2011 Group Project 11
| Note - This page is an undergraduate science embryology student group project 2011. |
Cleft Palate and Lip
Introduction
Cleft lip and cleft palate, whilst similar, have slightly differing pathogenesis. Cleft lip is more prevalent in males while cleft palate is more prevalent in females, the ratio between females and males being 60:40. From research, neither age nor parity appear to be directly related is both abnormalities. There is a higher occurance in Japanese people and lower occurance in Negro populations. The frequency of this birth defect is about 1 in 2,000 newborns in white populations.
History
In ancient times many congenital deformities, including the cleft lip and palate, were considered to be evidence of the existence of an evil spirit in the affected child. The reaction of the birth of a deformed child has varied widely from culture to culture where the infant was often removed from the tribe or cultural unit and left to die in the surrounding wilderness, a practise that was common in Antiquity and still happens today in certain parts of African tribes. In Sparta the unfortunate newborns were abandoned on Mount Tagete, while in Rome they were drowned in the Tarpeian rock. [1]
The renowned philosopher Plato discussed it in one of his dialogues in the Republic, explaining that it was indeed a means of eradicating evil omens and preserving the soundness of the race.[2]
This state of lack of knowledge is evident up until 1889 when Keating published his opinion that a series of congenital anomalies were provoked in each case, by the mother looking at a person with similar deformity during her pregnancy.[3] The first persuasive explanation regarding the actual causation of the condition offered by Philippe Frederick Blandin between 1838 to1896 has revolutionised the perception of the condition. It sparked interest among physicians to investigate further into embryological development and the possible origin of clefting.[4]
Since 1896 to the 19th century both understanding and the surgery of cleft lip witnessed remarkable improvements. Surgeons around the world continued to research and propose refinements on the early procedure striving to accomplish precise and reproducible methods.
Timeline
- In 1295- 1351, Jean Yperman noted that cleft had a congenital origin classifying the various forms of the condition and outlining corresponding treatment principles. [1]
- In 1460, Heinrich von Pfolsprundt passed stitches through all the layers to repair the cleft instead of simply suturing the skin accomplishing a better repair of the lip. [1]
- In 1537-1619, Fabricius ab Aquapendente first described the embryological basis of cleft lip. [1]
- In 1561, Pierre Franco and Ambroise Pare described the techniques of correction of both unilateral and bilateral cleft lips in Traite des Hernies using dry sutures, pins and a triangular bandage. He emphasized that an accurate surgery procedure can produce an inconspicuous scar, an outcome which was “particularly desirable when the patient was a girl”.[5]
- In 1795, Pierre Joseph DeSault, a French pioneer of bilateral cleft surgery at La Charité and Hôtel-Dieu in Paris developed a new method for teaching anatomy and taught the procedure of bilateral cleft surgery[6]
- In 1808, Meckel pubished his theory of the embryological development of the lips which stated that the lips formed from five distinct processes which eventually united, three for the upper lip and two for the lower lip. [1]
- In 1838, Philippe Frederick Blandin suggested that facial cleft resulted from a failure of the premaxilla and the maxillary segments to unite at a later stage in development. [7]
- In 1844, Germanicus Mirault introduced a triangular flap from the lateral side into a gap created by making a horizontal incision on the medial side of the lip creating a nostril floor and reducing the linear scar on the lip. [1]
- In 1872, Jacob August Estlander, a Finnish surgeon introduced a method to correct the mid-face retrusion that was left by the bilateral lip repair process. He recommended a wedge resection of the vomer which allowed the protruding premaxilla to be pushed back. [1]
- In 1935, Faltin, another Finnish surgeon recommended that the procedure described by Jacob A Estander be abandoned because it routinely left serious maxillary retrusion. [8]
- In 1960, Peter Randall standardized the triangular flap repair method with accurate and reproducible measurements. [9]
- In 1965, W. M. Manchester introduced a procedure for the bilateral cleft surgery. [10]
- In 2000, Hua Xi Kou Qiang demonstrated that simultaneous primary palate repair and alveolar bone grafting are safe for unoperated cleft palate patients, and this procedure should be performed in unoperated cleft palate patients above 8 years old. [11]
- In 2008, J Y Wong published a study describing that craniofacial anthropometry using the 3dMDface System is applicable and reliable. Application of software algorithms merging the different overlapping images into a single three-dimensional image can remarkably improve landmark identification. [12]
- In 2010, B Mishra along with a team of Indian doctors published a study concluding that Nasoalveolar molding can be a useful adjunct for management of cleft lip nasal deformity. It serves as a cost-effective technique that can diminish the number of future surgeries such as alveolar bone grafting and secondary rhinoplasties in under developed countries. [13]
Diagnosis
Cleft lip and palate occurrence varies among continents, races and populations from one in 700 to one in 1500 newborns. [14][15] Hence it is of clinical as well as social significance that effective diagnostic measures are available early enough into the pregnancy so as to offer families ample time to understand the consequences of having a baby with such deformity.
While the birth of a child with CLP can have a severe emotional impact on the parents, antenatal diagnosis with ultrasonography helps to reduce the impact and prepare the parents psychologically. Most parents that received prenatal counselling following the diagnosis of CLP antenatally (85%) felt that the information had prepared them psychologically for the birth of their child, and 92% indicated that they had never considered the possibility of voluntary termination of pregnancy. Prenatal diagnosis of CLP has the additional advantage of allowing plastic surgeons to achieve early lip repair.[16] Prenatal ultrasound detection of the fetal face remains a matter of clinical research due to several technical difficulties associated with the examination of the fetus.[17]
The common obstacles faced during CLP examination are as follows:
- shadowing effects of the superior alveolar ridge, and the fetal prone position[18]
- failure to identify the soft palate in three-dimensional (3D) ultrasound techniques as the soft palate is not in the same horizontal plane as the hard palate [19]
Cleft Soft Palate Detection
These technical challenges imply that the opportunity of prenatal screening for isolated defects of the soft palate is remote at present. Considering the additional time and effort involved in achieving satisfactory insonation and visualization, these methods are usually restricted to certain cases in which a cleft lip or hard palate is suspected. Isolated defects in the soft palate are relatively easier to repair surgically, and their emotional trauma on the parents if they are not diagnosed antenatally is far less severe than in newborns with cleft lip or cleft hard palate.[16]
Cleft Hard Palate Detection
A combination of three different methods of 3D ultrasounds has been tested to measure detection accuracy of hard palate anomalies (See Table 1 and 2). The three methods are:
- reverse face
- flipped face
- oblique face
To date, none of the three methods (reverse face, flipped face or oblique face) have been found to be notably superior to the others. Therefore a combination of all three tests should be carried out for any fetus provided that there are adequate volume acquisition and reasonable time to examine the images offline. However, in cases where an immediate diagnosis is required, the fastest method is the oblique-face approach. On the other hand when initial volume acquisition is inadequate and image quality is less than optimum, the reverse-face view or the oblique-face view in coronal planes is the most effective technique to use. [16]
Accurate ultrasound imaging of the secondary palate also requires the presence of fluid between the fetal tongue and palate, as well as curving of the plane so that the acquired volume follows the concave structure of the palate with either the oblique-face or flipped-face view. In some cases these views can reveal useful details about the soft palate which can be potentially be used in prenatal diagnosis of defects in future cases.
| Feature | Reverse-face view (% (n)) | Flipped-face view (% (n)) | Oblique-face view (% (n)) |
|---|---|---|---|
| Lip | 100 (10/10) | 100 (10/10) | 100 (10/10) |
| Alveolar ridge | 100 (9/9) | 100 (9/9) | 100 (9/9) |
| Hard palate | 71.4 (5/7) | 85.7 (6/7) | 100 (7/7) |
| Soft palate | 0 (0/7) | 14 (1/7) | 14 (1/7) |
Table 1. Percentage of fetuses with cleft lip and palate (n = 10) in which abnormal findings were well visualized using each technique [16]
| Feature | Reverse-face view (% (n)) | Flipped-face view (% (n)) | Oblique-face view (% (n)) |
|---|---|---|---|
| Lip | 100 (50/50) | 100 (50/50) | 100 (50/50) |
| Alveolar ridge | 100 (50/50)) | 100 (50/50) | 100 (50/50) |
| Hard palate | 78 (39/50) | 84 (42/50) | 86 (43/50) |
| Soft palate | 0 (0/50) | 16 (8/50) | 26 (13/50) |
Table 2. Percentage of fetuses with cleft lip and palate (n = 50) in which abnormal findings were well visualized using each technique [16]
Syndromes and Anomalies associated with cleft
CLP is implicated to be related to more than 100 syndromes, and trisomy 13 is the most commonly associated chromosomal anomaly. Associated malformations occur most commonly in the facial region (21%), followed by the ocular system, central nervous system, skeletal system, cardiovascular system, neck, auricular system, gastrointestinal system and urogenital system[20]
Median facial dysplasia-A facial anomaly associated with cleft
Median facial dysplasia is a definable group of patients typically featuring midline facial deficiencies often accompanied with unilateral or bilateral cleft lip with or without cleft palate. [21] This type of facial underdevelopment usually extends into the corpus callosum of the brain. If there is <90% abnormality in the circumference of the brain, these patients may have associated anomalies of the brain, specially in the frontal corpus callosum. This will then have compromised development of the midface which leads to very early dish face, Class III occlusion and severe maxillary hypoplasia. Early detection of these anomalies help to plan the course of treatment.
Van der Woude syndrome with lower lip pits-Most common syndrome associated with cleft
This syndrome has been identified as one of the most commonly occurring syndromes associated with oral cleft. It is known to be transmitted as an autosomal dominant allele within the gene. The lower lip pits as evident in the picture on the right is the characteristic of the condition. The locations of these pits are found to be situated bilaterally in the lower lip where the dry and wet vermilion. They can be either oval or transverse in form. [22]
The following conditions often coexist in patients with the syndrome:
- hypodontia-a condition where the patient has missing teeth
- missing maxillary
- mandibular second premolar teeth
- absent maxillary lateral incisor
- ankyloglossia- also known as tongue tie a congenital oral anomaly which results in limited mobility of the tongue tip
Other rare extra-oral symptoms that may be present include:
- accessory nipples
- congenital heart defects
- Hirschsprung disease(HSCR)-is a congenital disease which causes blockage of the large intestine due to improper muscle movement in the bowel[23]
- popliteal web
Velocardiofacial syndrome-An anomaly associated with cleft resulting from Chromosome 22q abnormality
Velocardiofacial syndrome (VCFS) is an autosomal dominant condition which results from a deletion on the long arm of Chromosome 22 in the“q11”region (deletion22q11). It is the most common sub-microscopic deletion syndrome and is known to occur in 1 out of 2000 live births. [24] In excess of a hundred phenotypic features can result from this defect as it affects every major part of the body.
VCFS is most frequently associated to:
- cleft palate
- cardiac anomaly
- characteristic facial appearance (vertical maxillary excess, malar flattening, relative mandibular retrusion, narrow palpebral fissure and small ears)
- minor learning problems
- speech and feeding problems
Close connections have been reported between VCFS and DiGeorge syndrome. The DiGeorge syndrome features small or absent thymus, tonsils, adenoids and hypocalcaemia. These children may have medial displacement of the carotid artery over the cervical vertebrae and this should be considered while planning any type of pharyngeal surgery like pharyngeal flap for Velo pharyngeal incompetence (VPI) correction. The majority of these patients will require continual support for their learning difficulties. [25]
Development
--Mark Hill 12:18, 8 September 2011 (EST) There is no text here.
Aetiology
--Mark Hill 12:18, 8 September 2011 (EST) There is no text here.
Cleft lip and cleft palate often occur together, however, they have different aetiologies. Many know and unknown factors contribute to these birth defects including possible interaction of indirect genetic factors with environmental factors or possibly just environmental factors. Some mutations are linked to this developmental abnormality as well. Cleft palate is caused by a one week delay in palatal shelve elevation, which often occurs in females at week 8 compared to week 7 in males. Errors in development include:
- inadequate growth of the palatine shelves
- failure of the shelves to elevate at the correct time
- an excessive wide head
- failure of the shelves to fuse
- secondary rupture after fusion
Cleft lip is the underdevelopment of the mesenchyme of the maxillary prominence and medial nasal process. Pathogenic factors include:
- inadequate migration or proliferation of neural crest cell ectomesenchyme
- excessive cell death during the developmental formation of the maxillary prominence and nasal placode.
Some common drugs which can cause the defect include:
- phenytoin
- dilantin
- vitamin A
- some vitamin A analogs
Developmental Staging
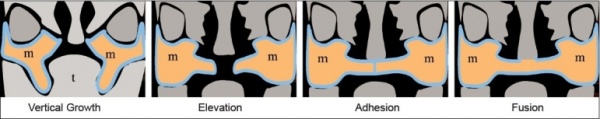
Development of the lip occurs during face development between weeks four and five. During this time the components that contribute to the face morphology come together and are fused to form a complete upper lip. These components include the maxillary processes and the medial and lateral nasal processes [26] At stage 15 the medial and nasal processes have started to fuse and the maxillary process lie inferior to them. During stage 16 the maxillary process and the medial nasal processes come in contact with each other and begin to fuse. Stage 18 is the later stage of lip formation. The maxillary process continue to grow and in doing so the force the medial nasal processes medialfrontally. It is between Stages 16 and 18 that a cleft lip is formed, after failure of the processes to fuse completely [27]
Development of the palate occurs between the seventh and tenth weeks. There are two main stages in palate development, primary and secondary. During the seventh week of development the intermaxillary processes are formed. These processes give rise to the primary palate. This primary palate contributes to the floor of the nasal cavity. Towards the end of the seventh week the palatine shelves, which were lying parallel to the tongue, start to move into a more horizontal position above the tongue. These palatine shelves begin to fuse with each other and with the primary palate. Fusion is complete by week ten and the secondary palate is formed. It is between weeks seven and ten of development that a cleft palate is formed as a cleft palate is the result of the palatine shelves failing to fuse properly. [28]
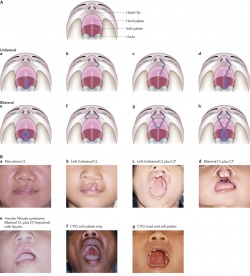
Types of Cleft Palate/Lip
There are different types of both cleft palates and cleft lips, each with varying degrees of severity. Cleft palates and lips may either occur together or individually. Variations of cleft palate/lips include:
- Unilateral
- Unilateral cleft lip
- Unilateral cleft palate
- Unilateral cleft lip with a cleft hard palate
- Unilateral cleft lip with cleft hard and soft palate
- Bilateral
- Bilateral cleft palate
- Bilateral cleft lip
- Bilateral cleft lip with cleft hard palate
- Bilateral cleft lip with cleft hard and soft palate [29]
A cleft palate may be either complete or incomplete. It may also be unilateral or bilateral, and involve just the soft palate or include the hard palate as well.
There are many variations of a cleft lip. Cleft lips may occur unilaterally or bilaterally. A unilateral cleft lip presents with only one cleft, either complete or incomplete. As with a cleft palate, a cleft lip may also be incomplete or complete. The bilateral cleft lip may also be further divided into Binderoid bilateral complete cleft lip and palate, Bilateral complete cleft lip and intact secondary palate, Bilateral incomplete cleft lip and Asymmetrical bilateral (complete/incomplete) cleft lip [30]
Lesser forms of incomplete bilateral cleft lips can be divided into micro-form, microform, and mini-microform depending on the degree of the disruption at the vermilion-cutaneous junction [30]
Pathophysiology
The Development of upper lip and nose in embryos involves a series of genetically programmed procedures. This includes the fusion of five major facial prominences, that occur in gestation period between the third and eighth week. The palate develops within the fifth and twelfth week while lip develops between the third and seventh week. DRAWING!!! To be added soon.
The cranio-facial development pathway is a very complex process. Since the several points of development at which “Clefting” might occur is based on the condition and the wide range of its phonotypical expression.
Neural crest cells forming craniofacial structures
| Structure Generated | Neural Crest Cell Zone |
|---|---|
| Premaxilla and Vomer | The rostral aspect of the second rhombomere (r2)[31] |
| The inferior turbinate, palatine bone, alisphenoid, maxilla and zygoma | The caudal aspect of the neural crest of rhombomere (r2))[31] |
| The squamous temporal, mandible, malleus and incus | The third rhombomere (r3)[31] |
Non neural crest cells forming craniofacial structures
| Structure Generated | Non Neural Crest Cell Zone |
|---|---|
| Cranial Base | PAM from somitomere 1[32] |
| Parietal Bone | Epaxial PAM from somitomeres 2 and 3 [33] |
In the case of Cleft Lip or palate, there’s a converge of maxillary, medial nasal and lateral prominences via a combination of few processes that include apoptosis ”programmed cell death”, epithelial bridging and subepithelial-mesenchymal penetration[insert photo for the convergence]. Cleft lip or palate are found to be secondary to a defect of mesenchymal growth or the epithelial bridging. There has been evidence stating that intracellular signalling pathways and a wide range of genetic loci may play a potential role in elucidating this abnormality. These possible proposals could cause the fusion of median nasal and maxillary prominences to be disturbed. Consequently, the blood supply in bilateral cleft lip and/or palate, especially the arterial network and musculature of the lateral elements seems to be similar to the lateral segment of the unilateral deformity. The blood vessels and muscle fibers run along the margins of the piriform aperture and prolabial segment toward the and run columella , where they anastomose with nearby vessels(pre-maxillary vessels.
In the unilateral cleft lip: the deep fibres of the orbicularis oris muscle are disturbed by the presence of the cleft and insert on the side of the defect (nasal base) as compared to the normal infant, these muscles will make their way around the mouth. Furthermore, the superficial component of the orbicularis oris changes direction and head superiorly, parallel to the edges of the cleft and insert inferior to the columella. This can be seen when infants smile as the base of the nose would splay laterally.
In cleft palate: Clefts of the palate are seem to be associated with bony and soft-tissue abnormalities. Usually, fusion of both lateral palatal shelves as well as nasal septum in the anterior posterior direction from incisive foreman ( key landmark in the bony palate ) to the uvula is essential for the palate development to progress[ADD photo]. As mentioned previously, the cleft palate is often formed the palatal development is disturbed between the 5th and 12th week of gestation. The occurrence of Cleft palate is often associated with a split uvula. Some issues are associated with the gap between both the nasal and oral cavities. These includes problems in speaking ear infections/hearing loss, aesthetic problem, dental anomalies, psychosocial problems and hyper-nasal voice resonance due to the leakage of air from the nasal cavity
[34]
[35]
[36]
Genetic Configuration
The origin of Cleft lip/ palate genetic and environmental factors arose since 1940s. A number of studies have been composed to elucidate the ambiguity behind this embryonic abnormality. It is believed that these combined factors contribute to the increase of Cleft lip/ palate incidence. Some of the genes that have been identified to have a vital role in the development of CL/CP include;
1) Transforming growth factor alpha(TGFA)
2) Transforming growth factor beta 3 (TGFB3)
3) Msh homeobox 1 (MSX1)
4) Activating protein 2 (AP2)
On the basis of animal models, particularly mice, have shown a closer relation in terms of these genes involved in this abnormality. The mechanism by which these genes affect and disturb the development of face is not entirely understood. However, the y are known as contributors to process of prominences fusion. This might be caused via the modification of a wide range of transcription factors, growth hormone or even signalling pathways.
Despite the fact that genes seem to contribute more towards the disturbance of the facial development, environmental factors have been found play a significant role in increasing the risk of cleft palate/cleft lip. They can be categorised into four sections:
1) Womb environment and 2) External environment
The consumption of alcohol by mothers when they were pregnant seems to be related to the cause of Cleft palate and cleft lip incidence. It has been noted that the migration of neural crest cells and their differentiation were disturbed in embryos who were exposed to alcohol. The Gene MSX1 is more likely to be altered with the consumption of four drinks or more. As a result, the likelihood of cleft palate and/ or cleft palate development is higher.
In addition, embryos who were exposed to smoke, were more likely to develop cleft palate and/ or cleft lip. Although the mechanism by which smoking cause facial development deficiency is unknown, it has been assumed that hypoxia might play a significant role in affecting the foetus face development. One of the genes which is affected by smoking is TFGA. Another altered form of it is Activation protein 2 (AP2), that seem to have an increase of 8 folds for Cleft lip and/ or Cleft palate.
3)Nutrition
Maternal nutrition has been found as one of the contributing impacts towards a high incidence of clefting. Folic acid which ,belongs to the Vitamin B family, goes back to the origin when it was firstly discovered in 1961 as reason to change the normal embryonic facial development in rats. This resulted in high rates of clefting. The deficiency of folic acid seems to have a potential impact on TGFA, particularly (AP2). How the interaction between the facial genes with the lack of folic acid is not fully understood.
4)Drugs
A number of drugs such as thalidomide, retinoic acid, antiepileptic drugs that contains the “teratogen” seem to be participating in birth defect, especially the Cleft lip and/ or Cleft palate. The current literature centre of attention is concentrated around the mechanism by which these drugs interact with the genes involved in facial development.
Neuroembryology and functional anatomy of craniofacial clefts
Embryological anatomy is the foundation upon which all treatment methods for craniofacial anomalies such as cleft must be based. Craniofacial clefts characterize states of inappropriate(excess or deficiency)distribution within and between specific developmental fields. The neuromeric mapping of the embryo is the primary denominator for understanding normal anatomy and pathology of the head and neck. [37]
The central nervous system of the human embryo develops in discrete segmental craniocaudal units called neuromeres. Specific genes known as Hox genes define the anatomical boundaries of each neuromere. [38]
The neural crest in each neromeric level is responsible for supplying it's specified zones of ectoderm and mesoderm; corresponding proteins are also expressed by the neural crest cells that complement the proteins secreted by the neural tube. The unique genetic markers associated with each neuromere can assist to identify the origins of craniofacial tissues that are eventually developed.[39]
The neuromeric model facilitates a precise mapping of the anatomical site of origin for the zones of ectoderm and endoderm supplied by a particular area of the nervous system. The density of neural crest cell population in these areas also can help us understand their individual roles in generating the structures they do.[40]
The neural crest cells migrate and differentiate to develop specific craniofacial tissues. These zones are called development fields. The neural crest cells from each neuromere also instigate the formation of nerves and arteries which will eventually supply the individual fields. [41] These fields migrate from their origin in a strictly synchronised spatio-temporal sequence. They then cluster around the developing brain and self assemble which defines the interconnection between fields. The correct development of one field might be reliant on the prescence and positioning of another field. As a consequence any abnormality in an individual field may influence the development of an otherwise regular adjacent field. Craniofacial clefts feature an excess, deficiency or absence of an embryonic development field and the resultant impact on its contiguous fields. [42]
Different mechanisms which cause interruption in neural crest cell movement give rise to different classes of field abnormalities evident in the developing face.
- Errors in the neuromere of origin result in premigratory deficiencies.
- Neural crest population dying before reaching their destination result in migratory deficiencies
- Faulty communication among mesenchyme and epithelial “target zone,” or a faulty epithelial “programme” result in post migratory deficiencies.
Thus the ultimate common pathway to field anomalies is inadequate stimulation of vascular support for emergent tissues in the specified zone.
There are four dimensions of development that field anomalies effect in a developing embryo’s facial morphology. These are known as the four Ds of cleft progression and are responsible for a specific dimension of cleft formation as listed below: [43]
- deficiency is axial
- displacement is coronal
- distortion is temporal
- division is saggital
The pathological sequence follows the order of axis specification in the embryo. [44]
A deficiency exists in the functional matrix to begin with which gives rise to the piriform margin. This is then followed by the formation of an abnormal development field. This results into a pattern of displacement in the soft tissue envelope on either side of the cleft. This gives rise to a deficiency in the soft tissue closure of the nasal floor and lip. The effects of such deficiency cause an abnormal anatomy of the septum. Subsequent growth of the osteocartilagenous nasal vault, uncoupling of regular interaction between the skeletal structures, as well as abnormal forces exerted by the per oral musculature result in the characteristic opening-up of the cleft site. [45]
Treatment
Generally palatoplasty should be performed between 6-12 months of age however, there are many centres performing palatoplasty between 12-18 month and few who perform at least a part of the palatoplasty as late as 10-12 years.
There are three major objectives of a cleft palate operation:
- To produce anatomical closure of the defect.
- To create an apparatus for development and production of normal speech.
- To minimize the maxillary growth disturbances and dento-alveolar deformities
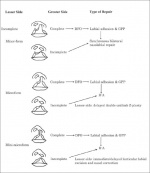
There are three general palatoplasty techniques:
1. Hard palate repair techniques
- Veau-Wardill-Kilner pushback (V-Y)
- von Langenbeck bipedicle flap (W)
- Bardach’s two-flap (V)
- Alveolar extension palatoplasty (AEP)
- Vomer flap Raw area free palatoplasty
2. Soft palate repair techniques
- Intravelar veloplasty
- Furlow double opposing Z-plasty
- Radical muscle dissection
- Primary pharyngeal flap
- Two stage palatal repair
3. Protocol based techniques
- Schweckendiek's
- Malek's
- Hole in one
There are many complications that can occur after the surgical procedure:
- Haemorrhage
- Respiratory obstruction
- Hanging Palate
- Dehiscence of the repair
- Oronasal fistula formation
- Bifid uvula
- Velopharyngeal Incompetence
- Abnormal speech
- Maxillary hypoplasia
- Dental malpositioning and malalignment
- Otitis media
Problems associated with Cleft Palate
Feeding Problems in Infants
- Inability to suck effectively while feeding on breast milk or bottled milk
- Milk entering the nasal cavity which may lead to choking or pulmonary aspiration
Teething Issues
- Missing teeth
- Increased number of cavities
- Malocclusion of teeth-This happens when teeth are bunched together or on top of each other.
Speech Issues
- Nasal voice
- May develop nodules on the vocal cord due to vocal abuse
- Delayed speech and language development
- Stuttering
- Cluttering
- Speed sound disorders
Ear Infections
- Most children with Cleft Palate are prone to middle ear infection
- Hearing Loss
- May be associated with repeated ear infection
- Psychological Problems
- To pay the price for looking and sounding different.
Social Problems
- Suffer isolation and alienation
- Depression related to social inacceptance
Current and Future Research
Maternal smoking and specific detoxification-gene variants are found to be increase risk of orofacial cleft
As we discussed earlier Van der Woude syndrome (VWS) - one of the most common cleft syndrome, is typically caused by the mutation of a dominant IRF6 gene. A recent study conducted in 2010 identified a mutation in 62.7% of the syndromic families tested and 3.3% of non syndromic patients.
Interestingly new insights into one of the non-syndromic families with an autosomal dominant inheritance (family B)the family history revealed the presence, at birth, of lower lip pits in two members and the diagnosis was revised as VWS one. A new sign in the lower lip was noticed in one subject within family B. Stimulating the intersts of the researchers a similar lower lip sign was also found in one individual from one other family(family A). The observation was the presence of two distinct nodules below the lower lip visible from the outside.
This triggered a reexamination of all other patients previously cleared as not having VMS syndrome.
It was concluded that IRF6 should be screened when:
any doubt rises about the normality of the lip and
when there is familial history of CL/Ps with a dominant inheritance
Glossary
Cleft Lip is the presence of one or two vertical fissures (clefts) in the upper lip
Cleft Palate refers to the congenital condition in which the palate at the roof of the mouth fails to fuse, resulting in direct communication between the nasal and oral cavities
Cluttering is a speech disorder similar to stuttering
Congenital present from birth
Lateral anatomical expression meaning of, at towards, or from the side or sides
Malformation abnormal or anomalous formation of structure
Palate the roof of the mouth, separating the cavities of the nose and the mouth in vertebrates
Posterior anatomical expression for towards the back in position or near the hind end of the body
Pulmonary Aspiration refers to the entry of material (such as food or drink, or stomach contents) from the oropharynx or gastrointestinal tract into the larynx (voice box) and lower respiratory tract
Speech sound disorders refer difficulty in producing particular speech sounds (most often certain consonants, such as /s/ or /r/)
Stuttering is a speech disorder where the speech is disrupted by involuntary repetitions and prolongations of sound
Dysplasia
Gallery
--Mark Hill 16:20, 20 September 2011 (EST) This is how to format a gallery of images.
- Cleft lip 3.jpg
Image shows Pre-Op Bilateral Cleft Lip
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 <pubmed>19884680</pubmed>
- ↑ Converse JM, Hogan VM, McCarthy JG. Cleft lip and palate. In: Converse JM, editor. Reconstructive Plastic Surgery. 2nd ed. Philadelphia: Saunders; 1977. p. 1930
- ↑ Keating JM. Cyclopaedia of the diseases of the children. Philadelphia: Lippincott; 1889.
- ↑ Blandin PF. Operation to remedy a division of the velum palati or cover of the palate. New York J Med. 1838;10:203
- ↑ Franco P. Traite des Hernies. Lyons: Thibauld Payan; 1561
- ↑ De Santo NG, Bisaccia C, De Santo LS, Cirillo M, Richet G. Pierre-Joseph Desault (1738-1795)--a forerunner of modern medical teaching. J Nephrol. 2003 Sep-Oct;16(5):742-53. PubMed PMID: 14733424
- ↑ Blandin PF. Operation to remedy a division of the velum palati or cover of the palate. New York J Med. 1838;10:203
- ↑ Faltin R. History of plastic surgery in Finland. Finsk Lak Sallsk Handl. 1937;80:97
- ↑ Randall P. Triangular flap operation for unilateral clefts of the lip. Plast Reconstr Surg. 1959;23:331
- ↑ Manchester WM. The repair of bilateral cleft lip and palate. Br J Surg. 1965 Nov;52(11):878-82. PubMed PMID: 5842977
- ↑ Mao C, Ma L, Li X. [Simultaneous primary palate repair and alveolar bone grafting in unoperated cleft palate patients over 8 years old]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2000 Oct;18(5):323-5. Chinese. PubMed PMID: 12539652
- ↑ Wong JY, Oh AK, Ohta E, Hunt AT, Rogers GF, Mulliken JB, Deutsch CK. Validity and reliability of craniofacial anthropometric measurement of 3D digital photogrammetric images. Cleft Palate Craniofac J. 2008 May;45(3):232-9. PubMed PMID: 18452351
- ↑ Mishra B, Singh AK, Zaidi J, Singh GK, Agrawal R, Kumar V. Presurgical nasoalveolar molding for correction of cleft lip nasal deformity: experience fromnorthern India. Eplasty. 2010 Jul 23;10. pii: e55. PubMed PMID: 20694165; PubMed Central PMCID: PMC2916669
- ↑ Gregg T, Bod D, Richardson A. The incidence of cleft lip and palate in Northern Ireland from 1980–1990. Br J Orthod 1994; 21: 387–392
- ↑ Coupland MA, Coupland AI. Seasonality, incidence and sex distribution of cleft lip and palate births in Trent region (1973–82). Cleft Palate J 1988; 25: 33–37
- ↑ 16.0 16.1 16.2 16.3 16.4 <pubmed>19109803</pubmed>
- ↑ Faure, J.-M., Bäumler, M., Boulot, P., Bigorre, M. and Captier, G. (2008), Prenatal assessment of the normal fetal soft palate by three-dimensional ultrasound examination: is there an objective technique?. Ultrasound in Obstetrics & Gynecology, 31: 652–656. doi: 10.1002/uog.5371
- ↑ Rotten D, Levaillant JM. Two- and three-dimensional sonographic assessment of the fetal face. 1. A systematic analysis of the normal face. Ultrasound Obstet Gynecol 2004; 23:224–231
- ↑ Faure JM, Captier G, B¨aumler M, Boulot P. Sonographic assessment of normal fetal palate using three-dimensional imaging: a new technique. Ultrasound Obstet Gynecol 2007; 29: 159–165
- ↑ Nyberg DA, Sickler GK, Hegge FN, Kramer DJ, Kropp RJ. Fetal cleft lip with or without cleft palate: ultrasound classification and correlation with outcome. Radiology 1995; 195: 677–684
- ↑ Noordhoff MS, Huang CS, Lo LJ. Median facial dysplasia in unilateral and bilateral cleft lip and palate: a subgroup of median cerebrofacial malformations. Plast Reconstr Surg. 1993;91:996–1005.[PubMed]
- ↑ Hercilo M, Marcelo R, Mario SO, Roseli TM. Clinical and Genetic Features of Van der Woude Syndromes in Two large families in Brazil. Cleft Palate Craniofac J. 2007;44:239–43. [PubMed]
- ↑ Kessmann J. Hirschsprung's Disease: Diagnosis and Management. Am Fam Phys. 2006;74:1319-1322. [PubMed: 17087425]
- ↑ Thomas JA, Graham JM., Jr Chromosomes 22q11 deletion syndrome: an update for the primary peditrician. Clin Pediatr (Phila) 1997;36:253–66. [PubMed]
- ↑ Thomas JA, Graham JM., Jr Chromosomes 22q11 deletion syndrome: an update for the primary peditrician. Clin Pediatr (Phila) 1997;36:253–66. [PubMed]
- ↑ <pubmed>16292776</pubmed>
- ↑ The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter Chapter 9 The Pharyngeal Apparatus pp201 - 240.
- ↑ The Developing Human: Clinically Oriented Embryology (8th Edition) by Keith L. Moore and T.V.N Persaud - Moore & Persaud Chapter Chapter 9 The Pharyngeal Apparatus pp201 - 240.
- ↑ M.J. Dixon, M. L. Marazita, T.H. Beaty , and J.C. Murray .Cleft lip and palate: synthesizing genetic and environmental influences. Nat Rev Genet. 2011 March; 12(3): 167–178
- ↑ 30.0 30.1 <pubmed>19884685</pubmed>
- ↑ 31.0 31.1 31.2 <pubmed>19109803</pubmed>
- ↑ Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: Genetic regulation of branchial arch skeletal pattern and development. J Anat. 2007;27:501–61.
- ↑ Nowicki JL, Burke AC. Testing Hox genes by surgical manipulation. Dev Biol. 1999. pp. 210–238
- ↑ <pubmed>2938624</pubmed>
- ↑ <pubmed>21331089</pubmed>
- ↑ <pubmed>3100859</pubmed>
- ↑ Indian J Plast Surg. 2009 October; 42(Suppl): S19–S34. doi: 10.4103/0970-0358.57184 PMCID: PMC2825068
- ↑ Graham A, Papalopulu N, Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–78[PubMed]
- ↑ Hunt P, Krumlauf R. Deciphering the Hox code: clues to patterning brachial regions of the head. Cell. 1991;66:1075–8. [PubMed]
- ↑ Indian J Plast Surg. 2009 October; 42(Suppl): S19–S34.doi: 10.4103/0970-0358.57184 PMCID: PMC2825068
- ↑ Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. [PubMed]
- ↑ Hall BK. The embryonic development of bone. Am Sci. 1988;76:174–81.
- ↑ Carstens MH. The sliding sulcus procedure: simultaneous repair of unilateral clefts of the lip and primary palate - a new technique. J Craniofac Surg. 1999;10:415–34. [PubMed]
- ↑ Precious DA, Delaire J. Surgical considerations in patients with cleft deformities. In: Bell WH, editor. Modern Practice in Orthognathic and Reconstructive Surgery. Philadelphia: WB Saunders; 1992. pp. 390–25.
- ↑ Delaire J. The potential role of facial muscles in monitoring maxillary growth and morphogenesis. In: DS Carlson, McNamara JA., Jr, editors. Muscle Adaptation and Craniofacial Growth. Craniofacial Growth Monograph 8. Ann Arbor, MI: Center for Human Growth and Development, University of Michigan; 1998. pp. 157–80
Textbooks
2011 Projects: Turner Syndrome | DiGeorge Syndrome | Klinefelter's Syndrome | Huntington's Disease | Fragile X Syndrome | Tetralogy of Fallot | Angelman Syndrome | Friedreich's Ataxia | Williams-Beuren Syndrome | Duchenne Muscular Dystrolphy | Cleft Palate and Lip