1987 Developmental Stages In Human Embryos - Stage 1
| Embryology - 20 Apr 2024 |
|---|
| Google Translate - select your language from the list shown below (this will open a new external page) |
|
العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Stage 1
- Approximately 0.1 - 0.15 mm in diameter
- Approximately 1 postovulatory day
- Characteristic feature: unicellularity
Embryonic life commences with fertilization, and hence the beginning of that process may be taken as the point de depart of stage 1. Despite the small size (ca. 0.1 mm) and weight (ca. 0.004 mg) of the organism at fertilization, the embryo is "schon ein individual-spezifischer Mensch" (Blechschmidt, 1972). The philosophical and ethical implications have been discussed briefly by O'Rahilly and Müller (1987).
Fertilization is the procession of events that begins when a spermatozoon makes contact with an oocyte or its investments and ends with the intermingling of maternal and paternal chromosomes at metaphase of the first mitotic division of the zygote (Brackett et al., 1972). Fertilization sensu stricto involves the union of developmentally competent gametes realized in an appropriate environment to result in the formation of a viable embryo capable of normal further development (Tesarík, 1986[1]).
Fertilization requires probably slightly longer than 24 hours in primates (Brackett et al., 1972). In the case of human oocytes fertilized in vitro, pronuclei were formed within 11 hours of insemination (Edwards, 1972).
Given the availability of a mature oocyte (first meiotic division completed) and capacitated spermatozoa (permitting the acrosomal reaction), the criteria for fertilization generally adopted are (1) the presence of two or more polar bodies in the perivitelline space, (2) the presence of two pronuclei within the ooplasm, and (3) the presence of remnants of the flagellum of the fertilizing spermatozoon within the ooplasm (Soupart and Strong, 1974).
Fertilization, which takes place normally in the ampulla of the uterine tube, includes (a) contact of spermatozoa with the zona pellucida of an oocyte, penetration of one or more spermatozoa through the zona pellucida and the ooplasm, swelling of the spermatozoal head and extrusion of the second polar body, (b) the formation of the male and female pronuclei, and (c) the beginning of the first mitotic division, or cleavage, of the zygote. The various details of fertilization, including such matters as capacitation, acrosomal reaction, and activation, are dealt with in special works.
When cortical granules are released, their contents appear to reinforce the structure of the zona pellucida (Sathananthan and Trounson, 1982). This is thought to be the morphological expression of the zonal reaction, and the cortical and zonal reactions may provide a block to polyspermy.
The three phases (a, b, and c) referred to above will be included here under stage 1, the characteristic feature of which is unicellularity. The sequence of events before and during the first three stages is summarized in Table 1-1.
The term "ovum," which has been used for such disparate structures as an oocyte and a 3-week embryo, has no scientific usefulness and is not used here. Indeed, strictly speaking, "the existence of the ovum ... is impossible" (Franchi, 1970). The term "egg" is best reserved for a nutritive object frequently seen on the breakfast table.
At ovulation, the oocyte is a large cell surrounded by a thick covering, the zona pellucida, which is believed to be produced (at least largely) by the surrounding follicular cells. Processes of the follicular cells and microvilli of the oocyte both extend into the zona. The diameter of such a mammalian cell, including its zona, ranges from 70 to 190 μm. In the human, the ooplasm measures about 100 μm, and the thickness of the zona ranges from 16 to 18 μm (Allen et al., 1930). Good photomicrographs and electron micrographs of human secondary oocytes are available (e.g., Baca and Zamboni, 1967, figs. 20 to 24; Kennedy and Donahue, 1969). The zona pellucida is covered externally by the corona radiata, which is a loose investment of granulosa cells from the ovarian follicle. On fixation and embedding, the oocyte undergoes shrinkage; this affects the cytoplasm more than the zona, so that a subzonal (or perivitelline) pace becomes accentuated. The polar bodies are found within that space. It is said that the first polar body may divide before the second is released, and it has been claimed that each of the three polar bodies is capable of being fertilized. Although it is not unusual for the second polar body to display a nucleus, the chromosomes of the first polar body are isolated and naked (Zamboni, 1971).
Fig. 1-1. (a) Phase contrast view of human ootid after fixation and staining. The zona pellucida had been dissolved during preparation of the specimen. (b) Phase contrast, oil immersion view of the pronuclei shown in (a). Both views, by courtesy of Dr. Z. Dickmann and Alan R. Liss, Inc. (1965)[2].
Table 1-1. Tabulaton of the First Three Stages
| Stage | Event | Products |
|---|---|---|
| meiosis 1 | Oocyte 2 and polar body 1 | |
| ovulation beginning meiosis 2 | Ovulated oocyte | |
| 1a | fertilization - Penetration | Penetrated oocyte |
| 1b | fertilization - Completion of meiosis 2 and formation of pronuclei | Ootid and polar body 2 |
| 1c | fertilization - Pronuclei enter cleavage division | zygote |
| 2 | Cleavage continues | 2 to about 16 cells morula |
| 3 | Formation of blastocystic cavity | blastocyst, from about 32 cells onward |
It is "likely that no more than one day intervenes between ovulation and fertilization, This time interval may be taken then as the possible error in age of [an] embryo when it is considered the same as ovulatory age" (Rock and Hertig, 1942).
(a) Penetrated oocyte
This term may conveniently be used once a spermatozoon has penetrated the zona pellucida and, strictly, "after gamete plasma membranes have become confluent" (Zamboni et al., 1966[3]). Penetration has been inferred from the presence of spermatozoa in the zona pellucida or in the subzonal space (Edwards, Bavister, and Steptoe, 1969). Moreover, in vitro examples showing portions of spermatozoa within the ooplasm are illustrated by Sathananthan, Trounson, and Wood (1986), in whose work are also detailed views showing the formation of the second polar body.
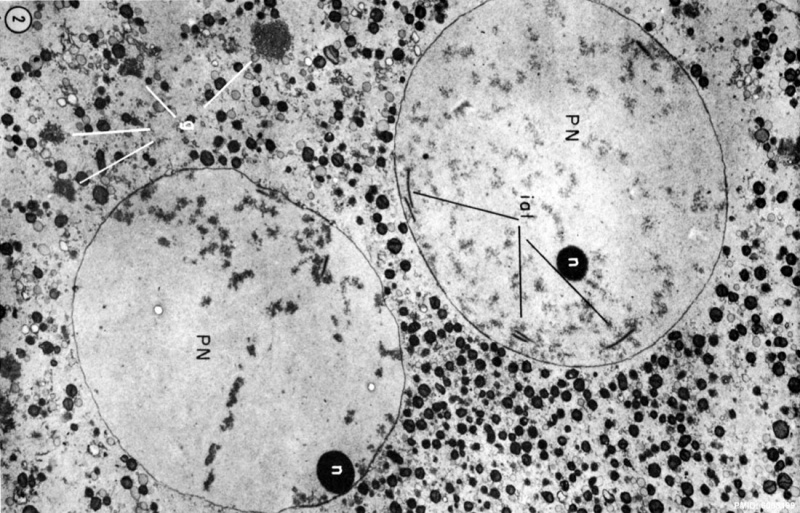
Fig. 1-2. Electron micrograph of the male and female pronuclei in a human ootid. The pronuclear material appears to be highly hydrated, although it is condensed in patches. A small black sphere, namely the nucleolus, and some annulate lamellae are visible within each pronucleus. Numerous organelles are present in the cytoplasm adjacent to the pronuclei, and portions of a Golgi complex are visible nearthe lower left-hand corner of the photograph. x 5,400.[3]
(b) Ootid
The cell characterized by the presence of the male and female pronuclei is termed an ootid (figs, l and 1-2). Several examples of human ootids have been described. They are probably about 12-24 hours in age. The diameter, including the zona pellucida, is about about 175 μm (Hamilton, 1946; Dickmann et al., 1965[2]), and the diameter of the subzonal space is approximately 140 μm. The cytoplasm of the ootid has a diameter Page 12 of about 100 μm (Hamilton, 1946; Noyes et al., 1965[4]); each of the pronuclei measures about 30 μm (Zamboni et al., 1966[3]). The various ultrastructural features of the ootid have been described and illustrated (Zamboni et al., 1966[3]; Sathananthan, Trounson, and Wood, 1986).
Although "in most mammalian species, the male pronucleus has been reported to be larger than the female pronucleus," the converse has been found in one human specimen and, in two others, the pronuclei appeared to be of equal size (Zamboni, 1971).
(c) Zygote
The cell that characterizes the last phase of fertilization is elusive. The first cleavage spindle forms rapidly and has been used in identification. Such cells have probably been seen in certain mammals, e.g., the pig, cow, hamster, rat, and mouse.
Pronuclear fusion does not occur. Rather, the two pronuclear envelopes break down ("post-apposition envelope vesiculation," Szabo and O'Day, 1983[5]), and the two groups of chromosomes move together and assume positions on the first cleavage spindle. Thus the zygote lacks a nucleus.
A human embryo "in syngamy just prior to cleavage" has been illustrated by Sathananthan and Trounson (1985[6], fig. 2). "The chromosomes, some associated in pairs, are located in an agranular zone in the central ooplasm."
In the human, the initial cleavage that heralds the onset of stage 2 occurs in the uterine tube "some time between twenty-four and thirty hours after [the beginning of] fertilization" (Hertig, 1968).
Specimens of Stage 1 Already Described
Embryos of stages 1-3 have been seen very frequently since the advent of in vitro fertilization in 1969.
Ootids have been described by the following authors:
- Hamilton (1946[7] and 1949[8]) - Tubal. Diameter (including zona pellucida), 173 μm. Diameter of ooplasm, 100 μm. Sectioned serially at 7 μm. Two pronuclei, one larger than the other. Many spermatozoa in zona pellucida. Dickmann et al. (1965)[2] have expressed some doubts about this specimen.
- Khvatov (1959)[9] - Tubal. Two pronuclei, claimed to be distinguished as male and female.
- Dickmann et al. (1965)[2] - Tubal. Diameter (including zona), 174 μm. Zona pellucida, 17.5 μm in thickness. Diameter of ooplasm, 103 μm (Noyes et al., 1965[4]). Two pronuclei, approximately equal in size (fig. 1-1b). Nucleoli visible. Tail of fertilizing spermatozoon identified over one pronucleus. Well illustrated (figs. 1-1a and b).
- Zamboni et al. (1966)[3] - Tubal. Ootid estimated to have a maximum diameter of about 150 μm, and 110-120 μm without the zona pellucida (Zamboni, personal communication, 1970). Fixed and sectioned for electron microscopy. Zona seen and three polar bodies identified. Two pronuclei, of about equal size (30 μm), each with a spheroidal nucleolus. Remnants of penetrating spermatozoon identified near one pronucleus. Ultrastructural findings described in detail and well illustrated (fig. 1-2).
- Edwards, Bavister, and Steptoe (1969)[10] - Seven ootids resulted from insemination in vitro of oocytes matured in vitro. Two had two pronuclei each, four had three each, and one had five. Photographs, but no cytological details, were provided.
- Soupart and Morgenstern (1973)[11] - Two pronuclei and two polar bodies obtained in vitro.
- Soupart and Strong (1974)[12] - Fourteen examples examined by electron microscopy. Two pronuclei (that near spermatozoal flagellum believed to be male) and two polar bodies.
- Lopata et al. (1978[13], 1980[14]) - Several in vitro examples.
- Sathananthan[15] and Trounson (1982) - studied the release of cortical granules at stages 1 and 2.
- Pereda and Coppo (1984)[16] - found, by electron microscopy, light and dark follicular cells surrounding an ootid from the uterine tube.
- Sathananthan, Trounson, and Wood (1986)[17] - Several in vitro examples are illustrated.
References
| Online Editor Note |
|---|
| O'Rahilly R. and Müller F. Developmental Stages in Human Embryos. Contrib. Embryol., Carnegie Inst. Wash. 637 (1987).
The original 1987 publication text, figures and tables have been altered in formatting, addition of internal online links, and links to PubMed. Original Document - Copyright © 1987 Carnegie Institution of Washington.
|
- ↑ Tesarík J, Kopecný V, Plachot M & Mandelbaum J. (1986). Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J. Reprod. Fertil. , 78, 463-70. PMID: 2433438
- ↑ 2.0 2.1 2.2 2.3 Dickmann Z, Chewe TH, Bonney WA & Noyes RW. (1965). The human egg in the pronuclear stage. Anat. Rec. , 152, 293-302. PMID: 5323255
- ↑ 3.0 3.1 3.2 3.3 3.4 Zamboni L, Mishell DR, Bell JH & Baca M. (1966). Fine structure of the human ovum in the pronuclear stage. J. Cell Biol. , 30, 579-600. PMID: 6008199
- ↑ 4.0 4.1 NOYES RW, DICKMANN Z, CLEWE TH & BONNEY WA. (1965). PRONUCLEAR OVUM FROM A PATIENT USING AN INTRAUTERINE CONTRACEPTIVE DEVICE. Science , 147, 744-5. PMID: 14242021
- ↑ Szabo SP & O'Day DH. (1983). The fusion of sexual nuclei. Biol Rev Camb Philos Soc , 58, 323-42. PMID: 6354284
- ↑ Trounson A, Rogers PA, Lutjen PJ, Sathananthan H, Hoppen HO, Yates C, de Kretser D, Leeton J, Healy D & Wood C. (1985). [Human in vitro fertilization and embryo transfer]. Nippon Sanka Fujinka Gakkai Zasshi , 37, 1231-40. PMID: 3897409
- ↑ HAMILTON WJ. (1946). A previllous human embryo. J. Anat. , 80, 215. PMID: 20286234
- ↑ HAMILTON WJ. (1949). Early stages of human development. Ann R Coll Surg Engl , 4, 281-94. PMID: 18121228
- ↑ KHVATOV BP. (1959). [New data on fertilization in man]. Arkh Anat Gistol Embriol , 36, 42-3. PMID: 13638115
- ↑ Edwards RG, Bavister BD & Steptoe PC. (1969). Early stages of fertilization in vitro of human oocytes matured in vitro. Nature , 221, 632-5. PMID: 4886881
- ↑ Soupart P & Morgenstern LL. (1973). Human sperm capacitation and in vitro fertilization. Fertil. Steril. , 24, 462-78. PMID: 4575635
- ↑ Soupart P & Strong PA. (1974). Ultrastructural observations on human oocytes fertilized in vitro. Fertil. Steril. , 25, 11-44. PMID: 4359040
- ↑ Lopata A, McMaster R, McBain JC & Johnston WI. (1978). In-vitro fertilization of preovulatory human eggs. J. Reprod. Fertil. , 52, 339-42. PMID: 564963
- ↑ Lopata A, Sathananthan AH, McBain JC, Johnston WI & Speirs AL. (1980). The ultrastructure of the preovulatory human egg fertilized in vitro. Fertil. Steril. , 33, 12-20. PMID: 7188691
- ↑ Lopata A, Sathananthan AH, McBain JC, Johnston WI & Speirs AL. (1980). The ultrastructure of the preovulatory human egg fertilized in vitro. Fertil. Steril. , 33, 12-20. PMID: 7188691
- ↑ Pereda J & Coppo M. (1984). Ultrastructure of the cumulus cell mass surrounding a human egg in the pronuclear stage. Anat. Embryol. , 170, 107-12. PMID: 6541005
- ↑ Sathananthan AH, Trounson A & Freeman L. (1987). Morphology and fertilizability of frozen human oocytes. Gamete Res , 16, 343-54. PMID: 3506921 DOI.
Search Pubmed
- 1987 Stages: Introduction | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | References | Appendix 1 | Appendix 2 | Historic Papers | Embryonic Development
| Historic Disclaimer - information about historic embryology pages |
|---|
| Pages where the terms "Historic" (textbooks, papers, people, recommendations) appear on this site, and sections within pages where this disclaimer appears, indicate that the content and scientific understanding are specific to the time of publication. This means that while some scientific descriptions are still accurate, the terminology and interpretation of the developmental mechanisms reflect the understanding at the time of original publication and those of the preceding periods, these terms, interpretations and recommendations may not reflect our current scientific understanding. (More? Embryology History | Historic Embryology Papers) |
Cite this page: Hill, M.A. (2024, April 20) Embryology 1987 Developmental Stages In Human Embryos - Stage 1. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/1987_Developmental_Stages_In_Human_Embryos_-_Stage_1
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G