Introduction
Blood develops initially within the core of "blood islands" in the mesoderm. During development, there follows a series of "relocations" of the stem cells to different organs within the embryo. In the adult, these stem cells are located in the bone marrow. At the time when blood first forms, there are no bones!
Note that blood vessel development is tightly coupled to development of other systems for example: osteogenesis (bone formation) that is dependent upon early capillary formation; endocrine development that requires blood vessels for hormone distribution.
| Vasculogenesis
|
Angiogenesis
|
formation of new blood vessels
(endothelium from mesoderm)
|
formation of blood vessels from pre-existing vessels
(occurs in development and adult)
|
Angioblasts initially form small cell clusters (blood islands) within the embryonic and extraembryonic mesoderm. These blood islands extend and fuse together making a primordial vascular network. Within these islands the peripheral cells form endothelial cells while the core cells form blood cells (haemocytoblasts).
Recent work has shown that the formation of the initial endothelial tube is by a process of coalescence of cellular vacuoles within the developing endothelial cells, which fuse together without cytoplasmic mixing to form the blood vessel lumen.
See also the related pages: artery, vein, placenta vascular bed, coronary circulation.
- Developmental Signals - Vascular Endothelial Growth Factor | Smooth Muscle Development
Some Recent Findings
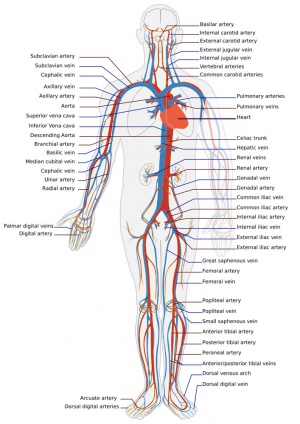
Adult human cardiovascular system
- Reconstructing the Vascular Developmental Milieu In Vitro[1] "Understanding human development has fascinated scientists for centuries. With advancements in stem cell technologies, this understanding has expanded beyond fascination to application towards informing the design of therapeutics in regenerative medicine. A focus on establishing a better grasp of the physicochemical cues governing differentiation and tissue assembly has continually enhanced engineered systems to an unprecedented level of biomimicry and, in doing so, has allowed the design of novel therapeutics. The vasculature has a critical role during early stages of development and regeneration events, and is responsive to a range of dynamic environmental cues. In this review, we present biomaterials systems capable of spatially and temporally controlling environmental signals that guide vascular fate and assembly, thereby further informing our understanding of differentiation schema."
- Review - Molecular identity of arteries, veins, and lymphatics[2] "Arteries, veins, and lymphatic vessels are distinguished by structural differences that correspond to their different functions. Each of these vessels is also defined by specific molecular markers that persist throughout adult life; these markers are some of the molecular determinants that control the differentiation of embryonic undifferentiated cells into arteries, veins, or lymphatics. The Eph-B4 receptor and its ligand, ephrin-B2, are critical molecular determinants of vessel identity, arising on endothelial cells early in embryonic development. Eph-B4 and ephrin-B2 continue to be expressed on adult vessels and mark vessel identity. However, after vascular surgery, vessel identity can change and is marked by altered Eph-B4 and ephrin-B2 expression. Vein grafts show loss of venous identity, with less Eph-B4 expression. Arteriovenous fistulas show gain of dual arterial-venous identity, with both Eph-B4 and ephrin-B2 expression, and manipulation of Eph-B4 improves arteriovenous fistula patency. Patches used to close arteries and veins exhibit context-dependent gain of identity, that is, patches in the arterial environment gain arterial identity, whereas patches in the venous environment gain venous identity; these results show the importance of the host infiltrating cells in determining vascular identity after vascular surgery."
- Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling[3] "Erythro-myeloid progenitors (EMPs) were recently described to arise from the yolk sac endothelium, just prior to vascular remodeling, and are the source of adult/post-natal tissue resident macrophages. Questions remain, however, concerning whether EMPs differentiate directly from the endothelium or merely pass through. We provide the first evidence in vivo that EMPs can emerge directly from endothelial cells (ECs) and demonstrate a role for these cells in vascular development. We find that EMPs express most EC markers but late EMPs and EMP-derived cells do not take up acetylated low-density lipoprotein (AcLDL), as ECs do. When the endothelium is labelled with AcLDL before EMPs differentiate, EMPs and EMP-derived cells arise that are AcLDL+. If AcLDL is injected after the onset of EMP differentiation, however, the majority of EMP-derived cells are not double labelled. We find that cell division precedes entry of EMPs into circulation, and that blood flow facilitates the transition of EMPs from the endothelium into circulation in a nitric oxide-dependent manner. In gain-of-function studies, we inject the CSF1-Fc ligand in embryos and found that this increases the number of CSF1R+ cells, which localize to the venous plexus and significantly disrupt venous remodeling. This is the first study to definitively establish that EMPs arise from the endothelium in vivo and show a role for early myeloid cells in vascular development."
- Quantitative comparison of cerebral artery development in human embryos with other eutherians[4] "Quantitative analysis of the internal radius of the aorta and cerebral arteries in a range of eutherian mammals has been used to compare arterial flow to the developing human brain with that to the brains of non-human eutherians....The findings suggest that the developing human brain may actually receive less blood flow at embryonic sizes (less than 22 mm body length) than do other mammalian embryos of a similar body size, but that internal carotid and vertebral flow is higher in human fetuses (body length greater than 30 mm) than in developing non-humans of the same body size. Increased flow to the developing human brain relative to non-humans is achieved by simultaneous increases in both aortic and cerebral feeder artery internal calibre."
- The relationship between human placental morphometry and ultrasonic measurements of utero-placental blood flow and fetal growth[5] "Placental area and weight are associated with uterine and umbilical blood flow, respectively, and both are associated with fetal growth rate."
|
| Older papers
|
| These papers originally appeared in the Some Recent Findings table, but as that list grew in length have now been shuffled down to this collapsible table.
See also the Discussion Page for other references listed by year and References on this current page.
- Cell-matrix signals specify bone endothelial cells during developmental osteogenesis[6] “Blood vessels in the mammalian skeletal system control bone formation and support haematopoiesis by generating local niche environments. Here, we report that embryonic and early postnatal long bone contains a specialized endothelial cell subtype, termed type E, which strongly supports osteoblast lineage cells and later gives rise to other endothelial cell subpopulations. The differentiation and functional properties of bone endothelial cells require cell-matrix signalling interactions." Bone Development | INTEGRIN BETA-1
- Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field[7] "Oxygenated blood from the heart is directed into the systemic circulation through the aortic arch arteries (AAAs). The AAAs arise by remodeling of three symmetrical pairs of pharyngeal arch arteries (PAAs), which connect the heart with the paired dorsal aortae at mid-gestation. Aberrant PAA formation results in defects frequently observed in patients with lethal congenital heart disease. How the PAAs form in mammals is not understood. The work presented in this manuscript shows that the second heart field (SHF) is the major source of progenitors giving rise to the endothelium of the pharyngeal arches 3 - 6, while the endothelium in the pharyngeal arches 1 and 2 is derived from a different source. During the formation of the PAAs 3 - 6, endothelial progenitors in the SHF extend cellular processes toward the pharyngeal endoderm, migrate from the SHF and assemble into a uniform vascular plexus. This plexus then undergoes remodeling, whereby plexus endothelial cells coalesce into a large PAA in each pharyngeal arch."
- Review - The Molecular Regulation of Arteriovenous Specification and Maintenance[8] "The formation of a hierarchical vascular network, composed of arteries, veins and capillaries, is essential for embryogenesis and is required for the production of new functional vasculature in the adult. Elucidating the molecular mechanisms that orchestrate the differentiation of vascular endothelial cells into arterial and venous cell fates is requisite for regenerative medicine, as the directed formation of perfused vessels is desirable in a myriad of pathological settings, such as in diabetes and following myocardial infarction. Additionally, this knowledge will enhance our understanding and treatment of vascular anomalies, such as arteriovenous malformations (AVMs). From studies in vertebrate model organisms, such as mouse, zebrafish and chick, a number of key signaling pathways have been elucidated that are required for the establishment and maintenance of arterial and venous fates. These include the Hedgehog, Vascular Endothelial Growth Factor (VEGF), Transforming Growth Factor-β (TGF-β), Wnt and Notch signaling pathways. In addition, a variety of transcription factor families acting downstream of-or in concert with-these signaling networks play vital roles in arteriovenous (AV) specification. These include Notch and Notch-regulated transcription factors (e.g. HEY and HES), SOX factors, Forkhead factors, β-Catenin, ETS factors and COUP-TFII. It is becoming apparent that AV specification is a highly coordinated process that involves the intersection and carefully orchestrated activity of multiple signaling cascades and transcriptional networks."
- Specification of arterial, venous, and lymphatic endothelial cells during embryonic development [9] "The groundbreaking discovery about arterial and venous expression of ephrinB2 and EphB4, respectively, in early embryonic development has led to a new paradigm for vascular research, providing compelling evidence that arterial and venous endothelial cells are established by genetic mechanisms before circulation begins. For arterial specification, vascular endothelial growth factor (VEGF) induces expression of Notch signaling genes, including Notch1 and its ligand, Delta-like 4 (Dll4), and Foxc1 and Foxc2 transcription factors directly regulate Dll4 expression. Upon activation of Notch signaling, the Notch downstream genes, Hey1/2 in mice or gridlock in zebrafish, further promote arterial differentiation. On the other hand, the orphan nuclear receptor COUP-TFII is a determinant factor for venous specification by inhibiting expression of arterial specific genes, including Nrp1 and Notch. After arterial and venous endothelial cells differentiate, a subpopulation of venous endothelial cells is thought to become competent to acquire lymphatic endothelial cell fate by progressively expressing the transcription factors Sox18 and Prox1 to differentiate into lymphatic endothelial cells."
- Developmental origin of smooth muscle cells in the descending aorta in mice[10] "Aortic smooth muscle cells (SMCs) have been proposed to derive from lateral plate mesoderm. ....(these results) suggested that all SMCs in the adult descending aorta derive from the somites, whereas no contribution was recorded from lateral plate mesoderm."
- Notch ligand Jagged1 is required for vascular smooth muscle development[11] "The Notch ligand Jagged1 (Jag1) is essential for vascular remodeling. ...Jag1 null phenotype. These embryos show striking deficits in vascular smooth muscle, whereas endothelial Notch activation and arterial-venous differentiation appear normal."
|
Endothelial Progenitors
Recent work has shown that the formation of the initial endothelial tube is by a process of coalescence of cellular vacuoles within the developing endothelial cells, which fuse together without cytoplasmic mixing to form the blood vessel lumen.[12]
Endothelial Tube Formation
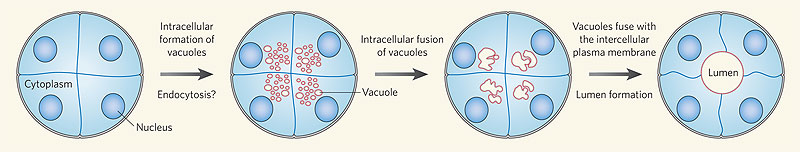
Blood vessel lumen formation
Vessel Specification
The following data is from a recent review.[9]
Arterial Specification
| Factor
|
Function
|
| Shh
|
Loss of Shh results in lack of arterial identity in zebrafish. Shh acts upstream of VEGF.
|
| VEGF
|
VEGF acts downstream of Shh signaling to activate Notch via the PLCγ/ERK pathway in zebrafish. Mutant mice expressing only VEGF188 lack arterial differentiation.
|
| Nrp1
|
Null mice display impaired arterial differentiation. Nrp1 is involved in a positive feedback loop of VEGF signaling.
|
| Notch
|
Notch acts downstream of Shh and VEGF signaling in zebrafish. Notch1; Notch4 mutant mice have abnormal vascular development.
|
| Dll4
|
Null mice lack arterial specification.
|
| Dll1
|
Null mice fail to maintain arterial identity.
|
| Hey1/2 (Grl)
|
Null mice lack arterial specification. Lack of grl in zebrafish results in loss of arterial specification.
|
| Foxc1/c2
|
Foxc1; Foxc2 mutant mice lack arterial specification. Foxc1 and Foxc2 directly regulate Dll4 and Hey2 expression. Foxc1 and Foxc2 are also involved in lymphatic vessel development.
|
| Sox7/18
|
Lack of Sox7/18 results in loss of arterial identity in zebrafish.
|
| Snrk-1
|
Snrk-1 acts downstream or parallel to Notch signaling in zebrafish.
|
| Dep1
|
Dep1 acts upstream of PI3K in arterial specification in zebrafish.
|
| Crlr
|
Shh regulates VEGF activity by controlling crlr expression in zebrafish.
|
| EphrinB2
|
Null mice lack boundaries between arteries and veins. EphrinB2 is involved in lymphatic vascular remodeling and maturation.
|
Venous Specification
| Factor
|
Function
|
| COUP-TFII
|
COUP-TFII suppresses arterial cell fate by inhibiting Nrp1 and Notch. COUP-TFII also interacts with Prox1 to regulate lymphatic gene expression.
|
| EphB4
|
Null mice lack boundaries between arteries and veins.
|
Lymphatic Specification
| Factor
|
Function
|
| Sox18
|
Null mice fail to specify lymphatic endothelial cells. Sox18 induces Prox1 expression.
|
| Prox1
|
Prox1 induces lymphatic markers and maintains lymphatic cell identity.
|
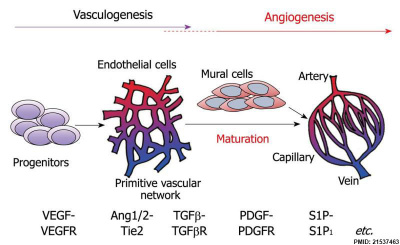
Vascular Endothelial Growth Factor
Growing blood vessels follow a gradient generated by tagret tissues/regions of Vascular Endothelial Growth Factor (VEGF) to establish a vascular bed. Recent findings suggest that Notch signaling acts as an inhibitor for this system, preventing sprouting of blood vessels.
Notch is a transmembrane receptor protein involved in regulating cell differentiation in many developing systems.
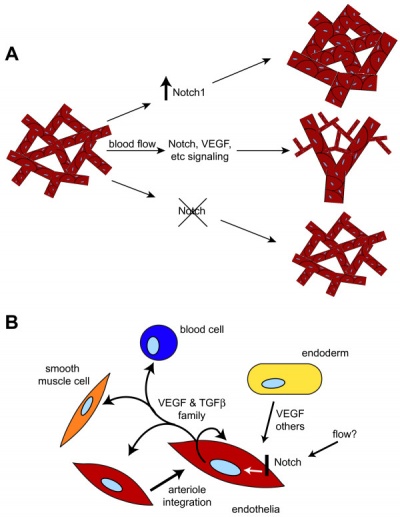
|
|
| Notch and yolk sac blood vessels model[13]
|
Vasculogenesis and angiogenesis[14]
|
Links: OMIM - VEGFA | OMIM - Notch
Regulators of Growth
The following data is from a review article on ovary vascular development.[15]
Stimulators of Angiogenisis
- Peptide growth factors
- Vascular endothelial growth factor-Aa,b, -Bb, -Cb, -D
- [-E], -F (VEGF-Aa,b, -Bb, -Cb, -Db, -E, -F)
- Placenta growth factor (PlGF)
- Angiopoietin-1 (Ang-1)
- Angiopoietin-2 (Ang-2) [modulator in the presence of angiogenic activity]
- Acidic fibroblast growth factor (FGF-1)
- Basic fibroblast growth factor (FGF-2)
- Platelet-derived growth factor (PDGF)
- Transforming growth factor-a (TGF-a)
- Transforming growth factor-b (TGF-b)
- Hepatocyte growth factor (HGF)
- Insulin-like growth factor-I (IGF-I)
- Multifunctional cytokines/immune mediators
- Tumour necrosis factor-a (low-dose)
- Monocyte chemoattractant protein-1 (MCP-1)
- CXC-chemokines
- Enzymes
- Platelet-derived endothelial cell growth factor
- (PD-ECGF; thymidine phosphorylase)
- Angiogenin (ribonuclease A homologue)
- Hormones
- Oestrogens
- Prostaglandin-E1, -E2
- Follistatin
- Proliferin
- Oligosaccharides
- Hyaluronan oligosaccharides
- Gangliosides
|
Inhibitors of Angiogenisis
- Peptide growth factors and proteolytic peptides
- Angiopoietin-2 (Ang-2) [in the absence of angiogenic activity]
- Angiostatin
- Endostatin
- 16 kDa prolactin fragment
- Laminin peptides
- Fibronectin peptides
- Inhibitors of enzymatic activity
- Tissue metalloproteinase inhibitors
- (TIMP-1, -2, -3, -4)
- Plasminogen activator inhibitors
- (PAI-1, -2)
- Multifunctional cytokines/immune mediators
- Tumour necrosis factor-a (high-dose)
- Interferons
- Interleukin-12
- CXC-chemokines
- Platelet factor-4 (PF-4)
- Interferon-gamma-inducible protein-10 (IP-10)
- Gro-beta
- Extracellular matrix molecules
- Hormones/metabolites
- 2-Methoxyestradiol (2-ME)
- Proliferin-related protein
- Oligosaccharides
- Hyaluronan, high-molecular-weight species
|
Histology
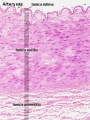
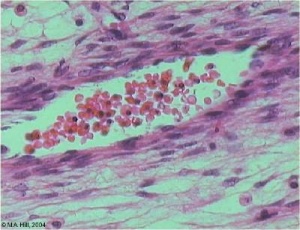
Vein Light Microscopy
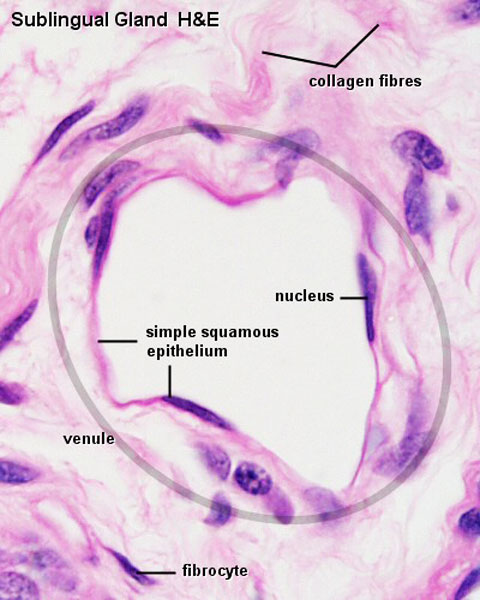
The entire developing and adult cardiovascular system (blood vessels and heart) is lined by a simple squamous epithelium. (Stain - Haematoxylin Eosin)
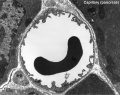
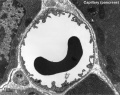
Capillaries
Type H
A developmental capillary endothelial cell subtype associated with osteogenesis, located at the metaphysis and endosteum of postnatal long bone, that couples angiogenesis with osteogenesis. This endothelial cell subtype expresses the markers CD31/PECAM1 and endomucin (CD31hi Emcnhi).
Type E
A newly identified endothelial cell subtype similar to type H in function, supporting osteoblast lineage cells and then gives rise to other endothelial cell subpopulations, but this subtype is found in embryonic and early postnatal long bone.[6]
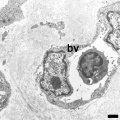
Electron Micrographs
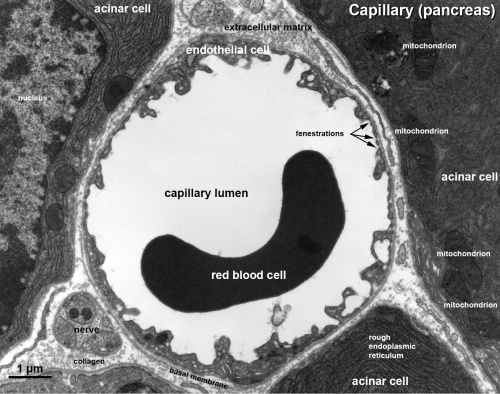
Capillary 1 large labeled
Capillary 1 large unlabelled
Capillary 1 small labeled
Capillary 1 small unlabelled
Containing white blood cell
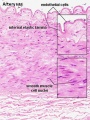
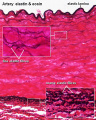
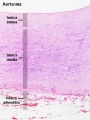
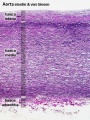
Arteries
Artery tunica media elastin
Cardiac Blood Vessels
Earliest vessels in the heart wall develop subepicardially (beneath the outside surface of the heart) near the apex at Carnegie stage 15, which then extends centripetally and at stage 17 coronary arterial stems communicate with the aortic lumen.[17]
Abnormalities
Due to the extensive embryonic, and ongoing, remodelling of the vascular system, there are many different vascular variations and anomalies.
Neural
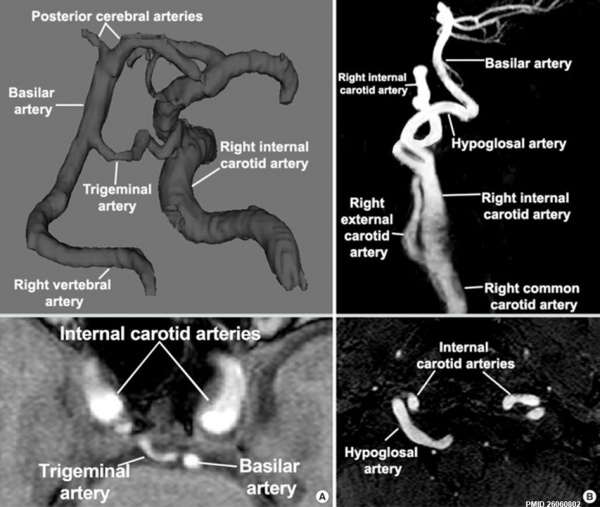
Persistent trigeminal and hypoglossal arteries[18]
- Links: Cerebrum Development | Head Development
References
- ↑ Blatchley MR & Gerecht S. (2019). Reconstructing the Vascular Developmental Milieu In Vitro. Trends Cell Biol. , , . PMID: 31718894 DOI.
- ↑ Wolf K, Hu H, Isaji T & Dardik A. (2019). Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. , 69, 253-262. PMID: 30154011 DOI.
- ↑ Kasaai B, Caolo V, Peacock HM, Lehoux S, Gomez-Perdiguero E, Luttun A & Jones EA. (2017). Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Sci Rep , 7, 43817. PMID: 28272478 DOI.
- ↑ Ashwell KW & Shulruf B. (2015). Quantitative comparison of cerebral artery development in human embryos with other eutherians. J. Anat. , 227, 286-96. PMID: 26183939 DOI.
- ↑ Salavati N, Sovio U, Mayo RP, Charnock-Jones DS & Smith GC. (2016). The relationship between human placental morphometry and ultrasonic measurements of utero-placental blood flow and fetal growth. Placenta , 38, 41-8. PMID: 26907381 DOI.
- ↑ 6.0 6.1 Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B, Sorokin L, Vaquerizas JM & Adams RH. (2017). Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. , 19, 189-201. PMID: 28218908 DOI.
- ↑ Wang X, Chen D, Chen K, Jubran A, Ramirez A & Astrof S. (2017). Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Dev. Biol. , 421, 108-117. PMID: 27955943 DOI.
- ↑ Fish JE & Wythe JD. (2015). The molecular regulation of arteriovenous specification and maintenance. Dev. Dyn. , 244, 391-409. PMID: 25641373 DOI.
- ↑ 9.0 9.1 Kume T. (2010). Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol. Histopathol. , 25, 637-46. PMID: 20238301 DOI.
- ↑ Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyürek LM, Partanen J & Lindahl P. (2008). Developmental origin of smooth muscle cells in the descending aorta in mice. Development , 135, 1823-32. PMID: 18417617 DOI.
- ↑ High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH & Epstein JA. (2008). Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. U.S.A. , 105, 1955-9. PMID: 18245384 DOI.
- ↑ Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH & Verfaillie CM. (2002). Origin of endothelial progenitors in human postnatal bone marrow. J. Clin. Invest. , 109, 337-46. PMID: 11827993 DOI.
- ↑ Copeland JN, Feng Y, Neradugomma NK, Fields PE & Vivian JL. (2011). Notch signaling regulates remodeling and vessel diameter in the extraembryonic yolk sac. BMC Dev. Biol. , 11, 12. PMID: 21352545 DOI.
- ↑ Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N & Yoshioka K. (2010). Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem , 1, 298-306. PMID: 21537463 DOI.
- ↑ Augustin HG. (2000). Vascular morphogenesis in the ovary. Baillieres Best Pract Res Clin Obstet Gynaecol , 14, 867-82. PMID: 11141338 DOI.
- ↑ Detry B, Bruyère F, Erpicum C, Paupert J, Lamaye F, Maillard C, Lenoir B, Foidart JM, Thiry M & Noël A. (2011). Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. , 12, 29. PMID: 21702933 DOI.
- ↑ Turner K & Navaratnam V. (1996). The positions of coronary arterial ostia. Clin Anat , 9, 376-80. PMID: 8915616 <376::AID-CA3>3.0.CO;2-9 DOI.
- ↑ Menshawi K, Mohr JP & Gutierrez J. (2015). A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J Stroke , 17, 144-58. PMID: 26060802 DOI.
Reviews
Wolf K, Hu H, Isaji T & Dardik A. (2019). Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. , 69, 253-262. PMID: 30154011 DOI.
Fish JE & Wythe JD. (2015). The molecular regulation of arteriovenous specification and maintenance. Dev. Dyn. , 244, 391-409. PMID: 25641373 DOI.
Articles
Davidson AJ & Zon LI. (2000). Turning mesoderm into blood: the formation of hematopoietic stem cells during embryogenesis. Curr. Top. Dev. Biol. , 50, 45-60. PMID: 10948449
McGrath KE, Koniski AD, Malik J & Palis J. (2003). Circulation is established in a stepwise pattern in the mammalian embryo. Blood , 101, 1669-76. PMID: 12406884 DOI.
Search Pubmed
Click on the listed keywords below (used to search the external database) the most current references on Medline will be displayed.
Search Pubmed: Blood Vessel Development | Blood Vessel embryology | Blood Vessel smooth muscle Development | Blood Vessel smooth muscle Development
Terms
| Cardiovascular Terms
|
Cardiovascular System Development See also Heart terms, Immune terms and Blood terms.
- angioblast - the stem cells in blood islands generating endothelial cells which will form the walls of both arteries and veins. (More? Blood Vessel)
- angiogenesis - the formation of new blood vessels from pre-existing vessels following from vasculogenesis in the embryo. (More? Blood Vessel)
- anlage (German, anlage = primordium) structure or cells which will form a future more developed or differentiated adult structure.
- blood islands - earliest sites of blood vessel and blood cell formation, seen mainly on yolk sac chorion.
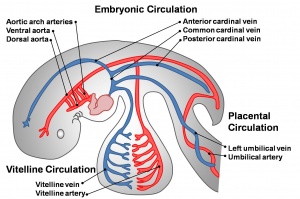
- cardinal veins - paired main systemic veins of early embryo, anterior, common, posterior.
- cardiogenic region - region above prechordal plate in mesoderm where heart tube initially forms.
- ectoderm - the layer (of the 3 germ cell layers) which form the nervous system from the neural tube and neural crest and also generates the epithelia covering the embryo.
- endoderm - the layer (of the 3 germ cell layers) which form the epithelial lining of the gastrointestinal tract (GIT) and accessory organs of GIT in the embryo.
- endocardium - lines the heart. Epithelial tissue lining the inner surface of heart chambers and valves.
- endothelial cells - single layer of cells closest to lumen that line blood vessels.
- extraembryonic mesoderm - mesoderm lying outside the trilaminar embryonic disc covering the yolk sac, lining the chorionic sac and forming the connecting stalk. Contributes to placental villi development.
- haemocytoblasts - stem cells for embryonic blood cell formation.
- anastomose - to connect or join by a connection (anastomosis) between tubular structures.
- chorionic villi - the finger-like extensions which are the functional region of the placental barrier and maternal/fetal exchange. Develop from week 2 onward as: primary, secondary, tertiary villi.
- estrogens - support the maternal endometrium.
- growth factor - usually a protein or peptide that will bind a cell membrane receptor and then activates an intracellular signaling pathway. The function of the pathway will be to alter the cell directly or indirectly by changing gene expression. (eg VEGF, shh)
- intra-aortic hematopoietic cluster - (IAHC) blood stem cells associated with the endothelial layer of aorta and large arteries.
- maternal decidua - region of uterine endometrium where blastocyst implants. undergoes modification following implantation, decidual reaction.
- maternal sinusoids - placental spaces around chorionic villi that are filled with maternal blood. Closest maternal/fetal exchange site.
- Megakaryocytopoiesis - the process of bone marrow progenitor cells developMENT into mature megakaryocytes.
- mesoderm - the middle layer of the 3 germ cell layers of the embryo. Mesoderm outside the embryo and covering the amnion, yolk and chorion sacs is extraembryonic mesoderm.
- myocardium - muscular wall of the heart. Thickest layer formed by spirally arranged cardiac muscle cells.
- pericardium - covers the heart. Formed by 3 layers consisting of a fibrous pericardium and a double layered serous pericardium (parietal layer and visceral epicardium layer).
- pericytes - (Rouget cells) cells located at the abluminal surface of microvessels close to endothelial cells, mainly found associated with CNS vessels and involved in vessel formation, remodeling and stabilization.
- pharyngeal arches (=branchial arches, Gk. gill) series of cranial folds that form most structures of the head and neck. Six arches form but only 4 form any structures. Each arch has a pouch, membrane and groove.
- placenta - (Greek, plakuos = flat cake) refers to the discoid shape of the placenta, embryonic (villous chorion)/maternal organ (decidua basalis)
- placental veins - paired initially then only left at end of embryonic period, carry oxygenated blood to the embryo (sinus venosus).
- protein hormone - usually a protein distributed in the blood that binds to membrane receptors on target cells in different tissues. Do not easliy cross placental barrier.
- sinus venosus - cavity into which all major embryonic paired veins supply (vitelline, placental, cardinal).
- splanchnic mesoderm - portion of lateral plate mesoderm closest to the endoderm when coelom forms.
- steroid hormone - lipid soluble hormone that easily crosses membranes to bind receptors in cytoplasm or nucleus of target cells. Hormone+Receptor then binds DNA activating or suppressing gene transcription. Easliy cross placental barrier.
- syncitiotrophoblast extraembryonic cells of trophoblastic shell surrounding embryo, outside the cytotrophoblast layer, involved with implantation of the blastocyst by eroding extracellular matrix surrounding maternal endometrial cells at site of implantation, also contribute to villi. (dark staining, multinucleated).
- truncus arteriosus - an embryological heart outflow structure, that forms in early cardiac development and will later divides into the pulmonary artery and aorta. Term is also used clinically to describe the malformation where only one artery arises from the heart and forms the aorta and pulmonary artery.
- vascular endothelial growth factor - (VEGF) A secreted protein growth factor family, which stimulates the proliferation of vasular endotheial cells and therefore blood vessel growth. VEGF's have several roles in embryonic development. The VEGF family has 7 members (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF) that have a common VEGF homology domain. PIGF is the placental growth factor. They act through 3 VEGF tyrosine kinase membrane receptors (VEGFR-1 to 3) with seven immunoglobulin-like domains in the extracellular domain, a single transmembrane region, and an intracellular tyrosine kinase sequence.
- vasculogenesis - the formation of new blood vessels from mesoderm forming the endothelium. Compared to angiogenesis that is the process of blood vessel formation from pre-existing vessels.
- vitelline blood vessels - blood vessels associated with the yolk sac.
- waste products - products of cellular metabolism and cellular debris, e.g.- urea, uric acid, bilirubin.
|
External Links
External Links Notice - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, M.A. (2024, April 23) Embryology Cardiovascular System - Blood Vessel Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cardiovascular_System_-_Blood_Vessel_Development
- What Links Here?
- © Dr Mark Hill 2024, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Code No. 00098G









![endothelium detail[16]](/embryology/images/thumb/6/6a/Blood_capillary_EM_01.jpg/120px-Blood_capillary_EM_01.jpg)