2010 Group Project 6: Difference between revisions
| Line 116: | Line 116: | ||
[[File:Karyotype_Down_syndrome.gif|thumb|right]] | [[File:Karyotype_Down_syndrome.gif|thumb|right]] | ||
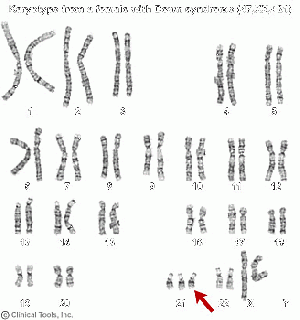
'''Down Syndrome''' is a chromosomal disorder where an extra 21st chromosome or part of it is present, this disorder associates with some impairment of cognitive ability, physical growth and a particular set of facial characteristics. | '''Down Syndrome''' is a chromosomal disorder where an extra 21st chromosome or part of it is present, this disorder associates with some impairment of cognitive ability, physical growth and a particular set of facial characteristics. | ||
Revision as of 00:11, 15 September 2010
Maternal serum alpha-fetoprotein
Introduction
brief outline on what will be covered
Background information
Maternal Serum Alpha Fetoprotein (MSAFP) screening is an non-invasive procedure in which the mother’s blood is taken and alpha-fetoprotein levels are measured. It is usually carried out during the 2nd trimester and is used to detect abnormalities such as neural tube defects, more specifically anencephaly spina bifida,encephalocele, open ventral wall defects such as gastroschisis as well as Down’s Syndrome.
What is Alpha fetoprotein
Alpha-Fetoprotein (AFP) is an embryo specific glycoprotein which is produced during the early stages of development by the liver, yolk sac as well as a small amount being produced by the gastrointestinal tract. AFP in adults is functionless as levels decrease drastically after birth with very low traces of AFP found in the average older adult with the only women experiencing spikes occurring in AFP levels during the onset of pregnancy and it is in fact through the testing of the blood of pregnant women, that AFP levels can be measured. The function of AFP itself is unknown but due to its similarity to albumin it has been hypothesized that AFP could be a carrier protein or may even play a role in the metabolism of bilirubin. Furthermore, it has been observed that it does play a role in the embryonic and early fetal stages of development as fluctuating levels of AFP indicate the presence of abnormalities within a fetus.
AFP has a molecular weight of around 70,000 daltons and is a single chain alpha globulin that has 590 amino acids and is estimated to have a 5% make up of carbohydrate content. It should be noted that it has an uncanny resemblance to another protein called albumin. The AFP level in human fetal serum is highest during the 13th week of gestation, where it may reach the level of several mg per ml, and accounts for almost a third of the total serum protein. Normal human serum also contains traces of AFP, however fetal AFP level is almost one million times higher than the adult level.
Ranges and Levels
AFP blood test ranges will vary between groups of people when factors such as age and sex come into play. However, a general trend for normal AFP levels in people is as follows:
Men: 0 - 20 ng/mL
Women: 0 - 20 ng/mL
Women (Pregnant): Ranges can be separated into First Trimester and Second Trimester Results as presented below.
First Trimester
- 200 - 400 mg/dL
Second Trimester
- 14 weeks of gestation: 25.6 ng/mL
- 15 weeks of gestation: 29.9 ng/mL
- 16 weeks of gestation: 34.8 ng/mL
- 17 weeks of gestation: 40.6 ng/mL
- 18 weeks of gestation:47.3 ng/mL
- 19 weeks of gestation: 55.1 ng/mL
- 20 weeks of gestation: 64.3ng/mL
- 21 weeks of gestation: 74.9 ng/mL
It should also be noted that 'normal' values are around 200% higher is women with twin pregnancies. Furthermore, it was found that the 'normal' value of AFP was 15% higher in African Americans when compared to Caucasians.
AFP in Pregnancy
Highest maternal AFP concentration occurs in the mid third trimester of the pregnancy where the mean level is 150-250ng/ml. The concentration of AFP in maternal serum at any moment of gestation development seems to be related to the AFP level in the fetal circulation as well as in the placental size.
Instances of abnormal AFP values (too high as well as too low) can partly been explained by physiological deviations from the expected normal pregnancy eg. in cases of under- or overestimated gestational age and multiple pregnancies. In other instances it have been found to indicate the presence of various fetal morphogenetic defects, such as open NTD (neural tube defect), hereditary congenital nephrosis (Finnish type), omphalocele, pilonidal sinus, esophageal atresia, and others.
The maternal AFP level has often reported to be increased in pregnancies where the fetus has a neural tube defect.
The Optimal practical time for detecting open spinabifida by measuring materal serum AFP is at 16-18 comepleted weeks of pregnancy. In Wald et el. (1977)’s sample of patients, 88% of cases of anencephaly, 79% of cases of open spina bifida, and 3% of unaffected singleton pregnancies had maternal serum AFP levels equal to or greater than 2.5 times the normal median. Because there is a certain degree of overlapping between the maternal AFP levels in pregnancies with and without fetal NTD, the AFP estimation in materal serum cannot per se serve as a specific diagnostic test, but it seems to be a useful screening test so as to select certain symptom-free women for further diagnostic procedures such as ultrasonography, amniocentesis, and amniography.
Maternal Serum Alpha Protein as a Screening Test
It should be made clear that MAFP is not a diagnostic test and is used only for screening purposes with further testing always necessary for any sort of accurate diagnosis to take place. Furthermore, MAFP is a screening test that is carried out during the second trimester whereas other tests may be carried out during the first trimester and are more accurate. It is part of two tests, one called the Triple Screen Test which is a battery of tests that measure AFP levels as well as human chorionic gonadotropin (hCG) and unconjugated estriol uE3 and a second series of tests known as the Quadruple Screen Test that tests AFP, hCG, uE3 as well as Inhibin A which is a hormone that is released by the placenta. These tests also take into account age, ethnic background, weight as well as the babys' gestational age. Currently, there are no known risks or side effects that have been associated with the MSAFP screening test except for any discomfort involved with the drawing of blood from the patient.
Previously, the use of MSAFP as a screening test was called into question in regards to its accuracy as well as its cost effectiveness as a medical program from the perspective of a managed health care system (note that this was from the view of an American health insurer). It was concluded that MSAFP would not result in a cost savings to the insurer however, it would be cost-justified when viewed from the perspective of society when other reasonable assumptions where taken into account. In Australia, the MSAFP screening test isn't as commonly used as other first trimester tests however, it is one of the few pre-natal tests that is covered by medicare whereas all the first trimester tests available are payed by the patients themselves.
MSAFP Screening Test Procedure
Advantages and Disadvantages of MSAFP
Advantages:
- In Australia, the MSAFP test is covered by medicare, thus, it is a financially viable test
- When used as part of the Triple or Quadruple Tests, MSAFP is a non-invasive screening test
Disadvantages:
- The accuracy of MSAFP screening is not as reliable as other pre-natal diagnostic tests due to the presence of false-positive results. The real danger, is the follow up of an invasive diagnostic test such as amniocentesis which has a 1 - 2% rate of fetal loss
- MSAFP can only be performed during the 2nd trimester between weeks 15 - 20
Accuracy of the MSAFP Sceening Test
The accuracy of MSFAP has always been a controversial issue with around a claim of a 5% false-positive rate, however more recent data suggests that around 80% of positive tests where the baby is in actual fact unaffected by any abnormalities that may have been expressed. Taking into account this discrepancy of results, the standard procedure is to repeat the MSAFP test and following a second positive result, ultrasound and/or amniocentesis is used.
Disorders that MSAFP indicates
Spina bifida and Anencephaly which are neural tube defects where the neural tube fail to close properly. When the neural tube fails to close at the cranial end is call anencephaly whilst failure to close at the caudal end is call spina bifida.
MSAFP level testing is good for detection for spinal bifida and anencephaly. While ultrasonic examination is capable of diagnosing anencephaly in utero, it is unlikely to be widely available as a screening procedure for all pregnant women, and there is no satisfactory way of diagnosing spina bifida in early pregnancy. AFP estimations can be performed early in pregnancies without the knowledge of the outcomes of the pregnancies. In Wald’s study (1974) it was found that the pregnancies which turn out to have either spina bifida or anencephaly have a higher level of MSAFP than those in the control pregnancies matched for maternal age, parity, and length of gestation. Even though it is impossible to say with complete confidence that a fetus is unaffected if the MSAFP did not rise above normal levels, by measuring the maternal serum AFP levels we can say with a defined degree of confidence the likelihood of a pregnancy leading to spina bifida or anencephaly.
Down Syndrome is a chromosomal disorder where an extra 21st chromosome or part of it is present, this disorder associates with some impairment of cognitive ability, physical growth and a particular set of facial characteristics.
MSAFP levels are also sufficient to form the basis of a screening test for fetus with Down syndrome as they are significantly lower in pregnancies associated with Down syndrome than in unaffected pregnancies. Using a MSAFP cut-off level of 0.5 multiples of median at 14-20 weeks of gestation, excluding any of these that ultrasound cephalometry shows to have been due to overestimation of gestational age, Cuckle (1984) identified 21% of pregnancies with Down syndrome as well as 5% of unaffected pregnancies.
If amniocentesis were offered to all women aged 38 or above and to younger women with serum AFP below specific maternal age-dependent cut-off levels the percentage would increase to 40% for picking up pregnancies with Down syndrome and 6.8% unaffected pregnancies.
The low AFP levels in pregnancies with Down syndrome cannot be explained by known factors associated with low AFP (i.e. maternal weight, birth weight, fetal sex, maternal diabetes mellitus). However it suggests that less AFP is produced by the fetal liver (being the main source of AFP at this time of the pregnancy) than in unaffected pregnancies.
Outside of pregnancy, the MSAFP test may be performed as part of a routine health screening especially if there is the potential of the presence of a disease or toxicity such as a liver carcinoma, or testicular cancer.
Other instances where maternal serum AFP levels are elevated includes:
1) Fetus effected by hereditary cogenital nephrosis of the Finnish type - an hereditary, autosomal recessive disease which leads to death in early infancy.
2) Meckel syndrome early enough in gestation to permit termination
3) Intrauterine death
4) Multiple gestations such as twin pregnancies or triplets
In conclusion, aberrant AFP values in maternal serum samples are to be regarded as unspecific warning signals, which sometimes may be observed weeks in advance of any other clinical or biochemical symptom of a deviant fetal development. Therefore, more specific diagnostic measures must be employed to verify and characterize the type of pregnancy disturbance that may exist. Nevertheless, the determination of AFP in maternal serum provides valuable information concerning the progress of pregnancy. All pregnant women having had a neural tube defect fetus before should be offered determination of amniotic fluid AFP at about the 16th week of gestation.
References
Nadel A. S, MD. Green J.K. Holmes L.B, MD. Frigoletto F.D,Jr,MD. Benacerraf B.R, MD. The New England Journal of Medicine; Absence of need for amniocentesis in parents with elevated levels of maternal alpha fetoprotein and normal ultrasonographic examinations. Volume 323 August 30, 1990.
Wald N.J., Cuckle H, Brock J.H., Peto R, Polani P.E., Woodford F.P. (1977). Report of the UK Collaborative Study on Alpha-Fetoprotein in Relation to Neural-Tube Defects: Maternal serum-alphafetoprotein measurement in antenatal screening for anencephaly and spina bifida in early pregnancy. Lancet, 1, 1323
Wald N.J., Brock J.H., J. Bonnar. (1974). Prenatal diagnosis of spina bifida and anencephaly by maternal serum-alpha-fetoprotein measurement: A controlled study. Lancet, 303, 7861, p765-767
2010 ANAT2341 Group Projects
Project 1 - Ultrasound | Project 2 - Chorionic villus sampling | Project 3 - Amniocentesis | Group Project 4 - Percutaneous Umbilical Cord Blood Sampling | Project 5 - Fetal Fibronectin | Project 6 - Maternal serum alpha-fetoprotein | Group Assessment Criteria
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 18) Embryology 2010 Group Project 6. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/2010_Group_Project_6
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G