1987 Developmental Stages In Human Embryos - Stage 2
| Embryology - 20 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Stage 2
- Approximately 0.1 - 0.2 mm in diameter
- Approximately 1½ - 3 postovulatory days
- Characteristic feature: more than 1 cell but no blastocystic cavity seen by light microscopy
Stage 2 (8698)
Summary
Stage 2 comprises specimens from 2 cells up to the appearance of the blastocystic (or segmentation) cavity. The more advanced examples (from about 12 cells on) of stage 2 are frequently called morulae (L., morus, a mulberry). The term morula is not historically appropriate for mammals, however, because the amphibian morula gives rise to embryonic tissues only, whereas in mammals non-embryonic structures (such as the chorion and the amnion) are also derived from the initial mass of cells.
Size And Age
The diameter at stage 2 before fixation is of the order of 175 μm; after fixation, it is approximately 120 μm (Hertig et al., 1954).[1] Indeed, shrinkage of as much as 50 percent may occur in some instances (Menkin and Rock, 1948[2]). Whether before or after fixation, the diameter at stage 2 may be expected to lie between 75 and 200 μm.
The volume of the protoplasmic mass diminishes during cleavage (O'Rahilly, 1973, table 5). The age at stage 2 is believed to be approximately 1½-3 postovulatory days. The range is probably 1-5 days (Sundström, Nilsson, and Liedholm, 1981[3]). In vitro, 2 cells may be found at 1½ days, 4 cells at 2 days, and 8 cells by about 2½ days.
General Features
The organism proceeds along the uterine tube by means not entirely understood (reviewed by Adams, 1960). It leaves the tube and enters the uterine cavity during the third or fourth day after ovulation, when probably 8-12 cells are present, and when the endometrium is early in the secretory phase (corresponding to the luteal phase of the ovarian cycle).
It has been shown experimentally (in the mouse, rat, and rabbit) that a blastomere isolated from the mammalian 2-cell organism is capable of forming a complete embryo. Separation of the early blastomeres is believed to account for about one-third of all cases of monozygotic twinning in the human (Corner, 1955[4]). Such twins should be dichorial and diamniotic (fig. 5-2). The fact that nearly 60 percent of dichorial twins (whether monozygotic or dizygotic) have two unfused placentae "indicates that the zona pellucida must have disappeared sufficiently long before implantation to allow the twins to become implanted in independent positions in the uterus" (Bulmer, 1970). Dizygotic twins, in contrast, are believed to arise from two oocytes, from a binucleate oocyte, or from a second polar body (Gedda, 1961[5]).
The successive cleavage divisions do not occur synchronously, so that (in the pig) specimens of anywhere from 1 to 8 cells can be found. It has been suggested that the more precociously dividing cells may be those that give rise to the trophoblast. Moreover, differences in the size, staining, and electron density of the blastomeres are observed.
There is reason to believe, however, that the blastomeres are not determined very early in development. For example, it has been shown experimentally in the mouse that the ability to develop into trophoblastic cells is inherent in all blastomeres of the first two stages. Up to 16 cells, none of the blastomeres is yet determined to give rise to cells of the inner mass. It may be that the primary factor responsible for the determination of one of the two alternative routes of differentiation (trophoblast or inner cell mass) is simply the position (peripheral or internal) that a given cell occupies.
|
|
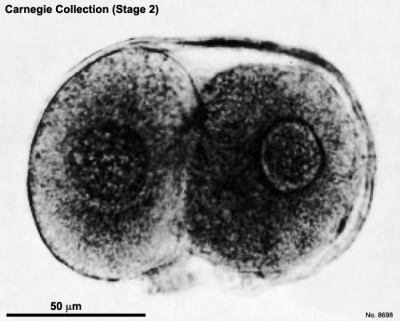
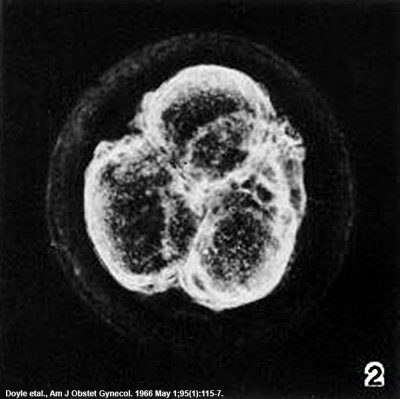
| Fig. 2-1. Intact 2-cell embryo showing zona pellucida and two polar bodies, the larger of which is clearly visible at the lower end of the cleavage line. No. 8698. | Fig. 2-2. Intact 4-cell embryo. The granular zona pellucida can be distinguished. By courtesy of Dr. J. Lippes (Doyle etal, 1966[6]). |
According to the "inside/outside hypothesis," microenvironmental differences influence the determination of blastomeres (between 8 and 16 cells in the mouse) so that those on the outside become more likely to form trophoblast (with more restricted potential) whereas those enclosed by other cells become more likely to form the inner cell mass, Another hypothesis accounting for early cellular diversity (in the mouse) is based on polarization of the larger, external cells, characterized by microvilli.
Furthermore, it has been possible in the mouse to unite two 16-cell organisms and obtain from them one giant, but otherwise perfectly normal, blastocyst. Fusion of mouse organisms with close to 32 cells each has also resulted in a single blastocyst.
It has been stressed that it is dangerous readily to infer normality on purely morphological grounds. In the human, two significant specimens of stage 2 (Hertig et al., 1954)[1] will be cited.
A 2-cell specimen (No. 8698) was spherical and surrounded by a transparent zona pellucida (fig. 2-1). Two polar bodies were present. Each blastomere was nearly spherical. It has been maintained that the larger blastomere would probably divide first and hence may perhaps be trophoblastic (Hertig, 1968).
A 12-cell specimen (No. 8904) was perfectly spherical and surrounded by a clear zona pellucida. One blastomere, central in position and larger than the others, was presumed to be embryogenic, whereas the smaller cells were thought to be trophoblastic.
A number of human specimens of stage 2 found in atretic ovarian follicles were considered to be parthenogenetic by their authors (Häggström, 1922; Krafka, 1939; Herranz and Vázquez, 1964; Khvatov, 1968). Such a claim, however, has been disputed (Ashley, 1959[7]), and it has been pointed out that polysegmentation, that is, cleavage-like conditions described as "pseudoparthenogenesis," are not infrequently encountered in moribund oocytes (Kampmeier, 1929). It is likely also that some instances of cleavage obtained in vitro may be pseudoparthenogenetic rather than caused by actual fertilization by spermatozoa.
The presence of a Y chromosome in a "spread from a replicating blastomere" (Jacobson, Sites, and Arias-Bernal, 1970[8]) has been claimed "but not convincingly demonstrated" (Brackett et al., 1972[9]).
The embryonic genome probably becomes functionally active during stage 2. Activation of transcription of rRNA genes (contributed to the embryonic genome by the male and female gametes at fertilization) is indicated in vitro by the pattern of nucleologenesis, which changes in 6- to 8-cell embryos and becomes typical in 10- to 12-cell embryos (Tesarík et al., 1986).
Specimens Of Stage 2 Already Described
(listed in order of numbers of cells present)
Specimens believed by their authors to have been parthenogenetic are indicated here by an asterisk.
- 2 cells Carnegie No. 8260. Described by Menkin and Rock (1948)[2]. Produced in vitro. Diameter, 153 x 155 μm; cytoplasm, Page 15 100 x 113 (50 x 75 after fixation); thickness of zona pellucida, 23 (8 after fixation); blastomeres, 88 x 58 and 105 x 58 (63 x 39 and 66 x 36 after fixation); polar body, 18 x 10 after fixation.
- 2 cells, Carnegie No. 8698 (fig. 2-1). Described by Hertig et al. (1954)[1]. Tubal Diameter, 178.5 μm (122 x 88 after fixation; 111.6 x 75 after sectioning); blastomeres, 71 (74 x 64 and 80 x 56 after fixation; 68.3 x 61.6 and 70 x 50 after sectioning); polar bodies, 20 x 18 after fixation. A few cells of the corona radiata were present. Thick zona pellucida (18 μm in thickness before fixation). No spermatozoa were seen in the zona, and the possibility of parthenogenetic cleavage"cannot be entirely ruled out" (Dickmann et al., 1965). Two polar bodies. Whether the larger blastomere "is the one of trophoblastic potential is unknown but it is probable" (Hertig, 1968). Believed to be about 1½ days old.
- 2 cells. Illustrated by Shettles (1958[10] and 1960). Produced in vitro.
- 2 cells. Undergoing cytolysis, produced parthenogenetically by Edwards et al. (1966)* from an oocyte cultured in vitro.
- 2 cells and 4 cells. Described by Häggström (1922),* who found them in atretic ovarian follicles of a 22-.year-old woman.
- 2 cells. Two in vitro embryos studied by electron microscopy (Dvorák et al., 1982), both round. Surprisingly, one was surrounded by a large mass of cumulus cells.
- 2 cells. Illustrated by Pereda and Coppo (1985); believed to be 37 hours.
- 2-8 cells. In vitro examples are illustrated by Sathananthan, Trounson, and Wood (1986).
- 3 cells, Carnegie No. 8500.1. Described by Menkin and Rock (1948). Produced in vitro. Diameter, 170 x 183 μm; cytoplasm, 103 x 127 (50 x 86 after fixation); thickness of zona pellucida, 21; blastomeres, 97 x 73,62 x 62, and 50 x 63 (66 x 49,35 x 38, and 33 x 34 after fixation); a possible polar body, 14 x 9 after fixation.
- 3 cells. Produced in vitro. Petrov (1958) found spermatozoal penetration of the zona pellucida after 2 hours, polyspermy in all cases, the first cleavage furrow after 20 hours, and three blastomeres after 26 hours.
- 4 cells. Illustrated by Krafka (I939),* who found it (within a zona pellucida) in an atretic ovarian follicle of a 1-year-old child.
- 4 cells. Described by Herranz and Vázquez (1964),* who found it (within a zona pellucida) in an atretic ovarian follicle of a 20-year-old woman.
- 4 cells. Illustrated by Way and Dawson (1959)[11]. Found in a routine vaginal smear. Well-marked zona pellucida.
- 4 cells. Illustrated by Shettles (1960). Produced in vitro.
- 4 cells. Illustrated by Doyle et al. (1966). Found in middle third of uterine tube. Devoid of corona radiata. Granular zona pellucida (fig. 2-2).
- 4-10 cells. Eight in vitro examples, studied by electron microscopy (Sundström, Nilsson, and Liedholm, 1981). They ranged from 60 to 120 hours, and the average cleavage time varied from 24 to 86 hours.
- 5-12 cells. Pathological specimens of 5 (No. 8630), 8 (No. 8450), 9 (No. 8190), and 11 or 12 (No. 8452) cells, found by Hertig et al. (1954)[1].
- 6 cells. Illustrated by Brackett et al. (1972). Produced in vitro. Other specimens consisted of 2-12 cells and "a questionable morula undergoing degeneration."
- 7 cells. Illustrated by Avendaño et al. (1975). Measured 201 x 197 μm. Zona intact (25 μm in thickness). Three polar bodies. Two of the seven blastomeres were metachromatic and electron-dense, and one polar body was metachromatic. Believed to be about 72 hours.
- 8 cells. Noted by Khvatov (1968)* in an atretic ovarian follicle. Diameter (after fixation), 110 x 95 μm.
- 8 cells. In vitro specimens of 8 cells and also some "early morulae and blastocysts" (Edwards and Fowler, 1970).
- 8 cells. Two in vitro specimens, studied by electron microscopy (Sathananthan, Wood, and Leeton, 1982). Zona intact "A small cleavage cavity was already apparent within each embryo" (their fig. 1).
- 12 cells, Carnegie No. 8904. Described by Hertig et al. (1954)[1]. Uterine. Diameter, 172.8 μm (115.2 after fixation); blastomeres, 38.4 x 19.2. Clear zona pellucida (10 μm in thickness before fixation). Polar bodies not identified. No evidence of a blastocystic cavity, A large, central blastomere was thought to be embryogenic, the other cells trophoblastic. Specimen lost during preparation. Believed to be about 3 days old.
- 14-26 cells. Lopata, Kohlman, and Johnston (1983) found complex intercellular junctions in the blastomeres that "suggest that compaction was occurring." Tinctorial differences were noted among the cells of specimens of 5-12 cells. Multinucleated (probably abnormal) examples were also recorded.
- 16 cells. Specimens ranging from 1 to "16 or more" cells were produced in vitro (Edwards, Steptoe, and Purdy, 1970). Photographs of a 4- and an 8-cell specimen were included.
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ 1.0 1.1 1.2 1.3 1.4 Hertig AT. Rock J. Adams EC. and Mulligan W.J. On the preimplantation stages of the human ovum: a description of four normal and four abnormal specimens ranging from the second to the fifth day of development. (1954) Carnegie Instn. Wash. Publ. 603, Contrib. Embryol., 35: 199-220.
- ↑ 2.0 2.1 MENKIN MF & ROCK J. (1948). In vitro fertilization and cleavage of human ovarian eggs. Am. J. Obstet. Gynecol. , 55, 440-52. PMID: 18903892
- ↑ Sundström P, Nilsson O & Liedholm P. (1981). Cleavage rate and morphology of early human embryos obtained after artificial fertilization and culture. Acta Obstet Gynecol Scand , 60, 109-20. PMID: 7246074
- ↑ CORNER GW. (1955). The observed embryology of human single-ovum twins and other multiple births. Am. J. Obstet. Gynecol. , 70, 933-51. PMID: 13258680
- ↑ GEDDA L & CAPRILLI F. (1961). [Sideremia in twins. (Study on 23 MZ pairs and 24 DZ pairs)]. Acta Genet Med Gemellol (Roma) , 10, 1-20. PMID: 13704137
- ↑ Doyle LL, Lippes J, Winters HS & Margolis AJ. (1966). Human ova in the fallopian tube. Am. J. Obstet. Gynecol. , 95, 115-7. PMID: 5934993
- ↑ ASHLEY DJ. (1959). Are ovarian pregnancies parthenogenetic?. Am. J. Hum. Genet. , 11, 305-10. PMID: 13794748
- ↑ Jacobson CB, Sites JG & Arias-Bernal LF. (1970). In vitro maturation and fertilization of human follicular oocytes. Int. J. Fertil. , 15, 103-14. PMID: 5430388
- ↑ Brackett BG, Mills JA & Jeitles GG. (1972). In vitro fertilization of rabbit ova recovered from ovarian follicles. Fertil. Steril. , 23, 898-909. PMID: 4644557
- ↑ SHETTLES LB. (1958). The living human ovum. Am. J. Obstet. Gynecol. , 76, 398-406. PMID: 13559329
- ↑ WAY S & DAWSON N. (1959). A human ovum in a vaginal smear. J Obstet Gynaecol Br Emp , 66, 491. PMID: 13843202
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 20) Embryology 1987 Developmental Stages In Human Embryos - Stage 2. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_2
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G