2011 Group Project 7: Difference between revisions
No edit summary |
|||
| (208 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{2011ProjectsMH}} | |||
='''Angelman Syndrome'''= | ='''Angelman Syndrome'''= | ||
| Line 20: | Line 6: | ||
==Introduction== | ==Introduction== | ||
Angelman syndrome (AS) is a rare neurogenetic disorder | [[File:Dr Harry Angelman.jpg|thumb|130px|right|Dr Harry Angelman.]] | ||
Angelman syndrome (AS) is a rare [[#Glossary | '''neurogenetic''']] [[#Glossary | '''disorder''']] first described by Dr. Harry Angelman in 1965.<ref name="PMID18754889"><pubmed>18754889</pubmed></ref> Dr. Angelman was an English [[#Glossary | '''paediatrician''']] who first diagnosed the disease in 3 children with a developmental delay.<ref>http://www.ncbi.nlm.nih.gov/books/NBK22221/</ref> AS occurs in 1 in 10000-20000 births, the exact number is still unknown.<ref name="PMID7573182"><pubmed>7573182</pubmed></ref><ref name="PMID8703225"><pubmed>8703225</pubmed></ref> It is caused by maternal [[#Glossary | '''allele''']] disruptions of a single gene- UBE3A. Either [[#Glossary | '''mutations''']] or [[#Glossary | '''deletions''']] of the UBE3A gene results in AS. | |||
AS presents with well known phenotypes during infancy and adulthood, such as microcephaly and maxillary hypoplasia. However, these features | AS presents with well known [[#Glossary | '''phenotypes''']] during infancy and adulthood, such as [[#Glossary | '''microcephaly''']] and [[#Glossary | '''maxillary hypoplasia''']]. However, these features become more marked with age. Most frequent clinical features include delayed development,[[#Glossary | '''seizures''']],[[#Glossary | '''ataxia''']], impairment of speech, happy demeanor, behavioural problems such as hyperactivity, short attention span and sleeping difficulty.<ref><pubmed>21592595</pubmed></ref><ref><pubmed>21484597</pubmed></ref><ref name="ASAwebsite">http://www.angelmansyndrome.org/home.html</ref> | ||
At present, there is no cure for the syndrome, though current research is focused at improving life quality of patients with Angelman syndrome through management of daily tasks.<ref>http://www.bbc.co.uk/health/physical_health/conditions/angelman1.shtml</ref> | |||
==History== | ==History== | ||
Dr. Harry Angelman first reported Angelman Syndrome on three handicapped children that were admitted to his ward in England in 1964. He reported how these three independent and unconnected clinical cases all had the same characteristic features of severe developmental delay, jerky movements, seizures and a happy disposition. However it was only when he stumbled upon an oil painting called ‘a Boy with a Puppet” while on a holiday in Italy he was able to connect the symptoms of all three children under one common cause. He linked the happy face of the boy depicted on the painting with the jerky movements and happy disposition he observed on his three patients back in England. He reported his findings on a paper entitled “Puppet children (a report on three cases)” which was published in 1965. | Dr. Harry Angelman first reported Angelman Syndrome on three handicapped children that were admitted to his ward in England in 1964.<ref name="PMID18754889" /> He reported how these three independent and unconnected clinical cases all had the same characteristic features of severe developmental delay, jerky movements, seizures and a happy disposition.<ref name="PMID19455185"/> However it was only when he stumbled upon an oil painting called ‘a Boy with a Puppet” while on a holiday in Italy he was able to connect the symptoms of all three children under one common cause.<ref name="PMID20981772"><pubmed>20981772</pubmed></ref> He linked the happy face of the boy depicted on the painting with the jerky movements and happy disposition he observed on his three patients back in England. He reported his findings on a paper entitled “Puppet children (a report on three cases)” which was published in 1965.<ref name="PMID18754889" /> | ||
The syndrome was | The syndrome was initially named ‘Puppet Syndrome’ but was later changed to Angelman Syndrome. Due to the extreme rarity of the syndrome and lack of sufficient clinical evidence the syndrome was soon forgotten.<ref name="PMID18754889" /> | ||
[[File:Dr Charles Williams.jpg|thumb|right|150px|Dr Charles Williams.]] | |||
In 1987, a physician named Ellen Magenis, came across children with deletions on chromosome 15 that were suffering from seizures and severe developmental delay. Even though genetically the children were expected to have Prader-Willi syndrome that was characterised by deletions on chromosome 15, their characteristic symptoms of seizures and lack of development did not correspond with that of the syndrome. This led to the realization that these children had deletions on the maternally derived chromosome 15 and not on the paternally derived chromosome as was observed in Prader-Willi syndrome. | In 1987, a physician named Ellen Magenis, came across children with deletions on [[#Glossary | '''chromosome''']] 15 that were suffering from seizures and severe developmental delay.<ref>http://www.angelman.org/stay-informed/facts-about-angelman-syndrome---7th-edition/harry-angelman-and-the-history-of-as/</ref> Even though genetically the children were expected to have [[#Diagnosis | '''Prader-Willi syndrome''']] that was characterised by deletions on chromosome 15, their characteristic symptoms of seizures and lack of development did not correspond with that of the syndrome. This led to the realization that these children had deletions on the maternally derived chromosome 15 and not on the paternally derived chromosome as was observed in Prader-Willi syndrome. Thus the deleted genetic code on maternally derived chromosome 15 was identified as the genetic marker for Angelman Syndrome. | ||
In 1997, UBE3A gene was isolated by Dr. Joseph Wagstaff and Dr. Arthur Beaudet as the cause of Angelman Syndrome.<ref name="PMID20981772" /> The discovery of this [[#Glossary | '''gene''']] led to the subsequent development of animal models and research aimed at finding a causal link between UBE3A gene abnormalities and characteristic features observed in Angelman Syndrome. | |||
Currently, four different genetic abnormalities for Angelman Syndrome have been confirmed by genetic testing. They include | Currently, four different genetic abnormalities for Angelman Syndrome have been confirmed by genetic testing. They include: Deletion, [[#Glossary | '''Uniparental Disomy''']] (UPD), [[#Glossary | '''Imprinting''']] and UBE3A mutation.<ref name="PMID:20211139"><pubmed>20211139</pubmed></ref> | ||
Below is a timeline, summarising the key discoveries and advancements of the Angelman Syndrome: | Below is a timeline, summarising the key discoveries and advancements of the Angelman Syndrome: | ||
| Line 48: | Line 34: | ||
|- bgcolor="lightpink" | |- bgcolor="lightpink" | ||
!Year | !Year | ||
! | !Milestone | ||
|-style="width:100%" | |-style="width:100%" | ||
|1964 | |'''1964''' | ||
|First observed by Dr Harry Angelman on three handicapped children | |First observed by Dr Harry Angelman on three handicapped children.<ref name="PMID20981772" /> | ||
|-style="width:100%" | |-style="width:100%" | ||
|1965 | |-bgcolor="mistyrose" | ||
|Harry Angelman publishes his finding in a report entitled 'Puppet Children' (a report on three cases) | |'''1965''' | ||
|Harry Angelman publishes his finding in a report entitled 'Puppet Children'(a report on three cases).<ref name="PMID18754889" /> | |||
|-style="width:100%" | |-style="width:100%" | ||
|1980s | |'''1980s''' | ||
|First reports of AS reaches US and research into the disorder being at the University of Florida under the direction of Dr. Charles Williams | |First reports of AS reaches US and research into the disorder being at the University of Florida under the direction of Dr. Charles Williams.<ref>http://www.angelmanproject.com/history.htm</ref> | ||
|-style="width:100%" | |-style="width:100%" | ||
|1982 | |-bgcolor="mistyrose" | ||
|Name changed from ' | |'''1982''' | ||
|Name changed from 'Happy Puppet' to Angelman syndrome by Williams and Frias. | |||
|-style="width:100%" | |-style="width:100%" | ||
|1987 | |'''1987''' | ||
|Discovery of a genetic marker for AS – an absent genetic code on maternally derived chromosome 15 | |Discovery of a genetic marker for AS – an absent genetic code on maternally derived chromosome 15. | ||
|-style="width:100%" | |-style="width:100%" | ||
|1997 | |-bgcolor="mistyrose" | ||
|The cause of AS discovered by Dr. Joseph Wagstaff and Dr. Arthur Beaudet – mutation or deletion in the UBE3A gene | |'''1997''' | ||
|The cause of AS discovered by Dr. Joseph Wagstaff and Dr. Arthur Beaudet – mutation or deletion in the UBE3A gene. | |||
|-style="width:100%" | |-style="width:100%" | ||
|1998 | |'''1998''' | ||
|Transgenic mice with absent maternal UBE3A created to illustrate motor and learning deficits in addition to seizures <ref><pubmed>9808466</pubmed></ref> | |Transgenic mice with absent maternal UBE3A created to illustrate motor and learning deficits in addition to seizures.<ref name="PMID9808466"><pubmed>9808466</pubmed></ref> | ||
|-style="width:100%" | |-style="width:100%" | ||
|2007 | |-bgcolor="mistyrose" | ||
|AS mouse model shows that neurological deficits can be reversed by decreasing levels of | |'''2007''' | ||
|AS mouse model shows that neurological deficits can be reversed by decreasing levels of [[#Glossary | '''alpha-CaMKII''']] inhibitory phosphorylation.<ref><pubmed>17259980</pubmed></ref> | |||
|-style="width:100%" | |-style="width:100%" | ||
| | |'''2010''' | ||
| | |Discovery of a another gene mutation in TC4 gene (on chromosome 18) in a small number of clinically diagnosed patients with no identifiable genetic alternation in UBE3A.<ref><pubmed>20184619</pubmed></ref> | ||
|-style="width:100%" | |-style="width:100%" | ||
|-bgcolor="mistyrose" | |||
|'''2010''' | |||
|Protein named Arc identified as a target of AS gene product; UBE3A.<ref name="PMID:20211139"><pubmed>20211139</pubmed></ref> | |||
|-style="width:100%" | |||
|-bgcolor="mistyrose" | |||
|} | |} | ||
==Epidemiology== | ==Epidemiology== | ||
'''Incidence''' | '''Incidence''' | ||
*The current population of people diagnosed with AS is unknown but studies have estimated the number to be around 1:10,000<ref name="PMID7573182" /> and 1:20,000<ref name="PMID8703225" />. | |||
'''Diagnosis''' | |||
*The mean age of diagnosis for patients with AS lies between 6 and 6.5 years of age.<ref><pubmed>18664077</pubmed></ref> <ref><pubmed>15741136</pubmed></ref> | |||
'''Gender''' | '''Gender''' | ||
* Males appear to be at higher risk for some aspects of early developmental delays in comparison to females with AS.<ref><pubmed>17019625</pubmed></ref> | |||
'''Demography''' | '''Demography''' | ||
: | *Europe: | ||
: | :Denmark- 1:10,000<ref name="PMID7573182" /> | ||
: | :Estonia- 1:52,000<ref><pubmed>16906556</pubmed></ref> | ||
:Sweden- 1:20,000<ref name="PMID8703225" /> | |||
*Australia: | |||
:Western Australia- 1:40,000<ref><pubmed>16492624</pubmed></ref> | |||
==Aetiology== | ==Aetiology== | ||
| Line 97: | Line 98: | ||
[[File:Chromosome 15 Deletion of 15q11.2 to 15q13.jpg|thumb|100px|Chromosome 15 - The deletion occurs at 15q11.2-15q13.]] | [[File:Chromosome 15 Deletion of 15q11.2 to 15q13.jpg|thumb|100px|Chromosome 15 - The deletion occurs at 15q11.2-15q13.]] | ||
'''Molecular Genetics''' | |||
* Location: 15q11-q13 | |||
* Protein product: Ubiquitin protein ligase E3A | |||
* Gene name: UBE3A | |||
* Phenotype: Angelman Syndrome | |||
'''Genetic Causes''' | |||
Different genetic mechanisms lead to different clinical phenotypes of AS. The most common genetic mechanism leading to AS is the deletion or re-arrangement of maternal chromosome at [[#Glossary | '''locus''']] 15q11.2-q13,as the chromosome image shows on the right, accounting for 70% of AS occurrences.<ref name="PMID8988171"><pubmed> 8988171</pubmed></ref> This leads to more severe clinical phenotypes of microcephaly, motor difficulties, seizures and impaired speech development. The next most common genetic mechanism is mutation in the UBE3A gene responsible for 10% of AS cases and paternal uniparental disomy and mutation in the imprinting centre (IC), both accounting for 2-5% of AS observed.<ref><pubmed>19455185</pubmed></ref> | |||
AS is caused by the 4 major genetic mechanisms mentioned above and are thus divided into Classes I to IV based on their underlying genetic mechanism. AS patients with the clinical features of AS but no [[#Glossary | '''cytogenetic''']] or molecular abnormality in Chromosome 15q11.2-13 are grouped under Class V (summarised in table below).<ref name="PMID8988171" /><ref><pubmed>10364509</pubmed></ref> | |||
{| style="width:100%; height:25px" | {| style="width:100%; height:25px" | ||
|-bgcolor="lightpink" | |-bgcolor="lightpink" | ||
! Class | |||
! Mechanism | |||
! Diagnostic Tests | |||
! Frequency | |||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| Ia | | '''Ia''' | ||
| De novo deletion | | De novo deletion | ||
| High resolution cytogenetics FISH | | High resolution cytogenetics [[#Glossary | '''FISH''']] | ||
| 70% | | 70% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| Ib | |-bgcolor="mistyrose" | ||
| '''Ib''' | |||
| Deletion due to chromosome rearrangement | | Deletion due to chromosome rearrangement | ||
| High resolution cytogenetics FISH | | High resolution cytogenetics FISH | ||
| <1% | | <1% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| II | | '''II''' | ||
| Paternal uniparental disomy | | Paternal uniparental disomy | ||
| RFLP analysis | | [[#Glossary | '''RFLP''']] analysis | ||
| 2% | | 2% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IIIa | |-bgcolor="mistyrose" | ||
| '''IIIa''' | |||
| Imprinting defect with IC mutation | | Imprinting defect with IC mutation | ||
| Screening of IC for mutations is positive | | Screening of [[#Glossary | '''IC''']] for mutations is positive | ||
| 2% | | 2% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IIIb | | '''IIIb''' | ||
| Imprinting defect withouth IC mutation | | Imprinting defect withouth IC mutation | ||
| Screening of IC for mutations is negative | | Screening of IC for mutations is negative | ||
| 2% | | 2% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IIIc | |-bgcolor="mistyrose" | ||
| '''IIIc''' | |||
| Mosaic imprinting defect | | Mosaic imprinting defect | ||
| Screening of IC for mutations is usually negative | | Screening of IC for mutations is usually negative | ||
| ? | | ? | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IV | | '''IV''' | ||
| ''UBE3A'' mutation | | ''UBE3A'' mutation | ||
| Screening of ''UBE3A'' for mutations | | Screening of ''UBE3A'' for mutations | ||
| 5-10% | | 5-10% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| V | |-bgcolor="mistyrose" | ||
| '''V''' | |||
| No identifiable genetic abnormality | | No identifiable genetic abnormality | ||
| Consider other diagnoses | | Consider other diagnoses | ||
| 5-26% | | 5-26% | ||
|} | |} | ||
[[File:Imprint defect inheritance in Angelman Syndrome.png|thumb|250px|Imprint defect inheritance in Angelman Syndrome]] | |||
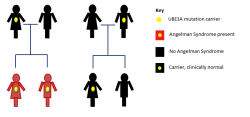
'''Inheritance''' | |||
Angelman Syndrome from UBE3A mutations and deletions in the Imprinting Centre follow an inheritance pattern. A carrier father with the genetic defect can pass it on to his children, however, his children will be clinically normal. On the other hand, a carrier mother can pass the genetic defect onto her children, who will then have Angelman Syndrome. | |||
The image highlights the inheritance of Angelman Syndrome, which only results after a carrier mother passes it on to her children. | |||
==Pathogenesis== | ==Pathogenesis== | ||
Chromosome 15 became associated with AS in the 1980s after the observation of many AS patients harboring [[#Glossary | '''microdeletions''']] of 15q11.2-15q13.<ref><pubmed>15668046</pubmed></ref> The last decade has shed even more light in the aetiology of AS,as Kishino and Matsuura first identified UBE3A as the causative gene for AS in 1997.<ref name="PMID:8988171"><pubmed>8988171</pubmed></ref><ref><pubmed>8988172</pubmed></ref> | |||
'''Normal gene product: E6-AP ubiquitin ligase''' | |||
The function of UBE3A is to encode for [[#Glossary | '''E6-AP''']] ubiquitin ligase, however its function in the development of the nervous system and the process of UBE3A mutation leading to cognitive impairment in AS patients is still vague.<ref name="PMID:20211139"><pubmed>20211139</pubmed></ref> Past and current research has elucidated that E6-AP ubiquitin ligase is involved in the degradation of four proteins, listed below. Currently no protein with a direct role in [[#Glossary | '''long-term potentiation (LTP)''']] as a target of E6-AP has been identified.<ref><pubmed>12684449</pubmed></ref> The identification of such target proteins would be a landmark discovery in further understanding of the pathogenesis, aetiology and prospective treatment strategies of AS, as LTP defects is one of the major neurological impairments of AS. | |||
* p53 tumor suppressor protein | |||
* yeast DNA repair protein Rad23 | |||
* multicopy maintenance protein 7 subunit | |||
* E6-AP | |||
'''Abnormal gene product''' | |||
Disruption to the gene UBE3A interferes with the normal functioning of the gene, which is its crucial role in UBE3A [[#Glossary | '''ubiquitylation''']]. Problems involving this pathway can lead to various diseases either from the loss or gain of function.<ref name="PMID:9857172"><pubmed>9857172</pubmed></ref> In the case of AS, E6-AP is affected, leading to detrimental cognitive impairment in addition to other deficits.<ref name="PMID:9857172"><pubmed>9857172</pubmed></ref> | |||
'''Regional functioning of UBE3A''' | |||
Despite the uncertain progression of UBE3A mutation and the neurological abnormalities characteristic of AS, animal models have illustrated the vital role of UBE3A in the normal functioning of the brain. This can be exemplified by a study which utilized a mouse model with target inactivation of the gene UBE3A resulting in the manifestation of classical features of AS.<ref><pubmed>11895368</pubmed></ref> In addition, the fact that imprinting of UBE3A takes place only in specific brain regions ([[#Glossary | '''cerebellum''']] , [[#Glossary | '''olfactory tracts''']] and the [[#Glossary | '''hippocampus''']]) confirms the hypothesis that the loss of this gene would be detrimental in cognitive development.<ref><pubmed>20398390</pubmed></ref> Most genes are inherited in copies of two, one paternal and the other maternal; however, some genes, such as UBE3A only have one functional copy as the other is silenced, coined 'imprinting'. Imprinting of the paternal copy of UBE3A takes place in some cell populations, such as the hippocampal [[#Glossary | '''neurons''']] and [[#Glossary | '''Purkinje cells''']] in the cerebellum.<ref><pubmed>9288101</pubmed></ref> This means only the maternal UBE3A is functionally expressed in these particular brain regions. Thus a [[#Glossary | '''de novo mutation''']] in the maternal copy of the gene will result in the complete absence of functional E6-AP in the associated brain areas.<ref><pubmed>9288088</pubmed></ref> | |||
It is also known that UBE3A plays an important role in synaptic transmission, but exactly how it does so is still not completely understood.<ref name="PMID:9808466"><pubmed>9808466</pubmed></ref> | |||
'''UBE3A ubiquitylation''' | |||
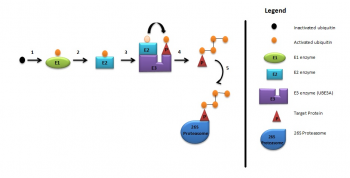
As briefly mentioned above, UBE3A is a member of the E3 ubiquitin ligase family of enzymes. It is responsible for the addition of ubiquitin to the target protein for degradation of the ubiquitinated protein, as illustrated in the diagram below. These processes are required for normal human cognitive function.<ref name="PMID:9808466"><pubmed>9808466</pubmed></ref> In this way synaptic protein [[#Glossary | '''Arc (activity-regulated cytoskeleton-associated protein)''']] is degraded to control synaptic function. Arc is a target protein of UBE3A in dendritic spines found in the hippocampal neurons.<ref name="PMID:20668179"><pubmed>20668179</pubmed></ref> Deletion of UBE3A leads to the accumulation of Arc in neurons leading to trafficking of [[#Glossary | '''AMPA ( α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)''']] receptors, resulting in impaired cognitive functions.<ref name="PMID:20211139"><pubmed>20211139</pubmed></ref> [[File:UBE3A Ubiquitylation Pathway.png|thumb|350px|right|UBE3A Ubiquitylation Pathway.]] | |||
UBE3A ubiquitylation consists of: | UBE3A ubiquitylation consists of:<ref name="PMID:20668179"><pubmed>20668179</pubmed></ref> | ||
# Activation of ubiquitin by E1 enzyme | # Activation of ubiquitin by E1 enzyme | ||
# E1 ezyme transfers ubiquitin to E2 enzyme | # E1 ezyme transfers ubiquitin to E2 enzyme | ||
# E2 enzyme transfers ubiquitin to UBE3A | # E2 enzyme transfers ubiquitin to UBE3A | ||
# UBE3A attaches activated ubiquitin to target protein which is then polyubiquitylated | # UBE3A attaches activated ubiquitin to target protein which is then polyubiquitylated | ||
# Target protein is then degraded by 26S proteosome. <ref><pubmed> | # Target protein is then degraded by 26S proteosome | ||
'''Phenotype-Genotype correlations''' | |||
[[File:Extent of microcephaly in Angelman Syndrome patients.png|thumb|250px|right|Extent of microcephaly in 20 AS patients with deletion and without deletion.<ref>http://www.nature.com/ejhg/index.html/</ref>]] | |||
All AS patients show differing extents of cognitive impairment, movement disorder, characteristic behaviours and difficulty in speech and language.<ref name="PMID:11748306"><pubmed>11748306</pubmed></ref> However, there seems to be some phenotype-genotype correlations: | |||
- 5-7Mb deletions result in the most severe phenotypes such as microcephaly, more sever [[#Glossary | '''epilepsy''']] and seizures, motor difficulties and language impairment.<ref><pubmed>9546330</pubmed></ref><ref name="PMID20445456"><pubmed>20445456</pubmed></ref> AS patients with large deletions also present with clinical [[#Glossary | '''hypopigmentation''']], light hair and eye colour due to the close association of OCA2 gene with UBE3A.<ref name="PMID16470747"><pubmed>16470747</pubmed></ref> Individuals under this category have in general no increased BMI<ref name="PMID:15253756"><pubmed>15253756</pubmed></ref> This is indicated by the graph on the left, which shows more cases of AS patients with deletions having larger deviations from the mean head circumference measurement than non-deletion AS patients. | |||
- AS patients with uniparental disomy (UPD) have better physical growth, fewer motor deficits and lower seizure occurrences.<ref><pubmed>16023557</pubmed></ref> | |||
- Individuals with AS resulting from imprinting defects have the least debilitating features, such as higher developmental and language ability than AS caused by other mechanisms.<ref name="PMID:11748306"><pubmed>11748306</pubmed></ref> | |||
- The Body Mass Index (BMI) of 33% of the UBE3A mutation patients, and 47–64% of the patients from the classes II (UPD) and III (Imprinting defect) is above the 95th centile.<ref name="PMID:15253756"><pubmed>15253756</pubmed></ref> | |||
'''Animal models''' | '''Animal models''' | ||
* ''Drosophila'' | * '''Drosophila''' | ||
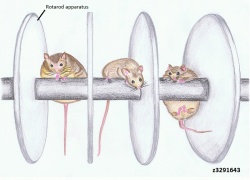
Drosophila is an excellent model to use for better understanding of genetic diseases in humans as they are highly homologous to | Drosophila (common fruit fly) is an excellent model to use for better understanding of genetic diseases in humans as they are highly homologous to human UBE3A (hUBE3A), illustrating a high evolutionary conservation. Studies using the Drosophila model have shown that the functional absence of UBE3A resulted in decreased morphogenesis of dendritic branches. This is of interest as dendritic branches cover over 90% of the neuronal surface where [[#Glossary | '''synapse''']] between neurons occur. Proper formation and maturation of dendritic spines is also required so they come in contact with other neurons for effective transmission of neuronal signals. Thus proper formation of dendritic branching is pivitol for effective neuronal function and hence cognitive function.<ref><pubmed>18996915</pubmed></ref> Interestingly, overexpression of dUBE3A also gave the same result, consisting of abnormal locomotion and decreased dendritic branching in sensory neurons.<ref><pubmed>18701717</pubmed></ref> This suggests a possible research field for some forms of [[#Glossary | '''autism''']] where the region containing UBE3A is duplicated, leading to delayed motor skills and seizures.<ref><pubmed>16433693</pubmed></ref>[[File:Normal and Angelman Syndrome mice models.jpg|thumb|250px|right|Normal and AS mice performance on the Rotarod apparatus.]] | ||
* ''Mice'' | * '''Mice''' | ||
Absence of UBE3A in mice manifest similar phenotype to human AS, as illustrated by motor and learning deficits with inducible seizures. <ref><pubmed>21235769</pubmed></ref> It has been shown through various mice model studies that larger deletions, ie. from UBE3A to GABRB3, more closely mirrors the large 6Mb deletions seen in human AS patients. Numerous tests, such as the Rotarod (for motor coordination and learning ability), Morris water maze test (for spatial learning and memory) and | Absence of UBE3A in mice manifest similar phenotype to human AS, as illustrated by motor and learning deficits with inducible seizures.<ref><pubmed>21235769</pubmed></ref> It has been shown through various mice model studies that larger deletions, ie. from UBE3A to GABRB3, more closely mirrors the large 6Mb deletions seen in human AS patients. Numerous tests, such as the Rotarod (for motor coordination and learning ability), Morris water maze test (for spatial learning and memory) and [[#Glossary | '''EEG''']] (electroencephalography), were carried out on UBE3A null mutants, which showed the results to be abnormal or impaired.<ref><pubmed>20808828</pubmed></ref> Other studies using mouse models have also outlined the obesity observed in 15% of AS children, albeit it is not the major characterisitics of AS.<ref><pubmed>17259980</pubmed></ref> The diagram on the right clearly highlights the results obtained from such mouse model studies of AS, where the absence of UBE3A accumulated in deficits of motor function and obesity. In addition, excessive drooling and feeding difficulties were also highlighted by mice models with inactivated UBE3A, reflected by defects in fluid consumption and licking.<ref><pubmed>18413322</pubmed></ref> | ||
==Signs and Symptoms== | ==Signs and Symptoms== | ||
Angelman Syndrome presents a variety of different | Angelman Syndrome presents a variety of different phenotypes, and the presented symptoms may vary at different ages.<ref name="PMID7625442"><pubmed>7625442</pubmed></ref> | ||
In 1995 Williams et al embraced the observed clinical features of AS in a consensus statement, in order to present an appliance for clinicians. | In 1995 Williams et al. embraced the observed clinical features of AS in a consensus statement, in order to present an appliance for clinicians. The diagnostic criteria were updated in 2005.<ref name="PMID16470747" /> The following table shows the most frequently occurring characteristics in AS patients: | ||
'''4 characteristics appear in 100% of the cases:''' | |||
*Delayed development: become apparent by the age of 6 to 12 months. | |||
*Motion and or balance malfunction | |||
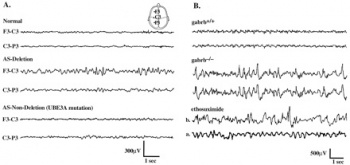
*Behaviour patterns like unmotivated laughter, frequent excitement; often including body movements,[[File:Electroencephalography of Angelman Syndrome.jpg|thumb|350px|right|EEG of AS and normal individuals.]] [[#Glossary | '''hypermotoric''']] behaviour | |||
*Impairment of speech | |||
'''3 characteristics appear in more than 80% of the cases:''' | |||
*Abnormalities in head circumference, microcephaly | |||
*Seizures | |||
*EEG shows specific abnormal patterns<ref name="PMID20445456" /><ref name="PMID16470747" /> | |||
Angelman Syndrome individuals show the most distinct disease pattern from 2 to 16 years of age. | Angelman Syndrome individuals show the most distinct disease pattern from 2 to 16 years of age. | ||
In most cases at least 8 of the symptom traits are shown. <ref | In most cases at least 8 of the symptom traits are shown.<ref name="PMID7625442" /> | ||
'''Behavioural Characteristics''' | '''Behavioural Characteristics''' | ||
A range of substantial characteristics appear in all AS cases, regardless of the genetic mechanisms, and are often responsible for diagnosing the syndrome. According to the original name, Happy Puppet | A range of substantial characteristics appear in all AS cases, regardless of the genetic mechanisms, and are often responsible for diagnosing the syndrome. According to the original name, Happy Puppet syndrome, they have shown to exhibit traits such as a happy demeanor, with frequent laughter or smiling that can easily be triggered.<ref name="PMID12566516"><pubmed>12566516</pubmed></ref> This behaviour can already be observed in 1 to 3 month old infants.<ref name="PMID20981772" /> Uplifted hand flapping or waving motions are associated with laughter and excitement. Other characteristics manifesting in early childhood are sleep abnormalities with reduced sleep demand, and feeding problems. Breast and bottle feeding can be difficult due to non efficient attachment to the breast, tongue thrusting and uncoordinated sucking. Furthermore, there is an affection to crinkly and reflective surfaces, like some papers or plastic materials, especially water.<ref name="PMID16470747" /><ref name="PMID12566516 "/> | ||
'''Communication Skills''' | '''Communication Skills''' | ||
Communication is challenging for AS patients due to the fact | Communication is challenging for AS patients due to the fact that speech impairment is present in all cases. In the majority of affected people a lack of speech development is present. Only some of them have a minimal vocabulary consisting of two or three words, a few can speak in basic sentences. Some patients use gestures to articulate themselves, the [[#Glossary | '''Picture Exchange Communication System (PECS)''']] or sign languages such as [[#Glossary | '''Makaton''']] can be used for communication. Simple demands regarding their everyday occurrence can be understood by most patients.<ref name="PMID16470747" /><ref name="PMID12566516 "/> | ||
'''Clinical and External Characteristics''' | '''Clinical and External Characteristics'''[[File:Angelman Syndrome patient.png|thumb|200px|right|4 year old AS patient.]] | ||
The most striking external | Developmental delays are present in all patients. Motor milestones are delayed with children managing to sit unsupported with 12 months and crawl or bottom shuffle at the age of 18-24 months. The average age for walking is 4 years,however, ranging from 18 months to 7 years. | ||
The [[#Glossary | '''gait''']] is characterised as slow, and flapping hands are a common trait. The legs remain stiff during walking, arms are inflected by the wrists and elbows. The movements can appear ataxic, jerky or lurching. The muscle tone shows abnormalities with truncal [[#Glossary | '''hypotonia''']] and [[#Glossary | '''hypertonicity''']] of the limbs and alert reflexes.<ref name="PMID16470747" /><ref name="PMID12566516 "/> | |||
The most striking external traits present in patients include broad-spaced teeth, and a wide mouth, light hair and eye colour, hypopigmented skin, deep set eyes, and a flat [[#Glossary | '''occiput''']].<ref name="PMID16470747" /> | |||
'''Seizures in Angelman Syndrome''' | '''Seizures in Angelman Syndrome''' | ||
85% of AS patients suffer from epileptic seizures during their first three years. The first appearance can be within one month to 20 year old patients. In infants with AS | 85% of AS patients suffer from epileptic seizures during their first three years. The first appearance can be within one month up to 20 year old patients. In infants with AS epilepsy manifests with [[#Glossary | '''febrile convulsions''']] and is hard to manage during childhood. | ||
The most common type of seizures within 25% of patients are myoclonic seizures, atonic seizures | The most common type of seizures within 25% of patients are [[#Glossary | '''myoclonic seizures''']], followed by [[#Glossary | '''atonic seizures''']] as the second most frequent type, then 21% experience generalised [[#Glossary | '''tonic-clonic seizures''']] and lastly [[#Glossary | '''atypical absences''']] occur in 12% of patients.<ref><pubmed>20398390</pubmed></ref> | ||
'''Angelman Syndrome in adults''' | '''Angelman Syndrome in adults''' | ||
Patients with Angelman syndrome | Patients with Angelman syndrome possess normal secondary sexual characteristics, and puberty sets in at a usual time. Limb hypertonicity, and [[#Glossary | '''thoracic scoliosis''']] lead to decreasing mobility. [[#Glossary | '''Oesophagus reflux''']] might induce severe problems, and the majority suffers from obesity. Furthermore, facial traits such as a prominent lower lip [[#Glossary | '''macrostomia''']] and [[#Glossary | '''mandibular prognathism''']] get manifested in adults. | ||
In some cases the communication barrier can lead to frustration, resulting in aggressive behaviour. Nonetheless, patients gain a higher concentration span with age, and their nature becomes more quiet.<ref name="PMID16470747" /> | |||
==Complications== | ===Complications=== | ||
'''Hypopigmentation and Ocular albinism''' | '''Hypopigmentation and Ocular albinism''' | ||
OCA2 gene, also known as P gene, is closely located to the UBE3A gene. It encodes a protein vital | OCA2 gene, also known as the P gene, is closely located to the UBE3A gene. It encodes a protein vital to tyrosine metabolism, which plays a role in pigmentation development of skin, hair and eyes. However, AS caused by the large deletion of UBE3A leads to haploinsufficiency of OCA2 gene, resulting in hypopigmentation of the skin and the eyes. In some children with severe hypopigmentation, some form of [[#Glossary | '''albinism''']] is suspected.<ref><pubmed>12749060</pubmed></ref> When AS is caused by another mechanism, no abnormality of the skin and eye pigmentation is observed. However, not all AS children with OCA2 gene deletion will present with obvious hypopigmentation, they may just exhibit lighter skin colour than their parents.<ref><pubmed>8302318</pubmed></ref> | ||
==Diagnosis== | ==Diagnosis== | ||
[[File: | The diagnosis of Angelman Syndrome is a clinical diagnosis confirmed by laboratory testing.<ref name="PMID19455185"><pubmed>19455185</pubmed></ref> This involves both prenatal and/or postnatal diagnosis. | ||
===Prenatal Diagnosis=== | |||
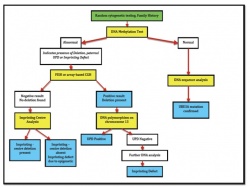
[[File:Angelman Syndrome Prenatal Diagnosis.jpg|thumb|250px|right|Flowchart of AS Diagnosis.]] | |||
Prenatal detection of deletions of 15q11.2-q13, [[#Glossary | '''Paternal UPD''']] (Uniparental Disomy), Imprinting defects and UBE3A mutations are done through DNA/chromosome/FISH analysis of fetal cells obtained by either [[#Glossary | '''Chorionic Villus Sampling''']](CVS) or [[#Glossary | '''Amniocentesis''']].<ref name="PMID20445456"/> | |||
DNA methylation analysis using [[#Glossary | '''Amniocytes''']] is usually the first test carried out in molecular diagnosis of AS.<ref name="PMID18059071"><pubmed>18059071</pubmed></ref> An abnormal result is followed up by either a FISH or array CGH analysis to search for a deletion on chromosome 15. If they produce a normal result, then UPD testing using DNA polymorphysims is carried out. If there is no evidence of UPD, other DNA analysis tests are used search for an imprinting centre deletion.<ref name="PMID9792887"><pubmed>9792887</pubmed></ref> A normal DNA methylation analysis is followed by a UBE3A sequence analysis.<ref name="PMID18059071"/> In addition, a parent of origin test is also done to determine if the genetic disruption is of maternal origin or paternal origin. If it is maternally derived the fetus is diagnosed with AS (a paternally derived genetic disruption is indicative of Prader-Willi Syndrome).<ref name="PMID17020468"><pubmed>17020468</pubmed></ref> | |||
The flow chart summarizes the normal procedure used for prenatal diagnosis of AS. More information on important laboratory tests used for the diagnosis of Angelman Syndrome are provided below: | |||
*'''DNA Methylation Analysis''' | |||
Identifies approximately 80% of AS affected individuals and is usually the first test to be ordered.<ref name="PMID7618904"><pubmed>7618904</pubmed></ref> Molecular disruptions such as deletions, paternal UPD and imprinting defects on chromosome 15 all demonstrate abnormal methylation patterns by either southern blot analysis or PCR amplification of bisulphate-treated DNA.<ref name="PMID20445456" /> An affected individual will only have an unmethylated (paternal) SNRPN (small nuclear ribonuclear protein-associated polypeptide N) allele compared with an unaffected individual who will have both a methylated and an unmethylated SNRPN allele.<ref name="PMID9634532"><pubmed>9634532</pubmed></ref> | |||
*'''FISH (Fluorescent In Situ Hybridization)''' | |||
[[File:Critical_region_of_Angelman_Syndrome_on_chromosome_15.png|thumb|150px|right|FISH (fluorescence in situ hybridisation) image showing the critical region of Angelman Syndrome on chromosome 15.]] | |||
FISH analysis detects 5-7 Mb deletions in approximately 68% of affected individuals using detecting probes such as D15S10 and/or SNRPN.<ref name="PMID7618904"><pubmed>7618904</pubmed></ref> It is also able to distinguish UPD from micro-deletions and imprinting defects. In simple terms, FISH analysis involves the binding of a fluorescent probe to a specific part of chromosome 15 and the detection of deletions within that region. FISH analysis is much more accurate in deletion detection compared to other high-resolution cytogenetics in the diagnosis of AS patients.<ref><pubmed>1511093</pubmed></ref> | |||
Array-based CGH is an advanced type of cytogenetic testing that can also be used to detect smaller deletions.<ref name="PMID19455185"/> | |||
*'''UPD (Uniparental Disomy) Detection''' | |||
DNA Polymorphism testing detects paternal UPD in approximately 7% of affected AS individuals.<ref name="PMID18059071"/> It is also able to distinguish between UPD and an Imprinting defect. It requires a DNA sample from the [[#Glossary | '''Proband''']] and from both parents. A homologous or non-homologous whole-arm translocation of the long arms of chromosome 15 is a sound indication for the need for prenatal UPD testing.<ref name="PMID18059071"/> | |||
*'''IC (Imprinting Center) Analysis''' | |||
This type of analysis is used when individuals have an abnormal DNA methylation pattern but show normal results for FISH, array CGH study and UPD analysis.<ref name="PMID9792887"/> It is used to differentiate between the Imprinting defects caused by either microdeletions in the AS imprinting centre or by epigenetic mutations (that occur in maternal oogenesis or early embryogenesis).<ref name="PMID9634532"/> | |||
Cytogenetic | *'''Cytogenetic Testing''' | ||
Is used to detect a cytogenetically visible chromosomal rearrangement (large deletion, translocation or inversion) involving the 15q11.2-q13 region, which occurs in less than 1% of affected individuals.<ref name="PMID19455185"/> | |||
*'''UBE3A Sequence Analysis''' | |||
Sequence analysis of the UBE3A gene detects mutations in about 10% of the patients.<ref name="PMID19455185"/> The test is used to detect either multiexonic (10 major coding exons of UBE3A) or whole gene deletions of UBE3A gene in individuals who produces a normal DNA methylation result but has characteristic clinical features of AS.<ref name="PMID9792887"/> | |||
This is recommended as a prenatal diagnostic tool in families that include multiple AS affected individuals because the imprinted pattern can be inherited from distant relatives.<ref name="PMID19455185"/> | |||
===Postnatal Diagnosis=== | |||
Postnatal diagnosis is made though a combination of common clinical features of AS and UBESA gene sequence analysis.<ref name="PMID18059071"/> The clinical features of AS include unsteady or shaky movements, muscle hypotonia, developmental delay and behavioral uniqueness such as frequent laughter/smiling and happy demeanor are features that are 100% consistent in all patients diagnosed with Angelman Syndrome.<ref name="PMID18830393"><pubmed>18830393</pubmed></ref> These clinical features become apparent between 6 months to 2 years of age. The onset of early or persistent smiling characteristic of this syndrome begin around the age of 1-3 months, but is often overlooked during infancy.<ref name="PMID20981772"/> Thus early diagnosis of AS during infancy period is difficult because the clinical indicators of the syndrome takes time to manifest and characteristic features such as finger and hand tremors and excessive smiling/laughter are often overlooked and not considered as possible indicators of this syndrome. | |||
===Differential Diagnosis=== | |||
*Rett Syndrome | *Rett Syndrome | ||
| Line 285: | Line 354: | ||
Genetic mechanism is heterozygous deletion or truncation in ''ZFHX1B'' (''SIP1'') gene on 2q22. This presents with clinical features of severe intellectual disability, micrcephaly and seizures.<ref><pubmed>17958891</pubmed></ref> | Genetic mechanism is heterozygous deletion or truncation in ''ZFHX1B'' (''SIP1'') gene on 2q22. This presents with clinical features of severe intellectual disability, micrcephaly and seizures.<ref><pubmed>17958891</pubmed></ref> | ||
[[File:Prader-Willi Syndrome patient.png|thumb|right|120px|12 year old PWS patient.]] | |||
*X-linked alpha-thalassemia/mental retardation syndrome (ATR-X) | *X-linked alpha-thalassemia/mental retardation syndrome (ATR-X) | ||
| Line 292: | Line 363: | ||
*Phelan-McDermid Syndrome/22q13 deletion syndrome | *Phelan-McDermid Syndrome/22q13 deletion syndrome | ||
Deletions are usually submicroscopic and thus requires special molecular cytogenetic methods to confirm this deletion. Patients show moderate to profound intellectual disability and delay in speech development. <ref><pubmed>18505557 | Deletions are usually submicroscopic and thus requires special molecular cytogenetic methods to confirm this deletion. Patients show moderate to profound intellectual disability and delay in speech development.<ref><pubmed>18505557</pubmed></ref> | ||
'''Related Disease''' | |||
*Prader-Willi Syndrome(PWS) | |||
PWS is a result from the absence of paternally expressed 15q11-q13 gene as a corollary of de novo deletion, maternal uniparental disomy of chromosome 15 or an imprinting defect on paternal chromosome 15 leading to the silencing of paternal alleles.<ref name="PMID:20668179 "/> It is characterized by [[#Glossary | '''hyperphagia''']], obesity in later infancy and early childhood and difficulty in feeding during early infancy.<ref name="PMID:20668179 "/> There is also cognitive impairment to some extent, though some PWS patients may exhibit intelligence in the normal IQ range. Behavioral phenotypes include stubbornness, manipulative tendency, obsessive compulsive characteristics.<ref name="PMID:20668179 "/> | |||
==Genetic Counselling== | ==Genetic Counselling== | ||
Genetic counselling is the process by which patients or relatives, at risk of an inherited disorder, are advised of the consequences and nature of the disorder, the probability of developing or transmitting it, and the options open to them in management and family planning. In the case for Angelman syndrome, types of risks associated are split into classes and formatted into the table below:<ref><pubmed>20459762</pubmed></ref> | |||
{| style="width:100%; height:25px" | {| style="width:100%; height:25px" | ||
|-bgcolor="lightpink" | |-bgcolor="lightpink" | ||
! Class | |||
! Mechanism | |||
! Risk to siblings | |||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
! Ia | |||
| De novo deletion | | De novo deletion | ||
| <1% | | <1% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| Ib | |-bgcolor="mistyrose" | ||
! Ib | |||
| Deletion due to chromosome rearrangement | | Deletion due to chromosome rearrangement | ||
| Up to 50% | | Up to 50% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
! II | |||
| Paternal uniparental disomy | | Paternal uniparental disomy (UPD) | ||
| <1% | | <1% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IIIa | |-bgcolor="mistyrose" | ||
! IIIa | |||
| Imprinting defect with IC mutation | | Imprinting defect with IC mutation | ||
| Close to 100% if father has 15;15 Robertsonian translocation | | Close to 100% if father has 15; 15 [[#Glossary | '''Robertsonian translocation''']] | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
! IIIb | |||
| Imprinting defect | | Imprinting defect without IC mutation | ||
| Up to 50% (if mother has IC deletion) | | Up to 50% (if mother has IC deletion) | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| IIIc | |-bgcolor="mistyrose" | ||
| Mosaic imprinting defect | ! IIIc | ||
| [[#Glossary | '''Mosaic''']] imprinting defect | |||
| <1% | | <1% | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
! IV | |||
| ''UBE3A'' mutation | | ''UBE3A'' mutation | ||
| Up to 50% ( | | Up to 50% (if mother has mutation) | ||
|-style="width:100%; height:25px" | |-style="width:100%; height:25px" | ||
| V | |-bgcolor="mistyrose" | ||
! V | |||
| No identifiable genetic abnormality | | No identifiable genetic abnormality | ||
| ? <ref><pubmed> | | ? | ||
|} | |||
'''Ia''': Individuals with de novo deletion have 1% risk to siblings | |||
'''Ib''': Risk to siblings in individuals with chromosomal rearrangements is up to 50% <ref><pubmed>9557895</pubmed></ref> | |||
'''II''': Paternal UPD with no Robertsonian translocation has a risk to sibling of less than 1% | |||
'''IIIa''': The risk to siblings in individuals with AS from an imprinting defect with a mutation in the imprinting center (IC) is close to 100% if father has 15;15 Robertsonian translocation | |||
'''IIIb''': Individuals with AS resulting from an imprinting defect without IC mutation, but with a carrier mother has a high risk to siblings of up to 50%. The mother could be phenotypically normal due to paternal inheritance of the genetic defect <ref><pubmed>11283796</pubmed></ref> | |||
'''IIIc''': Individuals who are mosaic for the imprinting defect have less than 1% risk to siblings | |||
'''IV''': UBE3A mutation can be either inherited or de novo mutation. If the mother is a carrier for this genetic mutation, the risk to siblings is up to 50% <ref><pubmed>11258627</pubmed></ref> | |||
'''V''': Individuals who present with AS clinically, but have no known underlying mechanism have an unknown risk to siblings. | |||
==Treatment and Management== | |||
Even though at present there is no known cure for Angelman syndrome, there are a number of treatments available that may alleviate symptons and improve daily life activities.<ref name="PMID19455185"><pubmed>19455185</pubmed></ref> The below table describes the problems and management options that are used: | |||
[[File:Subtle Finger tremors-Angelman Syndrome.jpg|right|thumb|150px|A five months old boy with Angelman syndrome showing signs of Subtle Finger tremor.]] | |||
{| style="height:50px" | |||
|-bgcolor="lightpink" | |||
! Problems encountered | |||
! Treatment and Management | |||
|-style="height:50px" | |||
| '''Difficulty in feeding newborn AS babies''' | |||
| Use of special nipples may improve feeding<ref name="PMID19455185" /> | |||
|-style="height:50px" | |||
|-bgcolor="mistyrose" | |||
| '''Oesophagus reflux''' | |||
| Special motility medications requiried or upright positioning<ref name="PMID19455185" /> | |||
|-style="height:50px" | |||
| '''Seizures''' | |||
| Anticonvulsant medications<ref><pubmed>19605773</pubmed></ref><ref><pubmed>19453717</pubmed></ref> | |||
|-style="height:50px" | |||
|-bgcolor="mistyrose" | |||
| '''Ocular problems''' | |||
| Visual assessment is vital to encourage interactions and minimize chances of self-harm and autism development<ref><pubmed>20656169</pubmed></ref> | |||
|-style="height:50px" | |||
| '''Drooling in developmental delayed AS patients''' | |||
| Difficult to treat, but new surgical procedure on salivary duct reimplantation seems to be a promising alternative<ref><pubmed>17699119</pubmed></ref> | |||
|-style="height:50px" | |||
|-bgcolor="mistyrose" | |||
| '''Unstable children''' | |||
| Physical therapy<ref name="PMID19455185" /> | |||
|-style="height:50px" | |||
| '''AS patients become less active with increasing age''' | |||
| Activity schedules to prevent scoliosis and obesity. Occupational therapy to stimulate fine motor and oral-motor control skills in conjunction to speech therapy<ref name="PMID16470747" /> | |||
|-style="height:50px" | |||
|-bgcolor="mistyrose" | |||
| '''Sleeping difficulty''' | |||
| Sedative medication in severe cases. [[#Glossary | '''Melatonin''']] may be used to promote sleeping but some studies have found that it loses its therapeutic effects after several weeks in most AS patients<ref><pubmed>19379289</pubmed></ref> | |||
|- | |||
|- | |||
|colspan="4" | | |||
|} | |} | ||
==Prognosis== | |||
Life expectancy of AS patients seems to be normal<ref><pubmed>19455185</pubmed></ref>, but there is still a high degree of developmental delay and limited expressive language skills.<ref><pubmed>17019625</pubmed></ref> This problem arises prominently in the assessment of the severity of coincidental medical conditions due to difficulties in communication. A visit to the dentist can become difficult if good communication is not conveyed between the parents, the dentist, and the child.<ref><pubmed>18271768</pubmed></ref> Some problems require extreme measures, such as the procedure of lens implantation was performed in both eyes of a person diagnosed with AS, due to the inability to wear spectacles.<ref><pubmed>20656169</pubmed></ref> | |||
During adulthood, menstruation and puberty begin at around average age evidenced by females with Angelman syndrome that are fully capable of conceiving children.<ref><pubmed>11258627</pubmed></ref> Most adult patients can feed themselves, but needed help with many other daily activities. Main troubles which have shown in adults are a tendency to become obese and start developing thoracic scoliosis, which some adults with AS begin wheelchair bounded.<ref><pubmed>9072912</pubmed></ref> | |||
| Line 391: | Line 491: | ||
Currently there is no cure for the treatment of AS, so much research is being carried out for therapeutic interventions. Findings in the last five years have suggested through various mice and fruit fly models that the absence of maternal UBE3A results in the same human AS features of neurological defects and seizures. However, more recently, studies have outlined that the symptoms of AS can be improved by modifying other gene products. In addition, restoring of a functional UBE3A protein into adult mice neurons have shown to reduce motor and neurological deficits and balance disorders. This shows promising research prospects in that improvements may be possible in human AS patients by restoring functional UBE3A proteins into their neurons. | Currently there is no cure for the treatment of AS, so much research is being carried out for therapeutic interventions. Findings in the last five years have suggested through various mice and fruit fly models that the absence of maternal UBE3A results in the same human AS features of neurological defects and seizures. However, more recently, studies have outlined that the symptoms of AS can be improved by modifying other gene products. In addition, restoring of a functional UBE3A protein into adult mice neurons have shown to reduce motor and neurological deficits and balance disorders. This shows promising research prospects in that improvements may be possible in human AS patients by restoring functional UBE3A proteins into their neurons. | ||
Although everyone is born with two copies of the gene UBE3A, one maternal and one paternal copy, it is only the maternal UBE3A that is used in the brain. Most AS patients have a genetic mutation on the maternal chromosome 15 that results in dysfunctional UBE3A.<ref>http://www.cureangelman.org/what-hope.html</ref> | ===Possible Treatment Pathways=== | ||
*'''Cognitive and physical impairment''' | |||
Although everyone is born with two copies of the gene UBE3A, one maternal and one paternal copy, it is only the maternal UBE3A that is used in the brain. Most AS patients have a genetic mutation on the maternal chromosome 15 that results in dysfunctional UBE3A.<ref>http://www.cureangelman.org/what-hope.html</ref> The above research focused on turning on the father's copy of UBE3A to restore its functions. Furthermore, mouse models were utilized to show that alpha-CaMKII could be increased to compensate the loss of UBE3A and restore compromised mental and physical abilities. However, this finding still requires much more research.<ref><pubmed>17259980</pubmed></ref> | |||
*'''Epilepsy''' | |||
There is research being done on improving certain conditions that are involved in AS by nutritional benefits. The use of [[#Glossary | '''corticosteroids''']]<ref><pubmed>19666884</pubmed></ref>, [[#Glossary | '''valproate''']]<ref><pubmed>19453717</pubmed></ref> and [[#Glossary | '''clonazepam''']]<ref><pubmed>19605773</pubmed></ref> and having a [[#Glossary | '''ketogenic diet''']]<ref><pubmed>20880305</pubmed></ref> has all been shown to aid in the treatment of epilepsy. It has also been reported to be efficient in the treatment of other conditions, such as behavioural disorders, reduction in seizures, ataxia, sleep patterns, developmental progress and sleep disturbances. [[#Glossary | '''Levetiracetam''']]<ref><pubmed>17326790</pubmed></ref> could be used in such a situation, especially when the patient has an intolerance to valproate and clonazepam. | |||
*'''Betaine and Folic acid''' | |||
This double-blind placebo-controlled study was done to examine the potential therapeutic role of [[#Glossary | '''betaine''']] and [[#Glossary | '''folic acid''']] in the treatment of the clinical phenotypes of AS. The aim of this protocol was to increase methylation and activate the paternally inherited UBE3A in hope to compensate for the inactive maternal UBE3A. Although there were no major differences in the control and treatment groups, a small number of patients in the treatment group showed some positive outcomes.<ref><pubmed>20635355</pubmed></ref> | |||
==Glossary== | ==Glossary== | ||
'''albinism''': A congenital disorder distincted by the complete or partial absence of pigment in the skin, hair and eyes due to defect of an enzyme involved in the production of melanin. | |||
'''allele''': Dissimilar variant of one gene. Organisms have two alleles for each gene inherited from their parents, one from each of them. | '''allele''': Dissimilar variant of one gene. Organisms have two alleles for each gene inherited from their parents, one from each of them. | ||
'''ataxia''': | '''alpha-CaMKII''': (alpha calcium calmodulin kinase II) Compound involved in synaptic plasticity, one of the most important theories behind learning and memory. Ability of a synapse between two neurons to change in strength due to the use or disuse of transmission. | ||
'''Amniocytes''': Cells that are shed by the fetus during fetal development to the surrounding amniotic fluid. They are a source of fetal DNA. | |||
'''Amniocentesis''': (AFT or amniotic fluid test) is a prenatal diagnostic test used to detect chromosomal abnormalities in the developing fetus by extracting amniotic fluid (a source of fetal DNA) surrounding the fetus. | |||
'''AMPA''': (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid) Compound that binds to the AMPA receptor to imitate glutamate neurotransmitter. | |||
'''Arc''': Activity-Regulated Cytoskeleton-associated protein; involved in learning and memory. | |||
'''ataxia''': Lack of coordination in muscle movements. | |||
'''atonic seizures''': A abrupt loss of muscle tone, this can lead to the limbs loosing strength, or the head may drop to the side. The patient remains conscious during this kind of epileptic seizure. | '''atonic seizures''': A abrupt loss of muscle tone, this can lead to the limbs loosing strength, or the head may drop to the side. The patient remains conscious during this kind of epileptic seizure. | ||
'''atypical absences''': Seizure leads to unconsciousness in most cases. | '''atypical absences''': Seizure leads to unconsciousness in most cases. | ||
'''autism''': A neural development disorder defined by limited social interaction and communication. | |||
'''betaine''': Also known as betaine anhydrous or trimethylglycine (TMG), assists the body in metabolising the amino acid, homocysteine. Can be consumed via beets, broccoli, grains, shellfish, and spinach. | |||
'''cerebellum''': Region of the brain that plays an important role in motor control. It is also involved in some cognitive functions such as attention and language. | |||
'''Chorionic Villus Sampling''': A prenatal diagnostic test used to detect chromosomal abnormalities in the developing fetus by extracting and testing chorionic villus (placental tissue). | |||
'''chromosome''': Structure consisting of coiled DNA with DNA associated proteins such as histones. | |||
'''clonazepam''': A psychoactive drug having muscle relaxant properties and treats anxiety and epilepsy. | |||
'''corticosteroid''': A type of steroid hormones that are produced in the adrenal cortex. | |||
'''cytogenetics''': Study of the structure, function and abnormality of the cell, especially the chromosome. | |||
'''deletion''': Lack of a part of the DNA (deoxyribonucleic acid is a nucleic acid containing information for protein synthesis), can vary form a single base to a hole gene. | '''deletion''': Lack of a part of the DNA (deoxyribonucleic acid is a nucleic acid containing information for protein synthesis), can vary form a single base to a hole gene. | ||
'''de novo mutation''': Change in a gene of a family member for the first time due to a mutation in the egg or sperm of the parents or in the fertilized egg (zygote). | |||
'''disorder''': A mental or physical health disturbance or dysfunction. | '''disorder''': A mental or physical health disturbance or dysfunction. | ||
'''EEG''': Electroencephalography is a means of measuring electrical activity along the scalp by measuring the total electrical charges fired by thousands or millions of neurons. | |||
'''epilepsy''': Neurological disorder characterised by recurrent impairment of brain function that can cause seizures or unconsciousness. | '''epilepsy''': Neurological disorder characterised by recurrent impairment of brain function that can cause seizures or unconsciousness. | ||
'''febrile convulsions''': The occurrence of high | '''E6-AP''': E6-AP ubiquitin-protein ligase is an enzyme encoded by UBE3A which adds ubiquitin chain to target proteins to be degraded by proteasomes. | ||
'''febrile convulsions''': The occurrence of high fever, especially in children. | |||
'''FISH''': Fluorescent in situ hybridization is a technique used to identify and localize the presence or absence of DNA sequences on the chromosome. This is achieved by using fluorescently labelled DNA sequences which then binds to complementary DNA sequence on the chromosome. After hybridization (attachment), chromosome of interest will fluoresce under the microscope. | |||
'''folic acid''': B vitamin that helps the body make healthy new cells. Can be consumed via leafy green vegetables, fruits, dried beans, peas and nuts. | |||
'''gait''': A manner of walking, stepping or running. | |||
'''gene''': A molecular unit of heredity of a living organism. | |||
'''hippocampus''': Area buried deep in the forebrain that helps regulate emotion and memory | |||
'''hyperphagia''': (Also referred to as Polyphagia) Excessive ingestion of food beyond the dietary and energy requirements of the body. | |||
'''hypermotoric''': | '''hypermotoric''': Sudden appearance of uncontrolled movements such as striking or kicking inappropriately. | ||
'''hypertonicity''': Increase of muscle tension and a disability to stretch leading to an impairment of motor activity. | '''hypertonicity''': Increase of muscle tension and a disability to stretch leading to an impairment of motor activity. | ||
''' | '''hypopigmentation''': A lack of melanin pigments or melanocytes affecting the colour of the skin, hair and iris. Features appear to be light-coloured due to loss of skin colour. | ||
'''hypotonia''': Decrease in the ability to stretch passively, due to a low muscle tone. | '''hypotonia''': Decrease in the ability to stretch passively, due to a low muscle tone. | ||
''' | '''imprinting''': This is a process to describe the modification of a maternally or paternally derived chromosome, which consequently influences the expression of certain genes on the altered chromosome. | ||
''' | '''ketogenic diet''': A high-fat, low-carbohydrate diet that is used to treat epilepsy in children. | ||
''' | '''levetiracetam''': An anticonvulsant drug used to treat epilepsy. | ||
''' | '''locus''': Location of a gene on a chromosome(loci: plural for locus). | ||
''' | '''LTP''': Long-term potentiation shares common features with long term memory. It strengthens and enhances signal transmission between neurons by improving the communication between two neurons. | ||
'''microcephaly''': The circumference of the head is less than | '''macrostomia''': A wide-spaced mouth. | ||
'''Makaton''': Language programme using symbols and signs to support speaking. | |||
'''mandibular prognathism''': The abnormal protrusion of the lower jaw. | |||
'''maxillary hypoplasia''': The underdevelopment of the upper jaw. | |||
'''melatonin''': A hormone secreted by the pineal gland. | |||
'''microcephaly''': The circumference of the head is less than average due to abnormalities of the nervous system. This abnormality can be present at birth or emerge during the first few years of life and reduces both life expectancy and the cognitive functions of the individual affected. | |||
'''microdeletion''': A genetic mutation in which a part of a chromosome or a sequence of DNA is missing. | |||
'''mosaic''': tissues or cells containing different mixture of genetic sequences | |||
'''mutation''': Event that changes the DNA (deoxyribonucleic acid is a nucleic acid, containing information for protein synthesis) or RNA (ribonucleic acid is a type of nucleic acid transmitting the information from the DNA to the proteins ) of a gene permanently. | '''mutation''': Event that changes the DNA (deoxyribonucleic acid is a nucleic acid, containing information for protein synthesis) or RNA (ribonucleic acid is a type of nucleic acid transmitting the information from the DNA to the proteins ) of a gene permanently. | ||
| Line 437: | Line 607: | ||
'''myoclonic seizures''': A quick jerk of the skeletal muscle, due to a irregular brain activity. | '''myoclonic seizures''': A quick jerk of the skeletal muscle, due to a irregular brain activity. | ||
'''neurogenetic''': Genetic basis of the nervous system | '''neurogenetic''': Genetic basis of the nervous system. | ||
'''neuron''': Cells of the nervous system that can be electrically excited to transmit information by electrical and chemical signaling | |||
'''neurotransmitter''': Transmits neuronal signals to a target cell. They are first packaged in vesicles just beneath the membrane of presynaptic axon terminals and released into the synaptic cleft (space between the two interacting neurons) and enter the postsynaptic dendrite. | |||
'''occiput''': The back portion of the head. | |||
''' | '''oesophagus reflux''': Impairment of a sphincter in the lower end of the oesophagus leads to the back flow of stomach acid into the oesophagus. Heartburn is a typical symptom. | ||
''' | '''olfactory tract''': It is a pathway for the sense of smell containing axons from some of the neurons in the olfactory bulb. | ||
'''paediatrician''': Physicians that | '''paediatrician''': Physicians that specialise on infants, children and adolescents. | ||
''' | '''paternal UPD''': Inheritance of two copies of a chromosome from the father and none from the mother. | ||
'''phenotype''': Visible traites of an organism determined by genetic and environmental factors. | |||
'''Picture Exchange Communication System (PECS)''': Individuals communicate using images instead of words. It is frequently used by children with autism, to articulate their needs and wishes or to make comments about something. | '''Picture Exchange Communication System (PECS)''': Individuals communicate using images instead of words. It is frequently used by children with autism, to articulate their needs and wishes or to make comments about something. | ||
'''seizure''': Abnormal electrical activity in the brain that can result in a variety of physical symptoms like convulsion, body | '''Proband''': Is the person (or animal) being studied or reported on in a genetic investigation. Most commonly it refers to the first affected family member who requires medical help for a genetic disorder. | ||
'''Purkinje cells''': Large neuron with many branching extensions that is found in the cortex of the cerebellum of the brain and plays a fundamental role in controlling motor movement | |||
'''Robertsonian translocation''': Rearrangement of the chromosome, occurs in 5 chromosome including 13,14,15,21 and 22. This type of mutation occurs in chromosomes with the centromere not at the centre, resulting in long and short arm chromosomes. Translocation breaks the chromosome at the centromere so the long arm attaches to the other long arm, and the same for the short arm happens. Cell division will result in the loss of the short arms as they do not contain important genes. | |||
'''seizure''': Abnormal electrical activity in the brain that can result in a variety of physical symptoms like convulsion, body shaking, loss of consciousness, confusion, and mood changes. | |||
'''synapse''': Allows transmission of signal between neurons for effective neuronal function. | |||
'''thoracic scoliosis''': The spine is curved laterally(from side to side) in the thoracic region. | |||
'''tonic-clonic seizures''': This type of seizure is associated with unconsciousness due to electrical discharges in a big proportion of the brain. | |||
'''ubiquitin''': A polypeptide found in all eukaryotic cells, that participates in a variety of cellular functions including protein recycling. | |||
'''Uniparental disomy''': (UPD) the inheritance of two copies of a chromosome pair or part of a chromosme from one parent and no copy from the other parent. | |||
'''valproate''': A mood-stabilizing drug that is also used for treatment for epilepsy and bipolar disorder. | |||
==Helpful Links== | |||
* Australia | |||
:* [http://www.angelmansyndrome.org/research.html Angelman Syndrome Association] | |||
:* [http://www.angelmansyndromeqld.org/ Queensland Angelman Association] | |||
* America | |||
:* [http://www.angelman.org/ Angelman Syndrome Foundation] | |||
:* [http://rarediseasesnetwork.epi.usf.edu/arpwsc/ Rare Clinical Diseases Research Network] | |||
:* [http://www.rarediseases.org/rare-disease-information/rare-diseases/byID/411/viewAbstract National Organisation for Rare Disorders] | |||
:* [http://www.epilepsyfoundation.org/ Epilepsy Foundation] | |||
:* [http://www.cureangelman.org/ Foundation for Angelman Syndrome Therapeutics] | |||
* Search Pubmed | |||
:* [http://www.ncbi.nlm.nih.gov/pubmed?term=angelman%20syndrome Angelman Syndrome] | |||
* OMIM entries for Angelman Syndrome | |||
:* [http://omim.org/entry/105830 ANGELMAN SYNDROME; AS] | |||
:* [http://omim.org/entry/601623 UBIQUITIN-PROTEIN LIGASE E3A; UBE3A] | |||
==Related Links== | |||
''' | '''Animal Models''' | ||
* [[Mouse Knockout]] - this page explains the current use of mouse models in disrupting genes to elucidate their genetic function, including the mouse gene knockouts for alpha calcium-calmodulin kinase II (alpha CaMKII) | |||
* [[2009 Group Project 4]] - this page gives an extensive overview on the history of the mouse model and its various use in embryology and genetics. In addition, it also describes mouse knockout and the role they play in current research of various human diseases | |||
* [[Fly Development]] - this page describes the use of drosophila flies as developmental models | |||
* [[2009 Group Project 2]] - this page gives an extensive overview on the history of the fly model and its various use in embryology and genetics | |||
'''Diagnosis''' | |||
* [[Chorionic villus sampling]] - this page explains the process of chorionic villus sampling, its disadvantages and gives a brief overview of recent findings | |||
* [[Amniocentesis]]- this page shows some of the recent findings in this area | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Latest revision as of 11:08, 19 October 2011
| Note - This page is an undergraduate science embryology student group project 2011. |
Angelman Syndrome
Introduction
Angelman syndrome (AS) is a rare neurogenetic disorder first described by Dr. Harry Angelman in 1965.[1] Dr. Angelman was an English paediatrician who first diagnosed the disease in 3 children with a developmental delay.[2] AS occurs in 1 in 10000-20000 births, the exact number is still unknown.[3][4] It is caused by maternal allele disruptions of a single gene- UBE3A. Either mutations or deletions of the UBE3A gene results in AS.
AS presents with well known phenotypes during infancy and adulthood, such as microcephaly and maxillary hypoplasia. However, these features become more marked with age. Most frequent clinical features include delayed development, seizures, ataxia, impairment of speech, happy demeanor, behavioural problems such as hyperactivity, short attention span and sleeping difficulty.[5][6][7]
At present, there is no cure for the syndrome, though current research is focused at improving life quality of patients with Angelman syndrome through management of daily tasks.[8]
History
Dr. Harry Angelman first reported Angelman Syndrome on three handicapped children that were admitted to his ward in England in 1964.[1] He reported how these three independent and unconnected clinical cases all had the same characteristic features of severe developmental delay, jerky movements, seizures and a happy disposition.[9] However it was only when he stumbled upon an oil painting called ‘a Boy with a Puppet” while on a holiday in Italy he was able to connect the symptoms of all three children under one common cause.[10] He linked the happy face of the boy depicted on the painting with the jerky movements and happy disposition he observed on his three patients back in England. He reported his findings on a paper entitled “Puppet children (a report on three cases)” which was published in 1965.[1]
The syndrome was initially named ‘Puppet Syndrome’ but was later changed to Angelman Syndrome. Due to the extreme rarity of the syndrome and lack of sufficient clinical evidence the syndrome was soon forgotten.[1]
In 1987, a physician named Ellen Magenis, came across children with deletions on chromosome 15 that were suffering from seizures and severe developmental delay.[11] Even though genetically the children were expected to have Prader-Willi syndrome that was characterised by deletions on chromosome 15, their characteristic symptoms of seizures and lack of development did not correspond with that of the syndrome. This led to the realization that these children had deletions on the maternally derived chromosome 15 and not on the paternally derived chromosome as was observed in Prader-Willi syndrome. Thus the deleted genetic code on maternally derived chromosome 15 was identified as the genetic marker for Angelman Syndrome.
In 1997, UBE3A gene was isolated by Dr. Joseph Wagstaff and Dr. Arthur Beaudet as the cause of Angelman Syndrome.[10] The discovery of this gene led to the subsequent development of animal models and research aimed at finding a causal link between UBE3A gene abnormalities and characteristic features observed in Angelman Syndrome.
Currently, four different genetic abnormalities for Angelman Syndrome have been confirmed by genetic testing. They include: Deletion, Uniparental Disomy (UPD), Imprinting and UBE3A mutation.[12]
Below is a timeline, summarising the key discoveries and advancements of the Angelman Syndrome:
| Year | Milestone |
|---|---|
| 1964 | First observed by Dr Harry Angelman on three handicapped children.[10] |
| 1965 | Harry Angelman publishes his finding in a report entitled 'Puppet Children'(a report on three cases).[1] |
| 1980s | First reports of AS reaches US and research into the disorder being at the University of Florida under the direction of Dr. Charles Williams.[13] |
| 1982 | Name changed from 'Happy Puppet' to Angelman syndrome by Williams and Frias. |
| 1987 | Discovery of a genetic marker for AS – an absent genetic code on maternally derived chromosome 15. |
| 1997 | The cause of AS discovered by Dr. Joseph Wagstaff and Dr. Arthur Beaudet – mutation or deletion in the UBE3A gene. |
| 1998 | Transgenic mice with absent maternal UBE3A created to illustrate motor and learning deficits in addition to seizures.[14] |
| 2007 | AS mouse model shows that neurological deficits can be reversed by decreasing levels of alpha-CaMKII inhibitory phosphorylation.[15] |
| 2010 | Discovery of a another gene mutation in TC4 gene (on chromosome 18) in a small number of clinically diagnosed patients with no identifiable genetic alternation in UBE3A.[16] |
| 2010 | Protein named Arc identified as a target of AS gene product; UBE3A.[12] |
Epidemiology
Incidence
- The current population of people diagnosed with AS is unknown but studies have estimated the number to be around 1:10,000[3] and 1:20,000[4].
Diagnosis
Gender
- Males appear to be at higher risk for some aspects of early developmental delays in comparison to females with AS.[19]
Demography
- Europe:
- Australia:
- Western Australia- 1:40,000[21]
Aetiology
Molecular Genetics
- Location: 15q11-q13
- Protein product: Ubiquitin protein ligase E3A
- Gene name: UBE3A
- Phenotype: Angelman Syndrome
Genetic Causes
Different genetic mechanisms lead to different clinical phenotypes of AS. The most common genetic mechanism leading to AS is the deletion or re-arrangement of maternal chromosome at locus 15q11.2-q13,as the chromosome image shows on the right, accounting for 70% of AS occurrences.[22] This leads to more severe clinical phenotypes of microcephaly, motor difficulties, seizures and impaired speech development. The next most common genetic mechanism is mutation in the UBE3A gene responsible for 10% of AS cases and paternal uniparental disomy and mutation in the imprinting centre (IC), both accounting for 2-5% of AS observed.[23]
AS is caused by the 4 major genetic mechanisms mentioned above and are thus divided into Classes I to IV based on their underlying genetic mechanism. AS patients with the clinical features of AS but no cytogenetic or molecular abnormality in Chromosome 15q11.2-13 are grouped under Class V (summarised in table below).[22][24]
| Class | Mechanism | Diagnostic Tests | Frequency |
|---|---|---|---|
| Ia | De novo deletion | High resolution cytogenetics FISH | 70% |
| Ib | Deletion due to chromosome rearrangement | High resolution cytogenetics FISH | <1% |
| II | Paternal uniparental disomy | RFLP analysis | 2% |
| IIIa | Imprinting defect with IC mutation | Screening of IC for mutations is positive | 2% |
| IIIb | Imprinting defect withouth IC mutation | Screening of IC for mutations is negative | 2% |
| IIIc | Mosaic imprinting defect | Screening of IC for mutations is usually negative | ? |
| IV | UBE3A mutation | Screening of UBE3A for mutations | 5-10% |
| V | No identifiable genetic abnormality | Consider other diagnoses | 5-26% |
Inheritance
Angelman Syndrome from UBE3A mutations and deletions in the Imprinting Centre follow an inheritance pattern. A carrier father with the genetic defect can pass it on to his children, however, his children will be clinically normal. On the other hand, a carrier mother can pass the genetic defect onto her children, who will then have Angelman Syndrome.
The image highlights the inheritance of Angelman Syndrome, which only results after a carrier mother passes it on to her children.
Pathogenesis
Chromosome 15 became associated with AS in the 1980s after the observation of many AS patients harboring microdeletions of 15q11.2-15q13.[25] The last decade has shed even more light in the aetiology of AS,as Kishino and Matsuura first identified UBE3A as the causative gene for AS in 1997.[26][27]
Normal gene product: E6-AP ubiquitin ligase
The function of UBE3A is to encode for E6-AP ubiquitin ligase, however its function in the development of the nervous system and the process of UBE3A mutation leading to cognitive impairment in AS patients is still vague.[12] Past and current research has elucidated that E6-AP ubiquitin ligase is involved in the degradation of four proteins, listed below. Currently no protein with a direct role in long-term potentiation (LTP) as a target of E6-AP has been identified.[28] The identification of such target proteins would be a landmark discovery in further understanding of the pathogenesis, aetiology and prospective treatment strategies of AS, as LTP defects is one of the major neurological impairments of AS.
- p53 tumor suppressor protein
- yeast DNA repair protein Rad23
- multicopy maintenance protein 7 subunit
- E6-AP
Abnormal gene product
Disruption to the gene UBE3A interferes with the normal functioning of the gene, which is its crucial role in UBE3A ubiquitylation. Problems involving this pathway can lead to various diseases either from the loss or gain of function.[29] In the case of AS, E6-AP is affected, leading to detrimental cognitive impairment in addition to other deficits.[29]
Regional functioning of UBE3A
Despite the uncertain progression of UBE3A mutation and the neurological abnormalities characteristic of AS, animal models have illustrated the vital role of UBE3A in the normal functioning of the brain. This can be exemplified by a study which utilized a mouse model with target inactivation of the gene UBE3A resulting in the manifestation of classical features of AS.[30] In addition, the fact that imprinting of UBE3A takes place only in specific brain regions ( cerebellum , olfactory tracts and the hippocampus) confirms the hypothesis that the loss of this gene would be detrimental in cognitive development.[31] Most genes are inherited in copies of two, one paternal and the other maternal; however, some genes, such as UBE3A only have one functional copy as the other is silenced, coined 'imprinting'. Imprinting of the paternal copy of UBE3A takes place in some cell populations, such as the hippocampal neurons and Purkinje cells in the cerebellum.[32] This means only the maternal UBE3A is functionally expressed in these particular brain regions. Thus a de novo mutation in the maternal copy of the gene will result in the complete absence of functional E6-AP in the associated brain areas.[33]
It is also known that UBE3A plays an important role in synaptic transmission, but exactly how it does so is still not completely understood.[34]
UBE3A ubiquitylation
As briefly mentioned above, UBE3A is a member of the E3 ubiquitin ligase family of enzymes. It is responsible for the addition of ubiquitin to the target protein for degradation of the ubiquitinated protein, as illustrated in the diagram below. These processes are required for normal human cognitive function.[34] In this way synaptic protein Arc (activity-regulated cytoskeleton-associated protein) is degraded to control synaptic function. Arc is a target protein of UBE3A in dendritic spines found in the hippocampal neurons.[35] Deletion of UBE3A leads to the accumulation of Arc in neurons leading to trafficking of AMPA ( α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, resulting in impaired cognitive functions.[12]
UBE3A ubiquitylation consists of:[35]
- Activation of ubiquitin by E1 enzyme
- E1 ezyme transfers ubiquitin to E2 enzyme
- E2 enzyme transfers ubiquitin to UBE3A
- UBE3A attaches activated ubiquitin to target protein which is then polyubiquitylated
- Target protein is then degraded by 26S proteosome
Phenotype-Genotype correlations
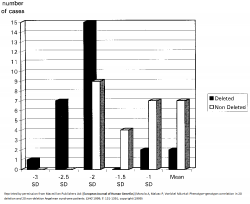
All AS patients show differing extents of cognitive impairment, movement disorder, characteristic behaviours and difficulty in speech and language.[37] However, there seems to be some phenotype-genotype correlations:
- 5-7Mb deletions result in the most severe phenotypes such as microcephaly, more sever epilepsy and seizures, motor difficulties and language impairment.[38][39] AS patients with large deletions also present with clinical hypopigmentation, light hair and eye colour due to the close association of OCA2 gene with UBE3A.[40] Individuals under this category have in general no increased BMI[41] This is indicated by the graph on the left, which shows more cases of AS patients with deletions having larger deviations from the mean head circumference measurement than non-deletion AS patients.
- AS patients with uniparental disomy (UPD) have better physical growth, fewer motor deficits and lower seizure occurrences.[42]
- Individuals with AS resulting from imprinting defects have the least debilitating features, such as higher developmental and language ability than AS caused by other mechanisms.[37]
- The Body Mass Index (BMI) of 33% of the UBE3A mutation patients, and 47–64% of the patients from the classes II (UPD) and III (Imprinting defect) is above the 95th centile.[41]
Animal models
- Drosophila
Drosophila (common fruit fly) is an excellent model to use for better understanding of genetic diseases in humans as they are highly homologous to human UBE3A (hUBE3A), illustrating a high evolutionary conservation. Studies using the Drosophila model have shown that the functional absence of UBE3A resulted in decreased morphogenesis of dendritic branches. This is of interest as dendritic branches cover over 90% of the neuronal surface where synapse between neurons occur. Proper formation and maturation of dendritic spines is also required so they come in contact with other neurons for effective transmission of neuronal signals. Thus proper formation of dendritic branching is pivitol for effective neuronal function and hence cognitive function.[43] Interestingly, overexpression of dUBE3A also gave the same result, consisting of abnormal locomotion and decreased dendritic branching in sensory neurons.[44] This suggests a possible research field for some forms of autism where the region containing UBE3A is duplicated, leading to delayed motor skills and seizures.[45]
- Mice
Absence of UBE3A in mice manifest similar phenotype to human AS, as illustrated by motor and learning deficits with inducible seizures.[46] It has been shown through various mice model studies that larger deletions, ie. from UBE3A to GABRB3, more closely mirrors the large 6Mb deletions seen in human AS patients. Numerous tests, such as the Rotarod (for motor coordination and learning ability), Morris water maze test (for spatial learning and memory) and EEG (electroencephalography), were carried out on UBE3A null mutants, which showed the results to be abnormal or impaired.[47] Other studies using mouse models have also outlined the obesity observed in 15% of AS children, albeit it is not the major characterisitics of AS.[48] The diagram on the right clearly highlights the results obtained from such mouse model studies of AS, where the absence of UBE3A accumulated in deficits of motor function and obesity. In addition, excessive drooling and feeding difficulties were also highlighted by mice models with inactivated UBE3A, reflected by defects in fluid consumption and licking.[49]
Signs and Symptoms
Angelman Syndrome presents a variety of different phenotypes, and the presented symptoms may vary at different ages.[50] In 1995 Williams et al. embraced the observed clinical features of AS in a consensus statement, in order to present an appliance for clinicians. The diagnostic criteria were updated in 2005.[40] The following table shows the most frequently occurring characteristics in AS patients:
4 characteristics appear in 100% of the cases:
- Delayed development: become apparent by the age of 6 to 12 months.
- Motion and or balance malfunction
- Behaviour patterns like unmotivated laughter, frequent excitement; often including body movements, hypermotoric behaviour
- Impairment of speech
3 characteristics appear in more than 80% of the cases:
- Abnormalities in head circumference, microcephaly
- Seizures
- EEG shows specific abnormal patterns[39][40]
Angelman Syndrome individuals show the most distinct disease pattern from 2 to 16 years of age. In most cases at least 8 of the symptom traits are shown.[50]
Behavioural Characteristics
A range of substantial characteristics appear in all AS cases, regardless of the genetic mechanisms, and are often responsible for diagnosing the syndrome. According to the original name, Happy Puppet syndrome, they have shown to exhibit traits such as a happy demeanor, with frequent laughter or smiling that can easily be triggered.[51] This behaviour can already be observed in 1 to 3 month old infants.[10] Uplifted hand flapping or waving motions are associated with laughter and excitement. Other characteristics manifesting in early childhood are sleep abnormalities with reduced sleep demand, and feeding problems. Breast and bottle feeding can be difficult due to non efficient attachment to the breast, tongue thrusting and uncoordinated sucking. Furthermore, there is an affection to crinkly and reflective surfaces, like some papers or plastic materials, especially water.[40][51]
Communication Skills
Communication is challenging for AS patients due to the fact that speech impairment is present in all cases. In the majority of affected people a lack of speech development is present. Only some of them have a minimal vocabulary consisting of two or three words, a few can speak in basic sentences. Some patients use gestures to articulate themselves, the Picture Exchange Communication System (PECS) or sign languages such as Makaton can be used for communication. Simple demands regarding their everyday occurrence can be understood by most patients.[40][51]
Clinical and External Characteristics
Developmental delays are present in all patients. Motor milestones are delayed with children managing to sit unsupported with 12 months and crawl or bottom shuffle at the age of 18-24 months. The average age for walking is 4 years,however, ranging from 18 months to 7 years.
The gait is characterised as slow, and flapping hands are a common trait. The legs remain stiff during walking, arms are inflected by the wrists and elbows. The movements can appear ataxic, jerky or lurching. The muscle tone shows abnormalities with truncal hypotonia and hypertonicity of the limbs and alert reflexes.[40][51]
The most striking external traits present in patients include broad-spaced teeth, and a wide mouth, light hair and eye colour, hypopigmented skin, deep set eyes, and a flat occiput.[40]
Seizures in Angelman Syndrome
85% of AS patients suffer from epileptic seizures during their first three years. The first appearance can be within one month up to 20 year old patients. In infants with AS epilepsy manifests with febrile convulsions and is hard to manage during childhood. The most common type of seizures within 25% of patients are myoclonic seizures, followed by atonic seizures as the second most frequent type, then 21% experience generalised tonic-clonic seizures and lastly atypical absences occur in 12% of patients.[52]
Angelman Syndrome in adults
Patients with Angelman syndrome possess normal secondary sexual characteristics, and puberty sets in at a usual time. Limb hypertonicity, and thoracic scoliosis lead to decreasing mobility. Oesophagus reflux might induce severe problems, and the majority suffers from obesity. Furthermore, facial traits such as a prominent lower lip macrostomia and mandibular prognathism get manifested in adults. In some cases the communication barrier can lead to frustration, resulting in aggressive behaviour. Nonetheless, patients gain a higher concentration span with age, and their nature becomes more quiet.[40]
Complications
Hypopigmentation and Ocular albinism
OCA2 gene, also known as the P gene, is closely located to the UBE3A gene. It encodes a protein vital to tyrosine metabolism, which plays a role in pigmentation development of skin, hair and eyes. However, AS caused by the large deletion of UBE3A leads to haploinsufficiency of OCA2 gene, resulting in hypopigmentation of the skin and the eyes. In some children with severe hypopigmentation, some form of albinism is suspected.[53] When AS is caused by another mechanism, no abnormality of the skin and eye pigmentation is observed. However, not all AS children with OCA2 gene deletion will present with obvious hypopigmentation, they may just exhibit lighter skin colour than their parents.[54]
Diagnosis
The diagnosis of Angelman Syndrome is a clinical diagnosis confirmed by laboratory testing.[9] This involves both prenatal and/or postnatal diagnosis.
Prenatal Diagnosis
Prenatal detection of deletions of 15q11.2-q13, Paternal UPD (Uniparental Disomy), Imprinting defects and UBE3A mutations are done through DNA/chromosome/FISH analysis of fetal cells obtained by either Chorionic Villus Sampling(CVS) or Amniocentesis.[39] DNA methylation analysis using Amniocytes is usually the first test carried out in molecular diagnosis of AS.[55] An abnormal result is followed up by either a FISH or array CGH analysis to search for a deletion on chromosome 15. If they produce a normal result, then UPD testing using DNA polymorphysims is carried out. If there is no evidence of UPD, other DNA analysis tests are used search for an imprinting centre deletion.[56] A normal DNA methylation analysis is followed by a UBE3A sequence analysis.[55] In addition, a parent of origin test is also done to determine if the genetic disruption is of maternal origin or paternal origin. If it is maternally derived the fetus is diagnosed with AS (a paternally derived genetic disruption is indicative of Prader-Willi Syndrome).[57]
The flow chart summarizes the normal procedure used for prenatal diagnosis of AS. More information on important laboratory tests used for the diagnosis of Angelman Syndrome are provided below:
- DNA Methylation Analysis
Identifies approximately 80% of AS affected individuals and is usually the first test to be ordered.[58] Molecular disruptions such as deletions, paternal UPD and imprinting defects on chromosome 15 all demonstrate abnormal methylation patterns by either southern blot analysis or PCR amplification of bisulphate-treated DNA.[39] An affected individual will only have an unmethylated (paternal) SNRPN (small nuclear ribonuclear protein-associated polypeptide N) allele compared with an unaffected individual who will have both a methylated and an unmethylated SNRPN allele.[59]
- FISH (Fluorescent In Situ Hybridization)
FISH analysis detects 5-7 Mb deletions in approximately 68% of affected individuals using detecting probes such as D15S10 and/or SNRPN.[58] It is also able to distinguish UPD from micro-deletions and imprinting defects. In simple terms, FISH analysis involves the binding of a fluorescent probe to a specific part of chromosome 15 and the detection of deletions within that region. FISH analysis is much more accurate in deletion detection compared to other high-resolution cytogenetics in the diagnosis of AS patients.[60] Array-based CGH is an advanced type of cytogenetic testing that can also be used to detect smaller deletions.[9]
- UPD (Uniparental Disomy) Detection
DNA Polymorphism testing detects paternal UPD in approximately 7% of affected AS individuals.[55] It is also able to distinguish between UPD and an Imprinting defect. It requires a DNA sample from the Proband and from both parents. A homologous or non-homologous whole-arm translocation of the long arms of chromosome 15 is a sound indication for the need for prenatal UPD testing.[55]
- IC (Imprinting Center) Analysis
This type of analysis is used when individuals have an abnormal DNA methylation pattern but show normal results for FISH, array CGH study and UPD analysis.[56] It is used to differentiate between the Imprinting defects caused by either microdeletions in the AS imprinting centre or by epigenetic mutations (that occur in maternal oogenesis or early embryogenesis).[59]
- Cytogenetic Testing
Is used to detect a cytogenetically visible chromosomal rearrangement (large deletion, translocation or inversion) involving the 15q11.2-q13 region, which occurs in less than 1% of affected individuals.[9]
- UBE3A Sequence Analysis
Sequence analysis of the UBE3A gene detects mutations in about 10% of the patients.[9] The test is used to detect either multiexonic (10 major coding exons of UBE3A) or whole gene deletions of UBE3A gene in individuals who produces a normal DNA methylation result but has characteristic clinical features of AS.[56] This is recommended as a prenatal diagnostic tool in families that include multiple AS affected individuals because the imprinted pattern can be inherited from distant relatives.[9]
Postnatal Diagnosis
Postnatal diagnosis is made though a combination of common clinical features of AS and UBESA gene sequence analysis.[55] The clinical features of AS include unsteady or shaky movements, muscle hypotonia, developmental delay and behavioral uniqueness such as frequent laughter/smiling and happy demeanor are features that are 100% consistent in all patients diagnosed with Angelman Syndrome.[61] These clinical features become apparent between 6 months to 2 years of age. The onset of early or persistent smiling characteristic of this syndrome begin around the age of 1-3 months, but is often overlooked during infancy.[10] Thus early diagnosis of AS during infancy period is difficult because the clinical indicators of the syndrome takes time to manifest and characteristic features such as finger and hand tremors and excessive smiling/laughter are often overlooked and not considered as possible indicators of this syndrome.
Differential Diagnosis
- Rett Syndrome
Some children with the clinical diagnosis of AS but no underlying genetic mechanism show mutations in MECP2 gene, corresponding to Rett Syndrome. This should be suspected in AS test negative girls in the first few years of life.[62]
- Mowat-Wilson Syndrome
Genetic mechanism is heterozygous deletion or truncation in ZFHX1B (SIP1) gene on 2q22. This presents with clinical features of severe intellectual disability, micrcephaly and seizures.[63]
- X-linked alpha-thalassemia/mental retardation syndrome (ATR-X)
This arises from mutations in XNP gene on Xq13. ATR-X presents with severe intellectual disability, no speech development and seizures are common.[64]
- Phelan-McDermid Syndrome/22q13 deletion syndrome
Deletions are usually submicroscopic and thus requires special molecular cytogenetic methods to confirm this deletion. Patients show moderate to profound intellectual disability and delay in speech development.[65]
Related Disease
- Prader-Willi Syndrome(PWS)
PWS is a result from the absence of paternally expressed 15q11-q13 gene as a corollary of de novo deletion, maternal uniparental disomy of chromosome 15 or an imprinting defect on paternal chromosome 15 leading to the silencing of paternal alleles.[35] It is characterized by hyperphagia, obesity in later infancy and early childhood and difficulty in feeding during early infancy.[35] There is also cognitive impairment to some extent, though some PWS patients may exhibit intelligence in the normal IQ range. Behavioral phenotypes include stubbornness, manipulative tendency, obsessive compulsive characteristics.[35]
Genetic Counselling
Genetic counselling is the process by which patients or relatives, at risk of an inherited disorder, are advised of the consequences and nature of the disorder, the probability of developing or transmitting it, and the options open to them in management and family planning. In the case for Angelman syndrome, types of risks associated are split into classes and formatted into the table below:[66]
| Class | Mechanism | Risk to siblings |
|---|---|---|
| Ia | De novo deletion | <1% |
| Ib | Deletion due to chromosome rearrangement | Up to 50% |
| II | Paternal uniparental disomy (UPD) | <1% |
| IIIa | Imprinting defect with IC mutation | Close to 100% if father has 15; 15 Robertsonian translocation |
| IIIb | Imprinting defect without IC mutation | Up to 50% (if mother has IC deletion) |
| IIIc | Mosaic imprinting defect | <1% |
| IV | UBE3A mutation | Up to 50% (if mother has mutation) |
| V | No identifiable genetic abnormality | ? |
Ia: Individuals with de novo deletion have 1% risk to siblings
Ib: Risk to siblings in individuals with chromosomal rearrangements is up to 50% [67]
II: Paternal UPD with no Robertsonian translocation has a risk to sibling of less than 1%
IIIa: The risk to siblings in individuals with AS from an imprinting defect with a mutation in the imprinting center (IC) is close to 100% if father has 15;15 Robertsonian translocation
IIIb: Individuals with AS resulting from an imprinting defect without IC mutation, but with a carrier mother has a high risk to siblings of up to 50%. The mother could be phenotypically normal due to paternal inheritance of the genetic defect [68]
IIIc: Individuals who are mosaic for the imprinting defect have less than 1% risk to siblings
IV: UBE3A mutation can be either inherited or de novo mutation. If the mother is a carrier for this genetic mutation, the risk to siblings is up to 50% [69]
V: Individuals who present with AS clinically, but have no known underlying mechanism have an unknown risk to siblings.
Treatment and Management
Even though at present there is no known cure for Angelman syndrome, there are a number of treatments available that may alleviate symptons and improve daily life activities.[9] The below table describes the problems and management options that are used:
| Problems encountered | Treatment and Management | ||
|---|---|---|---|
| Difficulty in feeding newborn AS babies | Use of special nipples may improve feeding[9] | ||
| Oesophagus reflux | Special motility medications requiried or upright positioning[9] | ||
| Seizures | Anticonvulsant medications[70][71] | ||
| Ocular problems | Visual assessment is vital to encourage interactions and minimize chances of self-harm and autism development[72] | ||
| Drooling in developmental delayed AS patients | Difficult to treat, but new surgical procedure on salivary duct reimplantation seems to be a promising alternative[73] | ||
| Unstable children | Physical therapy[9] | ||
| AS patients become less active with increasing age | Activity schedules to prevent scoliosis and obesity. Occupational therapy to stimulate fine motor and oral-motor control skills in conjunction to speech therapy[40] | ||
| Sleeping difficulty | Sedative medication in severe cases. Melatonin may be used to promote sleeping but some studies have found that it loses its therapeutic effects after several weeks in most AS patients[74] | ||
Prognosis
Life expectancy of AS patients seems to be normal[75], but there is still a high degree of developmental delay and limited expressive language skills.[76] This problem arises prominently in the assessment of the severity of coincidental medical conditions due to difficulties in communication. A visit to the dentist can become difficult if good communication is not conveyed between the parents, the dentist, and the child.[77] Some problems require extreme measures, such as the procedure of lens implantation was performed in both eyes of a person diagnosed with AS, due to the inability to wear spectacles.[78]
During adulthood, menstruation and puberty begin at around average age evidenced by females with Angelman syndrome that are fully capable of conceiving children.[79] Most adult patients can feed themselves, but needed help with many other daily activities. Main troubles which have shown in adults are a tendency to become obese and start developing thoracic scoliosis, which some adults with AS begin wheelchair bounded.[80]
Current and Future Research
Currently there is no cure for the treatment of AS, so much research is being carried out for therapeutic interventions. Findings in the last five years have suggested through various mice and fruit fly models that the absence of maternal UBE3A results in the same human AS features of neurological defects and seizures. However, more recently, studies have outlined that the symptoms of AS can be improved by modifying other gene products. In addition, restoring of a functional UBE3A protein into adult mice neurons have shown to reduce motor and neurological deficits and balance disorders. This shows promising research prospects in that improvements may be possible in human AS patients by restoring functional UBE3A proteins into their neurons.
Possible Treatment Pathways
- Cognitive and physical impairment
Although everyone is born with two copies of the gene UBE3A, one maternal and one paternal copy, it is only the maternal UBE3A that is used in the brain. Most AS patients have a genetic mutation on the maternal chromosome 15 that results in dysfunctional UBE3A.[81] The above research focused on turning on the father's copy of UBE3A to restore its functions. Furthermore, mouse models were utilized to show that alpha-CaMKII could be increased to compensate the loss of UBE3A and restore compromised mental and physical abilities. However, this finding still requires much more research.[82]
- Epilepsy
There is research being done on improving certain conditions that are involved in AS by nutritional benefits. The use of corticosteroids[83], valproate[84] and clonazepam[85] and having a ketogenic diet[86] has all been shown to aid in the treatment of epilepsy. It has also been reported to be efficient in the treatment of other conditions, such as behavioural disorders, reduction in seizures, ataxia, sleep patterns, developmental progress and sleep disturbances. Levetiracetam[87] could be used in such a situation, especially when the patient has an intolerance to valproate and clonazepam.
- Betaine and Folic acid
This double-blind placebo-controlled study was done to examine the potential therapeutic role of betaine and folic acid in the treatment of the clinical phenotypes of AS. The aim of this protocol was to increase methylation and activate the paternally inherited UBE3A in hope to compensate for the inactive maternal UBE3A. Although there were no major differences in the control and treatment groups, a small number of patients in the treatment group showed some positive outcomes.[88]
Glossary
albinism: A congenital disorder distincted by the complete or partial absence of pigment in the skin, hair and eyes due to defect of an enzyme involved in the production of melanin.
allele: Dissimilar variant of one gene. Organisms have two alleles for each gene inherited from their parents, one from each of them.
alpha-CaMKII: (alpha calcium calmodulin kinase II) Compound involved in synaptic plasticity, one of the most important theories behind learning and memory. Ability of a synapse between two neurons to change in strength due to the use or disuse of transmission.
Amniocytes: Cells that are shed by the fetus during fetal development to the surrounding amniotic fluid. They are a source of fetal DNA.
Amniocentesis: (AFT or amniotic fluid test) is a prenatal diagnostic test used to detect chromosomal abnormalities in the developing fetus by extracting amniotic fluid (a source of fetal DNA) surrounding the fetus.
AMPA: (2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl)propanoic acid) Compound that binds to the AMPA receptor to imitate glutamate neurotransmitter.
Arc: Activity-Regulated Cytoskeleton-associated protein; involved in learning and memory.
ataxia: Lack of coordination in muscle movements.
atonic seizures: A abrupt loss of muscle tone, this can lead to the limbs loosing strength, or the head may drop to the side. The patient remains conscious during this kind of epileptic seizure.
atypical absences: Seizure leads to unconsciousness in most cases.
autism: A neural development disorder defined by limited social interaction and communication.
betaine: Also known as betaine anhydrous or trimethylglycine (TMG), assists the body in metabolising the amino acid, homocysteine. Can be consumed via beets, broccoli, grains, shellfish, and spinach.
cerebellum: Region of the brain that plays an important role in motor control. It is also involved in some cognitive functions such as attention and language.
Chorionic Villus Sampling: A prenatal diagnostic test used to detect chromosomal abnormalities in the developing fetus by extracting and testing chorionic villus (placental tissue).
chromosome: Structure consisting of coiled DNA with DNA associated proteins such as histones.
clonazepam: A psychoactive drug having muscle relaxant properties and treats anxiety and epilepsy.
corticosteroid: A type of steroid hormones that are produced in the adrenal cortex.
cytogenetics: Study of the structure, function and abnormality of the cell, especially the chromosome.
deletion: Lack of a part of the DNA (deoxyribonucleic acid is a nucleic acid containing information for protein synthesis), can vary form a single base to a hole gene.
de novo mutation: Change in a gene of a family member for the first time due to a mutation in the egg or sperm of the parents or in the fertilized egg (zygote).
disorder: A mental or physical health disturbance or dysfunction.
EEG: Electroencephalography is a means of measuring electrical activity along the scalp by measuring the total electrical charges fired by thousands or millions of neurons.
epilepsy: Neurological disorder characterised by recurrent impairment of brain function that can cause seizures or unconsciousness.
E6-AP: E6-AP ubiquitin-protein ligase is an enzyme encoded by UBE3A which adds ubiquitin chain to target proteins to be degraded by proteasomes.
febrile convulsions: The occurrence of high fever, especially in children.
FISH: Fluorescent in situ hybridization is a technique used to identify and localize the presence or absence of DNA sequences on the chromosome. This is achieved by using fluorescently labelled DNA sequences which then binds to complementary DNA sequence on the chromosome. After hybridization (attachment), chromosome of interest will fluoresce under the microscope.
folic acid: B vitamin that helps the body make healthy new cells. Can be consumed via leafy green vegetables, fruits, dried beans, peas and nuts.
gait: A manner of walking, stepping or running.
gene: A molecular unit of heredity of a living organism.
hippocampus: Area buried deep in the forebrain that helps regulate emotion and memory
hyperphagia: (Also referred to as Polyphagia) Excessive ingestion of food beyond the dietary and energy requirements of the body.
hypermotoric: Sudden appearance of uncontrolled movements such as striking or kicking inappropriately.
hypertonicity: Increase of muscle tension and a disability to stretch leading to an impairment of motor activity.
hypopigmentation: A lack of melanin pigments or melanocytes affecting the colour of the skin, hair and iris. Features appear to be light-coloured due to loss of skin colour.
hypotonia: Decrease in the ability to stretch passively, due to a low muscle tone.
imprinting: This is a process to describe the modification of a maternally or paternally derived chromosome, which consequently influences the expression of certain genes on the altered chromosome.
ketogenic diet: A high-fat, low-carbohydrate diet that is used to treat epilepsy in children.
levetiracetam: An anticonvulsant drug used to treat epilepsy.
locus: Location of a gene on a chromosome(loci: plural for locus).
LTP: Long-term potentiation shares common features with long term memory. It strengthens and enhances signal transmission between neurons by improving the communication between two neurons.
macrostomia: A wide-spaced mouth.
Makaton: Language programme using symbols and signs to support speaking.
mandibular prognathism: The abnormal protrusion of the lower jaw.
maxillary hypoplasia: The underdevelopment of the upper jaw.
melatonin: A hormone secreted by the pineal gland.
microcephaly: The circumference of the head is less than average due to abnormalities of the nervous system. This abnormality can be present at birth or emerge during the first few years of life and reduces both life expectancy and the cognitive functions of the individual affected.
microdeletion: A genetic mutation in which a part of a chromosome or a sequence of DNA is missing.
mosaic: tissues or cells containing different mixture of genetic sequences
mutation: Event that changes the DNA (deoxyribonucleic acid is a nucleic acid, containing information for protein synthesis) or RNA (ribonucleic acid is a type of nucleic acid transmitting the information from the DNA to the proteins ) of a gene permanently.
myoclonic seizures: A quick jerk of the skeletal muscle, due to a irregular brain activity.
neurogenetic: Genetic basis of the nervous system.
neuron: Cells of the nervous system that can be electrically excited to transmit information by electrical and chemical signaling
neurotransmitter: Transmits neuronal signals to a target cell. They are first packaged in vesicles just beneath the membrane of presynaptic axon terminals and released into the synaptic cleft (space between the two interacting neurons) and enter the postsynaptic dendrite.
occiput: The back portion of the head.
oesophagus reflux: Impairment of a sphincter in the lower end of the oesophagus leads to the back flow of stomach acid into the oesophagus. Heartburn is a typical symptom.
olfactory tract: It is a pathway for the sense of smell containing axons from some of the neurons in the olfactory bulb.
paediatrician: Physicians that specialise on infants, children and adolescents.
paternal UPD: Inheritance of two copies of a chromosome from the father and none from the mother.
phenotype: Visible traites of an organism determined by genetic and environmental factors.
Picture Exchange Communication System (PECS): Individuals communicate using images instead of words. It is frequently used by children with autism, to articulate their needs and wishes or to make comments about something.
Proband: Is the person (or animal) being studied or reported on in a genetic investigation. Most commonly it refers to the first affected family member who requires medical help for a genetic disorder.
Purkinje cells: Large neuron with many branching extensions that is found in the cortex of the cerebellum of the brain and plays a fundamental role in controlling motor movement
Robertsonian translocation: Rearrangement of the chromosome, occurs in 5 chromosome including 13,14,15,21 and 22. This type of mutation occurs in chromosomes with the centromere not at the centre, resulting in long and short arm chromosomes. Translocation breaks the chromosome at the centromere so the long arm attaches to the other long arm, and the same for the short arm happens. Cell division will result in the loss of the short arms as they do not contain important genes.
seizure: Abnormal electrical activity in the brain that can result in a variety of physical symptoms like convulsion, body shaking, loss of consciousness, confusion, and mood changes.
synapse: Allows transmission of signal between neurons for effective neuronal function.
thoracic scoliosis: The spine is curved laterally(from side to side) in the thoracic region.
tonic-clonic seizures: This type of seizure is associated with unconsciousness due to electrical discharges in a big proportion of the brain.
ubiquitin: A polypeptide found in all eukaryotic cells, that participates in a variety of cellular functions including protein recycling.
Uniparental disomy: (UPD) the inheritance of two copies of a chromosome pair or part of a chromosme from one parent and no copy from the other parent.
valproate: A mood-stabilizing drug that is also used for treatment for epilepsy and bipolar disorder.
Helpful Links
- Australia
- America
- Search Pubmed
- OMIM entries for Angelman Syndrome
Related Links
Animal Models
- Mouse Knockout - this page explains the current use of mouse models in disrupting genes to elucidate their genetic function, including the mouse gene knockouts for alpha calcium-calmodulin kinase II (alpha CaMKII)
- 2009 Group Project 4 - this page gives an extensive overview on the history of the mouse model and its various use in embryology and genetics. In addition, it also describes mouse knockout and the role they play in current research of various human diseases
- Fly Development - this page describes the use of drosophila flies as developmental models
- 2009 Group Project 2 - this page gives an extensive overview on the history of the fly model and its various use in embryology and genetics
Diagnosis
- Chorionic villus sampling - this page explains the process of chorionic villus sampling, its disadvantages and gives a brief overview of recent findings
- Amniocentesis- this page shows some of the recent findings in this area
References
- ↑ 1.0 1.1 1.2 1.3 1.4 <pubmed>18754889</pubmed>
- ↑ http://www.ncbi.nlm.nih.gov/books/NBK22221/
- ↑ 3.0 3.1 3.2 <pubmed>7573182</pubmed>
- ↑ 4.0 4.1 4.2 <pubmed>8703225</pubmed>
- ↑ <pubmed>21592595</pubmed>
- ↑ <pubmed>21484597</pubmed>
- ↑ http://www.angelmansyndrome.org/home.html
- ↑ http://www.bbc.co.uk/health/physical_health/conditions/angelman1.shtml
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 9.8 9.9 <pubmed>19455185</pubmed>
- ↑ 10.0 10.1 10.2 10.3 10.4 <pubmed>20981772</pubmed>
- ↑ http://www.angelman.org/stay-informed/facts-about-angelman-syndrome---7th-edition/harry-angelman-and-the-history-of-as/
- ↑ 12.0 12.1 12.2 12.3 <pubmed>20211139</pubmed>
- ↑ http://www.angelmanproject.com/history.htm
- ↑ <pubmed>9808466</pubmed>
- ↑ <pubmed>17259980</pubmed>
- ↑ <pubmed>20184619</pubmed>
- ↑ <pubmed>18664077</pubmed>
- ↑ <pubmed>15741136</pubmed>
- ↑ <pubmed>17019625</pubmed>
- ↑ <pubmed>16906556</pubmed>
- ↑ <pubmed>16492624</pubmed>
- ↑ 22.0 22.1 <pubmed> 8988171</pubmed>
- ↑ <pubmed>19455185</pubmed>
- ↑ <pubmed>10364509</pubmed>
- ↑ <pubmed>15668046</pubmed>
- ↑ <pubmed>8988171</pubmed>
- ↑ <pubmed>8988172</pubmed>
- ↑ <pubmed>12684449</pubmed>
- ↑ 29.0 29.1 <pubmed>9857172</pubmed>
- ↑ <pubmed>11895368</pubmed>
- ↑ <pubmed>20398390</pubmed>
- ↑ <pubmed>9288101</pubmed>
- ↑ <pubmed>9288088</pubmed>
- ↑ 34.0 34.1 <pubmed>9808466</pubmed>
- ↑ 35.0 35.1 35.2 35.3 35.4 <pubmed>20668179</pubmed>
- ↑ http://www.nature.com/ejhg/index.html/
- ↑ 37.0 37.1 <pubmed>11748306</pubmed>
- ↑ <pubmed>9546330</pubmed>
- ↑ 39.0 39.1 39.2 39.3 <pubmed>20445456</pubmed>
- ↑ 40.0 40.1 40.2 40.3 40.4 40.5 40.6 40.7 40.8 <pubmed>16470747</pubmed>
- ↑ 41.0 41.1 <pubmed>15253756</pubmed>
- ↑ <pubmed>16023557</pubmed>
- ↑ <pubmed>18996915</pubmed>
- ↑ <pubmed>18701717</pubmed>
- ↑ <pubmed>16433693</pubmed>
- ↑ <pubmed>21235769</pubmed>
- ↑ <pubmed>20808828</pubmed>
- ↑ <pubmed>17259980</pubmed>
- ↑ <pubmed>18413322</pubmed>
- ↑ 50.0 50.1 <pubmed>7625442</pubmed>
- ↑ 51.0 51.1 51.2 51.3 <pubmed>12566516</pubmed>
- ↑ <pubmed>20398390</pubmed>
- ↑ <pubmed>12749060</pubmed>
- ↑ <pubmed>8302318</pubmed>
- ↑ 55.0 55.1 55.2 55.3 55.4 <pubmed>18059071</pubmed>
- ↑ 56.0 56.1 56.2 <pubmed>9792887</pubmed>
- ↑ <pubmed>17020468</pubmed>
- ↑ 58.0 58.1 <pubmed>7618904</pubmed>
- ↑ 59.0 59.1 <pubmed>9634532</pubmed>
- ↑ <pubmed>1511093</pubmed>
- ↑ <pubmed>18830393</pubmed>
- ↑ <pubmed>17980307</pubmed>
- ↑ <pubmed>17958891</pubmed>
- ↑ <pubmed>11449489</pubmed>
- ↑ <pubmed>18505557</pubmed>
- ↑ <pubmed>20459762</pubmed>
- ↑ <pubmed>9557895</pubmed>
- ↑ <pubmed>11283796</pubmed>
- ↑ <pubmed>11258627</pubmed>
- ↑ <pubmed>19605773</pubmed>
- ↑ <pubmed>19453717</pubmed>
- ↑ <pubmed>20656169</pubmed>
- ↑ <pubmed>17699119</pubmed>
- ↑ <pubmed>19379289</pubmed>
- ↑ <pubmed>19455185</pubmed>
- ↑ <pubmed>17019625</pubmed>
- ↑ <pubmed>18271768</pubmed>
- ↑ <pubmed>20656169</pubmed>
- ↑ <pubmed>11258627</pubmed>
- ↑ <pubmed>9072912</pubmed>
- ↑ http://www.cureangelman.org/what-hope.html
- ↑ <pubmed>17259980</pubmed>
- ↑ <pubmed>19666884</pubmed>
- ↑ <pubmed>19453717</pubmed>
- ↑ <pubmed>19605773</pubmed>
- ↑ <pubmed>20880305</pubmed>
- ↑ <pubmed>17326790</pubmed>
- ↑ <pubmed>20635355</pubmed>