Paper - The embryology of the human hip joint (1943)
| Embryology - 20 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Strayer MMJr. The embryology of the human hip joint. (1943) Yale J Biol. Med. 16(1): 13–26.6. PMCID: PMC2601352
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
The Embryology of the Human Hip Joint
Luther M. Strayer, Jr.
From the Department of Orthopedic Surgery, Harvard Medical School, and the Department of Pathology of the Children’s Hospital, Boston, Mass.
(1943)
Introduction
This paper has arisen from a need for more exact knowledge of the development of the normal human hip joint than could be gained from the literature and text-books of embryology. It was found that, in spite of Bardeen’s excellent work in 1901 and 1905, misconceptions of the details of development are to be found in texts and articles’ published in 1933 and 1935. The illustrations and photographs of models available do not show detail. It was therefore felt that a review of the subject and a presentation of photomicrographs would be of value. The observations have also been made with the hope of correlating the normal processes of development with the number of pathological affections of the hip in which the congenital factor has been thought to be of etiological significance.
The formation of the acetabulum, head and neck of the femur, the ligamentum teres, retinacula of Weitbrecht, glenoid labrum, the synovial membrane, and the transverse acetabular ligament are described in detail. The entry of blood vessels into the head and neck of the femur and the suggested predisposition of the head of the femur to dislocation in one embryo are noted in brief.
The illustrations of the 6.75 to 45 mm. embryos have been selected from the excellent material available in the Minot Embryological Collection of the Harvard Medical School. Six embryos, 45 mm., 70 mm., 81 mm., 90 mm., 167 mm., and 237 mm. respectively, were cut in serial sections in the Laboratory of Pathology of The Children’s Hospital, Boston. The observations on blood vessels and later development have been made from these sections. The crown rump measurement as correlated by Keibel and Mall has been used as a standard.
Literature
Writing on the embryology of the extremities Kolliker, in 1861, made a statement to the effect that “the ground work of all the bones of the extremities arises at first from one single undifferentiated body. When cartilage formation first begins throughout this mass, it will organize itself into several parts so that it will develop into just as many single cartilages or bones.” This simple forceful statement is the essence of skeletal embryology.
We now know that the “single undifferentiated body” includes all the connective tissue elements of the limb.
Much that has been written on the development of the hip is derived from comparative anatomy. Bland-Sutton?* concluded from such studies that the ligamentum teres femoris represents the remains of an extra-articular ligament or muscle related to the adductor group which was gradually enclosed within the joint as the femur became more adducted and the erect posture was assumed. Moser?” illustrated the hip joint of 30, 34, and 47 mm. human embryos, and in contrast to Bland-Sutton’s contention correctly concluded that whatever its origin of development in animals, in man this ligament develops in situ; also that the joint space is formed behind, and between, the ligament and the head of the femur at the same time as elsewhere in the joint. Keith’® presents his conception of the process as follows: “The ligamentum teres, the best example of an intra-articular ligament appears in the human fetus as part of the capsule of the joint; in reptiles this fetal form is retained. ‘The round ligament is isolated during development of the head of the femur, which expands as a wing on each side of the ligamentum teres and by fusion of the wings, isolates it from the capsule. The reflected ligament on the under surface of the neck is the part of the capsule with which the ligamentum teres was continuous.” Although cited by Keith, Walmsley? is in direct opposition to Keith when he concludes “In the human embryo the ligamentum teres is completely free at the first appearance of the joint cleft .. . We believe these facts to indicate that the inferior femoral retinaculum (of Weitbrecht) does not represent, in whole at least, the persistent remains of the ligamentum teres, but that this mesentery would and possibly does occur as the retinaculum of the acetabular fat pad (or Haversian gland) which arises in relation to the extra-acetabular part of the ligamentum teres and invests the blood vessels passing through the acetabular gap to the acetabular synovial pad.” The retinacula of Weitbrecht and the Haversian gland have no common origin according to our observations. Parsons supports Bland-Sutton’s contention.
There is another misconception in modern literature. Hagopoff® examined two sheep embryos 20.4 and 20.7 mm. in length and one human embryo of 25 mm. With this material in hand Hagopoff conceived of the diaphysis of the femur advancing, by enlargement, to meet the three elements of the pelvis; the head appears, and upon it is modeled the acetabulum except at those places where the cotyloid vessels by their presence prevent development, such as in the acetabular fossa and the transverse acetabular ligament. He also states that the apophysis of the pubis or pubic primordium remains independent of the other pelvic primordia so as to take practically no part in the formation of the acetabulum. ‘These erroneous conceptions have modern protagonists. Frazer® has explained the formation of the ligamentum teres as follows: “In its early stages, the ilium and ischium are alone concerned in the articulation, the synovial lining passing off them on to the capsule which is attached around their surfaces. The pubis is covered by these fibers and has no articular area. In the next stage the covering fibers are destroyed and the pubis has acquired an articular surface. This extends and the front part of the original ischial capsule is caught, so to speak, between the extending surface and the ischium. ‘These fibers persist and remain attached to the ischial region but on their surface the synovial cavity has extended as shown in the last diagram, and has joined the older cavity below as well, passing between the femur and the lower portion of attached capsule. Thus a synovial tunnel is formed wider below, where it includes the attachment of the fibers, and narrowed at its femoral end where it is fastened to the fovea.” Stewart" incorporates three separate misconceptions in his conclusions on the development of the hip. They are quoted here under the numbered headings in his article. “1. The femur and os innominatum are differentiated from a common skeletal anlage by a mesoblastic invasion ... 4. The head is pushed off toward the acetabulum on a cervical bud. 5. The ligamentum teres is formed by flanking growths of the rectangular head until finally the ends join leaving the central ligamentum teres and a hemispherical femoral head .. .”
The authors leading up to Bardeen’s excellent work published in 1901 and 1905 are: Hencke and Reyher,® who did not give an account of the development of the hip joint as is sometimes stated but described the extremities of 18-20 mm. human embryos including the articulation at the hip. They remarked that they could not observe the development of the os innominatum in three parts. Schulin®” supplied the first illustrations of the development of the hip, in schematic drawings of coronal sections, of 7 and 25 cm. embryos, 1- and 6-year-old children, and an adult, respectively. Moser,!7 with his interest focused on the ligamentum teres, illustrated the hips of 30, 34, and 47 mm. human embryos and described the joint at these stages.
The models and descriptions of Bardeen in his studies on the development of the human skeleton from human embryos, 4 to 50 mm. in length, are beautifully executed. His description of the hip is as follows: ‘The hip joint is represented at first by a dense mass of sclero-blastema. The development of the acetabulum by ingrowth and fusion of processes from the iliac, ischial and pubic cartilages has already been described.*
The cartilaginous joint cavity is at first quite shallow. But extension of the cartilage into the blastemal tissue which passes from the pelvis over the head of the femur serves greatly to deepen it on all sides except in the region of the cotyloid notch. “The joint cavity is at first completely filled with a dense blastemal tissue, while the embryo is growing from 20-30 mm. in length, cavity formation begins in the tissue between the cartilaginous floor of the acetabulum and the head of the femur. ‘The first stage in the process is marked by a condensation of the capsular tissue immediately bordering upon the joint and of the perichondral tissue which at this stage covers the cartilages on their articular surfaces as well as elsewhere. In the region of the ligamentum teres a fibrous band is likewise differentiated from the blastema of the joint. The rest of the tissue becomes looser in texture and ultimately is absorbed.”
“While the human embryo is growing from 15-20 mm. in length there occurs a rapid development of the pelvic cartilages. About the head of the femur each gives rise to a plate-like process. The fusion of these processes produces a shallow acetabulum. Those from the ilium and ischium are larger than that of the pubis and fuse with one another before the pubis fuses with them. The proportional areas of the acetabulum to which each pelvic cartilage contributes seem to be essentially the same as those later furnished by the corresponding pelvic bones, 2/5 ischium, 2/5 ilium, 1/5 pubis. While growing about the hip joint each of the pelvic cartilages has a centrifugal growth within the blastemal pelvis. . .”
The foregoing is all that the student of embryology has available for reference. ‘There are many papers on the development of the hip in the fetal period of intra-uterine life, some of which tabulate elaborate measurements of the articulating surfaces. One of the best of these writers is Harrenstein,” who subscribes in large part to LeDamany’s theory!” 1% ™ of congenital dislocation. However, none of these authors has undertaken the study of the embryo, and there are no articles in which the detail of hip embryology may be found.
Observations
The origin of the limb bud. In embryos 3 to 4 mm. in length a small protuberance appears on the anterior and lateral aspect of the body wall, at the level of the lumbar and Ist sacral segments. It is much more anterior than would be thought, and is in close relation to the coelom, the wolffian duct, and posteriorly with the somites in this region. The exact origin of the mesoderm which fills this protuberance has not been determined. Bardeen says it probably arises from the dorsal unsegmented mesoblast. Lewis states” that we know certainly that the myotomes do aot enter into the formation of limb muscles although they are responsible for those of the trunk.
The mass as a whole contains all the elements necessary to produce the skeleton, the synovia, the ligaments of the joint, the muscles, and their intermuscular septa and tendons, and at this stage it is devoid of blood vessels and nerves.
After the blastemal stage has begun, each primordium, such as the os innominatum or femur, should be recognized as existing in three or more kinds of tissue or stages of differentiation simultaneously. It is customary to distinguish the appearance of early skeletal tissue by the terms blastema, precartilage, cartilage, and fetal bone. Some authors have limited the outlines of the various primordia to the parts found composed of precartilage or cartilage. Taking the femur at 17-20 mm. as an example, the shaft is composed of cartilage-like cells, while the ends appear to be precartilage and in the region of the trochanter a mass of rapidly growing blastema is seen. All these parts of the bone are regarded as part of its primordium fully as much as any one part. The blastemal portions should not be disregarded.
The development of the elements of the joint. The first appearance of the skeletal primordium within the limb bud in the region of the hip is a group of densely packed cells in the form of a “truncated cone with an oblique base which is applied to the side of the body” (Bardeen’). The region of the future hip joint in this tissue is no different from the other cells—and remains a dense group of cells resembling the original blastema, until both the femur and os innominatum are differentiated into easily recognized precartilaginous tissue.
Femur
The upper end of the femur is indistinct until, beginning in the region of the middle of the shaft, an enlargement of each of the blastemal cells in this area with an increase in cytoplasm takes place. Gradually, contiguous cells proximally and distally undergo similar changes in the body of the shaft of the bone which is outlined by denser, smaller cells about the periphery. It is at this time in the shape of a dumb-bell whose longitudinal axis is directed perpendicularly to the acetabulum at a measured angle of 45 degrees to the sagittal midplane of the embryo. From this stage on there are three centers of enlargement, one each for the diaphysis and the two extremities. The central portion of the shaft is composed of the most differentiated tissue at all stages. There is a slight bulge in the contour of the cortex of the bone at the center. Just proximal and distal to the middle third are constrictions in the diameter of the shaft. At these points the cells are much more crowded than at the ends of the bone. The head of the femur follows the shaft and precedes the distal portion in the maturity of the cells composing it. There are, therefore, three groups of cells at different stages of development within the outlines of the femur. At the upper end blastemal projections are formed for the trochanters, and these cells then change into cartilage as do those of the shaft of the femur. The mechanism of angulation both of adduction and flexion of the femur at its junction with the neck can not be explained on morphological observations (Carey*). During its development layers of flattened cartilage cells are formed between the mature fetal cartilage at the center and the immature cartilage at each end of the bone. Bone salts are deposited within the degenerating cartilage of the center of the shaft, and a definite 2- to 3-celled layer of cortical bone is laid down before capillaries from a greatly thickened periosteum break through (37 mm.) the cortex at the distal end of the middle third to invade the cartilaginous bone matrix carrying with them fibroblasts (later endosteum) and blood-forming cells of the marrow. It should be noted that this first invasion of blood vessels occurs at the level of the nutrient artery in the adult femur.
In the 70 mm. embryo the blood vessels enter the cartilage of the head and neck of the femur. Capillary tufts of endothelium, surrounded by cells resembling immature connective tissue cells arise from the region of the retinaculae of Weitbrecht and enter numerous small lacunae. The cartilage cells bounding the lacunae are altered in staining reaction, but they are not compressed more than others of the region and the process seems to be one of active solution and disintegration of cells to form a passageway for the vessel.
Between the nutrient vessel of the shaft and these newly entered vessels in the head and neck there are no others entering beneath the periosteum, at this or the 167 mm. stage. Just before and simultaneous with the entry of blood vessels into the various parts, the character of the cartilage cells in the region changes from a polyhedral form to compact elongated spindle-shaped cells with long processes intertwined at angles with each other and to a dense homogeneous intercellular substance without vacuoles. This cartilage, of different appearance than that in the center of the shaft, neck, and the region of the epiphyseal line, is more highly differentiated than is that which precedes enchondral bone formation, and the time of this change suggests that its appearance is closely related to an altered nutrition.
These vessels have been demonstrated by injection and by the examination of serial sections to be part of a vascular anastomotic ring about the neck of the femur, enclosed by the retinaculae of Weitbrecht. This vascular ring is supplied in great part by the medial and lateral deep circumflex branches of the femoral artery, and are less directly connected with the obturator, and the superior and inferior gluteal vessels which ramify on the posterior and inferior portions of the capsule of the joint.
Only in one of the six larger embryos (No. 10, 167 mm.) are vessels shown entering the head from the ligamentum teres femoris, although the caliber of those in each ligament itself is fairly large.
Acetabulum
At the time of its first appearance as a line of cells of diminished density in the 14-15 mm. embryo, the depression proximal to the head of the femur in the innominate blastema is shallow and saucer-shaped, composing between 65 and 70 measured degrees of the arc of a circle drawn including it. This depression must be deepened and enclosed to form a full half circle of 180 degrees before the joint cavity opens. One of the factors involved in this process appears to be pressure from the head of the femur, as indicated by the fact that at 15 mm. it is marked by a line of cells of diminished density, and from 15-22 mm. by a similar line of increased density. At 23 mm. a line of diminished density appears proximal to the head of the femur and the differentiation of the ligamentum teres femoris and other capsular structures which protect the joint begins, although the head is only about two-thirds enclosed by the cartilage. Six embryos 17.5-20 mm. in length were studied with this observation in mind. Of these, three showed about onehalf of the head covered by precartilage and three showed approximately two-thirds of the head covered by cartilage. The gonads of these embryos were not differentiated sufficiently to determine sex. From 23-45 mm. the cartilage of the ilium grows out over the head of the femur with the labrum attached to its margin. The superior portion of the glenoid labrum at this stage is much larger in relation to the surrounding structure than it will be in the mature embryo. It covers more degrees of the arc of acetabulum at this stage than later in development. Hence it appears that an increase in the extent of the elements of the acetabulum is responsible for the lateral displacement of the labrum. This is the most important part of the deepening of the acetabulum, because, inferiorly the relations of the attachment of the ligamentum teres to the transverse ligament and the labrum do not cover much more of the head of the femur at 45 mm. than they did when present in the form of undifferentiated blastema.
Note that the centers of ossification in all three bones are far removed from this location.
The earliest differentiation of blastema in the innominate primordium begins in the ilium just above the acetabulum* at about the 15 mm. stage. The ilium lags behind the shaft and head of the femur in differentiation at all stages, even to the penetration of blood vessels into the cartilage of the acetabulum which occurs just after the entry of vessels into the head and neck of the femur. Shortly thereafter close to the acetabulum a similar center is established in the pubis, later in the ischium. These three centers become precartilage and then cartilage so that in the remaining blastema we first see the Y outline as found in the child. Chondrification radiates in the three primordia from these centers. The inferior limb of the Y passes through the middle of the acetabular fossa, which is formed by an opening in the lateral borders of the pubis and ischium as they join (see Fig. 10). The ilium and ischium fuse very quickly in fetal cartilage (13.3 mm.) to eliminate the posterior wing of the Y. Then the ilium and pubis begin to fuse in cartilage beginning at the medial and superior border and progressing downward toward the acetabulum (23 mm.). Finally, the pubis and ischium fuse from their inner margin outward so that by 45 mm. all that is left of the original blastemal junctions is a small angle open laterally at the apex of the acetabular fossa. In this fossa the Haversian gland develops as a mass of loose tissue permeated by many small capillaries. The fact that the pubic portion of the Y remains as blastema at the acetabulum until last, may relate as much to the joining of these bones at the symphysis (see Figs. 6, 7, and 8) and to the changes in direction of the bones necessary to accomplish this union as it does to the development of the acetabulum. In contrast to other authors we find that the portion of the acetabulum formed by the blastema and cartilage of the pubis remains the same in contour and size, in relation to the other two cartilages, throughout the development of the joint.
Glenoid Labrum, Transverse Acetabular Ligament
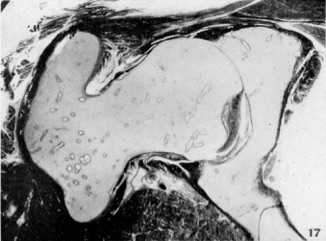
At the time of the first suggestion of the formation of an acetabulum, there is a condensation of blastema at the periphery of the depression which represents the glenoid labrum. At 19.3 mm. the oval cells may be seen under high power to be arranged concentrically with the rim of the cotyloid cavity. By comparison of the relative mass occupied by these cells to that of the innominate blastema it may be inferred that there is no extension of the cells, but that they reach their final position because of the enlargement of the rim of the ilium, ischium, and pubis which places the labrum out over the head of the femur. Inferiorly at the transverse ligament the circle is not supported by cartilage medially and at the time of the opening of the joint cavity the transverse ligament does not enclose the head of the femur distal to its greatest diameter so that displacement of the head could take place inferiorly at this place. Superiorly the labrum covers the greatest diameter and beyond, forming a firm protective covering for the femoral head. These anatomical features lead us to the surmise that the site of the transverse ligament of the hip joint is by far the weakest point in its structure. This is illustrated by the photomicrograph of the 167 mm. embryo in which one hip was flexed acutely (Fig. 18).
Joint Capsule and Synovia
Through the period of development up to 45 mm. the capsule is a loosely knit band of tissue which at 22-23 mm. can be distinguished as a layer of cells lying under the muscular primordia and over the glenoid labrum, and joining the perichondrium of the femur below. The nuclei of these cells which are round to oval in section become more and more spindle-shaped, but the cells are very loosely compacted at 44-45 mm. when a small condensation to form the zona orbicularis may be seen. The zona is clearly defined in the 167 mm. embryo (Fig. 17) half-way down the neck of the femur on its superior surface.
At the time of the opening of the joint space it is impossible morphologically to distinguish between a cell of the inner margin of the capsule which will eventually form synovial membrane and those cells of the capsule itself. This is also true of the 167 mm. embryo. At 237 mm. definite cuboidal cells can be seen in the region where the capsule is relaxed, but over the head of the femur, acetabulum, and stretched capsule they are spindle-shaped.
After the opening of the joint cavity the cells of the capsule develop more intercellular substance and fibrils, giving one the impression of increase in relative thickness and strength.
Ligamentum Teres Femoris
At 23 mm. the first suggestion of an orderly arrangement of cells in the region of the ligamentum teres is found. The individual cells appear as primitive fibroblasts with slightly oval nuclei closely applied to the head and in line with the long axis of the femur. Inferiorly these cells mingle with those of the transverse ligament. Sagittal sections (Fig. 9) show a triangular outline of cells attached along the length of the acetabular ligament and converging at the fovea. The head of the femur is round, as may be determined from sections made in sagittal, frontal, and transverse planes. There is at no stage a suggestion of a depression in the head to receive the ligamentum teres.
Subsequent development includes enlargement and slight lengthening as the femur becomes more adducted. Its separation to form a free mass within the joint occurs simultaneously with the opening of the remainder of the cavity, by vacuolization, degeneration, and splitting between the cells along its margin. Not until this occurs is it possible to see clearly that the ligament is to be attached to the medial border of the acetabular fossa behind the transverse acetabular ligament.
The three sites of origin of the ligament are not equally developed in all embryos. The ischium and medial aspect of the transverse ligament are points of origin in all the embryos examined. The pubic limb and the attachment passing under the base of the Haversian gland are variable. In sections of the two embryos illustrated in Figs. 13 and 15 there is a heavy band passing from the transverse ligament medial beneath the Haversian gland to the inferior margin of the acetabular notch which was not present in five others at this stage of development. In the planes of the illustrated sections the pubic and ischial bands are perpendicular to the section and their relative size varies in each embryo.
Blood vessels are present in the Haversian gland tissue and ligamentum teres at23 mm. As we have shown, the retinacular vessels enter the head and neck at 70 mm. Only one embryo (167 mm.) of the seven examined after the joint cavity opening showed vessels entering the head from the ligament. The largest fetus studied (237 mm.) did not show any vessels here.
Joint Cavity
The opening of the joint cavity is at once a degenerative and a mechanical process. There are observations by competent workers (Reyher, Schulin, Moser, Bardeen) to confirm the opinion that no ingrowth of tissue from outside provides a lining for the joint.
The evidences of degeneration are seen at 23 mm.; there is an increase in the intercellular spaces in the area of cells lying between the head of the femur, ligamentum teres, and acetabulum. At 36-42 mm., spaces filled with fluid are apparently formed where there are no opposing joint surfaces. Isolated cells suspended in this fluid show autolytic degeneration by the loss of their cellular outlines and the fading of their nuclear portion. Other nuclei are pyknotic. The spaces, also, contain long fibrils which probably result from cell degeneration.
Evidence of a mechanical factor is found in observing the closely applied cartilaginous joint surfaces. In these areas the first signs of discontinuity are long strips of well-preserved cells stretched diagonally from the acetabulum to the head of the femur. Processes of the cells are so strongly attached to one another as apparently to stand considerable tension. The splitting does not occur at one level between the arched lines of cells. One end of a strand crossing the space may arise on the outer layer of the acetabulum and be inserted or attached to a layer 3 to 4 cells deep within the head of the femur. That this appearance might be an artefact can not be denied; however, of six embryos between 36 and 45 mm. examined, the joint space of the 36 mm. (Figs. 11 and 12) is well under way with no splitting of the cell layers. All the others, 37, 42, 44.3, and 45 mm. respectively, show cell splitting along the joint margins as well as cell dissolution (Figs. 13 and 14). In Fig. 15 the space is well opened above and below the femoral neck. There are also strips of cells lying under the glenoid labrum. They are all larger than at 36 mm. where the opening appears to be pure cell autolysis. If it were artefact, it could be expected to be marked most in the younger, least-developed embryos.
It is suggested that probably factors of muscle innervation and function have some influence over the time of opening of the joint space and an early maturing neuromuscular mechanism may produce a joint opened early, while a slowly developing neuromuscular apparatus might allow the embryo to reach a greater length before this occurs.
The extension of the joint space down the neck of the femur occurs so as to leave the perichondrium and the reflected capsule which covers the retinacula of Weitbrecht. There is no possible explanation on purely morphological observations of how the selection of this plane occurs.
Discussion
These observations lead to one major conclusion, namely, that all the elements of the hip joint differentiate in situ from one mass of mesoderm. This point has already been brought out by Hencke and Reyher, Moser, Schulin, Walmsley, and Bardeen.
The evidence from comparative anatomy can not be applied to the actual development of the human hip. The modern authors using this source as a basis of statements are Keith and Frazer. The statements of these writers have to do with the formation of the ligamentum teres and the part of the pubic primordium in the formation of the acetabulum. Bardeen states, and we confirm the statement, that the proportion of the pelvic primordia entering into the formation of the acetabulum is essentially the same as in the adult. The confusion exists because the authors do not consider the blastemal portions of the primordia as in any way related to the cartilaginous portions, and regard the blastema as some actively migrating or ingrowing synovial special tissue. From our observations as well as those of others (Reyher, Schulin, Moser, Bardeen) synovia does not develop as an ingrowth but from cells in situ along a line of cleavage which appears between cells intimately a part of their respective primordia. A large portion of the pubic primordium remains as blastema longer than in any of the other pubic bones. That it does so may be related to the closure of the symphysis pubis. But with these facts in mind it can not be regarded as contributing less to the formation of the acetabulum than is proportional to its later part in composing the acetabulum. Likewise we are unable to find any evidence of the ligamentum teres being formed between wings extended from the head of the femur, as is stated by Keith.
The extension of cartilage into the blastemal portions of the various primordia can not be explained in the least part by these observations which have avoided dynamic interpretations.* The angulation of the shaft of the primarily straight femur to form a neck; the mechanical aspects of the opening of the joint cavity; and the extension of the pelvic cartilages into the blastema about the head of the femur closely approach the dynamic aspects of embryology which have been considered by Carey.* Nevertheless, it does not seem proper to regard the upper end of the femur as a bud or process (Stewart, Hagopoff). We must simply reiterate that each portion develops in situ from scleroblastema by contiguous growth and simple enlargement without gross changes in relationship.
Carey has written concerning the dynamic and physiological aspects of embryology pointing out some very interesting correlations.
We have been interested in the location and manner of penetration of the blood vessels into the primordia because the sites and process correspond to some of the congenital anomalies found in the region of the hip. Most of the clinical cases of congenital absence of the femur show absence of the proximal two-thirds of the shaft of the femur. The first blood vessel invasion of the shaft is at the junction of the middle and distal thirds of the femur and for a long period of development (36-70 mm.) constitutes the only vessel within the perichondrium and periosteum of the entire bone. At 70 mm. the blood vessels enter the head and neck of the femur from the retinacula of Weitbrecht at the location of the lesions found in the condition of so-called congenital coxa vara. These observations are valuable only in the light of what other observers may be able to add to them. The réle of the ligamentum teres femoris in supplying blood to that region is important in coxa plana and in fractures and infections of the hip. We have noted, as have many other authors, that the vessels in this region are variable in size and number.
The problem of congenital dislocation has engaged many writers but almost none have gone to embryology for the answers to their questions. The opportunity to observe the fetal position in a photomicrograph was fortuitous but is of value to emphasize the relationship of the head of the femur and acetabulum after the opening of the joint cavity.
Conclusions
All elements of the hip joint differentiate in situ in one mass of blastema.
The head of the femur is globular in shape at all times during its development.
The relative proportions of the primordia of pubic bones entering into the formation of the acetabulum are the same in early embryos as in later stages and in postnatal life.
The ligamentum teres shows no evidence of having arisen extraarticularly or within a groove in the head of the femur in the human embryo.
Congenital dislocation of the hip can not occur before the opening of the joint cavity and is related to the anatomy of the inferior portion of the hip joint and fetal position.
Thankful appreciation for helpful criticism and support in the preparation of this paper is extended to Dr. Frank R. Ober, Dr. William T. Greene, Dr. Sidney Farber of the Children’s Hospital, and Dr. J. L. Bremer of the Harvard Medical School.
References
1 Bardeen CR. Studies of the development of the human skeleton. (1905) Amer. J Anat. 4:265-302.
2. Bardeen CR. XI. Development of the Skeleton and of the Connective Tissues in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
3. Bardeen CR. and Lewis WH. The development of the limbs, body-wall and back. (1901) Amer. J Anat. 1: 1-36.
4. Carey, E. B.: Direct observations on the transformation of the mesenchyme in the thigh of the pig embryo. J. Morphol., 1922, 37, 1.
5. Frazer, J. E. S.: Anatomy of the Skeleton. 3rd ed., London, Churchill, 1933,. 128.
6. Hagopoff : De Vorigine et du mode de developpement embryonnaire de articulation de Ja hanche. Compt. rend. Soc. de biol., 1898, 50, 51.
7. Harrenstein, R. J.: Een kritieke periode in de ontwikkelung van het heupgewricht. Nederl. tijdschr. v. geneesk., 1924, 68, 2328.
8. Hencke, W., and Reyher, C.: Studien ueber die Entwickelung der Extremitaten des Menschen. Sitzungsber. d. k. Akad. d. Wissensch., Wien, 1874, 70, 217.
Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia. Keibel F. and Mall FP. Manual of Human Embryology II. (1912) J. B. Lippincott Company, Philadelphia.
Keith A. Human Embryology and Morphology. (1921) New York, Longmans, Green & Co. London: Edward Arnold.
Kélliker, A. von: Extwickelungsgeschichte des Menschen und der hiheren Thiere. Leipzig, Engelmann, 1861, p. 130. (Quoted by Schulin.)
Le Damany, P.: La cavité cotylide. J. de Vanat. et physiol., 1904, 40, 387.
Le Damany, P.: Die angeborene Hiiftgelenksverrenkung. Ztschr. f. orthop. Chir., 1908, 27, 129.
Le Damany, P.: La luxation congénitale de la hanche. Paris, Alcan, 1912.
Lewis WH. The development of the arm in man. (1902) Amer. J Anat. 1(2): 145-184.
Lewis WH. XII. The development of the muscular system pp 454-522 in Keibel F. and Mall FP. Manual of Human Embryology I. (1910) J. B. Lippincott Company, Philadelphia.
Moser, E.: Ueber die Ligamentum teres des Hiiftgelenks. Morphol. Arb., 1893, 2, 36.
Parsons, F. G.: The joints of mammals compared with those of man. Pt. II. The hip joint. J. Anat. & Physiol., 1900, 34, 301.
Reyher, C.: On the cartilages and synovial membranes of the joints. J. Anat. & Physiol., 1873-4, 8, 261.
Schulin, K.: Ueber die Entwickelung und weitere Ausbildung der Gelenke des menschlichen Kérpers. Arch. f. Anat. u. Physiol., Anat. Abt., 1879, 240.
Stewart, S. F.: The physiological treatment of congenital dislocation of the hip. J. Bone & Joint Surg., 1935, 33, 11.
Sutton, J. B.: The ligamentum teres. J. Anat. & Physiol., 1882-3, 17, 191.
Walmsley, T.: A note on the retinacula of Weitbrecht. J. Anat., 1917, 51, 61.
Figures
Fig. 1. Photomicrograph x55 H.E.C. Embryo 2300, length 6.75 mm., slide 420. The limb bud is formed. Its relation to the somites, coelom, Wolffian ducts are shown. The cells are indistinguishable from each other, except for the many mitoses. From this period on the limb bud is a distinct entity and displacement caudally and laterally occurs simultaneously with elongation of the embryo as a whole.
Fig. 2. Photomicrograph x30 H.E.C. Embryo 1000, length 10 mm., slide 657. The skeletal blastema retains its homogeneous character. The peripheral nerves have grown out in a sheet from the spinal cord and at the innominate blastema break up into three major masses which penetrate as far as the mid-thigh region. Muscular and osseous primordia are not separately distinguishable.
Fig. 3. Photomicrograph X21 H.E.C. Embryo 2051, length 15 mm., slide 1010. Under high power the dumb-bell shaped femur shows cells in lines perpendicular to the long axis, becoming convex at each end. The globular head of the femur is incongruous to the acetabular depression. Abduction 50° of the thigh places the femur at almost a right angle. 80° with the sockct, contributing to the stability of the junction.
Fig. 4. Photomicrograph x30 H.E.C. Embryo 2155, length 17.5 mm., slide 1169. A mass of deeply staining cells marks the joint. Muscle groups are outlined, some converge at a projection of blastema, locating the great trochanter. The femur, covered with perichondrium shows cartilage cells shrinking from matrix at the center and arcuate columns of precartilage cells compose the ends. The elements of the innominate primordia may be identified.
Fig. 5. Photomicrograph X30 H.E.C. Embryo 1597, length 19.3 mm., slide 807. The center of the femur shows mature fetal cartilage. An angulation of the upper end delineates the neck which forms an angle of 60° to the midline and 160° to the shaft. Note the cell distribution in transverse lines between neck and shaft. The joint region is densely stained.
Fig. 6. Photomicrograph x21 H.E.C. Embryo 2046, length 23 mm., slide 1606. The line of the joint is a zone of diminished density. The femur is becoming adducted to an angle of 30-40° with the midline. The neck appears longer and is more angulated on the shaft, 65-70° to the midline and 150-155° to the shaft. The false pelvis with the anterior superior spine of the ilium is shown as a projection of precartilage.
Fig. 7. Photomicrograph x30 H.E.C. Embryo 913, length 39 mm, slide 1064. Abduction of the femur to 20-28° to the midline is found. This appears to occur by some rotation of the head in the acetabulum and an increase in angulation of the neck to the shaft of the femur. Note well-developed glenoid labrum.
Fig. 8. Photomicrograph X30 H.E.C. Embryo 918, length 30 mm., slide 1094. Same embryo as Fig. 7. Blastema in the anterior limb of the Y between pubis and ilium is seen. Note location of ossification center in ilium as opposed to nucleus of chondrification near acetabulum in Figs. 3 and 6. Course of ligamentum teres from transverse ligament to fovea is prominent. The glenoid labrum and relationship of the capsule may be seen. Symphysis pubis is approximating.
Fig. 9. Photomicrograph X55 H.E.C. Embryo 1598, length 28.8 mm., slide 218. Sagittal section through the medial wall of the acetabulum shows the relative proportion of ischial and pubic attachments of the ligamentum teres, and the region of the acetabular fossa. At this stage the Y-shaped junction is not present, as fusion in cartilage has already taken place. This cartilage is that which will outline the Y in the child when the centers of ossification of the pelvic bones approximate the acetabulum. Thus we see the Y duplicated in both blastema and cartilage at different stages, although the points of origin of chondrification and ossification are not the same in the pelvic bones as they are in the femur.
Fig. 10. Photomicrograph x50 H.E.C. Embryo 787, length 22.8 mm., slide 406. Transverse section shows the recess between pubis and ischium to form the acetabular fossa. In it are seen the origins of the ligamentum teres and the Haversian gland. The anteversion of the neck of the femur has developed to produce an angle of 30° to the midline of the embryo. The ligamentum teres develops in situ without formation of a groove in the head of the femur. Short rotator muscles of the hip are well outlined.
Fig. 11. Photomicrograph x20 H.E.C. Embryo 2059, length 36 mm., slide 2531. Spaces are shown appearing in the tissue about the head of the femur. All the elements of the joint are defined. The acetabulum, glenoid labrum, and transverse acetabular ligament form 180° of a circle. Blood vessels are present in perichondrium, joint capsule, ligamentum teres, and Haversian gland, but none 1s present in the cartilage or shaft of the bones.
Fig. 12. Photomicrograph x 140 H.E.C. Embryo 2050, length 36 min., slide 2531. High power of space in Fig. 11. In the spaces float cells possessing normal-appearing nuclei and long fibrils. There are smaller cells with pyknotic nuclei as well as fragmented material suggesting shadows of nuclei. The spaces appear under the ligamentum teres, between it and the head of the femur and within the capsule distal to the glenoid labrum.
Fig. 13. Photomicrograph x20 H.E.C. Embryo 838, length 42 min., slide 1140. In two (Fig. 13 and 15) of 6 embryos examined at this stage a band from the ligamentum teres passes medially to the inferior margin of the acetabular fossa as well as to the transverse ligament, in addition to the usual ischial band. There is usually only a thin band of capsule arising from the pubis and ischium at this point.
Fig, 14. Photomicrograph x 140 H.E.C. Embryo 838, length 42 mm., slide 1149, High power of Fig. 13. The lamellae of cells reaching across the joint show no signs of degeneration as is seen in Fig. 12. They are spindle shaped and of the same character us those of the ligamentum teres. There are no cuboid shaped synovial cells.
Fig. 15. Photomicrograph x20 H.E.C. Embryo 1611, length 44.3 mm., slide 2623. Of note are the joint cavity; numerous vessels in the Haversian gland, ligamenturn teres, and inferior retinacula of Weitbrecht; the polyhedral cartilage cells of the neck opposed to the transverse arrangement of cartilage distally. The band from pubis to ligamentum teres is present. The glenoid labrum and transverse acetabular ligament reach beyond the greatest diameter of the head of the femur.
Fig. 16. Photomicrograph low power C.H. Embryo 2, length 70 mm., slide 117. The blood vessels enter through sinall lacunae hollowed out of the head and neck. In three embryos examined between 70 and 90 mm. those entering the inferior retinacula appear more advanced than those from the retinacular surfaces and the trochanteric fossa. The center of the neck is composed of rounded polyhedral cells. At the periphery the cells are elongated and flattened. This change occurs coincident with the entry of capillaries. Large vessels are shown in the ligamentum teres,
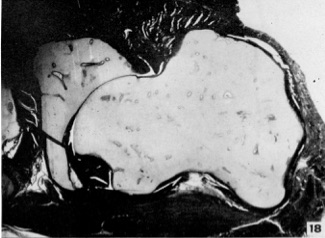
Fig. 17. Photomicrograph low power C.H. Embryo 10A, length 167 mm., slide 155. Frontal section showing the number of vessels present in the head and neck of the femur, which is in normal extra-uterine relation to the acetabulum. One vessel is shown entering through the ligamentum teres. All the cartilage is now composed of spindle-shaped cells with dense homogeneous intercellular substance. The synovial cells are spindle-shaped, flattened cells with a few of the transitional type over the relaxed capsular areas.
Fig. 18. Photomicrograph low power C.H. Embryo 10. length 167 mm., slide 245. Section with the femur in 90° flexion and external rotation. The head of the femur has been thrown inferiorly and laterally so that its greatest diameter is out of the circle of the glenoid labrum and cotyloid ligament. The inferior capsule is stretched and bulging. If force were applied in the long axis of the femur, the head would be forced outside the glenoid labrum to cause inferior dislocation.
Cite this page: Hill, M.A. (2024, April 20) Embryology Paper - The embryology of the human hip joint (1943). Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_embryology_of_the_human_hip_joint_(1943)
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G