Paper - A presomite human embryo (Shaw) - the implantation
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Hamilton WJ. and Gladstone RJ. A presomite human embryo (Shaw) - the implantation. (1942) J Anat. 76(2): 187-203 PMID 17104888
| Online Editor |
|---|
| Further descriptions of the "Shaw" Embryo characterised as (Carnegie Stage 8) can be found in the following papers:
Gladstone RJ. and Hamilton WJ. A presomite human embryo (Shaw) with primitive streak and chorda canal with special reference to the development of the vascular system. (1941) Amer. J Anat. 76(1): 9-44. Hamilton WJ. and Gladstone RJ. A presomite human embryo (Shaw) - the implantation. (1942) J Anat. 76(2): 187-203 PMID 17104888
|
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
A Presomite Human Embryo (Shaw): The Implantation
By W. J. Hamilton And R. J. Gladstone
Department Of Anatomy, St Bartholomew’s»Hospital Medical College, London
Introduction
In a previous communication (Gladstone & Hamilton, 1941) we gave a short description of the chorionic vesicle, and a detailed account of the structure of the intrachorionic rudiment of the ‘ Shaw ’ embryo. The present communication provides a more detailed account of the chorionic villi and their method of attachment to the -implantation cavity. An accountis also given of the structure of the endometrium adjacent to the implantation cavity. The chorionic vesicle has the following diameters: Maximum external, including villi: 11 mm. Vertical external: 4-04 mm. Maximum internal: 8 mm. Vertical internal: 3 mm.
The wall consists of an inner mesodermal layer (a description of this tissue is given in our previous paper), a cytotrophoblastic or Langhan’s layer, and a syncytiotrophoblastic layer. Projecting from the surface of the vesicle throughout its extent are villi; these are more differentiated and longer at the circumference than at the poles of the chorionic vesicle. The villi may be divided into three parts, a proximal part which has_ a mesodermal core, a short intermediate part which is densely cellular, some of the cells of which are continuous with the cytotrophoblast, and a distal part composed of loose columns of cytotrophoblastic tissue. There is a layer of syncytiotrophoblast of varying thickness on the sides of the villi.
As there is some confusion in the literature on the nomenclature of the different parts of the trophoblastic tissue we give here an account of the terms which we use:
Plasmoditrophoblast we regard as the primary plasmodium seen at the early stages of implantation, e.g. Miller, T.B. 1, Peters, and other early embryos; it corresponds to the implantation syncytium of Grosser’s classification (1927) and to the plasmo- dium of Florian (1928). After implantation it undergoes retrogression and, in the present specimen, only remnants of it are present.
Syncytiotrophoblast has been divided into villous syncytiotrophoblast, which covers the villi, and peripheral, which is found on the trophoblastic shell and lining the implantation cavity; it correspondsto the resorptive syncytium of Grosser (1927) and Florian (1928).
Cytotrophoblast, following the description of Ramsey (1938), has been subdivided into central cytotrophoblast, or Langhan’s layer, and peripheral, or cytotrophoblast of the cell columns and trophoblastic shell. This is regarded as a second generation of cytotrophoblast by Grosser (1927).
The trophoblastic shell is recognized as the layer of cytotrophoblastic tissue, of varying thickness, which lines the implantation cavity" including the decidua capsularis.
Peripheral syncytium is regarded as the tissue which invades the maternal tissue and which is situated outside the trophoblastic shell. It corresponds to the syncytial sprouts of Hill (1932) and the proliferation plasmodium of Florian (1928). It is typically found outside the border, or penetration zone, or the foetal-maternal border, of Wislocki-& Streeter (1938).
The Implantation Cavity
A A general view of the specimen shows that the walls of the implantation cavity have been torn, more especially on the superficial aspect and at the sides (Pl. 1, fig. 1). At one side the decidua capsularis has been torn away from the tips of the chorionic villi and has become folded and broken. A large fragment includes nearly the whole of the ‘operculum deciduae’ and the zone of the decidua capsularis surrounding the infiltrated fibrinous plug which has sealed the aperture of entry. On‘the deep aspect or placental side of the chorionic vesicle the wall of the implantation cavity is formed by an incomplete layer of cytotrophoblastic cells. Syncytiotrophoblast covers the foetal aspect of the cytotrophoblastic lamina, and scattered plasmoditrophoblast masses are found over and amongst these cells. A large cleft, the maternal sinus, separates the cytotrophoblastic lamina from the maternal decidua basalis. The maternal sinus, which is lined with endothelium in some areas and in others by flattened trophoblastic cells, opens into intervillous spaces.
Since considerable variations exist in the structure of different parts of the wall of the‘ implantation cavity, it is desirable to consider these parts under separate headings, namely: (1) A basal or placental area. (2) A marginal zone or circumferential part of the decidua capsularis, forming the lateral walls of the implantation cavity. (3) The central part of the decidua capsularis or superficial wall next the lumen of the uterus, comprising the ‘ operculum deciduae’, the infiltrated fibrinous plug, and the circular zone of the decidua capsularis surrounding the ‘aperture of entry’.
(1) The basal or placental area. A wide space, the basal sinus, separates the outer wall of the implantation cavity from an incomplete lamina which is formed by fused plates of cytotrophoblastic tissue uniting the tips of the villi. These plates arise from, and are continuous with, proliferating cells constituting the cell columns which grow outward from the tips of the villi. The cells at the base of the cell columns are continuous with the central cytotrophoblastic layer of the villi (Pl. 1, fig. 2). The central cytotrophoblastic cells lining the bases of the villi are cuboidal and are continuous with the similar cells in the chorionic wall. They have a large, round nucleus, with a distinct nucleolus, and a small amount of pale cytoplasm; mitoses are frequently found in these cells (Pl. 2, fig. 3). The cells of the peripheral cytotrophoblast which form the cell columns and the cytotrophoblastic lamina vary in size, those close to the tips of the villi being smaller than the more peripherally situated cells. The cell boundaries are less clearly defined, the nuclei are paler and often irregular and are sur- rounded by ‘a dark band‘ of cytoplasm outside of which there is a clear space (Pl. 2, fig. 5). Mitoses are not frequently observed in these cells.
The central cells are covered by a complete layer of syncytiotrophoblast, patches of which are occasionally included in the cytotrophoblast. Remnants of degenerating maternal tissue are rarely to be found among the cytotrophoblastic cells and occasional leucocytes are also present. The syncytiotrophoblast of the villi and chorionic wall varies in thickness in different villi; in some areas the outer border contains small vacuoles giving it a foamy appearance; in other areas large vacuoles are found (Pl. 2, figs. 3, 4). The syncytiotrophoblastic layer stains more darkly than the cytotrophoblast cells. A typical‘ brush border ’ was observed in many parts (Pl. 2, fig. 4); in others it is indistinct or absent.
Text-fig. 1. Remnants of the plasmoditrophoblast, or primary plasmodium of the implantation. Note large and small vacuoles and pycnotic nuclei in process of disintegration. x c. 550.
The nuclei vary in size and are irregular in shape; where the layer is thin they are flattened. Only very rarely are mitotic figures seen. A distinct nucleolus is present. In some situations the syncytiotrophoblastic layer shows indefinite cell boundaries (Pl. 2, fig. 3). The isolated masses of plasmoditrophoblast stain a deep pink colour with eosin, often with a lavender tint. Many small vacuoles and well-formed nuclei are present in certain of these masses, while other pieces contain large vacuoles and degenerating nuclei (Text-fig. 1). Large, apparently anuclear, masses are also present. Occasionally engulfed maternal blood cells and other maternal tissue are found within the masses. No transitional stages between the syncytiotrophoblast and the plasmoditrophoblast can be demon- strated.
The greater part of the uterine wall of the sinus is lined by a single layer of epithelioid cells continuous with the endothelium lining maternal vessels which open into the sinus (Pl. 1, fig. 2). Areas, however, are present which are covered by laminated masses of fibrin. In some places the epithelioid layer lies beneath a laminated fibrinous deposit, and in others the fibrinous deposit is infiltrated with nuclei indicating commencing organization of a_. clot which had been deposited during life. Where anchoring villi are seen directly attached to the decidua, near the circumference of the placental zone, there is no large continuous space or sinus, the intervals between the attachments of the villi being simply radiating clefts, lined by syncytiotrophoblast, which extend a short distance into the maternal tissue in the 'z5he of penetration. It is probable, therefore, that, in the living state, the space existed as a cleft which was capable of opening up into a sinus containing a variable amount of maternal blood which was in direct contact with the syncytiotrophoblast lining the uterine wall of the sinus as well as with that covering the villi and the outer wall of the chorionic vesicle.
The outer boundary of the sinus shows a number of rounded swellings separated by grooves or depressions into the bottom of which capillary vessels, and spaces lined by endothelium, are seen to open (Pl. 1, fig. 1; Pl. 4-, fig. 10).
The endothelium lining these vessels is continuous with a single layer of syncytiotrophoblast which in most places covers the summits of the swellings. The character of this epithelial lining is, in general, that of an attenuated layer, the nuclei and cytoplasm both being stained a deep purple colour and with no visible cell outlines; the nuclei of the tissue covering the summits of the swellings are as a rule larger and more rounded than the nuclei of the endo- thelium lining the capillary vessels and vascular spaces. Further, there is direct continuity of the thick layer of syncytiotrophoblast, which covers the sides of adherent ‘anchoring’ villi, with the thin stratum of epithelium lining
A the outer wall of the sinus. These anchoring villi are most numerous at the circumference of the sinus where the villi are firmly adherent to the decidua, whereas, in the central region, they are united together at their surfaces by expansions from the cell columns to form cytotrophoblastic laminae.
In some places the syncytiotrophoblast lining the uterine wall of the sinus is seen to be continuous with the epithelium of distended and degenerating uterine glands which have been opened up by the formation of the implantation cavity (Pl. 4-, fig. 8). Many of these glands are greatly elongated and displaced, so that their long axes lie parallel to the outer wall of the sinus. Since their much enlarged lumina often contain blood as well as secretion, it is probable that when, in the earlier stages of the embedding process, the inter glandular tissue in the ‘necrotic zone’ breaks down, the opening up of their lumina contributes to the formation of the cavity of the sinus. In many places groups of chorionic villi are attached to a thin plate of cytotrophoblastic tissue, covered by syncytiotrophoblast, which intervenes between the cavity of the sinus externally and the intervillous space internally, that is, on the side turned towards the wall of the chorionic vesicle. This clearly_ indicates, in our opinion, that a cleft has been formed by the breaking down of the spongytissue composing the ‘necrotic zone’ described by Bryce & Teacher (1908) in the earlier stage of development which follows the initial entry of the blastocyst. At the stage reached by the specimen we are describing, the necrotic tissue which formed the solid part of the sponge-work has broken down and has been‘ supplanted by thin plates of cytotrophoblast covered by syncytiotrophoblast, which join the tips of small groups of chorionic villi and which contribute to the formation of the trophoblastic shell. Also at this stage both walls of the sinus are covered by syncytiotrophoblast which is thicker and more typical in structure where it covers the outer surface of the thin plates attached to the tips of the villi than externally where it is attenuated and in some places defective.
In the region of the sinus, and in relation with the outer or parietal wall of the implantation cavity in the future placental area, we can distinguish three surfaces which are disposed in concentric planes relative to the outer wall of the chorionic vesicle. These surfaces are: (1) The outer wall of the intervillous space. The thin plates to which the tips of groups of anchoring villi are attached are, in the vicinity of the villi, lined by syncytiotrophoblast. (2) The outer surfaces of the thin plates of cytotrophoblast are also partly covered by syncytiotrophoblast, more particularly where this has apparently grown round the edges of the gaps between the plates; elsewhere it is bare or covered by a single layer of endothelium-like cells, suggesting the formation of the sinus by the opening up of vascular spaces as well as by the inclusion of dilated uterine glands and breaking down of necrotic tissue. Bare areas are also present which are sometimes covered by blood clot or a fibrinous coagulum tinged with eosin and containing disintegrated red blood corpuscles and leuco- cytes. The third surface is the outer wall of the sinus. The syncytiotrophoblast lining_the latter also varies in different places. In the clefts between the rounded swellings, where capillary vessels open into the sinus, the latter is lined by endothelium; where uterine glands have been opened up the epithelium lining the sinus is continuous with, and resembles, the glandular epithelium. In other places there are definite patches of plasmoditrophoblast giving origin to plasmodial strands which radiate outwards into the uterine tissue beyond the sinus where it may be seen opening up distended and degenerating glands, and extending into congested capillary vessels and vascular spaces. _ (2) The marginal zone or circumferential part of the decidua capsularis forming the lateral walls of the implantation cavity. The marginal or circum- ferential part of the decidua covering the sides of the chorionic vesicle differs from the decidua basalis in being more dense and uniform in general appearance.
The uterine epithelium on the surface is a simple layer of cubical and flattened cells beneath which are aconsiderable number of lymphocytes. The stroma dilfers from that of the decidua basalis in the sparseness of the glands, only remnants of which are present. The epithelium _of the glands shows varying stages of disintegration and in some cases has completely disappeared. In other places the walls consist merely of flattened cells of mesothelial type- On reaching the angle between the marginal and the parietal deciduae the lateral blood sinus divides into two branches of which one extends into the adjacent part of the decidua parietalis and the other is prolonged into the decidua marginalis and, by further extension and subdivision, into the peripheral part of the decidua capsularis. The sinuses are lined by flattened endothelial cells and contain blood coagulum, palely stained with eosin, in which are embedded scattered leucocytes and degenerated erythrocytes. The stroma contains a large number of round, oval and branched decidual cells which extend throughout the whole thickness of the stroma and are characterized by their large size, the pink colour of their cytoplasm, and their large, round nuclei (Pl. 3, fig. 7). The nucleus is palely stained by haematoxylin, the nuclear membrane is delicate and there is a fine nuclear reticulum showing usually a nucleolus and slight nodal thickenings. Numerous lymphocytes and leucocytes are present in the stroma and are "most numerous beneath the uterine epithelium and around the blood sinuses. The stroma nearer the chorionic vesicle is denser and more uniform in appearance than it is nearer the surface and beneath the uterine epithelium, where it appears to be oedematous. The decidua marginalis is limited on its inner or chorionic surface by an incomplete layer of cytotrophoblast and some plasmodial remains. The tips of the elongated circumferential villi have become detached in many places, the points of attachment being merely indicated by bare areas or a few cytotrophoblast cells adhering to the inner surface of the decidua (Pl. 3,_ fig. 6). Syncytial processes extending into the marginal decidua are frequent and are best seen near the junction of the marginal with the central part of the decidua capsularis (Pl. 3, ‘fig. 7). These syncytial processes vary in shape andlare darkly stained in contrast to the more lightly stained decidual cells.
(3) The central part of the decidua capsularis, including the ‘ operculum ’ and the region surrounding the aperture of entry. The epithelium on the surface of the decidua capsularis consists of a single layer of low cubical or polygonal cells which are continuous with similar cells of the marginal zone; the cell bodies are stained pink with eosin, the cytoplasm being coarsely granular and fre- quently showing vacuoles which are most frequent in the vicinity of the nucleus and near the free border of the cell. The outlines of the cells are ill-defined and there is no definite basement membrane. The nuclei are stained a deep purple and are round or oval in outline. Mitotic figures are rarely seen, but lympho- cytes lying in pairs close together are common immediately beneath the epithelial cells or, in some places, between the cells.
In the compact tissue beneath the epithelium are numerous decidual cells; these appear either as isolated cells or in groups and columns. The existence of the decidual cells is in most places associated with the presence of numerous lymphocytes and leucocytes in the supporting connective tissue. The inter- glandular connective tissue appears to have broken down in places, and in others to have been replaced by decidual cells. The presence of decidual cells at this stage of development is not limited to the zone immediately surrounding the implantation cavity, these cells being found also in the decidua parietalis, a small portion of which, in our specimen, is in continuity with the decidua capsularis.
On approaching the region of the closed aperture‘of entry the uterine epithelium tends to become flattened. Transitional stages occur between a single layer of evenly disposed cubical cells and one of irregularly shaped flattened cells which is spread out over the stratum compactum. The cells here show signs of degeneration and appear to be stretched over the underlying compact layer. In some places the epithelium is absent, leaving bare areas limited by a stratum of condensed connective tissue, or ‘ basement membrane ’.
Beneath the surface layer of uterine epithelium apparently isolated cells or small multinucleated masses of syncytium are occasionally visible and.also a considerable number of lymphocytes and polymorphonuclear leucocytes; the stroma contains numerous decidual cells (Pl. 3, fig. 7). The latter, however, diminish in number as the aperture of entry is approached. Further, in this region all traces of the uterine glands have disappeared and the stroma becomes denser and less vascular. It is infiltrated with extravasations of blood and fibrinoid material and‘ contains a large number of lymphocytes. The deep surface of the decidua capsularis is covered by cytotrophoblastic cells which are several layers thick in places, while in other areas they only appear as scattered cells. They line part of the trophoblastic shell.
The ‘operculum deciduae’, or closing plate. This is a flattened disc-like structure the margins of which spread out from the site of the original aperture of entry over the uterine epithelium which covers the stratum compactum of the decidua capsularis. The exact size of the operculum is diflicult to determine as, in the sections, the edge of the disc on one side is seen to be adherent to the surface of the decidua capsularis and to thin out gradually into an indeterminate border, whereas on the other side, where it is still separate from the decidua, the border is folded. Further, the lengths of the diameters at right angles to the planes of the sections cannot be estimated with certainty since the edge of the operculum extends on both sides beyond the area contained within the block. The number of serial sections (which were cut at a thickness of 10p.) is 263. This gives a minimum diameter in a plane at right angles to that of the sections of 2-63 mm. The greatest horizontal diameter of the disc, obtained by measurements from sections on the slides, is approximately 3 mm. Thus, assuming that the disc was roughly circular in outline, and allowing 0-37 mm. for the missing edges of the disc which were not included in the block, the average length of the diameters passing through the centre of the disc would be approximately 3 mm., or about one-third of the maximum diameter of the chorionic vesicle.
The operculum is seen in all the sections to rest on the surface of an accidentally detached fragment of the decidua capsularis. One edge of this fragment appears to have been torn from the circumferential part of the decidua capsularis a short distance from its junction with the decidua parietalis; here the torn edges of the two parts of the decidua capsularis which are slightly separated are seen to correspond. The other end of the fragment-gradually tapers to a free border which is at a considerable distance from the free border of the torn decidua on the corresponding side (Pl. 1, fig. 1). The wide gap between these two edges is occupied by a part of the wall of the chorionic vesicle, and from the latter project the elongated branched villi which characterize the circumferential zone of the vesicle. The tips of these villi are joined by thin plates of syncytiotrophoblast forming the trophoblastic shell, which are similar to the plates which have been previously described in the region of the decidua basalis. The superficial surface of these plates, however, although covered in some places by a syncytial layer, is in the greater part of its extent bare; it appears to have been torn away from the superficial layer of a missing part of the decidua capsularis which would have covered the exposed part of the chorionic vesicle.
The operculum consists of a hyaline fibrinoid material stained a yellowish pink colour with eosin and containing irregularly disposed, deeply stained, pycnotic nuclei (Pl. 4.-, fig. 9). These are round, oval, or rod-shaped, and vary in size, some being comparable to the nucleus of a small lymphocyte, though a cytoplasmic zone around the nucleus is seldom recognizable. Other nuclei are flattened and are seen mostly on the free surface; these resemble the nuclei of a mesothelium, while others which are larger in size appear to be the nuclei of degenerating plasmoditrophoblast. N_o vessels are visible, although where the operculum has become adherent to the decidua, ingrowths are present on its deep surface which resemble angioblastic strands and indicate a commencing organization of the fibrinoid material. Degenerating red blood corpuscles are visible in some places, and in some places also the ground substance or matrix is laminated, suggesting that the structure has originated as a blood coagulum which has become infiltrated with leucocytes and is undergoing degeneration and absorption. It is probable that the adherent portions of the operculum would have been replaced by scar tissue, while the outlying and circumferential free parts which do not receive any blood supply would have disintegrated and been cast off into the lumen of the uterus.
Comments and Comparisons
It is not our intention to enter into a detailed discussion of different phases of implantation, but only“ to deal with the more recent relevant literature. A statement on the age of the embryo was given in our previous communication. The assumed fertilization age of the embryo, from the patient’s history, was estimated to be between 20 and 23 days. The embryo was found, however, to be much younger than its supposed fertilization age and was estimated to be at about the 17th or 18th day of development, i.e. at approximately the same stage of development as the Wa 17 (Grosser, 1931) and younger than the Jones & Brewer (1935). The chorionic villi show a stage of development and difi"erentia- tion comparable to embryos of the Group E of Bryce’s classification (1924), and this group would also include the additional embryos, Hugo (Stieve, 1926), Goodwin (Kindred, 1933), that of Jones & Brewer (1935), of estimated "age of 18; days, and HRI (Johnston, 1941). The villi are found attached to the entire circumference of the chorion. The longest villi are found in the region of the circumference of the chorionic vesicle and in this respect it resembles the Goodwin embryo (Kindred, 1933). Stieve (1926) found, in the Hugo embryo, that the longest and thinnest villi were in the basal region and the thickest in the region of the decidua capsularis. In the embryo described by Brewer (1937) the villi were most numerous in the basalllregion. Johnston (1-94-1) found, in embryo HR 1, that they were longest in the basal region but less branched than in the lateral region.
Each villus may be differentiated into a basal part with a mesodermal core, a distal part composed of loosely arranged cell "columns, and an intermediate. part which has more regularly arranged cells and which stains more intensely than the distal part. In the mesoderm of the base of the villi developing blood vessels are found (Gladstone & Hamilton, 1941). No blood vessels were found in the mesodermal core of the Goodwin embryo but in it the chorionic vesicle is relatively small.
It is now generally agreed that the ‘cell columns growing from the villi are derived from the cells of the cytotrophoblast which covers the mesoderm. Frequent mitoses are found in the cytotrophoblastic cells at the bases of the columns,’ similar to those reported by Jones & Brewer (1935). In the cell columns, on the other hand, mitotic figures are rarely seen. The absence of mitotic figures in the cell columns in HR 1 embryo is noted by Johnston (1941), who infers that the cells may divide by amitosis as suggested by Florian (1928). We are of the opinion that the cellular activity of the cytotrophoblastic cells at the base of the columns is sufficient to account for the growth of the cell columns._ Jones & Brewer (1935) were able to demonstrate the presence of glycogen in these cells. They also point out that the cells become larger and frequently show degenerative changes near the periphery of the cell columns.
They further demonstrated, by using silver and Ma1lory’s connective tissue stain, that the cell columns are of foetal origin; no connective tissue fibres are found amongst the columns.
On the basal side of the chorionic vesicle of the Shaw embryo the cell columns unite to form a distinct cellular lamina; towards the circumference of the vesicle and decidua capsularis they are in continuity with the cytotrophoblastic tissue of the trophoblastic shell, as is the case in the embryos Hugo (Stieve, 1926), Bil (Florian, 1928), Falkiner (1932), and Yale (Ramsey, 1938).
Florian (1928) differentiates between two kinds of plasmodium, resorptive and proliferative. The former is subdivided into that in the villi and cell columns (corresponding to our syncytiotrophoblast), and isolated protoplasmic masses, corresponding to the plasmoditrophoblast of our description. The proliferative type is found in the penetration zone with elongated nuclei which are frequently pycnotic.
The syncytiotrophoblast forms an almost complete layer of varying thick- ness which covers the chorionic vesicle, the villi, the cell columns and the lacunae of the trophoblastic shell, as is the case in the Hugo embryo (Stieve, 1926). The amount of the syncytiotrophoblast varies in different areas; it is least in the trophoblastic shell and greatest on the sides of the villi. The transition of the cytotrophoblast into syncytiotrophoblast has been described in detail by Florian (1928); in some situations the transition from one tissue to the other has been observed by us. In many areas the syncytiotrophoblast shows a brush border or ciliated border as described by Jung (1908), Herzog (1909),.Stieve (1926), Florian (1928), Hill (1932), Jones & Brewer (1935), Brewer (1937; this author has also described and illustrated the development of this border), and Johnston (1941). In" many areas in our specimen the border of the syncytiotrophoblast shows a foamy appearance similar to that described by Johnston (1941).
There is fairly general agreement that the syncytiotrophoblast absorbs nutritive material from the maternal blood (Florian, 1928; Wislocki & Streeter, 1938; and Johnston, 1941). Florian (1928) also suggests that it may form a protection to the cytotrophoblast. Jones & Brewer (1935) suggest that the brush border is associated with phagocytic activity, as does Bartelmez (quoted by Jones & Brewer). Streeter (1926) states that there is no ingestion of maternal stroma cells in the Miller ovum. Herzog (1909), on the other hand, thinks that a ciliated border is associated with the secretion of an enzyme which causes 8 coagulation necrosis of the tissue.
In some of the cells of the syncytiotrophoblast vacuoles are found similar to those described by Streeter (1920) in the Mateer embryo, Stieve (1926) in the Hugo embryo, and by Florian (1928). What the significance of these vacuoles may be Stieve is unable to say. Florian is of the opinion that they are evidence of degenerative changes. Grosser, in the discussion in Florian’s paper (1928), points out that they are found in normal embryos. Politzer, on the other hand, regards them as pathological. We are unable to offer any definite opinion as to what their function may be, though possibly they are associated with absorption. Wislocki & Streeter (1938) believe that the large vacuoles (not quite similar to those described by us) in the macaque are a manifestation of absorptive activity of the trophoblast. The vacuoles in the syncytiotrophoblast should not be confused with the extensive vacuolation found in the degenerating masses of plasmoditrophoblast and described by many observers.
Outside the trophoblastic shell the proliferating syncytium sends out processes into the maternal tissue in the decidua capsularis, marginal and basal zones. Our observations confirm the View expressed by Florian.(1928) that these apparently isolated masses seen in the sections are connected together by fine strands. Herzog describes the syncytial masses as penetrating the border zone and statesithat they have a tendency to break up into individual detached pieces. In the Shaw embryo the maternal tissue is actively invaded by the strands as is shown by the destruction of uterine glands and blood vessels. It should be pointed out that in the region of the capsularis the syncytial masses frequently reach the lumen of the uterus (compare Brewer, 1.937).
There is no evidence of the necrotic zone which is such an outstanding feature in early embryos, e.g. in Teacher-Bryce no. 1, and in Sch of Von Mollendorff. A slight leucocytic infiltration round the implantation site is similar to that described by Jones '& Brewer (1935). We find no evidence in support of the statement of Meyer (1924) that syncytial masses develop from the endothelium of the maternal capillaries.
At the areas of attachment of the cell columns to the maternal tissue it is very difficult to state exactly what is maternal and what is foetal tissue (cf. statement on p. 192). We were not so fortunate as Brewer (1937) to be able to use differential stains to demonstrate the reticulum in the maternal tissue. The foetal cells generally stain more intensely and are larger than the maternal cells.
Owing to the small size of the block of tissue removed with the embryo, and to the damage to the capsularis, it has not been possible to give a detailed account of the arrangement of the venous sinuses and arterioles in relationship with the implahtation cavity.
A large basal sinus is situated in the basal part of the implantation cavity, as is typically found in most embryos. Capillaries are seen to open directly into the basal sinus, but no arterioles as shown by Stieve (1926) and F alkiner (1932) could be demonstrated to open into any part of the implantation cavity. Johnston (1941) states that a few capillaries in the decidua capsularis of the HR1 embryo undoubtedly establish connexions with the intervillous spaces, but he thinks that the number is negligible.
A lateral sinus as described by Herzog (1909) and Stieve (1926) was found on one side of the presentembryo; on approaching the marginal zone it divides into two, one branch running into the decidua capsularis, the other into the decidua parietalis;
There is a relatively small amount of blood in the intervillous spaces and in the surrounding glands compared with the amount present in the Peters, Teacher-Bryce II, Bil (Florian, 1923), Werner (Stieve, 1936) and Brewer embryos, all of which are younger than the present specimen. In our opinion there are several explanations to account for the small amount of blood present: either the intervillous circulation has been established, or the large amount of blood of the earlier stages has been absorbed by the syncytiotrophoblast, or the amount of haemorrhage in some specimens is excessive. That the intervillous circulation has been established is the most probable explanation.
In the Falkiner (1932) and Frassi (1907) specimens there is an absence of blood in the implantation cavity. These, according to Falkiner, ‘ support the View that even as early as the second or third week, a definite circulation through the implantation cavity is effected’. In the HR1 embryo, Johnston is of the opinion that the intervillous circulation has not been established and that the establishment of free circulation in the intervillous space at a later stage is not a gradual but rather a comparatively sudden process and ensures that increased erftry of blood into the space, no matter how extensive, will not overtax the carrying capacity of the outlets ’.
The point of entry of the ovum into the endometrium is Visible between the lips of the decidua capsularis. It is closed by a plug of fibrin in the meshes of which are many red corpuscles and some leucocytes. The plug is continuous with a large blood clot which extends over the surface of the decidua capsularis superficial to the uterine epithelium. It is, therefore, a ‘mushroom-shaped’ coagulum similar’ to that described by Peters (1899, 1925) and, in the Yale embryo, by Ramsey (1938).
It will be in place here to draw attention to a confusion which has arisen in the use of the terms, (1) operculum deciduae (Latin: ope1;culum—a small lid), (2) Gewebspilz (German: mushroom- or fungus-tissue), (3) Verschlusscoagulum (Gennan-Latin form: occlusion-clot or closing-clot), (4) Verschlusspfropf (German: occlusion plug), (5) Blutpfropf (German? blood plug). We would suggest that the term operculum be limited to the flattened or dome-shaped head of the fungus- or mushroom-shaped structure; that the part occupying and closing the ‘aperture of entry be called the ‘occlusion- or closing-plug’; that the name "mushroom-shaped plug’ be used to denote the whole structure including the head and stem of the mushroom; and, in order that there can be no confusion, the expression ‘fungoid tissue’ be replaced by the term ‘mycelioid tissue’ in the description of the microscopical structure of the infiltrating plasmodial tissue, with reference to the resemblance of the latter to the continuous network of a mycelial growth. It should be mentioned, however, that the word ‘fungoid’ was originally used by Peters to denote a resemblance in form of the object under discussion to that of a fungus and not to the structural resemblance of the plasmodial growth in the plug to a mycelial growth.
The fate of the superficial clot appears to be variable: (1) it may be accidentally lost in the removing or preparation of the specimen, (2) it may under natural conditions become organized and persist for a time as a minute scar, or (3) it may become separated and shed off into the cavity of the uterus. It also appears to vary- considerably in size and in degree of infiltration with plasmodium.
Covering the deep surface of the closing plug there is a layer of cytotrophoblastic tissue which is continuous with the trophoblastic shell. The structure of the closing plug is essentially similar to that found in the Herzog embryo. No trophoblastic elements are found in the closing plug in the Yale embryo. The closed canal in the Shaw embryo passes at an angle of approximately 45° to the surface of the capsularis and, therefore, obliquely as is the case in the Herzog, Teacher-Bryce I, Sch (Von Mollendorlf, 1921) and W0 (Von Möllendorff, 1925) embryos.
Remnants of the syncytium are present in the infiltrated blood clot, but not in the plug as described by Teacher (1924) in the Teacher-Bryce embryos I and II, and by Von Mollendorff in the Sch embryo. In the Peters ovum and in the ovum of Linzenmeier (1914) the plug contains migrating cells from the trophoblast. Schlagenhaufer &. Verocay (1916) found a plug comparable to that described by Teacher and regarded it as a method of closing the aperture in the decidua capsularis. In a young human embryo Dible & West (1941) find that the aperture of entry is closed with fibrinous material similar to that in the implantation cavity.
The chorionic vesicle is no longer adherent to the capsularis at the point of entry, as is described in the Hugo (Stieve, 1926), the Falkiner, and the Edwards-Jones-Brewer (Brewer, 1937) embryos, but is completely freed from it. Teacher states that the operculum later becomes detached from the chorion as is, partially, the case in the present embryo.
A brief comparison of the presentembryo with a corresponding stage of development of the macaque, as described by Wislocki & Streeter (1938), shows that the differentiation of the trophoblast, as pointed out by them, is essentially similar to that in man. They state that it is clear from perusal of the literature on early human embryos that the differentiation of the trophoblast in these imitates very closely the phases seen in the macaque.
Our embryo shows differentiation of the villi, trophoblastic shell, and penetration zone comparable to the"-macaque at about the 19th day of development. The vill'i in each case may be divided into three segments—a proximal, containing mesoderm, a distal, composed of loosely arranged cytotrophoblast, and an intermediate, which stains much more intensely than the distal segment. In the macaque, as in the human, the intervillous spaces. are bounded by a layer of syncytial trophoblast which, according to Wislocki & Streeter, absorbs nutriment from the maternal corpuscles which have become swollen and irregular, and have lost their haemoglobin. They are also of the opinion that the products of the necrotic junctional zone supply nourishment to the tropho- blastic shell.
The same difficulty is experienced in their beautifully graded macaque material, as in the human, in the junctional cellular zone, of differentiating accurately the precise boundary between degenerating maternal cells and the trophoblastic tissue. They state that ‘there is no sharply differentiating stain so far developed to distinguish the two, and on morphology of nucleus and cytoplasm they cannot be separated with precision’. They further state that ‘the cytotrophoblast in continuity with the cell columns extends deeply into the junctional zone spreading out below the intervillous space as “the trophoblastic shell ”.
Summary
An account is given of the implantation of the ‘Shaw’ embryo which is approximately at the same stage of development as the Wa 17 embryo‘(Gros ser, 1931); the estimated fertilization age of the ‘Shaw’ embryo would thus corre- spond to about the 17th or 18th day of development.
Villi are attached over the whole extent of the chorionic vesicle, being longest at the circumference.
A typical villus is differentiated into a basal part containing a mesodermal core, a distal part which consists of loosely arranged cell columns, and an intermediate part. The mesoderm of the basal segment contains angioblastic tissue some of which has advanced to the stage of capillary formation by the fusion of intracellular vacuoles to form the lumina of capillary vessels. No blood corpuscles are as yet present in these capillaries, proving that, in this situation, the vessel walls are not formed by the union of flattened outer cells of blood islands, since no blood islands are present in the wall of the chorionic vesicle.
Frequent mitoses are present in the cytotrophoblastie cells at the bases of the cell columns, but are rare in the columns themselves, and it seems probable that the cellular activity of the cytotrophoblastie cells at the bases of the columns is sufficient to account forithe growth of the cell columns. Appearances have been noted in the nuclei of the syncytiotrophoblast covering the villi, and the peripheral processes arising from these, which suggest amitotic divisions of the nuclei.
On the basal side of the chorionic vesicle the cell columns unite to form a distinct trophoblastic lamina, between the basal sinus and the intervillous space; towards the circumference of the chorionic vesicle and the decidua capsularis the cell columns are in continuity with the outer layer of the trophoblastic shell. The basal sinus communicates freely with the intervillous space by openings between the plates which form the cytotrophoblastic lamina.
Transitional stages between cytotrophoblast and syncytiotrophoblast of the villi have been observed, and in many places a definite ‘brush border’ is present and also minute vacuoles giving rise to the ‘foamy’ appearance described by Johnston. Large vacuoles and vacuoles of intermediate size are also present in the degenerating remnants of the plasmoditrophoblast.
Beyond the trophoblastic shell the proliferating peripheral syncytium sends out processes into the maternal tissue of the decidua capsularis, marginal and basal zones. These masses which appear, in sections, to be isolated, when traced through adjoining sections are frequently found to be connected and, although the connexions may be broken secondarily as a result of stretching or degenera- tion, it seems probable that the continuity, when present, is primary. Uterine blood vessels and uterine glands are invaded and destroyed by the peripheral syncytium.
Leucocytic infiltration, oedema, and degeneration of stroma cells, blood vessels and uterine glands are evident in places but no definite necrotic zone is visible. Some degenerating plasmodial tissue is found in the lumen of the uterus under cover of the free border of the operculum, between this and the uterine epithelium, as well as in the operculum itself.
A large basal sinus and a lateral sinus, in the marginal zone, are present. These are in free communication with the veins of the endometrium. Neither these nor the intervillous spaces contain much blood, and any red blood corpuscles or leucocytes which are found in recesses of the intervillous space are pale (lacking in haemoglobin), variable in size and partially disintegrated, giving the impression that they have been acted upon by some digestive ferment.
The point of entry of the ovum into the endometrium is closed by a plug of fibrin in the meshes of which are many red blood corpuscles and some leuco- cytes. The plug is continuous with a large blood clot which is flattened out over the surface of the decidua capsularis. The clot lies superficial to the uterine epithelium around the aperture of entry and on one side has blended with this, its edge being indeterminate, whereas, on the other side, the edge is free. On the deep surface of the plug, occupying the aperture of entry, there is a layer of cytotrophoblast which is continuous with the trophoblastic shell. The superficial fibrinous coagulum forming the head of the mushroom-shaped plug contains, in addition to degenerating red blood corpuscles and leucocytes, deeply stained pycnotic nuclei which appear to'be the only recognizable remnants here of the plasmoditrophoblast, the body plasm of the original elements having disappeared or being only indistinctly visible.
Comparison of our embryo with a corresponding stage of development, about the 19th day, of the macaque embryo, as described by Wislocki & Streeter, affords additional confirmation of their conclusion that the differentiation of the trophoblast is essentially the same in man and the macaque.
We wish to acknowledge our indebtedness to the Publications Fund of the University of London for a grant towards part of the cost of the illustrations in our previous and present communications; and also for the cost of blocks for the coloured plate of our previous communication (Gladstone & Hamilton, A 1941). We wish to thank Mr A. K. Maxwell for ‘working up ’ the photographs of Pl. 1, fig. 1; Pl. 2, figs. 3, 4 and 5; Pl. 3, figs. 6 and 7; and Text-fig.. 1. We wish also to thank our technicians, Messrs Westwood and Park, for their invaluable assistance.
Abbreviations Used in the Figures
ANG. Angioblast.
C. CY. TR. Central cytotrophoblast
BAS. SIN. Basal sinus.
C. COL. Cytotrophoblastic cell column.
CY. PL. Cytotrophoblastic plate(s).
CY. TR. Cytotrophoblast.
D. BAS. Decidua basalis.
D. CAPS. Decidua capsularis.
DEC. C. Decidua cell(s).
D. GL. 0. Degenerating gland cells.
END. I Endothelium.
IMP. C. Implantation cavity.
OP. Operculum.
PL. TR. Plasmoditrophoblast.
PR. SYN. Peripheral syneytium.
PR. TR. Primary plasmodium.
SYN. TR. Syncytiotrophoblast.
TB. Fibrin. - (Langhan’s Layer).
UT. EP. Uterine epithelium.
V.S. Vacuole(s) (small).
V.L. Vacuole (large).
V. SYN. TR. Villous syncytiotrophoblast.
References
BREWER, J. I. (1937). Amer. J. Anat. 61, 429.
BRYCE, T. H. (1924). Trans. Roy. Soc. Edinb. 53, 533.
BRYCE, T. H. & TEACHER, J. H. (1908). Contributions to tIw'Study of the Early Development and I mlmlding of the Human Ovum. Glasgow: Maclehouse.
DIBLE, J. H. & WEST, C. M. (1941). J. Anat., Land., 75, 269.
FALKINER, N. M. (1932). J. Obstet. Gymzec. 39, 471.
FLORIAN, J. (1928). Anat. Anz. Erg. 66, 211.
Fmssr, L. (1907). Arch. milcr. Anat. 70, 492.
GLADSTONE, R. J. & HAMILTON, W. J. (1941). J. Aruzt., Loru1., 76, 9.
GROSSER, 0. (1927). Friihentwicklurtg, Eihautbildung und Placentation des Menachen und der Sciugetiere. Munchen: Bergmann. (1931). Z. gas. Anal. 1. Z.'Am1t. EntwGesch. 94-, 275.
HEBZOG, M. (1909). Amer. J. Anat. 9, 361.
HILL, J. P. (1932). Philos. Trans. B, 221, 45.
JOHNSTON, T. B. (1941). J. Anat., Lond., 75, 153.
JONES, H. O. &. BREWER, J. I. (1935). Surg. Gyruac. Obstet. 60, 657.
JUNG, P. (1908). Beitrage zur frilhesten Eieinbettung beim mensohlichen Weibe. Berlin: Karger.
Kmmmn, J. E. (1933). Amer. J. Anat. 53, 221.
LINZENMEIER, G. (1914). Arch. 102, 1. -
MEYER, P. (1924). Arch. Gyndk. 122, 38.
M6LLENDORFF, W. VON (1921). Z. ges. Anal. 1. Z. Anat. EntwG'esch. 62, 406. X (1925). Z. ges. Aruzt. 1. Z. Aruzt. EntwGesch. 78, 16:
PETERS, H. (1899). Die Einbettung des Menschlichen Eies. Leipzig und Wein.
(1925). Arch. Gymik. 124-, 625.
RAMSEY, E. M. (1938). Contr. Embryol. Carneg. Instn, 27, 67.
SCHLAGENHAUFER, F. & VEROCAY, F. (1916). Arch. Gy-mile. 105, 151.
STIEVE, H. (1926). Z. mikr. Anat. 7, 295.
(1936). z. mikr. Anat. 40, 281.
Streeter GL. A human embryo (Mateer) of the pre-somite period. (1920) Contrib. Embryol., Carnegie Inst. Wash. Publ. 272, 9: 389-424.
(1926). Contr. Embryol. Carney. Instn, 18, 31.
TEACHER, J. H. (1924). J. Obstet. Gyruzec. 31, 166.
WISLOCKI, G. B. & STREETER, G. L. (1938). Contr. Embryol. Carney. Instn, 27, 1.
Explanation of Plates
Plate 1
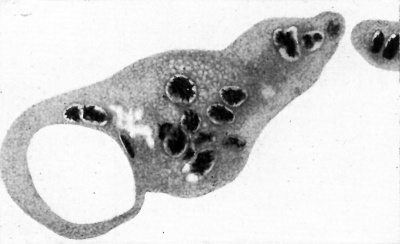
1. General view showing the relations of the embryonic rudiment and chorionic vesicle. The decidua basalis and a detached fragment of the decidua capsularis are also seen. The latter is traversed by the occlusion plug, and a part of the operculum is seen. The photograph was obtained from two original photographic prints which were pieced together and rephotographed so as to obtain a complete picture. An arrow indicates what we believe to be the point of entry. The interrupted line indicates the line of junction between the separate photographs. x c. 15.
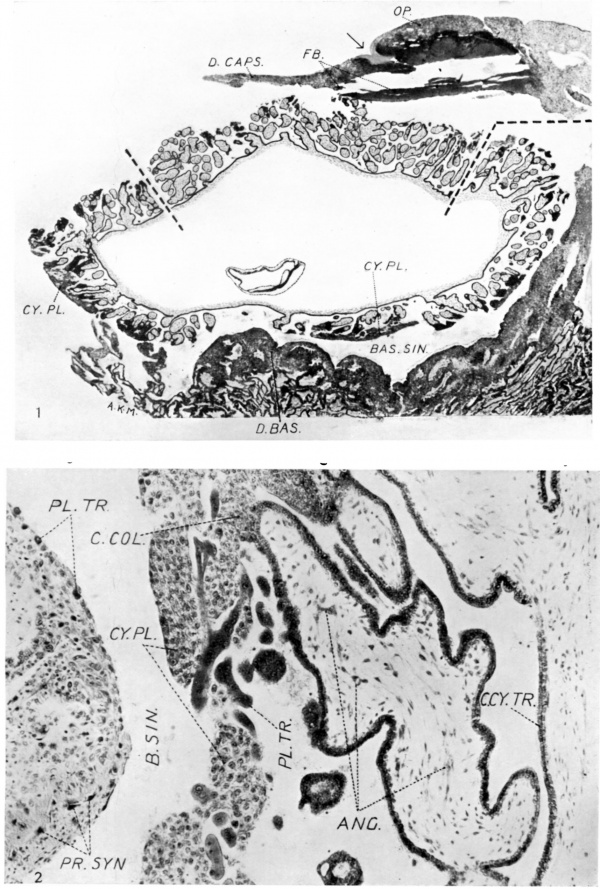
2. Section through the wall of the chorionic vesicle and a part of the decidua basalis; showing the general relations of the villi, cytotrophoblastic plates and basal sinus. x c. 140.
Plate 2
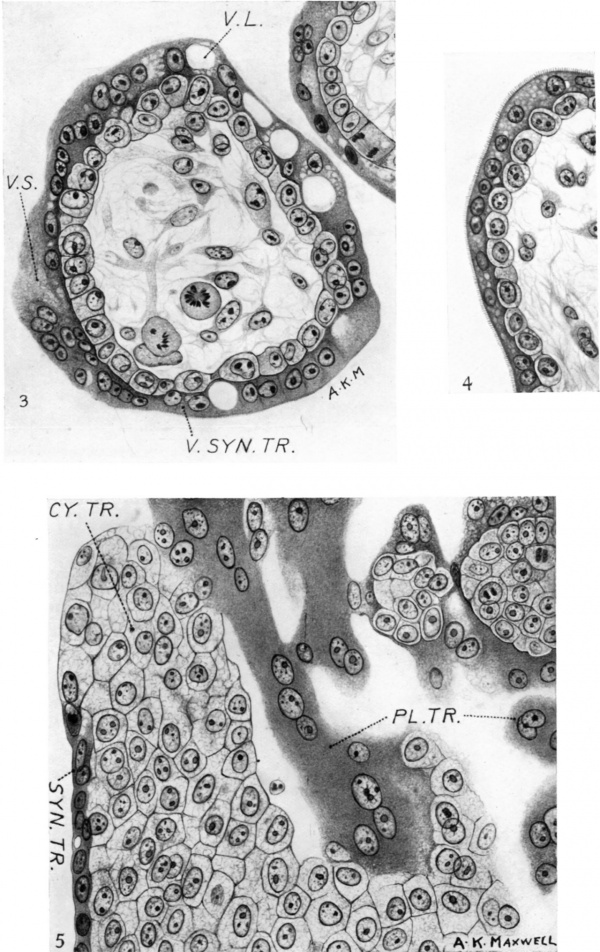
3. Transverse section through a typical villus showing the villous syncytiotrophoblast. Note large and small vacuoles and the ‘brush border’ on the bottom left-hand side of the figure. The nuclei aresmaller than those of the cytotrophohlast and tend to be disposed in pairs which are in contact or close together and are approximately of the same size. A conspicuous single nucleolus, or two true nucleoli, are present, more especially at the ends of a row of nuclei, suggesting amitotic division. x c. 550.
4. Typical ‘brush border’ on the syncytiotrophoblast of a villus. x c. 550.
5. Typical appearance of the cells of the cytotrophoblastic columns, showing bare areas and areas covered by syncytiotrophoblast. To the left and above in the figure are columns and masses which are probably remnants of the plasmoditrophoblast. x c. 550.
Plate 3
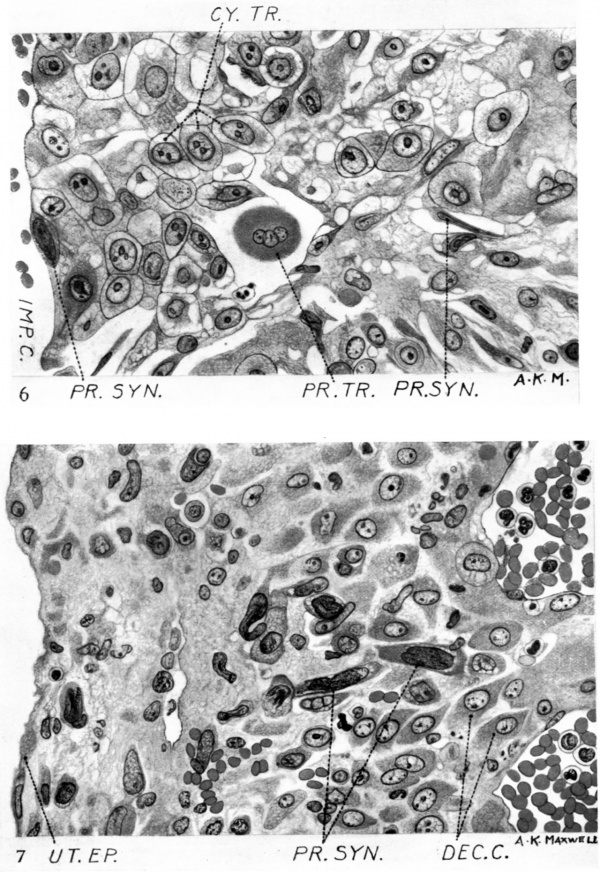
Fig. 6. Junctional penetration zone at the attachment of the cell columns to the endometrium. Note the invasion of the maternal tissue by both cytotrophoblast and peripheral syncytium. x c. 550.
Fig. 7. Showing the invasion of the decidua capsularis by peripheral syncytium. x c. 550.
Plate 4
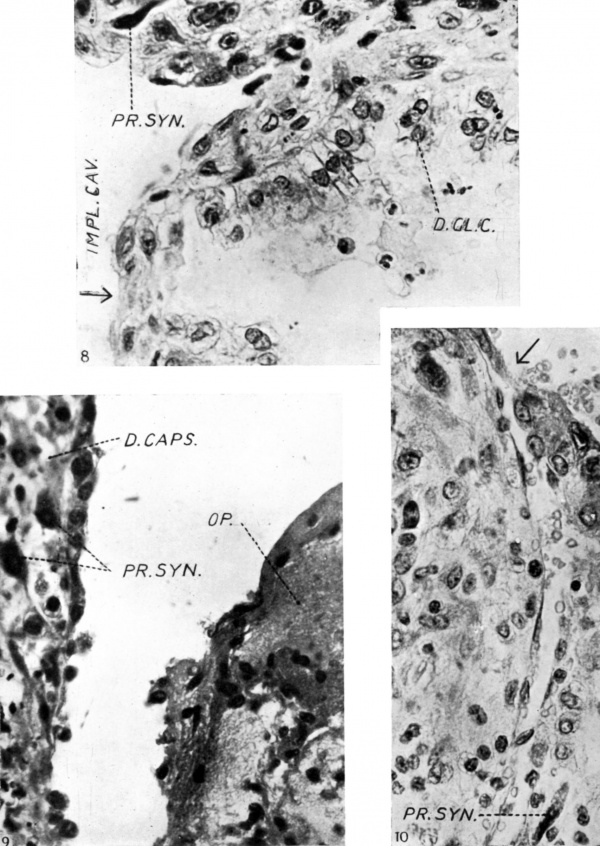
Fig. 8. Uterine gland opening into the implantation cavity at the point indicated by the arrow. The glandular epithelium is degenerating and leucocytes are visible V in the cell detritus occupying the lumen, and in the interglandular tissue which also contains strands of peripheral
syncytium. x c. 550.
Fig. 9. Showing the free edge of the operculum where it overlaps the decidua capsularis and covers remnants of the uterine epithelium which are degenerating an_d discontinuous. The operculum consists of a fibrinoid matrix containing degenerated plasmodial elements, red blood corpuscles and leucocytes. Peripheral syncytial elements are also seen in the stroma of the decidua capsularis which also contains many lymphocytes and leucocytes. x c. 550.
Fig. 10. Showing a capillary opening into the basal sinus at the point indicated by an arrow. Above the capillary is a strand of proliferating syncytium. x c. 550.
Reference
Hamilton WJ. and Gladstone RJ. A presomite human embryo (Shaw) - the implantation. (1942) J Anat. 76(2): 187-203 PMID 17104888
Cite this page: Hill, M.A. (2024, April 24) Embryology Paper - A presomite human embryo (Shaw) - the implantation. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Paper_-_A_presomite_human_embryo_(Shaw)_-_the_implantation
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G