ACADEMY OF PEDIATRICS Committee on Genetics (2001) Health Care Supervision for Children With Williams Syndrome Pediatrics Vol. 107 No. 5 May 1, 2001 pp. 1192 -1204
- ↑ <pubmed>11167112 </pubmed>
- ↑ <pubmed>15691415 </pubmed>
- ↑ <pubmed>12219071 </pubmed>
- ↑ National Human Genome Research Institute 2010, Knockout Mice, The National Human Genome Research Institute, viewed 11 October 2011, <http://www.genome.gov/12514551>