Paper - The Organization and Cell-Lineage of the Ascidian Egg 4
| Embryology - 19 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Conklin EG. The Organization and Cell-Lineage of the Ascidian Egg (1905) J. Acad., Nat. Sci. Phila. 13, 1.
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Conklin 1905 TOC: I. The Ovarian Egg | II. Maturation and Fertilization | III. Orientation of Egg and Embryo | IV. Cell-Lineage | V. Later Development | VI. Comparisons with A.mphioxus and Amphibia | VII. The Organization of the Egg | Summary | Literature Cited | Explanation of Figures
IV. Cell-Lineage
A. Nomenclature
In order to facilitate reference to the work of others, it is desirable that some good system of naming the individual cleavage cells be adopted and thereafter adhered to even if it he not ideally perfect. The system which has been employed with only slight modifications in all the recent cell-lineage work on annelids and mollusks is not well suited to the ascidian egg because in the latter there is no distinction of macromeres and micromeres, because there are no "quartets" which arise from stem cells, because this system is not well adapted to show the perfect bilateral symmetry of the egg and embryo, which is one of the must characteristic features of ascidian development, and finally because of the great number of figures which must be used as exponents in the representation of later stages {eg. the letter designating each of the cleavage cells of the ninth generation, figs. 140-143, wotdd need to be followed by no less than six exponents). Owing to these reasons I early saw the difficulty of attempting to apply this system to the cell-lineage of the ascidian egg. The most complete system of nomenclature which has heretofore been used in the study of the cell-lineage of ascidians is that of Castle, which is a modification of a system devised by Kofoid (1894). In this system, as is w^ell known, the four quadrants of the egg are designated by the letters A, B, C, D ; after the third cleavage the cells nearer the vegetal pole are designated by capitals, those near the animal pole by lower case letters. The first exponent following a letter indicates the generation to which a cell belongs, the second exponent the position of the cell relative to the vegetal pole. With this system it is always difficult to determine at a glance the lineage of any cell since "to ascertain the mother cell of any particular cell, its first exponent must be diminished by one; and its second exponent, if an even number, must be divided by two, but if an odd number it must be first increased by one and then divided by two. In order to determine the daughter cell of a particular cell, simply reverse this process ; that is, increase the first exponent by one and double the second exponent. To determine the other daughter cell diminish this second exponent by one" (Castle. L896, [>. 227). While it is thus difficult to determine at a glance the lineage of any cell, the number of exponents required is relatively small, and this fact, more than any other, has led me to adopt Castle's system, with the following modifications : The right and left halves of the embryo are designated by the same letters, the names of cells on the right being underscored as compared with those on the left. This method of designating the cells of the right and left sides is essentially similar to that employed by Chabry (1887). In this way but two letters are needed for the whole cell-lineage, one for the anterior and another for the posterior quadrants. For these I desired to use the letters employed by Van Beneden and Julin and by Chabry. viz. A and 1\ but owing to the difficulty of distinguishing between lower case and capital P. I finally chose the letter B instead of P. The right anterior quadrant is A, the left A ; the right posterior quadrant is B, the left B. After the third cleavage all cells lying on the polar body side of that cleavage plane are designated by lower case letters, while those on the opposite side of that plane continue to be designated by capitals. This modification not only emphasizes the bilateral character of the ascidian egg, but it also simplifies the nomenclature. Furthermore, it facilitates reference to Castle's work, for when in his orientation of the 48-eell stasre the egg is inverted as compared with earlier stages the right side is substituted for the left, and the letters A and D, which in the earlier stages designate the actual left side, are used after the 48-cell stage to designate the actual right and vice versa. In my modification of his system this substitution of one side for the other will be indicated only by the presence or absence of a line under the letter. In all stages later than the 48-cell stage I continue to use lower case letters to designate cells of the animal or ectodermal hemisphere, and capitals for those of the opposite hemisphere, whereas Castle reverses this rule.
B. Cleavage of the Egg
First to Seventh Generation of Cells (1-64 Cells).
Although the details of the early cleavages of the ascidian egg have been
treated at considerable length by previous writers on this subject, I have determined to present the subject here, cleavage by cleavage, both because my results
differ in many respects from the conclusions heretofore reached and because I wish
to call attention to certain features of these cleavages which have not as yet been'
noticed. I shall shorten the account wherever possible by references to previous
work.
As is well known, ascidian eggs develop with great rapidity; there are certainly few other eggs which develop so rapidly. Both Ciona and Cynthia reach the fully formed tadpole stage in about twelve hours, while Molgula reaches this stage in not more than eight hours after fertilization. Certainlv there are few things more wonderful than the origin of a complex animal. of a chordate , from an egg in the short space of from eight to twelve hours ! The portions of this brief period devoted to the different stages of development are interesting and suggestive. In Cynthia about 40 minutes elapse between the fertilization and the appearance of the first cleavage , furrow ; about 140 minutes between the first cleavage and the beginning of gastrulation ; and about 140 minutes between this stage and the young tadpole stage shown in figure 103. The different generations of cleavage cells are separated from one another by intervals about as follows : 1
Fertilization to first cleavage ( 1-2 Cells).
First to second cleavage
Second to third cleavage
Third to fourth cleavage
Fourth to fifth cleavage
Fifth to sixth cleavage
Sixth to seventh cleavage
Seventh to eighth cleavage (112-218
Eighth cleavage to young tadpole stage (fig. 163),
1. First Cleavage; 1-2 cells. (Figs. 18-27, 96-100, 177. 178. 181.)
After the meeting of the germ nuclei, which occurs about midway between the
center of the ci'i and the posterior pole (figs. 91, 92, 93), the cleavage spindle
develops rapidly and moves inward from the posterior pole until it lies almost
1 These figures are based upon the study of eggs laid and fertilized at 5 p.m. and put up at intervals thereafter "until 11 p.m. In each of these hits eggs were found in several different stages and the results can be aecejitr.l u- only approximately correct.
( 2-4 "
>.
I 4-8
),
| 8-16 "
),
t 16-32 "
),
i .",2-64 "
),
( 64-112
)
(112-218
)
40
min
30
a
30
u
20
n
20
a
20
a
20
u
20
a
2
hrs.
exactly in the center of the egg (fig. 96), always heing oriented so that it lies at
right angles to the plane of the first cleavage and with its equator in that plane.
At the same time the clear protoplasm and a small portion of the yellow protoplasm
move inward from the posterior pole toward the center of the egg (figs. 92, 96).
. The larger part of the yellow protoplasm remains at the surface in the form of a
crescent, but the clear protoplasm is entirely withdrawn from the surface except for
a narrow zone which lies just above (ventral to) the crescent on the posterior side
(figs. 13-18, 96). During the formation of the first cleavage furrow, even this
narrow zone of clear protoplasm is withdrawn from the surface to the center of the
egg, so that the yolk now covers the entire surface of the egg except for the area
of the crescent (tigs. 100-102 and 178-179). This condition is just the reverse of
that which prevailed at the beginning of development, when the yolk was central
in position and the protoplasm peripheral (figs. 76-79).
The centrosomes and asters are larger and more easily studied in Ciona than in Cynthia. Proceeding from the periphery to the center, the following parts of the aster may be recognized (figs. 177, 179) : (1) The deeply staining, peripheral layer of the aster, (2) the clear inner layer of the aster traversed by radiating fibres, (3) a granular central body upon which the astral fibres end. The latter is the centrosome, and is plainly composed of two parts, (a) an outer granular zone and (b) a central clear area from which the netrum arises. In Cynthia the outer and inner layers of the aster are not distinguishable and the centrosome itself is not so large as in Ciona ; the latter is, however, composed of the same parts, viz., a peripheral granular zone and a central clear area which gives rise to the netrum (figs. 98, 99). In these ascidians, just as in the gasteropods which I have studied (Conklin, 1902), the centrosome undergoes a decided growth and metamorphosis during the cycle of division ; in the early stages of the cycle it is a small, deeply staining body, in the Later stages it becomes much larger and differentiates into the outer granular zone and the central clear area (cf. figs. !)7, 98).
In Ciona both the first and second cleavage spindles are remarkable in that at all stages of the division the nuclear part of the spindle can be clearly distinguished from the polar or astral part (figs. 177, 17'.l). The portion of the spindle derived from the linin of the germ nuclei is short, deeply staining and barrel-shaped, and in all respects resembles a maturation spindle (compare figs. 177 and 179 with figs. 67. 70, 71). Even in the possession of a few peripheral fibres which radiate from the slightly rounded ends of the spindle toward the equator, this spindle resembles those of the maturation divisions. These peripheral fibres are not in line with the astral radiations, and hence are all the more striking. The astral rays which run from the centrosomes to the ends of this nuclear spindle are small and faintly staining as contrasted with the heavy, deeply staining fibres of the nuclear spindle. Nowhere else, so far as I am aware, is this double character of the mitotic figure so clearly shown as in these cleavages of Ciona. This is due to the small size of the nuclear spindle and to the large size of the astral systems, so that the ends of the nuclear spindle are not easily confused with the astral rays, and also to the great difference in the staining reactions of the two. Except for the presence of these astral systems, this cleavage spindle is almost exactly like a maturation spindle.
In Cynthia the distinction between these two parts of the cleavage spindle is not so clear because here the nuclear portion is much Longer and reaches nearly to the centrosomes (fig. 97); but even here the nuclear part can be distinguished from the astral by the stronger character of its fibres. In this genus also the astral systems are not so Large as in Ciona and the individual fibres are stronger, so that the contrast between the nuclear and the astral portions of the spindle are but faintly marked.
These cleavage spindles of Ciona, like the maturation spindles already described, are of especial interest for the study of the mechanics of mitosis. 1 have not attempted to make a detailed study of this subject, but it is quite evident that the separating chromosomes move only as far as the ends of the nuclear spindle fibres (fig. 99). They are never drawn up into contact witli the centrosomes, but remain at the border of the aster, where they are transformed into chromosomal vesicles. In the maturation divisions there are no centrosomes or asters at all to complicate the problem, and liere also the chromosomes move only to the ends of the nuclear spindle fibres. These fibres elongate somewhat in the later stages of mitosis (figs. 70, 71, 72), thus separating more widely the daughter chromosomes.
The fact that in the maturation divisions the chromosomes separate without the aid of centrosomes or asters may be taken as evidence that in the similar spindles of the first and second cleavages the centrosomes and asters, although present, take no part in this work. Anything which will explain the movements of the chromosomes in such spindles as those shown in figures 69 to 72 will also explain their movements in such cleavage spindles as those shown in figures 177 and 179. In the maturation mitoses there are neither centrosomes nor asters, and yet the separation of the chromosomes occurs in the usual manner. The spindle fibres apparently serve only as a guide for this movement, and must be considered the result rather than the cause of stresses in the cell substance. This is shown by the fact that when they first appear these spindle fibres are not parallel but run in all directions (figs. 02, G4). Later, under the influence of stresses in the cytoplasm, they become parallel. Under these circumstances there is no reason to believe that the movement of the chromosomes is caused by other factors than those which bring about movements in the cell body.
Tile constriction of the cell body first occurs, as indicated by figure 20. at the posterior pole. This is probably due, as Castle says, to the fact that at this stage tliis pole is the more richly protoplasmic one. Very soon the constriction extends all the way around the egg and, as the mitotic spindle lies in the middle, it follows that the constriction must be about as deep on one side as on another (fig. 99). This constriction divides the ooplasm with exact ecpiality, not only quantitatively but qualitatively also. During and immediately following this division the yellowcrescent undergoes some very remarkable transformations. These changes are shown in figures 21 to 26, which represent consecutive stages of the same egg drawn in intervals of about two or three minutes. The crescent is first constricted in the middle (figs. 20 and 21) ; then the gray yolk penetrates into the lower part of each half of the crescent and approaches near to the surface, being covered only by a thin layer of the yellow protoplasm (fig. 22). Above and below this intrusion of yolk the crescent remains deep yellow in color ; in the region of the intrusion the color is gray with a superficial covering of yellow pigment granules. The lower (dorsal) portion of the crescent thus cut off from the remainder is small as compared with the upper part, and is median in position. Not more than two minutes afterward this lower part unites with the upper along the median line (fig. 23), thus forming a deep yellow semicircle in each blastomere. The intrusion of yolk may still be seen entering this semicircle through its open half, which is dorsal and lateral in position. Then these two semicircles come into contact with each other along the first cleavage plane, the free ends enlarge into rounded knobs, and the intrusion of yolk is less large (fig. 24). Finally, the intrusion becomes still smaller, the open ends of each semicircle join, and the crescent is reestablished (figs. 25 and 26). Observation of the living egg during this period of division gives the impression of remarkable cytokenetic activity in all the ooplasm ; not only does the crescent take pai't in this activity but the yolk and the clear protoplasm undergo marked movements, in the course of which the clear protoplasm is divided into two areas which are entirely separated from each other by a partition of yolk (figs. 2527). I have been unable to analyze all of these movements; one thing, however, seems very probable, viz., that they are in the main of a vortical nature and that they are comparable with the movements in the constriction of the cell body which I have observed in gastropods (Conklin. 1902). I have not thoroughly studied these movements by the aid of serial sections ; since they take place with such rapidity, this would be possible only by sectioning and studying a very large number of eggs during the period of the first cleavage. In figure 99, which is an equatorial section of an egg of the stage shown in figure 22 or 23, the substance of the crescent (Cr) can be recognized on the posterior side of the egg; it does not. however, show any of the thickenings or thinnings indicated in the surface views mentioned. Beneath the superficial layer of yellow protoplasm in this figure is an area of clear alveolar protoplasm, while still deeper is the radiating protoplasm which constitutes the astral systems.
In the telophase of the first cleavage the centrosomes, daughter nuclei, and the surrounding areas of clear protoplasm rotate toward the animal pole (fig. 100) in a manner similar to that which occurs in the blastomeres of gasteropods (Conklin, 1902). Through the agency of these telokinetic movements of the first cleavage the nuclei, centrosomes and clear protoplasm are carried above the equator of the egg toward the animal pole. The pole at which the polar bodies lie thus becomes more richly protoplasmic than the opposite pole and ever after continues to be so (fig. 102.^7 seq.). Castle has observed this telokinetic movement in Ciona, and describes it in the following words (1896, p. 233): "The first cleavage spindle arises, as has been stated, not far from the center of the egg. As its first cleavage is nearing completion, however, the attraction spheres and nuclei begin to move toward the dorsal surface of the egg, me ay fro?) i its more richly protoplasmic animal pole, from which the plane of separation cuts it more rapidly." I find, however, that in Ciona, as in Cynthia, this telokinetic movement of the nuclei and spheres is not away from the more richly protoplasmic pole, unless the substance of the crescent he considered as constituting the protoplasmic pole, hut that the clear protoplasm also moves with the nuclei and spheres toward tin' animal (ventral) pole (fins. ITS. 182). Sobotta (1807) has also observed a similar bending of the spindle axis and movement of the daughter nuclei and centrosomes in the anaphase or telophase of the first cleavage of the egg of Amphioxus. He describes it as an attempt on the part of the centrosomes and nuclei to regain the center of the blastomeres ; but it is probable that this is only another case of the telokinetic rotation of the cell contents with the consequent establishment of a new cell axis [cf. Conklin, 1902).
2. Second Cleavage; 2-4 cells
(Figs. 28-30, 101-105. 179, 182, 183).
During the anaphase of the first cleavage each centrosome becomes elongated at right angles to the spindle axis and to the chief axis of the egg and gives rise to a minute centrosomal spindle or netrum at each pole of the mitotic figure (figs. 98, 99). These netra elongate in the antero-posterior axis until the daughter centrosomes come to lie at the anterior and posterior poles of the nucleus of each blastomere ; the nuclear membrane is then dissolved and the second cleavage spindles are formed (figs. 101, 102, 177, 179). These spindles, like those of the first cleavage, are composed of a nuclear and an astral portion, the two being distinguishable with especial ease in Ciona (fig. 179). The spindles lie in an area of clear protoplasm above the equator of the egg, and in Cynthia are slightly eccentric toward the posterior pole (fig. 102). The areas of clear protoplasm become elongated in the antero-posterior axis (figs. 28, 101, 102, 179) ; they are surrounded on all sides by yolk, which forms a peripheral laver over the whole surface of the egg, except in the region of the crescent. The substance of the crescent is directly continuous with the clear protoplasm in the region of the posterior pole of the second cleavage spindle (figs. 102, 17'.)). Although the yolk surrounds the areas of clear protoplasm it is not uniformly thick on all sides; on the upper or ventral side of these areas the layer of yolk is very thin, on the lower or dorsal side and anterior to the crescent it is especially thick. This distribution of the yolk can be seen not only in sections, such as figures 102 and 17!), but also in entire preparation and in living eggs. In the latter the light gray of the upper hemisphere (figs. 28, 29 and 30), as contrasted with the dark gray of the lower hemisphere, indicates that the layer of yolk which surrounds the areas of clear protoplasm is thin over the upper hemisphere and thick over the lower. The area at the vegetal pole, where the layer of yolk is thickest, gives rise to the endoderm cells which are always yolk laden : the upper hemisphere, where the yolk layer is thin. gives rise to the ectoderm cells which contain a relatively small amount of yolk ; the substance of the crescent gives rise to the muscle and mesenchyme cells, and in Cynthia is always characterized by the presence of the yellow spherules. In later stages the yolk ceases to be peripheral in position and comes to lie in the central portions of the cleavage cells (text figs. XVII XXIV) ; this change in position is brought about by the flowing of the peripheral layer of yolk inward along all the developing cleavage furrows (figs. 104-107, et seq.) until finally the yolk comes to occupy a central position in all the blastomeres, while the clear protoplasm is brought once more to the surface. These cytokinetic movements which accompany cell division do not change the relative distribution of yolk and cytoplasm in the different hemispheres and quadrants of the egg, but only its location in the individual cleavage cells.
In the second cleavage the constriction of the cell begins at the periphery or
free surface and proceeds inward through the cell body (figs. 104, 105). The
peripheral layer of yolk is thus carried inward along the cleavage furrow, as has
been said, and the middle of each spindle is bent in toward the center of the egg
(fig. 105). At the same time the yolk and yellow protoplasm continue to be carried in along the first cleavage furrow. The inflow of yolk along a developing
cleavage furrow leaves a protoplasmic connection between the two daughter cells,
into which the yolk does not penetrate for a considerable time ; this protoplasmic
connection is frequently of service in determining the lineage of cells since it always
connects daughter cells (figs. 104-107). Finally the inflow of yolk completely cuts off this connection.
The four cells which are formed by the second cleavage are all approximately
of the same size in Ciona ; in Cynthia the two anterior cells are slightly larger than
the posterior ones, just as Van Beneden and Julin found to be the case in Clavellina (text figs. VII, VIII). But though the daughter cells are of nearly the same
size they are of very different quality. The posterior cells contain about the same
quantity of clear protoplasm as the anterior ones, but they contain little yolk and
practically all of the yellow crescent substance; the anterior cells on the other hand
contain a great deal of yolk, but practically none of the crescent substance. The
second cleavage is therefore differential in a very marked degree (r/". figs. 29 and 30).
3. Third Cleavage; 4-8 cells
(Figs. 31-35, 106-100, 184).
In the anaphase of the second cleavage the centrosomes elongate in the vertical axis and the daughter centrosomes, moving to the upper and lower poles of the nuclei, form the centrosomes of the third cleavage spindles. In an abnormal egg shown in figure 103 this division of the centrosomes occurs in one of the blastomeres in the prophase of the second cleavage and not in a vertical but in a horizontal direction. The position of these third cleavage spindles is peculiar and of great prospective significance. They are slightly eccentric toward the animal pole, and accordingly the four cells which are cut oft* at this pole are smaller than those at the vegetal pole. When the egg is viewed from either the right or the left side the spindles in the anterior and posterior quadrants seem to be parallel and are both slanted forward at the upper pole ; accordingly the four upper cells, when formed, lie slightly anterior to the four lower ones (figs. 108, 184). When the egg is viewed from the anterior pole it is seen that the spindles in the anterior quadrants are not parallel. but that they converge toward the animal pole. The reverse is the case if the egg is viewed from the posterior pole, i.e., the spindles in the posterior quadrants diverge toward the animal pole. Thus it comes about that the nuclei in the anterior-vegetal cells (A 41 ) are relatively far apart, those in the anterior-animal cells (a 4 ' 2 ) (dose together (figs. 106 and 109); whereas the reverse is the case in the posterior cells, i.e., the nuclei in the posterior-vegetal cells (B 41 ) are near together, those in the posterior-animal cells (b 4-8 ) far apart (tig. 107). *
Every one of these matters is of prospective significance in the further development of the embryo; associated with the forward slant of the spindles toward the animal pole is the tact that the cells of the animal hemisphere overhang those of the vegetal hemisphere at the anterior pole; whereas the posterior cells of the vegetal hemisphere are not completely covered by those of the animal hemisphere when the egg is viewed exactly from the animal pole (figs. 110. 112, 116). Associated with the convergence of the spindles in the anterior quadrants toward the animal pole and the convergence of the spindles of the posterior quadrants toward the vegetal pole is the fact that in later stages the anterior half of the vegetal hemisphere is broad from side to side, its posterior half narrow, while the anterior half of the animal hemisphere is narrow from left to right, its posterior half broad (figs. 109-118, et seq.). While the position of these spindles is therefore indicative of important prospective characteristics of the embryo, it must not be regarded as the initial cause of these characteristics. Indications of these features may be seen in the distribution of the yolk and protoplasm at the four-cell stage, and there can be no doubt that the position of the spindles is itself the result of cytoplasmic localization.
One of the features of this stage to which Castle calls particular attention is the
presence of a " cross-furrow " on the right and left sides between the anterior dorsal
and the posterior ventral cells (A 4 - 1 and b 4 '", figs. SI, 32, 108, 184). I find, as did
Castle and Chabry, that this cross-furrow is constant in position and that it marks a
downward bend in the equator, which may be observed as late as the gastrula stage ;
in the region of this downward bend the ectoderm cells grow down over the cells of
the vegetal hemisphere in advance of the neighboring ectoderm cells (figs. 116-119,
123-126, 128, 130, 134, et seq.). I observed the process of formation of this crossfurrow r in the living egg, and have represented this in figure 31. When the third
cleavage furrows first appear, they are all in nearly the same plane, the furrows
between the daughter cells of the posterior quadrants being nearly perjiendicular to
the egg axis, as indicated by the faint line between the cells B 41 and b 4 8 of figure 31,
which line represents the position of the furrow between those cells when it first
appears. A minute or two afterward this furrow is tilted downward at its anterior
end and upward at its posterior, as indicated by the heavy line between those two
cells ; in this way the cross-furrow arises on the right and left sides of the egg
hetween the anterior dorsal and the posterior ventral cells.
- 1 Figs. 106 and 107 represent two sections of one and the same egg, in the 8-cell stage, the former through the nuclei of the anterior cells, the latter through the nuclei of the posterior ones.
During telokinesis the movements in the cell body are similar to those which
occur at the close of the second cleavage, i. e.. the middle of the spindle is carried
in toward the centre of the egg while the poles of the spindle move outward toward
the surface (figs. 106, 107). By this movement the spindle axis is much bent on
itself. I have not observed in these eggs any tendency for the sphere substance at
the poles of the spindles to be carried as near as possible to the animal pole, a
thing which is very apparent in gasteropod eggs.
I have already called attention to the fact that the four cells at the animal pole
are smaller than those at the vegetal pole; this disparity is most marked between
the upper and lower cells of the anterior quadrants (figs. 106, 107). The anterior
dorsal cells (A 41 ) are the largest in the egg at the eight-cell stage, the anterior
ventral cells (a 42 ) the smallest. The posterior dorsal cells (B 41 ) are but little, if any,
larger than the posterior ventral ones (h 42 ), and both are intermediate in size
between the upper and lower anterior cells.
The different cell substances are distributed to the eight cells as follows : The
clear protoplasm is found in all the cells, but is most abundant in the four ventral
cells and least abundant in the two posterior dorsal cells (B 41 ); yolk is found in
all of the cells, but is most abundant in the two anterior dorsal cells (A 41 ) and least
abundant in the four ventral cells ; the yellow protoplasm or crescent substance is
confined almost entirely to the two posterior dorsal cells (B 41 ), but a very small
amount of it is found around the nuclei of all the cells (figs. 106, 107, Cr. s.). My
attention was first drawn to the yellow protoplasm around the nuclei in my study
of the living eggs of Cynthia (figs. 32-40, et seq.)\ since then I have found, in
preserved material, a few of the spherules of this yellow protoplasm around the first
cleavage spindle and around the resting nuclei of the 2-cell, 4-cell and 8-cell stages
(figs. 96, 106, 107). In later stages of development it is found around the nuclei
of a few of the ectoderm cells even as late as the young tadpole stage (plates IV
and V). In spite of this perinuclear distribution of some of this crescent substance, it
is largely limited to the two posterior dorsal cells (B 41 ) of the 8-cell stage, where it
constitutes more than half of all the substance of those cells (figs. 31-35, 106, 107).
Van Beneden and Julin lirst observed that the four ventral cells of the
8-cell stage are smaller than the lour dorsal ones; Seeliger, Samassa, and Castle
observed this same fact, though they incorrectly called these smaller cells dorsal in
position. Castle is quite right when he says (1896, p. 228) the " four cells which Lie
nearest the polar globules are smaller than those more remote," but I cannot understand how it was possible for him to reach the conclusion that- the smaller cells are more abundantly supplied with yolk," while the larger cells are richer in protoplasm (pp. 234 and 235). According to my observations this is not true of Cz'ona,
Cynthia or Molgnla.
During the 8-cell stage two little bosses or caps of clear but deeply staining
protoplasm which will give rise at the 64-cell stage to the small posterior mesenchyme cells (B 7,; fig. 130 et seq.) are visible on the posterior surface of the cells B 41
and B 4 ' (fig. 184). These little caps lie in contact with each other on each side of the mid-line and right at the middle of the crescent, of which they form an
extremely small part. They arc formed by the aggregation at this point of a little
clear protoplasm which first appeared at the time of fertilization as a clear area
around the spermatozoon, and which afterwards lies at the middle of the crescent
(figs. 173, 17->. 17li). In Cynthia this area of clear protoplasm does not usually
take the form of the deeply staining hosses or caps before the 1 (i-cell stage (figs.
113. 110), though these may sometimes appear, as they do in Ciona, at the 8-cell
stage. Although they arise from the surface of the crescent they contain no yellow
pigment, and in the living egg this small spot of protoplasm ami the cells to which
it gives rise are almost perfectly transparent and are therefore difficult to see. In
stained preparations they always stain deeply and thus form an excellent landmark (figs. 116-120, et seq).
Chabry and Castle have called particular attention to these prominences of
clear protoplasm which are found at the posterior pole of the egg, and Castle traces
them hack to the 2-cell stage, and ives good reason for believing that this area
of clear protoplasm marks the point of entrance of the spermatozoon, and was
caused by it. I entirely agree with Castle that this aggregation of clear protoplasm
is caused by the entering spermatozoon, since I have seen it surrounding the spermatozoon immediately after its entrance (fig. 173); but it can scarcely be said to
mark the point of entrance, as it does not remain stationary hut moves with the
crescent from a point near the vegetal pole to one near the equator on the posterior
side of the erg. So far as I am able to determine from a study of Castle's figures and description, the area of finely granular protoplasm, which he represents in his
figures 17, 45, 40 and 47, is the middle portion of the crescent. The large area of
clear protoplasm represented in each of these figures and marked x gives rise to
the middle cells of the crescent (C 5 - 2 , D 52 of his fig. 49), therefore the small posterior mesenchyme cells, C" r ' and D 75 of later stages, can represent but a small part
of the area marked x in the earlier stages. The earliest stage in which Castle
represents the substance of these future mesenchyme cells is at the posterior pole
of the cells C 63 and D 63 in his figure 51. 1 conclude, therefore, that he observed
the middle portion of the crescent (x of his figures) in the earliest stages of the
development, but that be did not recognize the substance of the small mesenchyme
cells as distinguished from the substance x (crescent substance) before the 24-cell
stage (his fig. 51).
All students of ascidian embryology agree that the first plane of cleavage is
median in position, the second transverse, and the third horizontal or coronal, but
beyond this there are few agreements among them, as has been pointed out. In
the matter of the relations of these cleavage planes to the germ layers there are as
many opinions as there are concerning the orientation of the v^<j:. Van Beneden
and Julin (1884) maintained that the four ventral cells of the 8-cell stage are
purely ectodermal, but that the four dorsal cells are still mixed." each of them
containing ectoderm and endoderm, while not until the 44-cell stage is the separation of ectoderm and endoderm in these dorsal cells completed. Seeliger ( 1 885) held that the third cleavage plane separates the ectoderm from the endoderm, the four
ventral cells being ectodermal, the four dorsal endodermal. Davidoff (1891 ) found
that in Distaplia the tour ventral cells are ectodermal, the four dorsal endodermal.
and a similar view is maintained by Samassa (1S'.>4). Castle (189G), on the other
hand, maintains that both the ventral and the dorsal cells of the 8-cell stage are
mixed, the ventral cells containing ectoderm and mesoderm, and the dorsal cells
endoderm and mesoderm, and not until the 48-eell stage are the substances of these
layers finally separated.
My work, like that of Castle, places hut little weight upon the idea of germ
layers, since it undertakes to trace specific organs to certain cleavage cells, and
even to certain regions of the unsegmented egg. Emphasis is therefore placed upon
organs and upon organ-forming cells and substances rather than upon the more
indefinite germ layers. However, I find that the tour ventral cells of the 8-cell
stage are purely ectodermal, while the four dorsal cells are endodermal and mesodermal, save for the fact that four neural plate cells (A 74 , A 78 , figs. 120, 121, 123)
will arise from the anterior portion of the dorsal hemisphere at the 44-cell stage.
The mesoderm and endoderm are first completely separated at the 22-cell stage
(figs. 117, 118). I find that only four ectodermal (neural plate) cells come from the
dorsal hemisphere, whereas Van Beneden and Julin hold that at a corresponding
stage (44-cells), sixteen ectodermal cells have been derived from the dorsal hemisphere. Of these sixteen cells four only are really ectodermal (the neural plate
cells), eight are mesodermal, and four are endodermal. Castle's conclusion that a
portion of the mesoderm is derived from the ventral cells is due to his erroneous
lineage of the cells after the 48-cell stage; all of the ventral cells are ectodermal,
and all of the mesoderm and endoderm are derived from the dorsal cells. With ,
the exception therefore of these four neural plate cells, which arise at the 44-cell
stage on the dorsal side of the third cleavage plane, all of the ectoderm lies on the
ventral side of that plane, and all of the endoderm and mesoderm on its dorsal side.
This conclusion, it will he observed, is very similar to that of Seeliger, Davidoff,'
ami Samassa.
4. Fourth Cleavage; 8-16 cells
(Figs. 36-38, 110-115, 186-188.)
The spindles for the fourth cleavage appear in all of the eight cells at about
the same time, though the dorsal cells sometimes divide slightly in advance of the
ventral ones. All the spindles are approximately horizontal in position, and all are
oblique to the median and transverse planes (first and second cleavage planes).
As a result of the fact, stated on page 45, that the dorsal hemisphere is broad in front
and narrow behind, while the ventral hemisphere is broad behind and narrow in
front, we find that the obliquity of the spindles of one hemisphere is reversed as
compared with that of the other. Thus the spindles in the anterior-dorsal cells
approach in direction a transverse plane, in the posterior-dorsal cells they approach
an antero-posterior plane ; whereas in the anterior-ventral cells they approach an
autero-posterior plane, while in the posterior-ventral cells they approach a transverse plane (figs. 110-113, 180, 187).
Corresponding with these positions of the spindles the subsequent divisions of the cells are such as to lead to an inverse position of the cleavage cells in the dorsal as compared with the ventral hemisphere. In the anterior-dorsal cells the fourth cleavage furrows run from the anterior border of the cells to the transverse (second cleavage) plane, and are approximately antero-posterior in direction: in the posterior-dorsal cells these cleavage furrows run from the lateral borders of the cells to the median (first cleavage) plane and are approximately transverse in direction. In the ventral hemisphere the reverse is the ease; in the anterior-ventral cells the fourth cleavage furrows are approximately transverse in direction, in the posterior-ventral cells approximately antero-posterior. Thus it comes about that two of the anterior-dorsal cells do not reach the mid-line, while all of the posteriordorsal cells do; and that two of the posterior-ventral cells do not reach the midline, while all of the anterior-ventral ones do.
The fact that each hemisphere is thus the mirrored image of the other with
respect not only to the width of the anterior and posterior parts, but also as to
the direction of the fourth cleavage spindles and in the positions of the resulting
cleavage cells, this fact has contributed to the difficulties which most students of
ascidian embryology have experienced in distinguishing the dorsal and ventral
hemispheres, and has probably been responsible in some cases for the confusion of
those hemispheres. However, at this stage as at ever}- other, the two hemispheres
are easily distinguished by the relative amounts of yolk and protoplasm at the two
poles as well as by the position of the crescent and of the polar bodies.
All of the cell divisions of this cleavage are approximately equal, except that
of the posterior-dorsal cells, B 41 and B 41 . These cells divide very unequally, giving
rise to two small posterior cells, B 52 and B 52 , which are the smallest in the entire
egg (figs. Ill, 113, 180). Since the work of Van Beneden and Julin. these cells
have been observed by all who have studied the ascidian cleavage, and they have
served as the most important landmark in the orientation of the cleavage stages.
In this stage as in the preceding one. the yolk is most abundant in the cells
of the dorsal hemisphere; the protoplasm, in those of the ventral hemisphere; while
the yellow protoplasm is almost entirely confined to the posterior cells of the dorsal
hemisphere (figs. 36, 37, 38). In stained preparations the limits of the yolk and
protoplasm are sharp and distinct, and are represented in the drawings by a crenated line (figs. 108, et seq.). The relative amounts of yolk and protoplasm at the
two poles can he readily seen by comparing figure 110 with 111, and 112 with 113.
The yolk and protoplasm of the four ventral cells are about equally distributed to
their eight daughter cells; the same is true of the two anterior-dorsal cells, which
divide so that each of their four daughter cells contains about the same proportion
of yolk and protoplasm (A 51 , A 5 - 2 , Figs. 36, 37, 113). However, in the division of
the posterior-dorsal cells, the daughter cells are qualitatively very disimilar ; the
small posterior cells (B 5 - 2 ) consist almost entirely of yellow protoplasm, while their
larger sister cells (B 5 - 1 ) are about half and half, yellow protoplasm and yolk. The
outlines of the yellow protoplasm or crescent are perfectly distinct as shown in figure 37, and the formation of the .small posterior cells shows most beautifully that
the cleavage planes do not necessarily follow the lines of demarcation between the
yellow protoplasm and the yolk ; for in this case they cut across those lines so that
the small posterior cells contain a wedge of yolk in addition to the yellow protoplasm (figs. 37, 113, 117). This yolk is later obscured by being covered by the
yellow protoplasm (fig. 39, et seq.), but when the posterior cells are first formed it
is quite distinct. These small posterior cells contain not only yellow protoplasm
and yolk, but also those caps of clear superficial protoplasm which later go into the
small posterior mesenchyme cells. These cannot be seen in the living egg, but are
very evident in stained preparations (tigs. 113, 115, ISO, 187).
The localization of yolk and protoplasm at the vegetal pole is now practically
the same as at the beginning of gastrulation, and it is clearly indicative of the location of definitive organs. The relative positions of the yolk and yellow protoplasm
are the same in the 16-cell stage shown in figure 37, as in the early gastrula stage
shown in figure 46. The area of yolk, free from protoplasm, which surrounds the
vegetal pole (figs. 37. 111. 113), gives rise to the endoderm of the gastrula, the
tongue of yolk which runs back between the arms of the crescent (figs. 37. 113)
gives rise to the caudal endoderm cord of the larva, while the greater breadth of
the yolk in front of the second cleavage plane (fig. 37, 113) is indicative of the
great transverse extent of the endoderm of this region in later stages (fig. 4(i, ct
seq.). The protoplasm of the anterior-dorsal cells is located at the anterior borders
of those cells (figs. 37. 113). and in this region the notochord and neural plate cells
later arise. In all these respects the localization of these substances is of direct
prospective significance : in fact one may go further and say that all the regions of
the gastrula and certain important organs of the later larval stage are here
actually marked out on the egg at the i6-cell stage. This is no ideal mapping out
of the egg into organ forming germ regions, but an actual localisation of strikingly
different substances which need only to be followed through the development to
prove that they give rise to definite organs which occupy the same relative positions
in the larva, and are composed of the same peculiar substances, as in the early
cleavage stages or even in the unsegmented egg.
5. Fifth Cleavage; 16- 32 cells
(Figs. 3'.>-42, 116-11'.). 1S'.)-195).
The fifth cleavage does not occur simultaneously in all the cells of the fifth generation, but divisions appear in the cells of the vegetal or dorsal hemisphere before they do in those of the animal or ventral hemisphere digs, llli-118. lS'.i. I'M)). In Cynthia the anterior-dorsal cells divide a little earlier than the posteriordorsal ones (fig. 117). and the anterior-ventral cells a little in advance of the posterior-ventral ones (lii:. 118). In Cioua. also, the cells of the dorsal hemisphere divide before those of the vental, but there is practically no difference in the time of division of the anterior and posterior cells of this hemisphere. Neither at this stage nor at any preceding or succeeding one are the cleavages more rapid or the cells more numerous at the posterior than at the anterior pole, as claimed by Van Beneden and Juliti for Clavellina (1884, p. 13). Although this cleavage may be subdivided into 20-cell, 22-cell and 24-cell stages, the duration of each of those stages is very brief, and the fifth cleavage is completed in all the cells before the sixth appeal's.
Castle ( IS'.IC. p. 229), in particular, has described the differences in the time of
division of the cells of the dorsal and ventral hemispheres, and has made it a principal evidence in favor of his scheme of orientation. The fact that at this and at
the succeeding cleavage the cells of one hemisphere divide earlier than those of the
other has been accepted by him as proof that the earlier dividing hemisphere is
ventral and ectodermal, while the more slowly dividing one is dorsal and endodermal, since, at the time of gastrulation, the number of cells of the ectodermal
hemisphere is greater than that of the endodermal. But neither the fifth nor
the sixth cleavage results in the formation of more cells in one hemisphere than
in the other, since all the cells of both hemispheres divide before the next cleavage
begins; at the close of the fifth cleavage there are sixteen cells in each hemisphere,
and at the close of the sixth cleavage thirty-two cells in each hemisphere. In the
seventh cleavage, as we shall see. the hemisphere in which divisions were slower at
the two preceding cleavages becomes the more rapidly dividing one, and thereafter
the number of cells is more numerous in this hemisphere than in the opposite one.
In the anterior-dorsal cells the fifth cleavage spindles are parallel with the
median plane and are obliquely posterior-dorsal and anterior-ventral in direction
(fig. 117); four of the resulting daughter cells (A 6,2 , A 6 - 4 ) lie around the anterior
border of the egg just below the equator, while the other four (A 111 , A 6 - 3 ) form a rowacross the dorsal surface of the egg just in front of the second cleavage plane (fig.
117). The former are composed of yolk and protoplasm in about equal parts, and
give rise to chorda and neural plate cells; the latter are rich in yolk, but have little
protoplasm and give rise to endoderm.
The four posterior-dorsal cells divide a little later than the anterior ones, and the
spindles lie approximately in a transverse direction (figs. 117, 189). The protoplasm
of these cells is chiefly crescent substance ; the small posterior cells (B 52 ) are almost
entirely composed of this substance, while the larger cells (B 3-1 ) are composed of this
substance and yolk in about equal proportions, the former occupying the outer half
of the cell and the latter the median half. These larger cells divide equally so as to
cut off all of this crescent substance and a small amount of yolk in the lateral
daughter cells and to leave hut little protoplasm and much yolk in the median ones
(figs. 37. 39). This division occurs at the 20-cell stage, and when it is completed all
of the mesodermal or crescent substance is finally and completely separated from
the endoderm, and, except for a small amount of yellow protoplasm which lies close
around the nuclei of many of the blastomeres, all the crescent substance is contained
in the four cells which form the posterior border of the dorsal hemisphere (figs. 39,
40). The small posterior cells divide a little later than these larger ones and
unequally, the median daughter cells being smaller than the lateral ones (figs. 41,
\'i. 119). Thus there come to he six mesodermal cells, three on each side of the
mid-line, during this cleavage.
Divisions begin in the ventral hemisphere before they are finished in the dorsal (figs. 118, 19] ). Tn the most anterior and posterior pairs of cells of this hemisphere (a 5-3 , b 5-4 ) the spindles are nearly parallel with tlie median plane; in the two
remaining pairs of cells (a 5 - 4 and b 5-3 ) the spindles are oblique from posterior-ventral
to anterior-dorsal (figs. 118, 191). The division of the anterior pair of cells (a 53 ,
tins. 1 IS, 119) gives rise to a couple of cells (a 6-5 , a'"') which lie just above the equator and in contact with the chorda-neural-plate cells of the dorsal hemisphere.
Later development shows that these cells form part of the anterior portion of the
neural plate; the only other cells of the ventral hemisphere which enter into the
formation of this plate are portions of the cells a 6r , which lie on the lateral borders
of the cells a 6,5 . All of the cells of the ventral hemisphere are of practically the
same size and constitution ; each consists of a superficial layer of protoplasm, in
which the nucleus lies, and a deeper layer of yolk, the cells of this pole being
decidedly protoplasmic as compared with those of the opposite pole.
The result of this cleavage is the formation of sixteen cells in each hemisphere which may be tabulated as follows :
Ventral hemisphere
14 ectoderm cells, protoplasmic. 2 neural plate cells, protoplasmic. Dorsal hemisphere
6 endoderm cells, yolk laden.
4 chorda-nerve 1 cells, yolk and protoplasm.
6 mesoderm cells, yellow protoplasm or crescent substance.
32 cells.
At the (dose of this cleavage the cells of the ventral hemisphere are smaller in superficial area than those of the dorsal hemisphere; when viewed from the ventral pole the dorsal cells are seen around the entire periphery of the egg, except at a point on the right and left sides where a single ventral cell (b liD , b 6-B ) occupies the periphery; this is the only cell of the ventral hemisphere which can lie seen from the dorsal pole (figs. 11!), 192, 193). A similar condition prevailed at the close of the preceding cleaving (figs. 116, 117, 190), the only cells of the ventral hemisphere which could be seen from the dorsal pole being b 5:i and b 5 - 3 . This condition may be traced still farther back to the 8-cell stage (figs. 108, 1 L0, L84) where the ventral cells are smaller than the dorsal ones and where the only portion of the ventral hemisphere which lies below the general plane of the equator is that part of each of the posterior-ventral cells (b 4,2 , b 4 - 2 ) which meets the anterior-dorsal cell in the cross furrow (figs. 108, 184).
At the close of the fifth cleavage the superficial area of the ventral cells is
smaller than at any preceding stage and that of the dorsal cells is greater; this is
due to a change in t lie shape of the cells, the ventral cells becoming long and columnar, while the dorsal cells become relatively broad and lint (text figs. XI. XII,
XIX. XX). This change of shape has been observed and commented upon bj
Van Beneden amilulin. Samassa, and Castle, and both of the latter authors attribute the columnar form of the ventral cells to the pressure exerted upon them by
the overgrowth of the cells of the dorsal hemisphere; both regard this overgrowth
as the beginning of gastrulation (epibole). Whatever may he the cause of the
shapes of the cells at the two poles, whether purely mechanical or not. it is certain
that this is not the beginning of gastrulation, since, as I will show later, the
columnar cells of this stage become the flattened ectoderm cells of Later stages,
while the flattened cells of this stage become the columnar endoderm cells of the
gastrula.
- 1 Throughout this paper the cells which are to give rise to chorda, nerve, muscle and mesenchyme are, for the sake of brevity, frequently referred to as if they had already given rise to these
structures.
6. Sixth Cleavage
32-64 cell. (Figs. 4:5-45, 120-130, 194-197).
In this cleavage the divisions are not synchronous, the cells of the dorsal hemisphere dividing before those of the ventral as in the preceding cleavage, and some of the cells in the posterior half dividing later than those in the anterior one. Accordingly it would be possible to sub-divide the period between the 32-cell and (14 -cell stages into a 44-cell, a 46-celI and a 48-cell stage, as Castle does. These stages, however, are of brief duration and all the cells of the sixth generation divide before any of the seventh do; therefore, the sixth cleavage is distinct from preceding and succeeding ones.
The spindles appear in the four chorda-neural-plate cells at the anterior border of the dorsal hemisphere in a nearly dorso-ventral direction. The four ventral produets of this division (A' 4 , A 78 ) form a band of small cells around the anterior border of the egg just dorsal to the equator; these cells ultimately give rise to the posterior part of the neural plate; the dorsal products (A 7-3 , A 7 - 7 ) give rise to the chorda (figs. 119-123). The neural plate cells are small and contain little or no yolk, whereas the chorda cells are larger and are yolk-laden (text figs. XIX, XXI. XXIII); this cleavage of these cells is therefore markedly differential.
While these cells are dividing, all of the endoderm cells divide; these are the four median cells which meet at the vegetal pole (A" 1 . A" 1 . IV' 1 . ]\ M ). and a single pair of cells which lie lateral to these and in front of the transverse (second cleavage) plane (A 6-3 , A 6 - 3 ). The spindles in the median cells are antero-posterior in direction, while those in the lateral cells are nearly transverse (figs. 120, 121, 123). These divisions are equal and non-differential in the median cells; in the lateral cells the division is differential, the inner product (A 75 ) being rich in yolk, the outer (A 7 - 6 ) containing more protoplasm : the former is an endoderm cell, the latter, according to Castle, mesenchyme. By these divisions ten endoderm cells are produced, live on each side of the mid-line, and two mesenchyme cells (figs. 44-46).
While the divisions of the endoderm and chorda-neural-plate cells are occurring, the most anterior mesoderm cell (B 02 , B 1 '-). forming the point of the crescent on each side, divides, the spindle lying in a nearly dorso-ventral direction (figs. 43, 44, 120-129, 193, 194). This division, in fact, sometimes slightly precedes that of the anterior cells. The products of this division are nearly equal in size hut are qualitatively dissimilar, the dorsal one (B 7,3 ) containing less of the yellow protoplasm and more yolk than the ventral one (B 74 ). This difference between these daughter cells is plainly visible in the living condition, the dorsal cell being a fainter yellow than the ventral one (figs. 43-48, et seg.); in preparations the dorsal cells always stain more deeply than the ventral ones, owing to the greater quantity of clear protoplasm which they contain (text fig. XXII). This difference in the constitution of these cells corresponds to a difference in their fate; the dorsal cells give rise to mesenchyme, while the ventral ones produce some of the muscle cells of the tail of the tadpole.
The division of these twelve cells of the dorsal hemisphere are practically synchronous, and they advance the egg from the 32-cell to the 44-cell stage. A little later the second cell of the crescent on each side of the mid-line (B 6 - 4 ) divides, its spindle standing in a nearly dorso-ventral direction (figs. 45, 127-42'.>). The dorsal daughter cell (B 77 ) in this case also contains less yellow protoplasm and more yolk than the ventral one (B 78 ), and like the cell which immediately adjoins it anteriorly (B 73 ) gives rise to mesenchyme, while the ventral moiety becomes a muscle cell. By this division the mesenchyme and muscle substance of the crescent are finally and completely segregated into separate cells, and the number of cells in the crescent is advanced to ten, and in the entire egg to forty-six. This division of the cell B 64 is sometimes delayed until the cells of the ventral hemisphere are dividing (figs. 127-129), and a 46-cell stage is therefore not always present. The division of the last remaining cells of the dorsal hemisphere, the middle cells of the crescent (B 63 B 63 ), is delayed until divisions are well advanced in the ventral hemisphere, and it may occur even after the ventral cells have divided (fig. 47). I do not find, therefore, that there is commonly a 48-cell stage such as Castle describes.
The divisions of the cells of the ventral hemisphere are all synchronous, as figures 124 to 121) show. The direction of the spindles in the different cells is so different that it is difficult to give an exact description of them. In the four median cells which surround the animal pole (a 6-8 , b' is and their fellows of the right side) the spindles are transverse; the spindles are also nearly transverse in the most anterior and most posterior pairs of cells (a 65 . b a7 ); in the only other pair of cells which meet along the mid-line, the second pair in front of the animal pole (a 65 ), the spindles are nearly antero-posterior. In the other three pairs of cells of this hemisphere (a 6-7 , b 65 , b 66 ) the spindles are oblique in position, and their directions can he best appreciated by consulting the figures {v. figs. 124, L96). The most anterior pair of cells (a 6,5 ) are neural plate cells; these cells divide transversely (figs. 124126), forming a transverse band of four cells just above the equator; on each side of these a single cell (a 7 - 13 , fig. L30) is added at the close of this cleavage which completes the number of neural plate cells that are derived from the ventral hemisphere. In figure 130 the hand of six cell (a 7 ' 9 , a 710 , a 713 and their fellows of the right side) which lie around the anterior border of the ventral hemisphere are these neural plate cells.
All the divisions of the cells of the ventral hemisphere are equal, and all the daughter cells are similar in appearance. By this cleavage the cells of the ventral hemisphere are increased to thirty-two, and when the small posterior cells (B 6,8 , B 6-8 ) of the dorsal hemisphere have divided there are thirty-two cells in this hemisphere also, or sixty-four in the entire embryo, all in the seventh generation (figs. 130, 131). Tabulating these facts we find that there are at the close of the sixth cleavage the following cells : Ventral hemisphere
26 ectoderm cells, protoplasmic.
6 neural plate cells, protoplasmic. Dorsal hemisphere
Hi endoderm cells, yolk laden.
4 chorda cells, yolk laden.
4 neural plate cells, protoplasmic.
4 mesenchyme cells, light yellow protoplasm.
2 anterior mesenchyme cells, clear protoplasm.
2 posterior mesenchyme cells, clear protoplasm.
6 muscle cells, deep yellow protoplasm. ti4 cells. At the beginning of the sixth cleavage the cells of the ventral hemisphere are narrow and columnar, while those of the dorsal hemisphere are broad and flat (text figs. XI. XII, XIX, XX). This condition prevails up to the 44-cell stage when the cells of the ventral hemisphere begin to divide. During their division the ventral cells become shorter and broader, and at the same time the dorsal cells, which have passed into a resting stage, grow more columnar and much smaller in surface area, and before the close of this cleavage the cells at both poles are columnar and of about the same height (text figs. XIII. XIV, XXI). This change in the shape of the cells of the two hemispheres, which begins during the sixth cleavage, is not completed until the seventh cleavage of the ventral cells (figs. 133. 134. 198-204). During this change of shape there is no difficulty in distinguishing the two hemispheres, for the endoderm cells are filled with yolk ami the mesoderm cells with yellow protoplasm or crescent substance, whereas the cells of the ventral hemisphere are largely protoplasmic (text figs. XVII-XXIV). Moreover, the polar bodies are often attached to the egg at its animal pole throughout the whole of this period (cf. figs. 1U0-204).
I have already discussed the views of Van Beneden and Julin, of Samassa and of Castle relative to the shape of the cells of the two hemispheres. Although Van Beneden and Julin showed by their figures that the dorsal cells of the 32-cell stage of Clavellina are fiat and the ventral ones columnar, whereas the dorsal cells of the 44-cell stage are columnar and the ventral ones fiat, they did not observe nor attempt to explain this change of shape. On the other hand, as we have seen, Samassa and Castle denied that such a change of shape took place, and they therefore reversed Van Beneden and Julin's orientation of all stages before the 44-cell stage. I have already given what seems to me satisfactory and sufficient evidence in favor of the orientation of Van Beneden and Julin, and against that of Samassa and Castle, and I need not repeat that evidence here.
Turning now to a detailed study of the observations of Samassa and Castle during this critical sixth cleavage, we find that Samassa did not attempt to follow the cell-lineage further than the 48-cell stage (his tig. 9), but jumped at once from this stage to one with at least 76 cells (his fig. 10). His orientation of all stages up to and including the 48-cell stage (his fig. 9) is the reverse of that of Van Beneden and Julin, and is wrong; his orientation of the gastrula, shown in Lis figure 10, is right. Therefore, in the interval between his figures 9 and 10 he has inverted the egg so that the dorsal face of his figure 10 corresponds to the so-called ventral face of all preceding figures. 1
Castle, on the other hand, has traced the cell-lineage much further than the 48-cell stage, and it is therefore possible to follow in detail the manner in which lie passes from the erroneous orientation of earlier stages to the correct orientation of later ones. He has given correctly the lineage of every cell up to and including the 46-cell stage (his figs. 55 and 5(i), as I have convinced myself by comparing his figures, cell tor cell, with my own. but his orientation of these stages should be reversed. On the other hand his orientation of all stages later than the 46-cell stage is correct, but the cell lineage of these stages is wrong. This is due to the fact that between the 46-cell and the 48-cell stages (his figs. 56 and 57) he has inverted the egg so that the dorsal surface of all stages later than the 46-cell stage corresponds with the so-called ventral surface of all earlier stages. 2 This inversion of the egg introduces many profound errors in the cell-lineage after the 46-cell stage.
Considering in detail Castle's account of this sixth cleavage we find that be has correctly represented the divisions of the cells of the real dorsal hemisphere which bring these cells up to the seventh generation and the entire egg up to the 46-cell stage (his fig. 55). At this stage the cells of the ventral hemisphere are still in the sixth generation (:-. his fig. 56). and this stage is almost exactly comparable with my figures 1 1 9 to 1 23. Immediately after this, in the 4S-cell stage (his figs.
57 and 58), Castle supposes that the cells of the real dorsal hemisphere, which are now in the seventh generation, divide again, thus passing into the eighth generation, while the sixth generation cells at the opposite pole are supposed by him to remain undivided. It is absolutely essential to his scheme of orientation that the cells of one hemisphere should remain in the sixth generation, while those of the other hemisphere are advancing to the seventh and eighth generation. If it could be shown that all the cells of both hemispheres divide during this sixth cleavage it would completely break down Castle's orientation of the earlier stages and his cell-lineage of the later ones. In all of his figures of this cleavage (figs. 55, 56. 57, 58) Castle represents the cells at one pole in process of division while those at the other pole are in the resting condition. However, in two of my figures of this cleavage in Ciona (figs. L 96 and 197), which represent ventral and dorsal views of one and the same egg, the cells at both poles are seen to be in process of division, and the only cells in the entire embryo which are not dividing are the small posterior cells (B 6-3 ). The cells of the dorsal hemisphere are in the late anaphase or telophase, and their nuclei are still small and densely chromatic; the cells of the ventral hemisphere are all in the equatorial plate stage. These figures show most conclusively that all the cells of the embryo divide during this sixth cleavage and are advanced from the sixth to the seventh generation, and they therefore make impossible Castle's view that the eel Is of the dorsal hemisphere remain undivided, while those of the ventral hemisphere divide twice. Another evidence that the cells which are shown dividing in his figures 57 and 58 are not the same ones which have just divided in his figure 55 may he found in the fact that but sixteen of these cells are shown dividing in the former figures, whereas the other sixteen cells, which, according to Castle, belong to the ventral hemisphere, are in the resting stage, exactly as are the sixteen cells which immediately surround the dorsal pole; at the two previous cleavages, and as I have found also, at the two subsequent ones, all the cells of the ventral hemisphere divide simultaneously, and this fact speaks against Castle's view that at the 48-cell stage one-half of these cells divides ami the other half does not.
- 1 In his explanation of figures he says that figure '> is viewed from the cephalic pole; this is, of course, a verbal error, since his lettering of the cells shows plainly that the egg is viewed from the caudal pole.
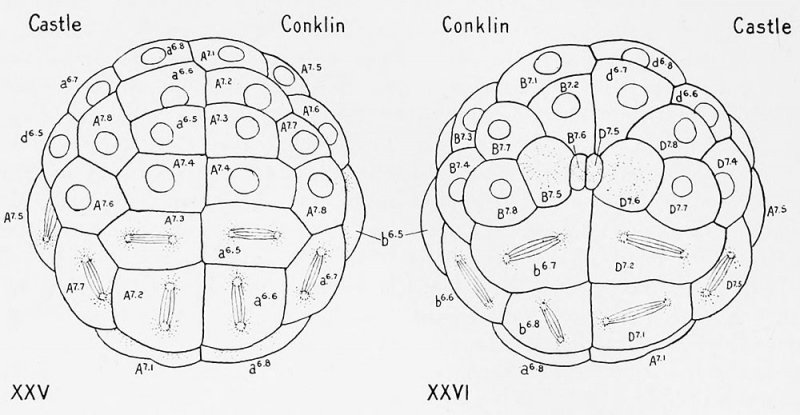
- 2 Unfortunately Castle gives no dorsal, ventral nor lateral views of this critical 48-cell stage at which the inversion occurs, but only an anterior and a posterior view (his figs. 57 and 58, reproduced in text tigs. XXV and XX VI (if this paper).
Since the dorsal hemisphere, shown in his figure 55, contains twenty-eight cells of the seventh generation and two of the sixth, while the ventral hemisphere shown in figure 56 contains only sixteen cells of the sixth generation, it is evident that if the egg is inverted in its orientation at this stage the equator must be shifted nearer to the dorsal hemisphere so as to reduce the number of dorsal cells to sixteen and to increase the number of ventral cells to thirty, or, after the division of the two small posterior cells, to thirty-two. This is just what Castle has done; in his description of the 48-cell stage (pp. 238, 239) he says that at this stage the embryo is composed of three zones of sixteen cells each, as follows :
Ventral hemisphere
16 cells of the seventh generation, ectodermal group.
16 cells of the seventh generation, equatorial hand. Dorsal hemisphere
16 cells of the sixth generation, endoderm, chorda and mesoderm.
1 8 cells. Immediately after this stage the 64-cell stage is reached by the division of the sixteen cells of the ectodermal group. Castle has tabulated the cells of this stage as follows :
Ventral hemisphere
32 cells in the eighth generation, ectodermal group. 16 cells in the seventh generation, the equatorial band.
48 Dorsal hemisphere
16 cells in the sixth generation. lil cells.
As it can be proved that no cells of this stage remain in the sixth generation, but all have passed into the seventh, it is certain that the equator both here and in the 48-cell stage is in the wrong place, that it really lies between his equatorial hand and the ectodermal group, and that there are therefore thirty-two cells in each hemisphere in the 64-cell stage.
Wholly apart, therefore, from the perfectly conclusive evidence as to the orientation of the egg and embryo which may be drawn from the histological character of the cells at the two poles, as well as from the location of the polar bodies, it can be shown by a detailed study of the cell-lineage that Castle has inverted the egg at the 48-cell stage, transferred sixteen cells from the dorsal to the ventral hemisphere and consequently shifted the equator of the embryo at least one cell row nearer the dorsal pole than it should be. Of course the lineage of every cell is thereby profoundly changed ; the oi
Figs. XXV and XXVI. Surface views of eggs of Cionn intestinalis; copied from Castle's figures 5T and 58 (1896). Fig. XXV represents an anterior view ; Fig. XXVI a posterior one of the same egg. Tlie orientation and cell-lineage, according to Castle, are indicated by the designations of the cells in that half of the egg under his name; the designations of the corresponding cells in the other half of each figure shows the system of orientation and cell-lineage adopted in this paper. Owing merely to differences in nomenclature the cells in the right half of Fig. XXVI are designated by the letter D, those on the left by the letter B. Everywhere lower-ease letters designate cells of the animal (maturation) hemisphere; capitals, cells of the hemisphere opposite the maturation pole. The equator lies between the cells designated by lower-case and capital letters.
lis which retain a semblance of their former names throughout this revolution arc the small posterior cells (C 75 . D 75 of Castle's system). and their sisters (C 7,li . D"' 1 ), the most anterior cells of the crescent of each side (C 74 , D 7 - 4 ), and the most anterior pair of cells of the dorsal hemisphere (A 7-4 , B 74 ). Even in the case of these four pairs of cells the right and left cells of each pair are interchanged, so that everywhere A should replace B, and C, D.
In subsequent stages Castle does not always preserve the same designations for
given cells. For example, the cell which in his figure 58 is labelled A 7-8 becomes
a 6,7 in fig. 00 ; a 6-7 of figure 58 becomes d 6 - 5 of figure GO, while the one labelled d' ! - 5
in the former figure becomes A 7-6 in the latter. Strangely enough this last cell
which had been variously located in the dorsal and ventral hemispheres, and in the anterior and posterior quadrants is finally brought back to its right position and
given its true designation. In all stages later than his figure 60 the designation
A" stands for the same cell in Castle's figures and in my own.
The changes in the designations of the cells which arc brought about by this
inversion of the orientation at the 48-cell stage may be most easily seen and appreciated by a reference to the accompanying text figures XXV and XXVI, where the
designations of the cells, according to my interpretation, are given on the righl side of figure XXV and on the left side of figure XXVI, while Castle's designations of the corresponding cells are given on the Left side of figure XXV and on the right side of figure XXVI. Barring the exceptions mentioned in the preceding paragraph. Castle has followed with substantial accuracy the subsequent lineage of the dorsal hemisphere up to a stage of about one hundred and twelve cells, though always upon the basis of his erroneous lineage of the 48-cell stage. With the exception of a single pair of cells, I need not further explain my departure from Castle's nomenclature of the later stages. This exception is the pair of small posterior mesenchyme cells which Castle designates C 7,5 , D 75 ; inasmuch as I find that they lie ventral to their sister cells. I shall designate them B' 6 , B 76 , and their more dorsally placed sister cells B 75 , J3 r ' 5 .
With the completion of the sixth cleavage we reach a period when the gastrulation is ready to begin. Already preparations for the gastrulation are apparent in the changing shapes of the cells of the dorsal and ventral hemispheres, in the relative positions of the cells and in the directions of their divisions. Even the peculiar type of the chordate gastrula, with its overgrowing anterior lip and its nearly stationary posterior one, is foreshadowed at a very early stage in the eccentric position of the animal and vegetal poles in the two hemispheres of the egg.
In the 32-cell and 64-cell stages it is apparent that the animal and vegetal poles do not mark the middle of the ventral and dorsal faces of the embryo. This was
first noticeable in the 4-cell stage of Cynthia where the two posterior cells are smaller than the anterior ones. In the 8-cell stage the anterior-ventral cells are elongated antero-posteriorly. while the posterior-ventral ones are elongated transversely: this brings the animal pole still farther back of the middle of the ventral face. In the 16-cell stage there are two pairs of cells adjoining the mid-line in front of the animal pole and but one pair behind it ; in the dorsal hemisphere there is one pair of such cells in front of the vegetal pole and two behind, but the most posterior pair is smaller than the others, so that the vegetal pole lies near the middle of the dorsal face. In the 32-cell stage three pairs of cells adjoining the midline are in front of the animal pole, two behind it; while in the dorsal hemisphere there are two pairs of such cells in front of the vegetal pole and two behind it. though here again the most posterior cells are very small ones. In the 64-cell stage (digs. 130, 131) there are four pairs of cells adjoining the mid-line in front of the animal pole and only two pairs behind it; while in the dorsal hemisphere there an- four pairs of such cells, both in front of and behind the vegetal pole, but the
most posterior pair are the tin\ mesenchyme cells (B 7 - 6 , B 7 - 6 ) which are partly covered by their sister cells (B 75 , B 75 ). Thus the vegetal pole is slightly posterior to
the middle of the dorsal lace and the animal pole is decidedly posterior to the middle of the ventral face in all of the stages mentioned, and this condition becomes
even more pronounced in later stages; thus in the 124-cell stage (fig. 139) there are,
ventral to the equator, six pairs of cells adjoining the mid-line in front of the animal
pole and four behind it. while in the 184-cell stage (fig. 143) there are eight pairs of such cells in front of the animal pole and five behind it; in the 2lN-cell stage (fig. 144-147) there are ten such pairs of cells in front of the animal pole and only five behind it. All of the cells of the ventral hemisphere are of approximately the same size, so that in these later stages it is evident that the animal pole lies far back of the middle of the ventral hemisphere. This location of the animal pole posterior to the middle of the ventral hemisphere is due in the first instance to the smaller size of the posterior cells in the 4-cell stage and then to the fact that the prevailing position of the spindles in the anterior cells of tins hemisphere is parallel with the median plane, while in the posterior cells it is transverse. It is not due. as might at first thought seem to be the case, to the more rapid growth and division of the anterior cells of the ventral hemisphere since all of these cells divide at nearly the same time and are of approximately the same size. The irrespective significance of this eccentric location of the animal pole maybe found in the greater length of the anterior lip of the blastopore, as compared with the posterior lip.
6. Gastrulation
Seventh to Ninth Generation of Cells, 64-218 Cells.
In both Ciona and Cynthia the gastrulation actually begins during the seventh
cleavage and it is far advanced by the close of the eighth, though the closure of the
blastopore and the completion of the gastrulation does not occur until about the end
of the tenth cleavage. 1 have followed the lineage of every cell through the seventh
cleavage and of almost all the cells through the eighth, and have therefore been able
to determine the part which each cell takes in the formation of the gastrula. At
no time after the 64-cell stage are all the cells of the embryo in the same generation.
From this time toward the endoderm cells lag behind the ectoderm and mesoderm
cells in division; the eighth cleavage occurs in the ectoderm and mesoderm before
the seventh is finished in the endoderm. Therefore the periods of the seventh and
eighth cleavages cannot be sharply separated, but for the sake of convenience we
shall consider these two cleavages as if they were distinct.
7. Seventh Cleavage
64-76, 76-112 cells. (Figs. 46-51, 130-139. 198-204.)
The seventh cleavage begins in the anterior quadrants of the dorsal hemisphere
in the two pairs of chorda cells (A 7:i , A 77 ) and in the two pairs of neural plate cells
(A 74 , A 78 ); in the posterior quadrants it begins in the two most anterior cells of the
m\scent on each side, the pair of muscle cells, B 74 . and the pair of mesenchyme
cells, B 73 (figs. 130-132). With the exception of the two mesenchyme cells the
spindles in all of these cases are parallel with the plane of the equator and with the
surface of the egg; in the mesenchyme cells the spindles, when seen from the dorsal side, are directed obliquely forward, outward and ventralward (figs. 131, 132).
These divisions, when completed, give rise in the anterior quadrants to four chorda
cells (A 8 - 5 , A 8 - 6 , A 818 , A. & "), and to four neural plate cells (A 87 , A 8 - 8 , A. 815 , A 818 ) on
each side of the mid-line, which are arranged in two rows of eight cells each
running around the anterior border of the dorsal hemisphere (figs. 134, 200). All
these divisions are equal and non-differential.
In the posterior quadrants the division of the anterior muscle cell on each side gives rise to two daughter cells (IT'. I'> ss ). one in front of the other, which are alike in size and quality. The mesenchyme cell (B 7r ) divides unequally and differentially giving rise to a small yolk-laden cell (B 88 ), lying anterior, lateral and ventral to its large sister cell (B 8 '). which is more protoplasmic (figs. 134. 200). The former is, according to Castle, the "posterior chorda fundament" while the latter is mesenchyme. I have been unable to find sufficient evidence that this small cell is later incorporated in the chorda, but on the other hand do not wish to deny that this is the case. Since it is derived from the mesenchyme I prefer to class it with the mesenchyme cells until its fate is more certainly known. These divisions advance the embryo to the 7G-cell stage and the distribution and generations of the cells may be summarized as follows : Ventral hemisphere
Ectoderm 7th generation 26 cells.
Neural plate 7th " G "
Dorsal hemisphere
Endoderm 7th " 10 "
Chorda . . . . 8th generation 8 cells.
Neural plate 8th " 8 "
. Muscle . . 8th " 4 " 7th " 2
Mesenchyme 8th " 4 " 7th " 8
a
sth generation 24 cells. 7th generation 52 cells.
o
76 cells. The classification of the cells of this stage into chorda, neural plate, muscle and mesenchyme. 1 must of course be based upon the later history of these cells, but even at this early stage important differences may be recognized in the histological characters of the cells named. In the living eggs of Cynthia the endoderm cells are slate-gray in color and are filled with yolk; the chorda cells are lighter gray and contain less yolk than the endoderm cells; the ectoderm and neural plate cells are clear and protoplasmic ; while the muscle and mesenchyme cells are yellow, the former being more deeply colored than the latter. In the main my classification of the cells of this stage agrees with that of Castle; the most important difference concerns the muscle cells which he classes as " neuro-muscular cells." Owing to the striking color of these cells in Cynthia their later history can be followed with relative ease; they ultimately give rise to the three rows of muscle cells in the tail of the tadpole (figs. 58, 59), and so far as 1 am able to determine do not contribute anything to the nervous system. In general the mesenchyme cells may also be traced by their faint yellow color until they give rise to the mesenchyme of the tadpole, and, with the exception of the small cell which Castle calls the posterior chorda fundament," I agree with him as to the fate of these cells. Castle figures and describes the cell, A 70 , as the most anterior of the mesenchyme cells. 1 do not find that it contains yellow protoplasm in Cynthia, but its histological structure is different from that of the other endoderm cells; I shall therefore follow Castle in classing it as a mesenchyme cell. The median cells of the crescent (B 7S ) resemble in their deep yellow color, the muscle cells rather more than the mesenchyme, and Castle reckons these cells with the " neuro-muscular ring." but the later history of these cells shows that they lie just beneath the notochord and at the hinder end of the ventral endodermal cord in the tail of the tadpole (figs. 161-165) ; therefore, they do no give rise to the lateral muscles, and they are probably to be counted as mesenchyme cells.
See note p. 52.
At this stage, therefore, the endoderm consists of four pairs of cells meeting
along the mid-line (figs. 134, 200), and of one pair of laterally placed cells which lie
just in front of the second cleavage plane ; the chorda consists of an arc of eight cells
bounding the endoderm in front; in front of the chorda cells and below the equator
is an arc of eight neural plate cells. Posteriorly the endoderm is bounded by an
arc of twelve mesenchyme cells, while just outside these is an arc of six muscle
cells (eight, in lig. 134). The neural arc in front is separated from the muscle arc
behind by the most dorsally situated of all the ectoderm cells (b 817 ). But for this
lateral interruption it would be possible to speak of a " neuro-muscular ring" as
Castle does. The chorda and mesenchyme arcs form a continuous chorda-mesenchynie ring, as Castle has shown.
Castle asserts (1896, p. 246) that the mesenchyme "is made up of cells derived from both hemispheres and all four quadrants," and again that two cells " vis. d' '- and c 712 [my B 85 ] are the sole contribution of the dorsal hemisphere to the mesoderm of the larva" (p. 242). This is certainly not the case; the mesenchyme and muscle cells are derived entirely and exclusively from the dorsal hemisphere and largely from the posterior quadrants. The most posterior cells of the crescent on each side (B S1,; and its mate) are counted b\ Caslle as part of the ectoderm; their histological structure, color in the living egg of Cynthia and later history show that they are the most posterior of the muscle cells. Of two other cells of tbis stage, Castle says (p. 242). " it is noticeable that d 65 and its mate c 65 have been shoved forward out of their own quadrants to a position beside the endoderm cells derived from the anterior quadrants." These cells are really A 75 and its mate, as is shown by their origin and later history (figs. 120, et set/.), and do not belong to the posterior but to the anterior quadrants.
The 76-cell stage is of very short duration, for immediately after those divisions in the dorsal hemisphere which advance the embryo from the 64-cell to the 76-cell stage, all the cells of the ventral hemisphere divide simultaneously. The direction of the spindles in these cells is indicated by the equatorial plates represented in figures L98 to 203. In the posterior quadrants all the spindles are nearly antero-posterior in direction, except in those three ventral cells on each side (b 7,9 , b rj ". W "i which lie nearest to the dorsal surface and between the muscle cells behind and the neural plate cells in front (figs. 201, 203); in these cells the spindles are nearly vertical. In the anterior quadrants the spindles are antero-posterior in a transverse row of four cells which lies just in front of the animal pole (a 7-16 , ai: in the row of four cell just in front of this the spindles are transverse (a ;i \ a' "l: they are also transverse in a single pair of cells which meet at the midline just in front of the last mentioned row (a'"); in the most anterior row of the ventral hemisphere, consisting of six neural plate cells (a 7 - 10 , a 7,9 , a 7-13 ), the spindles are dorso-ventral in position, therefore, at the close of this division there are twelve neural plate cells in the ventral hemisphere, arranged in two rows of six cells each.
All of these divisions of the cells of the ventral hemisphere are synchronous, equal and non-differential, and they increase the number of cells in the ventral hemisphere to sixty-four and bring the whole number of cells in the embryo up to one hundred and eight.
Very soon after these divisions in the ventral hemisphere the posterior muscle cell (B 7-8 ) and one of the mesenchyme cells (B 7,7 ) of each side divide; in the former the spindles converge posteriorly and ventrally toward the median plane, in the latter posteriorly and dorsally. By this division two mesenchyme and two muscle cells are added to the total in the embryo which at this stage consists of one bundred and twelve cells (fig. 133. 134 1 ) which may be tabulated as follows : Ventral hemisphere
Ectoderm . .8th generation, 52 cells.
Neural plate 8th generation, 12 cells. Dorsal hemisphere '
Endoderm 7th gen., 10 cells.
Chorda .... 8th generation, 8 cells.
Neural plate . 8th " 8
Muscle .... 8th 8 "
Mesenchyme 8th " 8 " 7th " 6 "
8th generation, 96 cells. 7th gen., lb cells.
112 cells. The only cells in the entire embryo which have not passed into the eighth generation at this stage are the ten endoderm cells, and six mesenchyme cells, two of which are anterior, and four median and posterior: all of these cells except two, the small posterior mesenchyme cells ( l? 7 !. divide soon after this stage and thus pass into the eighth generation, but not until after other cell- have passed into the ninth. This stage, therefore, max be taken as representing, as nearly as may be found, the close of the seventh cleavage ami the transition to the eighth.
'The division in a pair of mesenchyme cells B 7-7 ) of this stage i> not completed; therefore, in the explanation of figures, this called a LlO-cell stage.
This stage is important as marking the beginnings of gastrulation for which preparations were made in preceding stages. The endoderm cells and the four posterior mesenchyme cells which remain in the seventh generation now lie at a considerably lower level than the surrounding cells. This, although usually spoken of as an invagination, can scarcely with right be called such, for as sections show there is neither at this stage, nor at any preceding one, any considerable blastocoel ; since there is no cavity into which the cells can push it is scarcely permissible to speak of their invaginating. In reality the gastrulation is due to two factors, neither of which is invagination. The first and most important is the change of shape of the cells, which has been described in part already; the second is the overgrowth of the cells him:' around the endodermal area.
As to the first we have already seen that in the 16-cell stage the cells at both poles are of about equal height ; during the fifth cleavage the cells at the animal pole become long and columnar, while those at the vegetal pole are broad and flat; during the sixth cleavage the cells at the two poles change shape so that at the close of this cleavage (64-eell stage) the cells of both poles are of nearly equal height, those at the vegetal pole being perhaps slightly longer than those at the animal pole. At this stage the cells of the dorsal (vegetal) hemisphere still have a larger surface area than those of the ventral (animal) hemisphere, so that when viewed from the vegetal pole only cells of the dorsal hemisphere^ can be seen, but when viewed from the opposite pole a peripheral row of dorsal cells can be seen around those of the ventral hemisphere (figs. 130, 131). During the seventh cleavage this change of shape progresses rapidly so that at the 76-cell stage the surface area of the dorsal cells is less than that of the ventral ones ; the endoderm cells in particular grow long and narrow, whereas the ectoderm cells become broad and fiat (figs. 198-204). After the seventh cleavage of the ectoderm and mesoderm cells (112-cell stage) a row of ventral hemisphere cells is visible all around the periphery of the dorsal hemisphere when the embryo is viewed from the dorsal pole (figs, loo, 134). The remarkable reduction in the surface area of the endoderm cells, which occurs without any division in these cells, and wholly by their change of shape will be best appreciated by comparing figures I'll with 134, and figures 197 with 200; in all of these figures the endoderm cells are in the seventh generation, but the superficial area of these cells in the two older stages is not more than half as great as in the two earlier ones. In proportion as these cells decrease in surface area they increase in depth, their inner ends become enlarged, and at the same time their nuclei are withdrawn from the surface to a deeper level in the cells (text figs. XXI-XXIV). The flattening of the ectoderm cells leads to their covering a larger and larger surface area until they finalty overgrow the marginal cells of the dorsal hemisphere. Samassa undertook to explain the columnar form of the cells at the animal pole in the 32-cell stage by the pressure exerted on them by the overgrowing cells of the opposite pole, but it is obvious that neither at this early stage, nor during the later one just described, can the cause of this change of shape be located in the cells of one hemisphere rather than in those of the other.
The second factor of the gastrulation, viz., the overgrowth of the cells surrounding the endodermal area has been well described by Castle. It is the result
in part of the first factor and also of the more rapid division of the cells of the ectoderm and the corresponding retardation of division in the endodermal cells. This
overgrowth occurs in the anterior quadrants from in front, the chorda cells overgrowing the endoderni and the neural plate cells the chorda : in the posterior quadrants it occurs from the sides, the muscle cells overgrowing the mesenchyme, and
the ectoderm the muscle cells. At three points this overgrowth is long delayed, at
the posterior pole where there is a dee]) notch In the blastoporic rim which persists
until the blastopore has nearly closed, and at the light and left sides of the blastopore where the overgrowth is slow. This leads to the peculiar form of blastopore,
wide in front and narrow behind, which is found among ascidians.
8. Eighth Cleavage
112-132 cells, 132-218 cells. (Figs. 135-147, 205).
The eighth cleavage first appears in the two anterior muscle cells of each side ( B 88 , B 87 ), the spindles being nearly transverse to the antero-posterior axis of the embryo (figs. 135, 136). This division is equal and non-differential, and there result four muscle cells on each side, an anterior pair (B 915 , B 916 ) and a posterior pair (B 913 , B 914 ). When first formed the median cells of each of these pairs lie at a higher level than the lateral ones (fig. 135) ; soon afterward the lateral and median cells are at the same level (fig. 136); still later the lateral ones lie at a higher level than the median ones (fig. 140). This is, of course, a result of the overgrowth, whereby the cells which were lateral in the blastopore lip come to overly those which were median in position.
At the same time that these muscle cells are dividing the pair of large mesenchyme cells, B 85 , divides, the spindles being obliquely antero-posterior and dorsoventral in direction (figs. 135, 136). This division is approximately equal and
non-differential, and gives rise to the mesenchyme cells B", .B 910 , which lie on each
side of the caudal endoderm cells.
While these divisions are proceeding in the mesoderm, and thereby advancing
these cells to the ninth generation, the delayed seventh cleavage appears in the
mesenchyme and endoderm cells. The first of these cells to divide is the most
anterior mesenchyme cell (A 7 - 6 ) ; the- spindles are here nearly dorso-ventral in
direction, and the resulting daughter cells (A su , A 8 - 12 ) are of about the same size,
though the dorsal cell contains more protoplasm than the ventral one, as Castle has
shown.
Coincidently with these divisions two pairs of endoderm cells (A 72 and B 72 ) divide, the spindles being approximately transverse in the anterior pair and anteroposterior in the'posterior one (fig. 135).
A little later the four endoderm cells which meet at the vegetal pole (A 71 , B 71 , fig. 1 36) divide, the spindles being antero-posterior in direction. The last remaining pair of endoderm cells of the seventh generation to divide is the lateral one (A 75 , figs. 135, 136) ; I have not seen this cell in division, but it is probable from a study of later stages that the spindle lies in it also in an anteroposterior direction. All of these divisions of the endoderm are equal and non-differential-.
Finally the median mesenchyme cells, B 75 . divide, the spindles being anteroposterior (fig. 136), and the resulting daughter cells alike in size and quality.
With this division all the cells of the embryo have passed into the eighth generation, except the small posterior mesenchyme cells (B 7 - 6 ) which never again divide, so far as I have observed ; and excepting eight muscle cells and four mesenchyme cells which have passed into the ninth generation. The following tabular statement summarizes the character and location of the cells at the close of this stage: Ventral hemisphere
Ectoderm 8th gen., 52 cells.
Neural plate 8th " 12 "
Dorsal hemisphere
Endoderm 8th " 20 "
Chorda 8th " 8 "
Neural plate 8th " 8 "
Muscle .... 9th gen., 8 cells. . . 8th " 4 "
Mesenchyme. 9th " 4 " ...8th " 14 " 7th gen., 2 cells.
9th gen., 12 cells. 8th gen., 118 cells. 7th gen., 2 cells.
132 cells.
The 132-cell stage is not a sharply defined one. for before all the divisions which have been described above have been finished, other divisions are begun which lead to the 184-cell stage (figs. 136-143). The cells which divide first in this period are the four median neural plate cells A 87 , A 88 (fig. 136) ; shortly afterward the four lateral ones A 815 , A 816 (fig. 140), also divide. The spindles in all these cells lie in a radiating position around the blastopore, and as a result of this division there are produced in the dorsal hemisphere two rows of neural plate cells, eight cells in a row, situated at the anterior border of the blastopore and dorsal to the chorda cells.
About the same time forty-four of the fifty-two ectoderm cells divide ; the spindles are approximately transverse in all these cells, except in the most posterior row of the ventral hemisphere, where they are dorso-ventral, and in two transverse rows of four cells each, which are the third and fourth rows in front of the animal pole (figs. 139, 143), where the spindles are antero-posterior in direction.
By these divisions the dorsal neural plate cells are increased to sixteen, and the ectoderm to one hundred and eight cells, so that at this stage the entire embryo contains one hundred and eighty-four cells. Twenty cells, forming two rows often each around the anterior border of the embryo just ventral to the equator, remain undivided for some time and are conspicuous for the large size of their resting nuclei and their more deeply staining cytoplasm (figs. 138, 142). The four hindmost of these cells on each side belong to the posterior quadrants (b 8-17 , b 818 , b 819 ', b 8?0 ) ; the other six pairs (a 8-25 , a 826 , a 8 - 17 , a 818 , a 8-19 , a s ' 20 ) which form the median anterior portions of these rows, are derived from the anterior quadrants, and they form the anterior portion of the neural plate.
Finally in the stage shown in figures 144 to 147. these twenty cells divide, all the spindles being approximately dorso-ventral in direction. About the same time the eight chorda cells divide, the spindles standing in a dorso-ventral direction (fig. 1 Km. and a little later the six most anterior endoderm cells (A s -, A 83 , A 8,4 ) divide, the spindles being nearly transverse (fig. 147). These thirty -four divisions advance the embryo from 1S4 to 218 cells.
Beyond this point I have not attempted to follow the lineage of each and every cell; this could be done successfully only by a most exacting study of serial sections in connection with whole preparations. With sufficient labor and material I believe that the lineage of every cell could be traced through to the tadpole stage, but I have lacked both the time and the material for such a study. Tabulating the cells of this stage we find that there are : Ventral hemisphere
Ectoderm . . . 9th gen., 104 cells.
Neural plate . 9th " 24 " Dorsal hemisphere
Endoderm. . . 9th " 12 " . . . . 8th gen., 14 cells.
Chorda 9th " 16 "
Neural plate . 9th " 16 "
Muscle 9th " 8 " .... 8th " 4 "
Mesenchyme . 9th " 4 " . . . . 8th " 14 " 7th gen., 2 cells.
9th gen., 184 cells. 8th gen., 32 cells. 7th gen., 2 cells.
218 cells. At this stage the gastrula is nearly circular in outline when viewed from the dorsal or ventral pole ; the gastrocoel is a cavity deep and wide in front and narrow behind, where it opens to the exterior through a deep groove between the mesenchyme and muscle cells of each side. The endoderm lies at a deep level, in contact with the ectoderm of the ventral side; in the anterior quadrants it consists of nine pairs of cells which become the gastric endoderm of the larva, in the posterior quadrants are four pairs of endoderm cells which meet along the median plane; the two posterior pairs become the caudal endoderm of the tadpole, the two anterior pairs are added to the gastric endoderm. Three pairs of cells at the hinder end of this cord of caudal endoderm are mesenchyme, while on each side of these and running forward lateral to the caudal endoderm are seven pairs of large mesenchyme cells (in fig. 147 only five pairs are visible, since two lie at a deeper level, one beneath the cell B 814 and another beneath A 8 - 12 ). The muscle rudiment consists of six pairs of large cells, dorsal to the mesenchyme, on each side of the blastopore en >ove and still uncovered by ectoderm. The chorda consists of sixteen cells arranged in two rows of eight each, one ventral to the other, and covered superficially by cells of the neural plate. Sixteen cells of the neural plate which cover the chorda cells .and form the anterior border of the blastopore belong to the dorsal hemisphere ; the rest of this plate is composed of cells of the ventral hemisphere, arranged in four rows of six each, and lying just in front of the chorda region ; therefore in the 218-cell stage the nerve plate consists of forty cells arranged in six transverse rows, each containing six cells except, the two most posterior rows which contain eight each. The animal pole, with the polar bodies still attached, is still situated back of the middle of the ventral face, six cell rows posterior to the anterior edge of the neutral plate ; there are but five rows of cells of the ventral hemisphere posterior to the animal pole, while there are ten such rows in front of it.
Conklin 1905 TOC: I. The Ovarian Egg | II. Maturation and Fertilization | III. Orientation of Egg and Embryo | IV. Cell-Lineage | V. Later Development | VI. Comparisons with A.mphioxus and Amphibia | VII. The Organization of the Egg | Summary | Literature Cited | Explanation of Figures
Conklin EG. The Organization and Cell-Lineage of the Ascidian Egg (1905) J. Acad., Nat. Sci. Phila. 13, 1.
Cite this page: Hill, M.A. (2024, April 19) Embryology Paper - The Organization and Cell-Lineage of the Ascidian Egg 4. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_The_Organization_and_Cell-Lineage_of_the_Ascidian_Egg_4
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G