1987 Developmental Stages In Human Embryos - Stage 17
| Embryology - 24 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
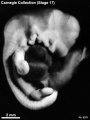
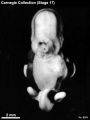
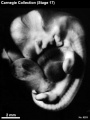
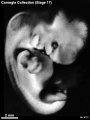
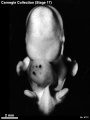
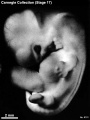
Stage 17
Stage 17 (8253)
Stage 17 (8253)
Stage 17 (8253)
Stage 17 (8253)
Stage 17 (8101)
Stage 17 (8101)
Stage 17 (8101)
Stage 17 (8101)
Summary
External: the head is relatively larger than previously; the main axis of the trunk has become straighter, and a slight indication of a lumbar curvature may be found; the nasal pit is further medial and is directed ventrally so that the nostril is not visible in profile views, and nasofrontal grooves are distinct; the full complement of auricular hillocks is present on the mandibular and hyoid arches; the hand plate exhibits definite digital rays, and the foot has acquired a rounded digital plate; surface elevations of individual somites are becoming limited mostly to the lumbosacral region.
Internal: in the heart, foramen secundum and semilunar cusps have appeared (stages 15–17), and foramen primum is being obliterated and the atrioventricular cushions are fusing (stages 16–18); the beginnings of the palate are appearing, the dorsal and ventral pancreas are fused, and the vermiform appendix is distinguishable; the bronchial tree shows segmental buds; the mesonephros is functional, calices are developing, and the urogenital sinus presents two divisions; chondrification begins in some of the vertebral centra and in the humerus and radius; the future olfactory bulb is indicated; the retinal fissure is largely closed and the lens cavity is becoming crescentic; semicircular ducts are imminent but none is yet present; the auditory ossicles are defined.
Size and Age
The size of 25 embryos at this stage ranged in greatest length from 10.0 to 14.5 mm. This does not include one that had a deformed head. If the five largest specimens (length 14.0–14.5 mm) and the three smallest ones (length 10.0–10.8 mm) be set aside, there remain seventeen embryos (68 percent) ranging from 11.0 to 13.6 mm in length. Embryos of stage 17 are, therefore, most likely to have a greatest length within this range. Taking the group as a whole, about half of them are under 13 mm and the other half measure 13 mm or more. On the basis of their inner structure, the largest embryos are more-advanced members of the group and belong on the borderline. Likewise, the three small ones belong among the least advanced members of the group.
One can speak less precisely of the size of the chorionic sac than of the size of the contained embryo. If the manifestly abnormal chorions be omitted, none has a greatest diameter less than 30 mm, and one-third have greatest diameters of 40–48 mm. These values differ little from those in the preceding stage, but they are somewhat smaller than those found in the next stage.
The age of the embryos of stage 17 is believed to be approximately 41 days.
External Form
(figs. 17-1 to 17-3) As a result of the precocious growth of the brain, the head of the embryo appears relatively large. The head, together with the adjacent part of the neck, now has about the same profile area as the remainder of the body. This is a greater proportion than existed at any previous stage. There is also a noticeable increase in the fronto-mesencephalic length of the head, measured from the most frontal point of the forehead to the caudal border of the colliculi, immediately in front of the precerebellar notch (isthmus). Furthermore, both the head and the thorax have become wider, whereas the lumbosacral region remains more slender. Up to this time, tinder normal conditions, embryos have had a curved dorsal contour interrupted only by the cervical flexure. The trunk in stage 17 becomes straighter and begins to acquire a slight lumbar flexure, the causative factors for which are to be found in the transformations of the inner structures. The caudal termination of the trunk, instead of tapering smoothly as heretofore, now begins to have an abrupt recessive character that will result in the caudal filament of later stages.
A distinct nasofrontal groove is constant in profiles of all specimens of the group. The nasal pit opens ventrally and cannot be seen in full-profile views. To examine the nostril it is necessary to have decapitated specimens or models.
The auricular hillocks exhibit their characteristic form at stage 17. Shown in figures 16-5 and 17-3, they consist of six circumscribed superficial condensations, three (Nos. 1–3) on the mandibular arch and three (Nos. 4–6) on the hyoid arch. The latter are more prominent and are destined to form the auricle of the ear. The three on the caudal surface of the mandibular bar are less sharply outlined. The ventralmost of them becomes the tragus, and the dorsal two join in the formation of the crus helicis. Between these two rows of hillocks the hyomandibular groove increases in width and depth, thereby initiating the formation of the concha and the external acoustic meatus. It is common for the hillocks to coalesce at this time and take the form of a key-plate. The opening for the key is encroached upon by hillocks 2 and 5, and the key slot is thus separated into dorsal and ventral parts, like the numeral 8.
The transformation of this region into the external ear has been described by Streeter (1922), Hochstetter (1948), and Blechschmidt (1965). According to Streeter, only minimal and superficial parts of the mandibular and hyoid arches participate in the building of the external ear. The importance of the auricular hillocks has long been in dispute. In spite of their apparent independence of other structures, the development of the external ear keeps in step with that of other organs, and one can fairly well determine, from the status of the external ear, the stage to which a given embryo belongs.
The upper limb bud at stage 17 is distinguished from that of the previous stage by the acquisition of finger rays. They are only slightly indicated in the less advanced members of the group, but are clearly demarcated in all others. The hand plate of the most advanced members begins to have a crenated rim caused by the projecting tips of the individual digits. This crenation becomes a prominent characteristic at the next stage.
An outline of the lower limb bud at stage 17 is shown in figure 16-4. Apart from having increased in length and general mass, it now has a rounded digital plate, set off from the tarsal region and leg. One can also begin to see, at the junction of the limb bud and trunk, evidence of slower-developing tissue that will eventually become the muscles and bones of the pelvic girdle.
Turning to the somites, it is found that these do not influence the surface markings except in the lumbar and sacral regions, where they stand out as definite elevations. In less-advanced members of the group they can be seen as far rostrally as mid-thoracic levels. Throughout the cervical and upper thoracic regions, the increase in superficial embryonic connective tissue obscures the spinal ganglia. When embryos are unusually translucent the ganglia can be seen even here.
Face
By stage 17 the face has emerged as a consequence of the enlargement and fusion of several growth centers which are generally spoken of as facial processes. As they merge, these produce structures recognizable as the definitive nose and upper jaw. The important steps in this transformation are illustrated in figure 17-3. Ever since the pioneer descriptions of His, they have been designated as facial processes. To call them that, however, has the disadvantage of oversimplification. In reality they are not prolongations having free ends that meet in the nasal region; nor is the ectoderm absorbed over their abutting surfaces. In transverse series of embryos the facial region is likely to be cut coronally (i.e., tangentially), which gives an exaggerated idea of their length. The alternate sections of the series used by His are now in the Carnegie Collection, catalogued as No. 7317. It is more precise to speak of these structures as swellings or ridges that correspond to centers of growth in the underlying common mesenchyme. The furrows that lie between them on the surface are smoothed out as the proliferation and fusion of the growth centers fill in beneath: i.e., merging occurs rather than fusion (Patten, 1961). Under these circumstances no ectoderm requires absorption; it is simply flattened out in adaptation to the changed surface. This is in confirmation of the observations of Peter (1913), who found that the grooves separating the facial processes do not disappear as a result of the fusion of their edges. Instead they become more shallow and eventually smooth, as the increase in mass produces a new surface level. This point is to be kept in mind in analysis of the factors involved in the deformities that accompany harelip.
In the formation of the face certain aspects require emphasis. First, the component growth centers are bilateral, and the right and left groups are originally widely separated from each other. Secondly, these growth centers on each side include ( l ) a ventromedial extension of the maxillary arch (maxillary process), (2) the ridge, or lip, forming the dorsolateral boundary of the nasal pit, destined to become the nasal wing on that side (lateral nasal process), (3) the rounded medial rim of the nasal pit (medial nasal process), which subsequently in cooperation with its mate and the intervening tissue unites in the formation of the septal elements of the nose, together forming a single median structure, the composite nasal septum, and (4) the growth center at the ventral end of the medial nasal rim, but discrete from it, destined to form the premaxilla (premaxillary center). The last is the globular process of His and is commonly included as part of the medial nasal process. It could equally well be termed the intermaxillary or incisive center. The term frontal process has been applied to the median field lying between and dorsal to the nasal pits. In stage 17 the frontal field is marked by the absence of any condensation, and it is premature to speak of a “process.” The latter will come considerably later as a part of the building of the elongated dorsum of the nose. Thirdly, the above-mentioned growth centers blend with each other and, in some cases, meet across the median plane. As they do so, they exhibit in each instance the ability to form the various structures and strata required in their particular region, such as lip, alveolar and palatine processes, and finally teeth.
According to Politzer (1936), two more or less parallel furrows are found cranial to the maxillary process. He maintained that the cranial is the nasolacrimal groove whereas, contrary to the usual description, the caudal, in his view, is merely the limiting furrow of the maxillary process (Grenzfurche des Oberkieferfortsatzes), or what is generally termed the nasomaxillary groove.
The establishment of the face can be traced in figures 16-5, 17-3, and 18-3. Scanning electron micrographs have been published by Hinrichsen (1985).
Nasal Passages
The transformations of the right and left nasal pits lead to the establishment of bilateral respiratory passages that by-pass the mouth. Already at stage 16 the epithelium of the nasal pit shows evidence of regional specialization. Because of this and the accompanying proliferation of the underlying mesenchyme, one can now recognize the outlines of the wings of the future nose and the median rim of each nostril.
From the edges of the nostril, and sharply demarcated from the abutting skin ectoderm, the nasal epithelium extends deeply into the preoptic region as a flattened pocket, constituting the nasal sac. Along the ventral fold of the latter, the epithelium exhibits a characteristic growth activity. It proliferates in the form of a plate-like fin or keel which maintains an epithelial continuity between the nasal sac and the roof of the mouth. The location of the nasal fin is indicated on the surface by a groove, at first shallow, later becoming deeper and marking the boundary between the premaxillary and maxillary growth centers. This is the primitive palatine groove of Peter (1913).
Except for that maintained by the nasal fin, the continuity between the nasal sac and the roof of the mouth becomes interrupted by the active proliferation of the mesenchyme of the premaxillary and maxillary growth centers, which blends across from one center to the other, in front of the nasal fin. There is thereby established the primordium of the palate. The right and left primary palates are derived from the premaxillary centers and unite in the median plane. That part (secondary palate) derived from the maxillary centers takes the form of lateral elevations on the two sides.
The developmental function of the nasal fin begins to express itself already in stage 16, at which time there is scarcely more than a nasal pit. In stage 17 the primary palate makes its appearance, and the nasal fin becomes transformed from an epithelial plate to an epithelium lined passage in consequence of the coalescence of its cleavage spaces and through adaptation of its rapidly proliferating cells. As long as the end of this passage is obstructed by incomplete cleavage of the epithelium, it constitutes a cul-de-sac, the hinteren Blindsack of Peter. In stage 18, among the less advanced members of the group, an epithelial membrane-like remnant (the bucconasal membrane of Hochstetter) still stretches across the opening. In more-advanced members, the last strand has been retracted and a free respiratory passage exists from the nostril through the choana to the nasopharynx. The steps in the laying down of the primary nasal passage can be seen in simplified form in sagittal sections, as is illustrated in figure 17-4. Sections taken from four embryos belonging to stage 17 are shown in greater detail in figure 17-5. These illustrate the nasal fin during its maximum prominence, as seen in both transverse and sagittal sections.
Cardiovascular System
Heart
The cleavage of the primary cardiac tube continues during stage 17, advancing the separation of the pulmonary and aortic channels. In addition, the right and left atrioventricular canals become completely separate. As shown in figure 17-6, the primary cardiac tube and its surrounding mesenchyme (gelatinous reticulum) still form a considerable part of the field and stand out in contrast with the trabeculated muscular sacs that are forming the right and left ventricles. As has been pointed out, the right and left ventricular pouches, as they increase in size and pulsating force, tend to give rise to two diverse currents corresponding to their separate directional axes. Apparently this finds its expression in the expansion of the endocardial lumen into two main channels (aortic and pulmonary), as seen in embryos Nos. 3385 and 6510 in figure 15-5. According to Streeter, the endocardium between them is consequently subject to less distention, resulting in its final absorption. Thereupon the supporting mesenchyme fills in along that spiral path of less stress. Under such circumstances the adaptation of endothelium to the existence of two blood currents must rank high as a factor in the cleavage of the aortic and pulmonary trunks, whereas conotruncal ridges are merely the remains of the cardiac mesenchyme, a filling-in behind the reshaping of the endothelium. An increase inthe supporting reticular cells is found, and such a condensation is seen particularly in the plane where the aortic and pulmonary channels are undergoing cleavage. An area of that kind is illustrated in section 6-2-2 in figure 17-6, and it signifies that one is close to an endothelial surface.
A complicated organ like the heart can be studied with advantage in three-dimensional reconstructions (fig. 17-7). In the embryo selected, the atria were contracted, thus favoring the viewing of the two atrioventricular canals. The reconstruction is shown at two levels, one (A) in front of and to be superimposed upon the other (B). The front model (A) shows the pulmonary and aortic trunks leaving the heart, and the part they take in the formation of the left dorsal aorta.
It also shows the progress of cleavage of the endocardial aortic and pulmonary channels. At the semilunar valves (which appeared already at stage 16) the endocardium preserves the continuity of the lumen, whereas the condensing mesenchyme fills in behind it, forming three characteristic rounded occluding masses. The narrow lumen through the valve appears on section as a three-limbed cleft. This, therefore, is a weak spot, and when one attempts to make a cast of the lumen of these trunks, a break at the semilunar sites is likely to result. Proximal to the valves the primary cardiac tube is still relatively voluminous, and the larger parts correspond more or less to channels suited to the two main blood currents. In the formation of the semilunar valves, if mechanical factors enter in, they certainly must be much more complicated than those suggested to account for the separation of two main blood channels. But even in these valves one would hesitate to rule out the possibility of some mechanical influence at these sites, associated, for instance, with the back-thrust of the systemic blood columns which would, already at this time, follow each heartbeat.
The posterior model (B) shown in figure 17-7 is visualized as dissected free from the more anteriorly situated part (A). The cut edge of the primary cardiac tube (B) is arranged so as to leave uncovered the entrance of the right atrioventricular canal. From examination of both views (A and B) one finds that the separation of the aortic and pulmonary channels is complete to the region proximal to the semilunar valves. From there proximally, the primary cardiac tube still consists of a single voluminous cavity, common to right and left sides. Into it the incoming blood enters separately from the right and left atria. These canals mark the sites of the mitral and tricuspid valves, which are still in the form of condensing mesenchyme. By stage 17 foramen primum is obliterated and foramen secundum is wide. The membranous part of the interventricular septum is formed from cushion material that does not close the primary interventricular foramen (McBride, Moore, and Hutchins, 1981)[1]. Interventricular foramen 2 is finally bounded by the conal septum (especially by the right conal ridge) and the fused atrioventricular cushions (Wenink, 1971). The outflow tract is said to have been undergoing a counterclockwise rotation. It should be stressed, however, that “the formation of the septal system of the truncus and conus is more complex than is usually recognized” (Kramer, 1942).
Digestive System
Six zones (four maxillary and two mandibular) of odontogenic epithelium have been identified (Nery, Kraus, and Croup, 1970)[2].
In figure 17-8 is shown in outline the epithelial alimentary canal that is characteristic of stage 17. Over and above its relative increase in length, various regional morphological details are making their appearance. In addition to a caecum, one can now distinguish the vermiform appendix. The primary intestinal loop projects further into the umbilical cord as the normal umbilical hernia, which persists until approximately 40 mm. The elongating colon seems to be providing the thrust that will aid in bringing the caecum to the right side of the body.
The duodenal region is an especially active center of epithelial proliferation, resulting in two fusiform enlargements, one proximal to the entrance of the bile and pancreatic ducts and the other distally. In the region of these enlargements the epithelium in its accentuated growth acquires a follicular arrangement in which the epithelium is oriented around small discrete cavities (Johnson, 1910). In some cases these constitute isolated follicles and in others they consist of diverticula, communicating with the partially obliterated original lumen of the gut (fig. 17-9) (Boyden, Cope, and Bill, 1967). Traces of this exuberant type of epithelial proliferation with its characteristic follicular tendency are found also in other parts of the canal, notably in the esophagus.
The relations of the ventral and dorsal pancreas and of the biliary ducts to the intestine are shown in figure 17-10. One can see that the ventral pancreas, which had sprouted from the bile duct, has now fused with the dorsal pancreas. Each pancreas, however, still retains its original duct, that of the ventral pancreas emptying into the bile duct and that of the dorsal pancreas, further rostrally, directly into the duodenum. Finally, following anastomosis of the ductal systems of the two parts, the more favored passage for the pancreatic secretions from the whole gland leads through the ventral pancreatic duct beside the bile duct, and the dorsal pancreatic duct may disappear in the process.
Liver
The liver has already taken form as the joint product of angioblastic tissue arising from the coelomic surface cells (fig. 11-6) and of epithelial columns coming from the opposite direction, sprouting from the hepatic evaginations of the gut epithelium (fig. 12-5). The two tissues in this combination undergo continued rapid proliferation, and there is soon produced the typical, large trabeculated epitheliovascular organ known as the liver. The liver shows signs of functional activity important to the embryo from the beginning of blood circulation and thereafter. If its first functional assignment were to be its sole function, there appears no reason that it should not become detached from the gut wall and carry on by itself. It does not, however, become detached. There remains an epithelial stem that preserves the continuity between the hepatic epithelium and that of the gut. This stem is partially plexiform, and by stage 17 it is readily recognized as the hepatic duct.
The nature of the abrupt terminal endings of the hepatic duct is illustrated in fig. 17-11. It does not yet penetrate far into the hepatic substance and is found only at the hilum in the connective tissue of the wall of the entering portal vein. At its elongating tips, it is directly continuous with the hepatic epithelium, and its further peripheral growth is accomplished at the expense of the latter, which cell by cell at these tips is transformed into smaller, darkly staining ductal cells. This differentiation keeps step with the formation of the connective tissue coat of the portal vein, along the branches of which the biliary ductal system spreads. Near the hepatic duct, but essentially separate in origin, is the gall bladder and its duct. The hepatic duct and the plexiform terminations of its biliary tributaries, in the reticulum along the wall of the portal vein, have been reconstructed in three dimensions and are shown in figure 17-10. Information on the development of the intrahepatic biliary ducts is available (Koga, 1971)[3].
Respiratory System
The separation of the lungs from the digestive system is essentially complete by stage 17, and the pseudoglandular phase of pulmonary development is under way. The form of the epithelial bronchial tree is illustrated in figures 17-8 and 17-14. One can more definitely recognize the groupings that will make up the three lobes on the right side and two on the left. Segmental buds represent the bronchopulmonary segments. In addition to the growth and branching of the epithelial tube, the surrounding visceral mesenchyme, which, in part at least, was derived from the coelomic epithelium, is advancing in its differentiation, and one can now recognize condensations that are to become tracheal cartilages. The vagus nerves have increased progressively insize. Figure 15-7c shows that the esophagus, lying behind the trachea, is acquiring a submucous coat enclosing the epithelium, which coat is practically absent from the trachea. It is also to be noted that the right and left pulmonary arteries have become more prominent.
Urinary System
(fig. 17-14) The mesonephros shows epithelial plaques in the visceral layer of the glomerular capsule and hence can produce urine (Silverman, 1969)[4]. The pelvis of the ureter usually shows three main divisions, and calices appear. The urogenital sinus presents a pelvic part (vesico-urethral canal) and a phallic part (definitive urogenital sinus).
Reproductive System
The structure of the gonad is shown in figure 18-9. The paramesonephric ducts, which may appear at the previous stage, arise as invaginations of the coelomic epithelium. The genital eminence forms the phallus at stage 17 or stage 18. The nipples appear as buds on the mammary crest (Bossy, 1980b).
Skeletal System
Some of the vertebral centra, which appear at stage 15, begin chondrification at stage 17 (Sensenig, 1949). The neural arch begins as right and left neural processes at stage 15. The skeleton of the upper and lower limbs becomes visible as mesenchyme at stages 15 and 16, respectively, and chondrification commences at stages 16–17 (e.g., in the humerus and radius) and 17-18 (e.g., in the femur) (O'Rahilly and Gardner, 1975)[5]. A general view of the mesenchymal skeleton at stage 17 shows also the beginning chondrocranium (Blechschmidt, 1963, plate 24).
Nervous System
(fig. 17-12) The rhombomeres are still visible, but the rhombic grooves are limited to the side and absorbed in the surrounding surface, so that they are no longer visible in a median view. Migration of cells from the medioventral cell column to the definitive sites of the special visceral motor nuclei is still proceeding. The first nucleus to settle down is that of the trigeminal nerve. Vestibulocerebellar and trigeminocerebellar fibers run toward the cerebellum, which grows in thickness.
The geniculate ganglion is now separated from that of the vestibulocochlear nerve. The cerebellar plate (innerer Kleinhirnwulst of Hochsetter) is present as a clear thickening. The mesencephalon now possesses an intermediate layer throughout most of its extent. The posterior commissure between the midbrain and the diencephalon is prominent. The ventral thalamus, lying rostral to the marginal ridge, is well in advance of the dorsal thalamus. The former possesses an intermediate layer, whereas the latter begins its development only now. The neurohypophysis is a distinct evagination in all embryos; the adenohypophysis is still open toward the pharyngeal cavity. The epiphysis cerebri, a thickening in the roof of the diencephalon, develops an intermediate layer. The corpus striatum bulges into the ventricular cavity, and, together with the ventral thalamus, delimits the interventricular foramen. The area overlying the corpus striatum is slightly flattened and represents the future insula. One-third of the length of the cerebral vesicles reaches more rostrally than the lamina terminalis. The olfactory tubercle is characterized by cellular islands; both the olfactory tubercle and the future olfactory bulb are marked by a slight elevation at the surface of the brain. The olfactory fibers form a compact bundle that runs from caudal to rostral, where the fibers enter the brain wall. A medial strand, containing the future vomeronasal and terminal nerves, can be distinguished from a lateral one (Bossy, 1980a). All parasympathetic ganglia of the cranial nerves, except the otic, are present (Wozniak and O'Rahilly, 1980). The dural limiting layer begins to form in basal areas of the brain, and pori durales for some of the cranial nerves are present (O'Rahilly and Müller, 1986b).
Eye
Retinal pigment is clearly visible even under low power (figs. 17-13C,D and 18-12B). The retinal fissure is largely closed. Further changes are occurring in retinal differentiation (O'Rahilly, 1983b), particularly in the future macular region. The cavity of the lens vesicle changes gradually from D-shaped to crescentic (fig. 17-13D).
Ear
The membranous labyrinth is shown in figure 17-14. The endolymphatic appendage is a relatively large, thin-walled, fusiform sac. The future cochlear duct is elongating at the tip of the labyrinth. The walls of the vestibular part are becoming thinner and approximated, making imminent the absorption of the contacting surfaces and the resulting formation of the semicircular ducts. Only those specimens in which absorption has not yet occurred are admitted to this stage, and consequently in none of them is a semicircular duct yet present. The auditory ossicles are defined in mesenchyme.
Specimen of Stage 17 Already Described
- 12-mm embryo. The peripheral nervous system was described in an stage 17 embryo.[6]
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ Mcbride RE, Moore GW & Hutchins GM. (1981). Development of the outflow tract and closure of the interventricular septum in the normal human heart. Am. J. Anat. , 160, 309-31. PMID: 7223678 DOI.
- ↑ Nery EB, Kraus BS & Croup M. (1970). Timing and topography of early human tooth development. Arch. Oral Biol. , 15, 1315-26. PMID: 5280132
- ↑ Koga A. (1971). Morphogenesis of intrahepatic bile ducts of the human fetus. Light and electron microscopic study. Z Anat Entwicklungsgesch , 135, 156-84. PMID: 5135745
- ↑ Silverman H. (1969). [The development of the epithelial plaques in the renal corpuscles of the human embryonic kidney]. Acta Anat (Basel) , 74, 36-43. PMID: 5374935
- ↑ O'Rahilly R & Gardner E. (1975). The timing and sequence of events in the development of the limbs in the human embryo. Anat. Embryol. , 148, 1-23. PMID: 1202989
- ↑ Volcher R. Le systeme nerveux pe'riphe'rique cninien d'un embryon humain de 12 mm. (1959) Arch. Biol. (Liege), 70:179-215.
See also Müller F & O'Rahilly R. (1989). The human brain at stage 17, including the appearance of the future olfactory bulb and the first amygdaloid nuclei. Anat. Embryol. , 180, 353-69. PMID: 2802187
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 24) Embryology 1987 Developmental Stages In Human Embryos - Stage 17. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_17
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G